Abstract
A study by Tcyganov et al.1 demonstrates that peroxynitrite, an oxidant abundant in the tumor microenvironment, changes the repertoire of MHC class I peptides presented by tumors and limits immune recognition. Peroxynitrite inhibition in combination with immune checkpoint blockade enhances efficacy preclinically.
A study by Tcyganov et al. demonstrates that peroxynitrite, an oxidant abundant in the tumor microenvironment, changes the repertoire of MHC class I peptides presented by tumors and limits immune recognition. Peroxynitrite inhibition in combination with immune checkpoint blockade enhances efficacy preclinically.
Main text
CD8 T cells are the primary mediators of cancer immunity, and enhancement of this response has been the focus of immunotherapy. When CD8 T cells recognize antigenic peptides presented by the major histocompatibility complex class I molecules (MHC class I) on the surface of tumor cells, they become activated and kill the tumor cells. Due to the presence of inhibitory signals in the tumor microenvironment (TME), these CD8 T cells are often dysfunctional.2 Immunotherapies that block the inhibitory pathways called immune checkpoints have shown profound and durable clinical activity, but only in a limited number of cancer patients. The presence of other immunosuppressive factors and reduced antigen presentation by tumor cells likely limit their activity. As a result, there is great interest in identifying the mechanisms of escape to immunotherapy, and design combination strategies to circumvent tumor resistance to immune checkpoint inhibitors.
The TME is characterized by the infiltration of a high number of myeloid cells with immunosuppressive functions. In particular, these cells are an important source of reactive oxygen species (ROS) and reactive nitrogen species (RNS).3 Peroxynitrite (PNT), the product of nitric oxide with superoxide, is well known for killing pathogens engulfed by macrophages through massive nitration and oxidation of the pathogen proteins. Similarly, early data suggest that PNT can limit T cell responses and intratumoral infiltration by altering protein function through nitration of the T cell receptor and chemokines.4, 5, 6 As tumor cells could also be the target of PNT nitration, Tcyganov et al.1 sought to explore the direct effect of PNT on the MHC class I peptidome presented by tumors and whether it influences response to immunotherapy.
First, the authors evaluated the impact of PNT treatment on the MHC class I peptide repertoire presented by tumor cell lines in vitro by mass spectrometry analysis and observed reduced presentation of a subset of peptides (PNT-sensitive peptides, PNT-S). How PNT alters the profile of presented peptides is not totally clear. PNT does not appear to have a direct impact on surface MHC class I expression, antigen expression or binding affinity of the peptides to MHC class I. PNT-S peptides unmodified and treated with PNT to induce their nitration have comparable binding affinity, suggesting peptide nitration does not alter binding. Interestingly, PNT-S peptides and PNT-resistant (PNT-R) peptides can be distinguished based on motif sequence specificities. Furthermore, the peptide/MHC class I (pMHC class I) complexes exhibit different properties, with PNT-S peptides having a higher dissociation rate. Then, the authors examined the impact of PNT on the proteasome activity, the protease complex required for the processing of proteins into peptides. They found that PNT reduces the proteasome activity thereby limiting MHC class I peptide generation. This result led the authors to postulate that slowing the rate of pMHC class I formation may have a higher impact on surface expression of PNT-S peptide bound MHC class I with shorter half-life.
Next, the authors evaluated the biological relevance of this phenomenon. In agreement to their in vitro data, they found preferential development of CD8 T cell responses against PNT-R peptides in mice bearing tumors producing high levels of PNT, confirming that PNT-S peptides are poorly presented by tumor cells in presence of PNT. This was in striking contrast to the comparable immunogenicity of PNT-S and PNT-R peptides after vaccination of healthy mice, and in absence of PNT. Furthermore, the authors assessed the therapeutic potential of PNT-S peptide- and PNT-R peptide-specific CD8 T cells. Adoptive transfer of PNT-S peptide-specific CD8 T cells from vaccinated healthy mice in combination with immune checkpoint blockade failed to reject tumors (Figure 1). In contrast, the same treatment but with infusion of PNT-R peptide-specific CD8 T cells resulted in the rejection of the majority of tumors. Furthermore, blocking PNT production restored tumor cell killing by PNT-S peptide-specific T cells and delayed tumor progression in combination with the blockade of the immune checkpoint programmed death-1 (PD-1). A limitation of this study was the use of inhibitors that are rather nonspecific and may have additional uncharacterized effects. Altogether, these results suggest that PNT limits anti-tumor responses and immunotherapy efficacy in part by narrowing the repertoire of tumor antigens presented on MHC class I.
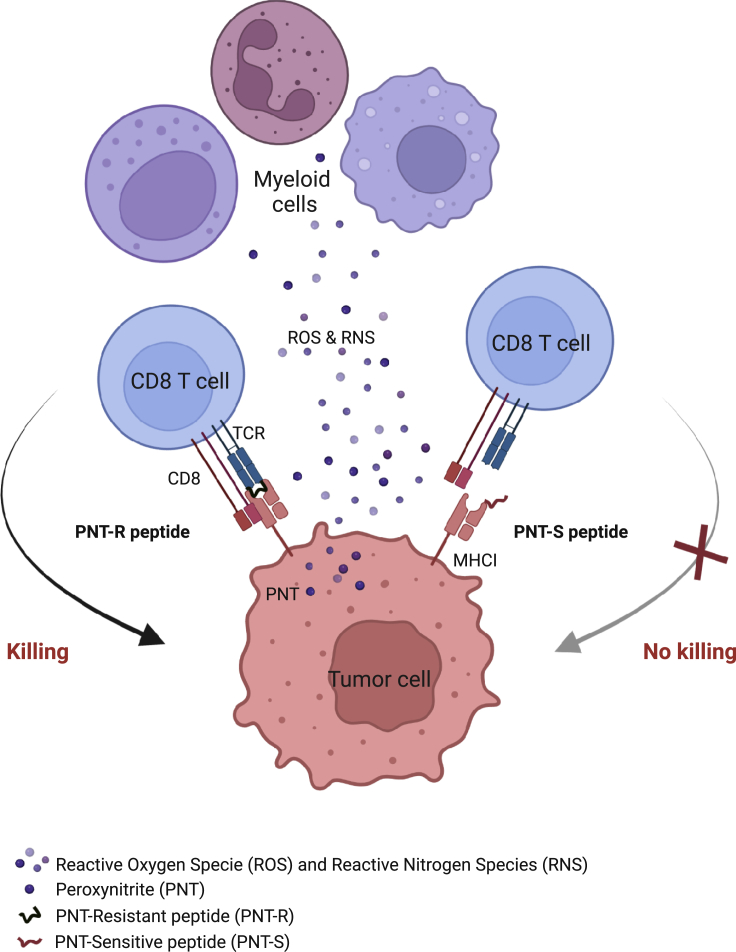
Figure 1.
Peroxynitrite limits tumor killing by reducing tumor antigenicity
PNT, produced by myeloid cells in the TME, inhibits proteasome activity and reduces MHC class I presentation of PNT-S peptides with high dissociation rate by tumor cells, thus limiting tumor cell recognition and killing CD8 T cells. Figure created with biorender.com
Finally, Tcyganov et al. assessed the link between PNT levels and clinical outcome to immunotherapy. They performed a retrospective analysis and found that low activity of PNT was associated with better overall survival and response to immunotherapies in two independent cohorts of patients with metastatic melanoma, who were either vaccinated against the Melan-A tumor antigen or received the immune checkpoint inhibitor pembrolizumab (anti-PD-1 antibody). While these results are encouraging, further validation is required with prospective clinical studies.
In summary, the study by Tcyganov et al. describes a novel mechanism by which PNT promotes tumor immune escape. It also suggests that therapeutic inhibition of PNT may enhance the therapeutic efficacy of immunotherapies. In addition, these findings have important implications for the design of tumor antigen-specific therapies, including vaccines and cell therapy. Indeed, most antigen-targeted therapies rely on evidence of antigen presentation by tumor cells cultured in vitro or prediction methods trained on MHC class I ligandome data from cells cultured in vitro. They do not take in consideration potential modifications of the presented repertoire by molecules within the TME. This study highlights some sequence specificities between PNT-S and PNT-R peptides. If key determinants that differentiate the two peptide populations can be identified in additional studies, they could be included in the prediction algorithms for neoantigen prioritization. Similarly, adoptive therapies using expanded T cells from the tumor may be more successful than from PBMCs.
Acknowledgments
Declaration of interests
The authors are employees of Genentech and declare no competing interests.
References
- 1.Tcyganov E., Sanseviero E., Marvel D., Beer T., Tang H.Y., Hembach P., Speicher D.W., Zhang Q., Donthireddy L.R., Mostafa A., et al. Peroxynitrite in tumor microenvironment changes the profile of antigens allowing escape from cancer immunotherapy. Cancer Cell. 2022;40:1173–1189.e6. doi: 10.1016/j.ccell.2022.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beatty G.L., Gladney W.L. Immune escape mechanisms as a Guide for cancer immunotherapy. Clin. Cancer Res. 2015;21:687–692. doi: 10.1158/1078-0432.CCR-14-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kennel K.B., Greten F.R. Immune cell - produced ROS and their impact on tumor growth and metastasis. Redox Biol. 2021;42 doi: 10.1016/j.redox.2021.101891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagaraj S., Gupta K., Pisarev V., Kinarsky L., Sherman S., Kang L., Herber D.L., Schneck J., Gabrilovich D.I. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molon B., Ugel S., del Pozzo F., Soldani C., Zilio S., Avella D., De Palma A., Mauri P., Monegal A., Rescigno M., et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J. Exp. Med. 2011;208:1949–1962. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu T., Ramakrishnan R., Altiok S., Youn J.I., Cheng P., Celis E., Pisarev V., Sherman S., Sporn M.B., Gabrilovich D. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J. Clin. Invest. 2011;121:4015–4029. doi: 10.1172/JCI45862. [DOI] [PMC free article] [PubMed] [Google Scholar]