Abstract
Osteoarthritis (OA) is a highly prevalent whole-joint disease that causes disability and pain and affects a patient's quality of life. However, currently, there is a lack of effective early diagnosis and treatment. Although stem cells can promote cartilage repair and treat OA, problems such as immune rejection and tumorigenicity persist. Extracellular vesicles (EVs) can transmit genetic information from donor cells and mediate intercellular communication, which is considered a functional paracrine factor of stem cells. Increasing evidences suggest that EVs may play an essential and complex role in the pathogenesis, diagnosis, and treatment of OA. Here, we introduced the role of EVs in OA progression by influencing inflammation, metabolism, and aging. Next, we discussed EVs from the blood, synovial fluid, and joint-related cells for diagnosis. Moreover, we outlined the potential of modified and unmodified EVs and their combination with biomaterials for OA therapy. Finally, we discuss the deficiencies and put forward the prospects and challenges related to the application of EVs in the field of OA.
Keywords: Extracellular vesicles, Osteoarthritis, Biomarkers, Diagnosis, Treatment
Graphical abstract
This review provides an overview highlighting the breakthrough of extracellular vesicles in pathogenesis, diagnosis, and treatment of osteoarthritis.
Highlights
-
•
The influence of extracellular vesicles on osteoarthritis progression through inflammation, senescence, and metabolism are discussed.
-
•
Extracellular vesicles from different sources have values of different dimensions in diagnosis, such asdiagnostic, monitoring, and predictive values.
-
•
The roles of extracellular vesicles from different sources and in different ways of engineering/modification are summarized.
-
•
The strategies for designing more intelligent and multifunctional extracellular vesicles are proposed.
1. Introduction
Osteoarthritis (OA) is a common and disabling disease that places a huge burden on individuals and the social economy [1]. With the aging population and increase in obesity, OA has become more common [2]. Traditional treatments for OA include nonsurgical therapies, such as nonsteroidal anti-inflammatory drugs (NSAIDs), and surgical therapies, such as joint replacement for advanced pain. Unfortunately, they do not reduce the incidence of the disease in its early stages [3], nor do they prevent cartilage degeneration or promote regeneration [4]. Emerging treatments, such as stem cell therapy, also have many risks, including immune rejection [5] and tumorigenicity [6]. Therefore, further understanding of the factors and mechanisms underlying OA pathophysiology can provide new methods to more effectively prevent and treat OA.
OA involves changes in the articular cartilage, subchondral bone, synovium, ligaments, and muscles [7]. OA was originally thought to be an age-related disease, but recent studies suggest that it is an active dynamic change caused by the imbalance between repair and destruction, and should be considered a syndrome rather than a single disease [8]. Increased levels of inflammatory components, metabolic changes, cellular senescence, and mechanical overload are thought to be related to the development of OA [2,9]. These factors lead to erosion of the cartilage surface, which in turn cause matrix degradation and the release of pro-inflammatory mediators as chondrocytes attempt repair. Inflammatory infiltration reduces chondrocyte function and promotes cell senescence, further stimulating the proliferation of the adjacent synovial membrane and ultimately leading to tissue hypertrophy and vascular proliferation [10]. Interestingly, the production, degradation, and content of extracellular vesicles (EVs) are associated with all these processes [[11], [12], [13]], indicating that EVs play an essential role in the pathogenesis of OA.
EVs are structures released by all cells and are currently considered a 'new word’ in the orthopedic field [14]. EVs were initially thought to be a kind of metabolic waste, but it was then realized that EVs carry many proteins and nucleic acids [15]. Thus, they may represent the state of their donor cells [16] and play an essential role in mediating cellular communication [17], which offers EVs pathological capability.
EVs are produced in various ways but they are all from the plasma membrane, which gives them low immunogenicity. Specifically, exosomes (a type of the EVs) are released when the multivesicular body is fused with the plasma membrane, whereas other EVs, such as microvesicles, microparticles, large oncosomes, and apoptotic bodies, could be released directly from the plasma membrane [18,19]. Besides, EVs have the same structure as cells: an extracellular domain of lipids and transmembrane proteins on the surface, and cytoplasmic components inside, which makes them relatively stable in body fluids [20]. Therefore, cargoes carried by EVs may also be used as potential biomarkers [21], which are urgently required for OA. The current diagnosis relies on symptoms and physical examination, but they are not typical in the early stage, resulting in severe damage to the joint structure of many patients [22]. Biomarkers, such as EVs, can be used to timeously predict and monitor changes within cells, contributing to early intervention.
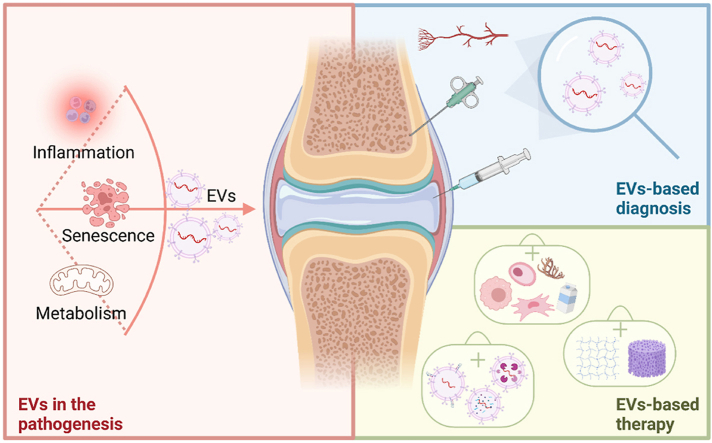
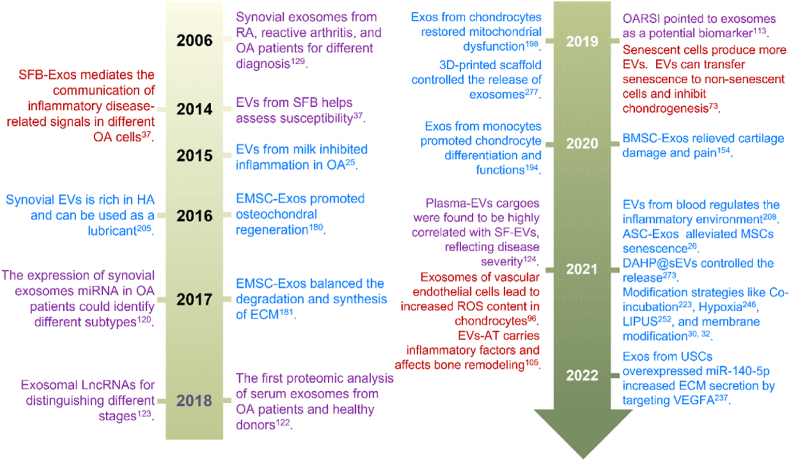
More attention has been paid to EVs as a cell-free treatment strategy for OA since traditional treatments and stem cell therapies have many limitations and risks [23]. EVs not only inherit most functions of parent cells but also avoid a series of problems caused by parent cells, such as immune compatibility, stability, heterogeneity and stemness maintenance [24]. Besides, the low immunogenicity of EVs makes it possible to use EVs derived from various biological sources, such as milk [25], antler [26], and marine organisms [27], to slow OA progression and promote regeneration. In addition, the efficiency, specificity, and safety of EVs can be improved by engineered-EVs through modifying parent cells and directly modifying EVs [[28], [29], [30], [31]]. They can also remain in the joint for a longer time and are evenly distributed around the target area, partly solving the problem of intra-articular drug delivery [32]. In the past decade, the research field of EVs has developed rapidly, covering all aspects from pathogenesis, diagnosis, to treatment of OA (Fig. 1).
Fig. 1.
Key events of EVs in the pathogenesis, diagnosis, and treatment of OA. Red: Typical examples of EVs in pathogenesis research. Purple: The signature events of EVs as markers for diagnosis and differential diagnosis. Blue: The therapeutic roles of EVs from different sources through different mechanisms, and all known modification strategies, as well as typical biomaterials for loading EVs.
As a hot topic, EVs have been widely studied in cancer, cardiovascular diseases, and neurological diseases [33]. To date, existing reviews on EVs in OA have mainly focused on the therapeutic effects. This review provides an overview of the role of EVs in OA, focusing on the pathogenesis, diagnosis, and treatment, while considering the associated challenges and limitations (Fig. 2). We aimed to summarize the following main aspects: (1) the role of EVs in OA progression by influencing inflammation, metabolism, and aging; (2) EVs from blood, synovial fluid (SF), and joint-related cells used for diagnosis and differential diagnosis; and (3) therapeutic values of modified and unmodified EVs for treating OA and biomaterial-loaded EVs for release control. Moreover, an outlook for the future development of EVs in the arthrosis fields is provided. It can be expected that this review might provide an exhaustive compendium with respect to the main aspects of EVs-related research in OA and might also stimulate its future development and applications.
Fig. 2.
EVs in the pathogenesis, diagnosis, and treatment of osteoarthritis.
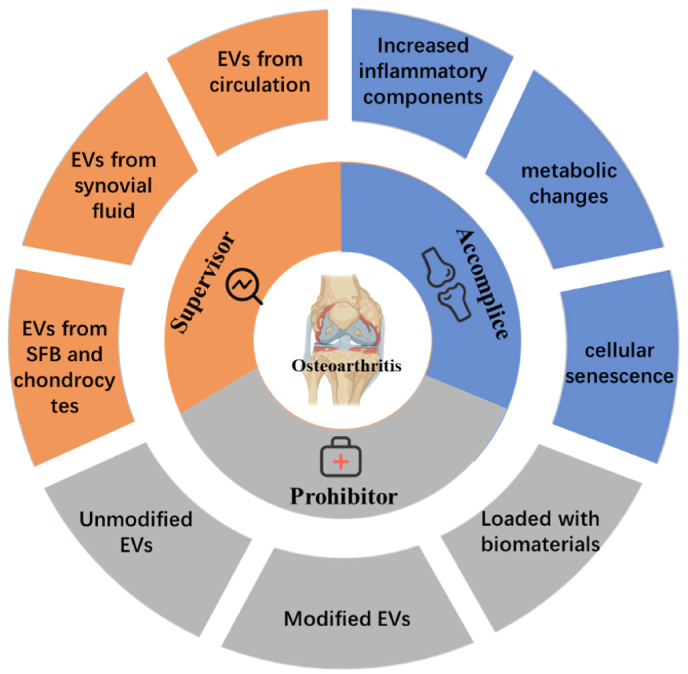
2. 'Accomplice'——EVs in the pathogenesis of osteoarthritis
OA is an active dynamic change caused by an imbalance between the repair and destruction of joint tissues [34]. The pathogenesis of the disease is complex and remains unclear, involving factors like inflammation, aging, and metabolism, which ultimately leads to structural destruction of joints [2,35]. EVs are important mediators of cell-to-cell communications in physiology and are also involved in disease progression, delivering pathological cargo from malfunctioning cells. Recently, an increasing number of studies have shown that EVs play an important role in all three parts, accelerating the progression of OA. In addition, EVs also affect other factors related to OA pathogenesis, such as angiogenesis and mechanical overload [36]. For instance, synovial fibroblast derived EVs increases the expression of VEGF, which stimulates neovascularization leading to OA progression [37]. While stimulated by fluid-flow shear stress (FFSS), the number of exosomes secreted by primary chondrocytes increased and the protein levels of MGP, TNAP, and NPP1 are abnormal, thus increasing cartilage calcification [38]. In this section, we specifically discuss the three major factors of inflammation, aging, and metabolism, overview the roles of EVs in promoting them, and point out the possible directions for further research (Table 1).
Table 1.
The characteristics and values of EVs in the pathogenesis of OA.
| Mechanisms | Sources | Characteristics | Functions | Ref. |
|---|---|---|---|---|
| Angiogenesis | FLS | High expression of VEGF | Stimulate neovascularization leading to OA progression | [37] |
| Mechanical overload | Chondrocytes | Increased number and abnormal levels of MGP, TNAP and NPP1 proteins | Increase cartilage calcification | [38] |
| Increased inflammatory component | M1 macrophage | High expression of miR-1246 | Transfer miR-1246 to chondrocytes and activate the Wnt/β-catenin pathway by inhibiting the expression of GSK3β and Axin2 | [39] |
| Chondrocytes | High expression of miR-449a-5p | Inhibit autophagy in LPS-induced macrophages by inhibiting ATG4B expression, and promote the production of mitoROS and mature IL-1β | [40] | |
| FLS | High expression of miR-142-5p and RUNX2 | Accelerate IL-1β-induced apoptosis and cartilage matrix degradation by miR-142-5p/RUNX2 in chondrocytes | [41] | |
| Chondrocytes | High expression of circ-BRWD1 and miR-1277 | Promote matrix degradation and cell apoptosis | [42] | |
| Chondrocytes | High expression of circ-001846 and miR-149-5p | Mediate chondrocyte injury through miR-149-5p/WNT5b | [43] | |
| Synovial fluid | Low expression of miR-193b-3p | Decrease inhibition of HDAC3 expression and promotion of H3 acetylation | [44] | |
| Chondrocytes | Carrying autophagy-associated tubulin 1A/1B LC3 | Cause cartilage calcification and degradation | [45] | |
| Osteoclast | High expression of miR-214-3p | Affect osteoblast activity and bone formation | [46] | |
| Osteoblast | High expression of miR-210-5p | Inhibit the oxygen consumption rate of chondrocytes and trigger catabolic gene expression of chondrocytes | [47] | |
| Cellular senescence | Senescent chondrocytes | High expression of miR-27b, −199a, −185 | Inhibit cartilage homeostasis and upregulate inflammations | [48] |
| Chondrocytes | High expression of connexin 43 | Promote inflammation and regulate cell senescence | [49] | |
| Senescent MSCs | Low expression of miR-21-5p | Reduce immunotherapy function | [50] | |
| SnCs | High expression of NF-κB | Participate in multiple innate and adaptive immune responses to spread inflammation | [51] | |
| Muscle | High expression of miR-34a-5p | Induce senescence of bone marrow stem cells | [52] | |
| MSCs | High expression of aging markers and miR-118-3p; | Affect the function and behavior of MSC | [53] | |
| low expression of pluripotent markers | ||||
| Senescent endothelial cells | High expression of miR-31 | Reduce osteogenesis by knocking down FZD3 mRNA | [54] | |
| Senescent bone marrow | High expression of miR-183-5p | Reduce cell proliferation and differentiation, promote oxidative stress, thus inhibiting the osteogenic activity of young MSCs | [55] | |
| Metabolic alterations | Vascular endothelial cells | Low expression of autophagy and p21 | Increase levels of ROS, thereby inducing apoptosis | [56] |
| Chondrocytes | High expression of miR-449a-5p | Inhibit macrophage autophagy by miR-449a-5p/ATG4B, cause ROS production, increase IL-1β production and ultimately aggravating synovitis and cartilage erosion | [40] | |
| Oxidative stress-EVs | / | Activate Toll-like receptor 4 through synergy between 15-lipoxygenase and secreted PLA 2, resulting in aseptic inflammation | [57] |
2.1. EVs in inflammation
Relatively low-grade inflammation could be found in most OA patients, characterized by synovitis, a pro-inflammatory/catabolic state of chondrocytes, and destruction of subchondral bone [35]. The knee SF was infiltrated by leukocytes, of which CD14+ leukocytes were the most abundant, followed by CD4+ leukocytes [58]. Further studies have shown that the degree of leukocyte infiltration correlates with the volume of fluid on magnetic resonance imaging (MRI) [59]. Systemic inflammation also plays a key role in pathogenesis [60], which can be reflected by abnormal peripheral leukocyte (PBL) [61]. Analysis of PBL's phenotypes in 114 patients with OA and healthy controls found that the proportion of CD8+ cells was increased in patients with OA, especially Treg and memory T cells [62]. NF-κB signal transduction is the main mechanism underlying the inflammation of OA [63], triggered by pattern recognition receptor (PRR), such as Toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) [64]. Inflammation has also been associated with pain [34]. Miller et al. [65]. found that injury-related molecular patterns produced in OA directly stimulate nociceptive neurons in mice via Toll-like receptor 4 (TLR-4), which leads to pain.
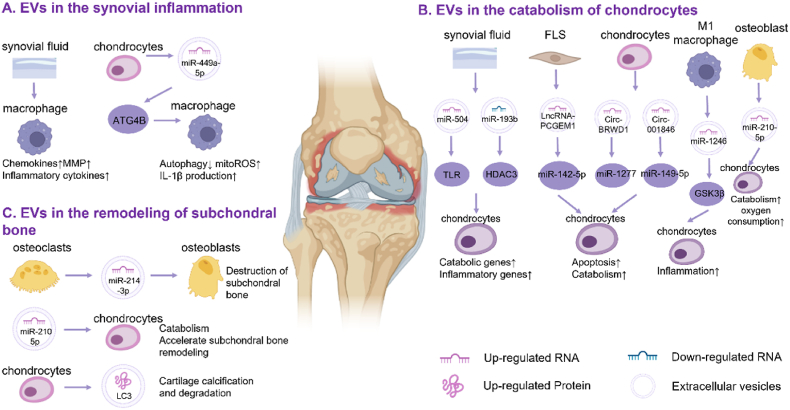
Although the role of EVs in inflammatory diseases has been well studied [11], little is known about their function in OA. Currently, it is believed that EVs aggravate inflammation in OA through three modes of action. First, EVs lead to the spread of synovial inflammation by activating macrophages in SF. Second, EVs from various cells in the joint microenvironment affect chondrocyte catabolism and promote cartilage destruction. Finally, subchondral bone remodeling is also regulated by EVs. (Fig. 3). Correspondingly, inflammation can also alter the amount and content of EVs. For example, IL-1β stimulated synovial fibroblasts and macrophages show increased production of exosomes [37], and inflammatory mediators such as TNF-α, affect the protein and RNA concentrations in EVs [19]. Thus, inflammation and EVs interact to promote the development of inflammation in OA.
Fig. 3.
EVs from different cells exacerbates the inflammation of osteoarthritis: (A) EVs from SF and chondrocytes affect macrophages, leading to the intensification of synovial inflammation. (B) EVs from the joint microenvironment affect chondrocyte metabolism, leading to increased catabolism and cartilage matrix destruction. (C) EVs from osteoclasts cause abnormal subchondral bone remodeling.
2.1.1. EVs in the synovial inflammation
Synovitis is a common feature of OA and is characterized by significant synovial cell proliferation, tissue hypertrophy, and vascular hyperplasia. Activation may occur secondary to the release of inflammatory mediators during the initial stage of injury. While synovial tissue attempts to repair, it produces additional inflammatory mediators, forming a positive feedback loop that drives joint degeneration [66]. EVs mediate crosstalk between synovial cells, chondrocytes, and immune cells in OA, promoting the progression of synovial inflammation.
Activated synovial macrophages have been confirmed to play an essential role in synovitis; however, the mechanism of activation remains unclear. Rossana et al. [67]. found that exosomes isolated from OA SF significantly stimulated macrophages to release various inflammatory cytokines, chemokines, and metalloproteinases, such as the CCL family and MMP family. The immunomodulatory properties of EVs isolated from OA SF have been demonstrated for the first time.
Similarly, Ni and colleagues showed that exosomes from OA chondrocytes enhance the maturation of IL-1β in macrophages [40]. They found that these vesicles inhibited ATG4B expression through miR-449a-5p, thereby inhibiting autophagy in LPS-induced macrophages, which promotes the production of mitoROS. MitoROS further enhances inflammasome activation and subsequent IL-1β processing. Finally, an increase in mature IL-1β may aggravate synovial inflammation and promote the progression of OA.
2.1.2. EVs in the catabolism of chondrocytes
The main component of cartilage is type II collagen, and the structure and biochemical composition of the cartilage are strictly regulated by chondrocytes. When activated by inflammatory signals, chondrocytes produce a variety of inflammation-related proteins, such as cytokines, interleukin, and tumor necrosis factor. Among them, collagenase (metalloproteinases 1, 3 and 13) and aggrecan-degrading enzymes (ADAMTS 4 and 5) play important pathogenic roles [68]. Simultaneously, chondrocytes express many Toll-like receptors on their surfaces and are activated by injury-related molecular patterns. This sequence of changes causes chondrocytes to exhibit a secretory phenotype and magnifies established cartilage degradation [69]. During this process, EVs from joint tissues and immune cells may play a role in transmitting inflammatory signals.
Exosomal PCGEM1 from fibroblast-like synoviocytes (FLS) of OA patients promotes IL-1β induced chondrocyte apoptosis and cartilage matrix degradation by upregulating miR-142-5p and RUNX2 [41]. EVs produced by primary chondrocytes treated with IL-1β adversely affected normal chondrocytes. High expression of circ-BRWD1 can act as a sponge for miR-1277, promoting matrix degradation and cell apoptosis [42]. Moreover, the increased expression of circ-001846 mediates chondrocyte injury through miR-149-5p/WNT5b [43]. Peng et al. found that EVs from M1 macrophage promote inflammation by transferring miR-1246 to chondrocytes. The mechanism may be activation of the Wnt/β-catenin pathway by inhibiting the expression of GSK3β and Axin2 [39]. Chondrocytes also internalize OA subchondral osteoblast-derived EVs with high miR-210-5p expression, which inhibits the oxygen consumption rate of chondrocytes and triggers catabolic gene expression in chondrocytes [47]. SF-derived exosomes (SF-Exos) in patients promote joint degeneration by recruiting inflammatory cells and inhibiting cartilage proliferation through the high expression of inflammatory factors and chemokines [70]. A study also found that SF-Exos loaded with higher levels of miR-193b-3p, which targets HDAC3 and promotes H3 acetylation, affects the catabolism of chondrocytes [44]. These studies indicate that EVs from different cells can be internalized by chondrocytes and induce chondrocyte degeneration by delivering various small molecules, such as miRNAs and circRNAs.
2.1.3. EVs in the remodeling of subchondral bone
Subchondral bone consists of a dense cortical plate (subchondral lamina) adjacent to the calcified cartilage and loose cancellous bone (trabecular bone) closer to the bone marrow cavity, which acts as shock absorption and load distribution. The structure and composition of cortical plates and trabeculae in osteoarthritis are significantly altered compared to those in normal conditions [71,72]. Some studies have shown that changes in subchondral bone precedence over cartilage degeneration, indicating that subchondral bone activity may determine cartilage loss [73]. The response of osteoblasts to mechanical stimulation leads to the expression of inflammatory factors and degrading enzymes, which may be the factors leading to subchondral bone destruction. Besides, the increased loading due to loss of cartilage integrity may be the cause of subchondral bone remodeling [74]. EVs might be associated with abnormal subchondral bone destruction and remodeling.
Previous studies have shown that chondrocyte-derived EVs are involved in mineral formation in OA [75]. Recently, the researchers found when the cartilage had pathological calcification, histone deacetylase 6 (HDAC6) would cause chondrocytes to release EVs carrying autophagy-associated tubulin 1A/1B light chain 3B (LC3), which can further cause cartilage calcification and degradation [45]. Besides, osteoclast-derived exosomes are also related to abnormal subchondral remodeling in OA. Li et al. [46]. found that osteoclasts release exosomes containing miR-214-3p, which are internalized by osteoblasts, affecting osteoblast activity and bone formation. Moreover, exosomes released from subchondral bone carry miR-210-5p, which can trigger the catabolism of chondrocytes, further accelerating subchondral bone remodeling [47]. At present, studies on EVs in subchondral bone changes are insufficient. Given that subchondral bone changes early and is rich in nerves that are closely associated with pain production [74], this area deserves further attention.
2.2. EVs in cellular senescence
Cellular senescence is defined as steady arrest of the cell cycle and resistance to apoptosis [76]. It is one of the senescence markers, characterized by permanent stagnation of the cell cycle and the production of senescence-associated secretory phenotypes (SASP) [77]. Although senescent cells (SnCs) are in a permanent state of growth stagnation, they are not dormant in tissues [78]. By contrast, SnCs remain metabolically active. SASP, secreted by SnCs, mainly includes pro-inflammatory cytokines, growth factors, chemokines, and matrix remodeling enzymes [79]. These molecules can induce a series of physiological responses in the surrounding microenvironment, including inflammation, growth stagnation, and tumor genesis. The critical regulator of SASP generation is mTOR, which regulates MAP kinase-activated protein kinase 2 [80] and IL-1α [81].
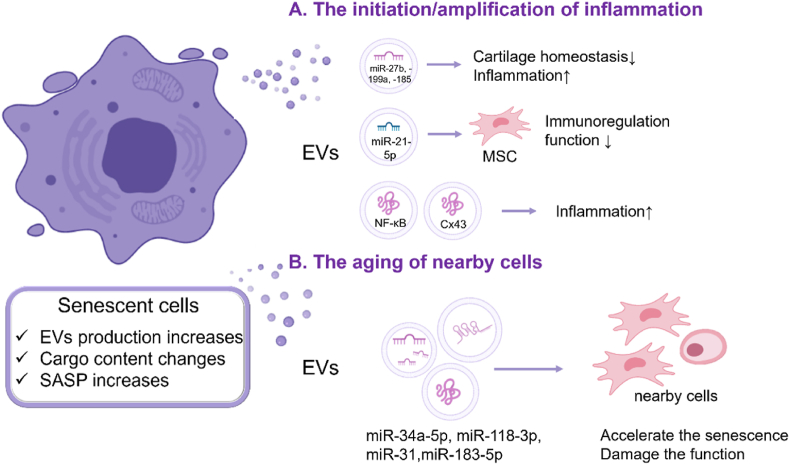
In the OA microenvironment, joint cells show common markers of aging, including telomere wear and increased expression of p16, p21, reactive oxygen species (ROS), and SASP [82]. The production of EVs increases [48], and the cargo of EVs secreted by SnCs, such as proteins and miRNAs, changes in number and type with age [53,50]. EVs with senescent characteristics affect adjacent cells through paracrine signaling, resulting in inflammatory infiltration and cell senescence. (Fig. 4).
Fig. 4.
Roles of EVs in the cell senescence: (A) Age-related EVs lead to the formation and spread of inflammation. (B) EVs from SnCs induce aging of nearby cells, including loss of normal function and increase of cell senescence.
2.2.1. EVs from SnCs cause the initiation/amplification of inflammation
SnCs can regulate the joint microenvironment, increasing inflammation and extracellular matrix (ECM) degradation in OA. Researchers speculated that EVs secreted by SnCs may be involved in this process [48]. First, they demonstrated that SnCs-derived EVs inhibited ECM formation in normal chondrocytes. To further investigate the role of aging related EVs in OA, the researchers eliminated SnCs from OA mice. They found that the secretion of EVs by chondrocytes was reduced and the miRNAs carried by EVs in SF were altered, including miR-27b, −199a, −185, and −23b. These are thought to be associated with catabolism and inflammatory responses. In addition, some immune cell-specific or enriched miRNAs such as miR-223, -150, and −123 were found in EVs, suggesting that senescence related EVs modulate the response, recruitment, and activation of cells in the bone marrow and lymphatic lineages. The researchers also found that sEVs secreted by OA-derived chondrocytes were enriched in transmembrane connexin 43 (Cx43). These sEVs can secrete SASP to promote inflammatory progression and can regulate cell senescence through NF-κB and ERK1/2 [49]. This series of studies shows that EVs from SnCs have a direct negative regulatory effect on inflammation, and the main mechanism might be through inflammation-related miRNAs.
EVs also affect the immunoregulatory function of stem cells, which is critical for maintaining microenvironmental homeostasis [83]. Studies have found that some miRNAs associated with the immune properties of MSCs, such as miR-21-5p, are reduced in EVs from elderly MSCs [50], which is thought to be related to the loss of immunotherapy function.
In addition, while different components of aging-related EVs have been identified and shown to play a role in the progression of multiple diseases, such as inducing tumor cell proliferation and paracrine senescence of primary human fibroblasts [84,85]. However, the downstream signaling pathway activated by EVs remains unclear. Juan et al. found that the NF-κB pathway may be an important component of this pathway [51]. As previously described, NF-κB is involved in multiple innate and adaptive immune responses and is regulated by phosphorylation of the cytoplasmic inhibitor, IκB. They found that the inhibition of IκB kinase (IKK) inhibits NF-κB activation and affects EVs function. These studies suggest that EVs from SnCs can influence the spread of inflammation.
2.2.2. EVs from SnCs accelerate the aging of nearby cells
Apart from aggravating inflammation, EVs from SnCs can transmit senescent phenotypes to nearby cells, thereby causing paracrine senescence. When aging MSC-EVs were co-cultured with young MSCs, down-regulation of pluripotent markers, such as Nanog and Oct4, and up-regulation of aging markers, such as Vinculin, LMNA, and mTOR, was observed [53]. Further studies have found that high expression of miR-118-3p may be associated with senescence. In addition, an increased number of EVs containing muscle-derived miR-34a-5p was detected in the serum of elderly mice. These EVs negatively affected MSCs vitality and increased aging [52]. Senescent phenotypes are like "pathogens" that spreads between cells and joints through EVs as "vectors", leading to systemic involvement and disease progression.
EVs also affect the functions of nearby normal cells. Researchers found that circulating EVs from elderly human donors negatively affected the osteogenic differentiation potential of adipose tissue-derived MSCs (ASCs) in an age-dependent manner [54]. They found that miR-31 was enriched in EVs produced by aging endothelial cells and reduced osteogenesis by knocking down FZD3 mRNA. Similarly, EVs isolated from the bone marrow of aged mice can reduce cell proliferation and differentiation while increasing oxidative stress through the high expression of miR-183-5p, thus inhibiting the osteogenic activity of young MSCs [55]. These studies demonstrated that EVs are extracellular factors, in addition to estrogen and growth factors, which reduce the osteogenic differentiation ability of stem cells with age.
2.3. EVs in metabolic alterations
Metabolism plays a key role in the physiological renewal of synovial joint tissues. Poor diet, obesity and lack of exercise are causes of metabolic changes [86,87]. According to the immune metabolism hypothesis, abnormal metabolism is closely related to many inflammatory responses [88]. Clinical evidence also shows that OA is often associated with metabolic diseases, such as diabetes, and leads to faster deterioration of OA [89]. In OA, chondrocytes and synovial cells undergo metabolic changes from a resting state to a highly active state, which is characterized by enhanced glycolysis and mitochondrial dysfunction. In addition, the tricarboxylic acid cycle, lipid metabolism, cholesterol metabolism, and amino acid metabolism are also closely related to the progression of OA [[90], [91], [92]], but the role of EVs in this process remains unclear. Herein, we introduce the characteristics of metabolic changes in chondrocytes during OA and summarize the role of EVs. We also discussed the effect of systemic metabolic disorders on OA and the characteristics of EVs derived from obesity.
2.3.1. EVs in mitochondrial damage and oxidative stress
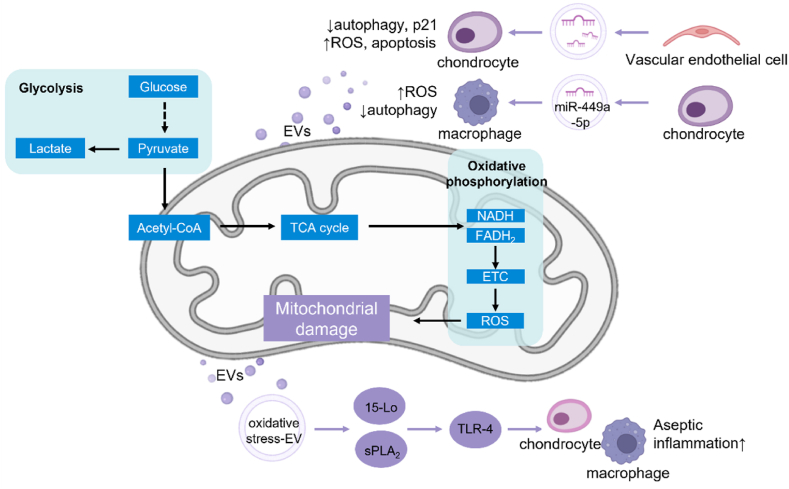
Joint cartilage contains a small number of cells, with no blood vessels, nerves, or lymph [93]. However, chondrocytes metabolize more actively than synovium and plasma, despite having less access to oxygen and glucose [94]. In cartilage, glucose is an essential metabolic fuel [95]. Chondrocytes express several glucose transporters, some of which are regulated by hypoxia and proinflammatory cytokines [94]. Normally, chondrocytes use glucose for energy production through glycolysis and oxidative phosphorylation. However, in the pathological environment of OA, excessive ROS production and mitochondrial damage inhibit AMPK signaling and reduce oxidative phosphorylation, leading to an increase in glycolysis [96]. Glycolysis produces less adenosine triphosphate (ATP) and leads to an accumulation of lactate, which reduces the pH in an already acidic microenvironment, further causing damage to chondrocytes [97]. Mitochondrial dysfunction and oxidative stress initiate a vicious cycle of cartilage metabolic disorders and are thus considered markers of OA [10].
EVs accelerate ROS accumulation, weaken the ability to resist oxidative stress, and cause damage to the mitochondria, thereby promoting the progression of OA (Fig. 5). Yang and colleagues demonstrated that exosomes from vascular endothelial cells reduce autophagy and p21 expression in chondrocytes, leading to increased levels of ROS, and thereby inducing apoptosis [56]. Similarly, exosomes from OA chondrocytes inhibit macrophage autophagy via miR-449a-5p/ATG4B and cause ROS production, which increases IL-1β production, ultimately aggravating synovitis and cartilage erosion in mouse models of OA [40]. Oxidative stress-induced EVs (stress-EVS) can also promote the progression of inflammation and lead to the spread of pain [98]. Ha et al. proposed that oxidative stress-EVs are distinct from lipopolysaccharide (LPS)-induced EVs. They activate Toll-like receptor 4 (a type of DAMP) via synergy between 15-lipoxygenase and secreted PLA 2, resulting in aseptic inflammation [57], which offers a new opportunity to limit aseptic inflammation. Raffaele et al. found that in the dorsal root ganglion (DRG), neuronal-immune crosstalk can be mediated by neuronal exosomes taken up by macrophages, which initiate a feedback loop of elevations in ROS and exosomes, propagating long-term pain [99]. These studies suggest that EVs can lead to an increase in ROS levels, which alters chondrocyte metabolism and leads to increased inflammation and pain.
Fig. 5.
Roles of EVs in mitochondrial damage and oxidative stress. In the pathological state of chondrocytes, oxidative phosphorylation decreases and glycolysis increases, leading to mitochondrial dysfunction and oxidative stress. The role of EVs in this change can be divided into two modes. First, EVs from cartilage and endothelial cells carry endogenous substances to accelerate ROS accumulation and enhance chondrocyte metabolic disorder. Second, EVs in the state of oxidative stress carry a lot of pathological contents, which aggravate the progression of OA.
2.3.2. EVs in systemic metabolic disorders
Apart from mitochondrial dysfunction, systemic metabolic disorders are also major risk factors for OA [[100], [101], [102]]. Obesity is the most vital risk factor for the onset of knee diseases [103]. Obese individuals have an increased risk of OA in non-load-bearing joints, such as hands and wrists, suggesting that adipose tissue and its derivatives, such as exosomes, may play a role in the development of OA [104]. EVs from adipose tissue (EVs-AT) carry adipokines, such as leptin and adiponectin, transfer lipids between adipocytes and macrophages, and contain most circulating miRNAs [105]. In the case of obesity, dysfunctional adipocytes affect the loading of exosome cargo, leading to a series of pathophysiological changes [106]. However, the role of EVs-AT in OA progression has rarely been studied.
Hyperglycemia, hypertension, and dyslipidemia can also regulate joint metabolism. The relationship between type 2 diabetes and OA is undoubtedly the most valuable and well-studied [107]. The effects of diabetes on joint tissues and cells can be divided into two types. First, the increase in glucose levels causes chondrocytes to undergo oxidative stress and release more inflammatory mediators and metalloproteinases [108]. Furthermore, hyperglycemia can induce the deposition of advanced glycation end products in joint tissues, which further increases the release of pro-inflammatory and pro-degradation mediators [109]. Second, insulin resistance weakens the protective effect of insulin on synovial cells. In type 2 diabetes, the synovium shows higher levels of TNF-α and macrophages, which also explains why OA is more severe in diabetics [110]. With regards to dyslipidemia, hypercholesterolemia and hypertriglyceridemia are associated with an increased risk of OA, whereas high-density lipoprotein may have a protective effect, which is consistent with the effect of lipid changes on other diseases. The CH25H-CYP7B1-RORα axis of cholesterol metabolism has recently been identified as a key catabolic regulator of OA, which leads to elevated cholesterol levels in OA chondrocytes [92]. Little attention has been paid to the association between hypertension and OA. Some studies have suggested that hypertension contributes to OA development by promoting atherosclerosis in chondrocytes [101]. It should be noted that the exact influence of blood glucose, lipids, and blood pressure on OA has not been determined, and the role of EVs has not been studied. By studying the role of EVs in hypertension, hyperglycemia, dyslipidemia, and other metabolic syndromes, such as intestinal flora disorder, circadian rhythm disruption, and osteoporosis, we can understand their relationship with OA and better control OA.
3. 'Supervisor'——EVs as a biomarker of OA
Currently, the clinical diagnosis of OA is based on symptoms, such as pain, brief morning stiffness, and functional limitations, and physical examinations, including crepitus, restricted or painful movement, joint tenderness, and bony enlargement. Auxiliary inspection, such as plain radiographs, can also be considered if necessary [2,111,112]. However, patients with OA have few symptoms in their early stages, but treatment becomes difficult as the disease progresses. Thus, early diagnosis using tools, such as biomarkers, is urgently required [22]. Biomarkers have many merits in terms of diagnostic, predictive and monitoring values [113,114]. Specifically, they can help with early diagnosis and differentiation of subgroups, identify people at high risk of developing OA, and monitor disease progression and response to interventions.
EVs are promising novel biomarkers because they contain specific information from donor cells and have a strong ability to circulate in body fluids [22]. The development of EVs-based biomarkers, whether based on nucleic acids, proteins, lipids or glycans, shows great promise, particularly for cancer, metabolic diseases and neurological diseases [33,115,116]. As early as 2004, polycystin-1 was shown to be detectable in urine-derived exosomes, making exosomes a potential tool for detecting early kidney disease [117]. Shortly after, a group led by Jan Lotvall discovered that exosomes can deliver mRNAs and miRNAs in 2007, which is considered a new mechanism of genetic exchange between cells [118], leading to a series of related studies. ExoDx™ Lung (ALK) is the first biomarker, pass clinical trials in 2016, to diagnose lung cancer by detecting EMLA-ALK mutation [119]. As a milestone, ExoDx™ demonstrates the superiority and value of EVs as biomarkers.
In OA, the earliest progress in the use of EVs as biomarkers came from mechanistic studies. Researchers have found that miRNAs and other nucleic acid substances are differentially expressed between the normal population and OA patients [37]. Subsequently, EVs were found to help distinguish between different subgroups of OA, such as different genders, bringing an interesting area for clinical diagnosis [120,121]. In 2018, the first proteomic analysis of serum exosomes from OA patients and healthy donors showed that exosomal proteins also have potential as biomarkers [122]. Subsequent studies have shown that synovial EVs can distinguish between the different stages of OA [123], expanding the value of EVs as biomarkers. Moreover, recent studies have shown that the content of plasma EVs has a strong correlation with SF-EVs [124], further enhancing the value of plasma EVs as a non-invasive biomarker of OA.
In this section, we analyzed the different roles of EVs from different sources in OA diagnosis and discussed the possibility of EVs as differential markers for inflammatory joint disease. The deficiency of EVs as a biomarker and their future development were also discussed in the last part.
3.1. EVs in the diagnosis of osteoarthritis
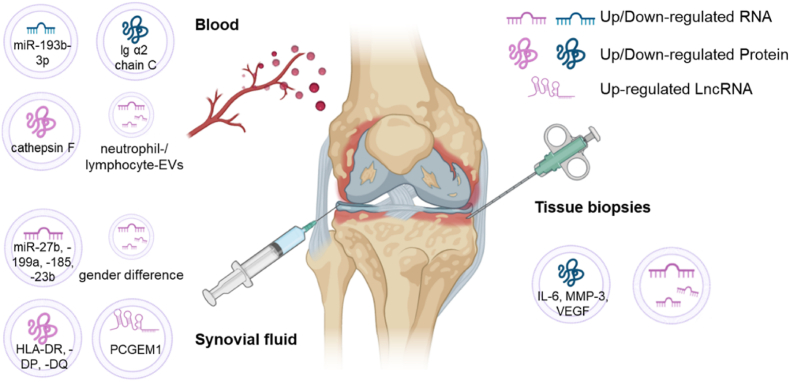
EVs have been studied as biomarkers based on the differences between patients with OA and healthy participants. Owing to their differences in access and content, EVs from different sources play different roles in early diagnosis, identification of inflammation types, and assessment of disease susceptibility (Fig. 6, Table 2).
Fig. 6.
EVs from different sources loaded with different cargoes plays different roles in the diagnosis of OA.
Table 2.
The characteristics and values of EVs in the diagnosis of OA.
| Sources | Characteristic | Diagnosis value | Relationship with OA | Ref. | |
|---|---|---|---|---|---|
| Blood | Plasma | Lower expression of miR-193b | Early diagnosis; | miR-193b is negatively correlated with inflammation | [44] |
| Reflect the phenotypes | |||||
| Serum | Higher expression of cathepsin F and lower lg α2 chain C | Early diagnosis | Cathepsin F may involve in the pathogenesis of OA | [122] | |
| Plasma | Ratios of neutrophil-EVs to lymphocyte-EVs were positively correlated between plasma and SF | Early diagnosis; | Reflect OA joint inflammation and disease severity | [124] | |
| Reflect the severity of OA | |||||
| synovial fluid | Synovial fluid | Higher expression of HLA-DR,-DP, and -DQ | Reflect the severity of OA | Mainly originated from the immune cell infiltration in the OA joint | [124] |
| Synovial fluid | Higher expression of PCGEM1 in late OA than in early OA | Reflect the stages of OA | PCGEM1 inhibited apoptosis, induced autophagy, and stimulated proliferation | [123,125] | |
| Synovial fluid | Differential expression of miRNA between men and women | Reflect the subtype of OA | female specific miRNAs are estrogen responsive and target TLR signaling pathways. | [120] | |
| Synovial fluid | Differential expression of miRNA before and after treatment | Evaluate the effect of treatment | Correlated with cartilage production (in young mice) and immune (in aged mice) | [48] | |
| Tissue biopsies | Synovial fibroblasts | Differential expression of fifty miRNAs | Reflect the susceptibility of OA | miRNA expression patterns in exosomes are altered and miRNAs are selectively released; | [37] |
| Chondrocytes | Nine proteins only present in OA-ACVs | Reflect the presence of stromal changes and predict OA | Differences in ECM proteins in OA and normal ACVs largely reflect known changes in OA ECM and increased catabolism | [126] | |
*ACV: articular cartilage vesicles.
3.1.1. EVs from circulation can be used as a screen for early OA
As mentioned previously, the early diagnosis of OA is essential. However, to our knowledge, there are no validated diagnostic criteria for early-stage OA. Blood sampling is less invasive, and EVs are stable in body fluids, such as blood. Thus, we believe that EVs can be used as an early screening tool. They can use less invasive methods to assess joint burden and potential injury factors when symptoms are present, but not yet confirmed by imaging. Moreover, the cellular and molecular basis can be understood based on early diagnosis to help select appropriate interventions.
Meng et al. [44]. found that there was a significant decrease in the expression of exosomal miR-193b-3p in the plasma of OA patients compared to normal participants, which was parallel to the decline observed in degenerate cartilage samples. Similarly, previous studies have shown that serum miR-193b expression is negatively correlated with inflammation [127]. Therefore, plasma exosomal miR-193b is a potential biomarker.
In addition to nucleic acids, the differential expression of proteins in plasma EVs may also serve as diagnostic evidence. Based on proteomic findings of serum exosomes from patients with OA and healthy controls, serum cathepsin F levels in exosomes were increased, while Ig α2 chain C region levels were decreased in patients with OA [122]. However, as in other experiments, how to avoid the influence of serum proteins on the results is a problem that needs to be solved.
In addition, further studies found that EVs subsets in plasma have significant associations with those in SF, such as CD34+EVs, CD29+LEVs, and CD15+ and CD19+MEVs [124]. The ratio of neutrophil-EVs to lymphocyte-EVs (representing a pro-inflammatory marker) was also positively correlated, indicating that plasma EVs could also reflect the severity of OA like SF-EVs besides being useful for early diagnosis.
3.1.2. EVs from SF can provide information about the stages and subtypes of OA and analyze the treatment efficacy
EVs from the SF are rich in substances secreted by cells in the joint structure, which can timely reflect the state of OA. In addition, the differential expression of EVs cargoes also shows potential for identifying different subtypes of OA. Moreover, by comparing the EVs content before and after treatment, they can be used to assess efficacy.
The number of EVs carrying HLA-DR, -DP, and -DQ from SF is 25–50 times than that from blood (with a significant difference), suggesting that infiltrated immune cells mainly contribute to the SF-EV pool [124]. They reflect antigen-presenting cells and activated t-nucleus pro-inflammatory fibroblasts, which can be used to assess disease severity. Additionally, Zhao et al. [123]. demonstrated that exosomal long non-coding RNA (lncRNAs) could also be used to distinguish different stages of OA. They found that SF exosomal lncRNA prostate-specific transcription 1 (PCGEM1), which acts as a sponge lncRNA targeting miR-770, stimulating the proliferation of synoviocytes [125], was significantly differentially expressed. Exosomal lncRNA PCGEM1 not only differs between OA patients and healthy people but also shows significant differences between late OA and early OA. In addition, it was highly correlated with the WOMAC Index, an osteoarthritis index score. Therefore, exosomal lncRNAs from SF can be used not only to distinguish OA from the normal population but also to distinguish different stages of OA.
SF-EVs can also be used to distinguish between OA subtypes. Kolhe et al. [120]. showed that exosomal miRNAs might be expressed differently in patients with OA according to gender. By analyzing SF exosomal miRNAs, they found 69 down-regulated miRNAs and 45 up-regulated miRNAs in men and 91 and 53 in women respectively, of which miR-504-3p was the only miRNA up-regulated in both male and female OA patients. Further research found that exosomal miRNAs expressed explicitly in female OA patients respond to estrogen and target the toll-like receptor (TLR) signaling pathways. The decrease in miRNA in targeted immunity and TLR-related genes may reduce the ability to prevent inflammation, which may explain the prevalence of OA in females. Other subgroups of interest include supervised exercise, specific comorbidities, and the use of NSAIDs drugs. The identification of subgroups is helpful for targeted management and in-depth understanding of the mechanism of OA.
Besides, Jeon et al. [48]. found that the miRNA and protein content of EVs isolated from SF in OA were similar to those of senescent chondrocytes. Senolytic treatment led to changes in miRNAs such as miR-34a, −30C, −125a, −24, −92A, −150, and −186, which are associated with chondrogenesis. This suggests that EVs can be used not only to diagnose OA but also to evaluate the effects of treatment.
It is important to note that whether biomarkers are for stages, subtypes, or therapeutic effects, they are always focused on a specific joint, whereas studies that have focused on multiple joints have not looked at their potential as biomarkers. Joint specificity affects patient management, such as regulated exercise and drug targeting; thus, developing biomarkers for specific joints is a problem that needs to be solved. In addition, in recent years, an increasing number of biomarker studies on OA have focused on different phenotypes, such as the inflammatory driven and chondrogenic driven phenotypes [128]. Identification of phenotype is an important step in understanding disease, diagnosis, and treatment. However, the role of EVs in phenotypes remains unclear and requires further study.
3.1.3. EVs of synovial fibroblasts (SFB) and chondrocytes in healthy individuals can provide information on susceptibility to OA
EVs can reflect the characteristics and status of donor cells, such as synovial fibroblasts (SFB) and chondrocytes, and help predict disease susceptibility. Tomohiro found that EVs isolated from normal SFB had low expression of inflammatory cytokines, IL-6, MMP-3, and VEGF, while IL-1ß, TNF-α, MMP-9 and MMP-13 could not be detected. Compared to normal SFB, a total of 50 miRNAs were differentially expressed in EVs of SFB in the state of inflammation [37]. It is expected that early detection of changes in these contents can aid in predicting the probability of OA and provide opportunities for early intervention and personalized treatment.
Similarly, proteomic analysis of normal and OA patients' articular cartilage vesicles (ACVs, extracellular organelles at 50–150 nm found in articular cartilage) revealed nine proteins only present in OA-ACVs, many of which are typical markers of inflammation, such as immunoglobulin and fibrinogen [126]. This differential expression is largely quantitative, reflecting the known changes and increased catabolism in the extracellular matrix of OA, which may serve as a basis for diagnosis and prediction.
Until now, the number of studies on susceptibility has been limited, which may be due to the limitations on available datasets and sampling difficulty. However, for OA, a disease with limited intervention, susceptibility assessment is extremely important. It is believed that with an in-depth understanding of the OA mechanism and the enhancement of EVs isolation detection methods, EVs can be developed into kits in the future and combined with proven diagnostic methods to improve risk assessment and clinical decision making.
3.2. EVs in the differential diagnosis of osteoarthritis
EVs can also be used as biomarkers to distinguish between different types of joint diseases (Table 3). As early as 2006, Skriner et al. [129]. compared synovial exosomes from patients with rheumatoid arthritis (RA), reactive arthritis, and OA. They found that all exosomes carried citrullinated proteins, but fibronectin was only found in the exosomes of patients with RA. They pointed out that the specific identification of these proteins could help identify different types of arthritis. Further studies compared the protein profiles of RA-, OA-, and normal serum-derived exosomes. Researchers found that the exosomes from RA patients contained 1.4 times pro-neuregulin-3, 1.3 times alpha-1-antitypain, 6.3 times TLR3, and 0.7 times type Ⅱ cytoskeletal 1 than those in normal people (each has significantly different), while compared with OA the number was 1.4, 1.4, 5.7, and 0.8 times, respectively [122]. Among these, TLR3 differed the most significantly. TLR3 is a member of the innate immune system pattern recognition receptor TLR family that activates NF-κB and interferon regulator 3, which may be involved in the pathogenesis of RA. Therefore, proteins in exosomes, such as TLR3 fragments, can be used as markers of RA activity to differentiate RA from OA.
Table 3.
The characteristics of EVs in the differential diagnosis of osteoarthritis.
| Sources | Characteristic | Diseases | Ref. |
|---|---|---|---|
| Serum | RA patients had an high expression of pro-neuregulin-3, alpha-1-antitypain and TLR3, while a low expression of type Ⅱ cytoskeletal 1. | RA, OA | [122] |
| Serum | Circulating exosomal miRNAs are differentially expressed, and miR-151a, −199a, −370, −589, and −769 are believed to be involved in co-pathogenesis. | PsA, RA, GA | [130] |
| SFB | Membrane-bound form of TNF in EVs was found only in RA patients. | RA, OA | [131] |
| SF | Platform-derived EVs were found only in RA patients. | RA, OA | [132] |
| SF | Citrullinated proteins were found in all exosomes, and fibronectin was found only in exosomes from RA patients. | RA, OA, reactive arthritis | [129] |
*Abbreviation: OA, osteoarthritis; RA, rheumatoid arthritis; PsA, psoriatic arthritis; GA, gouty arthritis; SFB, synovial fibroblasts; SF, synovial fluid.
Chen et al. [130]. reported that plasma exosomal miRNAs in patients with psoriatic arthritis (PsA), RA, and gout arthritis (GA) had 230,198, 141 up-regulation and 108, 31, 73 down-regulation, respectively, compared with healthy people. The co-detected miRNAs include miR-151a, −199a, −370, −589, and −769, which are believed to be related to the common pathogenesis of these diseases. Further bioinformatic analysis revealed that these miRNAs are associated with immune disorders and bone damage. However, the authors did not compare the expression of these miRNAs in the three types of joint diseases in detail, thus failing to provide a reference range for identifying these diseases. Moreover, patients with OA were not included in the study. Thus, improving the above questions will facilitate the application of exosomal miRNAs in the differential diagnosis of joint diseases.
Zhang and colleagues found a membrane-bound form of TNF in the EVs of RA patients, but not in OA patients [131]. TNF can activate NF-κB, induce collagenase 1 formation, and lead to T-cell apoptosis. Similarly, platform-derived EVs were found only in the SF of patients with RA [132], causing a series of confirmatory reactions. These specific EVs and their contents have the potential to be used as markers of OA and RA. In summary, these results suggest that studying the differences in EVs from SF, plasma, or other tissues between patients with joint disease and healthy people may help develop diagnostic methods for OA.
3.3. Challenges of clinical transformation of EVs as biomarkers for OA
Although EVs show exciting potential as biomarkers, there is still a gap in the use of EVs for clinical diagnosis. First, the significance of biomarkers lies in early diagnosis, but to the best of our knowledge, there are no validated early markers of OA, including EVs. A possible reason for this is the lack of a single/unified molecule associated with the early disease process owing to the heterogeneity of OA. In addition, EVs derived from serum and urine may reflect the condition of all joints in the whole body, rather than a certain joint of interest. Moreover, the stability and reproducibility of EVs in different populations, environments, and laboratories is also a challenge.
In view of these challenges, we believe that a combination of multiple biomarkers can solve the difficulties of early diagnosis. The combination of several biomarkers reflecting different pathophysiological pathways can better predict disease progression than any single biomarker or clinical factor alone. Moreover, biomarkers can be combined with traditional diagnostic methods, such as imaging, to improve accuracy and sensitivity. In addition, because EVs can reflect the status of parent cells in real time, continuous monitoring to construct time-integrated concentrations or time-dynamic curves can more sensitively reflect the status of the disease and predict disease progression. In addition, with the continuous improvement of EVs isolation and characterization technology, such as gradually emerging kits that can compete with traditional isolation methods, such as ultracentrifugation and size exclusion chromatography (SEC), we believe that the stability and reproducibility of EVs as biomarkers will continue to improve.
Although a number of EVs-based biomarkers have entered clinical trials, covering the fields of tumors [133,134], cardiovascular diseases [135], and even infectious diseases [136], there is no relevant research in the field of OA. EVs have been used in clinical studies to diagnose diseases [137], predict progression [133], and evaluate efficacy and prognosis [135,138,139]. These studies further demonstrate the potential of EVs as biomarkers. We believe that overcoming the above problems, we can witness EVs-based biomarkers for OA from bench to bed in the near future.
4. 'Prohibitor'——EVs as a treatment of OA
The ability of EVs to shuttle bioactive cargoes has led to the extensive exploitation of their functions in pathogenesis and diagnostics. EVs are also considered to have potential for treatment because of their inherent characteristics such as non-toxicity and targeting ability. Although the therapeutic effect is not as good as that of the secretory group, such as the conditioned medium, the composition of EVs is more controllable [140]. In addition, EVs are more stable due to their low immunogenicity and prolonged presence in the blood. EVs are not recognized and eliminated as foreign objects by the immune system, which may be related to their surface adhesion proteins and carrier ligands [141]. The weak nonspecific interaction between EVs and circulating proteins makes them difficult to be cleared in blood circulation, making EVs potential drug delivery tools [142]. EVs have also been shown to stably shuttle in a joint microenvironment. The absorption dynamics of EVs can be observed using real-time quantitative multimodal nonlinear optical imaging [143]. Therefore, an increasing number of studies have started to explore the potential of EVs for OA therapy.
Compared to parent cells, EVs have certain natural advantages, especially in terms of long-term efficacy, which is partly due to their biological characteristics. First, EVs can avoid the risks of using cell products, such as cell immune compatibility, stability, heterogeneity, and the influence of dead cells on the joint microenvironment. At the same time, because of the low immunogenicity of EVs, they could be derived from a wide range of sources, including mammals related to humans as well as marine animals and plants. Moreover, EVs exhibit good programmability. Through engineering, we could enhance the efficacy of EVs and overcome the problems of short residence time and unequal distribution caused by the special structure of joints, which can enhance long-term efficacy.
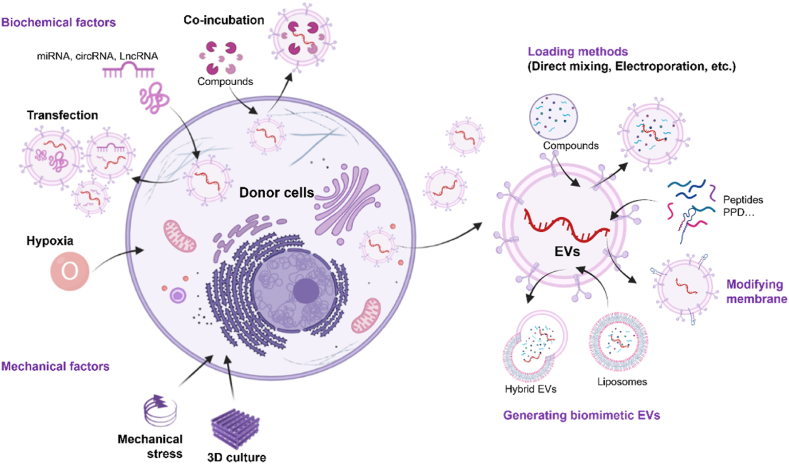
Natural EVs are the most commonly studied type of OA treatment because they are easy to obtain and have been shown to have good efficacy. However, they also have many limitations, such as insufficient targeting ability, difficulty in scaling up production, and potential safety risks owing to heterogeneity. Therefore, engineering has gradually become a trend in the field of EVs applications. Currently, there are two main strategies for modifying EVs. First, target cargoes are integrated into the cells to acquire modified EVs through naturally occurring processes. Second, direct modification of EVs including using biotechnology to introduce cargoes into EVs and modification of EV membranes, could improve loading efficiency and targeting capability while increasing yield. Simultaneously, the delivery and release of EVs is also a concern. There is an increasing interest in combining EVs with biomaterials or nanomaterials to achieve better therapeutic effects. Herein, we discussed the application of unmodified and modified EVs in OA treatment and summarize the current strategies to improve EVs delivery and release.
4.1. Unmodified extracellular vesicles
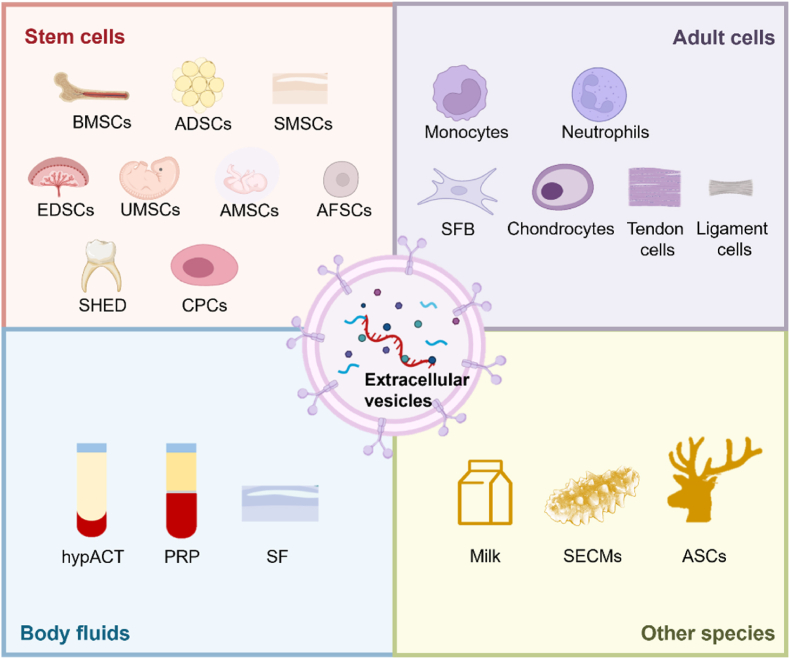
EVs from various cells without modification play different roles in OA therapy (Fig. 7). Normally, the processes of restoration and reconstruction in OA include immune regulation, pain inhibition, improvement of chondrocyte senescence and metabolic disorders, and promotion of chondrocyte regeneration. EVs from different cells exhibit different characteristics. Specifically, EVs derived from stem cells, especially pluripotent stem cells, play an important role in maintaining normal cell homeostasis due to their multidirectional differentiation potential. EVs from adult cells and body fluids help regulate OA inflammation and reduce chondrocyte damage. In addition, EVs derived from other species, such as milk, sea cucumber and deer antlers, have the characteristics of easy acquisition, strong regeneration, and immune regulation ability, and are regarded as promising biological source.
Fig. 7.
EVs from various sources without modification for OA therapy. Abbreviation: BMSCs, Bone marrow mesenchymal stem cells; ADSCs, adipose mesenchymal stem cells; SMSCs, synovial mesenchymal stem cells; EMSCs, embryonic mesenchymal stem cells; UMSCs, umbilical cord mesenchymal stem cells; AMSCs, amniotic mesenchymal stromal cells; AFSCs, amniotic fluid stem cells; SHED, stem cells from human exfoliated deciduous teeth; CPCs, chondrogenic progenitor cells; SFB, synovial fibroblasts; hypACT, hyperacute serum; PRP, platelet-rich plasma; SECMs, sea cucumber extracellular matrices; ASCs, antler stem cells.
Hence, in this section, the application of EVs from different sources in the treatment of OA was discussed, and representative research results were specifically analyzed (Table 4). Meanwhile, it is important to note that, although EVs from multiple sources have reported promising results in small animals, few studies to date have examined the effects of EVs in large animals and clinical settings. The existence of decreased OA progression also needs to be confirmed in a longer time dimension. Besides, clinical studies should be conducted to compare the effects of EVs from different sources, to help select the optimal source for clinical transformation. Additionally, EVs research in OA treatment can be combined with genomics, proteomics, and lipidomics to better predict and transform cell-free clinical products.
Table 4.
The roles of unmodified EVs in the treatment of OA.
| Sources | Targeting cells | Cargos | Delivery methods | Effect | Ref. | |
|---|---|---|---|---|---|---|
| EVs from stem cells | BMSCs | Chondrocytes | / | Intra-articular injection | Mitigated IL-1β-induced COL2A1 and ACAN downregulation, and increased the PWL | [144] |
| Chondrocytes | / | Co-culture in vitro | Inhibited the activation of pro-inflammatory signaling pathways (ERK1/2, AKT, P38, TAK1 and NF-κB etc.) | [145] | ||
| Chondrocytes | LncRNA MEG-3 | Intra-articular injection | Reduced IL-1β-induced chondrocyte senescence and apoptosis | [146] | ||
| Chondrocytes | LYRM4-AS1/miR-6515-5p | Co-culture in vitro | Alleviated OA inflammation | [147] | ||
| Chondrocytes | miR-136-5p | Intra-articular injection | Inhibited chondrocyte degeneration in OA by targeting ELF3 | [148] | ||
| Chondrocytes | / | Intra-articular injection | Induced cartilage reconstruction of TMJ-OA via Autotaxin-YAP Signaling Axis | [149] | ||
| Synovial macrophage | / | Intra-articular injection | Regulated synovial macrophage polarization to prevent OA | [150] | ||
| pBMSCs | Chondrocytes | BMP4 | Intra-articular injection | Alleviated OA by promoting chondrocyte proliferation | [151] | |
| ADSCs | Chondrocytes, M1 macrophages | miR-199a, 125b, 221 and 92a) | Intra-articular injection | Attenuated cartilage degeneration, and inhibited the infiltration of M1 macrophages into the synovium | [152] | |
| Chondrocytes | / | Co-culture in vitro | Attenuated the destruction of inflammatory response by inhibiting the NF-κB signaling pathway | [153] | ||
| IPFP MSCs | Chondrocytes | miR-100-5p | Intra-articular injection | Inhibited mTOR to protect chondrocytes and improve gait abnormalities | [154] | |
| SMSCs | Chondrocytes | miR-129-5p | Co-culture in vitro | Targeted HMGB1 to alleviate the inflammatory phenotype of chondrocytes | [155] | |
| Chondrocytes | miR-26a-5p | Co-culture in vitro | Inhibited the expression of PTEN, thereby inhibiting apoptosis and inflammation | [156] | ||
| CPCs | Chondrocytes | miR-221-3p | Intra-articular injection | Promoted the proliferation and migration of chondrocytes | [157] | |
| SHED | Chondrocytes | miR-100-5p | Intra-articular injection | Suppressed inflammation in TMJ chondrocytes | [158] | |
| EMSCsEMSCsEMSCs | Chondrocytes | / | Intra-articular injection | Promote osteochondral regeneration | [159] | |
| Chondrocytes | / | Intra-articular injection | Maintained the chondrocyte phenotype by increasing type II collagen synthesis and decreasing ADAMTS5 expression | [160] | ||
| Chondrocytes | / | Intra-articular injection | Enhanced IL-1β-blocked S-GAG synthesis and inhibited IL-1β-induced production of NO and MMP13 | [161] | ||
| UMSCsUMSCsUMSCs | Chondrocytes | miR-100-5p | Co-culture in vitro | Inhibited ROS production and apoptosis in human articular chondrocytes | [162] | |
| Macrophages | PI3K-Akt | Co-culture in vitro | Promoted the polarization of M2 macrophages and regulate the immune level of OA to slow down cartilage degradation. | [163] | ||
| Chondrocytes | miR-1208 | Intra-articular injection | Inhibited the secretion of pro-inflammatory factors and the degradation of cartilage ECM | [164] | ||
| AMSCs | Macrophages, | miRNA | Co-culture in vitro | Induced polarization of macrophages and inhibited inflammatory T cells | [165] | |
| T cells | ||||||
| AFSCs | Chondrocytes | TGF-β | Intra-articular injection | Induced cartilage recovery (achieving similar effects as stem cells) | [166] | |
| EVs from adult cells | Monocytes | Chondrocytes | Sox9 | Co-culture in vitro | Promoted chondrocyte differentiation and functions | [167] |
| Neutrophils | FLS | / | Co-culture in vitro | Inhibited the secretion of a broad spectrum of pro-inflammatory cytokines stimulated by TNF-α | [168] | |
| Chondrocytes | Chondrocytes | protein | Intra-articular injection | Restored mitochondrial dysfunction and produced anti-inflammatory macrophages | [169] | |
| SFB | Chondrocytes | LncRNA H19 | Co-culture in vitro | Enhanced cell proliferation and migration, and reduced expression of MMP13 and ADAMTS5 | [170] | |
| Tendon cells | MSCs | TGF-β | Co-culture in vitro | Regulate the tenogenic differentiation of MSCs | [171] | |
| EVs from body fluid | Synovia | / | HA | / | EVs in synovial fluid are rich in HA and provide lubrication and protection | [172] |
| hypACT | M1 macrophages | / | Co-culture in vitro | Increased the expression of COL2A1 and ACAN, enhancing the anabolism of chondrocytes | [173] | |
| PRP | Chondrocytes | / | Intra-articular injection | Inhibited TNF-α release and activated the Wnt/β-catenin signaling pathway | [174] | |
| EVs from other species | MilkMilk | Macrophages, Splenocytes | / | Oral administration | Inhibited inflammation, reduced adaptive immune response and attenuated OA | [25] |
| Chondrocytes | TGF-β, miR-148a | Co-culture in vitro | Reduced the release of sGAGs and catabolism of chondrocytes | [175] | ||
| SECMs | Synovial cells | / | Co-culture in vitro | Reduced synovial inflammation | [27] | |
| ASCs | Chondrocytes, MSCs | / | Intra-articular injection | Alleviated MSCs senescence and osteoarthritis by promoting cell division and inhibiting aging-related inflammation | [26] | |
4.1.1. EVs from stem cells
Stem cells, such as MSCs, have proven to be a promising approach for the treatment of OA by reducing inflammation, protecting cartilage from degradation in non-clinical models, and improving pain and function in clinical trials [176,177]. However, it is thought that the observed efficacy was derived from secretory factors rather than the cells themselves [178], which led to concerns regarding stem cell-derived EVs as potential therapeutic agents. Besides, the use of stem cells has many problems, including safety concerns, such as immune rejection [5] and tumorigenicity [6], and the limitation of survival and function due to exposure to chronic inflammation and catabolic environments [179]. The fate of the cells after drug administration, especially the long-term survival of allogeneic cells during treatment, affects their efficacy. At the same time, there are always some dead cells in cell products, which themselves and their secretions have a worrying impact on the health of patients [180]. Although many methods have been designed to pretreat MSCs before transplantation to solve these problems, including inflammatory cytokines, hypoxia, drugs, biomaterials and different culture conditions [181], it is difficult to provide quality assurance for clinical MSC [182]. The use of EVs is a perfect solution to these problems because they are non-neoplastic and have a structure similar to that of cells [183]. Herein, we summarized the characteristics of EVs therapy with different stem cell sources in detail and pointed out their shortcomings and potential development directions.
4.1.1.1. EVs from bone marrow mesenchymal stem cells (BMSCs)
BMSCs have the potential to differentiate into bone, cartilage, muscle, and fat [184], and are used for immune repair and tissue regulation [185]. BMSCs derived EVs (BMSC-EVs) are the earliest and most widely studied treatment for OA. BMSC-EVs mediated OA repair is characterized by an early reduction in inflammation, inhibition of cartilage degeneration, and pain production. After that, they promote chondrocyte proliferation, matrix expression, and subchondral bone structure improvement, leading to overall joint recovery and regeneration.
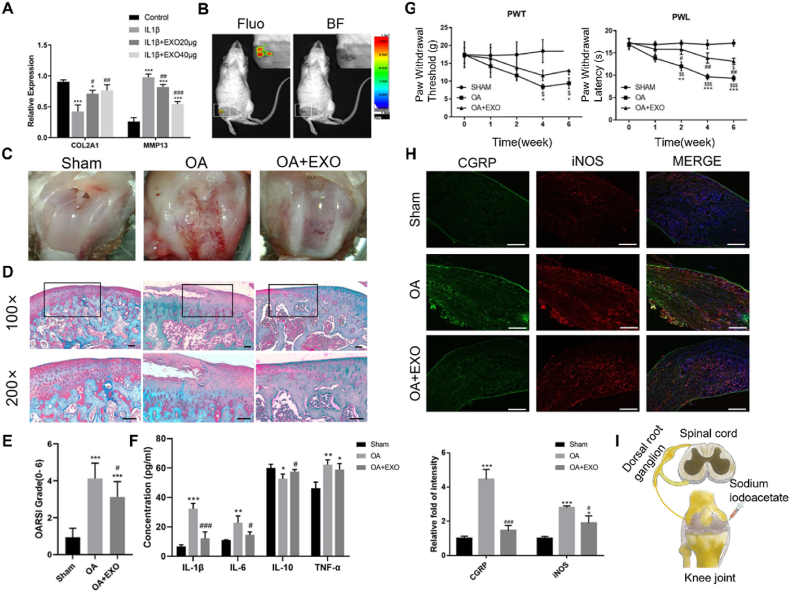
He et al. [144] found that BMSC-Exos protected against cartilage damage and relieved pain by affecting the dorsal root ganglion (DRG). (Fig. 8I). Firs, BMSC-Exos co-cultured with chondrocytes upregulated COL2A1 expression and down-regulated MMP13 expression, indicating that it could inhibit chondrocyte catabolism and maintain ECM stability (Fig. 8A). Besides, by intra-articular injection, BMSC-Exos accumulated in the articular cavity of OA rats (Fig. 8B), rescuing cartilage damage, which is characteristic of restoration of cartilage structure, reduction of OSAID score, and decrease in the expression of inflammatory factors in the serum (Fig. 8C–F). More importantly, they found that the claw contraction threshold (PWT) and claw contraction latency (PWL) of OA rats treated with exosomes were significantly improved (Fig. 8G), suggesting that exosomes help alleviate pain in OA rats. Further studies found that the mechanism may be related to exosomes attenuating the downregulation of CGRP and iNOS in the DRG (Fig. 8H). However, the specific molecular mechanisms underlying pain suppression remain to be studied in detail.
Fig. 8.
BMSCs-Exos relieved cartilage damage and pain in OA rats. (A) Western blot analysis of COL2A1 and MMP13 protein levels (* compared with the control group, # compared with the IL-1β group). (B) In vivo imaging after intra-articular injection of exosome (Fluo, Fluorescence, BF, brightfield). (C) General morphology of the knee joint in rats. (D) Saffron solid green staining of the knee joint (scale = 50 μm). (E) OARSI scores among different groups. (F) ELISA of inflammatory factors in cartilage. (* compared with Sham group, # compared with OA group, n = 8 for each group). (G) PWT and PWL of rats at different time. (H) Immunofluorescence staining of CGRP and iNOS proteins in DRG tissues (scale = 200 μm, * compared with Sham group, # compared with OA group, n = 4 for each group). (I) A model of knee OA induced pain in rats. Reproduced under the terms of the CC-BY 4.0 [144]. Copyright 2020, The Authors, published by Springer Nature.
Further studies suggest that BMSC-Exos attenuate inflammation mediated cartilage damage by inhibiting the activation of pro-inflammatory signaling pathways such as ERK1/2, AKT, P38, TAK1, and NF-κB [145]. In addition, BMSC-EVs contain cargoes, such as lncRNA MEG-3 [146], miR-6515-5p [147], and miR-136-5p [148], which inhibit aging and apoptosis of chondrocytes. BMSC-EVs could also regulate the expression of inflammatory factors by autotaxin-YAP [149] and regulate the polarization of synovial macrophages [150], which play an essential role in immune regulation. Similarly, exosomes from congenital multi-finger tissue BMSCs (pBMSCs) can regulate chondrogenesis, possibly through the BMP4 signaling pathway [151]. These studies demonstrate the potential of BMSC-EVs as a treatment for OA.
4.1.1.2. EVs from adipose mesenchymal stem cells (ADSCs)
ADSCs have a similar potential to BMSCs. Previous studies have shown that injecting adipose stem cells into the joints can have anti-inflammatory and cartilage-protective effects [186]. Besides, they are more readily available and productive after separation [187]. A number of studies have shown that ADSCs also have a strong immunosuppressive effect and can secrete immune regulatory factors, such as IL-4, -10, −13, and transforming growth factors. Thus, ADSCs have been widely used to study a variety of diseases, including rheumatoid arthritis [188].
According to recent studies, ADSC-EVs play a protective role mainly by inhibiting inflammation. Mortati et al. [143]. found that ADSC-sEVs could be taken up by chondrocytes. Further studies found that ADSC-sEVs reduced the catabolism of chondrocytes in OA patients, inhibited M1 macrophage infiltration in the OA synovium, and protected cartilage degradation in both subacute and chronic OA models [152]. In particular, intra-articular injection of ADSC-EVs at the early stage of OA showed a significant therapeutic effect on cartilage regeneration. Early OA is characterized by increased catabolic activity mediated by inflammatory mediators and cartilage-degrading proteases, suggesting that the early use of ADSC-EVs can effectively regulate inflammation and promote tissue repair, thus slowing down the progression of OA.
Cavallo et al. [153]. compared the effects of ADSC-sEVs on chondrocytes and synovial cells. Synovial cells absorb sEVs earlier and in greater quantities, leading to more significant regulation of synovial cell secretion of cytokines, catabolic enzymes, angiogenic factors, and pain factors by sEVs. In addition, sEVs may lead to the secondary release of new vesicles by synovial cells, forming positive feedback to enhance the biological activity of sEVs. Moreover, sEVs neutralized the inflammatory effects of IL-1β by affecting p65 in the NF-κB signaling pathway.
Infrapatellar fat pad (IPFP) derived MSCs (IPFP-MSCs) are a type of ADSCs that have been previously shown to promote chondrogenesis [189]. The researchers found that exosomes may also play a role in this process. Exosomes inhibit apoptosis and enhanced matrix synthesis in vitro, while preventing cartilage destruction and improving gait abnormalities in DMM mouse models. The mechanism may be related to the up-regulation of miR-100-5p, thereby inhibiting the mTOR autophagy pathway [154]. It is relatively feasible to obtain IPFP from OA patients through arthroscopic surgery, but it is more complicated than ADSCs. Therefore, we believe that there is a way before the clinical translation of IPFP-MSCs before proving to have a significantly better therapeutic potential than ADSCs.
4.1.1.3. EVs from synovial mesenchymal stem cells (SMSCs)
SMSCs have been shown to inhibit OA progression [190]. Moreover, compared to BMSCs and ADSCs, SMSCs differentiate into cartilage more easily [191]. A possible reason for this is that the synovium and cartilage originate from a common cell cisterna [192]. However, it is more difficult to obtain SMSCs as they can only be acquired through invasive procedures.
Researchers have found that high miR-129-5p expression in EVs from SMSCs can target HMGB1 to alleviate the inflammatory phenotype of chondrocytes [155]. SMSC-EVs are also rich in miR-26a-5p, which can inhibit the expression of phosphatase and tensin homologue (PTEN) [156]. The overexpression of PTEN was associated with increased apoptosis. Therefore, SMSC-EVs can inhibit chondrocyte apoptosis, reduce inflammation, and improve chondrocyte proliferation.
Zhu and his colleagues compared the effects of exosomes from SMSCs and induced pluripotent stem cell-derived MSCs (iMSCs) on OA treatment [193]. They found that both iMSC-Exos and SMSC-Exos can reduce inflammation; however, iMSC-Exos have a better therapeutic effect than SMSC-Exos, which may be related to their stronger stimulation of chondrocyte migration and proliferation, offering a new cell-free strategy.
4.1.1.4. EVs from cartilage progenitor cells (CPCs)
CPCs are a class of oligopotent stem cells with MSC characteristics together with cartilage stem cells, which are considered to be involved in chondrogenesis and regulation. After damage to healthy cartilage, cartilage progenitor/stem cells (CSPCs) migrate to the site of injury and participate in repair. Notably, CSPCs exhibit different phenotypes in early and late OA. Changes in their distribution during OA progression suggest that they may be responsible for communication between the cartilage, subchondral bone, and other articular tissues [194].
Wang et al. [157]. compared the application of CPC-EVs in OA treatment between MRL/MpJ mice (MRL-EVs) and CBA mice (CBA-EVs). Both CBA-EVs and MRL-EVs enhanced chondrocyte proliferation and migration, and promoted repair and regeneration in surgically induced models. Unsurprisingly, MRL-EVs showed a better repair effect than CBA-EVs. Further analysis of miRNAs expression in the two groups revealed that 180 miRNAs were differentially expressed. Among them, three miRNAs, miR-148a-3p, miR-221-3p, and miR-222-3p, were significantly upregulated, while four miRNAs, let-7b-5p, miR-22-3p, miR-125a-5p, and miR-26a-5p, were significantly downregulated. Inhibition experiments showed that miR-221-3p is a key mediator of CPC-EVs induced chondrocyte proliferation and migration in vitro. This is the first study to investigate the application of CPC-EVs in OA and to compare the therapeutic differences between MRL-EVs and EVs from normal mice, which will contribute to the development of new methods to identify and treat OA in the future.
4.1.1.5. EVs from stem cells from human exfoliated deciduous teeth (SHED)
The residual pulp of deciduous teeth contains a population of pluripotent stem cells, called SHED, which have a higher proliferation rate, stronger osteogenic induction ability and are easier to obtain than pulp stem cells [195]. SHED is considered an ideal cell source for regenerative medicine because it is highly proliferative, pluripotent, and immunosuppressant [196]. Luo et al. [158]. found that SHED-Exos enriched with miR-100-5p could reduce the degradation products of chondrocytes under inflammatory conditions, possibly through a mechanism similar to the IPFP-Exos described above. These results suggest that SHED-Exos may be a novel therapeutic agent for OA inflammation.
4.1.1.6. EVs from perinatal stem cells
Perinatal stem cells, such as embryonic MSCs (EMSCs), umbilical cord MSCs (UMSCs), amniotic MSCs (AMSCs), and amniotic fluid stem cells (AFSCs) have strong self-renewal and differentiation abilities and are widely used in cartilage repair. Similar to other stem cells, perinatal stem cells can restore joint micro-environment stability by promoting proliferation and regulating immunity, thereby alleviating OA progression. Recent omics studies, such as miRNA omics, have systematically explained the contents of EVs derived from perinatal stem cells and highlighted future research priorities.
EMSC-derived exosomes (EMSC-Exos) were studied for the first time in OA. Zhang et al. [159]. found that EMSC-Exos could repair cartilage and subchondral bone during inflammation. By intra-articular injection of EMSC-Exos weekly in a rat model of osteochondral defects, they found improvements in appearance and histological scores compared to PBS treatment. Surprisedly, complete recovery of cartilage and subchondral bone was observed at 12th weeks. Furthermore, EMSC-Exos balanced ECM synthesis and degradation by increasing type II collagen synthesis and decreasing ADAMTS5 expression [160]. These studies demonstrate the efficacy of EMSC-Exos in cartilage repair and ECM protection, revealing its potential as a cell-free replacement therapy.
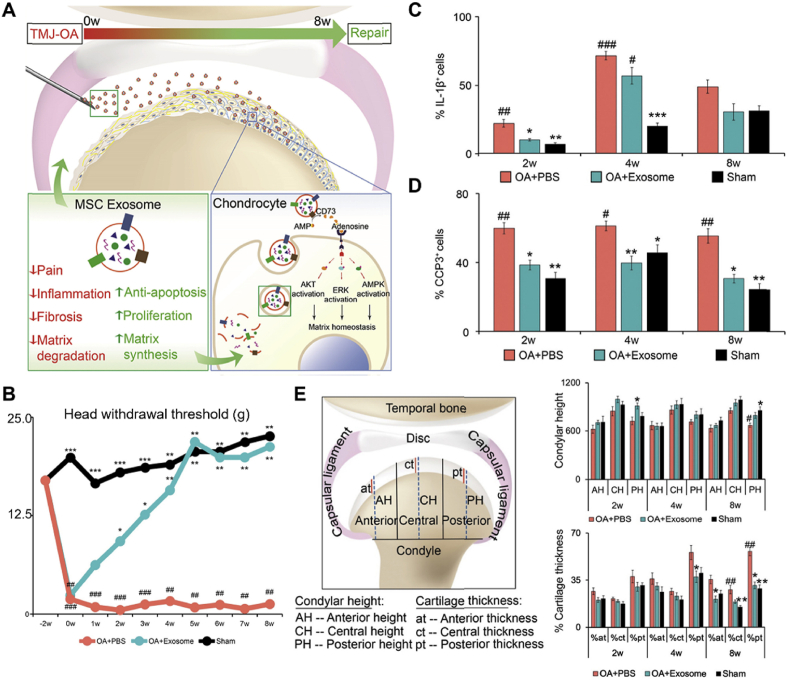
The researchers also found that EMSC-Exos relieved OA by reducing inflammation, inhibiting pain, and restoring matrix homeostasis (Fig. 9A). First, the researchers compared gene expression differences in cartilage tissue after two weeks of treatment with exosomes. Compared with the OA + PBS group, the expression of pro-inflammatory, apoptotic, and pain-related genes was significantly decreased, whereas the expression of matrix regulation-related genes was significantly increased in the EMSC-EVs group, suggesting that the early characteristics of EMSC-Exos mediated repair may be pain inhibition and inflammation reduction. To further prove this hypothesis, they first demonstrated that after five weeks, the head withdrawal threshold of the EMSC-Exos group was similar to that of the sham group (Fig. 9B). Subsequently, they found that EMSC-Exos reversed TMJ degeneration in OA and reduced subchondral bone degeneration (Fig. 9E). During this process, exosomes reduced the expression of il-1β+ and iNOS+ cells (Fig. 9C) and reduced apoptosis (Fig. 9D). Finally, they found that EMSC-Exos might have therapeutic effects through adenosine receptor-mediated phosphorylation of AKT, ERK, and AMPK, restoring s-GAG matrix synthesis and inhibiting NO and MMP13 production [161]. This study demonstrates the potential of EMSC-EXOs in the treatment of pain and degeneration. Although the role of individual molecules in therapy remains to be determined, the function of the overall combination provides a compelling reason for their efficacy.
Fig. 9.
EMSC-Exos relieved osteoarthritis by reducing inflammation, inhibiting pain, and restoring matrix homeostasis. (A) The mechanism of EMSC-Exos promoting joint repair. (B) Time-dependent injury response after treatment indicated the effect of Exos treatment on pain. (C) Exos treatment suppressed inflammation. Expression of IL-1β+ cells in cartilage at 2, 4, and 8 weeks. (D) Exos treatment reduced apoptosis. Expression of CCP3+ cells in cartilage at 2, 4, and 8 weeks. (E) Exos reversed TMJ degeneration in OA patients. Schematic model of joint condyle head (left), condyle height in different areas (upper right), percentage of cartilage thickness in different areas (lower right). * Compared to OA + PBS group, # compared to Sham group, n = 6–8/group. Reproduced with permission [161]. Copyright 2019, Elsevier.
UMSCs have the advantages of easy access, small immune rejection, and strong immune regulation, which have been used in the treatment of OA [197]. Researchers found that exosomes from UMSCs could inhibit cyclic strain-induced ROS production and apoptosis, which is an important cause of chondrocyte injury in OA. This might owing to exosomal miR-100-5p inhibiting the expression of NOX4 [162]. UMSCs-sEVs could also promote the polarization of M2 macrophages and the expression of IL-10, thereby regulating the immune level of OA to slow down cartilage degradation. Through miRNA sequencing and protein mass spectrometry analysis, the authors found that the mechanism may be related to the activation of PI3K-Akt signaling pathway [163]. Besides, Zhou et al. [164]. found that UMSCs-sEVs targeted METTL3 by miR-1208, thereby reducing the m6A level of NLRP3 mRNA and reducing the release of inflammatory factors. These studies demonstrate that UMSC- sEVs is a reliable treatment that can safely and effectively replace UMSCs.
AMSCs are readily available MSCs because the placenta is usually discarded after birth. AMSCs have strong immunomodulatory abilities and can inhibit the proliferation and function of T lymphocytes, monocytes and natural killer cells [198], the efficacy of which has been demonstrated in orthopedic diseases, such as OA and tendinopathy. Considering that AMSCs may play a role in EVs-related paracrine signaling, Ragni et al. [165]. analyzed miRNA in EVs. They identified 336 miRNAs with different expression levels, among which miR-24-3p and miR-146a-5p showed the most significant differences. miR-146a-5p is a determinant of OA related joint homeostasis and pain status, while miR-24-3p promotes proliferation and inhibits chondrocyte apoptosis. Further compared with miRNAs involved in the pathogenesis of OA, 14 protective miRNAs (33.12% of EVs genetic weight) and five destructive miRNAs (6.85%) were screened out. Among them, 10 miRNAs were associated with the proliferation, activation, and differentiation of T cells, such as miR-146a-5p and miR-125b-5p, which accounted for 19% of the EVs genetic weight. Five miRNAs were involved in M2 polarization (39.78%), and three were involved in M1 phenotypic regulation (3.94%). By studying the miRNA lineage of AMSC-EVs, the potential mechanism of AMSC-EVs in the regulation of OA matrix remodeling was revealed.
Since amniotic fluid can be obtained through routine prenatal diagnosis, there is no ethical or technical resistance to obtaining AFSCs. AFSCs are considered a valuable source for cell therapy for degenerative and inflammatory diseases because of their low tumorigenesis and easy storage [199]. Previous studies have shown that AFSCs can promote bone tissue repair and regeneration [200]. Researchers have compared AFSCs with exosomes from AFSCs for the treatment of the MIA-induced OA model. They found that higher levels of pain tolerance and histological scores were observed in the exosome group, possibly due to the effect of exosomal TGF-β on macrophage polarization [166]. This study is the first to demonstrate the efficacy of AFSC-EVS in the treatment of OA by offsetting cartilage damage.
4.1.2. EVs from adult cells
EVs from adult cells, such as immune cells (macrophages and neutrophils) and joint structure-related cells (synoviocytes, chondrocytes, tendon cells, and ligament cells), influence the inflammatory environment as well as the aging and metabolism of chondrocytes in OA. Their function is usually simpler than that of EVs from stem cells, which also means that their adverse effects are likely to be smaller.
During the development of OA, activated innate immune cells, especially macrophages, participate in OA progression [201]. Macrophages have been observed to accumulate and polarize in the synovium and articular lumen [202]. Phosphorylated STAT3 and STAT6 expression increases in macrophages under inflammatory conditions, resulting in increased upstream MMP2 expression. These cells release exosomes rich in SOX9 mRNA and protein, which promote the differentiation and function of chondrocytes [167]. This is the first study to elucidate the role of EVs derived from macrophages in the regulation of cartilage repair. Neutrophil-derived microvesicles (NDMVs) promote inflammation and regulate monocytes and macrophages. In addition, NDMVs can reduce IL-8 and prostaglandin E2 release to maintain chondrocyte function [203]. Dong et al. [168]. found that NDMVs can be internalized by FLS in OA patients, thus playing an anti-inflammatory role. NDMVs down-regulated the expression of TNF-α induced inflammatory factors such as IL-5, -6, -8, and MCP-1. Notably, the absence of significant changes in IL-2 and IL-4 levels suggests that this downregulation was selective. This study demonstrated that NDMVs can play a role in local synovitis and may have potential as a treatment strategy for OA.
Structures, such as cartilage, synovium, subchondral bone, tendons, and ligaments are important components of the joints. EVs from these structures can be internalized by the corresponding cells, thereby promoting the maintenance of joint homeostasis. Chondrocytes are resident cells responsible for maintaining a dynamic balance between the catabolism and anabolism of the ECM [204]. The quantity and state of chondrocytes are closely related to the disease. Exos from primary chondrocytes contain more mitochondria proteins and are involved in immune processes. Lin et al. [169]. found that exosomes from chondrocytes can prevent the development of OA by regulating mitochondria and immune response. By comparing the exosomal proteins in chondrocytes under normal and inflammatory conditions, 50 mitochondria-related proteins were found to be differentially expressed. In addition, exosome treatment resulted in improved morphological and histological scores, and increased M2 macrophage infiltration. Further research can be combined with genomics and proteomics to explore key proteins and elucidate the mechanisms that eliminate mitochondrial dysfunction.
The synovium is composed mainly of FLS, which provides nutrients to articular cartilage and protects the articular structure of the synovium [205]. Synovial inflammation is involved in the occurrence and progression of OA by releasing a large number of pro-inflammatory factors and affecting chondrocyte metabolism [206]. EVs are thought to play an important role in mediating crosstalk between chondrocytes and FLS. Tan et al. [170]. found that Exos from FLS (FLS-Exos) enhanced cell proliferation and migration, which is currently the main strategy for controlling OA. In addition, FLS-Exos through the lncRNA H19-mediated miR-106b-5p/TIMP2 axis reduced the expression of MMP13 and ADAMTS5, which alleviated matrix degradation. FLS-Exos have been proven to have a cartilage preservation effect, and H19 might be a potential target for the treatment of OA in the future.
Tendons and ligaments are the structures that maintain joint stability, and their destruction often induces and exacerbates OA. Tendon abnormalities have been observed in OA and are associated with OA progression [207]. Similarly, degenerative changes in ligaments are highly associated with cartilage damage in OA [208]. Therefore, repairing and maintaining the stability of the tendons and ligaments is a strategy to prevent and control OA. Recent studies have shown that exosomes play an important role in this process. Xu et al. [171]. found that exosomes secreted by the tendon could promote the tendon differentiation of MSCs through TGF-β, which enriched the protective effect of exosomes on the joint.
To date, still no studies have compared EVs derived from adult cells and stem cells for the treatment of OA. Further understanding of the role of EVs from different sources in the treatment of OA is conducive to the development of a complete treatment system and an in-depth understanding of OA pathogenesis.
4.1.3. EVs from body fluids
EVs from body fluids, such as blood, SF, and urine, are easily available and thus have better clinical transformation prospects. SF is an important lubricant in the joint cavity, which is rich in hyaluronic acid (HA). However, inflammation and oxidative stress can accelerate HA degradation. Studies have found that HA can be carried by EVs in SF, and its possible source is FLS [172]. This opens the possibility for us to supplement HA exogenously.
Blood-derived products, including whole blood derivatives based on plasma or serum, have been intra-articularly injected to reduce OA pain and inflammation [209]. Flow cytometry showed that EVs from citrate-anticoagulated platelet-rich plasma and hyperacute serum (hypACT) were primarily from platelets [210]. The expression of COL2A1 and ACAN increased after hypACT-EVs were used to treat the inflammatory model of chondrocytes co-cultured with M1 macrophages in OA patients, suggesting that EVs of blood products can enhance the anabolism of chondrocytes and reduce the damage caused by OA inflammation [173]. Liu and colleagues compared platelet-rich plasma exosomes (PRP-Exos) with activated PRP (PRP-As). They found that PRP-Exos significantly inhibited TNF-α release and decreased the apoptosis rate of OA chondrocytes. The mechanism may be related to the inhibition of the Wnt/β-catenin signaling pathway, which is activated by inflammation [174]. However, the exact molecular mechanisms remain unclear, and the optimal concentration, frequency, and interval of dosing remain to be determined.
4.1.4. EVs from other species
The use of biomaterials from different species to treat human diseases has many inherent advantages, such as plentiful, easily available, and inexpensive. Moreover, owing to the donor cells, they have some characteristics that conventional sources do not have. For example, milk is rich in various immunoglobulins and nutrients, such as calcium and phosphorus; therefore, their EVs are rich in miRNAs associated with immunity and cell growth [211]. However, EVs of different species may face a number of safety issues, such as immune rejection, tumor promotion, cytokine release syndrome, and infusion toxicity. Although these occurrences are rare, further studies on safety are needed in future.
Most studies have demonstrated a beneficial relationship between milk and OA. Lu et al. [212]. found that frequent milk intake (≥7 cups/week) may be associated with reduced progression of OA in women and can be quantified by a reduction in joint space width over time. Bovine milk-derived EVs (BMEVs) contain many immunoregulatory miRNAs, such as miR-124a, which directly targets MCP-1 mRNA; miR-21, -146, −126, −155, and −199a, which target TLR/IL-1 inflammatory pathways; and proteins, such as lactoferrin and TGF-β. Therefore, BMEVs may play a role in OA treatment. Onno et al. [25]. observed that oral BMEVs attenuated the progression of OA and decreased the serum levels of MCP-1 and IL-6 in both spontaneous polyarthritis and collagen-induced arthritis models. Further studies showed that BMEVs reduced the damage to sGAGs and the expression of MMP-1, -3, -13, and other catabolic products. The TGF-β carried by BMEVs may play a crucial role in this process. Moreover, BMEVs can also transfer miR-148a to chondrocytes and inhibit the hypertrophic differentiation of chondrocyte [175]. These studies suggest that BMEVs have the potential to be used to treat OA. However, it is worth noting that there may be potential adverse reactions with BMEVs. Samuel et al. [213]. found that BMEVs could accelerate tumor metastasis while reducing the size of primary tumors. Therefore, the safety of BMEVs requires further study.
Marine organisms are considered alternative biomaterial sources for terrestrial vertebrates, and marine bioderived components are used for OA treatment [214]. Echinoderms, such as sea cucumbers, have an incredible ability to regenerate. Moreover, sea cucumbers are rich in bioactive ingredients, such as chondroitin sulfate, collagen, amino acids, and phenols, which are thought to play anti-inflammatory and anti-tumor roles. Jo et al. [27]. isolated sEVs from lyophilized sea cucumber extracellular matrices (SECMs) and co-cultured them with synovial cells to reduce synovial inflammation. SECM-EVs reduced the expression of IL-1β, IL-6 and MMP-1 by 26.4%, 52.4%, and 34.9%, respectively. In addition, the expression of COX-2, which is associated with pain, was decreased by approximately 3.5 times (p < 0.05). This study demonstrated the potential of ocean derived EVs for the treatment of OA. Although the mechanism remains unclear, future studies on cargoes from ocean derived EVs will help clarify the mechanism and clinical transformation.
Antler velvet is the only mammalian organ known to be regenerated entirely annually [215]. Compared to other mammalian stem cells, antler stem cells (ASCs) show a higher ability to proliferate and regenerate. Lei et al. [26]. found that ASCs may be a good source of Exos, which can effectively reduce cartilage aging and alleviate OA (Fig. 10A). ASC-Exos can slow the aging of human ESC-derived MSCs (hMSCs) by including reducing β -galactosidase activity, protein levels of P16 and P21, and SASP-related gene expression (Fig. 10B). Further studies revealed that ASC-Exos could also improve bone erosion in OA mice (Fig. 10C) and rescue cartilage degeneration (Fig. 10D). Exosome treatment also reduced the expression of the aging marker P16 and increased the expression of the proliferation marker Ki67 in the articular cartilage region (Fig. 10E). These results suggest that ASC-Exos can improve OA, which is characterized by aging and cartilage degeneration. Proteomic analysis was performed to investigated the underlying mechanisms (Fig. 10F). They found that ASC-Exos were rich in protein effectors in response to growth factors, contributing to the protective role of Exos in anti-aging and OA models.
Fig. 10.
ASC-Exos alleviate MSCs senescence and osteoarthritis. (A) Schematic diagram of ASC-Exos restoring stem cell senescence in vitro and alleviating OA progression in vivo. (B) hMSCs were treated with vector (Veh) or exosome (Exo) for SA-β-Gal staining (scale, 50 μm), and quantification. Western blot and quantification of P16 and P21 expression. Heat map of relative mRNA expression levels of SASP-related genes (n = 3). (C) Bone mineral density analysis of joints of OA mice treated with Veh or Exo (n = 15). (D) Saffron solid green staining (scale, 200 μm) and OARSI grade of articular cartilages (n = 15). (E) Immunohistochemical staining (scale, 60 μm) of Ki67 and P16 and quantitative analysis of articular cartilage in OA mice treated with Veh or Exo (n = 15). (F) Venn diagram showing the number of differentially expressed genes in OA mice after Exo treatment. Reproduced under the terms of the CC-BY 4.0 [26]. Copyright 2022, The Authors, published by Springer Nature.
EVs derived from other species provide different approaches for OA treatment. Plants and honey are also interesting research sources. Although they themselves may not contribute to the maintenance of joint homeostasis, they are viable sources of EVs production in large quantities and may achieve good efficacy through further engineered modifications. As the development of any new treatment, there are a number of issues that need to be resolved before the results can be brought to the clinic. The long-term efficacy should be evaluated using animal models. Moreover, comprehensive studies, such as long-term safety tests, should be conducted to exclude side effects, such as tumor promoting, due to their origin from different species.
4.2. Modified extracellular vesicles
Appropriate modification of EVs can improve their delivery efficiency, targeting accuracy, and therapeutic effects [216,217]. As shown in Fig. 11 and Table 5, current strategies fall into two broad categories [218,219]. One category modifies the donor cells to load exogenous cargo into EVs. Common methods include biochemical factors (such as co-incubation, transfection, and hypoxia) and mechanical factors (such as mechanical stress and 3D culture). Another category modifies EVs directly, including loading exogenous cargoes (such as direct mixing and electroporation) and modification of the membrane of EVs. In this section, we summarized the known modification strategies used for the treatment of OA and compared their advantages and disadvantages. It is worth noting that although some new strategies, such as MIMIC EVs, have been widely used in tumor treatment, there are no similar studies in the field of OA, which may be the direction of future research.
Fig. 11.
Main strategies for EVs modification. Left: Strategies for modifying donor cells. Co-incubation, transfection and hypoxia are biochemical factors, which are used to load cargoes into donor cells. Mechanical stress and 3D-culture are mechanical factors that increase yield and change contents. Right: Strategies for directly modifying EVs. Direct mixing and electroporation are used to introduce cargoes into EVs. Fusion with membrane proteins and reverse surface charge are used to modify EVs membrane to improve targeting, distribution, retention, and bioavailability. Nanomaterials including liposomes can be mixed with EV membranes to generate hybrid EVs.
Table 5.
Different strategies to modify EVs for OA therapy.
| Strategies | Methods | Advantages | Disadvantages | Examples | Ref. | |
|---|---|---|---|---|---|---|
| Modifying donor cells | Biochemical factors | Co-incubation | Simple; | Cytotoxicity; | Delivery of Curcumin | [220] |
| No significant impact on EV structures; | Susceptible loading efficiency; | |||||
| Transfection | High specificity; | Induce donor cell apoptosis; | Overexpression of miR-140 | [221] | ||
| No significant impact on EVs structures; | Low loading efficiency; | |||||
| Hypoxia | Maintain stemness; | Complex; Low specificity; Affect donor cell proliferation; |
Hypo-sEVs had high expression of miR-216a | [222] | ||
| Influence the differentiation of stem cells; | ||||||
| Mechanical factors | Mechanical stress | Simulated internal environment; No damage to membrane integrity; |
Complex; | Stimulated by LIPUS | [223] | |
| Low specificity; | ||||||
| 3D culture | Mass production; | Complex; | Cultured in hollow-fiber bioreactor | [224] | ||
| Increase the cargoes and ability of EVs; | Low specificity; | |||||
| Modifying EVs directly | Loading cargoes | Direct mixing | Simple and quick; | Only suitable for hydrophobic compounds; | Delivery of COS | [225] |
| electroporation | Simple and quick; | Affect integrity; | Delivery of KGN | [30] | ||
| High efficiency; | Not suitable for RNAs with special structures; | |||||
| Modifying membrane | Fusion with proteins | Improve the targeting; | Affect the functions of cargoes; | Fused with MSCs binding peptide E7 | [30,31] | |
| Reverse surface charge | Improved distribution, retention ability and bioavailability; | Affects integrity and cargoes; | Modified with PPD | [32] | ||
| Low homogeneity; | ||||||
| Generating biomimetic EV | Hybrid EVs | High load capacity; Controllable production process; Scalability |
Increase the difficulty of preparation; | Fused with liposomes to carry Cas9 sgMMP-13 | [31] | |
| Low homogeneity | ||||||
4.2.1. Modifying donor cells
Studies have shown that the type and state of donor cells affect the yield and contents of EVs [226]. The stimulation of donor cells to enhance the therapeutic potential of EVs has great application prospects, because external stimulation is controllable and quantifiable.
4.2.1.1. Application biochemical factors to modify donor cells
-
(1)
Co-incubation method
Compounds, such as small-molecule drugs, growth factors, and anti-inflammatory factors, can be introduced into EVs by co-incubation. EVs can protect these hydrophobic or easily destroyed compounds and deliver them to target cells. This method is simple and has no significant impact on the EVs structure; however, the loading efficiency is easily affected by compound characteristics and other factors [227].
In the treatment of OA, current strategies mainly use co-incubation with anti-inflammatory substances, such as small molecule drugs and anti-inflammatory factors, to enhance the ability of EVs to regulate inflammation. Interestingly, EVs produced by donor cells co-incubated with pro-inflammatory factors can also improve their cartilage protection ability. This may be related to adaptive changes in donor cells caused by moderate stimulation. However, the exact amount of stimulation and its long-term efficacy remains to be investigated further.
The polyphenols of curcumin from traditional Chinese medicine, such as quercetin and icariin, possess anti-inflammatory activity. However, the bioavailability of these compounds is usually limited due to their low hydrophobicity and stability [228]. Li et al. [220]. found that the co-incubation of curcumin with BMSCs enhanced the cartilage protective effect of BMSC-EVs (Fig. 12A). Specifically, Cur-EVs promoted the viability of OA chondrocyte (OA-CH) cartilage, reduced apoptosis, and improved metabolism (Fig. 12B). Further studies revealed that miR-126-3p might play a role in this mechanism (Fig. 12C). Cur-EVs up-regulated the expression of miR-126-3p, thereby reducing the phosphorylation levels of Erk, PI3K/Akt, and p38/MAPK in OA chondrocytes. Thus, Cur-EVs reversed the damage induced by IL-1β. In addition, Chen et al. [229]. found that Cur-EVs could also enhance the ability of chondrocytes to resist oxidative stress, thus significantly enhancing the therapeutic effect of chondrocytes and reducing the frequency of injection, further expanding the therapeutic prospects of Cur-EVs. Similarly, sEVs from curcumin-pretreated ADSC more effectively downregulated TBHP-induced oxidative stress and chondrocyte apoptosis in vitro and showed stronger cartilage protection in vivo [229]. However, further research regarding cargo is required to reveal the specific mechanisms. Similarly, exosomes from MSCs co-incubated with TGF-β1 are rich in miR-135b, which promotes the polarization of synovial macrophages. Mechanistically, miR-135b promotes the polarization of macrophages M2 by targeting MAPK6 [230]. These series of studies revealed the changes in EVs function after pretreatment with anti-inflammatory molecules, most of which are related to inflammatory regulation and oxidative stress, which provides a direction for further optimization of EVs.
Fig. 12.
Co-incubation as a modification strategy of donor cells. (A) Pattern diagram of Cur-EVs reversing IL-1β induced catabolism of OA-CH. (B) qRT-PCR analysis of the effect of Cur-EVs on OA-CH gene expression. n = 4; * compared with the control group; # difference between groups. C. Effects of Cur-EVs on expression of miR-126-3p gene in OA-CH. Reproduced under the terms of the CC-BY 4.0 [220]. Copyright 2021, The Authors, published by Springer Nature.
As for pro-inflammatory factors, for example, EVs from cells co-incubated with IL-1β reach the cartilage region more quickly and carry more chondroprotective miRNAs, such as miR-146a, −520c, −155, etc. [231], and anti-inflammatory factors, including SOCS3, SOCS6, and miR-147b [232]. Similarly, EVs from SMSCs co-incubated with lipopolysaccharide (LPS) inhibited ECM degradation in OA. LPS-EVs inhibited the decrease in ACAN and COL2A1 via let-7b, promoted chondrocyte proliferation and migration, and significantly prevented the development of OA [233]. These experiments proved that the stimulation of pro-inflammatory factors could promote MSCs to up-regulate or produce EVs with chondroprotective effect, which to some extent proved the process of self-repair of articular cartilage in pathogenesis and provided a new treatment strategy.
-
(2)
Gene transfection method
Gene transfection is a common strategy for loading cargo into donor cells. A large number of studies have obtained EVs with high expression of specific miRNAs, circRNAs or lncRNAs through transfection for the treatment of OA. The most common cargoes are miRNAs, which are encapsulated in EVs and pass between cells to perform biological functions. For example, the overexpression of miR-26a-5p [234], miR-126-3p [235], miR-127-3p [236], miR-155-5p [237], and miR-361-5p [238] can alleviate chondrocyte inflammation and cartilage degradation. Overexpression of miR-140-5p inhibits chondrocyte apoptosis [239] and promotes chondrocyte proliferation and migration [3]. Similarly, overexpression of miR-31 can also enhance cell proliferation and migration [240]. In terms of the mechanism, exosomal miR-31 can target histone demethylase KDM2A, bind to transcription factor E2F1, and inhibit transcriptional activity. Moreover, overexpression of miR-206 can inhibit the apoptosis of osteoblasts induced by Elf3 and increase the expression of OCN and BMP2 in bone tissue [241]. Therefore, the overexpressed miRNAs in EVs can affect the proliferation, migration, and apoptosis of chondrocytes and other cells, thus slowing down the progression of OA. It is worth mentioning that recent studies have shown that exosomes from urinary-derived stem cells (USCs) that overexpress miR-140-5p not only retain the original advantages of USC-Exos in promoting chondrocyte proliferation and migration, but also increase ECM secretion by targeting VEGFA [221]. This proves that transfecting specific exogenous goods, such as miRNAs, can precisely regulate the functions of EVs to meet the needs of OA therapy.
Exosomal circRNAs can regulate OA as competitive endogenous RNA (ceRNA) regulates downstream target gene expression by binding to miRNAs. By analyzing the difference in circRNA expression before and after chondrogenic differentiation of MSCs, circRNA-0001236 was found due to 5.29-fold upregulation (p < 0.001). CircRNA-0001236 can act as a sponge for miR-3677-3p. By overexpressing it, the expression of COL2A1 and Sox9 was enhanced, while the expression of MMP13 was inhibited, suggesting that it improved the protective effect of MSC-Exos [242]. Similarly, EVs overexpressing circ-HIPK3 significantly improved IL-1β induced chondrocyte injury via the miR-124-3p/MYH9 axis [243]. These studies demonstrate that overexpression of circRNA, a stable non-coding RNA, might be a potential new cell-free therapy for OA.
LncRNAs can also function as ceRNA. To enhance the therapeutic ability of UMSC-Exos, Yan et al. [29]. transferred the lncRNA H19 (Fig. 13A) and found that exosomes carrying lncRNA H19 (H19-Exos) were taken up by the chondrocytes. Subsequently, H19-Exos enhanced the inhibition of chondrocyte senescence and anabolism by directly inhibiting miR-29b-3p (Fig. 13B). Finally, the protective role of H19-Exos in OA was confirmed by in vivo analysis of the gross images (Fig. 13C and D) and histological staining (Fig. 13E and F). Further studies have found that lncRNA H19 can also improve pain and central sensitization in advanced OA, helping enhance the quality of life of patients [244]. However, the gene transfection method has limitations, including low loading efficiency and may induce donor cell apoptosis, which requires further study to improve efficiency and find suitable donor cells.
-
(3)
Hypoxic method
Fig. 13.
Gene transfection as a modification strategy of donor cells. (A) Pattern diagram of H19-Exos playing a protective role in cartilage. (B) Quantitative analysis of SA-β-Gal staining of chondrocytes and protein and mRNA levels of chondrocyte-associated genes. (C, D) Gross, MRI images and International Cartilage Repair Society (ICRS) score of regenerated tissues at weeks of 4 and 8, (n = 5). (E, F) Histological staining and Wakitani score after 4 weeks of cartilage repair (n = 5). Reproduced under the terms of the CC-BY 4.0 [29]. Copyright 2021, The Authors, published by Wiley-VCH.
Oxygen concentration is believed to be critical for proliferation, differentiation, and self-renewal of MSCs [245,246]. Although MSCs are located near vascular structures, they are often exposed to hypoxic conditions. Hypoxia helps maintain stemness, determine cell fate, and promote migration [247]. For example, hypoxia leads to increased chondrogenic potential, normal osteogenic potential, and reduced adipogenic potential of ADSCs, which are believed to be affected by HIF-1α [248]. The sEVs from MSCs under hypoxia (hypo-sEVs) also have similar properties. EVs secreted by urine-derived stem cells under hypoxia highly expressed miR-26a-5p, which promoted the proliferation and migration of chondrocytes by targeting PTEN [249]. Similarly, Yu and colleagues found that hypo-sEVs had better cartilage repair than sEVs under normal oxygen conditions [222]. Further studies showed that miR-216a-5p was highly expressed in hypo-sEVs and affected chondrocyte proliferation and migration through the JAK2/STAT3 signaling pathway. Zhang et al. [250]. sequenced the miRNA of hypo-EVs and found that the expression of miR-181c-5p, miR-18a-3p, miR −376a-5p, and miR -337-5p was reduced, further explaining the possible reasons for its promotion of proliferation and anti-apoptosis. These studies provide a new approach to enhance the therapeutic efficacy of EVs.
4.2.1.2. Application physical factors to modify donor cells
-
(1)
Mechanical stress method
Mechanical stress is a crucial factor affecting cell proliferation and differentiation [251]. Appropriate dynamic stimulation can increase chondrocyte proliferation and ECM deposition [252,253]. Low intensity pulsed ultrasound (LIPUS) is a sound pressure wave that provides local mechanical stimulation to the cells. Previous studies have shown that LIPUS regulates MSCs autophagy, promotes chondrogenesis, and improves the synthesis of ECM [254,255]. Further study found that by activating autophagy, MSCs release more exosomes thereby enhancing the ability to promote cartilage repair [256]. Besides, Liao et al. [223]. found that exosomes from LIPUS-stimulated BMSCs inhibited inflammation, increased chondrocyte proliferation, and promoted extracellular matrix synthesis by inhibiting the IL-1β induced NF-κB signaling pathway. Although articular cartilage is an extremely mechanically sensitive tissue [257], few studies have combined mechanical stimulation with EVs. This is the first trial to combine LIPUS with exosomes, demonstrating that mechanical stimulation can potentially improve EVs function in restoring articular cartilage integrity and function, thereby preventing and reversing the cascade of progression to OA.
-
(2)
3D culture method
3D culture can increase the size, yield, and function of EVs. Compared to 2D culture, EVs yield in 3D culture was 20 times higher (p = 0.0009), and siRNA delivery efficiency was 5 times higher (p < 0.0001) [258]. Similarly, Yan et al. [224] found that MSCs cultured in a circulating hollow fiber bioreactor produced considerably more exosomes (approximately 7.5-fold, p < 0.01) and possessed stronger cartilage repair ability, possibly related to the TGF-β1 and Smad 2/3 signaling pathway. These results suggest the possibility of large-scale production of EVs and can effectively facilitate the transition of EVs technology to clinical applications.
4.2.2. Modifying EVs directly
4.2.2.1. Loading cargoes to modify EVs
By mixing EVs directly with various compounds under different conditions, these compounds can be encapsulated. This method is more suitable for hydrophobic compounds because they are more likely to pass through the EVs membranes [259]. As a natural source of polysaccharides, chitosan oligosaccharides (COS) have antibacterial, anticoagulant and immune function enhancement effects [260]. Li et al. [225]. encapsulated COS in ADSC-EVs and found that EVs-COS had a stronger ability to promote cartilage migration and inhibit cartilage apoptosis and matrix degradation. Bioinformatics analysis revealed 760 differentially expressed genes associated with the classic Wnt, PI3K-Akt, AMPK, and MAPK signaling pathways. However, the encapsulation efficiency was not mentioned in their work, and chondrocytes were treated with different concentrations of EVs and COS to determine the optimal concentration, which could not ensure the optimal therapeutic effect after mixing EVs and COS at this concentration. Further research is needed to evaluate the efficiency of direct mixing, as well as its advantages and disadvantages compared to other methods.
Electroporation creates a transport hole in the EVs membrane through an electric field, allowing the entry of exogenous cargo into EVs [261]. Kartogenin (KGN) was loaded into Exos via electroporation (Fig. 14A) [30]. KGN is a small molecule that can induce the differentiation of SFSCs into chondrocytes. However, owing to its low water solubility, the clinical applications are limited [262]. Encapsulation of EVs can solve this problem. Nevertheless, this approach may induce EVs aggregation, thereby affecting the integrity of EVs.
Fig. 14.
Modifications of EVs membrane. Left: E7-Exos delivered KGN to SFSCs to enhance cartilage regeneration. Right: PPD-sEVs reversed the surface charge and enhanced OA treatment. (A) Diagram of exosome engineering to enhance the delivery of KGN to SFSCs, and for cartilage regeneration. (B) Fluorescence images show that KGN was selectively delivered to SFSCs instead of chondrocytes (n = 3), scale bar = 10 μm. (C) Typical microscopic images of cartilage tissues in different treatment groups by HE staining and SO-FG staining. Reproduced with permission [30]. Copyright 2021, Elsevier. (D) Schematic diagram of sEVs reverse surface charge modification strategy by PPD. (E) DiO (green) labeled sEVs or PPD-sEVs chondrocyte uptake, scale bar = 50 μm. (F) Positive chondrocyte rate for uptake of DiO labeled sEVs or PPD-sEVs. (G) Cartilage degradation assessed by H&E and SO-FG staining, scale bar = 200 μm; Reproduced under the terms of the CC-BY 4.0 [32]. Copyright 2021, The Authors, published by Wiley-VCH.
4.2.2.2. Modifying membrane of EVs
Apart from loading exogenous cargo into EVs, the EVs membrane can be modified to enhance their targeting and delivery capabilities. Common membrane modification methods include chemical modification and genetic engineering [217]. Fusion of target proteins with EVs membrane proteins can enhance the specificity of EVs. To target SFSCs, Xu et al. [30]. fused MSCs binding peptide E7 with the exosomal membrane protein Lamp 2B to form E7-Exos. KGN delivered by E7-Exos was evenly distributed in the cytoplasm of SFSCs (Fig. 14B), increased their effective concentration, and strongly promoted the chondrogenesis of SFSCs (Fig. 14C). Similarly, chondrocyte affinity peptide (CAP) can be fused with exosomal surface membrane protein 2B to obtain chondrotargeted CAP-Exos [263]. CAP-Exos can specifically deliver cargoes, such as miR-140, to chondrocytes and prolong their retention in the joint, thereby better inhibiting chondrodegrading proteases. However, fusion with membrane proteins may affect the function of cargo, which requires further study.
Intra-articular injection of MSC-sEVs is a common method of administration; however, a highly negatively charged cartilage matrix may generate electrostatic obstruction to sEVs composed of anions on the surface, which results in limited distribution and low bioavailability of sEVs. To overcome this, Feng et al. [32]. modified sEVs with ε-polylysine-polyethylene-distearyl phosphatidylethanolamine (PPD). PPD-sEVs with positive charge were obtained (Fig. 14D). Fluorescence images of chondrocyte uptake (Fig. 14E) and flow cytometry (Fig. 14F) showed that PPD-sEVs were more easily taken up by chondrocytes. In vivo, PPD-sEVs showed better cartilage uptake, penetration, and retention, leading to a longer half-life than that of sEVs (approximately 2-fold, p < 0.0001). Subsequently, the therapeutic ability of PPD-sEVs was tested using the ACLT model. H&E and saffron O staining showed that the degeneration and wear degree of cartilage in the PPD-sEVs group were significantly lower (Fig. 14G). Similar results were obtained for the OARSI histological score. The enhancement of retention ability reduces the injection time while ensuring efficacy, thus reducing adverse injection reactions such as infection, soft tissue injury, and pain, which is conducive to realizing the application potential of sEVs for OA therapy.
4.2.2.3. Generating biomimetic EVs
Biomimetic EVs, such as EV-mimetic NVs, hybrid EVs, and EV-like NPs, have recently been developed. These biomimetic EVs have similar physical and chemical properties to natural EVs, but they can be expanded in production, have a higher load capacity and more precise targeting ability, which enriches the application of EVs.
Hybrid EVs are fabricated by fusing EVs with common biomaterials, such as liposomes. Hybrid EVs improve the stability and loading capacity of vesicles while retaining their biological characteristics. In 2018, Lin et al. [264]. delivered CRISPR/Cas9 to MSCs via hybrid EVs for the first time. In 2022, Liang et al. [31]. used hybrid EVs-loaded CRISPR/Cas9 tool for the first time to target MMP-13 gene knockdown in chondrocytes and showed significant therapeutic effects in both in vitro and in vivo OA models (Fig. 15A). The authors first developed exosomes targeting chondrocytes (CAP-Exo) and then fused them with liposomes to construct the hybrid CAP-Exo (Fig. 15B). After that they designed Cas9 sgMMP-13, a CRISPR/Cas9 gene editing system targeting MMP-13, and assessed its efficacy by qRT-PCR (Fig. 15C). The authors then performed in vivo experiments. They delivered hybrid EVs by intra-articular injection and found that hybrid EVs were enriched in the knee joint and remained there for at least 7 days (Fig. 15D). After weekly injection for 4 weeks, the authors found that hybrid Cap-Exo/Cas9 sgMMP-13 effectively inhibited cartilage destruction (Fig. 15E) and prevented collagen II degradation and aggrecan reduction (Fig. 15F). This study groundbreakingly makes use of the rich data accumulated from previous studies on the mechanism of OA and provides a direction worthy of further exploration for a more efficient and safe treatment of OA.
Fig. 15.
Generating biomimetic EVs containing Cas9 sgMMP-13. (A) Schematic diagram of chondrocyte specific gene editing by hybrid CAP-Exo construction. (B) TEM images of CAP-Exo, liposomes, and hybrid CAP-Exo. Scale bar: 200 nm. (C) Schematic image of the Cas9 sgMMP-13 system, and detection of MMP-13 mRNA levels by qRT-PCR in chondrocytes co-incubated with Cas9 sgMMP-13 loaded in different forms. (D) Schematic diagram of intra-articular injection and chondrocyte uptake of Hybrid CAP-Exo, and distribution of Hybrid Exo and Hybrid CAP-Exo in vivo. (E) Schematic illustration of the in vivo procedure and modified OARSI score of cartilage tissue after four weeks. (F) Quantification of fluorescence signals of MMP-13, Aggrecan, and Collagen II in different treatment groups after four weeks. Reproduced under the terms of the CC-BY 4.0 [31]. Copyright 2022, The Authors, published by IVYSPRING INT PUBL.
Meanwhile, in the treatment of OA, other biomimetic EVs, such as EV-mimetic NVs and EV-like NPs, have not yet been applied, which may have stronger targeting, yield and therapeutic effects. It is believed that with the gradual development of future research, the comparison and mixing of various biomimetic EVs will provide a more feasible solution for OA treatment.
4.3. Biomaterials loading EVs for releasing control in OA treatment
Although modified and unmodified EVs have been proven to be therapeutic in OA, they still face a series of problems such as transportation, storage, and release. Controlling release in the joint cavity helps reduce the number of intra-articular administrations, thereby relieving pain and reducing injections adverse reactions. Hence, strategies to better release EVs are also the focus of research. Biomaterials, including hydrogels, scaffolds, membranes, sponges and so on, have been widely used to control the release of EVs. Although many studies have investigated the application of biomaterial-loaded EVs in bone regeneration [265,266], myocardial remodeling [267], and tumor therapy [268], few studies have focused on the treatment of OA.
Hydrogels are crosslinked polymer network materials with high water content and are the most studied biomaterials [269]. The ultrastructure of the synovial membrane is not conducive to long-term retention of nanoparticles. Studies have shown that nanoparticles of 300 nm can quickly escape [270]. Therefore, EVs need to be combined with hydrogels to achieve sustained release. Zhang et al. [271]. combining PRP-Exo with heat-sensitive hydrogel (Gel-Exo) increases retention in joints. They verified that Gel-Exo could be released continuously for 28 days and promoted the proliferation and migration of chondrocytes, promoted the differentiation of BMSCs, and suppressed inflammation. At the same time, Gel-Exo could also recruit stem cells and delay the progression of OA. Similarly, Sang et al. [272]. combined chondrocyte derived Exos with Gel and prolonged the efficacy of Exos.
Yang et al. [273]. developed an injectable Diels-Alder crosslinked hyaluronic acid/PEG (DAHP) hydrogel as an intra-articular delivery platform for MSC-sEVs. The diffusion release of EVs was very limited after 14 days, indicating good retention ability. In addition, compared with traditional methods, such as photo-crosslinking, which may produce extra free radicals to affect EVs contents, Diels-Alder crosslinking with low interference characteristics can help preserve EVs functions. Furthermore, the DAHP-controlled-release group achieved similar effects to multiple injections of sEVs, indicating that the same therapeutic ability could be achieved with fewer injections.
Tao et al. [274]. successfully isolated sEVs loaded with circRNA3503 from SMSCs and used poly(D, l-lactide)-b-poly(ethylene glycol)-b-poly(D, l-lactide) (PDLLA-PEG-PDLLA, PLEL) as the carrier. PLEL@circRNA3505-sEVs alleviated inflammation-induced apoptosis and the imbalance between ECM synthesis and degradation by acting as a sponge of hsa-miR-181c-3p and hsa-let-7b-3p. This study was the first to demonstrate the potential of PLEL triblock copolymer gels in sustained release sEVs. Similarly, chondrocyte-derived EVs loaded with chitosan-hyaluronic acid (CS-HA) hydrogel promoted the differentiation of ADSCs into cartilage and increased the expression of Sox9 and COL2A1 [275]. The results were superior to those of EVs alone, CS-HA hydrogel alone, and CS@EVs, suggesting that the CS-HA hydrogel provides a favorable microenvironment and prolongs the therapeutic effect of EVs.
3D-printed scaffolds are another vehicle of interest because they provide better mechanical support than hydrogels [276]. In addition, 3D printing can accurately control the internal architecture and topology to create a scaffold that is more suitable for the complex structure within the joint, assisting EVs in maintaining joint homeostasis while controlling the release behavior. Chen et al. [277]. found that a 3D-printed ECM/GelMA/exosome scaffold effectively restored the mitochondrial function of chondrocytes, enhanced chondrocyte migration, and polarized synovial macrophages toward the M2 subtype. The 3D-printed stent effectively controlled drug delivery, providing a more convenient mode than repeated intra-articular injections of exosomes. In addition to EVs loading, 3D-printed scaffolds can also be used to construct in vitro culture models to study the interaction between ADSC-EVs, chondrocytes, and fibroblast-like synovial cells from OA patients [278]. Compared to 2D models, 3D models can better study molecular mechanisms and provide effective tools for clinical EV release measurement and potency prediction.
It should be noted that there are still many limitations in the combination with biomaterials. First, it is necessary to further elucidate whether biomaterials affect EVs cargo and function. Second, due to the relatively independent metabolism of the joint microenvironment, the impact of the material itself and degradation need to be considered. Finally, standardized techniques are needed to assess EVs release and post-release coordination effects.
4.4. Challenges of clinical transformation in implementing EV therapy for OA
Currently, the EVs used to treat OA are almost exclusively tested in small animals (rats or mice). However, the selection of different animal models affects the conclusion [279]. Among animal models related to OA, small animal models, such as mice, rats, and rabbits, are commonly used to study the mechanisms of disease. However, large animal models, such as dogs, sheep, horses and monkeys, are often used to study the process and treatment of diseases because their anatomical structures are similar to those of humans [280].
Compared with MSCs, there are few studies on EV-based therapeutics for OA in large animals [[281], [282], [283]]. We speculate that this might be because EV-based treatment strategies are still in their early stages. Small animal models could shorten the period to screen for better EVs and discover the underlying mechanisms. Subsequently, the selected EVs will be further verified for their curative effect, especially the long-term effect, by large animal models to help clinical transformation.
There have been several EV-based therapeutics in preclinical/clinical trials [33]. Most focus on cardiovascular diseases [[284], [285], [286]], lung injuries [287,288], and neoplasms (NCT04592484). Generally, a meaningful basic study translating into a safe and feasible treatment requires five stages, which is called the T model [289]. When it comes to the EV-based therapeutics, there is currently no clear regulatory framework for EV therapeutics. Besides therapeutic efficacy, pharmacokinetics, safety, and even economic value also need to be demonstrated before approval, which is still in its infancy in the field of EVs.
Except for one study on Wharton's jelly in which the therapeutic component may include EVs, there has been no clinical trial related to EVs in the field of OA treatment [290]. We analyzed the possible reasons and concluded that specific challenges in implementing EV therapy for OA exists in at least two aspects:1) The specificity of OA pathogenesis. Currently, the mechanism of OA is unclear. OA was initially considered an age-related disease. It was later considered that the formation of OA was jointly determined by a variety of factors and thus had a variety of phenotypes. The heterogeneity of the disease makes it difficult to identify a specific target for intervention, resulting in therapeutic challenges. In addition, when we focus on a single pathogenic factor, taking inflammation as an example, there are also some challenges. Inflammation in OA is usually chronic and low-grade; therefore, traditional systemic anti-inflammatory approaches have only a small benefit. Therefore, new approaches must be adopted to treat OA inflammation and the resulting cartilage degeneration. 2) The particularity of joint structure. The rapid clearance of drugs from the joint and therapeutic targets deep within the cartilage that drugs cannot reach pose significant delivery challenges for many promising drugs [291,292]. Possible approaches include A) changing the size or membrane potential of EVs to combat the steric and electrostatic hindrance of the cartilage matrix; B) improving the structure and targeting ability of EVs to prolong the residence time in the joint cavity; and C) combined with biomaterials to enhance the penetration ability of EVs into the cartilage and achieve sustained release. However, some engineering approaches are contradictory. For example, reducing the size can enhance the cartilage penetration of EVs but also increase the chance to be clear out of the joint cavity. It is believed that with the continuous development of research, we will find appropriate targets and effectively solve these problems. Within a decade or more, we would witness the first in-human trials of EV-based therapeutics for OA.
5. Conclusions and further perspectives
EVs have shown great value in OA research because of their biocompatibility, bioactivity, immunogenicity and other characteristics. In the past decade, exciting advances have been made in the biology, isolation, characterization, therapeutic use, and engineering of EVs. EVs can carry genetic materials from donor cells and mediate cell communication, thus playing a series of roles in the development of OA disease, such as spreading inflammation, changing metabolism, and promoting cell senescence. Because EVs can remain in body fluids such as blood for a long time and reflect the status of donor cells, they might be used as biomarkers for the early diagnosis of OA and evaluation of pathological typing and severity. Modified and unmodified EVs can also be used as emerging treatments to delay disease progression, relieve pain, and improve prognosis, but there are still limitations and challenges.
5.1. EVs research needs to be more systematic and normative
EVs are a relatively new concept. They have received significant attention as potential mediators of disease remission after demonstrating that they can deliver proteins and genes from donor cells to recipient cells. However, rapid development has led to a lack of standardization in terms of nomenclature, separation techniques, and storage conditions.
-
a)
Nomenclature. Many studies refer to vesicles as ‘exosomes’ after simply identifying their shape, size, and surface markers. However, the physical properties of exosomes may overlap with other EVs. Thus, without proof of their origin from the multivesicular body, the International Extracellular Vesicles Association (ISEV) has recommended describing them by physical characteristics, biochemical composition, conditions, or cell of origin [293].
-
b)
Separation techniques. Currently, various methods are available for isolating EVs, such as differential ultracentrifugation (dUC), density gradients, filtration, SEC, and immune isolation. Different separation methods impact the components and functions of EVs, especially in the study of soluble SASP in EVs. Commonly used dUC cannot separate EVs from soluble protein, while SEC is one approach that helps to address this issue. Therefore, different separation methods are required for various research purposes. There are two obvious benefits to improve separation techniques: increased purity and yield. First, a potentially feasible measure is to improve the centrifugation rate to obtain smaller vesicles, such as exomeres and supermeres, which have been proven to be more biologically active, more easily absorbed, and can reduce problems caused by EVs heterogeneity. Second, by combining EVs with biological reactors or changing separation conditions, such as illumination, mechanical force, and drug stimulation, the yield of EVs can be effectively increased to achieve mass production.
-
c)
Storage conditions. Storage at −80 °C is a widely used method. However, there is increasing evidence that storage affects EVs concentration, physical properties, and function. Moreover, the impact will be intensified with an increase in the number of freeze-thaw cycles. Therefore, one-time use after separation may be a more reasonable choice at present. However, storage methods with less impact require further investigation, including changing the storage medium, altering the pH, modifying the EVs surface, and combining EVs with other NPs. In future, storage methods should be selected according to different uses to meet clinical needs.
5.2. Mechanism studies should be more specific
EVs plays an irreplaceable role in OA progression including inflammation, aging, and metabolism. However, the exact targets of disease progression require further study to provide clear directions for disease treatment. For example, secretory phospholipase A2 (sPLA2i) is an effective inflammatory mediator in OA development. Thus, the specific targeting of sPLA2i could mitigate OA progression. At the same time, the discovery of similarities and differences in the pathogenesis of different subtypes of OA will help us systematically understand diseases progression and establish a comprehensive database to provide early and precise intervention for patients in future.
Interestingly, inflammation, senescence, and metabolism appear to play synergistic and mutually reinforcing roles in the progression of OA. For example, aging cells and microenvironment produce a large number of inflammatory mediators and cause metabolic changes, and the progression of inflammation also induces cell senescence and destroys mitochondrial function. Therefore, EVs, as an important component of paracrine signaling, may play a role in communication in this network, which initiates a vicious cycle by transferring key molecules, promoting the continuous deterioration of OA in the cycle of inflammation, aging, and metabolism. Simultaneously, EVs may play a role in other causes of OA, such as excessive mechanical load.
At the same time, given that EVs could carry pathogenic factors and transfer them between cells, inhibition of EVs formation, release, or uptake may reduce vesicle-mediated pathogenesis. At present, possible ways include inhibiting the release of EVs by targeting essential components and inhibiting the uptake of pathological EVs by regulating receptor cells.
5.3. Studies of EVs in OA diagnosis needs to improve sensitivity, specificity, and operability
EVs from the blood, SF, synovial fibroblasts, or chondrocytes have shown great potential as biomarkers. Not only do EVs contribute to the early diagnosis of OA and the differentiation of OA subtypes, but they have predictive value in identifying people at high risk of developing OA as well. However, translating biomarkers based on EVs into clinical settings requires technological advances, including improved sensitivity, specificity, and maneuverability.
The sensitivity of a single biomarker is limited because of the complexity of OA pathophysiology. Therefore, multiple diagnostic methods are required to support each other. Besides, EVs may lack specificity for joint tissue. Specifically, there are differences in biomarkers between the knee, hip, hand and spine, and further research is needed to explore whether specific biomarkers exist in specific joints. Engineered exogenous EVs may also play a role in solving these problems.
Another challenge of using EVs as a biomarker of OA is the complex and lacks standardized isolation and characterization methods, which limits their operability. Therefore, high-throughput methods are required to rapidly isolate EVs from many samples or to increase EVs production by the improving Separation techniques mentioned above. Simultaneously, standardized protocols on how EVs biomarkers can be validated in different laboratories and in different samples are also essential, which will accelerate the clinical transformation of EVs as markers.
5.4. Strategies for improving treatment
For the application of EVs as drug delivery agents, one issue that needs to be addressed is how to load the required exogenous cargo more efficiently into EVs and improve the targeting ability. Many emerging methods of engineering EVs, such as EV-mimetic NVs and EV-like NPs, can be attempted. In addition, further deepening the understanding of the EVs delivery mechanism may help to solve the problem. However, at the same time, the question of whether loading exogenous goods interferes with the original contents and whether this has off-target effects may become a huge barrier to EVs applications.
Another problem is the determination of the dose, dosage form, and cell source of EVs to avoid toxicity and immunogenicity. To solve this problem, preclinical models, for examples, should be used to assess efficacy in relation to EVs dose and frequency. Although previous studies have shown that transplanting human EVs in animal models is safe, the immunogenicity of EVs from allogeneic sources still needs to be verified. Further understanding of the mechanisms of action of different doses and dosage forms of EVs in treatment can help guide the selection of these parameters.
Long-term therapeutic effect is also a challenge. Engineered EVs can remain in the joint spaces for longer period by changing their size and membrane structure. Naturally derived EVs could also achieve this through at least four ways: 1) Increase the times of administration to maintain the drug concentration in the joint cavity. However, the increased pain and decreased compliance should not be ignored. 2) Select more appropriate parent cells for EVs. Because of the low immunogenicity of EVs, we can screen for sources with better therapeutic efficacy. Potential parent cells include perinatal stem cells with greater regenerative capacity, ADSCs with greater immunomodulatory capacity, MSCs from younger individuals, and even marine organisms. EVs from different sources can even be combined to cover different pathogenic mechanisms to enhance the efficacy. 3) Select EVs with more suitable size or structure. Because of the particularity of joint lumen structure, screening EVs that can not only penetrate cartilage steric hindrance but not be rapidly metabolized is very important to maintain long-term therapeutic effect. 4) Combine with biomaterials. As discussed in Section 4.3, with the biomaterials that have been approved by clinical trials, we can increase the targeting and residence capability of EVs without compromising safety and achieve sustained release through the slow degradation of the material. Ensuring the long-term efficacy of EVs can contribute to better clinical transformation of EVs.
Moreover, EVs may play a synergistic role with existing OA interventions. For example, exosomes combining HA with PRP may further reduce pain. EVs from different sources can also be used simultaneously because crosstalk may occur among their cargoes.
EVs also have potential for personalized and precise treatment. As we all know, EVs in synovium can be used to diagnose disease stage. With the understanding of the progress of EVs in OA, we can obtain sufficient information from the database in future to conduct personalized analysis of patients' joints. Thus, the required compounds can be loaded into EVs to achieve personalized treatment.
Overall, this review summarizes the studies on EVs in the pathogenesis, diagnosis, and treatment of OA, which are important links in disease progression and intervention. In general, EVs contributes to the further understanding of OA pathogenesis, and the concept of utilizing EVs as biomarkers and treatment options for OA is attractive and promising. As expected, in future, the solutions to key problems will promote the clinical transformation of EVs and become a novel strategy to control OA.
Ethics approval and consent to participate
This review manuscript does not involve animal experiments or clinical trials, so there is no Ethics approval and consent to participate.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (82072396, 81871490), Program of Shanghai Academic/Technology Research Leader (20XD1433100), Science and Technology Commission of Shanghai Municipality (21490711700, 21DZ2294600), Interdisciplinary Program of Shanghai Jiao Tong University (YG2021ZD12), CAMS Innovation Fund for Medical Sciences (CIFMS) (2019-I2M-5–037), and Key Laboratory of Spine and Spinal Cord Injury Repair and Regeneration (Tongji University), Ministry of Education.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2022.10.012.
Contributor Information
Changyong Yuan, Email: yuanchangyong1983@foxmail.com.
Xudong Wang, Email: xudongwang70@hotmail.com.
Kaili Lin, Email: linkaili@sjtu.edu.cn, lklecnu@aliyun.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hunter D.J., Schofield D., Callander E. The individual and socioeconomic impact of osteoarthritis, Nature reviews. Rheumatology. 2014;10(7):437–441. doi: 10.1038/nrrheum.2014.44. [DOI] [PubMed] [Google Scholar]
- 2.Hunter D.J., Bierma-Zeinstra S. Osteoarthritis, Lancet. 2019;393(10182):1745–1759. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 3.Tao S.-C., Yuan T., Zhang Y.-L., Yin W.-J., Guo S.-C., Zhang C.-Q. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7(1):180–195. doi: 10.7150/thno.17133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martel-Pelletier J., Barr A.J., Cicuttini F.M., Conaghan P.G., Cooper C., Goldring M.B., Goldring S.R., Jones G., Teichtahl A.J., Pelletier J.-P. Osteoarthritis. Nat. Rev. Dis. Prim. 2016;2 doi: 10.1038/nrdp.2016.72. [DOI] [PubMed] [Google Scholar]
- 5.Ankrum J.A., Ong J.F., Karp J.M. Mesenchymal stem cells: immune evasive, not immune privileged. Nat. Biotechnol. 2014;32(3):252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damjanov I., Andrews P.W. Teratomas produced from human pluripotent stem cells xenografted into immunodeficient mice - a histopathology atlas. Int. J. Dev. Biol. 2016;60(10–11-12):337–419. doi: 10.1387/ijdb.160274id. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandt K.D., Radin E.L., Dieppe P.A., van de Putte L. Yet more evidence that osteoarthritis is not a cartilage disease. Ann. Rheum. Dis. 2006;65(10):1261–1264. doi: 10.1136/ard.2006.058347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deveza L.A., Loeser R.F. Is osteoarthritis one disease or a collection of many? Rheumatology. 2018;57(suppl_4):iv34–iv42. doi: 10.1093/rheumatology/kex417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appleton C.T. Osteoarthritis year in review 2017: biology. Osteoarthritis Cartilage. 2018;26(3):296–303. doi: 10.1016/j.joca.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Loeser R.F., Collins J.A., Diekman B.O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016;12(7):412–420. doi: 10.1038/nrrheum.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asghar S., Litherland G.J., Lockhart J.C., Goodyear C.S., Crilly A. Exosomes in intercellular communication and implications for osteoarthritis. Rheumatology. 2020;59(1):57–68. doi: 10.1093/rheumatology/kez462. [DOI] [PubMed] [Google Scholar]
- 12.Withrow J., Murphy C., Liu Y., Hunter M., Fulzele S., Hamrick M.W. Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis. Arthritis Res. Ther. 2016;18(1):286. doi: 10.1186/s13075-016-1178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie F., Liu Y.-L., Chen X.-Y., Li Q., Zhong J., Dai B.-Y., Shao X.-F., Wu G.-B. Role of MicroRNA, LncRNA, and exosomes in the progression of osteoarthritis: a review of recent literature. Orthop. Surg. 2020;12(3):708–716. doi: 10.1111/os.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paschos N.K. Editorial commentary: exosomes-A new word in the orthopaedic vocabulary? Arthroscopy. 2020;36(8):2229–2230. doi: 10.1016/j.arthro.2020.05.046. [DOI] [PubMed] [Google Scholar]
- 15.Théry C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 16.Lo Cicero A., Stahl P.D., Raposo G. Extracellular vesicles shuffling intercellular messages: for good or for bad. Curr. Opin. Cell Biol. 2015;35:69–77. doi: 10.1016/j.ceb.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 17.van Niel G., D'Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19(4):213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 18.Kalluri R., LeBleu V.S. The biology function and biomedical applications of exosomes. Science. 2020;367(6478) doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colombo M., Raposo G., Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 20.Cocozza F., Grisard E., Martin-Jaular L., Mathieu M., Théry C. SnapShot: extracellular vesicles. Cell. 2020;182(1) doi: 10.1016/j.cell.2020.04.054. [DOI] [PubMed] [Google Scholar]
- 21.Karimi N., Cvjetkovic A., Jang S.C., Crescitelli R., Hosseinpour Feizi M.A., Nieuwland R., Lötvall J., Lässer C. Detailed analysis of the plasma extracellular vesicle proteome after separation from lipoproteins. Cell. Mol. Life Sci. 2018;75(15):2873–2886. doi: 10.1007/s00018-018-2773-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maehara M., Toyoda E., Takahashi T., Watanabe M., Sato M. Potential of exosomes for diagnosis and treatment of joint disease: towards a point-of-care therapy for osteoarthritis of the knee. Int. J. Mol. Sci. 2021;22(5) doi: 10.3390/ijms22052666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin B., Ni J., Witherel C.E., Yang M., Burdick J.A., Wen C., Wong S.H.D. Harnessing tissue-derived extracellular vesicles for osteoarthritis theranostics. Theranostics. 2022;12(1):207–231. doi: 10.7150/thno.62708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou T., Yuan Z., Weng J., Pei D., Du X., He C., Lai P. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021;14(1):24. doi: 10.1186/s13045-021-01037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arntz O.J., Pieters B.C.H., Oliveira M.C., Broeren M.G.A., Bennink M.B., de Vries M., van Lent P.L.E.M., Koenders M.I., van den Berg W.B., van der Kraan P.M., van de Loo F.A.J. Oral administration of bovine milk derived extracellular vesicles attenuates arthritis in two mouse models. Mol. Nutr. Food Res. 2015;59(9):1701–1712. doi: 10.1002/mnfr.201500222. [DOI] [PubMed] [Google Scholar]
- 26.Lei J., Jiang X., Li W., Ren J., Wang D., Ji Z., Wu Z., Cheng F., Cai Y., Yu Z.-R., Belmonte J.C.I., Li C., Liu G.-H., Zhang W., Qu J., Wang S. Exosomes from Antler Stem Cells Alleviate Mesenchymal Stem Cell Senescence and Osteoarthritis. Protein Cell. 2022;13(3):220–226. doi: 10.1007/s13238-021-00860-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jo S.-H., Kim C., Park S.-H. Novel marine organism-derived extracellular vesicles for control of anti-inflammation. Tissue Eng Regen Med. 2021;18(1):71–79. doi: 10.1007/s13770-020-00319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X., Zhang H., Gu J., Zhang J., Shi H., Qian H., Wang D., Xu W., Pan J., Santos H.A. Engineered extracellular vesicles for cancer therapy. Adv Mater. 2021;33(14) doi: 10.1002/adma.202005709. [DOI] [PubMed] [Google Scholar]
- 29.Yan L., Liu G., Wu X. The umbilical cord mesenchymal stem cell-derived exosomal lncRNA H19 improves osteochondral activity through miR-29b-3p/FoxO3 axis. Clin. Transl. Med. 2021;11(1):e255. doi: 10.1002/ctm2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu X., Liang Y., Li X., Ouyang K., Wang M., Cao T., Li W., Liu J., Xiong J., Li B., Xia J., Wang D., Duan L. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2021;269 doi: 10.1016/j.biomaterials.2020.120539. [DOI] [PubMed] [Google Scholar]
- 31.Liang Y., Xu X., Xu L., Iqbal Z., Ouyang K., Zhang H., Wen C., Duan L., Xia J. Chondrocyte-specific genomic editing enabled by hybrid exosomes for osteoarthritis treatment. Theranostics. 2022;12(11):4866–4878. doi: 10.7150/thno.69368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng K., Xie X., Yuan J., Gong L., Zhu Z., Zhang J., Li H., Yang Y., Wang Y. Reversing the surface charge of MSC-derived small extracellular vesicles by εPL-PEG-DSPE for enhanced osteoarthritis treatment. J. Extracell. Vesicles. 2021;10(13) doi: 10.1002/jev2.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng L., Hill A.F. Therapeutically harnessing extracellular vesicles. Nat. Rev. Drug Discov. 2022;21(5):379–399. doi: 10.1038/s41573-022-00410-w. [DOI] [PubMed] [Google Scholar]
- 34.Fu K., Robbins S.R., McDougall J.J. Osteoarthritis: the genesis of pain. Rheumatology. 2018;57(suppl_4):iv43–iv50. doi: 10.1093/rheumatology/kex419. [DOI] [PubMed] [Google Scholar]
- 35.van den Bosch M.H.J. Osteoarthritis year in review 2020: biology. Osteoarthritis Cartilage. 2021;29(2):143–150. doi: 10.1016/j.joca.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Todorova D., Simoncini S., Lacroix R., Sabatier F., Dignat-George F. Extracellular vesicles in angiogenesis. Circ. Res. 2017;120(10):1658–1673. doi: 10.1161/CIRCRESAHA.117.309681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kato T., Miyaki S., Ishitobi H., Nakamura Y., Nakasa T., Lotz M.K., Ochi M. Exosomes from IL-1β stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res. Ther. 2014;16(4):R163. doi: 10.1186/ar4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Q., Wang R., Hou S., He F., Ma Y., Ye T., Yu S., Chen H., Wang H., Zhang M. Chondrocyte-derived exosomes promote cartilage calcification in temporomandibular joint osteoarthritis. Arthritis Res. Ther. 2022;24(1):44. doi: 10.1186/s13075-022-02738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng S., Yan Y., Li R., Dai H., Xu J. Extracellular vesicles from M1-polarized macrophages promote inflammation in the temporomandibular joint via miR-1246 activation of the Wnt/β-catenin pathway. Ann. N. Y. Acad. Sci. 2021;1503(1):48–59. doi: 10.1111/nyas.14590. [DOI] [PubMed] [Google Scholar]
- 40.Ni Z., Kuang L., Chen H., Xie Y., Zhang B., Ouyang J., Wu J., Zhou S., Chen L., Su N., Tan Q., Luo X., Chen B., Chen S., Yin L., Huang H., Du X., Chen L. The exosome-like vesicles from osteoarthritic chondrocyte enhanced mature IL-1β production of macrophages and aggravated synovitis in osteoarthritis. Cell Death Dis. 2019;10(7):522. doi: 10.1038/s41419-019-1739-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng G., Deng G., Xiao S., Li F. Fibroblast-like synoviocytes-derived exosomal PCGEM1 accelerates IL-1β-induced apoptosis and cartilage matrix degradation by miR-142-5p/RUNX2 in chondrocytes. Immunol. Invest. 2022;51(5):1284–1301. doi: 10.1080/08820139.2021.1936010. [DOI] [PubMed] [Google Scholar]
- 42.Guo Z., Wang H., Zhao F., Liu M., Wang F., Kang M., He W., Lv Z. Exosomal circ-BRWD1 contributes to osteoarthritis development through the modulation of miR-1277/TRAF6 axis. Arthritis Res. Ther. 2021;23(1):159. doi: 10.1186/s13075-021-02541-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu C., Shen K., Zhou W., Wu H., Lu Y. Exosome-mediated circ_0001846 participates in IL-1β-induced chondrocyte cell damage by miR-149-5p-dependent regulation of WNT5B. Clin Immunol. 2021 doi: 10.1016/j.clim.2021.108856. [DOI] [PubMed] [Google Scholar]
- 44.Meng F., Li Z., Zhang Z., Yang Z., Kang Y., Zhao X., Long D., Hu S., Gu M., He S., Wu P., Chang Z., He A., Liao W. MicroRNA-193b-3p regulates chondrogenesis and chondrocyte metabolism by targeting HDAC3. Theranostics. 2018;8(10):2862–2883. doi: 10.7150/thno.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan J., Shen M., Sui B., Lu W., Han X., Wan Q., Liu Y., Kang J., Qin W., Zhang Z., Chen D., Cao Y., Ying S., Tay F.R., Niu L.-N., Jiao K. Autophagic LC3 calcified extracellular vesicles initiate cartilage calcification in osteoarthritis. Sci. Adv. 2022;8(19) doi: 10.1126/sciadv.abn1556. eabn1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li D., Liu J., Guo B., Liang C., Dang L., Lu C., He X., Cheung H.Y.-S., Xu L., Lu C., He B., Liu B., Shaikh A.B., Li F., Wang L., Yang Z., Au D.W.-T., Peng S., Zhang Z., Zhang B.-T., Pan X., Qian A., Shang P., Xiao L., Jiang B., Wong C.K.-C., Xu J., Bian Z., Liang Z., Guo D.-A., Zhu H., Tan W., Lu A., Zhang G. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat. Commun. 2016;7 doi: 10.1038/ncomms10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu X., Crawford R., Xiao Y., Mao X., Prasadam I. Osteoarthritic subchondral bone release exosomes that promote cartilage degeneration. Cells. 2021;10(2) doi: 10.3390/cells10020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeon O.H., Wilson D.R., Clement C.C., Rathod S., Cherry C., Powell B., Lee Z., Khalil A.M., Green J.J., Campisi J., Santambrogio L., Witwer K.W., Elisseeff J.H. Senescence cell-associated extracellular vesicles serve as osteoarthritis disease and therapeutic markers. JCI Insight. 2019;4(7) doi: 10.1172/jci.insight.125019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varela-Eirín M., Carpintero-Fernández P., Guitián-Caamaño A., Varela-Vázquez A., García-Yuste A., Sánchez-Temprano A., Bravo-López S.B., Yañez-Cabanas J., Fonseca E., Largo R., Mobasheri A., Caeiro J.R., Mayán M.D. Extracellular vesicles enriched in connexin 43 promote a senescent phenotype in bone and synovial cells contributing to osteoarthritis progression. Cell Death Dis. 2022;13(8):681. doi: 10.1038/s41419-022-05089-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fafián-Labora J., Lesende-Rodriguez I., Fernández-Pernas P., Sangiao-Alvarellos S., Monserrat L., Arntz O.J., van de Loo F.J., Mateos J., Arufe M.C. Effect of age on pro-inflammatory miRNAs contained in mesenchymal stem cell-derived extracellular vesicles. Sci. Rep. 2017;7 doi: 10.1038/srep43923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fafián-Labora J.A., O'Loghlen A. NF-κB/IKK activation by small extracellular vesicles within the SASP. Aging Cell. 2021;20(7) doi: 10.1111/acel.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fulzele S., Mendhe B., Khayrullin A., Johnson M., Kaiser H., Liu Y., Isales C.M., Hamrick M.W. Muscle-derived miR-34a increases with age in circulating extracellular vesicles and induces senescence of bone marrow stem cells. Aging (Albany NY) 2019;11(6):1791–1803. doi: 10.18632/aging.101874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fafián-Labora J., Morente-López M., Sánchez-Dopico M.J., Arntz O.J., van de Loo F.A.J., De Toro J., Arufe M.C. Influence of mesenchymal stem cell-derived extracellular vesicles in vitro and their role in ageing. Stem Cell Res. Ther. 2020;11(1):13. doi: 10.1186/s13287-019-1534-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weilner S., Schraml E., Wieser M., Messner P., Schneider K., Wassermann K., Micutkova L., Fortschegger K., Maier A.B., Westendorp R., Resch H., Wolbank S., Redl H., Jansen-Dürr P., Pietschmann P., Grillari-Voglauer R., Grillari J. Secreted microvesicular miR-31 inhibits osteogenic differentiation of mesenchymal stem cells. Aging Cell. 2016;15(4):744–754. doi: 10.1111/acel.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davis C., Dukes A., Drewry M., Helwa I., Johnson M.H., Isales C.M., Hill W.D., Liu Y., Shi X., Fulzele S., Hamrick M.W. MicroRNA-183-5p increases with age in bone-derived extracellular vesicles, suppresses bone marrow stromal (stem) cell proliferation, and induces stem cell senescence. Tissue Eng. 2017;23(21–22):1231–1240. doi: 10.1089/ten.tea.2016.0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang R.-Z., Zheng H.-L., Xu W.-N., Zheng X.-F., Li B., Jiang L.-S., Jiang S.-D. Vascular endothelial cell-secreted exosomes facilitate osteoarthritis pathogenesis by promoting chondrocyte apoptosis. Aging (Albany NY) 2021;13(3):4647–4662. doi: 10.18632/aging.202506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ha V.T., Lainšček D., Gesslbauer B., Jarc-Jovičić E., Hyötyläinen T., Ilc N., Lakota K., Tomšič M., van de Loo F.A.J., Bochkov V., Petan T., Jerala R., Manček-Keber M. Synergy between 15-lipoxygenase and secreted PLA promotes inflammation by formation of TLR4 agonists from extracellular vesicles. Proc. Natl. Acad. Sci. U. S. A. 2020;117(41):25679–25689. doi: 10.1073/pnas.2005111117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moradi B., Rosshirt N., Tripel E., Kirsch J., Barié A., Zeifang F., Gotterbarm T., Hagmann S. Unicompartmental and bicompartmental knee osteoarthritis show different patterns of mononuclear cell infiltration and cytokine release in the affected joints. Clin. Exp. Immunol. 2015;180(1):143–154. doi: 10.1111/cei.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCabe P.S., Parkes M.J., Maricar N., Hutchinson C.E., Freemont A., O'Neill T.W., Felson D.T. Brief report: synovial fluid white blood cell count in knee osteoarthritis: association with structural findings and treatment response. Arthritis Rheumatol. 2017;69(1):103–107. doi: 10.1002/art.39829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glyn-Jones S., Palmer A.J.R., Agricola R., Price A.J., Vincent T.L., Weinans H., Carr A.J. Osteoarthritis, Lancet. 2015;386(9991):376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 61.Scanzello C.R., Loeser R.F. Editorial: inflammatory activity in symptomatic knee osteoarthritis: not all inflammation is local. Arthritis Rheumatol. 2015;67(11):2797–2800. doi: 10.1002/art.39304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ponchel F., Burska A.N., Hensor E.M.A., Raja R., Campbell M., Emery P., Conaghan P.G. Changes in peripheral blood immune cell composition in osteoarthritis. Osteoarthritis Cartilage. 2015;23(11):1870–1878. doi: 10.1016/j.joca.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scanzello C.R. Role of low-grade inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2017;29(1):79–85. doi: 10.1097/BOR.0000000000000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olivotto E., Otero M., Marcu K.B., Goldring M.B. Pathophysiology of osteoarthritis: canonical NF-κB/IKKβ-dependent and kinase-independent effects of IKKα in cartilage degradation and chondrocyte differentiation. RMD Open. 2015;1(Suppl 1) doi: 10.1136/rmdopen-2015-000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller R.E., Belmadani A., Ishihara S., Tran P.B., Ren D., Miller R.J., Malfait A.-M. Damage-associated molecular patterns generated in osteoarthritis directly excite murine nociceptive neurons through Toll-like receptor 4. Arthritis Rheumatol. 2015;67(11):2933–2943. doi: 10.1002/art.39291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scanzello C.R., Goldring S.R. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51(2):249–257. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Domenis R., Zanutel R., Caponnetto F., Toffoletto B., Cifù A., Pistis C., Di Benedetto P., Causero A., Pozzi M., Bassini F., Fabris M., Niazi K.R., Soon-Shiong P., Curcio F. Characterization of the proinflammatory profile of synovial fluid-derived exosomes of patients with osteoarthritis. Mediat. Inflamm. 2017;2017 doi: 10.1155/2017/4814987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vincent T.L. Targeting mechanotransduction pathways in osteoarthritis: a focus on the pericellular matrix. Curr. Opin. Pharmacol. 2013;13(3):449–454. doi: 10.1016/j.coph.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 69.Rasheed Z., Akhtar N., Haqqi T.M. Advanced glycation end products induce the expression of interleukin-6 and interleukin-8 by receptor for advanced glycation end product-mediated activation of mitogen-activated protein kinases and nuclear factor-κB in human osteoarthritis chondrocytes. Rheumatology. 2011;50(5):838–851. doi: 10.1093/rheumatology/keq380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao K., Zhu W., Li H., Ma D., Liu W., Yu W., Wang L., Cao Y., Jiang Y. Association between cytokines and exosomes in synovial fluid of individuals with knee osteoarthritis. Mod. Rheumatol. 2020;30(4):758–764. doi: 10.1080/14397595.2019.1651445. [DOI] [PubMed] [Google Scholar]
- 71.Cox L.G.E., van Donkelaar C.C., van Rietbergen B., Emans P.J., Ito K. Decreased bone tissue mineralization can partly explain subchondral sclerosis observed in osteoarthritis. Bone. 2012;50(5):1152–1161. doi: 10.1016/j.bone.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 72.Weinans H., Siebelt M., Agricola R., Botter S.M., Piscaer T.M., Waarsing J.H. Pathophysiology of peri-articular bone changes in osteoarthritis. Bone. 2012;51(2):190–196. doi: 10.1016/j.bone.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 73.Buckland-Wright C. Subchondral bone changes in hand and knee osteoarthritis detected by radiography. Osteoarthritis Cartilage. 2004;12(Suppl A):S10–S19. doi: 10.1016/j.joca.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 74.Sanchez C., Pesesse L., Gabay O., Delcour J.-P., Msika P., Baudouin C., Henrotin Y.E. Regulation of subchondral bone osteoblast metabolism by cyclic compression. Arthritis Rheum. 2012;64(4):1193–1203. doi: 10.1002/art.33445. [DOI] [PubMed] [Google Scholar]
- 75.Kirsch T., Swoboda B., Nah H. Activation of annexin II and V expression, terminal differentiation, mineralization and apoptosis in human osteoarthritic cartilage. Osteoarthritis Cartilage. 2000;8(4):294–302. doi: 10.1053/joca.1999.0304. [DOI] [PubMed] [Google Scholar]
- 76.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fafián-Labora J.A., Rodríguez-Navarro J.A., O’Loghlen A. Small extracellular vesicles have GST activity and ameliorate senescence-related tissue damage. Cell Metabol. 2020;32(1):71–86. doi: 10.1016/j.cmet.2020.06.004. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Coryell P.R., Diekman B.O., Loeser R.F. Mechanisms and therapeutic implications of cellular senescence in osteoarthritis. Nat. Rev. Rheumatol. 2021;17(1):47–57. doi: 10.1038/s41584-020-00533-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Basisty N., Kale A., Jeon O.H., Kuehnemann C., Payne T., Rao C., Holtz A., Shah S., Sharma V., Ferrucci L., Campisi J., Schilling B. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020;18(1) doi: 10.1371/journal.pbio.3000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Herranz N., Gallage S., Mellone M., Wuestefeld T., Klotz S., Hanley C.J., Raguz S., Acosta J.C., Innes A.J., Banito A., Georgilis A., Montoya A., Wolter K., Dharmalingam G., Faull P., Carroll T., Martínez-Barbera J.P., Cutillas P., Reisinger F., Heikenwalder M., Miller R.A., Withers D., Zender L., Thomas G.J., Gil J. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat. Cell Biol. 2015;17(9):1205–1217. doi: 10.1038/ncb3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Laberge R.-M., Sun Y., Orjalo A.V., Patil C.K., Freund A., Zhou L., Curran S.C., Davalos A.R., Wilson-Edell K.A., Liu S., Limbad C., Demaria M., Li P., Hubbard G.B., Ikeno Y., Javors M., Desprez P.-Y., Benz C.C., Kapahi P., Nelson P.S., Campisi J. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 2015;17(8):1049–1061. doi: 10.1038/ncb3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boulestreau J., Maumus M., Jorgensen C., Noel D. Extracellular vesicles from mesenchymal stromal cells: therapeutic perspectives for targeting senescence in osteoarthritis. Adv. Drug Deliv. Rev. 2021 doi: 10.1016/j.addr.2021.113836. [DOI] [PubMed] [Google Scholar]
- 83.Naik S., Larsen S.B., Cowley C.J., Fuchs E. Two to tango: dialog between immunity and stem cells in health and disease. Cell. 2018;175(4):908–920. doi: 10.1016/j.cell.2018.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takasugi M., Okada R., Takahashi A., Virya Chen D., Watanabe S., Hara E. Small extracellular vesicles secreted from senescent cells promote cancer cell proliferation through EphA2. Nat. Commun. 2017;8 doi: 10.1038/ncomms15728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Borghesan M., Fafián-Labora J., Eleftheriadou O., Carpintero-Fernández P., Paez-Ribes M., Vizcay-Barrena G., Swisa A., Kolodkin-Gal D., Ximénez-Embún P., Lowe R., Martín-Martín B., Peinado H., Muñoz J., Fleck R.A., Dor Y., Ben-Porath I., Vossenkamper A., Muñoz-Espin D., O’Loghlen A. Small extracellular vesicles are key regulators of non-cell autonomous intercellular communication in senescence via the interferon protein IFITM3. Cell Rep. 2019;27(13):3956–3971. doi: 10.1016/j.celrep.2019.05.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van der Kraan P., Matta C., Mobasheri A. Age-related alterations in signaling pathways in articular chondrocytes: implications for the pathogenesis and progression of osteoarthritis - a mini-review. Gerontology. 2017;63(1):29–35. doi: 10.1159/000448711. [DOI] [PubMed] [Google Scholar]
- 87.Mobasheri A., Matta C., Zákány R., Musumeci G. Chondrosenescence: definition, hallmarks and potential role in the pathogenesis of osteoarthritis. Maturitas. 2015;80(3):237–244. doi: 10.1016/j.maturitas.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 88.Early J.O., Curtis A.M. Immunometabolism: is it under the eye of the clock? Semin. Immunol. 2016;28(5):478–490. doi: 10.1016/j.smim.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 89.Calders P., Van Ginckel A. Presence of comorbidities and prognosis of clinical symptoms in knee and/or hip osteoarthritis: a systematic review and meta-analysis. Semin. Arthritis Rheum. 2018;47(6):805–813. doi: 10.1016/j.semarthrit.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 90.Mobasheri A., Rayman M.P., Gualillo O., Sellam J., van der Kraan P., Fearon U. The role of metabolism in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2017;13(5):302–311. doi: 10.1038/nrrheum.2017.50. [DOI] [PubMed] [Google Scholar]
- 91.Zheng L., Zhang Z., Sheng P., Mobasheri A. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res. Rev. 2021;66 doi: 10.1016/j.arr.2020.101249. [DOI] [PubMed] [Google Scholar]
- 92.Choi W.-S., Lee G., Song W.-H., Koh J.-T., Yang J., Kwak J.-S., Kim H.-E., Kim S.K., Son Y.-O., Nam H., Jin I., Park Z.-Y., Kim J., Park I.Y., Hong J.-I., Kim H.A., Chun C.-H., Ryu J.-H., Chun J.-S. The CH25H-CYP7B1-RORα axis of cholesterol metabolism regulates osteoarthritis. Nature. 2019;566(7743):254–258. doi: 10.1038/s41586-019-0920-1. [DOI] [PubMed] [Google Scholar]
- 93.Bora F.W., Miller G. Joint physiology, cartilage metabolism, and the etiology of osteoarthritis. Hand Clin. 1987;3(3):325–336. [PubMed] [Google Scholar]
- 94.Mobasheri A., Bondy C.A., Moley K., Mendes A.F., Rosa S.C., Richardson S.M., Hoyland J.A., Barrett-Jolley R., Shakibaei M. Facilitative glucose transporters in articular chondrocytes. Expression, distribution and functional regulation of GLUT isoforms by hypoxia, hypoxia mimetics, growth factors and pro-inflammatory cytokines. Adv. Anat. Embryol. Cell Biol. 2008;200:1. p following vi, 1-1 pp. following vi,84. [PubMed] [Google Scholar]
- 95.Mobasheri A. Glucose: an energy currency and structural precursor in articular cartilage and bone with emerging roles as an extracellular signaling molecule and metabolic regulator. Front Endocrinol (Lausanne) 2012;3:153. doi: 10.3389/fendo.2012.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maneiro E., Martín M.A., de Andres M.C., López-Armada M.J., Fernández-Sueiro J.L., del Hoyo P., Galdo F., Arenas J., Blanco F.J. Mitochondrial respiratory activity is altered in osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003;48(3):700–708. doi: 10.1002/art.10837. [DOI] [PubMed] [Google Scholar]
- 97.Richardson S.M., Hoyland J.A., Mobasheri R., Csaki C., Shakibaei M., Mobasheri A. Mesenchymal stem cells in regenerative medicine: opportunities and challenges for articular cartilage and intervertebral disc tissue engineering. J. Cell. Physiol. 2010;222(1):23–32. doi: 10.1002/jcp.21915. [DOI] [PubMed] [Google Scholar]
- 98.Ni Z., Zhou S., Li S., Kuang L., Chen H., Luo X., Ouyang J., He M., Du X., Chen L. Exosomes: roles and therapeutic potential in osteoarthritis. Bone Res. 2020;8(1):25. doi: 10.1038/s41413-020-0100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Simeoli R., Montague K., Jones H.R., Castaldi L., Chambers D., Kelleher J.H., Vacca V., Pitcher T., Grist J., Al-Ahdal H., Wong L.-F., Perretti M., Lai J., Mouritzen P., Heppenstall P., Malcangio M. Exosomal cargo including microRNA regulates sensory neuron to macrophage communication after nerve trauma. Nat. Commun. 2017;8(1):1778. doi: 10.1038/s41467-017-01841-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sellam J., Berenbaum F. Is osteoarthritis a metabolic disease? Joint Bone Spine. 2013;80(6):568–573. doi: 10.1016/j.jbspin.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 101.Zhuo Q., Yang W., Chen J., Wang Y. Metabolic syndrome meets osteoarthritis. Nat. Rev. Rheumatol. 2012;8(12):729–737. doi: 10.1038/nrrheum.2012.135. [DOI] [PubMed] [Google Scholar]
- 102.Courties A., Sellam J., Berenbaum F. Metabolic syndrome-associated osteoarthritis. Curr. Opin. Rheumatol. 2017;29(2):214–222. doi: 10.1097/BOR.0000000000000373. [DOI] [PubMed] [Google Scholar]
- 103.Felson D.T., Anderson J.J., Naimark A., Walker A.M., Meenan R.F. Obesity and knee osteoarthritis. The framingham study. Ann. Intern. Med. 1988;109(1):18–24. doi: 10.7326/0003-4819-109-1-18. [DOI] [PubMed] [Google Scholar]
- 104.Grotle M., Hagen K.B., Natvig B., Dahl F.A., Kvien T.K. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Muscoskel. Disord. 2008;9:132. doi: 10.1186/1471-2474-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang Y., Zhang C., Wang J., Liu H., Wang M. Bone-adipose tissue crosstalk: role of adipose tissue derived extracellular vesicles in bone diseases. J. Cell. Physiol. 2021;236(11):7874–7886. doi: 10.1002/jcp.30414. [DOI] [PubMed] [Google Scholar]
- 106.Kwan H.Y., Chen M., Xu K., Chen B. The impact of obesity on adipocyte-derived extracellular vesicles. Cell. Mol. Life Sci. 2021;78(23):7275–7288. doi: 10.1007/s00018-021-03973-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Veronese N., Cooper C., Reginster J.-Y., Hochberg M., Branco J., Bruyère O., Chapurlat R., Al-Daghri N., Dennison E., Herrero-Beaumont G., Kaux J.-F., Maheu E., Rizzoli R., Roth R., Rovati L.C., Uebelhart D., Vlaskovska M., Scheen A. Type 2 diabetes mellitus and osteoarthritis. Semin. Arthritis Rheum. 2019;49(1):9–19. doi: 10.1016/j.semarthrit.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Laiguillon M.C., Courties A., Houard X., Auclair M., Sautet A., Capeau J., Fève B., Berenbaum F., Sellam J. Characterization of diabetic osteoarthritic cartilage and role of high glucose environment on chondrocyte activation: toward pathophysiological delineation of diabetes mellitus-related osteoarthritis. Osteoarthritis Cartilage. 2015;23(9):1513–1522. doi: 10.1016/j.joca.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 109.Rosa S.C., Rufino A.T., Judas F.M., Tenreiro C.M., Lopes M.C., Mendes A.F. Role of glucose as a modulator of anabolic and catabolic gene expression in normal and osteoarthritic human chondrocytes. J. Cell. Biochem. 2011;112(10):2813–2824. doi: 10.1002/jcb.23196. [DOI] [PubMed] [Google Scholar]
- 110.Franke S., Sommer M., Rüster C., Bondeva T., Marticke J., Hofmann G., Hein G., Wolf G. Advanced glycation end products induce cell cycle arrest and proinflammatory changes in osteoarthritic fibroblast-like synovial cells. Arthritis Res. Ther. 2009;11(5):R136. doi: 10.1186/ar2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Altman R., Asch E., Bloch D., Bole G., Borenstein D., Brandt K., Christy W., Cooke T.D., Greenwald R., Hochberg M. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 112.Zhang W., Doherty M., Peat G., Bierma-Zeinstra M.A., Arden N.K., Bresnihan B., Herrero-Beaumont G., Kirschner S., Leeb B.F., Lohmander L.S., Mazières B., Pavelka K., Punzi L., So A.K., Tuncer T., Watt I., Bijlsma J.W. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann. Rheum. Dis. 2010;69(3):483–489. doi: 10.1136/ard.2009.113100. [DOI] [PubMed] [Google Scholar]
- 113.van Spil W.E., Szilagyi I.A. Osteoarthritis year in review 2019: biomarkers (biochemical markers) Osteoarthritis Cartilage. 2020;28(3):296–315. doi: 10.1016/j.joca.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 114.Malda J., Boere J., van de Lest C.H.A., van Weeren P.R., Wauben M.H.M. Extracellular vesicles — new tool for joint repair and regeneration. Nat. Rev. Rheumatol. 2016;12(4):243–249. doi: 10.1038/nrrheum.2015.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jafari N., Llevenes P., Denis G.V. Exosomes as novel biomarkers in metabolic disease and obesity-related cancers. Nat. Rev. Endocrinol. 2022;18(6):327–328. doi: 10.1038/s41574-022-00666-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abhange K., Makler A., Wen Y., Ramnauth N., Mao W., Asghar W., Wan Y. Small extracellular vesicles in cancer. Bioact. Mater. 2021;6(11):3705–3743. doi: 10.1016/j.bioactmat.2021.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pisitkun T., Shen R.-F., Knepper M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. U. S. A. 2004;101(36):13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 119.Sheridan C. Exosome cancer diagnostic reaches market. Nat. Biotechnol. 2016;34(4):359–360. doi: 10.1038/nbt0416-359. [DOI] [PubMed] [Google Scholar]
- 120.Kolhe R., Hunter M., Liu S., Jadeja R.N., Pundkar C., Mondal A.K., Mendhe B., Drewry M., Rojiani M.V., Liu Y., Isales C.M., Guldberg R.E., Hamrick M.W., Fulzele S. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci. Rep. 2017;7(1):2029. doi: 10.1038/s41598-017-01905-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Watt F.E. Osteoarthritis biomarkers: year in review. Osteoarthritis Cartilage. 2018;26(3):312–318. doi: 10.1016/j.joca.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 122.Tsuno H., Arito M., Suematsu N., Sato T., Hashimoto A., Matsui T., Omoteyama K., Sato M., Okamoto K., Tohma S., Kurokawa M.S., Kato T. A proteomic analysis of serum-derived exosomes in rheumatoid arthritis. BMC Rheumatol. 2018;2:35. doi: 10.1186/s41927-018-0041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhao Y., Xu J. Synovial fluid-derived exosomal lncRNA PCGEM1 as biomarker for the different stages of osteoarthritis. Int. Orthop. 2018;42(12):2865–2872. doi: 10.1007/s00264-018-4093-6. [DOI] [PubMed] [Google Scholar]
- 124.Zhang X., Huebner J.L., Kraus V.B. Extracellular vesicles as biological indicators and potential sources of autologous therapeutics in osteoarthritis. Int. J. Mol. Sci. 2021;22(15) doi: 10.3390/ijms22158351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kang Y., Song J., Kim D., Ahn C., Park S., Chun C.-H., Jin E.-J. PCGEM1 stimulates proliferation of osteoarthritic synoviocytes by acting as a sponge for miR-770. J. Orthop. Res. 2016;34(3):412–418. doi: 10.1002/jor.23046. [DOI] [PubMed] [Google Scholar]
- 126.Rosenthal A.K., Gohr C.M., Ninomiya J., Wakim B.T. Proteomic analysis of articular cartilage vesicles from normal and osteoarthritic cartilage. Arthritis Rheum. 2011;63(2):401–411. doi: 10.1002/art.30120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang H.-j., Zhang P.-j., Chen W.-j., Feng D., Jia Y.-h., Xie L.-x. Four serum microRNAs identified as diagnostic biomarkers of sepsis. J Trauma Acute Care Surg. 2012;73(4):850–854. doi: 10.1097/TA.0b013e31825a7560. [DOI] [PubMed] [Google Scholar]
- 128.Henrotin Y. Osteoarthritis in year 2021: biochemical markers. Osteoarthritis Cartilage. 2022;30(2):237–248. doi: 10.1016/j.joca.2021.11.001. [DOI] [PubMed] [Google Scholar]
- 129.Skriner K., Adolph K., Jungblut P.R., Burmester G.R. Association of citrullinated proteins with synovial exosomes. Arthritis Rheum. 2006;54(12):3809–3814. doi: 10.1002/art.22276. [DOI] [PubMed] [Google Scholar]
- 130.Chen X.-M., Zhao Y., Wu X.-D., Wang M.-J., Yu H., Lu J.-J., Hu Y.-J., Huang Q.-C., Huang R.-Y., Lu C.-J. Novel findings from determination of common expressed plasma exosomal microRNAs in patients with psoriatic arthritis, psoriasis vulgaris, rheumatoid arthritis, and gouty arthritis. Discov. Med. 2019;28(151):47–68. [PubMed] [Google Scholar]
- 131.Zhang H.-G., Liu C., Su K., Su K., Yu S., Zhang L., Zhang S., Wang J., Cao X., Grizzle W., Kimberly R.P. A membrane form of TNF-alpha presented by exosomes delays T cell activation-induced cell death. J. Immunol. 2006;176(12):7385–7393. doi: 10.4049/jimmunol.176.12.7385. [DOI] [PubMed] [Google Scholar]
- 132.Boilard E., Nigrovic P.A., Larabee K., Watts G.F.M., Coblyn J.S., Weinblatt M.E., Massarotti E.M., Remold-O'Donnell E., Farndale R.W., Ware J., Lee D.M. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327(5965):580–583. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McKiernan J., Donovan M.J., Margolis E., Partin A., Carter B., Brown G., Torkler P., Noerholm M., Skog J., Shore N., Andriole G., Thompson I., Carroll P. A prospective adaptive utility trial to validate performance of a novel urine exosome gene expression assay to predict high-grade prostate cancer in patients with prostate-specific antigen 2-10ng/ml at initial biopsy. Eur. Urol. 2018;74(6):731–738. doi: 10.1016/j.eururo.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 134.Dobra G., Bukva M., Szabo Z., Bruszel B., Harmati M., Gyukity-Sebestyen E., Jenei A., Szucs M., Horvath P., Biro T., Klekner A., Buzas K. Small extracellular vesicles isolated from serum may serve as signal-enhancers for the monitoring of CNS tumors. Int. J. Mol. Sci. 2020;21(15) doi: 10.3390/ijms21155359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Oggero S., Godec T., van Gorp R., Pinto A.L., Schurgers L.J., Reutelingsperger C., Sever P., Norling L.V., Perretti M., Gupta A. Role of plasma extracellular vesicles in prediction of cardiovascular risk and alterations in response to statin therapy in hypertensive patients. J. Hypertens. 2022;40(8):1522–1529. doi: 10.1097/HJH.0000000000003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Meningher T., Lerman G., Regev-Rudzki N., Gold D., Ben-Dov I.Z., Sidi Y., Avni D., Schwartz E. Schistosomal MicroRNAs isolated from extracellular vesicles in sera of infected patients: a new tool for diagnosis and follow-up of human schistosomiasis. J. Infect. Dis. 2017;215(3):378–386. doi: 10.1093/infdis/jiw539. [DOI] [PubMed] [Google Scholar]
- 137.Krug A.K., Enderle D., Karlovich C., Priewasser T., Bentink S., Spiel A., Brinkmann K., Emenegger J., Grimm D.G., Castellanos-Rizaldos E., Goldman J.W., Sequist L.V., Soria J.C., Camidge D.R., Gadgeel S.M., Wakelee H.A., Raponi M., Noerholm M., Skog J. Improved EGFR mutation detection using combined exosomal RNA and circulating tumor DNA in NSCLC patient plasma. Ann. Oncol. : Official Journal of the European Society For Medical Oncology. 2018;29(3):700–706. doi: 10.1093/annonc/mdx765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Athauda D., Gulyani S., Karnati H.K., Li Y., Tweedie D., Mustapic M., Chawla S., Chowdhury K., Skene S.S., Greig N.H., Kapogiannis D., Foltynie T. Utility of neuronal-derived exosomes to examine molecular mechanisms that affect motor function in patients with Parkinson disease: a secondary analysis of the exenatide-PD trial. JAMA Neurol. 2019;76(4):420–429. doi: 10.1001/jamaneurol.2018.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Braun F., Rinschen M., Buchner D., Bohl K., Plagmann I., Bachurski D., Richard Späth M., Antczak P., Göbel H., Klein C., Lackmann J.-W., Kretz O., Puelles V.G., Wahba R., Hallek M., Schermer B., Benzing T., Huber T.B., Beyer A., Stippel D., Kurschat C.E., Müller R.-U. The proteomic landscape of small urinary extracellular vesicles during kidney transplantation. J. Extracell. Vesicles. 2020;10(1) doi: 10.1002/jev2.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Giannasi C., Niada S., Magagnotti C., Ragni E., Andolfo A., Brini A.T. Comparison of two ASC-derived therapeutics in an in vitro OA model: secretome versus extracellular vesicles. Stem Cell Res. Ther. 2020;11(1):521. doi: 10.1186/s13287-020-02035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kordelas L., Rebmann V., Ludwig A.K., Radtke S., Ruesing J., Doeppner T.R., Epple M., Horn P.A., Beelen D.W., Giebel B. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28(4):970–973. doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- 142.Peng Q., Mu H. The potential of protein-nanomaterial interaction for advanced drug delivery. J. Contr. Release. 2016;225:121–132. doi: 10.1016/j.jconrel.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 143.Mortati L., de Girolamo L., Perucca Orfei C., Viganò M., Brayda-Bruno M., Ragni E., Colombini A. Vitro study of extracellular vesicles migration in cartilage-derived osteoarthritis samples using real-time quantitative multimodal nonlinear optics imaging. Pharmaceutics. 2020;12(8) doi: 10.3390/pharmaceutics12080734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.He L., He T., Xing J., Zhou Q., Fan L., Liu C., Chen Y., Wu D., Tian Z., Liu B., Rong L. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res. Ther. 2020;11(1):276. doi: 10.1186/s13287-020-01781-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li S., Stöckl S., Lukas C., Götz J., Herrmann M., Federlin M., Grässel S. hBMSC-derived extracellular vesicles attenuate IL-1β-induced catabolic effects on OA-chondrocytes by regulating pro-inflammatory signaling pathways. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.603598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jin Y., Xu M., Zhu H., Dong C., Ji J., Liu Y., Deng A., Gu Z. Therapeutic effects of bone marrow mesenchymal stem cells-derived exosomes on osteoarthritis. J. Cell Mol. Med. 2021;25(19):9281–9294. doi: 10.1111/jcmm.16860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang X., Li Z., Cui Y., Cui X., Chen C., Wang Z. Exosomes isolated from bone marrow mesenchymal stem cells exert a protective effect on osteoarthritis via lncRNA LYRM4-AS1-GRPR-miR-6515-5p. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.644380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen X., Shi Y., Xue P., Ma X., Li J., Zhang J. Mesenchymal stem cell-derived exosomal microRNA-136-5p inhibits chondrocyte degeneration in traumatic osteoarthritis by targeting ELF3. Arthritis Res. Ther. 2020;22(1):256. doi: 10.1186/s13075-020-02325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang Y., Zhao M., Li W., Yang Y., Zhang Z., Ma R., Wu M. BMSC-derived small extracellular vesicles induce cartilage reconstruction of temporomandibular joint osteoarthritis autotaxin-YAP signaling Axis. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.656153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang J., Rong Y., Luo C., Cui W. Bone marrow mesenchymal stem cell-derived exosomes prevent osteoarthritis by regulating synovial macrophage polarization. Aging (Albany NY) 2020;12(24):25138–25152. doi: 10.18632/aging.104110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhou X., Liang H., Hu X., An J., Ding S., Yu S., Liu C., Li F., Xu Y. BMSC-derived exosomes from congenital polydactyly tissue alleviate osteoarthritis by promoting chondrocyte proliferation. Cell Death Dis. 2020;6(1):142. doi: 10.1038/s41420-020-00374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Woo C.H., Kim H.K., Jung G.Y., Jung Y.J., Lee K.S., Yun Y.E., Han J., Lee J., Kim W.S., Choi J.S., Yang S., Park J.H., Jo D.-G., Cho Y.W. Small extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. J. Extracell. Vesicles. 2020;9(1) doi: 10.1080/20013078.2020.1735249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cavallo C., Merli G., Borzì R.M., Zini N., D'Adamo S., Guescini M., Grigolo B., Di Martino A., Santi S., Filardo G. Small Extracellular Vesicles from adipose derived stromal cells significantly attenuate in vitro the NF-κB dependent inflammatory/catabolic environment of osteoarthritis. Sci. Rep. 2021;11(1):1053. doi: 10.1038/s41598-020-80032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wu J., Kuang L., Chen C., Yang J., Zeng W.-N., Li T., Chen H., Huang S., Fu Z., Li J., Liu R., Ni Z., Chen L., Yang L. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials. 2019:206. doi: 10.1016/j.biomaterials.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 155.Qiu M., Liu D., Fu Q. MiR-129-5p shuttled by human synovial mesenchymal stem cell-derived exosomes relieves IL-1β induced osteoarthritis via targeting HMGB1. Life Sci. 2021;269 doi: 10.1016/j.lfs.2020.118987. [DOI] [PubMed] [Google Scholar]
- 156.Lu L., Wang J., Fan A., Wang P., Chen R., Lu L., Yin F. Synovial mesenchymal stem cell-derived extracellular vesicles containing microRN555A-26a-5p ameliorate cartilage damage of osteoarthritis. J. Gene Med. 2021:e3379. doi: 10.1002/jgm.3379. [DOI] [PubMed] [Google Scholar]
- 157.Wang R., Jiang W., Zhang L., Xie S., Zhang S., Yuan S., Jin Y., Zhou G. Intra-articular delivery of extracellular vesicles secreted by chondrogenic progenitor cells from MRL/MpJ superhealer mice enhances articular cartilage repair in a mouse injury model. Stem Cell Res. Ther. 2020;11(1):93. doi: 10.1186/s13287-020-01594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Luo P., Jiang C., Ji P., Wang M., Xu J. Exosomes of stem cells from human exfoliated deciduous teeth as an anti-inflammatory agent in temporomandibular joint chondrocytes via miR-100-5p/mTOR. Stem Cell Res. Ther. 2019;10(1):216. doi: 10.1186/s13287-019-1341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang S., Chu W.C., Lai R.C., Lim S.K., Hui J.H.P., Toh W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24(12):2135–2140. doi: 10.1016/j.joca.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 160.Wang Y., Yu D., Liu Z., Zhou F., Dai J., Wu B., Zhou J., Heng B.C., Zou X.H., Ouyang H., Liu H. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res. Ther. 2017;8(1):189. doi: 10.1186/s13287-017-0632-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang S., Teo K.Y.W., Chuah S.J., Lai R.C., Lim S.K., Toh W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35–47. doi: 10.1016/j.biomaterials.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 162.Li X., Wang Y., Cai Z., Zhou Q., Li L., Fu P. Exosomes from human umbilical cord mesenchymal stem cells inhibit ROS production and cell apoptosis in human articular chondrocytes via the miR-100-5p/NOX4 axis. Cell Biol. Int. 2021;45(10):2096–2106. doi: 10.1002/cbin.11657. [DOI] [PubMed] [Google Scholar]
- 163.Li K., Yan G., Huang H., Zheng M., Ma K., Cui X., Lu D., Zheng L., Zhu B., Cheng J., Zhao J. Anti-inflammatory and immunomodulatory effects of the extracellular vesicles derived from human umbilical cord mesenchymal stem cells on osteoarthritis via M2 macrophages. J. Nanobiotechnol. 2022;20(1):38. doi: 10.1186/s12951-021-01236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhou H., Shen X., Yan C., Xiong W., Ma Z., Tan Z., Wang J., Li Y., Liu J., Duan A., Liu F. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate osteoarthritis of the knee in mice model by interacting with METTL3 to reduce m6A of NLRP3 in macrophage. Stem Cell Res. Ther. 2022;13(1):322. doi: 10.1186/s13287-022-03005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ragni E., Papait A., Perucca Orfei C., Silini A.R., Colombini A., Viganò M., Libonati F., Parolini O., de Girolamo L. Amniotic membrane-mesenchymal stromal cells secreted factors and extracellular vesicle-miRNAs: anti-inflammatory and regenerative features for musculoskeletal tissues. Stem Cells Transl Med. 2021;10(7):1044–1062. doi: 10.1002/sctm.20-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zavatti M., Beretti F., Casciaro F., Bertucci E., Maraldi T. Comparison of the therapeutic effect of amniotic fluid stem cells and their exosomes on monoiodoacetate-induced animal model of osteoarthritis. Biofactors. 2020;46(1):106–117. doi: 10.1002/biof.1576. [DOI] [PubMed] [Google Scholar]
- 167.Bai J., Zhang Y., Zheng X., Huang M., Cheng W., Shan H., Gao X., Zhang M., Sheng L., Dai J., Deng Y., Zhang H., Zhou X. LncRNA MM2P-induced, exosome-mediated transfer of Sox9 from monocyte-derived cells modulates primary chondrocytes. Cell Death Dis. 2020;11(9):763. doi: 10.1038/s41419-020-02945-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhan D., Cross A., Wright H.L., Moots R.J., Edwards S.W., Honsawek S. Internalization of neutrophil-derived microvesicles modulates tnfα-stimulated proinflammatory cytokine production in human fibroblast-like synoviocytes. Int. J. Mol. Sci. 2021;22(14) doi: 10.3390/ijms22147409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zheng L., Wang Y.Y., Qiu P.C., Xia C., Fang Y.F., Mei S., Fang C., Shi Y.L., Wu K.W., Chen Z.J., Fan S.W., He D.W., Lin X.F., Chen P.F. Primary chondrocyte exosomes mediate osteoarthritis progression by regulating mitochondrion and immune reactivity. Nanomedicine-Uk. 2019;14(24):3193–3212. doi: 10.2217/nnm-2018-0498. [DOI] [PubMed] [Google Scholar]
- 170.Tan F., Wang D., Yuan Z. The fibroblast-like synoviocyte derived exosomal long non-coding RNA H19 alleviates osteoarthritis progression through the miR-106b-5p/TIMP2 Axis. Inflammation. 2020;43(4):1498–1509. doi: 10.1007/s10753-020-01227-8. [DOI] [PubMed] [Google Scholar]
- 171.Xu T., Xu M., Bai J., Lin J., Yu B., Liu Y., Guo X., Shen J., Sun H., Hao Y., Geng D. Tenocyte-derived exosomes induce the tenogenic differentiation of mesenchymal stem cells through TGF-β. Cytotechnology. 2019;71(1):57–65. doi: 10.1007/s10616-018-0264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mustonen A.-M., Nieminen P., Joukainen A., Jaroma A., Kääriäinen T., Kröger H., Lázaro-Ibáñez E., Siljander P.R.M., Kärjä V., Härkönen K., Koistinen A., Rilla K. First in vivo detection and characterization of hyaluronan-coated extracellular vesicles in human synovial fluid. J. Orthop. Res. 2016;34(11):1960–1968. doi: 10.1002/jor.23212. [DOI] [PubMed] [Google Scholar]
- 173.Otahal A., Kramer K., Kuten-Pella O., Moser L.B., Neubauer M., Lacza Z., Nehrer S., De Luna A. Effects of extracellular vesicles from blood-derived products on osteoarthritic chondrocytes within an inflammation model. Int. J. Mol. Sci. 2021;22(13) doi: 10.3390/ijms22137224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liu X., Wang L., Ma C., Wang G., Zhang Y., Sun S. Exosomes derived from platelet-rich plasma present a novel potential in alleviating knee osteoarthritis by promoting proliferation and inhibiting apoptosis of chondrocyte via Wnt/β-catenin signaling pathway. J. Orthop. Surg. Res. 2019;14(1):470. doi: 10.1186/s13018-019-1529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pieters B.C.H., Arntz O.J., Aarts J., Feitsma A.L., van Neerven R.J.J., van der Kraan P.M., Oliveira M.C., van de Loo F.A.J. Bovine milk-derived extracellular vesicles inhibit catabolic and inflammatory processes in cartilage from osteoarthritis patients. Mol. Nutr. Food Res. 2022;66(6) doi: 10.1002/mnfr.202100764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Song Y., Zhang J., Xu H., Lin Z., Chang H., Liu W., Kong L. Mesenchymal stem cells in knee osteoarthritis treatment: a systematic review and meta-analysis. J Orthop Translat. 2020;24:121–130. doi: 10.1016/j.jot.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Toh W.S., Lai R.C., Hui J.H.P., Lim S.K. MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin. Cell Dev. Biol. 2017;67:56–64. doi: 10.1016/j.semcdb.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 178.Eleuteri S., Fierabracci A. Insights into the secretome of mesenchymal stem cells and its potential applications. Int. J. Mol. Sci. 2019;20(18) doi: 10.3390/ijms20184597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ragni E., Colombini A., De Luca P., Libonati F., Viganò M., Perucca Orfei C., Zagra L., de Girolamo L. miR-103a-3p and miR-22-5p are reliable reference genes in extracellular vesicles from cartilage, adipose tissue, and bone marrow cells. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.632440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hoang D.M., Pham P.T., Bach T.Q., Ngo A.T.L., Nguyen Q.T., Phan T.T.K., Nguyen G.H., Le P.T.T., Hoang V.T., Forsyth N.R., Heke M., Nguyen L.T. Stem cell-based therapy for human diseases. Signal Transduct. Targeted Ther. 2022;7(1):272. doi: 10.1038/s41392-022-01134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fričová D., Korchak J.A., Zubair A.C. Challenges and translational considerations of mesenchymal stem/stromal cell therapy for Parkinson's disease. NPJ Regenerative Medicine. 2020;5(1):20. doi: 10.1038/s41536-020-00106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yin J.Q., Zhu J., Ankrum J.A. Manufacturing of primed mesenchymal stromal cells for therapy. Nature Biomedical Engineering. 2019;3(2):90–104. doi: 10.1038/s41551-018-0325-8. [DOI] [PubMed] [Google Scholar]
- 183.Qian X., An N., Ren Y., Yang C., Zhang X., Li L. Immunosuppressive effects of mesenchymal stem cells-derived exosomes. Stem Cell Rev Rep. 2021;17(2):411–427. doi: 10.1007/s12015-020-10040-7. [DOI] [PubMed] [Google Scholar]
- 184.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 185.Maxson S., Lopez E.A., Yoo D., Danilkovitch-Miagkova A., Leroux M.A. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med. 2012;1(2):142–149. doi: 10.5966/sctm.2011-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.ter Huurne M., Schelbergen R., Blattes R., Blom A., de Munter W., Grevers L.C., Jeanson J., Noël D., Casteilla L., Jorgensen C., van den Berg W., van Lent P.L.E.M. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. 2012;64(11):3604–3613. doi: 10.1002/art.34626. [DOI] [PubMed] [Google Scholar]
- 187.Gimble J.M., Katz A.J., Bunnell B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007;100(9):1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Baharlou R., Ahmadi-Vasmehjani A., Faraji F., Atashzar M.R., Khoubyari M., Ahi S., Erfanian S., Navabi S.-S. Human adipose tissue-derived mesenchymal stem cells in rheumatoid arthritis: regulatory effects on peripheral blood mononuclear cells activation. Int. Immunopharm. 2017;47:59–69. doi: 10.1016/j.intimp.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 189.Huang S., Song X., Li T., Xiao J., Chen Y., Gong X., Zeng W., Yang L., Chen C. Pellet coculture of osteoarthritic chondrocytes and infrapatellar fat pad-derived mesenchymal stem cells with chitosan/hyaluronic acid nanoparticles promotes chondrogenic differentiation. Stem Cell Res. Ther. 2017;8(1):264. doi: 10.1186/s13287-017-0719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ozeki N., Muneta T., Koga H., Nakagawa Y., Mizuno M., Tsuji K., Mabuchi Y., Akazawa C., Kobayashi E., Matsumoto K., Futamura K., Saito T., Sekiya I. Not single but periodic injections of synovial mesenchymal stem cells maintain viable cells in knees and inhibit osteoarthritis progression in rats. Osteoarthritis Cartilage. 2016;24(6):1061–1070. doi: 10.1016/j.joca.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 191.Archer C.W., Dowthwaite G.P., Francis-West P. Development of synovial joints. Birth Defects Res C Embryo Today. 2003;69(2):144–155. doi: 10.1002/bdrc.10015. [DOI] [PubMed] [Google Scholar]
- 192.Sakaguchi Y., Sekiya I., Yagishita K., Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52(8):2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 193.Zhu Y., Wang Y., Zhao B., Niu X., Hu B., Li Q., Zhang J., Ding J., Chen Y., Wang Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 2017;8(1):64. doi: 10.1186/s13287-017-0510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jiang Y., Tuan R.S. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat. Rev. Rheumatol. 2015;11(4):206–212. doi: 10.1038/nrrheum.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Miura M., Gronthos S., Zhao M., Lu B., Fisher L.W., Robey P.G., Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. U. S. A. 2003;100(10):5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shimojima C., Takeuchi H., Jin S., Parajuli B., Hattori H., Suzumura A., Hibi H., Ueda M., Yamamoto A. Conditioned medium from the stem cells of human exfoliated deciduous teeth ameliorates experimental autoimmune encephalomyelitis. J. Immunol. 2016;196(10):4164–4171. doi: 10.4049/jimmunol.1501457. [DOI] [PubMed] [Google Scholar]
- 197.Park Y.-B., Ha C.-W., Lee C.-H., Yoon Y.C., Park Y.-G. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 Years of extended follow-up. Stem Cells Transl Med. 2017;6(2):613–621. doi: 10.5966/sctm.2016-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Silini A.R., Magatti M., Cargnoni A., Parolini O. Is immune modulation the mechanism underlying the beneficial effects of amniotic cells and their derivatives in regenerative medicine? Cell Transplant. 2017;26(4):531–539. doi: 10.3727/096368916X693699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Harrell C.R., Gazdic M., Fellabaum C., Jovicic N., Djonov V., Arsenijevic N., Volarevic V. Therapeutic potential of amniotic fluid derived mesenchymal stem cells based on their differentiation capacity and immunomodulatory properties. Curr. Stem Cell Res. Ther. 2019;14(4):327–336. doi: 10.2174/1574888X14666190222201749. [DOI] [PubMed] [Google Scholar]
- 200.Maraldi T., Riccio M., Pisciotta A., Zavatti M., Carnevale G., Beretti F., La Sala G.B., Motta A., De Pol A. Human amniotic fluid-derived and dental pulp-derived stem cells seeded into collagen scaffold repair critical-size bone defects promoting vascularization. Stem Cell Res. Ther. 2013;4(3):53. doi: 10.1186/scrt203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Utomo L., Bastiaansen-Jenniskens Y.M., Verhaar J.A.N., van Osch G.J.V.M. Cartilage inflammation and degeneration is enhanced by pro-inflammatory (M1) macrophages in vitro, but not inhibited directly by anti-inflammatory (M2) macrophages. Osteoarthritis Cartilage. 2016;24(12):2162–2170. doi: 10.1016/j.joca.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 202.Zhang H., Lin C., Zeng C., Wang Z., Wang H., Lu J., Liu X., Shao Y., Zhao C., Pan J., Xu S., Zhang Y., Xie D., Cai D., Bai X. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Ann. Rheum. Dis. 2018;77(10):1524–1534. doi: 10.1136/annrheumdis-2018-213450. [DOI] [PubMed] [Google Scholar]
- 203.Headland S.E., Jones H.R., Norling L.V., Kim A., Souza P.R., Corsiero E., Gil C.D., Nerviani A., Dell'Accio F., Pitzalis C., Oliani S.M., Jan L.Y., Perretti M. Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Sci. Transl. Med. 2015;7(315):315ra190. doi: 10.1126/scitranslmed.aac5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhou Z.-B., Huang G.-X., Fu Q., Han B., Lu J.-J., Chen A.-M., Zhu L., circRNA 33186 contributes to the pathogenesis of osteoarthritis by sponging miR-127-5p. Mol. Ther. 2019;27(3):531–541. doi: 10.1016/j.ymthe.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ding X., Zhang Y., Huang Y., Liu S., Lu H., Sun T. Cadherin-11 involves in synovitis and increases the migratory and invasive capacity of fibroblast-like synoviocytes of osteoarthritis. Int. Immunopharm. 2015;26(1):153–161. doi: 10.1016/j.intimp.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 206.Nair A., Kanda V., Bush-Joseph C., Verma N., Chubinskaya S., Mikecz K., Glant T.T., Malfait A.-M., Crow M.K., Spear G.T., Finnegan A., Scanzello C.R. Synovial fluid from patients with early osteoarthritis modulates fibroblast-like synoviocyte responses to toll-like receptor 4 and toll-like receptor 2 ligands via soluble CD14. Arthritis Rheum. 2012;64(7):2268–2277. doi: 10.1002/art.34495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ibrahim M., Kartus J.-T., Steigen S.E., Olsen R., Meknas K. More tendon degeneration in patients with shoulder osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2019;27(1):267–275. doi: 10.1007/s00167-018-5186-x. [DOI] [PubMed] [Google Scholar]
- 208.Schulze-Tanzil G. Intraarticular ligament degeneration is interrelated with cartilage and bone destruction in osteoarthritis. Cells. 2019;8(9) doi: 10.3390/cells8090990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tassara M., De Ponti A., Barzizza L., Zambelli M., Parisi C., Milani R., Santoleri L. Autologous conditioned serum (ACS) for intra-articular treatment in Osteoarthritis: retrospective report of 28 cases. Transfus. Apher. Sci. 2018;57(4):573–577. doi: 10.1016/j.transci.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 210.Otahal A., Kramer K., Kuten-Pella O., Weiss R., Stotter C., Lacza Z., Weber V., Nehrer S., De Luna A. Characterization and chondroprotective effects of extracellular vesicles from plasma- and serum-based autologous blood-derived products for osteoarthritis therapy. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.584050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Benmoussa A., Provost P. Milk MicroRNAs in health and disease. Compr. Rev. Food Sci. Food Saf. 2019;18(3):703–722. doi: 10.1111/1541-4337.12424. [DOI] [PubMed] [Google Scholar]
- 212.Lu B., Driban J.B., Duryea J., McAlindon T., Lapane K.L., Eaton C.B. Milk consumption and progression of medial tibiofemoral knee osteoarthritis: data from the Osteoarthritis Initiative. Arthritis Care Res. 2014;66(6):802–809. doi: 10.1002/acr.22297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Samuel M., Fonseka P., Sanwlani R., Gangoda L., Chee S.H., Keerthikumar S., Spurling A., Chitti S.V., Zanker D., Ang C.-S., Atukorala I., Kang T., Shahi S., Marzan A.L., Nedeva C., Vennin C., Lucas M.C., Cheng L., Herrmann D., Pathan M., Chisanga D., Warren S.C., Zhao K., Abraham N., Anand S., Boukouris S., Adda C.G., Jiang L., Shekhar T.M., Baschuk N., Hawkins C.J., Johnston A.J., Orian J.M., Hoogenraad N.J., Poon I.K., Hill A.F., Jois M., Timpson P., Parker B.S., Mathivanan S. Oral administration of bovine milk-derived extracellular vesicles induces senescence in the primary tumor but accelerates cancer metastasis. Nat. Commun. 2021;12(1):3950. doi: 10.1038/s41467-021-24273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ohta N., Sato M., Ushida K., Kokubo M., Baba T., Taniguchi K., Urai M., Kihira K., Mochida J. Jellyfish mucin may have potential disease-modifying effects on osteoarthritis. BMC Biotechnol. 2009;9:98. doi: 10.1186/1472-6750-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wang D., Berg D., Ba H., Sun H., Wang Z., Li C. Deer antler stem cells are a novel type of cells that sustain full regeneration of a mammalian organ-deer antler. Cell Death Dis. 2019;10(6):443. doi: 10.1038/s41419-019-1686-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.El Andaloussi S., Mäger I., Breakefield X.O., Wood M.J.A. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013;12(5):347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 217.Armstrong J.P.K., Holme M.N., Stevens M.M. Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano. 2017;11(1):69–83. doi: 10.1021/acsnano.6b07607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Liao W., Du Y., Zhang C., Pan F., Yao Y., Zhang T., Peng Q. Exosomes: the next generation of endogenous nanomaterials for advanced drug delivery and therapy. Acta Biomater. 2019;86:1–14. doi: 10.1016/j.actbio.2018.12.045. [DOI] [PubMed] [Google Scholar]
- 219.Esmaeili A., Hosseini S., Baghaban Eslaminejad M. Engineered-extracellular vesicles as an optimistic tool for microRNA delivery for osteoarthritis treatment. Cell. Mol. Life Sci. 2021;78(1):79–91. doi: 10.1007/s00018-020-03585-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Li S., Stöckl S., Lukas C., Herrmann M., Brochhausen C., König M.A., Johnstone B., Grässel S. Curcumin-primed human BMSC-derived extracellular vesicles reverse IL-1β-induced catabolic responses of OA chondrocytes by upregulating miR-126-3p. Stem Cell Res. Ther. 2021;12(1):252. doi: 10.1186/s13287-021-02317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Liu Y., Zeng Y., Si H.-B., Tang L., Xie H.-Q., Shen B. Exosomes derived from human urine-derived stem cells overexpressing miR-140-5p alleviate knee osteoarthritis through downregulation of VEGFA in a rat model. Am. J. Sports Med. 2022;50(4):1088–1105. doi: 10.1177/03635465221073991. [DOI] [PubMed] [Google Scholar]
- 222.Rong Y., Zhang J., Jiang D., Ji C., Liu W., Wang J., Ge X., Tang P., Yu S., Cui W., Cai W. Hypoxic pretreatment of small extracellular vesicles mediates cartilage repair in osteoarthritis by delivering miR-216a-5p. Acta Biomater. 2021;122:325–342. doi: 10.1016/j.actbio.2020.12.034. [DOI] [PubMed] [Google Scholar]
- 223.Liao Q., Li B.J., Li Y., Xiao Y., Zeng H., Liu J.M., Yuan L.X., Liu G. Low-intensity pulsed ultrasound promotes osteoarthritic cartilage regeneration by BMSC-derived exosomes via modulating the NF-κB signaling pathway. Int. Immunopharm. 2021;97 doi: 10.1016/j.intimp.2021.107824. [DOI] [PubMed] [Google Scholar]
- 224.Yan L., Wu X. Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow-fiber bioreactor show improved osteochondral regeneration activity. Cell Biol. Toxicol. 2020;36(2):165–178. doi: 10.1007/s10565-019-09504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Li S., Liu J., Liu S., Jiao W., Wang X. Chitosan oligosaccharides packaged into rat adipose mesenchymal stem cells-derived extracellular vesicles facilitating cartilage injury repair and alleviating osteoarthritis. J. Nanobiotechnol. 2021;19(1):343. doi: 10.1186/s12951-021-01086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ni Z., Zhou S., Li S., Kuang L., Chen H., Luo X., Ouyang J., He M., Du X., Chen L. Exosomes: roles and therapeutic potential in osteoarthritis. Bone Res. 2020;8:25. doi: 10.1038/s41413-020-0100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Smyth T., Kullberg M., Malik N., Smith-Jones P., Graner M.W., Anchordoquy T.J. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J. Contr. Release. 2015;199:145–155. doi: 10.1016/j.jconrel.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Bhawana R.K. Basniwal, Buttar H.S., Jain V.K., Jain N. Curcumin nanoparticles: preparation, characterization, and antimicrobial study. J. Agric. Food Chem. 2011;59(5):2056–2061. doi: 10.1021/jf104402t. [DOI] [PubMed] [Google Scholar]
- 229.Xu C., Zhai Z., Ying H., Lu L., Zhang J., Zeng Y. Curcumin primed ADMSCs derived small extracellular vesicle exert enhanced protective effects on osteoarthritis by inhibiting oxidative stress and chondrocyte apoptosis. J. Nanobiotechnol. 2022;20(1):123. doi: 10.1186/s12951-022-01339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wang R., Xu B. TGF-β1-modified MSC-derived exosomal miR-135b attenuates cartilage injury via promoting M2 synovial macrophage polarization by targeting MAPK6. Cell Tissue Res. 2021;384(1):113–127. doi: 10.1007/s00441-020-03319-1. [DOI] [PubMed] [Google Scholar]
- 231.Colombini A., Ragni E., Mortati L., Libonati F., Perucca Orfei C., Viganò M., Brayda-Bruno M., de Girolamo L. Adipose-derived mesenchymal stromal cells treated with interleukin 1 beta produced chondro-protective vesicles able to fast penetrate in cartilage. Cells. 2021;10(5) doi: 10.3390/cells10051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kim M., Shin D.I., Choi B.H., Min B.-H. Exosomes from IL-1β-primed mesenchymal stem cells inhibited IL-1β- and TNF-α-mediated inflammatory responses in osteoarthritic SW982 cells. Tissue Eng Regen Med. 2021;18(4):525–536. doi: 10.1007/s13770-020-00324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Duan A., Shen K., Li B., Li C., Zhou H., Kong R., Shao Y., Qin J., Yuan T., Ji J., Guo W., Wang X., Xue T., Li L., Huang X., Sun Y., Cai Z., Liu W., Liu F. Extracellular vesicles derived from LPS-preconditioned human synovial mesenchymal stem cells inhibit extracellular matrix degradation and prevent osteoarthritis of the knee in a mouse model. Stem Cell Res. Ther. 2021;12(1):427. doi: 10.1186/s13287-021-02507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Jin Z., Ren J., Qi S. Human bone mesenchymal stem cells-derived exosomes overexpressing microRNA-26a-5p alleviate osteoarthritis via down-regulation of PTGS2. Int. Immunopharm. 2020;78 doi: 10.1016/j.intimp.2019.105946. [DOI] [PubMed] [Google Scholar]
- 235.Zhou Y., Ming J., Li Y., Li B., Deng M., Ma Y., Chen Z., Zhang Y., Li J., Liu S. Exosomes derived from miR-126-3p-overexpressing synovial fibroblasts suppress chondrocyte inflammation and cartilage degradation in a rat model of osteoarthritis. Cell Death Dis. 2021;7(1):37. doi: 10.1038/s41420-021-00418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Dong J., Li L., Fang X., Zang M. Exosome-encapsulated microRNA-127-3p released from bone marrow-derived mesenchymal stem cells alleviates osteoarthritis through regulating CDH11-mediated wnt/β-catenin pathway. J. Pain Res. 2021;14:297–310. doi: 10.2147/JPR.S291472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wang Z., Yan K., Ge G., Zhang D., Bai J., Guo X., Zhou J., Xu T., Xu M., Long X., Hao Y., Geng D. Exosomes derived from miR-155-5p-overexpressing synovial mesenchymal stem cells prevent osteoarthritis via enhancing proliferation and migration, attenuating apoptosis, and modulating extracellular matrix secretion in chondrocytes. Cell Biol. Toxicol. 2021;37(1):85–96. doi: 10.1007/s10565-020-09559-9. [DOI] [PubMed] [Google Scholar]
- 238.Tao Y., Zhou J., Wang Z., Tao H., Bai J., Ge G., Li W., Zhang W., Hao Y., Yang X., Geng D. Human bone mesenchymal stem cells-derived exosomal miRNA-361-5p alleviates osteoarthritis by downregulating DDX20 and inactivating the NF-κB signaling pathway. Bioorg. Chem. 2021;113 doi: 10.1016/j.bioorg.2021.104978. [DOI] [PubMed] [Google Scholar]
- 239.Lin T., Wu N., Wang L., Zhang R., Pan R., Chen Y.-F. Inhibition of chondrocyte apoptosis in a rat model of osteoarthritis by exosomes derived from miR-140-5p-overexpressing human dental pulp stem cells. Int. J. Mol. Med. 2021;47(3) doi: 10.3892/ijmm.2020.4840. [DOI] [PubMed] [Google Scholar]
- 240.Wang K., Li F., Yuan Y., Shan L., Cui Y., Qu J., Lian F. Synovial mesenchymal stem cell-derived EV-packaged miR-31 downregulates histone demethylase KDM2A to prevent knee osteoarthritis. Mol. Ther. Nucleic Acids. 2020;22:1078–1091. doi: 10.1016/j.omtn.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Huang Y., Zhang X., Zhan J., Yan Z., Chen D., Xue X., Pan X. Bone marrow mesenchymal stem cell-derived exosomal miR-206 promotes osteoblast proliferation and differentiation in osteoarthritis by reducing Elf3. J. Cell Mol. Med. 2021;25(16):7734–7745. doi: 10.1111/jcmm.16654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Mao G., Xu Y., Long D., Sun H., Li H., Xin R., Zhang Z., Li Z., Yang Z., Kang Y. Exosome-transported circRNA_0001236 enhances chondrogenesis and suppress cartilage degradation via the miR-3677-3p/Sox9 axis. Stem Cell Res. Ther. 2021;12(1):389. doi: 10.1186/s13287-021-02431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Li S., Liu J., Liu S., Jiao W., Wang X. Mesenchymal stem cell-derived extracellular vesicles prevent the development of osteoarthritis via the circHIPK3/miR-124-3p/MYH9 axis. J. Nanobiotechnol. 2021;19(1):194. doi: 10.1186/s12951-021-00940-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Yang Q., Yao Y., Zhao D., Zou H., Lai C., Xiang G., Wang G., Luo L., Shi Y., Li Y., Yang M., Huang X. LncRNA H19 secreted by umbilical cord blood mesenchymal stem cells through microRNA-29a-3p/FOS axis for central sensitization of pain in advanced osteoarthritis. Am J Transl Res. 2021;13(3):1245–1256. [PMC free article] [PubMed] [Google Scholar]
- 245.Hu X., Wu R., Shehadeh L.A., Zhou Q., Jiang C., Huang X., Zhang L., Gao F., Liu X., Yu H., Webster K.A., Wang J.a. Severe hypoxia exerts parallel and cell-specific regulation of gene expression and alternative splicing in human mesenchymal stem cells. BMC Genom. 2014;15:303. doi: 10.1186/1471-2164-15-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zhuang Y., Zhao Z., Cheng M., Li M., Si J., Lin K., Yu H. HIF-1α regulates osteogenesis of periosteum-derived stem cells under hypoxia conditions modulating POSTN expression. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.836285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Mohyeldin A., Garzón-Muvdi T., Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7(2):150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 248.Malladi P., Xu Y., Chiou M., Giaccia A.J., Longaker M.T. Hypoxia inducible factor-1alpha deficiency affects chondrogenesis of adipose-derived adult stromal cells. Tissue Eng. 2007;13(6):1159–1171. doi: 10.1089/ten.2006.0265. [DOI] [PubMed] [Google Scholar]
- 249.Wan S., Bao D., Li J., Lin K., Huang Q., Li Q., Li L. Extracellular vesicles from hypoxic pretreated urine-derived stem cells enhance the proliferation and migration of chondrocytes by delivering miR-26a-5p. Cartilage. 2022;13(2) doi: 10.1177/19476035221077401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zhang B., Tian X., Qu Z., Hao J., Zhang W. Hypoxia-Preconditioned extracellular vesicles from mesenchymal stem cells improve cartilage repair in osteoarthritis. Membranes (Basel) 2022;12(2) doi: 10.3390/membranes12020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Li D., Zhou J., Chowdhury F., Cheng J., Wang N., Wang F. Role of mechanical factors in fate decisions of stem cells. Regen. Med. 2011;6(2):229–240. doi: 10.2217/rme.11.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ouyang X., Xie Y., Wang G. Mechanical stimulation promotes the proliferation and the cartilage phenotype of mesenchymal stem cells and chondrocytes co-cultured in vitro. Biomed. Pharmacother. 2019;117 doi: 10.1016/j.biopha.2019.109146. [DOI] [PubMed] [Google Scholar]
- 253.Colombo V., Cadová M., Gallo L.M. Mechanical behavior of bovine nasal cartilage under static and dynamic loading. J. Biomech. 2013;46(13):2137–2144. doi: 10.1016/j.jbiomech.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 254.Wang X., Lin Q., Zhang T., Wang X., Cheng K., Gao M., Xia P., Li X. Low-intensity pulsed ultrasound promotes chondrogenesis of mesenchymal stem cells via regulation of autophagy. Stem Cell Res. Ther. 2019;10(1):41. doi: 10.1186/s13287-019-1142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Naito K., Watari T., Muta T., Furuhata A., Iwase H., Igarashi M., Kurosawa H., Nagaoka I., Kaneko K. Low-intensity pulsed ultrasound (LIPUS) increases the articular cartilage type II collagen in a rat osteoarthritis model. J. Orthop. Res. 2010;28(3):361–369. doi: 10.1002/jor.20995. [DOI] [PubMed] [Google Scholar]
- 256.Xia P., Wang Q., Song J., Wang X., Wang X., Lin Q., Cheng K., Chen A., Li X. Low-intensity pulsed ultrasound enhances the efficacy of bone marrow-derived MSCs in osteoarthritis cartilage repair by regulating autophagy-mediated exosome release. Cartilage. 2022;13(2) doi: 10.1177/19476035221093060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sanchez-Adams J., Leddy H.A., McNulty A.L., O'Conor C.J., Guilak F. The mechanobiology of articular cartilage: bearing the burden of osteoarthritis. Curr. Rheumatol. Rep. 2014;16(10):451. doi: 10.1007/s11926-014-0451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Haraszti R.A., Miller R., Stoppato M., Sere Y.Y., Coles A., Didiot M.-C., Wollacott R., Sapp E., Dubuke M.L., Li X., Shaffer S.A., DiFiglia M., Wang Y., Aronin N., Khvorova A. Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol. Ther. 2018;26(12):2838–2847. doi: 10.1016/j.ymthe.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Fuhrmann G., Serio A., Mazo M., Nair R., Stevens M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Contr. Release. 2015;205:35–44. doi: 10.1016/j.jconrel.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 260.Wang W., Meng Q., Li Q., Liu J., Zhou M., Jin Z., Zhao K. Chitosan derivatives and their application in biomedicine. Int. J. Mol. Sci. 2020;21(2) doi: 10.3390/ijms21020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kooijmans S.A.A., Stremersch S., Braeckmans K., de Smedt S.C., Hendrix A., Wood M.J.A., Schiffelers R.M., Raemdonck K., Vader P. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J. Contr. Release. 2013;172(1):229–238. doi: 10.1016/j.jconrel.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 262.Kang M.L., Ko J.-Y., Kim J.E., Im G.-I. Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration. Biomaterials. 2014;35(37):9984–9994. doi: 10.1016/j.biomaterials.2014.08.042. [DOI] [PubMed] [Google Scholar]
- 263.Liang Y., Xu X., Li X., Xiong J., Li B., Duan L., Wang D., Xia J. Chondrocyte-targeted MicroRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl. Mater. Interfaces. 2020;12(33):36938–36947. doi: 10.1021/acsami.0c10458. [DOI] [PubMed] [Google Scholar]
- 264.Lin Y., Wu J., Gu W., Huang Y., Tong Z., Huang L., Tan J. Exosome-liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv Sci (Weinh) 2018;5(4) doi: 10.1002/advs.201700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Liu A., Lin D., Zhao H., Chen L., Cai B., Lin K., Shen S.G. Optimized BMSC-derived osteoinductive exosomes immobilized in hierarchical scaffold via lyophilization for bone repair through Bmpr2/Acvr2b competitive receptor-activated Smad pathway. Biomaterials. 2021;272 doi: 10.1016/j.biomaterials.2021.120718. [DOI] [PubMed] [Google Scholar]
- 266.Liu L., Yu F., Li L., Zhou L., Zhou T., Xu Y., Lin K., Fang B., Xia L. Bone marrow stromal cells stimulated by strontium-substituted calcium silicate ceramics: release of exosomal miR-146a regulates osteogenesis and angiogenesis. Acta Biomater. 2021;119:444–457. doi: 10.1016/j.actbio.2020.10.038. [DOI] [PubMed] [Google Scholar]
- 267.Pezzana C., Agnely F., Bochot A., Siepmann J., Menasché P. Extracellular vesicles and biomaterial design: new therapies for cardiac repair. Trends Mol. Med. 2021;27(3):231–247. doi: 10.1016/j.molmed.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 268.Caballero D., Abreu C.M., Lima A.C., Neves N.N., Reis R.L., Kundu S.C. Precision biomaterials in cancer theranostics and modelling. Biomaterials. 2022;280 doi: 10.1016/j.biomaterials.2021.121299. [DOI] [PubMed] [Google Scholar]
- 269.Li J., Mooney D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016;1(12) doi: 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Pradal J., Maudens P., Gabay C., Seemayer C.A., Jordan O., Allémann E. Effect of particle size on the biodistribution of nano- and microparticles following intra-articular injection in mice. Int J Pharm. 2016;498(1–2):119–129. doi: 10.1016/j.ijpharm.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 271.Zhang Y., Wang X., Chen J., Qian D., Gao P., Qin T., Jiang T., Yi J., Xu T., Huang Y., Wang Q., Zhou Z., Bao T., Zhao X., Liu H., Zheng Z., Fan J., Zhao S., Li Q., Yin G. Exosomes derived from platelet-rich plasma administration in site mediate cartilage protection in subtalar osteoarthritis. J. Nanobiotechnol. 2022;20(1):56. doi: 10.1186/s12951-022-01245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Sang X., Zhao X., Yan L., Jin X., Wang X., Wang J., Yin Z., Zhang Y., Meng Z. Thermosensitive hydrogel loaded with primary chondrocyte-derived exosomes promotes cartilage repair by regulating macrophage polarization in osteoarthritis. Tissue Eng Regen Med. 2022;19(3):629–642. doi: 10.1007/s13770-022-00437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Yang Y., Zhu Z., Gao R., Yuan J., Zhang J., Li H., Xie Z., Wang Y. Controlled release of MSC-derived small extracellular vesicles by an injectable Diels-Alder crosslinked hyaluronic acid/PEG hydrogel for osteoarthritis improvement. Acta Biomater. 2021;128:163–174. doi: 10.1016/j.actbio.2021.04.003. [DOI] [PubMed] [Google Scholar]
- 274.Tao S.-C., Huang J.-Y., Gao Y., Li Z.-X., Wei Z.-Y., Dawes H., Guo S.-C. Small extracellular vesicles in combination with sleep-related circRNA3503: a targeted therapeutic agent with injectable thermosensitive hydrogel to prevent osteoarthritis. Bioact. Mater. 2021;6(12):4455–4469. doi: 10.1016/j.bioactmat.2021.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Heirani-Tabasi A., Hosseinzadeh S., Rabbani S., Ahmadi Tafti S.H., Jamshidi K., Soufizomorrod M., Soleimani M. Cartilage tissue engineering by co-transplantation of chondrocyte extracellular vesicles and mesenchymal stem cells, entrapped in chitosan-hyaluronic acid hydrogel. Biomed Mater. 2021;16(5) doi: 10.1088/1748-605X/ac0cbf. [DOI] [PubMed] [Google Scholar]
- 276.Vijayavenkataraman S., Yan W.-C., Lu W.F., Wang C.-H., Fuh J.Y.H. 3D bioprinting of tissues and organs for regenerative medicine. Adv. Drug Deliv. Rev. 2018;132:296–332. doi: 10.1016/j.addr.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 277.Chen P., Zheng L., Wang Y., Tao M., Xie Z., Xia C., Gu C., Chen J., Qiu P., Mei S., Ning L., Shi Y., Fang C., Fan S., Lin X. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics. 2019;9(9):2439–2459. doi: 10.7150/thno.31017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Ragni E., Palombella S., Lopa S., Talò G., Perucca Orfei C., De Luca P., Moretti M., de Girolamo L. Innovative visualization and quantification of extracellular vesicles interaction with and incorporation in target cells in 3D microenvironments. Cells. 2020;9(5) doi: 10.3390/cells9051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.van der Kraan P.M. Factors that influence outcome in experimental osteoarthritis. Osteoarthritis Cartilage. 2017;25(3):369–375. doi: 10.1016/j.joca.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 280.Kuyinu E.L., Narayanan G., Nair L.S., Laurencin C.T. Animal models of osteoarthritis: classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 2016;11:19. doi: 10.1186/s13018-016-0346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Veronesi F., Berni M., Marchiori G., Cassiolas G., Muttini A., Barboni B., Martini L., Fini M., Lopomo N.F., Marcacci M., Kon E. Evaluation of cartilage biomechanics and knee joint microenvironment after different cell-based treatments in a sheep model of early osteoarthritis. Int. Orthop. 2021;45(2):427–435. doi: 10.1007/s00264-020-04701-y. [DOI] [PubMed] [Google Scholar]
- 282.Lv X., He J., Zhang X., Luo X., He N., Sun Z., Xia H., Liu V., Zhang L., Lin X., Lin L., Yin H., Jiang D., Cao W., Wang R., Zhou G., Wang W. Comparative efficacy of autologous stromal vascular fraction and autologous adipose-derived mesenchymal stem cells combined with hyaluronic acid for the treatment of sheep osteoarthritis. Cell Transplant. 2018;27(7):1111–1125. doi: 10.1177/0963689718773333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Feng C., Luo X., He N., Xia H., Lv X., Zhang X., Li D., Wang F., He J., Zhang L., Lin X., Lin L., Yin H., He J., Wang J., Cao W., Wang R., Zhou G., Wang W. Efficacy and persistence of allogeneic adipose-derived mesenchymal stem cells combined with hyaluronic acid in osteoarthritis after intra-articular injection in a sheep model, tissue engineering. Part. Accel. 2018;24(3–4):219–233. doi: 10.1089/ten.TEA.2017.0039. [DOI] [PubMed] [Google Scholar]
- 284.Femminò S., D’Ascenzo F., Ravera F., Comità S., Angelini F., Caccioppo A., Franchin L., Grosso A., Thairi C., Venturelli E., Cavallari C., Penna C., De Ferrari G.M., Camussi G., Pagliaro P., Brizzi M.F. Percutaneous coronary intervention (PCI) reprograms circulating extracellular vesicles from ACS patients impairing their cardio-protective properties. Int. J. Mol. Sci. 2021;22(19) doi: 10.3390/ijms221910270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Monguió-Tortajada M., Prat-Vidal C., Martínez-Falguera D., Teis A., Soler-Botija C., Courageux Y., Munizaga-Larroudé M., Moron-Font M., Bayes-Genis A., Borràs F.E., Roura S., Gálvez-Montón C. Acellular cardiac scaffolds enriched with MSC-derived extracellular vesicles limit ventricular remodelling and exert local and systemic immunomodulation in a myocardial infarction porcine model. Theranostics. 2022;12(10):4656–4670. doi: 10.7150/thno.72289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Lin Y.-N., Mesquita T., Sanchez L., Chen Y.-H., Liu W., Li C., Rogers R., Wang Y., Li X., Wu D., Zhang R., Ibrahim A., Marbán E., Cingolani E. Extracellular vesicles from immortalized cardiosphere-derived cells attenuate arrhythmogenic cardiomyopathy in desmoglein-2 mutant mice. Eur. Heart J. 2021;42(35):3558–3571. doi: 10.1093/eurheartj/ehab419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Shi M.-M., Yang Q.-Y., Monsel A., Yan J.-Y., Dai C.-X., Zhao J.-Y., Shi G.-C., Zhou M., Zhu X.-M., Li S.-K., Li P., Wang J., Li M., Lei J.-G., Xu D., Zhu Y.-G., Qu J.-M. Preclinical efficacy and clinical safety of clinical-grade nebulized allogenic adipose mesenchymal stromal cells-derived extracellular vesicles. J. Extracell. Vesicles. 2021;10(10) doi: 10.1002/jev2.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Sengupta V., Sengupta S., Lazo A., Woods P., Nolan A., Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cell. Dev. 2020;29(12):747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Khoury M.J., Gwinn M., Yoon P.W., Dowling N., Moore C.A., Bradley L. The continuum of translation research in genomic medicine: how can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention? Genet. Med. 2007;9(10):665–674. doi: 10.1097/GIM.0b013e31815699d0. [DOI] [PubMed] [Google Scholar]
- 290.Gupta A., Maffulli N., Rodriguez H.C., Lee C.E., Levy H.J., El-Amin S.F. Umbilical cord-derived Wharton's jelly for treatment of knee osteoarthritis: study protocol for a non-randomized, open-label, multi-center trial. J. Orthop. Surg. Res. 2021;16(1):143. doi: 10.1186/s13018-021-02300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Wei Y., Yan L., Luo L., Gui T., Jang B., Amirshaghaghi A., You T., Tsourkas A., Qin L., Cheng Z. Phospholipase A inhibitor-loaded micellar nanoparticles attenuate inflammation and mitigate osteoarthritis progression. Sci. Adv. 2021;7(15) doi: 10.1126/sciadv.abe6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.McHugh J. Promising drug delivery system. Nat. Rev. Rheumatol. 2019;15(2):64. doi: 10.1038/s41584-019-0158-1. [DOI] [PubMed] [Google Scholar]
- 293.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., Ayre D.C., Bach J.-M., Bachurski D., Baharvand H., Balaj L., Baldacchino S., Bauer N.N., Baxter A.A., Bebawy M., Beckham C., Bedina Zavec A., Benmoussa A., Berardi A.C., Bergese P., Bielska E., Blenkiron C., Bobis-Wozowicz S., Boilard E., Boireau W., Bongiovanni A., Borràs F.E., Bosch S., Boulanger C.M., Breakefield X., Breglio A.M., Brennan M.Á., Brigstock D.R., Brisson A., Broekman M.L., Bromberg J.F., Bryl-Górecka P., Buch S., Buck A.H., Burger D., Busatto S., Buschmann D., Bussolati B., Buzás E.I., Byrd J.B., Camussi G., Carter D.R., Caruso S., Chamley L.W., Chang Y.-T., Chen C., Chen S., Cheng L., Chin A.R., Clayton A., Clerici S.P., Cocks A., Cocucci E., Coffey R.J., Cordeiro-da-Silva A., Couch Y., Coumans F.A., Coyle B., Crescitelli R., Criado M.F., D'Souza-Schorey C., Das S., Datta Chaudhuri A., de Candia P., De Santana E.F., De Wever O., Del Portillo H.A., Demaret T., Deville S., Devitt A., Dhondt B., Di Vizio D., Dieterich L.C., Dolo V., Dominguez Rubio A.P., Dominici M., Dourado M.R., Driedonks T.A., Duarte F.V., Duncan H.M., Eichenberger R.M., Ekström K., El Andaloussi S., Elie-Caille C., Erdbrügger U., Falcón-Pérez J.M., Fatima F., Fish J.E., Flores-Bellver M., Försönits A., Frelet-Barrand A., Fricke F., Fuhrmann G., Gabrielsson S., Gámez-Valero A., Gardiner C., Gärtner K., Gaudin R., Gho Y.S., Giebel B., Gilbert C., Gimona M., Giusti I., Goberdhan D.C., Görgens A., Gorski S.M., Greening D.W., Gross J.C., Gualerzi A., Gupta G.N., Gustafson D., Handberg A., Haraszti R.A., Harrison P., Hegyesi H., Hendrix A., Hill A.F., Hochberg F.H., Hoffmann K.F., Holder B., Holthofer H., Hosseinkhani B., Hu G., Huang Y., Huber V., Hunt S., Ibrahim A.G.-E., Ikezu T., Inal J.M., Isin M., Ivanova A., Jackson H.K., Jacobsen S., Jay S.M., Jayachandran M., Jenster G., Jiang L., Johnson S.M., Jones J.C., Jong A., Jovanovic-Talisman T., Jung S., Kalluri R., Kano S.-I., Kaur S., Kawamura Y., Keller E.T., Khamari D., Khomyakova E., Khvorova A., Kierulf P., Kim K.P., Kislinger T., Klingeborn M., Klinke D.J., Kornek M., Kosanović M.M., Kovács Á.F., Krämer-Albers E.-M., Krasemann S., Krause M., Kurochkin I.V., Kusuma G.D., Kuypers S., Laitinen S., Langevin S.M., Languino L.R., Lannigan J., Lässer C., Laurent L.C., Lavieu G., Lázaro-Ibáñez E., Le Lay S., Lee M.-S., Lee Y.X.F., Lemos D.S., Lenassi M., Leszczynska A., Li I.T., Liao K., Libregts S.F., Ligeti E., Lim R., Lim S.K., Linē A., Linnemannstöns K., Llorente A., Lombard C.A., Lorenowicz M.J., Lörincz Á.M., Lötvall J., Lovett J., Lowry M.C., Loyer X., Lu Q., Lukomska B., Lunavat T.R., Maas S.L., Malhi H., Marcilla A., Mariani J., Mariscal J., Martens-Uzunova E.S., Martin-Jaular L., Martinez M.C., Martins V.R., Mathieu M., Mathivanan S., Maugeri M., McGinnis L.K., McVey M.J., Meckes D.G., Meehan K.L., Mertens I., Minciacchi V.R., Möller A., Møller Jørgensen M., Morales-Kastresana A., Morhayim J., Mullier F., Muraca M., Musante L., Mussack V., Muth D.C., Myburgh K.H., Najrana T., Nawaz M., Nazarenko I., Nejsum P., Neri C., Neri T., Nieuwland R., Nimrichter L., Nolan J.P., Nolte-'t Hoen E.N., Noren Hooten N., O'Driscoll L., O'Grady T., O'Loghlen A., Ochiya T., Olivier M., Ortiz A., Ortiz L.A., Osteikoetxea X., Østergaard O., Ostrowski M., Park J., Pegtel D.M., Peinado H., Perut F., Pfaffl M.W., Phinney D.G., Pieters B.C., Pink R.C., Pisetsky D.S., Pogge von Strandmann E., Polakovicova I., Poon I.K., Powell B.H., Prada I., Pulliam L., Quesenberry P., Radeghieri A., Raffai R.L., Raimondo S., Rak J., Ramirez M.I., Raposo G., Rayyan M.S., Regev-Rudzki N., Ricklefs F.L., Robbins P.D., Roberts D.D., Rodrigues S.C., Rohde E., Rome S., Rouschop K.M., Rughetti A., Russell A.E., Saá P., Sahoo S., Salas-Huenuleo E., Sánchez C., Saugstad J.A., Saul M.J., Schiffelers R.M., Schneider R., Schøyen T.H., Scott A., Shahaj E., Sharma S., Shatnyeva O., Shekari F., Shelke G.V., Shetty A.K., Shiba K., Siljander P.R.M., Silva A.M., Skowronek A., Snyder O.L., Soares R.P., Sódar B.W., Soekmadji C., Sotillo J., Stahl P.D., Stoorvogel W., Stott S.L., Strasser E.F., Swift S., Tahara H., Tewari M., Timms K., Tiwari S., Tixeira R., Tkach M., Toh W.S., Tomasini R., Torrecilhas A.C., Tosar J.P., Toxavidis V., Urbanelli L., Vader P., van Balkom B.W., van der Grein S.G., Van Deun J., van Herwijnen M.J., Van Keuren-Jensen K., van Niel G., van Royen M.E., van Wijnen A.J., Vasconcelos M.H., Vechetti I.J., Veit T.D., Vella L.J., Velot É., Verweij F.J., Vestad B., Viñas J.L., Visnovitz T., Vukman K.V., Wahlgren J., Watson D.C., Wauben M.H., Weaver A., Webber J.P., Weber V., Wehman A.M., Weiss D.J., Welsh J.A., Wendt S., Wheelock A.M., Wiener Z., Witte L., Wolfram J., Xagorari A., Xander P., Xu J., Yan X., Yáñez-Mó M., Yin H., Yuana Y., Zappulli V., Zarubova J., Žėkas V., Zhang J.-Y., Zhao Z., Zheng L., Zheutlin A.R., Zickler A.M., Zimmermann P., Zivkovic A.M., Zocco D., Zuba-Surma E.K. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7(1) doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.