This nonrandomized trial assesses the clinical workflow feasibility and treatment-related toxic effects of FLASH radiotherapy and pain relief at the treatment sites.
Key Points
Question
Is proton FLASH radiotherapy, delivered at 1000 times the dose rate of conventional-dose-rate photon radiotherapy for its potential normal tissue-sparing effects, feasible for the palliation of painful bone metastases in the extremities?
Findings
This nonrandomized trial of 10 patients with bone metastases in the extremities found that proton FLASH was clinically feasible, and its safety was supported by the minimal severity of related adverse events. In this small sample size, the efficacy of FLASH treatment for pain relief appeared to be similar to that of conventional-dose-rate photon radiotherapy.
Meaning
The results of this study confirm the workflow feasibility of delivering ultra-high-dose-rate proton FLASH radiation treatment in a routine clinical setting and support the further exploration of proton FLASH radiotherapy.
Abstract
Importance
To our knowledge, there have been no clinical trials of ultra-high-dose-rate radiotherapy delivered at more than 40 Gy/sec, known as FLASH therapy, nor first-in-human use of proton FLASH.
Objectives
To assess the clinical workflow feasibility and treatment-related toxic effects of FLASH and pain relief at the treatment sites.
Design, Setting, and Participants
In the FAST-01 nonrandomized trial, participants treated at Cincinnati Children’s/UC Health Proton Therapy Center underwent palliative FLASH radiotherapy to extremity bone metastases. Patients 18 years and older with 1 to 3 painful extremity bone metastases and life expectancies of 2 months or more were eligible. Patients were excluded if they had foot, hand, and wrist metastases; metastases locally treated in the 2 weeks prior; metal implants in the treatment field; known enhanced tissue radiosensitivity; and implanted devices at risk of malfunction with radiotherapy. One of 11 patients who consented was excluded based on eligibility. The end points were evaluated at 3 months posttreatment, and patients were followed up through death or loss to follow-up for toxic effects and pain assessments. Of the 10 included patients, 2 died after the 2-month follow-up but before the 3-month follow-up; 8 participants completed the 3-month evaluation. Data were collected from November 3, 2020, to January 28, 2022, and analyzed from January 28, 2022, to September 1, 2022.
Interventions
Bone metastases were treated on a FLASH-enabled (≥40 Gy/sec) proton radiotherapy system using a single-transmission proton beam. This is consistent with standard of care using the same prescription (8 Gy in a single fraction) but on a conventional-dose-rate (approximately 0.03 Gy/sec) photon radiotherapy system.
Main Outcome and Measures
Main outcomes included patient time on the treatment couch, device-related treatment delays, adverse events related to FLASH, patient-reported pain scores, and analgesic use.
Results
A total of 10 patients (age range, 27-81 years [median age, 63 years]; 5 [50%] male) underwent FLASH radiotherapy at 12 metastatic sites. There were no FLASH-related technical issues or delays. The average (range) time on the treatment couch was 18.9 (11-33) minutes per patient and 15.8 (11-22) minutes per treatment site. Median (range) follow-up was 4.8 (2.3-13.0) months. Adverse events were mild and consistent with conventional radiotherapy. Transient pain flares occurred in 4 of the 12 treated sites (33%). In 8 of the 12 sites (67%) patients reported pain relief, and in 6 of the 12 sites (50%) patients reported a complete response (no pain).
Conclusions and Relevance
In this nonrandomized trial, clinical workflow metrics, treatment efficacy, and safety data demonstrated that ultra-high-dose-rate proton FLASH radiotherapy was clinically feasible. The treatment efficacy and the profile of adverse events were comparable with those of standard-of-care radiotherapy. These findings support the further exploration of FLASH radiotherapy in patients with cancer.
Trial Registration
ClinicalTrials.gov Identifier: NCT04592887
Introduction
The unique biologic effects of ultra-high-dose-rate radiotherapy delivered at more than 40 Gy/sec, now known as FLASH therapy, were first reported more than 50 years ago.1 Interest in this modality began re-emerging recently, concurrent with the advent of commercial radiotherapy equipment capable of delivering these ultra-high dose rates in a clinical setting.
Multiple animal experiments have demonstrated that FLASH can increase the therapeutic ratio of radiotherapy by decreasing normal tissue injury while maintaining the tumoricidal effects of conventional-dose-rate radiotherapy (conventional radiotherapy) or by allowing for dose escalation and improved tumor control probability without increasing normal tissue injury.2,3,4,5,6,7 The mechanisms by which FLASH spares normal tissues are not fully understood, but there are data that suggest FLASH produces lower levels of toxic oxygen reactive species in normal tissues compared with conventional radiotherapy.8
In the seminal study for the contemporary use of FLASH, Favaudon et al2 irradiated mouse lungs in vivo with both FLASH therapy and conventional-dose-rate radiotherapy.2 Using a single dose of 17 Gy, 100% of mice receiving conventional radiotherapy developed pneumonitis and fibrosis, whereas none of the mice given FLASH therapy developed these toxic effects. FLASH therapy doses were escalated to 30 Gy delivered in a single dose before the mice exhibited evidence of radiation pneumonitis and fibrosis. The same investigators treated orthotopic lung tumors in mice with single-dose FLASH therapy and conventional radiotherapy. A dose of 15 Gy using conventional radiotherapy controlled tumors in only 20% of mice, most of which developed considerable radiation pneumonitis. In contrast, 27 Gy FLASH therapy controlled tumors in 70% of mice, none of which developed radiation pneumonitis.
The normal tissue-sparing effects of FLASH have also been observed in several other tissues and animal models, including mouse brain and neurocognition,3 mouse intestine,4 mouse skin,5 mouse extremities,6 and cat and pig skin.7 In preclinical studies of proton FLASH, we irradiated mouse extremities with 35 Gy using either FLASH or conventional proton radiotherapy.6 Subsequent leg contracture, skin toxic effects, and serum transforming growth factor β 1 levels were significantly decreased in the FLASH group. In this same study, tumor control of mouse head and neck cancer cell lines was equivalent between FLASH and conventional radiotherapy. A separate mouse extremity proton irradiation study by Velalopoulou et al8 demonstrated similar results with diminished normal mesenchymal tissue toxic effects and inflammatory response with FLASH compared with conventional proton therapy and preservation of sarcoma tumor control.
To date, and to our knowledge, there has been 1 published case report of the use of FLASH therapy in a human—a single patient with cutaneous T-cell lymphoma and extensive prior radiotherapy to the skin who was safely and effectively treated with electron FLASH therapy for a recurrent cutaneous lymphoma lesion.9 A single dose of FLASH therapy delivered with electron radiotherapy to 15 Gy was delivered. This resulted in a complete response of the lesion with minimal toxic effects of the heavily pretreated surrounding skin.
Electron radiotherapy is limited to superficial targets such as skin lesions. In contrast, proton radiotherapy can deliver FLASH at depth, for example, to bone, lymph node metastases, or visceral organ tumors.10,11,12,13,14,15 In addition, proton FLASH may provide superior uniformity of dose distribution compared with electrons.
We conducted a prospective nonrandomized clinical study to assess the workflow feasibility and clinical outcomes of proton FLASH therapy based on the extensive preclinical data suggesting that use of FLASH could reduce normal tissue toxic effects while achieving equivalent efficacy of treatment. To mitigate potential risks for toxic effects in, to our knowledge, this first-in-human clinical trial of FLASH, the clinical study focused on treatment of painful bone metastasis sites in the extremities.
Methods
Enrollment and treatment of 10 patients was approved by the US Food and Drug Administration as an investigational device exemption for a FLASH-enabled proton therapy system (ProBeam [Varian Medical Systems]) and by the Cincinnati Children’s Hospital institutional review board (Supplement 1). Study conduct and progress were monitored by an external Data Safety Monitoring Committee. Patients provided informed consent before undergoing treatment. Where applicable to the study design, Transparent Reporting of Evaluations With Nonrandomized Designs (TREND) reporting guidelines were followed.
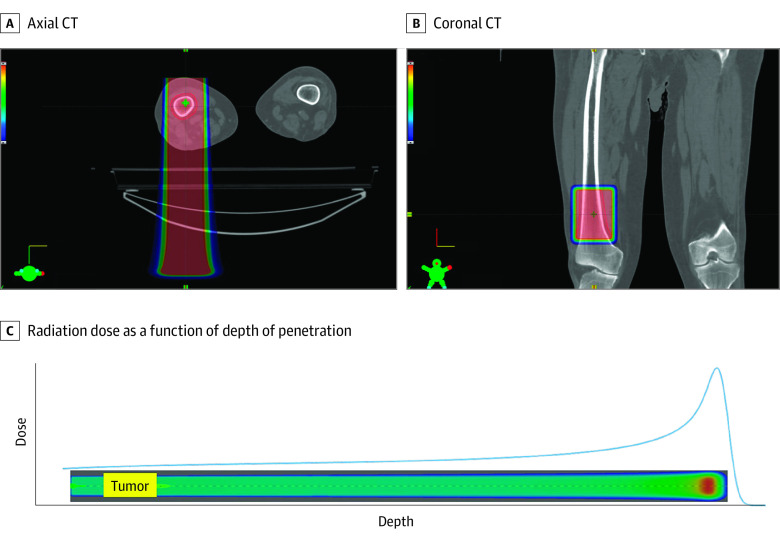
Patients with up to 3 painful metastases in the extremities (excluding hands, wrists, and feet) were evaluated for enrollment. Eligibility criteria also included age of 18 years or older, no prior radiotherapy to the intended target lesion(s), and a life expectancy of more than 2 months. Patients were excluded if there was tumor lysis of more than 50% of the circumferential bone cortex, there were fracture and/or metal implants in the treatment field, they had undergone cytotoxic chemotherapy within 1 week prior to or following FLASH therapy, they had local therapy to the treated sites within 2 weeks prior to FLASH therapy, they were pregnant or breastfeeding, they had an implanted pacemaker, or they had known risk of enhanced normal tissue sensitivity to radiotherapy. In addition, it was required that the target lesion(s) could be treated with 1 of a library of 7 precalculated rectangular radiotherapy fields. Following signing of informed consent, patients underwent computed tomography simulation of the affected site(s). A treatment plan was generated using 1 of these predefined, transmission, high-energy, pencil-beam scanning proton therapy FLASH fields (eAppendix in Supplement 2). A transmission proton therapy field enters and exits the patient, treating the target lesion with the entrance region of a Bragg peak, whereas conventional proton therapy treats the target lesion with Bragg peaks. A dose of 8 Gy in a single fraction was planned with a nominal dose rate of 60 Gy/sec. An example treatment plan is shown in Figure 1, and the proton spot list and dose distribution for this same field are illustrated in eFigure 2 in Supplement 2.
Figure 1. Sample FLASH Treatment Plan and Bragg Curve Showing Radiation Dose in Color Wash.
A, Axial computed tomography (CT) through a lesion treated in the right distal femur. B, Coronal CT through the same lesion. C, The radiation dose, drawn as a blue line, as a function of depth of penetration into the body for FLASH delivery with a 250-MeV transmission beam. The radiation dose is represented on the vertical axis, with depth of penetration into the body on the horizontal axis. The yellow box on the aqua-colored bar represents the position of the tumor in the radiation field, and the brighter red spot is the location of the increased dose at the Bragg peak, occurring outside of the patient’s body. In all panels, darker red color indicates higher dose.
Day-of-treatment study activities included taking photographs of the treatment site(s), performance status evaluation, and a physical examination. Additionally, multiple pain questionnaires were administered, including Brief Pain Inventory (BPI), treated site pain, and pain flare questionnaires. Pain medication use was recorded. Patients were positioned on the treatment couch and, using radiographic image guidance, the target lesions were localized. Treatment was delivered using a modified Varian ProBeam proton system with a dose monitoring chamber specifically developed to accurately measure delivered radiation dose given at FLASH dose rates (IDE No. G200155). In addition to quality assurance guidance from the American Association of Physicists in Medicine task group 224,16 dose-rate quality assurance was performed before and after treatment as per the methodology in Folkerts et al.17 Total time for patient alignment and treatment was recorded. Telephone follow-up to assess for pain flares and changes in pain medications was carried out daily for 10 days following treatment.
Posttreatment, patients were evaluated at day 1 (day of treatment), day 15, month 1, month 2, month 3, and every 2 months thereafter until patients died or were lost to follow-up. Follow-up visits were conducted in person when possible (according to the patient’s medical condition and COVID-19 restrictions in effect at the time) and alternatively by telemedicine visit. The BPI and treated site pain questionnaires were administered, and pain medications were recorded at each follow-up. A spaghetti plot and corresponding table of the average pain score obtained from the treated sites pain questionnaire (which is a subset of the BPI form and particularly relevant for assessing pain relief at the treated site) is included in eFigure 1 in Supplement 2. The methodology of Hartsell et al in the Radiation Therapy Oncology Group (RTOG) 9714 trial,18 which assessed pain response at the 3-month time point following treatment, was used to determine pain response rates. Photographs of the treated site(s) were taken at in-person visits; photographs were also taken by a caregiver when the follow-up visit was conducted by telemedicine, whenever possible.
All adverse events (AEs) were graded using the Common Terminology Criteria of Adverse Events, version 5.0, and recorded independent of their relationship to the treated metastasis. A serious AE was defined per International Organization for Standardization 14155 criteria. All AEs were scored by 1 of the investigators as being definitely related, probably related, possibly related, probably not related, or definitely not related to the FLASH treatment. Stopping rules would have been triggered if 3 patients experienced a serious AE related to treatment, if 3 patients had a delay of more than 7 business days from simulation or required more than 1 hour of time on the treatment couch, or if a major malfunction of the dose-monitoring device occurred.
Results
Patient Population
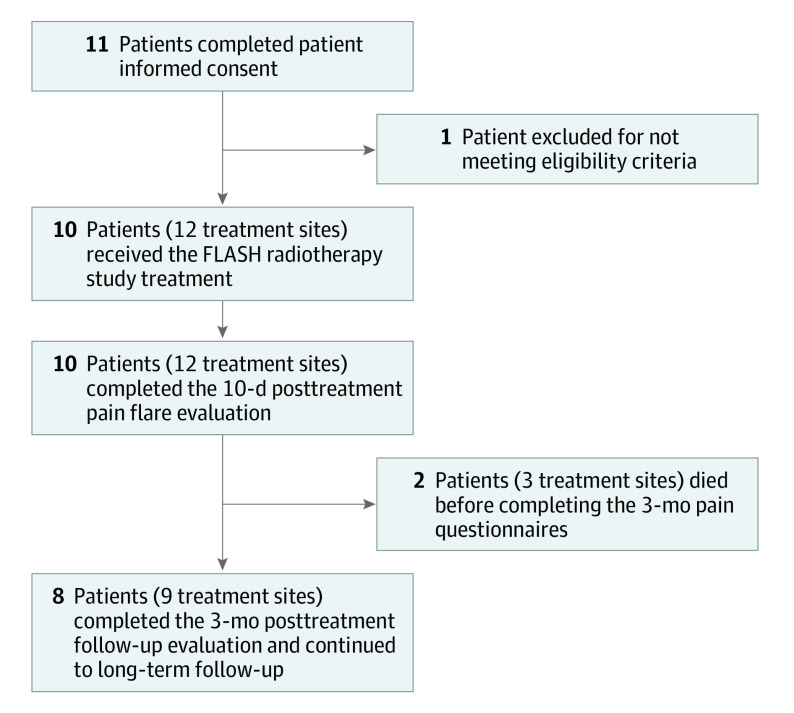
Eleven patients were included in the study; however, 1 patient did not meet eligibility criteria, and subsequently 10 were enrolled (Figure 2). No patients were lost to follow-up. Median (range) follow-up was 4.8 (2.3-13.0) months. Patient ages ranged from 27 to 81 years (median, 63 years). An equal number of men and women were enrolled. All patients reported their ethnicity as not Hispanic/Latino and their race as White. There was a spectrum of histologic diagnoses, the most frequent being non–small cell lung cancer, breast cancer, and multiple myeloma. The clinical characteristics of the study population are summarized in Table 1.
Figure 2. CONSORT Participant Flow Diagram.
Table 1. Baseline Demographics and Clinical Characteristics.
| Demographics and characteristics | No. (%) |
|---|---|
| Patient demographics | |
| Total No. | 10 |
| Sex | |
| Male | 5 (50) |
| Female | 5 (50) |
| Race and ethnicity | |
| White, non-Hispanic/Latino | 10 (100) |
| Clinical characteristics | |
| Histologic diagnosis, No. | 10 |
| Breast | |
| Adenocarcinoma | 1 (10) |
| Unspecified malignant neoplasm | 1 (10) |
| Epithelioid hemangioendothelioma | 1 (10) |
| Lung | |
| Adenocarcinoma | 1 (10) |
| Neuroendocrine carcinoma | 1 (10) |
| Unspecified malignant neoplasm | 1 (10) |
| Multiple myeloma | 2 (20) |
| Prostate adenocarcinoma | 1 (10) |
| Thyroid squamous cell carcinoma | 1 (10) |
| FLASH treatment sites, No. | 12 |
| Femur, lower proximal | 5 (42) |
| Humerus | |
| Upper distal | 2 (17) |
| Upper proximal | 3 (25) |
| Tibia | |
| Lower anterior | 1 (8) |
| Lower distal | 1 (8) |
There was a total of 12 treatment sites for the 10 enrolled patients. Eight patients received treatment to 1 anatomic site, and 2 patients received treatment to 2 distinct anatomical sites. All metastases fit within the predefined treatment field sizes and met the required dosimetric constraints specified in the study protocol. All treatments were delivered at a FLASH dose rate (range, 51-61 Gy/sec) normalized at 5-cm depth (eFigure 3 in Supplement 2).
FLASH Treatment Workflow Feasibility
The elapsed time the patient spent on the treatment couch (including the time for patient setup and positioning, imaging, and FLASH treatment delivery) was an average (range) of 15.8 (11-22) minutes per treatment site and 18.9 (11-33) minutes per patient. The 2 patients who received treatment to 2 anatomical treatment sites were on the treatment couch for 32 and 33 minutes, respectively. There were no delays in treatment, and FLASH treatment was delivered without any device-related problems.
Adverse Events
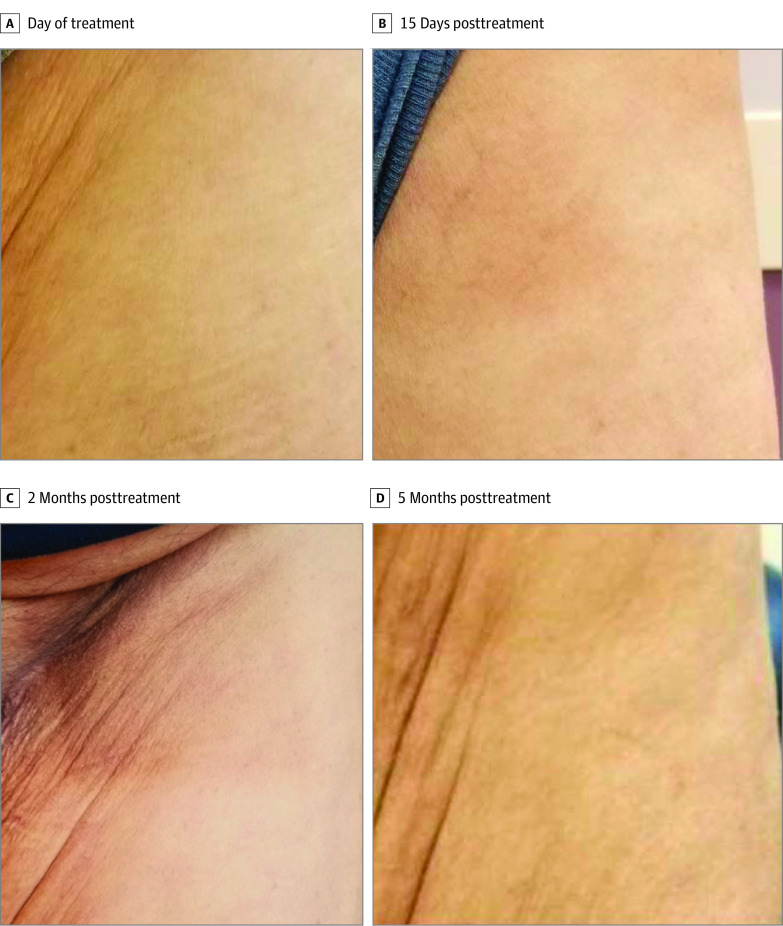
There were 12 AEs attributed as being possibly/probably/definitely related to FLASH treatment noted in 6 patients, with no serious AEs (Table 2). Most AEs (8 of 12) were related to skin changes, with 5 consisting of mild hyperpigmentation. Representative photographs of skin changes in 1 patient are shown in Figure 3.
Table 2. Adverse Events (Possibly, Probably, or Definitely) Attributed to FLASH Treatment (N = 10).
| Adverse eventsa | Patient, No. (%) |
|---|---|
| Acute (≤3 mo posttreatment) | |
| Edema, limb (grade 1) | 1 (10) |
| Erythema (grade 1) | 1 (10) |
| Extremity pain (grade 2) | 1 (10) |
| Fatigue (grade 1) | 1 (10) |
| Pruritus (grade 1) | 2 (20) |
| Skin hyperpigmentation (grade 1) | 4 (40) |
| Long term (>3 mo posttreatment) | |
| Skin discoloration (grade 1) | 1 (10) |
Adverse events were graded using the Common Terminology Criteria of Adverse Events, version 5.0.
Figure 3. Posttreatment Hyperpigmentation.
Photographs of a transient, mild hyperpigmentation adverse event in the area of FLASH treatment in a single patient. The photographs may have been taken under different lighting conditions and/or with different cameras. To facilitate comparison across images, the brightness was uniformly decreased and the warmth uniformly increased in panel C, and the brightness was uniformly increased in panel D.
Eleven of these 12 AEs were grade 1, and 1 of these AEs was grade 2 pain in the extremity. This patient received FLASH treatment to the distal tibia; they experienced grade 1 edema at 3 months posttreatment, grade 1 trace-mild hyperpigmentation at 2 months posttreatment, and grade 2 pain in the extremity at 1 month posttreatment. At the time of publication, the pain in the extremity was ongoing and being managed with medications.
Bone fractures in or near a treated site occurred in 2 patients. Approximately 4 weeks after treatment, 1 patient with approximately 40% cortical destruction pretreatment experienced a humeral fracture in the setting of mild trauma to the FLASH treatment site. This lesion was classified as Mirels score 8 prior to treatment, indicating a moderate risk for impending pathologic fracture. Due to the short time interval after FLASH and the associated trauma, the principal investigator (J.C.B.) assessed the event as probably unrelated to the patient’s treatment. A second patient who received FLASH therapy for a tibial metastasis experienced a fracture of the ipsilateral ankle after a fall. The fracture was located outside of the FLASH treatment field and was attributed as probably unrelated to the study treatment.
There were 352 AEs that were attributed as being probably not related or definitely not related to study treatment (eTable 1 in Supplement 2). Of these, 23 AEs were serious (22 were definitely not and 1 probably not related to FLASH treatment) in 7 patients. Each of these AEs was attributed to systemic cancer treatments or due to disease activity and/or progression.
Pain Flare Following Treatment
Applying the criteria of Chow et al,19 a pain flare following treatment was recorded by patients in 4 of 12 (33%) treated lesions. The pain flares occurred from 2 to 9 days following FLASH treatment. Additionally, 1 patient’s pain management regimen was changed on day 11 from a modest dose of acetaminophen to low-dose hydrocodone at the recommendation of the patient’s pharmacist, but this medication change was not attributed to a flare in pain symptoms.
Pain Relief
Patient-reported pain scores were collected for the 12 treated metastatic sites. Three-month pain scores were available for 9 of the 12 treated metastatic sites in 8 of the 10 patients. One patient, treated at 2 metastatic sites, died shortly after the 2-month posttreatment evaluation. This patient had a complete response of pain at 1 treated site and a partial response at the other site at the 1-month follow-up visit. A second patient died 3 months following FLASH treatment. This patient had a pain complete response at the 2-month follow-up visit.
In 6 of the 12 treated sites, patients reported a complete relief of pain, for a complete response rate of 50%. In 2 of the 12 treated sites patients reported a partial relief of pain for a partial response rate of 16.7%. Two of 12 sites were referred for retreatment with conventional photon radiation to the FLASH irradiated site due to recurrence/progression of pain. Including all 10 patients, a complete or partial pain response following FLASH therapy was seen in 8 of 12 treated sites (7 of 10 patients) for an overall response rate of 66.7%. The temporal response of patient-reported average pain rating at treated sites is shown in eFigure 1 in Supplement 2, and pain response scoring for each patient is shown in eTable 2 in Supplement 2.
Discussion
Herein we describe, to our knowledge, the first-in-human study of FLASH proton radiotherapy. Patients with bone metastases in the extremities were selected with the expectation that these patients were likely to receive benefit equivalent to the same treatment with conventional-dose-rate radiotherapy. Additionally, extremity treatment sites have minimal risk of unexpected toxic effects due to their distance from organs with the greatest sensitivity to radiation. The choice of workflow feasibility as a primary end point was selected in recognition of the need to validate this technically complex new modality in a routine clinical setting. Many components of FLASH implementation are more demanding than radiation delivered at conventional dose rates. A FLASH treatment occurs in milliseconds rather than minutes, which requires more rigorous validation of beam delivery, advanced dosimetry (incorporating dose-rate information into treatment planning), quality assurance, patient positioning, and safety interlocks. Confirming that all of these elements can be seamlessly integrated to deliver FLASH within a conventional timeslot for patient treatment seemed an appropriate end point for this first-in-human study. This prospective study confirmed that FLASH is clinically feasible and appears to be safe in this patient group.
Treatment-related AEs were mild, and most were transient hyperpigmentation and pruritus in the treatment field. It should be noted that, unlike megavoltage photon radiotherapy, transmission FLASH proton radiotherapy as used in this study has no skin-sparing effects so that the skin received the full 8 Gy of prescribed radiotherapy. No patient experienced fibrosis or visible vascular changes in the treatment field. There have been no serious AEs attributable to the study treatment, and administration of FLASH therapy did not result in delays in treatment planning or delivery. Long-term toxic effects may be underrepresented in this investigation owing to the advanced cancer stages and related mortality in this study population. However, in light of the modest radiation dose used in the FAST-01 study, one would anticipate predominantly short-term toxic effects.
Although the study was not powered to evaluate pain relief as a primary objective, most patients experienced pain relief in the range expected for this 8-Gy single-fraction regimen of palliative proton FLASH radiotherapy (8 of 12 treatments sites for an overall response rate of 67%). This rate is comparable with the outcomes of 8-Gy single-fraction conventional-dose-rate radiotherapy administered for painful bone metastases, such as the 65% overall response rate achieved in the RTOG 9714 trial.18 It should be noted that the present study enrolled 2 patients with multiple myeloma, which is generally more radiosensitive compared with carcinomas, and this diagnosis was not included in the RTOG 9714 study. With regard to pain flare, the incidence in this study was 33%, again comparable with other reports using similar, non-FLASH photon techniques.20
Strengths and Limitations
Strengths of this study include its prospective design, meticulous tracking of AEs, and thorough workflow analysis. In addition, because concurrent systemic therapies were not permitted, there is confidence that the results are directly attributable to FLASH radiotherapy. Study limitations include the small study population size and that treatment sites were limited to extremity bone metastases. Consequently, assessment of normal tissue effects was limited to skin, bone, muscle, and lymphatic/vascular and connective tissues. Prior to granting approval for the study, these limitations to the study population were developed and agreed on in consultation with the regulatory agencies to minimize risk for study participants in this, to our knowledge, first-in-human clinical trial. Additionally, owing to the underlying diseases of the patient population, long-term follow-up data are limited. These limitations can be addressed in future clinical trials by including other patient groups.
Considerable further study and technology development will be required to elucidate what place FLASH may eventually have in the radiotherapy armamentarium. Much additional work remains to be done to find optimal dose regimens for the FLASH effect, technologies to deliver conformal FLASH, and to better elicit the biologic mechanisms at work.21
Conclusions
In this nonrandomized trial, we provide, to our knowledge, a first experience in humans showing minimal toxic effects and the desired therapeutic benefit for most patients. Based on clinical workflow metrics, treatment efficacy and safety data, we conclude that ultra-high-dose-rate proton FLASH therapy is feasible in a clinical setting. Future clinical trials of proton FLASH should extend these findings to other parts of the body (eg, thorax, pelvis, head and neck) to demonstrate the applicability of this technology to multiple cancers.
Trial Protocol
eTable 1. Adverse Events, Grade, and Attribution for the FAST-01 Study
eTable 2. Patient Reported Average Pain Rating at Treated Sites and Individual Pain Response Scoring
eFigure 1. Spaghetti Plot of Temporal Response of Pain at Treated Sites
eFigure 2. Schematic of Proton Spot List and Dose Distribution for Same Field
eFigure 3. Scatter Plot of Dose Rate Quality Assurance (Pre- and Post-Treatment) of Dose Rate by Patient
eAppendix. Additional Details Regarding the Planning and Delivery of FLASH Radiotherapy
Data Sharing Statement
References
- 1.Prempree T, Michelsen A, Merz T. The repair time of chromosome breaks induced by pulsed x-rays on ultra-high dose-rate. Int J Radiat Biol Relat Stud Phys Chem Med. 1969;15(6):571-574. doi: 10.1080/09553006914550871 [DOI] [PubMed] [Google Scholar]
- 2.Favaudon V, Caplier L, Monceau V, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med. 2014;6(245):245ra93. doi: 10.1126/scitranslmed.3008973 [DOI] [PubMed] [Google Scholar]
- 3.Montay-Gruel P, Petersson K, Jaccard M, et al. Irradiation in a flash: unique sparing of memory in mice after whole brain irradiation with dose rates above 100Gy/s. Radiother Oncol. 2017;124(3):365-369. doi: 10.1016/j.radonc.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 4.Loo JB, Schuler E, Lartey F, et al. Delivery of ultra-rapid flash radiation therapy and demonstartion of normal tissue sparing after abdominal irradiation of mice. Int J Radiat Oncol Biol Phys. 2017;98(2)(suppl):E16. doi: 10.1016/j.ijrobp.2017.02.101 [DOI] [Google Scholar]
- 5.Field SB, Bewley DK. Effects of dose-rate on the radiation response of rat skin. Int J Radiat Biol Relat Stud Phys Chem Med. 1974;26(3):259-267. doi: 10.1080/09553007414551221 [DOI] [PubMed] [Google Scholar]
- 6.Cunningham S, McCauley S, Vairamani K, et al. FLASH proton pencil beam scanning irradiation minimizes radiation-induced leg contracture and skin toxicity in mice. Cancers (Basel). 2021;13(5):1012. doi: 10.3390/cancers13051012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vozenin MC, De Fornel P, Petersson K, et al. The advantage of FLASH radiotherapy confirmed in mini-pig and cat-cancer patients. Clin Cancer Res. 2019;25(1):35-42. doi: 10.1158/1078-0432.CCR-17-3375 [DOI] [PubMed] [Google Scholar]
- 8.Velalopoulou A, Karagounis IV, Cramer GM, et al. FLASH proton radiotherapy spares normal epithelial and mesenchymal tissues while preserving sarcoma response. Cancer Res. 2021;81(18):4808-4821. doi: 10.1158/0008-5472.CAN-21-1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourhis J, Sozzi WJ, Jorge PG, et al. Treatment of a first patient with FLASH-radiotherapy. Radiother Oncol. 2019;139:18-22. doi: 10.1016/j.radonc.2019.06.019 [DOI] [PubMed] [Google Scholar]
- 10.van Marlen P, Dahele M, Folkerts M, Abel E, Slotman BJ, Verbakel W. Ultra-high dose rate transmission beam proton therapy for conventionally fractionated head and neck cancer: treatment planning and dose rate distributions. Cancers (Basel). 2021;13(8):1859. doi: 10.3390/cancers13081859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mou B, Beltran CJ, Park SS, Olivier KR, Furutani KM. Feasibility of proton transmission-beam stereotactic ablative radiotherapy versus photon stereotactic ablative radiotherapy for lung tumors: a dosimetric and feasibility study. PLoS One. 2014;9(6):e98621. doi: 10.1371/journal.pone.0098621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei S, Lin H, Choi JI, et al. FLASH radiotherapy using single-energy proton PBS transmission beams for hypofractionation liver cancer: dose and dose rate quantification. Front Oncol. 2022;11:813063. doi: 10.3389/fonc.2021.813063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Marlen P, Dahele M, Folkerts M, Abel E, Slotman BJ, Verbakel WFAR. Bringing FLASH to the clinic: treatment planning considerations for ultrahigh dose-rate proton beams. Int J Radiat Oncol Biol Phys. 2020;106(3):621-629. doi: 10.1016/j.ijrobp.2019.11.011 [DOI] [PubMed] [Google Scholar]
- 14.Verhaegen F, Wanders R-G, Wolfs C, Eekers D. Considerations for shoot-through FLASH proton therapy. Phys Med Biol. 2021;66(6):06NT01. doi: 10.1088/1361-6560/abe55a [DOI] [PubMed] [Google Scholar]
- 15.van Marlen P, Verbakel WFAR, Slotman BJ, Dahele M. Single-fraction 34 Gy lung stereotactic body radiation therapy using proton transmission beams: FLASH-dose calculations and the influence of different dose-rate methods and dose/dose-rate thresholds. Adv Radiat Oncol. 2022;7(4):100954. doi: 10.1016/j.adro.2022.100954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arjomandy B, Taylor P, Ainsley C, et al. AAPM task group 224: comprehensive proton therapy machine quality assurance. Med Phys. 2019;46(8):e678-e705. doi: 10.1002/mp.13622 [DOI] [PubMed] [Google Scholar]
- 17.Folkerts MM, Abel E, Busold S, Perez JR, Krishnamurthi V, Ling CC. A framework for defining FLASH dose rate for pencil beam scanning. Med Phys. 2020;47(12):6396-6404. doi: 10.1002/mp.14456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97(11):798-804. doi: 10.1093/jnci/dji139 [DOI] [PubMed] [Google Scholar]
- 19.Chow E, Ling A, Davis L, Panzarella T, Danjoux C. Pain flare following external beam radiotherapy and meaningful change in pain scores in the treatment of bone metastases. Radiother Oncol. 2005;75(1):64-69. doi: 10.1016/j.radonc.2004.12.016 [DOI] [PubMed] [Google Scholar]
- 20.McDonald R, Chow E, Rowbottom L, DeAngelis C, Soliman H. Incidence of pain flare in radiation treatment of bone metastases: a literature review. J Bone Oncol. 2014;3(3-4):84-89. doi: 10.1016/j.jbo.2014.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang M, Wei S, Choi JI, Lin H, Simone CB 2nd. A universal range shifter and range compensator can enable proton pencil beam scanning single-energy Bragg peak FLASH-RT treatment using current commercially available proton systems. Int J Radiat Oncol Biol Phys. 2022;113(1):203-213. doi: 10.1016/j.ijrobp.2022.01.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Adverse Events, Grade, and Attribution for the FAST-01 Study
eTable 2. Patient Reported Average Pain Rating at Treated Sites and Individual Pain Response Scoring
eFigure 1. Spaghetti Plot of Temporal Response of Pain at Treated Sites
eFigure 2. Schematic of Proton Spot List and Dose Distribution for Same Field
eFigure 3. Scatter Plot of Dose Rate Quality Assurance (Pre- and Post-Treatment) of Dose Rate by Patient
eAppendix. Additional Details Regarding the Planning and Delivery of FLASH Radiotherapy
Data Sharing Statement