Abstract
Volunteer studies with Vibrio cholerae O1 have shown that the best correlate of a vaccine's protective efficacy is its propensity to elicit serum bactericidal responses in its recipients. Attempts to detect such responses following infection with V. cholerae O139, however, have met with varying success. Using a tube-based assay which involves viable counting, we now report that strains of serogroup O139 can appear to be sensitive or resistant to a fixed concentration of complement in the presence of antibody, depending on assay conditions. Susceptibility to lysis is critically dependent on the availability of complement, but with O139 indicator strains this is not simply determined by the concentration of serum added to the reaction mix. The nature of the assay diluent and the concentration of indicator bacteria can also dramatically affect bactericidal end points, whereas such variables have minimal significance with O1 indicator bacteria. Although some laboratories use unencapsulated mutant strains to seek evidence of seroconversion following exposure to V. cholerae O139, this is not necessary, and our findings question the significance of capsule expression as a determinant of complement sensitivity when antibody is present. The medium used for growth of the indicator strain and the particular strain used appeared to be unimportant. Each of seven O139 isolates tested was found to be lysed by antibody and complement in our standard assay system, which allowed the detection of significant serum bactericidal responses in 9 of 11 cases of O139 disease.
Until recently, only Vibrio cholerae strains of the O1 serogroup had been associated with epidemics of cholera. However, in 1992, outbreaks in India and Bangladesh were attributed to isolates of a newly recognized serogroup, O139 (5, 16). Subsequent investigation suggested that this serogroup arose from an O1 strain of the El Tor biotype by acquisition of foreign DNA involved in the synthesis of serogroup-determining O antigen (4, 20). The resulting lipopolysaccharide (LPS) structure differs not only in the composition of the O-antigen repeat unit but also in that only 1 such subunit is linked to the core, compared with almost 20 in the case of the O1 serogroup (14). In addition, V. cholerae O139 strains produce a capsule which is thought to comprise additional polymerized O-repeat units which are not attached to the core structure (10, 23). This capsule has been associated with serum resistance (10, 23).
In some locations, V. cholerae O139 displaced V. cholerae O1 as the primary cause of cholera (5, 16). In contrast to the age-related incidence of O1 disease normally seen in regions where cholera is endemic, the majority of O139-related cholera victims were adults (5, 16). This indicated that the natural immunity to V. cholerae O1 acquired by older inhabitants of areas of endemicity afforded no protection against V. cholerae O139, suggesting that vaccines targeted against the O1 serogroup would be similarly ineffective against O139 strains. The rate at which the new serogroup spread to neighboring countries prompted fears of an eighth cholera pandemic, superimposed upon the continuing seventh pandemic caused by O1 El Tor strains (16). Accordingly, researchers were quick to begin the quest for an effective O139 cholera vaccine.
Volunteer studies revealed that the clinical profile of O139 disease was similar to that previously observed with O1 strains. An initial immunizing infection with pathogenic O139 vibrios (15), or administration of the live O139 vaccine candidate CVD112 (21), conferred a high degree of immunity to subsequent homologous rechallenge. In neither case, however, was this state of immunity accompanied by a detectable increase in the titer of serum bactericidal antibodies. This contrasts with earlier volunteer studies with V. cholerae O1, in which a vaccine's capacity to elicit bactericidal responses provided the best indicator of its protective efficacy (12). The failure of O139 strains to induce such responses was suggested to be the result of the capsule shielding underlying LPS and thereby reducing its immunogenicity (15, 21).
In other studies, however, oral immunization with live attenuated (7) or chemically inactivated (8) V. cholerae O139 has resulted in detectable serum bactericidal responses. Since the vaccine strains used in these studies also produce capsules, the basis for this inconsistency is unclear. The present report addresses the possibility that studies in which bactericidal responses have not been reported reflect a failure to detect, rather than a failure to elicit, antibodies with complement-dependent lytic activity. Whereas V. cholerae O1 is readily and reliably lysed by complement in the presence of specific antibody, this is not the case with O139 strains. One report (18) suggested that only certain O139 isolates were sensitive to antibody-dependent bacteriolysis, while other groups have used unencapsulated mutants as indicator strains to assist measurement of bactericidal responses following O139 infection (7, 13).
The apparently conflicting reports concerning the induction of serum bactericidal responses following exposure to V. cholerae O139 emanate from laboratories which employ microtiter plate-based bactericidal assay systems. The ability to assess killing spectrophotometrically makes it feasible for these groups to screen large numbers of serum samples, but it seems possible that this assay system might itself be a limiting factor in the detection of complement-fixing antibodies. For many years we have used a bactericidal assay which is performed in tubes and involves viable counting to assess residual bacterial survival. Using this method, we have been able to consistently demonstrate lysis of wild-type O139 strains. Given the potential value of serum bactericidal responses as an aid to O139 vaccine development, it seemed worthwhile to consider the ways in which the assay systems differ, in the hope of identifying those variables of greatest significance with respect to the susceptibility of O139 to antibody and complement. We now report that, depending on the conditions of the assay system, O139 vibrios can appear to be sensitive or resistant to antibody and complement. It has not been possible to correlate this phenotype with differential expression of surface capsule. Using our standard bactericidal assay, each of seven O139 isolates was sensitive to lysis, and significant serum responses were detected in 9 of 11 cases of O139 disease.
MATERIALS AND METHODS
Bacterial strains.
V. cholerae AI1837, AI1838, AI1841, AI1852, AI1854, AI1855, AI4260, and AI4450 are strains of serogroup O139 isolated in Bangladesh. V. cholerae H1 and 174 are of serogroup O1 and El Tor biotype. Bacteria were grown with vigorous shaking for 3 to 4 h at 37°C in either Oxoid Nutrient Broth (OX) (10 g of Oxoid Bacteriological Peptone per liter, 10 g of Oxoid Lab-Lemco Powder per liter, 5 g of NaCl per liter), Luria-Bertani medium (LB) (10 g of Difco Bacto Tryptone per liter, 5 g of Difco Bacto Yeast Extract per liter, 10 g of NaCl per liter, pH 7.0), or Difco brain heart infusion (BHI).
MAbs and patient sera.
Five monoclonal antibodies (MAbs) directed against LPS of serogroup O139 were used. Four (ICL 9 [immunoglobulin M {IgM} isotype], ICL11 [IgG3], ICL12 [IgG2b], and ICL13 [IgM]) have been described elsewhere and shown by a variety of assays to be sensitive diagnostic reagents for V. cholerae O139 (17); ICL 16 is of the IgM isotype. MAb 20B is specific for the A determinant of O1 serogroup LPS and from previous studies (S. R. Attridge, unpublished data) is known to be lytic for V. cholerae O1 in the presence of complement. An IgG fraction of a polyclonal rabbit antiserum raised against viable 569B bacteria (V. cholerae O1) was also used in bactericidal assays; this was absorbed with a 569B-165 hybrid strain to make it specific for O1 LPS as described previously (1). Serum and plasma samples were obtained from patients with bacteriologically confirmed O139 or O1 cholera. An initial acute-phase sample was taken on day 2, and a later sample (following convalescence) was taken on day 11 or 22; for convenience these are referred to as pre- and postinfection samples.
Bactericidal assays.
MAbs and patient sera were tested for their potential to effect complement-dependent lysis of V. cholerae. Antibodies were serially diluted (three- or fourfold dilutions in 150 to 200 μl) in assay diluent (see below) in 7.5- by 1.0-cm glass tubes. The indicator strain was usually grown to ca. 5 × 108 cells per ml in OX or LB, although killing end points were similar regardless of the growth phase at which the bacteria were harvested. After centrifugation, bacteria were resuspended in homologous culture medium (CM) and then diluted to ca. 4 × 103 cells per ml in CM containing guinea pig serum (usually at 20% vol/vol) as a source of complement. An equal volume of this suspension was added to the serial dilutions of the test antibodies (so that the final concentration of complement was generally 10%, vol/vol). The contents of the tubes were mixed, incubated at 37°C for 60 min, and transferred to an ice bath, and 50-μl aliquots were plated to determine residual viability (relative to control tubes lacking antibody). Bactericidal titers were obtained by interpolation of plots of percent survival versus log10 (reciprocal) antibody dilution and represent reciprocals of the dilutions capable of killing 50% of the indicator vibrios.
Generally the assay diluent was the CM used to grow the indicator strain. This represented an attempt to maintain the phenotype of the vibrios during the period of the assay, since initial experiments suggested the CM might be an important determinant of bacterial susceptibility to lysis by antibody and complement. In some experiments, however, bacteria were tested for sensitivity to MAb and complement in either phosphate-buffered saline (PBS) (pH 7.2) or heterologous CM.
Some assays were performed using mixed bacterial suspensions. We have previously reported that introduction of a chromosomal tcpA::Kan mutation does not alter the sensitivity of O1 serogroup V. cholerae to antibody-dependent, complement-mediated lysis (3). A similar mutant was available for the O139 strain AI1838 (2), and a preliminary test confirmed that it also showed unaltered susceptibility to lysis when both mutant and parent strains were grown and assayed in OX (data not shown). The presence of the Kan marker made it possible to separately enumerate vibrios grown in different CM in bactericidal assays performed on mixed suspensions.
Hemolysis assay.
Assay diluents (CM, PBS, or Mg2+-saline [2 mM MgCl2 in 0.85% {wt/vol} saline, pH 7.0]) were compared for possible inhibitory affects on complement activity by using a hemolysis assay. Guinea pig serum (as a complement source) was serially titrated, in duplicate in each diluent, across the rows of a plastic microtiter tray. An equal volume (50 μl) of rabbit hemolysin-sensitized sheep erythrocytes (at 1% [vol/vol] in each diluent) was added, and the tray was shaken briefly. After incubation at 37°C for 60 min, the tray was centrifuged and a multichannel pipettor was used to transfer 50-μl supernatant samples to a clean tray. Hemoglobin release was assessed by measuring the optical density at 405 nm in an enzyme-linked immunosorbent assay plate reader, and these readings used to plot percent lysis versus reciprocal serum dilution.
EM.
Bacteria were sectioned and examined by electron microscopy (EM) essentially as described elsewhere (9). Vibrios were harvested from shaking cultures by centrifugation, washed once in CM, and then fixed with glutaraldehyde (5% [vol/vol] in CM) for 90 min at 37°C and then overnight at 4°C. After three washes in PBS, the bacteria were incubated with polycationic ferritin (Sigma) (1 mg/ml), an electron-dense negative stain for capsular material, for 60 min at room temperature. Bacteria were washed twice in PBS, fixed with 1% osmium tetroxide, washed again in PBS, and then immobilized in 4% agarose. The agarose was sliced into small fragments, and then samples were dehydrated by exposure to increasing concentrations (30 to 100%) of ethanol. After being washed in propylene oxide, the samples were embedded in resin and sectioned. The thin sections were placed on grids, stained with uranyl acetate, and examined under a Philips transmission EM at 80 kV.
Immunoblotting.
AI1838 was grown in OX or LB for the normal period of 3 to 4 h, or overnight to achieve greater cell densities. After centrifugation, the bacteria were resuspended in PBS, with volumes being adjusted on the basis of the optical densities of the cultures at the time of harvest. An equal volume of 2× lysis buffer was added, and the suspensions were boiled for 5 min before overnight incubation at 56°C with proteinase K (0.1 mg/ml). Samples were stored frozen until sodium dodecyl sulfate-polyacrylamide gel electrophoresis (ca. 5 × 109 bacteria were loaded per lane) and transfer to nitrocellulose as described elsewhere (22). MAbs to O139 polysaccharide were applied, and binding was detected using horseradish peroxidase-conjugated anti-mouse IgG followed by enhanced chemiluminescence (22).
RESULTS
Susceptibility of V. cholerae O139 to lysis by antibody and complement.
Preliminary bactericidal assays confirmed that MAbs directed against the O antigens of serogroup O1 or O139 mediate complement-dependent lysis of vibrios of the homologous serogroup only (data not shown). These assays were performed using our normal growth medium, OX, as CM and assay diluent. However, BHI is commonly used to culture O139 indicator bacteria for use in plate-based bactericidal assays (8, 19), while others have used LB (7). Surprisingly, O139 vibrios grown (and assayed) in LB or BHI were resistant to killing by antibody and complement (data not shown, but see below). Each of five O139 strains showed a similar medium-dependent susceptibility to complement in the presence of any of five MAbs to O139 LPS, whereas O1 strains H1 and 174 were equally sensitive whether grown in OX, LB, or BHI (data not shown).
Capsule expression by OX- and LB-grown V. cholerae O139.
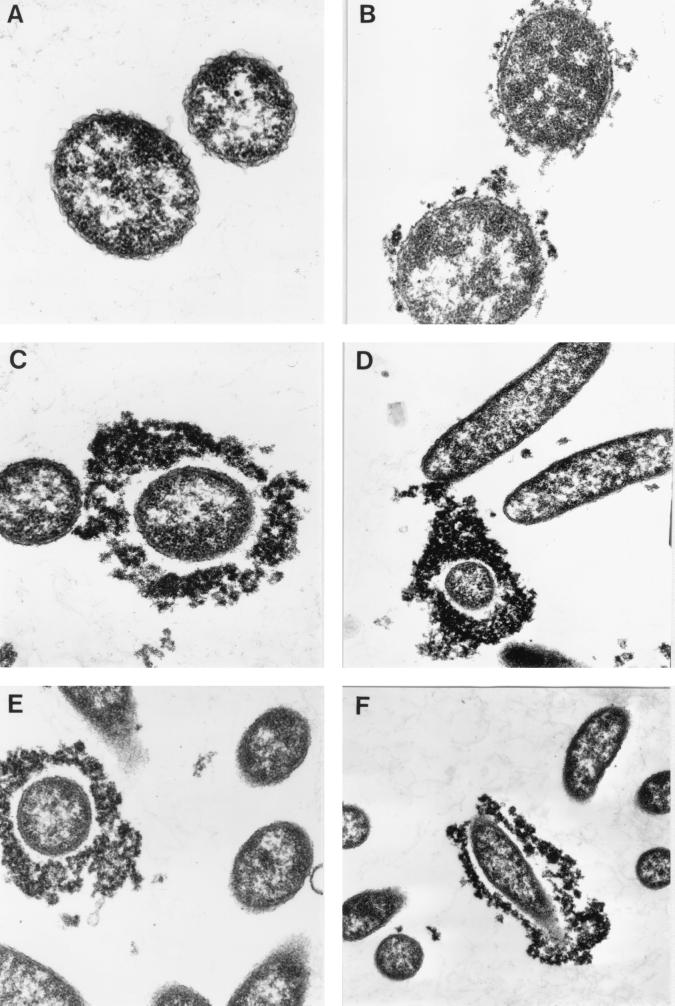
Strains of serogroup O139 have been reported to produce a capsule following growth in LB, and this has been associated with complement resistance (23). Moreover, some groups use unencapsulated mutants to facilitate detection of bactericidal antibodies to O139 (7, 13). It was therefore of great interest to ascertain whether the level of capsule synthesis might vary in different CM and, if so, whether such variation parallels observed differences in susceptibility to antibody and complement. AI1838, as well as the O1 strain H1 as a control, was grown in OX, LB, and BHI. Washed bacteria were glutaraldehyde fixed, incubated with polycationic ferritin for detection of capsular material, and examined by EM.
Little or no ferritin binding was seen with H1 bacteria, regardless of the CM used (Fig. 1A). In contrast, the vast majority of AI1838 bacteria were labeled after growth in BHI or LB (Fig. 1B), although the level of ferritin binding was much less dramatic than that shown in other reports (6). AI1838 grown in OX showed great variation in ferritin binding, as shown in Fig. 1C to F. While most bacteria showed no labeling, a significant minority resembled the heavily encapsulated O139 vibrios described by others (6). The latter were consistently present in OX-AI1838 preparations but were not seen in LB- or BHI-grown suspensions of the same strain.
FIG. 1.
Capsule production by V. cholerae. Ferritin labeling in sections of OX-grown H1 (A) (magnification, ×52,000) LB-grown AI1838 (B) (×39,000), and OX-grown AI1838 (C to F) (×39,000, ×21,000, ×21,000, and ×16,000, respectively) are shown.
Waldor et al. (23) reported that O139 capsular material could be visualized by immunoblotting whole-cell lysates electrophoresed on acrylamide gels. Using this approach, it was possible to detect polysaccharide running in the “medium migrating” position of the gel (23); similar amounts of this material were associated with AI1838 bacteria following growth in LB or OX (not shown).
Significance of assay diluent.
Other experiments examined the impact of varying the diluent used in the bactericidal assay. Initially AI1838 was cultured in OX or LB, and the organisms were harvested by centrifugation and then washed and resuspended in PBS. The bacteria were then tested for susceptibility to antibody and complement, either immediately or after incubation in PBS at 37°C for 30 min, in bactericidal assays using PBS as the diluent. All four suspensions were equally sensitive to lysis by ICL11, with the titers (5.8 × 104 to 8.2 × 104) being typical of those previously seen with AI1838 vibrios grown and assayed in OX. Similar results were obtained in a subsequent experiment. OX- and LB-AI1838 cultures were subdivided, with one aliquot diluted in homologous CM for estimation of the ICL11 bactericidal titer. The other aliquots were spun, and the bacteria were resuspended and immediately diluted in PBS for exposure to the same antibody. As shown in Table 1 (experiment A), LB-AI1838 was effectively lysed when assayed in PBS, but no killing was observed in LB diluent.
TABLE 1.
Assay diluent as a critical determinant of bactericidal titers against V. cholerae O139a
| Expt | Culture medium | Assay diluent | Titer of ICL11 |
|---|---|---|---|
| A | OX | OX | 1.7 × 105 |
| OX | PBS | 5.8 × 104 | |
| LB | LB | <103 | |
| LB | PBS | 3.1 × 104 | |
| B | OX | OX | 4.6 × 105 |
| OX | LB | <400 | |
| LB | OX | 3.6 × 105 | |
| LB | LB | <400 | |
| C | OX | OX | 2.8 × 105 |
| OX | BHI | <400 | |
| BHI | OX | 2.0 × 105 | |
| BHI | BHI | <400 |
The O139 serogroup strain AI1838 was cultured in OX, LB, or BHI and then tested for sensitivity to ICL11-mediated, complement-dependent lysis. Assay diluents were either PBS compared to homologous CM (experiment A) or homologous CM compared to heterologous CM (experiments B and C).
Further experiments showed that lysis of OX-grown AI1838 was also diluent dependent. Strain AI1838 was cultured in OX or LB, and then each suspension was diluted (ca. 105-fold) in both media for assessment of MAb (ICL11)-dependent, complement-mediated lysis. As shown in Table 1 (experiment B), bacteria grown in either CM were readily lysed if the assays were performed in OX, but no killing was detected in LB diluent. A similar result was obtained when AI1838 was grown in BHI then assayed in BHI or OX; OX diluent was supportive of bacteriolysis, but BHI was not (Table 1, experiment C).
These experiments indicated that susceptibility to lysis was determined by the nature of the assay diluent rather than the CM. Confirmation that LB-grown AI1838 bacteria were not inherently more resistant to lysis came from assays performed on mixed suspensions of LB- and OX-grown vibrios. AI1838 and its tcpA::Kan mutant, which displays unaltered susceptibility to antibody and complement (see Materials and Methods), were each cultured in OX and LB. Two mixed suspensions were prepared in OX diluent, one with approximately equal concentrations of OX-grown AI1838 and LB-grown AI1838 tcpA::Kan and the other with LB-grown AI1838 and OX-grown AI1838 tcpA::Kan. Aliquots of both suspensions were incubated, in duplicate, with complement alone or additionally with either of two concentrations of ICL12. After incubation for 60 min at 37°C, all suspensions were plated in triplicate on nutrient agar with or without kanamycin. The ratios of wild-type to mutant bacteria recovered from tubes containing antibody (at concentrations resulting in ∼65 or ∼90% killing) were not consistently different from those in control tubes. For the mixed OX-AI1838–LB-AI1838 tcpA::Kan suspension, these ratios were 0.84, 0.87, and 0.78 respectively; for the reciprocal suspension, they were 0.97, 1.36, and 1.58, respectively.
The assay diluent influences efficiency of complement activation.
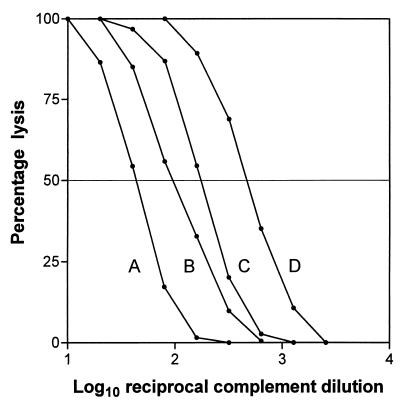
One possible explanation for the variable, diluent-dependent lysis of V. cholerae O139 is that the assay diluent somehow influences the availability or function of one or more of the complement subcomponents. This possibility was addressed in a different assay system which also depends on complement activation. Two batches of complement were separately titrated for lytic activity against antibody-sensitized erythrocytes in a hemolysis assay. To assess the significance of the assay diluent, parallel titrations were performed in OX, LB, PBS, or Mg2+-saline. Representative data are shown in Fig. 2 and confirm that lytic activity is indeed dependent upon the diluent used. Of greatest relevance in the present context, LB medium was less supportive of complement function than OX (a two- to threefold difference in lytic end points [e.g., titers of 45 and 100, respectively, in Fig. 2]).
FIG. 2.
Effect of assay diluent on sensitivity of complement-mediated hemolysis. Percent hemolysis versus reciprocal complement dilution using various assay diluents is shown. A, LB (50% hemolytic titer, 45); B, OX (100); C, PBS (175); D, Mg2+-saline (470).
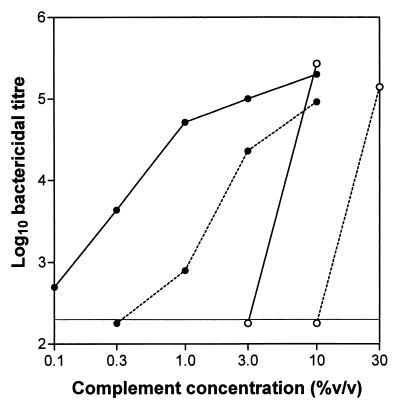
This result offered an explanation for the apparent resistance of LB-grown (and assayed) O139 to lysis by antibody and complement. It also suggested that it might be possible to achieve lysis of such bacteria by increasing the concentration of complement; conversely, lysis of OX-O139 might be less efficient if the complement level was reduced. The effect of complement concentration on bactericidal titers was therefore examined, using indicator strains of either serogroup and LB or OX as the assay diluent. These results are shown in Fig. 3.
FIG. 3.
Effect of complement concentration on bactericidal titer. The effect of varying complement concentrations on bactericidal end points is shown. Antibodies of the IgG isotype (ICL11 versus AI1838; polyclonal anti-V. cholerae versus H1) were titrated against the appropriate indicator strain (AI1838 [○] or H1 [●]) using OX (solid lines) or LB (dotted lines) as the assay diluent.
The antibodies used were both of the IgG isotype and showed similar bactericidal activities against the appropriate indicator strain under our standard assay conditions (OX diluent, 10% complement). This experiment (which was subsequently repeated with very similar results) shows that, by increasing the concentration of complement to 30%, it is indeed possible to lyse AI1838 even when LB is used as the assay diluent. Moreover, the titer observed is similar to that seen at a level of 10% complement in OX. In either case, a threefold reduction in complement concentration is sufficient to completely prevent antibody-dependent lysis (Fig. 3).
In contrast, bactericidal titers against the O1 indicator strain H1 were less dramatically affected by reductions in the level of complement. In OX diluent an initial 10-fold fall in complement concentration resulted in only a fourfold reduction in lytic titer, although further reductions had more marked effects on titers (Fig. 3). In LB diluent the effects of reducing complement levels were more pronounced, in line with the reduced efficiency of complement fixation in this medium (Fig. 3).
Other variables which may influence bactericidal end points.
In addition to the CM, the assay diluent, and the level of complement added to the reaction mix, other parameters which vary in bactericidal assays performed in different laboratories include the nature of the indicator strain and the concentration of indicator bacteria. Of eight O139 strains examined, seven were serum resistant, i.e., able to survive incubation with complement in the absence of antibody. Each of these strains was susceptible to lysis by MAb to O139 LPS in our standard assay system.
Plate-based bactericidal assays use much higher concentrations of indicator bacteria than that employed in our system. To assess the significance of this variable, parallel assays were performed (in OX diluent) using OX-grown vibrios of the O1 or O139 serogroup present at ∼2 × 103 or ∼2 × 107 per ml. As shown in Table 2, bactericidal titers were reduced at the higher concentration of indicator organisms, whether the antibodies were of the IgG or IgM isotype. Although this effect was fairly limited in assays with O1 bacteria, dramatic (16 to 53-fold) reductions were observed in bactericidal titers against O139 strains.
TABLE 2.
Concentration of indicator organisms influences bactericidal titersa
| Indicator strain | Antibody isotype | Expt. no. | Titer with the following concn of indicator bacteria:
|
Titer ratio (a/b) | |
|---|---|---|---|---|---|
| 2 × 103 (a) | 2 × 107 (b) | ||||
| H1 | IgG | 1 | 1.4 × 105 | 4.2 × 104 | 3.3 |
| 2 | 1.4 × 105 | 3.0 × 104 | 4.7 | ||
| IgM | 1 | 7.4 × 105 | 3.3 × 105 | 2.2 | |
| 2 | 3.8 × 105 | 2.0 × 105 | 1.9 | ||
| AI1838 | IgG | 1 | 3.2 × 105 | 1.7 × 104 | 19 |
| 2 | 3.7 × 105 | 7.0 × 103 | 53 | ||
| IgM | 1 | 4.2 × 105 | 2.7 × 104 | 16 | |
| 2 | 4.6 × 105 | 9.7 × 103 | 47 | ||
Antibodies of the IgG or IgM isotype were titrated for their capacities to lyse OX-grown H1 or OX-grown AI1838, using indicator bacteria at concentrations of ca. 2 × 103 or ca. 2 × 107 per ml. Each comparison was performed twice. Antibodies tested against H1 were an IgG fraction from a polyclonal rabbit antiserum and MAb 20B (IgM); those tested against AI1838 were ICL11 (IgG) and ICL16 (IgM).
Assessment of bactericidal responses following O139 cholera.
Paired serum samples from six bacteriologically confirmed cases of cholera caused by strains of V. cholerae O139 were tested for evidence of bactericidal responses, using OX-grown AI1838 as the indicator organism in our standard assay system (OX diluent, 10% complement, ca. 2 × 103 bacteria per ml). Increases in bactericidal titer ranging from 7- to over 80-fold were detected (Table 3, group A). No significant increases in titer were observed when the sera were tested against OX-grown H1 (data not shown). Subsequently, paired plasma samples from five O1 and five O139 cholera victims were also tested for bactericidal activity against indicator strains of the homologous serogroup. All five O1 patients, and three of five O139 patients, mounted significant responses (Table 3, groups B and C).
TABLE 3.
Bactericidal responses following V. cholerae O139 or O1 diseasea
| Group | Patient | Bactericidal titer
|
Fold rise in titer | |
|---|---|---|---|---|
| Preinfection | Postinfection | |||
| A | 1 | <20 (<10) | 1,200 (40) | 60 (4.0) |
| 2 | 630 | 4,400 | 7.0 | |
| 3 | 45 (<10) | 2,800 (50) | 62 (5.0) | |
| 4 | 30 | 420 | 14 | |
| 5 | 52 (<10) | 4,300 (86) | 83 (8.6) | |
| 6 | 42 | 320 | 7.6 | |
| B | 7 | 42 (<10) | 890 (12) | 21 (1.2) |
| 8 | 126 | 86 | 0.68 | |
| 9 | 14 | 16 | 1.1 | |
| 10 | 19 (<10) | 320 (14) | 17 (1.4) | |
| 11 | <10 (<10) | 2,000 (70) | 200 (7.0) | |
| C | i | 2,400 | 11,600 | 4.8 |
| ii | 25 | 30,000 | 1,200 | |
| iii | 1,660 | 162,000 | 98 | |
| iv | 280 | 66,000 | 236 | |
| v | 170 | 15,000 | 88 | |
Groups A and B (patients 1 to 11) include O139 patients, while group C (patients i to iv) includes O1 patients. Samples were either serum (group A) taken at day 2 (preinfection) and day 22 (postinfection) or plasma (groups B and C) taken at day 2 (preinfection) and day 11 (postinfection); the latter samples were diluted 1 in 2 during collection, but titers have not been adjusted. Assays were performed using OX-grown AI1838, (groups A and B) or OX-grown H1 (group C) as indicator bacteria, generally at a concentration of ∼2 × 103 per ml. Some samples were reassayed with indicators at ∼2 × 107 per ml; titers under these conditions are shown in parentheses.
The six pairs of serum samples which showed the greatest increase in bactericidal titer against V. cholerae O139 were reassayed, using a higher concentration of OX-grown AI1838 indicator organisms (ca. 2 × 107 per ml). The postinfection samples now showed dramatically reduced titers, resulting in much weaker apparent responses (Table 3, groups A and B). The geometric mean fold rise among this limited cohort was now only 3.6 (range, 1.2 to 8.6), compared with 53 (range, 17 to 200) under standard assay conditions.
DISCUSSION
Whether grown in OX, LB, or BHI, V. cholerae O139 was readily lysed in assays performed using OX diluent but not in those using LB or BHI (Table 1). EM (Fig. 1) and immunoblotting studies failed to reveal a difference in capsule expression which correlated with susceptibility to antibody and complement. Paradoxically, it was only in samples prepared from OX-grown O139 that the thick capsules described by others (6) were observed. The presence of a significant minority of heavily encapsulated bacteria in suspensions which showed uniform sensitivity to lysis suggested that capsular material might not be responsible for the apparent resistance of LB- and BHI-grown O139 strains to lysis by antibody and complement.
Other experiments suggested an alternative explanation for the link between assay diluent and susceptibility to complement. Hemolysis assays demonstrated an effect of the diluent on the efficiency of complement activation (Fig. 2). In particular, lytic titers were higher in OX than in LB, prompting an assessment of the consequences of titrating complement levels in bactericidal assays performed using these diluents. This showed that, even with LB as the assay diluent, LB-grown AI1838 was indeed susceptible to antibody-dependent lysis if the concentration of complement was increased threefold to a final concentration of 30%. Under these conditions, the lytic titer of ICL12 was similar to that seen in our standard assay system, using OX-grown AI1838 indicators (at ∼2 × 103 per ml), OX diluent, and 10% complement (Fig. 3). In either case a threefold decrease in complement level completely abrogated bacteriolysis.
In the same experiments, lytic titers against the O1 indicator strain were less dramatically affected by reductions in complement concentration (Fig. 3). Clearly, the level of complement added to the assay system is a prime determinant of lytic sensitivity, but the significance of these experiments lies in their implications for the development of O139 bactericidal assays. Compared to the lysis of O1 vibrios, the demonstration of O139 lysis is much less tolerant of suboptimal levels of complement. A similar difference between the serogroups was evident when we assessed the effect of varying the concentration of indicator organisms used in bactericidal assays. Antibody titers against the O1 strain H1 fell ∼3-fold in response to a 104-fold increase in bacterial concentration, whereas titers against AI1838 fell ∼30-fold (Table 2). Evidently complement becomes limiting at the higher concentration of the O139 strain, confirming the notion that this serogroup is comparatively resistant to killing by antibody and complement. The reason for this remains to be elucidated, but clearly one possibility is that the presence of capsular material competitively blocks binding of antibody to the underlying core-linked O-antigen subunit. This might lead to nonproductive fixation of complement on the bacterial surface, reducing the availability of complement components to antibodies whose location confers the potential for lysis.
When our standard assay system was used to seek evidence of bactericidal responses following O139 or O1 cholera, seroconversion was detected in 9 of 11 O139 patients and 5 of 5 O1 patients (Table 3). Pre- and postinfection titers were generally lower against V. cholerae O139, but among responders the titer increases were of similar magnitude with the two serogroups. This is consistent with the findings of a recent, more comprehensive study (19). The impact of using a higher concentration of indicator bacteria in the O139 assay system was revealed by repeating the titration of the serum pairs showing the greatest increases in lytic titer. The apparent bactericidal responses were now dramatically lower (Table 3). The requirement of plate-based assays for much higher concentrations of indicator bacteria is therefore likely to seriously compromise detection of bactericidal responses (Tables 2 and 3). This probably explains why the use of selected mutant or variant strains of O139 is sometimes necessary to demonstrate lysis by antibody and complement.
Using our system, however, all seven (serum-resistant) O139 isolates examined were lysed in the presence of MAb to O139 LPS. Qadri et al. (18) reported that AI1837 and AI1838, two of the strains checked by us, did not allow the detection of bactericidal responses following O139 infection, but theirs was a plate-based assay, with indicator bacteria at 0.27 × 107 per ml and a final complement concentration of only 5%. These workers used strain 4260B as an indicator, following the demonstration that A and B variants can be isolated from V. cholerae O139 strains. The A variants resist killing by antibody and complement, whereas the B variants are susceptible, even though both retain the capacity to produce a capsule (11). This is consistent with the finding that in our assay system, capsule production does not appear to be the major impediment to lysis by antibody and complement.
The tube-based assay used in the present studies is suitable for demonstrating lysis of V. cholerae O139 by antibody and complement and for the detection of serum bactericidal responses after infection by strains of this serogroup. Although comparatively labor intensive, this assay can nevertheless be applied to 10 to 15 serum pairs at a time and could therefore prove useful as an additional measure of immunogenicity during testing of O139 candidate vaccines. Clearly, however, plate-based assays are much more convenient than the viable counting approach used here. Studies are under way to define an optimal combination of assay parameters which would allow us to combine the sensitivity of the tube-based method with the screening power of the plate-based assays.
ACKNOWLEDGMENTS
This study was supported by the Australian National Health and Medical Research Council.
We thank Marilyn Henderson (Centre for Electron Microscopy and Micro Analysis, The University of Adelaide) and Uwe Stroeher for assistance with EM and Ann-Mari Svennerholm and Kevin Killeen for detailed descriptions of their bactericidal assay systems.
REFERENCES
- 1.Attridge S R, Daniels D, Morona J K, Morona R. Surface co-expression of Vibrio cholerae and Salmonella typhi O-antigens on Ty21a clone EX210. Microb Pathog. 1990;8:177–188. doi: 10.1016/0882-4010(90)90045-r. [DOI] [PubMed] [Google Scholar]
- 2.Attridge S R, Manning P A, Holmgren J, Jonson G. Relative significance of mannose-sensitive hemagglutinin and toxin-coregulated pili in colonization of infant mice by Vibrio cholerae El Tor. Infect Immun. 1996;64:3369–3373. doi: 10.1128/iai.64.8.3369-3373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attridge S R, Voss E, Manning P A. The role of toxin-coregulated pili in the pathogenesis of Vibrio cholerae O1 El Tor. Microb Pathog. 1993;15:421–431. doi: 10.1006/mpat.1993.1091. [DOI] [PubMed] [Google Scholar]
- 4.Bik E M, Bunschoten A E, Gouw R D, Mooi F R. Genesis of the novel epidemic Vibrio cholerae O139 strain: evidence for horizontal transfer of genes involved in polysaccharide synthesis. EMBO J. 1995;14:209–216. doi: 10.1002/j.1460-2075.1995.tb06993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cholera Working Group. Large epidemic of cholera-like disease in Bangladesh caused by Vibrio cholerae O139 synonym Bengal. Lancet. 1993;342:387–390. [PubMed] [Google Scholar]
- 6.Comstock L E, Maneval D, Jr, Panigrahi P, Joseph A, Levine M M, Kaper J B, Morris J G, Jr, Johnson J A. The capsule and O antigen in Vibrio cholerae O139 Bengal are associated with a genetic region not present in Vibrio cholerae O1. Infect Immun. 1995;63:317–323. doi: 10.1128/iai.63.1.317-323.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coster T S, Killeen K P, Waldor M K, Beattie D T, Spriggs D R, Kenner J R, Trofa A, Sadoff J C, Mekalanos J J, Taylor D N. Safety, immunogenicity, and efficacy of live attenuated Vibrio cholerae O139 vaccine prototype. Lancet. 1995;345:949–952. doi: 10.1016/s0140-6736(95)90698-3. [DOI] [PubMed] [Google Scholar]
- 8.Jertborn M, Svennerholm A-M, Holmgren J. Intestinal and systemic immune responses in humans after oral immunization with a bivalent B subunit-O1/O139 whole cell cholera vaccine. Vaccine. 1996;15:1459–1465. doi: 10.1016/s0264-410x(96)00071-0. [DOI] [PubMed] [Google Scholar]
- 9.Johnson J A, Panigrahi P, Morris J G., Jr Non-O1 Vibrio cholerae NRT365 produces a polysaccharide capsule that determines colony morphology, serum resistance, and virulence in mice. Infect Immun. 1992;60:864–869. doi: 10.1128/iai.60.3.864-869.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson J A, Salles C A, Panigrahi P, Albert M J, Wright A C, Johnson R J, Morris J G., Jr Vibrio cholerae O139 synonym Bengal is closely related to Vibrio cholerae El Tor but has important differences. Infect Immun. 1994;62:2108–2110. doi: 10.1128/iai.62.5.2108-2110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jonson G, Osek J, Svennerholm A-M, Holmgren J. Immune mechanisms and protective antigens of Vibrio cholerae serogroup O139 as a basis for vaccine development. Infect Immun. 1996;64:3778–3785. doi: 10.1128/iai.64.9.3778-3785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine M M, Tacket C O. Recombinant live cholera vaccines. In: Wachsmuth I K, Blake P A, Olsvik O, editors. Vibrio cholerae and cholera. Molecular to global perspectives. Washington, D.C.: ASM Press; 1994. pp. 395–413. [Google Scholar]
- 13.Losonsky G A, Lim Y, Motamedi P, Comstock L E, Johnson J A, Morris J G, Jr, Tacket C O, Kaper J B, Levine M M. Vibriocidal antibody responses in North American volunteers exposed to wild-type or vaccine Vibrio cholerae O139: specificity and relevance to immunity. Clin Diagn Lab Immunol. 1997;4:264–269. doi: 10.1128/cdli.4.3.264-269.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manning P A, Stroeher U H, Morona R. Molecular basis for O-antigen biosynthesis in Vibrio cholerae O1: Ogawa-Inaba switching. In: Wachsmuth I K, Blake P A, Olsvik O, editors. Vibrio cholerae and cholera. Molecular to global perspectives. Washington, D.C.: ASM Press; 1994. pp. 77–94. [Google Scholar]
- 15.Morris J G, Jr, Losonsky G E, Johnson J A, Tacket C O, Nataro J P, Panigrahi P, Levine M M. Clinical and immunologic characteristics of Vibrio cholerae O139 Bengal infection in North American volunteers. J Infect Dis. 1995;171:903–908. doi: 10.1093/infdis/171.4.903. [DOI] [PubMed] [Google Scholar]
- 16.Nair G B, Ramamurthy T, Bhattacharya S K, Mukhopadhyay A K, Garg S, Bhattacharya M K, Takeda T, Shimada T, Takeda Y, Deb B C. Spread of Vibrio cholerae O139 Bengal in India. J Infect Dis. 1994;169:1029–1034. doi: 10.1093/infdis/169.5.1029. [DOI] [PubMed] [Google Scholar]
- 17.Qadri F, Azim T, Chowdhury A, Hossain J, Sack R B, Albert M J. Production, characterization, and application of monoclonal antibodies to Vibrio cholerae O139 synonym Bengal. Clin Diagn Lab Immunol. 1994;1:51–54. doi: 10.1128/cdli.1.1.51-54.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qadri F, Mohi G, Hossain J, Azim T, Khan A M, Salam M A, Sack R B, Albert M J, Svennerholm A-M. Comparison of the vibriocidal antibody response in cholera due to Vibrio cholerae O139 Bengal with the response in cholera due to Vibrio cholerae O1. Clin Diagn Lab Immunol. 1995;2:685–688. doi: 10.1128/cdli.2.6.685-688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qadri F, Wenneras C, Albert M J, Hossain J, Mannoor K, Begum Y A, Mohi G, Salam M A, Sack R B, Svennerholm A-M. Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect Immun. 1997;65:3571–3576. doi: 10.1128/iai.65.9.3571-3576.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stroeher U H, Jedani K E, Dredge B K, Morona R, Brown M H, Karageorgos L E, Albert M J, Manning P A. Genetic rearrangements in the rfb regions of Vibrio cholerae O1 and O139. Proc Natl Acad Sci USA. 1995;92:10374–10378. doi: 10.1073/pnas.92.22.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tacket C O, Losonsky G, Nataro J P, Comstock L, Michalski J, Edelman R, Kaper J B, Levine M M. Initial clinical studies of CVD112 Vibrio cholerae O139 live oral vaccine: safety and efficacy against experimental challenge. J Infect Dis. 1995;172:883–886. doi: 10.1093/infdis/172.3.883. [DOI] [PubMed] [Google Scholar]
- 22.Voss E, Attridge S R. In vitro production of toxin-coregulated pili by Vibrio cholerae El Tor. Microb Pathog. 1993;15:255–268. doi: 10.1006/mpat.1993.1076. [DOI] [PubMed] [Google Scholar]
- 23.Waldor M K, Colwell R, Mekalanos J J. The Vibrio cholerae O139 serogroup antigen includes an O-antigen capsule and lipopolysaccharide virulence determinants. Proc Natl Acad Sci USA. 1994;91:11388–11392. doi: 10.1073/pnas.91.24.11388. [DOI] [PMC free article] [PubMed] [Google Scholar]