Abstract
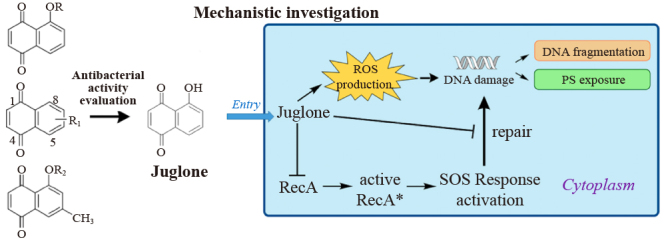
The diverse and large-scale application of disinfectants posed potential health risks and caused ecological damage during the 2019-nCoV pandemic, thereby increasing the demands for the development of disinfectants based on natural products, with low health risks and low aquatic toxicity. In the present study, a few natural naphthoquinones and their derivatives bearing the 1,4-naphthoquinone skeleton were synthesized, and their antibacterial activity against selected bacterial strains was evaluated. In vitro antibacterial activities of the compounds were investigated against Escherichia coli and Staphylococcus aureus. Under the minimum inhibitory concentration (MIC) of ⩽ 0.125 µmol/L for juglone (1a), 5,8-dimethoxy-1,4-naphthoquinone (1f), and 7-methyl-5-acetoxy-1,4-naphthoquinone (3c), a strong antibacterial activity against S. aureus was observed. All 1,4-naphthoquinone derivatives exhibited a strong antibacterial activity, with MIC values ranging between 15.625 and 500 µmol/L and EC50 values ranging between 10.56 and 248.42 µmol/L. Most of the synthesized compounds exhibited strong antibacterial activities against S. aureus. Among these compounds, juglone (1a) showed the strongest antibacterial activity. The results from mechanistic investigations indicated that juglone, a natural naphthoquinone, caused cell death by inducing reactive oxygen species production in bacterial cells, leading to DNA damage. In addition, juglone could reduce the self-repair ability of bacterial DNA by inhibiting RecA expression. In addition to having a potent antibacterial activity, juglone exhibited low cytotoxicity in cell-based investigations. In conclusion, juglone is a strong antibacterial agent with low toxicity, indicating that its application as a bactericidal agent may be associated with low health risks and aquatic toxicity.
Electronic Supplementary Material
Supplementary material is available in the online version of this article at 10.1007/s11783-023-1631-2 and is accessible for authorized users.
Keywords: 1, 4-naphthoquinone derivatives; Antibacterial; Action mechanism; RecA
Acknowledgements
This work was co-supported by the Startup Fund for Youngman Research at SJTU (No. 19X100040082) and the Medical and Engineering Interdisciplinary Research Fund of Shanghai Jiao Tong University (No. 20X190020002).
Supporting information
Synthesis of 1,4-naphthoquinone derivatives as antibacterial agents: activity and mechanistic studies
Footnotes
Highlights
• All 1,4-naphthoquinone hybrids exhibited significant antimicrobial activity.
• Presence of a hydroxyl group on aromatic B-ring of juglone was crucial for activity.
• Juglone can cause DNA damage by producing ROS and downregulation of RecA.
• Juglone has the potential to become a disinfectant.
Contributor Information
Jiahua Cui, Email: cpucjh@sjtu.edu.cn.
Jinping Jia, Email: jpjia@sjtu.edu.cn.
References
- Ahmed Y, Zhong J, Yuan Z, Guo J. Roles of reactive oxygen species in antibiotic resistant bacteria inactivation and micropollutant degradation in Fenton and photo-Fenton processes. Journal of Hazardous Materials. 2022;430:128408. doi: 10.1016/j.jhazmat.2022.128408. [DOI] [PubMed] [Google Scholar]
- Boga C, Delpivo C, Ballarin B, Morigi M, Galli S, Micheletti G, Tozzi S. Investigation on the dyeing power of some organic natural compounds for a green approach to hair dyeing. Dyes and Pigments. 2013;97(1):9–18. doi: 10.1016/j.dyepig.2012.11.020. [DOI] [Google Scholar]
- Bove G E, Jr, Rogerson P A, Vena J E. Case control study of the geographic variability of exposure to disinfectant byproducts and risk for rectal cancer. International Journal of Health Geographics. 2007;6(1):18. doi: 10.1186/1476-072X-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Guo J, Jiang Y, Shao Y. High concentration and high dose of disinfectants and antibiotics used during the COVID-19 pandemic threaten human health. Environmental Sciences Europe. 2021;33(1):11. doi: 10.1186/s12302-021-00456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen V, Faltermann S, Brun N R, Kunz P Y, Fent K. Cytotoxicity and molecular effects of biocidal disinfectants (quaternary ammonia, glutaraldehyde, poly(hexamethylene biguanide) hydrochloride PHMB) and their mixtures in vitro and in zebrafish eleuthero-embryos. Science of the Total Environment. 2017;586:1204–1218. doi: 10.1016/j.scitotenv.2017.02.114. [DOI] [PubMed] [Google Scholar]
- Collivignarelli M, Abbà A, Alloisio G, Gozio E, Benigna I. Disinfection in wastewater treatment plants: evaluation of effectiveness and acute toxicity effects. Sustainability (Basel) 2017;9(10):1704. doi: 10.3390/su9101704. [DOI] [Google Scholar]
- Cui J, Jia J. Discovery of juglone and its derivatives as potent SARS-CoV-2 main proteinase inhibitors. European Journal of Medicinal Chemistry. 2021;225:113789. doi: 10.1016/j.ejmech.2021.113789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Li S, Jia J. A regioselective synthesis of 7-methyl juglone and its derivatives. Natural Product Research. 2022;36(1):18–25. doi: 10.1080/14786419.2020.1761356. [DOI] [PubMed] [Google Scholar]
- DeLeo P C, Huynh C, Pattanayek M, Schmid K C, Pechacek N. Assessment of ecological hazards and environmental fate of disinfectant quaternary ammonium compounds. Ecotoxicology and Environmental Safety. 2020;206:111116. doi: 10.1016/j.ecoenv.2020.111116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas O, Varraso R, Boggs K M, Quinot C, Zock J P, Henneberger P K, Speizer F E, Le Moual N, Camargo C A J. Association of occupational exposure to disinfectants with incidence of chronic obstructive pulmonary disease among US female nurses. JAMA Network Open. 2019;2(10):e1913563. doi: 10.1001/jamanetworkopen.2019.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiguchi A, Tian J, Komatsu S. Proteomic contributions to medicinal plant research: from plant metabolism to pharmacological action. Proteomes. 2017;5(4):35. doi: 10.3390/proteomes5040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibis C, Tuyun A F, Ozsoy-Gunes Z, Bahar H, Stasevych M V, Musyanovych R Y, Komarovska-Porokhnyavets O, Novikov V. Synthesis and biological evaluation of novel nitrogen- and sulfur-containing hetero-1,4-naphthoquinones as potent antifungal and antibacterial agents. European Journal of Medicinal Chemistry. 2011;46(12):5861–5867. doi: 10.1016/j.ejmech.2011.09.048. [DOI] [PubMed] [Google Scholar]
- Jakopic J, Colaric M, Veberic R, Hudina M, Solar A, Stampar F. How much do cultivar and preparation time influence on phenolics content in walnut liqueur? Food Chemistry. 2007;104(1):100–105. doi: 10.1016/j.foodchem.2006.11.008. [DOI] [Google Scholar]
- Janeczko M, Demchuk O M, Strzelecka D, Kubiński K, Masłyk M. New family of antimicrobial agents derived from 1,4-naphthoquinone. European Journal of Medicinal Chemistry. 2016;124:1019–1025. doi: 10.1016/j.ejmech.2016.10.034. [DOI] [PubMed] [Google Scholar]
- Jha B K, Jung H J, Seo I, Suh S I, Suh M H, Baek W K. Juglone induces cell death of Acanthamoeba through increased production of reactive oxygen species. Experimental Parasitology. 2015;159:100–106. doi: 10.1016/j.exppara.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Ji Q, Zhang C, Li D. Influences and mechanisms of nanofullerene on the horizontal transfer of plasmid-encoded antibiotic resistance genes between E. coli strains. Frontiers of Environmental Science & Engineering. 2020;14(6):108. doi: 10.1007/s11783-020-1287-0. [DOI] [Google Scholar]
- Kapoor N, Kandwal P, Sharma G, Gambhir L. Redox ticklers and beyond: naphthoquinone repository in the spotlight against inflammation and associated maladies. Pharmacological Research. 2021;174:105968. doi: 10.1016/j.phrs.2021.105968. [DOI] [PubMed] [Google Scholar]
- Kim S, Ji K, Shin H, Park S, Kho Y, Park K, Kim K, Choi K. Occurrences of benzalkonium chloride in streams near a pharmaceutical manufacturing complex in Korea and associated ecological risk. Chemosphere. 2020;256:127084. doi: 10.1016/j.chemosphere.2020.127084. [DOI] [PubMed] [Google Scholar]
- Kimura S Y, Ortega-Hernandez A. Formation mechanisms of disinfection byproducts: Recent developments. Current Opinion in Environmental Science & Health. 2019;7:61–68. doi: 10.1016/j.coesh.2018.11.002. [DOI] [Google Scholar]
- Kiran K, Patil K N. Expression and characterization of the Staphylococcus aureus RecA protein: a mapping of canonical functions. Protein Expression and Purification. 2022;189:105967. doi: 10.1016/j.pep.2021.105967. [DOI] [PubMed] [Google Scholar]
- Kohanski M A, Dwyer D J, Hayete B, Lawrence C A, Collins J J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130(5):797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- Kurz T, Stossel S, Spiller A (1996). Skin-coloring preparation: US05569460A
- Le Roux J, Plewa M J, Wagner E D, Nihemaiti M, Dad A, Croué J P. Chloramination of wastewater effluent: toxicity and formation of disinfection byproducts. Journal of Environmental Sciences (China) 2017;58:135–145. doi: 10.1016/j.jes.2017.04.022. [DOI] [PubMed] [Google Scholar]
- Lee S, Lee J S, Kho Y, Ji K. Effects of methylisothiazolinone and octylisothiazolinone on development and thyroid endocrine system in zebrafish larvae. Journal of Hazardous Materials. 2022;425:127994. doi: 10.1016/j.jhazmat.2021.127994. [DOI] [PubMed] [Google Scholar]
- Lee Y S, Lee D Y, Kim Y B, Lee S W, Cha S W, Park H W, Kim G S, Kwon D Y, Lee M H, Han S H. The mechanism underlying the antibacterial activity of shikonin against methicillin-resistant Staphylococcus aureus. Evidence-based complementary and alternative medicine: eCAM. 2015;2015:520578. doi: 10.1155/2015/520578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žgur-Bertok D. DNA damage repair and bacterial pathogens. PLoS Pathogens. 2013;9(11):e1003711. doi: 10.1371/journal.ppat.1003711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R A, McDonald J A, Sathasivan A, Khan S J. Disinfectant residual stability leading to disinfectant decay and by-product formation in drinking water distribution systems: a systematic review. Water Research. 2019;153:335–348. doi: 10.1016/j.watres.2019.01.020. [DOI] [PubMed] [Google Scholar]
- Liang Y, Dong B, Pang N, Hu J. ROS generation and DNA damage contribute to abamectin-induced cytotoxicity in mouse macrophage cells. Chemosphere. 2019;234:328–337. doi: 10.1016/j.chemosphere.2019.06.031. [DOI] [PubMed] [Google Scholar]
- Melin V E, Melin T E, Dessify B J, Nguyen C T, Shea C S, Hrubec T C. Quaternary ammonium disinfectants cause subfertility in mice by targeting both male and female reproductive processes. Reproductive Toxicology (Elmsford, N.Y.) 2016;59:159–166. doi: 10.1016/j.reprotox.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Memar M Y, Yekani M, Celenza G, Poortahmasebi V, Naghili B, Bellio P, Baghi H B. The central role of the SOS DNA repair system in antibiotics resistance: a new target for a new infectious treatment strategy. Life Sciences. 2020;262:118562. doi: 10.1016/j.lfs.2020.118562. [DOI] [PubMed] [Google Scholar]
- Nautiyal A, Patil K N, Muniyappa K. Suramin is a potent and selective inhibitor of Mycobacterium tuberculosis RecA protein and the SOS response: RecA as a potential target for antibacterial drug discovery. The Journal of antimicrobial chemotherapy. 2014;69(7):1834–1843. doi: 10.1093/jac/dku080. [DOI] [PubMed] [Google Scholar]
- Nazari A. Efficient mothproofing of wool through natural dyeing with walnut hull and henna against Dermestes maculatus. Journal of the Textile Institute. 2017;108(5):755–765. doi: 10.1080/00405000.2016.1186340. [DOI] [Google Scholar]
- Novais J S, Moreira C S, Silva A C J A, Loureiro R S, Sá Figueiredo A M, Ferreira V F, Castro H C, da Rocha D R. Antibacterial naphthoquinone derivatives targeting resistant strain Gram-negative bacteria in biofilms. Microbial Pathogenesis. 2018;118:105–114. doi: 10.1016/j.micpath.2018.03.024. [DOI] [PubMed] [Google Scholar]
- Ojha D, Patil K N. p-Coumaric acid inhibits the Listeria monocytogenes RecA protein functions and SOS response: an antimicrobial target. Biochemical and Biophysical Research Communications. 2019;517(4):655–661. doi: 10.1016/j.bbrc.2019.07.093. [DOI] [PubMed] [Google Scholar]
- Ozma M A, Khodadadi E, Pakdel F, Kamounah F S, Yousefi M, Yousefi B, Asgharzadeh M, Ganbarov K, Kafil H S. Baicalin, a natural antimicrobial and anti-biofilm agent. Journal of Herbal Medicine. 2021;27:100432. doi: 10.1016/j.hermed.2021.100432. [DOI] [Google Scholar]
- Paul P, Chakraborty P, Chatterjee A, Sarker R K, Dastidar D G, Kundu T, Sarkar N, Das A, Tribedi P. 1,4-Naphthoquinone accumulates reactive oxygen species in Staphylococcus aureus: a promising approach towards effective management of biofilm threat. Archives of Microbiology. 2021;203(3):1183–1193. doi: 10.1007/s00203-020-02117-1. [DOI] [PubMed] [Google Scholar]
- Pavela R. Efficacy of naphthoquinones as insecticides against the house fly, Musca domestica L. Industrial Crops and Products. 2013;43:745–750. doi: 10.1016/j.indcrop.2012.08.025. [DOI] [Google Scholar]
- Pavlopoulou A. RecA: a universal drug target in pathogenic bacteria. Frontiers in bioscience (Landmark edition) 2018;23(1):36–42. doi: 10.2741/4580. [DOI] [PubMed] [Google Scholar]
- Pereira S P, Oliveira R, Coelho S, Musso C, Soares A M, Domingues I, Nogueira A J. From sub cellular to community level: toxicity of glutaraldehyde to several aquatic organisms. Science of the Total Environment. 2014;470–471:147–158. doi: 10.1016/j.scitotenv.2013.09.054. [DOI] [PubMed] [Google Scholar]
- Prati F, Bergamini C, Molina M T, Falchi F, Cavalli A, Kaiser M, Brun R, Fato R, Bolognesi M L. 2-Phenoxy-1,4-naphthoquinones: From a Multitarget Antitrypanosomal to a Potential Antitumor Profile. Journal of Medicinal Chemistry. 2015;58(16):6422–6434. doi: 10.1021/acs.jmedchem.5b00748. [DOI] [PubMed] [Google Scholar]
- Sharma N, Ghosh P, Sharma U K, Sood S, Sinha A K, Gulati A. Microwave-assisted efficient extraction and stability of juglone in different solvents from juglans regia: quantification of six phenolic constituents by validated RP-HPLC and evaluation of antimicrobial activity. Analytical Letters. 2009;42(16):2592–2609. doi: 10.1080/00032710903202055. [DOI] [Google Scholar]
- Wang J, Cheng Y, Wu R, Jiang D, Bai B, Tan D, Yan T, Sun X, Zhang Q, Wu Z. Antibacterial activity of juglone against Staphylococcus aureus: From apparent to proteomic. International Journal of Molecular Sciences. 2016;17(6):965. doi: 10.3390/ijms17060965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Li Q, Ge F, Hu Z, He P, Chen D, Xu D, Wang P, Zhang Y, Zhang L, Wu Z, Zhou Q. Responses of aquatic organisms downstream from WWTPs to disinfectants and their by-products during the COVID-19 pandemic. Science of the Total Environment. 2022;818:151711. doi: 10.1016/j.scitotenv.2021.151711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Yin C, Huang R, He M, Duan X, Jiang Y, Li T. Enhanced resistance in ‘shatang’ mandarin fruit against Penicillium italicum caused by 2-methoxy-1,4-naphthoquinone. Physiological and Molecular Plant Pathology. 2022;119:101828. doi: 10.1016/j.pmpp.2022.101828. [DOI] [Google Scholar]
- Wu Z X, Huang S, Gao Y, Jin Z X, Zhang Q, Xu Y P. Juglone on oxidative induced damage in Escherichia coli. Food Science & Technology (London) 2012;2:247–250. [Google Scholar]
- Yang P, Luo J B, Wang Z Z, Zhang L L, Feng J, Xie X B, Shi Q S, Zhang X G. Synthesis, molecular docking, and evaluation of antibacterial activity of 1,2,4-triazole-norfloxacin hybrids. Bioorganic Chemistry. 2021;115:105270. doi: 10.1016/j.bioorg.2021.105270. [DOI] [PubMed] [Google Scholar]
- Ye L, Zhang J, Xiao W, Liu S. Efficacy and mechanism of actions of natural antimicrobial drugs. Pharmacology & Therapeutics. 2020;216:107671. doi: 10.1016/j.pharmthera.2020.107671. [DOI] [PubMed] [Google Scholar]
- Zeng J, Hu Y, Jia T, Zhang R, Su T, Sun J, Gao H, Li G, Cao M, Song M. Chemoenzymatic synthesis of sialylated lactuloses and their inhibitory effects on Staphylococcus aureus. PLoS One. 2018;13(6):e0199334. doi: 10.1371/journal.pone.0199334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, An J, Zhou Q. Single and joint effects of HHCB and cadmium on zebrafish (Danio rerio) in feculent water containing bedloads. Frontiers of Environmental Science & Engineering. 2012;6(3):360–372. doi: 10.1007/s11783-011-0353-z. [DOI] [Google Scholar]


