Abstract
Pyrimidine nucleosides (PN) are abundant in mammalian milk and mainly involved in glycogen deposition and lipid metabolism. To investigate the effects of maternal supplementation with pyrimidine nucleoside on glucose, fatty acids (FAs), and amino acids (AAs) metabolism in neonatal piglets. Forty pregnant sows were randomly assigned into the control (CON) group (fed a basal diet, n = 20) or the PN group (fed a basal diet supplemented with PN at 150 g/t, n = 20). Litter size, born alive and birth litter weight were recorded. The serum and placenta of sows, and jejunum and liver of neonatal piglets were sampled. The results indicated that supplementing sow diets with PN decreased birth mortality and increased the birth weight of piglets (P < 0.05). In addition, neonates from sows supplemented with PN had higher glucose levels in serum and liver compared with the CON group (P < 0.05). Moreover, maternal PN supplementation regulated the ratio of saturated FAs and polyunsaturated FAs, and AAs content in serum and liver of piglets (P < 0.05). Furthermore, an up-regulation of mRNA expression of genes related to glucose and AA transport were observed in the neonatal jejunum from the PN group (P < 0.05). Additionally, hepatic protein expressions of phosphorylated hormone-sensitive lipase (P-HSL), HSL, sterol regulatory element-binding transcription factor 1c (SREBP-1c), and phosphorylated protein kinase B (P-AKT) was higher in the piglets from the PN group than the CON group (P < 0.05). Together, maternal PN supplementation may regulate nutrient metabolism of neonatal piglets by modulating the gene expression of glucose and AA transporters in placenta and jejunum, and the gene and protein expression of key enzymes related to lipid metabolism in liver of neonatal piglets, which may improve the reproductive performance of sows.
Keywords: Pyrimidine nucleoside, Glucose, Fatty acid, Amino acid, Neonatal piglet, Sow milk
1. Introduction
Maternal nutrition regulates mothers' health and their infants at birth and the neonatal period (Wu et al., 2004). Nutrient requirements of sows increase significantly in late gestation due to the rapid growth of the fetal-placental unit (Kim et al., 2013). As the most important factors of maternal nutrition, glucose (GLU), fatty acids (FAs), and amino acids (AAs) are of great significance to the health and growth of the fetus (Han et al., 2010; Peng et al., 2010; Wu et al., 2015). Therefore, promoting piglet health by regulating the nutrition of sows in late gestation is crucial for improving productive and reproductive performance in the pig industry.
Nucleotides play a crucial role in biological processes, such as promoting growth (Daneshmand et al., 2017a, Daneshmand et al., 2017b), improving immunity (Waititu et al., 2017), and regulating AAs and FAs composition in animals (Tie et al., 2019). Previous studies showed that nucleotide supplementation regulated AAs and FAs metabolism in sow-piglet and fish models (Gao et al., 2021a, Gao et al., 2021b, Gao et al., 2021c; Tie et al., 2019). As the most abundant nucleotide in mammalian milk (Mateo et al., 2004), it is believed that pyrimidine nucleoside (PN) including uridine (Ur) and cytidine (Cy) may play an important role in regulating the health of piglets. Nucleosides are the only forms absorbed in the intestine of the animal; thus, dietary nucleotides require to be enzymatically hydrolyzed to nucleosides in vivo (Ricardo et al., 1994). A study showed that supplementation of adenosine, Ur and Cy in broiler chickens was found to improve their growth performance (Daneshmand et al., 2017a, Daneshmand et al., 2017b). Similarly, dietary supplementation with Ur improves the growth performance of weaned piglets (Li et al., 2019; Xie et al., 2019). In addition, Ur also plays an important role in regulating AAs, FAs and bile acid metabolism, and is conducive to maintaining energy homeostasis (Deng et al., 2017; Liu et al., 2019; Zhang et al., 2019; Gao et al., 2021a, Gao et al., 2021b, Gao et al., 2021c), as it can also produce pyrimidine-lipid and pyrimidine–sugar conjugates that are substrates for the deposition of glycogen (Yamamoto et al., 2011). Furthermore, Cy and Ur in the body can be transformed into each other, and Cy plays an important role in the nucleoside-lipids metabolism, DNA methylation, antidepressant, and toll-like receptor 8 (Ramiro et al., 2015; Alies et al., 2018; Furusho et al., 2019). Previous work also indicates that Ur affects body temperature and feeding behavior (Deng et al., 2017). For example, elevated serum Ur promotes food intake by increasing brain levels of uridine diphosphate (UDP; Steculorum et al., 2015). Therefore, this suggests that the growth-promoting effects of Ur may be related to its effects on nutrient metabolism and energy homeostasis.
However, there is no report about the impact of adding PN to the sow's diet on the development of neonatal piglets. Based on the previous research results, it could be inferred that maternal PN supplementation may regulate the absorption and utilization of nutrients in offspring. Thus, the current study was conducted to explore the effects of dietary PN supplementation to sows during late pregnancy on the reproductive performance, and the absorption and metabolism of GLU, FAs, and AAs of neonatal piglets.
2. Materials and methods
2.1. Animal ethics
All animal experiments were approved by the Animal Care Committee of the Institute of Subtropical Agriculture, Chinese Academy of Science (Beijing, China), and all procedures in this study were conducted according to the guidelines established by the committee (2015-8A).
2.2. Experimental design
Forty pregnant sows (Large White × Landrace) with similar parity (3.23 ± 0.247 parities), back-fat thickness (BFT, approximately 17.24 ± 0.384 mm), and close parturition date were assigned to 1 of the 2 dietary treatments (n = 20): (1) control (CON) and (2) PN groups (PN, 150 g/t) from 85 d of gestation to parturition. In this study, single-column feeding was used, and sows in the CON group received the basal diet, and sows in the PN group received the basal diet supplemented with PN at 150 g/t (Ur:Cy = 1:1, the dose was selected based on the preliminary experiment). Uridine (purity ≥99%) and Cy (purity ≥99%) were provided by Meiya Co., Ltd., (Hangzhou, China).
Experimental diets were based on corn, sorghum, barley, wheat germ and soybean meal. The experimental diet was corn-soybean-based, and the nutrients in the basal diet met the nutrient recommendation of the NRC 2012 for sows (Table 1). All sows in this study were fed twice a day at 06:30 and 14:30, and each sow was housed in slatted-floor enclosures with free access to drinking water during the experiment period. The average daily feed intake of each sow was approximately 3.0 kg from 85 to 109 d of pregnancy, and 1.8 kg from 110 to 114 d of pregnancy. The breeding management of animals was described in detail (Gao et al., 2019). The animal experiment was carried out at a large-scale breeding farm at Henan Guang’ an Biology Technology Co., Ltd., (Zhengzhou, China).
Table 1.
Ingredients and nutrient levels of the basal diet for late gestating sows (air-dry basis, %).
| Item | Content |
|---|---|
| Ingredients | |
| Yellow corn | 65.0 |
| Soybean meal | 24.3 |
| Soybean oil | 2.5 |
| Steam fish meal | 3.0 |
| Fine stone powder | 1.2 |
| Glucose | 1.0 |
| DL-Methionine | 0.08 |
| L-Threonine | 0.07 |
| L-Lysine•HCl | 0.35 |
| CaHPO4 | 1.0 |
| NaCl | 0.5 |
| Vitamin-mineral premix1 | 1.0 |
| Total | 100.0 |
| Nutrient levels2 | |
| ME, MJ/kg | 13.4 |
| Crude protein | 17.0 |
| Total P | 0.66 |
| Ca | 0.85 |
The vitamin-mineral premix provided the following per kilogram of the basal diets: sweetening agent 200 mg, antioxidant 100 mg, vitamin D3 3,000 IU, vitamin E 20 IU, vitamin K3 1.8 mg, vitamin A 6,000 IU, riboflavin 6.0 mg, thiamine 2.0 mg, pyridoxine 4.0 mg, vitamin B12 0.02 mg, niacin 26.0 mg, pantothenic acid 18.0 mg, folic acid 3.2 mg, biotin 0.4 mg, Zn (as ZnSO4⋅H2O) 100 mg, Cu (as CuSO4⋅5H2O) 20 mg, Mn (as MnSO4⋅H2O) 50 mg, Se (as Na2SeO3) 0.30 mg, I (as KI) 1.2 mg.
The nutrient levels were calculated values.
2.3. Sample collection
On delivery day, the litter size, numbers of born alive, stillborn piglets, intrauterine growth restriction (IUGR) (Myrie et al., 2012), and individual piglet weight of each sow were recorded. Moreover, during parturition, seven sows per group were randomly selected for sample collection. An approximately 5 mL blood sample was collected from the ear vein of the sow, and the blood samples were centrifuged at 3,000 × g at 4 °C for 10 min, and then serum samples were stored at −80 °C for further analysis. Furthermore, 2 slices of placenta per sow were collected and frozen in liquid nitrogen, and then stored at −80 °C until analysis (Gao et al., 2019).
Seven new born male piglets (1.54 ± 0.05 kg) were randomly selected from seven sows per group immediately after they were born as previously described (Wu et al., 2020). Following blood collection (5 mL), piglets were anesthetized with sodium pentobarbital (50 mg/kg BW) via IV injection and bled by exsanguination (Deng et al., 2009). Liver and kidney weights were measured, then 2 liver samples and one jejunal sample were collected, one of the hepatics and jejunal samples were immediately frozen in liquid nitrogen, and stored at −80 °C, and the other liver tissue was stored at - 20 °C.
2.4. Sample analysis
2.4.1. Serum chemistry
According to the manufacturer's instructions, serum total protein (TP), albumin (ALB), alkaline phosphatase (ALP), aspartate transaminase (AST), alanine aminotransferase (ALT), urea nitrogen (UN), GLU, cholesterol (CHOL), total triglyceride (TG), ammonia (NH3), high-density lipoprotein (HDL), low-density lipoprotein (LDL), total bile acid (TBA) and lactate dehydrogenase (LDH) were determined by Roche Diagnostics kits (Division of Hoffman–la Roche Limited, Montreal, QC, Canada), using an Automated Biochemistry Analyzer (Synchron CX Pro, Beckman Coulter, Fullerton, CA, USA).
2.4.2. Hepatic GLU analysis
The hepatic GLU level of neonatal piglets was determined using the CheKine Glucose Assay Kit (Abbkine Scientific Co., Ltd., Wuhan, China) according to the manufacturer's instructions.
2.4.3. Amino acids in the serum and liver analysis
Free AA profiles in the serum and liver were detected by a L-8800 automatic AA analyzer (L8800; Hitachi, Tokyo, Japan), using standards from Sigma Chemicals (St Louis, MO, USA). The methods were described by Gao et al., 2021a, Gao et al., 2021b, Gao et al., 2021c.
2.4.4. Medium- and long-chain FAs proportion in the serum and liver analysis
For FAs proportion analysis, 1 mL serum sample was extracted overnight at 50 °C in a water bath with 5% acetyl chloride/methanol solution. And then, 1 mL of n-hexane was added and centrifuged at 2,500×g at 4 °C for 5 min. Finally, the supernatant was collected and used to determine FAs composition. In addition, the FA in the liver of piglets was extracted with chloroform-methanol, and transmethylated with boron trifluoride (BF3) and methanolic KOH. The gas chromatography (Agilent 6890, Boston, MA) was used to analyze the FA profiles, and the results were expressed as a percentage of total FA.
2.4.5. Quantitative real-time PCR (qRT-PCR)
The placental, hepatic and jejunal tissues were homogenized under liquid nitrogen, and the messenger RNA (mRNA) was extracted as previously described by Gao et al., 2021a, Gao et al., 2021b, Gao et al., 2021c. According to the manufacturer's instructions, the relative mRNA expression levels of β-actin (reference gene), and the target genes related to AA, and glycolipid metabolism were determined by a Luminaris Color HiGreen High ROX (Thermo Scientific, Waltham, MA, USA) on a Bio-Rad iCycler. The primers used are shown in Table 2. The fold change in mRNA expression levels was calculated using the 2−ΔΔCt method.
Table 2.
Sequence of primers for quantitative real-time PCR.
| Genes | Nucleotide sequence of primers (5′-3′) | Accession no. | Length, bp |
|---|---|---|---|
| Glucose metabolism | |||
| GLUT1 | F: GCAGGAGATGAAGGAGGAGAGC | XM_021096908.1 | 258 |
| R: ACGAACAGCGACACGACAGT | |||
| GLUT3 | F: GCCCTGAAAGTCCTCGGTTCCT | XM_021092392.1 | 252 |
| R: ACACGGCGTTGATGCCAGAGA | |||
| OGT | F: TTGACTGCCCCTCTATGCTG | NM_001039748.2 | 131 |
| R: CCTGGGATTTCATTTCTGCTG | |||
| GSK3B | F: AGGTCCTAGGGACACCAACA | XM_021068187.1 | 161 |
| R: TGTGTACTCCAACAGACGGC | |||
| G6PC | F: AAGCCAAGCGAAGGTGTGAGC | NM_001113445.1 | 165 |
| R: GGAACGGGAACCACTTGCTGAG | |||
| GR | F: CCAAACTCTGCCTTGTGTGTTC | XM_021080412.1 | 108 |
| R: TGTGCTGTCCTTCCACTGCT | |||
| FOXO1 | F: GCAAATCGAGTTACGGAGGC | NM_214014.3 | 95 |
| R: AATGTCATTATGGGGAGGAGAGT | |||
| Lipid metabolism | |||
| FABPpm | F: ATGGGCTTATACGGTGAGCG | NM_213928.1 | 127 |
| R: CGTTGACAGGAGGGTTGGAA | |||
| FAT/CD36 | F: CTGGTGCTGTCATTGGAGCAGT | XM_021102279.1 | 161 |
| R: CTGTCTGTAAACTTCCGTGCCTGTT | |||
| FBP1 | F: TCCACCGCACGCTGGTCTAT | NM_213979.1 | 129 |
| R: CCAGTCCTCCTGCCTTCTCCAT | |||
| FATP4 | F: AGACACACGTTGGACCTTCC | XM_013993903.2 | 188 |
| R: GCAGGTTGGTGTTGATGAGC | |||
| FATP3 | F: CAGCATAGGGTGACGGTGTT | XM_021089805.1 | 194 |
| R: CCCTCTGTCAGTCCGTAGGT | |||
| CYP7a1 | F: ACCTGACCAGTTCCGAGATG | XM_013996745.2 | 200 |
| R: TATAGGGCACGATGCACAGA | |||
| FADS2 | F: ACGGCCTTCATCCTTGCTAC | NM_001171750.1 | 144 |
| R: GTTGGCAGAGGCACCCTTTA | |||
| SREBP-1c | F: GACCGGCTCTCCATAGACAA | XM_021066226.1 | 229 |
| R: CCTCTGTCTCTCCTGCAACC | |||
| ELOVL5 | F: TACCACCATGCCACTATGCT | XM_021098832.1 | 102 |
| R: GACGTGGATGAAGCTGTTGA | |||
| ELOVL2 | F: ATTCTTCACCACCAGCGAGG | XM_013977421.1 | 131 |
| R: TGCCTGGCTGTTATCACTCG | |||
| Amino acid metabolism | |||
| LAT1 | F: TTTGTTATGCGGAACTGG | XM_013978228.2 | 155 |
| R: AAAGGTGATGGCAATGAC | |||
| CAT-1 | F: TGCCCATACTTCCCGTCC | NM_001012613.1 | 192 |
| R: GGTCCAGGTTACCGTCAG | |||
| PAT1 | F: TGTGGACTTCTTCCTGATTGTC | XM_021077073.1 | 125 |
| R: CATTGTTGTGGCAGTTATTGGT | |||
| EAAT1 | F: GATGGGACCGCCCTCTAT | XM_021076550.1 | 105 |
| R: CGTGGCTGTGATGCTGATG | |||
| EAAT2 | F: GGCTGCTGGACAGGATGA | XM_021085278.1 | 154 |
| R: TAAATGGACTGGGTCTTGGT | |||
| ASCT2 | F: GATTGTGGAGATGGAGGATGTGG | XM_003355984.4 | 127 |
| R: GCGAGTGAAGAGGAAGTAGATGA | |||
| SNAT1 | F: AAGAACCTGGGCTATCTCGG | XM_003355629.4 | 138 |
| R: TGTTGCGTTAGGACTCGTTG | |||
| SNAT4 | F: GCTGTGGCAATCCTGTCACT | XM_021092582.1 | 105 |
| R: CCATCCAAATGCTTTTTCACCCA | |||
| GLUD1 | F: GGAGGCTGACTGTGACATACTGATTC | NM_001244501.2 | 81 |
| R: TTGGCTTTGACTCTAGGTGCATTGG | |||
| GAD1 | F: GGAAGAGAAGAGCAGGCTTGTGAG | XM_005671944.3 | 132 |
| R: CGAGCGAACAGGTTGGAGAAGTC | |||
| OGDH | F: AGACCAGCAGCAGCCAGGAC | XM_021079066.1 | 113 |
| R: GCGTAGTACATCTCCTCCACATAGTTG | |||
| GSTA1 | F: CATCGCCACCAAGTACAACCTCTAC | NM_214389.2 | 91 |
| R: TTCACCCAAATCTGCCACACCTTC | |||
| β-actin | F: CGTTGGCTGGTTGAGAATC | XM_003357928.4 | 132 |
| R: CGGCAAGACAGAAATGACAA | |||
GLUT1 = glucose transporter-1; GLUT3 = glucose transporter-3; OGT = O-linked N-acetylglucosamine transferase; GSK3B = glycogen synthase kinase 3 beta; G6PC = glucose-6-phosphatase catalytic subunit 1; GR = glucocorticoid receptor; FOXO1 = forkhead box O1; FABPpm = plasma membrane fatty acid binding protein; FAT/CD36 = fatty acid transporter/CD36; FABP1 = fatty acid binding protein 1; FATP3 = fatty acid transport protein 3; FATP4 = fatty acid transport protein 4; CYP7a1 = cholesterol-7a-hydroxylase; FADS2 = fatty acid desaturase 2; SREBP-1c = sterol regulatory element binding transcription factor 1c; ELOVL5 = fatty acid elongase 5; ELOVL2 = fatty acid elongase 2; LAT1 = neutral AA transporter 1; CAT-1 = cationic amino acid transporter 1; PAT1 = proton-coupled amino acid transporter 1; EAAT1 = excitatory amino acid transporters 1; EAAT2 = excitatory amino acid transporters 2; ASCT2 = amino-acid transporter 2; SNAT1 = sodium-dependent neutral amino acid transporter 1; SNAT4 = sodium-dependent neutral amino acid transporter 4; GLUD1 = glutamate dehydrogenase 1; GAD1 = glutamate decarboxylase 1; OGDH = recombinant oxoglutarate dehydrogenase; GSTA1 = glutathione S-transferase alpha 1.
2.4.6. Total protein extraction and automated capillary Western blotting (WES)
Total protein was extracted as described in detail (Xie et al., 2016), and WES was performed as previously described (Zhou et al., 2019). Briefly, hepatic protein lysates were mixed with 5 × fluorescent master mix and boiled for 5 min. Then, the samples, wash buffer, primary antibodies including phosphorylated protein kinase B (P-AKT; #9271; Cell Signaling Technology), protein kinase B (AKT; #9272; Cell Signaling Technology), phosphorylated hormone-sensitive lipase (P-HSL; ab109400; Abcam), hormone-sensitive lipase (HSL; ab45422; Abcam), sterol regulatory element-binding transcription factor 1c (SREBP-1c; ab28481; Abcam), cytochrome P450 family 27 subfamily a member 1 (CYP27A1; ab126785; Abcam), GAPDH (# 2118; Cell Signaling Technology), secondary antibodies, blocking reagent, and then, in a manufacturer-provided microplate, chemiluminescent substrate was dispensed into the designated wells. In the individual capillaries, protein separation was performed automatically using the default settings. Compass software 3.1 (Protein Simple, San Jose, CA, USA) was used to analyze the data.
2.5. Statistical analysis
All statistical analyses were performed using SPSS 21.0 (2015, IBM-SPSS Inc., Chicago, Illinois, USA), and all data were presented as the mean ± standard error of the mean (SEM). Pearson's correlation between serum biochemical parameters of sows and neonatal piglets was carried out by GraphPad Prism 7.00 for Windows (GraphPad Software, San Diego, CA, USA). The differences between the 2 groups were identified using an independent samples T-test. The probability value < 0.05 was considered statistically significant, and trends were identified when P < 0.10.
3. Results
3.1. Litter size and litter weights
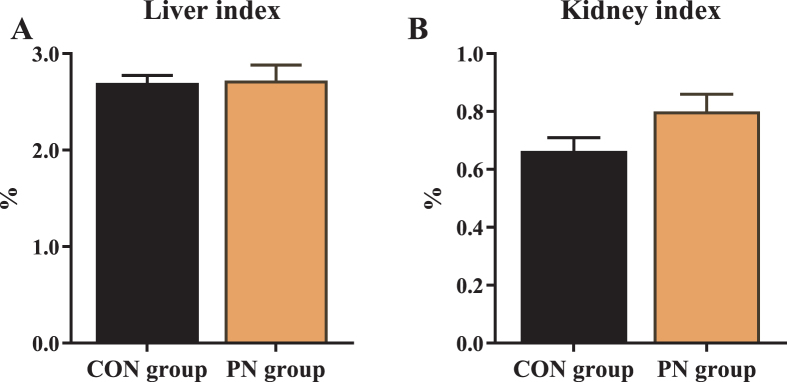
The reproductive performance of sows is summarized in Table 3. Maternal PN supplementation increased the birth weight of piglets (1.40 vs. 1.53 kg; P = 0.02), and decreased birth mortality compared with those in the CON group (P < 0.05). Similarly, compared to the CON group, the birth litter weight in the PN group tended to be higher (P = 0.08). In addition, the renal index tended to increase in the neonatal piglets from the PN group than in the CON group (P = 0.09, Fig. 1).
Table 3.
Influence of PN supplementation in the diet of sows from d 85 to 114 of gestation on reproductive performance.
| Item | Groups (n = 20) |
P-value | |
|---|---|---|---|
| CON group | PN group | ||
| Daily feed intake per sow, kg | 2.12 ± 0.07 | 2.18 ± 0.08 | 0.60 |
| Reproductive performance | |||
| FARPLA, h | 4.66 ± 0.44 | 4.55 ± 0.29 | 0.83 |
| Total born | 11.16 ± 0.37 | 11.15 ± 0.39 | 0.99 |
| Born alive | 9.74 ± 0.29 | 10.00 ± 0.33 | 0.56 |
| Birth mortality1, % | 7.35 ± 1.63 | 3.01 ± 1.12 | 0.03 |
| IUGR rate2, % | 2.62 ± 1.12 | 3.28 ± 1.11 | 0.68 |
| Birth litter weight, kg | 15.26 ± 0.64 | 16.99 ± 0.71 | 0.08 |
| Birth weight, kg | 1.40 ± 0.03 | 1.53 ± 0.04 | 0.02 |
PN = pyrimidine nucleoside; FARPLA = duration of farrowing and placenta expulsion of sows; IUGR = intrauterine growth restriction.
Birth mortality (%) = (total litter size – alive)/total × 100.
IUGR rate (%) = the number of IUGR/total × 100.
Fig. 1.
Influence of PN supplementation in the diet of sows from 85 d of gestation to parturition on the (A) liver and (B) kidney indexes of piglets. Data are presented as mean ± SEM, n = 7. PN = pyrimidine nucleoside.
3.2. Serum biochemical parameters
The serum biochemical parameters are summarized in Table 4. Compared with the CON group, maternal PN supplementation significantly decreased sow's serum ALT, AST, LDH, LDL and UN contents (P < 0.05), and tended to increase sow's serum GLU contents (P = 0.08). However, sows supplemented with PN significantly increased serum GLU, AST and BUN contents of neonatal piglets compared with the CON group's piglets (P < 0.05).
Table 4.
Influence of PN supplementation in the diet of sows from 85 d of gestation to parturition on serum biochemical parameters.1
| Item | The serum of sows (n = 7) |
P-value | The serum of piglets (n = 7) |
P-value | ||
|---|---|---|---|---|---|---|
| CON group | PN group | CON group | PN group | |||
| TP, g/L | 71.10 ± 1.39 | 72.93 ± 1.15 | 0.33 | 25.01 ± 1.38 | 31.88 ± 7.02 | 0.36 |
| ALB, g/L | 36.89 ± 0.80 | 34.79 ± 1.71 | 0.29 | 5.54 ± 0.55 | 6.23 ± 0.60 | 0.42 |
| ALT, U/L | 41.59 ± 3.77 | 30.77 ± 2.24 | 0.03 | 21.17 ± 2.67 | 24.00 ± 4.75 | 0.61 |
| AST, U/L | 44.29 ± 7.83 | 25.78 ± 1.34 | 0.04 | 39.00 ± 4.78 | 71.04 ± 12.06 | 0.03 |
| ALP, U/L | 34.86 ± 3.83 | 29.57 ± 3.53 | 0.33 | 1,863.57 ± 360.62 | 1,338.71 ± 295.00 | 0.28 |
| UN, mmol/L | 6.67 ± 1.31 | 3.86 ± 0.19 | 0.04 | 3.46 ± 0.33 | 5.89 ± 0.99 | 0.04 |
| LDH, U/L | 335.57 ± 26.07 | 237.00 ± 18.86 | 0.01 | 451.86 ± 33.20 | 494.43 ± 82.93 | 0.64 |
| GLU, mmol/L | 4.49 ± 0.24 | 5.25 ± 0.32 | 0.08 | 3.61 ± 0.28 | 4.62 ± 0.25 | 0.02 |
| TG, mmol/L | 0.34 ± 0.04 | 0.38 ± 0.04 | 0.49 | 0.39 ± 0.06 | 0.39 ± 0.04 | 0.93 |
| CHOL, mmol/L | 1.54 ± 0.13 | 1.32 ± 0.10 | 0.23 | 1.27 ± 0.14 | 1.68 ± 0.41 | 0.37 |
| HDL, mmol/L | 0.46 ± 0.03 | 0.51 ± 0.05 | 0.37 | 0.54 ± 0.10 | 0.65 ± 0.16 | 0.58 |
| LDL, mmol/L | 0.96 ± 0.12 | 0.66 ± 0.07 | 0.05 | 0.68 ± 0.08 | 0.95 ± 0.31 | 0.41 |
| HDL/LDL | 0.52 ± 0.07 | 0.76 ± 0.10 | 0.07 | 0.92 ± 0.27 | 0.80 ± 0.11 | 0.68 |
| HDL/CHO | 0.32 ± 0.04 | 0.41 ± 0.02 | 0.06 | 0.48 ± 0.13 | 0.38 ± 0.02 | 0.48 |
| LDL/CHO | 0.62 ± 0.04 | 0.54 ± 0.04 | 0.19 | 0.55 ± 0.04 | 0.51 ± 0.05 | 0.60 |
| TBA, mmol/L | 18.17 ± 2.75 | 15.08 ± 1.47 | 0.34 | 19.51 ± 3.95 | 17.56 ± 3.99 | 0.73 |
TP = total protein; ALB = albumin; ALT = alanine aminotransferase; AST = aspartate transaminase; ALP = alkaline phosphatase; UN = urea nitrogen; LDH = lactate dehydrogenase; GLU = glucose; TG = total triglyceride; CHOL = total cholesterol; HDL = high-density lipoprotein; LDL = low-density lipoprotein; TBA = total bile acid; PN = pyrimidine nucleoside.
Data are presented as mean ± SEM.
3.3. Serum FA proportion of sows and neonatal piglets
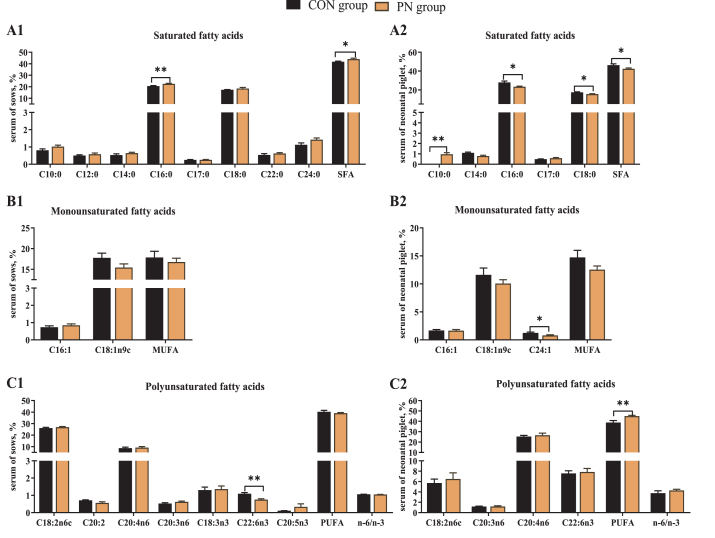
To test whether PN supplementation regulates FA metabolism, we analyzed FA composition in the serum of sows and neonates. The results showed that dietary PN supplementation increased sow's serum C16:0 and total saturated FA (SFA) proportion, and decreased sow's serum C22:6n3 compared with the CON group (P < 0.05). Additionally, some FAs such as C10:0 and C24:0 showed an uptrend in the serum of sows in the PN group (P < 0.10, Fig. 2, A1-C1). In neonatal piglets, maternal dietary PN supplementation increased serum C10:0 and total polyunsaturated FA (PUFA) proportion, and decreased the C16:0, C18:0, C24:1 and SFA proportion (P < 0.05, Fig. 2, A2-C2).
Fig. 2.
Influence of PN supplementation in the diet of sows from 85 d of gestation to parturition on serum FA proportion of (A1–C1) sows and (A2–C2) piglets. Data are presented as mean ± SEM, n = 7. Statistical significance was set at ∗P < 0.05 or ∗∗P < 0.01. FA = fatty acid; SFA = saturated fatty acids; MUFA = monounsaturated fatty acids; PUFA = polyunsaturated fatty acids. Total SFA includes C10:0, C12:0, C14:0, C16:0, C17:0, C18:0, C22:0 and C24:0; total MUFA includes C16:1, C18:1n9t; total PUFA includes C18:2n6c, C18:3n6, C20:3n6, C20:4n6, C18:3n3, C20:2, C22:6n3 and C20:5n3; PN = pyrimidine nucleoside.
3.4. Serum AA profiles of sows and neonatal piglets
Free AAs in the serum of sows and neonatal piglets were assessed, and the results are shown in Table 5. Compared with the CON group, dietary PN supplementation decreased isoleucine, phenylalanine, valine, serine, citrulline, β-alanine and increased glutamic acid contents (P < 0.05) and tended to increase methionine content in the sow's serum (P < 0.10).
Table 5.
Influence of PN supplementation in the diet of sows from 85 d of gestation to parturition on AAs in serum of sows and neonatal piglets (mg/L).
| Item | Serum of sows (n = 7) |
P-value | Serum of neonatal piglet (n = 7) |
P-value |
||
|---|---|---|---|---|---|---|
| CON group | PN group | CON group | PN group | |||
| Histidine | 7.41 ± 1.02 | 5.98 ± 0.61 | 0.24 | 3.46 ± 0.42 | 2.22 ± 0.38 | 0.05 |
| Isoleucine | 11.40 ± 2.18 | 6.28 ± 0.82 | 0.04 | 2.98 ± 1.01 | 2.50 ± 0.38 | 0.76 |
| Leucine | 16.45 ± 3.41 | 9.08 ± 1.42 | 0.06 | 2.79 ± 0.53 | 4.83 ± 0.70 | 0.04 |
| Lysine | 17.68 ± 2.44 | 16.62 ± 3.60 | 0.82 | 11.29 ± 1.91 | 18.28 ± 2.36 | 0.05 |
| Methionine | 2.50 ± 0.52 | 1.53 ± 0.22 | 0.09 | 1.06 ± 0.39 | 1.26 ± 0.31 | 0.69 |
| Phenylalanine | 9.09 ± 0.88 | 6.71 ± 0.64 | 0.04 | 4.62 ± 1.15 | 6.05 ± 1.26 | 0.42 |
| Threonine | 10.56 ± 1.74 | 8.53 ± 1.32 | 0.36 | 3.89 ± 0.86 | 3.27 ± 0.35 | 0.60 |
| Valine | 22.40 ± 3.20 | 13.44 ± 1.61 | 0.05 | 13.55 ± 3.57 | 12.92 ± 1.57 | 0.87 |
| EAA | 99.39 ± 15.29 | 68.18 ± 9.83 | 0.10 | 43.66 ± 8.41 | 47.25 ± 5.16 | 0.72 |
| Alanine | 20.61 ± 1.67 | 23.97 ± 1.85 | 0.21 | 44.94 ± 5.58 | 45.02 ± 4.34 | 0.99 |
| Aspartic acid | 0.23 ± 0.08 | 0.30 ± 0.14 | 0.69 | 0.69 ± 0.11 | 0.63±0.11 | 0.69 |
| Arginine | 17.81 ± 1.42 | 18.23 ± 2.27 | 0.88 | 2.26 ± 0.46 | 5.22±0.90 | 0.01 |
| Glutamic acid | 6.94 ± 0.40 | 10.73 ± 1.51 | 0.04 | 10.87 ± 1.82 | 6.82±1.17 | 0.08 |
| Glycine | 18.83 ± 1.95 | 21.35 ± 1.51 | 0.32 | 33.28 ± 3.11 | 25.76 ± 0.73 | 0.05 |
| Serine | 7.58 ± 0.78 | 5.36 ± 0.52 | 0.03 | 9.41 ± 0.29 | 8.94 ± 1.02 | 0.68 |
| Tyrosine | 11.36 ± 1.81 | 9.42 ± 1.46 | 0.41 | 6.12 ± 1.35 | 7.60 ± 2.11 | 0.58 |
| Cysteine | 3.56 ± 1.16 | 2.42 ± 0.52 | 0.37 | 2.78 ± 0.32 | 2.62 ± 0.15 | 0.64 |
| Proline | 13.91 ± 1.85 | 12.79 ± 2.10 | 0.70 | 8.20 ± 0.96 | 8.15 ± 0.61 | 0.96 |
| NEAA | 105.61 ± 8.63 | 103.34 ± 9.64 | 0.86 | 121.99 ± 10.42 | 115.53 ± 5.20 | 0.59 |
| Taurine | 6.91 ± 0.82 | 7.95 ± 0.57 | 0.31 | 8.68 ± 0.63 | 12.54±1.70 | 0.05 |
| α-Aminoadipic acid | 2.39 ± 0.63 | 1.74±0.23 | 0.32 | 1.77 ± 0.48 | 1.28 ± 0.27 | 0.37 |
| Citrulline | 2.05 ± 0.26 | 2.99 ± 0.28 | 0.03 | 3.44 ± 0.25 | 4.79 ± 0.22 | <0.01 |
| β-Alanine | 0.54 ± 0.03 | 0.42 ± 0.03 | 0.01 | 1.72 ± 0.19 | 1.48 ± 0.23 | 0.43 |
| β-Aminoisobutyric acid | 0.15 ± 0.07 | 0.10 ± 0.01 | 0.49 | 0.13 ± 0.03 | 0.14 ± 0.06 | 0.89 |
| Ethanolamine | 1.17 ± 0.21 | 0.79 ± 0.20 | 0.21 | 0.40 ± 0.04 | 0.51 ± 0.14 | 0.48 |
| Hydroxylysine | 0.41 ± 0.07 | 0.44 ± 0.14 | 0.85 | 0.19 ± 0.10 | 0.65 ± 0.19 | 0.05 |
| Ornithine | 5.09 ± 0.84 | 4.46 ± 0.81 | 0.60 | 5.95 ± 1.43 | 6.91 ± 0.84 | 0.60 |
| 3-Methyl-histidine | 3.22 ± 0.42 | 2.64 ± 0.36 | 0.31 | 0.59 ± 0.06 | 0.75 ± 0.06 | 0.09 |
| Carnosine | 1.30 ± 0.29 | 1.22 ± 0.20 | 0.83 | 1.17 ± 0.29 | 0.95 ± 0.16 | 0.50 |
| Total AA | 239.52 ± 22.63 | 199.89 ± 20.54 | 0.23 | 190.78 ± 17.94 | 190.60 ± 9.93 | 0.99 |
Data are presented as mean ± SEM. AAs = amino acids; EAA = essential amino acid; NEAA = non-essential amino acid; PN = pyrimidine nucleoside.
On the other hand, maternal dietary PN supplementation increased serum leucine, lysine, arginine, citrulline and hydroxylysine contents, and decreased serum histidine and glycine contents of neonatal piglets (P < 0.05). Moreover, serum glutamic acid content tended to decrease, and serum taurine and 3-methyl-histidine contents tended to increase in neonatal piglets from the PN group (P < 0.10).
3.5. Hepatic FA proportion, GLU and AA constituents of neonatal piglets
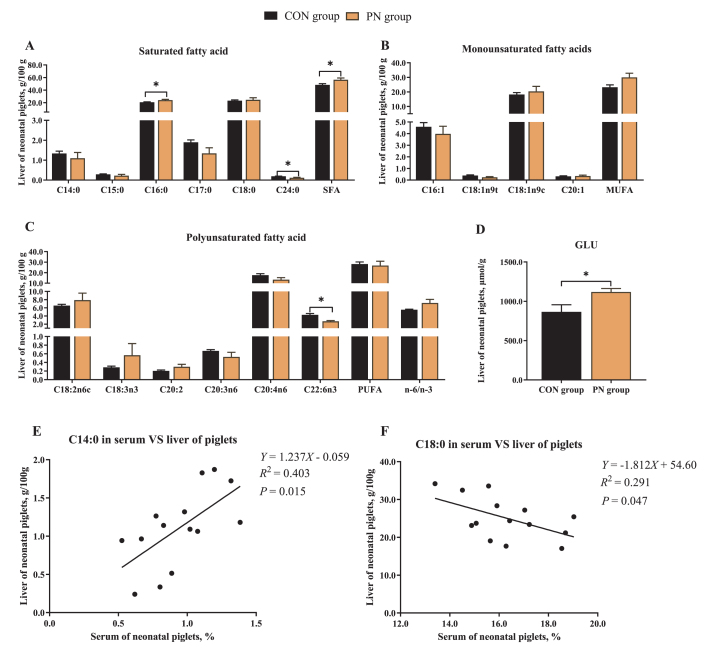
To evaluate the effects of PN on hepatic nutrient composition, hepatic GLU, FA, and AA constituents of neonatal piglets were analyzed (Fig. 3, Table 6). Compared with the CON group, maternal dietary PN supplementation increased hepatic C16:0 and SFA proportion (P < 0.05, Fig. 3 A), and decreased hepatic C24:0 and C22:6n3 proportion of piglets (P < 0.05, Fig. 3A and C). In the liver of neonatal piglets from the PN group, the C18:1n9t and C20:4n6 proportion tended to increase, while the monounsaturated FA (MUFA) and n-6/n-3 proportion tended to decrease (P < 0.10, Fig. 3B and C). Furthermore, a positive correlation in the C14:0 proportion, and a negative correlation in C18:0 proportion between serum and liver of piglets were observed in this study (P < 0.05, Fig. 3E and F).
Fig. 3.
Influence of PN supplementation in the diet of sows from 85 d of gestation to parturition on hepatic (A–C) FA proportion and (D) GLU content of piglets. Data are presented as mean ± SEM, n = 7. Statistical significance was set at ∗P < 0.05. (A) Hepatic saturated fatty acids proportion. (B) Hepatic monounsaturated fatty acids proportion. (C) Hepatic polyunsaturated fatty acids proportion. (D) Hepatic GLU. (E) Correlation analysis for C14:0 in serum vs. liver of piglets. (F) Correlation analysis for C18:0 in serum vs. liver of piglets. PN = pyrimidine nucleoside; FA = fatty acid; GLU = glucose.
Table 6.
Influence of PN supplementation in the diet of sows from 85 d of gestation to parturition on AAs in liver of neonatal piglets (mg/kg).
| Item | Groups (n = 7) |
P-value | |
|---|---|---|---|
| CON group | PN group | ||
| Histidine | 0.95 ± 0.40 | 0.87 ± 0.24 | 0.87 |
| Isoleucine | 0.52 ± 0.14 | 2.47 ± 0.69 | 0.02 |
| Leucine | 3.49 ± 0.25 | 6.13 ± 0.89 | 0.01 |
| Lysine | 0.72 ± 0.12 | 3.34 ± 1.03 | 0.03 |
| Methionine | 0.23 ± 0.05 | 0.35 ± 0.10 | 0.25 |
| Phenylalanine | 2.09 ± 0.43 | 4.37 ± 1.11 | 0.08 |
| Threonine | 1.01 ± 0.18 | 1.82 ± 0.31 | 0.04 |
| Valine | 1.32 ± 0.17 | 3.07 ± 0.83 | 0.06 |
| EAA | 10.32 ± 0.76 | 22.38 ± 3.86 | 0.01 |
| Alanine | 11.49 ± 2.79 | 12.81 ± 4.26 | 0.80 |
| Aspartic acid | 6.19 ± 1.10 | 7.10 ± 1.39 | 0.62 |
| Arginine | 0.65 ± 0.27 | 0.66 ± 0.25 | 0.97 |
| Glutamic acid | 13.53 ± 2.19 | 12.09 ± 2.09 | 0.64 |
| Glycine | 4.11 ± 0.79 | 5.27 ± 1.59 | 0.53 |
| Serine | 2.82 ± 0.56 | 2.97 ± 0.59 | 0.86 |
| Tyrosine | 3.06 ± 0.25 | 7.51 ± 1.39 | < 0.01 |
| Cysteine | 0.47 ± 0.14 | 0.72 ± 0.21 | 0.36 |
| Proline | 4.37 ± 0.97 | 3.88 ± 1.27 | 0.77 |
| NEAA | 46.63 ± 6.40 | 53.03 ± 10.50 | 0.61 |
| Total AA | 61.20 ± 6.49 | 82.10 ± 14.40 | 0.21 |
Data are presented as mean ± SEM. AAs = amino acids; EAA = essential amino acid; NEAA = non-essential amino acid; PN = pyrimidine nucleoside.
On the other hand, the inclusion of PN in the sow's diet significantly increased hepatic GLU content (1,119.18 μmol/g) of neonatal piglets compared to those in the CON group (866.50 μmol/g; P = 0.03, Fig. 3D). Moreover, sows fed a PN supplemented diet had higher hepatic essential AA (EAA) including isoleucine, leucine, lysine, threonine, and tyrosine contents of neonatal piglets than those in the CON group (P < 0.05). In addition, compared to those in the CON group, an increment in hepatic phenylalanine and valine contents from neonatal piglets in the PN group was observed, but not statistically significant (P < 0.10, Table 6).
3.6. Relative gene expression level of GLU, FA and AA transporters in the jejunum of neonatal piglets
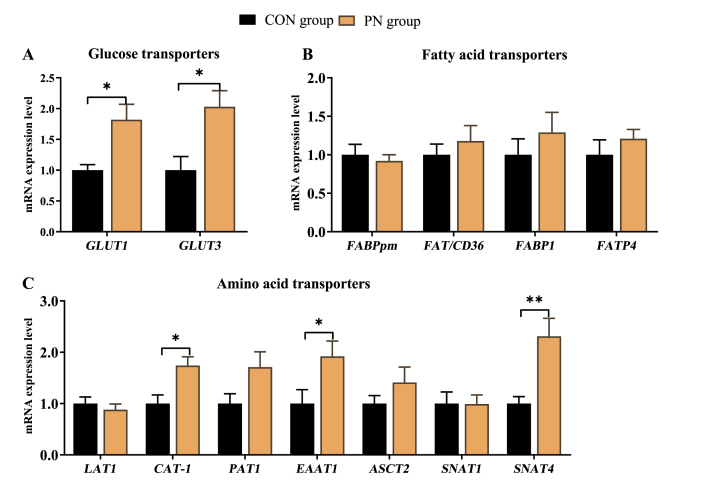
To study the effects of PN on nutrient transport in the small intestine, the gene expressions related to AA, FA, and GLU transporters was detected in the jejunum of neonatal piglets, and the results are depicted in Fig. 4. Compared with the CON group, glucose transporter (GLUT) 1 and GLUT3, which encode GLU transporters, were highly expressed in the jejunum of neonatal piglets from the PN group (P < 0.05, Fig. 4A). Furthermore, the genes encoding AA transporters, including cationic AA transporter 1 (CAT-1), excitatory AA transporters 1 (EAAT1), and sodium-dependent neutral AA transporter 4 (SNAT4) manifested expression patterns similar to GLUT1 and GLUT3 (P < 0.05, Fig. 4C). In addition, the jejunal mRNA expression of proton-coupled AA transporter 1 (PAT1) in the neonatal piglets from the PN group tended to upregulate (P = 0.07).
Fig. 4.
Influence of PN supplementation in the diet of sows from 85 d of gestation to parturition on mRNA expression of (A) GLU, (B) FA and (C) AA transporters in the jejunum of neonatal piglets. Data are presented as mean ± SEM, n = 7. Statistical significance was set at ∗P < 0.05 or ∗∗P < 0.01. GLUT1 = glucose transporter-1; GLUT3 = glucose transporter-3; FABPpm = plasma membrane fatty acid binding protein; FAT/CD36 = fatty acid transporter/CD36; FABP1 = fatty acid binding protein 1; FATP4 = fatty acid transport protein 4; LAT1 = neutral AA transporter 1; CAT-1 = cationic amino acid transporter 1; PAT1 = proton-coupled amino acid transporter 1; EAAT1 = excitatory amino acid transporters 1; ASCT2 = amino-acid transporter 2; SNAT1 = sodium-dependent neutral amino acid transporter 1; SNAT4 = sodium-dependent neutral amino acid transporter 4; GLU = glucose; FA = fatty acid; AA = amino acid; PN = pyrimidine nucleoside.
3.7. Hepatic relative gene expression related to AA and glycolipid metabolism of neonatal piglets
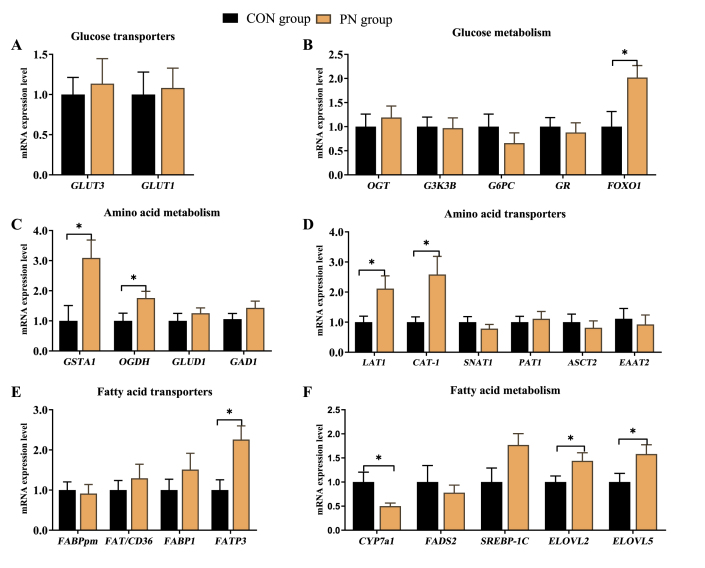
Based on the above results, the relative expression of key enzymes related to AA and glycolipid metabolism in the hepatic tissue was investigated. As shown in Fig. 5, glutathione S-transferase alpha 1 (GSTA1) and recombinant oxoglutarate dehydrogenase (OGDH), which encode the rate-limiting enzymes for AA metabolism, were highly expressed in neonatal piglets from the PN group than in the CON group (P < 0.05, Fig. 5C). Similarly, the genes encoding rate-limiting enzymes for long-chain fatty acids (LCFAs) elongation reaction, FA elongase (ELOVL) 2 and ELOVL5 manifested expression patterns similar to GSTA1 in neonatal piglets' liver from the PN group (P < 0.05, Fig. 5F). Conversely, cholesterol-7a-hydroxylase (CYP7a1), which encodes the rate-limiting enzymes for TBA metabolism, was down-regulated in neonatal piglets' liver from the PN group (P < 0.05). Furthermore, maternal supplementation with PN up-regulated hepatic gene expression of neutral AA transporter 1 (LAT1), CAT-1, and fatty acid transport protein 3 (FATP3), which are involved in AA and FA transport (P < 0.05). Also, the genes encoding the regulation factor in protein and glycolipid metabolism, forkhead box O1 (FOXO1) showed higher expression in neonatal piglets from the PN group than those piglets from the CON group (P < 0.05). Maternal PN supplementation tended to increase the hepatic mRNA expression level of SREBP-1c in neonatal piglets from the PN group compared to those from the CON group (P < 0.10).
Fig. 5.
Influence of PN supplementation in the diet of sows from 85 d of gestation to parturition on mRNA expression of (A-B) GLU, (C-D) AA, and (E-F) FA metabolism in liver of neonatal piglets. Data are presented as mean ± SEM, n = 7. Statistical significance was set at ∗P < 0.05. GLUT3 = glucose transporter-3; GLUT1 = glucose transporter-1; OGT = O-linked N-acetylglucosamine transferase; GSK3B = glycogen synthase kinase 3 beta; G6PC = glucose-6-phosphatase catalytic subunit 1; GR = glucocorticoid receptor; FOXO1 = forkhead box O1; GSTA1 = glutathione S-transferase alpha 1; OGDH = recombinant oxoglutarate dehydrogenase; GAD1 = glutamate decarboxylase 1; GLUD1 = glutamate dehydrogenase 1; LAT1= neutral AA transporter 1; CAT-1 = cationic amino acid transporter 1; SNAT1 = sodium-dependent neutral amino acid transporter 1; PAT1 = proton-coupled amino acid transporter 1; ASCT2 = amino-acid transporter 2; EAAT2 = excitatory amino acid transporters 2; FABPpm= plasma membrane fatty acid binding protein; FAT/CD36= fatty acid transporter/CD36; FABP1= fatty acid binding protein 1; FATP3= fatty acid transport protein 3; CYP7a1 = cholesterol-7a-hydroxylase; FADS2 = fatty acid desaturase 2; SREBP-1c = sterol regulatory element binding transcription factor 1c; ELOVL5 = fatty acid elongase 5; ELOVL2 = fatty acid elongase 2; GLU = glucose; AA = amino acid; FA = fatty acid; PN = pyrimidine nucleoside.
3.8. Hepatic relative protein expression level related to lipid metabolism in neonatal piglets
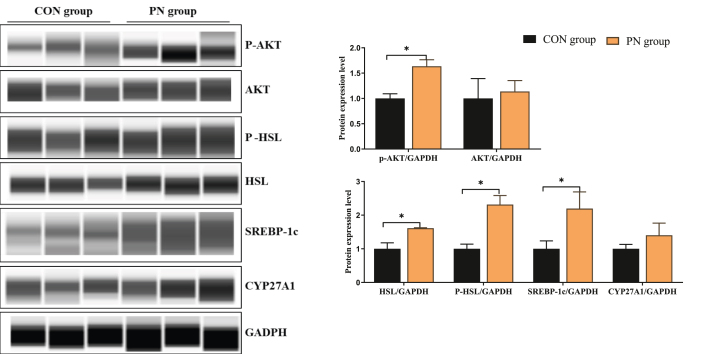
The hepatic protein levels related to lipid metabolism of neonatal piglets are shown in Fig. 6. As the rate-limiting enzyme for fat decomposition, the relative protein expression of HSL and P-HSL was increased in the liver of neonatal piglets from the PN group compared to those in the CON group (P < 0.05). In addition, maternal dietary PN supplementation up-regulated the hepatic protein level of SREBP-1c and P-AKT in neonatal piglets (P < 0.05).
Fig. 6.
Influence of PN supplementation in the diet of sows from 85 d of gestation to parturition on the protein expression of key enzymes for lipid metabolism in liver of neonatal piglets. Data are presented as mean ± SEM, n = 3. Statistical significance was were set at ∗P < 0.05. AKT = protein kinase B; P-AKT = phosphorylated protein kinase B; HSL = hormone-sensitive lipase; P-HSL = phosphorylated HSL; SREBP-1c = sterol regulatory element binding transcription factor 1c; CYP27A1 = cytochrome P450 family 27 subfamily a member 1; GADPH = glyceraldehyde-3-phosphate dehydrogenase; PN = pyrimidine nucleoside.
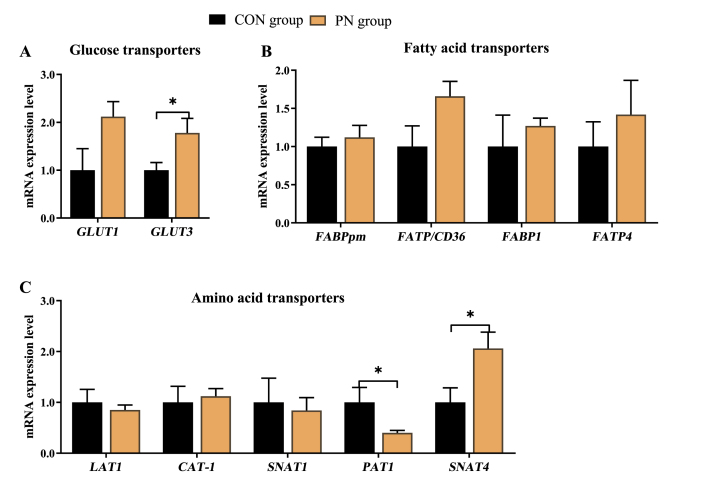
3.9. Placental gene expression related to AA, FA and GLU transport
To determine whether PN regulates nutrient metabolism of neonatal piglets partially through placental transport, placental gene expression related to AA, FA, and GLU transport was analyzed and presented in Fig. 7. Compared to those in the CON group, maternal dietary PN supplementation up-regulated placental mRNA expression of GLUT3 and SNAT4, and down-regulated placental mRNA expression of PAT1 (P < 0.05). Furthermore, compared with the CON group, maternal dietary PN supplementation tended to upregulate GLUT1 and FA transporter/CD36 (FAT/CD36) mRNA expression in the placenta (P < 0.10).
Fig. 7.
Influence of PN supplementation in the diet of sows from 85 d of gestation to parturition on mRNA expression of (A) GLU, (B) FA, and (C) AA transporters in the placenta. Data are presented as mean ± SEM, n = 7. Statistical significance was set at ∗P < 0.05. GLUT1 = glucose transporter-1; GLUT3 = glucose transporter-3; FABPpm= plasma membrane fatty acid binding protein; FAT/CD36= fatty acid transporter/CD36; FABP1= fatty acid binding protein 1; FATP4= fatty acid transport protein 4; LAT1= neutral AA transporter 1; CAT-1 = cationic amino acid transporter 1; SNAT1 = sodium-dependent neutral amino acid transporter 1; PAT1 = proton-coupled amino acid transporter 1; SNAT4 = sodium-dependent neutral amino acid transporter 4; GLU = glucose; FA = fatty acid; AA = amino acid; PN = pyrimidine nucleoside.
4. Discussion
4.1. Exogenous PN increased birth weight and decreased birth mortality
During late pregnancy, more nutrients are required for fast fetal growth and thus endogenous nucleotide synthesis may not satisfy the demand of the body (Martinez-Puig et al., 2007). Nucleotides include purine nucleotides and pyrimidine nucleotides, such as uridine 5′monophosphate (UMP), cytidine 5′monophosphate (CMP), and their phosphorylated forms or coenzymes are the most abundant nucleotides in sow's milk (Mateo et al., 2004). The research has shown that nucleosides are the only forms that can be absorbed in the intestine, thus, dietary nucleotides must be enzymatically hydrolyzed to nucleosides before they are utilized (Ricardo et al., 1994). Consistently, our previous study showed that dietary nucleosides may have a higher utilization rate than nucleotides in pigs (Li et al., 2019). Previous studies have shown that supplementation of sow’ diet with yeast extract, live yeast, yeast RNA, or NuPro during late pregnancy did not affect the reproductive performance of sows (Plante et al., 2011; Jang et al., 2013; Vitagliano et al., 2014; Hung, 2015). In contrast to the previous report, dietary nucleotide supplementation improves the growth performance, and healthy FA proportion in fish (Tie et al., 2019). In addition, sows supplemented with yeast-based nucleotides or Ur during late pregnancy have a lower stillbirth rate (Gao et al., 2021a, Gao et al., 2021b, Gao et al., 2021c). Furthermore, the components of nucleotides participate in energy metabolism, protein metabolism, and blood GLU regulation suggesting that the combination of Cy and Ur might meet the high demand for genetic materials (Daneshmand et al., 2017a, Daneshmand et al., 2017b). In the present study, the supplementation of PN to sows diet in late-gestation periods could improve the birth weight of piglets and decrease the stillbirth rate, which may be related to fetal nutritional metabolism. Although the detailed mechanism is unclear, improved reproductive performance is a process tightly linked to the function of PN.
4.2. Exogenous PN regulated GLU metabolism of sows and piglets
As the main energy source, the production and utilization of blood GLU are in a state of dynamic balance to maintain the needs of the body. In addition, GLU metabolism during late gestation is related to the number of stillborn piglets (Weldon et al., 1994), because GLU is essential for aerobic metabolism in the growing fetus (Novakovic et al., 2013). Plasma Ur improves GLU metabolism (Peters et al., 1987), however, on the flip side, oral GLU regulates the level of Ur in the blood (Yamamoto et al., 1999). Our results unveil pivotal roles in the interaction between PN and GLU. For example, maternal PN supplementation tends to increase sow's serum GLU content in the present study, consistent with the previous results that dietary Ur or UMP supplementation regulates serum GLU in sows (Gao et al., 2021a, Gao et al., 2021b, Gao et al., 2021c), rats (Zhang et al., 2018; Liu et al., 2019), and calves (Katoh et al., 2019). On the other hand, maternal PN supplementation markedly increases GLU content in the serum and liver of neonatal piglets. The previous study suggested that maternal yeast-based nucleotide (YN) supplementation increased serum GLU content of neonatal piglets (Gao et al., 2021a, Gao et al., 2021b, Gao et al., 2021c), while dietary Ur supplementation to sows does not affect serum GLU content of piglets, implying that the combination of Ur and Cy can regulate the serum GLU level of neonatal piglets. It was believed that Ur and Cy may have a synergistic effect in regulating the metabolism of nutrients because oral Cy is metabolized into Ur in the body (Wurtman et al., 2000), and Ur is phosphorylated to uridine triphosphate (UTP), then converted to cytidine triphosphate (CTP; Richardson et al., 2003). Furthermore, the elevated serum Ur could increase UDP (Steculorum et al., 2015), and UDP and CMP are important substrates for the synthesis of nucleotide-sugar (Roach et al., 2012), and exogenous nucleotides could regulate the synthesis of nucleotide-sugar (Mironova et al., 2018). Thus, the above results showed that the combination of Ur and Cy increased GLU level in sows and neonatal piglets, possibly affecting nucleotide-sugar synthesis, which is conducive to the energy supply of sows and fetuses during farrowing.
The sources of GLU of the neonatal piglet are placental transport (Novakovic et al., 2013), absorption from amniotic fluid (Sangild et al., 2002) and hepatic gluconeogenesis. Studies have shown that the piglet's prenatal intestine could absorb and utilize large molecules during the final weeks of gestation (Gao et al., 2021a, Gao et al., 2021b, Gao et al., 2021c). In addition, the transport of GLU across the placenta or intestine is mediated by a family of facilitative transporter proteins, known as the glucose transporters (GLUTs) (Novakovic et al., 2013). Our findings indicates that maternal PN supplementation up-regulated mRNA expression of GLUT1 and GLUT3 in the placenta and jejunum of neonatal piglets, implying that PN may increase the GLU level of neonatal piglets by promoting its transport and absorption from the placenta and small intestines. Higher GLU levels in serum and liver may be one of the reasons for lower stillbirths and higher birth weight in the present study (Han et al., 2010).
4.3. Exogenous PN regulated fatty acid metabolism of sows and piglets
A good energy supply is very important for both sows and fetuses since sows are in a catabolic state during the last third of gestation. As the members of the apolipoprotein family, LDL transports CHOL from the liver to the periphery, and HDL transports CHOL from the periphery to the liver for catabolism (Sun et al., 2019). Our findings showed that PN promoted hepatic utilization of CHOL in the liver of piglets, as evidenced by significantly lower serum LDL content and higher HDL/LDL and HDL/CHOL levels in PN supplemented group, in agreement with our previous study that maternal dietary Ur supplementation decreased LDL (Gao et al., 2021a, Gao et al., 2021b, Gao et al., 2021c).
As one of the PN, Ur or UMP can regulate hepatic lipid metabolism in rats (Le et al., 2014; Liu et al., 2019) and piglets (Zhang et al., 2019). Uridine is catabolized to alanine and acetyl-CoA, and acetyl-CoA plays an important role in energy metabolism (Wellen et al., 2012); thus, PN may affect the synthesis of FA. Dietary nucleotides or Ur have been shown to regulate total SFA proportion in grass carp (Tie et al., 2019), rats (Liu et al., 2019), and piglets (Gao et al., 2021a, Gao et al., 2021b, Gao et al., 2021c). Our results indicated that maternal dietary PN supplementation increased sow's serum total SFA proportion, such as C16:0, suggesting that PN has a synergistic effect on regulating FA composition. Dietary nucleotides may stimulate PUFA in newborn infants and rats (Sánchez-Pozo et al., 1994; Sato et al., 1995), while dietary nucleotides could reduce the relative proportion of omega 6 and 3 PUFAs (Delucchi et al., 1987). Similarly, we found that maternal dietary PN supplementation decreased C22:6n3 proportion in sow's serum and piglet's liver. In addition, maternal dietary PN supplementation regulated serum C10:0, C18:0, C24:1, PUFA and hepatic C24:0 proportion of neonatal piglets, suggesting the combination of Ur and Cy participate in the elongation, desaturation, and oxidation process of FAs, which is consistent with the previous research that dietary nucleotide play a significant role in the desaturation and elongation of FA during early life (Delucchi et al., 1987). Interestingly, the serum C16:0 and SFA proportion of piglets from the PN group were lower than those of piglets from the CON group. On the contrary, hepatic C16:0 and SFA proportion in the piglets from the PN group were higher than in piglets from the CON group, which suggested that the combination of Ur and Cy may regulate FA transport in the piglets.
The liver is the major organ for FA supply, and many key enzymes play a key role in lipid metabolism. The present study showed that maternal dietary PN supplementation improved the protein expression of P-HSL and HSL, suggesting dietary PN supplementation to sows may promote lipolysis of neonatal piglets, which may be related to the energy demand of neonatal piglets during delivery. In addition, our data demonstrated that dietary PN could up-regulate the gene expression of ELOVL5, ELOVL2, suggesting that dietary PN may affect the prolongation of PUFA. Moreover, SREBP-1c is a key transcriptional regulator of fat synthesis genes. In this study, dietary PN supplementation to pregnant sows increased hepatic gene and protein expression of SREBP-1c, indicating that the effects of PN on lipid metabolism may be associated with SREBP-1c. Interestingly, AKT could stimulate de novo lipid synthesis through activating SREBP-1c, and the protein expression of P-AKT in this study was increased in the PN groups, suggesting the combination of Ur and Cy may promote the prolongation of FAs by AKT- SREBP-1c pathway (Gao et al., 2021a, Gao et al., 2021b, Gao et al., 2021c). On the other hand, nucleotides, especially Ur, regulate CHOL and TBA metabolism (Deng et al., 2017; Zhang et al., 2018; Gao et al., 2021a, Gao et al., 2021b, Gao et al., 2021c). CYP7a1 is a rate-limiting enzyme during cholesterol and bile acid metabolism (Cai et al., 2014). The present study showed that dietary PN supplementation to pregnant sows down-regulated hepatic genes expression of CYP7a1, suggesting that the combination of Ur and Cy may regulate the hepatic conversion of CHOL and TBA. The above results showed that maternal dietary PN supplementation promotes hepatic lipolysis, and prolongation of FA chains in neonatal piglets during delivery, consistent with the lower stillbirth rate observed in the present study, since medium- and long-chain FAs are beneficial for improving the survival rate of piglets due to the high utilization rate (Enke et al., 2008).
4.4. Exogenous PN regulated AAs metabolism of sow and piglets
Some AAs, especially glutamate, aspartic acid, alanine, etc. are substrates for de novo nucleotide synthesis. The present study demonstrated that maternal PN supplementation mainly decreased sows' serum ALT and AST contents, implying PN could regulate AA metabolism that may contribute to hepatic health (Zhao et al., 2020). Moreover, the plasma UN reflects the protein metabolism and AA balance, thus, the utilization of protein for sows in the PN group may be better as AA balance improves with a consequent decrease in plasma UN level (Sohail et al., 1978). These data reveal a strong correlation between nucleotide and AA metabolism.
Serum AA of pregnant sows play a huge role in their pregnancy health, as well as in the development of the fetus and mammary gland. Thus, we further evaluated the effects of PN supplementation on the serum AA composition and found that PN mainly decreased sows' serum isoleucine, valine, phenylalanine and serine. Those results were in line with a previous study that showed nucleotide supplementation regulates the relative amount of glutamic, aspartic acid, serine, alanine, glycine, leucine and valine in grass carp muscle (Tie et al., 2019). Similarly, maternal nucleotide supplementation regulates the relative amount of sows' serum phenylalanine, valine, leucine and isoleucine (Gao et al., 2021a, Gao et al., 2021b, Gao et al., 2021c). However, the diet with Ur alone increases sows' serum glutamic acid, valine, leucine, isoleucine, serine, and methionine during late pregnancy (Gao et al., 2021a, Gao et al., 2021b, Gao et al., 2021c), suggesting that different ratios and forms of nucleotides have different effects on AA metabolism, and Cy also plays a regulatory role in AA metabolism. Furthermore, the diet with PN mainly increased glutamic acid and decreased β-alanine of sows' serum in the present study, suggesting the diet with PN may promote the utilization or salvage synthesis pathway of nucleotides because glutamic acid participates in de novo synthesis of pyrimidine nucleotides, and β-alanine is involved in the metabolism of nucleotides (Le et al., 2014). Also, maternal PN supplementation during late pregnancy increased sows' serum citrulline, which indicates that PN may affect the urea cycle.
On the other hand, maternal PN supplementation regulated AA metabolism in neonatal piglets. In the present study, maternal PN supplementation mainly increased piglets' serum AST and UN. The young animals have intense AA metabolism due to rapid growth, which is in line with the higher serum AST and UN contents. Further analysis revealed that maternal supplementation with PN regulated serum leucine, lysine, arginine, citrulline, histidine and glycine, and hepatic EAA, glycine, leucine, lysine, threonine and tyrosine of neonatal piglets. The changes in these AAs may be explained by three main reasons. Firstly, as a semi-essential AA, histidine is especially important for the growth of young animals, and 3-methyl-histidine is formed by methylation of histidine, which indicates that maternal PN supplementation regulates the metabolism of histidine and 3-methyl-histidine in neonatal piglets, consistent with previous research (Gao et al., 2021a, Gao et al., 2021b, Gao et al., 2021c). Secondly, lysine is the first limiting AA in mammals, thus, the higher serum lysine content in the neonates from the PN group indicates that maternal PN supplementation may promote placental AA transport. Thirdly, arginine and citrulline are the key substances of the urea cycle; the higher arginine and citrulline revealed that maternal supplementation with PN may regulate the urea cycle, which may be closely related to carbamoyl phosphate synthase, since carbamoyl phosphate synthase I present in mitochondria is involved in the ornithine cycle (Adam et al., 2019), while carbamoyl phosphate synthetase II enzymes present in the cytoplasm are used for pyrimidine synthesis (Bourget et al., 1971). Lastly, maternal supplementation with the combination of Ur and Cy also regulated branched-chain AAs metabolism including isoleucine, leucine and tyrosine, which is consistent with the result of our previous research, however, the mechanism needs further study. The aforementioned results suggested maternal PN supplementation might stimulate an AA sparing effect (Daneshmand et al., 2017a, Daneshmand et al., 2017b) that promotes the utilization of these AA by other organs that require them for growth, which is helpful for the fetal growth and development.
To meet fetal nutritional needs, the expression of nutritional transporters in the placenta could be altered with the change in fetal nutritional needs, because placental nutrient supply is adaptive (Sibley et al., 2010). EAAT3, LAT1, CAT-1, and PAT1 are responsible for transporting acidic AAs, branched-chain aromatic AAs, basic AAs, and small neutral AAs, respectively (Suryawan et al., 2013; Liu et al., 2016). While as a neutral AA transporter, SNAT4 has a lower activity for neutral AAs, such as histidine, and plays a crucial role in fetal growth and development (Matoba et al., 2019). In the present study, maternal PN supplementation regulated the AA transport in the placenta and jejunum of piglets. For example, maternal PN supplementation down-regulated placental mRNA expression of PAT1 and SNAT4, and up-regulated jejunal mRNA expression of PAT1 and SNAT4, which may be related to the glycine and histidine content in the serum of sow and piglet. In addition, maternal dietary PN supplementation up-regulated jejunal and hepatic CAT-1, and jejunal LAT1 mRNA expression of piglets, suggesting that it promotes the absorption of AAs in the small intestine and liver of piglets, especially isoleucine, arginine, and lysine. Furthermore, glutathione S-transferase A1 (GSTA1) is used in glutathione synthesis and is involved in glycine, glutamic acid, and cysteine metabolism. Thus, the present study indicates that maternal PN supplementation may promote glycine and glutamic acid utilization by up-regulating the mRNA expression level of GSTA1. The higher expression of oxoglutarate dehydrogenase (OGDH) may also be a reason for the decrease of glutamic acid, glycine and histidine.
5. Conclusion
In summary, maternal dietary Ur and Cy supplementation altered neonatal AA profiles by increasing leucine, lysine, arginine, and citrulline content, and decreasing serum histidine and glycine content. In addition, maternal PN supplementation improved serum GLU content and up-regulated the mRNA expression of GLUT1, GLUT3 in the placenta, and jejunum of piglets. Furthermore, it regulated serum and hepatic FA profiles by promoting the extension of the FA chain and fat decomposition. Together, our results demonstrated that maternal dietary Ur and Cy supplementation during late pregnancy decreased birth mortality and improved the birth weight of piglets.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Author contributions
Lu-min Gao: Conceptualization, Methodology, Investigation, Formal analysis, Data curation, Writing - original draft. Gang-yi Liu: Conceptualization, Methodology. Hong-ling Wang: Conceptualization, Methodology. Teketay Wassie: Writing - original draft. Xin Wu: Supervision, Writing - review & editing.
Acknowledgement
This paper was jointly supported by grants from National key R&D program of China (2021YFD1301002) and the Tianjin Synthetic Biotechnology Innovation Capacity Improvement Project (TSBICIP-CXRC-031).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Adam A.A.A., van der Mark V.A., Ruiter J.P.N., Wanders R.J.A., Oude Elferink R.P.J., Chamuleau R.A.F.M., et al. Overexpression of carbamoyl-phosphate synthase 1 significantly improves ureagenesis of human liver heparg cells only when cultured under shaking conditions. Mitochondrion. 2019;47:298–308. doi: 10.1016/j.mito.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Alies B., Ouelhazi M.A., Patwa A., Verget J., Navailles L., Desvergnes V., et al. Cytidine- and guanosine-based nucleotide-lipids. Org Biomol Chem. 2018;16(26):4888–4894. doi: 10.1039/c8ob01023d. [DOI] [PubMed] [Google Scholar]
- Bourget P.A., Natale P.J., Tremblay G.C. Pyrimidine biosynthesis in rat liver: studies on the source of carbamoylphosphate. Biochem Bioph Res Co. 1971;45(4):1109–1114. doi: 10.1016/0006-291x(71)90452-9. [DOI] [PubMed] [Google Scholar]
- Cai D., Jia Y., Lu J., Yuan M., Sui S., Song H., et al. Maternal dietary betaine supplementation modifies hepatic expression of cholesterol metabolic genes via epigenetic mechanisms in newborn piglets. Br J Nutr. 2014;112(9):1459–1468. doi: 10.1017/S0007114514002402. [DOI] [PubMed] [Google Scholar]
- Daneshmand A., Kermanshahi H., Danesh Mesgaran M., King A.J., Ibrahim S.A. Effects of pyrimidine nucleosides on growth performance, gut morphology, digestive enzymes, serum biochemical indices and immune response in broiler chickens. Livest Sci. 2017;204:1–6. doi: 10.1080/00071668.2017.1335859. [DOI] [PubMed] [Google Scholar]
- Daneshmand A., Kermanshahi H., Mesgaran M., King A.J., Ibrahim S.A., Klasing K.C. Combination of purine and pyrimidine nucleosides influences growth performance, gut morphology, digestive enzymes, serum biochemical indices and immune functions in broiler chickens. Anim Feed Sci Technol. 2017;228:186–193. [Google Scholar]
- Delucchi C., Pita M.L., Faus M.J., Molina J.A., Uauy R., Gil A. Effects of dietary nucleotides on the fatty acid composition of erythrocyte membrane lipids in term infants. J Pediatr Gastroenterol Nutr. 1987;6(4):568–574. doi: 10.1097/00005176-198707000-00014. [DOI] [PubMed] [Google Scholar]
- Deng D., Yao K., Chu W., Li T., Huang R., Yin Y., et al. Impaired translation initiation activation and reduced protein synthesis in weaned piglets fed a low-protein diet. J Nutr Biochem. 2009;20(7):544–552. doi: 10.1016/j.jnutbio.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Deng Y., Wang Z.V., Gordillo R., An Y., Zhang C., Liang Q., et al. An adipo-biliary-uridine axis that regulates energy homeostasis. Science. 2017;355(6330) doi: 10.1126/science.aaf5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enke U., Seyfarth L., Schleussner E., Markert U.R. Impact of PUFA on early immune and fetal development. Br J Nutr. 2008;100(6):1158–1168. doi: 10.1017/S000711450801413X. [DOI] [PubMed] [Google Scholar]
- Furusho K., Shibata T., Sato R., Fukui R., Motoi Y., Zhang Y., et al. Cytidine deaminase enables Toll-like receptor 8 activation by cytidine or its analogs. Int Immunol. 2019;31(3):167–173. doi: 10.1093/intimm/dxy075. [DOI] [PubMed] [Google Scholar]
- Gao L., Lin X., Xie C., Zhang T., Wu X., Yin Y. The time of calcium feeding affects the productive performance of sows. Animals. 2019;9(6):337. doi: 10.3390/ani9060337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., Xie C., Liang X., Li Z., Li B., Wu X., Yin Y. Yeast-based nucleotide supplementation in mother sows modifies the intestinal barrier function and immune response of neonatal pigs. Anim Nutr. 2021;7(1):84–93. doi: 10.1016/j.aninu.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., Liu Y., Zhou X., Zhang Y., Wu X., Yin Y. Maternal supplementation with uridine influences fatty acids and amino acids constituents of offspring in a sow-piglet model. Br J Nutr. 2021;125(7):743–756. doi: 10.1017/S0007114520003165. [DOI] [PubMed] [Google Scholar]
- Gao L., Zhou T., Chen Z., Wassie T., Li B., Wu X., Yin Y. Maternal yeast-based nucleotides supplementation decreased stillbirth by regulating nutrient metabolism. J Sci Food Agric. 2021;101(10):4018–4032. doi: 10.1002/jsfa.11037. [DOI] [PubMed] [Google Scholar]
- Han Y.K., Wang Q., Cho J.H., Chen Y.J., Yoo J.S., Shin S.O., et al. Effects of dietary glucose level during late gestation on litter performance and glucose concentration in sows. Anim Sci J. 2010;80(1):57–61. doi: 10.1111/j.1740-0929.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- Hung I. Dissertations & Theses - Gradworks; 2015. The effect of dietary nucleotides in sow and nursery piglet diets on reproduction, growth, and immune response. [Google Scholar]
- Jang Y.D., Kang K.W., Piao L.G., Jeong T.S., Auclair E., Jonvel S., et al. Effects of live yeast supplementation to gestation and lactation diets on reproductive performance, immunological parameters and milk composition in sows. Livest Sci. 2013;152(2–3):167–173. [Google Scholar]
- Katoh K., Yoshioka K., Hayashi H., Mashiko T., Yoshida M., Kobayashi Y., et al. Effects of 5-uridylic acid feeding on postprandial plasma concentrations of GH, insulin and metabolites in young calves. J Endocrinol. 2019;186(1):157–163. doi: 10.1677/joe.1.06043. [DOI] [PubMed] [Google Scholar]
- Kim S.W., Weaver A.C., Shen Y.B., Zhao Y. Improving efficiency of sow productivity: nutrition and health. J Anim Sci Biotechnol. 2013;4(3) doi: 10.1186/2049-1891-4-26. 26-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T.T., Urasaki Y., Pizzorno G. Uridine prevents fenofibrate-induced fatty liver. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0087179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Xie C., Wang Q., Wan D., Zhang Y., Wu X., et al. Uridine/UMP metabolism and their function on the gut in segregated early weaned piglets. Food Funct. 2019;10(7):4081–4089. doi: 10.1039/c9fo00360f. [DOI] [PubMed] [Google Scholar]
- Liu Y., Kong X., Li F., Tan B., Li Y., Duan Y., et al. Co-dependence of genotype and dietary protein intake to affect expression on amino acid/peptide transporters in porcine skeletal muscle. Amino Acids. 2016;48(1):75–90. doi: 10.1007/s00726-015-2066-2. [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhang Y., Yin J., Ruan Z., Wu X., Yin Y. Uridine dynamic administration affects circadian variations in lipid metabolisms in the liver of high-fat-diet-fed mice. Chronobiol Int. 2019;36(9):1258–1267. doi: 10.1080/07420528.2019.1637347. [DOI] [PubMed] [Google Scholar]
- Martinez-Puig D., Manzanilla E.G., Morales J., Borda E., Perez J.F., Pineiro C., et al. Dietary nucleotide supplementation reduces occurrence of diarrhoea in early weaned pigs. Livest Sci. 2007;108(1–3):276–279. [Google Scholar]
- Matoba S., Nakamuta S., Miura K., Hirose M., Shiura H., Kohda T., et al. Paternal knockout of Slc38a4/SNAT4 causes placental hypoplasia associated with intrauterine growth restriction in mice. Proc Natl Acad Sci U S A. 2019;116(42):21047–21053. doi: 10.1073/pnas.1907884116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo C.D., Peters D.N., Stein H.H. Nucleotides in sow colostrum and milk at different stages of lactation. J Anim Sci. 2004;82(5):1339–1342. doi: 10.2527/2004.8251339x. [DOI] [PubMed] [Google Scholar]
- Mironova G.D., Khrenov M.O., Yu T.E., Glushkova O.V., Parfenyuk S.B., Novoselova T.V., et al. The role of mitochondrial KATP channel in anti-inflammatory effects of uridine in endotoxemic mice. Arch Biochem Biophys. 2018;654:70–76. doi: 10.1016/j.abb.2018.07.006. [DOI] [PubMed] [Google Scholar]
- Myrie S.B., Mackay D.S., Van Vliet B.N., Bertolo R.F. Early programming of adult blood pressure in the low birth weight Yucatan miniature pig is exacerbated by a post-weaning high-salt-fat-sugar diet. Br J Nutr. 2012;108(7):1218–1225. doi: 10.1017/S0007114511006696. [DOI] [PubMed] [Google Scholar]
- Novakovic B., Gordon L., Robinson W.P., Desoye G., Saffery R. Glucose as a fetal nutrient: dynamic regulation of several glucose transporter genes by DNA methylation in the human placenta across gestation. J Nutr Biochem. 2013;24(1):282–288. doi: 10.1016/j.jnutbio.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Peng Y., Ren F., Yin J.D., Fang Q., Li D.F. Transfer of conjugated linoleic acid from sows to their offspring and its impact on the fatty acid profiles of plasma, muscle, and subcutaneous fat in piglets. J Anim Sci. 2010;88(5):1741–1751. doi: 10.2527/jas.2009-2354. [DOI] [PubMed] [Google Scholar]
- Peters G.J., van Groeningen C.J., Laurensse E.J., Lankelma J., Leyva A., Pinedo H.M. Uridine-induced hypothermia in mice and rats in relation to plasma and tissue levels of uridine and its metabolites. Cancer Chemother Pharmacol. 1987;20(2):101–108. doi: 10.1007/BF00253962. [DOI] [PubMed] [Google Scholar]
- Plante P.A., Laforest J.P., Farmer C. Effect of supplementing the diet of lactating sows with NuPro® on sow lactation performance and piglet growth. Canadian Veterinary Journal La Revue Veterinaire Canadienne. 2011;91(2):295–300. [Google Scholar]
- Ramiro A.R., Barreto V.M. Activation-induced cytidine deaminase and active cytidine demethylation. Trends Biochem Sci. 2015;40(3):172–181. doi: 10.1016/j.tibs.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Ricardo U., Quan R., Angel G. Role of nucleotides in intestinal development and repair: implications for infant nutrition. J Nutr. 1994;124(8 Suppl):1436S–1441S. doi: 10.1093/jn/124.suppl_8.1436S. [DOI] [PubMed] [Google Scholar]
- Richardson U.I., Watkins C.J., Pierre C., Ulus I.H., Wurtman R.J. Stimulation of CDP-choline synthesis by uridine or cytidine in PC12 rat pheochromocytoma cells. Brain Res. 2003;971(2):161–167. doi: 10.1016/s0006-8993(03)02333-3. [DOI] [PubMed] [Google Scholar]
- Roach P.J., Depaoli-Roach A.A., Hurley T.D., Tagliabracci V.S. Glycogen and its metabolism: some new developments and old themes. Biochem J. 2012;441(3):763–787. doi: 10.1042/BJ20111416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Pozo A., Morillas J., MoltóL, Robles R., Gil A. Dietary nucleotides influence lipoprotein metabolism in newborn infants. Pediatr Res. 1994;35(1):112–116. doi: 10.1203/00006450-199401000-00023. [DOI] [PubMed] [Google Scholar]
- Sangild P.T., Schmidt M., Elnif J., Björnvad C.R., Weström B.R., Buddington R.K. Prenatal development of gastrointestinal function in the pig and the effects of fetal esophageal obstruction. Pediatr Res. 2002;52(3):416–424. doi: 10.1203/00006450-200209000-00019. [DOI] [PubMed] [Google Scholar]
- Sato N., Murakami Y., Nakano T., Sugawara M., Kawakami H., Idota T., Nakajima I. Effects of dietary nucleotides on lipid metabolism and learning ability of rats. Biosci Biotechnol Biochem. 1995;59(7):1267–1271. doi: 10.1271/bbb.59.1267. [DOI] [PubMed] [Google Scholar]
- Sibley C.P., Brownbill P., Dilworth M., Glazier J.D. Review: adaptation in placental nutrient supply to meet fetal growth demand: implications for programming. Placenta. 2010;31(Suppl):S70–S74. doi: 10.1016/j.placenta.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Steculorum S., Paeger L., Bremser S., Evers N., Hinze Y., Idzko M., et al. Hypothalamic udp increases in obesity and promotes feeding via p2y6-dependent activation of agrp neurons. Cell. 2015;162(6):1404–1417. doi: 10.1016/j.cell.2015.08.032. [DOI] [PubMed] [Google Scholar]
- Sohail M.A., Cole D.J., Lewis D. Amino acid requirements of the breeding sow: the dietary lysine requirement during pregnancy. Br J Nutr. 1978;40(2):369–376. doi: 10.1079/bjn19780133. [DOI] [PubMed] [Google Scholar]
- Sun S., Meng Q., Luo Z., Shi B., Bi C., Shan A. Effects of dietary resveratrol supplementation during gestation and lactation of sows on milk composition of sows and fat metabolism of sucking piglets. J Anim Physiol Anim Nutr. 2019;103(3):813–821. doi: 10.1111/jpn.13064. [DOI] [PubMed] [Google Scholar]
- Suryawan A., Nguyen H.V., Almonaci R.D., Davis T.A. Abundance of amino acid transporters involved in mTORC1 activation in skeletal muscle of neonatal pigs is developmentally regulated. Amino Acids. 2013;45(3):523–530. doi: 10.1007/s00726-012-1326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tie H.M., Wu P., Jiang W.D., Liu Y., Kuang S.Y., Zeng Y.Y., et al. Dietary nucleotides supplementation affect the physicochemical properties, amino acid and fatty acid constituents, apoptosis and antioxidant mechanisms in grass carp (Ctenopharyngodon idellus) muscle. Aquaculture. 2019;502:312–325. [Google Scholar]
- Vitagliano L.A., Bonato M.A., Barbalho R., Santos G.D., Arajo L.F. Adsa-asas-csas Joint Meeting; 2014. Nucleotide supplementation in the diet of farrowing sows and its effect on milk quality, litter weight gain, and mortality. [Google Scholar]
- Waititu S.M., Yin F., Patterson R., Yitbareka A., Rodriguez-Lecompte J.C., Nyachoti C.M. Dietary supplementation with a nucleotide-rich yeast extract modulates gut immune response and microflora in weaned pigs in response to a sanitary challenge. Animal. 2017;11(12):2156–2164. doi: 10.1017/S1751731117001276. [DOI] [PubMed] [Google Scholar]
- Wellen K.E., Thompson C.B. A two-way street: reciprocal regulation of metabolism and signalling. Nat Rev Mol Cell Biol. 2012;13(4):270–276. doi: 10.1038/nrm3305. [DOI] [PubMed] [Google Scholar]
- Weldon W.C., Lewis A.J., Louis G.F., Kovar J.L., Miller P.S. Postpartum hypophagia in primiparous sows. 2. effects of feeding level during gestation and exogenous insulin on lactation feed-intake, glucose-tolerance, and epinephrine stimulated release of nonesterified fatty-acids and glucose. J Anim Sci. 1994;72(2):395–403. doi: 10.2527/1994.722395x. [DOI] [PubMed] [Google Scholar]
- Wu X., Xie C., Zhang Y., Fan Z., Yin Y., Blachier F. Glutamate-glutamine cycle and exchange in the placenta-fetus unit during late pregnancy. Amino Acids. 2015;47(1):45–53. doi: 10.1007/s00726-014-1861-5. [DOI] [PubMed] [Google Scholar]
- Wu X., Gao L., Liu Y., Xie C., Cai L., Xu K., et al. Maternal dietary uridine supplementation reduces diarrhea incidence in piglets by regulating the intestinal mucosal barrier and cytokine profiles. J Sci Food Agric. 2020;100(9):3709–3718. doi: 10.1002/jsfa.10410. [DOI] [PubMed] [Google Scholar]
- Wu G., Bazer F.W., Cudd T.A., Meininger C.J., Spencer T.E. Mother nutrition and fetal development. J Nutr. 2004;134(9):2169–2172. doi: 10.1093/jn/134.9.2169. [DOI] [PubMed] [Google Scholar]
- Wurtman R.J., Regan M., Ulus I., Yu L. Effect of oral CDP-choline on plasma choline and uridine levels in humans. Biochem Pharmacol. 2000;60(7):989–992. doi: 10.1016/s0006-2952(00)00436-6. [DOI] [PubMed] [Google Scholar]
- Xie C., Wu X., Long C., Wang Q., Fan Z., Li S., et al. Chitosan oligosaccharide affects antioxidant defense capacity and placental amino acids transport of sows. BMC Vet Res. 2016;12(1):243. doi: 10.1186/s12917-016-0872-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C., Wang Q., Li G., Fan Z., Wang H., Wu X. Dietary supplement with nucleotides in the form of uridine monophosphate or uridine stimulate the intestinal development and promote nucleotide transport in weaned piglets. J Sci Food Agric. 2019;99(13):6108–6113. doi: 10.1002/jsfa.9850. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Koyama H., Kurajoh M., Shoji T., Tsutsumi Z., Moriwaki Y. Biochemistry of uridine in plasma. Clin Chim Acta. 2011;412(19–20):1712–1724. doi: 10.1016/j.cca.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Moriwaki Y., Takahashi S., Tsutsumi Z., Higashino K. Effect of glucose on the plasma concentration and urinary excretion of uridine and purine bases. Metabolism. 1999;48(3):338–341. doi: 10.1016/s0026-0495(99)90082-3. [DOI] [PubMed] [Google Scholar]
- Zhang K., Liu Y., Zhang Y., Zhang J., Deng Z., Wu X., Yin Y. Dynamic oral administration of uridine affects the diurnal rhythm of bile acid and cholesterol metabolism-related genes in mice. Biol Rhythm Res. 2018;50(4):543–552. [Google Scholar]
- Zhang Y., Guo S., Xie C., Wang R., Zhang Y., Zhou X., et al. Short-Term oral UMP/UR administration regulates lipid metabolism in early-weaned piglets. Animals. 2019;9(9) doi: 10.3390/ani9090610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Niu Y., He J., Gan Z., Ji S., Zhang L., et al. Effects of dietary dihydroartemisinin supplementation on growth performance, hepatic inflammation, and lipid metabolism in weaned piglets with intrauterine growth retardation. Anim Sci J. 2020;91(1) doi: 10.1111/asj.13363. [DOI] [PubMed] [Google Scholar]
- Zhou J., Wang Z., Zhang S., Lin H., Gao C., Zhao J., et al. Methionine and its hydroxyl analogues improve stem cell activity to eliminate deoxynivalenol-induced intestinal injury by reactivating Wnt/beta-catenin signaling. J Agric Food Chem. 2019;67(41):11464–11473. doi: 10.1021/acs.jafc.9b04442. [DOI] [PubMed] [Google Scholar]