Abstract
The health risks associated with consumption of water from river Gomti polluted with potentially toxic elements (PTEs), including As, Fe, Pb, Cd, Mn, Cr, Ni, and Hg were investigated at the initiation of unlocking of COVID-19 lockdown and compared with pre-COVID-19 lockdown status. In the current investigation, the total hazard index (THI) values exceeded the acceptable limit of “unity” at all sampling stations. The use of river water for drinking and domestic purposes by millions of people with high THI values has emerged as a matter of huge concern. The individual hazard quotients associated with Cd and Pb were found to be most severe (> 1). A vivid difference between the THI values during the two study phases indicated the positive impact of COVID-19 lockdown signifying the prominent impact of anthropogenic activities on the PTE concentrations. The closure of local manufacturing units (textile, battery, etc.) emerged as a potential reason for decreased health risks associated with PTE levels. The higher susceptibility of children to health risks in comparison with adults through the values of THI and HQs was interpreted across the study area. Potential remedial measures for PTE contamination have also been suggested in the study.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10661-022-10562-2.
Keywords: COVID-19, Lockdown, River Gomti, Lucknow city, Hazard quotient
Introduction
Human existence and growth have been witnessed in the vicinity of river channels since the advent of settlements (Shukla & Ganguly, 2020; Shukla et al., 2020). Rivers have been recognized as very effective indicators of variation in the natural state of a landscape. The conducive impact of natural and anthropogenic processes on the water quality of surface water sources has been a matter of concern across the globe (Panneerselvam et al., 2021). The rapid pace of development through urbanization and industrialization for fulfilling the growing demands consequent to population growth is a serious concern in developing nations (Haji et al., 2021; Shukla & Saxena, 2020a). The surface sources of water have visibly undergone quantitative and qualitative deterioration consequent to industrial discharges. The release of untreated and partially treated domestic and industrial effluents has led to elevated levels of potentially toxic elements (PTEs) in water bodies. The noxious nature of PTEs is a probable health hazard for all biotic components of our environment (Soujanya Kamble et al., 2020). The lack of proper sanitation facilities and wastewater remediation systems intensifies the health risk hazard associated with PTEs (Kumar et al., 2021). The assessment of potential health risks associated with PTEs in drinking water enables the consumers, involved stakeholders, policymakers, and researchers to understand and evaluate the health hazards and, subsequently, propose suitable mitigation measures. Since, water from river Gomti is utilized for drinking, and other domestic purposes, the human health risk assessment for the river water becomes very vital and critical for the residents. The development of riverfront along river Gomti in Lucknow city has cut off the possibility of sorption of PTEs on the natural river bank sediments. River bank sediments act as a potential sink for PTEs, but due to the concrete lining along the river channel, this natural mechanism has been totally disrupted. Instead, the vehicular movement, organization of fairs, exhibitions in the vicinity of developed riverfront has led to increased sources of river water pollution, and halted natural processes in the riverine ecosystem.
The novel coronavirus (SARS-CoV-2) has emerged as a major challenge for both developing and developed nations across the world (Khan et al., 2021a; Shukla et al., 2021c). A nationwide lockdown was extended in multiple phases from 24 March 2020 to 31 May 2020, triggering research on assessing the impact of lockdown on natural resources. There were various reports stating the positive impact of lockdown on the quality of water resources, including surface and groundwater (Dutta et al., 2020; Selvam et al., 2020). Several studies and reports also stated water quality depletion in various rivers (Ganga, Beas, Chambal, Sutlej, Svarnarekha, and Gomti) during the lockdown period (DTE, 2020; Khan et al., 2021b). However, none of these studies have reported the possible human health risks and the impacts of COVID-19 lockdown on the concentration of these PTEs in the river water with potential measures towards remediation.
The removal techniques for heavy metal contamination primarily include sorption (Pal et al., 2021; Rahmani-Sani et al., 2020), chemical precipitation (Pohl, 2020; Zhang & Duan, 2020), using filtration membrane (Azad & Mohsennia, 2020; Peng & Guo, 2020), catalytic degradation (Dayanidhi et al., 2020; Zhang et al., 2020), ion-exchange processes (Kobayashi et al., 2020; Park et al., 2020), solvent extraction (Halli et al., 2020; Pino et al., 2020), and oxidation–reduction reactions (Tofighy & Mohammadi, 2020; Xiao et al., 2020). Among all these methods, sorption is one of the most commonly accepted and used technique for removal of heavy metals owing to low operating cost, simple and eco-friendly operation, and easy regeneration methods for the adsorbents (Ke et al., 2021; Maranhão et al., 2021). However, with the advent of technology, the use of novel nanomaterials, demonstrating superior removal performance, is also increasing. Nanoscale zero-valent irons (nZVI), metal–organic frameworks (MOFs), MXenes, etc. have offered varying degrees of mobility, high reactivity, low toxicity, and large surface areas and, hence, are very effective in reducing PTEs from the environment (Esrafili et al., 2021; Gan et al., 2020; Liang et al., 2021).
Thus, in the present study, we attempt to analyze the health risks due to PTE pollution in river Gomti through non-carcinogenic health risk assessment for both children and adults. Efficient techniques for removal of PTEs, using novel-nanomaterials, are also suggested. This study also aims to provide insights for establishing baseline database, which will prove to be a resourceful reference for future studies across the globe for the evaluation of the impacts of lockdown and unlocking on surface water bodies.
Methodology
Study area
The Gomti River Basin (GRB) is in the north-west parts of the Indo-Gangetic alluvial plain and has a catchment area of 30,437 km2. The slope of GRB is generally flat, with the altitude varying between 200m and 62m above the mean sea level (Khan et al., 2021a, b, c). GRB can be characterized with low surface runoff and low water storage capacity. GRB can be suitably divided into two basic geological units, viz. younger alluvium and older alluvium of quaternary age. The older alluvial plain lies in the higher elevations, underlain by thick alluvial deposits which comprise clay, kankar, and sand, whereas the younger is alluvium which lies all along the river forming a wide flood plain supporting agricultural activities as well. River Gomti originates from Fulhar lake (near Madho Tanda, Pilibhit), traversing a distance of more than 960 km through the state of Uttar Pradesh, and meets river Ganga in Ghazipur city. River Gomti, a rain- and groundwater-fed river, is more like a thin stream until it reaches Mohamadi, a town about 100 km downstream the origin, where Saryu river joins it (Khan et al., 2022). Another major tributary, river Sai, joins the river Gomti near Jaunpur city. After traversing for about 240 km, the river enters Lucknow city, where water is lifted from the river to cater to the city’s water supply at Aishbagh waterworks. The discharge of river Gomti is very low during the summers (30 m3/s), which gets as high as 600 m3/s during the monsoon season. The mineralogical composition of the bedload sediments constitutes of quartz (the highest), mica, feldspar, and rock fragments, with illite being the major clay mineral (Khan et al., 2022).
The city of Lucknow with a population of 3.5 million (Census, 2011) is situated at the bank of river Gomti in the central parts of Indo-Gangetic alluvial plain between 26.30°–27.10°N and 80.30°–81.13° E. The city’s terrain is almost flat, with depression recorded in the north-east parts of the city, the highest elevation of 123.5 m above mean sea level (msl) and a lowest elevation of 110 m above msl in the flood plain of the river. River Gomti flows from north-west (Gaughat) towards south-east (Pipraghat) parts of the city. The cool, dry winters in the city are experienced in the months of December to February, whereas hot summers in the months of April to June. River Gomti not only acts as a major drinking water source in Lucknow city, but also serves as a major source of industrial and agricultural water usage. Increasing population and subsequently increasing domestic water demand, industrial and agricultural activities have created immense pressure on the river.
Sampling and analysis
Sampling campaign
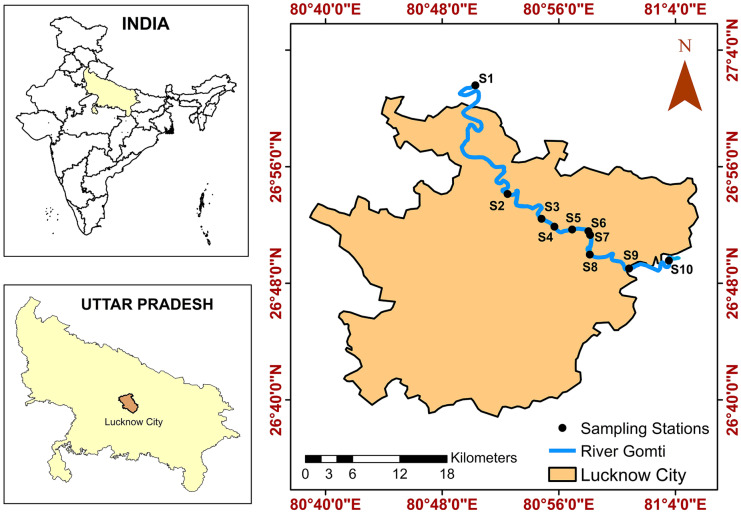
In the current study, thirty samples (three samples at each sampling station) were collected across a total stretch of ~ 61 km from River Gomti in the monsoon season (year 2020), successive to the COVID-19 unlocking. The sampling stations included S1 (Chandrika Devi), S2 (IIM Road), S3 (Harding Bridge), S4 (Arti Sthal), S5 (UP RERA), S6 (Kukrail Drain-Gomti Confluence), S7 (Gomti Barrage), S8 (Dilkusha Bridge), S9 (Shahid Path), and S10 (Bharwara STP discharge point-Gomti Confluence). The sampling stations are illustrated in Fig. 1 and Fig. S1.
Fig. 1.
Sampling locations in the study area
Quality assurance of results
Strict quality control and assurance standards were maintained through analytical-grade chemicals with more than 97% purity (Sigma-Aldrich). All the glassware were soaked in diluted nitric acid solution (1%) followed by cleaning using distilled water. The collection, storage, and analysis of water samples were done in strict accordance with (APHA, 2012). The samples were collected in high-density polyethylene bottles, using a cylindrical bailer with a rope attached to it. The data quality was maintained through blank, duplicate, and procedural spiked samples. To prevent any inaccuracy during analysis, an internal quality assurance system is implemented in the laboratory regularly. Approximately 2 mL of 65% nitric acid (HNO3) was added to individual water samples at all sites to prevent precipitation of metals and stored in an incubator at 4 °C.
Analytical methods
The levels of PTEs arsenic (As), iron (Fe), cadmium (Cd), lead (Pb), manganese (Mn), chromium (Cr), nickel (Ni), and mercury (Hg) in all the water samples after digestion were analyzed through atomic absorption spectrophotometer. The PTEs were selected for analysis in coherence with the possible anthropogenic intrusion, with reference to results of previous studies on surface water, and availability of laboratory resources.
Non-carcinogenic human health risk assessment
The non-degradable nature of PTEs and the subsequent possibility of their bioaccumulation and biomagnification make assessment of their impact on human health very critical. The PTEs may enter the human body through various pathways such as inhalation (through air), dermal contact, food chain (through biomagnification), and oral intake (through drinking water) (Shukla & Saxena, 2020a, b), where oral and dermal pathways pose the maximum risk. Non-carcinogenic human health risk assessment (HHRA) is an efficient technique to assess the probable risk of exposure of these PTEs on humans. The health risk assessment helps in providing valuable insights to propose suitable remedial actions. The potential non-carcinogenic health risks through oral intake of the PTEs can be done through estimation of hazard quotients (HQs) and total hazard index (THI). Initially, the chronic daily dose (CDD) of the PTEs through drinking water was computed using Eq. (1) (Shukla & Saxena, 2020a, c; USEPA, 1989; Wang et al., 2020).
| 1 |
where Cw (expressed in µg/L) is the concentration of a particular PTE in water, DI (expressed in L/day) is considered the daily average intake of water in the study region, EF (expressed in days/year) is the annual exposure frequency of the intake source, EP (expressed in years) is the total exposure period, BW (expressed in kg/person) is the average body weight, and AT (expressed in days) is the average time.
The values of DI, EP, and BW vary greatly according to the region’s climatic conditions and residents’ habit under consideration. In this study, the values of these parameters were taken from previous studies (Adimalla & Rajitha, 2018; Rishi et al., 2020; Shukla & Saxena, 2020b) for the arid-climatic conditions which prevail in northern India. Further, HQ was computed using the following equation:
| 2 |
where the oral toxicity reference dose values (RfD) can be taken as 0.3 μg/kg/day for As, 700 μg/kg/day for Fe, 0.5 μg/kg/day for Cd, 1.4 μg/kg/day for Pb, 24 μg/kg/day for Mn, and 3 μg/kg/day for Cr, in strict accordance with previous studies (Muhammad et al., 2011; USEPA, 2005). In general, the hazard quotient expresses the potential exposure towards a substance and the levels at which no adverse effects are expected on human health. Hence, when the value of HQ is less than unity, water can be assumed to be safe for the residents in the study area (Li et al., 2018). In the final step, the THI is calculated for various PTEs, which helps in recognition and quantification of the probable non-carcinogenic health risks. It is computed through Eq. (3):
| 3 |
Further, the average values of hazard quotient for all the parameters calculated for children and adults were subjected to cluster analysis through “Q-mode Hierarchical Cluster Analysis” (Q-HCA). In a Q-HCA, the similarities/dissimilarities among the dataset are used to classify the datasets into various clusters. These clusters represent the sites/variables with similar chemical composition and geochemistry (Selvakumar et al., 2017; Shukla & Saxena, 2020d). Ward’s linkage with squared Euclidean distance was incorporated for the assessment of Q-HCA, and the results are represented through dendrogram (Table 1).
Table 1.
Hazard quotient (HQ) for adults and children with respect to different PTEs at all the sampling locations
| Sites | HQ (As) | HQ (Fe) | HQ (Cd) | HQ (Pb) | HQ (Mn) | HQ (Cr) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adults/Children | Adults | Children | Adults | Children | Adults | Children | Adults | Children | Adults | Children | Adults | Children |
| S1 | 0.000 | 0.000 | 0.015 | 0.020 | 0.015 | 0.021 | 1.236 | 1.671 | 0.019 | 0.026 | 0.012 | 0.016 |
| S2 | 1.090 | 1.473 | 0.005 | 0.007 | 0.385 | 0.520 | 1.291 | 1.746 | 0.021 | 0.028 | 0.038 | 0.052 |
| S3 | 0.923 | 1.248 | 0.007 | 0.010 | 2.308 | 3.120 | 2.418 | 3.269 | 0.136 | 0.184 | 0.044 | 0.059 |
| S4 | 0.000 | 0.000 | 0.006 | 0.008 | 2.231 | 3.016 | 2.170 | 2.934 | 0.127 | 0.171 | 0.037 | 0.050 |
| S5 | 1.038 | 1.404 | 0.010 | 0.013 | 3.846 | 5.200 | 2.610 | 3.529 | 0.128 | 0.173 | 0.027 | 0.036 |
| S6 | 0.974 | 1.317 | 0.009 | 0.013 | 2.538 | 3.432 | 2.253 | 3.046 | 0.136 | 0.184 | 0.269 | 0.364 |
| S7 | 0.000 | 0.000 | 0.008 | 0.011 | 2.154 | 2.912 | 2.610 | 3.529 | 0.016 | 0.022 | 0.244 | 0.329 |
| S8 | 0.269 | 0.364 | 0.005 | 0.007 | 0.231 | 0.312 | 2.253 | 3.046 | 0.016 | 0.022 | 0.109 | 0.147 |
| S9 | 1.667 | 2.253 | 0.009 | 0.012 | 0.308 | 0.416 | 2.363 | 3.194 | 0.104 | 0.141 | 0.101 | 0.137 |
| S10 | 1.795 | 2.427 | 0.009 | 0.013 | 1.000 | 1.352 | 3.846 | 5.200 | 0.136 | 0.184 | 0.095 | 0.128 |
| Mean | 0.776 | 1.049 | 0.008 | 0.011 | 1.502 | 2.030 | 2.305 | 3.116 | 0.084 | 0.114 | 0.098 | 0.132 |
| Minimum | 0.000 | 0.000 | 0.005 | 0.007 | 0.015 | 0.021 | 1.236 | 1.671 | 0.016 | 0.022 | 0.012 | 0.016 |
| Maximum | 1.795 | 2.427 | 0.015 | 0.020 | 3.846 | 5.200 | 3.846 | 5.200 | 0.136 | 0.184 | 0.269 | 0.364 |
Results and discussion
Spatial distribution of potentially toxic elements
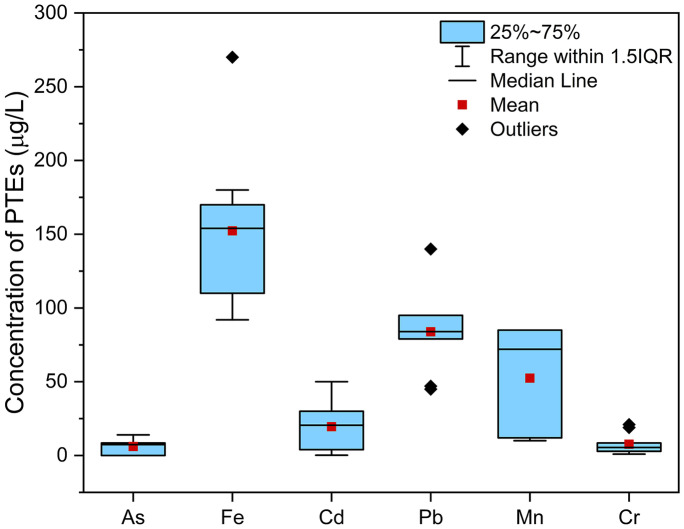
A total of six out of eight PTEs were detected in the water samples collected from the river Gomti. Only nickel and mercury were not reported, whereas the analytical summary of these six PTEs, viz. arsenic, iron, cadmium, lead, manganese, and chromium, is represented through box and whisker plot (Fig. 2). The concentration of As varied between 0 and 14.0 µg/L, with the highest value being reported at site S10. Similarly, the maximum value for Fe was reported at S1 (270.0 µg/L), which is attributed towards the gathering of a large number of devotees considering religious significance of the site. All the values of As and Fe were found to be within the permissible limits as per BIS norms (BIS, 2012). With respect to Cd, more than 60% of the sites had Cd levels exceeded the BIS limit of 10 µg/L, with a maximum reported concentration of 50.0 µg/L. The concentration of Pb exceeded the BIS permissible limit at all the sites and varied between the ranges of 45.0–140.0 µg/L. The local battery manufacturing units, automobile garages discharging their waste into the river system, are the probable sources contributing to high Pb concentrations in river water. The other probable sources of Pb contamination in river water across the study area are automobile emissions, pesticides through agricultural runoff, and paints (Paul, 2017). The concentration of Mn was found to be within the permissible limits at all the sampling stations, with a mean value of 52.4 µg/L, whereas the concentration of Cr was more than the BIS limit at 90% of the sampling stations, with a maximum value of 21.0 µg/L. The release of effluents from small-scale manufacturing units, electroplating operations, and the contribution of stabilizing pigments used in paint industries can be considered the reason behind elevated levels of these PTEs in river water. In a previous study by Singh et al. (2005), elevated levels of Pb and Cd were reported across the Lucknow city. Similarly, elevated levels of Cr, Cu, Ni, Cd, Pb, and Zn were reported at Lucknow city, owing to small-scale enterprises and battery manufacturing units in another study by Gaur et al. (2005).
Fig. 2.
Concentration of potentially toxic elements (PTEs) across the sampling locations
Human health risk assessment
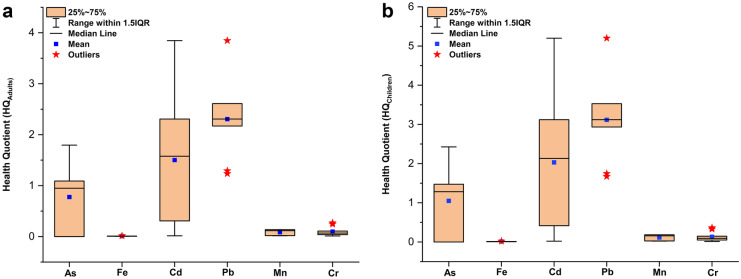
The current noxious state of river Gomti considering the elevated levels of PTEs across the sampling stations was clearly highlighted in our study. However, the COVID-19 lockdown visibly caused a reduction in the current (monsoon season, 2020) health hazards in comparison with pre-COVID-19 lockdown phase (post monsoon season, 2019). The value of HQ > 1 is considered to be unacceptable and indicates higher risk (Li et al., 2019). The HQ associated with various PTEs in the river water samples exceeded the permissible value of “unity” in both children and adults. The Box and Whisker plot for HQ in adults (Fig. 3a) and children (Fig. 3b) respectively showed the range of values and outliers, i.e., values which are not in coherence with values at other sampling stations. The highest health risk was seen to be associated with Pb with HQ > 1 at all the sampling stations. The values of HQ for Pb in adults and children ranged between 1.236–3.846 and 1.671–5.200, respectively. After the intake of Pb, the human body shows a rate of absorption varying between 20 and 70%. The disorders related to Pb ingestion include issues in the nervous system, reproductive system, nephrology, and heart ailments. The negative impact of Pb intake on the growth and development of the fetus in pregnant women has also been highlighted in a study by Kumar et al. (2020). The severity of health risk due to these PTEs can be arranged as Pb > Cd > As. The values of HQ for Cd and As at 60% and 40% of the sampling stations were found to be greater than “unity,” highlighting the health risk associated with their intake. The range of HQ for Cd varied between 0.015–3.846 for adults and 0.021–5.200 children. Constant exposure to cadmium can have detrimental impacts on human health (cardiovascular diseases, anemia, and nephrological disorders, etc.). The HQ values for As varied between 0–1.795 and 0–2.427 for adults and children, respectively. The oral intake of As can have detrimental impacts on human health, including neurological issues, reproductive system, skin allergies/infections, etc. The other PTEs (Fe, Mn, Cr) did not show any health risk, and the associated value of HQ was found to be less than unity at all sampling stations.
Fig. 3.
a Variation of health quotient (HQ) for adults with reference to PTEs. b Variation of health quotient (HQ) for children with reference to PTEs
The value of THI in both adults and children exceeded the acceptable limit of “unity” at all sampling stations. The results of THI are presented in Fig. 4. The highest to lowest risk for human health at various sampling stations can be stated as S5 > S10 > S6 > S3 > S7 > S4 > S9 > S8 > S2 > S1. The range of THI in adults and children was found to be “1.284–9.907” and “1.736–13.395” respectively. The highest hazard index was “7.660” and “10.356” for adults and children respectively at S5, highlighting the severe status of pollution. The potential impact of the crematorium “Baikund Dham” and associated anthropogenic activities which did not stop during the lockdown also emerged as a possible cause of elevated levels of PTEs and consequent health hazards. The downstream sampling station S10 which is the confluence of the outlet of Bharwara sewage treatment plant and river Gomti also showed high health hazards with THI values “6.881” and “9.304” in adults and children, respectively. The functioning efficiency of the STP also needs to be reassessed considering the elevated THI values. The overflow of discharge from the “Kukrail drain” with or without treatment is a potential cause of raised THI values for both adults and children at sampling station S6. The lowest health hazard was witnessed at site S1 probably due to its distant location ~ 30 km upstream of Lucknow city and lower impact of anthropogenic activities.
Fig. 4.
Total hazard index (THI) for adults and children at all the sampling locations
The classification of the dataset into different clusters depends greatly upon the homogeneity or non-homogeneity among the datasets (Fig. 5). It can be observed that the sampling stations were divided into four clusters based on the similarities of HQ values within the sampling locations of River Gomti:
Cluster I: In this cluster, there are two sub-clusters. The first sub-cluster represents the site S1 and S8 with respect to similar HQ values of Fe and Mn. This cluster represents the sites with the lowest values of HQ and THI for both children and adults. The second sub-cluster contains sites S2 and S9. This sub-cluster represents relatively “higher pollution” with respect to HQ and THI values. The sub-cluster represents sites with similar HQ values for Fe, Mn, Cd, and Cr.
Cluster II: This cluster contains only site S10 and considerable pollution in terms of very high HQ and THI values. The HQ value for Pb was highest at this site.
Cluster III: This cluster also has two sub-clusters. The first sub-cluster represents sites S3 and S6, which have similar HQ values for As, Fe, Cd, Pb, and Mn. The second sub-cluster has two sites S4 and S7, grouped together based upon similar HQ values for Cd, Fe, and Pb. This cluster signifies “critical pollution” levels. The reduced river flow because of riverfront development in the city has probably affected the pollution levels in this cluster.
Cluster IV: This cluster represents sites S5 highlighting “severe pollution” with respect to HQ and THI values. The midstream location and sluggish flow conditions at this stretch of the river can be considered the possible cause of elevated HQ and THI values. This site had HQ values of As, Fe, and Pb to be greater than 1, which may pose severe health risks.
Fig. 5.
Dendrogram representing Q-HCA based upon average HQ values for children and adults
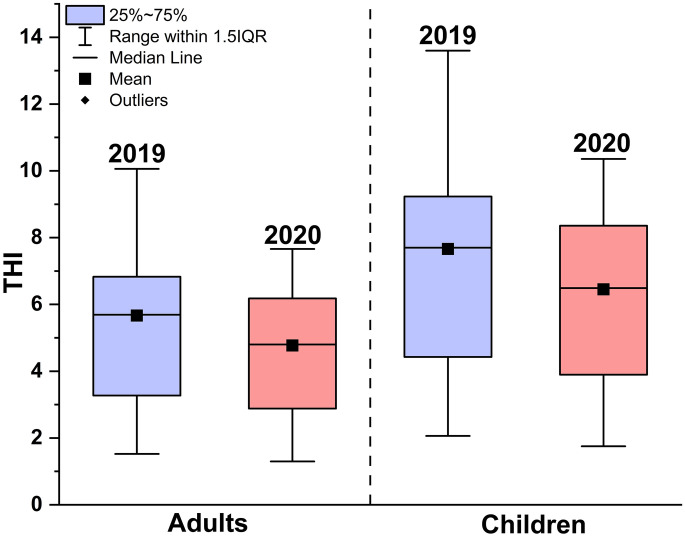
Variation of THI pre- and post-COVID-19 lockdown
A noticeable reduction in the health risks for both children and adults associated with HM pollution in river Gomti was observed at the initiation of unlocking (monsoon season, 2020) in comparison to its status highlighted in pre COVID-19 lockdown (post monsoon, 2019) as represented through Fig. 6. This decrease confirmed the impact of anthropogenic enterprises, including local manufacturing units, vehicular movement, activities along river channel (washing, waste dumping), etc. The order of decrease in THI values was found to be S10 > S9 > S4 > S1 > S2 > S3 > S8 > S6 > S7 > S5. The highest decrease in THI was observed at S10 (~31.587%) for both children and adults. Sampling stations S9 and S10 are downstream sites of Lucknow city, and the influence of the closure of various small-scale industries including battery production, textile units, dyes, etc., emerged as the probable reason for reduced hazards. The least improvement was seen at S5 with 6.998% and 7% for adults and children, respectively. The situation of S5 near the crematorium “Baikund Dham,” which was working during the COVID-19 lockdown, could also be the possible reason for minimum improvement.
Fig. 6.
Total hazard index pre- and post-COVID-19 lockdown for adults and children at all the sampling sites
Advancements in nanotechnology for remediation of PTEs
Nanoscale zero-valent iron (nZVI) is a highly used adsorbent, which offers efficient remediation of PTEs due to its reductive potential, large surface area, wide availability, low cost, no secondary pollutant, and eco-friendliness (Khan et al., 2021d; Shukla et al., 2021a, b). nZVI offers 1000 times more reactivity in removing various PTEs from water, especially arsenic (Hussain & Mishra, 2018a, b). Liquid-phase reducing method is the most efficient preparation method for nZVI; however, the use of expensive reagents limits their large-scale/industrial applications (Liu et al., 2021; Xu et al., 2020).
Similarly, magnetic organic frameworks (MOFs), formed through a combination of a metal precursor such as Al(III), Ca(II), Cd(II), Co(II), Cu(II), Fe(III), and Mg(II) and an organic ligand (e.g., amines, benzoic acid, carboxylates, imidazole, and piperazine) (Shukla et al., 2021a, b, c), have been proved to be very efficient for removal of these PTEs from aquatic environments. MOFs have large surface areas, a high porosity, a well-defined structure, and an easy preparation and hence are much more used than sorption (Gong et al., 2020; Zhao et al., 2021). Generation of MOFs through solvothermal methods is the most accepted and used technique to aid in the removal of PTEs like Cr(VI), Cu(II), and Hg(II) (Liang et al., 2016; Yang et al., 2016; Zhang et al., 2015).
Carbon nitride (C3N4) also finds its place as one of the best adsorbents for the removal of PTEs from aquatic environments (Aggarwal et al., 2022), among all the allotropes, namely cubic-phase nitrides (c-C3N4), quasi-cubic phase nitrides (q-C3N4, α-C3N4, β-C3N4), and graphite phase nitrides (g-C3N4). Among these, g-C3N4 is the most stable allotrope demonstrating diverse physico-chemical properties, controllable structure, eco-friendly products, and excellent thermal as well as chemical stability (Teter & Hemley, 1996; Wu et al., 2019). Thermal polymerization, pyrolytic organic compound, deposition methods, and solvothermal methods are some of the most commonly adapted methods for the preparation of g-C3N4, and thermal polymerization is highly adapted among these (Zou et al., 2017). In general, a nano-sheet structure is obtained through thermal polymerization technique which has a large specific surface area, improving the structure and sorption performance (Wu et al., 2019).
Among all the nanomaterials, MXenes are one of the latest and most novel inventions used in wastewater remediation (Hussain, 2020). MXenes are a novel class of two-dimensional inorganic materials that consist of layers of transition metals (carbides, nitrides, or carbonitrides) and appropriate etching agents (Ihsanullah, 2020). MXenes are usually synthesized through a top-down process adopting selective etching (Gan et al., 2020). The surface of MXenes is usually attached with various functional groups (e.g., eO, eF, and eOH), which significantly improve its sorption performance (Chaudhery Hussain, 2021). MXenes have been used in various studies for efficient removal of Cd2+, Cr2+−, Cu2+, and Pb2+ etc. However, high preparation cost and cumbersome preparation methods have limited their large-scale applications and requires extensive research in the preparation methods of MXenes (Wang et al., 2021; Yang et al., 2021).
Conclusion
The COVID-19 lockdown did act as a “ventilator” with respect to health hazards associated with PTE pollution in river Gomti. The highest risk was found at S5 for adults (7.660) and children (10.356) highlighting the influence of anthropogenic activities in the vicinity of the site. The least improvement was seen at S5 (31.587%) highlighting the impact of various anthropogenic activities due to the presence of a crematorium in the vicinity of the sampling station. The high levels of THI in adults (9.907) and children (13.395) at S10 (Bharwara STP discharge point-Gomti Confluence) create an urgent need to analyze the working efficiency of STPs in Lucknow city. The lowest health hazards were seen at S1 considering the upstream location and least anthropogenic intrusion at the location. The susceptibility of children to health risks at all sampling stations was significantly higher in comparison to adults. Thus, this study highlights the urgent need for remediation activities and authoritarian intervention to reinstate the river to its natural state.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are thankful to Dr. A. K. Singh, Vice-Chancellor, Shri Ramswaroop Memorial University, for his guidance during this study. We thank Central Water Commission (CWC), Central Pollution Control Board (CPCB), Uttar Pradesh Pollution Control Board (UPPCB) for their support and providing secondary data towards conduction of this study. The support of our families during the preparation of this manuscript is highly acknowledged.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ramsha Khan, Email: ramshaokhan@gmail.com.
Abhishek Saxena, Email: abhisheksaxena79@gmail.com.
References
- Adimalla N, Rajitha S. Spatial distribution and seasonal variation in fluoride enrichment in groundwater and its associated human health risk assessment in Telangana State, South India. Human and Ecological Risk Assessment. 2018;24(8):2119–2132. doi: 10.1080/10807039.2018.1438176. [DOI] [Google Scholar]
- Aggarwal, M., Shetti, N. P., Basu, S., & Aminabhavi, T. M. (2022). Two-dimensional ultrathin metal-based nanosheets for photocatalytic CO2 conversion to solar fuels. Journal of Environmental Management, 313, 114916, ISSN 0301-4797. 10.1016/j.jenvman.2022.114916 [DOI] [PubMed]
- APHA. (2012). Standard methods for the examination of water and wastewater. American Public Health Association.
- Azad H, Mohsennia M. A novel free-standing polyvinyl butyral-polyacrylonitrile/ZnAl-layered double hydroxide nanocomposite membrane for enhanced heavy metal removal from wastewater. Journal of Membrane Science. 2020;615:118487. doi: 10.1016/J.MEMSCI.2020.118487. [DOI] [Google Scholar]
- BIS. (2012). Bureau of Indian Standards, Drinking Water-Specification, Second Revision. IS10500.
- Census. (2011). Indian Districts by population, sex ratio, literacy. Accessed February 5, 2021, from https://www.census2011.co.in/district.php
- Chaudhery Hussain VK. Handbook of Functionalized Nanomaterials. Elsevier; 2021. [Google Scholar]
- Dayanidhi K, Vadivel P, Jothi S, Eusuff NS. Facile synthesis of Silver@Eggshell nanocomposite: A heterogeneous catalyst for the removal of heavy metal ions, toxic dyes and microbial contaminants from water. Journal of Environmental Management. 2020;271:110962. doi: 10.1016/J.JENVMAN.2020.110962. [DOI] [PubMed] [Google Scholar]
- DTE. (2020). No major improvement in river water quality during COVID-19 lockdown: CPCB report. Accessed February 23, 2021, from https://www.downtoearth.org.in/news/water/no-major-improvement-in-river-water-quality-during-covid-19-lockdown-cpcb-report-73520
- Dutta V, Dubey D, Kumar S. Cleaning the River Ganga: Impact of lockdown on water quality and future implications on river rejuvenation strategies. Science of the Total Environment. 2020;743:140756. doi: 10.1016/j.scitotenv.2020.140756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esrafili L, Firuzabadi FD, Morsali A, Hu ML. Reuse of predesigned dual-functional metal organic frameworks (DF-MOFs) after heavy metal removal. Journal of Hazardous Materials. 2021;403:123696. doi: 10.1016/J.JHAZMAT.2020.123696. [DOI] [PubMed] [Google Scholar]
- Gan D, Huang Q, Dou J, Huang H, Chen J, Liu M, et al. Bioinspired functionalization of MXenes (Ti3C2TX) with amino acids for efficient removal of heavy metal ions. Applied Surface Science. 2020;504:144603. doi: 10.1016/J.APSUSC.2019.144603. [DOI] [Google Scholar]
- Gaur VK, Gupta SK, Pandey SD, Gopal K, Misra V. Distribution of heavy metals in sediment and water of river Gomti. Environmental Monitoring and Assessment. 2005;102(1–3):419–433. doi: 10.1007/s10661-005-6395-6. [DOI] [PubMed] [Google Scholar]
- Gong XY, Huang ZH, Zhang H, Liu WL, Ma XH, Xu ZL, Tang CY. Novel high-flux positively charged composite membrane incorporating titanium-based MOFs for heavy metal removal. Chemical Engineering Journal. 2020;398:125706. doi: 10.1016/J.CEJ.2020.125706. [DOI] [Google Scholar]
- Haji M, Karuppannan S, Qin D, Shube H, Kawo NS. Potential human health risks due to groundwater fluoride contamination: A case study using multi-techniques approaches (GWQI, FPI, GIS, HHRA) in Bilate River Basin of Southern Main Ethiopian Rift, Ethiopia. Archives of Environmental Contamination and Toxicology. 2021;80(1):277–293. doi: 10.1007/S00244-020-00802-2. [DOI] [PubMed] [Google Scholar]
- Halli P, Agarwal V, Partinen J, Lundström M. Recovery of Pb and Zn from a citrate leach liquor of a roasted EAF dust using precipitation and solvent extraction. Separation and Purification Technology. 2020;236:116264. doi: 10.1016/J.SEPPUR.2019.116264. [DOI] [Google Scholar]
- Hussain, C. M. (2020). The ELSI handbook of nanotechnology: Risk, safety, ELSI and commercialization, 464. Retrieved January 5, 2022, from https://www.wiley.com/en-us/The+ELSI+Handbook+of+Nanotechnology%3A+Risk%2C+Safety%2C+ELSI+and+Commercialization-p-9781119591603
- Hussain, C. M., & Mishra, A. K. (2018). Nanotechnology in environmental science. Retrieved January 5, 2022, from https://www.wiley.com/en-in/Nanotechnology+in+Environmental+Science%2C+2+Volumes-p-9783527342945
- Ihsanullah I. MXenes (two-dimensional metal carbides) as emerging nanomaterials for water purification: Progress, challenges and prospects. Chemical Engineering Journal. 2020;388:124340. doi: 10.1016/J.CEJ.2020.124340. [DOI] [Google Scholar]
- Ke B, Nguyen H, Bui XN, Bui HB, Choi Y, Zhou J, et al. Predicting the sorption efficiency of heavy metal based on the biochar characteristics, metal sources, and environmental conditions using various novel hybrid machine learning models. Chemosphere. 2021;276:130204. doi: 10.1016/J.CHEMOSPHERE.2021.130204. [DOI] [PubMed] [Google Scholar]
- Khan, R., Shukla, S., Daverey, A., & Hussain, C. M. (2021d). Chapter 23:Future of functionalized magnetic nanoparticles in analytical chemistry. Analytical Applications of Functionalized Magnetic Nanoparticles (pp. 574–595). 10.1039/9781839162756-00574
- Khan, R., Saxena, A., & Shukla, S. (2021a). Assessment of the impact of COVID-19 lockdown on the heavy metal pollution in the River Gomti, Lucknow city, Uttar Pradesh, India. Environmental Quality Management. 10.1002/TQEM.21746
- Khan R, Saxena A, Shukla S, et al. Effect of COVID-19 lockdown on the water quality index of River Gomti, India, with potential hazard of faecal-oral transmission. Environmental Science and Pollution Research. 2021;28:33021–33029. doi: 10.1007/s11356-021-13096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, R., Saxena, A., Shukla, S., et al. (2022). Appraisal of water quality and ecological sensitivity with reference to riverfront development along the River Gomti, India. Applied Water Science,12(13). 10.1007/S13201-021-01560-9
- Khan R, Saxena A, Shukla S, Sekar S, Senapathi V, Wu J. Environmental contamination by heavy metals and associated human health risk assessment: A case study of surface water in Gomti River Basin India. Environmental Science and Pollution Research. 2021;28(40):56105–56116. doi: 10.1007/S11356-021-14592-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Ogata F, Nakamura T, Kawasaki N. Synthesis of novel zeolites produced from fly ash by hydrothermal treatment in alkaline solution and its evaluation as an adsorbent for heavy metal removal. Journal of Environmental Chemical Engineering. 2020;8(2):103687. doi: 10.1016/J.JECE.2020.103687. [DOI] [Google Scholar]
- Kumar, A., Kumar, A., Cabral-Pinto, M., Chaturvedi, A. K., Shabnam, A. A., Subrahmanyam, G., et al. (2020). Lead toxicity: Health hazards, influence on food Chain, and sustainable remediation approaches. International Journal of Environmental Research and Public Health. MDPI AG. 10.3390/ijerph17072179 [DOI] [PMC free article] [PubMed]
- Kumar A, Taxak AK, Mishra S, Pandey R. Environmental technology & innovation long term trend analysis and suitability of water quality of River Ganga at Himalayan hills of Uttarakhand. India. Environmental Technology & Innovation. 2021;22:101405. doi: 10.1016/j.eti.2021.101405. [DOI] [Google Scholar]
- Li P, He X, Guo W. Spatial groundwater quality and potential health risks due to nitrate ingestion through drinking water: A case study in Yan’an City on the Loess Plateau of northwest China. Human and Ecological Risk Assessment. 2019;25(1–2):11–31. doi: 10.1080/10807039.2018.1553612. [DOI] [Google Scholar]
- Li P, Wu J, Tian R, He S, He X, Xue C, Zhang K. Geochemistry, hydraulic connectivity and quality appraisal of multilayered groundwater in the Hongdunzi Coal Mine, Northwest China. Mine Water and the Environment. 2018;37(2):222–237. doi: 10.1007/s10230-017-0507-8. [DOI] [Google Scholar]
- Liang L, Chen Q, Jiang F, Yuan D, Qian J, Lv G, et al. In situ large-scale construction of sulfur-functionalized metal–organic framework and its efficient removal of Hg(II) from water. Journal of Materials Chemistry A. 2016;4(40):15370–15374. doi: 10.1039/C6TA04927C. [DOI] [Google Scholar]
- Liang Li, Li X, Guo Y, Lin Z, Su X, Liu B. The removal of heavy metal cations by sulfidated nanoscale zero-valent iron (S-nZVI): The reaction mechanisms and the role of sulfur. Journal of Hazardous Materials. 2021;404:124057. doi: 10.1016/J.JHAZMAT.2020.124057. [DOI] [PubMed] [Google Scholar]
- Liu K, Li F, Zhao X, Wang G, Fang L. The overlooked role of carbonaceous supports in enhancing arsenite oxidation and removal by nZVI: Surface area versus electrochemical property. Chemical Engineering Journal. 2021;406:126851. doi: 10.1016/J.CEJ.2020.126851. [DOI] [Google Scholar]
- Maranhão FS, de Souza Junior FG, Filho ST, de Oliveira Athayde BH, de Carvalho FF, Lino A, Malm O. Magnetic porous geopolymer: A cheaper and efficient environmental tool for heavy metal sorption. Macromolecular Symposia. 2021;398(1):2000182. doi: 10.1002/MASY.202000182. [DOI] [Google Scholar]
- Muhammad S, Shah MT, Khan S. Health risk assessment of heavy metals and their source apportionment in drinking water of Kohistan region, northern Pakistan. Microchemical Journal. 2011;98(2):334–343. doi: 10.1016/j.microc.2011.03.003. [DOI] [Google Scholar]
- Pal DB, Singh A, Jha JM, Srivastava N, Hashem A, Alakeel MA, et al. Low-cost biochar adsorbents prepared from date and delonix regia seeds for heavy metal sorption. Bioresource Technology. 2021;339:125606. doi: 10.1016/J.BIORTECH.2021.125606. [DOI] [PubMed] [Google Scholar]
- Panneerselvam B, Muniraj K, Pande C, Ravichandran N, Thomas M, Karuppannan S. Geochemical evaluation and human health risk assessment of nitrate-contaminated groundwater in an industrial area of South India. Environmental Science and Pollution Research. 2021;2021(1):1–18. doi: 10.1007/S11356-021-17281-0. [DOI] [PubMed] [Google Scholar]
- Park JH, Eom JH, Lee SL, Hwang SW, Kim SH, Kang SW, et al. Exploration of the potential capacity of fly ash and bottom ash derived from wood pellet-based thermal power plant for heavy metal removal. Science of the Total Environment. 2020;740:140205. doi: 10.1016/J.SCITOTENV.2020.140205. [DOI] [PubMed] [Google Scholar]
- Paul D. Research on heavy metal pollution of river Ganga: A review. Annals of Agrarian Science. 2017;15(2):278–286. doi: 10.1016/j.aasci.2017.04.001. [DOI] [Google Scholar]
- Peng H, Guo J. Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption electrocoagulation, electrochemical reduction, electrodialysis, electrodeionization, photocatalysis and nanotechnology: A review. Environmental Chemistry Letters. 2020;18(6):2055–2068. doi: 10.1007/S10311-020-01058-X. [DOI] [Google Scholar]
- Pino L, Beltran E, Schwarz A, Ruiz MC, Borquez R. Optimization of nanofiltration for treatment of acid mine drainage and copper recovery by solvent extraction. Hydrometallurgy. 2020;195:105361. doi: 10.1016/J.HYDROMET.2020.105361. [DOI] [Google Scholar]
- Pohl A. Removal of heavy metal ions from water and wastewaters by sulfur-containing precipitation agents. Water, Air, and Soil Pollution. 2020;231(10):1–17. doi: 10.1007/S11270-020-04863-W/TABLES/2. [DOI] [Google Scholar]
- Rahmani-Sani A, Singh P, Raizada P, Claudio Lima E, Anastopoulos I, Giannakoudakis DA, et al. Use of chicken feather and eggshell to synthesize a novel magnetized activated carbon for sorption of heavy metal ions. Bioresource Technology. 2020;297:122452. doi: 10.1016/J.BIORTECH.2019.122452. [DOI] [PubMed] [Google Scholar]
- Rishi MS, Kaur L, Sharma S. Groundwater quality appraisal for non-carcinogenic human health risks and irrigation purposes in a part of Yamuna sub-basin. India. Human and Ecological Risk Assessment. 2020;26(10):2716–2736. doi: 10.1080/10807039.2019.1682514. [DOI] [Google Scholar]
- Selvakumar S, Chandrasekar N, Kumar G. Hydrogeochemical characteristics and groundwater contamination in the rapid urban development areas of Coimbatore. India. Water Resources and Industry. 2017;17(February):26–33. doi: 10.1016/j.wri.2017.02.002. [DOI] [Google Scholar]
- Selvam S, Jesuraja K, Venkatramanan S, Chung SY, Roy PD, Muthukumar P, Kumar M. Imprints of pandemic lockdown on subsurface water quality in the coastal industrial city of Tuticorin, South India: A revival perspective. Science of the Total Environment. 2020;738(June):139848. doi: 10.1016/j.scitotenv.2020.139848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla, S., & Ganguly, R. (2020). Hazardous wastes – Types and sources. In C. M. Hussain (Ed.), The Handbook of environmental remediation: Classic and modern techniques. 10.1039/9781788016261-00024
- Shukla, S., Khan, R., & Daverey, A. (2021a). Synthesis and characterization of magnetic nanoparticles, and their applications in wastewater treatment: A review. Environmental Technology & Innovation, 101924. 10.1016/J.ETI.2021.101924
- Shukla, S., Khan, R., & Hussain, C. M. (2020). Chapter 16. Nanoremediation. The Handbook of Environmental Remediation (pp. 443–467). Royal Society of Chemistry. 10.1039/9781788016261-00443
- Shukla, S., Khan, R., Saxena, A., & Hussain, C. M. (2021b). Chapter 9. Use of functionalized magnetic nanoparticles in modern separation techniques. Analytical Applications of Functionalized Magnetic Nanoparticles (pp. 237–261). 10.1039/9781839162756-00237
- Shukla S, Khan R, Tech M, Ahmed Y, Memish ZA. Conducting mass gathering events during the COVID-19 pandemic: A case study of Kumbh Mela 2021 as a potential ‘super spreader event’. Journal of Travel Medicine. 2021;2021:1–7. doi: 10.1093/JTM/TAAB160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S, Saxena A. Sources and leaching of nitrate contamination in groundwater. Current Science. 2020;118(6):883–891. doi: 10.18520/cs/v118/i6/883-891. [DOI] [Google Scholar]
- Shukla, S., & Saxena, A. (2020b). Appraisal of groundwater quality with human health risk assessment in parts of Indo-Gangetic Alluvial Plain, North India. Archives of Environmental Contamination and Toxicology, (0123456789). 10.1007/s00244-020-00771-6 [DOI] [PubMed]
- Shukla, S., & Saxena, A. (2020c). Groundwater quality and associated human health risk assessment in parts of Raebareli district, Uttar Pradesh, India. Groundwater for Sustainable Development 10, Elsevier B.V. 10.1016/j.gsd.2020.100366
- Shukla S, Saxena A. Water quality index assessment of groundwater in the Central Ganga Plain with reference to Raebareli district, Uttar Pradesh, India. Current Science. 2020;119(8):1308–1315. doi: 10.18520/cs/v119/i8/1308-1315. [DOI] [Google Scholar]
- Singh KP, Malik A, Sinha S, et al. Estimation of source of heavy metal contamination in sediments of Gomti River (India) using principal component analysis. Water, Air, and Soil Pollution. 2005;166:321–341. doi: 10.1007/s11270-005-5268-5. [DOI] [Google Scholar]
- Soujanya Kamble B, Saxena PR, Kurakalva RM, Shankar K. Evaluation of seasonal and temporal variations of groundwater quality around Jawaharnagar municipal solid waste dumpsite of Hyderabad city India. SN Applied Sciences. 2020;2(3):1–22. doi: 10.1007/S42452-020-2199-0/FIGURES/12. [DOI] [Google Scholar]
- Teter DM, Hemley RJ. Low-compressibility carbon nitrides. Science. 1996;271(5245):53–55. doi: 10.1126/SCIENCE.271.5245.53. [DOI] [Google Scholar]
- Tofighy MA, Mohammadi T. Divalent heavy metal ions removal from contaminated water using positively charged membrane prepared from a new carbon nanomaterial and HPEI. Chemical Engineering Journal. 2020;388:124192. doi: 10.1016/J.CEJ.2020.124192. [DOI] [Google Scholar]
- USEPA. (1989). Risk Assessment Guidance for Superfund (RAGS). Human Health Evaluation Manual Part A (vol. 1). Washington, DC: Office of Emergency and Remedial Response.
- USEPA. (2005). Guidelines for carcinogen risk assessment. United States Environmental Protection Agency. Retrieved September 15, 2021, from https://www3.epa.gov/airtoxics/cancer_guidelines_final_3-25-05.pdf
- Wang D, Wu J, Wang Y, Ji Y. Finding high-quality groundwater resources to reduce the hydatidosis incidence in the Shiqu County of Sichuan Province, China: Analysis, Assessment, and Management. Exposure and Health. 2020;12(2):307–322. doi: 10.1007/s12403-019-00314-y. [DOI] [Google Scholar]
- Wang S, Wang F, Jin Y, Meng X, Meng B, Yang N, et al. Removal of heavy metal cations and co-existing anions in simulated wastewater by two separated hydroxylated MXene membranes under an external voltage. Journal of Membrane Science. 2021;638:119697. doi: 10.1016/J.MEMSCI.2021.119697. [DOI] [Google Scholar]
- Wu Y, Pang H, Liu Y, Wang X, Yu S, Fu D, et al. Environmental remediation of heavy metal ions by novel-nanomaterials: A review. Environmental Pollution. 2019;246:608–620. doi: 10.1016/J.ENVPOL.2018.12.076. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Tan S, Wang D, Wu J, Jia T, Liu Q, et al. CeO2/BiOIO3 heterojunction with oxygen vacancies and Ce4+/Ce3+ redox centers synergistically enhanced photocatalytic removal heavy metal. Applied Surface Science. 2020;530:147116. doi: 10.1016/J.APSUSC.2020.147116. [DOI] [Google Scholar]
- Xu W, Hu X, Lou Y, Jiang X, Shi K, Tong Y, et al. Effects of environmental factors on the removal of heavy metals by sulfide-modified nanoscale zerovalent iron. Environmental Research. 2020;187:109662. doi: 10.1016/J.ENVRES.2020.109662. [DOI] [PubMed] [Google Scholar]
- Yang Q, Zhao Q, Ren SS, Lu Q, Guo X, Chen Z. Fabrication of core-shell Fe3O4@MIL-100(Fe) magnetic microspheres for the removal of Cr(VI) in aqueous solution. Journal of Solid State Chemistry. 2016;244:25–30. doi: 10.1016/J.JSSC.2016.09.010. [DOI] [Google Scholar]
- Yang X, Liu Y, Hu S, Yu F, He Z, Zeng G, et al. Construction of Fe3O4@MXene composite nanofiltration membrane for heavy metal ions removal from wastewater. Polymers for Advanced Technologies. 2021;32(3):1000–1010. doi: 10.1002/PAT.5148. [DOI] [Google Scholar]
- Zhang LP, Liu Z, Zhou XL, Zhang C, Cai QW, Xie R, et al. Novel composite membranes for simultaneous catalytic degradation of organic contaminants and adsorption of heavy metal ions. Separation and Purification Technology. 2020;237:116364. doi: 10.1016/J.SEPPUR.2019.116364. [DOI] [Google Scholar]
- Zhang Y, Duan X. Chemical precipitation of heavy metals from wastewater by using the synthetical magnesium hydroxy carbonate. Water Science and Technology. 2020;81(6):1130–1136. doi: 10.2166/WST.2020.208. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhao X, Huang H, Li Z, Liu D, Zhong C. Selective removal of transition metal ions from aqueous solution by metal–organic frameworks. RSC Advances. 2015;5(88):72107–72112. doi: 10.1039/C5RA09897A. [DOI] [Google Scholar]
- Zhao X, Yu X, Wang X, Lai S, Sun Y, Yang D. Recent advances in metal-organic frameworks for the removal of heavy metal oxoanions from water. Chemical Engineering Journal. 2021;407:127221. doi: 10.1016/J.CEJ.2020.127221. [DOI] [Google Scholar]
- Zou Y, Wang P, Yao W, Wang X, Liu Y, Yang D, et al. Synergistic immobilization of UO22+ by novel graphitic carbon nitride @ layered double hydroxide nanocomposites from wastewater. Chemical Engineering Journal. 2017;330:573–584. doi: 10.1016/J.CEJ.2017.07.135. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.