Abstract
Blood pressure variability (BPV) is essential in hypertensive patients and is frequently associated with organ damage. As of today, hypertension is still the most common comorbidity in COVID-19, but the impact of BPV and the therapeutic target of BPV on outcomes in COVID-19 patients with hypertension remain unclear. Therefore, this study investigated the relationship between BPV and severity of COVID-19, in-hospital mortality, hypertensive status, and efficacy of antihypertensives in suppressing hypertensive covid-19 patient BPV. This cohort retrospective study enrolled 351 patients hospitalized with COVID-19. Subjects were classified according to the severity of COVID-19, the presence of hypertension, and their BPV status. During hospitalization, mean arterial pressure (MAP) was measured at 6 a.m. and 6 p.m., and BPV was calculated as the coefficient of variation of MAP (MAPCV). MAPCV values above the median were defined as high BPV. In addition, we compared the hypertensive status, COVID-19 severity, in-hospital mortality, and antihypertensive agents between the BPV groups. The mean age was 53.85 ± 18.84 years old. Hypertension was significantly associated with high BPV with prevalence ratio (PR) = 1.38 (95% CI = 1.13–1.70; p = 0.003) or severe COVID-19 (PR = 1.39; 95% CI = 1.09–1.76; p = 0.005). In laboratory findings, high BPV group had lower Albumin, higher WBC, serum Cr, CRP, and creatinine to albumin ratio. High BPV status also significantly increased risk of mortality (HR = 2.30; 95% CI = 1.73–3.86; p < 0.001). Patients with a combination of severe COVID-19 status, hypertension, and high BPV status had the highest risk of in-hospital mortality (HR = 3.51; 95% CI = 2.32–4.97; p < 0.001) compared to other combination status groups. In COVID-19 patients with hypertension, combination therapy with calcium channel blockers (CCB) as well as CCB monotherapy significantly develop low BPV (PR = 2.002; 95 CI% = 1.33–3.07; p = 0.004) and low mortality (HR = 0.17; 95% CI = 0.05–0.56; p = 0.004). Hypertensive status and severe COVID-19 were significantly associated with high BPV, and these factors increased in-hospital mortality. CCBs might be antihypertensive agents that potentially effectively suppressing BPV and mortality in COVID-19 patients.
Keywords: Hypertension, Blood pressure variability, Calcium channel blockers, COVID-19
Study design and outcomes of Therapeutic Target And Clinical Impact Of Day-To-Day Blood Pressure. Variability In Hypertensive Patients With Covid-19 study.
Background
Various reports have shown a high prevalence of hypertension in patients with COVID-19, where it is reported that patients with hypertension will be more susceptible to COVID-19. Conversely, hypertensive patients with COVID-19 are more likely to experience worsening conditions, even death [1–3]. Studies on COVID-19 patients showed a significant association between higher blood pressure (BP) levels at baseline at hospital admission and higher mortality [4, 5]. Poor BP control status was also independently associated with higher risk. However, a recent study comparing BP < 130 mmHg with <140 mmHg reported that patients with BP < 130 mmHg treated with COVID-19 were associated with higher mortality, especially in geriatric patients [6–8] In the end, how is the BP target in COVID-19 patients still unclear.
In COVID-19, there will be a phase of excess inflammation called a “cytokine storm” that causes immune cell activation, the release of inflammatory cytokines, and further cell recruitment from the immune system [9]. If this immune response occurs in excess, inflammation will cause final tissue damage and organ failure. Overall these cytokine storms are thought to be caused by an imbalance of the autonomic nervous system (ANS) [9], which is associated with overexcitation of the sympathetic nervous system (SNS) as an adaptive response to maintain homeostasis [9, 10]. Where imbalanced ANS regulation contributes to the pathophysiology of hypertensive patients, such as fluctuating BP [11].
BP variability (BPV) is a parameter that represents the dynamic physiological condition of the functioning of the cardiovascular system. The value of this BPV varies between individuals because it depends on their needs in carrying out their daily activities. It is also determined by the reactivity of the characteristics of control mechanisms. Their cardiovascular effects include the contribution of the ANS [12, 13]. From a clinical perspective, BPV has now been widely studied and is thought to have the potential to be an independent predictor of cardiovascular risk [14]. Recent studies have also hypothesized that BPV levels could potentially be a new therapeutic target in hypertensive patients to provide good outcomes [13].
Because in COVID-19 patients, there is systemic inflammation, impaired cardiopulmonary coupling, and cardiac insufficiency that will interfere with BP regulation, all of which have the potential to increase BPV that precedes the general condition worsening in some patients with COVID-19, especially in hypertensive patients who have already ANS regulation disturbance occurs [15]. Thereby, BPV can predict clinical outcomes and a therapeutic target for hypertensive patients with COVID-19 infection.
Design and method
This study is a single-center, retrospective cohort study conducted at the IGNG Prof. Dr. Ngoerah, Bali, Indonesia, from 2020 to 2021. We included hypertensive patients aged >18 with ICD-X coding: I10 in medical records suffering from COVID-19, patients with an incomplete recording of BP and laboratory parameters during treatment, and patients with shock or sepsis since the first day of hospitalization were excluded. They were confirmed positive for COVID-19 based on their symptoms and the results of the PCR swab test positive and isolated in the inpatient room of our hospital. Patient data during hospitalization in the form of socio-demographic characteristics, comorbid diseases, vital signs during treatment, results of laboratory tests, antihypertensive drugs used, and patient outcomes were reviewed by four authors through medical records, which were then inputted and analyzed. All patient’s BP were measured in a supine position, the patient was needed to lie down for 5 min to stabilize sympathetic activity. Each measurement was taken twice and then the mean value was calculated to be reported. The measurement was using a digital BP device twice a day by nurses during treatment, in the morning at 6 a.m before taking antihypertensive drugs and another one in the afternoon at 6 p.m after taking antihypertensive drugs. The device itself has been validated according to our hospital standards and is constantly checked for recalibration, and all patients were tested for blood on the first day of treatment. Patients were followed-up and BP measurement is carried out until the patient went home or the patient died during the treatment (mean 11.4 + 2.1 days), which we made as the patient’s outcome. Then we compared the outcomes based on the grouping of hypertension control status, the severity of COVID-19 based on its clinical presentation, and whether the patient’s BPV status was categorized as low or high BPV.
We grouped hypertension control status into controlled and uncontrolled hypertension based on the target pressure according to the International Society of Hypertension Global Hypertension Practice Guidelines [16]. Where if the systolic BP (SBP) is 140 mmHg or diastolic BP (DBP) is 90 mmHg, then the patient is categorized as uncontrolled hypertension, and if the systolic BP < 140 mmHg and diastolic BP < 90 mmHg, then the patient is categorized as controlled hypertension. BPV status was determined based on mean arterial pressure (MAP), mean (MAP mean), SD (MAPD), and coefficient of variation [equal to (SD × 100)/mean, MAPCV]. These were measured and calculated for each individual during treatment. In our study, MAPCV was considered a BPV parameter, and BPV is classified as high BPV when the MAPCV value is above the median and low BPV when the values are below the median. For severe COVID-19, we divide it into severe COVID-19 and non-severe COVID-19, where the definition of severe COVID-19 is when patients with COVID-19 had SpO2 < 94% on room air at sea level, PaO2/FiO2 < 300 mmHg, a respiratory rate >30 breaths/min, or lung infiltrates >50% and called critical illness if patients may have acute respiratory distress syndrome, septic shock that may represent virus-induced distributive shock, cardiac dysfunction, an exaggerated inflammatory response, and exacerbation of underlying comorbidities. In contrast, non-severe COVID-19 is a spectrum of COVID-19 with mild and moderate illnesses.
Categorical variables are shown as frequencies or percentages, and continuous variables as mean ± SD. Categorical variables were compared using the x2 test. Continuous variables were compared using the Mann–Whitney U test. Kaplan–Meier method was used to estimate the survival rate, and Cox proportional hazard regression analysis was applied to determine the potential risk factors associated with in-hospital mortality. Variables that showed a univariate relationship with in-hospital mortality (P < 0.05) were included in the multivariate regression analysis, and the results are reported as hazard ratios (HR) and 95% confidence interval (CI). All data were analyzed through the statistical package SPSS 22 (SPSS Inc.).
Results
A total of 351 hypertensive patients with COVID-19 were included in this study for analysis. Of the 351 patients, 67.2% had uncontrolled hypertension status, and during treatment, 39.1% experienced severe COVID-19, with a total of 37.8% deaths. We divided the patients according to their hypertension control status and severity of COVID-19 during treatment, as shown in Table 1. On clinical characteristics, if the groups were divided according to their hypertension control status, patients with uncontrolled hypertension were mainly male with a proportion of 58.8% (p = 0.007) and had SBP and DBP were significantly higher at baseline (144 ± 31 vs. 126 ± 18, p = 0.007; 92 ± 18 vs. 81 ± 11, p = 0.043) respectively. However, if divided based on the severity of COVID-19, the two groups had no significant differences in demographic data. However, in the patient’s comorbidities, diabetes mellitus was significantly higher in the severe COVID-19 group, but there was no significant difference in comorbidities when compared based on hypertension control status. The use of major antihypertensive drugs such as Angiotensin Converting Enzyme Inhibitors (ACEi), Angiotensin II Receptor Blockers (ARB), Beta blockers, Calcium Channel Blockers (CCBs), and diuretics, was evenly distributed in all groups. However, other antihypertensive drugs were significantly higher in uncontrolled hypertension and severe COVID-19 groups.
Table 1.
Demographics, blood pressure profiles, treatments and outcomes of patients
| Controlled Hypertension (n = 115) |
Uncontrolled Hypertension (n = 236) |
P value | Non-Severe Covid-19 (n = 137) |
Severe covid-19 (n = 214) |
P value | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, year | 52 ± 17 | 55 ± 26 | 0.473 | 50 ± 27 | 56 ± 30 | 0.053 |
| Sex, men | 41.2% | 58.8% | 0.033 | 51.2% | 49.9% | 0.231 |
| BP on admission | ||||||
| SBP, mmHg | 126 ± 18 | 144 ± 31 | 0.007 | 128 ± 10 | 130 ± 22 | 0.144 |
| DBP, mmHg | 81 ± 11 | 92 ± 18 | 0.043 | 80 ± 9 | 80 ± 15 | 0.556 |
| MAP, mmHg | 96 ± 13 | 97 ± 16 | 0.632 | 99 ± 20 | 97 ± 23 | 0.473 |
| Comobidities | ||||||
| Diabetes mellitus | 32.9% | 25.6% | 0.063 | 14.6% | 34.5% | 0.003 |
| CAD | 32.9% | 17.6% | 0.556 | 32.9% | 27.6% | 0.556 |
| CKD | 14.1% | 33.3% | 0.188 | 14.1% | 33.3% | 0.188 |
| Heart failure | 10.6% | 17.6% | 0.631 | 10.6% | 17.6% | 0.631 |
| Antihypertensive drugs on admission | ||||||
| AdCEis/ARBs | 60.5% | 64.2% | 0.152 | 60% | 62.3% | 0.209 |
| Beta blockers | 18.5% | 25% | 0.131 | 25.2% | 22.3% | 0.440 |
| CCBs | 42% | 43.1% | 0.665 | 44.5% | 46.8% | 0.556 |
| Diuretics | 22.3% | 19.3% | 0.154 | 23.1% | 18,3% | 0.188 |
| Any of drugs above | 12.3% | 27.9% | 0.045 | 33.4% | 27% | 0.052 |
| Other medications | ||||||
| Glucocorticoid | 86.9% | 83.8% | 0.822 | 87.5% | 92.5% | 0.082 |
| Anticoagulant | 52.1% | 55.1% | 0.898 | 36.49% | 84.1% | 0.000 |
| BP Profile during hospitalization | ||||||
| MAPCV | 2.1 ± 2.85 | 3.27 ± 4.9 | 0.002 | 2.9 ± 3.77 | 1.88 ± 2.42 | 0.000 |
| High BPV | 40.2% | 59.8% | 0.003 | 68.4% | 31.6% | 0.005 |
| Outcome | ||||||
| In-hospital mortality | 16.52% | 46.08% | 0.003 | 8.02% | 37.8% | 0.000 |
ABP blood pressure, SBP systolic blood pressure, DBP diastolic blood pressure, MAP mean arterial pressure, CAD coronary artery disease, CKD chronic kidney disease, ACEis angiotensin converting enzyme inhibitors, ARBs angiotensin receptor blockers, CCBs calcium channel blockers, MAPCV mean arterial pressure coefficient of variation, BPV blood pressure variability
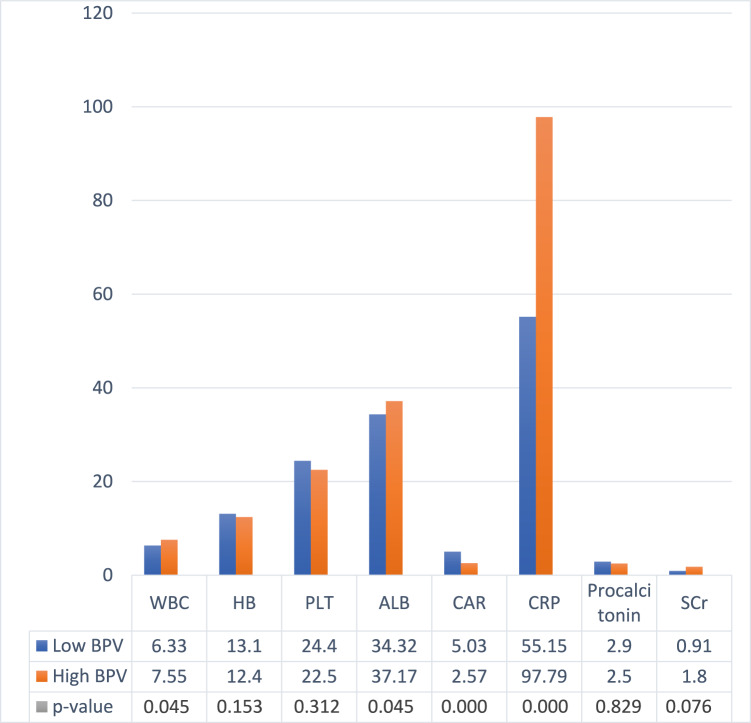
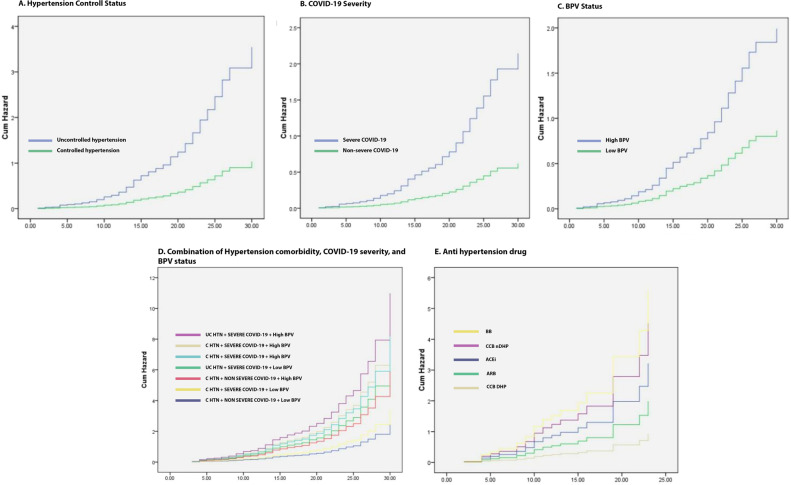
Based on laboratory data taken on the first day of patient admission (Table 2), patients with lower albumin (34.43 ± 5.52 vs. 37.17 ± 12.35), higher white blood cells (WBC) (7.55 ± 11.21 vs. 6.33 ± 4.51), serum creatinine (SCr) (1.80 ± 1.5 vs. 0.91 ± 0.55), C-Reactive Protein (CRP) (97.79 ± 77.17 vs. 55.15 ± 50.80), and creatinine to albumin ratio (CAR) (5.03 ± 1.54 vs. 2.57 ± 0.89) had higher BPV during treatment. Patients with uncontrolled hypertension (PR = 1.38; 95% CI = 1.13–1.70; p = 0.003) and severe COVID-19 (PR = 1.39; 95% CI = 1.09–1.76; p = 0.005) were also significantly more likely to have high BPV when compared to controlled hypertension and non-severe COVID-19. Likewise, the SBP and DBP values were higher in the uncontrolled hypertension group, but there was no significant difference based on the severity of COVID-19 (Table 1.). From the outcome section, the frequency of mortality was higher in the uncontrolled hypertension group (16.52% vs. 46.07%; p = 0.003) and severe COVID-19 (37.8% vs. 8 l.02%; p = 0.000). In the HR of deaths in the hospital during treatment, both groups were uncontrolled hypertension (HR = 3.42; 95% CI = 2.24–5.23; p < 0.001), severe COVID-19 (HR = 3.46; 95% CI = 1.84–6.53; p < 0.001), and high BPV (HR = 2.30; 95% CI = 1.73–3.86; p = 0.001), was significantly associated with mortality during treatment, and when all three groups were combined, patients with severe COVID-19 + uncontrolled hypertension + high BPV had the highest risk of in-hospital mortality (HR = 3.51; 95% CI = 2.32–4.97; p < 0.001) (Fig. 1).
Table 2.
Laboratory findings based on BPV status
WBC White blood cell, HB hemoglobin, PLT platelet, ALB albumin, CAR C-reactive protein to albumin ratio, CRP C-reactive protein, SCr Serum Creatinine
Fig. 1.
Hazzard Ratio Base on: (A) Hypertension controlled status; (B) COVID-19 severity; (C) BPV status; (D) Combination of patients category; (E) Antihypertension drug. UC HTN Uncontrolled hypertension, C HTN Controlled hypertension, BPV Blood pressure. variability, BB Beta blockers, ACEi Angiotensin converting enzyme inhibitors, ARBs Angiotensin receptor blockers, CCBs calcium channel blockers
In Table 3, patients on dihydropyridine CCB medication, either monotherapy or in combination with other drugs, had significantly lower BPV (PR = 2.002; 95 CI% = 1.33–3.07; p = 0.004) (Table 3), and mortality was also lower (HR = 0.17; 95% CI = 0.05–0.56; p = 0.004) (Fig. 1E). Kaplan–Meyer curves (Fig. 2) show that patients with low BPV, who in our study used a daily MAPcv cut-off below 1.4, had a protective effect on mortality as evidenced by higher survival during hospitalization (HR = 0.466; 95% CI = 0.289–0.721) (Fig. 2). Then we performed a regression analysis of all variables on BPV and found that age, CAR, severity of COVID-19, and hypertension control status contributed to high BPV. At the same time, using CCBs had a protective effect on high BPV (Table 4).
Table 3.
Association of antihypertensive drugs, BPV status, and mortality
| Low BPV | High BPV | p value | |
|---|---|---|---|
| Antihypertension drug monoteraphy | |||
| Beta Blockers | 25% | 75% | 0.005 |
| CCBs Non DHP | 16.7% | 83.3% | |
| CCBs DHP | 63.6% | 36.4% | |
| ACEi | 13.3% | 86.7% | |
| ARB | 88.9% | 11.1% | |
| Antihypertension drug combination teraphy | |||
| ACEi/ARB + CCB | 61.5% | 38.5% | 0.084 |
| ACEi/ARB + Diuretic | 25% | 75% | |
| ACEi/ARB + BB | 40% | 60% | |
| BB + Diuretic | 25% | 75% | |
| CCBs + Diuretic | 83% | 16,7% | |
| CCB DHP medication | |||
| CCB DHP | 66.7% | 33.3% | 0.004 |
| Other antihypertensive drugs | 33% | 67% | |
| Mortality | |||
| Death | 71.7% | 28.3% | 0.000 |
| Survive | 28.3% | 41.7% | |
ACEis angiotensin converting enzyme inhibitors, ARBs angiotensin receptor blockers, CCBs calcium channel blockers, DHP Dihidropyridin
Fig. 2.
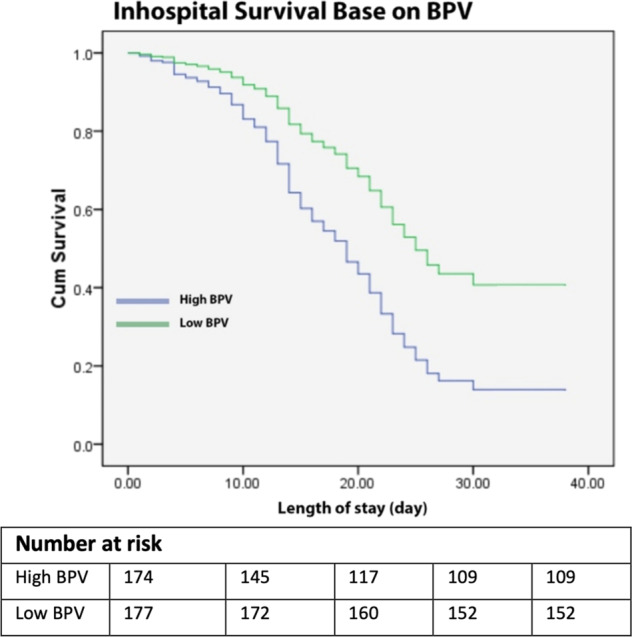
Kaplan–Meier curve for in-hospital survaival base on BPV Status. BPV Blood pressure variability
Table 4.
Cox regression analysis of factors that influence high BPV
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | 1.321 (1.112–1.572) | 0.017 | 1.178 (1.091–1.272) | 0.026 |
| COVID-19 Severity | 1.465 (1.265–2.055) | 0.015 | 1.515 (1.332–2.389) | 0.003 |
| CRP | 1.489 (0.986–0.993) | 0.012 | 1.323 (0.844–1.621) | 0.090 |
| CAR | 1.758 (1.334–2.266) | 0.063 | 2.141 (1.118–3.198) | 0.002 |
| Hypertension control status | 0.178 (0.114–0.192) | 0.000 | 0.128 (0.118–0.147) | 0.000 |
| Antihypertensive drug | 0.237 (0.124–0.312) | 0.000 | 0.469 (0.292–0.721) | 0.000 |
| WBC | 1.113 (1.011–2.551) | 0.044 | 2.562 (0.364–3.091) | 0.155 |
| Sex (male) | 0.787 (0.621–1.435) | 0.787 | Not included | |
| Albumin | 0.232 (0.146–1.058) | 0.454 | Not included | |
| Procalcitonin | 1.046 (0.971–1.121) | 0.424 | Not included | |
| SCr | 1.032 (0.542–1.932) | 0.263 | Not included | |
CAR C-Reactive protein to albumin ratio, WBC White blood cells, SCr Creatinin cerum, COVID-19 Corona virus disease 19
Discussion
The main finding in our study is that uncontrolled hypertension, severe COVID-19, and CAR contribute to high BPV, and all of these parameters together promote poorer clinical outcomes, especially mortality during treatment. However, CCB use was significantly associated with low BPV, and maintaining BP under low BPV with daily fluctuations MAPcv < 1.4 had a protective effect on mortality in hypertensive patients with COVID-19.
In the baseline characteristic, patients with uncontrolled hypertension were mostly male, whereas there was no difference based on the severity of COVID-19. We found that the male sex is significantly more in uncontrolled hypertension patients, and the mechanism is thought to be due to a lack of estrogen, which modulates vascular endothelial function to perform vasodilation so that BP will decrease [17, 18]. There is a correspondence between the reports from these studies and our study, namely that the population we used was hypertensive patients, and severe COVID-19 was significantly associated with some comorbid like diabetes mellitus [19]. The pathogenesis of COVID-19 in diabetes mellitus is said to be caused by glucotoxicity, oxidative stress, endothelial damage by inflammation, and cytokine production, which results in the risk of thromboembolic complications and organ damage to patients with diabetes mellitus. Some treatments used in the clinical care of COVID-19 patients, such as systemic corticosteroids or antiviral agents to suppress excessive inflammation, can cause hyperglycemia that worsens the patient’s condition [20].
Besides factors influencing uncontrolled hypertension and severe COVID-19, these two conditions were significantly associated with high BPV in our study. Over the years, several studies have demonstrated independent associations between the various components of BPV and organ damage or cardiovascular events [13]. From the prognostic point of view, some series of studies have provided evidence that a day-to-day increase in BPV, regardless of mean BP level, is a predictor of the development, formation, and evolution of damage to the heart, blood vessels, and kidneys that result in an increased risk of fatal and nonfatal cardiovascular events [15, 21–24]. Furthermore, in our study, high BPV significantly increased mortality, and its combination with the status of uncontrolled hypertension and severe COVID-19 gave the highest mortality. Although the mechanism underlying abnormal BPV is still under debate, autonomic nervous factors, particularly SNS overactivity, which also occurs in hypertension and COVID-19, are thought to be involved. In the condition of being infected with SARS-CoV-2, an excessive immune reaction will be induced, which causes a cytokine storm that leads to multi-organ failure. This cytokine storm involves the release of a large number of proinflammatory cytokines, such as IL-6 and TNF- α which can further cross the blood-brain barrier, thereby increasing SNS activation through dysregulation of the central autonomic network consisting of the insular cortex, anterior/mid-cingulate cortices, amygdala, hypothalamus, periaqueductal gray matter, parabrachial complex, the nucleus of the solitary tract, and rostral ventrolateral medulla [9, 25–27]. Increased activation of the ordinarily dominant and reactive resting SNS can have detrimental effects on several physiological systems, including changes in cardiac contraction [28], impaired vascular functions [29], and reduced blood flow capacity [30]. Although the levels of IL-6 and TNF- α which are markers of cytokine storm, were not examined in our study, the CAR levels associated with high BPV in our study, also reported in previous studies, can be a marker of the cytokine storm of COVID-19 patients [31].
The sensitivity of baroreceptor function is one of the primary determinants of BPV. It is known that structural vascular changes can reduce baroreceptor sensitivity (BRS), especially in hypertension [32]. It has also been reported that there is a decrease in the compliance of the great arteries, which contributes to the decrease in BRS in young hypertension, increasing the fluctuations in BP associated with minor changes in stroke volume, due to ANS instability [32]. Thus, it makes sense in our study that patients with uncontrolled hypertension had high BPV. The study reported by Barbaro et al. [33] also reported similar findings, but in resistant hypertension without a known history of infection or other inflammation. In his study, the condition of resistant hypertension had a higher arterial stiffness than the usual group, and it is suspected that this is influenced by a series of inflammatory processes that occur in the hypertension condition itself, causing a heavier arterial stiffness, as evidenced by the relationship between pulse wave velocity disturbances and hypertension. Hs-CRP, IL-10, IL-1β, and TNF-α are higher in patients with resistant hypertension, which triggers structural remodeling and functional changes in the arterial wall [33]. There is also evidence that the renin-angiotensin-aldosterone system (RAAS) and aldosterone cause vascular injury by inducing stress. Oxidative stress and inflammation by activating mineralocorticoid receptors [34, 35]. Thus, in hypertensive conditions without SARS-CoV-2 infection or its combination, it is possible that high BPV is initiated by an increase in chronic inflammatory mediators and is exacerbated by acute infection.
Another important finding that should be noted is that the use of CCBs is associated with low BPV and significantly lowers mortality. These findings are consistent with a study in Japan, a randomized efficacy study about a combined treatment with olmesartan and a CCB versus olmesartan and diuretics in 207 hypertensive patients to compare the effect of hydrochlorothiazide treatment with Azelnidipine after 12 weeks of Olmesartan monotherapy [36]. The study found that between the two groups, a significantly higher reduction in day-to-day BPV was found in the CCB/ARB combination use compared to the diuretic/ARB group. This indicates the potential of CCBs in improving significant arterial stiffness, not only peripheral muscular artery relaxation [37], where the relationship of day-to-day BPV with sizeable arterial stiffness and BRS has been described previously. In contrast, in the study with a longer follow-up with a median of 7.3 years from the HOMED-BP Study, a multicenter study of hypertension treatment based on measurement by electrical devices of blood pressure. The first-line treatment with CCB, ARB, or ACE inhibitors has no significant difference in reducing day-to-day BPV [38]. Recent evidence in a sub-analysis from a meta-analysis suggests CCBs may provide a benefit in reducing mortality in hypertensive patients with COVID-19 [39].
CCBs could block calcium entry, inhibiting the virus entry. Some have evidenced MERS-CoV and SARS-CoV, which utilize calcium ions to bind to cell membranes through the Spike protein [40, 41], where this protein is also found in SARS-CoV-2. So it makes sense that SARS-CoV-2 also uses calcium to enter the virus. Another study reported that dihydropyridines CCBs could inhibit the entry of SARS-CoV-2 into lung epithelial cells [42]. Furthermore, CCBs were also associated with a pseudo-increase of serum calcium, related to the binding of unsaturated fatty acids that may play a role in preventing organ failure [39]. In pharmacokinetics, CCBs also induce relaxation of pulmonary smooth muscle resulting in pulmonary vasodilation and amelioration of hypoxic conditions in COVID-19 patients [39]. Moreover, nifedipine, a relatively widely available drug in our population sample, is known to have an anti-inflammatory effect by suppressing production. IL-1a, IL-6, and IFN-γ from peripheral blood mononuclear cells [43, 44],, whose roles in COVID-19 severity, uncontrolled hypertension, and high BPV have been described previously. It makes sense that these alleged mechanisms together contribute positively to the reduction of BPV in hypertensive patients with COVID-19. Still, this incredible benefit may not be obtained in COVID-19 only conditions without hypertension.
Although CCB has been associated with low BPV, and this condition has a protective effect on mortality during treatment, the therapeutic target of BPV is still a gray zone. In any guidelines, it has not even been the therapy target for hypertensive patients. In this study, we propose daily MAPcv fluctuations of less than 1.4 to be a target for BP control that should be achieved in hypertensive patients with COVID-19, following the limits on the mean MAP value for categorizing the BPV status that we use. There were several indices of BPV, however coefficient of variation (CV) that we used in this study express a normalized measure of BPV. CV measures the variability of numeric data independently of the unit of measurement. Therefore, CV can be used to compare distribution data with different measurement units [45–48]. To the best of our knowledge, no studies have assessed fluctuations in BP as a therapeutic target because most may assume that BPV is a marker or result of organ damage, ignoring the effects of BP fluctuations. However, various studies have reported that not only as a predictor but high BPV is also suspected of worsening multiple patient conditions, such as exacerbating endothelial dysfunction and plaque rupture in coronary artery disease [49], increasing the incidence of cerebrovascular disease [50], thus contributing significantly to the increase in the incidence of major adverse cardiac events. As a marker and outcome, the event is evidence of its contribution to endothelial dysfunction, atherogenesis, atheroma development, plaque rupture, inflammation, and myocardial remodeling to arterial stiffness [51]. So, it makes sense that BPV can be an optional therapeutic target in hypertensive patients, especially in conditions prone to worsening, such as SARS-CoV-2 infection, if BP is controlled at tolerable limits. However, it should be noted that fluctuations in BP are normal in healthy patients because they are related to activity and circadian needs such as morning surges and night dips, which are physiological fluctuations in humans [13], the measurement must be carried out twice outside these times.
Conclusions
The control status of hypertension and the severity of COVID-19 infection is crucial in mortality because it increases BPV through various mechanisms. Where not only as a predictor, BPV also has the potential to be a novel therapeutic target for hypertensive patients, especially patients who are prone to worsening conditions. CCBs dihydropyridine, either as monotherapy or in combination with other antihypertensive drugs, are antihypertensive agents that promise to provide more benefits in terms of controlling BPV in hypertensive patients, especially those with COVID-19 because the inhibitory pathway of action is thought to be similar to the pathophysiology of COVID-19 and increase in BPV in hypertensive patients.
Acknowledgements
The authors would like to thank the Prof. I.G.N.G Ngoerah Public General Hospiotal for their permission and support in research. We also thank AAADAY, for providing medical writing support.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–81. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323:2052–9. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geng L, He C, Kan H, Zhang K, Mao A, Zhang C, et al. The association between blood pressure levels and mortality in critically ill patients with COVID-19 in Wuhan, China: a case-series report. Hypertens Res. 2021;44:368–70. doi: 10.1038/s41440-020-00594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ran J, Song Y, Zhuang Z, Han L, Zhao S, Cao P, et al. Blood pressure control and adverse outcomes of COVID-19 infection in patients with concomitant hypertension in Wuhan, China. Hypertens Res. 2020;43:1267–76. doi: 10.1038/s41440-020-00541-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koudelka M, Sovová E. COVID-19 causing hypotension in frail geriatric hypertensive patients? Medicina. 2021;57:633. doi: 10.3390/medicina57060633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun B, Wang H, Lv J, Pei H, Bai Z. Predictors of mortality in hospitalized COVID-19 patients complicated with hypotension and hypoxemia: a retrospective cohort Study. Front Med. 2021;8:753035. [DOI] [PMC free article] [PubMed]
- 8.Angus DC, vander Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:2063. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein DS. Theext ended autonomic system, dyshomeostasis, and COVID-19. Clin Auton Res. 2020;30:299–315. doi: 10.1007/s10286-020-00714-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldberger JJ, Arora R, Buckley U, Shivkumar K. Autonomicnervous system dysfunction. J Am Coll Cardiol. 2019;73:1189–206. [DOI] [PMC free article] [PubMed]
- 11.Wyss JM. The role of the sympathetic nervous system in hypertension. Curr Opin Nephrol Hypertens. 1993;2:265–73. doi: 10.1097/00041552-199303000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Bilo G, Parati G. Blood pressure variability and kidney disease. J Hypertens. 2018;36:1019–21. doi: 10.1097/HJH.0000000000001707. [DOI] [PubMed] [Google Scholar]
- 13.Parati G, Stergiou G, Dolan E, Bilo G. Blood pressure variability: clinical relevance and application. J Clin Hypertension. 2018;20:1133–7. doi: 10.1111/jch.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood-pressure variability. Nat Rev Cardiol. 2013;10:143–55. doi: 10.1038/nrcardio.2013.1. [DOI] [PubMed] [Google Scholar]
- 15.Li FK, An DW, Guo QH, Zhang YQ, Qian JY, Hu WG, et al. Day-by-day blood pressure variability in hospitalized patients with COVID-19. J Clin Hypertens (Greenwich). 2021;23:1675–80. [DOI] [PMC free article] [PubMed]
- 16.Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75:1334–57. doi: 10.1161/HYPERTENSIONAHA.120.15026. [DOI] [PubMed] [Google Scholar]
- 17.Kapoor M, Dhar M, Mirza A, Saxena V, Pathania M. Factors responsible for uncontrolled hypertension in the adults over 50 years of age: a pilot study from Northern India. Indian Heart J. 2021;73:644–6. doi: 10.1016/j.ihj.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reckelhoff JF, Granger JP. Role of androgens in mediating hypertension and renal injury. Clin Exp Pharmacol Physiol. 1999;26:127–31. [DOI] [PubMed]
- 19.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–7. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim S, Bae JH, Kwon HS, Nauck MA. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17:11–30. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, et al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ. 2016;354:i4098. doi: 10.1136/bmj.i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mena LJ, Felix VG, Melgarejo JD, Maestre GE. 24-Hour Blood Pressure Variability Assessed by Average Real Variability: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2017;6:e006895 [DOI] [PMC free article] [PubMed]
- 23.Wang J, Shi X, Ma C, Zheng H, Xiao J, Bian H, et al. Visit-to-visit blood pressure variability is a risk factor for all-cause mortality and cardiovascular disease. J Hypertens. 2017;35:10–7. doi: 10.1097/HJH.0000000000001159. [DOI] [PubMed] [Google Scholar]
- 24.Mancia G, DiRienzo M, Parati G, Grassi G. Sympatheticactivity,blood pressure variability and end organ damage in hypertension. J Hum Hypertens. 1997;11(Suppl. 1):S3–8. [PubMed] [Google Scholar]
- 25.Channappanavar R, Perlman S. Pathogenichumancoronavirusinfections: causes and consequences of cytokine storm and immunopathol- ogy. Semin Immunopathol. 2017;39:529–39. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pongratz G, Straub RH. The sympathetic nervous response in inflammation. Arthritis Res Ther. 2014;16:504. doi: 10.1186/s13075-014-0504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagai M, Scheper V, Lenarz T, Förster CY. The insular cortex as a vestibular area in relation to autonomic function. Clin Auton Res. 2021;31:179–85. doi: 10.1007/s10286-020-00744-8. [DOI] [PubMed] [Google Scholar]
- 28.Moreira HG, Lage RL, Martinez DG, Ferreira-Santos L, Rondon M, Negrão CE, et al. Sympatheticnervousactivity in patients with acute coronary syndrome: a comparative study of inflammatory biomarkers. Clin Sci. 2017;131:883–95. doi: 10.1042/CS20170049. [DOI] [PubMed] [Google Scholar]
- 29.Thijssen DH, deGroot P, Kooijman M, Smits P, Hopman MT. Sympathetic nervous system contributes to the age-related impairment of flow-mediated dilation of the superficial femoral artery. Am J Physiol Heart Circ Physiol. 2006;291:H3122–9. doi: 10.1152/ajpheart.00240.2006. [DOI] [PubMed] [Google Scholar]
- 30.Stuckless TJ, Pyke KE. The impact of a cold pressor test on brachial artery handgrip exercise induced flow-mediated dilation. Vasc Med. 2015;20:409–16. doi: 10.1177/1358863X15586473. [DOI] [PubMed] [Google Scholar]
- 31.El-Shabrawy M, Alsadik ME, El-Shafei M, Abdelmoaty AA, Alazzouni AS, Esawy MM, et al. Interleukin-6 and C-reactive protein/albumin ratio as predictors of COVID-19 severity and mortality. Egypt J Bronchol. 2021;15:5.
- 32.Kingwell BA, Cameron JD, Gillies KJ, Jennings GL, Dart AM. Arterial compliance may influence baroreflex function in athletes and hyper- tensives. Am J Physiol. 1995;268:H411–8. doi: 10.1152/ajpheart.1995.268.1.H411. [DOI] [PubMed] [Google Scholar]
- 33.Barbaro NR, Fontana V, Modolo R, De Faria AP, Sabbatini AR, Fonseca FH, et al. Increased arterial stiffness in resistant hypertension is associated with inflammatory biomarkers. Blood Press. 2015;24:7–13. doi: 10.3109/08037051.2014.940710. [DOI] [PubMed] [Google Scholar]
- 34.Briet M, Schiffrin EL. Vascular actions of aldosterone. J Vasc Res. 2012;50:89–99. doi: 10.1159/000345243. [DOI] [PubMed] [Google Scholar]
- 35.Luther JM, Gainer JV, Murphey LJ, Yu C, Vaughan DE, Morrow JD, et al. Angiotensin II induces interleukin-6 in humans through a mineralocorticoid receptor-dependent mechanism. Hypertension. 2006;48:1050–7. doi: 10.1161/01.HYP.0000248135.97380.76. [DOI] [PubMed] [Google Scholar]
- 36.Matsui Y, Kario K, Shimada K, Hoshide S, Ishikawa J, O’Rourke MF. Combined efect of angiotensin II receptor blocker and either a calcium channel blocker or diuretic on day-by-day variability of home blood pressure. Hypertension. 2012;59:1132–8. doi: 10.1161/HYPERTENSIONAHA.111.189217. [DOI] [PubMed] [Google Scholar]
- 37.Nardin C, Rattazzi M, Pauletto P. Blood pressure variability and therapeutic implications in hypertension and cardiovascular diseases. High Blood Press Cardiovasc Prev. 2019;26:353–9. doi: 10.1007/s40292-019-00339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asayama K, Ohkubo T, Hanazawa T, Watabe D, Hosaka M, Satoh M, et al. Does antihypertensive drug class affect day-to-day vari-ability of self-measured home blood pressure? the HOMED-BP Study. J Am Heart Assoc. 2016;5:1–15. doi: 10.1161/JAHA.115.002995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alsagaff MY, Mulia E, Maghfirah I, Luke K, Nugraha D, Rachmi DA, et al. Association of calcium channel blocker use with clinical outcome of COVID-19: a meta-analysis. Diabetes Metab Syndr. 2021;15:102210. doi: 10.1016/j.dsx.2021.102210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Straus MR, Tang T, Lai AL, Flegel A, Bidon M, Freed JH, et al. Ca2+ Ions Promote Fusion of Middle East Respiratory Syndrome Coronavirus with Host Cells and Increase Infectivity. J Virol. 2020;94:e00426–20. [DOI] [PMC free article] [PubMed]
- 41.Lai AL, Millet JK, Daniel S, Freed JH, Whittaker GR. The SARS-CoV fusion peptide forms an extended bipartite fusion platform that perturbs membrane order in a calcium-dependent manner. J Mol Biol. 2017;429:3875.e92–92. doi: 10.1016/j.jmb.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Straus MR, Bidon MK, Tang T, Jaimes JA, Whittaker GR, Daniel S. Inhibitors of L-Type Calcium Channels Show Therapeutic Potential for Treating SARS-CoV-2 Infections by Preventing Virus Entry and Spread. ACS Infect Dis. 2021;7:2807–15. [DOI] [PubMed]
- 43.Matsumori A, Nishio R, Nose Y. Calcium channel blockers differentially modulate cytokine production by peripheral blood mononuclear cells. Circ J. 2010;74:567e71–71. doi: 10.1253/circj.CJ-09-0467. [DOI] [PubMed] [Google Scholar]
- 44.Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abdi H, Edelman B, Valentin D, Dowling WJ. Coefficient of Variation. In: Salkind N, editor. Encyclopedia of Research Design, vol. 36, 2010. p. 21–32.
- 46.Mancia G, Bombelli M, Facchetti R, Madotto F, Corrao G, Trevano FQ, et al. Long-term prognostic value of blood pressure variability in the general population: results of the Pressioni Arteriose Monitorate e Loro Associazioni Study. Hypertension. 2007;49:1265–70. doi: 10.1161/HYPERTENSIONAHA.107.088708. [DOI] [PubMed] [Google Scholar]
- 47.Kato T, Kikuya M, Ohkubo T, Satoh M, Hara A, Obara T, et al. Factors associated with day-by-day variability of self-measured blood pressure at home: the Ohasama study. Am J Hypertens. 2010;23:980–6. doi: 10.1038/ajh.2010.94. [DOI] [PubMed] [Google Scholar]
- 48.Imai Y, Aihara A, Ohkubo T, Nagai K, Tsuji I, Minami N, et al. Factors that affect blood pressure variability. A community-based study in Ohasama, Japan. Am J Hypertens. 1997;10:1281–9. doi: 10.1016/S0895-7061(97)00277-X. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, Luo X, Jia H, Yu B. The Effect of Blood Pressure Variability on Coronary Atherosclerosis Plaques. Front Cardiovasc Med. 2022;9:803810. [DOI] [PMC free article] [PubMed]
- 50.Brickman AM, Reitz C, Luchsinger JA, Manly JJ, Schupf N, Muraskin J, et al. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol. 2010;67:564–9. doi: 10.1001/archneurol.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park CH, Kim HW, Joo YS, Park JT, Chang TI, Yoo TH, et al. Association Between Systolic Blood Pressure Variability and Major Adverse Cardiovascular Events in Korean Patients With Chronic Kidney Disease: Findings From KNOW-CKD. J Am Heart Assoc. 2022;11:e025513. [DOI] [PMC free article] [PubMed]