Abstract
Background
With increasing prevalence of overweight and obesity, it is important to study how body mass index (BMI) change may affect lung function among subjects with asthma. There are few prospective studies on this topic, especially with separate analyses of those with normal and high BMI. The aim of the present study was to prospectively study the association between annual BMI change and annual lung function decline, separately among those with normal initial BMI and overweight/obesity, in an adult asthma cohort.
Methods
A population-based adult asthma cohort was examined at study entry between 1986 and 2001 and at follow-up between 2012 and 2014 (n=945). Annual BMI change was analysed in association with annual decline in forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) and FEV1/FVC separately in those with normal weight (BMI 18.5–24.9) and overweight/obese subjects (BMI ≥25) at study entry. Regression models were used to adjust for sex, age, smoking, inhaled corticosteroids use and occupational exposure to gas, dust or fumes.
Results
Overweight/obese subjects had lower FEV1 and FVC but slower annual FEV1 and FVC decline compared to those with normal weight. After adjustment through regression modelling, the association between BMI change with FEV1 and FVC decline remained significant for both BMI groups, but with stronger associations among the overweight/obese (FEV1 B[Overweight/obese]=−25 mL versus B[normal weight]=−15 mL). However, when including only those with BMI increase during follow-up, the associations remained significant among those with overweight/obesity, but not in the normal-weight group. No associations were seen for FEV1/FVC.
Conclusions
BMI increase is associated with faster FEV1 and FVC decline among overweight and obese adults with asthma in comparison with their normal-weight counterparts.
Short abstract
BMI increase is associated with faster FEV1 and FVC decline in adults with asthma, and this association is stronger among overweight and obese adults with asthma than among their normal-weight counterparts https://bit.ly/3PDxOk0
Introduction
Asthma is characterised by a variable expiratory airflow limitation which may become persistent [1] and lead to a more accelerated FEV1 decline [2]. Impaired lung function in asthma is known to be associated with multiple factors such as current smoking [3] and smoking pack-years [4] but also obesity [5]. Consequently, these factors relate to a worse asthma control [6] and severity [7], which in turn associate with lower quality of life [8–10].
Overweight and obesity are becoming more common worldwide [11], and increased body mass index (BMI ≥25) [12, 13] is known to be related to lower FEV1. There are cross-sectional studies that have shown that obese adults with asthma have lower FEV1 than adults with asthma having normal weight [5]. However, there are few population-based longitudinal studies on this topic [14], and specifically those focusing on adults with asthma have been lacking. One prospective study [15] showed that BMI increase indeed was related to FEV1 decline among young adults with non-obstructive asthma, but this study did not separate overweight and obese subjects from those with normal weight, and thus it is not known if the association differs depending on initial BMI.
Asthma treatment response has been shown to vary depending on the underlying asthma phenotypes and should ideally be tailored based on clinical and molecular traits [1, 16]. Despite the available pharmacological therapies, obese asthma patients often remain symptomatic, with more exacerbations and hospitalisations, although these results are less clear regarding overweight asthma patients [17–19]. Overweight and obese subjects with asthma are more commonly resistant to the standard treatment with inhaled corticosteroids (ICS), likely related to frequent lack of the eosinophilic airway inflammation and also to dysregulation of some metabolic signalling pathways [20–22]. Thus, non-pharmacological interventions such as weight loss and smoking cessation may be particularly important for this group [1, 3, 23, 24].
In summary, prospective studies focusing on the association between longitudinal changes in BMI and lung function in asthma are important but scarce [15], especially with sample sizes allowing for stratification by baseline BMI [25]. Thus, our aim is to study the association between annual BMI change and annual lung function decline, separately among those with normal weight and overweight/obesity, in a 10- to 28-year follow-up of a population-based adult asthma cohort.
Methods
Study design
This study is part of the Obstructive Lung Disease in Northern Sweden (OLIN) research programme. A population-based cohort of adults with asthma living in the northernmost county of Sweden, Norrbotten (n=2055, 55% women, aged 19–72 years) was identified from five original population-based cohorts during 1986–2001. The inclusion criteria were strictly predefined based on data from clinical examinations and detailed structured interviews.
Participants from cohorts I–IV with at least one of the following criteria were included:
1) Physician-diagnosed asthma or report of ever having asthma;
2) Asthmatic wheeze without a cold in the last 12 months in combination with attacks of shortness of breath/wheeze or use of asthma medication;
3) Attacks of shortness of breath in the last 12 months with FEV1 reversibility of both ≥12% and ≥200 mL; and
4) Attacks of shortness of breath or wheeze in the last 12 months with methacholine bronchial hyperresponsiveness.
All participants from cohort V were included as they were physician-diagnosed with adult-onset asthma from primary care and had a medical history of asthma together with methacholine bronchial hyperresponsiveness [26].
At study entry in 1986–2001, a detailed structured interview was performed including questions regarding asthma diagnosis, potential risk factors, treatment of obstructive respiratory diseases and occupation. Clinical examinations included spirometry and assessment of height and weight.
The follow-up was performed during 2012–2014 in which those still alive and living in the county (n=1425) were invited. In total, n=1006 (71%) participated in similar clinical examinations that included structured interviews, spirometry and measurement of height and weight [26].
Participants with BMI ≥18.5 at study entry and not missing FEV1 or BMI measurements both at study entry and at follow-up were included in the study sample (n=945) (supplementary figure S1).
Lung function
At study entry, spirometry was performed with a Vicatest VCT-5 dry volume spirometer (Mijnhardt, Bunnik, The Netherlands) following the 1987/1994 American Thoracic Society (ATS) guidelines with a repeatability criterion of the two best measurements of ≤5% and ≤100 mL when the best FEV1 was ≤1 L [27, 28].
Spirometry at follow-up was performed with a pneumotach spirometer (Jaeger Masterscope, Hoechberg, Germany) following the 2005 European Respiratory Society (ERS)/ATS guidelines with a repeatability criterion of ≤150 mL [29].
FEV1 and FVC were measured (before bronchodilatation), expressed both in millilitres and as per cent of predicted (pp) using the OLIN reference values [30]. The ratio between FEV1 and FVC was also calculated. Post-bronchodilatory values were not included in the current study as they were available only in certain subsamples.
Changes in FEV1, FVC and FEV1/FVC were calculated as the value at follow-up minus the value at study entry. The annual decline was calculated as the change divided by the numbers of years between examinations (10–28 follow-up years), expressed in terms of pp per year and millilitres per year, respectively, as ΔFEV1pp/y, ΔFVCpp/y, ΔFEV1mL/y, ΔFVCmL/y and ΔFEV1/FVC/y.
BMI
At study entry, participants were categorised based on BMI (kg·m−2) as normal weight (18.5≤BMI<25) and overweight/obese (BMI≥25). Additional analyses were also performed by dividing overweight/obese into the categories overweight (25≤BMI<30) and obese (BMI≥30); these results are presented in the supplementary material.
BMI change was calculated as the value at follow-up minus the value at study entry. The annual BMI change was calculated by dividing the BMI change with the number of years between examinations (ΔBMI/y). The sample was also divided by quartiles for ΔBMI/y with cut-offs of Q1<0.042 (n=236), 0.042≤Q2<0.142 (n=237), 0.142≤Q3<0.274 (n=236) and Q4≥0.274 (n=236).
Other definitions
Data at study entry included sex, age, ICS use in the last 12 months, smoking habits (categorised as nonsmoker, ex-smoker and current smoker) and original cohort (I–V).
Follow-up data included smoking pack-years, which was calculated by multiplying the number of packs of cigarettes smoked per day by the number of years of smoking, as well as ICS use in the last 12 months. Occupational exposure to gas, dust or fumes (GDF) at follow-up was defined by the question “Have you been heavily exposed to dust, gases or fumes at your work (not including tobacco)?”.
Changes in smoking habits from study entry to follow-up were defined as: never-smokers (nonsmokers on both occasions), ex-smokers (from nonsmokers or ex-smokers to ex-smokers), quitters (from current smokers to ex-smokers), current smokers (from nonsmokers or ex-smokers or current smokers to current smokers) and inconsistent (from ex-smokers or current smokers to nonsmokers).
Statistical analyses
The analyses were made with IBM Statistical Package for the Social Sciences (SPSS) software version 26. Results were stratified by BMI categories (normal and overweight/obese) at study entry. The assumption of normal distributions for annual changes in BMI and lung function were assessed by histograms. Spearman correlation coefficients (rho) were used to evaluate correlations between annual changes in BMI and lung function. Comparisons of proportions of female sex, smoking categories and ICS use across BMI categories were done by Chi-squared test. Comparisons of means of BMI and lung function variables between BMI categories were done by independent T-test, and across quartiles of ΔBMI/y by ANOVA. p-values <0.05 were considered statistically significant. Separate linear regression models with each lung function value as dependent variable were constructed including ΔBMI/y, sex, age, changes in smoking habits, pack-years, ICS use, occupational GDF exposure at follow-up and original cohort as independent variables. Subgroup analyses were performed by additionally separating overweight from obese, by stratifying for sex and also by including only those gaining BMI during follow-up (ΔBMI/y >0).
Results
Basic characteristics
In total, 62.5% were women among those with normal weight, compared to 48.5% of the overweight/obese. The overweight/obese were older, while non-smoking was more common in the normal-weight group. Mean pack-years was similar in both BMI groups. ICS use at study entry was uncommon, 11.1% (normal weight) and 13.3% (overweight/obese), while at follow-up it increased to 42.1% and 45.9%, respectively (table 1). Basic characteristics are presented separately for overweight and obese subgroups in supplementary table S1.
TABLE 1.
Basic characteristics at study entry and follow-up by BMI groups at study entry
| Normal weight | Overweight/obese | p-value | ||
| Subjects n | 485 | 460 | ||
| Sex, female | 303 (62.5) | 223 (48.5) | <0.001 | |
| Age at study entry years, mean±sd | 37.7±11.4 | 43.3±11.2 | <0.001 | |
| Age at follow-up years, mean±sd | 56.6±12.3 | 61.4±11.9 | <0.001 | |
| Overweight/obese at follow-up | 283 (58.4) | 442 (96.1) | <0.001 | |
| Smoking habits at study entry | ||||
| Nonsmoker | 238 (49.1) | 186 (40.4) | ||
| Ex-smoker | 113 (23.3) | 158 (34.3) | ||
| Smoker | 134 (27.6) | 116 (25.2) | 0.001 | |
| Smoking habits at follow-up | ||||
| Nonsmoker | 251 (51.8) | 202 (43.9) | ||
| Ex-smoker | 177 (36.5) | 209 (45.4) | ||
| Smoker | 57 (11.8) | 49 (10.7) | 0.019 | |
| Mean pack-years at follow-up, mean±sd # | 16.5±14.9 | 17.6±16.6 | 0.436 | |
| ICS use at study entry | 54 (11.1) | 61 (13.3) | 0.318 | |
| ICS use at follow-up | 204 (42.1) | 211 (45.9) | 0.238 |
Frequencies presented as n (%) unless otherwise stated. Bold values indicate p<0.05. Normal weight: BMI 18.5–24.9; overweight/obese: BMI ≥25. BMI: body mass index. ICS: inhaled corticosteroids. #: among ever-smokers with: normal weight n=234, overweight/obese n=258.
BMI and lung function
The overweight/obese had lower FEV1 at study entry (pp and mL) and follow-up (pp) than the normal-weight group (table 2). However, subjects with normal weight had a larger ΔBMI/y and tended to have faster (i.e. worse) ΔFEV1pp/y than the overweight/obese, while there was no difference in ΔFEV1mL/y. When studying the overweight and obese separately, the obese presented the lowest FEV1 (pp and mL) at study entry but slower ΔBMI/y and ΔFEV1/y (pp and mL) than both the overweight and the normal-weight groups. Similar results were seen regarding FVC at study entry and follow-up, while no significant differences between BMI groups were seen regarding ΔFVC/y (pp or mL) and ΔFEV1/FVC/y (table 2, supplementary table S2). Distributions of changes in BMI, FEV1, FVC and FEV1/FVC among subjects with normal weight and overweight/obesity, respectively, are shown in supplementary figure S2.
TABLE 2.
BMI and lung function by BMI groups at study entry
| Normal weight | Overweight/obese | p-value | |
| Subjects n | 485 | 460 | |
| Years between examinations | 18.8±4.4 | 18.1±4.3 | 0.011 |
| BMI at study entry | 22.6±1.7 | 28.9±3.3 | <0.001 |
| BMI at follow-up | 25.9±3.3 | 31.5±4.9 | <0.001 |
| ΔBMI/y | 0.179±0.165 | 0.149±0.242 | 0.025 |
| FEV1pp at study entry | 90.4±13.7 | 86.6±13.7 | <0.001 |
| FEV1pp at follow-up | 88.4±16.6 | 85.8±15.7 | 0.015 |
| ΔFEV1pp/y | −0.099±0.571 | −0.023±0.678 | 0.062 |
| FEV1mL at study entry | 3 272±818 | 3 163±804 | 0.040 |
| FEV1mL at follow-up | 2 749±824 | 2 651±797 | 0.061 |
| ΔFEV1mL/y | −27.3±20.0 | −27.7±23.1 | 0.788 |
| FVCpp at study entry | 89.0±11.5 | 85.3±11.8 | <0.001 |
| FVCpp at follow-up | 95.5±15.1 | 91.7±14.9 | <0.001 |
| ΔFVCpp/y | 0.377±0.603 | 0.386±0.785 | 0.847 |
| FVCmL at study entry | 4 032±953 | 3 939±957 | 0.133 |
| FVCmL at follow-up | 3849±1057 | 3 706±1060 | 0.038 |
| ΔFVCmL/y | −8.3±27.0 | −11.5±36.0 | 0.119 |
| FEV1/FVC at study entry | 0.812±0.076 | 0.805±0.074 | 0.143 |
| FEV1/FVC at follow-up | 0.714±0.093 | 0.717±0.082 | 0.643 |
| ΔFEV1/FVC/y | −0.005±0.004 | −0.005±0.004 | 0.122 |
Results presented as mean±sd unless otherwise stated. Bold values indicate p<0.05. Normal weight: BMI 18.5–24.9; overweight/obese: BMI ≥25. BMI: body mass index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; ΔBMI/y: annual BMI change; ΔFEV1pp/y: annual FEV1pp decline; ΔFVCpp/y: annual FVCpp decline; pp: % of predicted; ΔFEV1mL/y: annual FEV1mL decline; ΔFVCmL/y: annual FVCmL decline; ΔFEV1/FVC/y: annual FEV1/FVC decline.
Associations between changes in BMI and lung function
Correlation analysis between annual changes in BMI and FEV1 were performed separately among those with normal weight and overweight/obesity. These analyses yielded a rho in the overweight/obese of −0.246 (p<0.001) for ΔFEV1pp/y and −0.196 (p<0.001) for ΔFEV1mL/y, compared to −0.093 (p=0.040) for ΔFEV1pp/y and −0.030 (p=0.516) for ΔFEV1mL/y in the normal weight group (figure 1). Correlation analysis of ΔBMI/y with ΔFVCpp/y, ΔFVCmL/y and ΔFEV1/FVC/y showed the same pattern as for FEV1 with significant stronger negative correlations for FVC (pp and mL) in the overweight/obese. In contrast, ΔFEV1/FVC/y had a significant but weak negative correlation with ΔBMI/y among those with normal weight (rho=−0.104, p=0.022) but not among the overweight/obese (rho=−0.026, p=0.572) (supplementary figure S2).
FIGURE 1.
Correlations of ΔBMI/y with ΔFEV1pp/y and FEV1mL/y by BMI group presented as scatterplots with rho coefficients and p-values. Grey circles: normal weight (BMI 18.5–24.9), n=485; black circles: overweight/obese (BMI ≥25), n=460. BMI: body mass index; y: year; FEV1: forced expiratory volume in 1 s; pp: per cent of predicted.
To compare those who gained most BMI and those who gained the least or even decreased their BMI, the sample was divided by quartiles of ΔBMI/y. Among the overweight/obese, a linear trend was seen where the larger the ΔBMI/y (from Q1 to Q4), the faster the ΔFEV1/y (pp and mL). No linear trend was seen among those with normal weight (table 3). Similar results were seen for ΔFVC/y (pp and mL), while no linear trends in ΔFEV1/FVC/y or pack-years were observed (supplementary table S3).
TABLE 3.
Mean changes in BMI and changes in lung function within quartiles based on ΔBMI/y, by BMI groups at study entry
| Quartiles of ΔBMI/y | |||||
| Q1 | Q2 | Q3 | Q4 | p-value | |
| Normal weight | |||||
| Subjects n | 90 | 140 | 134 | 121 | |
| ΔBMI/y | −0.024±0.055 | 0.091±0.029 | 0.206±0.037 | 0.402±0.119 | <0.001 |
| ΔFEV1pp/y | −0.003±0.577 | −0.132±0.535 | −0.096±0.589 | −0.136±0.584 | 0.322 |
| ΔFEV1mL/y | −23.6±20.2 | −29.8±18.9 | −27.9±18.7 | −26.6±21.9 | 0.127 |
| Overweight/obese | |||||
| Subjects n | 146 | 97 | 102 | 115 | |
| ΔBMI/y | −0.091±0.137 | 0.095±0.029 | 0.195±0.036 | 0.457±0.193 | <0.001 |
| ΔFEV1pp/y | 0.125±0.695 | 0.030±0.625 | −0.036±0.674 | −0.244±0.651 | <0.001 |
| ΔFEV1mL/y | −24.4±21.2 | −26.8±20.2 | −27.6±26.2 | −32.8±24.0 | 0.032 |
Results presented as mean±sd unless otherwise stated. Bold values indicate p<0.05. p-values are used to test differences in mean values across quartiles of ΔBMI/y by ANOVA. Quartile 1: ΔBMI/y <0.042, Quartile 2: 0.042≤ΔBMI/y<0.142, Quartile 3: 0.142≤ΔBMI/y<0.274, Quartile 4: ΔBMI/y ≥0.274. Normal weight: BMI 18.5–24.9; overweight/obese: BMI ≥25. BMI: body mass index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; ΔBMI/y: annual BMI change; ΔFEV1pp/y: annual FEV1pp decline; ΔFEV1mL/y: annual FEV1mL decline; pp: per cent of predicted.
Adjusted associations between changes in BMI and lung function
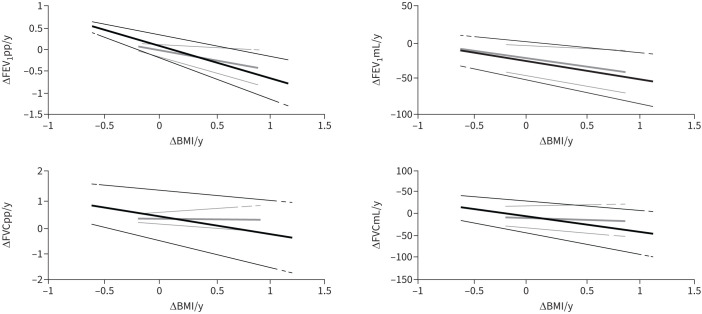
To further analyse the relationship between ΔBMI/y and annual lung function decline, adjusted regression models were constructed. The associations between ΔBMI/y and respectively ΔFEV1pp/y and ΔFEV1mL/y remained significant after adjustment but were about twice as strong among the overweight/obese compared to those with normal weight. Regarding associations between ΔBMI/y with ΔFVCpp/y and ΔFVCmL/y, significance remained only in the overweight/obese, while no associations were found with ΔFEV1/FVC/y (table 4). Adjusted regression curves for associations between ΔBMI/y and lung function outcomes are illustrated in figure 2 (regression coefficients are shown in supplementary table S4).
TABLE 4.
Association between ΔBMI/y and annual decline in lung function after adjusting for other factors among BMI groups
| ΔBMI/y | ||||
| Normal weight | Overweight/obese | |||
| B | 95% confidence interval | B | 95% confidence interval | |
| ΔFEV1pp/y | −0.356 | (−0.663– −0.049) | −0.747 | (−1.003– −0.491) |
| ΔFEV1mL/y | −14.662 | (−24.903– −4.420) | −24.809 | (−33.608– −16.010) |
| ΔFVCpp/y | −0.102 | (−0.436–0.232) | −0.719 | (−1.026– −0.413) |
| ΔFVCmL/y | −10.091 | (−23.509–3.328) | −34.422 | (−48.073– −20.772) |
| ΔFEV1/FVC/y | −0.002 | (−0.004–0.000) | −0.000 | (−0.002–0.002) |
Significant values are given in bold. Normal weight: BMI 18.5–24.9; overweight/obese: BMI ≥25. Adjusting factors: sex, age, changes in smoking habits, pack-years, inhaled corticosteroid use, occupational gas, dust or fumes exposure at follow-up and original cohort. BMI: body mass index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; B: β-coefficient from linear regression models; ΔBMI/y: annual BMI change; ΔFEV1pp/y: annual FEV1pp decline; ΔFVCpp/y: annual FVCpp decline; pp: per cent of predicted; ΔFEV1mL/y: annual FEV1mL decline; ΔFVCmL/y: annual FVCmL decline; ΔFEV1/FVC/y: annual FEV1/FVC decline.
FIGURE 2.
Adjusted regression curves of ΔBMI/y with ΔFEV1pp/y, ΔFEV1mL/y, ΔFVCpp/y and ΔFVCmL/y by BMI groups. Grey lines: normal weight (BMI 18.5–24.9), n=485; black lines: overweight/obese (BMI ≥25), n=460. Regression curves are presented along with 95% confidence intervals. Only significant factors were included in the models for each BMI group (supplementary table S2). BMI: body mass index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity.
Regression analyses in subgroups
As BMI increase was of particular interest, analyses including only subjects with a BMI increase between study entry and follow-up (n=782) were performed. The overweight/obese had strong associations between ΔBMI/y with ΔFEV1/y and ΔFVC/y (pp and mL) while no significance was seen in their normal-weight counterparts. No significant associations were seen regarding ΔFEV1/FVC/y (table 5).
TABLE 5.
Association between ΔBMI/y and annual decline in lung function after adjusting for other factors among only BMI gainers by BMI groups
| ΔBMI/y | ||||
| Normal weight (n=432) | Overweight/obese (n=350) | |||
| B | 95% confidence interval | B | 95% confidence interval | |
| ΔFEV1pp/y | −0.287 | (−0.642–0.069) | −0.731 | (−1.098– −0.364) |
| ΔFEV1mL/y | −10.745 | (−22.882–1.392) | −22.437 | (−35.813– −9.062) |
| ΔFVCpp/y | −0.043 | (−0.430–0.343) | −0.718 | (−1.164– −0.271) |
| ΔFVCmL/y | −4.857 | (−20.482–10.767) | −35.251 | (−55.969– −14.533) |
| ΔFEV1/FVC/y | −0.002 | (−0.005–0.001) | 0.000 | (−0.002–0.003) |
Significant values in bold. Normal weight: BMI 18.5–24.9; overweight/obese: BMI ≥25. BMI gainer refers to those with ΔBMI/y >0. Adjusting factors: sex, age, changes in smoking habits, pack-years, inhaled corticosteroid use, occupational gas, dust or fumes exposure at follow-up and original cohort. BMI: body mass index; B: β-coefficient from linear regression models; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; ΔBMI/y: annual BMI change; ΔFEV1pp/y: annual FEV1pp decline; ΔFVCpp/y: annual FVCpp decline; pp: per cent of predicted; ΔFEV1mL/y: annual FEV1mL decline; ΔFVCmL/y: annual FVCmL decline; ΔFEV1/FVC/y: annual FEV1/FVC decline.
Stratified analyses were also performed to see if the observed associations were present in both sexes. All lung function values, now also with the addition of ΔFEV1/FVC/y, were associated to ΔBMI/y among both women and men with overweight/obesity (supplementary table S5).
Additionally overweight and obese subjects were studied separately. Here, the adjusted associations between ΔBMI/y with ΔFEV1/y and ΔFVC/y (pp and mL) remained significant and stronger compared to the normal-weight group for both subgroups. No associations were seen for ΔFEV1/FVC/y (supplementary table S6).
Discussion
This long-term prospective asthma cohort study highlights BMI increase as a risk factor for a worse decline in both FEV1 and FVC particularly among those who are overweight or obese at study entry, independently of ICS use and several other factors. BMI increase associated weakly with the decline in FEV1, but not FVC, also in those with normal weight, while BMI change (increase or decrease) did not associate with the decline in FEV1/FVC regardless of having normal weight, overweight or obesity at study entry.
A BMI ≥25 is associated with higher incidence and prevalence of asthma [31, 32] and often a more severe clinical presentation among adults with asthma [7, 10, 18]. Lower lung function levels in overweight/obese than in normal-weight adults have been observed, however with a greater difference among those without asthma than among those with asthma [33], probably related to the fact that adults with asthma generally have lower lung function and thus are less likely to have a large further reduction. In our study, overweight and obese subjects were grouped together in the main analyses not only to maintain equal sample size as those with normal weight, but also as it is known that features of silent systemic inflammation as well as other morbidities associated with obesity can be present not only in obese but also in overweight subjects [34]. Whether BMI change affects lung function decline in the long-term among adults with asthma, and whether this association differs between those with overweight/obesity and those with normal weight, has however not been studied before [25]. Our study enabled such analyses and revealed that BMI increase associates stronger with faster decline in both FEV1 and FVC among those who were overweight or obese than among those who were normal weight at study entry, which are novel results. Interestingly, when overweight and obese subjects were studied separately, the association between BMI change and lung function decline tended to be equally strong or even stronger for the overweight subgroup compared to the obese. Thus, although there is emerging evidence that overweight may associate with a more favourable asthma prognosis in terms of less mortality compared to normal weight [35], this was not observed in our study, which assessed prognosis in terms of lung function.
The effect of BMI increase on lung function has been studied prospectively among healthy adults using the 10-year follow-up of the US CARDIA study [14] and the 6-year follow-up of the Humboldt cohort recruited from the general population [36]. These two studies showed that both BMI level at study entry as well as weight gain during follow-up related to changes in lung function. In the CARDIA study, obesity at study entry associated strongly with excess decline in FVC but not FEV1, while the Humboldt study showed such associations for decline in both FVC and FEV1. Although not stratifying for baseline BMI group, both studies showed that large weight gain during follow-up associated with excess decline in FEV1 as well as FVC [14, 36], which is well in line with our results for adults with asthma with an even longer follow-up period.
Regarding the association between changes in BMI and lung function among adults with asthma, there are few prospective studies. Within the ECRHS [15], 638 asthmatic patients were followed from 1998 to 2002 and were stratified by having airflow obstruction or not at study entry. In line with our results, they showed that those with normal weight had faster FEV1 decline during follow-up than the obese. They also showed that those with largest BMI increase also had the fastest FEV1 decline. In contrast to our study, they did not stratify for BMI at study entry and did not perform analysis of association between BMI change and decline in FEV1, and further they did not analyse changes in FVC or FEV1/FVC [15]. Our study confirmed a strong association between BMI increase and FEV1 decline in adults with asthma, but also revealed that this association was twice as strong among the overweight/obese at study entry compared to normal-weight subjects. In addition, regarding FVC the corresponding associations in our study were only seen in those with overweight and obesity at study entry. As no associations between BMI change and FEV1/FVC were seen in our study, this implies that BMI increase may mostly relate to the development of a restrictive rather than obstructive lung function pattern.
Asthma is a heterogeneous condition with different phenotypes, including the non-Th2-related (obesity-related and neutrophilic) [16]. The obesity-related asthma phenotype is often complicated with the presence of several obesity-related comorbidities such as diabetes or ischaemic heart disease [17, 18]. It is also known that, for example, obstructive sleep apnoea syndrome and gastro-esophageal reflux, which are common comorbidities particularly in severe asthma [37], may impact the association between BMI and lung function. Smoking also has adverse effects on lung function among adults with asthma [4] and was more common among the overweight/obese than those with normal weight in our study. Further, obesity-related asthma is known more often to be therapy-resistant with regard to corticosteroid use due to less eosinophilic inflammation [19–21]. Obesity also has physiological effects on lung mechanics as the increase in thoracic and abdominal fat tissue increases the compression of the lungs and therefore reduces lung volumes [12, 38]. These premises highlight the potential of weight loss as one of the non-pharmacological treatment alternatives for asthma due to the potential positive long-term effect on lung function in overweight and obese subjects [23, 39].
Limitations of our study include that BMI may not be the most accurate indicator for determining overweight and obesity as it may account poorly for the amount and distribution of fat tissue [40]. However, BMI is widely used, and results are directly applicable to clinical practice. Further, different guidelines and types of spirometers were used at study entry and follow-up resulting in potential systematic differences in lung function measurements between examinations [41]. One example is the implementation of 6 s of forced expiratory time during FVC measuring at follow-up, which can be a possible explanation for why some subjects presented with a higher FVC at follow-up. Still, as this applies for all subjects, the association with change in BMI and the comparability between BMI groups should not be affected.
The long follow-up time may yield a healthy survivor effect both regarding lung function and BMI. This was addressed in previous publications that implied that non-participants at follow-up were more frequently overweight or obese and had lower lung function at study entry than participants. Therefore, subjects with lower lung function and overweight/obesity may be underrepresented at follow-up [26, 42]. This means that the observed associations between changes in BMI and lung function, which in our study seem to be moderate, in fact might be stronger if the healthy survivor effect were not present.
That study entry was between 1986 and 2001 from five different cohorts can be another potential weakness as treatment guidelines changed during the recruitment period. A cohort effect of successively improved lung function in the general population may be present as large-scale studies have presented such evidence [43]. In order to account for this, the regression analyses were adjusted for original cohorts. Further, the exclusion of 61 subjects from the study as they were underweight (BMI <18.5) or lacked valid measurements of BMI or FEV1pp at follow-up may reduce the statistical power and could affect the results. However, we prioritised using a complete case method as we could use real measurements and exclusively focus on the normal weight and overweight/obese categories.
Strengths of our study include high participation rate and large sample size which allows for stratification while maintaining a similar number of subjects in both BMI groups. The long follow-up can also be regarded as a strength as there are few prospective studies allowing for 10- to 28-year follow-ups. The large amount of data collected enabled us to adjust for several factors that could act as potential confounders or mediators, e.g. smoking habits, which is known to be strongly associated with decreased lung function. Finally, validation studies regarding self-reported physician-diagnosed asthma within OLIN have shown that the positive predictive value is >90% among adults [44].
In conclusion, BMI increase associates with a faster decline in FEV1 and FVC but not in FEV1/FVC in adults with asthma. The association between BMI change and decline in FEV1 and FVC is stronger among those with overweight or obesity in comparison with their normal-weight counterparts. Maintaining or achieving a normal weight should be considered as an important cornerstone in asthma management to avoid excess lung function decline in the long run.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00110-2022.SUPPLEMENT (909.4KB, pdf)
Acknowledgements
The participants in the OLIN studies and all the OLIN research staff that have been collecting data throughout the years are acknowledged for their contributions. In addition, Bo Lundbäck is specially acknowledged as founder of the OLIN studies, and we want to express our gratitude for important scientific input to this study.
Provenance: Submitted article, peer reviewed.
Conflict of interest: H. Kankaanranta has received consulting fees from AstraZeneca, Chiesi Pharma, GlaxoSmithKline, SanofiGenzyme, MSD and Novartis, outside the submitted work; and personal fees for lectures at educational events outside the submitted work from AstraZeneca, Boehringer Ingelheim and Orion Pharma. C. Stridsman has received personal fees for lectures at educational events outside the submitted work from AstraZeneca, Boehringer Ingelheim and Novartis. A. Lindberg has received personal fees for advisory boards from AstraZeneca, GlaxoSmithKline, Novartis and Boehringer Ingelhem, outside the submitted work. E. Rönmark has received support for the present manuscript from the Swedish Heart and Lung Foundation, Swedish Asthma and Allergy Foundation, Swedish Research Council, ALF (a regional agreement between Umeå University and Västerbotten County Council), Norrbotten County Council, AstraZeneca, SanofiEnzyme, GlaxoSmithKline and AstraZeneca. H. Backman has received personal fees for presentation at scientific meetings from AstraZeneca and Boehringer Ingelheim, outside the submitted work. The remaining authors have nothing to disclose.
Support statement: This study was supported by Centrala ALF-medel and Norrbottens Läns Landsting. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention. 2021. Available from: http://ginasthma.org
- 2.Lange P, Parner J, Vestbo J, et al. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med 1998; 339: 1194–1200. doi: 10.1056/NEJM199810223391703 [DOI] [PubMed] [Google Scholar]
- 3.Chaudhuri R, Livingston E, McMahon AD, et al. Effects of smoking cessation on lung function and airway inflammation in smokers with asthma. Am J Respir Crit Care Med 2006; 174: 127–133. doi: 10.1164/rccm.200510-1589OC [DOI] [PubMed] [Google Scholar]
- 4.Tommola M, Ilmarinen P, Tuomisto LE, et al. The effect of smoking on lung function: a clinical study of adult-onset asthma. Eur Respir J 2016; 48: 1298–1306. doi: 10.1183/13993003.00850-2016 [DOI] [PubMed] [Google Scholar]
- 5.Pakhale S, Doucette S, Vandemheen K, et al. A comparison of obese and nonobese people with asthma. Chest 2010; 137: 1316–1323. doi: 10.1378/chest.09-2491 [DOI] [PubMed] [Google Scholar]
- 6.De Vries MP, van Den Bemt L, Lince S, et al. Factors associated with asthma control. J Asthma 2005; 42: 659–665. doi: 10.1080/02770900500264903 [DOI] [PubMed] [Google Scholar]
- 7.Taylor B, Mannino D, Brown C, et al. Body mass index and asthma severity in the National Asthma Survey. Thorax 2008; 63: 14–20. doi: 10.1136/thx.2007.082784 [DOI] [PubMed] [Google Scholar]
- 8.Braido F, Brusselle G, Guastalla D, et al. Determinants and impact of suboptimal asthma control in Europe: The INTERNATIONAL CROSS-SECTIONAL AND LONGITUDINAL ASSESSMENT ON ASTHMA CONTROL (LIAISON) study. Respir Res 2016; 17: 51. doi: 10.1186/s12931-016-0374-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ilmarinen P, Juboori H, Tuomisto LE, et al. Effect of asthma control on general health-related quality of life in patients diagnosed with adult-onset asthma. Sci Rep 2019; 9: 16107. doi: 10.1038/s41598-019-52361-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vortmann M, Eisner MD. BMI and health status among adults with asthma. Obesity 2008; 16: 146–152. doi: 10.1038/oby.2007.7 [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Obesity and overweight. www.who.int/news-room/fact-sheets/detail/obesity-and-overweight Date last updated: 9 June 2021. Date last accessed: 1 February 2022.
- 12.Jones RL, Nzekwu M-MU. The effects of body mass index on lung volumes. Chest 2006; 130: 827–833. doi: 10.1378/chest.130.3.827 [DOI] [PubMed] [Google Scholar]
- 13.King GG. The effects of body weight on airway calibre. Eur Respir J 2005; 25: 896–901. doi: 10.1183/09031936.05.00104504 [DOI] [PubMed] [Google Scholar]
- 14.Thyagarajan B, Jacobs DR, Apostol GG, et al. Longitudinal association of body mass index with lung function: The CARDIA Study. Respir Res 2008; 9: 31. doi: 10.1186/1465-9921-9-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcon A, Corsico A, Cazzoletti L, et al. Body mass index, weight gain, and other determinants of lung function decline in adult asthma. J Allergy Clin Immunol 2009; 123: 1069–1074.e4. doi: 10.1016/j.jaci.2009.01.040 [DOI] [PubMed] [Google Scholar]
- 16.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 2012; 18: 716–725. doi: 10.1038/nm.2678 [DOI] [PubMed] [Google Scholar]
- 17.Ilmarinen P, Tuomisto LE, Niemelä O, et al. Cluster analysis on longitudinal data of patients with adult-onset asthma. J Allergy Clin Immunol Pract 2017; 5: 967–978.e3. doi: 10.1016/j.jaip.2017.01.027 [DOI] [PubMed] [Google Scholar]
- 18.Ilmarinen P, Pardo A, Tuomisto LE, et al. Long-term prognosis of new adult-onset asthma in obese patients. Eur Respir J 2021; 57: 2001209. doi: 10.1183/13993003.01209-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med 2008; 178: 218–224. doi: 10.1164/rccm.200711-1754OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCravy M, Ingram JL, Que LG. Dysregulated metabolism in the pathophysiology of non-allergic obese asthma. J Asthma Allergy 2021; 14: 179–186. doi: 10.2147/JAA.S282284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Telenga ED, Tideman SW, Kerstjens HAM, et al. Obesity in asthma: more neutrophilic inflammation as a possible explanation for a reduced treatment response. Allergy 2012; 67: 1060–1068. doi: 10.1111/j.1398-9995.2012.02855.x [DOI] [PubMed] [Google Scholar]
- 22.Peters-Golden M. Influence of body mass index on the response to asthma controller agents. Eur Respir J 2006; 27: 495–503. doi: 10.1183/09031936.06.00077205 [DOI] [PubMed] [Google Scholar]
- 23.Pakhale S, Baron J, Dent R, et al. Effects of weight loss on airway responsiveness in obese adults with asthma. Chest 2015; 147: 1582–1590. doi: 10.1378/chest.14-3105 [DOI] [PubMed] [Google Scholar]
- 24.Hakala K, Stenius-Aarniala B, Sovijärvi A. Effects of weight loss on peak flow variability, airways obstruction, and lung volumes in obese patients with asthma. Chest 2000; 118: 1315–1321. doi: 10.1378/chest.118.5.1315 [DOI] [PubMed] [Google Scholar]
- 25.Kankaanranta H, Kauppi P, Tuomisto LE, et al. Emerging comorbidities in adult asthma: risks, clinical associations, and mechanisms. Mediators Inflamm 2016; 2016: 1–23. doi: 10.1155/2016/3690628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Backman H, Hedman L, Stridsman C, et al. A population-based cohort of adults with asthma: mortality and participation in a long-term follow-up. Eur Clin Respir J 2017; 4: 1334508. doi: 10.1080/20018525.2017.1334508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Standardization of Spirometry, 1987 Update. Statement of the American Thoracic Society. Am Rev Respir Dis 1987; 136: 1285–1298. [DOI] [PubMed] [Google Scholar]
- 28.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med 1994; 152: 1107–1136. [DOI] [PubMed] [Google Scholar]
- 29.Miller MR. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 30.Backman H, Lindberg A, Odén A, et al. Reference values for spirometry – report from the Obstructive Lung Disease in Northern Sweden studies. Eur Clin Respir J 2015; 2: 26375. doi: 10.3402/ecrj.v2.26375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rönmark E. Obesity increases the risk of incident asthma among adults. Eur Respir J 2005; 25: 282–288. doi: 10.1183/09031936.05.00054304 [DOI] [PubMed] [Google Scholar]
- 32.Nystad W. Body mass index in relation to adult asthma among 135,000 Norwegian men and women. Am J Epidemiol 2004; 160: 969–976. doi: 10.1093/aje/kwh303 [DOI] [PubMed] [Google Scholar]
- 33.Forno E, Han Y-Y, Mullen J, et al. Overweight, obesity, and lung function in children and adults—a meta-analysis. J Allergy Clin Immunol Pract 2018; 6: 570–581.e10. doi: 10.1016/j.jaip.2017.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ilmarinen P, Tuomisto LE, Niemelä O, et al. Comorbidities and elevated IL-6 associate with negative outcome in adult-onset asthma. Eur Respir J 2016; 48: 1052–1062. doi: 10.1183/13993003.02198-2015 [DOI] [PubMed] [Google Scholar]
- 35.Bellia V, Pedone C, Catalano F, et al. Asthma in the elderly. Chest 2007; 132: 1175–1182. doi: 10.1378/chest.06-2824 [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Horne SL, Dosman JA. Body weight and weight gain related to pulmonary function decline in adults: a six year follow up study. Thorax 1993; 48: 375–380. doi: 10.1136/thx.48.4.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porsbjerg C, Ulrik C, Skjold T, et al. Nordic consensus statement on the systematic assessment and management of possible severe asthma in adults. Eur Clin Respir J 2018; 5: 1440868. doi: 10.1080/20018525.2018.1440868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol 2018; 141: 1169–1179. doi: 10.1016/j.jaci.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stenius-Aarniala B. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. BMJ 2000; 320: 827–832. doi: 10.1136/bmj.320.7238.827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y, Rennie D, Cormier YF, et al. Waist circumference is associated with pulmonary function in normal-weight, overweight, and obese subjects. Am J Clin Nutr 2007; 85: 35–39. doi: 10.1093/ajcn/85.1.35 [DOI] [PubMed] [Google Scholar]
- 41.Milanzi EB, Koppelman GH, Oldenwening M, et al. Considerations in the use of different spirometers in epidemiological studies. Environ Health 2019; 18: 39. doi: 10.1186/s12940-019-0478-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bermúdez Barón N, Lindberg A, Stridsman C, et al. Among respiratory symptoms, wheeze associates most strongly with impaired lung function in adults with asthma: a long-term prospective cohort study. BMJ Open Respir Res 2021; 8: e000981. doi: 10.1136/bmjresp-2021-000981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allinson JP, Afzal S, Çolak Y, et al. Changes in lung function in European adults born between 1884 and 1996 and implications for the diagnosis of lung disease: a cross-sectional analysis of ten population-based studies. Lancet Respir Med 2022; 10: 83–94. doi: 10.1016/S2213-2600(21)00313-1 [DOI] [PubMed] [Google Scholar]
- 44.Lundbäck B, Rönmark E, Jönsson E, et al. Incidence of physician-diagnosed asthma in adults—a real incidence or a result of increased awareness? Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med 2001; 95: 685–692. doi: 10.1053/rmed.2001.1126 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00110-2022.SUPPLEMENT (909.4KB, pdf)