Abstract
Background
Although the initial use of combination treatment has been proven to be beneficial for patients’ clinical outcomes, there are scarce data on its haemodynamic effects. The objective of the present study was to evaluate the effect of an initial combination of pulmonary arterial hypertension (PAH)-targeted therapies on haemodynamic parameters in treatment-naïve PAH patients.
Methods
A systematic search of PubMed, Cochrane Central Register of Controlled Trials and Web of Science was performed. We considered eligible studies with an intervention of initial PAH-targeted combination therapy in treatment-naïve PAH patients with or without monotherapy control. A random-effects meta-analysis was performed for the difference between baseline and follow-up in pulmonary vascular resistance (PVR) and other haemodynamic parameters.
Results
In 880 patients receiving initial combination therapy PVR was reduced by −6.5 Wood Units (95% CI −7.4–−5.7 Wood Units) or by −52% (95% CI −56%–−48%, I2=0%) compared to baseline. Initial triple therapy including a parenteral prostanoid resulted in significantly greater PVR reduction (−67% versus −50% with all other combination therapies, p=0.01). The effect was more pronounced in younger patients (p=0.02). Compared to baseline, there was −12.2 mmHg (95% CI −14.0–−10.4 mmHg) decrease in mean pulmonary artery pressure, 0.9 L·min−1·m−2 (95% CI 0.8–1.1 L·min−1·m−2) increase in cardiac index, −3.2 mmHg (95% CI −4.1–−2.3 mmHg) decrease in right atrial pressure and 8.6% (95% CI 6.9–10.3%) increase in mixed venous oxygen saturation. In the controlled studies, initial combination therapy reduced PVR by −4.2 Wood Units (95% CI −6.1–−2.4 Wood Units) compared to monotherapy.
Conclusion
Initial combination therapy leads to remarkable haemodynamic amelioration. Parenteral prostanoids should be considered early, especially in more severely affected patients, to enable right ventricular reverse remodelling.
Short abstract
Initial combination therapy in PAH results in >50% reduction in pulmonary vascular resistance compared to baseline. Parenteral prostanoids accentuate this response and should be considered early to enable timely right ventricular reverse remodelling. https://bit.ly/3uG3k8H
Introduction
Pulmonary arterial hypertension (PAH) is a chronic condition with great pathobiological complexity, which involves multiple pathogenetic pathways, including the endothelin, nitric oxide and prostacyclin pathway. Current management strategies depend on a multivariable-based risk stratification in order to guide pathway-targeted therapy titration, and both initial and sequential combination therapies are now the proposed treatment strategy [1, 2]. The Ambrisentan and Tadalafil in Patients with Pulmonary Arterial Hypertension (AMBITION) trial showed that among participants with PAH who are treatment-naïve, an initial combination therapy of ambrisentan and tadalafil resulted in a significantly reduced risk of clinical failure events than monotherapy with each one of the combination's components [3]. Hence, although the initial use of combination treatment has been proven beneficial for patients’ clinical outcome, there are scarce data on its haemodynamic effects.
The aim of this study was to systematically evaluate the effect of an initial combination of PAH-targeted therapies on haemodynamic parameters in treatment-naïve PAH patients.
Methods
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines and was registered in PROSPERO (www.crd.york.ac.uk/PROSPERO identifier CRD42021283091) [4]. The PubMed, Cochrane Central Register of Controlled Trials and Web of Science databases were searched from inception up until August 2021. The search terms included the currently approved targeted medication classes and substances for the treatment of PAH, a term for PAH, and the keywords “initial” and “upfront”. The search strategy is available in the supplementary material.
We considered eligible both prospective and retrospective studies with an intervention of initial PAH-targeted combination therapy (endothelin receptor antagonists (ERA), phosphodiesterase-5 inhibitors (PDE5i), soluble guanylyl cyclase stimulators (sGC), prostanoids and prostacyclin receptor agonists) in treatment-naïve PAH patients with or without a comparison with PAH-targeted monotherapy. The primary efficacy outcome was the mean difference between baseline and follow-up in pulmonary vascular resistance (PVR). Other haemodynamic parameters regarded as efficacy outcomes included the mean difference between baseline and follow-up in mean pulmonary arterial pressure (mPAP), cardiac index, right atrial pressure (RAP) and mixed venous oxygen saturation (SvO2) as assessed by right heart catheterisation. In addition, we assessed the mean difference between baseline and follow-up in the 6-min walk distance (6MWD) and brain natriuretic peptide (BNP). Safety outcomes included serious adverse events.
Study selection and data extraction were performed according to standard procedures as described in the Cochrane Handbook, namely, both processes were performed independently by two investigators (I.T. Farmakis, E. Vrana) and in case of disagreement a third investigator (S. Zafeiropoulos) was consulted. We used the Cochrane Collaboration tool for assessing the risk of bias for nonrandomised studies of interventions (ROBINS-I) [5].
We performed a single-arm random-effects model meta-analysis of the eligible studies to evaluate the effect of initial PAH-targeted combination therapy on the haemodynamic efficacy outcomes, and additionally we performed a second analysis with the studies providing a control monotherapy arm. The effect measure for all outcomes was the mean difference (MD) or the standardised mean difference (SMD) as appropriate, with corresponding 95% confidence intervals. We used the standard DerSimonian–Laird equations to produce estimates of variance in the summary measures. Heterogeneity was assessed with Cochran's Q test and the I2 statistic. Publication bias was assessed with the use of funnel plots and Egger's test. A sensitivity analysis was performed to exclude studies not providing the variance of difference between baseline and follow-up in the outcomes. Subgroup analyses included the use of double or triple therapy and the use of parenteral prostanoids in the combination therapy. Meta-regression analysis was performed adjusting for the age and sex of participants (each baseline variable evaluated in separate univariate analyses). All analyses were performed by I.T. Farmakis using the “meta” package in R (The R Project for Statistical Computing, version 4.0.2).
Results
The search strategy resulted in 681 identified studies after duplicates were removed. Subsequently, 59 full-text articles were assessed for eligibility and, eventually, we considered 13 studies eligible [6–18]. The study selection process and the corresponding flow chart is presented in the supplementary material. In total, 880 patients received initial combination therapy (comprising 17 treatment arms), while four studies reported a control monotherapy group comprising a total of 194 patients. Eight studies reported the initial use of an ERA+PDE5i combination, three studies the use of mono-oral+parenteral prostanoid combination, one studied the ERA+sGC combination and three reported the use of an initial triple combination therapy (ERA+PDE5i+prostanoids). The mean age of participants was 52±16 years and 74% were women, while 80.7% of patients belonged to World Health Organization functional class III or IV. The mean baseline mPAP was 53±13 mmHg and mean baseline 6MWD was 334±123 m. Baseline characteristics of eligible studies are presented in table 1.
TABLE 1.
Baseline characteristics of included studies
| First author, year [reference] | Participants | PAH subgroup | Mean age years | Follow-up duration | WHO functional class | Interventional group treatment (upfront therapy) | Control group treatment | Outcome measures | Adverse events |
| Badagliacca,# 2018 [6] | 165 | IPAH | 54 | 155±65 days | 3.2±0.4 | Group 1: prostanoids+ERAs or PDE5i Group 2: ERAs+PDE5i |
Group 3: ERAs or PDE5i Group 4: prostanoids |
PVR, mPAP, CI, RAP, 6MWD | NR |
| Badagliacca, 2021 [7] | 181 | IPAH, CTD-PAH, CHD-PAH with closed shunt | 53 | 180 (144–363) days | I–II: 37 (20.4) III: 127 (70.2) IV: 17 (9.4) |
Ambrisentan-tadalafil or ambrisentan-sildenafil or bosentan-tadalafil or bosentan-sildenafil or macitentan-tadalafil or macitentan-sildenafil | NA | PVR, mPAP, CI, RAP, 6MWD | NR |
| Chin, 2021 [ 8 ] | 247 | PAH | 51.9 | 26 weeks | I–II: 50 (20.2) III–IV: 197 (79.8) |
Macitentan-tadalafil-selexipag | Macitentan-tadalafil-placebo | PVR, mPAP, CI, RAP, SvO2, NT-proBNP, 6MWD | Headache, diarrhoea, nausea, pain in extremity, jaw pain, vomiting |
| D'Alto, 2020 [9] | 21 | IPAH | 44 | 24±14 months | III: 12 (57) IV: 9 (43) |
Ambrisentan-tadalafil-treprostinil | Bosentan or ambrisentan or sildenafil or tadalafil; | PVR, mPAP, CI, RAP, SvO2, NT-proBNP, 6MWD | Peripheral oedema, nasal congestion, flushing |
| Hassoun, 2015 [ 10 ] | 24 | SSc-PAH | 59.9 | 36 weeks | II: 8 (35) III: 15 (65) |
Ambrisentan-tadalafil | NA | PVR, mPAP, CI, RAP, NT-proBNP, 6MWD | Peripheral oedema, nasal congestion, dyspnoea, cough, headache, dizziness, fatigue, abdominal pain, nausea-vomiting, hypotension, diarrhoea |
| Kemp, 2012 [11] | 23 | IPAH, HPAH, DPAH | 43 | 4±1 months | III: 16 (70) IV: 7 (30) |
Epoprostenol-bosentan | Epoprostenol monotherapy | PVR, mPAP, CI, RAP, SvO2, 6MWD | Jaw pain, facial flushing, headache, gastrointestinal disturbance, leg pain, catheter infections, increase in liver enzymes |
| Rinaldi, 2018 [ 12 ] | 19 | PAH | NR | NR | II–III: 100 | Ambrisentan-tadalafil | Bosentan or sildenafil monotherapy or other ERAs or PDE5i monotherapy or bosentan-sildenafil or macitentan-sildenafil | PVR, mPAP, CI, RAP, SvO2, 6MWD | |
| Sitbon, 2014 [13] | 19 | IPAH, HPAH, DPAH | 39.4 | 4 months | III: 8 (42) IV: 11 (58) |
Bosentan-sildenafil-epoprostenol | NA | PVR, mPAP, CI, RAP, SvO2, 6MWD | Jaw pain, headache, diarrhoea, flushing, increase in liver enzymes |
| Sitbon, 2016 [ 14 ] | 97 | PAH | 54.1 | 4.1 (3.5–4.9) months | II: 15 (15) III: 70 (72) IV: 12 (12) |
Bosentan-sildenafil or bosentan-tadalafil or ambrisentan-sildenafil or ambrisentan-tadalafil | NA | PVR, mPAP, CI, RAP, SvO2, BNP, 6MWD | Peripheral oedema, increase in liver enzymes, blurred vision |
| Sitbon, 2020 [15] | 46 | PAH | 57.4 | 16 weeks | II: 10 (21.7) III: 36 (78.3) |
Macitentan-tadalafil | NA | PVR, mPAP, CI, RAP, SvO2, NT-proBNP, 6MWD | Peripheral oedema, headache, diarrhoea, dyspnoea, anaemia, asthenia, fatigue, increase in liver enzymes |
| Sulica, 2019 [ 16 ] | 15 | PAH | 55.8 | 13.7±3.6 months | III: 93 IV: 7 |
Macitentan-riociguat | NA | PVR, mPAP, CI, RAP, SvO2, BNP, 6MWD | Peripheral oedema, nasal congestion, headache, hypotension |
| van de Veerdonk, 2017 [17] | 80 | IPAH, HPAH, DPAH | 49 | 12 months | II: 24 (30) III: 56 (70) |
Bosentan-sildenafil or bosentan-tadalafil or ambrisentan-sildenafil or ambrisentan-tadalafil | Bosentan or ambrisentan or macitentan or sitaxentanor or sildenafil or tadalafil | PVR, mPAP, CI, RAP, SvO2, NT-proBNP, 6MWD | Increase in liver enzymes |
| Zhang, 2014 [ 18 ] | 68 | CHD-PAH | NR | 6 months | NR | Upfront iloprost-tadalafil | Sequential iloprost-tadalafil | PVR, SvO2, 6MWD |
Data are presented as n, mean±sd, median (interquartile range) or n (%). PAH: pulmonary arterial hypertension; WHO: World Health Organization; IPAH: idiopathic pulmonary arterial hypertension; ERA: endothelin receptor antagonist; PDE5i: phosphodiesterase type 5 inhibitor; PVR: pulmonary vascular resistance; mPAP: mean pulmonary arterial pressure; CI: cardiac index; RAP: right atrial pressure; 6MWD: 6-min walk distance; NR: not reported; CTD: connective tissue disorder; CHD: congenital heart disease; NA: not applicable; SvO2: mixed venous oxygen saturation; NT-proBNP: N-terminal pro-brain natriuretic peptide; SSc: systemic sclerosis; HPAH: heritable pulmonary arterial hypertension; DPAH: drug-induced pulmonary arterial hypertension. #: in this study, group 1 was treated with treprostinil-tadalafil or treprostinil-ambrisentan or treprostinil-bosentan or epoprostenol-tadalafil or epoprostenol-bosentan or iloprost-ambrisentan; group 2 was treated with ambrisentan-tadalafil or ambrisentan-sildenafil or bosentan-tadalafil or bosentan-sildenafil or macitentan-tadalafil or macitentan-sildenafil; group 3 was treated with bosentan or ambrisentan or sildenafil or tadalafil; group 4 was treated with treprostinil or epoprostenol.
In the quality assessment, the majority of studies were at moderate risk of bias, while the most frequent category of serious bias was bias due to confounding. The summary risk-of-bias plot, as well as the traffic light plot of individual study assessment are presented in the supplementary material.
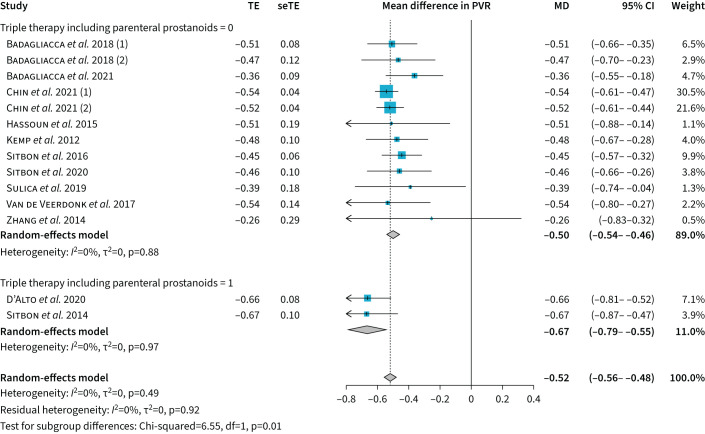
In the single-arm meta-analysis, initial combination therapy reduced PVR by −6.5 Wood Units (95% CI −7.4–−5.7 Wood Units) or by −52% (95% CI −56–−48%) compared to the baseline value, with no heterogeneity in the model (I2=0%, p=0.49) (figure 1). Compared to baseline, there was −12.2 mmHg (95% CI −14.0–−10.4 mmHg) decrease in mPAP, 0.9 L·min−1·m−2 (95% CI 0.8–1.1 L·min−1·m−2) increase in cardiac index, −3.2 mmHg (95% CI −4.1–−2.3 mmHg) decrease in RAP and 8.6% (95% CI 6.9–10.3%) increase in SvO2 (figure 2). The was a decrease in BNP levels (SMD −1046.7, 95% CI −1511–−581.4) and an increase in 6MWD (MD 86 m, 95% CI 67.2–104.8 m). No significant differences were observed in the sensitivity analysis.
FIGURE 1.
Forest plot of the effects of initial combination therapy on the pulmonary vascular resistance (PVR) expressed as percentage difference compared to baseline. TE: treatment effect; seTE: standard error of treatment effect; MD: mean difference.
FIGURE 2.
Effects of upfront combination therapy on haemodynamic outcomes in the single-arm meta-analysis. MD: mean difference; PVR: pulmonary vascular resistance; mPAP: mean pulmonary arterial pressure; RAP: right atrial pressure; SvO2: mixed venous oxygen saturation.
Initial triple therapy including a parenteral prostanoid resulted in significantly greater PVR reduction (−67% versus −50% with all other combination therapies, p=0.01). The inclusion of a parenteral prostanoid in any combination treatment resulted in greater numerical PVR reduction (−58% with versus −50% without), although this was not statistically significant (p=0.15). The meta-regression analysis showed that the effect was more pronounced in younger patients in all outcomes (p=0.02) (figure 3 presents the effect of age on the PVR outcome). There was no evidence of publication bias for the primary efficacy outcome PVR (supplementary material), but there was publication bias for the cardiac index and SvO2 outcomes.
FIGURE 3.

Bubble plot for the effect of participants’ mean age on the pulmonary vascular resistance (PVR) outcome.
In the controlled-arm meta-analysis, initial combination therapy reduced PVR by −4.2 Wood Units (95% CI −6.1–−2.4 Wood Units) compared to monotherapy, with substantial heterogeneity in the model (I2=87%, p<0.01). Compared to monotherapy, there was −6.7 mmHg (95% CI −8.6–−4.8 mmHg) decrease in mPAP, 0.4 L·min−1·m−2 (95% CI 0.2–0.6 L·min−1·m−2) increase in cardiac index, −1.7 mmHg (95% CI −2.6; −0.8 mmHg) decrease in RAP and 4% (95% CI 0.5–7.4%) increase in SvO2 (supplementary material).
In general, most adverse events were similar across the included studies and were consistent with the most frequently presented adverse events of different PAH regimens.
Discussion
In this meta-analysis, initial combination therapy was associated with remarkable haemodynamic changes in treatment-naïve PAH patients. In particular, we observed a −52% reduction in the PVR after the initiation of initial combination therapy; this reduction was even more prominent, reaching −67%, when a parenteral prostanoid treatment component was included in a triple initial PAH-targeted combination therapy.
In severe PAH, pressure overload, represented by PVR, results in an “adaptive” remodelling of the right ventricle; however, its constant increase inevitably leads to ventriculoarterial uncoupling, right ventricle distension and decompensation [19]. Our analysis of pooled results estimates a −48% to −56% reduction of PVR with the use of initial combination therapy and may also suggest an opportunity for reverse remodelling of the right ventricle [6, 17]. The inclusion of a parenteral prostanoid treatment compound results in a more pronounced haemodynamic effect. Survival rates have been shown to be higher with initial triple therapy (two oral medications and a parenteral prostacyclin) compared with dual therapy or monotherapy [20], which is in line with the greater PVR reduction with the use of an initial triple therapy suggested by the current analysis, favouring timely initiation [21]. Conversely, initial double oral combination produces similar PVR drop compared to initial triple oral combination [8].
A more prominent effect of PVR reduction was observed in younger patients with more typical PAH and lesser comorbidities, probably underlying the treatment effect on their vascular pathology. There seems to be a significant variation in the haemodynamic response even in patients receiving similar PAH drug treatment [7]. Patients with significant PVR lowering (>45–50%) are those who obtain the reverse right heart remodelling and improve their right ventricle systolic function [17, 22].
A limitation of this analysis is that it did not have access to individual patient data and, therefore, effects of therapy could not be stratified by risk profiles and selection bias of severely affected patients could exist. In addition, there was great variability in the components of the initial combination therapy and not all comparisons between them were possible. For instance, a known pharmacokinetic interaction between sildenafil and bosentan leads to reduction of plasma levels of sildenafil and increase of the plasma levels of bosentan and may lead to a blunted haemodynamic response [14]. The contribution of the specific components in PVR reduction (mPAP lowering versus cardiac index increase) was not studied in the current analysis. Right ventricle function assessed by RVEF change is a better predictor of survival than PVR; however, the association between PVR reduction and RVEF improvement could not be assessed in this analysis [22]. Lastly, since no hard end-points were assessed, absolute value of PVR at follow-up after initial combination treatment may be more important predictor of outcome than PVR drop rate.
Conclusion
Initial combination therapy leads to remarkable haemodynamic amelioration. Parenteral prostanoids should be considered early, especially in more severely affected patients, to enable right ventricle reverse remodelling. Treatment delays have deleterious effects in patients’ functional capacity and outcomes, therefore a “watch-and-wait” approach does not help achieving low-risk status and should be avoided.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00313-2022.SUPPLEMENT (2.1MB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Conflict of interest: I.T. Farmakis has nothing to disclose.
Conflict of interest: E. Vrana has nothing to disclose.
Conflict of interest: S-A. Mouratoglou has nothing to disclose.
Conflict of interest: S. Zafeiropoulos has nothing to disclose.
Conflict of interest: S. Zanos has nothing to disclose.
Conflict of interest: G. Giannakoulas has received fees for lectures and/or consultation from Actelion, Bayer, ELPEN Pharmaceuticals, GlaxoSmithKline, Janssen, MSD, Pfizer, Lilly and United Therapeutics.
References
- 1.Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019; 53: 1801899. doi: 10.1183/13993003.01889-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022; 53: 1801913. 10.1093/eurheartj/ehac237. [DOI] [PubMed] [Google Scholar]
- 3.Galiè N, Barberà JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373: 834–844. doi: 10.1056/NEJMoa1413687 [DOI] [PubMed] [Google Scholar]
- 4.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355: i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badagliacca R, Raina A, Ghio S, et al. Influence of various therapeutic strategies on right ventricular morphology, function and hemodynamics in pulmonary arterial hypertension. J Heart Lung Transplant 2018; 37: 365–375. doi: 10.1016/j.healun.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 7.Badagliacca R, D'Alto M, Ghio S, et al. Risk reduction and hemodynamics with initial combination therapy in pulmonary arterial hypertension. Am J Respir Crit Care Med 2021; 203: 484–492. doi: 10.1164/rccm.202004-1006OC [DOI] [PubMed] [Google Scholar]
- 8.Chin KM, Sitbon O, Doelberg M, et al. Three- versus two-drug therapy for patients with newly diagnosed pulmonary arterial hypertension. J Am Coll Cardiol 2021; 78: 1393–1403. doi: 10.1016/j.jacc.2021.07.057 [DOI] [PubMed] [Google Scholar]
- 9.D'Alto M, Badagliacca R, Argiento P, et al. Risk reduction and right heart reverse remodeling by upfront triple combination therapy in pulmonary arterial hypertension. Chest 2020; 157: 376–383. doi: 10.1016/j.chest.2019.09.009 [DOI] [PubMed] [Google Scholar]
- 10.Hassoun PM, Zamanian RT, Damico R, et al. Ambrisentan and tadalafil up-front combination therapy in scleroderma-associated pulmonary arterial hypertension. Am J Respir Crit Care Med 2015; 192: 1102–1110. doi: 10.1164/rccm.201507-1398OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kemp K, Savale L, O'Callaghan DS, et al. Usefulness of first-line combination therapy with epoprostenol and bosentan in pulmonary arterial hypertension: an observational study. J Heart Lung Transplant 2012; 31: 150–158. doi: 10.1016/j.healun.2011.11.002 [DOI] [PubMed] [Google Scholar]
- 12.Rinaldi A, Dardi F, Albini A, et al. Haemodynamic and exercise effects of different types of initial oral combination therapy in pulmonary arterial hypertension. Eur Heart J 2018; 39: P6241. doi: 10.1093/eurheartj/ehy566.P6341 [DOI] [Google Scholar]
- 13.Sitbon O, Jaïs X, Savale L, et al. Upfront triple combination therapy in pulmonary arterial hypertension: a pilot study. Eur Respir J 2014; 43: 1691–1697. doi: 10.1183/09031936.00116313 [DOI] [PubMed] [Google Scholar]
- 14.Sitbon O, Sattler C, Bertoletti L, et al. Initial dual oral combination therapy in pulmonary arterial hypertension. Eur Respir J 2016; 47: 1727–1736. doi: 10.1183/13993003.02043-2015 [DOI] [PubMed] [Google Scholar]
- 15.Sitbon O, Cottin V, Canuet M, et al. Initial combination therapy of macitentan and tadalafil in pulmonary arterial hypertension. Eur Respir J 2020; 56: 2000673. doi: 10.1183/13993003.00673-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sulica R, Sangli S, Chakravarti A, et al. Clinical and hemodynamic benefit of macitentan and riociguat upfront combination in patients with pulmonary arterial hypertension. Pulm Circ 2019; 9: 2045894019826944. doi: 10.1177/2045894019826944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van de Veerdonk MC, Huis In T Veld AE, Marcus JT, et al. Upfront combination therapy reduces right ventricular volumes in pulmonary arterial hypertension. Eur Respir J 2017; 49: 170007. doi: 10.1183/13993003.00007-2017 [DOI] [PubMed] [Google Scholar]
- 18.Zhang C, Huang Y, Huang T, et al. [Effects of iloprost combined with low dose tadalafil in adult congenital heart disease patients with severe pulmonary arterial hypertension: a single-center, open-label controlled study]. Zhonghua Xin Xue Guan Bing Za Zhi 2014; 42: 474–480. [PubMed] [Google Scholar]
- 19.Sanz J, Sánchez-Quintana D, Bossone E, et al. Anatomy, function, and dysfunction of the right ventricle: JACC state-of-the-art review. J Am Coll Cardiol 2019; 73: 1463–1482. doi: 10.1016/j.jacc.2018.12.076 [DOI] [PubMed] [Google Scholar]
- 20.Boucly A, Savale L, Jaïs X, et al. Association between initial treatment strategy and long-term survival in pulmonary arterial hypertension. Am J Respir Crit Care Med 2021; 204: 842–854. doi: 10.1164/rccm.202009-3698OC [DOI] [PubMed] [Google Scholar]
- 21.Humbert M, Lau EMT. POINT: should initial combination therapy be the standard of care in pulmonary arterial hypertension? Yes. Chest 2019; 156: 1039–1042. doi: 10.1016/j.chest.2019.07.005 [DOI] [PubMed] [Google Scholar]
- 22.van de Veerdonk MC, Kind T, Marcus JT, et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol 2011; 58: 2511–2519. doi: 10.1016/j.jacc.2011.06.068 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00313-2022.SUPPLEMENT (2.1MB, pdf)