Abstract
Introduction
Treatment with biologics for severe asthma is informed by international and national guidelines and defined by national regulating bodies, but how these drugs are used in real-life is unknown.
Materials and methods
The European Respiratory Society (ERS) SHARP Clinical Research Collaboration conducted a three-step survey collecting information on asthma biologics use in Europe. Five geographically distant countries defined the survey questions, focusing on seven end-points: biologics availability and financial issues, prescription and administration modalities, inclusion criteria, continuation criteria, switching biologics, combining biologics and evaluation of corticosteroid toxicity. The survey was then sent to SHARP National Leads of 28 European countries. Finally, selected questions were submitted to a broad group of 263 asthma experts identified by national societies.
Results
Availability of biologics varied between countries, with 17 out of 28 countries having all five existing biologics. Authorised prescribers (pulmonologists and other specialists) also differed. In-hospital administration was the preferred deliverance modality. While exacerbation rate was used as an inclusion criterion in all countries, forced expiratory volume in 1 s was used in 46%. Blood eosinophils were an inclusion criterion in all countries for interleukin-5 (IL-5)-targeted and IL-4/IL-13-targeted biologics, with varying thresholds. There were no formally established criteria for continuing biologics. Reduction in exacerbations represented the most important benchmark, followed by improvement in asthma control and quality of life. Only 73% (191 out of 263) of surveyed clinicians assessed their patients for corticosteroid-induced toxicity.
Conclusion
Our study reveals important heterogeneity in the use of asthma biologics across Europe. To what extent this impacts on clinical outcomes relevant to patients and healthcare services needs further investigation.
Short abstract
This study, based on a three-step survey among 28 European countries, has demonstrated some similarities but also great disparities in the availability and use of biologics for severe asthma in Europe https://bit.ly/3pqwlC5
Introduction
Asthma is a chronic disease characterised by variable airway obstruction, underpinned by airway inflammation and bronchial hyperresponsiveness, and occasional acute exacerbations. It affects 1–18% of the world population [1], with 3.5% to 5% of patients having severe disease [2], displaying higher morbidity and representing >50% of the direct total cost of asthma management [1] due to increased use of medications, emergency department visits and hospitalisations. The European Respiratory Society/American Thoracic Society (ERS/ATS) consensus defines severe asthma as a pattern of disease requiring high-dose inhaled corticosteroids (ICS) and a second controller, such as oral corticosteroids (OCS) or long-acting β-agonists, to prevent it from being uncontrolled, or that remains uncontrolled despite well-applied therapy [1, 3].
The main aims of asthma treatment are reduction in exacerbations, improvement in quality of life and lung function, and minimisation of long-term adverse events from corticosteroids [1]. Improved characterisation of severe asthma pathophysiology [4, 5] has led to development of biological therapies aiming to modulate the airway inflammatory processes driven by IgE and type 2 interleukins (IL)-4, -5 and -13 [6]. Five biologics are currently available: omalizumab, mepolizumab, reslizumab, benralizumab and dupilumab (table 1).
TABLE 1.
Biologics available in severe asthma
| Biologics | Type | Route and dosing | Mechanism of action | FDA–EMA recommendations |
| Omalizumab (Xolair) | Anti-IgE | SC 0.016 mg·kg−1 per IU·mL−1 of IgE per month, injected every 2 to 4 weeks |
|
FDA approval: 2002 |
| Mepolizumab (Nucala) | Anti-IL-5 | SC 100 mg every 4 weeks |
|
FDA approval: 2015 |
| Reslizumab (Cinquaero) | Anti-IL-5 | IV (intravenous) 3 mg·kg−1 |
|
FDA approval: 2016 |
| Benralizumab (Fasenra) | Anti-IL-5R | SC 30 mg every 4 weeks for the first three doses, then 30 mg every 8 weeks |
|
FDA approval: 2017 |
| Dupilumab (Dupixent) | Anti-IL-4/IL-13 | SC 400–600 mg for loading dose, then 200–300 mg every 2 weeks |
|
FDA approval: 2018 |
FDA: Food and Drug Administration; EMA: European Medicines Agency; SC: subcutaneous; GINA: Global Initiative for Asthma; IV: intravenous; IL: interleukin; OCS: oral corticosteroids; FENO: fractional exhaled nitric oxide.
The “Severe Heterogeneous Asthma Research collaboration, Patient-centered” (SHARP), is a Clinical Research Collaboration (CRC) of the ERS with an overall ambition to improve asthma care and wellbeing of patients with severe asthma across Europe, through a patient-centred approach [7]. Discussions between four stakeholders (patients, clinicians, scientists and pharmaceutical companies) have identified the use of biologics as an important issue to study so as to inform best practice and ensure that the right patient is given the most effective biological treatment.
While evidence-based guidance on treatment with biologics is provided by international (European Medicines Agency (EMA)) or national agencies (e.g. National Institute for Health and Care Excellence (NICE) in the UK), pricing and reimbursement criteria are defined at national level in each country, potentially leading to heterogeneity in their use. A previous study performed by the SHARP/ERS research group indicated that the severe asthmatic population in Europe is heterogeneous and differs in both clinical characteristics and treatment regimen before initiation of any biologics [8]. Harmonising the use of biologics in Europe is of interest for reproducible clinical practice and effective comparison of treatments in longitudinal and multicentric real-life studies. In order to understand current and influence future practice, we conducted a survey in 28 European nations to investigate how biologics are currently employed, focusing on key treatment indicators: availability, inclusion criteria, administration modalities and continuation criteria.
Materials and methods
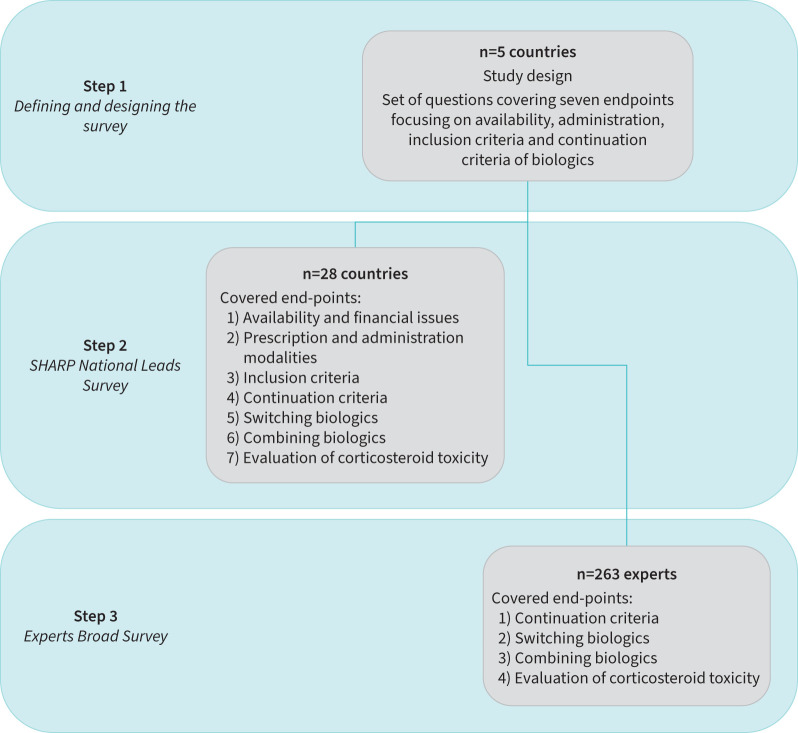
We applied a three-step survey (figure 1) to describe the use of biologics across Europe between June 2020 and April 2021. In the first step (June 2020 to November 2020), five geographically distant countries (Belgium, Estonia, Romania, Sweden, UK) were selected to define the survey questions during virtual meetings, assisted by the SHARP support team, focusing on availability of biologics, their administration and criteria for inclusion or continuation of treatment. In the second step (November 2020 to April 2021), the survey was extended (by e-mail correspondence) to all SHARP National Leads in 28 European countries (SHARP National Leads survey, table 2). In the third step (January 2021 to April 2021), a selection of questions was submitted to a larger audience of 263 asthma experts across Europe, identified by their national societies (Experts Broad Survey).
FIGURE 1.
Study flow chart: a three-step survey.
TABLE 2.
Availability of biologics in Europe (as of April 2021)
| Country | Number of available biologics | Omalizumab | Mepolizumab | Reslizumab | Benralizumab | Dupilumab |
| Austria | 5 | 1 | 1 | 1 | 1 | 1 |
| Belgium | 4 | 1 | 1 | 1 | 1 | 0 |
| Croatia | 4 | 1 | 1 | 1 | 1 | 0 |
| Czech Republic | 5 | 1 | 1 | 1 | 1 | 1 |
| Denmark | 5 | 1 | 1 | 1 | 1 | 1 |
| Estonia | 5 | 1 | 1 | 1 | 1 | 1 |
| Finland | 5 | 1 | 1 | 1 | 1 | 1 |
| France | 5 | 1 | 1 | 1 | 1 | 1 |
| Germany | 5 | 1 | 1 | 1 | 1 | 1 |
| Greece | 2 | 1 | 1 | 0 | 0 | 0 |
| Hungary | 5 | 1 | 1 | 1 | 1 | 1 |
| Iceland | 5 | 1 | 1 | 1 | 1 | 1 |
| Ireland | 4 | 1 | 1 | 1 | 1 | 0 |
| Italy | 3 | 1 | 1 | 0 | 1 | 0 |
| Latvia | 5 | 1 | 1 | 1 | 1 | 1 |
| Lithuania | 3 | 1 | 1 | 0 | 1 | 0 |
| Netherlands | 5 | 1 | 1 | 1 | 1 | 1 |
| Poland | 3 | 1 | 1 | 0 | 1 | 0 |
| Portugal | 5 | 1 | 1 | 1 | 1 | 1 |
| Romania | 2 | 1 | 0 | 0 | 1 | 0 |
| Russia | 5 | 1 | 1 | 1 | 1 | 1 |
| Serbia | 3 | 1 | 0 | 1 | 1 | 0 |
| Slovenia | 4 | 1 | 1 | 1 | 1 | 0 |
| Spain | 5 | 1 | 1 | 1 | 1 | 0 |
| Sweden | 5 | 1 | 1 | 1 | 1 | 1 |
| Switzerland | 5 | 1 | 1 | 1 | 1 | Off label |
| Turkey | 2 | 1 | 1 | 0 | 0 | 0 |
| UK | 5 | 1 | 1 | 1 | 1 | 1 |
Of note, this table summarises the availability of biologics at the time the survey was conducted (November 2020 to April 2021). Since survey completion, dupilumab has become available in Spain, Switzerland, Croatia and Slovenia.
The survey involving SHARP National Leads covered seven end-points.
1) Availability and financial issues. Lists of biologics available in individual countries and financial issues (patient contribution or fixed hospital budget dedicated to biologics) were requested. We analysed the number of available biotherapies related to per capita gross domestic product (GDP), comparing countries with ≤3 and those with >3 biologics.
2) Prescription and administration modalities. Information on prescribers (pulmonologist, allergologist, ENTs (ear–nose–throat, otorhinolaryngologist), paediatrician, team of experts) was collected, together with prescription and administration modalities (home or hospital administration).
3) Inclusion criteria. Participants provided the criteria for prescribing biologics, focusing on each biologic individually, singling out common basic criteria among nations, together with additional criteria specifically required in some countries.
4) Continuation criteria. Details were requested for how and when effectiveness of biologics was evaluated and how decisions were made whether to continue treatment. After the initial survey returned multiple and diverse criteria, a second survey, containing 10 objective criteria commonly used to assess efficacy in severe asthma clinical trials, was undertaken. National Leads were asked to rank those criteria in order of importance. We separated two groups of patients to apply those criteria: those on and those not on maintenance OCS. The survey participants ranked criteria between 1 and 10 (from most to least important). The mean value was calculated for each criterion's rank in order to obtain a complete ranking of objective criteria.
5) Switching biologics. SHARP National Leads were asked to describe how easy it was to switch from one biotherapy to another.
6) Combining biologics. SHARP National Leads were asked whether combining biologics was possible in their country.
7) Evaluation of corticosteroid toxicity. Participants were asked whether and how they evaluated corticosteroid toxicity in patients with severe asthma.
After completing the National Leads survey, the broad panel of European asthma experts (Experts Broad Survey) were surveyed to validate the following end-points: 4) Continuation criteria, 5) Switching biologics, 6) Combining biologics and 7) Evaluation of corticosteroid toxicity.
Statistics
Parametric unpaired t-tests compared GDP per capita and number of available biologics. Objective continuation criteria were ranked by calculating the mean value for each criterion's rank: the lower the mean item score, the higher the importance of the criterion.
Results
Availability and financial issues
The details of biologics available in the surveyed countries are shown in table 2. Availability was related to per capita GDP, which was significantly (p=0.0072) lower in countries with ≤3 biologics (n=7) than in those with >3 biologics (n=21) (USD 16 831±9102 versus USD 41 302±21 327) (figure 2). Patient financial contribution was required in nine countries: patients had to pay a percentage of total cost in six countries, whereas those from the three others had a fixed amount to pay. Six countries worked with a fixed hospital budget dedicated to biologics.
FIGURE 2.
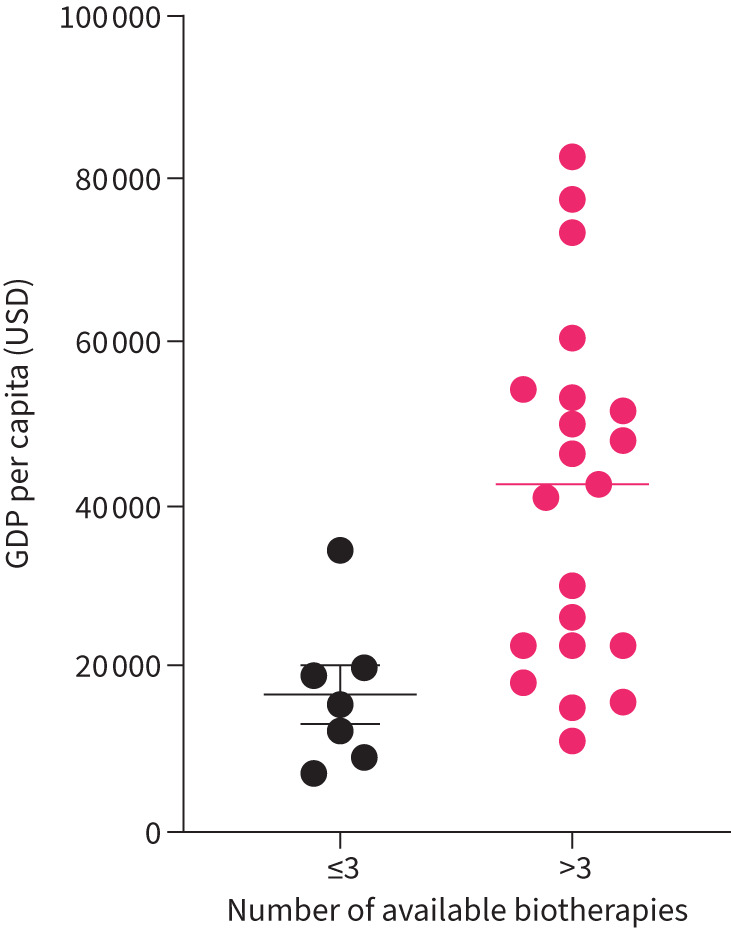
Comparison of the number of available biotherapies according to gross domestic product (GDP) per capita. Results are expressed as mean±sem; data analysed by parametric unpaired t-tests.
Prescription and administration modalities
Biologics could be prescribed by a single prescriber in all but three countries, where decisions were taken by a team/panel of experts (including pulmonologists, allergologists, paediatricians and ENTs). Pulmonologists were sole prescribers in four countries, whereas allergologists and paediatricians could also prescribe biologics for severe asthma in the other countries. Initial administration was performed in hospitals in all countries, with subsequent home administration possible in 20.
Inclusion criteria
Exacerbation rates were inclusion criterion in all (n=28) countries, with a threshold of ≥2/year, except in two countries for which cut-off was 1/year (table 3). Forced expiratory volume in 1 s (FEV1) was used in 46% of countries, mainly for omalizumab, with a threshold at FEV1 <80% of predicted value, except for one country with a threshold <50% of predicted. Blood eosinophil counts were used in all countries for IL-5- and IL-4- targeted medications, but threshold values differed between biologics and countries, ranging from 150 to 500·µL−1. To prescribe omalizumab, serum IgE levels were used in all countries with an inclusion threshold varying from 30 to 148 IU·mL−1, and evidence of sensitisation to at least one aero-allergen, as judged by serum-specific IgE or prick tests, was required in 21 countries (75%). Additional criteria had to be met for certain biologics, such as asthma control and quality of life questionnaires, good adherence to existing treatment, evidence of non-smoking status (e.g. saliva cotinine levels), fractional exhaled nitric oxide (FENO), sputum eosinophil levels and expert consensus meeting prior to initiation of treatment.
TABLE 3.
Inclusion criteria for severe asthma biotherapies in Europe
| Biotherapy | Common criteria | Disparities noted between countries | Additional criteria for some countries |
| Omalizumab (n=26 countries) | Severe asthma definition (ATS/ERS) (24/26) Exacerbation rate (24/26) • Four countries required >2 exacerbations/year • 20 countries considered ≤2 exacerbations/year IgE levels (25/26), with very variable threshold (majority >30 or >76 IU·mL−1) |
Age threshold: adults versus children for some countries FEV1 levels: used as criterion in 13/26 countries (50%) |
Adherence is an absolute criterion in two countries Non-smoking status is an absolute criterion in three countries Questionnaire for quality of life (QOL) was used as criterion in one country |
|
Mepolizumab
(n=24 countries) |
Severe asthma definition (ATS/ERS) (21/24) Exacerbation rate (24/24) • Five countries required >2 exacerbations/year • 19 countries considered ≤2 exacerbations/year Blood eosinophils (24/24), with a threshold variable between 150 and 500 cells·µL−1) Of note, one country separated maintenance OCS patients and not on maintenance OCS patients to define threshold |
Age: adult versus children. This was not clearly defined as criterion in some countries FEV1 levels: used as criterion in 5/24 countries (20.8%) |
Adherence is an absolute criterion in four countries Non-smoking status is an absolute criterion in three countries Questionnaire for QOL was used as criterion in one country Two countries offer the possibility of inclusion if sputum eosinophils are high, even if blood eosinophils are below the fixed threshold Cotinine level in saliva is a criterion in one country |
| Benralizumab (n=24 countries) | Severe asthma definition (ATS/ERS) (20/24) Exacerbation rate (22/24) • Five countries required >2 exacerbations/year • 17 countries considered ≤2 exacerbations/year Blood eosinophils (24/24), with a threshold variable between 150 and 500 cells·µL−1) |
Age: adult versus children. This was not clearly defined as criterion in some countries FEV1 levels: used as criterion in 6/24 countries (25%) |
Adherence is an absolute criterion in three countries Non-smoking status is an absolute criterion in three countries Questionnaire for QOL was used as criterion in one country Two countries offer the possibility of inclusion if sputum eosinophils are high, even if blood eosinophils are below the fixed threshold Cotinine level in saliva is a criterion in one country |
| Reslizumab (n=16 countries) | Severe asthma definition (ATS/ERS) (16/16) Exacerbation rate (16/16) • Seven countries required >2 exacerbations/year • Nine countries considered ≤2 exacerbations/year Blood eosinophils (16/16), with a threshold variable between 150 and 400 cells·µL−1) |
Age: adult versus children. This was not clearly defined as criterion in some countries FEV1 levels: used as criterion in 5/16 countries (31.2%) |
Adherence is an absolute criterion in three countries Non-smoking status is an absolute criterion in three countries Questionnaires for QOL were not used Two countries offer the possibility of inclusion if sputum eosinophils are high, even if blood eosinophils are below the fixed threshold Three countries granted the biotherapy if patient was on OCS >50% of the year Cotinine level in saliva was not used |
| Dupilumab (n=11 countries) | Severe asthma definition (ATS/ERS) (10/11) Exacerbation rate (10/11) • One country required >2 exacerbations/year • Nine countries considered ≤2 exacerbations/year • One country had no threshold Blood eosinophils (11/11), with a threshold variable between 150 and 300 cells·µL−1) |
Age: adult versus children. This was not clearly defined as criterion in some countries FEV1 levels: used as criterion in 1/11 countries (9.1%) FENO levels: used in 5/11 countries (45.5%), with threshold >25 ppb for all countries |
Adherence is an absolute criterion in two countries Non-smoking status is an absolute criterion in two countries Questionnaires for QOL were not used Three countries granted the biotherapy if patient was on OCS >50% of the year (without needing exacerbations or high blood eosinophilic count) Cotinine level in saliva is a criterion in one country |
ATS: American Thoracic Society; ERS: European Respiratory Society; FEV1: forced expiratory volume in 1 s; QOL: quality of life; OCS: oral corticosteroids; FENO: fractional exhaled nitric oxide.
Assessment of effectiveness and criteria for continuation
Recommendations for treatment duration before first evaluation after starting therapy and modalities to assess treatment effectiveness differed between countries and biologics. In 13 countries, clinicians were required to perform an assessment at 4–6 months and continue medication only if effectiveness was proven, whereas in three countries this was done at 1 year. However, in 12 countries, unlimited initial reimbursement was directly granted.
Although effectiveness evaluation was mandatory in most countries, assessment criteria were not strictly defined. Some countries had few objective criteria and mainly assessed patients’ subjective responses, whereas others needed demonstration of improvement in objective benchmarks, such as reduction in exacerbation rate and/or maintenance OCS dose or positive change in the Global Evaluation of Treatment Effectiveness (GETE) score (for omalizumab).
After analysing these preliminary results, a predefined ranking questionnaire was sent back to National Leads and to the wider group of 263 asthma experts (table 4). For patients on maintenance OCS, the four most important criteria were similar in the SHARP National Leads survey and the Experts Broad Survey. For participants, the most meaningful criteria of effectiveness were the reductions in exacerbation rate by 75% and 50% and reduction in maintenance OCS dose. For patients not on maintenance OCS, the four most important criteria were identical between the two groups, represented by reduction in exacerbation rate (by 75%, 50% then 25%) then improvement of Asthma Control Questionnaire (ACQ) (by one on 1 year). Overall, the reduction in exacerbations was the most important criterion, followed by improvements in asthma control and quality of life scores. The least important criterion was 5% improvement in FEV1 both for patients on and those not on OCS.
TABLE 4.
Assessment of effectiveness in biologics: ranking of objective criteria
| Ranking | Patients on maintenance OCS | Item score | Patients not on maintenance OCS | Item score |
| National Leads Survey (n=28) | ||||
| 1 | Reduction of exacerbation rate by 75% over 1 year | 3.4 | Reduction of exacerbation rate by 75% over 1 year | 2.5 |
| 2 | Reduction of chronic dose of OCS by 50% | 3.4 | Reduction of exacerbation rate by 50% over 1 year | 2.8 |
| 3 | Stopping chronic maintenance OCS | 3.5 | Reduction of exacerbation rate by 25% over 1 year | 4.8 |
| 4 | Reduction of exacerbation rate by 50% over 1 year | 4.1 | Reduction in ACQ by 1 at 4/6 months | 5 |
| 5 | Reduction of exacerbation rate by 25% over 1 year | 6 | Increase in AQLQ by 1 at 4/6 months | 5 |
| 6 | Increase in AQLQ by 1 at 4/6 months | 6.1 | Reduction in ACQ by 0.5 at 4/6 months | 5.5 |
| 7 | Reduction in ACQ by 1 at 4/6 months | 6.2 | Increase in AQLQ by 0.5 at 4/6 months | 5.7 |
| 8 | Reduction in ACQ by 0.5 at 4/6 months | 6.6 | Reduction of chronic dose of ICS by 50% | 6.2 |
| 9 | Increase in AQLQ by 0.5 at 4/6 months | 6.9 | Improvement of 5% predicted FEV1 | 7.6 |
| 10 | Improvement of 5% predicted FEV1 | 8.5 | ||
| Experts Broad Survey (n=263) | ||||
| 1 | Reduction of exacerbation rate by 75% over 1 year | 2.6 | Reduction of exacerbation rate by 75% over 1 year | 1.9 |
| 2 | Reduction of exacerbation rate by 50% over 1 year | 3.1 | Reduction of exacerbation rate by 50% over 1 year | 3.1 |
| 3 | Stopping chronic maintenance OCS | 3.5 | Reduction of exacerbation rate by 25% over 1 year | 4.5 |
| 4 | Reduction of chronic dose of OCS by 50% | 4.1 | Reduction in ACQ by 1 at 4/6 months | 4.6 |
| 5 | Reduction in ACQ by 1 at 4/6 months | 4.8 | Reduction in ACQ by 0.5 at 4/6 months | 5.2 |
| 6 | Reduction of exacerbation rate by 25% over 1 year | 5.1 | Reduction of chronic dose of ICS by 50% | 5.6 |
| 7 | Reduction in ACQ by 0.5 at 4/6 months | 6.3 | Increase in AQLQ by 1 at 4/6 months | 6.3 |
| 8 | Increase in AQLQ by 1 at 4/6 months | 7.3 | Increase in AQLQ by 0.5 at 4/6 months | 6.8 |
| 9 | Increase in AQLQ by 0.5 at 4/6 months | 8.5 | Improvement of 5% predicted FEV1 | 7.8 |
| 10 | Improvement of 5% predicted FEV1 | 8.9 | ||
These items are clinical efficacy criteria used in most RCTs to assess effectiveness of biologics in severe asthma. The lower the mean score of the item is, the higher its importance as effectiveness criteria is, according to participants. OCS: oral corticosteroids; AQLQ: Asthma Quality of Life Questionnaire; ACQ: Asthma Control Questionnaire; ICS: inhaled corticosteroids; FEV1: forced expiratory volume in 1 s.
Switching biologics
The survey in SHARP National Leads revealed that it is easy to switch between biologics, although one country (1 out of 28) reported difficulties and one had no experience. The Experts Broad Survey showed that 202 out of 263 participants (77%) found it easy to switch between biologics. For those who faced difficulties in switching, the main reasons were lack of experience, formal prohibition, need for a wash-out period, health insurance issues and cost.
Combining biologics
The SHARP National Leads survey revealed that combining biologics was not authorised in 16 countries, while there were no formal restrictions in the other 12, but experience was lacking. This was verified by the Experts Broad Survey, which showed that combining biologics was not allowed or not-tested for 81% of participants.
Evaluation of corticosteroids toxicity
The SHARP National Leads survey revealed that corticosteroid-induced toxicity was assessed in 20 countries (mainly by clinical evaluation and cortisol blood levels). This was supported by data extracted from the Experts Broad Survey, which showed evaluation by 70% of experts. Cortisol blood level and clinical evaluation were also the most commonly used assessment modalities.
Discussion
Our study shows that availability of biologics, especially those most recently licensed, varies across Europe. This was also recently observed in the International Severe Asthma Registry [9]. In our survey, the wealthier countries (by GDP per capita) offer a greater choice of biologics. Large variation was observed in medical criteria for reimbursing the patients. Although any financial contributions imposed on the patient vary greatly between countries, it was encouraging to see that health insurance covers the majority of the cost in all countries. Our survey also demonstrated differences in prescription and administration modalities, albeit without negative impact on initiation of treatment. Three countries required an expert panel to initiate treatment; whether or not this impacts on clinical outcomes is unclear, but we speculate that expert panels discussing each case individually, especially during multi-disciplinary meetings, may be more likely to pick up other unmet needs for which alternative treatment modalities could be offered. We also speculate that this could reduce unnecessary premature introduction of biologics. We also noticed differences in prescribers (pulmonologist, allergologist, paediatrician). Furthermore, while hospital administration of biologics is standard in every country, home administration was possible in 71% of countries, making it easier for patients living at distance from the hospital to access those medications in some but not all European countries.
Surprisingly, our survey revealed marked differences in treatment inclusion criteria. Remarkably, some countries have established reimbursement criteria that do not strictly follow the clinical or laboratory criteria used in monoclonal antibodies randomised controlled trials (RCTs). While all countries agreed on the need to meet the strict definition of severe asthma, the required minimal exacerbation rate in the past year was highly variable, ranging from 1 to 4, the greatest proportion of countries choosing 2. Arguably, doctors should strive to prevent all exacerbations, but the financial realities make this a difficult objective to achieve. We would argue that duration and severity of an exacerbation should also be included in the decision-making process. Omalizumab and anti-IL-5 or anti-IL-4/IL-13 biologics all required specific laboratory biomarkers, namely total serum IgE concentration and blood eosinophil counts, respectively. While these biomarkers are used in most European countries, there were marked differences in threshold values, for unclear reasons that need elucidation. Of note, in two countries, it was possible to bypass the blood eosinophil threshold and offer anti-IL-5 therapy if sputum eosinophil counts were high. In three countries, dupilumab was offered to patients on OCS for >50% of the year irrespective of immunological T2 profile. Lung function has long been a measure of treatment efficacy but has often been considered as the secondary end-point in severe asthma clinical trials studying biologics. In keeping with this established concept, FEV1 was mostly used as an inclusion criterion for omalizumab (in 50% of countries) but was only used in 9% of countries for dupilumab. This is paradoxical given that dupilumab was found to be more effective than other biologics at improving FEV1 [10]. Furthermore, considerable improvement in FEV1 has been reported with benralizumab in severe eosinophilic asthma and nasal polyposis [11]. Arguably, requiring an already altered FEV1 as inclusion criteria might not be suitable as it may limit access to the medication in patients who might have benefited from prevention of remodelling and subsequent decrease in lung function. Indeed, there are some data on long-term follow-up suggesting that biologics may prevent decline in lung function [12–14]. Finally, there was an obligation in some countries to prove non-smoking status or treatment adherence, or completion of a questionnaire of quality of life. Concerning exposure to tobacco, there is an exclusion of smokers or sometimes significant ex-smokers in severe asthma clinical trials, which may explain why some countries also apply this restriction when prescribing biologics. While there are no RCTs including smokers, a recent work focusing on ex-smokers suggested that a significant smoking history did not preclude effectiveness of anti-IL-5/anti-IL-5R therapy regarding exacerbations and asthma control [15]. In addition, a recent real-life study has indicated that anti-IL-5 was able to attenuate lung function decline in severe eosinophilic asthmatic individuals independently of smoking status [13]. While we recognise the need to encourage smoking cessation, we question the morality of withholding treatment in people who are unable to quit. In addition, it is worth highlighting that most countries do not need strict proof of compliance regarding background ICS treatment, which is surprising as it is known that non-adherence is an important cause of uncontrolled asthma.
An important but very contentious point for treatment with biologics is its effectiveness evaluation. GINA (Global Initiative for Asthma) guidelines recommend a 4-month trial period before assessing effectiveness in respect of asthma control [1]. A recent panel of European experts recommended a traffic lights assessment system that offers three decision pathways: 1) continue treatment in super-responders; 2) stop if there is no evidence of response; and 3) extend for 1 year in intermediate responders in case the response is delayed [16]. Our survey noted divergence between countries in timing between first administration of biologics and first assessment of effectiveness. While in most countries evaluation was done at 4–6 months (depending on biologic), others only assessed at 1 year, and some allowed immediate access to an unlimited reimbursement without strict evaluation of effectiveness. The most appropriate timing of effectiveness assessment needs to be addressed, as early evaluation might not show clinically relevant reduction in exacerbations, whereas late evaluation might delay any treatment discontinuation or switch between biologics. Similarly, we noted large differences in the benchmarks used to assess effectiveness. Indeed, many countries did not take into account efficacy criteria obtained in severe asthma monoclonal antibodies RCTs. In an attempt to reach a consensus on optimal assessment, we asked asthma experts to rank the clinical efficacy criteria used in those RCTs. Not surprisingly, the reduction in exacerbation rate and the burden of maintenance OCS were rated as the most important, followed by improvements in asthma control and quality of life. Of note, the least important criterion was improvement of 5% predicted in FEV1, although this level of improvement has been achieved in most RCTs and real-life studies with biologics [17, 18]. Overall, there is yet no general consensus about criteria defining significant response to treatment with biologics, even if this is an active area of discussion [19].
The question of switching or combining biologics emerges when asthma remains uncontrolled despite the biologic. There are reports indicating that switching from anti-IgE to anti-IL-5 or anti-IL-5R may improve asthma control [20–22]. Our survey showed that it is easy in most countries to switch between biologics if standard inclusion criteria for the alternative biologic are met. Combining biologics in cases of uncontrolled asthma is a plausible option since biologics act through different mechanisms. However, combination therapy has not been studied extensively so its efficacy–safety profile is not established. Indeed, relevant data on this subject are mainly derived from case reports [23, 24]. This is not surprising, as our survey showed that combining biotherapies is not authorised in 16 of 28 countries, while 12 countries have no formal restrictions but also have no experience. A recent study has shown that combining dupilumab with an anti-IL-33 did not bring further advantage [25]. Formal studies are necessary to define the benefit–risk balance of combining asthma biologics that target different molecular pathways [26].
Despite known side-effects, OCS treatment remains a cornerstone in the management of a substantial proportion of severe asthmatics. Some biologics have been shown to have a corticosteroid-sparing potential, such as mepolizumab [27], benralizumab [28] or dupilumab [29]. Our survey revealed that corticosteroid-related side-effects were assessed in 70% of countries, which is quite considerable, but this leaves almost one third of patients without appropriate assessment. The methods used to evaluate OCS side-effects also varied, with cortisol blood levels and clinical evaluation being most frequently used. There are accurate tools, including the Glucocorticoid Toxicity Index [30], that can be used. Standardisation is essential for patient-centred care to improve evaluation of corticosteroid-induced toxicity, as it might lead to a more rapid identification of side-effects on the one hand, and a better structured and consistent approach to corticosteroid weaning on the other hand.
This survey has several limitations. Firstly, it did not take into account patient engagement. As a patient-centred CRC, we recognise the importance of patients’ perspectives of effectiveness, but patient involvement in SHARP in some countries is still lacking either completely or is suboptimal. Thus, patient engagement to define the inclusion and exclusion criteria would have been insufficiently representative of all the European countries. To follow-up on this study, an additional European collaborative and patient-centred survey led by the SHARP/ERS expert group, involving patient organisations and networks, is needed. Secondly, our study being essentially descriptive, it has not investigated how between-nation differences in inclusion or continuation criteria may result in disparities in real-life effectiveness and patient care. Thirdly, the study has not assessed the impact of corticosteroid monitoring on patient outcomes, particularly on the weaning of steroids, which is one of the fundamental aims when starting biologics in patients receiving maintenance OCS.
Conclusion
Our survey has demonstrated some similarities but also great disparities in the use of biologics for severe asthma, which need to be understood better and remedied to achieve best possible practice for patients. Harmonising the use of biologics is also of interest for reproducible clinical practice and effective comparison of treatments in longitudinal and multicentric real-life studies. While harmonisation of practice across Europe requires further analysis, we can now, as members of a strongly patient-centred clinical research collaboration, appeal to healthcare providers of all countries where the full complement of biologics is not available to explore ways of improving availability. From a patient and public health perspective, we also strongly recommend that formal criteria for effectiveness assessment should be established.
Acknowledgements
The SHARP CRC would like to acknowledge the support and expertise of the following individuals without whom the study would not have been possible: Emmanuelle Berret (European Respiratory Society, Lausanne, Switzerland) and Daniel Doberer (Vienna General Hospital, Vienna, Austria).
Footnotes
Provenance: Submitted article, peer reviewed.
Conflict of interest: L.G. Heaney reports the following relationships outside the submitted work: academic lead for the UK MRC Consortium for Stratified Medicine in Severe Asthma (industrial pharma partners: Amgen, AstraZeneca, Medimmune, Janssen, Novartis, Roche/Genentech, GlaxoSmithKline and Boehringer Ingelheim); project grant funding from Medimmune, Novartis UK, Roche/Genentech and GlaxoSmithKline; travel funding support to international respiratory meetings received from AstraZeneca, Chiesi, Novartis, Boehringer Ingelheim, Teva and GlaxoSmithKline; participated on advisory boards for AstraZeneca, Novartis, GlaxoSmithKline, Chiesi, Teva, Theravance and Vectura.
Conflict of interest: B. Dahlén reports the following relationships outside the submitted work: grant received from GSK and Novartis to support Karolinska Severe Asthma Centre; personal consulting fees received from GSK, Novartis, Teva and AstraZeneca; consulting fees, paid to the institution, received from Region Stockholm; personal honoraria received from GSK, Novartis, Teva and AstraZeneca.
Conflict of interest: S. Popović-Grle reports the following relationships outside the submitted work: payment for educational events from Abbot, Alkaloid, AstraZeneca, BerlinChemie Menarini, Boehringer Ingelheim, Medis, Novartis, Pliva-Teva, PharmaS, Providens, Salvus, Sandoz, Sanofi-Aventis and SwixxPharma; participation on advisory boards for AstraZeneca, Boehringer Ingelheim, BerlinChemie Menarini, GSK, Novartis, Pliva-Teva, PharmaS, Sanofi-Aventis and SwixxPharma.
Conflict of interest: V. Sedlák reports the following relationships outside the submitted work: payment for presentations received from AstraZeneca, Novartis, GlaxoSmithKline, TEVA and Sanofi; payment for expert testimony payments received from AstraZeneca, Novartis, GlaxoSmithKline, TEVA and Sanofi; payment for participation on a data safety monitoring board or advisory board received from AstraZeneca, Novartis, GlaxoSmithKline, TEVA and Sanofi.
Conflict of interest: L. Lehtimäki reports the following relationships outside the submitted work: fees for lectures or advisory board meetings received from ALK, AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, GSK, Mundipharma, Novartis, Orion Pharma and Sanofi; owner of shares for Ausculthing OY.
Conflict of interest: A. Bourdin reports the following relationships outside the submitted work: unrestricted grants received from AstraZeneca and Boehringer Ingelheim; consulting fees received from Astra Zeneca, GSK, Novartis, Sanofi Regeneron, Boehringer Ingelheim and Chiesi; payment or honoraria received for lectures, presentations, speakers bureaus, manuscript writing or educational events received from AstraZeneca, GSK, Novartis, Sanofi Regeneron, Boehringer Ingelheim and Chiesi; support for attending meetings and/or travel received from Astra Zeneca, GSK, Novartis, Sanofi Regeneron, Boehringer Ingelheim and Chiesi.
Conflict of interest: S. Korn reports the following relationships outside the submitted work: consulting fees received from AstraZeneca, Novartis, GlaxoSmithKline and Sanofi; payment or honoraria received for lectures and presentations from AstraZeneca, Novartis, GlaxoSmithKline and Sanofi; participation on a data safety monitoring board or advisory board for AstraZeneca, Novartis and GlaxoSmithKline.
Conflict of interest: M.W. Butler reports the following relationships outside the submitted work: payment or honoraria received for lectures and presentations from AstraZeneca, Novartis and GlaxoSmithKline; support received for international meeting attendance received from AstraZeneca; participation on Advisory Boards for ALK Abello, AstraZeneca, GlaxoSmithKline and Novartis; Chair of Medical Advisory Group, board member of Asthma Society of Ireland, Ireland member of GINA Assembly, President of Irish Thoracic Society (2022 to present).
Conflict of interest: G.W. Canonica reports the following relationships outside the submitted work: payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events received from AstraZeneca, GSK, Novartis and Sanofi; participation on a data safety monitoring board or advisory board for AstraZeneca, GSK, Novartis and Sanofi; leadership or fiduciary role in other board, society, committee or advocacy group for EAACI Methodology Committee, REG Vice President and SANI Steering Committee.
Conflict of interest: K. Beiksiene reports the following relationships outside the submitted work: payment or honoraria received for lectures, presentations, speakers’ bureaus, manuscript writing or educational events received from AstraZeneca and Berlin Chemie.
Conflict of interest: A. ten Brinke reports the following relationships outside the submitted work: unrestricted grants received from TEVA, GSK and AstraZeneca; consulting fees paid to the institution from GSK, Sanofi, TEVA, AstraZeneca and Boehringer Ingelheim; payments for lectures, paid to the institution, received from GSK, TEVA, AstraZeneca and Sanofi; participation on research advisory boards for GSK, Sanofi, AstraZeneca, Boehringer Ingelheim and TEVA; Chair of Dutch severe asthma registry RAPSODI.
Conflict of interest: P. Kuna reports the following relationships outside the submitted work: personal fees received for lectures, presentations, speakers’ bureaus, manuscript writing or educational events received from Adamed, AstraZeneca, Boehringer Ingelheim, Berlin Chemie Menarini, Alvogen, Glenmark, Novartis, GSK, Chiesi, Polpharma and Teva.
Conflict of interest: C. Chaves Loureiro reports the following relationships outside the submitted work: Consulting fees received from AstraZeneca and GSK. Payment for r presentations and speakers bureaus received from AstraZeneca, GSK, Novartis and Sanofi-Aventis. Support for attending meetings received from AstraZeneca, Novartis, Nippon, Sanofi-Aventis, Tecnifarma and VitalAire. Participation on Advisory Boards for AstraZeneca, GSK, Novartis and Sanofi-Aventis.
Conflict of interest: Z. Lazic reports the following relationships outside the submitted work: payment or honoraria for educational events received from AstraZeneca, BerlinChemie Menarini, Boehringer Ingelheim, Novartis, PharmaS, Providens, Sandoz and Actavis-Teva Serbia; participation on Advisory Boards for AstraZeneca, Boehringer Ingelheim, BerlinChemie Menarini, Novartis and Actavis-Teva Serbia.
Conflict of interest: S. Škrgat reports the following relationships outside the submitted work: honoraria received for lectures and educational events from Astra Zeneca, Pliva Teva, Berlin Chemie, Chiesi and Medis; participation on local advisory boards organized by AstraZeneca.
Conflict of interest: J. Leuppi reports the following relationships outside the submitted work: J. Leuppi is supported by grants from the Swiss National Science Foundation (SNF 160072 and 185592) and by Swiss Personalised Health Network (SPHN 2018DR108); J. Leuppi has also received unrestricted grants from AstraZeneca AG Switzerland, Boehringer Ingelheim GmbH Switzerland, GSK AG Switzerland, and Novartis AG Switzerland.
Conflict of interest: B. Gemicioglu reports the following relationships outside the submitted work: grants or contracts received from AstraZeneca, Novartis, GlaxoSmithKline and Sanofi; payment or honoraria received for lectures, presentations, speakers’ bureaus, manuscript writing or educational events received from Novartis; support for attending meetings received from Novartis; participation on a data safety monitoring board or advisory board for GlaxoSmithKline.
Conflict of interest: A. Bossios reports the following relationships outside the submitted work: payment or honoraria received for lectures, presentations, speakers’ bureaus, manuscript writing or educational events received from Astra Zeneca, GSK and TEVA; support for attending meetings and/or travel received from Novartis; participation on a data safety monitoring board or advisory board for AstraZeneca, Novartis, GlaxoSmithKline, TEVA and Sanofi; member of the steering committee of SHARP, Secretary of Assembly 5 (Airway diseases, asthma, COPD and chronic cough), European Respiratory Society; vice-chair of Nordic Severe Asthma Network (NSAN).
Conflict of interest: C.M. Porsbjerg reports the following relationships outside the submitted work: grants or contracts, paid to the institution, received from AZ, GSK, Novartis, TEVA, Sanofi, Chiesi and ALK; consulting fees received from AZ, GSK, Novartis, TEVA, Sanofi, Chiesi and ALK; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events received from AZ, GSK, Novartis, TEVA, Sanofi, Chiesi and ALK; participation on a data safety monitoring board or advisory board for AZ, GSK, Novartis, TEVA, Sanofi, Chiesi and ALK.
Conflict of interest: E.H. Bel reports the following relationships outside the submitted work: research grants received from GSK and Teva; consulting fees received from Teva, Sanofi, AstraZeneca, GSK, Sterna and Chiesi; participation on a data safety monitoring board or advisory board for AstraZeneca.
Conflict of interest: R. Djukanovic reports the following relationships outside the submitted work: funding received for the SHARP CRC from ERS, TEVA, GSK, Novartis, Sanofi and Chiesi; consultancy fees paid to the author received from Synairgen plc, Sanofi-Genzyme Corporation and Galapagos; honorarium for lectures paid to the author received from GlaxoSmithKline, Congress of Interasma, AstraZeneca and Airways Vista, Seoul; personal shares owned for Synairgen.
Conflict of interest: L. Renaud reports receiving support for the present manuscript from SHARP CRC supported by GSK, Novartis, Sanofi, Chiesi and Teva. The following relationships are reported outside the submitted work: grants or contracts received from GSK, AZ and Chiesi; consulting fees received from AZ and Sanofi; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events, received from AZ, GSK and Chiesi; patents planned, issued or pending WO 2017/050527 A1 “Method for the diagnosis of airway disease inflammatory subtype”.
Conflict of interest: The remaining authors have nothing to disclose.
Support statement: The SHARP CRC has been supported by financial and other contributions from the following consortium partners: European Respiratory Society, GlaxoSmithKline Research and Development Limited, Chiesi Farmaceutici SPA, Novartis Pharma AG, Sanofi-Genzyme Corporation, and Teva Branded Pharmaceutical Products R&D, Inc. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2021. Available from: http://ginasthma.org/
- 2.Hekking PPW, Wener RR, Amelink M, et al. The prevalence of severe refractory asthma. J Allergy Clin Immunol 2015; 135: 896–902. doi: 10.1016/j.jaci.2014.08.042 [DOI] [PubMed] [Google Scholar]
- 3.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–373. doi: 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 4.Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med 2017; 377: 965–976. doi: 10.1056/NEJMra1608969 [DOI] [PubMed] [Google Scholar]
- 5.Pavord ID, Beasley R, Agusti A, et al. After asthma: redefining airways diseases. Lancet 2018; 391: 350–400. doi: 10.1016/S0140-6736(17)30879-6 [DOI] [PubMed] [Google Scholar]
- 6.Brusselle GG, Koppelman GH. Biologic therapies for severe asthma. N Engl J Med 2022; 386: 157–171. doi: 10.1056/NEJMra2032506 [DOI] [PubMed] [Google Scholar]
- 7.Djukanovic R, Adcock IM, Anderson G, et al. The severe heterogeneous asthma research collaboration, patient-centred (SHARP) ERS clinical research collaboration: a new dawn in asthma research. Eur Respir J 2018; 52: 1801671. doi: 10.1183/13993003.01671-2018 [DOI] [PubMed] [Google Scholar]
- 8.van Bragt JJMH, Adcock IM, Bel EHD, et al. Characteristics and treatment regimens across ERS SHARP severe asthma registries. Eur Respir J 2020; 55: 1901163. doi: 10.1183/13993003.01163-2019 [DOI] [PubMed] [Google Scholar]
- 9.Porsbjerg CM, Menzies-Gow AN, Tran TN, et al. Global variability in administrative approval prescription criteria for biologic therapy in severe asthma. J Allergy Clin Immunol Pract 2022; 10: 1202–1216.e23. doi: 10.1016/j.jaip.2021.12.027 [DOI] [PubMed] [Google Scholar]
- 10.Calzetta L, Matera MG, Rogliani P. Monoclonal antibodies in severe asthma: is it worth it? Expert Opin Drug Metab Toxicol 2019; 15: 517–520. doi: 10.1080/17425255.2019.1621837 [DOI] [PubMed] [Google Scholar]
- 11.Canonica GW, Harrison TW, Chanez P, et al. Benralizumab improves symptoms of patients with severe, eosinophilic asthma with a diagnosis of nasal polyposis. Allergy 2022; 77: 150–161. doi: 10.1111/all.14902 [DOI] [PubMed] [Google Scholar]
- 12.Menzella F, Fontana M, Contoli M, et al. Efficacy and safety of omalizumab treatment over a 16-year follow-up: when a clinical trial meets real-life. J Asthma Allergy 2022; 15: 505–515. doi: 10.2147/JAA.S363398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graff S, Brusselle G, Hanon S, et al. Anti-interleukin-5 therapy is associated with attenuated lung function decline in severe eosinophilic asthma patients from the Belgian severe asthma registry. J Allergy Clin Immunol Pract 2022; 10: 467–477. doi: 10.1016/j.jaip.2021.09.023 [DOI] [PubMed] [Google Scholar]
- 14.Wechsler ME, Ford LB, Maspero JF, et al. Long-term safety and efficacy of dupilumab in patients with moderate-to-severe asthma (TRAVERSE): an open-label extension study. Lancet Respir Med 2022; 10: 11–25. doi: 10.1016/S2213-2600(21)00322-2 [DOI] [PubMed] [Google Scholar]
- 15.Hansen S, Ulrik C, Hilberg O, et al. The effectiveness of anti-IL5 biologics is comparable in previous-smokers and never-smokers with severe asthma. Eur Respir J 2021; 58: Suppl. 65, PA3742. [Google Scholar]
- 16.Buhl R, Humbert M, Bjermer L, et al. Severe eosinophilic asthma: a roadmap to consensus. Eur Respir J 2017; 49: 1700634. doi: 10.1183/13993003.00634-2017 [DOI] [PubMed] [Google Scholar]
- 17.Frix AN, Schleich F, Paulus V, et al. Effectiveness of omalizumab on patient reported outcomes, lung function, and inflammatory markers in severe allergic asthma. Biochem Pharmacol 2020; 179: 113944. doi: 10.1016/j.bcp.2020.113944 [DOI] [PubMed] [Google Scholar]
- 18.Schleich F, Graff S, Nekoee H, et al. Real-world experience with mepolizumab: does it deliver what it has promised? Clin Exp Allergy 2020; 50: 687–695. doi: 10.1111/cea.13601 [DOI] [PubMed] [Google Scholar]
- 19.Upham JW, Le Lievre C, Jackson DJ, et al. Defining a severe asthma super-responder: findings from a Delphi process. J Allergy Clin Immunol Pract 2021; 9: 3997–4004. doi: 10.1016/j.jaip.2021.06.041 [DOI] [PubMed] [Google Scholar]
- 20.Pelaia C, Crimi C, Nolasco S, et al. Switch from omalizumab to benralizumab in allergic patients with severe eosinophilic asthma: a real-life experience from southern Italy. Biomedicines 2021; 9: 1822. doi: 10.3390/biomedicines9121822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carpagnano GE, Pelaia C, D'Amato M, et al. Switching from omalizumab to mepolizumab: real-life experience from Southern Italy. Ther Adv Respir Dis 2020; 14: 175346662092923. doi: 10.1177/1753466620929231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Numata T, Araya J, Miyagawa H, et al. Effectiveness of switching biologics for severe asthma patients in Japan: a single-center retrospective study. J Asthma Allergy 2021; 14: 609–618. doi: 10.2147/JAA.S311975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortega G, Tongchinsub P, Carr T. Combination biologic therapy for severe persistent asthma. Ann Allergy Asthma Immunol 2019; 123: 309–311. doi: 10.1016/j.anai.2019.06.013 [DOI] [PubMed] [Google Scholar]
- 24.Dedaj R, Unsel L. Case study: a combination of Mepolizumab and Omaluzimab injections for severe asthma. J Asthma 2019; 56: 473–474. doi: 10.1080/02770903.2018.1471706 [DOI] [PubMed] [Google Scholar]
- 25.Wechsler ME, Ruddy MK, Pavord ID, et al. Efficacy and safety of Itepekimab in patients with moderate-to-severe asthma. N Engl J Med 2021; 385: 1656–1668. doi: 10.1056/NEJMoa2024257 [DOI] [PubMed] [Google Scholar]
- 26.Menzies-Gow AN, McBrien C, Unni B, et al. Real world biologic use and switch patterns in severe asthma: data from the international severe asthma registry and the US CHRONICLE study. J Asthma Allergy 2022; 15: 63–78. doi: 10.2147/JAA.S328653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of Mepolizumab in eosinophilic asthma. N Engl J Med 2014; 371: 1189–1197. doi: 10.1056/NEJMoa1403291 [DOI] [PubMed] [Google Scholar]
- 28.Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid–sparing effect of benralizumab in severe asthma. N Engl J Med 2017; 376: 2448–2458. doi: 10.1056/NEJMoa1703501 [DOI] [PubMed] [Google Scholar]
- 29.Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med 2018; 378: 2475–2485. doi: 10.1056/NEJMoa1804093 [DOI] [PubMed] [Google Scholar]
- 30.McDowell PJ, Stone JH, Zhang Y, et al. Quantification of glucocorticoid-associated morbidity in severe asthma using the glucocorticoid toxicity index. J Allergy Clin Immunol Pract 2021; 9: 365–372. doi: 10.1016/j.jaip.2020.08.032 [DOI] [PubMed] [Google Scholar]
- 31.E uropean Medicines Agency. XOLAIR (omalizumab). www.ema.europa.eu/en/medicines/human/EPAR/xolair
- 32.Food and Drug Administration. www.accessdata.fda.gov/drugsatfda_docs/label/2016/103976s5225lbl.pdf XOLAIR (omalizumab) for injection, for subcutaneous use.
- 33.European Medicines Agency. NUCALA (mepolizumab). www.ema.europa.eu/en/medicines/human/EPAR/nucala
- 34.Food and Drug Administration. NUCALA (mepolizumab) for injection, for subcutaneous use. www.accessdata.fda.gov/drugsatfda_docs/label/2015/125526Orig1s000Lbl.pdf
- 35.European Medicines Agency. CINQAERO (reslizumab). www.ema.europa.eu/en/documents/overview/cinqaero-epar-summary-public_en.pdf
- 36.Food and Drug Administration. CINQAIR (reslizumab) injection, for intravenous use. www.accessdata.fda.gov/drugsatfda_docs/label/2016/761033lbl.pdf
- 37.European Medicines Agency. www.ema.europa.eu/en/documents/overview/fasenra-epar-medicine-overview_en.pdf FASENRA (benralizumab). An overview of Fasenra and why it is authorized in the EU.
- 38.Food and Drug Administration. www.accessdata.fda.gov/drugsatfda_docs/label/2017/761070s000lbl.pdf FASENRA (benralizumab) injection, for subcutaneous use.
- 39.Food and Drug Administration. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761055s007lbl.pdf DUPIXENT (dupilumab) injection, for subcutaneous use.
- 40.European Medicines Agency. www.ema.europa.eu/en/documents/product-information/dupixent-epar-product-information_en.pdf DUPIXENT (dupilumab).