Highlights
-
•
PERTINENT is a European active hospital-based surveillance system for pertussis in infants.
-
•
We measured the effectiveness of maternal and primary vaccination in infants <2 and 2–11 months.
-
•
Maternal vaccination reduced severe pertussis incidence in infants too young for vaccination.
-
•
Infants’ vaccination reduced severe pertussis irrespective of the mother’s vaccination status.
-
•
Only network allowing for independent multi-country pertussis vaccine effectiveness studies.
Keywords: Pertussis, Vaccine effectiveness, Vaccine in pregnancy, Whooping cough, Hospital surveillance
Abbreviations: PV, primary vaccination; PERTINENT, Pertussis in Infants European Network
Abstract
Background
PERTINENT is an active hospital-based surveillance system for pertussis in infants. In 2019, four of the six participating European countries recommended pertussis vaccination in pregnancy. Among infants aged <2 months, we measured the vaccine effectiveness (VE) in pregnancy; among infants aged 2–11 months, VE of vaccination in pregnancy and of primary vaccination (PV).
Methods
From December 2015 to 2019, we included all infants aged <1 year presenting with pertussis-like symptoms. Using a test-negative-design, cases were infants testing positive for Bordetella pertussis by PCR or culture. Controls were those testing negative for all Bordetella species. Vaccinated mothers were those who received vaccine in pregnancy. Vaccinated infants were those who received ≥1 dose of PV > 14 days before symptom onset. We excluded infants with unknown maternal or PV status or with mothers vaccinated ≤14 days before delivery. We calculated pooled VE as 100 * (1-odds ratio of vaccination) adjusted for study site, onset date in quarters and infants’ age group.
Results
Of 829 infants presenting with pertussis-like symptoms, 336 (41%) were too young for PV. For the VE in pregnancy analysis, we included 75 cases and 201 controls. Vaccination in pregnancy was recorded for 9 cases (12%) and 92 controls (46%), adjusted VE was between 75% [95%CI: 35–91%] and 88% [95%CI: 57–96%].
Of 493 infants eligible for PV, we included 123 cases and 253 controls. Thirty-one cases and 98 controls recorded both PV with ≥ 1 dose and vaccination in pregnancy, adjusted VE was between 74% [95%CI: 33–90] and 95% [95%CI: 69–99]; 27 cases and 53 controls recorded PV only, adjusted VE was between 68% [95%CI: 27–86] and 94% [95%CI: 59–99].
Conclusion
Our findings suggest that vaccination in pregnancy reduces pertussis incidence in infants too young for PV. In infants aged 2–11 months, PV only and both PV and vaccination in pregnancy provide significant protection against severe pertussis.
1. Introduction
Pertussis (whooping cough) is a highly contagious acute respiratory infection caused by the bacterial pathogen Bordetella pertussis. In 2019, across the 30 European Union/European Economic Area (EU/EEA) Member States reporting pertussis data to the European Centre for Disease Prevention and Control (ECDC), infants aged <1 year were the most affected age group (46.8 per 100,000 population). Three deaths were reported that year in infants, all were too young to have received the first dose of primary vaccination (PV) [1].
Pertussis PV includes three doses in the first year of life and aims to reduce the risk of severe pertussis in infants.
After the introduction in the 1950s of pertussis vaccination with whole-cell (wP) vaccine in children in Europe, pertussis incidence and mortality markedly decreased [2]. Most European countries replaced wP with acellular-pertussis (aP) containing vaccine in the 1990s, which is less reactogenic. After a continued decline, reported cases have progressively increased again in recent years with the last peak incidence in 2012 with >42,000 reported cases in EU/EEA [3]. The World Health Organization (WHO) estimated that pertussis was still responsible for around 63,000 deaths in children aged <5 years worldwide in 2013, despite a global vaccination coverage estimated at 86% in 2014 [4]. Even with immunisation achievements, pertussis remains a major public health concern worldwide.
In September 2012, in response to an increase of hospitalisations and deaths in unvaccinated infants aged <3 months, the United Kingdom recommended for each pregnancy a single dose of aP-containing vaccine between 28 and 32 weeks of gestation. The programme was based on the evidence of transplacental transfer of maternal antibodies known to be maximal from the 34th week of gestation. One year after the programme was introduced, pertussis mortality decreased and VE in pregnancy remained stable around >90% in the following years [5], [6]. Since 2012, an increasing number of EU/EEA countries introduced vaccination in pregnancy: Belgium, Czech Republic, Ireland, Italy, Portugal, Slovenia and Spain [1], [7].
However, recent immunological studies suggest that vaccination in pregnancy could interfere with PV and reduce infants’ immune response. But little evidence exists about the clinical implications of this potential “blunting effect” of vaccination in pregnancy with infants’ PV [8].
From September 2015 to January 2020, ECDC created and funded PERTINENT, “Pertussis in Infants European Network”, a multi-country hospital-based active sentinel surveillance system to measure pertussis incidence and VE in infants aged <1 year [3]. For the first time in Europe, a prospective test-negative design (TND) [9] in hospital settings was used to estimate pertussis VE in a multi-country study.
In this study, we estimate VE in pregnancy in infants aged <2 months (i.e., too young to be eligible for PV) and investigate the effect of vaccination in pregnancy and PV in infants aged 2–11 months (i.e., eligible for PV).
2. Methods
2.1. Study sites
In 2019, four of the six European countries participating in PERTINENT recommended pertussis vaccination in pregnancy to protect infants too young for PV: Czech Republic, Ireland, Italy and Spain with two participating regions, Catalonia and Navarra (five study sites, 14 hospitals).
All sites complied with the generic PERTINENT sentinel surveillance and vaccine effectiveness protocol and laboratory guidelines [10] allowing to pool the data across sites.
All sites used the aP-containing vaccine for both PV and vaccination in pregnancy. Even though recommended schedules vary across countries, infants were eligible for the first dose of the primary series from their 61st day of life (2 months of age) in the four participating countries, including the two countries with a 3, 5, 11-month-old schedule.
The Czech Republic and Italy introduced vaccination in pregnancy during the course of the PERTINENT study and were included in both analyses only from that point onwards. Vaccine coverage estimates were not available for these two sites, and ranged from 50% to 90% in the other three sites (Table 1).
Table 1.
Characteristics of PERTINENT study sites, vaccination strategy during pregnancy, in adulthood, primary schedule in infants, Europe, 1 December 2015–31 December 2019.
| Study sites | Vaccination strategy |
Number of participating hospitals | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pregnancy |
Cocooning |
Adult | Primary schedule in infants (age in months) |
||||||
| Year of introduction | Gestational age (in weeks) | Estimated vaccination coverage | Year of introduction | 1st dose | 2nd dose | 3rd dose | |||
| Czech Republic | 2016 | 28–36 | 1.6% in 2021 a | No | At least once | 3 b | 5 b | 11–13b | 6 |
| Ireland | 2013 | 16–36 | 49.9% in 2017/2018 [11] | 2013 | No | 2 | 4 | 6 | 2 |
| Italy | 2017 | ≥ 27 | NA | No | Every 10 years | 3 | 5 | 11 | 1 |
| Spain, Catalonia | 2014 | 27–36 | 82.8% in 2019 [12] | No | No | 2 c | 4 c | 11c | 1 |
| Spain, Navarra | 2015 | 27–36 | 91.1% in 2019 [12] | No | No | 2 c | 4 c | 11c | 4 |
NA: not available.
Estimates from the final report of the project “Monitoring the vaccination of pregnant women against pertussis and influenza, 2020–2021” financed from NIPH Prague internal institutional funds. In this pilot prospective observational hospital-based study in the maternity hospital in Prague, 4617 women (84%) were included in the analysis out of the 5475 women who gave birth in 2021.
Before 2018: doses at 2, 3, 4 and 10 months.
Before 2016: doses at 2, 4 and 6 months.
2.2. Study population and eligibility criteria
The study population consisted of all infants aged <1 year, likely to be hospitalised in one of the participating hospitals if developing pertussis-like symptoms.
All infants attending one of the participating hospitals and presenting with apnoea or cough associated with at least one of paroxysms, whoop or post-tussive vomiting were tested for pertussis. Infants with any respiratory symptoms and an epidemiological link with a pertussis confirmed case or those not meeting the above clinical presentation but diagnosed as pertussis by a physician were also tested for pertussis.
We included all infants who were tested for pertussis and invited their parents to participate in the study. When required by site-specific ethical committee, infants’ legal guardians provided with an informed consent.
2.3. Laboratory methods
We recommended to the hospital laboratories to ensure an accurate identification of the Bordetella species using, as much as possible, a triplex quantitative PCR (qPCR): first targeting IS481 gene (in B. pertussis, B. holmesii, and some Bordetella bronchiseptica strains), pIS1001 (B. parapertussis-specific) and RNase P as the human internal control and two confirmatory singleplex tests for B. pertussis (ptxA-Pr) and B. holmesii (hIS1001) if IS481 was positive. Diagnostic algorithm was detailed in the PERTINENT laboratory guidelines [10].
2.4. Test-negative case control study
We conducted a multi-centre case control study using TND in the 14 participating hospitals.
We defined a laboratory-confirmed Bordetella pertussis case as an infant testing positive for Bordetella pertussis by PCR (DNA detection of Bordetella pertussis using PCR or real-time PCR in a nasopharyngeal aspirate or swab) or culture (isolation of Bordetella pertussis from the prior-mentioned clinical specimen) regardless of the clinical criteria. Test-negative controls were those testing negative to all Bordetella species by PCR or culture. In the Catalan hospital, due to heavy workload, we selected systematically the next three controls per case matched for date of specimen collection.
2.5. Exposures
We defined infants as vaccinated with PV if they had received at least one dose of pertussis vaccine >14 days before symptoms onset. Unvaccinated infants were those who had not received any dose or who had received the first dose ≤14 days before symptom onset.
We defined an infant as having a mother vaccinated during her pregnancy if she had received a pertussis vaccine dose >14 days before delivery. We defined an infant as having a mother not vaccinated if she did not receive any dose during adulthood.
2.6. Exclusion criteria
We excluded all infants with missing information for laboratory results, date of onset, or vaccination status. We also excluded infants sampled >4 weeks after symptoms onset, those testing positive to other Bordetella species than Bordetella pertussis, those with previous laboratory confirmed pertussis episode and those whose legal guardian did not give consent.
For both analyses, we excluded infants with unknown maternal vaccination status, those whose mothers were vaccinated ≤14 days before delivery or before/after pregnancy or had contra-indication for pertussis vaccination.
2.6.1. Effectiveness of vaccination in pregnancy in infants too young for vaccination (<2 months)
To estimate VE in pregnancy, we restricted the analysis to infants too young to be vaccinated and aged <61 days of life. Additionally, we excluded infants too young to develop the disease and aged <4 days of life (4 days being commonly known as the minimum incubation period for pertussis [2]).
2.6.2. Effectiveness of vaccination in pregnancy and PV in infants (2–11 months)
To explore the effect of both vaccinations, we restricted the analysis to infants eligible for PV and aged 2–11 months. We excluded all infants with unknown PV status or with contra-indication for pertussis vaccination.
2.7. Analysis
For both analyses, we described cases and controls by clinical presentations, severity, risk and protective factors. We used Fisher’s exact test to compare those characteristics between cases and controls.
2.7.1. Effectiveness of vaccination in pregnancy in infants too young for vaccination (<2 months)
We compared the odds of vaccination of the infants’ mother between cases and controls. We used a logistic regression to model the odds ratio (OR), including study site as fixed effect. We adjusted for time of onset in quarter and age group (4–30 days; 31–60 days). We computed VE as 1 minus the OR, expressed as a percentage.
2.7.2. Effectiveness of vaccination in pregnancy and PV in infants (2–11 months)
We conducted an indicator analysis based on four categories: (1) infants recording no vaccination in pregnancy nor PV (reference category); (2) infants recording PV only (at least one dose); (3) infants recording vaccination in pregnancy only; (4) infants recording both vaccination in pregnancy and PV (at least one dose).
Using infants recording no vaccination in pregnancy nor PV (1) as reference category, we compared the odds of each category of vaccination exposure (2), (3) and (4) between cases and controls and estimated the corresponding OR using logistic regression. We refer to this analysis as the indicator analysis. We included study site as fixed effect in the model and adjusted for time of onset in quarter and age group (2 months; 3–11 months). We computed VE as 1 minus the OR, expressed as a percentage.
2.7.3. Sensitivity analyses
Bordetella species can be isolated from both nasopharyngeal swabs (NPS) or aspirates (NPA). However, a 15% gain in the isolation rate can be obtained by using aspirates in neonates and infants [13].
Additionally, the Czech Republic and Italy encountered difficulties of adherence to the maternal immunisation programme in the first years of its implementation. National vaccine coverage in both sites were assumed to be very low and the mothers of the children enrolled in the analysis were not vaccinated.
Therefore, we conducted sensitivity analyses: (I) excluding the two sites with no mother vaccinated in pregnancy included in the study, (II) excluding all infants sampled with NPS, (III) excluding both the two sites and the infants with NPS.
If the number of events per parameter was lower than 10, we conducted an additional sensitivity analysis using Firth’s method of penalised logistic regression to assess small sample bias [14].
2.8. Data collection
Using a standardised questionnaire we collected demographic, epidemiological, clinical, and laboratory data, vaccination status of the infant and the mother, risk and protective factors. Hospital teams collected data through the review of clinical case-patient notes, vaccination cards, interviews with parents or legal guardians, and extraction from patient registries.
2.9. Ethical statement
Each site complied with the local ethical procedures. The planning, conduct and reporting of the study was in line with the Declaration of Helsinki [15]. Ethical approval was not needed in Navarra as the PERTINENT study was considered part of the mandatory surveillance system. Other study sites sought ethical approval from a review board according to country-specific regulations (Catalonia: PIC-31-16, Czech Republic: SZU/05992/2019, Ireland: Royal College of Physicians in Ireland REC reference number 16.058 and Gen/499/16, Italy: Bambino Gesù Children's Hospital Ethical Committee: protocol n. 1064_OPBG_2016).
3. Results
From December 2015 to December 2019, 829 infants aged less than one year were tested for Bordetella pertussis. Among them, 336 (40.5%) were too young to receive the first dose of PV (aged < 2 months) and 493 (59.5%) were eligible for PV (aged 2–11 months). No death was reported during the study period.
3.1. Effectiveness of vaccination in pregnancy in infants too young for vaccination (<2 months)
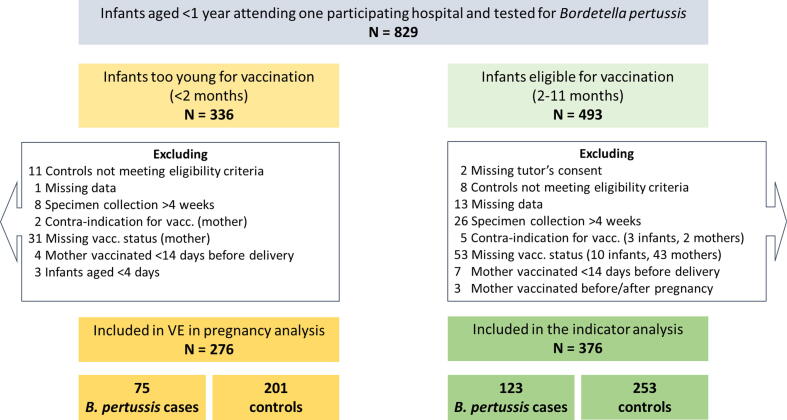
After applying the exclusion criteria for VE in pregnancy analysis, we included 276 infants aged <2 months with 75 Bordetella pertussis laboratory confirmed cases (27%) and 201 test-negative controls (73%). Among excluded infants, 31 had a missing maternal vaccination status or date of vaccination (Fig. 1).
Fig. 1.
Flowchart of hospitalised infants aged <1 year inclusion in or exclusion from the analysis of the effectiveness of vaccination in pregnancy, and the analysis of the effectiveness of vaccination in pregnancy combined with primary vaccinations after at least one dose, PERTINENT study, Europe, 1 December 2015–31 December 2019 (N = 829).
Twenty-six cases (35%) and 53 controls (26%) were aged 4–30 days (p = 0.181). The median-birthweight was 3320 g for cases (range: 1740–4925; interquartile range (IQR): 800) and 3260 g for controls (range: 1000–5150; IQR: 649) (p = 0.412). The median gestational week at birth was 39 for both cases (range: 29–42; IQR: 2) and controls (range: 28–42; IQR: 2) (p = 0.671).
Information on the type of specimen collection was available for the 75 cases and 199 controls with 18 cases (24%) and 71 controls (36%) only diagnosed based on NPS collection (p = 0.043) (Table 2).
Table 2.
Characteristics of Bordetella pertussis cases and controls by analysis (left: effectiveness of vaccination in pregnancy analysis in infants aged <2 months; right: vaccination in pregnancy and primary vaccination analysis in infants aged 2–11 months) and by sex, laboratory components, clinical presentation, severity and risk/protective factors, hospitalised infants aged <1 year, PERTINENT study, Europe, 1 December 2015–31 December 2019.
| Characteristics | Cases <2mo (n = 75) |
Controls <2mo (n = 201) |
p value | Cases 2-11mo (n = 123) |
Controls 2-11mo (n = 253) |
p value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | ||||
| Demographic | |||||||||||
| Sex |
Female | 39 | 52.0 | 91 | 45.3 | 0.345 | 57 | 46.3 | 126 | 49.8 | 0.583 |
| Male | 36 | 48.0 | 110 | 54.7 | 66 | 53.7 | 127 | 50.2 | |||
| Laboratory | |||||||||||
| Nasopharyngeal specimen collection | Aspirate or both aspirate and swab | 57 | 76.0 | 128 | 64.3 | 0.043 | 92 | 75.4 | 159 | 62.9 | 0.019 |
| Swab only | 18 | 24.0 | 71 | 35.7 | 30 | 24.6 | 94 | 37.2 | |||
| Clinical criteria | |||||||||||
| Cough | Yes | 72 | 96.0 | 186 | 92.5 | 0.415 | 121 | 98.4 | 249 | 98.4 | 1.000 |
| No | 3 | 4.0 | 15 | 7.5 | 2 | 1.6 | 4 | 1.6 | |||
| Cough with paroxysms | Yes | 64 | 85.3 | 120 | 59.7 | <0.001 | 112 | 91.1 | 179 | 70.8 | <0.001 |
| No | 11 | 14.7 | 81 | 40.3 | 11 | 8.9 | 74 | 29.2 | |||
| Whoop | Yes | 35 | 47.9 | 36 | 18.6 | <0.001 | 66 | 53.7 | 47 | 18.9 | <0.001 |
| No | 38 | 52.1 | 158 | 81.4 | 57 | 46.3 | 202 | 81.1 | |||
| Post-tussive vomiting | Yes | 39 | 52.0 | 77 | 38.5 | 0.055 | 56 | 45.5 | 124 | 49.0 | 0.582 |
| No | 36 | 48.0 | 123 | 61.5 | 67 | 54.5 | 129 | 51.0 | |||
| Apnoea | Yes | 50 | 67.6 | 92 | 46.0 | 0.002 | 61 | 49.6 | 62 | 25.0 | <0.001 |
| No | 24 | 32.4 | 108 | 54.0 | 62 | 50.4 | 186 | 75.0 | |||
| Cyanosis | Yes | 47 | 63.5 | 62 | 30.8 | <0.001 | 53 | 43.1 | 49 | 19.4 | <0.001 |
| No | 27 | 36.5 | 139 | 69.2 | 70 | 56.9 | 203 | 80.6 | |||
| Epidemiological link | Yes | 43 | 58.9 | 3 | 1.5 | <0.001 | 64 | 54.7 | 10 | 4.0 | <0.001 |
| No | 30 | 41.1 | 192 | 98.5 | 53 | 45.3 | 241 | 96.0 | |||
| Diagnosis by a clinician | Yes | 71 | 94.7 | 74 | 36.8 | <0.001 | 113 | 93.4 | 137 | 54.6 | <0.001 |
| No | 4 | 5.3 | 127 | 63.2 | 8 | 6.6 | 114 | 45.4 | |||
| Severity | |||||||||||
| Death | Yes | 0 | 0.0 | 0 | 0.0 | NA | 0 | 0.0 | 0 | 0.0 | NA |
| No | 75 | 100.0 | 199 | 100.0 | 123 | 100.0 | 249 | 100.0 | |||
| ICU | Yes | 26 | 34.7 | 24 | 12.0 | <0.001 | 14 | 11.4 | 13 | 5.2 | 0.035 |
| No | 49 | 65.3 | 176 | 88.0 | 109 | 88.6 | 238 | 94.8 | |||
| ECMO | Yes | 0 | 0.0 | 0 | 0.0 | NA | 0 | 0.0 | 0 | 0.0 | NA |
| No | 75 | 100.0 | 200 | 100.0 | 123 | 100.0 | 249 | 100.0 | |||
| Pneumonia | Yes | 4 | 5.3 | 5 | 2.5 | 0.262 | 3 | 2.5 | 14 | 5.6 | 0.289 |
| No | 71 | 94.7 | 195 | 97.5 | 119 | 97.5 | 237 | 94.4 | |||
| Encephalopathy | Yes | 0 | 0.0 | 1 | 0.5 | 1.000 | 0 | 0.0 | 0 | 0.0 | NA |
| No | 75 | 100.0 | 199 | 99.5 | 122 | 100.0 | 251 | 100.0 | |||
| Seizure | Yes | 1 | 1.3 | 2 | 1.0 | 1.000 | 1 | 0.8 | 0 | 0.0 | 0.327 |
| No | 74 | 98.7 | 198 | 99.0 | 121 | 99.2 | 251 | 100.0 | |||
| Eating difficulties | Yes | 22 | 29.3 | 66 | 33.2 | 0.566 | 32 | 26.2 | 83 | 33.2 | 0.190 |
| No | 53 | 70.7 | 133 | 66.8 | 90 | 73.8 | 167 | 66.8 | |||
| Kidney failure | Yes | 0 | 0.0 | 2 | 1.0 | 1.000 | 0 | 0.0 | 0 | 0.0 | NA |
| No | 75 | 100.0 | 198 | 99.0 | 123 | 100.0 | 250 | 100.0 | |||
| Dehydration | Yes | 6 | 10.2 | 5 | 3.2 | 0.075 | 12 | 10.8 | 15 | 6.8 | 0.208 |
| No | 53 | 89.8 | 151 | 96.8 | 99 | 89.2 | 207 | 93.2 | |||
| Risk factors | |||||||||||
| Premature <37 weeks | Yes | 7 | 9.5 | 23 | 11.4 | 0.828 | 14 | 11.4 | 43 | 17.0 | 0.170 |
| No | 67 | 90.5 | 178 | 88.6 | 109 | 88.6 | 210 | 83.0 | |||
| Delivery type | Vaginal | 53 | 70.7 | 145 | 73.6 | 0.649 | 90 | 73.8 | 182 | 74.3 | 1.000 |
| C-section | 22 | 29.3 | 52 | 26.4 | 32 | 26.2 | 63 | 25.7 | |||
| Episode in pregnancy | Yes | 3 | 4.1 | 0 | 0.0 | 0.020 | 2 | 1.8 | 1 | 0.4 | 0.233 |
| No | 71 | 95.9 | 197 | 100.0 | 112 | 98.2 | 248 | 99.6 | |||
| Infant going to day care | Yes | 5 | 6.7 | 6 | 3.0 | 0.178 | 6 | 4.9 | 26 | 10.3 | 0.114 |
| No | 70 | 93.3 | 194 | 97.0 | 117 | 95.1 | 226 | 89.7 | |||
| Infant with babysitter | Yes | 1 | 1.4 | 11 | 7.3 | 0.109 | 5 | 4.5 | 11 | 5.7 | 0.793 |
| No | 69 | 98.6 | 139 | 92.7 | 105 | 95.5 | 182 | 94.3 | |||
| Infant staying regularly with grandparents | Yes | 24 | 32.0 | 40 | 20.1 | 0.054 | 38 | 31.9 | 64 | 25.5 | 0.214 |
| No | 51 | 68.0 | 159 | 79.9 | 81 | 68.1 | 187 | 74.5 | |||
| Protective factors | |||||||||||
| Breastfeeding | Yes | 55 | 73.3 | 157 | 78.5 | 0.421 | 84 | 68.3 | 174 | 69.3 | 0.905 |
| No | 20 | 26.7 | 43 | 21.5 | 39 | 31.7 | 77 | 30.7 | |||
| Mother vaccination in pregnancy | Yes | 9 | 12.0 | 92 | 45.8 | <0.001 | 40 | 32.5 | 136 | 53.8 | <0.001 |
| No | 66 | 88.0 | 109 | 54.2 | 83 | 67.5 | 117 | 46.2 | |||
| Vaccinated at least 1 dose | Yes | 0 | 0 | 0 | 0 | NA | 58 | 47.2 | 151 | 59.7 | 0.027 |
| No | 0 | 0 | 0 | 0 | 65 | 52.8 | 102 | 40.3 | |||
| Number of doses | 1 dose | 0 | 0 | 0 | 0 | NA | 30 | 24.4 | 73 | 28.9 | 0.151 |
| 2 doses | 0 | 0 | 0 | 0 | 22 | 17.9 | 62 | 24.5 | |||
| 3 doses | 0 | 0 | 0 | 0 | 6 | 4.9 | 16 | 6.3 | |||
Out of the 75 cases, 20 cases (27%) were both PCR and culture-confirmed, 17 cases (23%) were PCR-confirmed but culture-negative, 37 cases (49%) were PCR-confirmed (no culture result) and one case (1%) was culture-confirmed (no PCR performed). Out of the 201 controls, 6 (3%) were confirmed by culture only.
The proportion of cases and controls by risk and protective factors such as prematurity, delivery type, child care, breastfeeding was similar. Three cases (4%) and no controls had their mother experiencing pertussis in pregnancy. The mothers did not receive pertussis vaccine during their pregnancy.
The median gestational age at vaccination was 30.4 weeks for cases (range: 23–36; IQR: 4) and 30.1 for controls (range: 20–37; IQR: 3.5) (p = 0.741).
Out of the 276 infants too young to be vaccinated, nine cases (12%) and 92 controls (46%) had their mother vaccinated in pregnancy. VE in pregnancy adjusted for study site and time of onset (in quarter) was 76% (95% CI: 38–91) and 75% (95% CI: 35–91) when also adjusted for age group (Table 3).
Table 3.
Adjusted vaccine effectiveness of pertussis vaccination in pregnancy in hospitalised infants too young to be vaccinated (aged < 2 months), PERTINENT study, Europe, 1 December 2015–31 December 2019 (n = 276).
| Adjustment variables | Df | N | Cases | Controls | VE (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Vacc. | N | Vacc. | N | ||||
| All infants, 5 sites (N = 276) | |||||||
| Site; Onset quarter | 9 | 276 | 9 | 75 | 92 | 201 | 76 (38–91) |
| Site; Onset quarter; Age group (4-30d; 31-60d) |
10 | 276 | 9 | 75 | 92 | 201 | 75 (35–91) |
| All infants, 3 sites* (N = 165) | |||||||
| Site; Onset quarter | 7 | 165 | 9 | 30 | 92 | 135 | 76 (39–91) |
| Site; Onset quarter; Age group (4-30d; 31-60d) |
8 | 165 | 9 | 30 | 92 | 135 | 75 (35–90) |
| Infants sampled with NPA, 5 sites (N = 185) | |||||||
| Site; Onset quarter | 9 | 185 | 6 | 57 | 51 | 128 | 88 (59–96) |
| Site; Onset quarter; Age group (4-30d; 31-60d) |
10 | 185 | 6 | 57 | 51 | 128 | 87 (55–96) |
| Infants sampled with NPA, 3 sitesa (N = 88) | |||||||
| Site; Onset quarter | 7 | 88 | 6 | 20 | 51 | 68 | 88 (57–96) |
| Site; Onset quarter; Age group (4-30d; 31-60d) |
8 | 88 | 6 | 20 | 51 | 68 | 87 (53–96) |
CI: confidence interval; Df: degree of freedom; NPA: nasopharyngeal aspirate; VE: vaccine effectiveness.
Excluding 2 sites due to the absence of vaccinated women.
In the sensitivity analysis excluding infants sampled only with NPS (N = 185), VE adjusted for site and time of onset (in quarter) and VE also adjusted for age group were, respectively 88% (95% CI: 59–96) and 87% (95% CI: 55–96).
The results were similar when excluding the two sites with no infant with mother vaccinated in pregnancy, or when using penalised logistic regression.
3.2. Effectiveness of vaccination in pregnancy and PV in infants (2–11 months)
After applying the exclusion criteria for the effectiveness of both vaccinations analysis, we included 376 infants eligible for PV (aged 2–11 months) with 123 Bordetella pertussis laboratory confirmed cases (33%) and 253 test-negative controls (67%). Among excluded infants, 43 had a missing maternal vaccination status or vaccination date and 10 had a missing PV status or vaccination date (Fig. 1).
Thirty-six cases (29%) and 97 controls (38%) were in their third month of life (p = 0.053). The median-birthweight was 3250 g for cases (range: 1160–4780; IQR: 750) and 3200 g for controls (range: 640–4500; IQR: 770) (p = 0.186). The median gestational week at birth was 39 for both cases (range: 28–42; IQR: 2) and controls (range: 24–43; IQR: 2) (p = 0.220).
Information on the type of specimen collection was available for 122 cases and the 253 controls with 30 cases (25%) and 94 controls (37%) only diagnosed based on NPS (p = 0.019) (Table 2). Out of the 123 cases, 32 cases (26%) were both PCR and culture-confirmed, 22 cases (18%) were PCR-confirmed but culture-negative, 65 cases (53%) were PCR-confirmed (no culture result) and four cases (3%) were culture-confirmed (no PCR performed). Two controls (<1%) were confirmed by culture only.
The median gestational age at vaccination was 30.1 weeks for cases (range: 19–36; IQR: 4) and 30.6 for controls (range: 14–36; IQR: 3) (p = 1.000).
Out of the 376 infants eligible for PV, 40 cases (33%) and 136 controls (54%) had their mother vaccinated in pregnancy (p < 0.001), 58 cases (47%) and 151 controls (60%) were vaccinated with at least one dose of PV (p = 0.027) (Table 2). Thirty-one cases (25%) and 98 controls (39%) had received both PV and vaccination in pregnancy, 27 cases (22%) and 53 controls (21%) had received PV only, 9 cases (7%) and 38 controls (15%) had received vaccination in pregnancy only (Table 4).
Table 4.
Adjusted effectiveness of three combinations of vaccine exposures in hospitalised infants eligible for vaccination (2–11 months): (1) mother vaccinated in pregnancy and infant vaccinated with at least one dose of PV; (2) infant vaccinated with PV only (at least one dose); (3) mother vaccinated in pregnancy only. PERTINENT study, Europe, 1 December 2015–31 December 2019 (n = 376).
| Vaccination status | N | Cases | Controls | VE (95% CI)a | |
|---|---|---|---|---|---|
| Infant | Mother | ||||
| All infants, 5 sites (N = 376) | |||||
| Unvaccinated | Unvaccinated | 120 | 56 | 64 | Ref. |
| Vaccinated | Vaccinated | 129 | 31 | 98 | 74 (33–90) |
| Vaccinated | Unvaccinated | 80 | 27 | 53 | 68 (27–86) |
| Unvaccinated | Vaccinated | 47 | 9 | 38 | 36 (-85–78) |
| All infants, 3 sitesb (N = 257) | |||||
| Unvaccinated | Unvaccinated | 29 | 13 | 16 | Ref. |
| Vaccinated | Vaccinated | 129 | 31 | 98 | 90 (64–97) |
| Vaccinated | Unvaccinated | 52 | 11 | 41 | 92 (69–98) |
| Unvaccinated | Vaccinated | 47 | 9 | 38 | 63 (−29–89) |
| Infants sampled with NPA, 5 sites (N = 251) | |||||
| Unvaccinated | Unvaccinated | 84 | 43 | 41 | Ref. |
| Vaccinated | Vaccinated | 90 | 24 | 66 | 88 (62–96) |
| Vaccinated | Unvaccinated | 52 | 19 | 33 | 81 (46–93) |
| Unvaccinated | Vaccinated | 25 | 6 | 19 | 44 (−109–85) |
| Infants sampled with NPA, 3 sitesb (N = 164) | |||||
| Unvaccinated | Unvaccinated | 21 | 11 | 10 | Ref. |
| Vaccinated | Vaccinated | 90 | 24 | 66 | 95 (69–99) |
| Vaccinated | Unvaccinated | 28 | 7 | 21 | 94 (59–99) |
| Unvaccinated | Vaccinated | 25 | 6 | 19 | 61 (−89–92) |
CI: confidence interval; NPA: nasopharyngeal aspirate; PV: primary vaccination; VE: vaccine effectiveness.
Adjusted for site, onset quarters and age group (2; 3–11 months).
Excluding 2 sites due to the absence of vaccinated women.
In the main analysis (N = 376), using unvaccinated infants and mothers as the reference group, VE adjusted for site, time of onset and age group was 74% (95% CI: 33–90) for infants with both PV and vaccination in pregnancy; 68% (95% CI: 27–86) for those with PV only; 36% (95% CI: -85–78) for those with vaccination in pregnancy only (Table 4).
In the sensitivity analysis excluding the two sites with no infants with vaccination in pregnancy (N = 257), VE adjusted for site, time of onset and age group was 90% (95% CI: 64–97) for infants with both PV and vaccination in pregnancy; 92% (95% CI: 69–98) for those with PV only; 63% (95% CI: -29–89) for those with vaccination in pregnancy only. When excluding infants sampled with NPS only (N = 251), VE adjusted for site, time of onset and age group was 88% (95% CI: 62–96) for infants with both PV and vaccination in pregnancy; 81% (95% CI: 46–93) for those with PV only; 44% (95% CI: -109–85) for those with vaccination in pregnancy only. Applying both exclusions (N = 164) provided with similar results (Table 4).
4. Discussion
After four years of PERTINENT data collection in 14 participating hospitals from four EU/EEA countries, we included 276 infants aged <2 months in the VE in pregnancy analysis and 373 infants aged 2–11 months in the indicator analysis of both vaccination in pregnancy and PV. Our results suggest that vaccination in pregnancy reduces the risk of being hospitalised for pertussis by 75–88% in infants aged <2 months too young to be vaccinated with PV. In the indicator analysis, regardless of the recommended schedule, when the infants are aged 2–11 months and eligible for vaccination, at least one dose of PV in infants whose mother had received vaccination in pregnancy would reduce the risk of hospitalisation for confirmed pertussis by 74–95%. Using the same reference group, at least one dose of PV in infants with unvaccinated mother would reduce the risk by 68–94%. Even though those results are based on small sample sizes, they suggest a good VE in pregnancy, consistent with existing literature [16] and also a similarly good VE after at least one dose of PV only and receiving both PV and mother vaccination.
However, these findings need to be interpreted with caution due to some existing limitations. Despite four years of active pertussis surveillance, the achieved sample sizes for maternal vaccination studies did not allow for more precise estimates. Increasing data collection in this multicentre study is needed to consolidate our results and to allow additional adjustments for potential confounding factors or stratification by effect modifiers (e.g., breastfeeding, repeated vaccination in pregnancy). Due to this substantial limitation in our study, we could not compute VE in pregnancy by site and estimate sites’ heterogeneity. The current sample size also prevented us to explore VE in pregnancy according to time of and since vaccination in pregnancy, VE for one dose of primary series only, VE by dose or VE by time since vaccination. As described by Barug et al. [17], pertussis antibody responses in infants may differ depending on the infant vaccination schedule. Their study suggested a higher immunological effect when PV is starting at 2 months compared with starting at 3 months of age. Interaction between vaccination in pregnancy and PV may also differ according to the number of doses received, the time of and since vaccination of the mother and other additional factors [18].
Implementation and compliance to the maternal immunisation programme was very heterogeneous across sites during the study period. It was very well established in Spain with Catalonia and Navarra regions. Conversely, immunisation programmes for pregnant women were not being fully implemented in Czech Republic and Italy. Vaccine acceptance aspects were documented in Italy [19].
Hospital teams had to test for pertussis and include in the study any infants suspected for pertussis, even though some typical symptoms were missing [20]. However, clinicians may be more likely or less likely to test suspected pertussis cases according to vaccination status leading to selection bias. We believe this bias may had a very limited impact at least on the VE in pregnancy analysis as we assume that clinicians may not have direct access to the mother vaccination status at the infant’s admission.
Vaccination status data were obtained by reviewing clinical case notes, vaccination cards, interviews with parents or legal guardians, and extraction from patient registries. The current small sample size did not allow to compare VE estimates by source of information for the vaccine status. In the VE in pregnancy analysis, 31 infants were excluded due to missing values for mother vaccination status or vaccination date. Out of them, 21 were excluded due to missing vaccination date (2 cases and 19 controls), assuming that the mother was vaccinated. In the indicator analysis, 43 infants were excluded due to missing values for mother vaccination status or vaccination date. Out of them, 35 were excluded due to missing vaccination date (4 cases and 31 controls), assuming that the mother was vaccinated. For both analyses, this suggests that motheŕs vaccinations may be better documented among cases than among controls, which could lead to underestimation of VE in pregnancy.
A large proportion of the exclusions in the study are due to lack of information on vaccination status and vaccination date from the mother. Even though efforts done for an enhanced data collection at hospital level were successful, more efforts are needed to retrieve information outside of the hospital setting.
In our study population, the clinical case definition was associated with confirmed pertussis. Even if this lends support to the definition used, discussing pertussis clinical presentation was however not part of our study objectives.
To validate our findings, we would need to further study and confirm that TND in hospital settings is a proper study design for pertussis VE estimation in infants. This is the first time that a prospective TND is used in Europe in hospital settings for estimating pertussis vaccine effectiveness in infants. The rationale for TND is that the control group (infants hospitalised for pertussis-like symptoms but with other respiratory disease than pertussis) are representative of the vaccine coverage in the source population of pertussis cases. The risk of hospitalisation for non-pertussis respiratory infections should then be equal between vaccinated and unvaccinated infants. There is a need to validate this assumption using large cohorts in Europe. Unfortunately, we could not compare the proportion of our controls that were vaccinated to the vaccine coverage in the catchment area of the participating hospitals. Aiming to validate TND for pertussis, a recent Canadian study compared their results with a frequency-matched design (FMD) for pertussis VE studies estimating waning immunity. In both designs, VE estimates were high and consistent with clinical trials at early stage after vaccination and in early years of life [21].
In both our analyses, we included all infants tested for pertussis and classified them as cases and controls according to PCR or culture results. Although PCR has a high sensitivity, culture sensitivity is only about 60% with the highest among unvaccinated infants [22]. Including false-negative, especially among vaccinated infants, could lead to overestimate VE in both analyses. However, only six controls (3%) aged <2 months and two controls (<1%) aged 2–11 months were confirmed by culture only, which lead us to assume a very minor impact on our results.
Even though our VE estimates for both analyses are consistent with existing literature [23], they tend to be in the lower range. In our study, controls were more likely than cases to have been diagnosed based on the laboratory results of a NPS only (Table 2). Since NPS can be less sensitive than NPA in infants to isolate Bordetella pertussis by PCR or culture [13], we cannot prevent inclusion of false-negative among controls. Misclassification of unvaccinated cases as controls would lead to underestimating the corresponding VE. Despite the very low sample size, when excluding infants sampled with NPS, we observed higher VE estimates, closer to existing literature. Overall, our results are in the range of VE observed in other studies reporting VE in pregnancy between 70% and 90% in infants aged <2 months [8], [24] and additional protection from vaccination in pregnancy during the first year of life [16]. In our indicator analysis, we also observed a good VE after at least one dose of PV only and after at least one dose of PV in infants whose mother was vaccinated. However, our limited sample size did not allow a robust stratified analysis to investigate whether vaccination in pregnancy modifies VE after at least one dose of PV. It did not allow either to measure the interaction between the two vaccinations. Therefore, even if our results may indicate a similarly good VE of at least one dose of PV irrespective of the vaccination status of the mother, we cannot conclude about the absence of clinical significance of the immunological blunting effect of maternal vaccination in infants’ immune response to PV.
5. Conclusion
The PERTINENT network is the only EU/EEA collaboration that allows for large, independent and multi-country pertussis vaccine effectiveness studies.
Despite PV starting at 2 months of age, infants too young to be eligible for vaccination still harbour the highest risk of illness and related deaths.
Our findings suggest that vaccination in pregnancy is an effective strategy to fill the immunisation gap of the first two months of life, when infants are not eligible for vaccination and the disease is the most life-threatening. From 2 months of age onwards, despite existing immunological studies suggesting a possible lower immunological response after PV in infants whose mother had received vaccination in pregnancy [8], our results suggest a good effectiveness of at least one dose PV in infants aged 2–11 months irrespective of the vaccination status of the mother.
In making decisions about vaccination strategies, countries take into account various factors, including cost-effectiveness evaluations. As health economic analyses are sensitive to local circumstances and are not easily generalisable, national health-economic studies may need to be conducted as part of such comprehensive evaluations.
In the up-coming post-acute COVID-19 pandemic times where an increase of vaccine-preventable respiratory infections such as bronchiolitis and pertussis is to be expected [25], consideration should be given to increase disease awareness, to improve pertussis surveillance and laboratory diagnosis [3] but, above all, to enhance maternal vaccination in pregnancy, as well as ensuring that these recommendations are effectively implemented in accordance with national guidelines.
Funding
This work was supported by the European Centre for Disease Prevention and Control (ECDC) funding the PERTINENT study (Framework contract n° ECDC/2015/017).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We are grateful to all patients and their legal tutor, nurses, clinicians, microbiologists and epidemiologists from the seven study sites of the PERTINENT Network who actively participated in the study. Thanks to the epidemiologists, clinicians, microbiologists, biostatisticians and other researchers from the four expert sites who were engaged in the study: Wiebke Hellenbrand, Kai Michaelis, Robert Koch Institute, Berlin, Germany; Odette Popovici, National Institute of Public Health, Bucharest, Romania; Bernice Aronsson, Public Health Agency of Sweden, Stockholm, Sweden; Heather Murdoch, Alison Smith-Palmer, Joe Jasperse, Health Protection Scotland, Glasgow, Scotland.
PERTINENT group.
Epiconcept, France:
Lore Merdrignac, Epiconcept;
Camelia Savulescu, Epiconcept;
Marta Valenciano, Epiconcept;
Alain Moren, Epiconcept.
Czech Republic:
Pavla Křížová, National Institute of Public Health, Prague;
Kateřina Fabiánová, National Institute of Public Health, Prague;
Jana Zavadilová, National Institute of Public Health, Prague;
Zuzana Blechová, University Hospital Na Bulovce, Prague;
Květa Bláhová, University Hospital Motol, Prague;
Pavel Kosina, University Hospital, Hradec Králové;
Josef Sýkora, University Hospital, Pilsen;
Alena Holčíková, University Hospital, Brno;
Petr Širůček, University Hospital, Ostrava.
France:
Daniel Lévy-Brühl, Santé Publique France, Saint-Maurice;
Fatima Aït El Belghiti, Santé Publique France, Saint-Maurice;
Adèle Grembombo, Santé Publique France, Saint-Maurice;
Sophie Guillot, Institut Pasteur, Paris;
Sylvain Brisse, Institut Pasteur, Paris;
Julie Toubiana, Institut Pasteur, Paris.
Ireland:
Suzanne Cotter, HSE-Health Protection Surveillance Centre, Dublin;
Jane Murphy, Temple Street Children’s University Hospital, Dublin;
Robert Cunney, Temple Street Children’s University Hospital, Dublin;
Norma O’Shaughnessy, Temple Street Children’s University Hospital, Dublin;
Adele Habington, Our Lady’s Children’s hospital Crumlin, Dublin;
Niamh O’Sullivan, Our Lady’s Children’s hospital Crumlin, Dublin.
Italy:
Elisabetta Pandolfi, Bambino Gesù Children Hospital, Rome;
Alberto E Tozzi, Bambino Gesù Children Hospital, Rome;
Caterina Rizzo, Bambino Gesù Children Hospital, Rome;
Luisa Russo, Bambino Gesù Children Hospital, Rome;
Ilaria Campagna, Bambino Gesù Children Hospital, Rome;
Francesco Gesualdo, Bambino Gesù Children Hospital, Rome;
Sara Ciampini, Bambino Gesù Children Hospital, Rome;
Valentina Annarosa Ferro, Bambino Gesù Children Hospital, Rome;
Elena Boccuzzi, Bambino Gesù Children Hospital, Rome.
Norway:
Håkon Bøås, Norwegian Institute of Public Health, Oslo;
Terese Bekkevold, Norwegian Institute of Public Health, Oslo;
Liliana Vazquez Fernandez, Norwegian Institute of Public Health, Oslo.
Catalonia, Spain:
Carmen Muñoz-Almagro, Instituto de Recerca Pediatrica Hospital Sant Joan de Deu, Barcelona; Universitat Internacional de Catalunya and CIBER of Epidemiology and Public Health CIBERESP;
Cristina Esteva, Instituto de Recerca Pediatrica Hospital Sant Joan de Deu, Barcelona; CIBER of Epidemiology and Public Health CIBERESP;
Mireia Jané, Public Health Agency of Catalonia, Barcelona; CIBER of Epidemiology and Public Health CIBERESP; University of Barcelona;
Gloria Carmona, Public Health Agency of Catalonia, Barcelona;
Lesly Acosta, Universitat Politècnica de Catalunya - BarcelonaTech (UPC), Public Health Agency of Catalonia, Barcelona;
Yolanda Jordan Garcia, Instituto de Recerca Pediatrica Hospital Sant Joan de Deu, Barcelona; CIBER of Epidemiology and Public Health CIBERESP.
Navarra, Spain:
Manuel García Cenoz, Instituto de Salud Pública de Navarra, IdiSNA – Navarre Institute for Health Research, Pamplona;
Ana Navascués, Complejo Hospitalario de Navarra, Pamplona;
Leticia Fernandino Zubieta, Instituto de Salud Pública de Navarra, IdiSNA – Navarre Institute for Health Research, Pamplona;
Jesús Castilla, Instituto de Salud Pública de Navarra, IdiSNA - Navarre Institute for Health Research, Pamplona.
Sentinelles, France:
Thomas Hanslik, Sorbonne Université, INSERM, Institut Pierre Louis d’Epidémiologie et de Santé Publique (IPLESP UMRS 1136), Paris.
ECDC:
Sabrina Bacci, European Centre for Disease Prevention and Control, Stockholm, Sweden;
Gianfranco Spiteri, European Centre for Disease Prevention and Control, Stockholm, Sweden;
Lucia Pastore Celentano, European Centre for Disease Prevention and Control, Stockholm, Sweden.
*The members of the network are listed in the Acknowledgments.
References
- 1.Pertussis: Annual Epidemiological Report for 2018 2018:8.
- 2.Plotkin’s Vaccines – 7th Edition n.d. <https://www.elsevier.com/books/T/A/9780323357616> [accessed November 4, 2021].
- 3.Merdrignac L., Belghiti F.A.E., Pandolfi E., Jané M., Murphy J., Fabiánová K., et al. Incidence and severity of pertussis hospitalisations in infants aged less than 1 year in 37 hospitals of six EU/EEA countries, results of PERTINENT sentinel pilot surveillance system, December 2015 to December 2018. Eurosurveillance. 2021;26:1900762. doi: 10.2807/1560-7917.ES.2021.26.4.1900762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pertussis vaccines: WHO position paper – September 2015. Releve Epidemiol Hebd 2015;90:433–58. [PubMed]
- 5.Dabrera G., Amirthalingam G., Andrews N., Campbell H., Ribeiro S., Kara E., et al. A case-control study to estimate the effectiveness of maternal pertussis vaccination in protecting newborn infants in England and wales, 2012–2013. Clin Infect Dis. 2015;60:333–337. doi: 10.1093/cid/ciu821. [DOI] [PubMed] [Google Scholar]
- 6.Amirthalingam G., Campbell H., Ribeiro S., Fry N.K., Ramsay M., Miller E., et al. Sustained Effectiveness of the maternal pertussis immunization program in England 3 years following introduction. Clin Infect Dis Off Publ Infect Dis Soc Am. 2016;63:S236–S243. doi: 10.1093/cid/ciw559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Centre for Disease Prevention and Control. Vaccine Scheduler 2022. <https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=3&SelectedCountryIdByDisease=-1> [accessed March 21, 2022].
- 8.Abu-Raya B., Edwards K.M. Interference with pertussis vaccination in infants after maternal pertussis vaccination. Pediatrics. 2020;146 doi: 10.1542/peds.2019-3579. [DOI] [PubMed] [Google Scholar]
- 9.Fukushima W., Hirota Y. Basic principles of test-negative design in evaluating influenza vaccine effectiveness. Vaccine. 2017;35:4796–4800. doi: 10.1016/j.vaccine.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Valero-Rello A., Henares D., Acosta L., Jane M., Jordan I., Godoy P., et al. Validation and implementation of a diagnostic algorithm for DNA detection of bordetella pertussis, B. parapertussis, and B. holmesii in a Pediatric Referral Hospital in Barcelona, Spain. J Clin Microbiol. 2019;57:e01231–e1318. doi: 10.1128/JCM.01231-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quattrocchi A., Mereckiene J., Fitzgerald M., Cotter S. Determinants of influenza and pertussis vaccine uptake in pregnant women in Ireland: a cross-sectional survey in 2017/18 influenza season. Vaccine. 2019;37:6390–6396. doi: 10.1016/j.vaccine.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Ministerio de Sanidad - Profesionales - Salud pública - Prevención de la salud - Vacunaciones - Programa vacunación - Coberturas de Vacunación. Dartos Estadísticos n.d. <https://www.sanidad.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/calendario-y-coberturas/coberturas/> [accessed August 30, 2022].
- 13.World Health Organization. Laboratory Manual for the diagnosis of whooping cough caused by bordetella pertussis/bordetella parapertussis : update 2014. World Health Organization; 2014.
- 14.Peduzzi P., Concato J., Kemper E., Holford T.R., Feinstein A.R. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 15.World Medical Association World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 16.Baxter R., Bartlett J., Fireman B., Lewis E., Klein N.P. Effectiveness of vaccination during pregnancy to prevent infant pertussis. Pediatrics. 2017;139 doi: 10.1542/peds.2016-4091. [DOI] [PubMed] [Google Scholar]
- 17.Barug D., Pronk I., van Houten M.A., Versteegh F.G.A., Knol M.J., van de Kassteele J., et al. Maternal pertussis vaccination and its effects on the immune response of infants aged up to 12 months in the Netherlands: an open-label, parallel, randomised controlled trial. Lancet Infect Dis. 2019;19:392–401. doi: 10.1016/S1473-3099(18)30717-5. [DOI] [PubMed] [Google Scholar]
- 18.Campbell H., Gupta S., Dolan G.P., Kapadia S.J., Kumar Singh A., Andrews N., et al. Review of vaccination in pregnancy to prevent pertussis in early infancy. J Med Microbiol. 2018;67:1426–1456. doi: 10.1099/jmm.0.000829. [DOI] [PubMed] [Google Scholar]
- 19.Scatigna M., Appetiti A., Pasanisi M., D’Eugenio S., Fabiani L., Giuliani A.R. Experience and attitudes on vaccinations recommended during pregnancy: survey on an Italian sample of women and consultant gynecologists. Hum Vaccines Immunother. 2021:1–8. doi: 10.1080/21645515.2021.1894061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heininger U., André P., Chlibek R., Kristufkova Z., Kutsar K., Mangarov A., et al. Comparative epidemiologic characteristics of pertussis in 10 central and eastern European Countries, 2000–2013. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0155949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crowcroft N.S., Schwartz K.L., Savage R.D., Chen C., Johnson C., Li Y., et al. A call for caution in use of pertussis vaccine effectiveness studies to estimate waning immunity: a canadian immunization research network study. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020;73:83–90. doi: 10.1093/cid/ciaa518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wirsing von König C-H Pertussis diagnostics: overview and impact of immunization. Expert Rev Vacc. 2014;13:1167–1174. doi: 10.1586/14760584.2014.950237. [DOI] [PubMed] [Google Scholar]
- 23.Kandeil W., van den Ende C., Bunge E.M., Jenkins V.A., Ceregido M.A., Guignard A. A systematic review of the burden of pertussis disease in infants and the effectiveness of maternal immunization against pertussis. Expert Rev Vacc. 2020;19:621–638. doi: 10.1080/14760584.2020.1791092. [DOI] [PubMed] [Google Scholar]
- 24.Skoff T.H., Blain A.E., Watt J., Scherzinger K., McMahon M., Zansky S.M., et al. The impact of the U.S. maternal tdap vaccination program on preventing pertussis in infants <2 months of age: a case-control evaluation. Clin Infect Dis Off Publ Infect Dis Soc Am. 2017;65:1977–1983. doi: 10.1093/cid/cix724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reicherz F., Golding L., Lavoie P.M., Abu-Raya B. Decay of anti-Bordetella pertussis antibodies in women of childbearing age following COVID-19 non-pharmaceutical measures. Vaccine. 2022;40:3746–3751. doi: 10.1016/j.vaccine.2022.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]