Abstract
Using the microwave-assisted method, novel Fe3O4/Zn-metal organic framework magnetic nanostructures were synthesized. The crystallinity, thermal stability, adsorption/desorption isotherms, morphology/size distribution, and magnetic hysteresis of synthesized Fe3O4/Zn-metal organic framework magnetic nanostructures were characterized by XRD patterns, TGA curve, BET adsorption/desorption technique, SEM image, and VSM curve, respectively. After confirming the Fe3O4/Zn-metal organic framework magnetic nanostructures, its antimicrobial properties against Gram-positive bacterial, Gram-negative bacterial, and fungal strains based on minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), and minimum fungicidal concentration (MFC) values were studied. The MIC values in antimicrobial activity for Gram-positive and Gram-negative bacterial strains, between 16–128 μg/ml, and for fungal strain, 128 μg/ml were observed. The results showed that the high specific surface area of Fe3O4/Zn-metal organic framework magnetic nanostructures caused the antimicrobial power of nanoparticles to be high, and the observed antimicrobial effects were higher than some known commercial antimicrobial drugs. Another advantage of the specific surface area of Fe3O4/Zn-metal organic framework magnetic nanostructures was its high catalytic properties in the three-component reaction of isatin, malononitrile, and dimedone. New spiro [indoline-pyranopyrimidines] derivatives were synthesized with high efficiency. The catalytic activity results of Fe3O4/Zn-metal organic framework magnetic nanostructures showed that, in addition to recyclability, derivatives could be synthesized in less time than previously reported methods. The results of investigating the catalytic activity of Fe3O4/Zn-metal organic framework magnetic nanostructures showed that the spiro [indoline-pyranopyrimidines] derivatives were synthesized in the time range of 10–20 min with an efficiency of over 85%. As a final result, it can be concluded that the microwave synthesis method improves the unique properties of magnetic nanostructures, especially its specific surface area, and has increased its efficiency.
Keywords: Fe3O4/Zn-metal organic framework magnetic nanostructures, microwave assisted, antimicrobial agent, MIC and MBC value, spiro[indoline-pyranopyrimidines
1 Introduction
Organometallic framework compounds with crystalline structures and unique properties have attracted the attention of chemists. These efficient nanostructures have been synthesized by different methods, and various applications have been reported for them (Ren et al., 2015; Al-Rowaili et al., 2018). These nanostructures have excellent physical and chemical properties, based on these properties; they show broad and diverse applications. High stability against heat, high porosity, high resistance on the surface, and high reactivity was the capabilities of these compounds (Ghanbari et al., 2020). Recently, the use of MOF nanostructures as a catalyst in synthesizing organic compounds, especially heterocycles and their derivatives, has been expanding (Pascanu et al., 2019; Dhameliya et al., 2020; Goetjen et al., 2020; Purohit et al., 2020). MOF compounds with magnetic properties have also been reported so far. The importance of these compounds in the synthesis of organic compounds is their easy separation after performing the reaction by a magnet (Yao et al., 2016; Ghorbani-Choghamarani and Taherinia, 2020). Biological properties such as enzyme immobilization, enantioselective hydrolysis of (R, S)-naproxen methyl ester, and immobilization of proline activated lipase from magnetic MOF compounds have been reports (Nadar and Rathod, 2018; Nadar and Rathod, 2020; Ozyilmaz et al., 2021).
In the synthesizing of organic compounds, spiro heterocycles play a unique role, and the synthesis of these compounds has been widely reported in modern chemistry (Fatahpour et al., 2017; Pogosyan et al., 2018). Investigations show that spiroheterocycle compounds have biological properties such as anticancer, antianaphylactic, anticoagulant, spasmolytic activities, and the synthesis procedure of these compounds has been considered due to these properties (Chen et al., 1999; Saraswat et al., 2016; Harichandran et al., 2018; Faroughi Niya et al., 2021; Al-Obaidi et al., 2022).
Pyrimidines are the main building blocks of nucleic acid. In addition, a literature review shows that pyrimidines have many biological properties such as anti-HIV agents, antitumor activity, anti-inflammatory activity, antimalarial activity, anti-microbial activity, antihypertensive activity, potassium channel antagonists, etc. (Bhat, 2017; Ahmad et al., 2021; Ahmad et al., 2022).
Pyran derivatives also have many biological properties. Biological properties such as anticancer (Sayed et al., 2019), antiviral activity (Morahan et al., 1972; Shehab and Ghoneim, 2016), antibacterial (Shehab and Ghoneim, 2016; Dutta et al., 2021), etc. Have also been reported from heterocyclic compounds containing pyran derivatives.
Pyranopyrimidines are one of the essential polycyclic heterocyclic compounds with high biological properties. Biological properties such as antimicrobial activities (Bedair et al., 2001), anti-HIV activity (Maddila et al., 2020), antioxidant activity (Yousefi et al., 2015), anticancer activity (Bhosle et al., 2019), and antitumor activity (Haggam et al., 2020) from polycyclic heterocyclic compounds contain pyranopyrimidines derivatives have been reported.
Multicomponent reactions are an essential method in synthesizing of heterocyclic, and organic compounds and many ways have been reported in this regard (Biswas and Das, 2022; Coppola et al., 2022; Wang et al., 2022). One of the crucial factors in multicomponent reactions is the selection of the appropriate catalyst. Several catalysts such as nanoparticles and magnetic nanoparticles have been reported to synthesize organic and heterocyclic compounds in multicomponent reactions (Amoozadeh et al., 2016; Chen et al., 2019; Ghorbani-Choghamarani et al., 2019; Harikrishna et al., 2020; Arlan et al., 2021).
Considering the importance of the synthesis of new nano compounds with high capabilities, in this research, novel Fe3O4/Zn-metal organic framework magnetic nanostructures were synthesized. After confirming their structure and determination of antimicrobial properties, they were used as magnetic catalysts in the three-component synthesis of new spiro [indoline-pyranopyrimidines] derivatives. Significant results of nanoparticles in antimicrobial and catalytic properties were observed.
2 Experimental
2.1 General
All reagents and solvents were purchased from Merck and Sigma without further purification. XRD pattern using a Philips XPERT PRO Cu-Kα radiation was performed. TGA curves in an N2 atmosphere, by Netzsch Thermal analyzer STA 409°at a heating rate of 10°C/min, were recorded. Hitachi S-4800 FESEM (Field Emission Scanning Electron Microscope) for SEM image was used. Vibrating Sample Magnetometer curves (VSM) by using Meghnatis Daghigh Kavir Co (Kashan, Iran), MDKB model were recorded. The FT-IR spectra were recorded by Nicolet AVATAR 360 FT-IR spectrophotometer. An advanced microwave synthesis lab station (MICROSYNTH, Milestone Co.) was used to synthesize Fe3O4/Zn-metal organic framework magnetic nanostructures. By Bruker FT-NMR Ultra Shield-spectrometer (300 and 75 MHz), the 1H- and 13C-NMR spectra were recorded. Uncorrected melting points of derivatives by KSP1N melting point meter of Krus’s type were determined.
2.2 Synthesis of Fe3O4/Zn-metal organic framework magnetic nanostructures by using microwave irradiation
For the synthesis of Fe3O4/Zn-metal organic framework magnetic nanostructures by microwave method, ZnCl2 (2°mmol), pyridine-2,6-dicarboxylic acid (4°mmol), and Fe3O4 nanoparticle (1°mmol), were added to the mixture including double-distilled water/acetic acid (30°ml, 1:1) and stirred quickly 15°min at 70 °C. In the next step, the mixture was subjected to microwave irradiation with a power of 350°W for 20°min at room temperature. Finally, by using a magnet, the Fe3O4/Zn-metal organic framework magnetic nanostructures were separated and washed several times with water and acetic acid and dried under vacuum at ambient temperature (Sargazi et al., 2018).
2.3 Antimicrobial activity of Fe3O4/Zn-metal organic framework magnetic nanostructures
To obtain the antimicrobial property of Fe3O4/Zn-metal organic framework magnetic nanostructures based on MIC, MBC, and MFC on Gram-negative, Gram-positive, and fungal strains, the clinical and laboratory standards institute (CLSI) guidelines M07-A9, M26-A, M02-A11, M44-A, and M27-A2, and previously reported methods were used (Moghaddam-Manesh et al., 2020; Abdieva et al., 2022; Zeraati et al., 2022).
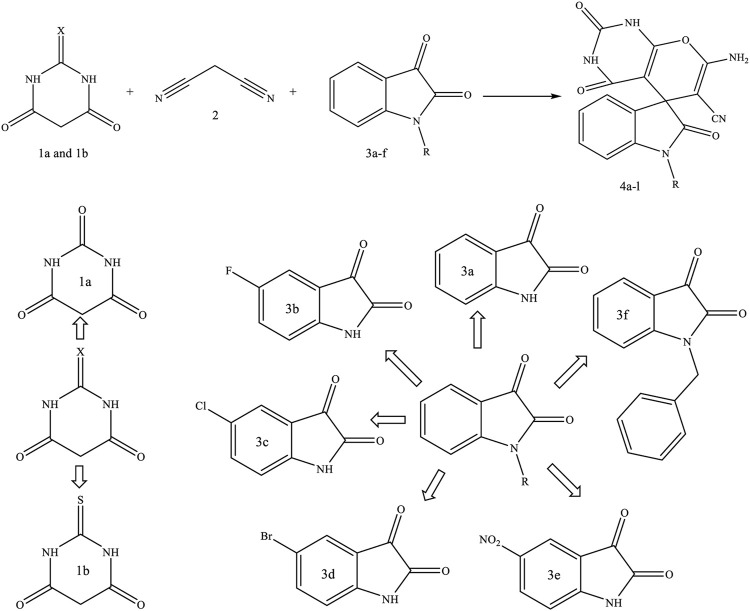
2.4 Synthesis of 1-benzylindoline-2,3-dione
By using indoline-2,3-dione, benzyl halide, potassium carbonate, and potassium iodide in acetonitrile and the method reported by Auria-Luna et al. Auria-Luna et al. (2015) and Tehrani et al. Tehrani et al. (2016) 1-benzylindoline-2,3-dione (3f) was synthesized. 1-Benzylindoline-2,3-dione was used as a reactant to synthesize new spiro [Indoline-pyranopyrimidine] derivatives.
2.5 General procedure for the synthesis of spiro [Indoline-pyranopyrimidine] derivatives
For the synthesis of spiro [indoline-pyranopyrimidines] derivatives, malononitrile (1 mmol), indoline-2,3-dione derivatives (1 mmol), barbituric acid or thiobarbituric acid (1 mmol), and 0.03 g catalyst (Fe3O4/Zn-metal organic framework magnetic nanostructures) added to 2 ml EtOH. The resultant was stirred at room temperature (optimal condition). The progress of the reaction was monitored by thin layer chromatography (TLC). After completion of the reaction, the catalyst was separated using a magnet. Finally, recrystallization of the mixture in water and ethanol was used to purify the sediments.
2.5.1 7′-amino-1-benzyl-2,2′,4′-trioxo-1′,2′,3′,4′-tetrahydrospiro [indoline-3,5′-pyrano [2,3-d]pyrimidine]-6′-carbonitrile (4K)
IR (KBr, ν, cm−1): 3290, 3350 (NH2), 3156 (NH), 2940 (CH), 2193 (CN), 1726 (CO), 1487 (C=C).
1H NMR (300 MHz, DMSO-d 6): δ 4.25 (s, 2H, CH2), 6.73 (d, 1H, J = 8.4 Hz, ArH), 6.88–6.89 (t, 1H, ArH), 7.01–7.12 (m, 4H, ArH), 7.24 (d, 2H, J = 8.4 Hz, ArH), 7.37–7.8 (t, 1H, ArH), 7.45 (s, 2H, NH2), 10.64 (s, 1H, NH), 12.41 (s, 1H, NH) ppm.
13C NMR (75 MHz, DMSO-d 6): δ 184.17, 163.29, 158.34, 154.07, 149.73, 140.86, 136.12, 134.57, 129.36, 128.75, 128.42, 127.46, 127. 55, 126.48, 124.55, 122.07, 117.45, 109.18, 88.52, 58.24, 55.01, 47.76 ppm.
Elemental analysis (C22H15N5O3S): Calculated; C, 61.53; H, 3.52; N, 16.31; S, 7.47. Found: C, 61.57; H, 3.49; N, 16.32; S, 7.50.
2.5.2 7′-amino-1-benzyl-2,4′-dioxo-2′-thioxo-1′,2′,3′,4′-tetrahydrospiro [indoline-3,5′-pyrano [2,3-d]pyrimidine]-6′-carbonitrile (4L)
IR (KBr, ν, cm−1): 3420, 3364 (NH2), 3171 (NH), 2958 (CH), 2195 (CN), 1717 (CO), 1522 (C=C).
1H NMR (300 MHz, DMSO-d 6): δ 4.32 (s, 2H, CH2), 6.77 (d, 1H, J = 8.7 Hz, ArH), 6.84–6.91 (t, 1H, ArH), 7.04–7.10 (m, 4H, ArH), 7.21 (d, 2H, J = 8.4 Hz, ArH), 7.30–7.37 (t, 1H, J = 8 Hz, ArH), 7.42 (s, 2H, NH2), 11.04 (s, 1H, NH), 12.01 (s, 1H, NH) ppm.
13C NMR (75 MHz, DMSO-d 6): δ 172.38, 162.45, 158.02, 154.17, 149.28, 141.01, 135.74, 134.28, 129.37, 128.64, 128.31, 127.31, 127. 01, 126.29, 124.29, 122.76, 117.69, 109.47, 88.31, 58.44, 54.67, 47.23 ppm.
Elemental analysis (C22H15N5O4): Calculated; C, 63.92; H, 3.66; N, 16.94; O, 15.48. Found: C, 63.90; H, 3.69; N, 16.95, O, 15.46.
3 Results and discussion
3.1 Characterization of Fe3O4/Zn-metal organic framework magnetic nanostructures
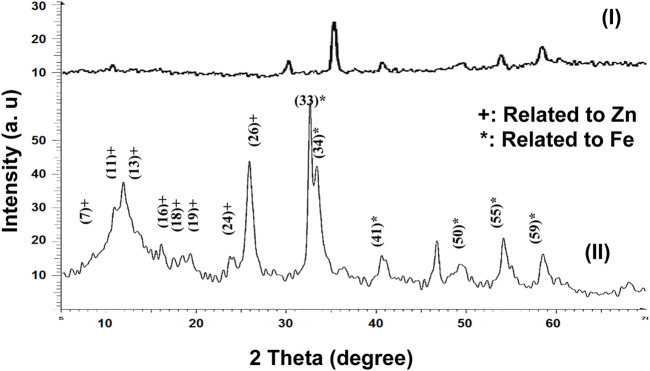
The pattern given in Figure 1 showed the XRD pattern of Fe3O4/Zn-metal organic framework magnetic nanostructures. The obtained XRD pattern was similar to the standard pattern reported for zinc and Fe3O4 nanoparticles (Hosseinzadegan et al., 2020; Rafiee, 2021; Zeraati et al., 2022). The calculation of Debby Scherer’s equation for Fe3O4/Zn-metal organic framework magnetic nanostructures showed that the size of the synthesized nanoparticles is 25 nm, which proves the importance of the synthesis method using microwaves.
FIGURE 1.
XRD patterns of Fe3O4 (I) Fe3O4/Zn-metal organic framework magnetic nanostructures (II).
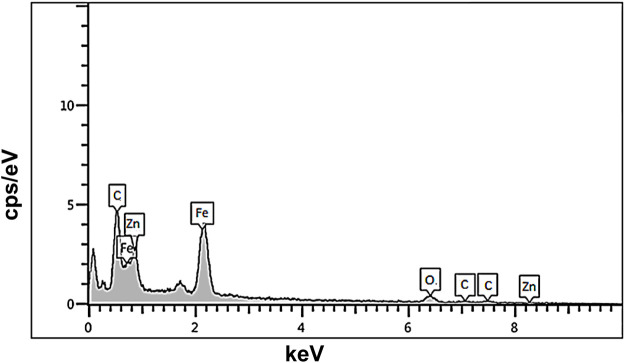
In Figure 2, EDX spectrum of Fe3O4/Zn-metal organic framework magnetic nanostructures were given which confirmed the successful synthesis of products.
FIGURE 2.
EDX spectrum of Fe3O4/Zn-metal organic framework magnetic nanostructures.
Based on the EDX spectrum of Fe3O4/Zn-metal organic framework magnetic nanostructures, the elements in the raw materials, including C, Fe, Zn, and O, were observed in the final product.
The results obtained from the thermal stability curve of Fe3O4/Zn-metal organic framework magnetic nanostructures show that in the temperature range of 90–100 °C, the partial weight decreases due to the evaporation of the solvent on the surface of the sample (Figure 3). The partial weight loss in the second stage in the temperature range of about 190 C is related to the evaporation of the solvent trapped in the nanoparticle structure. In the third stage, a noticeable weight loss in the sample was observed first in the range of 392 C of the pure Fe3O4/Zn-metal organic framework magnetic nanostructures. Then, at near 600 C, we see the beginning of the degradation and collapse of the final Fe3O4/Zn-metal organic framework magnetic nanostructures. From the observations, it can be concluded that Fe3O4/Zn-metal organic framework magnetic nanostructures had high thermal stability. The high thermal stability can be provided the advantages and potential applications of these synthesized compounds in the optimal microwave method.
FIGURE 3.
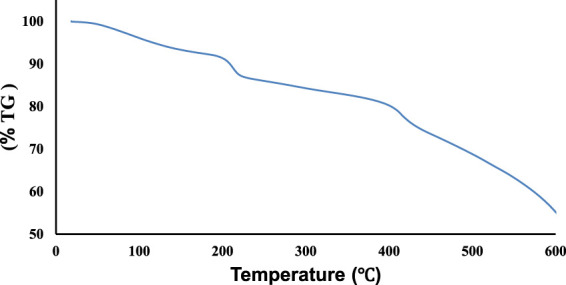
Thermal stability curve of Fe3O4/Zn-metal organic framework magnetic nanostructures.
The following graphs (Figure 4) were obtained using N2 adsorption and desorption techniques from core-shell nanostructures of Fe3O4/Zn-metal organic framework magnetic nanostructures. The BET diagram of Fe3O4/Zn-metal organic framework magnetic nanostructures shows that the effective specific surface area was corresponded to the final core-shell nanostructure.
FIGURE 4.
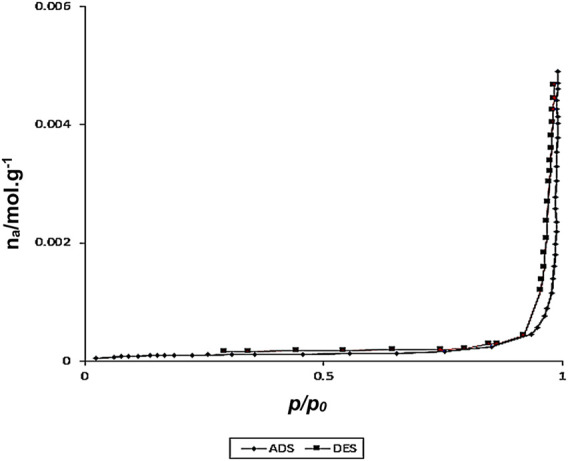
N2 adsorption/desorption isotherm of Fe3O4/Zn-metal organic framework magnetic nanostructures.
The specific surface area for the Fe3O4/Zn-metal organic framework magnetic nanostructures was about 37,500 m2/g. The high specific surface area is an essential factor in the effectiveness of nanoparticles in catalytic reactions and biological properties. It can be related that the use of microwave method in the synthesis of these structures was an important reason for the high specific surface as well as high thermal stability of Fe3O4/Zn-metal organic framework magnetic nanostructures.
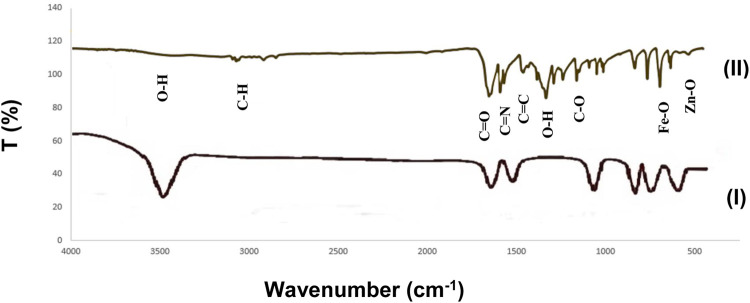
The figure below (Figure 5) shows the FTIR spectrum of Fe3O4/Zn-metal organic framework magnetic nanostructures synthesized by the microwave route. According to the obtained spectrum, the broad peak in the 3500 cm−1 was related to water molecules and OH groups coordinated to the Fe3O4/Zn-metal organic framework magnetic nanostructures. The absorption around the 3100 and 2900 cm−1 region is related to the C-H stretching bond in the aromatic ring. The frequency in the region of about 1653 cm−1 corresponds to the -COO- group present in the final structure of Fe3O4/Zn-metal organic framework magnetic nanostructures. The peaks in the region ∼1590 cm−1 were due to stretching bond of C=N, C=C stretching bond appears in 1450 cm−1, the peak in area 1335 cm−1 for O-H bending, C-O groups appear in 1162 cm−1. The peck in rejoins 630 cm−1 was related to Fe-O (Togashi et al., 2011), and finally, the peak in rejoin 500 cm−1 was attributed to Zn-O (Rafiee, 2021).
FIGURE 5.
FTIR spectrum of Fe3O4 (I) and Fe3O4/Zn-metal organic framework magnetic nanostructures (II).
Figure 6 shows the SEM image and particle size histogram of Fe3O4/Zn-metal organic framework magnetic nanostructures. It seems that development of the microwave method with optimal conditions has led to the production of the MOF sample with uniform morphology and high stability surface. According to the SEM and size histogram, no effect of agglomeration of particles were observed, but nanoparticles were observed in a one-dimensional form (average particle size of 24 nm) with a clear correlation which can be attributed to the use of the efficient microwave synthesis method. The evidence shows that the type of synthesis method has a significant effect on the morphology and particle size distribution. As an important result, synthesis of nanostructures with uniform size distribution and homogeneous surfaces have special applications in medical science.
FIGURE 6.
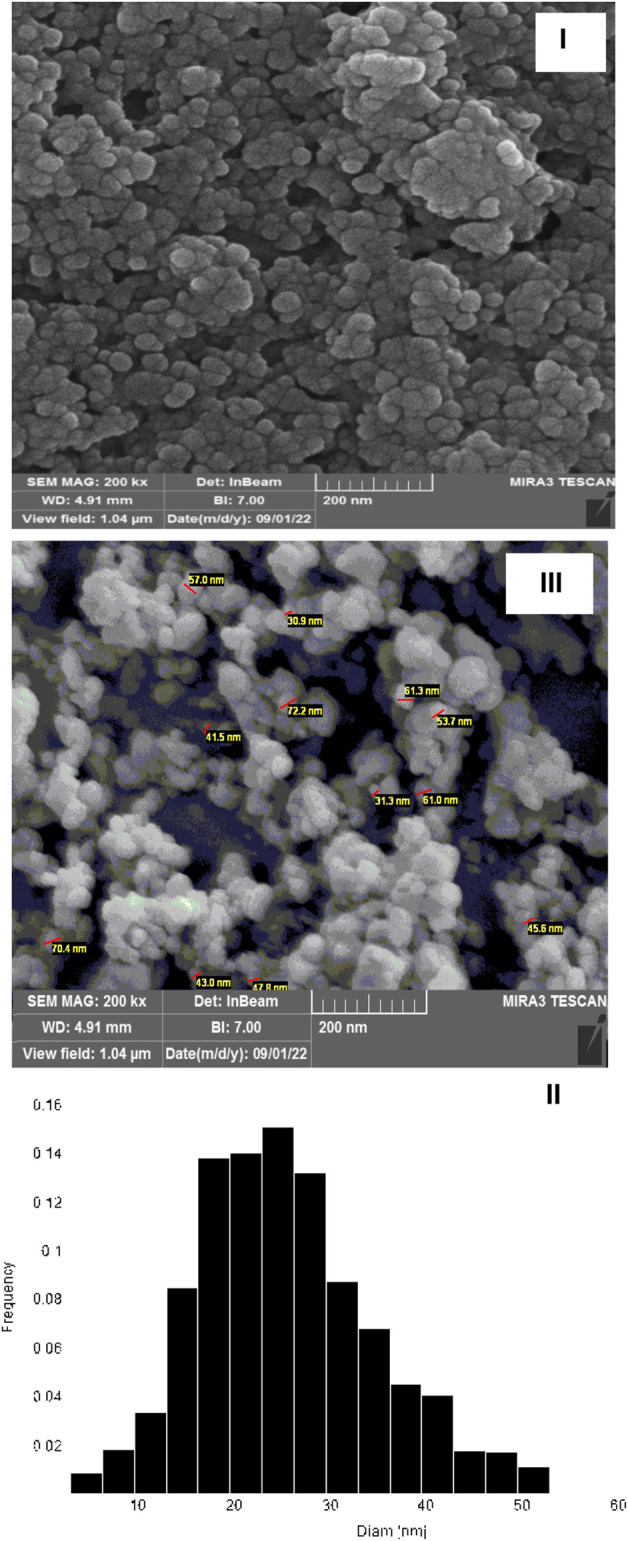
SEM image (I) and size histogram (II) of Fe3O4/Zn-metal organic framework magnetic nanostructures and SEM image of Fe3O4 (III).
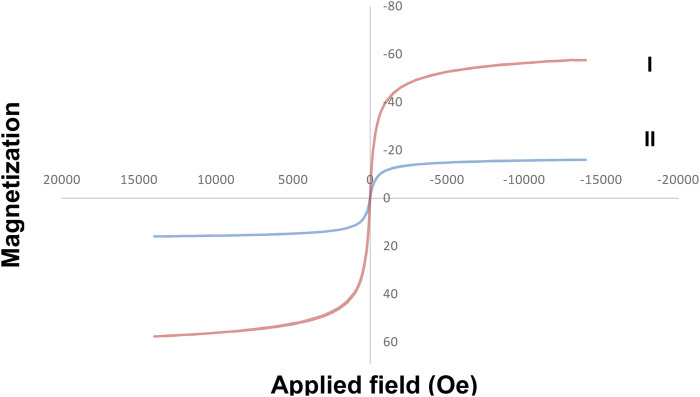
The figure below (Figure 7) shows the magnetic property of Fe3O4/Zn-metal organic framework magnetic nanostructures. The magnetic property for Fe3O4 MNPs 57 emu/g was reported (Shiri et al., 2018). The magnetic property of Fe3O4/Zn-metal organic framework magnetic nanostructures 16.1 emu/g was obtained and proved that the core (Fe3O4) were covered with Zn-metal organic framework magnetic nanostructures as a shell. The importance of magnetic properties in the synthesized nanostructures was revealed when they were separated from the reaction medium. The magnetic property makes the catalyst easily separated by a magnet after the reaction is finished.
FIGURE 7.
Magnetic property curve of Fe3O4 (I) and Fe3O4/Zn-metal organic framework magnetic nanostructures (II).
Based on the obtained spectral data, the structure of Figure 8 was proposed for Fe3O4/Zn-metal organic framework magnetic nanostructures.
FIGURE 8.
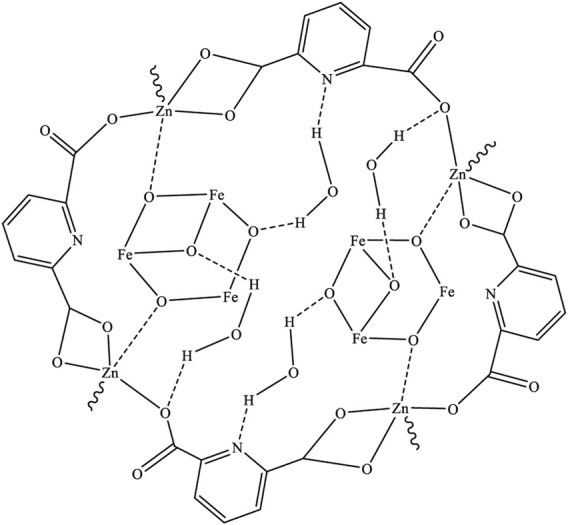
Proposed structure for Fe3O4/Zn-metal organic framework magnetic nanostructures.
3.2 Antimicrobial activity of Fe3O4/Zn-metal organic framework magnetic nanostructures
In the investigation of antimicrobial activities, Gram-negative strains including, Pseudomonas aeruginosa and Shigella dysenteriae; Gram-positive bacteria strains including, Rhodococcus equi and Streptococcus agalactiae; Fungi including, Candida albicans were used.
Investigations showed that nanoparticles were effects on all Gram-positive and Gram-negative bacterial and fungal strains studied. The obtained results were given in Table 1.
TABLE 1.
Antibacterial and Antifungal activity of Fe3O4/Zn-metal organic framework magnetic nanostructures.
| Sample | Gram-negative bacteria | Gram-positive bacteria | Fungi | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pseudomonas aeruginosa | Shigella dysenteriae | Rhodococcus equi | Streptococcus agalactiae | Candida albicans | ||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MFC | |
| (μg/ml) | (μg/ml) | (μg/ml) | (μg/ml) | (μg/ml) | ||||||
| A | 64 | 128 | 32 | 64 | 32 | 64 | 16 | 32 | 128 | 256 |
| B | 32 | 64 | 0.5 | 1 | 4 | 16 | - | - | 32 | 64 |
| C | - | - | - | - | - | - | 8 | 16 | - | - |
A: Fe3O4/Zn-metal organic framework magnetic nanostructures; B: gentamicin for bacteria, Terbinafine for Fungi; C: cefazolin for bacteria, Tolnaftate for Fungi.
Fe3O4/Zn-metal organic framework magnetic nanostructures were effective against all study bacterial and fungal strains, and MBC values of 32–256 μg/ml were obtained. The effectiveness of Fe3O4/Zn-metal organic framework magnetic nanostructures against Gram-positive strains was more than Gram-negative strains and fungi. The effectiveness of Fe3O4/Zn-metal organic framework magnetic nanostructures was compared with the efficacy of commercial drugs in the market. More effectiveness of nanoparticles compared to drugs was observed. In general, the efficacy of Fe3O4/Zn-metal organic framework magnetic nanostructures can be attributed to their high specific surface that engages with bacterial and fungal strains.
3.3 Synthesis of spiro [indoline-pyranopyrimidines] derivatives by Fe3O4/Zn-metal organic framework magnetic nanostructures
In this study, based on Scheme 1, using Fe3O4/Zn-metal organic framework magnetic nanostructures as magnetic nanocatalyst during the three-component reaction of malononitrile, indoline-2,3-dione derivatives, and barbituric acid or thiobarbituric acid, new spiro [indoline-pyranopyrimidines] derivatives were synthesized.
SCHEME 1.
Fe3O4/Zn-metal organic framework magnetic nanostructures as magnetic nanocatalyst in synthesis spiro [indoline-pyranopyrimidines] derivatives.
SCHEME 2.
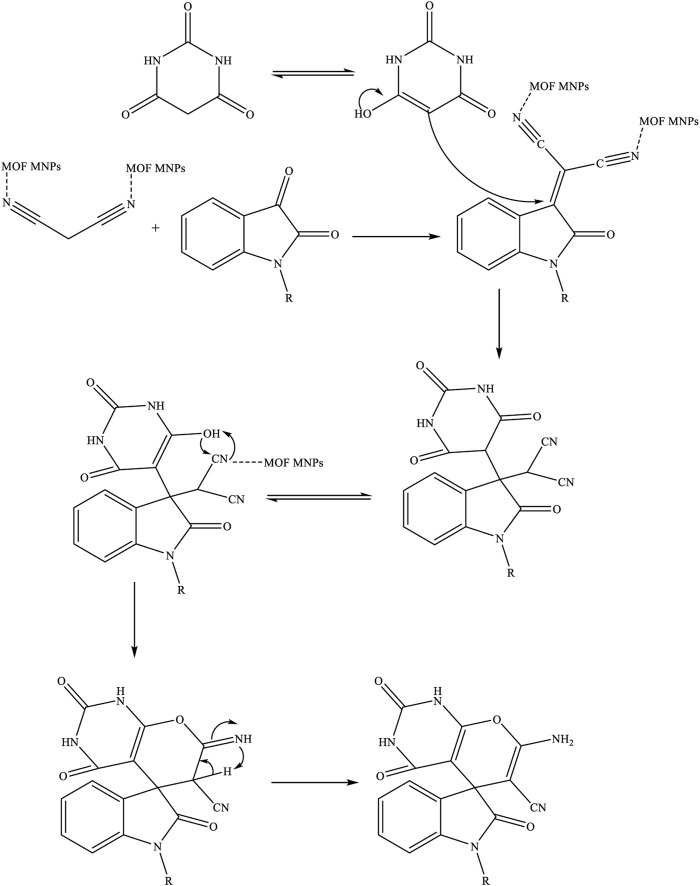
Proposed mechanisms for the synthesis of spiro [indoline-pyranopyrimidines] derivatives by Fe3O4/Zn-metal organic framework magnetic nanostructures
For the synthesis of spiro [indoline-pyranopyrimidines] derivatives, the optimal conditions of solvent, amount of catalyst, and temperature were studied according to Table 2 (for compound 4a).
TABLE 2.
Determination of optimal conditions in synthesis of spiro [indoline-pyranopyrimidines] derivatives.
| Product | Solvent | Catalyst (g) | Temperature (oC) | Time (min) | Yield (%) | TON | TOF (min−1) |
|---|---|---|---|---|---|---|---|
| 4a | EtOH | 0.01 | r. t | 30 | 81 | 45.76 × 105 | 152,500 |
| 4a | H2O | 0.01 | r. t | 60 | 35 | 19.77 × 105 | 32,950 |
| 4a | H2O:EtOH (1:1) | 0.01 | r. t | 30 | 72 | 40.68 × 105 | 135,600 |
| 4a | MeOH | 0.01 | r. t | 60 | 64 | 36.16 × 105 | 60,270 |
| 4a | EtOH | 0.02 | r. t | 30 | 89 | 25.21 × 105 | 84,030 |
| 4a | EtOH | 0.03 | r. t | 10 | 97 | 18.30 × 105 | 183,000 |
| 4a | EtOH | 0.04 | r. t | 10 | 94 | 13.30 × 105 | 133,000 |
| 4a | EtOH | 0.05 | r. t | 10 | 88 | 9.97 × 105 | 99,700 |
| 4a | EtOH | 0.03 | 40 | 10 | 90 | 16.98 × 105 | 169,800 |
| 4a | EtOH | 0.03 | 50 | 10 | 83 | 15.66 × 105 | 156,600 |
The results of ICP showed that 0.03 g of Fe3O4/Zn-metal organic framework magnetic nanostructures contain ×3.4710–3 g of zinc or 5.3 × 10–5 mol or Zn, therefore in optimal conditions, TON and TOF were obtained, 18×105 and 180,000 min−1, respectively.
According to Table 3, using optimal conditions studied in Table 1 (EtOH as a solvent, 0.03 mg of Fe3O4/Zn-metal organic framework magnetic nanostructures and room temperature), 12 spiro [indoline-pyranopyrimidines] derivatives were synthesized, and derivatives 4k and 4L were novel and reported for the first time.
TABLE 3.
Synthesized derivatives of spiro [indoline-pyranopyrimidines] derivatives (4a-l) by Fe3O4/Zn-metal organic framework magnetic nanostructures.
| Product | Structure | Time (min) | Yield (%) | TON | TOF (min−1) | M.p. (°C) | |
|---|---|---|---|---|---|---|---|
| Found | Reported | ||||||
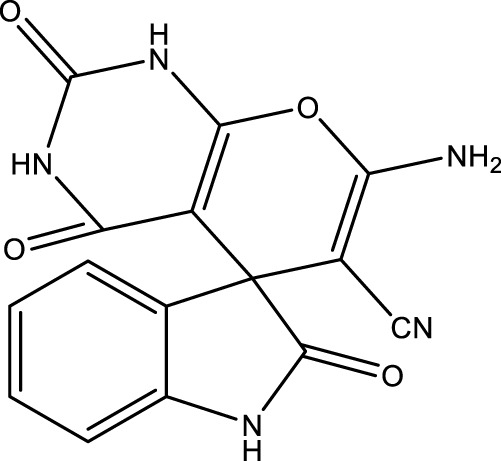
|
10 | 97 | 18.30 × 105 | 183,000 | 272–275 | 275 (Keshavarz and Farahi, 2022) | |
| b |
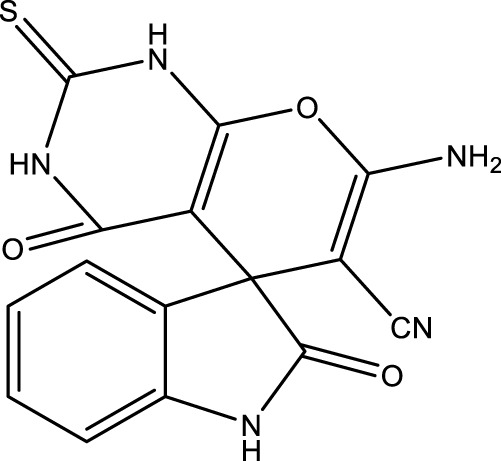
|
12 | 96 | 18.11 × 105 | 150,900 | 241–242 | 240–242 (Azimi and Lasemi, 2022) |
| 4c |
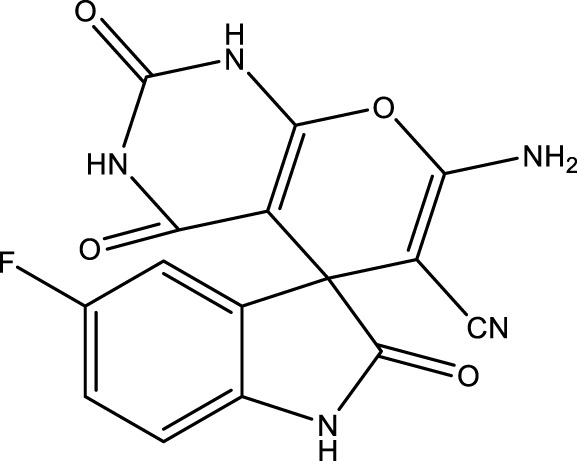
|
12 | 91 | 17.17 × 105 | 143,080 | 235–237 | 235 (Joshi et al., 1988) |
| 4d |
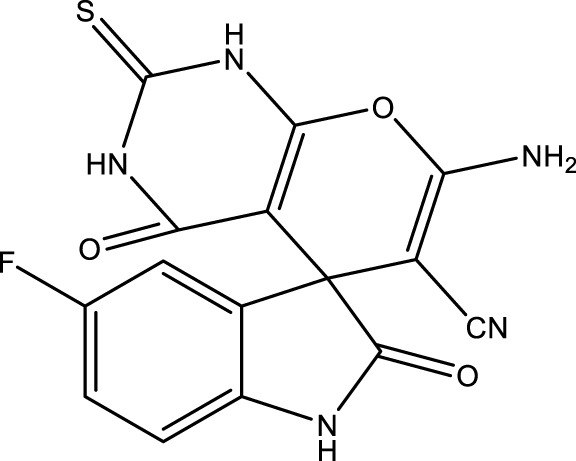
|
15 | 92 | 17.36 × 105 | 115,730 | 255–256 | 252–254 (Keshavarz, 2016) |
| 4e |
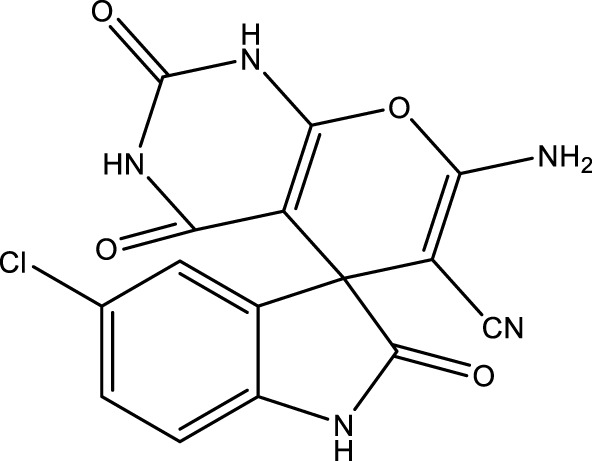
|
13 | 94 | 17.74 × 105 | 136,460 | 241–244 | 242–245 (Dadaei and Naeimi, 2021) |
| 4f |
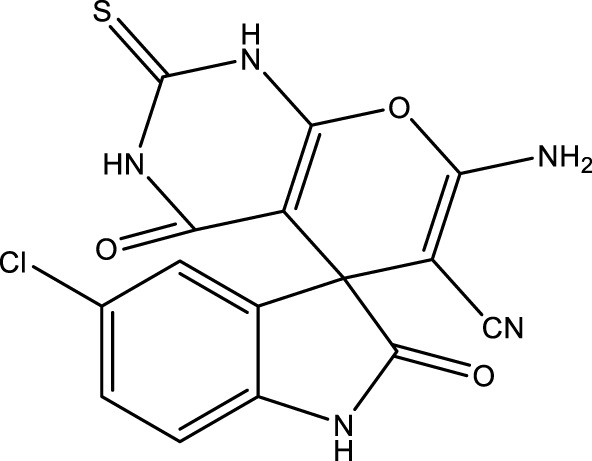
|
13 | 93 | 17.55 × 105 | 135,000 | 231–232 | 228–230 (Dadaei and Naeimi, 2021) |
| 4g |
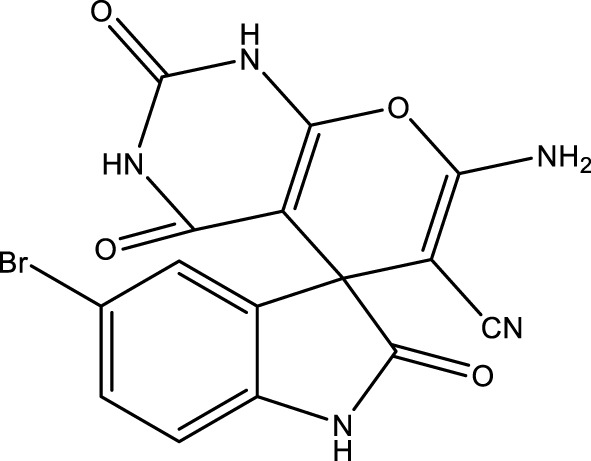
|
15 | 90 | 16.98 × 105 | 113,200 | 262–264 | 263–265 (Bodaghifard and Mousavi, 2020) |
| 4h |
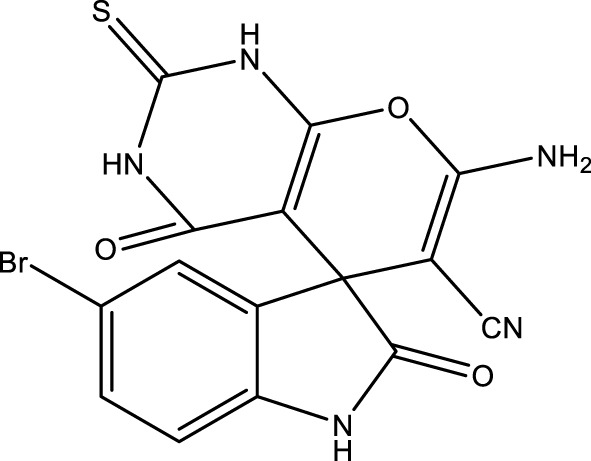
|
15 | 92 | 17.35 × 105 | 115,600 | 240–243 | 243–245 (Bodaghifard and Mousavi, 2020) |
| 4i |
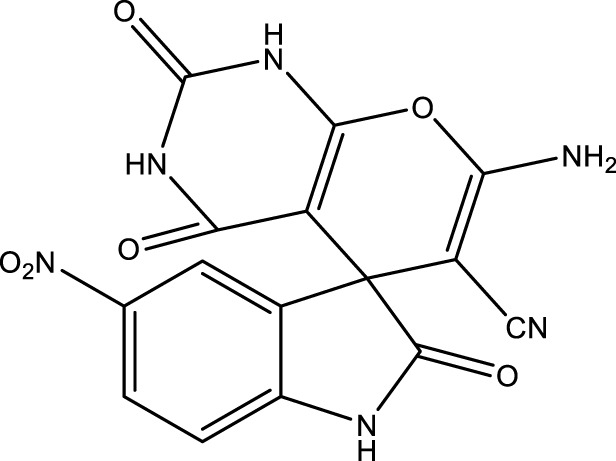
|
14 | 94 | 17.74 × 105 | 126,700 | 285–287 | 288–289 (Keshavarz, 2016) |
| 4j |
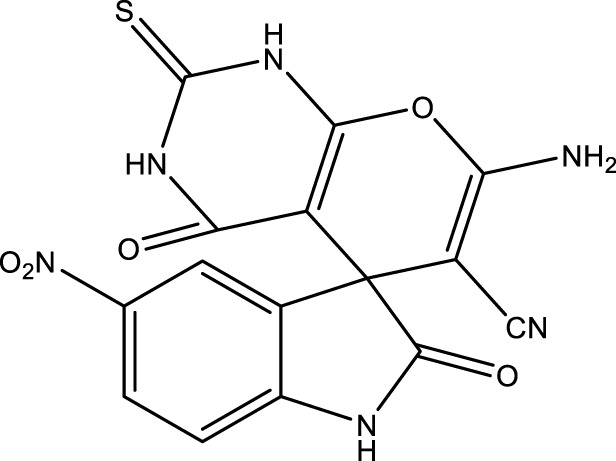
|
15 | 95 | 17.92 × 105 | 119,460 | 249–252 | 252–254 (Azimi and Lasemi, 2022) |
| 4k |
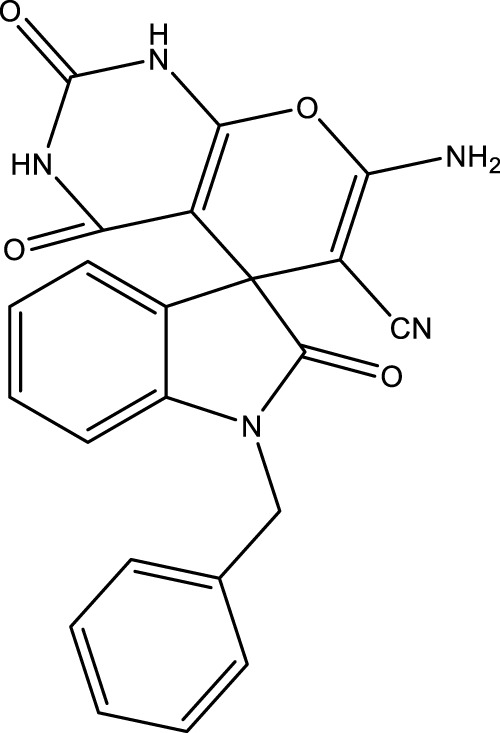
|
17 | 89 | 16.79 × 105 | 98,760 | 279–280 | In this study |
| 4L |
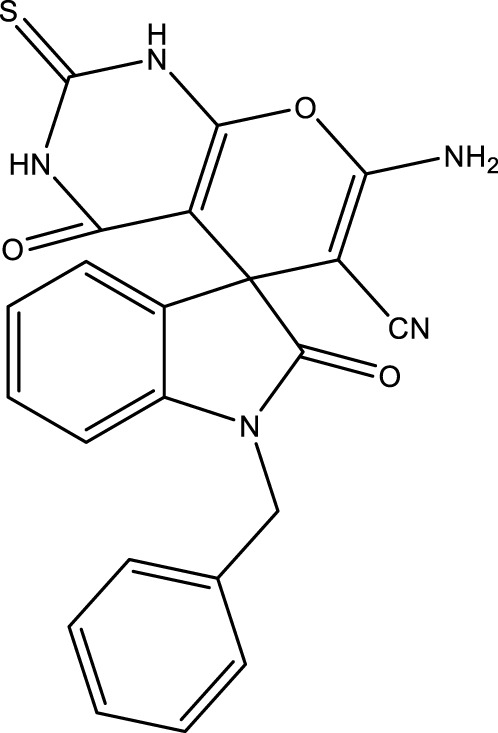
|
20 | 85 | 16.04 × 105 | 80,200 | 286–288 | In this study |
The proposed mechanism for synthesizing spiro [indoline-pyranopyrimidines] derivatives using Fe3O4/Zn-metal organic framework magnetic nanostructures as a magnetic nano-catalyst was given in Scheme 1.
There have been several reports of the three-component reaction of malononitrile, indoline-2,3-dione derivatives, and barbituric acid or thiobarbituric acid for the synthesis of spiro [indoline-pyranopyrimidines] derivatives. Some of them that were reported recently, were listed in Table 4 and compared with this study.
TABLE 4.
Reported methods for the synthesis of compound 4a.
| Entry | Catalyst | Time (min) | Temperature (°C)/Condition | Yield (%) |
|---|---|---|---|---|
| 1 | Silica-supported organocatalyst | 60 | 80 | 98 (Khalafi-Nezhad et al., 2013) |
| 2 | Glutathione functionalized Fe3O4 nanoparticles | 15 | 80 | 97 (Jamatia et al., 2016) |
| 3 | Tin dioxide in ethanol | 90 | 20 | 96 (Moradi et al., 2019) |
| 4 | C3H6N6*3C5H9NO2 | 10 | 20 | 95 (Nagaraju et al., 2017) |
| 5 | Pyridine-2,3-dicarboxylic acid | 6 | 70 | 95 (Oudi et al., 2019) |
| 6 | Zinc (II) immobilized on poly (ureaformaldehyde)-functionalized silica-coated CoFe2O4 | 30 | Reflux (water) | 91 (Bodaghifard and Mousavi, 2020) |
| 7 | 1-Amino-2-naphthol-4-sulfonic acid supported on magnetic nano-CoFe2O4 nanocatalyst | 10 | 40 | 89 (Faroughi Niya et al., 2021) |
| 8 | Fe3O4/Zn-metal organic framework magnetic nanostructures (this work) | 10 | r.t | 97 |
The comparison between the results proves that the catalyst of this study has better conditions for the synthesis of derivatives and high efficiency, and less time was its advantages.
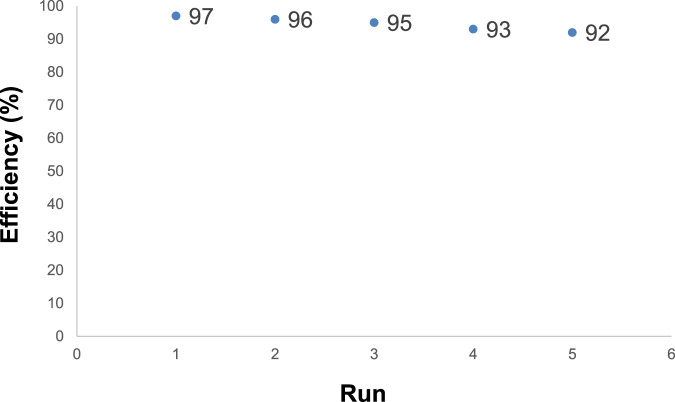
Magnetic Fe3O4/Zn-metal organic framework magnetic nanostructures showed significant recycling properties. The Fe3O4/Zn-metal organic framework magnetic nanostructures, after acting as a catalyst, were collected by a magnet, and washed several times with water and ethanol then reused in the reaction. The results of Figure 9 showed that Fe3O4/Zn-metal organic framework magnetic nanostructures could be reused up to 5 times.
FIGURE 9.
Fe3O4/Zn-metal organic framework magnetic nanostructures reusability in the synthesis of compound 4a.
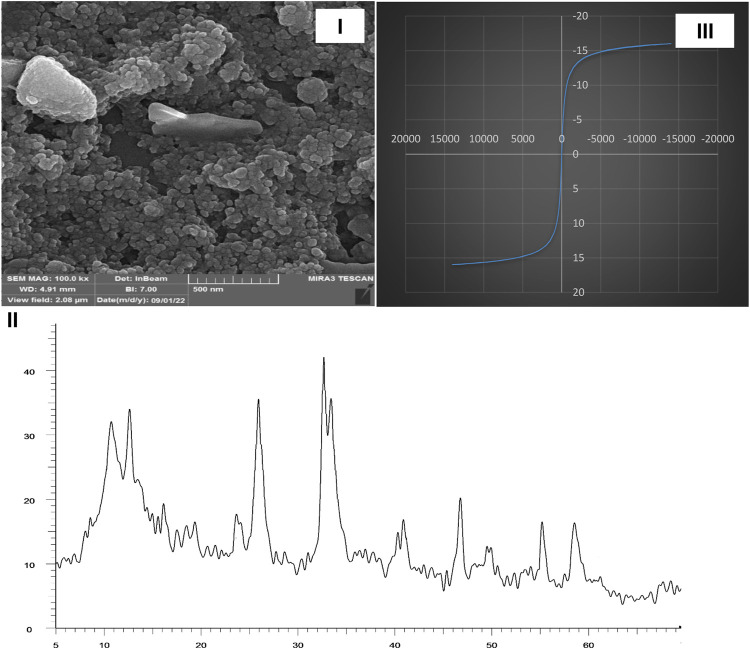
A hot filtration test was done based on previous reports, and no enhancement in conversion was noticed in the filtrate (Babaei and Mirjalili, 2020). Characterization data such as SEM, XRD, and VSM from catalyst after recycling was done, and it was confirmed that the structure of the Fe3O4/Zn-metal organic framework magnetic nanostructures was the same as before recycling (Figure 10).
FIGURE 10.
SEM (I), XRD (II), and VSM (III) of Fe3O4/Zn-metal organic framework magnetic nanostructures reusability in the synthesis of compound 4a.
4 Conclusion
In short, in this research, new Fe3O4/Zn-metal organic framework magnetic nanostructures were synthesized using the microwave method and characterization of their structure. It seems that the microwave irradiation synthesis route has a significant effect on the particle size distribution, and morphology, increased specific surface area, and heat stability of samples. In fact, this efficient route can produce samples in a short time with uniform morphology. The effect of the microwave synthesis routes on the morphology and particle-sized distribution is in compared with previous studies.
The high specific surface area of the synthesized nanoparticles made it have high catalytic properties and novel spiro [indoline-pyranopyrimidines] derivatives were synthesized with higher efficiency and less synthesis time than previously reported methods. In this study, spiro [indoline-pyranopyrimidines] derivatives in the 10–20 min with an efficiency of over 85% were synthesized.
Another advantage of the specific surface area of Fe3O4/Zn-metal organic framework magnetic nanostructures was its high antimicrobial properties. In antimicrobial activity on Gram-positive and Gram-negative bacterial strains, MIC values between 16–128 μg/ml, and for fungal strain, MIC value of 128 μg/ml were observed. The results obtained on antibacterial and antifungal activity proved that, Fe3O4/Zn-metal organic framework magnetic nanostructures, in some cases, had more effective than commercial drugs.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through the Research Group Program under grant number RGP. 2/195/43.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.1014731/full#supplementary-material
References
- Abdieva G. A., Patra I., Al-Qargholi B., Shahryari T., Chauhan N. P. S., Moghaddam-Manesh M. (2022). An efficient ultrasound-assisted synthesis of Cu/Zn hybrid MOF nanostructures with high microbial strain performance. Front. Bioeng. Biotechnol. 10, 861580. 10.3389/fbioe.2022.861580 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ahmad N., Almahdawi S., Alshehri A., Shkir M., Ahmad I., Hasan P., et al. (2022). Facile low temperature synthesis of homogeneous CuS nanosheets: An effect of Ga loading on structural, optical, nonlinear and antimicrobial properties. Mater. Chem. Phys. 277, 125552. 10.1016/j.matchemphys.2021.125552 [DOI] [Google Scholar]
- Ahmad N., Alshehri A., Ahmad I., Shkir M., Hasan P., Melaibari A. A. (2021). In doping effect on the structural, morphological, optical and enhanced antimicrobial activity of facilely synthesized novel CuS nanostructures. Surfaces Interfaces 27, 101536. 10.1016/j.surfin.2021.101536 [DOI] [Google Scholar]
- Al-Obaidi Z. M. J., Hussein Y. A., Al-Duhaidahawi D., Al-Aubaidy H. A., et al. (2022). Molecular docking studies and biological evaluation of luteolin on cerebral ischemic reperfusion injury. Egypt. J. Chem. 65, 1–2. 10.21608/ejchem.2021.106753.4898 [DOI] [Google Scholar]
- Al-Rowaili F. N., Jamal A., Ba-Shammakh M. S., Rana A. (2018). A review on recent advances for electrochemical reduction of carbon dioxide to methanol using metal–organic framework (MOF) and non-MOF catalysts: Challenges and future prospects. ACS Sustain. Chem. Eng. 6, 15895–15914. 10.1021/acssuschemeng.8b03843 [DOI] [Google Scholar]
- Amoozadeh A., Rahmani S., Bitaraf M., Abadi F. B., Tabrizian E. (2016). Nano-zirconia as an excellent nano support for immobilization of sulfonic acid: A new, efficient and highly recyclable heterogeneous solid acid nanocatalyst for multicomponent reactions. New J. Chem. 40, 770–780. 10.1039/c5nj02430g [DOI] [Google Scholar]
- Arlan F. M., Marjani A. P., Javahershenas R., Khalafy J. (2021). Recent developments in the synthesis of polysubstituted pyridines via multicomponent reactions using nanocatalysts. New J. Chem. 45, 12328–12345. 10.1039/d1nj01801a [DOI] [Google Scholar]
- Auria-Luna F., Marqués-López E., Mohammadi S., Heiran R., Herrera R. P. (2015). New organocatalytic asymmetric synthesis of highly substituted chiral 2-oxospiro-[indole-3, 4′-(1′, 4′-dihydropyridine)] derivatives. Molecules 20, 15807–15826. 10.3390/molecules200915807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimi R., Lasemi Z. (2022). Environmentally friendly synthesis of Ag/SiO2 nanoparticles using Thymus kotschyanus extract and its application as a green catalyst for synthesis of spirooxindoles. Res. Chem. Intermed. 48, 1615–1630. 10.1007/s11164-022-04667-z [DOI] [Google Scholar]
- Babaei E., Mirjalili B. B. F. (2020). Fe3O4@ nano-dextrin/Ti (IV) as a bio-based magnetic nano-catalyst for facile synthesis of 2, 3-dihydroquinazolin-4 (1H)-ones. Iran. J. Catal. 10, 219–226. [Google Scholar]
- Bedair A. H., Emam H. A., El-Hady N. A., Ahmed K. A., El-Agrody A. M. (2001). Synthesis and antimicrobial activities of novel naphtho [2, 1-b] pyran, pyrano [2, 3-d] pyrimidine and pyrano [3, 2-e] [1, 2, 4] triazolo [2, 3-c]-pyrimidine derivatives. Il Farm. 56, 965–973. 10.1016/s0014-827x(01)01168-5 [DOI] [PubMed] [Google Scholar]
- Bhat A. R. (2017). Biological activity of pyrimidine derivativies: A review. Org. Med. Chem. Int. J. 2, 23–26. 10.19080/omcij.2017.02.555581 [DOI] [Google Scholar]
- Bhosle M. R., Andil P., Wahul D., Bondle G. M., Sarkate A., Tiwari S. V. (2019). Straightforward multicomponent synthesis of pyrano [2, 3-d] pyrimidine-2, 4, 7-triones in β-cyclodextrin cavity and evaluation of their anticancer activity. J. Iran. Chem. Soc. 16, 1553–1561. 10.1007/s13738-019-01633-2 [DOI] [Google Scholar]
- Biswas S. K., Das D. (2022). One-pot synthesis of pyrano [2, 3-c] pyrazole derivatives via multicomponent reactions (MCRs) and their applications in medicinal chemistry. Mini. Rev. Org. Chem. 19, 552–568. 10.2174/1570193x19666211220141622 [DOI] [Google Scholar]
- Bodaghifard M. A., Mousavi Z. (2020). Zinc (II)-poly (urea-formaldehyde) supported on magnetic nanoparticles: A hybrid nanocatalyst for green synthesis of spiropyrans, spiroxanthenes, and spiropyrimidines. Appl. Organomet. Chem. 34, e5859. 10.1002/aoc.5859 [DOI] [Google Scholar]
- Chen M.-H., Pollard P. P., Patchett A. A., Cheng K., Wei L., Chan W. W.-S., et al. (1999). Synthesis and biological activities of spiroheterocyclic growth hormone secretagogues. Bioorg. Med. Chem. Lett. 9, 1261–1266. 10.1016/s0960-894x(99)00183-3 [DOI] [PubMed] [Google Scholar]
- Chen M.-N., Mo L.-P., Cui Z.-S., Zhang Z.-H. (2019). Magnetic nanocatalysts: Synthesis and application in multicomponent reactions. Curr. Opin. Green Sustain. Chem. 15, 27–37. 10.1016/j.cogsc.2018.08.009 [DOI] [Google Scholar]
- Coppola G. A., Pillitteri S., Van Der Eycken E. V., You S.-L., Sharma U. K. (2022). Multicomponent reactions and photo/electrochemistry join forces: Atom economy meets energy efficiency. Chem. Soc. Rev. 51, 2313–2382. 10.1039/d1cs00510c [DOI] [PubMed] [Google Scholar]
- Dadaei M., Naeimi H. (2021). Guanidine functionalized core–shell structured magnetic cobalt-ferrite: An efficient nanocatalyst for sonochemical synthesis of spirooxindoles in water. RSC Adv. 11, 15360–15368. 10.1039/d1ra00967b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhameliya T. M., Donga H. A., Vaghela P. V., Panchal B. G., Sureja D. K., Bodiwala K. B., et al. (2020). A decennary update on applications of metal nanoparticles (MNPs) in the synthesis of nitrogen-and oxygen-containing heterocyclic scaffolds. RSC Adv. 10, 32740–32820. 10.1039/d0ra02272a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A., Rahman N., Kumar J. E., Rabha J., Phukan T., Nongkhlaw R. (2021). Catalyst-free UV365-assisted synthesis of pyran annulated heterocyclic scaffolds and evaluation of their antibacterial activities. Synth. Commun. 51, 263–278. 10.1080/00397911.2020.1825741 [DOI] [Google Scholar]
- Faroughi Niya H., Hazeri N., Fatahpour M. (2021). Synthesis, characterization, and application of CoFe2O4@ amino-2-naphthol-4-sulfonic acid as a novel and reusable catalyst for the synthesis of spirochromene derivatives. Appl. Organomet. Chem. 35, e6119. 10.1002/aoc.6119 [DOI] [Google Scholar]
- Fatahpour M., Noori Sadeh F., Hazeri N., Maghsoodlou M. T., Lashkari M. (2017). Aspirin: An efficient catalyst for synthesis of bis (pyrazol-5-ols), dihydropyrano [2, 3-c] pyrazoles and spiropyranopyrazoles in an environmentally benign manner. J. Iran. Chem. Soc. 14, 1945–1956. 10.1007/s13738-017-1133-x [DOI] [Google Scholar]
- Ghanbari T., Abnisa F., Daud W. M. A. W. (2020). A review on production of metal organic frameworks (MOF) for CO2 adsorption. Sci. Total Environ. 707, 135090. 10.1016/j.scitotenv.2019.135090 [DOI] [PubMed] [Google Scholar]
- Ghorbani-Choghamarani A., Mohammadi M., Shiri L., Taherinia Z. (2019). Synthesis and characterization of spinel FeAl2O4 (hercynite) magnetic nanoparticles and their application in multicomponent reactions. Res. Chem. Intermed. 45, 5705–5723. 10.1007/s11164-019-03930-0 [DOI] [Google Scholar]
- Ghorbani-Choghamarani A., Taherinia Z. (2020). Fe3O4@ GlcA@ Cu-MOF: A magnetic metal–organic framework as a recoverable catalyst for the hydration of nitriles and reduction of isothiocyanates, isocyanates, and isocyanides. ACS Comb. Sci. 22, 902–909. 10.1021/acscombsci.0c00178 [DOI] [PubMed] [Google Scholar]
- Goetjen T. A., Liu J., Wu Y., Sui J., Zhang X., Hupp J. T., et al. (2020). Metal–organic framework (MOF) materials as polymerization catalysts: A review and recent advances. Chem. Commun. 56, 10409–10418. 10.1039/d0cc03790g [DOI] [PubMed] [Google Scholar]
- Haggam R. A., Assy M. G., Mohamed E. K., Mohamed A. S. (2020). Synthesis of pyrano [2, 3-d] pyrimidine-2, 4-diones and pyridino [2, 3-d] pyrimidine-2, 4, 6, 8-tetraones: Evaluation antitumor activity. J. Heterocycl. Chem. 57, 842–850. 10.1002/jhet.3830 [DOI] [Google Scholar]
- Harichandran G., Devi K. S., Shanmugam P., Jesse M. I., Kathiravan K. (2018). Amberlite IRA-400 Cl resin catalyzed multicomponent organic synthesis in water: Synthesis, antimicrobial and docking studies of spiroheterocyclic 2-oxindoles and acenaphthoquinone. Curr. Organocatal. 5, 13–24. 10.2174/2213337205666180316170023 [DOI] [Google Scholar]
- Harikrishna S., Robert A. R., Ganja H., Maddila S., Jonnalagadda S. B. (2020). A green, facile and recyclable Mn3O4/MWCNT nano-catalyst for the synthesis of quinolines via one-pot multicomponent reactions. Sustain. Chem. Pharm. 16, 100265. 10.1016/j.scp.2020.100265 [DOI] [Google Scholar]
- Hosseinzadegan S., Hazeri N., Maghsoodlou M. T., Moghaddam-Manesh M., Shirzaei M. (2020). Synthesis and evaluation of biological activity of novel chromeno [4, 3-b] quinolin-6-one derivatives by SO 3 H-tryptamine supported on Fe 3 O 4@ SiO 2@ CPS as recyclable and bioactive magnetic nanocatalyst. J. Iran. Chem. Soc. 17, 3271–3284. 10.1007/s13738-020-01990-3 [DOI] [Google Scholar]
- Jamatia R., Gupta A., Pal A. K. (2016). Nano-FGT: A green and sustainable catalyst for the synthesis of spirooxindoles in aqueous medium. RSC Adv. 6, 20994–21000. 10.1039/c5ra27552k [DOI] [Google Scholar]
- Joshi K., Jain R., Sharma K., Bhattacharya S., Goel R. (1988). ChemInform abstract: Studies in spiro-heterocycles. Part 12. Synthesis of some fluorine containing spiro(3H-indole-3, 4′(4H)-pyrano(2, 3-d)pyrimidine)-2, 5′, 7′(1H)-triones as CNS agents. ChemInform 19, 202–204. 10.1002/chin.198838220 [DOI] [Google Scholar]
- Keshavarz M. (2016). Ion-pair immobilization of l-prolinate anion onto cationic polymer support and a study of its catalytic activity for one-pot synthesis of spiroindolones. J. Iran. Chem. Soc. 13, 553–561. 10.1007/s13738-015-0765-y [DOI] [Google Scholar]
- Keshavarz R., Farahi M. (2022). Novel cellulose supported 1, 2-bis (4-aminophenylthio) ethane Ni (ii) complex (Ni II (BAPTE)(NO 3) 2-Cell) as an efficient nanocatalyst for the synthesis of spirooxindole derivatives. RSC Adv. 12, 3584–3592. 10.1039/d1ra08182a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalafi-Nezhad A., Shahidzadeh E. S., Sarikhani S., Panahi F. (2013). A new silica-supported organocatalyst based on L-proline: An efficient heterogeneous catalyst for one-pot synthesis of spiroindolones in water. J. Mol. Catal. A Chem. 379, 1–8. 10.1016/j.molcata.2013.07.009 [DOI] [Google Scholar]
- Maddila S., Nagaraju K., Jonnalagadda S. B. (2020). Synthesis and antimicrobial evaluation of novel pyrano [2, 3-d]-pyrimidine bearing 1, 2, 3-triazoles. Chem. Data Collect. 28, 100486. 10.1016/j.cdc.2020.100486 [DOI] [Google Scholar]
- Moghaddam-Manesh M., Ghazanfari D., Sheikhhosseini E., Akhgar M. (2020). Synthesis of bioactive magnetic nanoparticles spiro [indoline-3, 4′-[1, 3] dithiine]@ Ni (NO3) 2 supported on Fe3O4@ SiO2@ CPS as reusable nanocatalyst for the synthesis of functionalized 3, 4-dihydro-2H-pyran. Appl. Organomet. Chem. 34, e5543. 10.1002/aoc.5543 [DOI] [Google Scholar]
- Moradi L., Ataei Z., Zahraei Z. (2019). Convenient synthesis of spirooxindoles using SnO2 nanoparticles as effective reusable catalyst at room temperature and study of their in vitro antimicrobial activity. J. Iran. Chem. Soc. 16, 1273–1281. 10.1007/s13738-019-01598-2 [DOI] [Google Scholar]
- Morahan P. S., Regelson W., Munson A. E. (1972). Pyran and polyribonucleotides: Differences in biological activities. Antimicrob. Agents Chemother. 2, 16–22. 10.1128/aac.2.1.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadar S. S., Rathod V. K. (2020). Immobilization of proline activated lipase within metal organic framework (MOF). Int. J. Biol. Macromol. 152, 1108–1112. 10.1016/j.ijbiomac.2019.10.199 [DOI] [PubMed] [Google Scholar]
- Nadar S. S., Rathod V. K. (2018). Magnetic-metal organic framework (magnetic-MOF): A novel platform for enzyme immobilization and nanozyme applications. Int. J. Biol. Macromol. 120, 2293–2302. 10.1016/j.ijbiomac.2018.08.126 [DOI] [PubMed] [Google Scholar]
- Nagaraju S., Paplal B., Sathish K., Giri S., Kashinath D. (2017). Synthesis of functionalized chromene and spirochromenes using l-proline-melamine as highly efficient and recyclable homogeneous catalyst at room temperature. Tetrahedron Lett. 58, 4200–4204. 10.1016/j.tetlet.2017.09.060 [DOI] [Google Scholar]
- Oudi M., Sanchooli Tazeh K., Hazeri N., Fatahpour M., Ahmadi R. (2019). A convenient route toward one‐pot multicomponent synthesis of spirochromenes and pyranopyrazoles accelerated via quinolinic acid. J. Chin. Chem. Soc. 66, 1721–1728. 10.1002/jccs.201800470 [DOI] [Google Scholar]
- Ozyilmaz E., Ascioglu S., Yilmaz M. (2021). Preparation of one-pot immobilized lipase with Fe3O4 nanoparticles into metal-organic framework for enantioselective hydrolysis of (R, S)-Naproxen methyl ester. ChemCatChem 13, 3687–3694. 10.1002/cctc.202100481 [DOI] [Google Scholar]
- Pascanu V., González Miera G., Inge A. K., Martín-Matute B. (2019). Metal–organic frameworks as catalysts for organic synthesis: A critical perspective. J. Am. Chem. Soc. 141, 7223–7234. 10.1021/jacs.9b00733 [DOI] [PubMed] [Google Scholar]
- Pogosyan S., Pogosyan M., Aleksanyan L., Safaryan A., Arakelyan A. (2018). Synthesis and biological activity of substituted spiro [chromene-4, 3′-indoles] and spiro [indole-3, 4′-quinolines]. Russ. J. Org. Chem. 54, 1860–1863. 10.1134/s1070428018120254 [DOI] [Google Scholar]
- Purohit G., Rawat D. S., Reiser O. (2020). Palladium nanocatalysts encapsulated on porous silica@ magnetic carbon‐coated cobalt nanoparticles for sustainable hydrogenation of nitroarenes, alkenes and alkynes. ChemCatChem 12, 569–575. 10.1002/cctc.201901371 [DOI] [Google Scholar]
- Rafiee Z. (2021). Fabrication of efficient Zn-MOF/COF catalyst for the Knoevenagel condensation reaction. J. Iran. Chem. Soc. 18, 2657–2664. 10.1007/s13738-021-02221-z [DOI] [Google Scholar]
- Ren J., Langmi H. W., North B. C., Mathe M. (2015). Review on processing of metal–organic framework (MOF) materials towards system integration for hydrogen storage. Int. J. Energy Res. 39, 607–620. 10.1002/er.3255 [DOI] [Google Scholar]
- Saraswat P., Jeyabalan G., Hassan M. Z., Rahman M. U., Nyola N. K. (2016). Review of synthesis and various biological activities of spiro heterocyclic compounds comprising oxindole and pyrrolidine moities. Synth. Commun. 46, 1643–1664. 10.1080/00397911.2016.1211704 [DOI] [Google Scholar]
- Sargazi G., Afzali D., Mostafavi A. (2018). An efficient and controllable ultrasonic-assisted microwave route for flower-like Ta (V)–MOF nanostructures: Preparation, fractional factorial design, DFT calculations, and high-performance N2 adsorption. J. Porous Mat. 25, 1723–1741. 10.1007/s10934-018-0586-3 [DOI] [Google Scholar]
- Sayed G. H., Azab M. E., Anwer K. E. (2019). Conventional and microwave‐assisted synthesis and biological activity study of novel heterocycles containing pyran moiety. J. Heterocycl. Chem. 56, 2121–2133. 10.1002/jhet.3606 [DOI] [Google Scholar]
- Shehab W. S., Ghoneim A. A. (2016). Synthesis and biological activities of some fused pyran derivatives. Arabian J. Chem. 9, S966–S970. 10.1016/j.arabjc.2011.10.008 [DOI] [Google Scholar]
- Shiri L., Narimani H., Kazemi M. (2018). Synthesis and characterization of sulfamic acid supported on Fe3O4 nanoparticles: A green, versatile and magnetically separable acidic catalyst for oxidation reactions and knoevenagel condensation. Appl. Organomet. Chem. 32, e3927. 10.1002/aoc.3927 [DOI] [Google Scholar]
- Tehrani K. H. M. E., Hashemi M., Hassan M., Kobarfard F., Mohebbi S. (2016). Synthesis and antibacterial activity of Schiff bases of 5-substituted isatins. Chin. Chem. Lett. 27, 221–225. 10.1016/j.cclet.2015.10.027 [DOI] [Google Scholar]
- Togashi T., Naka T., Asahina S., Sato K., Takami S., Adschiri T. (2011). Surfactant-assisted one-pot synthesis of superparamagnetic magnetite nanoparticle clusters with tunable cluster size and magnetic field sensitivity. Dalton Trans. 40, 1073–1078. 10.1039/c0dt01280g [DOI] [PubMed] [Google Scholar]
- Wang C., Yu B., Li W., Zou W., Cong H., Shen Y. (2022). Effective strategy for polymer synthesis: Multicomponent reactions and click polymerization. Mater. Today Chem. 25, 100948. 10.1016/j.mtchem.2022.100948 [DOI] [Google Scholar]
- Yao X., Bai C., Chen J., Li Y. (2016). Efficient and selective green oxidation of alcohols by MOF-derived magnetic nanoparticles as a recoverable catalyst. RSC Adv. 6, 26921–26928. 10.1039/c6ra01617k [DOI] [Google Scholar]
- Yousefi A., Yousefi R., Panahi F., Sarikhani S., Zolghadr A. R., Bahaoddini A., et al. (2015). Novel curcumin-based pyrano [2, 3-d] pyrimidine anti-oxidant inhibitors for α-amylase and α-glucosidase: Implications for their pleiotropic effects against diabetes complications. Int. J. Biol. Macromol. 78, 46–55. 10.1016/j.ijbiomac.2015.03.060 [DOI] [PubMed] [Google Scholar]
- Zeraati M., Moghaddam-Manesh M., Khodamoradi S., Hosseinzadegan S., Golpayegani A., Chauhan N. P. S., et al. (2022). Ultrasonic assisted reverse micelle synthesis of a novel Zn-metal organic framework as an efficient candidate for antimicrobial activities. J. Mol. Struct. 1247, 131315. 10.1016/j.molstruc.2021.131315 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.