Abstract
Little is known about how exposure to limited socioeconomic resources (SER) in childhood gets “under the skin” to shape brain development, especially using rigorous whole-brain multivariate methods in large, adequately powered samples. The present study examined resting state functional connectivity patterns from 5821 youth in the Adolescent Brain Cognitive Development (ABCD) study, employing multivariate methods across three levels: whole-brain, network-wise, and connection-wise. Across all three levels, SER was associated with widespread alterations across the connectome. However, critically, we found that parental education was the primary driver of neural associations with SER. These parental education associations with the developing connectome exhibited notable concentrations in somatosensory and subcortical regions, and they were partially accounted for by home enrichment activities, child’s cognitive abilities, and child’s grades, indicating interwoven links between parental education, child stimulation, and child cognitive performance. These results add a new data-driven, multivariate perspective on links between household SER and the child’s developing functional connectome.
Keywords: Socioeconomic resources, Socioeconomic status, Parental education, Household income, Neighborhood disadvantage, Resting state fMRI, Functional connectivity, Connectomics, Adolescent Brain Cognitive Development (ABCD) Study, Predictive modeling, Intrinsic connectivity networks, Neurodevelopment
Graphical Abstract
Highlights
-
•
How does access to socioeconomic resources (SER) shape the developing brain?
-
•
Examined resting state connectomes from 5821 youth in ABCD Study.
-
•
Used multivariate methods across 3 levels: brain-wide, networks, and connections.
-
•
SER was associated with widespread alterations across the connectome.
-
•
Parental education was the primary driver of these SER associations.
1. Introduction
Childhood socioeconomic resources (SER), measured by parental education and economic resources within home and neighborhood contexts, shape adult outcomes in economic (e.g., earnings, employment), educational (e.g., cognitive skills, college completion), and physical and mental health domains (Green et al., 2010; Cohen et al., 2010; Duncan et al., 2010). Disparities in access to SER (e.g., by racialized identity, age, urbanicity) are larger in the United States than other industrialized countries (Reardon and Bischoff, 2011), have grown over time (Piketty and Saez, 2003; Hoffmann et al., 2020), and reflect broader structural inequities (e.g., residential segregation, wealth accumulation). Thus, though measured imperfectly, variation in education, income, and neighborhood resources exerts wide-ranging impacts on wellbeing across the life course (Cooper and Pugh, 2020). This implication has encouraged neuroscientists to investigate pathways through which SER influences the developing brain (Hackman and Farah, 2009; Johnson et al., 2016). Yet our current understanding of these pathways remains highly incomplete, particularly during critical developmental windows such as early adolescence, marked by extensive neural reorganization (Paus, 2005) and when many serious psychosocial challenges (e.g., problems in interpersonal, academic, and mental health domains) first emerge (Paus et al., 2008).
The human brain is organized as a complex network (Sporns, 2011, Sporns, 2014), with interconnections among regions implicated in diverse cognitive and socioemotional functions (Laird et al., 2011). Task-free “resting state” functional magnetic resonance imaging (fMRI) uses coherence in spontaneous activity across brain regions to yield maps of functional connectivity patterns (Smith et al., 2013), which, in turn, can be linked to individual difference variables such as such cognition, personality traits, or psychopathology (Castellanos et al., 2013).
Previous studies of the impact of SER on resting state functional connectivity patterns have mostly relied on region-specific approaches that focus on individual connections (e.g., amygdala-ventromedial prefrontal connectivity) (Rakesh and Whittle, 2021), requiring strong a priori knowledge about which connections are (and are not) implicated. There is, however, convergent evidence that characteristics of social, psychological, and clinical interest often involve distributed and wide-ranging changes at tens of thousands of connections distributed across the entire brain (Woo et al., 2017), rather than focal changes involving individual pairs of regions. Additionally, previous studies often used small samples consisting of tens to hundreds of subjects. Recent widely discussed results (Marek et al., 2022) demonstrate that these studies are liable to produce spurious findings, and several thousand subjects are typically needed to derive statistically reliable conclusions. At the present time, however, no previous studies have investigated SER-associated functional connectivity patterns using multivariate methods across tens of thousands of brain connections in large, adequately powered samples.
Additionally, SER is a multi-dimensional construct that incorporates features of parental education, household income, and neighborhood disadvantage (Ensminger et al., 2003). Different components of SER implicate different underlying environmental mechanisms (e.g., maternal education may impact cognitive stimulation in the home, whereas neighborhood disadvantage may operate through school quality). Understanding which component(s) of SER affect the developing brain is critical for designing targeted interventions and for informing housing, school, and redistributive policies (Raver and Blair, 2020, Hyde et al., 2020a). But the unique effects of each dimension of SER in shaping brain-wide connectivity patterns remains unclear.
To address these gaps in understanding, we leveraged the Adolescent Brain and Cognitive Development (ABCD) Study (Volkow et al., 2018, Karcher and Barch, 2020), a population-based study of 11,875 9- and 10-year-olds from 22 sites across the United States with substantial sociodemographic diversity (Garavan et al., 2018). ABCD is the largest developmental neuroimaging study ever undertaken, providing a unique opportunity to study how SER shapes connectivity patterns of the developing brain. To convergently establish results at multiple levels of analysis, we employed three complementary multivariate methods: a whole-brain approach (multivariate predictive modeling) (Sripada et al., 2019); a network-wise approach (network contingency analysis) (Sripada et al., 2014a, Sripada et al., 2021a, Donoho and Jin, 2004), and a connection-wise approach (quantile-quantile modeling) (Schweder and Spjøtvoll, 1982).
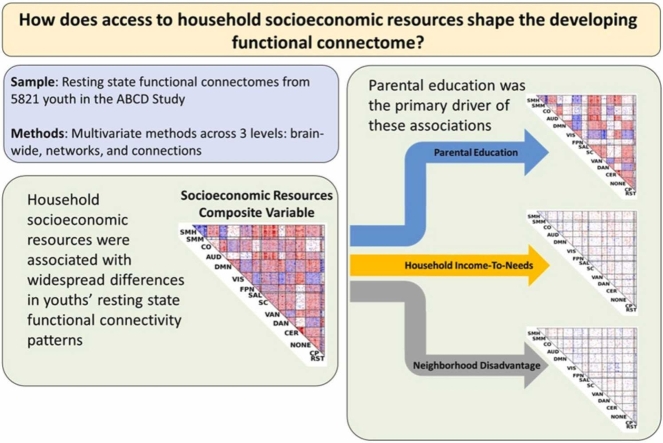
These analyses jointly indicated that a combination of all the three dimensions of SER (i.e., parental education, household income-to-needs, and neighborhood disadvantage) was associated with widespread individual variation in connectivity across the entire brain, with significant effects observed in 78 out of 120 “cells”, i.e., sets of connections linking pairs of large-scale brain networks. In additional analyses that dissected the unique contribution of individual components of SER, we found the most potent associations with parental education, even after controlling for the contributions of household income-to-needs and neighborhood disadvantage. Moreover, functional connectivity patterns associated with parental education were uniquely concentrated in sensorimotor and subcortical networks. Our results may help to illuminate why SER is associated with a variety of outcomes across the life course, while also highlighting the need for more research to explore proximal biopsychosocial mechanisms of SER-connectome associations.
2. Methods
2.1. Sample and data
The ABCD study is a multisite longitudinal study with 11,875 children between 9 and 10 years of age from 22 sites across the United States. The study conforms to the rules and procedures of each site’s Institutional Review Board, and all participants provide informed consent (parents) or assent (children). Data for this study are from ABCD Release 3.0.
2.2. Data acquisition, fMRI preprocessing, and connectome generation
High spatial (2.4 mm isotropic) and temporal resolution (TR=800 ms) resting state fMRI was acquired in four separate runs (5 min per run, 20 min total). Preprocessing was performed using fMRIPrep version 1.5.0 (Esteban et al., 2019). Briefly, T1-weighted (T1w) and T2-weighted images were run through recon-all using FreeSurfer v6.0.1, spatially normalized, rigidly coregistered to the T1, motion corrected, normalized to standard space, and transformed to CIFTI space.
Connectomes were generated for each functional run using the Gordon 333 parcel atlas (Gordon et al., 2016), augmented with parcels from high-resolution subcortical (Tian et al., 2020) and cerebellar (Diedrichsen et al., 2011) atlases. Volumes exceeding a framewise displacement (FD) threshold of 0.5 mm were marked to be censored. Covariates were regressed out of the time series in a single step, including: linear trend, 24 motion parameters (original translations/rotations + derivatives + quadratics, aCompCorr 5 CSF and 5 WM components and ICA-AROMA aggressive components, high pass filtering at 0.008 Hz, and censored volumes. Next, correlation matrices were calculated. Full details of preprocessing and connectome generation can be found in the Supplement as well as the automatically-generated FMRI Prep Supplement.
2.3. Inclusion/exclusion
There are 11,875 subjects in the ABCD Release 3.0 dataset. Subjects were excluded for: failing ABCD QC, insufficient number of runs each 4 min or greater, failing visual QC of registrations and normalizations, and missing data required for regression modeling. This left us with N = 5821 subjects across 19 sites for the main sample analysis, and details of exclusions are provided in the Supplement.
2.4. Neuroimaging analysis
To quantify brain-wide relationships between functional connectivity patterns and outcome variables of interest including SER, we used principal component regression (PCR) predictive modeling (Sripada et al., 2019, Sripada et al., 2020a) (see Fig. S2). In brief, this method performs dimensionality reduction on resting state connectomes, fits a regression model on the resulting components, and applies this model out of sample in a leave-one-site-out cross-validation framework. To identify network-wise brain-behavior relationships, we used network contingency analysis (NCA) (Sripada et al., 2014a, Sripada et al., 2021a, Donoho and Jin, 2004). In brief, for each cell (set of connections linking pairs of large-scale networks), this method identifies whether the count of connections significantly related to an outcome variable of interest exceeds what is expected by chance. To quantify connection-wise brain-behavior relationships, we used quantile-quantile modeling (Schweder and Spjøtvoll, 1982). In brief, we first calculated the p-value at each connection for the association between that connection and an outcome variable of interest. We then rank ordered these p-values and compared them to the rank-ordered distribution of p-values expected under the global null hypothesis.
In implementing the three preceding methods, we control for the effect of a number of nuisance covariates, specifically sex assigned at birth, parent-reported race-ethnicity, age, age squared, mean FD and mean FD squared. For all three methods, we assessed statistical significance with non-parametric permutation tests, in which the procedure of Freedman and Lane (Freedman et al., 1983) was used to account for covariates. In addition, exchangeability blocks were used to account for twin, family, and site structure and were entered into Permutation Analysis of Linear Models (PALM) (Winkler et al., 2014) to produce permutation orderings. Details on all the preceding neuroimaging analyses are provided in the Supplement.
2.5. Latent variable modeling
We constructed a latent variable for socioeconomic resources by applying exploratory factor analysis to household income-to-needs, parental education, and neighborhood disadvantage. Household income-to-needs represents the ratio of a household’s income relative to its need based on family size, and details on its calculation are provided in the Supplement. Parental education was the average educational achievement of parents or caregivers. Neighborhood disadvantage scores reflect an ABCD consortium-supplied variable (reshist_addr1_adi_wsum). In brief, participant’s primary home address was used to generate Area Deprivation Index (ADI) values (Fan et al., 2021), which were weighted based on results from Kind et al (Kind et al., 2014). to create an aggregate measure.
The general psychopathology factor (P-factor) was produced from bifactor modeling of the parent-rated Child Behavior Checklist (CBCL) (Achenbach and Ruffle, 2000), and was described in detail in our previous studies in ABCD (Clark et al., 2021, Brislin et al., 2021). The general cognitive ability (GCA) variable was produced from bifactor modeling of the ABCD neurocognitive battery, and was also described in detail in our previous studies in ABCD (Sripada et al., 2021b, Clark et al., 2021). Additional details on construction of the preceding latent variables are provided in the Supplement.
2.6. Code availability
The ABCD data used in this report came from NDA Study 901, 10.15154/1520591, which can be found at https://nda.nih.gov/study.html?id= 901. Code for running analyses can be found at https://github.com/SripadaLab/ABCD_Resting_Socioeconomic_Resources.
3. Results
3.1. Across three levels of analysis (i.e., whole-brain, network-wise, and connection-wise), socioeconomic resources are associated with large, brain-wide changes in functional connectivity
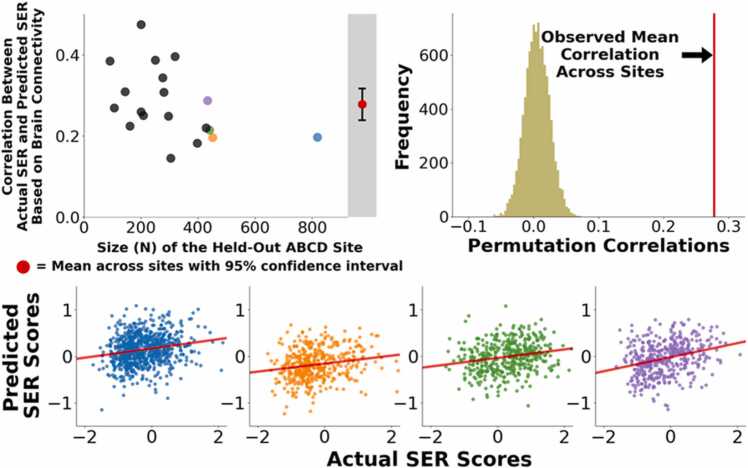
Whole-Brain-Level Analysis: We built and assessed multivariate predictive models for socioeconomic resource (SER) scores using a leave-one-site-out cross-validation approach. At each fold of the cross-validation, we trained a multivariate predictive model to use individual differences in brain connectivity patterns to predict SER scores. We then applied the trained model to brain connectivity data from subjects at the held-out site, yielding predictions of their SER, and we repeated this sequence with each site held out once. We accounted for nuisance covariates (youth sex assigned at birth, age, parent-reported race-ethnicity [a social construct linked to historical and present structural discrimination], and head motion) by applying regression coefficients for covariates learned in the train data to covariates in the test data, thus preserving complete independence between train and test datasets. We found that the correlation between actual versus predicted SER, controlling for covariates and averaging across the 19 folds of the cross-validation, was 0.28 (Fig. 1, upper left panel). That is, after accounting for covariates, brain connectivity patterns accounted for 9.0% of the variance in SER in held-out samples of youth (cross-validated r2). Cross-site generalizability was remarkably consistent: Correlations between predicted and actual scores were statistically significant in all 19 out of 19 held-out sites (all 19 site-specific permutation p-values < 0.0001; observed correlations were higher than all 10,000 correlations in the permutation distribution).
Fig. 1.
Correlations Between Actual Socioeconomic Resources Scores and Scores That Are Predicted Based on Whole-Brain Connectivity Patterns. We applied multivariate predictive models to 5821 subjects at 19 sites to identify brain-wide connectivity patterns that are associated with SER scores. (Upper Left Panel) In leave-one-site-out cross-validation, functional connectivity patterns associated with SER scores generalized to 19 out of 19 held out sites. (Upper Right Panel) The overall mean correlation between observed SER scores and predicted SER scores (predicted exclusively from brain connectivity patterns) was 0.28, pPERM< 0.0001 (observed correlation was higher than all 10,000 correlations in the permutation distribution). (Lower Panel) Scatter plots for four largest held-out sites (blue, orange, green, purple) show consistent performance at individual sites.
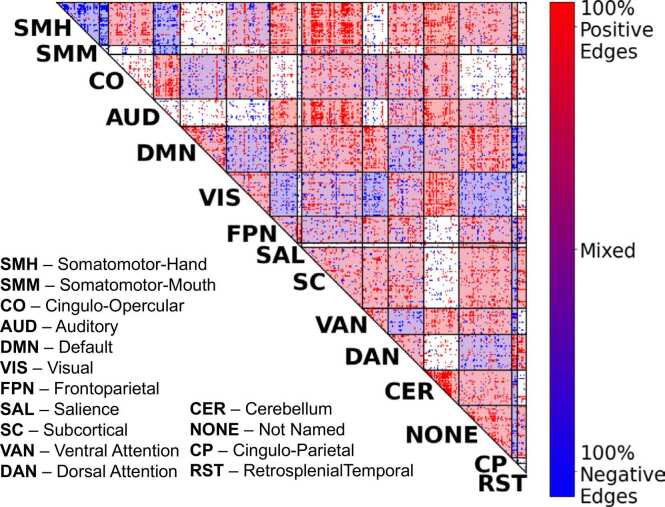
Network-Level Analysis: The human brain is organized into a number of large-scale networks (Power et al., 2011) defining a set of “cells”, which are sets of connections linking pairs of large-scale networks. We performed network contingency analysis (NCA) (Sripada et al., 2014a, Sripada et al., 2021a, Donoho and Jin, 2004), which identifies cells in which the count of connections associated with SER (controlling for covariates) exceeds the count expected by chance, established by non-parametric permutation tests. As shown in Fig. 2 and Supplemental Table S3, a total of 78 out of 120 cells exhibited significant associations with SER (FDR<0.05; shaded in the Fig. 2), and these cells were notably widespread throughout the brain spanning all large-scale networks.
Fig. 2.
Network-to-Network Connections Exhibiting Significant Associations with Socioeconomic Resources. We performed network contingency analysis (NCA) which identifies cells (i.e., sets of connections linking pairs of large-scale networks) where the number of edges related to SER scores exceeds the number expected by chance. A total of 78 out of 120 cells exhibited significant effects of SER (FDR<0.05; shaded in the figure), and these cells were notably widespread throughout the brain.
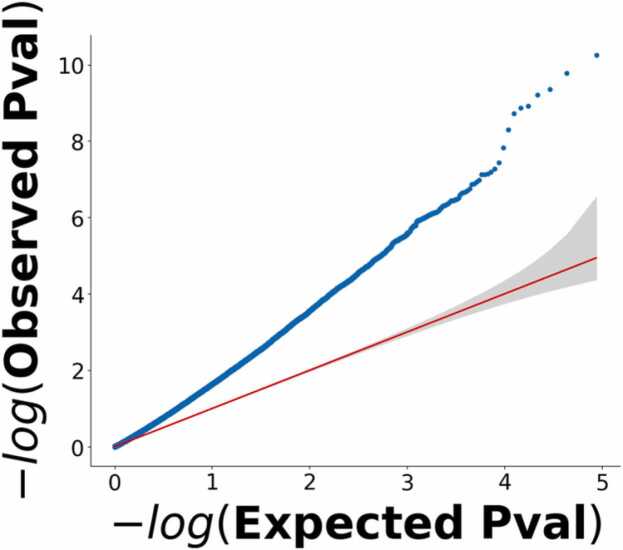
Connection-Level Analysis: We additionally assessed associations with SER on a connection-by-connection basis using quantile-quantile modeling (Schweder and Spjøtvoll, 1982). We first calculated the p-value at each connection for the association between that connection and SER (controlling for covariates). We then rank ordered these p-values and plotted them against the rank-ordered distribution of p-values expected under the global null hypothesis, which was calculated with non-parametric permutation-based methods (Fig. 3). If there is no association between SER and brain functional connections, this plot should follow the 45° line shown in in red in Fig. 3. But the observed plot strongly deviated from this line. Moreover, “lift off”, where the observed distribution deviates from the 95% confidence interval of the null line, occurred very early and persisted through the range of the x-axis. This result is consistent with widespread associations of SER with most functional connections across the brain.
Fig. 3.
Quantile-Quantile Model of Associations between Socioeconomic Resources and Functional Connections of the Connectome. The red line and its associated 95% confidence interval (in gray) represent the global null hypothesis that household SER scores are unrelated to functional connections of children’s connectomes. The plot shows strong deviation of the observed data (blue line) from the red line with a pattern of early “lift-off”, in which the deviation occurs towards the left of the plot and is sustained throughout. This pattern is consistent with widespread, diffuse influences of SER scores throughout the connectome.
3.2. Parental education is the primary driver of brain-wide changes in functional connectivity, with household income-to-needs and neighborhood disadvantage having weaker (or absent) unique associations
The preceding results established strong and widespread associations between SER and children’s connectomes across whole-brain, network-wise, and connection-wise levels of analysis. We next sought to identify unique contributions of the three components of the SER variable: parental education, household income-to-needs, and neighborhood disadvantage. We thus repeated the preceding analyses, this time with one of these three dimensions of SER as the variable of interest and the other dimensions entered as additional covariates. We repeated these analyses three times in total with each SER dimension as the variable-of-interest.
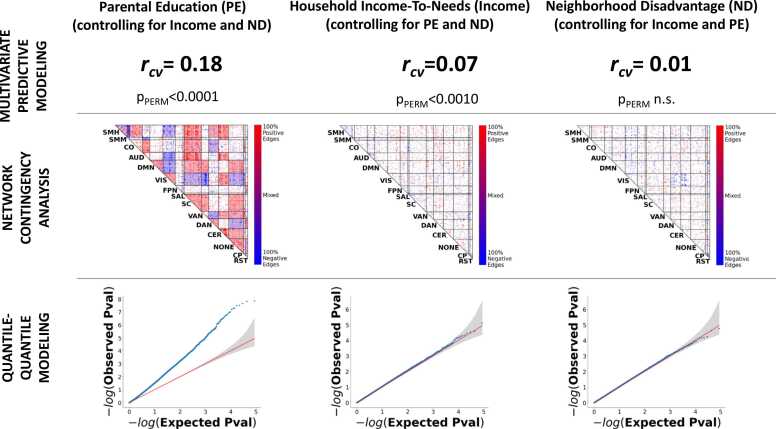
Results showed that parental education (controlling for household income-to-needs and neighborhood disadvantage; left column in Fig. 4; Supplemental Table S4) exhibited consistently strong effects at all three levels of analysis. Household income-to-needs (controlling for parental education and neighborhood disadvantage; middle column of Fig. 4) showed modest but statistically significant effects in multivariate predictive modeling analysis, no significant cells in network analysis, and did not deviate from the null hypothesis line in quantile-quantile analysis. Neighborhood disadvantage (controlling for parental education and household income-to-needs; right column of Fig. 4) did not show any statistically significant effects in any of the three analyses.
Fig. 4.
Parental education has widespread and unique associations with children’s resting state connectomes. We used whole-brain-level (top row), network-level (middle row), and connection-level (bottom row) methods to identify unique effects on children’s resting state connectomes of three SER variables: parental education (left column), household income-to-needs (middle column), and neighborhood disadvantage (right column), Across the three-levels of analysis, parental education (controlling for household income-to-needs and neighborhood disadvantage) demonstrated consistent strong effects. Household income-to-needs (controlling for parental education and neighborhood disadvantage) showed modest, statistically significant effects in multivariate predictive modeling analysis, but showed no significant cells in network analysis and did not deviate from the null hypothesis line in quantile-quantile analysis. Neighborhood disadvantage (controlling for parental education and household income-to-needs) did not show any statistically significant effects in all three analyses. rcv = cross-validated out-of-sample correlation between actual scores and predicted scores using resting state connectivity data; * = observed correlation higher than all 10,000 correlations in the permutation distribution.
The preceding analysis was performed with an ABCD-furnished neighborhood disadvantage variable, based on area deprivation index weightings from Kind and colleagues (Kind et al., 2014), which was used in previous studies using ABCD data of effects of SER on resting state connectivity (Rakesh et al., 2021a, Rakesh et al., 2021b). We in addition repeated the preceding analysis with an alternative measure of neighborhood disadvantage used in another recent ABCD Study (Taylor et al., 2020), which uses a subset of ADI metrics that emphasize core aspects of disadvantage. Results (see Supplemental Fig. S5) continued to show a relatively weak neighborhood disadvantage effect, while effects of parental education and income-to-needs were virtually unchanged.
3.3. Parental education exhibits a sensorimotor/subcortical pattern that differentiates it from other youth phenotypes also associated with resting state connectivity
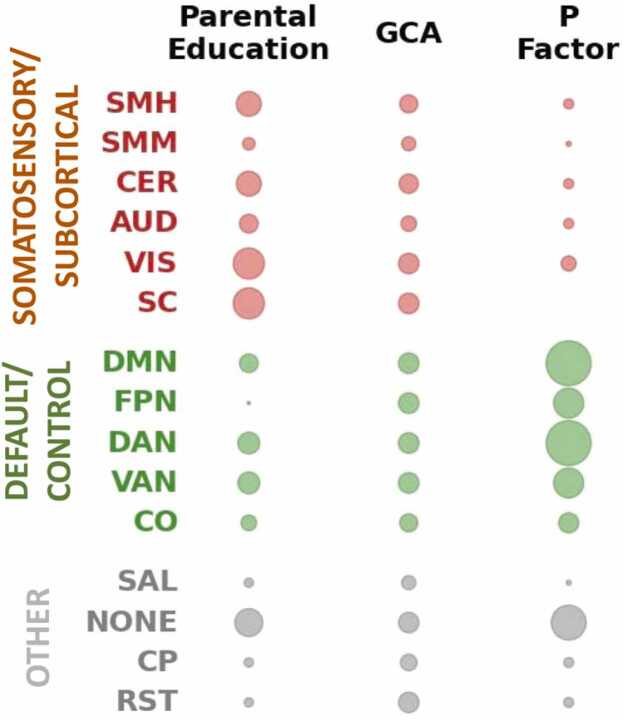
Given evidence of robust associations between parental education and resting state functional connectivity, we next compared the parental education connectivity pattern with corresponding connectivity patterns observed for general cognitive ability and the general factor of psychopathology, i.e., “P factor”, which were recently studied by our group in this same ABCD sample (Sripada et al., 2021a, Sripada et al., 2021b). We focused on connectivity patterns observed with network contingency analysis, which is well-suited for localizing associations to sets of connections linking pairs of networks. Bubble graphs (Fig. 5) capture the proportion of significant cells associated with each network for each variable. Consistent with our previous reports, the general factor of psychopathology implicated interconnections between the default mode network and a number of control networks (Sripada et al., 2021a), while general cognitive ability implicated highly distributed connectivity patterns involving all networks (Sripada et al., 2021b). Though there was some overlap across the three variables, parental education preferentially implicated somatosensory/subcortical networks, with especially prominent involvement of the visual and subcortical networks. Non-parametric tests of concentration showed that the proportion of significant cells within somatosensory/subcortical networks exceeded what is expected by chance (pPERM=0.003).
Fig. 5.
Parental Education Associations are Concentrated in Somatosensory/Subcortical Networks. We compared parental education connectivity patterns with corresponding patterns observed for general cognitive ability and the general factor of psychopathology (P Factor). The bubble graph captures the proportion of significant cells, i.e. sets of connections linking large-scale networks, associated with each network. The graph shows each variable has a distinct profile across the connectome, with parental education preferentially implicating somatosensory/subcortical networks.
3.4. Parental education’s associations with the functional connectome are related to home enrichment activities, child’s grades, and child’s cognitive abilities
Previous literature, reviewed in (Conger and Donnellan, 2007a), suggests that parental education may impact children’s behavioral and cognitive outcomes through provision of greater cognitive stimulation and parenting behaviors that promote academic and social competence (e.g., warmth and support). To gain some initial insight into why parental education was associated with children’s brain connectivity patterns, we performed an exploratory analysis that accounted for potential additional explanatory variables: 1) parent-reported enrichment activities (e.g., having intellectual discussions, reading with the child); 2) child-reported family support (e.g., smiling at the child, discussing the child’s worries, providing support); 3) child-reported school support (e.g., availability of extracurricular activities, praise when the child does a good job); 4) child’s grades in school; and 5) child’s general cognitive ability. This analysis was performed in a subsample of 3223 children for whom all the preceding variables were available. We focused on whole-brain predictive modeling, which affords a single dependent measure (out-of-sample brain-phenotype association) as well as quantitative bootstrap-based tests for attenuation of this association (see Supplement). A finding of significant attenuation of brain-parental education associations with the inclusion of contextualizing variables in the model indicates overlapping variance among these variables (though it does not indicate directionality of the relationships among these variables). Results showed that home enrichment activities, child’s grades, and child’s general cognitive ability each individually significantly attenuated the association between parental education and youth brain connectivity patterns (Table 1). In contrast, these associations were not meaningfully attenuated by controlling for family supportiveness and school supportiveness. In combination, 29% of the multivariate association between parental education and the functional connectome was accounted for by these five candidate explanatory variables.
Table 1.
Associations Between Parental Education and Brain Connectivity Patterns Controlling for Aspects of the Family and School Environment and Child Characteristics. We quantified the attenuation of the relationship between parental education and brain connectivity patterns after controlling individually and jointly for six candidate explanatory variables. Overall, 1/3 of the multivariate association between parental education and brain connectivity patterns was accounted for by the six candidate explanatory variables. rcv = cross-validated out-of-sample correlation between actual scores and predicted scores using resting state connectivity data.
| Contextualizing Variable | Parental Education rcv | Attenuation Magnitude | Attenuation p value |
|---|---|---|---|
| No Contextualizing Variables | 0.247 | – | – |
| Enrichment Activities‡ | 0.230 | 0.016 | 0.0439 |
| Family Support§ | 0.247 | -0.001 | n.s. |
| School Support¶ | 0.246 | 0.000 | n.s. |
| Child’s Grades | 0.219 | 0.028 | 0.0001* |
| Child’s General Cognitive Ability# | 0.189 | 0.058 | 0.0001* |
| All Contextualizing Variables | 0.175 | 0.071 | 0.0001* |
‡ Intellectual/Cultural Orientation subscale of the Family Environment Scale
§ Acceptance subscale of the Children’s Report of Parental Behavior Inventory
¶ School Environment subscale of the School Risk and Protective Factors Survey
# Latent Variable Created from ABCD Neurocognitive Battery
% Latent Variable Created from Child Behavior Checklist – Parent Report
* Observed attenuation larger than all 10,000 in the permutation distribution.
4. Discussion
Using data from a large population-based sample of 5821 9- and 10-year-olds in the ABCD Study, we evaluated associations between socioeconomic resources (SER) and youth functional connectomes using whole-brain, network-wise, and connection-wise approaches. Across these three levels of analysis, we observed widespread associations between SER and the developing connectome, with convergent evidence that parental education was the primary driver of these associations. Moreover, these parental education associations with the functional connectome were concentrated in somatosensory and subcortical networks, suggesting a spatial profile for parental education effects that is somewhat distinct from other recently studied constructs (i.e., general cognitive ability and general psychopathology). Overall, these results add a new data-driven multivariate perspective on links between household SER and the child’s developing functional connectome. Moreover, they potentially illuminate a primary role for parental education in explaining how socioeconomic adversity gets “under the skin” to shape the developing brain.
Previous examinations of the associations between SER and functional connectivity patterns in the brain have largely relied on regionalist and apriorist methods (e.g., 31, 32), see (Rakesh and Whittle, 2021) for a review. That is, SER-brain associations have been examined within individual pre-selected regions (e.g., amygdala – ventromedial prefrontal connectivity) based on prior theory. In addition, previous studies have generally been small, involving tens to hundreds of subjects (Rakesh and Whittle, 2021), and these sample sizes may generally be too small for reliable statistical inference (Marek et al., 2022). Against the backdrop of this previous work, our study takes breaks new ground in taking a multivariate, brain-wide, data-driven approach. In whole-brain analysis, we found functional connectivity patterns across the entire connectome captured 9.0% of the variance in SER in held-out subjects, with statistically significant generalization of these SER-associated connectivity patterns to all 19 out of 19 held-out sites. In network-wise analysis, we found SER has distributed effects throughout the brain, with statistically significant effects observed at 78 of 120 cells (i.e., sets of connections linking large-scale networks). In connection-wise analysis, we found strong deviations between observed versus expected p-value distributions, with a notable pattern of “early lift-off” (see Fig. 3), indicating the presence of highly distributed SER-related associations throughout the connectome.
Interestingly, our results are reminiscent of the distributed architecture of complex traits now recognized in genetics (Goddard et al., 2016). There too, it was initially assumed that complex traits were represented by few genetic loci, each of large effect, leading to the popularity of candidate genes studies in which loci were selected for study based on prior theory. However, the polygenic nature of complex human traits is now considered the norm, wherein phenotypes result from the cumulative impact of hundreds of thousands of genetic variants, each of very small effect (Visscher et al., 2017, Boyle et al., 2017). We here demonstrate a similar pattern in which SER-brain associations with the brain are analogously highly “poly-connectic”, implicating thousands of connections across the connectome, see also (Zhao et al., 2021). Our results highlight the need for developmental neuroscience research on the associations between environmental conditions and brain function to expand the toolkit of analysis methods beyond region-specific approaches to better capture what are likely to be highly distributed brain-wide associations (Woo et al., 2017, Varoquaux and Poldrack, 2019, Rosenberg et al., 2018).
We disentangled neural patterns of three indices of SER (parental education, household income-to-needs, and neighborhood disadvantage) using three different multivariate methods, each focused on different levels of analysis (whole-brain, network-wise, and connection-wise). These three methods convergently supported the conclusion that parental education has the most potent relationships with brain connectivity patterns in this large, population-based sample. Each of the three preceding method has its own strengths and weaknesses. For example, predictive modeling is optimized for aggregating widespread signals across the brain (Zhao et al., 2021), but it is poor at localizing signals (Tian and Zalesky, 2021). Meanwhile, NCA is better for localizing effects but makes assumptions about network boundaries that may turn out to be suboptimal. Quantile-quantile modeling makes no assumptions about network boundaries but is not well suited for quantifying multivariate effect sizes. By using these three methods in combination, however, we address the limitations of each method and provide evidence of the robustness of our results across different analytic choices.
Parental education neural associations were preferentially concentrated in somatosensory/subcortical regions. This result agrees with previous seed-based studies that also found SER associations with connectivity in amygdala, hippocampus, and striatum (Barch et al., 2016, Hanson et al., 2019, Dégeilh et al., 2019). The present study nonetheless goes beyond previous work in providing a brain-wide comparative perspective (i.e., parental education-associated connectivity is more concentrated in somatosensory/subcortical networks compared to other networks)—something that cannot be readily revealed by seed-based methods. Moreover, we showed this pattern of preferential somatosensory/subcortical concentration contrasts with general psychopathology (which is concentrated in default network and control networks; 11) and general cognitive ability (which is widespread across all networks; 12). Taken together, these results point to a distinctive spatial profile across the connectome of parental education that should be the focus of further investigation.
Several recent studies have also examined associations between SER and resting state functional connectivity in the same ABCD study dataset and reached somewhat different conclusions (Rakesh et al., 2021a, Rakesh et al., 2021b, DeJoseph et al., 2022). These studies generally found much weaker brain-behavior associations than what is reported here. In addition, one study (Rakesh et al., 2021b) found significant unique effects of neighborhood disadvantage, which were not observed here. Key differences in analytic approach may shed light on these divergent findings. The present study used connection-resolution connectomic data for each subject, encompassing 87,153 connections per connectome, as the input datatype for all analyses. In contrast, these other studies used summary statistics (available through the ABCD Data Exploration and Analysis Portal; https://deap.nimhda.org) in which each subject’s connectome is reduced to 78 numbers representing mean connectivity between each pair among 12 large-scale networks. In Supplement Fig. S6 and Table S2, we report results from the current study alongside results that would have been seen were we to have used these summary statistics. We found that for SER, as well as for parental education, household income-to-needs, and neighborhood disadvantage, between 68% and 80% of the multivariate signal associated with these variables is lost when using the cell mean summary data (with 78 features per subject) rather than the connection resolution data (with 87,153 features per subject). It is likely that this signal loss, as well as other differences in analysis choices (discussed in Supplement §10), explain the weaker effect sizes observed in these studies and the different pattern of effects. Of note, the use of summary statistics in place of connection-level data has not been extensively validated, and the preceding results suggest the need for caution in adopting this approach.
One pathway through which SER (and particularly parental education) is thought to shape youth cognitive and socioemotional development is through the provision of cognitively-stimulating activities (Conger and Donnellan, 2007b, Bradley et al., 2001, Ursache and Noble, 2016) that shape cognitive skills (e.g., general cognitive abilities and academic skills (Hyde et al., 2020b, Hackman and Farah, 2009, Rosen et al., 2019) and non-cognitive skills including socioemotional skills (e.g., emotion regulation and impulse control). To better understand these pathways, we conducted additional multivariate predictive modeling analyses with additional control variables. Home enrichment activities, child’s grades, and child’s cognitive abilities partially attenuated the multivariate association between parental education and resting-state connectivity patterns. The full set of five contextualizing variables accounted for nearly 30% of the multivariate association between parental education and resting-state connectivity patterns. Overall, these findings suggests there are rich, interwoven links between parental education, cognitive stimulation, and child cognitive/academic outcomes (Conger and Donnellan, 2007a, Rosen et al., 2019). However, given the cross-sectional, observational nature of the data, the directional natures of these associations cannot be determined. Moreover, though the provision of cognitively stimulating activities in the home is one example of a proximal mechanism through which parental education is associated with brain connectivity, access to educational resources (i.e., through libraries, transportation, information) is filtered through policies and other structural forces (e.g., spatial allocation of resources).
Regarding the remaining 70% of the multivariate association between parental education and resting-state connectivity patterns, it is likely that the contextualizing variables that we assessed were measured imperfectly, and improved measurement would lead to larger attenuation. Alternatively, it is possible that other contextualizing variables that were not assessed at the current time in the ABCD dataset play an important role in the parental education-functional connectivity association.
One such variable is parental educational expectations, which are found to predict youth educational outcomes longitudinally and over-and-above SER (Pinquart and Ebeling, 2020). Another may be genotype (Krapohl and Plomin, 2016), wherein genetic variants shared by parent and child may account for some of the parental education-functional connectivity association (note: children’s genetic data are collected in the ABCD study but parents’ are not). A third possibility is that these connectivity patterns linked to parental education have behavioral sequalae at later ages (e.g., mid- to late adolescence), and thus they do not share variance with the contextualizing variables measured at ages 9 and 10. Finally, parental education is one node in a dense, complex matrix of contextual influences that span multiple units of analysis (individual, family, neighborhood, district, region, etc.), where many elements of this matrix are unmeasured in the current study. Notable constructs include small area-level measures of structural inequality, which can be captured (albeit imperfectly) by historical maps of race-based redlining policies and inequities in the density of local services (e.g., libraries, healthcare) (Lynch et al., 2021). Ongoing efforts by ABCD-associated workgroups will expand measures of neighborhood resources to include aspects of the built and natural environments (Fan et al., 2021), which in the United States are also linked to structural racism through purposeful spatial patterning (Lynch et al., 2021, Braveman et al., 2022). Thus, overall, although the present study establishes that distributed functional connectivity patterns are associated with levels of parental education, the larger causal matrix that contextualizes and explains this association, and the directional relationships between key nodes, await further elucidation (Hyde et al., 2020a).
This study has additional limitations and caution should be taken in interpreting its results. A key limitation is that this is a correlational study that uses cross-sectional data from the baseline wave of the ABCD study. This type of study cannot be used to infer causal relationships between modeled variables (MacKinnon et al., 2012). Thus, while we performed analyses that identify shared connectivity-related variance among parental education, home enrichment activities, child’s grades, and child’s cognitive abilities, this study cannot identify directional relationships among these variables. In particular, stronger inferences about “mediation” and/or causal relationships require other kinds of data, such as longitudinal data or experimental manipulations (MacKinnon et al., 2012, Dearing and Hamilton, 2006). Another limitation is that the ABCD Study sample is disproportionately higher in SER relative to the national population, and inclusion criteria for our analysis (especially cutoffs for head motion) exacerbate this overrepresentation (Cosgrove et al., 2022), so care should be exercised in extrapolating our results to the general population.
Finally, the results of the current study should not be used to perpetuate static, deficit interpretations of development (Simmons et al., 2021). The brain is a highly plastic organ with abundant capacities to learn/relearn, modify, and adjust, consistent with the observation that substantial neural change and reorganization extends through late adolescence up to young adulthood (Casey et al., 2005, Grayson and Fair, 2017). Additionally, it is altogether possible that many of the SER-associated neural patterns we observed represent compensatory adjustments that help youth adaptively navigate features of their local environmental milieu (e.g., constrained opportunities, uncertainty) (Nketia et al., 2021).
In sum, in a large, rigorously characterized sample of youth, we identified highly distributed, brain-wide functional connectivity patterns linked to SER. Moreover, we demonstrated that parental education was the primary driver of these associations, advancing our understanding of how socio-environmental factors are linked to the developing connectome.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive
Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9–10 and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, and U24DA041147. A full list of supporters is available at https://abcdstudy.org/nih-collaborators. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/principal-investigators.html. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from NDA Study 721, 10.15154/1504041, which can be found at https://nda.nih.gov/study.html?id= 721. NDA Study 901, 10.15154/1520591, which can be found at https://nda.nih.gov/study.html?id= 901.
Footnotes
This work was supported by the following grants from the United States National Institutes of Health, the National Institute on Drug Abuse, and the National Institute on Alcohol Abuse and Alcoholism: R01MH123458 (CS), U01DA041106 (CS, MH, LH), T32 AA007477 (KC), K23 DA051561 (AW). This research was supported in part through computational resources and services provided by Advanced Research Computing at the University of Michigan, Ann Arbor.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2022.101164.
Appendix A. Supplementary material
Supplementary material
.
Data Availability
Data availability is provided at attach file step. The ABCD data used in this report came from NDA Study 901, 10.15154/1520591, which can be found at https://nda.nih.gov/study.html?id= 901. Code for running analyses can be found at https://github.com/SripadaLab/ABCD_Resting_Socioeconomic_Resources.
References
- Achenbach T.M., Ruffle T.M. The child behavior checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr. Rev. 2000;21:265–271. doi: 10.1542/pir.21-8-265. [DOI] [PubMed] [Google Scholar]
- Barch D., et al. Effect of hippocampal and amygdala connectivity on the relationship between preschool poverty and school-age depression. Am. J. Psychiatry. 2016;173:625–634. doi: 10.1176/appi.ajp.2015.15081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle E.A., Li Y.I., Pritchard J.K. An expanded view of complex traits: from polygenic to omnigenic. Cell. 2017;169:1177–1186. doi: 10.1016/j.cell.2017.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R.H., Corwyn R.F., McAdoo H.P., García Coll C. The home environments of children in the United States part I: Variations by age, ethnicity, and poverty status. Child Dev. 2001;72:1844–1867. doi: 10.1111/1467-8624.t01-1-00382. [DOI] [PubMed] [Google Scholar]
- Braveman P.A., Arkin E., Proctor D., Kauh T., Holm N. Systemic and structural racism: definitions, examples, health damages, and approaches to dismantling: study examines definitions, examples, health damages, and dismantling systemic and structural racism. Health Aff. 2022;41:171–178. doi: 10.1377/hlthaff.2021.01394. [DOI] [PubMed] [Google Scholar]
- Brislin S., et al. Differentiated nomological networks of internalizing, externalizing, and the general factor of psychopathology (“P factor”) in emerging adolescence in the ABCD study. Psychol. Med. 2021 doi: 10.1017/S0033291720005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Tottenham N., Liston C., Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn. Sci. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Castellanos F.X., Di Martino A., Craddock R.C., Mehta A.D., Milham M.P. Clinical applications of the functional connectome. NeuroImage. 2013;80:527–540. doi: 10.1016/j.neuroimage.2013.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.A., et al. The general factor of psychopathology in the adolescent brain cognitive development (ABCD) study: a comparison of alternative modeling approaches. Clin. Psychol. Sci. 2021 doi: 10.1177/2167702620959317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Janicki-Deverts D., Chen E., Matthews K.A. Childhood socioeconomic status and adult health. Ann. N. Y. Acad. Sci. 2010;1186:37–55. doi: 10.1111/j.1749-6632.2009.05334.x. [DOI] [PubMed] [Google Scholar]
- Conger R.D., Donnellan M.B. An interactionist perspective on the socioeconomic context of human development. Annu. Rev. Psychol. 2007;58:175–199. doi: 10.1146/annurev.psych.58.110405.085551. [DOI] [PubMed] [Google Scholar]
- Conger R.D., Donnellan M.B. An interactionist perspective on the socioeconomic context of human development. Annu Rev. Psychol. 2007;58:175–199. doi: 10.1146/annurev.psych.58.110405.085551. [DOI] [PubMed] [Google Scholar]
- Cooper M., Pugh A.J. Families Across the Income Spectrum: A Decade in Review. J. Marriage Fam. 2020;82:272–299. [Google Scholar]
- Cosgrove K.T., et al. Limits to the generalizability of resting-state functional magnetic resonance imaging studies of youth: an examination of ABCD Study® baseline data. Brain Imaging Behav. 2022:1–7. doi: 10.1007/s11682-022-00665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearing E., Hamilton L.C. Contemporary advances and classic advice for analyzing mediating and moderating variables. Monogr. Soc. Res. Child Dev. 2006;71:88–104. [Google Scholar]
- Dégeilh F., Beauchamp M.H., Leblanc É., Daneault V., Bernier A. Socioeconomic status in infancy and the developing brain: functional connectivity of the hippocampus and amygdala. Dev. Neurosci. 2019;41:327–340. doi: 10.1159/000507616. [DOI] [PubMed] [Google Scholar]
- DeJoseph M.L., Herzberg M.P., Sifre R.D., Berry D., Thomas K.M. Measurement matters: an individual differences examination of family socioeconomic factors, latent dimensions of children’s experiences, and resting state functional brain connectivity in the ABCD sample. Dev. Cogn. Neurosci. 2022;53 doi: 10.1016/j.dcn.2021.101043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J., et al. Imaging the deep cerebellar nuclei: a probabilistic atlas and normalization procedure. Neuroimage. 2011;54:1786–1794. doi: 10.1016/j.neuroimage.2010.10.035. [DOI] [PubMed] [Google Scholar]
- Donoho D., Jin J. Higher criticism for detecting sparse heterogeneous mixtures. Ann. Stat. 2004;32:962–994. [Google Scholar]
- Duncan G.J., Ziol-Guest K.M., Kalil A. Early-Childhood Poverty and Adult Attainment, Behavior, and Health. Child Dev. 2010;81:306–325. doi: 10.1111/j.1467-8624.2009.01396.x. [DOI] [PubMed] [Google Scholar]
- Ensminger M.E., Fothergill K.E., Bornstein M.H., Bradley R.H. A decade of measuring SES: what it tells us and where to go from here. Socioecon. Status Parent. Child Dev. 2003;13:27. [Google Scholar]
- Esteban O., et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat. Methods. 2019;16:111. doi: 10.1038/s41592-018-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C.C., et al. Adolescent Brain Cognitive Development (ABCD) study Linked External Data (LED): protocol and practices for geocoding and assignment of environmental data. Dev. Cogn. Neurosci. 2021;52 doi: 10.1016/j.dcn.2021.101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman D., Lane D., Nonstochastic A. Interpretation of reported significance levels. J. Bus. Econ. Stat. 1983;1:292–298. [Google Scholar]
- Garavan H., et al. Recruiting the ABCD sample: design considerations and procedures. Dev. Cogn. Neurosci. 2018;32:16–22. doi: 10.1016/j.dcn.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard M.E., Kemper K.E., MacLeod I.M., Chamberlain A.J., Hayes B.J. Genetics of complex traits: prediction of phenotype, identification of causal polymorphisms and genetic architecture. Proc. R. Soc. B Biol. Sci. 2016;283:20160569. doi: 10.1098/rspb.2016.0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E.M., et al. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb. Cortex N. Y. N. 1991. 2016;26:288–303. doi: 10.1093/cercor/bhu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson D.S., Fair D.A. Development of large-scale functional networks from birth to adulthood: a guide to the neuroimaging literature. NeuroImage. 2017;160:15–31. doi: 10.1016/j.neuroimage.2017.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J.G., et al. Childhood Adversities and Adult Psychiatric Disorders in the National Comorbidity Survey Replication I: Associations With First Onset of DSM-IV Disorders. Arch. Gen. Psychiatry. 2010;67:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman D.A., Farah M.J. Socioeconomic status and the developing brain. Trends Cogn. Sci. 2009;13:65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., et al. A family focused intervention influences hippocampal‐prefrontal connectivity through gains in self‐regulation. Child Dev. 2019;90:1389–1401. doi: 10.1111/cdev.13154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann F., Lee D.S., Lemieux T. Growing income inequality in the United States and other advanced economies. J. Econ. Perspect. 2020;34:52–78. [Google Scholar]
- Hyde L.W., et al. An ecological approach to understanding the developing brain: examples linking poverty, parenting, neighborhoods, and the brain. Am. Psychol. 2020;75:1245. doi: 10.1037/amp0000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde L.W., et al. An ecological approach to understanding the developing brain: examples linking poverty, parenting, neighborhoods, and the brain. Am. Psychol. 2020 doi: 10.1037/amp0000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S.B., Riis J.L., Noble K.G. State of the art review: poverty and the developing brain. Pediatrics. 2016;137 doi: 10.1542/peds.2015-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcher N.R., Barch D.M. The ABCD study: understanding the development of risk for mental and physical health outcomes. Neuropsychopharmacology. 2020:1–13. doi: 10.1038/s41386-020-0736-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kind A.J., et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann. Intern. Med. 2014;161:765–774. doi: 10.7326/M13-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapohl E., Plomin R. Genetic link between family socioeconomic status and children’s educational achievement estimated from genome-wide SNPs. Mol. Psychiatry. 2016;21:437–443. doi: 10.1038/mp.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird A.R., et al. Behavioral interpretations of intrinsic connectivity networks. J. Cogn. Neurosci. 2011;23:4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch E.E., et al. The legacy of structural racism: associations between historic redlining, current mortgage lending, and health. SSM-Popul. Health. 2021;14 doi: 10.1016/j.ssmph.2021.100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon D.P., Cheong J., Pirlott A.G. Statistical mediation analysis. APA Handb. Res. Methods Psychol. 2012;2:313–331. [Google Scholar]
- Marek S., et al. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022:1–7. doi: 10.1038/s41586-022-04492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nketia J., Amso D., Brito N.H. Towards a more inclusive and equitable developmental cognitive neuroscience. Dev. Cogn. Neurosci. 2021;52 doi: 10.1016/j.dcn.2021.101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn. Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piketty T., Saez E. Income inequality in the United States, 1913–1998. Q. J. Econ. 2003;118:1–41. [Google Scholar]
- Pinquart M., Ebeling M. Parental educational expectations and academic achievement in children and adolescents—a meta-analysis. Educ. Psychol. Rev. 2020;32:463–480. [Google Scholar]
- Power J.D., et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakesh D., Whittle S. Socioeconomic status and the developing brain–A systematic review of neuroimaging findings in youth. Neurosci. Biobehav. Rev. 2021;130:379–407. doi: 10.1016/j.neubiorev.2021.08.027. [DOI] [PubMed] [Google Scholar]
- Rakesh D., Zalesky A., Whittle S. Similar but distinct–effects of different socioeconomic indicators on resting state functional connectivity: Findings from the Adolescent Brain Cognitive Development (ABCD) Study®. Dev. Cogn. Neurosci. 2021;51 doi: 10.1016/j.dcn.2021.101005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakesh D., Seguin C., Zalesky A., Cropley V., Whittle S. Associations between neighborhood disadvantage, resting-state functional connectivity, and behavior in the Adolescent Brain Cognitive Development (ABCD) StudyⓇ: Moderating role of positive family and school environments. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2021;6:877–886. doi: 10.1016/j.bpsc.2021.03.008. [DOI] [PubMed] [Google Scholar]
- Raver C.C., Blair C. Developmental science aimed at reducing inequality: maximizing the social impact of research on executive function in context. Infant Child Dev. 2020 [Google Scholar]
- Reardon S.F., Bischoff K. Income inequality and income segregation. Am. J. Sociol. 2011;116:1092–1153. doi: 10.1086/657114. [DOI] [PubMed] [Google Scholar]
- Rosen M.L., Amso D., McLaughlin K.A. The role of the visual association cortex in scaffolding prefrontal cortex development: a novel mechanism linking socioeconomic status and executive function. Dev. Cogn. Neurosci. 2019;39 doi: 10.1016/j.dcn.2019.100699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M.D., Casey B.J., Holmes A.J. Prediction complements explanation in understanding the developing brain. Nat. Commun. 2018;9:1–13. doi: 10.1038/s41467-018-02887-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweder T., Spjøtvoll E. Plots of p-values to evaluate many tests simultaneously. Biometrika. 1982;69:493–502. [Google Scholar]
- Simmons C., et al. Responsible use of open-access developmental data: the adolescent brain cognitive development (ABCD) study. Psychol. Sci. 2021;32:866–870. doi: 10.1177/09567976211003564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., et al. Functional connectomics from resting-state fMRI. Trends Cogn. Sci. 2013;17:666–682. doi: 10.1016/j.tics.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. The human connectome: a complex network. Ann. N. Y. Acad. Sci. 2011;1224:109–125. doi: 10.1111/j.1749-6632.2010.05888.x. [DOI] [PubMed] [Google Scholar]
- Sporns O. Contributions and challenges for network models in cognitive neuroscience. Nat. Neurosci. 2014;17:652–660. doi: 10.1038/nn.3690. [DOI] [PubMed] [Google Scholar]
- Sripada C., et al. Disrupted network architecture of the resting brain in attention-deficit/hyperactivity disorder. Hum. Brain Mapp. 2014;35:4693–4705. doi: 10.1002/hbm.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada C., et al. Basic units of inter-individual variation in resting state connectomes. Sci. Rep. 2019;9:1900. doi: 10.1038/s41598-018-38406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada C., et al. Prediction of neurocognition in youth from resting state fMRI. Mol. Psychiatry. 2020;25:3413–3421. doi: 10.1038/s41380-019-0481-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada C., et al. Widespread attenuating changes in brain connectivity associated with the general factor of psychopathology in 9- and 10-year-olds. Transl. Psychiatry. 2021 doi: 10.1038/s41398-021-01708-w. (https:/doi.org/https://doi.org/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada C., et al. Brain-wide functional connectivity patterns support general cognitive ability and mediate effects of socioeconomic status in youth. Transl. Psychiatry. 2021 doi: 10.1038/s41398-021-01704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R.L., Cooper S.R., Jackson J.J., Barch D.M. Assessment of neighborhood poverty, cognitive function, and prefrontal and hippocampal volumes in children. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.23774. e2023774–e2023774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Zalesky A. Machine learning prediction of cognition from functional connectivity: are feature weights reliable? NeuroImage. 2021;245 doi: 10.1016/j.neuroimage.2021.118648. [DOI] [PubMed] [Google Scholar]
- Tian Y., Margulies D.S., Breakspear M., Zalesky A. Hierarchical organization of the human subcortex unveiled with functional connectivity gradients. bioRxiv. 2020 doi: 10.1038/s41593-020-00711-6. [DOI] [PubMed] [Google Scholar]
- Ursache A., Noble K.G. Neurocognitive development in socioeconomic context: multiple mechanisms and implications for measuring socioeconomic status. Psychophysiology. 2016;53:71–82. doi: 10.1111/psyp.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoquaux G., Poldrack R.A. Predictive models avoid excessive reductionism in cognitive neuroimaging. Curr. Opin. Neurobiol. 2019;55:1–6. doi: 10.1016/j.conb.2018.11.002. [DOI] [PubMed] [Google Scholar]
- Visscher P.M., et al. 10 years of GWAS discovery: biology, function, and translation. Am. J. Hum. Genet. 2017;101:5–22. doi: 10.1016/j.ajhg.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., et al. The conception of the ABCD study: from substance use to a broad NIH collaboration. Dev. Cogn. Neurosci. 2018;32:4–7. doi: 10.1016/j.dcn.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. Permutation inference for the general linear model. Neuroimage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C.-W., Chang L.J., Lindquist M.A., Wager T.D. Building better biomarkers: brain models in translational neuroimaging. Nat. Neurosci. 2017;20:365–377. doi: 10.1038/nn.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., et al. Individual differences in cognitive performance are better predicted by global rather than localized BOLD activity patterns across the cortex. Cereb. Cortex. 2021;31:1478–1488. doi: 10.1093/cercor/bhaa290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data availability is provided at attach file step. The ABCD data used in this report came from NDA Study 901, 10.15154/1520591, which can be found at https://nda.nih.gov/study.html?id= 901. Code for running analyses can be found at https://github.com/SripadaLab/ABCD_Resting_Socioeconomic_Resources.