Abstract
Background
Uncomplicated urinary tract infections (uUTIs) are among the most common community-acquired infections for women worldwide. Treatment options are increasingly limited by antibiotic resistance; novel oral antibiotics are urgently needed. Gepotidacin is a novel, bactericidal, first-in-class triazaacenaphthylene antibiotic that inhibits bacterial deoxyribonucleic acid (DNA) replication by a distinct mechanism of action, which confers activity against most strains of target pathogens, such as Escherichia coli and Staphylococcus saprophyticus, including those resistant to current antibiotics. Here, we describe the designs of two near-identical phase III clinical trials (EAGLE-2 and EAGLE-3) evaluating gepotidacin for the treatment of uUTI.
Methods
These are phase III, randomized, multicenter, parallel-group, double-blind, double-dummy, comparator-controlled, noninferiority studies, comparing the efficacy and safety of gepotidacin to nitrofurantoin in the treatment of uUTI. Eligible participants are women aged ≥ 12 years with ≥ 2 uUTI symptoms, randomized (1:1) to receive oral gepotidacin (1500 mg) plus placebo or nitrofurantoin (100 mg) plus placebo, twice daily for 5 days. The primary therapeutic endpoint is composite clinical and microbiological efficacy, with noninferiority comparisons made in individuals with a qualifying (≥ 105 colony-forming units/mL urine) nitrofurantoin-susceptible uropathogen.
Results
These trials were designed in accordance with US Food and Drug Administration (2019) and European Medicines Agency (2018) guidance, particularly the composite endpoint and microbiological evaluability requirements. Across the trials ~ 5000 participants are planned to be enrolled from > 200 centers globally.
Conclusions
Gepotidacin represents an important potential treatment option being evaluated to address the need for novel oral antibiotics to treat uUTI.
These trials are registered at ClinicalTrials.gov (https://clinicaltrials.gov/) where the full protocols can be accessed under trial IDs: NCT04020341 (EAGLE-2) and NCT04187144 (EAGLE-3).
Digital features
This article includes a video abstract. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.22015349.
Design of Two Phase III, Randomized, Multicenter Studies Comparing Gepotidacin with Nitrofurantoin for the Treatment of Uncomplicated Urinary Tract Infection in Female Participant (MP4 1,04,082 kb)
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-022-00706-9.
Keywords: Acute cystitis, Antibiotic, Antimicrobial, Gepotidacin, Nitrofurantoin, Phase III clinical trial, Uncomplicated urinary tract infection
Key Summary Points
| This article describes rationale and design of two phase III clinical trials of novel oral antibiotic gepotidacin versus nitrofurantoin for treatment of uncomplicated urinary tract infection. |
| The trials were designed in accordance with latest FDA (2019) and EMA (2018) guidance. |
| The primary endpoint is therapeutic response, which is combined microbiological and clinical response. |
| Approximately 5000 participants are planned to be enrolled from > 200 centers globally. |
| Collectively, these are some of the largest trials of an antibiotic for the treatment of uUTI ever performed. |
Introduction
Uncomplicated urinary tract infections (uUTIs) are among the most common infections in female patients, affecting approximately 50–60% of adult women in their lifetime [1]. The primary uropathogen in uUTI is Escherichia coli, and uUTIs are often treated empirically with oral antibiotics and without urine culture or antimicrobial susceptibility testing [1, 2].
To inform empiric treatment strategies, primary care physicians may rely on local and national guidelines. Some of the current first-line therapies recommended by the Infectious Diseases Society of America and European Association of Urology include nitrofurantoin, trimethoprim-sulfamethoxazole (SXT), fosfomycin, and pivmecillinam [3, 4]. Effective treatments (especially oral agents) for uUTI are, however, increasingly limited by antibiotic resistance. Multidrug resistance (MDR) has emerged at the community level, including extended-spectrum β-lactamase-producing (ESBL) Enterobacterales and fluoroquinolone-resistant pathogens [5].
The World Health Organization has highlighted the increasing prevalence of antibiotic resistance as one of the biggest threats currently facing global health [6]. The US Centers for Disease Control and Prevention has also raised concerns about increasing community-acquired infections, and the emergence and spread of new forms of antibiotic resistance [7]. ESBL-producing Enterobacterales are recognized as a serious threat as these may not be treatable without hospital admission for parenteral therapy. Antibiotic-resistant Enterobacterales have been classed by the World Health Organization as critical priority pathogens [8]. MDR E. coli has emerged as a prominent cause of uUTIs and bacteremia globally [5, 9].
Of the recommended first-line therapies for uUTI, nitrofurantoin, SXT, and fosfomycin were first approved for uUTI by the US Food and Drug Administration (FDA) in 1953, 1973, and 1996, respectively [10]. Pivmecillinam is approved for the treatment of adults with uUTI only in Canada and some European countries, and is pending review and approval from the FDA [11, 12]. Furthermore, where treatment fails owing to antibiotic resistance or drug allergy/intolerance, the limited oral options can result in patients requiring hospitalization to receive intravenous antibiotics.
There is, therefore, a substantial unmet need for novel oral antibiotics in the treatment of uUTI. Despite this, relatively few novel-class antibiotics are in development, and < 5% of those are being studied by large pharmaceutical firms [13]. Furthermore, historical clinical trials of antibiotics for uUTI were conducted with protocols employing inclusion criteria and endpoints that were arguably less stringent than, and unaligned with, current regulatory guidance [14]. The European Medicines Agency (EMA) and FDA uUTI guidance were updated in 2018 and 2019, respectively, with requirements for inclusion of patients and the use of a composite primary endpoint including both microbiological eradication and complete clinical symptom resolution [15, 16]. Previous guidance did not advise the use of a composite endpoint, recommending microbiological eradication as the primary endpoint. Furthermore, while ≥ 105 colony-forming units (CFU)/mL is the criterion for microbiological evaluation, the cutoff for microbiological eradication was previously suggested as < 104 CFU/mL and the cutoff for microbiological eradication is now < 103 CFU/mL. A category of “improvement” was also previously allowed for clinical response; however, complete resolution of symptoms is now necessary.
Gepotidacin is a novel, bactericidal, first-in-class triazaacenaphthylene antibiotic that inhibits bacterial deoxyribonucleic acid (DNA) replication by a distinct mechanism of action [17, 18], which confers activity against most strains of target pathogens, such as E. coli, Staphylococcus saprophyticus, and Neisseria gonorrhoeae, including those resistant to current antibiotics [19–21]. In this article, we describe the rationale and design behind two ongoing phase III randomized comparator-controlled clinical trials [the Efficacy of Antibacterial Gepotidacin Evaluated (EAGLE)-2 (NCT04020341) and EAGLE-3 (NCT04187144) trials] of gepotidacin for the treatment of uUTI among adult and adolescent female participants, following the most recent EMA and FDA uUTI guidance documents for industry [15, 16]. Collectively, these trials are some of the largest ever conducted for an antibiotic targeting uUTI. Furthermore, these global trials are being conducted in the context of an unprecedented pandemic. During the unique circumstances created by the SARS-CoV-2 (COVID-19) pandemic, every effort was made to adhere to protocol-specified assessments for participants on study treatment, including follow-up assessments. Where this was not possible, measures were implemented to minimize the amount of time that participants spent at the clinic. Electronic consent and remote collection of study-related data were utilized where local regulations permitted.
Gepotidacin
Gepotidacin selectively inhibits bacterial DNA gyrase and topoisomerase IV by a unique mechanism, which is not utilized by any currently approved human therapeutic agents [13, 17, 18].
Nonclinical Activity and Efficacy Data
Gepotidacin has demonstrated in vitro activity against fastidious, nonfastidious, aerobic, and anaerobic Gram-positive and Gram-negative pathogens, including E. coli, and maintains activity against most isolates resistant to established antibacterial classes [19].
Dose Justification
The gepotidacin dose and duration for these studies was selected based on in vitro pharmacokinetic (PK)/pharmacodynamic (PD) and in vivo studies, including a rat pyelonephritis model simulating human PK exposures of gepotidacin to determine efficacy against isolates of E. coli, including MDR strains [19, 22–24].
A 5-day dosing duration aligns with current treatment guidelines for efficacious antibacterial treatment of uUTI, which typically ranges from 3 to 7 days [3, 4]. The safety and tolerability at this oral dose and duration have been evaluated in phase I and phase II studies [25]. Furthermore, high urine concentrations of gepotidacin are expected based on a healthy volunteer phase I study (NCT02853435) [26], and a phase II study (NCT03568942) in patients with uUTI [25] where gepotidacin urine exposures were found to exceed plasma levels by > 600-fold.
Safety
To date, oral and/or intravenous gepotidacin has been investigated in 15 completed clinical studies, in total, oral and intravenous gepotidacin has been administered to approximately totaling ~1200 study participants [25–33].
From these studies, reports of adverse events (AEs) were generally nonserious, and mild-to-moderate in intensity. In phase I and phase II studies, two and three serious AEs (SAEs) were reported, respectively, with none considered related to the study drug [25, 27, 29]. Across the previous clinical studies of gepotidacin, very common (≥ 10%) AEs were diarrhea and nausea, and common (≥ 1% and < 10%) AEs were vomiting and headache. Gastrointestinal (GI) tolerability has been seen to improve when gepotidacin is taken with food [34].
Concentration-dependent QT (QTc) prolongation has been observed during clinical trials with gepotidacin [31]; however, to date this mild QTc prolongation has not translated into clinically concerning QTc changes or cardiovascular AEs [30]. Predicted QTc interval prolongation from the thorough QT/QTc study approaches 20 ms with concentration ≥ 14 μg/mL; furthermore, mild and transient non-GI AEs and AEs potentially related to acetylcholinesterase inhibition have also been associated with maximum concentration (Cmax) ≥ 14 μg/mL [31]. Therefore, dosing to keep the geometric mean Cmax < 14 μg/mL is expected to minimize adverse effects of QT prolongation [31]. The oral, 1500 mg twice-daily (BID) dosing regimen has associated Cmax levels well under this threshold.
Efficacy
In a phase II single-center clinical study (NCT03568942), 22 adult female patients with uUTI were treated with oral gepotidacin 1500 mg BID for 5 days [25] and evaluated for PK and efficacy as inpatients. Of these patients, eight had qualifying baseline uropathogens (≥ 105 CFU/mL) for inclusion in the microbiological intent-to-treat population. At test-of-cure (TOC) on days 10–13, 86% (n = 19/22) had complete symptom resolution and 88% (n = 7/8) were considered a microbiological success (eradication). At follow-up, 82% (n = 18/22) had sustained complete symptom resolution and 75% (n = 6/8) were a microbiological success. Therapeutic success (combined microbiological and clinical success) was achieved in 75% (n = 6/8) at TOC and 63% (n = 5/8) at follow-up.
Trial Design
EAGLE-2 and EAGLE-3 are near-identical phase III, randomized, multicenter, parallel-group, double-blind, double-dummy, comparator-controlled, noninferiority studies in adolescent and adult female participants comparing the efficacy and safety of gepotidacin with nitrofurantoin in the treatment of uUTI. The comparator antibiotic in EAGLE-2 and EAGLE-3 is nitrofurantoin, which is recommended in both USA and European guidelines as a first-line empiric therapy for uUTI [3, 4]. EAGLE-2 examines the PK/PD properties of gepotidacin. EAGLE-2 is being conducted across approximately 95 sites in the USA, UK, Mexico, Spain, Germany, Greece, Hungary, Poland, Bulgaria, Romania, Slovakia, Czech Republic, and India; while EAGLE-3 is being conducted across approximately 110 sites in the USA, Australia, Bulgaria, India, South Korea, and Poland. Participating sites in both studies were community-based outpatient clinics.
The pragmatic study design, with ability to enroll at screening based on symptoms and urine dipstick or microscopy, of EAGLE-2 and EAGLE-3 is based on recent FDA guidance for industry for developing antibiotic treatments for uUTIs [15], the EMA addendum to the guideline on the evaluation of medicinal products indicated for treatment of bacterial infections [16], and feedback from the FDA and EMA.
Study Populations
Adults (≥ 18 years of age) providing written informed consent (and/or eConsent, if applicable) and adolescents (≥ 12 to < 18 years of age) providing written informed assent (or eConsent, if applicable) are eligible for the study if they are women with a body weight ≥ 40 kg and with at least two of the following symptoms of uUTI with onset < 96 h prior to study entry: dysuria, frequency, urgency, or lower abdominal pain, and dipstick evidence of nitrite or pyuria (3+/large leukocyte esterase); or pyuria by microscopy (> 15 white blood cell/high-power field) from a pretreatment clean-catch midstream urine sample.
While no upper age limit is placed on participants, residence in a nursing home or dependent-care-type facility is part of the exclusion criteria. Participants as young as 12 years of age are eligible, enabling adolescents to be included so as to better reflect the wide spectrum of female patients who may experience uUTIs and who may benefit from novel treatments such as gepotidacin.
Participants are excluded if they have specific urinary tract, renal, urogenital, cardiac, or hepatic medical conditions; are immunocompromised; require medication that may be impacted by inhibition of acetylcholinesterase; have acute porphyria; have any condition that may interfere with drug absorption, distribution, metabolism, or excretion of gepotidacin; have known glucose-6-phosphate dehydrogenase deficiency; have received treatment with other systemic antibiotics or antifungals within 1 week before study entry; were previously enrolled in a gepotidacin study; or are pregnant (Table 1 and Supplementary Material).
Table 1.
Schedule of study activities for EAGLE-2 and EAGLE-3
| Visita | Baseline | On-therapyb | TOCb | Follow-up | Early withdrawal | |
|---|---|---|---|---|---|---|
| Study day | 1 | 2–4 | 10–13 | 28 ± 3 | NA | |
| Procedure | Predose | Postdose | ||||
| Written informed consent/assent | X | – | – | – | – | – |
| Inclusion and exclusion criteria | X | – | – | – | – | – |
| Participant demography | X | – | – | – | – | – |
| Physical examination (including height and weight at Baseline only) | X | – | – | Xc | – | – |
| Record uUTI signs and symptomsd | X | – | X | X | X | X |
| Investigator assessment of clinical responsee | – | – | X | X | X | |
| Medical/surgical history | X | – | – | – | – | – |
| Diagnosis of presumptive uUTIf | X | – | – | – | – | – |
| Bacteriology samplesg | X | – | Xh | X | X | X |
| Randomization | X | – | – | – | – | – |
| 12-lead electrocardiogram | X | – | Xi | – | – | – |
| Vital sign measurementsj | X | – | X | X | – | – |
| Hematology, chemistry, and urinalysis | X | – | X | X | – | – |
| Serology (hepatitis B and C and HIV)k | X | – | – | – | – | – |
| Urine pregnancy testl | Xm | – | Xm | X | – | X |
| Drug and alcohol screen | X | – | – | – | – | – |
| UTI activity impairment assessmentl | X | – | X | X | X | X |
| Administer oral dose of study treatmentn | X | Xo | – | – | – | |
| Serious adverse eventsp | X | X | X | X | X | X |
| Adverse eventsq | X | X | X | X | X | |
| Concomitant medication review | X | X | X | X | X | X |
| Interactive response technology | X | – | – | – | – | – |
| PK blood sampler | – | – | Xs | – | – | – |
| PK urine sampler | – | – | Xs | – | – | – |
| Study treatment compliancet | – | – | X | X | – | – |
| Schedule next visit | Xu | – | Xv | Xv | Xv | – |
| Genetic samplew | X | – | – | – | – | – |
HIV human immunodeficiency virus, HPF high-power field, NA not applicable, PK pharmacokinetic, TOC test-of-cure, uUTI uncomplicated urinary tract infection, WBC white blood cell
aFor all study visits, to minimize the amount of time that participants spend at the clinic, eConsent may be utilized and remote collection of study-related data may take place. Thus, in some cases, visit data may be collected through a combination of telemedicine and the scheduled on-site study visit (note that telemedicine will not be used as a substitute for a scheduled on-site visit). Collection of information via telemedicine will be performed only where local regulations permit. Prescreening activities may also be conducted, including a prescreening informed consent and urine testing
bFor the on-therapy (days 2–4) visit: participants will be instructed to return to the study site within 1–3 days postrandomization. Each treatment day will be assessed over 24 h starting with the first dose of study treatment. For the TOC (days 10–13) visit: participants will be instructed to return to the study site 5–8 days after completion of study treatment
cAt the TOC visit, the physical examination may be symptom directed and is only required if indicated for a specific participant
dIndividual clinical signs and symptoms scores of uUTI will be recorded by a study physician or otherwise appropriately medically trained staff based on participant interview and using the scoring system (Table 2). The same scorer will be used at all assessment time points for each participant, on all occasions, whenever possible
eThe investigator will provide an assessment of clinical response (clinical success, clinical failure, or indeterminate) for each participant at the TOC and follow-up visits, and at early withdrawal (if applicable). This assessment should be completed after the clinical signs and symptoms score is determined by the same study physician or otherwise appropriately medically trained staff who performed the clinical scoring assessment
fBased on confirmation of nitrite or pyuria (> 15 WBC/HPF or the presence of 3+/large leukocyte esterase) from a pretreatment clean-catch midstream urine sample per local laboratory procedures
gParticipants will provide a clean-catch midstream urine sample at each scheduled on-site visit for Gram stain, quantitative bacteriology culture, and in vitro antimicrobial susceptibility testing by a designated laboratory(ies)
hA bacteriology urine sample will be collected at the on-therapy visit
iEAGLE-3 only
jTake measurement of temperature, blood pressure, and pulse rate
kIf serology testing was performed within 3 months prior to the first dose of study treatment and the results were positive, testing at baseline is not required. If testing was performed within 3 months and any result was negative, testing at baseline is required
lThe UTI activity impairment assessment will be administered to participants by study site staff at the baseline, on-therapy, TOC, and follow-up visits, and at early withdrawal (if applicable). This assessment should be done by a different study staff member than the person who determines the clinical signs and symptoms score, and should be performed at the end of the study visit
mFor women of childbearing potential, a negative high-sensitivity urine pregnancy test is sufficient for eligibility. Pregnancy testing should also be performed after dose 4 and before dose 8
nParticipants will receive oral study treatment twice daily for 5 days under double-blind, double-dummy conditions. The first oral dose will be administered at the study site during the baseline visit; participants will self-administer as outpatients thereafter. Each dose should be taken after food consumption and with water
oParticipants should continue taking study treatment per their planned dosing schedule. If at all possible, the appointment time of the on-therapy visit should be approximately 1–2 h after the participant’s most recent dose is expected to be taken
pRecord serious adverse events from the time of consent/assent to fulfill international regulatory requirements
qRecord adverse events from the time of the first dose of study treatment
rEAGLE-2 only
sAt the On-therapy visit, PK samples will be collected
tDetermine study treatment compliance by performing pill count
uConfirm return day/time for the on-therapy, TOC, and follow-up visits. Refer to footnote o for scheduling the on-therapy visit
vPrevisit reminder: study site staff will contact the participant 24 ± 4 h before the scheduled on-therapy, TOC, and follow-up visits
wCollect sample only if the participant has a signed consent/assent specific for this purpose. The baseline visit is the recommended time to collect the sample, but it can be collected at any time during the study
Study Procedures
For the purposes of randomization, participants are stratified by age category (< 18 years, ≥ 18 to 50 years, or > 50 years) and history of uUTI recurrence (recurrent infection defined as a confirmed infection with ≥ 1 prior episode within the past 3 months, ≥ 2 prior episodes within the past 6 months, or ≥ 3 prior episodes within the past 12 months before study entry).
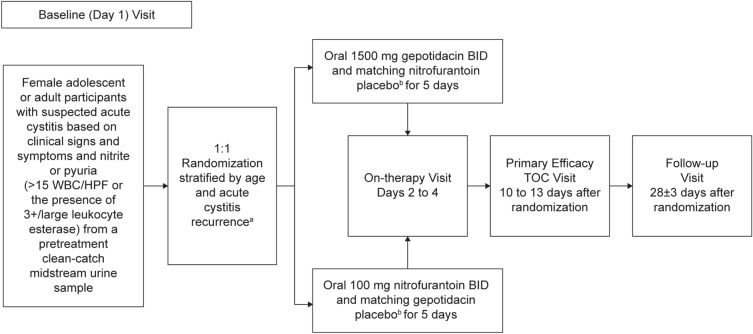
Participants are centrally randomized, in a 1:1 ratio to receive gepotidacin 1500 mg orally BID for 5 days or nitrofurantoin 100 mg orally BID for 5 days (Fig. 1). To maintain the double-blind nature of the study, participants receive a placebo in the form of the active treatment to which they have not been assigned. The first oral dose will be administered at the study site during the baseline visit (day 1) and subsequent doses will be self-administered by participants as outpatients.
Fig. 1.
EAGLE-2 and EAGLE-3 study design. aThere will be central randomization with stratification by age category (< 18 years, ≥ 18 to 50 years, or > 50 years) and uUTI recurrence [nonrecurrent infection or recurrent infection, defined as a confirmed infection (not including the current infection in the calculation) with at least one prior episode within the past 3 months, at least two prior episodes within the past 6 months, or at least three prior episodes within the past 12 months before study entry]. bStudy treatment will be administered under double-blind, double-dummy conditions. Each dose should be taken after food consumption and with water. BID twice daily, HPF high-power field, TOC test-of-cure, uUTI uncomplicated urinary tract infection, WBC white blood cell
Safety, clinical, and microbiological assessments are conducted at the baseline visit (day 1) and repeated at on-therapy (days 2–4), TOC (days 10–13), and follow-up (day 28 ± 3) visits (Table 1). Clinical signs and symptoms of uUTI will be recorded by appropriately trained medical staff based on participant interview per the schedule of activities (Table 1) using the scoring system in Table 2. At baseline, the participant must present with ≥ 2 signs and symptoms and have a total cumulative symptom score ≥ 2. The TOC assessment will be performed by the same investigator who conducted the baseline signs and symptoms assessment where possible and before any other questionnaire-based evaluations required by the protocol.
Table 2.
Clinical scoring tool for assessing clinical signs and symptoms of uUTI
| Clinical signs and symptoms | None | Mild Symptom is easily tolerated, causing minimal discomfort, and not interfering with everyday activities |
Moderate Symptom is sufficiently discomforting to interfere with normal everyday activities |
Severe Symptom prevents normal everyday activities |
|---|---|---|---|---|
| SCORE 0 | SCORE 1 | SCORE 2 | SCORE 3 | |
| Dysuria | ||||
| Frequency | ||||
| Urgency | ||||
| Lower abdominal or suprapubic pain |
uUTI uncomplicated urinary tract infection
To help minimize the amount of time that participants spend at the study site when attending the four scheduled study visits, some study-related data may be collected remotely using telemedicine (i.e., telemedicine used in parallel with, not in place of, on-site study visits). The collection of information via telemedicine will be performed only where local regulations permit.
In each of the studies, the planned enrollment of ~2500 participants aims to fulfill the target evaluable sample size of 884 participants in the analysis of the primary efficacy endpoint performed using the microbiological intent-to-treat nitrofurantoin-susceptible (micro-ITT NTF-S) population. The final number of randomized participants may vary based on the evaluability rate and qualifying bacterial uropathogens defined at ≥ 105 CFU/mL.
Objective and Endpoints
The primary objective of both trials is to assess the combined clinical and microbiological efficacy of gepotidacin versus nitrofurantoin at TOC, in female participants with uUTI and qualifying bacterial uropathogen(s) at baseline that are susceptible to nitrofurantoin.
Per FDA guidance, the primary endpoint is therapeutic response (combined per participant microbiological and clinical response) at TOC. Therapeutic success refers to patients who have been deemed both a microbiological success [reduction of all qualifying bacterial uropathogens (≥ 105 CFU/mL) recovered at baseline to < 103 CFU/mL at TOC as observed on quantitative urine culture without the patient receiving other systemic antibiotics] and a clinical success (normal presentation with resolution of baseline signs and symptoms with cumulative symptom score of 0 and no new signs and symptoms of uUTI and without the patient receiving other systemic antibiotics) at or prior to the TOC Visit in the micro-ITT NTF-S population, irrespective of pre-morbid symptomology and regardless of treatment discontinuation.
Secondary endpoints include components of the primary endpoint at TOC and follow-up, i.e., clinical outcome and response at TOC and follow-up visits, microbiological outcome and response at TOC and follow-up visits, therapeutic response at follow-up visit, gepotidacin plasma and urine concentrations (EAGLE-2 only), treatment-emergent AEs, SAEs, and change from baseline results for clinical laboratory tests, electrocardiograms (EAGLE-3 only), and vital sign measurements.
Details of PK/PD analyses and exploratory endpoints can be found in the Supplementary Material.
Safety
The severity of AEs and SAEs is determined by the investigator according to the US National Institute of Allergy and Infectious Diseases Division of Microbiology and Infectious Diseases criteria for adult toxicity assessment [35], with the exception of serum creatinine adolescent laboratory data, which will be assessed using pediatric toxicity criteria [36]. Predefined AEs of special interest for these studies are cardiovascular events, GI events, Clostridium difficile infection, and AEs potentially related to acetylcholinesterase inhibition. All reported AEs are coded using Medical Dictionary for Regulatory Activities and summarized by system organ class and preferred terms.
Ethical Considerations
These studies are conducted in accordance with the protocols and consensus ethical principles derived from international guidelines including the Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethical Guidelines, International Council on Harmonization Good Clinical Practice Guidelines, and applicable laws and regulations. Protocol and amendments, informed consent form/adolescent assent form/eConsent (if applicable), investigator’s brochure, and other relevant documents (e.g., advertisements) were submitted to an institutional review board/independent ethics committee (IRB/IEC) by the investigator and reviewed and approved by the IRB/IEC before the study was initiated. The master IRB was Advarra Institutional Review Board (Columbia, MD, USA), other IRBs/IECs are listed in Supplementary Table 1. Adolescent participants will only be enrolled at study sites where investigators have experience in this population and if allowed per the study site’s institutional ethics committees and local country/national regulatory guidelines; enrollment will be contingent upon such approvals.
Statistical Considerations
These studies are designed to demonstrate noninferiority of gepotidacin to nitrofurantoin for the primary efficacy endpoint. A noninferiority margin of –10% is used in accordance with current FDA guidance [37]. If noninferiority is declared, superiority will be assessed. Assuming a 76% therapeutic success rate for both nitrofurantoin and gepotidacin, a sample size of 884 participants (allowing for an interim analysis) in the micro-ITT NTF-S population is required to provide approximately 90% power to demonstrate noninferiority in the therapeutic response rate of gepotidacin compared with nitrofurantoin with a 0.025 one-sided alpha level and a −10.0% noninferiority margin. Qualifying uropathogen susceptibilities for the micro-ITT NTF-S population will be monitored in stream to help ensure sufficient and balanced enrollment of participants into the studies.
Discussion
The development of gepotidacin is important in the fight against antibiotic resistance, providing a unique mechanism of action that is effective against most target pathogens resistant to many currently available antibiotics. The phase III uUTI trials of gepotidacin, among the largest planned trials for the antibiotic treatment of uUTI ever performed, have been designed in accordance with FDA and EMA uUTI guidance and in consultation with these organizations. These trials are taking place during the COVID-19 pandemic, which places additional challenges on data collection and study processes (e.g., the switch from face-to-face consultation to telemedicine for the management of community-based infections); the challenges have been mitigated by protocol amendments allowing for remote collection of data and reduced time required for site visits.
Notably, the primary endpoint of therapeutic response combines clinical and microbiological assessment, providing a composite endpoint which avoids potential limitations of each individual assessment [15, 16]. Clinical success relies on investigator assessment and patient-reported symptoms that are somewhat subjective, employing an instrument that is not fully validated (in terms of internal consistency, symptoms assessed, and relationship to other measures of treatment success). This method of measuring clinical response is, however, similar to previous studies with clinical success as an endpoint [38]. Microbiological success is objective but relies on culture of the causative pathogen at baseline and reproducible culture at later visits, and it does not necessarily correlate with clinical success, which is the ultimate aim of therapy, nor is it representative of clinical management in the real world, where symptom resolution is a key marker of treatment success. A urine culture defined by ≥ 105 CFU/mL of a uropathogen and more stringent assessment of microbiological success than used in historical studies of reduction to < 103 CFU/mL is being used for primary population definition and endpoint analysis [15, 16]. Additionally, earlier trials included an “improvement” category in assessing clinical response, while new guidance requires complete resolution of symptoms to achieve clinical success; this does not consider the presence of baseline symptoms in patients who may not be fully asymptomatic between uUTI episodes. The greater rigor required of new agents by regulatory bodies may reflect that uUTIs often self-resolve, or require over-the-counter treatment only. Ibuprofen, for example, can be used to manage the symptoms of uUTI until resolution; however, the time to resolution is longer, the symptom burden is larger, and the likelihood of patients developing pyelonephritis is greater than with antibiotics [39, 40]. Treating to manage the symptoms of uUTI is, therefore, often inadequate for reducing the burden of disease, and in clinical trials many patients still required antibiotics as secondary therapy [39, 40]. Importantly, some data suggest that the more stringent clinical trial guidelines for uUTIs could yield less robust findings versus historical studies, and between-study comparisons should be made with caution [14].
Among bacterial topoisomerase inhibitors, gepotidacin is structurally and mechanistically unique, inhibiting gyrase-catalyzed DNA supercoiling and relaxation of positive supercoiled substrates via single-stranded DNA breaks mediated by gyrase [17–19]. The comparator antibiotic in EAGLE-2 and EAGLE-3 is nitrofurantoin, which is recommended in both USA and European guidelines as a first-line empiric therapy for uUTI [3, 4]. FDA guidelines advise that for noninferiority trials, isolates susceptible to the investigational drug and comparator are studied [15]. Nitrofurantoin has relatively low rates of resistance in the community setting, meaning that a greater number of patients have susceptible isolates compared with other antibiotics such as SXT where rates of resistance are very high in some areas [5, 41]. Rates of resistance are also high for fluoroquinolones [5, 41], and the use of these antibiotics is no longer recommended for the treatment of uUTI following safety concerns [42]. Nitrofurantoin was, therefore, considered the most clinically feasible and appropriate comparator for gepotidacin.
Gepotidacin has the potential to become an important oral antibiotic in the treatment of uUTI, and its evaluation in phase III trials is an important step in addressing the critical need for novel oral antibiotics that are effective against uropathogens resistant to current treatments.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
These studies and journal’s Rapid Service Fee are funded by GSK (EAGLE-2: GSK study 204989, NCT04020341; EAGLE-3: GSK study 212390, NCT04187144). Studies were also supported in whole or in part with Federal funds from the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority (HHSO100201300011C).
Medical writing, editorial, and other assistance
Medical writing support for the development of this manuscript, under the guidance of the authors, was provided by Fraser Shearer, PhD, of Ashfield MedComms, Manchester, UK, an Inizio company and was funded by GSK. The authors would like to thank PPD, part of Thermo Fisher Scientific, for its role in running the clinical trials.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author contributions
Caroline Perry: Concept and/or design of the work, Drafting the manuscript and/or revising it critically for important intellectual content; Mohammad Hossain: Concept and/or design of the work, Drafting the manuscript and/or revising it critically for important intellectual content; Marcy Powell: Concept and/or design of the work; Drafting the manuscript and/or revising it critically for important intellectual content; Aparna Raychaudhuri: Concept and/or design of the work; Drafting the manuscript and/or revising it critically for important intellectual content; Nicole Scangarella-Oman: Concept and/or design of the work, Drafting the manuscript and/or revising it critically for important intellectual content; Courtney Tiffany: Concept and/or design of the work, Drafting the manuscript and/or revising it critically for important intellectual content; Sherry Xu: Concept and/or design of the work, Drafting the manuscript and/or revising it critically for important intellectual content; Etienne Dumont: Concept and/or design of the work, Drafting the manuscript and/or revising it critically for important intellectual content; Salim Janmohamed: Concept and/or design of the work, Drafting the manuscript and/or revising it critically for important intellectual content.
Disclosures
Caroline Perry—employee of and shareholder in GSK. Mohammad Hossain—employee of GSK at time of study and current shareholder in GSK; currently an employee of Servier Pharmaceuticals, Boston, MA, USA. Marcy Powell—employee of and shareholder in GSK. Aparna Raychaudhuri—employee of GSK at time of study and current shareholder in GSK; currently an employee of CSL Behring, King of Prussia, PA, USA. Nicole Scangarella-Oman—employee of and shareholder in GSK. Courtney Tiffany—employee of GSK at time of study and current shareholder in GSK; currently an employee of Spark Therapeutics, Inc., Philadelphia, PA, USA. Sherry Xu—employee of and shareholder in GSK at time of study; currently an employee of IQVIA, Plymouth Meeting, PA, USA. Etienne Dumont—employee of GSK at time of study and current shareholder in GSK; currently an employee of Boston Pharmaceuticals, Cambridge, MA, USA. Salim Janmohamed—employee of and shareholder in GSK.
Compliance with ethics guidelines
These studies are conducted in accordance with the protocols and consensus ethical principles derived from international guidelines including the Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethical Guidelines, International Council on Harmonization Good Clinical Practice Guidelines, and applicable laws and regulations. Protocol and amendments, informed consent form/adolescent assent form/eConsent (if applicable), investigator’s brochure, and other relevant documents (e.g., advertisements) were submitted to an institutional review board/independent ethics committee (IRB/IEC) by the investigator and reviewed and approved by the IRB/IEC before the study was initiated. The master IRB was Advarra Institutional Review Board (Columbia, MD, US), other IRBs/IECs are listed in Supplementary Table 1. Adolescent participants will only be enrolled at study sites where investigators have experience in this population and if allowed per the study site’s institutional ethics committees and local country/national regulatory guidelines; enrollment will be contingent upon such approvals.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Footnotes
Mohammad Hossain, Aparna Raychaudhuri, Courtney Tiffany, Sherry Xu, Etienne Dumont: At time of study.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
2/25/2023
A peer-reviewed video abstract was retrospectively added to this publication.
Contributor Information
Caroline Perry, Email: caroline.r.perry@gsk.com.
Salim Janmohamed, Email: salim.g.janmohamed@gsk.com.
References
- 1.Medina M, Castillo-Pino E. An introduction to the epidemiology and burden of urinary tract infections. Ther Adv Urol. 2019;11:1756287219832172. doi: 10.1177/1756287219832172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010;7:653–660. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 3.Bonkat G, Bartoletti R, Bruyère F, et al. Urological infections. Arnhem: EAU Guidelines Office; 2020. [Google Scholar]
- 4.Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 5.Kaye KS, Gupta V, Mulgirigama A, et al. Antimicrobial resistance trends in urine Escherichia coli isolates from adult and adolescent females in the United States from 2011–2019: rising ESBL strains and impact on patient management. Clin Infect Dis. 2021;73:1992–1999. doi: 10.1093/cid/ciab560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Ten threats to global health in 2019. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019. Accessed 1 Mar 2022.
- 7.Centers for Disease Control and Prevention . Antibiotic Resistance Threats in the United States, 2019. Atlanta: US Department of Health and Human Services; 2019. [Google Scholar]
- 8.World Health Organization . Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. Geneva: World Health Organization; 2017. [Google Scholar]
- 9.Nicolas-Chanoine MH, Bertrand X, Madec JY. Escherichia coli ST131, an intriguing clonal group. Clin Microbiol Rev. 2014;27:543–574. doi: 10.1128/CMR.00125-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Food and Drug Administration. Drugs@FDA: FDA-approved drugs. https://www.accessdata.fda.gov/scripts/cder/daf/. Accessed 7 Oct 2021.
- 11.Lodise TP, Henriksen AS, Hadley T, Patel N. US-focused conceptual health care decision-analytic models examining the value of pivmecillinam relative to current standard-of-care agents among adult patients with uncomplicated urinary tract infections due to Enterobacterales. Open Forum Infect Dis. 2021;8:ofab380. doi: 10.1093/ofid/ofab380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Health Canada. Selexid product information. https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=94365. Accessed 7 Oct 2021.
- 13.The Pew Charitable Trusts. Tracking the global pipeline of antibiotics in development, March 2021. https://www.pewtrusts.org/en/research-and-analysis/issue-briefs/2021/03/tracking-the-global-pipeline-of-antibiotics-in-development. Accessed 7 Oct 2021.
- 14.Henriksen AS, Nicolle L, Das AF. Impact of 2019 US Food and Drug Administration (FDA) guidance on developing drugs for urinary tract infection (UTI) on the perceived efficacy of antibiotics for the treatment of uncomplicated UTI (uUTI) [abstract 1433] Open Forum Infect Dis. 2021;8(Suppl 1):S798. doi: 10.1093/ofid/ofab466.1625. [DOI] [Google Scholar]
- 15.US Food and Drug Administration . Uncomplicated urinary tract infections: developing drugs for treatment guidance for industry. Beltsville: US Department of Health and Human Services; 2019. [Google Scholar]
- 16.European Medicines Agency. Guideline on the evaluation of medicinal products indicated for treatment of bacterial infections, Rev 3. https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-evaluation-medicinal-products-indicated-treatment-bacterial-infections-revision-3_en.pdf. Accessed 1 Feb 2022.
- 17.Bax BD, Chan PF, Eggleston DS, et al. Type IIA topoisomerase inhibition by a new class of antibacterial agents. Nature. 2010;466:935–940. doi: 10.1038/nature09197. [DOI] [PubMed] [Google Scholar]
- 18.Gibson EG, Bax B, Chan PF, Osheroff N. Mechanistic and structural basis for the actions of the antibacterial gepotidacin against Staphylococcus aureus gyrase. ACS Infect Dis. 2019;5:570–581. doi: 10.1021/acsinfecdis.8b00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biedenbach DJ, Bouchillon SK, Hackel M, et al. In vitro activity of gepotidacin, a novel triazaacenaphthylene bacterial topoisomerase inhibitor, against a broad spectrum of bacterial pathogens. Antimicrob Agents Chemother. 2016;60:1918–1923. doi: 10.1128/AAC.02820-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobsson S, Golparian D, Scangarella-Oman N, Unemo M. In vitro activity of the novel triazaacenaphthylene gepotidacin (GSK2140944) against MDR Neisseria gonorrhoeae. J Antimicrob Chemother. 2018;73:2072–2077. doi: 10.1093/jac/dky162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scangarella-Oman N, Hossain M, Tiemeyer TJ Jr, et al. Microbiological analysis from a phase IIa study evaluating gepotidacin (GSK2140944) in the treatment of uncomplicated urinary tract infections. In: IDWeek 2019; 2019 October 2–6. Washington DC, USA.
- 22.Hoover JL, Singley CM, Elefante P, Rittenhouse S. Efficacy of human exposures of gepotidacin (GSK2140944) against Escherichia coli in a rat pyelonephritis model. Antimicrob Agents Chemother. 2019;63:e00086–e119. doi: 10.1128/AAC.00086-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VanScoy BD, Lakota EA, Conde H, et al. Gepotidacin pharmacokinetics-pharmacodynamics against Escherichia coli in the one-compartment and hollow-fiber in vitro infection model systems. Antimicrob Agents Chemother. 2021;65:e0012221. doi: 10.1128/AAC.00122-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scangarella-Oman NE, Hossain M, Hoover JL, et al. Dose selection for phase III clinical evaluation of gepotidacin (GSK2140944) in the treatment of uncomplicated urinary tract infections. Antimicrob Agents Chemother. 2022;66:e0149221. doi: 10.1128/aac.01492-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Overcash JS, Tiffany CA, Scangarella-Oman NE, et al. Phase 2a pharmacokinetic, safety, and exploratory efficacy evaluation of oral gepotidacin (GSK2140944) in female participants with uncomplicated urinary tract infection (acute uncomplicated cystitis) Antimicrob Agents Chemother. 2020;64:e00199–e220. doi: 10.1128/AAC.00199-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barth A, Hossain M, Brimhall DB, et al. Pharmacokinetics of oral formulations of gepotidacin (GSK2140944), a triazaacenaphthylene bacterial type II topoisomerase inhibitor, in healthy adult and adolescent participants. Antimicrob Agents Chemother. 2021;66:e0126321. doi: 10.1128/AAC.01263-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hossain M, Tiffany C, Tao Y, et al. Pharmacokinetics of gepotidacin in subjects with normal hepatic function and hepatic impairment. Clin Pharmacol Drug Devel. 2021;10:588–597. doi: 10.1002/cpdd.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hossain M, Tiffany C, Raychaudhuri A, et al. Pharmacokinetics of gepotidacin in renal impairment. Clin Pharmacol Drug Devel. 2020;9:560–572. doi: 10.1002/cpdd.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Riordan W, Tiffany C, Scangarella-Oman N, et al. Efficacy, safety, and tolerability of gepotidacin (GSK2140944) in the treatment of patients with suspected or confirmed Gram-positive acute bacterial skin and skin structure infections. Antimicrob Agents Chemother. 2017;61:e02095–e2116. doi: 10.1128/AAC.02095-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor SN, Morris DH, Avery AK, et al. Gepotidacin for the treatment of uncomplicated urogenital gonorrhea: a phase 2, randomized, dose-ranging, single-oral dose evaluation. Clin Infect Dis. 2018;67:504–512. doi: 10.1093/cid/ciy145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hossain M, Zhou M, Tiffany C, Dumont E, Darpo B. A phase I, randomized, double-blinded, placebo- and moxifloxacin-controlled, four-period crossover study to evaluate the effect of gepotidacin on cardiac conduction as assessed by 12-lead electrocardiogram in healthy volunteers. Antimicrob Agents Chemother. 2017;61:e02385–e2416. doi: 10.1128/AAC.02385-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Negash K, Andonian C, Felgate C, et al. The metabolism and disposition of GSK2140944 in healthy human subjects. Xenobiotica. 2016;46(8):683–702. doi: 10.3109/00498254.2015.1112933. [DOI] [PubMed] [Google Scholar]
- 33.Tiffany C, Dumont EF, Hossain M, et al. Pharmacokinetics, safety, and tolerability of gepotidacin administered as single or repeat ascending doses, in healthy adults and elderly subjects. Clin Transl Sci. 2022 doi: 10.1111/cts.13359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tiffany CA, Hossain M, McDonald M, Dumont EF. Effect of food on safety, tolerability and pharmacokinetics in healthy volunteers >65 years following multiple doses of GSK2140944, a novel bacterial topoisomerase inhibitor. In: 55th Interscience conference of antimicrobial agents and chemotherapy; 2015 September 17–21. San Diego, CA, USA.
- 35.US National Institute for Allergy and Infectious Diseases Division of Microbiology and Infectious Diseases . Adult toxicity table. Bethesda: US Department of Health and Human Services; 2007. [Google Scholar]
- 36.US National Institute for Allergy and Infectious Diseases Division of Microbiology and Infectious Diseases . Pediatric Toxicity Table. Bethesda: US Department of Health and Human Services; 2007. [Google Scholar]
- 37.U.S. Food and Drug Administration. Non-Inferiority Clinical Trials to Establish Effectiveness. Guidance for Industry. 2016. https://www.fda.gov/media/78504/download. Accessed 2 Aug 2022.
- 38.Nicolle LE, Madsen KS, Debeeck GO, et al. Three days of pivmecillinam or norfloxacin for treatment of acute uncomplicated urinary infection in women. Scand J Infect Dis. 2002;34:487–492. doi: 10.1080/00365540110080728. [DOI] [PubMed] [Google Scholar]
- 39.Gágyor I, Bleidorn J, Kochen MM, Schmiemann G, Wegscheider K, Hummers-Pradier E. Ibuprofen versus fosfomycin for uncomplicated urinary tract infection in women: randomised controlled trial. BMJ. 2015;351:h6544. doi: 10.1136/bmj.h6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vik I, Bollestad M, Grude N, et al. Ibuprofen versus pivmecillinam for uncomplicated urinary tract infection in women—a double-blind, randomized non-inferiority trial. PLOS Med. 2018;15:e1002569. doi: 10.1371/journal.pmed.1002569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanchez GV, Babiker A, Master RN, Luu T, Mathur A, Bordon J. Antibiotic resistance among urinary isolates from female outpatients in the United States in 2003 and 2012. Antimicrob Agents Chemother. 2016;60:2680–2683. doi: 10.1128/AAC.02897-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yarrington ME, Anderson DJ, Dodds Ashley E, et al. Impact of FDA black box warning on fluoroquinolone and alternative antibiotic use in southeastern US hospitals. Infect Control Hosp Epidemiol. 2019;40:1297–1300. doi: 10.1017/ice.2019.247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.