Abstract
Background
Osseointegrated implants for patients with transfemoral amputations (TFAs) are a novel treatment under development, and prospective long-term evidence is lacking. The objectives were to determine patient-reported outcomes (PROs) and complications after ten years compared to before treatment and to compare the first five-year period with the later five-year period with regard to the outcomes.
Methods
In a nonrandomized, prospective cohort study, patients with TFAs treated between 1999 and 2007 with the Osseointegrated Prosthesis for the Rehabilitation of Amputees (OPRA) system (n = 51) (28 men/23 women; mean age at amputation: 32 years old; mean age at treatment: 44 years old in a single university hospital were followed for ten years. PROs included the Questionnaire for Persons with a Transfemoral Amputation (Q-TFA, four scores 0–100) and the Short Form 36 Health Survey (SF-36, ten scores 0–100) and were answered before treatment and until the ten-year follow-up after treatment. Analyses of differences in PRO scores were conducted using Wilcoxon's signed rank test. The implant survival and revision-free rates with respect to adverse events (implant revision, mechanical complications, and deep infections) were presented as Kaplan–Meier graphs with 95% confidence intervals (CIs). The incidences of events per ten and five person-years were calculated. Spearman's correlation analysis was used for analyses of associations between adverse events.
Results
PROs showed statistically significant mean improvements between baseline and the ten-year follow-up with regard to all Q-TFA scores: the prosthetic use score (+36), prosthetic mobility score (+18), problem score (−28) and global score (+38) (all p < 0.001), and the SF-36 physical functioning score (+26, p < 0.001) and physical component score (+6, p < 0.01). No PROs showed a statistically significant deterioration. Over the ten years, 12 patients were lost (one lost to follow-up, one dropped out of the study, two died, and eight had implants removed (four before five years and four between five and ten years). At ten years, the revision-free survival rates were 83% (CI: 69%–91%), 65% (CI: 49%–77%) and 17% (CI: 7%–29%) for implant revision, deep infection and mechanical complications, respectively. Mechanical complications, 3.9 per 10 person-years (CI: 2.2–5.1) constituted the most common serious adverse event and were more common during the last five years than during the first five years (p < 0.001). No significant difference in the incidence of deep infections was observed between the earlier and the later five-year periods: 0.3 per 5 person-years (CI: 0.1–0.5) vs. 0.3 per person-years (CI: 0.1–0.5) (p = 0.740). Correlation analyses between the earlier and later five years revealed a positive association between deep infections and implant removal (0.57, p < 0.001) and between mechanical complications and adverse events (0.65, p < 0.001).
Conclusion
Improved PROs were demonstrated ten years after the introduction of a novel principle for bone anchorage of amputation prostheses. Nevertheless, an increasing rate of mechanical complications is of concern.
Keywords: Adverse effects, Bone-anchored prosthesis, Osseointegration, Patient-reported outcome measures, Percutaneous, Quality of life
Translational potential of this article
This study provides compelling evidence that patients with TFAs benefit long term from osseointegration. Nevertheless, knowledge gaps for ensuring long-term sustainability were identified, mainly related to mechanical complications and prosthetic activity, as well as mechanistic understanding of the role of implant properties in deep infections.
1. Introduction
Osseointegration, i.e., the integration of an implant into bone, has led to major advances in medical treatment. A prime example is bone-anchored teeth [1], which generally show long-term survival and functional restoration. In recent decades, significant efforts have been focused on the possibility of treating patients with amputated limbs by attaching a prosthesis to an osseointegrated implant [2].
Traditionally, individuals with transfemoral amputations (TFAs) are given socket-suspended prostheses (SPs), in which the socket is individually fit to the residual limb [3]. However, discomfort, pain and unreliable suspension frequently complicate the use of SPs, thereby affecting mobility and health-related quality of life (HRQoL) [4,5].
The Osseointegrated Prosthesis for the Rehabilitation of Amputees (OPRA) implant system (Integrum AB, Molndal, Sweden) was introduced in 1998 and consists of three main components: an intramedullary fixture, a percutaneous abutment and an abutment screw (Fig. 1A). Since 1999, the so-called OPRA study has followed 51 patients treated with 55 implants and reported prospective short-term (two years) [6] and mid-term (five years) [7] results. Other studies have reported on the benefits [[8], [9], [10], [11]] and complications [10,12,13] following OPRA treatment. Considered together, the findings have shown large and important improvements in everyday life, mobility and HRQoL, accompanied by Adverse events (AEs) in terms of infection and other implant complications. Today, other implant systems for missing limbs are in clinical use worldwide, and benefits alongside AEs have been reported for these systems [[14], [15], [16], [17], [18], [19], [20]]. Nevertheless, prospective outcomes beyond five years have not yet been reported from these treatment modalities.
Figure 1.
Schematic of the location of the intramedullary fixture, abutment and abutment screw in the residual femur (A). Radiograph showing the location of the implant components in the femur (B). Photograph showing the residual limb and the macroscopic appearance of the percutaneous abutment with abutment screw (C). Photograph of a patient with a prosthesis attached to the osseointegrated implant in the femur (reproduced with permission and copyright © of The British Editorial Society of Bone & Joint Surgery [10]) (D). Flowchart and timeline description of the study cohort (E).
The aim of this study was, first, to report the ten-year follow-up results from the OPRA study with regard to patient-reported outcomes (PROs) and complications. Second, given the opportunity to determine time-dependent changes in the results, the aim was also to compare the early period (first five years after treatment) with the late (last five years) period with respect to outcomes.
This study reports the ten-year results from the first-ever prospective study following individuals with TFAs being supplied with bone-anchored artificial limbs.
2. Methods
2.1. Study population
In this nonrandomized, prospective clinical study, patients treated with OPRA implants at a single university hospital in Sweden were followed for ten years after treatment. The study was in accordance with the European standard for clinical investigations of medical devices (EN-540). Fifty-one patients were included between 1999 and 2007 based on the following criteria: having a TFA with adequate residual skeletal conditions, being 20–70 years of age and suffering from obvious problems related to the use of an SP. Patients with TFA due to severe vascular disease, including diabetes mellitus, as well as patients prescribed drugs that could negatively affect the treatment (e.g., chemotherapy), were excluded. In brief, the treatment was performed in two surgical sessions six months apart, followed by a period of graded prosthetic rehabilitation (Fig. 1A–D). Details of the study design, implant details and treatment protocol were previously reported [6,7]. This manuscript was conducted in accordance with the STROBE guidelines.
The Regional Ethical Board approved the study (R402-98) to follow the patients for the first two years, and additional approval enabled long-term follow-up after two years (T216-03). All of the patients provided their written informed consent.
The study group consisted of 28 men and 23 women (mean age: 44 years old [SD: 12]) [6]. Reasons for amputation included trauma (n = 33), tumour (n = 12), infection (n = 4) and arterial embolus (n = 2), and treatment was performed a mean of 12 (SD 11) years after amputation. Among the 51 patients, 45 had a unilateral TFA, and six had bilateral TFAs. Four of the six patients with bilateral TFAs were treated bilaterally, yielding a total of 51 patients with 55 limbs treated.
Amputation-specific and general HRQoL were captured by two questionnaires answered before treatment initiation (baseline) and at defined time points over the follow-up. In the current study, we report PROs at five- and ten-year follow-ups compared to preoperative baseline values.
2.2. Clinical complications and definitions
The clinical data were prospectively collected during the first two years and thereafter retrospectively collected from medical records by an unbiased surgeon on the osseointegration team. AEs and severe adverse events (SAEs) were captured from medical records. AEs included infections, soft tissue sequelae, mechanical complications of outer components (i.e., abutment or abutment screw) and fixture loosening. When AEs required patient hospitalization and initiation of treatment, they were categorized as SAEs. The definitions of superficial infection, deep infection, mechanical complications, soft tissue revision, fixture removal, AEs and SAEs are provided in Table 1.
Table 1.
Definitions of superficial infection, deep infection, mechanical complications, fixture removal, adverse events and serious adverse events as captured from patients’ medical records.
| Event | Definition |
|---|---|
| Superficial infection (SI) | Clinical signs that involve redness, increased secretion from the skin penetration area and/or pain; with or without a positive result of swabbing; and An antibiotic treatment is prescribed; and A 3-months antibiotic-free period before and after any SI event is required to be counted as a separate SI event. |
| Deep infection (DI) | Clinical signs that involve pain while loading the implant system, pain while not using the prosthesis/during the night and/or oedema and increased secretion; with or without fever or X-ray verification; and The positive result of a deep culture from the bone marrow canal through the central screw in the operation room in at least three samples with identical pathogens or positive result of the soft tissue culture from a fistula duct or bone tissue in the operation room; and An antibiotic treatment is prescribed. |
| Mechanical complication (MC) | Fracture or bending of any of the outer components such as abutment screw or abutment, or any signs of wear (e.g., black deposits, insufficient press-fit), leading to a change of a component (using a permanent or a temporary abutment, the so-called dummy). |
| Fixture removal (FR) | The extraction of the fixture due to fracture or aseptic or septic loosening. |
| Adverse event (AE) | Any undesirable clinical occurrence in a subject on the limb treated with the OPRA Implant System
|
| Serious adverse event (SAE) | Any AE necessitating patient hospitalization and initiation of treatment due to:
|
2.3. Patient-reported outcome measures
The PROs were collected prospectively over the entire ten-year period. Two validated, reliable questionnaires: the Questionnaire for Persons with a Transfemoral Amputation (Q-TFA) [21] and the Short Form 36 Health Survey (SF-36) [22], were used to assess efficacy outcomes. The Q-TFA captures current aspects of prosthetic use, mobility and problems in patients with TFAs. The results are presented in four main scores: the prosthetic use score (0–100), prosthetic mobility score (0–100), problem score (100–0, reversed score) and global score (0–100). If the patient reports not wearing a prosthesis for at least one day per week, the prosthetic use score will be zero, and the other three scores cannot be reported. A prosthetic use score of 100 means that the prosthesis is normally used seven days per week for >15 h/day. In addition, the Q-TFA includes a single question regarding the overall situation as an amputee (scale of five degrees, ranging from “extremely poor” to “extremely good”) that can be reported regardless of prosthetic use. Finally, to capture problems specifically related to the bone-anchored prosthesis, which was not included in the original version of the Q-TFA, two questions were added at follow-up assessments. These non-validated questions asked about the degree of problems perceived at the skin-penetration area and the degree of worry regarding complications with the prosthetic anchorage (i.e., osseointegration) [7].
General HRQoL was captured by the SF-36 [22], providing results on eight subscales (0–100) and two component scores (physical component score and mental component score (0–100, for which a score of 50 (SD 10) represents general population norms) [23].
The primary efficacy variable was the Q-TFA prosthetic use score, and the second efficacy variables were all of the other scores from the Q-TFA and SF-36. For all scores, except for the Q-TFA Problem score, a higher figure represents a better outcome.
2.4. Statistical analysis
The descriptive statistics for the patients' demographic data are presented as the means, standard deviations, medians and ranges for continuous variables, whereas the nominal variables are reported as numbers and percentages. The demographic data included the intention-to-treat (ITT) population at inclusion (baseline) as well as the per-protocol (PP) population, providing PROs at the five- and ten-year follow-ups. The descriptive PROs (Q-TFA and SF-36 scores) are presented as the means, standard deviations, 95% confidence intervals (CIs), medians, ranges and numbers. For the statistical evaluations of the changes from baseline to each follow-up, as well as between the follow-ups, Q-TFA results are presented in boxplots, and the analysis was conducted using Wilcoxon's signed rank test. The implant survival and revision-free rates with respect to implant removal, mechanical complications, deep infections and SAEs are presented as Kaplan–Meier graphs, with CIs. Bar graphs are used to illustrate the incidences of events per ten and five person-years, with 95% CIs of the mean number of events. Statistical comparisons of the events were performed between the early (baseline-five years) and late (five years-ten years) periods using Wilcoxon's signed rank test. Finally, Spearman's correlation analysis was conducted to evaluate possible associations between results. Missing data were excluded pairwise in the comparison and correlation analyses. All statistical significance tests were two sided and were performed at the 5% significance level. The statistical analyses were conducted with the SPSS software package (IBM SPSS Inc., New York, NY, USA), version 25.0. All graphs were created in GraphPad Prism software, version 9.0 (GraphPad Software, La Jolla, CA, USA).
3. Results
3.1. Study participants
During the entire period from baseline to ten years, one patient with one implant was lost to follow-up, one patient with one implant dropped out of the study, and two patients, each with one implant died. Eight patients had eight implants removed. Four of these patients had the implants removed before five years due to aseptic loosening (n = 3) or deep infection (n = 1), whereas four patients had the implants removed between five and ten years due to fixture fracture (n = 4). The number of patients at each follow-up, as well as the time points for implant removal and missing PROs, are provided in a flowchart (Fig. 1E). The demographics of the ITT baseline population (n = 51) and the PP population who completed PROs after five years (n = 40) and ten years (n = 37) are shown in Table 2.
Table 2.
Demographic description. The descriptive data show the demographics of the intention-to-treat patient (ITT) population at inclusion (baseline), as well as the per-protocol (PP) patients, providing patient-reported outcomes at the five- and ten-year follow-ups.
| Continuous variables Mean [STDEV; Median] (Range) |
Baseline (ITT) (N = 51a) |
5 years (PP) (N = 40a) |
10 years (PP) (N = 37a) |
|
|---|---|---|---|---|
| Age at amputation (years) | 32 [14; 31] (12–64) | 32 [15; 31] (12–64) | 32 [14; 31] (12–60) | |
| Age at inclusion (years) | 44 [12; 46] (20–65) | 44 [13; 46] (20–65) | 44 [12; 46] (20–62) | |
| Time from amputation to S1 (years) | 12 [11; 7] (1–42) | 12 [11; 7] (1–42) | 12 [11; 8] (1–42) | |
| Weight at inclusion (kg) | 74 [17; 75] (40–115) | 74 [17; 75] (45–115) | 74 [17; 75] (45–115) | |
| Height at inclusion (cm) | 172 [10; 174] (154–194) | 173 [11; 173] (155–194) | 173 [10; 174] (155–194) | |
| BMI at inclusion |
25 [4; 24] (16–38) |
25 [5; 24] (16–38) |
25 [5; 24] (16–38) |
|
|
Nominal variables n (%) |
Baseline |
5 years |
10 years |
|
| Sex | Female | 23 (45%) | 19 (47.5%) | 18 (49%) |
| Male | 28 (55%) | 21 (52.5%) | 19 (51%) | |
| Reason for amputation | Trauma | 33 (64.7%) | 26 (65%) | 25 (68%) |
| Tumour | 12 (23.5%) | 9 (22.5%) | 9 (24%) | |
| Infection | 4 (7.8%) | 3 (7.5%) | 1 (2.7%) | |
| Arterial embolus | 2 (3.9%) | 2 (5%) | 2 (5.4%) | |
| Unilateral/bilateral TFAb | Unilateral | 45 (88%) | 35 (87.5%) | 32 (86%) |
| Bilateral | 6 (12%) | 5 (12.5%) | 5 (14%) | |
| Smoker at inclusion | Smoker | 11 (22%) | 8 (20%) | 6 (16%) |
| Nonsmoker | 40 (78%) | 32 (80%) | 31 (84%) | |
| Socket-prosthesis user at inclusionc | User | 42 (82%) | 34 (85%) | 31 (84%) |
| Nonuser | 9 (18%) | 6 (15%) | 6 (16%) | |
| Nationality | Swedish | 25 (49%) | 20 (50%) | 18 (49%) |
| Other nationality | 26 (51%) | 20 (50%) | 19 (51%) | |
N refers to the number of individuals who have completed PROs.
TFA = Transfemoral amputation
A prosthetic user was defined as one normally wearing a prosthesis at least one day per week.
3.2. Patient-reported outcomes
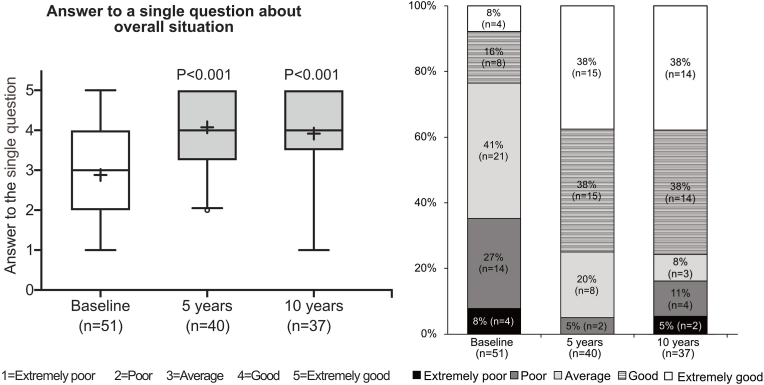
The PROs showed statistically significant improvements in all four Q-TFA scores between baseline and the five-year and ten-year follow-ups (all p < 0.001) (Fig. 2). The differences for each Q-TFA score at between five and ten years were not statistically significant (all p > 0.05). The single question on the overall situation improved in the same way between baseline and each follow-up (all p < 0.001). At the ten-year follow-up, 76% stated their overall situation to be good or extremely good compared to 24% at baseline (Fig. 3). All details of the Q-TFA results are presented in Supplementary Table 1. The results from the two osseointegration-specific questions at the five- and ten-year follow-ups showed that 28% and 20% of the patients, respectively, reported moderate to considerable trouble with regard to problems from the skin-penetration area, and 18% and 17%, respectively, reported moderate to considerable trouble regarding worries about the prosthetic anchorage (Supplementary Fig. S1).
Figure 2.
Questionnaire for Persons with Transfemoral Amputation (Q-TFA). The boxplots show the four Q-TFA scores at baseline and at five and ten years of follow-up (A, C, E, G) and the changes in the Q-TFA scores between baseline and five and ten years of follow-up (B, D, F, H). (A and B) Prosthetic Use score (0–100) (C and D) Prosthetic mobility score (0–100) (E and F) Problem score (100–0) (G and H) Global score (0–100). If the prosthetic use score is 0, the prosthetic mobility score, the problem score and the global score cannot be determined given a lower number of these three scores. The boxplots show the median (line), mean (plus), first and third quartiles (box), 5th and 95th percentile (whiskers) and the minimum and maximum data values. For comparisons over time (changes), Wilcoxon's signed rank test was used.
Figure 3.
Q-TFA single question on the overall situation as an amputee, rated as extremely poor, poor, average, good or extremely good and answered at baseline and at the five- and ten-year follow-ups, regardless of prosthetic use.
The SF-36 physical functioning score and physical component score were significantly improved between baseline and the five- and ten-year follow-ups (all p < 0.01). The role physical score was improved at the five-year follow-up (p = 0.02). No other differences between baseline and follow-ups were statistically significant. When changes in SF-36 results between follow-up points were analysed, a deterioration was noted for the general health score at between 5 and 10 years (p = 0.02). All of the SF-36 results are detailed in Supplementary Table S2.
3.3. Clinical complications
After ten years, the revision-free survival of the implant was 83% (CI: 69%-91%) (Fig. 4A), and the percentage of revision-free cases with respect to changes in the abutment and/or the abutment screw was 17% (CI: 7%-29%) (Fig. 4B). The rate of revision-free deep infections was 65% (CI: 49%-77%) (Fig. 4C). The overall SAEs demonstrated a revision-free rate of 11% (CI: 4%-22%) (Fig. 4D).
Figure 4.
Kaplan–Meier survival and revision-free rates. The graphs show the survival of the implant over time (A), the revision-free rates over time with respect to mechanical complications (B) and deep infections (C) and the overall serious adverse events (D).
Mechanical complications constituted the most common AE, with an incidence of (mean) 3.94 per 10 person-years (95% CI: 2.19-5.11) (Fig. 5A). The incidence rates for superficial and deep infections were 1.88 per 10 person-years (CI: 1.27-2.21) and 0.59 per 10 person-years (CI: 0.21-0.87), respectively. Overall, the AEs and SAEs were 6.69 per 10 person-years (CI: 4.32-8.08) and 4.57 per 10 person-years (CI: 2.67-5.80), respectively (Fig. 5A).
Figure 5.
Clinical complications. A. Bar graphs show the incidences of clinical adverse events per 10 person-years. B. Bar graphs show the incidences of adverse events per 5 person-years. Statistical comparisons in (B) were performed on the number of events of each category between the early (baseline-5 years) and late (5–10 years) periods. For comparisons, Wilcoxon's signed rank test was used. The error lines show the 95% CIs of the mean number of adverse events in each category. SI = superficial infections; DI = deep infections; MC = mechanical complications; STR = soft tissue revision; FR = fixture removal; AE = adverse events; SAE = serious adverse events.
Differences in the incidence of events between the earlier and later five-year periods are shown in Fig. 5B. A significant reduction in superficial infections was shown between the earlier and later five-year periods, with means of 1.43 per 5 person-years (CI: 0.98-1.66) and 0.53 per 5 person-years (CI: 0.24-0.73) (p < 0.001), respectively. No significant difference in the incidence of deep infections was observed between the earlier and later periods: 0.31 per 5 person-years (CI: 0.12-0.46) vs. 0.33 per 5 person-years (CI: 0.05-0.54) (p = 0.74). In contrast, a significant increase in mechanical complications was demonstrated between the earlier (0–5) and later (5–10) periods: 1.37 per 5 person-years (CI: 0.54-2.00) vs. 3.05 per 5 person-years (CI: 1.81-3.75) (p = 0.001). Overall, the results revealed that the AEs did not increase significantly between the time periods, that is, 3.33 per 5 person-years (CI: 2.13-4.04) and 4.00 per 5 person-years (CI: 2.44-4.87) (p = 0.61), whereas the incidence of SAEs increased significantly between the first (1.76 per 5 person-years; CI: 0.87-2.39) and the second five-year periods (3.3 per 5 person-years; CI: 1.99-4.05) (p = 0.004).
In total, 16 patients were diagnosed with deep infections during the ten-year period. Twelve patients received a diagnosis of deep infection and subsequent antibiotic treatment on one to four occasions (total number of deep infection events = 18) from baseline to five years (Supplementary Table S3). Eight patients had deep infections and received antibiotic treatment on one to four occasions (total number of deep infection events = 15) at five years to ten years of follow-up (Supplementary Table S4). One patient required removal of the implant due to deep infection with a methicillin-resistant Staphylococcus aureus strain during the early period. Altogether, four implants (four patients) were removed due to fixture fractures during the later five-year period.
3.4. Correlation analyses
Correlation analyses showed that events regarding superficial infections, deep infections, and mechanical complications, pooled over the 10 years, all shared a common significant association with the Q-TFA prosthetic mobility score (Supplementary Table S5). For mechanical complications, a positive correlation with prosthetic mobility was found for both follow-up periods (five and ten years). Furthermore, deep infections and mechanical complications revealed positive associations with patients' worries regarding prosthetic anchorage at 10 years. No correlations were found between soft tissue revision or fixture removal events and any PROs (Supplementary Table S5).
When the events were correlated with each other, several associations were found over the ten years (Supplementary Table S6). A positive association was shown between superficial and deep infections (Rho = 0.5; p < 0.001), between superficial infections and soft tissue revisions (Rho = 0.35; p = 0.01), and between deep infections and mechanical complications (Rho = 0.35; p = 0.009). In addition, superficial infections and mechanical complications in the early five-year period correlated positively with the same type of events in the late period (Supplementary Table S6). Furthermore, a strong, positive association was demonstrated between deep infection in the early period and fixture removal in the late period (Rho = 0.57; p < 0.001) (Supplementary Table S7), whereas a modest, significant correlation was found between early mechanical complications and late fixture removal (Rho = 0.36; p = 0.01) (Supplementary Table S7).
Patient-related variables (gender, age at amputation, time between amputation and OPRA treatment, age at inclusion, weight, height, BMI or smoking at inclusion) did not reveal any significant associations with either PROs or complications. Having bilateral TFAs showed negative associations with Q-TFA prosthetic mobility at five years (Rho = −0.55 p < 0.001) and number of AEs over the full ten-year period (Rho = −0.3, p = 0.01).
4. Discussion
To the authors’ knowledge, this study is the first ten-year follow-up study of a defined cohort of TFA patients treated with a bone-anchored prosthetic system to report both PROs and complications. The principal finding in this ten-year follow-up study of 51 patients with TFAs treated with an OPRA implant for direct bone anchorage of the artificial limb is the stable improvement in the patient-reported benefits with this treatment. The primary efficacy variable, the prosthetic use score, demonstrates that the group of patients followed for ten years still wore a prosthesis to a considerably greater degree than in their preoperative situation. However, a substantial number of mechanical implant complications, especially during the later five-year period, accompanied PRO improvements.
The available literature has shown major benefits from having a bone-anchored TFA prosthesis compared to an SP, regardless of the treatment protocol. Several publications with short-term follow-ups (one year - three years) have revealed that treated patients report more prosthetic use, better mobility and an improved overall situation compared to before treatment [[24], [25], [26], [27]].
Nevertheless, the duration of improved PRO in these studies must be regarded as short term. To the authors’ knowledge, four studies have reported five-year HRQoL results for bone-anchored TFA prostheses [7,17,28,29]. Matthew et al. (2018) [28] and Rojas et al. (2021) [29] reported similar results in OPRA-treated patients in the UK (n = 13) and Chile (n = 21), respectively, as in the Swedish OPRA study, i.e., robust and statistically significant improvements in all four Q-TFA scores and in part of the SF-36 scores after five years. Similarly, Reetz et al. (2020) [17] reported on 39 patients treated with the Integral Leg Prosthesis (ILP; Orthodynamics) in the Netherlands and showed improvements in the Q-TFA prosthetic use and global scores, while the other Q-TFA scores and SF-36 were not part of their protocol. In the present ten-year OPRA follow-up, proof of long-term PRO improvements added to this base of knowledge. Moreover, the SF-36 general health score results (mean 74) after ten years were on par with results previously reported in a cross-sectional study of patients with unilateral TFA for reasons other than severe vascular disease [4]. The small but statistically significantly lower score noted for the SF-36 general health score during the later five-year period should, however, not be ignored and requires further attention. Regrettably, long-term prospective studies of the development of general HRQoL in patients with lower limb amputation are currently not available. Hence, the causes of the decreased general health score between five and ten years are difficult to discern.
When interpreting the outcomes, it is important to consider that the group of treated TFA patients generally had additional difficulties with their SPs; thus, the improvements in PROs and mobility tests must be viewed in light of a worse-than-normal preoperative situation that possibly yielded low baseline values. This point was recently supported by Pospiech et al. (2021), who compared a group of high-mobility TFA bone-anchored prosthesis users (treated with the German ESKA Endo-Exo-system) (n = 22) to a matched SP control group (n = 17) [30]. Favourable results for the treated patients were found in the Q-TFA problem and global scores, but the prosthetic use and mobility scores did not differ between the groups [30]. This outcome emphasizes the importance of careful patient selection, especially in light of the risk for complications accompanying surgical interventions with implants.
The present study demonstrated 83% implant survival after ten years, which is less favourable than the rate observed in the same cohort after five years (92%) [7] and on par with the survival rates reported in Chile (81% five-year) [29] and the UK (80% 12-year) [28]. In our study, four implants were removed at between five and ten years due to fatigue and fracture originating from incorporated tantalum balls in the fixture for the purpose of roentgen stereophotogrammetry analysis (RSA) [31].
Although not part of the OPRA study, we can conclude that seven of the eight patients with fixture removal were treated a second time at our hospital (three of four patients with removal before five years and all four patients with removal at between five and ten years). Currently, five of these seven patients have an implant, while two do not. Future studies focused on the outcomes in this small group of patients undergoing repeated treatments will be of interest.
Amputees, particularly those with TFAs, have reduced bone mineral density in the residual limb compared with the intact side [32]. Furthermore, since TFA patients with removed implants have reduced periprosthetic bone mineral density after 30 months compared with baseline [33], the success of these implants is founded on achieving and maintaining a high degree of osseointegration despite compromised local conditions. Support for this assumption has been found in morphological studies of retrieved osseointegrated TFA implants after eleven years, demonstrating 81% direct bone-to-implant contact and a similarly large proportion (87%) of remodelled bone filling the threaded part [34]. Furthermore, RSA suggests at least short-to mid-term stable biomechanical conditions [31]. Considered together, the available data indicate that long-term stable osseointegration of the fixture is achievable. A pertinent downside of the osseointegrated state is that high prosthetic mobility and load impact primarily affect the distal and percutaneous components, resulting in abrasion, wear, or bent or broken outer components, often necessitating a change in these parts [10]. The present observations indicate that improvements of the junction between the fixture and the abutment are needed.
Superficial skin infection, normally treated with a short, oral antibiotic regimen, has been described as the most common AE from baseline to five years [7]. In contrast, the present results showed that mechanical complications of the abutment and/or the abutment screw constituted the most common event from baseline until the ten-year follow-up. An increase in mechanical complications was previously observed after five years [7], with the largest numbers found after eight years in a retrospective study of a large cohort of patients with unilateral TFA (n = 111) [10]. The present finding of an increase in SAEs (i.e., mechanical complications) during the last five years compared with the first five-year period is of major concern. The large number of abutment exchanges, requiring the implementation of personalized measures prior to and during interventions in a sterile surgical environment, imposes considerable strain and has necessitated revised patient instructions for the reduction of excessive prosthetic mobility. The interventions for exchanging the percutaneous implant components appear tolerable by patients, as judged by the current PRO results. Nevertheless, the correlative statistics revealed associations between greater prosthetic mobility and a larger number of mechanical complications of percutaneous implant components, especially during the later five years, challenging the long-term sustainability of the implant system. Surgical interventions due to mechanical complications have also been reported for so-called press-fit TFA implant systems [[17], [18], [19], [20],27]. However, the results are difficult to compare to the current study due to shorter follow-up periods or changes in implant versions or treatment regimens over time. Future studies of these treatment modalities will be of great interest, especially for learning whether any implant system has better long-term sustainability combined with high prosthetic mobility among treated patients.
Importantly, in the current study, the incidence of deep infections was stable throughout the ten-year period, whereas a reduction in superficial infections was shown between the earlier and later five-year periods. Nevertheless, a strong, positive association was demonstrated between deep infection in the early period and fixture removal in the late period. Furthermore, although neither proven nor disproven mechanistically, the identified associations between deep infection and mechanical complications, between superficial and deep infections and between deep infection in the early period with fixture removal in the late period point towards unresolved scientific and clinical challenges posed by these bone-anchored implants. This circumstance also connects to the overall risk for reduced long-term sustainability and increased costs [35].
The breach of the soft tissue structural barrier for percutaneous implants and the colonization of multiple potential pathogenic microorganisms detected on and around the percutaneous abutment of the implant system [36] imply that the risk of inward migration and spread of bacteria cannot be disregarded. The strains causing deep infections in these patients (mainly S. aureus, Staphylococcus epidermidis and Enterococcus faecalis) demonstrate biofilm-producing properties [37]. Moreover, S. aureus and S. epidermidis associated with bone-anchored amputation prostheses removed due to osteomyelitis have the ability to modulate the immune system by virtue of secreted extracellular vesicles [38]. Although the diagnostic criteria for infection await consensus, prosthetic use in five of seven patients with deep infections was reported by Tillander and coworkers not to be affected [13]. Considered together, the risks for chronic, low-virulence biofilm-associated infection, delayed infection diagnosis and challenges to eradicating biomaterial-related infections with antibiotics are critical issues that require further research.
Limitations of this study exist. Since any SAEs were solely managed at our hospital, the authors are confident that no fixture removal or changes in any implant part were handled elsewhere and thus missing from our data. However, the number of superficial infections should be interpreted with caution since this AE did not need to be diagnosed or treated at our hospital, and information might be lacking in the records. Other limitations in the OPRA study design include the lack of a comparable control group with SPs, the relatively small number of patients included, the mixture of patients having both unilateral and bilateral TFAs and the absence of systematically registered prosthetic device details (i.e., type of prosthetic knee and foot components). Finally, this ten-year follow-up did not include details about other complications commonly reported among individuals living for decades with a lower-limb amputation, such as low back pain, phantom limb pain, falls, and arthrosis in the lower extremity [39,40].
5. Conclusion
At the ten-year follow-up, patients with transfemoral amputation treated with an osseointegrated implant system reported improved physical HRQoL, more hours of prosthesis use, better mobility, fewer problems and an improved situation overall compared to their situation before treatment. The findings of lowered implant survival, increased numbers of mechanical complications of the percutaneous modular parts, and associations between mechanical complications and prosthetic mobility and other complications, e.g., deep infection, suggest that the implant system and treatment still require improvements, further research and follow-up periods beyond ten years.
Ethics statement
The patients provided written informed consent to participate in the study, which was approved by the Regional Ethical Board (R402–98 and T216-03).
Data availability statement
Data are available upon reasonable request.
Funding
This study was funded by noncommercial grants from the Swedish state under an agreement between the Swedish government and the county councils; the ALF agreement ALFGBG-725641, ALFGBG-766480); the Swedish Research Council (2018–02891), the Eivind o Elsa K:son Sylvan Foundation; the Johan Jansson Foundation; the IngaBritt and Arne Lundberg Foundation; the Hjalmar Svensson Foundation; the Adlerbertska Foundation; and the Area of Advance Materials of Chalmers and GU Biomaterials within the Strategic Research Area Initiative, launched by the Swedish government.
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Authorship contribution
Conception and design of study: KH, SAG, OO, PT; Acquisition of data: KH, SAG, OO, PT, Analysis and/or interpretation of data: KH, SAG, OO, PT, Drafting the manuscript: KH, PT, Revising the manuscript critically for important intellectual content: KH, SAG, OO, PT. Approval of the version of the manuscript to be published: KH, SAG, OO, PT.
Declaration of competing interest
Peter Thomsen reports patent fees received in 2010 from Integrum AB, not related to this study. Kerstin Hagberg, Shadi Afarin Ghasemi Jahani and Omar Omar declare no conflicts of interest.
Acknowledgements
The authors would like to thank Statistiska Konsultgruppen, Gothenburg, Sweden, for valuable advice related to statistical analyses.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2022.09.004.
Contributor Information
Kerstin Hagberg, Email: kerstin.hagberg@vgregion.se.
Shadi Afarin Ghasemi Jahani, Email: Ghasemi.shadi@gmail.com.
Omar Omar, Email: Omar.omar@biomaterials.gu.se.
Peter Thomsen, Email: Peter.thomsen@biomaterials.gu.se.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Branemark P.I., Hansson B.O., Adell R., Breine U., Lindstrom J., Hallen O., et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl. 1977;16:1–132. [eng] [PubMed] [Google Scholar]
- 2.Li Y., Fellander-Tsai L. The bone anchored prostheses for amputees - historical development, current status, and future aspects. Biomaterials. 2021;273 doi: 10.1016/j.biomaterials.2021.120836. [DOI] [PubMed] [Google Scholar]
- 3.Gholizadeh H., Abu Osman N.A., Eshraghi A., Ali S. Transfemoral prosthesis suspension systems: a systematic review of the literature. Am J Phys Med Rehabil. 2014;93(9):809–823. doi: 10.1097/PHM.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 4.Hagberg K., Branemark R. Consequences of non-vascular trans-femoral amputation: a survey of quality of life, prosthetic use and problems. Prosthet Orthot Int. 2001;25(3):186–194. doi: 10.1080/03093640108726601. [DOI] [PubMed] [Google Scholar]
- 5.Gholizadeh H., Abu Osman N.A., Eshraghi A., Ali S., Yahyavi E.S. Satisfaction and problems experienced with transfemoral suspension systems: a comparison between common suction socket and seal-in liner. Arch Phys Med Rehabil. 2013;94(8):1584–1589. doi: 10.1016/j.apmr.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Branemark R., Berlin O., Hagberg K., Bergh P., Gunterberg B., Rydevik B. A novel osseointegrated percutaneous prosthetic system for the treatment of patients with transfemoral amputation: a prospective study of 51 patients. The bone & joint journal. 2014;96-B(1):106–113. doi: 10.1302/0301-620X.96B1.31905. [DOI] [PubMed] [Google Scholar]
- 7.Branemark R.P., Hagberg K., Kulbacka-Ortiz K., Berlin O., Rydevik B. Osseointegrated percutaneous prosthetic system for the treatment of patients with transfemoral amputation: a prospective five-year follow-up of patient-reported outcomes and complications. J Am Acad Orthop Surg. 2019;27(16):e743–e751. doi: 10.5435/JAAOS-D-17-00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagberg K., Haggstrom E., Uden M., Branemark R. Socket versus bone-anchored trans-femoral prostheses: hip range of motion and sitting comfort. Prosthet Orthot Int. 2005;29(2):153–163. doi: 10.1080/03093640500238014. [DOI] [PubMed] [Google Scholar]
- 9.Hagberg K., Hansson E., Branemark R. Outcome of percutaneous osseointegrated prostheses for patients with unilateral transfemoral amputation at two-year follow-up. Arch Phys Med Rehabil. 2014;95(11):2120–2127. doi: 10.1016/j.apmr.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Hagberg K., Ghassemi Jahani S.A., Kulbacka-Ortiz K., Thomsen P., Malchau H., Reinholdt C. A 15-year follow-up of transfemoral amputees with bone-anchored transcutaneous prostheses. The bone & joint journal. 2020;102-B(1):55–63. doi: 10.1302/0301-620X.102B1.BJJ-2019-0611.R1. [English] [DOI] [PubMed] [Google Scholar]
- 11.Lundberg M., Hagberg K., Bullington J. My prosthesis as a part of me: a qualitative analysis of living with an osseointegrated prosthetic limb. Prosthet Orthot Int. 2011;35(2):207–214. doi: 10.1177/0309364611409795. [eng] [DOI] [PubMed] [Google Scholar]
- 12.Tillander J., Hagberg K., Berlin O., Hagberg L., Branemark R. Osteomyelitis risk in patients with transfemoral amputations treated with osseointegration prostheses. Clin Orthop Relat Res. 2017;475(12):3100–3108. doi: 10.1007/s11999-017-5507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tillander J., Hagberg K., Hagberg L., Branemark R. Osseointegrated titanium implants for limb prostheses attachments: infectious complications. Clin Orthop Relat Res. 2010;468(10):2781–2788. doi: 10.1007/s11999-010-1370-0. [Eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thesleff A., Branemark R., Hakansson B., Ortiz-Catalan M. Biomechanical characterisation of bone-anchored implant systems for amputation limb prostheses: a systematic review. Ann Biomed Eng. 2018;46(3):377–391. doi: 10.1007/s10439-017-1976-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hebert J.S., Rehani M., Stiegelmar R. Osseointegration for lower-limb amputation: a systematic review of clinical outcomes. JBJS Rev. 2017;5(10):e10. doi: 10.2106/JBJS.RVW.17.00037. [DOI] [PubMed] [Google Scholar]
- 16.Gerzina C., Potter E., Haleem A.M., Dabash S. The future of the amputees with osseointegration: a systematic review of literature. J Clin Orthop Trauma. 2020;11(Suppl 1):S142–S148. doi: 10.1016/j.jcot.2019.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reetz D., Atallah R., Mohamed J., van de Meent H., Frolke J.P.M., Leijendekkers R. Safety and performance of bone-anchored prostheses in persons with a transfemoral amputation: a 5-year follow-up study. J Bone Joint Surg Am. 2020;102(15):1329–1335. doi: 10.2106/JBJS.19.01169. [DOI] [PubMed] [Google Scholar]
- 18.Al Muderis M., Khemka A., Lord S.J., Van de Meent H., Frolke J.P. Safety of osseointegrated implants for transfemoral amputees: a two-center prospective cohort study. J Bone Joint Surg Am. 2016;98(11):900–909. doi: 10.2106/JBJS.15.00808. [DOI] [PubMed] [Google Scholar]
- 19.Atallah R., van de Meent H., Verhamme L., Frolke J.P., Leijendekkers R.A. Safety, prosthesis wearing time and health-related quality of life of lower extremity bone-anchored prostheses using a press-fit titanium osseointegration implant: a prospective one-year follow-up cohort study. PLoS One. 2020;15(3) doi: 10.1371/journal.pone.0230027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juhnke D.L., Beck J.P., Jeyapalina S., Aschoff H.H. Fifteen years of experience with Integral-Leg-Prosthesis: cohort study of artificial limb attachment system. J Rehabil Res Dev. 2015;52(4):407–420. doi: 10.1682/JRRD.2014.11.0280. [DOI] [PubMed] [Google Scholar]
- 21.Hagberg K., Branemark R., Hagg O. Questionnaire for Persons with a Transfemoral Amputation (Q-TFA): initial validity and reliability of a new outcome measure. J Rehabil Res Dev. 2004;41(5):695–706. [eng] [PubMed] [Google Scholar]
- 22.Ware J.E., Jr., Sherbourne C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 23.Ware J.E., Jr., Kosinski M., Bayliss M.S., McHorney C.A., Rogers W.H., Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33(4 Suppl):AS264–A279. [PubMed] [Google Scholar]
- 24.Al Muderis M., Lu W., Li J.J. Osseointegrated prosthetic limb for the treatment of lower limb amputations: experience and outcomes. Unfallchirurg. 2017;120(4):306–311. doi: 10.1007/s00113-016-0296-8. [DOI] [PubMed] [Google Scholar]
- 25.Van de Meent H., Hopman M.T., Frolke J.P. Walking ability and quality of life in subjects with transfemoral amputation: a comparison of osseointegration with socket prostheses. Arch Phys Med Rehabil. 2013;94(11):2174–2178. doi: 10.1016/j.apmr.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 26.Leijendekkers R.A., van Hinte G., Frolke J.P., van de Meent H., Atsma F., Nijhuis-van der Sanden M.W., et al. Functional performance and safety of bone-anchored prostheses in persons with a transfemoral or transtibial amputation: a prospective one-year follow-up cohort study. Clin Rehabil. 2019;33(3):450–464. doi: 10.1177/0269215518815215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orgel M., Ranker A., Harb A., Krettek C., Aschoff H.H. [Transcutaneous osseointegrated prosthetic systems after major amputation of the lower extremity : a retrospective 3-year analysis] Orthopä. 2021;50(1):4–13. doi: 10.1007/s00132-020-04031-2. [DOI] [PubMed] [Google Scholar]
- 28.Matthews D.J., Arastu M., Uden M., Sullivan J.P., Bolsakova K., Robinson K., et al. UK trial of the osseointegrated prosthesis for the rehabilitation for amputees: 1995-2018. Prosthet Orthot Int. 2019;43(1):112–122. doi: 10.1177/0309364618791616. [DOI] [PubMed] [Google Scholar]
- 29.Rojas C., Laso J., Valiente D., Olivieri R., Gaggero N. Outcome of percutaneous osseointegrated prostheses for patients with transfemoral amputation at 5-year follow-up. Acta Scientific Orthopaedics. 2021;4(5):44–50. [Google Scholar]
- 30.Pospiech P.T., Wendlandt R., Aschoff H.H., Ziegert S., Schulz A.P. Quality of life of persons with transfemoral amputation: comparison of socket prostheses and osseointegrated prostheses. Prosthet Orthot Int. 2021;45(1):20–25. doi: 10.1177/0309364620948649. [DOI] [PubMed] [Google Scholar]
- 31.Nebergall A., Bragdon C., Antonellis A., Karrholm J., Branemark R., Malchau H. Stable fixation of an osseointegated implant system for above-the-knee amputees: titel RSA and radiographic evaluation of migration and bone remodeling in 55 cases. Acta Orthop. 2012;83(2):121–128. doi: 10.3109/17453674.2012.678799. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherk V.D., Bemben M.G., Bemben D.A. BMD and bone geometry in transtibial and transfemoral amputees. J Bone Miner Res. 2008;23(9):1449–1457. doi: 10.1359/jbmr.080402. [DOI] [PubMed] [Google Scholar]
- 33.Hansen R.L., Langdahl B.L., Jorgensen P.H., Petersen K.K., Soballe K., Stilling M. Changes in periprosthetic bone mineral density and bone turnover markers after osseointegrated implant surgery: a cohort study of 20 transfemoral amputees with 30-month follow-up. Prosthet Orthot Int. 2019;43(5):508–518. doi: 10.1177/0309364619866599. [DOI] [PubMed] [Google Scholar]
- 34.Palmquist A., Windahl S.H., Norlindh B., Branemark R., Thomsen P. Retrieved bone-anchored percutaneous amputation prosthesis showing maintained osseointegration after 11 years-a case report. Acta Orthop. 2014;85(4):442–445. doi: 10.3109/17453674.2014.919559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansson E., Hagberg K., Cawson M., Brodtkorb T.H. Patients with unilateral transfemoral amputation treated with a percutaneous osseointegrated prosthesis: a cost-effectiveness analysis. The bone & joint journal. 2018;100-B(4):527–534. doi: 10.1302/0301-620X.100B4.BJJ-2017-0968.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lenneras M., Tsikandylakis G., Trobos M., Omar O., Vazirisani F., Palmquist A., et al. The clinical, radiological, microbiological, and molecular profile of the skin-penetration site of transfemoral amputees treated with bone-anchored prostheses. J Biomed Mater Res. 2017;105(2):578–589. doi: 10.1002/jbm.a.35935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaborowska M., Tillander J., Branemark R., Hagberg L., Thomsen P., Trobos M. Biofilm formation and antimicrobial susceptibility of staphylococci and enterococci from osteomyelitis associated with percutaneous orthopaedic implants. J Biomed Mater Res B Appl Biomater. 2017;105(8):2630–2640. doi: 10.1002/jbm.b.33803. [DOI] [PubMed] [Google Scholar]
- 38.Zaborowska M., Vazirisani F., Shah F.A., Firdaus R., Omar O., Ekstrom K., et al. Immunomodulatory effects exerted by extracellular vesicles from Staphylococcus epidermidis and Staphylococcus aureus isolated from bone-anchored prostheses. Biomaterials. 2021;278 doi: 10.1016/j.biomaterials.2021.121158. [DOI] [PubMed] [Google Scholar]
- 39.Kim J., Major M.J., Hafner B., Sawers A. Frequency and circumstances of falls reported by ambulatory unilateral lower limb prosthesis users: a secondary analysis. Pharm Manag PM R. 2019;11(4):344–353. doi: 10.1016/j.pmrj.2018.08.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oosterhoff M., Geertzen J.H.B., Dijkstra P.U. More than half of persons with lower limb amputation suffer from chronic back pain or residual limb pain: a systematic review with meta-analysis. Disabil Rehabil. 2020:1–21. doi: 10.1080/09638288.2020.1783377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.