Abstract
Background
Anti-eosinophilic therapy with interleukin-5/interleukin-5-receptor antibodies represents an established treatment for patients with severe eosinophilic asthma (SEA) but did not show clinical efficacy in patients with COPD. The objective of the present study was to evaluate treatment response to anti-eosinophilic antibody therapy in patients with asthma and COPD.
Methods
A retrospective comparison of pulmonary function testing, oral corticosteroid intake, quality of life and pulmonary symptom control in patients with SEA and COPD and 1:1 propensity score matched patients suffering from SEA alone was performed. All patients received treatment with either mepolizumab or benralizumab. Data were assessed prior to antibody treatment start and after 6 months of therapy.
Results
Data from 84 patients (42 patients with SEA and COPD and 42 patients with SEA) were analysed. After 6 months of treatment, patients in both groups showed improved forced expiratory volume in 1 s (improvement by 11% (IQR 5–18) in the SEA and COPD group versus 15% (IQR −3–23); p=0.637) and decreased oral corticosteroid dosages (median reduction by 3 mg in the SEA and COPD group versus 5 mg; p=0.070), without significant differences between groups. Pulmonary symptom control and quality of life improved in both groups. A significant decrease in eosinophils could be measured in both groups with similar cell numbers prior to treatment initiation (600 cells·µL−1 in the SEA and COPD group versus 500 cells·µL−1).
Conclusion
Anti-eosinophilic therapy with interleukin-5/interleukin-5-receptor antibodies shows clinical efficacy in patients with SEA and COPD comparable to treatment response in patients with SEA alone.
Short abstract
Anti-eosinophilic therapy with interleukin-5/interleukin-5 receptor antibodies shows clinical efficacy in patients with severe eosinophilic asthma (SEA) and COPD comparable to treatment response in patients with SEA alone https://bit.ly/3KAPlI7
Introduction
Bronchial asthma and COPD are common chronic pulmonary diseases and affect millions of people worldwide [1]. Both diseases are characterised by chronic airway inflammation and can coexist in one patient [2]. The term asthma–COPD overlap (ACO) has been used to identify patients with features of both diseases; however the topic and especially the term remain controversial and no uniformly agreed definition for ACO exists. The Global Initiative for Asthma (GINA) describe ACO as persistent airflow limitation with several features associated with asthma and several features usually associated with COPD [3], whereby the Global Initiative for Chronic Obstructive Lung Disease (GOLD) no longer refers to ACO but emphasises that asthma and COPD are different diseases that can coexist in one patient but may come along with common clinical features [4]. Most of these features, which include pulmonary symptoms and airflow limitation in lung function, are not suitable to clearly distinguish between both diseases, and therefore discrimination between asthma and COPD is on the one hand challenging but on the other hand essential, as choice of therapy differs. For treatment of patients with severe asthma, which is defined as asthma being uncontrolled despite adherence to optimised maximal therapy or which requires high dosages of inhaled corticosteroids to prevent it from being uncontrolled [5], five different antibodies have been approved [6]. Three of these antibodies operate by interfering with the interleukin-5 (IL-5)/interleukin-5 receptor alpha (IL-5Rα) axis and thus interfere with the pathological function of eosinophils which play a leading role in the pathogenesis of patients with severe asthma and eosinophilic phenotype. But also in COPD eosinophilic inflammation seems to be an important driver of disease progression because increased blood eosinophils are associated with an increased risk of exacerbations [7]. The clinical benefit of anti-eosinophilic treatment with IL-5/IL-5Rα antibodies in patients with severe eosinophilic asthma (SEA) has been proven in various clinical trials, showing a decrease of the exacerbation rate, an increase in lung function and a reduction of systemic oral corticosteroids (OCS) [8–10]. As in most of the IL-5/IL-5Rα antibodies licensing trials patients with COPD or a smoking history of ≥10 pack-years (PY) were excluded, no prediction could be made concerning treatment efficacy of anti-IL-5 treatment in patients with asthma and COPD. Four randomised controlled trials (RCTs) in patients with COPD could show that treatment with mepolizumab or benralizumab was only associated with a very slight reduction of the exacerbation rate in patients with COPD and blood eosinophilia [11, 12]. To date, anti-eosinophilic treatment has only been tested in patients with either bronchial asthma or COPD, disregarding the fact that asthma patients may also suffer from COPD. Therefore, the aim of our study was to further analyse the effect of IL-5/IL-5Rα treatment in patients with SEA and COPD in clinical practice.
Methods
Aim, design and setting
In this single-centre, retrospective analysis, clinical efficacy of anti-IL-5/IL-5Rα therapy with mepolizumab or benralizumab was analysed in patients with SEA and COPD. The study was conducted in accordance with the principles of the Declaration of Helsinki. This retrospective analysis was performed with approval of the local ethics committee of the Hannover Medical School (9567_BO_K_2021). All patients provided written informed consent allowing the use of their data for scientific research.
Patient selection and treatment
All patients included in the study were diagnosed with severe asthma, according to American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines with an eosinophilic phenotype. All patients in our outpatient clinic for severe asthma were referred from pneumologists for confirmation of the asthma diagnosis and evaluation of treatment. Confirmation of the SEA diagnosis was based on symptoms (wheezing, coughing, shortness of breath, chest tightness, reduced capacity) or often worsening by certain trigger factors and reversible central airway obstruction in lung function, whereby positive bronchodilator reversibility tests might be historical. Comorbidities such as allergies, allergic rhinitis or atopic dermatitis as well as a positive family history for bronchial asthma were assessed. According to GINA/GOLD guidelines, additional COPD diagnosis was based on patients’ age (>40 years), smoking history (>10 PY), persistence of pulmonary symptoms despite optimised asthma treatment, optimised inhaler technique and adherence, and impaired lung function (post-bronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) <0.7). A reduced diffusing capacity of the lung for carbon monoxide (DLCO <80%) and emphysema on chest computed tomography (CT) scan were not mandatory but used as optional criteria to validate diagnosis of COPD, as CT scans of the chest were not available in all patients. Without uniformly agreed upon diagnostic criteria for patients with asthma and COPD, the diagnoses were based on GINA description of ACO and physician's clinical assessment. A SEA control group with 1:1-propensity score matching regarding age, sex and timepoint of treatment initiation was created. All patients were treated with medium to high-dose inhaled glucocorticoids and a long-acting β2-agonist and could receive a second or third controller and/or additional OCS therapy. Thereby, all patients fulfilled requirements for anti-IL-5/anti-IL-5Rα therapy according to the Food and Drug Administration (FDA) and European Medicines Agency (EMA) and were treated as add-on therapy with either mepolizumab subcutaneously once every 4 weeks or benralizumab once every 8 weeks, after an initial loading of three doses every 4 weeks. The choice of antibody was made by the treating physician.
Routine follow-up
Routine follow-up included spirometry or body plethysmography standardised to ERS/ATS guidelines, capillary blood gas analysis, measurement of exhaled nitric oxide (eNO) and laboratory testing (differential blood count), if indicated. Structured questionnaires assessing for pulmonary symptoms and asthma control (asthma control test (ACT)), number of exacerbations over the last 12 months and changes in medication were also completed at each follow-up visit. Exacerbations were defined as worsening of asthma symptoms requiring OCS for at least 3 days or an increase in the OCS dose. Quality of life (QoL) was assessed using a visual analogue scale (VAS) ranging from 0 points (worst imaginable health state) to 10 points (best imaginable health state). Moreover, patients were asked whether their subjective condition under antibody therapy had improved, worsened or was unchanged (categorical answer). For their answer, which was based on subjective judgement, patients were asked to consider pulmonary symptoms, QoL and improvement of subjective physical fitness, measured as flight of stairs or a distance a patient is able to walk until a break is needed.
Data collection
Data was assessed at two different time points: 1st “baseline” visit within 3 months prior to treatment start with anti-IL-5/IL-5Rα therapy and 2nd time point “follow-up” after 6 months (±1 month) of IL-5/IL-5Rα therapy. All pulmonary function tests (PFT) were performed under continued stable inhaled therapy. Information concerning number of exacerbations, QoL-VAS, ACT and change in patients’ subjective condition were assessed at the same time points. Information concerning smoking history, allergies, nasal polyposis and further comorbidities was assessed prior to start of anti-IL-5 treatment.
CT imaging
The subjects underwent clinically indicated volumetric chest CT examinations at full inspiration. These scans were reconstructed with a slice thickness of 0.625–0.9 mm depending on the manufacturer of the CT unit. Standard soft tissue reconstruction kernels, depending on the manufacturer, were used. Image analysis of all clinical CT examinations was performed using specialised software (AVIEW; Coreline Soft, Seoul, South Korea). Automated segmentation of the right and left lungs from the chest wall and mediastinum was performed for total lung volume calculation. Emphysema was defined as the percentage of lung pixels with an attenuation of −950 HU or less on inspiratory CT (i.e., low-attenuation areas (LAA)-950).
Statistical analysis
IBM SPSS Statistics 27.0 (IBM Corp, Armonk, NY, USA) and STATA 13.0 (State Corp LP, College Station, TX, USA) statistical software were used for analysis of the data. Delta-values between the two time points were calculated for continuous variables in order to compare the slope. Categorical variables are stated as numbers (n) and percentages (%). Depending on distribution, continuous variables are shown as median with interquartile ranges (IQR) or as mean±standard deviation (sd) unless indicated otherwise. For group comparisons, Fisher's exact test, Chi-squared test, two-sided t-test or Mann–Whitney U-test were used, as appropriate. For intergroup comparisons between time points paired t-test or Wilcoxon signed-rank test were used as appropriate. To account for multiple comparisons, we used the Benjamini–Hochberg method to adjust p-values [13]. A post hoc power calculation was conducted to determine the needed sample size to prove non-inferiority for end-point variables FEV1, ACT and QoL-VAS with a power set to 0.8 and alpha to 0.05. To account for missing data in regard to COPD diagnosis, a subgroup analysis was conducted for patients having data on DLCO or chest CT. Group discrimination was done using a DLCO below 80% predicted or showing signs of emphysema (LAA-950). All reported p-values are two-sided. p-values <0.05 were considered statistically significant.
Propensity score matching
Propensity score matching was used to minimise confounding or bias in this retrospective analysis. A 1:1 nearest neighbour model using logistic regression models controlling for patients’ current age, sex and date of anti-IL-5/IL-5Rα therapy initiation was conducted. Propensity scores before and after matching were compared using mean±sd propensity score per group.
Results
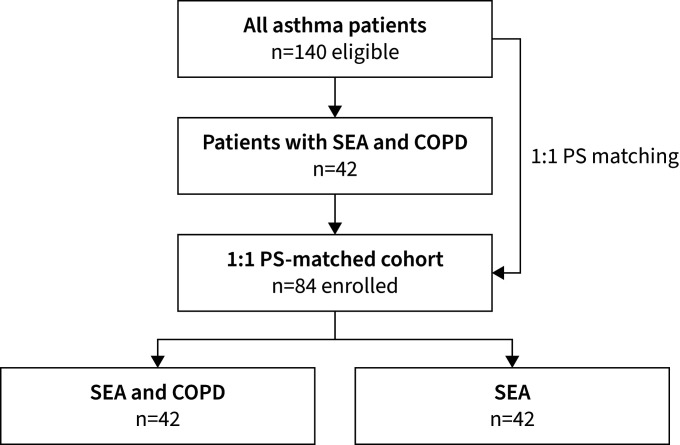
Data from 84 patients treated in our outpatient clinic between November 2016 and July 2020 were included (figure 1). 42 patients were diagnosed with SEA and COPD and 42 belonged to the SEA group diagnosed with SEA only. All patients received anti-IL-5 or anti-IL-5Rα therapy (42% mepolizumab and 58% benralizumab), whereby mepolizumab predominated in the SEA and COPD group (55%) and benralizumab predominated in the SEA group (71%). Smoking history stated as pack-years differed significantly between both groups showing a median of 20 PY in the SEA and COPD group versus 7 PY in the SEA group (p<0.001). DLCO was 20% lower (74% versus 94%, p=0.116) and the transfer coefficient of the lung for carbon monoxide (KCO) was 19% lower (93% versus 112%, p=0.005) in the SEA and COPD group. CT-derived %LAA-950 showed no significant difference between groups (p=0.099). Comorbidities were similar between both groups. Demographics are displayed in table 1. Propensity scores from available controls were 0.21±0.14 before matching and 0.51±0.06 after matching. Matched propensity score in the SEA and COPD group was 0.49±0.05 accordingly. Subgroup analysis of n=57 patients having data on DLCO and chest CT are shown in supplementary tables S1 and S2, confirming results of the overall cohort.
FIGURE 1.
Flowchart of patient inclusion. COPD: chronic obstructive pulmonary disease; PS: propensity score; SEA: severe eosinophilic asthma.
TABLE 1.
Baseline demographics
| Item | All | SEA and COPD | SEA | p-value |
| Subjects | 84 | 42 (50) | 42 (50) | |
| Age years | 61 (55–66) | 61 (55–65) | 61 (55–66) | 0.917 |
| Sex | ||||
| Female | 34 (41) | 17 (41) | 17 (41) | 1.000 |
| Male | 50 (59) | 25 (59) | 25 (59) | |
| BMI kg·m−2 | 29 (25–34) | 28 (25–34) | 29 (25–34) | 0.512 |
| Therapy | ||||
| Anti-IL-5 (mepolizumab) | 35 (42) | 23 (55) | 12 (29) | 0.015 # |
| Anti-IL-5Rα (benralizumab) | 49 (58) | 19 (45) | 30 (71) | |
| OCS | 43 (51) | 23 (55) | 20 (48) | 0.513# |
| ICS, medium–high dose | 100 (100) | 42 (100) | 42 (100) | 1.000# |
| IL-5 responder | 64 (76) | 32 (76) | 32 (76) | 1.000# |
| Smoking | ||||
| Never | 28 (33) | 0 (0) | 28 (67) | <0.001 # |
| Former | 56 (67) | 42 (100) | 14 (33) | |
| Pack-years | 20 (9–30) | 20 (15–30) | 7 (4–9) | <0.001 |
| PFT | ||||
| DLCO % predicted | 79 (72–97) | 74 (70–90) | 94 (75–101) | 0.116 |
| KCO % predicted | 107 (89–115) | 93 (80–112) | 112 (105–117) | 0.005 |
| FEV1/FVC post-BDT | 66 (54–73) | 66 (58–73) | 68 (51–73) | 0.785 |
| Positive bronchodilator test ¶ | 21 (38) | 13 (43) | 8 (31) | 0.333# |
| Total IgE IU·mL−1 | 177 (74–751) | 446 (87–1442) | 126 (41–259) | 0.040 |
| CT imaging, mean±sd+ | ||||
| Total lung volume mL | 5878±1479 | 6041±1461 | 5681±1515 | 0.427 |
| LAA-950 cc | 188±379 | 271±494 | 88±101 | 0.113 |
| LAA-950 % | 2.8±5.2 | 3.9±6.7 | 1.4±1.6 | 0.099 |
| Comorbidities | ||||
| Obesity (BMI ≥30 kg m−2) | 33 (39) | 36 (38) | 17 (41) | 0.823# |
| CRSwNP | 2 (2) | 1 (2) | 1 (2) | 1.000# |
| CRSsNP | 14 (17) | 4 (10) | 10 (24) | 0.079# |
| ASA intolerance | 4 (5) | 2 (5) | 2 (5) | 1.000# |
| Atopic dermatitis | 4 (5) | 1 (2) | 3 (7) | 0.305# |
| Cardiovascular diseases | 11 (13) | 6 (14) | 5 (12) | 0.746# |
| Diabetes | 3 (4) | 0 (0) | 3 (7) | 0.078# |
| Reflux | 5 (6) | 2 (5) | 3 (7) | 0.645# |
| Allergic rhinitis | 2 (2) | 0 (0) | 2 (5) | 0.152# |
Data are presented as n (%) or median (interquartile range), unless otherwise stated. p-values were derived using t-test, if not indicated otherwise. SEA: severe eosinophilic asthma; BMI: body mass index; IL: interleukin; OCS: oral corticosteroids; ICS: inhaled corticosteroids; PFT: pulmonary function testing; DLCO: diffusing capacity for carbon monoxide; KCO: carbon monoxide transfer coefficient; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; BDT: bronchodilator test; CT: computed tomography; LAA: low-attenuation areas; CRSwNP: chronic rhinosinusitis with nasal polyps; CRSsNP: chronic rhinosinusitis without nasal polyps; ASA: acetylsalicylic acid. #: Chi squared test; ¶: data from n=56 patients available; +: data from n=45 patients available. Significant values are marked as bold.
Changes in lung function, eosinophil counts, oral corticosteroids and exacerbations
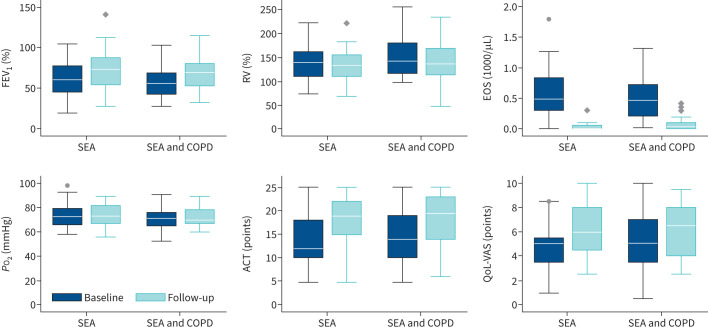
Results are displayed in tables 2 (delta) and 3 (absolute values). PFT showed improvement in both groups with increase of FEV1% by 11% (IQR 5–18) in the SEA and COPD group and 15% (IQR −3–23) in the SEA group and without significant difference between both groups (p=0.637, table 2). FVC% increased significantly and residual volume % and eNO decreased in both groups, both parameters not being significantly different between groups. Total lung capacity (TLC), oxygen tension (PO2) and carbon dioxide tension (PCO2) remained stable. Eosinophil counts showed similar values prior to treatment initiation (median of 600 cells·µL−1 (IQR 400–800) in the SEA and COPD group versus 500 cells·µL−1 (200–800) in the SEA group) and dropped to near zero without differences between both groups (p=0.571). Dosage of OCS was reduced from a median of 10 mg (IQR 5–15 mg) to 8 mg (IQR 4–18) (p=0.665) in the SEA and COPD group and from 10 mg (IQR 5–20 mg) to 5 mg (IQR 3–6 mg) in the SEA only group (p=0.002). OCS therapy could be stopped in three SEA and COPD patients (7%) and in six SEA patients (14%) respectively (p=0.069). Mean number of exacerbations over the last 12 months decreased from 2±2.8 to 0.9±1.8 (p=0.011) in the SEA and COPD group and from 1.9±2.5 to 0.4±0.7 (p=0.001) in the SEA group.
TABLE 2.
Comparison of baseline and follow-up visits (delta)
| Item | Δ total | Δ SEA and COPD | Δ SEA | p-value |
| Δ of lung function | ||||
| FVC % predicted | 8 (−2–18) | 6 (−1–16) | 12 (−2–23) | 0.216 |
| FEV1 % predicted | 11 (3–22) | 11 (5–18) | 15 (−3–23) | 0.637 |
| RV % predicted | −10 (−23–6) | −11 (−24–5) | −10 (−22–12) | 0.472 |
| TLC % predicted | 0 (−6–7) | −1 (−6–5) | 0 (−6–10) | 0.128 |
| eNO ppb | −6 (−30–9) | −3 (−25–10) | −7 (−32–8) | 0.612 |
| Δ of blood gases | ||||
| PO2 mmHg | 1 (−4–8) | −2 (−5–10) | 2 (−3–7) | 0.740 |
| PCO2 mmHg | 1 (−1–2) | 0 (−1–1) | 1 (−1–3) | 0.229 |
| Δ of laboratory | ||||
| Eosinophils 103·µL−1 | −6 (−10– −4) | −6 (−10– −4) | −6 (−12– −3) | 0.571 |
| Δ of OCS therapy | ||||
| OCS dosage mg | −5 (−11–0) | −3 (−6–0) | −5 (−20– −1) | 0.070 |
| OCS therapy, n (%) | −9 (11) | −3 (7) | −6 (14) | 0.069# |
| Δ of quality-of-life scores | ||||
| QoL-VAS | 1 (0–3) | 0 (−1–3) | 2 (0–3) | 0.019 |
| ACT score | 4 (0–8) | 3 (0–6) | 6 (1–11) | 0.097 |
| Stair climbing (flights of stairs) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.274¶ |
| Annual exacerbations, mean±sd | −1.4±2.4 | −1.1±2.3 | −1.5±2.6 | 0.505 |
| Δ of subjective condition, n (%) | ||||
| Worsened | 12 (14) | 4 (11) | 8 (22) | 0.453 |
| Stable | 41 (49) | 17 (47) | 16 (44) | |
| Improved | 31 (37) | 15 (42) | 12 (33) |
Data are presented as median (interquartile range), unless otherwise stated. p-values were derived using t-test, if not indicated otherwise. SEA: severe eosinophilic asthma; FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s; RV: residual volume; TLC: total lung capacity; eNO: exhaled nitric oxide; PO2: oxygen tension; PCO2: carbon dioxide tension; OCS: oral corticosteroids; QoL-VAS: quality of life visual analogue scale; ACT: asthma control test. #: Chi squared test; ¶: Mann–Whitney U-test. Significant values are marked as bold.
TABLE 3.
Comparison of baseline and follow-up visits (absolute)
| Item | SEA and COPD | SEA | ||||||
| Baseline | Follow-up | p-value | Adjusted p-value | Baseline | Follow-up | p-value | Adjusted p-value | |
| Lung function | ||||||||
| FVC % predicted | 81 (73–92) | 92 (76–99) | <0.001 | <0.001 | 77 (60–92) | 89 (74–102) | <0.001 | <0.001 |
| FEV1 % predicted | 56 (42–69) | 70 (53–81) | <0.001 | <0.001 | 62 (44–75) | 75 (55–91) | <0.001 | <0.001 |
| RV % predicted | 141 (112–179) | 134 (110–168) | 0.005 | 0.011 | 138 (105–159) | 128 (104–151) | 0.069 | 0.080 |
| TLC % predicted | 104 (95–110) | 104 (95–114) | 0.502 | 0.502 | 99 (84–113) | 102 (93–110) | 0.156 | 0.169 |
| Blood gases | ||||||||
| PO2 mmHg | 71 (65–77) | 71 (67–79) | 0.275 | 0.358 | 74 (66–79) | 73 (67–80) | 0.334 | 0.334 |
| PCO2 mmHg | 39 (36–41) | 38 (36–41) | 0.572 | 0.620 | 38 (35–39) | 40 (37–41) | 0.031 | 0.040 |
| Laboratory | ||||||||
| Eosinophils 103·µL−1 | 0.6 (0.4–0.8) | 0.01 (0–0.08) | <0.001 | <0.001 | 0.5 (0.2–0.8) | 0 (0–0.06) | <0.001 | <0.001 |
| OCS therapy | ||||||||
| OCS dosage mg | 10 (5–15) | 8 (4–18) | 0.665 | 0.665 | 10 (5–20) | 5 (3–6) | 0.002 | 0.003 |
| OCS therapy, n (%) | 23 (55) | 20 (48) | <0.250# | <0.271# | 20 (48) | 14 (33) | <0.109# | <0.177# |
| QoL scores | ||||||||
| QoL-VAS | 5 (4–7) | 7 (4–8) | 0.047 | 0.076 | 5 (3–6) | 7 (5–9) | <0.001 | <0.001 |
| ACT score | 14 (11–19) | 19 (13–23) | <0.001 | 0.003 | 12 (9–18) | 20 (17–23) | <0.001 | <0.001 |
| Stair climbing (flights of stairs) | 1 (1–2) | 2 (1–3) | 0.183 | 0.264 | 2 (1–2) | 2 (1–3) | 0.008 ¶ | 0.012 |
| Annual exacerbations, mean±sd | 2.0±2.8 | 0.9±1.8 | 0.011 | 0.020 | 1.9±2.5 | 0.4±0.7 | 0.001 | 0.002 |
Data are presented as median (interquartile range), unless otherwise stated. p-values were derived using paired t-test, if not indicated otherwise. SEA: severe eosinophilic asthma; FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s; RV: residual volume; TLC: total lung capacity; PO2: oxygen tension; PCO2: carbon dioxide tension; OCS: oral corticosteroids; QoL-VAS: quality of life – visual analogue scale; ACT: asthma control test. #: McNemar test; ¶: Wilcoxon signed-rank test. Significant values are marked as bold.
Changes in asthma control and quality of life
Comparison of baseline and follow-up data are displayed in tables 2 (delta) and 3 (absolute values) and figure 2. ACT and QoL-VAS improved significantly in both groups. Asthma control improved slightly more in the SEA group (improvement of 6 points (IQR 1–11) versus 3 points (IQR 0–6), p=0.097). QoL-VAS increased significantly by 2 points in both groups. In both groups the majority of patients reported stable or improved subjective condition (89% in the SEA and COPD group and 77% in the SEA group, p=0.453, table 2). Post hoc power analysis showed sufficient sample size to conduct this research: to prove non-inferiority for FEV1 n=13 patients per group were needed (n=26 total). For ACT n=10 (n=20 in total) and for QoL-VAS n=4 per group (n=8 in total) were needed.
FIGURE 2.
Change of parameters from baseline to follow-up by group. SEA: severe eosinophilic asthma; COPD: chronic obstructive pulmonary disease; ACT: asthma control test; EOS: eosinophils; FEV1: forced expiratory volume in 1 s; RV: residual volume; QoL-VAS: quality of life – visual analogue scale; PO2: oxygen tension.
Discussion
In this retrospective real-life study, we could show that treatment with anti-IL-5/anti-IL-5R antibodies is highly clinically effective not only in patients with SEA but also in patients with SEA and COPD. After 6 months of treatment, patients with SEA and COPD also showed significant improvement in PFT, asthma control and QoL.
Differentiating asthma from COPD is challenging, especially in older patients with severe or long persisting asthma. Asthma is characterised by variable airflow limitation, but especially in patients with long-standing asthma, fixed airflow limitation as can typically be seen in COPD can also be found [14]. On the other hand, a certain degree of bronchodilator reversibility of airway obstruction can also be detected in some COPD patients [15]. Patients with asthma and COPD combine clinical features of both diseases and represent a highly heterogeneous group. Major and minor criteria to diagnose ACO in COPD patients were published in 2012 and included a positive and significant bronchodilator response (>400 mL and >15% increase in FEV1), sputum eosinophilia or a previous diagnosis of asthma as major criteria, and increased total serum IgE, previous history of atopy or a positive bronchodilator test (>200 mL and >12% in FEV1) on at least two occasions as minor criteria [16]. To reduce the number of missing ACO diagnoses due to the criteria's restrictive character, variations of these criteria were analysed and a diagnostic algorithm for identifying patients with ACO was established [17]. This algorithm includes a smoking history of ≥10 PY, an airflow limitation (FEV1/FVC <0.7) persisting after treatment with bronchodilators and inhaled corticosteroids or after a course of OCS and a current asthma diagnosis. All our SEA plus COPD patients were diagnosed with severe asthma, had a smoking history of ≥10 PY and showed fixed airflow limitation at time of antibody start, whereby not all patients received bronchodilator testing in our outpatient clinic, as some patients were diagnosed outside our university clinic. All patients in our study received a primary diagnosis of SEA while the COPD was rather mild. This is reflected by the preserved DLCO in most patients and the small number of patients with emphysema. The missing consensus on the ACO diagnosis criteria and difficulties concerning treatment decisions in this very heterogeneous patient cohort led to exclusion of COPD patients in many asthma studies. In the MENSA, SIRIUS and DREAM study which led to approval of mepolizumab in SEA, patients with a smoking history of >10 PY (and thus most patients with coexisting COPD) were excluded from the trials [9, 18, 19]. In the ZONDA, CALIMA and SIROCCO licensing trials for benralizumab, patients with a diagnosis of COPD were excluded [10, 20, 21]. In 2017 two RCTs (METREX and METREO) were published investigating the effect of mepolizumab in patients with COPD with regard to the reduction of exacerbations [11]. Differences between treatment groups and placebo were significant in METREX but not in METREO. Since the differences between groups in METREX were also small (1.40 exacerbations per year in the treatment group versus 1.71 in the placebo group), the clinical relevance of these findings remains unclear. Benralizumab was also tested in COPD patients in two RCTs, and in both trials no significant reduction of the exacerbation rate could be shown [12]. So far, none of the existing IL-5/anti-IL-5R antibodies is approved for patients with the primary diagnosis of COPD. In our study we could show that anti-IL-5/anti-IL-5R therapy is as effective in patients with SEA and COPD as it is in patients with SEA only. PFT, symptom control and QoL improved significantly despite a median smoking history of 20 PY in the SEA and COPD group. In our cohort only few patients had radiological evidence of emphysema according to CT imaging. Subanalysis from the ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points) study revealed that COPD patients with persisting blood eosinophilia showed slower progression of emphysema [22]. According to proposed COPD phenotypes by Han et al. [23], our cohort represents the airway-phenotype with eosinophilia associated with frequent exacerbations and steroid response but without enhanced emphysema. Meta-analysis of the METREX, METREO, GALATHEA and TERRANOVA trials indicate that COPD patients with elevated eosinophils (>300 cells·µL−1) responded to anti-eosinophilic antibody treatment in terms of a reduced exacerbation rate [24, 25]. These results are supported by the results of our study, suggesting that IL-5/anti-IL-5R treatment might be an option for COPD patients with a specific clinical phenotype without pronounced hyperinflation but with airway inflammation and eosinophilia. Impact of eosinophilic airway inflammation in COPD has not yet been fully understood, but it has been recognised that eosinophils play an important role in inflammatory processes at least in some COPD patients. In a large study 19% of COPD patients had blood eosinophil counts >300 cells·µL−1 with higher values in patients with a history of asthma [26]. In COPD as well as in asthma the number of eosinophils in peripheral blood represents a predictor of exacerbations and response to corticosteroid therapy [27, 28]. As mechanisms of eosinophilia in COPD are not yet certain and it remains unclear why only a minority of COPD patients show significant eosinophilia, an association with asthma and allergies in COPD patients with eosinophilia has been explored. In the COPDGene study which examined 4915 COPD patients, early-life asthma was detected as a risk factor for increased disease activity with more frequent exacerbations [29], highlighting the disease interaction of COPD and asthma. Our study results confirm recent findings by Morobeid et al. [30] analysing antibody treatment in patients with allergic or eosinophilic asthma and a smoking status of >10 PY, showing treatment efficacy of IL-5 antibodies. In contrast to our study only limited information concerning patient characteristics were provided, not allowing further phenotyping of patients. In our view, the more detailed clinical characterisation of our COPD patients represents a strength of our study as COPD patients show an accelerated decline in DLCO over time and inclusion of DLCO/KCO values and CT scan reconstruction helps to confirm COPD diagnosis in affected patients and to support our conclusion [4].
Our study is primarily limited by its single-centre, retrospective design and limited number of patients included. Diagnosis of COPD could not always be supported by DLCO values and CT scans due to missing data. However, subgroup analysis showed very similar results to the main cohort of this research. In most patients COPD was mild, limiting transferability of the results to patients with moderate to severe COPD. Differences in treatment effects of benralizumab and mepolizumab in COPD patients cannot be ruled out. Follow-up amounted to only 6 months, representing a rather short follow-up period, e.g., not fully covering annual exacerbations. Strengths of our study are the clinically highly relevant but understudied cohort of asthma and COPD patients and the inclusion of a propensity score matched SEA group.
Conclusion
In summary, anti-eosinophilic treatment is highly efficient in patients with SEA and also in patients with SEA and COPD but, according to existing literature, not in patients with COPD alone.
Acknowledgements
We wish to thank all patients who participated in this project. We would also like to thank the clinical staff caring for the patients and administering the questionnaires, and last but not least, the families of the authors who have given up a lot of time with their loved ones to make this research possible.
Provenance: Submitted article, peer reviewed.
Ethics approval and consent to participate: Approval of the local ethic committee of the Hannover Medical School (9567_BO_K_2021) was given and all patients provided written informed consent allowing the use of their data for scientific research.
Availability of data and material: Data used in this study are available on reasonable request by the corresponding author.
Author contributions: N. Drick was responsible for implementation of the study, data interpretation and drafting the manuscript. J. Fuge was responsible for study design, implementation of the study, data collection, statistical analysis, data interpretation and drafting the manuscript. B. Seeliger was responsible for data interpretation and critically revising the manuscript. M. Speth was responsible for implementation of the study, data collection and revising the manuscript. J. Vogel-Claussen was responsible for implementation of the study, data collection and revising the manuscript. T. Welte was responsible for study design, data interpretation and critically revising the manuscript. H. Suhling was responsible for study design, implementation of the study, data interpretation and critically revising the manuscript.
Conflict of interest: N. Drick reports no conflict of interest.
Conflict of interest: J. Fuge reports personal fees/speaker honoraria from AstraZeneca, outside the submitted work.
Conflict of interest: B. Seeliger reports no conflict of interest.
Conflict of interest: M. Speth reports no conflict of interest.
Conflict of interest: J. Vogel-Claussen received grants, advisory/lecture/clinical trial fees and nonfinancial support from BMBF (German Ministry of Research and Education), Siemens Healthineers, AstraZeneca, Bayer, Boehringer Ingelheim, GSK, MSD, Novartis, AstraZeneca and Coreline Soft all outside the submitted work
Conflict of interest: T. Welte and/or his institution received grants, advisory/lecture/clinical trial fees and nonfinancial support from DFG (German Research Council), BMBF, BMG (German Ministry of Health), EU, WHO, AstraZeneca, Basilea, Biotest, Bayer, Boehringer, Berlin Chemie, GSK, Infectopharm, MSD, Novartis, Pfizer, Roche, Gilead, and Janssen, all outside the submitted work.
Conflict of interest: H. Suhling reports personal fees/speaker honoraria from AstraZeneca, GSK, Novartis and Sanofi, outside the submitted work.
References
- 1.James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1789–1858. doi: 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Postma DS, Rabe KF. The Asthma-COPD overlap syndrome. N Engl J Med 2015; 373: 1241–1249. doi: 10.1056/NEJMra1411863 [DOI] [PubMed] [Google Scholar]
- 3.Global Initiative for Asthma (GINA ). Global Strategy for Asthma Management and Prevention. 2022. Available from: https://ginasthma.org/gina-reports/
- 4.Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global Strategy for the Diagnosis, Management, and Prevention of COPD. 2022. Available from: https://goldcopd.org/2022-gold-reports-2/
- 5.Lommatzsch M, Buhl R, Korn S. The treatment of mild and moderate asthma in adults. Dtsch Arztebl Int 2020; 117: 434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edris A, De Feyter S, Maes T, et al. Monoclonal antibodies in type 2 asthma: a systematic review and network meta-analysis. Respir Res 2019; 20: 179. doi: 10.1186/s12931-019-1138-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yun JH, Lamb A, Chase R, et al. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 2018; 141: 2037–2047 e10. doi: 10.1016/j.jaci.2018.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med 2015; 3: 355–366. doi: 10.1016/S2213-2600(15)00042-9 [DOI] [PubMed] [Google Scholar]
- 9.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012; 380: 651–659. doi: 10.1016/S0140-6736(12)60988-X [DOI] [PubMed] [Google Scholar]
- 10.Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med 2017; 376: 2448–2458. doi: 10.1056/NEJMoa1703501 [DOI] [PubMed] [Google Scholar]
- 11.Pavord ID, Chanez P, Criner GJ, et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med 2017; 377: 1613–1629. doi: 10.1056/NEJMoa1708208 [DOI] [PubMed] [Google Scholar]
- 12.Criner GJ, Celli BR, Brightling CE, et al. Benralizumab for the prevention of COPD exacerbations. N Engl J Med 2019; 381: 1023–1034. doi: 10.1056/NEJMoa1905248 [DOI] [PubMed] [Google Scholar]
- 13.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc: Series B (Methodol) 1995; 57: 289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 14.Contoli M, Baraldo S, Marku B, et al. Fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease: 5-year follow-up. J Allergy Clin Immunol 2010; 125: 830–837. doi: 10.1016/j.jaci.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 15.Janson C, Malinovschi A, Amaral AFS, et al. Bronchodilator reversibility in asthma and COPD: findings from three large population studies. Eur Respir J 2019; 54: 1900561. doi: 10.1183/13993003.00561-2019 [DOI] [PubMed] [Google Scholar]
- 16.Soler-Cataluna JJ, Cosio B, Izquierdo JL, et al. Consensus document on the overlap phenotype COPD-asthma in COPD. Arch Bronconeumol 2012; 48: 331–337. doi: 10.1016/j.arbr.2012.06.017 [DOI] [PubMed] [Google Scholar]
- 17.Miravitlles M, Alvarez-Gutierrez FJ, Calle M, et al. Algorithm for identification of asthma-COPD overlap: consensus between the Spanish COPD and asthma guidelines. Eur Respir J 2017; 49: 1700068. doi: 10.1183/13993003.00068-2017 [DOI] [PubMed] [Google Scholar]
- 18.Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 2014; 371: 1198–1207. doi: 10.1056/NEJMoa1403290 [DOI] [PubMed] [Google Scholar]
- 19.Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med 2014; 371: 1189–1197. doi: 10.1056/NEJMoa1403291 [DOI] [PubMed] [Google Scholar]
- 20.FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016; 388: 2128–2141. doi: 10.1016/S0140-6736(16)31322-8 [DOI] [PubMed] [Google Scholar]
- 21.Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016; 388: 2115–2127. doi: 10.1016/S0140-6736(16)31324-1 [DOI] [PubMed] [Google Scholar]
- 22.Singh D, Kolsum U, Brightling CE, et al. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J 2014; 44: 1697–1700. doi: 10.1183/09031936.00162414 [DOI] [PubMed] [Google Scholar]
- 23.Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med 2010; 182: 598–604. doi: 10.1164/rccm.200912-1843CC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pavord ID, Chapman KR, Bafadhel M, et al. Mepolizumab for eosinophil-associated COPD: analysis of METREX and METREO. Int J Chron Obstruct Pulmon Dis 2021; 16: 1755–1770. doi: 10.2147/COPD.S294333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Criner GJ, Celli BR, Singh D, et al. Predicting response to benralizumab in chronic obstructive pulmonary disease: analyses of GALATHEA and TERRANOVA studies. Lancet Respir Med 2020; 8: 158–170. doi: 10.1016/S2213-2600(19)30338-8 [DOI] [PubMed] [Google Scholar]
- 26.Landis S, Suruki R, Maskell J, et al. Demographic and clinical characteristics of COPD patients at different blood eosinophil levels in the UK clinical practice research datalink. COPD 2018; 15: 177–184. doi: 10.1080/15412555.2018.1441275 [DOI] [PubMed] [Google Scholar]
- 27.Bafadhel M, Peterson S, De Blas MA, et al. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med 2018; 6: 117–126. doi: 10.1016/S2213-2600(18)30006-7 [DOI] [PubMed] [Google Scholar]
- 28.Zeiger RS, Tran TN, Butler RK, et al. Relationship of blood eosinophil count to exacerbations in chronic obstructive pulmonary disease. J Allergy Clin Immunol Pract 2018; 6: 944–954 e5. doi: 10.1016/j.jaip.2017.10.004 [DOI] [PubMed] [Google Scholar]
- 29.Hayden LP, Hardin ME, Qiu W, et al. Asthma is a risk factor for respiratory exacerbations without increased rate of lung function decline: five-year follow-up in adult smokers from the COPDGene study. Chest 2018; 153: 368–377. doi: 10.1016/j.chest.2017.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morobeid H, Pizarro C, Biener L, et al. Impact of prior smoking exposure and COPD comorbidity on treatment response to monoclonal antibodies in patients with severe asthma. ERJ Open Res 2021; 7: 00190-2021. doi: 10.1183/23120541.00190-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]