Abstract
Plant-derived vesicles (PDVs) are membranous structures that originate from plant cells and are responsible for multiple physiological and pathological functions. In the last decade, PDVs have gained much attention for their involvement in different biological processes, including intercellular communication and defense response, and recent scientific evidence has opened a new avenue for their applications in cancer treatment. Nevertheless, much remains unknown about these vesicles, and current research remains inconsistent. This review aims to provide a comprehensive introduction to PDVs, from their biological characteristics to purification methods, and to summarize the status of their potential development for cancer therapy.
Keywords: Plant-derived vesicles, Extracellular vesicles, Cancer therapy, Anti-cancer, Drug delivery
Graphical abstract
Highlights
-
•
Extracellular vesicles are involved in many physiological processes in plants.
-
•
Plant-derived vesicles exhibit promising anticancer activity in vitro and in vivo.
-
•
Plant-derived vesicles emerge as a drug delivery system for cancer therapy.
1. Introduction
Extracellular vesicles (EVs) are defined as “particles naturally released from the cell that are delimited by a lipid bilayer and cannot replicate, i.e., do not contain a functional nucleus” by the International Society of Extracellular Vesicles (ISEV) [1]. These EVs are released by all types of cells, including those of plants, animals, and microorganisms [2]. Emerging evidence has revealed the significant role of extracellular vesicles in many processes, ranging from toxic disposal to intercellular communication and defense against pathogens. Among the various types of vesicles, plant extracellular vesicles are anticipated to be less risky as most of which are extracted from edible and medicinal plants. They are now gaining attention not only for their biological roles but also for their simple accessibility, cost-effectiveness, and scalability in the context of therapeutic application.
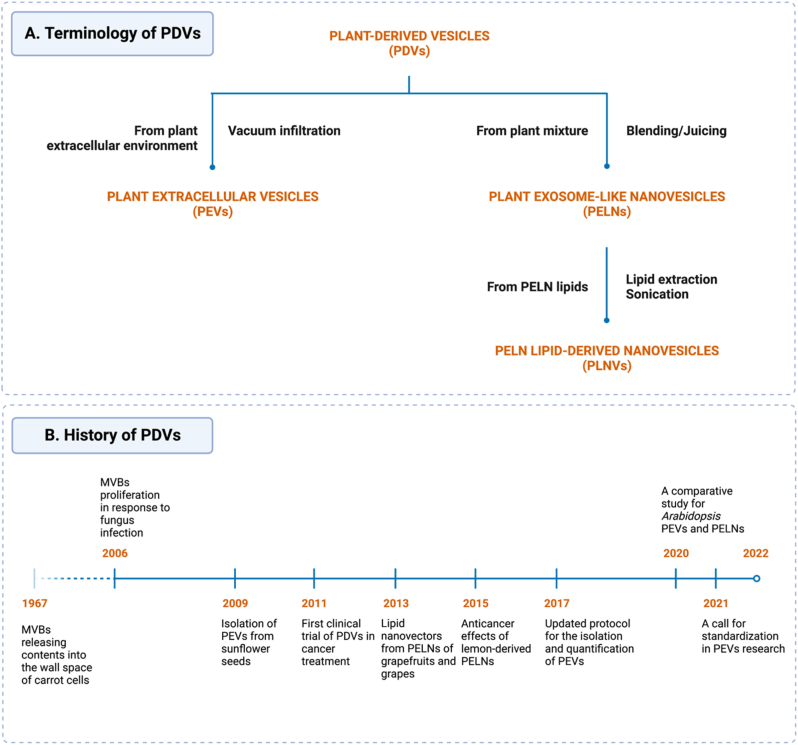
Plant extracellular vesicles (PEVs) were first recorded using transmission electron microscopy (TEM) in the 1960s [3,4]. Despite these early observations, follow-up studies could not demonstrate their importance, and they were consequently overshadowed by the discovery of mammalian extracellular vesicles (e.g., mammalian exosomes). Up until the early 2000s, when evidence for plant ultrastructure secretion upon pathogen infection emerged [5,6], many researchers succeeded in extracting PEVs from the apoplastic fluid of different plants, such as Arabidopsis thaliana and sunflower [7,8]. Since then, efforts have been made to elucidate the biological characteristics and functions of PEVs. In 2013, Zhang's team successfully synthesized vesicles from grapefruits and grapes using a scalable method without prioritizing their “extracellular” origin [9,10]. Following this, plant-derived vesicle (PDV) research began to branch into distinct paths and clusters (Fig. 1). The protocol followed by Zhang et al. produced intact vesicles with similar morphology and composition to exosomes; however, without the presence of specific markers, clear biogenesis, and characterization, these vesicles should only be referred to as plant-derived exosome-like nanovesicles (PELNs). Furthermore, Zhang's group innovated drug delivery nanoparticles using lipids extracted from PELNs (referred to hereafter as PLNVs as to distinguish them from whole PELNs). Owing to its high reproducibility and promising results, research surrounding PELNs has attracted considerable attention (Fig. 1B).
Fig. 1.
The terminology of PDVs used in this review and a brief history of PDV research. (A) All nano-sized vesicles derived from plants are called plant-derived vesicles (PDVs). Based on how the original materials are handled, these vesicles can be categorized into two groups: plant extracellular vesicles (PEVs) and plant exosome-like nanovesicles (PELNs). PEVs are directly extracted from the extracellular fluid of plants, while PELNs are produced by simply blending or juicing plant materials (Details in section 3.1). PLNVs are nanovesicles containing only lipid components of PELNs in this review. (B) MVBs: multivesicular bodies. References from left to right: [4,5,7,[9], [10], [11], [12], [13], [14], [15]].
At present, PEV and PELN research are evolving simultaneously. PEV research has been focused on exposing their nature, including biogenesis, identification, and characterization, and thus strictly separates vesicles from the extracellular environment (leaf apoplast, root exudates, etc.). On the other hand, PELN studies have investigated the risk-benefit ratio for therapeutic applications while optimizing their production and scalability. PELNs are primarily extracted from fruit juice or plant-blended mixtures, which may also lead to the recovery of not only EVs but also other intracellular components. Due to this cluttered situation, nomenclature relating to plant-derived vesicle research is recommended to be used with great caution and transparency. As no consensus on PDV-related terminology has been built yet, we use different terms for better comprehension as shown in Fig. 1A. Briefly, in this review, we discuss plant-derived vesicles (PDVs), which encompasses PEVs, PELNs, and PLNVs.
In recent years, pioneering studies have highlighted the potential roles PDVs may play in intercellular signaling, defense responses, and cross-kingdom communication. Other appealing innate features, such as its anti-inflammatory, antioxidant, and regenerative properties, have also been gradually revealed [[16], [17], [18], [19]]. Based on these fundamentals, scientists have endeavored to utilize PDVs for the treatment of many medical conditions, including skin diseases, inflammatory bowel diseases, and cancer. Despite enormous dedication from oncology researchers, cancer therapy continues to face many challenges, such as therapeutic efficiency, target specificity, and adverse effects. The discovery of extracellular vesicles has shifted the paradigm of the fight against cancer and has allowed the development of a new approach to overcome these obstacles. Over the past decade, PDVs have emerged as natural-based tools with ideal intrinsic values and high flexibility for advanced engineering applications. In particular, PDVs can be novel candidates for cancer treatment owing to their advantages such as large-scale production, minimal cytotoxicity and reduced immunogenicity and efficient cellular uptake [10,18,19]. Therefore, a timely review of relevant research progress is of great significance for the continuous development of PDVs for cancer therapy. This review is to patch up the tapestry of PDV research - a newly arisen field full of scattered pieces of information and hurdles. From the fundamental background, this review could raise the possibility of PDV applications that could be beneficial for cancer therapy in the long term. Herein, we attempt to comprehensively review the current status of PDV research, including its main characteristics and evidence for cancer therapeutic applications. Finally, we highlight the current limitations and future directions of this research area.
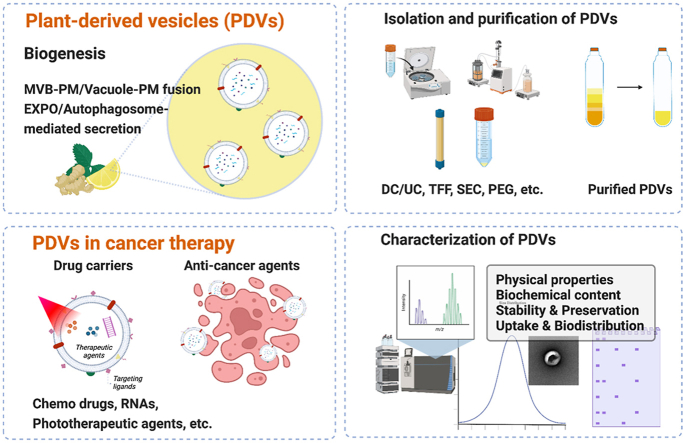
2. Biogenesis of PDVs
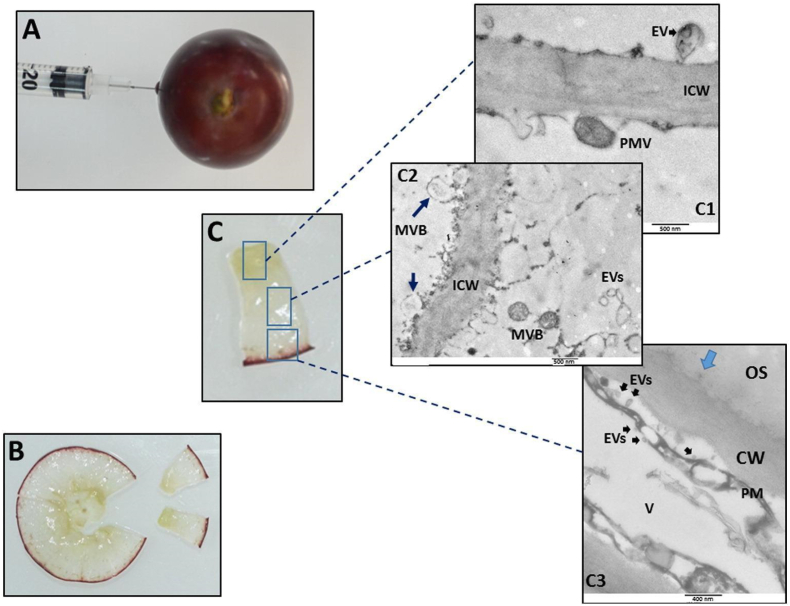
Multivesicular bodies (MVBs) were first observed in the 1960s using TEM [3,4]. Since then, many studies on various topics, such as unconventional protein secretion (UPS) and plant-pathogen interactions, have contributed to confirming the existence of PEVs as well as their mechanisms of biogenesis [[5], [6], [7],20,21]. The most common pathway proposed for the origin of PEVs is multivesicular–plasma membrane (MVB–PM) fusion, in which MVBs fuse into the PM to release their intraluminal vesicles into the extracellular matrix. In addition, evidence also exists for other pathways, such as EXPO-mediated secretion, autophagosome-mediated secretion, and vacuole-PM fusion. Exocyst-positive organelles (EXPOs) and autophagosomes are spherical double-membrane organelles. Despite their similar morphology, EXPOs do not colocalize with autophagosomes, nor are they affected by nutrient starvation (as autophagosomes are). Therefore, these two organelles are considered distinct. Vacuoles are single-membrane organelles that play an important role in plant defense owing to their hydrolytic enzyme content [22]. Fig. 2 shows TEM images of grape berry cuts, confirming the presence of MVBs and EVs around the plasma membrane, in which some were detected fusing to the intercellular wall (C1, C2), implying their delivery function [23].
Fig. 2.
EVs are produced by different cells in grape berries. Whole grape berries were fixed in situ to preserve fine structures by injecting the fixative (A). After fixation, equatorial slices of grape berries were obtained (B); and three different cuts were processed for TEM (C): from the most internal part (C1) to an intermediate cut (C2), and the most external cut (C3). TEM magnification is shown accordingly to size bars. OS: outermost space; EV: extracellular vesicle; ICW: intercellular wall; PMW: paramural bodies; MVB: multivesicular bodies; V: vacuole. Adapted with permission [23]. Copyright 2017, Elsevier.
Most of the evidence for PEV secretion pathways is based on plant infection responses [5,21,[23], [24], [25]]. When a plant cell is infected with a pathogen, MVBs, vacuoles, and EXPOs fuse with the plasma membrane to release internal vesicles into the extracellular environment, and these vesicles burst and secrete defense agents to inhibit pathogen proliferation. Autophagosomes first fuse with lytic compartments such as vacuoles, which later combine with MVBs and then with PMs to release vesicles.
Although the cell wall of plant cells may prevent the formation and functions of PDVs, there is much evidence that plants can produce PDVs intending to mediate a wide range of cellular and physiological functions. A few studies demonstrated the possibility of the PDV secretion passing over the cell wall: PDVs contain cell wall-remodeling proteins that contribute to a transient destabilization of cell wall structure, and reversible stretching of plant cell wall determined by its natural plasticity or local breaks may help PDVs to facilitate their passage through the cell wall.
3. Isolation and purification of PDV
3.1. Sample processing
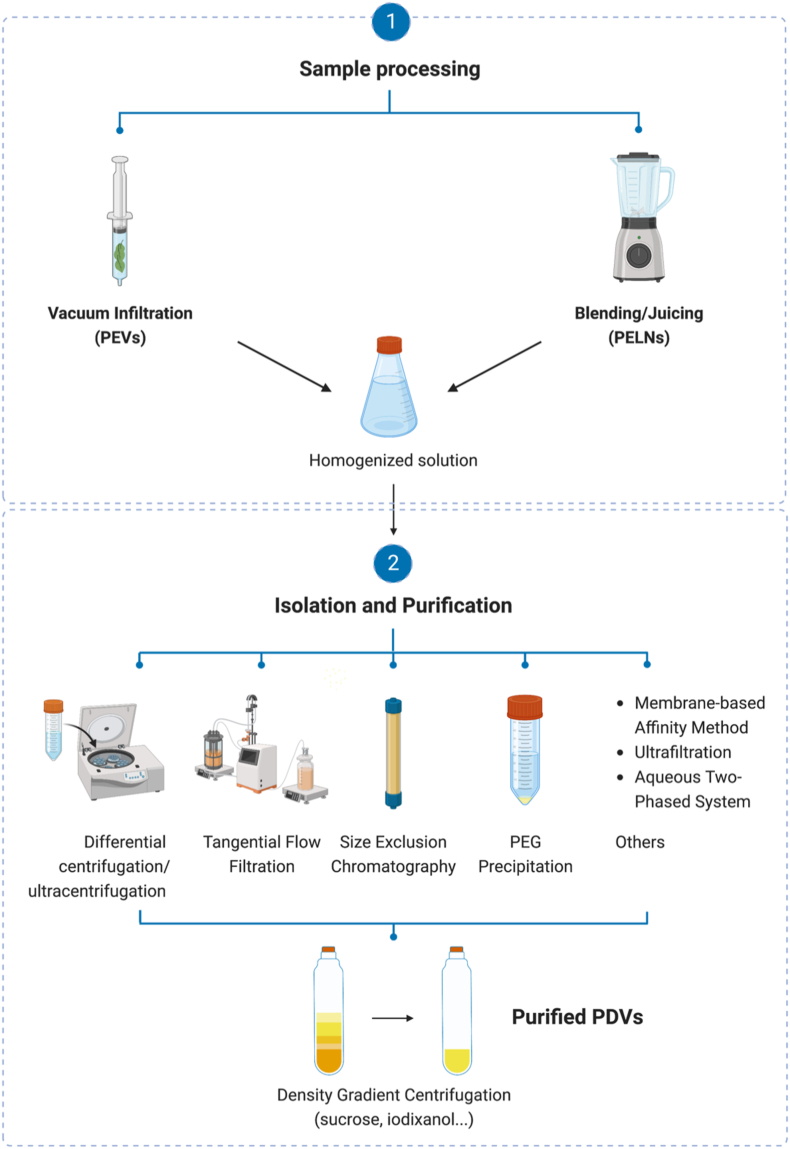
PEVs are extracted directly from the extracellular environment, such as from apoplastic fluids (e.g., Arabidopsis thaliana and sunflower) or root exudate (e.g., tomato), in which healthy plant samples are immersed in an infiltration buffer and subjected to vacuum pulses to yield sample solutions [7,13,26]. On the other hand, in the case of PELNs, the samples are first washed and handled physically by squeezing, pressing, and blending in buffer solutions. As PDVs are expected to share some common metabolites with their original plants, it is suggested that the sample processing step might affect vesicle content and activity, and therefore should be carefully screened according to each type of plant. For example, Uckoo et al. recommended that blending is a better option for obtaining higher levels of phytochemicals from grapefruits than juicing or hand-squeezing [27]. Meanwhile, Wang et al. concluded that between blending, high-speed centrifugal juicing, and low-speed juicing, the latter two conserved the strongest phytochemical profiles and antioxidant activities of 19 vegetables including kale, turnip, radish, beetroot, and carrot [28].
3.2. Isolation and purification
After the sample processing step, PDVs are isolated using a wide range of techniques. In this study, we collected information from more than 50 studies on PDV production. Fig. 3 and Table 1 present an overview of PDV separation techniques and physical characterizations. Differential centrifugation/ultracentrifugation is the most common method for PDV isolation, owing to its flexible adjustability and cost-effectiveness. Generally, low-speed centrifugation (<20 000×g) is applied to remove dead cells, cell debris, and large particles; and high-speed centrifugation and/or ultracentrifugation (>100 000×g) are used to precipitate nano-sized vesicles. One concern with this method is that it requires several hours to complete. The extended duration of repeated centrifugation may lead to vesicle deformation and increase the amount of other non-vesicle contaminants. After successive rounds of centrifugation, density gradient centrifugation with sucrose or iodixanol media is frequently used to refine vesicles. From a technical perspective, to optimize PDV production, many studies have added a filtration step using 0.22–0.45 μm pore size filters in between steps. However, this microfiltration step could unintentionally lead to manipulating the size range of PDVs, therefore it should be considered conscientiously in the case of studies that aim at defining intrinsic biological characteristics of PDVs.
Fig. 3.
The common protocol for PDV production using several methods.
Table 1.
Preparation and physical characterization of plant-derived vesicles.
| Types | Sources | Isolation | Purification | Morphology check | Size (nm) | Ref |
|---|---|---|---|---|---|---|
| PEV | Arabidopsis and Brassica | VI-C | Sucrose DGC | TEM, Cryo-EM, NTA | 60–200 | [14] |
| Arabidopsis thaliana | VI-C | Iodixanol DGC | TEM, Cryo-EM, DLS | 165 | [8] | |
| Nicotiana tabacum L. | VI-C | Agarose gel electrophoresis | Cryo-EM, DLS | [37] | ||
| Vinca minor L. | ||||||
| Viscum album L. | ||||||
| Sunflower seeds | VI-C | DC | TEM | 50–200 | [7] | |
| Sunflower seeds | VI-C | DC | TEM | 30–150 | [25] | |
| Tomato | DC | SEM, MRPS, DLS | 50–300 (MRPS) | [26] | ||
| 40–100 (DLS) | ||||||
| PELN | Acerola cherry | Filtration-exoEasy Maxi Kit | DC | TEM, NTA | 245 ± 132 | [30] |
| Aloe vera | DC-Filtration | TFF | TEM, NTA | 50–200 | [17] | |
| Aloe vera | DC | TEM, AFM, NTA, DLS | 138.7 (gel) | [34] | ||
| 220 (rind) | ||||||
| Apple | DC | TEM, TRPS | 170 | [38] | ||
| Apple | DC-Filtration | UC | TEM, TRPS | 90–180 | [39] | |
| Arabidopsis and Brassica | DC | Sucrose DGC | TEM, Cryo-EM, NTA | 100–300 | [14] | |
| Asparagus cochinchinensis | DC | TEM, DLS | 119 | [40] | ||
| Bitter melon | DC-ELD | TEM, NTA | 100–200 | [41] | ||
| Bitter melon | DC | Sucrose DGC-UC-Filtration | TEM, NTA | 120 | [42] | |
| Bitter melon | DC | Sucrose DGC-Filtration | TEM, NTA | 106 | [43] | |
| Blueberry | DC-UF | Filtration-UC | SEM, DLS, LTS | 114 ± 36 (SEM) | [44] | |
| 198 ± 112 (DLS) | ||||||
| Blueberry | DC-Filtration-PEG-precipitation-Centrifugation | TEM, DLS | 150–250 | [45] | ||
| Blueberry, coconut, ginger, grapefruit, Hami melon, kiwifruit, orange, pea, pear, soybean, and tomato | DC | Filtration-UC | AFM, NTA, DLS | 100–1000 | [46] | |
| Broccoli | DC | Sucrose DGC | EM | 18.3–118.2 | [47] | |
| Cabbage | PEG-precipitation | SEC | TEM | 100 | [29] | |
| DC | ||||||
| UF | ||||||
| Carrot | UF | SEC | TEM, NTA, DLS | 143.9 | [48] | |
| Coconut | DC | Filtration | SEM, DLS | 13.16 (SEM) | [49] | |
| 59.72 (DLS) | ||||||
| Corn | DC-Filtration | Sucrose DGC | TEM, DLS | 80 | [18] | |
| Dendropanax morbifera | DC-Filtration | TEM, NTA, DLS | 90 | [50] | ||
| Fingerroot | DC | SEC | TEM, NTA | 100 | [51] | |
| Garlic | ATPS | SEM, NTA, DLS | 50–150 | [52] | ||
| Ginger | DC | Sucrose DGC | AFM, DLS | 102.3–998.3 | [53] | |
| Ginger | DC | Sucrose DGC | TEM, AFM, DLS | 292.5 (band 1) | [16] | |
| 231.6 (band 2) | ||||||
| Ginger | DC | Iodixanol DGC | NTA, DLS | 110.5 ± 27.49 | [54] | |
| Ginger | DC | Sucrose DGC | TEM, DLS | 146 ± 1.8 | [55] | |
| Ginger | DC | Dialysis | DLS | 400 | [56] | |
| DC-PEG-precipitation | ||||||
| Ginseng | DC | Sucrose DGC | TEM, DLS | 344.8 | [57] | |
| Ginseng | DC-Sucrose cushion UC | Iodixanol DGC | TEM, Cryo-EM, DLS | 92.04 ± 4.85 | [58] | |
| Ginseng | DC-Sucrose cushion UC | Sucrose DGC | TEM, DLS | 105.8 ± 47.85 | [59] | |
| Grape | DC | Sucrose DGC | Cryo-EM, DLS | 380.5 ± 37.47 | [9] | |
| Grape | DC-Filtration | UC | TEM | 30–200 | [23] | |
| Grape, grapefruit, ginger, carrot | DC | Sucrose DGC | TEM, DLS | 100–1000 | [60] | |
| Grapefruit | DC | Sucrose DGC | TEM, DLS | 253.7 (PELN) | [10] | |
| Grapefruit | DC | Sucrose DGC | TEM, DLS | 105.7–396.1 | [61] | |
| Grapefruit | DC | Sucrose DGC | SEM, DLS | 102.4 | [62] | |
| Grapefruit | APTS | Filtration | AFM, NTA | 82–239 | [63] | |
| Grapefruit | DC | Sucrose DGC | TEM, DLS | 135 ± 5 | [64] | |
| Lemon | DC | TEM, NTA | <200 | [31] | ||
| DC-ELD | ||||||
| Lemon | DC | Sucrose DGC | TEM, DLS | 151.63 ± 5.20 | [65] | |
| Lemon | DC | Sucrose DGC | TEM, DLS | 50–70 | [12] | |
| Mulberry | DC | Sucrose DGC | TEM, NTA | 151.3 ± 45.4 | [66] | |
| Nicotiana tabacum L. | DC | Agarose gel electrophoresis | TEM, DLS | 70 ± 20/520 ± 170 | [37] | |
| Vinca minor L. | 380 ± 200 | |||||
| Viscum album L. | 280 ± 115 | |||||
| Onion | DC | Filtration | DLS | 288.1 (low speed) | [67] | |
| 185.3 (high speed) | ||||||
| Petasites japonicus | DC-Filtration | UC-Filtration | TEM, DLS | 122.6 | [68] | |
| Pomegranate | APTS | NTA, ESEM, DLS | 91–234 | [32] | ||
| Shiitake mushroom | DC | Filtration | SEM, NTA | 100–140 | [69] | |
| Strawberry | DC-Filtration | TEM | 30–191 | [70] | ||
| Tartary buckwheat | DC | Filtration | TEM, NTA | 30–200 | [71] | |
| Tea flowers | DC | Sucrose DGC | TEM, DLS | 131.6 | [72] | |
| Tea leaves | DC | Sucrose DGC | TEM, AFM, DLS | 134.0–145.6 | [73] | |
| Thai black ginger | DC | Sucrose DGC-Filtration | TEM, DLS | 200–300 | [74] | |
| Tomato | DC | Sucrose DGC SEC | TEM, NTA, DLS | 110 | [75] | |
| Turmeric | DC | Sucrose DGC | TEM, AFM, DLS | 177.9 | [19] | |
| Wheat | DC | Filtration | SEM, DLS | 40–100 | [76] | |
| PLNV | Ginger | DC | Sucrose DGC | TEM, AFM, DLS | 188.5 | [77] |
| Ginger | DC | Sucrose DGC | TEM, DLS | 204–284 | [78] | |
| Grapefruit | DC | Sucrose DGC | TEM, DLS | 186.8 | [10] |
VI-C: vacuum infiltration-centrifugation; DC: differential centrifugation; DGC: density gradient centrifugation; UC: ultracentrifugation; ELD: electrophoresis and dialysis; UF: ultrafiltration; APTS: aqueous two-phase system; SEC: size exclusion chromatography; TEM: transmission electron microscopy; SEM: scanning electron microscopy; Cryo-EM: cryogenic electron microscopy; DLS: dynamic light scattering; NTA: nanoparticle tracking analysis; AFM: atomic force microscopy; TRPS: tunable resistive pulse sensing; ESEM: environmental scanning electron microscope; MRPS: microfluidic resistive pulse sensing; LTS: laser electron microscopy.
In addition to the above putative standard strategy, some research groups have attempted other innovations for PDV separation, such as ultrafiltration, size exclusion chromatography (SEC), aqueous two-phase system (ATPS), polyethylene glycol (PEG) precipitation, sucrose single/double cushion ultracentrifugation, electrophoretic techniques, and membrane-based affinity methods. You et al. compared cabbage-derived vesicles isolated using three methods: PEG-precipitation, ultracentrifugation, and ultrafiltration-SEC [29]. They reported that the vesicle yields of the three were not significantly different, while vesicles from the ultrafiltration-SEC method had the highest purity level. Umezu et al. successfully isolated acerola-derived nanovesicles using an exoEasy midi kit (membrane-based affinity method) with high quality, uniform size, and quick recovery time compared to vesicles using only ultracentrifugation or ExoQuick reagent [30]. The electrophoretic technique combined with a dialysis step (ELD) separated PELNs from lemon juice with intact morphology and a suitable size for therapeutic applications. This stands out as a time-saving method that does not require special equipment [31]. The combination of ultracentrifugation and tangential flow filtration (TFF) also showed great potential for the successful refinement of pure vesicles from Aloe vera [17]. ATPS-based isolation has been reported to purify PELNs from different impurities (contaminant proteins, fatty acids, phenol red, etc.), which may affect quantification experiments (e.g., BCA assay) [32].
Each method has its own flaws. For instance, in ATPS, dextran remains in the final volume of vesicles, which can affect vesicle activity and interfere with downstream analysis. SEC requires a lengthy run time and is difficult to scale up. TFF is infamous for its complex setup and high costs, whereas PEG-precipitation has low specificity and is likely to co-precipitate contaminants. It should be noted that there is no standardized protocol or guideline for PDV production. In addition to the different preferences in methods among the studies we collected, there were also large variations in protocols even when the same methods were selected. These differences included specifications such as time, speed, and buffer. For instance, Regente et al. observed a higher density of sunflower seed apoplastic vesicles in the 40 000×g pellet compared to that in the 100 000×g fraction, which suggested that a medium centrifugation speed is more effective for pelleting sunflower seed-derived vesicles [7]. Meanwhile, studies on Arabidopsis thaliana leaves showed that a speed of 100 000×g had a greater EV separation efficiency. Nevertheless, it is worth noting that PEN1-positive EVs represent the majority of EVs in the 40 000×g fraction (72%), indicating that this intermediate speed is suitable for PEN1-related EV isolation. This also suggests that the target population of EVs is also a factor for centrifugation speed selection [8,33]. Zeng et al. studied the optimization of ultracentrifugation conditions in a time-dependent manner and recommended that 10–20 min of centrifugation at 100 000×g was ideal for extraction of Aloe vera PELNs, as the yield was homogenous at around 200 nm diameter with low polydispersity indexes (0.14 and 0.21, respectively). In contrast, centrifugation for 60 min at the same speed led to a highly disparate population with a swollen size above 500 nm and a polydispersity value of 0.59 [34]. In fact, the centrifugation method could be tricky, as using a low speed might be inefficient to pellet the desired vesicles, while overlong ultracentrifugation may result in non-vesicle contaminants and inflate the size distributions [35]. Furthermore, pH is a factor to be considered when developing a PDV production strategy. It was reported that using the PEG-precipitation method under low pH conditions (pH 4 and 5) to isolate ginger-derived nanovesicles resulted in a 4-to 5-fold higher vesicle yield and higher polyphenolic content compared to using neutral and alkaline pH environments [36]. Overall, isolation and purification procedures should be meticulously adjusted according to many factors, such as laboratory conditions, research purposes, and experimental targets for optimal results.
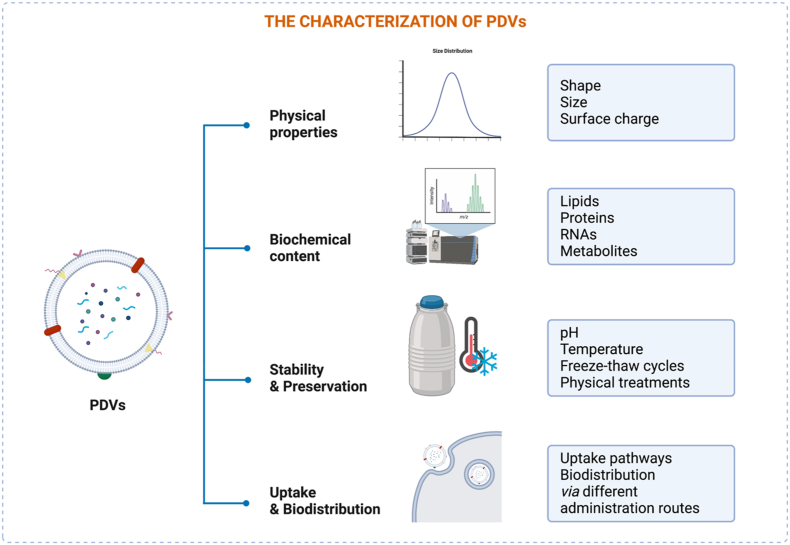
4. Characterization of PDVs
In this section, we introduce studies carried out to elucidate the properties of PDVs in various aspects, from their physical and biochemical characteristics, stability, and storage to their uptake pathways and biodistribution through different administration routes (Fig. 4).
Fig. 4.
Different characterization of PDVs.
4.1. Physical characteristics
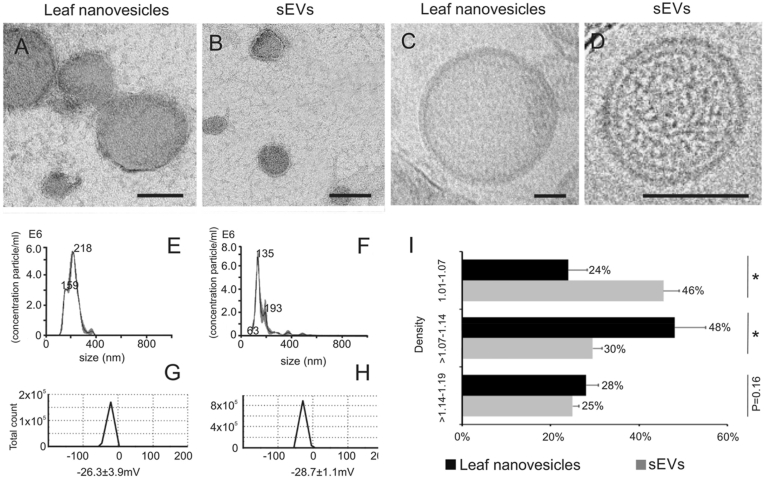
In most studies, plant-derived vesicles have been physically investigated based on their shape, size, and surface charge. Cumulative electron microscopy studies have since revealed the presence of round or cup-shaped particles with lipid bilayers. For PEVs, the sizes remain within 30–300 nm, while for those separated by the disruption process (PELNs), the size distributions vary greatly from 18 to 1000 nm, and in either case, the vesicles have negative surface charges (Table 1). Fig. 5 presents the morphology of Arabidopsis thaliana PEV and PELN for comparison; here, PELNs are slightly larger in size but covered by thinner lipid bilayers compared to PEVs [14]. Interestingly, the size and charge of PDVs can be altered by modulating environmental pH or temperature. This is further discussed in the stability and preservation sections.
Fig. 5.
Characterization of leaf nanovesicles and small EVs (sEVs) from Arabidopsis. (A–B) TEM images of leaf nanovesicles and sEVs (bar = 100 nm). (C–D) Cryo-EM image of leaf nanovesicles and sEVs (bar = 50 nm). (E–F) Leaf nanovesicle and sEVs size distributions, as assessed by nanoparticle tracking analysis. (G–H) Zeta potential of leaf nanovesicles and sEVs. (I) The protein distributed over the sucrose density fractions of the leaf nanovesicles (black bars) and sEVs (gray bars); mean value is shown in the graph. Adapted with permission [14]. Copyright 2020, Elsevier.
Morphology evaluation methods play a crucial role in vesicle studies as they provide straightforward information about what researchers have in hand. At present, dynamic light scattering (DLS), nanoparticle tracking analysis (NTA), and electron microscopy (EM) are used to characterize the PDV. Depending on the testing method, the size results may vary as each method works on different principles and requires unique preparations. For instance, visualization with SEM revealed smaller vesicles than did DLS visualization of the same vesicles [44,49]. This could be attributed to the SEM preparation requirements, including sample dehydration and vacuum conditions, which evidently shrink the lipid-based ultra-structures. This discrepancy in morphology should be crosschecked by adopting different methods to achieve an insightful physical analysis of the particles and ensure the reliability of the study.
4.2. Biochemical content
Owing to their physiological origin, the composition of PDVs is intrinsically complex compared to artificial carriers. According to recent studies, although the exact compartments differ greatly between species, PDVs mainly consist of lipids, proteins, RNAs, and other metabolites (Table 2).
Table 2.
The main biochemical content of various PDVs.
| Types | Sources | Biochemical content |
Ref | |||
|---|---|---|---|---|---|---|
| Lipid | Protein | RNA | Others | |||
| PEV | Arabidopsis thaliana | 1438 (PELN) | [14] | |||
| 787 (PEV) | ||||||
| Arabidopsis thaliana | GIPC, PA | [79] | ||||
| Arabidopsis thaliana | 598 | [8] | ||||
| PELN | Aloe vera | Glucosyl-ceramide (40%) | Aloe-emodin, aloesin β-sitosterol | [34] | ||
| Ceramide (10%) | ||||||
| Bitter melon | 400 | [41] | ||||
| Bitter melon | 81 miRNAs | [42] | ||||
| Blueberry, coconut, ginger, grapefruit, Hami melon, kiwifruit, orange, pea, pear, soybean, and tomato | 418 miRNAs (32–127 per species) | [46] | ||||
| Broccoli | Sulforaphane | [47] | ||||
| Citrus fruits | 600–800 | [80] | ||||
| Clementine | 1018 | [81] | ||||
| Coconut | 47 known miRNAs | [49] | ||||
| 14 unknown miRNAs | ||||||
| Corn | PC, PG, PE | [18] | ||||
| Fingerroot | Phenolic compounds (naringenin chalcone, pinostrobin, pinocembrin) | [51] | ||||
| Ginger | PA, DGDG | 6-shogaol | [53] | |||
| Ginger | PA, DGDG, MGDG | [82] | ||||
| Ginger | Low content | 125 miRNAs | 6-gingerol, 6-shogaol | [16] | ||
| Ginger | 116 miRNAs | [83] | ||||
| Ginseng | DGMG (59.4%) | 3129 | Ginsenoside Rg3 | [57] | ||
| PE (16.8%) Ceramide (13.8%) | ||||||
| Grape | PA (53.2%) | [9] | ||||
| PE (26.1%) | ||||||
| Grapefruit | PE, PC, PI | [10] | ||||
| Grapefruit | PE, PC, PI | Naringin, naringenin | [61] | |||
| Grapefruit | Carbohydrates; amino acids (leucine, isoleucine); alpha hydroxy acids (glycolic acids, citric acids); fatty acids (palmitic acid, doconexent); myo-inositol; quininic acid; aucubin; doconexent | [84] | ||||
| Lemon | 580 | [12] | ||||
| Strawberry | Ascorbic acid | [70] | ||||
| Tea leaves | PA, PG, PC | Polyphenols (gallic acid, caffeine, EGCG); flavones (quercetin) | [73] | |||
| Turmeric | DGDG (41.6%) | [19] | ||||
| PA (19.7%) | ||||||
| Orange | PE (40%) | Carbohydrates (glucose, fructose, sucrose); amino acids (alanine, asparagine isoleucine, threonine, leucine) | [85] | |||
| PC (25%) | ||||||
| PI (12%) | ||||||
| PA (5%) | ||||||
PC: phosphatidylcholine; PA: phosphatidic acid; PE: phosphatidylethanolamine; PI: phosphatidylinositol; PG: phosphatidylglycerol; DGMG: digalactosyl monoacylglycerol; DGDG: digalactosyl diacylglycerol; GIPC: glycosylinositol phosphoceramides; EGCG: epigallocatechin gallate.
4.2.1. Lipids
Lipids are one of the most essential components of PDVs, as the lipid bilayer composition determines their potential to be internalized by recipient cells and utilized for therapeutic development. Accumulated data show that PDVs are enriched in phospholipids, such as phosphatidylcholine (PC), phosphatidic acid (PA), phosphatidylethanolamine (PE), phosphatidylinositol (PI), and phosphatidylglycerol (PG) [88]. Unlike mammalian exosomes and synthetic liposomes, lipid content in PDVs has been reported in the absence of cholesterol.
Triple quadrupole mass spectrometry (TQMS) has revealed that PE, PC, and PI are the major lipid components of grapefruit-derived nanoparticles [10,61]. PELNs from orange juice exhibit similar characteristics, mostly containing PE (40%), PC (25%), PI (12%), and PA (5%) [85]. Lipidomic data from two different research groups have consistently indicated that PA (more than 30% of total lipids) and digalactosyl diacylglycerol (DGDG) are crucial constituents of ginger-derived nanovesicles [53,82]. Similarly, PELNs from turmeric rhizomes (ginger family) are most enriched in DGDG (41.6%) and PA (19.7%) [19]. These analyses suggest that vesicles extracted from the same families, Rutaceae and Zingiberaceae, share common lipid compositions. Other PELNs from tea leaves and corn are also abundant in phospholipids such as PG and PC [18,73]. Lipid analysis of grape PELNs showed that PA and PE accounted for 53.2% and 26.1% of the total lipid content, respectively. Interestingly, observations from the same sample of grapes showed that the percentage of PA in whole grapes was much lower than that in its nanovesicles (approximately 2.6-fold lower), which supports the idea that PA might be selectively loaded into the vesicles [9]. In addition, some PELNs contain high levels of ceramide. Specifically, aloe PELNs are rich in glucosylceramide (40%) and ceramide (10%), and ginseng-derived nanoparticles were reported with 13.8% of ceramide in total lipids [34,57].
In the case of PEVs, lipidomic analysis of Arabidopsis rosette leaf EVs has revealed a high abundance of sphingolipids (46%), mostly glycosylinositol phosphoceramides (GIPCs). This percentage was much higher than that in whole leaf tissues (0.5%). As much evidence has demonstrated the role of sphingolipids in the distribution of cell membranes, the enrichment of GIPCs (99% of total sphingolipids) in these EVs could imply a signaling function of the EVs membrane. This study also reported that approximately 21% of the total lipids in EVs are phospholipids. Notably, PA accounted for 32% of phospholipids, which was much higher than the percentage in leaf tissues (4%) [79]. Similarly, extracellular fluids from tomato and sunflower seeds contain a high percentage of PA and phosphatidylinositol-4-phosphate (PI4P) [75,86].
Phosphatidic acid has been recognized as a signaling messenger involved in plant cell regulation, including membrane fusion and protein binding [87]. Teng et al. revealed that ginger-derived PELNs were preferentially absorbed by Lactobacillus rhamnosus because of the high PA content (35.2%), whereas PC-enriched (36.2%) grapefruit nanovesicles were preferred for internalization into Ruminococcaceae. This study also reported that PA (34.4%) and PC (52.6%) were the most abundant lipids in vesicles from turmeric and garlic. Further analysis revealed the potential of PA lipids to modulate the duration and amount of intestinal PDVs accumulation with PC lipids, enhancing the migration of vesicles from the intestine to the liver [88]. In another study, PA lipids were demonstrated to support the uptake of ginger nanoparticles by the pathogen Porphyromonas gingivalis [78]. Although the abundant PA content in PDVs can be beneficial for its application in drug internalization and delivery, it should be noted that the abnormally high content of PA in PDVs could be due to the activation of phospholipase D during the experimental processes [79,89]. This enzyme catalyzes glycerophospholipids (e.g., PC) to generate PA. Thus, the conversion likely occurred because both phospholipase proteins and PA were detected in PDVs (e.g., Arabidopsis thaliana leaf EVs) [8,79].
4.2.2. Proteins
A limited number of studies have identified the various proteins involved in PDVs. Unfortunately, these studies remain inconsistent and lack concrete evidence for specific PDV protein markers. Some researchers have revealed diverse proteomic data from PDV samples. Cao et al. identified 3129 proteins in ginseng-derived nanoparticles using mass spectrometry, which were later classified into three groups by Gene Ontology consisting of biological processes, cellular compartments, and molecular functions [57]. Among the 1018 identified proteins from clementine (Citrus clementina) juice nanovesicles, 162 were under the category of Gene Ontology, including 71 transmembrane transport-related, 53 vesicle-mediated, and 50 intracellular transporters [81]. In contrast, bitter melon PELNs contained more than 400 proteins [41]. Many proteins were detected in aloe PELNs, some of which are commonly found in PDVs, including HSP 70, glutathione S-transferase, annexin families, adenosylhomocysteinase, and glyceraldehyde 3 phosphate dehydrogenase (GAPDH) [34]. Conversely, proteomic analysis of ginger PELNs reported a relatively low protein content, mostly cytosolic (e.g., actin and proteolytic enzymes) and a few membrane proteins (e.g., aquaporin and chloride channels) [16].
Rutter and Innes first analyzed the proteome of apoplastic EVs from Arabidopsis leaves and indicated that these EVs were enriched in proteins associated with stress responses. A total of 598 proteins were detected in two replicates, 170 of which were found in both replicates [8]. In Liu group's comparative proteomic study between apoplastic EVs and whole leaf PELNs from Arabidopsis, the total number of proteins identified was 787 and 1438, respectively. As expected, vesicles prepared with tissue rupture processes contained higher amounts of proteins, whereas vesicles extracted strictly from extracellular fluid were less diverse in protein composition [14]. These independent studies from Rutter, Innes and Liu et al. reported similar proteins in Arabidopsis EVs including PENETRATION1 (PEN1), Patellin1 (PATL1), and Patellin2 (PATL2). In addition, some proteins in mammalian exosomes have also been detected in PDVs, such as annexins, SNARES, GPI-anchored proteins in Arabidopsis leaf EVs, heat shock protein HSP70, and aquaporin proteins in grape PELNs [9]. In a proteomic analysis of lemon-derived nanovesicles, it was determined that approximately 56.7% of proteins overlapped with proteins from mammalian exosomes, regardless of cell origin [12].
From scarce sources of proteomic data, some authors have emphasized three protein families that are commonly found in PEVs and are also present in mammalian EVs: HSP70, S-adenosyl-homocysteinase, and GAPDH. Evidently, it is crucial to establish a reliable list of PDV protein markers for precise identification and characterization. Based on the frequency of detection in various types of PDV and their expression patterns, some recommended proteins are syntaxin PEN1, ABC transporter PEN3, tetraspanin-8 TET8, annexin, heat shock proteins (HSP70, HSP90), GAPDH, PATL-1, and PATL-2 [15].
4.2.3. RNAs
Several studies have confirmed the presence of RNAs in PDVs, which are usually small RNA molecules (sRNAs) with less than 30 nucleotides in length. It has been proposed that plant microRNAs (miRNAs), which are single-stranded non-coding sRNAs, are protected from degradation during transportation by their association with micro- or nanovesicles. Teng et al. suggested that PELNs could mediate the communication between the gut microbiota and host immune systems while altering gut microbial compositions because they carry a wide variety of sRNAs [88].
A total of 418 miRNAs (32–127 per species) were identified from 11 species of PELNs, in which the highly expressed miRNAs were predicted to be involved in inflammatory responses and cancer-related pathways [46]. In addition, considerable amounts of RNAs have been confirmed in grape-derived nanoparticles using various methods such as gel electrophoresis and mass spectrometry [9]. Deep sequencing reads of ginger-derived vesicles by Zhang et al. confirmed 125 different miRNAs 15–27 nucleotides in length [16], whose results were aligned with those of another research group that detected 116 miRNAs in ginger PELNs with predicted immunomodulatory or metabolic regulation roles [83]. Arabidopsis EVs contain a variety of sRNAs and show preference for loading distinct miRNAs [90]. They also carried sRNAs that were believed to play an important role in cross-kingdom interactions between Arabidopsis and the fungal pathogen Botrytis cinerea and eventually silenced virulence genes [91]. Remarkably, a recent study suggested that many sRNAs in Arabidopsis leaf EVs are more likely to be associated with proteins outside the membranes instead of being fully encapsulated inside EVs [92].
4.2.4. Metabolites
Plants have long been known to produce primary and secondary bioactive compounds for metabolism. As many studies have proposed a possible role for PDVs in plant defense and communication, PDVs are expected to carry a wide range of plant metabolites. For instance, PELNs in orange juice have been shown to contain primary metabolites, such as carbohydrates (glucose, fructose, and sucrose) and amino acids (alanine, asparagine isoleucine, threonine, leucine) [85]. Similarly, grapefruit-derived nano- and microvesicles have been found to contain carbohydrates, amino acids (leucine and isoleucine), alpha hydroxy acids (glycolic and citric acids), and fatty acids (palmitic acid and doconexent). Interestingly, compounds known to be involved in anti-cancer activities, including myo-inositol, quininic acid, aucubin, and doconexent, were also identified in the gas chromatography-mass spectrometry (GC-MS) profile of grapefruit vesicles [84].
Regarding secondary metabolites, large amounts of 6-gingerol and 6-shogaol have been detected in ginger-derived nanoparticles [16]. Thin layer chromatography (TLC) analysis indicated that most of the shogaols were associated with ginger vesicles, as ginger extracts left no trace of shogaol on the TLC plate after the depletion of vesicles [53]. High performance liquid chromatography (HPLC) results of broccoli-derived nanoparticles also consistently indicated that sulforaphane, a major component of broccoli with anti-inflammatory effects, was more enriched than that in the microparticle fraction, and little sulforaphane existed in the free form in broccoli extracts [47]. Naringin and naringenin, the major flavonoids in grapefruit, were identified in the HPLC data of grapefruit PELNs [61]. Electrospray ionization (ESI) scanning revealed that ginsenoside Rg3, a valuable active compound in ginseng, was highly concentrated in ginseng-derived nanoparticles [57]. HPLC-MS analysis showed that tea leaf PELNs contained various types of polyphenols, such as gallic acid, caffeine, epigallocatechin gallate (EGCG), and flavones, such as quercetin. These bioactive constituents are well known for their antioxidant, anti-inflammatory, and anti-cancer properties, suggesting that tea leaf nanovesicles have potential for therapeutic applications [73]. Similarly, HPLC results showed that Aloe vera PELNs were enriched in bioactive compounds such as aloe-emodin, a strong anti-cancer candidate, and aloesin and β-sitosterol, which are known for their antioxidative effects [34].
However, there are cases where major phytochemicals were not present in the PDVs of the same plant. For example, in the case of orange-derived nanovesicles, free fatty acids, including palmitic acid, oleic acid, and linoleic acid, have been identified, but the main bioactive compounds of orange, such as vitamin C and naringenin, have not been identified [85]. In an attempt to clarify whether plant metabolites were packaged inside PDVs, Woith et al. believed that secondary metabolites were more likely to be associated with vesicle membranes because of lipophilicity rather than being actively incorporated inside [93].
Overall, the current literature has contributed to the identification of PDV components, including but not limited to lipids, proteins, RNAs, and other plant metabolites. Especially when they tend to have synergistic interactions in many ways, the components should be comprehensively investigated. For example, some sRNAs selectively bind to proteins for their attachment to PDVs, whereas plant secondary metabolites seem to be associated with PDV membranes because of their lipophilicity. Another crucial need for PDV research now is the determination of PDV protein markers. Even though many protein candidates have been proposed, an agreement on the list of PDV protein markers must be established in the near future to support the identification and characterization of PDVs, which would help improve the consistency and transparency of this research field.
4.3. Stability and preservation
Unlike mammalian exosomes and synthetic liposomes, PDV stability and optimal preservation have not been intensively studied, although these steps are essential to elucidate the potential of PDVs for biological administration and scalability. Several studies have been conducted to test the durability of PDVs under different conditions by altering factors such as temperature, pH, freeze-thaw cycles, and external physical treatment.
4.3.1. Stability
Several types of PDV (e.g., citrus fruit, ginger, carrot, and grape) have been reported to be stable over a wide range of pH values at physiological temperatures [60]. Notably, the size and surface charge of these PELNs were pH dependent. Specifically, grapefruit-derived nanovesicles remained unchanged in size and surface charge in neutral and alkaline pH environments, whereas in acidic solution, both corresponding values slightly increased [61]. Ginger PELNs showed the same pattern, with an enlarged size and surface charge shifting from negative to weakly positive in a gastric-like solution [16,53]. Moreover, PELNs from grapefruit and lemon juice were highly resistant to gastric and intestinal digestion, both in a simulated environment and in mouse models [31,61].
Owing to their good stability and adjustable size, PDVs have been widely utilized as drug delivery vehicles. It is recommended that binding hydrophobic agents to grapefruit PLNVs would help enhance the overall stability and bioavailability of the delivery system. In fact, grapefruit PLNVs can be modified with agents such as curcumin, folic acid, or zymosan A without affecting the biological activities of these agents. Furthermore, these lipid particles were found to be more stable than cationic DOTAP:DOPE liposomes at 37 °C in a 10% bovine serum solution [10].
Some studies have shown that PDVs are susceptible to boiling and sonication. Nanovesicles derived from Citrus fruits lost their antiproliferative effects after boiling or sonication [12]. Similarly, boiling and sonication destroyed the nanoparticles extracted from apples [38]. Sonicated ginseng PELNs inadequately shifted macrophage polarization compared with unsonicated ginseng [57]. In contrast, some studies have successfully modified and loaded therapeutic agents into PDVs by sonication [10,62,82,94]. Researchers should be aware of this as sonication is a common method used for drug loading; hence, the morphology of PDVs must be carefully examined before and after loading cargo.
4.3.2. Preservation
Kim et al. investigated Dendropanax morbifera leaf PELN stability at various temperatures: -20 °C, 4 °C, 25 °C, and 45 °C [95]. After a purification process, these PELNs were diluted in distilled water, stored at different temperatures, and assessed in terms of their pH, size distribution, and total protein. Firstly, the storage pH values remained stable after 4 weeks of storage at 4 °C and −20 °C; however, they slightly dropped over time when stored at higher temperatures (25 °C and 45 °C). Besides that, long-term storage affected vesicle morphology as the size distribution of vesicles stored at all four temperatures increased over time. This could be due to the aggregation during the storage period. Notably, the −20 °C condition showed superiority over the other three temperatures in maintaining vesicle size within the range of 30–500 nm, specifically between 30 and 200 nm. Moreover, total protein levels in these PELNs decreased over time in all temperature conditions. Lastly, this research group tested the effect of freeze-thaw cycles (−20 °C to room temperature) on ELN storage. TEM results exhibited spherical-shaped vesicles at first and unclear aggregations from cycle 1 to 3. A study on vesicles extracted from Kaempferia parviflora rhizomes reported similar results: that −20 °C and −80 °C conditions effectively preserved the properties of samples, such as size, shape, surface charge, and fatty acid content after 8 weeks, compared to 4 °C and room temperature conditions. Repeated freeze-thaw cycles led to enlarged vesicle sizes and decreased surface potentials [74]. Another group showed that ginger-derived nanovectors maintained their sizes and charges after storage for 25 days at 4 °C in pH 7.4 buffer [82]. In addition, PLNVs from grapefruit were highly stable while preserving the biological activity of its curcumin cargo for over a month at 4 °C [10]. PELNs from Aloe vera gel were able to maintain their integrated morphology after 90 days stored at −20 °C, outperforming control liposomes with gradually fragmented membrane structures over time when preserved with the same condition [34].
A freeze-drying method for ginseng-derived nanoparticles was proposed, in which fresh particles were first stored in a deep freezer for 24 h and then transferred to a freeze-drying chamber under a vacuum for 96 h. Particles were obtained in powder form and exhibited no significant damage or loss in terms of morphology, RNA, or protein content after up to 60 days of storage at room temperature. These results suggested that freeze-drying is a potential option for facile vesicle storage after mass production [59].
4.4. Uptake mechanism and biodistribution
In recent years, various internalization pathways have been proposed for PDVs in various cell lines. Using several assays that block specific pathways, researchers can detect the endocytosis mechanisms involved in particle internalization. The most frequently reported pathways include macropinocytosis, clathrin-dependent endocytosis, and caveolae-dependent endocytosis. Macrophage RAW 264.7 cells were shown to uptake tea leaf nanoparticles via galactose-mediated endocytosis and infuse onion PELNs through caveolae-dependent endocytosis and macropinocytosis [67,73]. These macrophages also absorbed turmeric PELNs via caveolae-mediated endocytosis, while taking up grapefruit-derived vesicles via both macropinocytosis and clathrin-dependent pathways [19,61]. Meanwhile, HepG2 cells internalized Asparagus cochinchinensis PELNs via phagocytosis, and CT-26 cells absorbed vesicles extracted from grapes through macropinocytosis [9,40]. In addition, apple-derived nanoparticles utilized a clathrin-dependent mechanism in two intestinal cell lines, Caco-2 and LS180 [96]. Ginger PELNs are taken up by Caco-2 cells via macropinocytosis and caveolin-mediated endocytosis [83]. Grapefruit PLNVs were taken up by lung cancer cells A549 through different mechanisms, including phagocytosis, clathrin-mediated endocytosis, and microtubule polymerization [10]. Sasaki et al. demonstrated that PELNs from corn are taken up by colon-26 cells via a cholesterol-dependent pathway. Interestingly, when comparing the internalization of these PELNs with that of control liposomes, PELNs showed superiority with a higher rate of cancer cell targeting [18]. Significantly, it has been reported that surface proteins play a role in garlic PELN internalization, as trypsin-digested vesicles (surface proteins removed) showed less uptake by HepG2 cells than did undigested ones. This study also indicated that the interaction between CD98 and lectin II could have a significant effect on vesicle internalization [97]. Among the above cases, it is noteworthy that turmeric and ginger PELNs uptake rates were affected by temperature. In particular, uptake efficiency was higher at 37 °C, relative to 4 and 20 °C, suggesting that the uptake process was energy-dependent. Interestingly, a comparative experiment on the cancer cell uptake of Arabidopsis thaliana PEVs and PELNs showed that the uptake efficiency of PEVs was higher than that of PELNs (99.5% and 31.3%, respectively) [14].
Regarding biodistribution, grapefruit PELNs were mainly retained in the small and large intestines after 2 h of oral administration in mice; these vesicles were still visible in the cecum after 4 h [61]. Turmeric PELNs were preferentially distributed in the inflamed colon 24 h after oral administration [19]. It is worth noting that the biodistribution of PDV depends on the injection route. Grapefruit PLNVs accumulated the most in the liver, lung, kidney, and splenic tissues when injected through the tail vein (i.v.) or peritoneum (i.p.). They were predominantly localized in the muscle via intramuscular injections, and in the lungs and brain via intranasal administration. Follow-up in vivo experiments showed that these particles remained stable in the liver and spleen 20 days after intravenous injection. Surprisingly, these lipid nanovesicles did not pass through the placenta of i.v.-injected mice, suggesting their potential for drug delivery in pregnant individuals [10]. Furthermore, Cao et al. reported varied distribution tendencies in mouse models injected with DiR-labeled ginseng PELNs through different routes. After 72 h after injection, i.p. and i.v. administered particles showed the strongest fluorescent signals in the lung and spleen, while the intragastrically injected particles were mainly retained in the stomach and intestines. Importantly, i.p. injected nanoparticle signals remained strong in the liver and spleen on day 7 [57]. Collectively, PDVs would be retained in organs such as lung, liver, and spleen; the duration for degradation of PDV varies from 2 h up to 20 days depending on the different administration routes (e.g., i.v., i.p., oral gavage). Nevertheless, more in-depth studies regarding the biodistribution of PDVs via various administration routes must be carried out to carefully assess their biosafety and therapeutic applications.
5. PDVs in cancer therapy
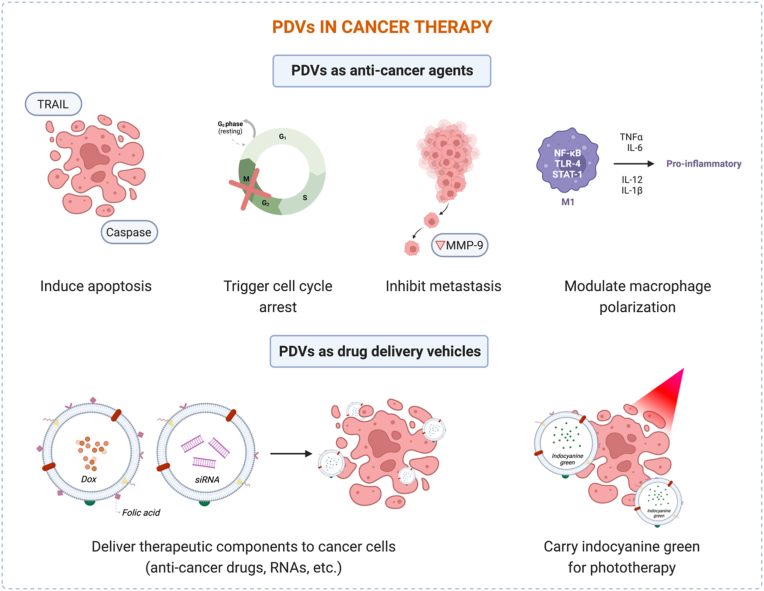
In recent years, scientists have accelerated PDV research in order to elucidate the potential wider application of more biocompatible materials in cancer treatment, as opposed to synthetic drugs which are currently commonplace. For therapeutic applications, PDVs must be internalized by target cells without triggering harmful side effects in healthy cells or systemic toxicity. Based on the current literature, PDVs exhibit promising capabilities for cancer therapy in two ways: (1) to express their innate anti-cancer activities as natural bioactive phytomedicine, or (2) to deliver active agents to targeted tumors as drug delivery vesicles. As shown in Fig. 6 and Table 3, this section highlights PDVs obtained from various sources and their applications for cancer therapy.
Fig. 6.
Potential applications of PDVs in cancer therapy.
Table 3.
Plant-derived vesicle research related to cancer therapy.
| Category | Source | Target cancer | Findings | Ref |
|---|---|---|---|---|
| Anti-cancer activity | Asparagus cochinchinensis | Liver | SRs blockade or PEGylation of vesicles increase vesicle circulation time and accumulation in tumor sites | [40] |
| Bitter melon | Oral | Induce S-phase cell cycle arrest and apoptosis Downregulate NLRP3 expression Reduce the resistance of cancer cells to 5-Fluorouracil | [41] | |
| Bitter melon | Brain | Inhibit glioma growth via regulating PI3K/AKT pathway | [42] | |
| Prevent metastasis via downregulating MMP9 | ||||
| Corn | Colon | Inhibit proliferation of cancer cells | [18] | |
| Activate tumor necrosis factor-α release in macrophages | ||||
| Dendropanax morbifera | Skin | Reduce cancer-associated fibroblasts in tumor environment | [98] | |
| Inhibit metastasis | ||||
| Dendropanax morbifera Pinus densiflora | Breast and skin | Exert synergistic anti-cancer effects when in combination | [99] | |
| Fingerroot | Colorectal | Disrupt intracellular redox homeostasis and induce apoptosis | [51] | |
| Garlic | Kidney and lung | Cause caspase mediated apoptosis | [52] | |
| Ginseng | Skin | Alter macrophage polarization | [57] | |
| Ginseng | Colon and breast | Combined with programmed cell death protein-1 monoclonal antibody | [100] | |
| Alter cold tumor environment | ||||
| Grapefruit | Skin | Induce cell cycle arrest at G2/M checkpoint | [84] | |
| Reduce cyclins B1 and B2 expression levels | ||||
| Upregulate cell cycle inhibitor p21 Inhibit Akt and ERK signaling | ||||
| Lemon | Colorectal, blood and lung | Induce TRAIL-mediated cell death | [12] | |
| Lemon | Colorectal, blood | Mediate lipid metabolism inhibition Downregulate Acetyl-CoA Carboxylase 1 | [101] | |
| Lemon | Gastric | Induce S-phase cell cycle arrest and apoptosis | [31] | |
| Moringa oleifera | Blood and cervical | Increase apoptosis levels | [102] | |
| Decrease B-cell lymphoma 2 protein expression | ||||
| Reduce mitochondrial membrane potential | ||||
| Tea flowers | Breast | Trigger breast tumors apoptosis | [72] | |
| Inhibit lung metastasis | ||||
| Modulate gut microbiota | ||||
| Therapeutic agents delivery | Aloe vera | Skin | Deliver indocyanine green for phototherapy | [34] |
| Cabbage | Colon | Deliver Dox, miR-184 | [29] | |
| Ginger | Colon | Carry FA + Dox | [82] | |
| Ginger | Colon | Coated with fucoidan and poly-lysine Carry Dox | [55] | |
| Ginger | Skin | Carry FA and siRNA (manipulated angle and orientation) | [54] | |
| Grapefruit | Colon and brain | Carry FA + PTX, FA + siRNA, JSI-124 Do not pass the placental barrier | [10] | |
| Grapefruit | Colon and breast | Coated with plasma membrane of leukocytes Carry curcumin and Dox | [103] | |
| Grapefruit | Brain | Coated with FA + polyethylenimine Carry miR17 | [62] | |
| Grapefruit | Colon | Carry miR-18a | [94] | |
| Inhibit liver metastasis | ||||
| Grapefruit | Brain | Loaded with heparin + Dox | [64] | |
| Lemon | Ovarian | Carry heparin-cRGD + Dox Overcome drug resistance | [65] |
FA: folic acid; Dox: doxorubicin; PTX: paclitaxel.
5.1. PDVs as anti-cancer agents
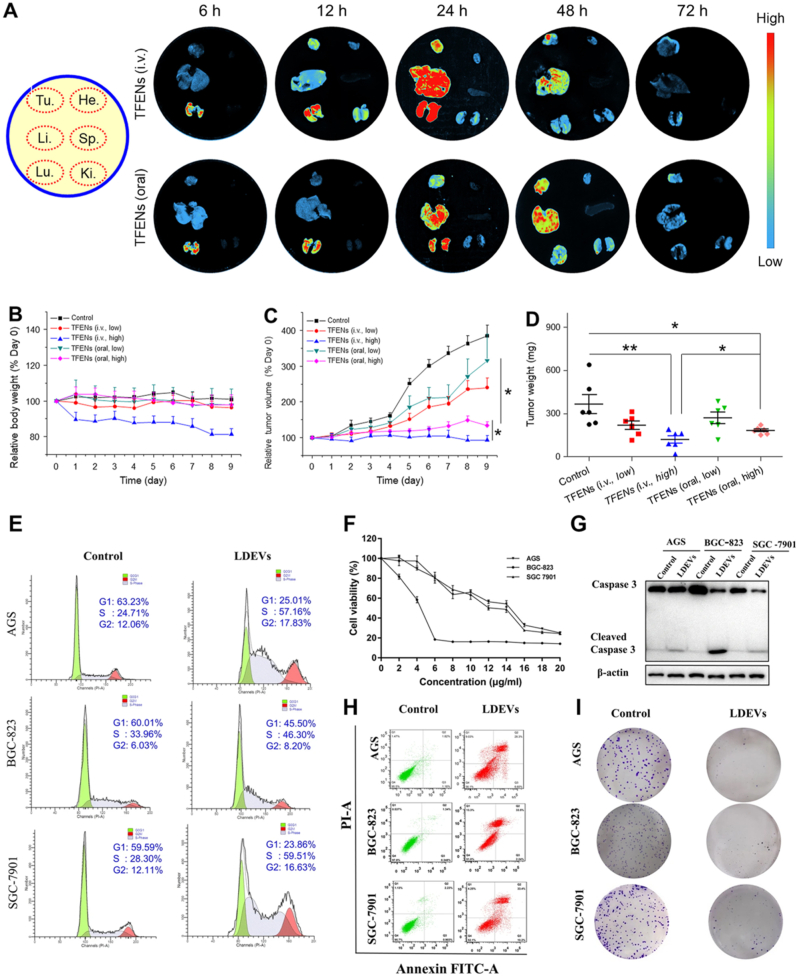
The anti-cancer properties of various types of PDVs have been investigated and reported to be involved in various mechanisms. In general, PDVs inhibit cancer cell proliferation and promote cancer cell death without inducing harmful effects in non-cancer cells. Citrus-derived nanovesicles have been reported to inhibit the viability of tumor cell lines, including A549, SW480, and LAMA84, in a dose- and time-dependent manner. Other normal cell lines (HS5, HUVEC, and PBMC) treated under the same conditions showed no signs of damage. Follow-up analysis suggested that these vesicles stimulated cancer cell death by activating TRAIL-mediated apoptosis, which was further confirmed in the in vivo LAMA84 xenograft model [12]. A proteomic study performed by the same research group demonstrated that the antitumor effect of these vesicles, particularly on colorectal tumors, was related to the downregulation of acetyl-CoA carboxylase 1 (ACACA) [101]. The effects of PELNs from lemon juice on gastric cancer cells (AGS, BGC-823, and SGC-7901) were also examined (Fig. 7). The results showed that the vesicles caused S-phase arrest and induced apoptosis in all cell lines, which could be attributed to the upregulation of GADD45a. The biosafety of lemon PELNs on major organs (heart, liver, spleen, lung, and kidney) was confirmed, as no significant abnormality was detected after vesicle administration into mice models [31]. Stanly et al. indicated that micro- and nano-sized vesicles from lemon, grapefruit, and orange specifically obstructed the proliferation of lung, skin, and breast cancer cells (A549, A375, and MCF7), but not of non-cancer cells (HaCat). In particular, grapefruit-derived vesicles triggered cell cycle arrest at the G2/M checkpoint associated with reduced cyclin B1 and B2 expression levels and upregulation of the cell cycle inhibitor p21 [84]. Garlic-derived vesicles effectively suppressed the proliferation of kidney and lung cancer cells (A498 and A549) by inducing cell cycle arrest in the S phase along with caspase-mediated apoptosis [52]. Interestingly, while exerting no detectable toxic effect on the normal human dermal fibroblasts (HDF), these vesicles were likely to increase HDF cell viability in the experiments by protecting the cells from apoptosis. Likewise, tumor cell viability (Jurkat E6-1 and HeLa) was reduced when treated with Moringa oleifera microvesicles compared with that of healthy human peripheral blood mononuclear cells (PBMCs). Stimulation of these vesicles by apoptosis was associated with the downregulation of B-cell lymphoma 2 protein expression and reduction of mitochondrial membrane potential [102]. Sasaki et al. examined the anti-cancer activity of nanoparticles extracted from corn and revealed that these particles significantly reduced the number of cells in various cell lines, such as colon26, MDA-MB-231, and Panc-1, but did not affect non-cancer cells (NIH3T3, RAW264.7). This anti-tumor effect was also expressed in colon26 tumor-bearing mice models, in which tumor growth was restrained after subcutaneous injection of corn-derived particles, and no severe toxicity was detected in mouse organs [18].
Fig. 7.
Studies on PDVs anti-cancer activities. (A–D) In vivo bio-distribution and anti-cancer activities of TFENs (tea flower PELNs) based on a subcutaneous xenograft breast tumor model. (A) In vivo bio-distribution of DiR-loaded TFENs in tumor, heart, liver, spleen, lung, and kidney at different time points (6, 12, 24, 48, and 72 h). (B) Relative body weight variations and (C) tumor volume variations over 9 days after the treatment of TFENs via i.v. injection and oral route. (D) Tumor weights on day 9. Each point represents the mean ± SEM (n = 6). ∗P < 0.05, ∗∗P < 0.01. ns, no significance. (E–I) Assessment of LDEVs (lemon PELNs) effects on gastric cancer cells. (E) Flow cytometry analysis of cell cycle phases of AGS, BGC-823 and SGC-7901 treated with LDEVs. (F) CCK-8 assay to evaluate the cell viability of AGS, BGC-823, and SGC-7901 cells treated with different concentration LDEVs. (G) Western blot analysis the expression of caspase 3 and cleaved caspase 3 proteins in gastric cancer cells. (H) Flow cytometry analysis the apoptosis of three gastric cancer cells induced by LDEVs. (I) Plate colony formation assay of AGS, BGC-823, and SGC-7901 cells with or without LDEVs treatment; These experiments were performed three times. (A–D) Adapted with permission [72]. Copyright 2022, Elsevier. (E–I) Adapted with permission [31]. Copyright 2020, Springer Nature.
In addition to the PDVs from common edible plants, those from resinous trees and medicinal herbs have been evaluated. PELNs from Dendropanax morbifera and Pinus densiflora plant sap exhibited cytotoxic effects on malignant breast cells (MDA-MB-231 and MCF7) and skin tumor cells (A431) relative to normal cell lines (MCF10A and HNF). Importantly, the combination of these two types of vesicles produced synergistic cytotoxic activities against tumor cells and minimized the side effects [99]. The same research group also demonstrated that Dendropanax morbifera PELNs exerted suppressive effects on cancer-associated fibroblasts in a tumor microenvironment, suggesting potential antimetastatic activity [98]. PEGylated nanovesicles from Asparagus cochinchinensis effectively inhibited liver cancer cell proliferation without inducing systemic toxic effects in xenograft models. This study also suggested that PEGylation or blockade of scavenger receptors could improve the biodistribution and accumulation of vesicles at tumor sites [40]. Recently, Chen et al. revealed that nanovesicles extracted from tea flowers exhibit impressive anti-cancer activity in vitro by suppressing three factors: tumor proliferation, cell migration, and cell invasion (Fig. 7). Furthermore, the vesicles could also inhibit breast cancer as well as its metastasis and modulate the gut microbiota after i.v. and oral administration in mice. It is worth noting that, compared to oral administration which is relatively safe, i.v. injection tended to stimulate immune responses, alter hemograms, and induce hepatorenal toxicity [72].
In addition to apoptosis induction and cell cycle arrest, PDVs also appear to modulate the tumor environment. Ginseng-derived nanoparticles have been shown to alter macrophage polarization both in vitro and in vivo via a TLR4-MyD88-dependent mechanism, which eventually inhibits tumor growth. In addition, these particles were validated by their biocompatibility, as they showed no adverse effects on healthy cells and mouse models. Interestingly, the depletion of proteins in these vesicles reduced their uptake by ovarian cancer cells and attenuated the upregulation of M1-related surface markers compared with normal vesicles, which supported the vital role of proteins in the bioactivity of ginseng PELNs [57]. Moreover, ginseng PELNs were also evaluated for their combination with the programmed cell death protein-1 monoclonal antibody. This combination showed an ability to alter the cold tumor environment and subsequently induce sustained systemic antitumor immunity in vivo [100].
Another key point is that PDVs may employ distinct mechanisms in different types of tumors. In vitro assays of bitter melon-derived vesicles showed reduced viability and proliferation while suppressing the invasion and migration of U251 glioma cells; however, flow cytometry results showed no signs of apoptosis stimulation. Further experiments elucidated vesicle blood-brain barrier penetration and anti-cancer mechanisms that inhibited glioma growth via the PI3K/AKT pathway and suppressed metastasis by downregulating MMP9 [42]. Another study on bitter melon vesicles reported that they exhibited both apoptotic and anti-inflammatory activities in oral cancer cells. These PELNs also showed synergistic therapeutic effects with 5-fluorouracil. Specifically, they remarkably enhanced the anti-cancer effect of 5-fluorouracil by downregulating NLRP3 expression, which eventually reduced the drug resistance of 5-fluorouracil in oral cancer cells both in vitro and in vivo [41].
Overall, recent studies demonstrate that anti-cancer activities of PDVs are likely associated with one or more mechanisms of action: i) cancer-selective apoptosis induction (e.g., TRAIL-mediated and caspase-mediated apoptosis) which could be attributed to the regulation of various factors like ACACA, GADD45a, B-cell lymphoma 2 protein, and mitochondrial membrane potential; ii) cell cycle arrest at S phase or G2/M checkpoint; iii) tumor microenvironment modulation by altering macrophage polarization via a TLR4-MyD88-dependent mechanism or shifting cold to hot tumor.
5.2. PDVs as drug delivery vehicles
PDVs have been studied for their cargo transporting ability owing to their physiological composition and flexibility for advanced engineering, such as surface modification and size alteration. In most cases, whole PELNs are loaded with therapeutic agents by incubation alone. PLNVs are formed using lipids extracted from PELNs and then assembled with drugs via a sonication process. Currently, grapefruit PELNs are among the most studied PDVs because of their promising cargo delivery efficiency. Wang et al. demonstrated that grapefruit PLNVs exhibit high performance in the co-delivery of folic acid and the chemotherapeutic drug paclitaxel (PTX) to colon tumor sites. In vivo experimental results showed that the treatment not only enhanced the therapeutic effect of PTX by targeting tumor tissues and inhibiting tumor growth, but also improved siRNA delivery to tumors. Importantly, these PLNVs were efficiently taken up by different cell types without causing detectable cytotoxicity or inflammatory responses. While increasing the pH level from 6.5 to 9.0 did not apparently affect vesicle internalization, raising temperature values from 4 °C or 20 °C–37 °C accelerated the uptake. Comparing uptake efficiency between PLNVs and synthetic DOTAP:DOPE liposomes, the former showed superiority with an over 80% uptake rate, double that of the latter, contributing to improving the therapeutic effects of the entire drug system [10]. In another study, these PLNVs were coated with inflammatory-related receptor-enriched membranes of activated leukocytes, and this delivery system showed advantages in transporting the chemotherapy drug doxorubicin (Dox) to breast and colon tumors compared to free Dox [103]. In addition, PLNVs could be beneficial as therapeutic miRNA carriers. For instance, PLNVs encapsulating miR-18a target Kupffer cells and induce M1 macrophages, which eventually suppress liver metastasis [94]. Modification of PLNVs coated with folic acid and polyethyleneimine lead to a more effective intranasal delivery of miR17 to GL-26 tumor cells in mice, which in turn effectively inhibits brain tumor progression [62]. PLNVs in combination with aptamers LA1, P-gp siRNA, and Dox appear to be a novel strategy to strengthen the anti-tumor activity of therapeutic components against multidrug-resistant LoVo cells [104]. In addition, Niu et al. designed a delivery system for grapefruit PELNs connected to Dox-loaded heparin-based nanoparticles via PELN-heparin chemical conjugation. This system was proven to maximize the brain tumor penetration of the drug and significantly enhance the anti-glioma efficacy of the treatment [64].
PLNVs fabricated from ginger PELNs could load Dox with high efficiency. Specifically, they exhibited better drug release in a pH-dependent manner than commercial liposomal-Dox. The binding of folic acid to Dox-carrying vesicles formed a delivery system that enhanced both the targeting ability and anti-tumor activity against colon-26 cells in vivo compared to free Dox. With respect to biosafety, these ginger vesicles could be internalized by colon cancer cells without causing considerable toxicity both in vitro and in vivo and had a milder effect on cell proliferation when compared to cationic liposomes at equivalent lipid concentrations [82]. In another study, ginger exosome-like nanovesicles were modified with folic acid-displaying arrowtail RNA nanoparticles. Manipulation of RNA orientation on vesicle surfaces improved the specific delivery of siRNA to KB cancer cells, thereby increasing cancer suppression efficiency through intravenous administration [54]. Zhang et al. designed an encapsulation model for Dox delivery to colon cancer cells by layering different components from inside out, starting with Dox, ginger vesicles, ϵ-poly-lysine, and fucoidan. This model showed significant tumor inhibition in Luc-HT-29 and HCT-116 xenografts, which was superior to free Dox in terms of therapeutic effect and biocompatibility [55].
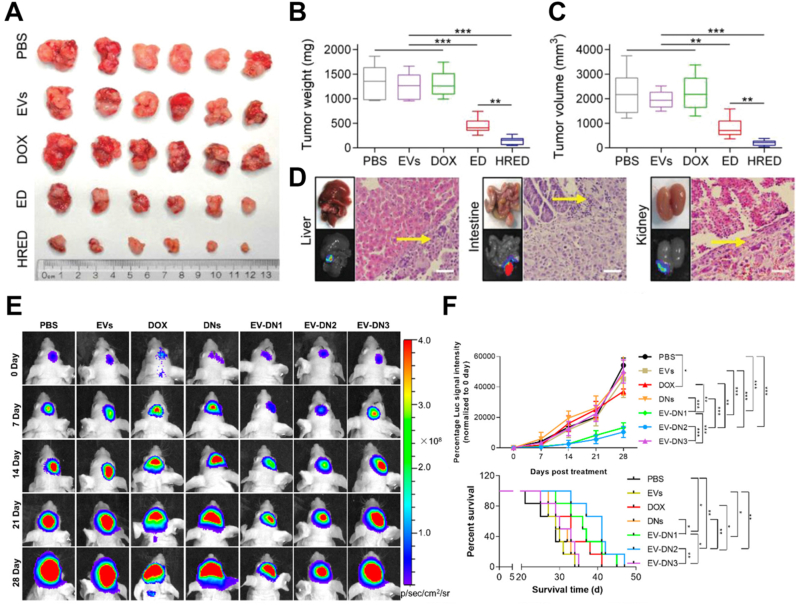
Furthermore, cabbage-derived vesicles were also shown to successfully deliver Dox to SW480 cells without altering the vehicles themselves or the anti-cancer effect of the drug [29]. Fingerroot vesicles were selectively taken up by colorectal cancer cells (HT-29 and HCT116) but not by normal colon epithelial cells. These vesicles disrupt intracellular redox homeostasis and induce apoptosis in cancerous cells [51]. Nanoparticles from Aloe vera loaded with indocyanine green (ICG) showed greater stability and inhibition of melanoma growth via phototherapy than free ICG and ICG liposomes. Seven days after i.v. injection of nanoparticles, no significant inflammatory response or organ damage was detected. Significantly, the leak-proof performance test showed that aloe vera vesicles had better packaging capability than reference liposomes after 30 days of loading ICG, with ICG retention rates of approximately 90% and 73%, respectively [34]. A novel system for multidrug-resistant cancers (Fig. 8) was developed using lemon PELNs. Heparin-cRGD was attached to the surface of the vesicles loaded with Dox. This system exhibited strong anti-proliferative effects on Dox-resistant ovarian cancer cells, which overcame drug resistance both in vitro and in vivo [65].
Fig. 8.
Studies on PDV applications in drug delivery for cancer therapy. (A–D) Anti-tumor effects of lemon PELNs (named EVs in this figure), Dox, PELN-Dox combination (ED), and heparin-PELN-Dox (HRED) model in the SKOV3/DOX-Luc orthotopic ovarian cancer xenograft nude mice model. (A) Excised tumor of the mice from each group. Scale: 1 unit = 1 cm. (B) Tumor weights of the mice in all groups. (C) Tumor volume of the mice in all groups. Results are represented as mean ± SD (n = 6). *p < 0.05, **p < 0.01, and ***p < 0.001. (D) Excised organ images and H&E staining of metastatic nodules of liver in PBS group, intestine in EVs group, and kidney in Dox group. yellow arrows point to tumor metastasis. Scale bar: 50 μm. (E–F) Antitumor effect of grapefruit PELNs (named EVs in this figure), Dox, DNs (Dox-loaded heparin-based nanoparticles), EV-DN (DNs patched on PELN surface) including EV-DN1 (166 ± 4 nm), EV-DN2 (192 ± 7 nm), and EV-DN3 (352 ± 22 nm) in the LN229-luc intracranial glioma model. (E) IVIS bioluminescent imaging of a representative glioma-luc-bearing mouse from each treatment group at different time and the bioluminescent signal intensity curve of treatment groups (n = 6). Normalized to 0 day of glioma-luc-bearing mice in all groups. (F) Kaplan–Meier survival curves of the mice in all groups. (A–D) Adapted from Ref. [65]. Copyright, 2022. Wiley Online Library. (E–F) Adapted from Ref. [64]. Copyright, 2021. American Chemical Society.
5.3. Clinical trials of PDVs for cancer therapy
Given the promising anticancer effects achieved both in vitro and in vivo, PDVs-based cancer therapy has been considered to be an encouraging approach for cancer treatment. Currently, as shown in Table 4, two early-phase clinical trials have already been performed or are ongoing [11]. One newly completed trial aims to investigate the ability of grape-derived vesicles to prevent oral mucositis associated with chemotherapy for head and neck cancer. This trial is simultaneously investigating the influence of vesicles on cytokine production, immune responses to tumor exosomal antigens, and metabolic and molecular markers in patients. The other clinical trial currently being conducted is on the capability of PDVs to deliver curcumin to normal and colon cancer tissues and improve curcumin bioavailability after administration (NCT01294072). Both completed and ongoing studies suggested the fact that PDV-based therapies are safe in a clinical setting, while their therapeutic outcomes have not been released yet and are still under evaluation.
Table 4.
The clinical state of PDV studies.
| Name | Condition | Intervention | Status | Clinical State (Clinical Trial. Gov Identifier) |
|---|---|---|---|---|
| Edible plant exosome ability to prevent oral mucositis associated with chemoradiation treatment of head and neck cancer | Head and neck cancer, oral mucositis | Grape extracts | Phase 1 Completed (2022) | NCT01668849 |
| Study investigating the ability of plant exosomes to deliver curcumin to normal and colon cancer tissue | Colon cancer | Curcumin conjugated with plant exosomes | Phase 1 Recruiting (2021) | NCT01294072 |
6. Discussion
Research surrounding plant-derived vesicles is still in its early stages. Despite being discovered in the 1960s, PDVs have attracted the most attention in the last decade. Though efforts have been made, only a few studies have reached the clinical stage. PEV and PELN studies have been accelerating for distinct purposes, and their confluence has filled the gap in PDV research. Depending on the goals of researchers, many studies have been conducted with different priorities. For studies focusing on PEVs to elucidate their nature, stringent isolation and purification processes must be prioritized. For instance, PEV extraction should be performed directly from the extracellular fluid without harsh physical treatment of plant samples such as blending and grinding, and the refining process should not be manipulated by microfiltration, which actively controls the size distribution of the vesicles. In addition, to demonstrate extracellular vesicle biological activities driven by its content (for example, RNAs and proteins) the origin of the components should be clearly identified to avoid incorrect assumptions based on non-vesicle structures. Meanwhile, for studies focusing on plant-derived vesicles to utilize their biocompatibility for therapeutic purposes, vesicle production and bioactive efficiency are more crucial criteria. Therefore, various techniques and modifications have been proposed based on plant species and laboratory conditions to optimize research outcomes.
As PDV research has only begun to emerge, misconceptions and inconsistencies are inevitable. Several problematic aspects of this field have been discussed in recent studies. Rutter and Innes emphasized several possible pitfalls in PEV research, focusing on the experimental challenges of obtaining genuine EVs [35]. Pinedo, de la Canal, and de Marcos Lousa indicated confusion regarding PDV nomenclature and characterization [15]. To build a reliable foundation for PDVs, researchers have been encouraged to provide transparent information in their scientific reports. Sharing and communication are critical issues. Scientists may consider sharing their work through EV-TRACK (http://evtrack.org/index.php), an online database established to improve the transparency of EV research. One reason for the inconsistency in PDV research could be the variability in plant cultivation. Plant sources and cultivation conditions should be considered to optimize mass production and quality control for uniform PDVs. In this case, smart-farming models might be a good option to standardize plant cultivation. Considering all the aspects of PDV mentioned in this review, scientists will be able to outline the PDV research strategy with a more holistic approach.
The field of targeted cancer therapy continues to thrive; however, clinical translation of synthetic delivery systems faces challenges regarding targeting efficacy, adverse side effects, and rapid clearance. Therefore, PDVs are anticipated to be a suitable alternative because of their unique properties, such as cell-derived origin that can possibly hinder them from systemic clearance and the signaling role that improves their internalization at tumor sites. Several studies on the therapeutic potential of PDVs in cancer have been conducted over the last decade. Regarding the anti-cancer activities of PDVs, various mechanisms of action have been proposed, yet most are related to cancer-selective apoptosis induction [41,52,84], while a minority of research reports suggest interference with tumor environments, such as macrophage polarization and cold-to-hot tumor shift [57,98]. In addition, PDVs have been progressively developed as novel nanoplatforms that can effectively deliver therapeutic agents to tumor sites, especially multidrug-resistant cells. Other outstanding aspects of PDVs include bioavailability and biocompatibility. With an innate targeting ability, PDVs are selectively directed to cancerous cells, thus improving therapeutic efficacy.
Comparisons between PDVs and other engineered nanoparticles (e.g., liposomes) or other types of extracellular vesicles (e.g., autologous EVs, mammalian EVs) remain unclear and unbalanced as PDV research remains relatively novel, and most comparisons rely on risk-benefit assessment on a case-by-case basis. Nevertheless, PDVs hold significant potential as therapeutic agents and drug carriers for several advantages as follows: i) large-scale production from many beneficial sources [54] and ii) minimal cytotoxicity and reduced immunogenicity by naturally evolved constituents in plant cells [47], iii) efficient cellular uptake [10,88], and iv) high biocompatibility [16], thereby meeting the requirements for the production of high-grade vesicles.
From these valuable properties, PDVs seem to share some similar biological effects with mammalian-derived EV; however, differences exist in many aspects. For example, the lipid layers of mammalian EV are mainly composed of cholesterol, glycoshingolipids, ceramides, and phosphatidylserine. On the other hand, PDVs are enriched in phospholipids such as phosphatidic acid (PA), and phosphatidylcholines (PC) [88]. These lipid characteristics provide inherent mammalian-cell-regulating activities. In particular, PA is notable owing to its ability to target and stimulate the mammalian target of rapamycin (mTOR) pathway responsible for cell growth, proliferation, and recovery. Furthermore, PC, a source of choline in the body, may also protect large intestine cell walls [88]. Therefore, these distinct properties of PDVs are anticipated to provide outstanding therapeutic advantages compared with mammalian EVs or synthetic nanoparticles.
Although some studies reported the characteristics and treatment efficacy of PDVs, many aspects of these vesicles, such as biogenesis, protein markers, and targeting ability, are not fully understood. In this regard, more in-depth investigations are needed to better understand the bioactivities and applications of PDVs. In addition, there are still some challenges related to biosafety and toxicity because of the unknown bioactive components of plants. For example, one report expressed concerns about the potential risk of PDV when they are intravenously administered [72]. Mice treated intravenously with high doses of tea flower PDVs suffered severe body weight loss on day 9 compared to the control group and groups treated orally. Further hemolysis and hepatotoxicity analysis demonstrated that intravenously administered PDVs could trigger immune responses and induce toxicities, while the oral route appeared to be a safer way of administration, indicating no significant side effects at both low and high doses. This result implies that behind the promising results lies a potential risk due to the complex high-level structures, which pose many challenges in fully elucidating the whole story of PDVs and their clinical translations. Therefore, the generalization that PDVs are non-toxic because of their physiological origin should be avoided unless comprehensive evaluations, including dose, administration routes, biosafety, toxicity, and half-life, are carried out.
In summary, PDVs and their applications in cancer treatment have much room for development. Along with their unique properties, the plentiful source of plants and their relatively undemanding large-scale production can provide PDV research with an advantage in terms of cost efficiency. Fostering research on PDVs and their cancer therapeutic effects from bench to bedside may be a long way off, yet the field is open and full of uncovered opportunities.
Declaration of competing interest
The authors have no competing interests to declare.
CRediT authorship contribution statement
Ngoc Phung Ly: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. Hwa Seung Han: Writing – review & editing. Myungsuk Kim: Writing – original draft. Jae Hyung Park: Conceptualization, Funding acquisition. Ki Young Choi: Conceptualization, Funding acquisition, Project administration, Writing – original draft, Writing – review & editing, Supervision.
Acknowledgments
This research was supported by Korea Institute of Science and Technology (KIST) intramural research grant and the Korean Fund for Regenerative Medicine (21A0503L1) of the Korea government (the Ministry of Science and ICT, the Ministry of Health & Welfare). Graphical illustrations were created with BioRender.com.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Jae Hyung Park, Email: jhpark1@skku.edu.
Ki Young Choi, Email: kiyoungchoi@kist.re.kr.
Abbreviations
- ACACA
Acetyl-CoA carboxylase 1
- AFM
Atomic force microscopy
- ATPS
Aqueous two-phase system
- Cryo-EM
Cryogenic electron microscopy
- DC
Differential centrifugation
- DGC
Density gradient centrifugation
- DGDG
Digalactosyl diacylglycerol
- DGMG
Digalactosyl monoacylglycerol
- DLS
Dynamic light scattering
- Dox
Doxorubicin
- EGCG
Epigallocatechin gallate
- ELD
Electrophoresis and dialysis
- ESEM
Environmental scanning electron microscope
- ESI
Electrospray ionization
- EXPO
Exocyst-positive organelle
- FA
Folic acid
- GAPDH
Glyceraldehyde 3 phosphate dehydrogenase
- GC-MS
Gas chromatography-mass spectrometry
- GIPC
Glycosylinositol phosphoceramide
- HPLC
High performance liquid chromatography
- HSP 70
Heat shock protein 70
- ICG
Indocyanine green
- ISEV
International Society of Extracellular Vesicles
- LTS
Laser electron microscopy
- MRPS
Microfluidic resistive pulse sensing
- MVB–PM
Multivesicular–plasma membrane
- MVB
Multivesicular bodie
- NTA
Nanoparticle tracking analysis
- PA
Phosphatidic acid
- PBMC
Peripheral blood mononuclear cell
- PC
Phosphatidylcholine
- PDV
Plant-derived vesicle
- PE
Phosphatidylethanolamine
- PEG
Polyethylene glycol
- PELN
Plant exosome-like nanovesicle
- PEV
Plant extracellular vesicle
- PG
Phosphatidylglycerol
- PI
Phosphatidylinositol
- PI4P
Phosphatidylinositol-4-phosphate
- PLNV
PELN lipid-derived nanovesicle
- PTX
Paclitaxel
- SEC
Size exclusion chromatography
- SEM
Scanning electron microscopy
- TEM
Transmission electron microscopy
- TFF
Tangential flow filtration
- TLC
Thin layer chromatography
- TRAIL
TNF-related apoptosis-inducing ligand
- TRPS
Tunable resistive pulse sensing
- UC
Ultracentrifugation
- UF
ltrafiltration
- UPS
Unconventional protein secretion
- VI-C
Vacuum infiltration-centrifugation
References
- 1.Théry C., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7 doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Niel G., et al. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 3.Manocha M.S., Shaw M. Occurrence of lomasomes in mesophyll cells of ‘khapli’ wheat. Nature. 1964;203:1402–1403. [Google Scholar]
- 4.Halperin W., Jensen W.A. Ultrastructural changes during growth and embryogenesis in carrot cell cultures. J. Ultra. Res. 1967;18:428–443. doi: 10.1016/s0022-5320(67)80128-x. [DOI] [PubMed] [Google Scholar]
- 5.An Q., et al. Multivesicular compartments proliferate in susceptible and resistant MLA12‐barley leaves in response to infection by the biotrophic powdery mildew fungus. New Phytol. 2006;172:563–576. doi: 10.1111/j.1469-8137.2006.01844.x. [DOI] [PubMed] [Google Scholar]
- 6.An Q., et al. Do plant cells secrete exosomes derived from multivesicular bodies? Plant Signal. Behav. 2007;2:4–7. doi: 10.4161/psb.2.1.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regente M., et al. Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS (Fed. Eur. Biochem. Soc.) Lett. 2009;583:3363–3366. doi: 10.1016/j.febslet.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 8.Rutter B.D., Innes R.W. Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins. Plant Physiol. 2016;173:728–741. doi: 10.1104/pp.16.01253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ju S., et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 2013;21:1345–1357. doi: 10.1038/mt.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q., et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat. Commun. 2013;4:1867. doi: 10.1038/ncomms2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu K., et al. Extracellular vesicles as emerging targets in cancer: recent development from bench to bedside, Biochimica et biophysica acta. Rev. Cancer. 2017;1868:538–563. doi: 10.1016/j.bbcan.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raimondo S., et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget. 2015;6:19514–19527. doi: 10.18632/oncotarget.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rutter B.D., et al. Isolation and quantification of plant extracellular vesicles. Bio Protoc. 2017;7:e2533. doi: 10.21769/BioProtoc.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y., et al. Characterization of and isolation methods for plant leaf nanovesicles and small extracellular vesicles. Nanomed. Nanotechnol. Biol. Med. 2020;29 doi: 10.1016/j.nano.2020.102271. [DOI] [PubMed] [Google Scholar]
- 15.Pinedo M., et al. A call for Rigor and standardization in plant extracellular vesicle research. J. Extracell. Vesicles. 2021;10 doi: 10.1002/jev2.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang M., et al. Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321–340. doi: 10.1016/j.biomaterials.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim M.K., et al. The antioxidant effect of small extracellular vesicles derived from aloe vera peels for wound healing. Tissue Eng. Regen. Med. 2021;18:561–571. doi: 10.1007/s13770-021-00367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasaki D., et al. Development of nanoparticles derived from corn as mass producible bionanoparticles with anticancer activity. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-02241-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu C., et al. Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy. J. Nanobiotechnol. 2022;20:206. doi: 10.1186/s12951-022-01421-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Regente M., et al. Apoplastic exosome-like vesicles: a new way of protein secretion in plants? Plant Signal. Behav. 2012;7:544–546. doi: 10.4161/psb.19675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer D., et al. Extracellular transport and integration of plant secretory proteins into pathogen-induced cell wall compartments. Plant J. 2009;57:986–999. doi: 10.1111/j.1365-313X.2008.03743.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang X., et al. Protein secretion in plants: conventional and unconventional pathways and new techniques. J. Exp. Bot. 2017;69:21–37. doi: 10.1093/jxb/erx262. [DOI] [PubMed] [Google Scholar]
- 23.Pérez-Bermúdez P., et al. Extracellular vesicles in food: experimental evidence of their secretion in grape fruits. Eur. J. Pharmaceut. Sci. 2017;98:40–50. doi: 10.1016/j.ejps.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 24.Movahed N., et al. Turnip mosaic virus components are released into the extracellular space by vesicles in infected leaves. Plant Physiol. 2019;180:1375–1388. doi: 10.1104/pp.19.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regente M., et al. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J. Exp. Bot. 2017;68:5485–5495. doi: 10.1093/jxb/erx355. [DOI] [PubMed] [Google Scholar]
- 26.De Palma M., et al. Plant roots release small extracellular vesicles with antifungal activity. Plants. 2020;9:1777. doi: 10.3390/plants9121777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uckoo R.M., et al. Grapefruit (citrus paradisi macfad) phytochemicals composition is modulated by household processing techniques. J. Food Sci. 2012;77:C921–C926. doi: 10.1111/j.1750-3841.2012.02865.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang J., et al. Untargeted chemometrics evaluation of the effect of juicing technique on phytochemical profiles and antioxidant activities in common vegetables. ACS Food Sci. Technol. 2021;1:77–87. [Google Scholar]
- 29.You J.Y., et al. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioact. Mater. 2021;6:4321–4332. doi: 10.1016/j.bioactmat.2021.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Umezu T., et al. Acerola exosome-like nanovesicles to systemically deliver nucleic acid medicine via oral administration. Mol. Ther. Methods Clin. Dev. 2021;21:199–208. doi: 10.1016/j.omtm.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang M., et al. An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J. Nanobiotechnol. 2020;18:100. doi: 10.1186/s12951-020-00656-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kırbaş O.K., et al. Optimized isolation of extracellular vesicles from various organic sources using aqueous two-phase system. Sci. Rep. 2019;9:19159. doi: 10.1038/s41598-019-55477-0. 19159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Y., et al. Effective methods for isolation and purification of extracellular vesicles from plants. J. Integr. Plant Biol. 2021;63:2020–2030. doi: 10.1111/jipb.13181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng L., et al. Aloe derived nanovesicle as a functional carrier for indocyanine green encapsulation and phototherapy. J. Nanobiotechnol. 2021;19:439. doi: 10.1186/s12951-021-01195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rutter B.D., Innes R.W. Growing pains: addressing the pitfalls of plant extracellular vesicle research. New Phytol. 2020;228:1505–1510. doi: 10.1111/nph.16725. [DOI] [PubMed] [Google Scholar]
- 36.Suresh A.P., et al. Low pH-based method to increase the yield of plant-derived nanoparticles from fresh ginger rhizomes. ACS Omega. 2021;6:17635–17641. doi: 10.1021/acsomega.1c02162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woith E., Melzig M.F. Extracellular vesicles from fresh and dried plants—simultaneous purification and visualization using gel electrophoresis. Int. J. Mol. Sci. 2019;20:357. doi: 10.3390/ijms20020357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujita D., et al. Apple-derived nanoparticles modulate expression of organic-anion-transporting polypeptide (OATP) 2B1 in caco-2 cells. Mol. Pharm. 2018;15:5772–5780. doi: 10.1021/acs.molpharmaceut.8b00921. [DOI] [PubMed] [Google Scholar]
- 39.Trentini M., et al. An apple a day keeps the doctor away: potential role of miRNA 146 on macrophages treated with exosomes derived from apples. Biomedicines. 2022;10:415. doi: 10.3390/biomedicines10020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L., et al. Engineering exosome-like nanovesicles derived from Asparagus cochinchinensis can inhibit the proliferation of hepatocellular carcinoma cells with better safety profile. Int. J. Nanomed. 2021;16:1575–1586. doi: 10.2147/IJN.S293067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang M., et al. Bitter melon derived extracellular vesicles enhance the therapeutic effects and reduce the drug resistance of 5-fluorouracil on oral squamous cell carcinoma. J. Nanobiotechnol. 2021;19:259. doi: 10.1186/s12951-021-00995-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang B., et al. Momordica charantia-derived extracellular vesicles-like nanovesicles inhibited glioma proliferation, migration, and invasion by regulating the PI3K/AKT signaling pathway. J. Funct.Foods. 2022;90 [Google Scholar]
- 43.Cui W.-W., et al. Momordica. Charantia-derived extracellular vesicles-like nanovesicles protect cardiomyocytes against radiation injury via attenuating DNA damage and mitochondria dysfunction. Front. Cardiovasc. Med. 2022;9 doi: 10.3389/fcvm.2022.864188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Robertis M., et al. Blueberry-derived exosome-like nanoparticles counter the response to TNF-α-induced change on gene expression in EA.hy926 cells. Biomolecules. 2020;10:742. doi: 10.3390/biom10050742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao W.-j., et al. Blueberry-derived exosomes-like nanoparticles ameliorate nonalcoholic fatty liver disease by attenuating mitochondrial oxidative stress. Acta Pharmacol. Sin. 2022;43:645–658. doi: 10.1038/s41401-021-00681-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao J., et al. Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. PeerJ. 2018;6 doi: 10.7717/peerj.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deng Z., et al. Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell AMP-activated protein kinase. Mol. Ther. 2017;25:1641–1654. doi: 10.1016/j.ymthe.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim D.K., Rhee W.J. Antioxidative effects of carrot-derived nanovesicles in cardiomyoblast and neuroblastoma cells. Pharmaceutics. 2021;13:1203. doi: 10.3390/pharmaceutics13081203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao Z., et al. Isolation of exosome-like nanoparticles and analysis of MicroRNAs derived from coconut water based on small RNA high-throughput sequencing. J. Agric. Food Chem. 2018;66:2749–2757. doi: 10.1021/acs.jafc.7b05614. [DOI] [PubMed] [Google Scholar]
- 50.Lee R., et al. Anti-melanogenic effects of extracellular vesicles derived from plant leaves and stems in mouse melanoma cells and human healthy skin. J. Extracell. Vesicles. 2020;9 doi: 10.1080/20013078.2019.1703480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wongkaewkhiaw S., et al. Induction of apoptosis in human colorectal cancer cells by nanovesicles from fingerroot (Boesenbergia rotunda (L.) Mansf.) PLoS One. 2022;17 doi: 10.1371/journal.pone.0266044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ozkan I., et al. Garlic (Allium sativum)-derived SEVs inhibit cancer cell proliferation and induce caspase mediated apoptosis. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-93876-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhuang X., et al. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles. 2015;4 doi: 10.3402/jev.v4.28713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Z., et al. Arrowtail RNA for ligand display on ginger exosome-like nanovesicles to systemic deliver siRNA for cancer suppression. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-32953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang M., et al. Highly biocompatible functionalized layer-by-layer ginger lipid nano vectors targeting P-selectin for delivery of doxorubicin to treat colon cancer. Adv. Therapeut. 2019;2 doi: 10.1002/adtp.201900129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalarikkal S.P., et al. A cost-effective polyethylene glycol-based method for the isolation of functional edible nanoparticles from ginger rhizomes. Sci. Rep. 2020;10:4456. doi: 10.1038/s41598-020-61358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cao M., et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J. ImmunoTher. Cancer. 2019;7:326. doi: 10.1186/s40425-019-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cho E.-G., et al. Panax ginseng-derived extracellular vesicles facilitate anti-senescence effects in human skin cells: an eco-friendly and sustainable way to use ginseng substances. Cells. 2021;10:486. doi: 10.3390/cells10030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim J., et al. Isolation and characterization of ginseng-derived exosome-like nanoparticles with sucrose cushioning followed by ultracentrifugation. SN Appl. Sci. 2022;4:63. [Google Scholar]
- 60.Mu J., et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014;58:1561–1573. doi: 10.1002/mnfr.201300729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang B., et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol. Ther. : J. Am. Soc. Gene Ther. 2014;22:522–534. doi: 10.1038/mt.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhuang X., et al. Grapefruit-derived nanovectors delivering therapeutic miR17 through an intranasal route inhibit brain tumor progression. Mol. Ther. 2016;24:96–105. doi: 10.1038/mt.2015.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Savcı Y., et al. Grapefruit-derived extracellular vesicles as a promising cell-free therapeutic tool for wound healing. Food Funct. 2021;12:5144–5156. doi: 10.1039/d0fo02953j. [DOI] [PubMed] [Google Scholar]
- 64.Niu W., et al. A biomimetic drug delivery system by integrating grapefruit extracellular vesicles and doxorubicin-loaded heparin-based nanoparticles for glioma therapy. Nano Lett. 2021;21:1484–1492. doi: 10.1021/acs.nanolett.0c04753. [DOI] [PubMed] [Google Scholar]
- 65.Xiao Q., et al. Lemon-derived extracellular vesicles nanodrugs enable to efficiently overcome cancer multidrug resistance by endocytosis-triggered energy dissipation and energy production reduction. Adv. Sci. 2022;9:2105274. doi: 10.1002/advs.202105274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sriwastva M.K., et al. Exosome-like nanoparticles from Mulberry bark prevent DSS-induced colitis via the AhR/COPS8 pathway. EMBO Rep. 2022;23 doi: 10.15252/embr.202153365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamasaki M., et al. Onion (Allium cepa L.)-Derived nanoparticles inhibited LPS-induced nitrate production, however, their intracellular incorporation by endocytosis was not involved in this effect on RAW264 cells. Molecules. 2021;26:2763. doi: 10.3390/molecules26092763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han J.M., et al. Immunostimulatory potential of extracellular vesicles isolated from an edible plant, petasites japonicus, via the induction of murine dendritic cell maturation. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms221910634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu B., et al. Protective role of shiitake mushroom-derived exosome-like nanoparticles in D-galactosamine and lipopolysaccharide-induced acute liver injury in mice. Nutrients. 2020;12:477. doi: 10.3390/nu12020477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perut F., et al. Strawberry-derived exosome-like nanoparticles prevent oxidative stress in human mesenchymal stromal cells. Biomolecules. 2021;11:87. doi: 10.3390/biom11010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Y., et al. In vitro effects of tartary buckwheat-derived nanovesicles on gut microbiota. J. Agric. Food Chem. 2022;70:2616–2629. doi: 10.1021/acs.jafc.1c07658. [DOI] [PubMed] [Google Scholar]
- 72.Chen Q., et al. Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. Acta Pharm. Sin. B. 2022;12:907–923. doi: 10.1016/j.apsb.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zu M., et al. ‘Green’ nanotherapeutics from tea leaves for orally targeted prevention and alleviation of colon diseases. Biomaterials. 2021;279 doi: 10.1016/j.biomaterials.2021.121178. [DOI] [PubMed] [Google Scholar]
- 74.Nemidkanam V., Chaichanawongsaroj N. Characterizing Kaempferia parviflora extracellular vesicles, a nanomedicine candidate. PLoS One. 2022;17 doi: 10.1371/journal.pone.0262884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bokka R., et al. Biomanufacturing of tomato-derived nanovesicles. Foods. 2020;9:1852. doi: 10.3390/foods9121852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Şahin F., et al. In vitro wound healing activity of wheat-derived nanovesicles. Appl. Biochem. Biotechnol. 2019;188:381–394. doi: 10.1007/s12010-018-2913-1. [DOI] [PubMed] [Google Scholar]
- 77.Zhang M., et al. Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy. Mol. Ther. : J. Am. Soc. Gene Ther. 2016;24:1783–1796. doi: 10.1038/mt.2016.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sundaram K., et al. Plant-derived exosomal nanoparticles inhibit pathogenicity of Porphyromonas gingivalis. iScience. 2019;21:308–327. doi: 10.1016/j.isci.2019.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu N.-J., et al. Lipidomic analysis reveals the importance of GIPCs in Arabidopsis leaf extracellular vesicles. Mol. Plant. 2020;13:1523–1532. doi: 10.1016/j.molp.2020.07.016. [DOI] [PubMed] [Google Scholar]
- 80.Pocsfalvi G., et al. Protein biocargo of citrus fruit-derived vesicles reveals heterogeneous transport and extracellular vesicle populations. J. Plant Physiol. 2018;229:111–121. doi: 10.1016/j.jplph.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 81.Stanly C., et al. Membrane transporters in citrus clementina fruit juice-derived nanovesicles. Int. J. Mol. Sci. 2019;20:6205. doi: 10.3390/ijms20246205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang M., et al. Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy. Mol. Ther. 2016;24:1783–1796. doi: 10.1038/mt.2016.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yin L., et al. Characterization of the MicroRNA profile of ginger exosome-like nanoparticles and their anti-inflammatory effects in intestinal caco-2 cells. J. Agric. Food Chem. 2022;70 doi: 10.1021/acs.jafc.1c07306. [DOI] [PubMed] [Google Scholar]
- 84.Stanly C., et al. Grapefruit-derived micro and nanovesicles show distinct metabolome profiles and anticancer activities in the A375 human melanoma cell line. Cells. 2020;9:2722. doi: 10.3390/cells9122722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Berger E., et al. Use of nanovesicles from orange juice to reverse diet-induced gut modifications in diet-induced obese mice. Mol. Ther. Methods Clin. Dev. 2020;18:880–892. doi: 10.1016/j.omtm.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Regente M., et al. Phospholipids are present in extracellular fluids of imbibing sunflower seeds and are modulated by hormonal treatments. J. Exp. Bot. 2008;59:553–562. doi: 10.1093/jxb/erm329. [DOI] [PubMed] [Google Scholar]
- 87.Li M., et al. Phospholipase D- and phosphatidic acid-mediated signaling in plants. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2009;1791:927–935. doi: 10.1016/j.bbalip.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 88.Teng Y., et al. Plant-derived exosomal MicroRNAs shape the gut microbiota. Cell Host Microbe. 2018;24:637–652. doi: 10.1016/j.chom.2018.10.001. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brown H.A., et al. Targeting phospholipase D in cancer, infection and neurodegenerative disorders. Nat. Rev. Drug Discov. 2017;16:351–367. doi: 10.1038/nrd.2016.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baldrich P., et al. Plant extracellular vesicles contain diverse small RNA species and are enriched in 10- to 17-nucleotide “tiny” RNAs. Plant Cell. 2019;31:315–324. doi: 10.1105/tpc.18.00872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cai Q., et al. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science. 2018;360:1126–1129. doi: 10.1126/science.aar4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zand Karimi H., et al. The Plant Cell; 2022. Arabidopsis Apoplastic Fluid Contains sRNA- and Circular RNA–Protein Complexes that Are Located outside Extracellular Vesicles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Woith E., et al. Plant extracellular vesicles and nanovesicles: focus on secondary metabolites, proteins and lipids with perspectives on their potential and sources. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22073719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Teng Y., et al. Grapefruit-derived nanovectors deliver miR-18a for treatment of liver metastasis of colon cancer by induction of M1 macrophages. Oncotarget. 2016;7:25683–25697. doi: 10.18632/oncotarget.8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim K., et al. Stability of plant leaf-derived extracellular vesicles according to preservative and storage temperature. Pharmaceutics. 2022;14:457. doi: 10.3390/pharmaceutics14020457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arai M., et al. Uptake pathway of apple-derived nanoparticle by intestinal cells to deliver its cargo. Pharmaceut. Res. 2021;38:523–530. doi: 10.1007/s11095-021-03018-8. [DOI] [PubMed] [Google Scholar]
- 97.Song H., et al. Internalization of garlic-derived nanovesicles on liver cells is triggered by interaction with CD98. ACS Omega. 2020;5:23118–23128. doi: 10.1021/acsomega.0c02893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim K., et al. Anti-metastatic effects of plant sap-derived extracellular vesicles in a 3D microfluidic cancer metastasis model. J. Funct. Biomater. 2020;11:49. doi: 10.3390/jfb11030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim K., et al. Cytotoxic effects of plant sap-derived extracellular vesicles on various tumor cell types. J. Funct. Biomater. 2020;11:22. doi: 10.3390/jfb11020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Han X., et al. Ginseng-derived nanoparticles potentiate immune checkpoint antibody efficacy by reprogramming the cold tumor microenvironment. Mol. Ther. 2022;30:327–340. doi: 10.1016/j.ymthe.2021.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Raimondo S., et al. Label-free quantitative proteomic profiling of colon cancer cells identifies acetyl-CoA carboxylase alpha as antitumor target of Citrus limon-derived nanovesicles. J. Proteonomics. 2018;173:1–11. doi: 10.1016/j.jprot.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 102.Potestà M., et al. Effect of microvesicles from Moringa oleifera containing miRNA on proliferation and apoptosis in tumor cell lines. Cell Death Discov. 2020;6:43. doi: 10.1038/s41420-020-0271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Q., et al. Grapefruit-derived nanovectors use an activated leukocyte trafficking pathway to deliver therapeutic agents to inflammatory tumor sites. Cancer Res. 2015;75:2520–2529. doi: 10.1158/0008-5472.CAN-14-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yan W., et al. Overcoming drug resistance in colon cancer by aptamer-mediated targeted Co-delivery of drug and siRNA using grapefruit-derived nanovectors. Cell. Physiol. Biochem. 2018;50:79–91. doi: 10.1159/000493960. [DOI] [PubMed] [Google Scholar]