Abstract
Purpose of Review
The incidence of type 1 diabetes (T1D) is rising in all age groups. T1D is associated with chronic microvascular and macrovascular complications but improving glycemic trends can delay the onset and slow the progression of these complications. Utilization of technological devices for diabetes management, such as continuous glucose monitors (CGM) and insulin pumps, is increasing, and these devices are associated with improvements in glycemic trends. Thus, device use may be associated with long-term prevention of T1D complications, yet few studies have investigated the direct impacts of devices on chronic complications in T1D. This review will describe common diabetes devices and combination systems, as well as review relationships between device use and cardiovascular outcomes in T1D.
Recent Findings
Findings from existing cohort and national registry studies suggest that pump use may aid in improving cardiovascular risk factors such as hypertension and dyslipidemia. Furthermore, pump users have been shown to have lower arterial stiffness and better measures of myocardial function. In registry and case–control longitudinal data, pump use has been associated with fewer cardiovascular events and reduction of cardiovascular disease (CVD) and all-cause mortality.
Summary
CVD is the leading cause of morbidity and mortality in T1D. Consistent use of diabetes devices may protect against the development and progression of macrovascular complications such as CVD through improvement in glycemic trends. Existing literature is limited, but findings suggest that pump use may reduce acute cardiovascular risk factors as well as chronic cardiovascular complications and overall mortality in T1D.
Keywords: Type 1 diabetes, Continuous glucose monitor, Insulin pump, Hybrid closed loop, Cardiovascular health, Diabetes complications
Introduction
Type 1 diabetes (T1D) is the most common form of diabetes in the pediatric population but is diagnosed in all ages, and incidence rates are continuing to rise. Currently, 1.6 million people are estimated to have T1D in the USA [1] and this figure is predicted to increase to 5 million people by the year 2050 [2]. T1D is a result of permanent autoimmune destruction of insulin-producing pancreatic β-cells leading to an absolute insulin deficiency, and thus requires treatment with insulin for the remainder of the lifetime [3]. Insulin is administered subcutaneously via injection with a syringe or pen or via infusion with an insulin pump. Injection therapy combines long-acting insulin (LAI, also referred to as basal insulin) and short- or rapid-acting insulin (RAI) to create a multiple daily injection (MDI) regimen. LAI is administered once or twice daily to inhibit gluconeogenesis and ketogenesis and RAI is administered multiple times per day to correct acute hyperglycemia and/or with meals to prevent hyperglycemia from carbohydrate intake [4].
Chronic hyperglycemia increases risk for microvascular and macrovascular complications, as well as resultant increased morbidity and mortality in T1D. The landmark 1993 Diabetes Control and Complications Trial (DCCT) demonstrated in both pediatric and adult populations alike that intensive insulin treatment and subsequent improvement in glycemic trends delay the onset and slow the progression of these complications, but these improvements came at the expense of higher rates of hypoglycemia [5, 6]. Hypoglycemia is associated with acute complications such as cognitive impairment and seizures and can contribute to chronic vascular and neurocognitive complications. Consequently, T1D treatment guidelines recommend achievement of > 70% time in goal glycemic range (TIR), considered to be between 70 and 180 mg/dL), and targeting a hemoglobin A1c (HbA1c) of 7% or less [4, 7–10]. Adjunct TIR goals include minimizing the amount of time that blood glucoses exceed goal range and targeting < 4% of time per day with glucoses below the goal range [10].
As diabetes technologies continue to undergo rapid advancement, utilization rates are increasing across many national registries, particularly for devices related to glucose monitoring and insulin delivery [11–15]. Incorporating devices such as insulin pumps and continuous glucose monitors (CGM) into diabetes management is shown to help persons with diabetes (PwD) reduce risk of hypoglycemia and improve HbA1c and TIR [16–20], and thus may contribute to delaying onset and slowing progression of T1D-associated complications. This review will provide an overview of commonly utilized diabetes devices and combination systems, as well as review relationships between technology use and T1D-associated cardiovascular outcomes.
Devices
Continuous Glucose Monitors
A subcutaneous CGM estimates blood glucose concentrations by measuring glucose concentration in the interstitial fluid via a sensor inserted directly under the skin. This device serves as an alternative to self-monitoring of blood glucose (SMBG) with a single measurement “fingerstick” glucometer [21]. CGM sensors are inserted by the user and adhered directly to the skin with adhesive. In 2000, the MiniMed CGM System was the first to obtain US Food and Drug Administration (FDA) approval [22], and since that time, newer generations have continued to improve upon accuracy, functionality, and ease of use. CGMs can relay glucose values to a designated receiver, cellphone, and/or an insulin pump, and multiple brands now hold FDA approval to replace fingerstick glucose measurements for decision-making in insulin dosing in pediatric and adult populations with diabetes [23]. Current devices have varying durations of wear, but typically require removal and replacement every 7 to 14 days. These devices are typically equipped with optional and customizable alerts for hypoglycemia, hyperglycemia, and rapid glycemic change.
CGMs can be divided into two categories based on data type: “real time” and “flash.” Real-time CGMs (rtCGM) report glucose every 1–5 min through Bluetooth communication to the designated receiver, cellphone, or insulin pump. Flash CGMs, also referred to as intermittently scanned CGMs (isCGM), report glucose concentrations every 1–15 min, but only download the data to the designated reader when the user “flashes” the Near Field Communication tag, at which time the previous 8 h of data is downloaded [21]. In 2018, the first 90-day implantable real-time glucose sensor received FDA approval for use in adults 18 years and older with diabetes, and then in 2019 also received approval for use in insulin dosing decision-making [24]. This device is implanted under the skin during an outpatient procedure, requires users to wear a removable transmitter on the skin atop the sensor location, and is replaced every 90–180 days. This CGM glucose concentration values every 5 min and transmits data via Bluetooth to a cellphone app [19, 25•]. CGMs may also be categorized based on calibration need, including factory-calibrated and calibration-requiring devices. Older CGM devices required 2–3 SMBG values per day to calibrate the sensor value against a reference glucose concentration. Many newer CGM devices are factory calibrated, allowing advanced calibration algorithms to ensure accuracy without the need for user SMBG entry. Table 1 provides an overview of commonly used CGMs.
Table 1.
Comparison of select continuous glucose monitors
| Medtronic Guardian Sensor 3 | Medtronic Guardian Sensor 4* | Senseonics Eversense | Dexcom G6 | Abbott Freestyle Libre 2 | Abbott Freestyle Libre 3 | |
|---|---|---|---|---|---|---|
| Sensor type | rtCGM | rtCGM | rtCGM | rtCGM | isCGM | rtCGM |
| Age of approval (years) | 2+ | 7+ | 18+ | 2+ | 4+ | 4+ |
| Subcutaneous or implanted | Subcutaneous | Subcutaneous |
Surgically Implanted Transmitter adhered to skin over implanted sensor |
Subcutaneous | Subcutaneous | Subcutaneous |
| Duration of wear (days) | 7 | 7 | Maximum 180 | 10 | 14 | 14 |
| Calibration status | User calibration with blood glucose meter at least 2 × /day | Factory calibrated | User calibration with blood glucose meter 2 × /day | Factory calibrated | Factory calibrated | Factory calibrated |
| Approved for use for insulin dosing | No | Yes | Yes | Yes | Yes | Yes |
| Compatible in HCL system | MiniMed 670G, 770G | MiniMed 780G | No |
Tandem t:slim X2 CIQ Omnipod 5 CamAPS** Diabeloop** |
No | No |
References for Table 1 information: [25•, 138–140]
rtCGM real-time continuous glucose monitor, isCGM intermittently scanned continuous glucose monitor, HCL hybrid closed loop, CIQ Control IQ
*At the time of writing, CE marked in Europe but still under review for United States FDA approval
**System not available in the USA
CGMs are beneficial for all ages of people with T1D, regardless of insulin delivery method. Studies performed around the world utilizing various CGMs have associated CGM use with reductions in hypoglycemia [18, 26–31] and HbA1c [12, 14, 19, 30, 32–34], improvements in TIR [19], fewer episodes of diabetic ketoacidosis (DKA) [14, 35], and improvements in psychosocial outcomes [36–38]. Furthermore, early initiation of CGM (i.e., within 1 year of T1D diagnosis) has shown association with lower HbA1c and fewer diabetes-related emergency visits [39, 40]. Few studies have directly compared rtCGM and isCGM, but limited evidence suggests that rtCGM has greater benefit than isCGM in reducing hypoglycemia and improving TIR [41–43, 44•]. CGM has become standard of care in diabetes management around the world; indeed, US and international clinical guidelines for both youth and adults with T1D support use of CGM, stating that CGMs are safe and effective in both populations. The American Diabetes Association (ADA) recommends CGM be considered from the time of diagnosis and implementation of insulin therapy [41]. The International Society for Pediatric and Adolescent Diabetes (ISPAD) guidelines include that rtCGMs are effective in lowering HbA1c, reducing glucose variability, reducing hypoglycemia, and increasing TIR [45••]. Similarly, a joint statement by from the ADA and the European Association for the Study of Diabetes (EASD) describes CGM as the standard for glucose monitoring for most adults with T1D and an effective method to improve HbA1c and reduce hypoglycemia [4].
Insulin Pumps
Increasing numbers of PwD are utilizing insulin pumps, also referred to as continuous subcutaneous insulin infusion (CSII) systems, for insulin delivery [13, 46]. The first insulin pump prototype was designed in 1963 and was a large system that was worn by the user similarly to a backpack. Wearable insulin pumps have now been commercially available since 1976 and have continued to undergo reductions in size and advancement in ease of use and capabilities [47]. Use of modern CSII replaces the need for insulin injections, as these devices continuously infuse RAI into the subcutaneous tissue via a small cannula and allow for bolus dosing to be administered with carbohydrate intake at meals or to correct hyperglycemia. When utilized as a singular device without associated CGM, insulin dosing parameters for basal and bolus insulin are programmed into the pump. Users then input blood glucoses and carbohydrate counts for the pump to calculate and deliver the appropriate insulin bolus dose. CSII devices can be divided into two categories: tubed and patch. Tubed pumps store insulin in a reservoir within the pump device. Insulin is then delivered through tubing to a small subcutaneous infusion cannula adhered to the skin. Patch pumps are an adhesive patch device that includes an insulin reservoir that is directly connected to an infusion cannula. The cannula is inserted under the skin at the time the device is adhered to the body. Most insulin pumps require the entire patch or the infusion site to be changed every 3 days, though there are now tubed infusion sets approved for 7 days of continuous wear.
CSII is also beneficial for all ages of those with T1D, as it is associated with lower HbA1c [14, 20, 28, 33, 48–52]. One pediatric study showed that when compared to MDI users, pump users had lower HbA1cs for 6 years of treatment follow-up [53]. CSII use is also associated with lower rates of hypoglycemia [33, 48, 54, 55], lower total daily insulin doses [48], less glycemic variability [56], and improved sleep [57] as compared to MDI therapy. In older adults with T1D, people using CSII were less likely to exhibit cognitive dysfunction compared to those using MDI [33]. Recent data from diabetes registries and cohort studies also demonstrate associations between insulin pump use and reduced rates of DKA [13, 14, 48, 58], although two meta-analyses analyzing results of clinical trials found higher incidence of DKA in people using CSII when compared to MDI use [28, 52]. Like CGMs, insulin pump use is supported by US and international T1D clinical treatment guidelines for both pediatric and adult populations. Both ADA and ISPAD guidelines recommend consideration of insulin pump therapy at the time of T1D diagnosis, as CSII is safe and effective and helps to achieve glycemic targets, reduce risk of hypoglycemia and DKA, improve quality of life, and prevent T1D-associated complications [41, 45••].
Evolution of Device Collaboration
CGM and CSII devices may be used as independent devices; however, in recent years, technology has advanced to include real-time CGM data as a factor in user-directed and automated pump dosing decisions. Sensor-augmented insulin pump therapy (SAP) describes when a PwD uses CGM data to inform user-driven real-time decisions in insulin dose adjustment via CSII pump. SAP use is associated with a lower HbA1c without increasing rates of hypoglycemia when compared to MDI [59–61] and CSII alone [62]. Automated insulin suspension systems allow the insulin pump to suspend basal insulin delivery in response to either a current low glucose concentration or prediction of an impending hypoglycemic event, as identified by CGM. Automated insulin suspension has been shown to reduce HbA1c [63, 64], hypoglycemia [65–68], and patient-reported fear of hypoglycemia [69].
The concept of a completely closed-loop insulin pump and glucose monitoring system has existed since 1974 when Dr. Ernst Friedrich Pfeiffer developed a system that combined an intravenous insulin infusion and continuous glucose monitoring [47, 70]. Dr. Pfeiffer’s system at that time was too large and complex for commercial use but served as a foundation for advancements in diabetes devices. Current closed-loop systems are termed automated insulin delivery (AID) devices wherein CGM data is incorporated in real time into insulin dosing algorithm software to automatically modulate (i.e., increase or decrease) basal insulin delivery via CSII pump. Some systems also include AID for hyperglycemia correction. The most advanced commercial systems currently available are the hybrid closed loop (HCL) devices, which require user input of carbohydrate intake at mealtimes as well as some user-initiated correction doses. The first of such devices (Medtronic MiniMed 670G) obtained FDA approval in 2016 (Fig. 1). Since the novel MiniMed device’s market appearance, multiple other AID systems have obtained FDA approval. These devices continually undergo rapid advancements in functionality and ease of use. Two systematic review and meta-analysis studies from 2017 and 2020, respectively, found AID system use to be the most effective treatment strategy for achieving target range blood glucose concentrations [71, 72]. Figure 2 depicts a current HCL system.
Fig. 1.
Medtronic 670G insulin pump (left) with Guardian Sensor 3 continuous glucose monitor (right)
Fig. 2.
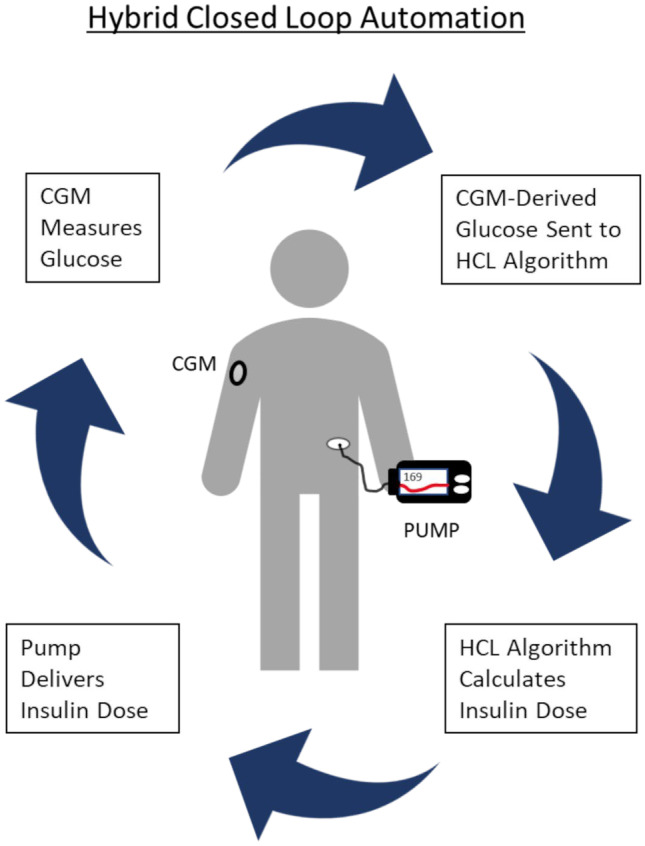
Illustration of hybrid closed-loop system. A continuous glucose monitor measures the interstitial glucose concentration and sends the glucose measurement to the control algorithm. The algorithm calculates the dose of insulin required based on the glucose received. The insulin pump then delivers the insulin dose. The cycle repeats
AID Systems
The first commercial HCL system, the Medtronic MiniMed 670G, consists of the Medtronic 670G insulin pump paired with the Guardian 3 sensor. It received FDA approval in 2016 based on pivotal trial data demonstrating an average TIR of 68.8% in adults and 67.2% in adolescents with T1D [73]. The 670G system was subsequently approved in children with an average TIR of 65% and the updated 770G system later received approval in young children with an average TIR of 63.8% [74, 75]. While the 670G and 770G systems brought HCL technology from research to real-world use, the systems were limited by frequent fingerstick testing requirements, excessive system alerts, and frequent exits from automation [76–79]. A 12-month analysis of real-world use of the Medtronic 670G at a single center found a significant decrease in time spent in HCL mode over time, with a decrease from 70.7% at 1 month of use to 49.3% at 12 months of use in children with T1D [77]. This same analysis demonstrated showed that adults had a higher time in HCL mode which was maintained in the 78–76% range over 12 months. The updated version of the Medtronic MiniMed design, the advanced hybrid closed-loop 780G system, appears to have resolved these issues. This system includes automated basal insulin delivery based upon total daily insulin requirements over previous days as well as automatic correction dose delivery. The approval trial of the 780G system demonstrated a 75.1% average TIR for adults and 72.7% average TIR for adolescents, with adults spending 95.2% time in HCL mode and adolescents spending 93.8% time in HCL mode over 3 months of system use [80]. Initial trials were conducted using the Guardian 3 CGM but the commercially available system pairs with the Guardian 4 CGM. At the time of writing, 780G is CE marked in Europe but is still under review by the FDA for approval in the USA.
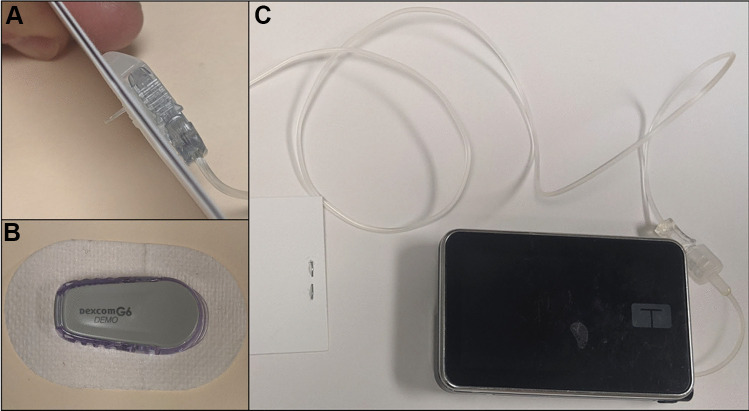
The second HCL system to come to market was the Tandem Control-IQ (CIQ) HCL system, which includes the Tandem t:slim X2 insulin pump paired with Dexcom G6 CGM (Fig. 3). This system expanded on a decade of previous research involving the University of Virginia Diabetes Assistant algorithm and can adjust basal insulin delivery rates as well as administer automatic correction doses according to current and predicted future glucose concentrations [81–83]. The National Institute of Health (NIH)–sponsored randomized controlled trial of the CIQ system demonstrated an average 71% TIR for adults and adolescents and a 67% average TIR for children [84, 85•]. These studies resulted in FDA approval for adults and adolescents in 2019 followed by approval in children in 2020. Pilot testing of the CIQ system in young children demonstrated an average TIR of 71.3% during a brief hotel study with additional at-home use [86]. The approval trial of this system in young children has been completed but not yet published.
Fig. 3.
Tandem Control-IQ hybrid closed-loop system. A Insulin infusion set with subcutaneous cannula. B Dexcom G6 continuous glucose monitor. C Tandem t:slim X2 insulin pump with infusion tubing
The European market has several phone-based HCL designs which have received CE mark. The CamAPS FX system demonstrated an average TIR of 65% in adults and was the first system approved to be controlled from the user’s cell phone [87]. The algorithm runs on an Android phone and works with the Dexcom G6 CGM and the DANA Diabecare RS insulin pump [88]. In an approval trial completed in 2017, the Diabeloop system demonstrated an average TIR of 68.5% in adults [89] and the commercial version of the system is compatible with the Roche Accu-Chek Insight, Vi Centra Kaleido, SOOIL Dana-I, and Cellnovo insulin pumps [88].
The most recently approved HCL system is the Insulet Omnipod 5 patch-pump system which pairs the Omnipod tubeless patch pump with the Dexcom G6 CGM (Fig. 4). This system can modulate basal insulin delivery rates based upon customizable glucose targets and current and predicted glucoses, with further basal insulin rate automation over the 3-day period of wear as the system recognizes glucose trends [90]. The approval trial for Omnipod 5 demonstrated an average 73.9% TIR for adults and adolescents and an average 68% TIR for children over the course of 3 months of use [91]. Additional studies completed in the young child age group demonstrated an average 68.1% TIR [92]. Table 2 provides an overview of HCL systems that are currently available and under FDA review in the USA.
Fig. 4.
Omnipod patch pump (top right) with personal diabetes manager (left) and Dexcom G6 continuous glucose monitor (bottom right)
Table 2.
Comparison of select hybrid closed-loop systems
| Medtronic 670G/770G | Medtronic 780G* | t:slim X2 Control IQ | Omnipod 5 | |
|---|---|---|---|---|
| Term for automated insulin delivery | “Auto Mode” | “Smart Guard” | “Control IQ” | “Automated Mode” |
| Age of approval (years) |
7+ (670G) 2+ (770G) |
7+ | 6+ | 6+ |
| Basal rate automation |
“Auto Basal” Basal rates based on total daily insulin from previous 2–6 days |
“Auto Basal” Basal rates based on total daily insulin from previous 2–6 days |
“Control IQ” Can increase or decrease the programmed basal rates |
“Adaptive Basal” Basal rates determined from total daily insulin since last pump change |
| Correction bolus dose automation | No |
Yes, IF: Glucose > 120 mg/dL AND at maximum “auto basal” delivery |
Yes, IF: Glucose predicted to reach > 180 mg/dL Maximum 1 dose per hour Delivers 60% of calculated dose from programmed settings |
No |
| Algorithm target glucose | 120 mg/dL | Select 100, 110, or 120 mg/dL | 112.5–160 mg/dL range | Select 110, 120, 130, 140, or 150 mg/dL |
| Special features | “Temporary Target”: changes target glucose to 150 mg/dL (30 min–12 h) |
“Temporary Target”: changes target glucose to 150 mg/dL (30 min–24 h) Extended wear (7 days) insulin infusion set |
“Exercise Activity”: changes target glucose range to 140–160 mg/dL “Sleep Activity”: changes target range to 112.5–120 mg/dL and prevents automated correction bolus |
“Activity Feature”: changes target glucose to 150 mg/dL and reduces insulin delivery (1–24 h) User can control basal or bolus dosing remotely from cell phone |
Diabetes Technology and Cardiovascular Outcomes
Current T1D treatment strategies and goals are largely founded upon results from numerous studies from the DCCT and its epidemiological follow-up study, the Epidemiology of Diabetes Interventions and Complications (EDIC), which demonstrated that intensive insulin therapy aimed at achieving glycemic control approximating normoglycemia is effective at delaying the onset and slowing the progression of microvascular and macrovascular complications seen in T1D [5]. Delaying and slowing these chronic complications are critical, as they contribute significantly to morbidity and mortality in T1D.
Macrovascular complications, specifically atherosclerotic cardiovascular disease (ASCVD), are the leading cause of morbidity and mortality in diabetes [93, 94]. T1D significantly increases the risk for cardiovascular disease (CVD) and this occurs independently of other common CVD risk factors. Notably, people with T1D are more than twice as likely to exhibit cardiovascular mortality than the general population, even when meeting glycemic targets [95, 96]. Known cardiovascular risk factors also contribute to this risk but are not entirely responsible for the excess mortality associated with diabetes [97]. Development of atherosclerosis begins in childhood, and youth with T1D may develop subclinical CVD even within the first 10 years of diabetes diagnosis [98]. CVD contributes to 25–50% of deaths in those with T1D of less than 20 years diabetes duration, and that percentage increases with longer diabetes duration [93, 99, 100].
Glycemic status is a modifiable risk factor for CVD, and glycemic status has been shown to predict coronary heart disease events independently of other risk factors [101, 102]. Chronic hyperglycemia may promote atherosclerosis, endothelial dysfunction, and arterial stiffness [103]. Studies also demonstrate associations between glucose variability, CVD, and all-cause mortality, regardless of mean glucose concentration [103–107]. Alongside chronic hyperglycemia and glucose variability, hypoglycemia also contributes to cardiovascular complications. Hypoglycemia-induced changes in hemodynamics, hemostasis, and coagulation, arterial wall stiffness, and cardiac electrophysiology and autonomic function are postulated to explain the associations seen between hypoglycemia and cardiovascular complications including myocardial ischemia and cardiac arrhythmias [108]. Studies have found that a history of recurrent hypoglycemia was associated with reduced survival after a major CVD event such as myocardial infarction or stroke [109], and those with T1D who report history of repeated hypoglycemia events had a higher prevalence of CVD [110]. DCCT/EDIC showed that tighter glycemic control can improve cardiovascular risk factors such as hypertension, carotid intima-media thickness, and coronary artery calcium scores, and even reduce cardiovascular events [111–114].
As diabetes device use may improve glycemic trends and stability, use of diabetes technologies may also have favorable impacts on T1D-associated complications. Indeed, a recent prospective cohort study including 515 adults with T1D utilizing CGMs and insulin pumps found that TIR and HbA1c were independent risk factors for microvascular and macrovascular complications, respectively [115••]. Yet, few existing studies have assessed for relationships between technology use and complication onset or severity in T1D. Limited studies suggest that CSII use may reduce microvascular complications seen in T1D, such as retinopathy, neuropathy, and diabetic kidney disease [116–123]. There is also evidence suggesting insulin pump use may be beneficial for cardiovascular risk factors and CVD. A large study from the Diabetes-Patienten-Verlaufsdokumentation (DPV) registry involving multiple diabetes centers in Germany, Austria, Switzerland, and Luxembourg found that initiation of insulin pump therapy within 6 months of diagnosis in people with childhood onset T1D was associated with a better cardiovascular risk profile compared to those with delayed CSII initiation within 2–3 years of T1D diagnosis. Specifically, they reported lower mean systolic blood pressure and higher high-density lipoprotein cholesterol (HDL-C), although no significant relationships were seen with diastolic blood pressure, low-density lipoprotein cholesterol (LDL-C), or triglycerides [124]. A 12-month, randomized, multicenter case–control study found that PwD using insulin pumps demonstrated increased HDL-C and decreased total cholesterol, LDL-C, and triglycerides as compared to MDI users. This finding persisted after 8 years of follow-up [56, 125]. During the follow-up study, CSII use was also associated with fewer cardiovascular events, specifically atrial fibrillation, premature ventricular contractions, acute coronary infarction, angina pectoris, peripheral vascular ischemia, and heart failure, as compared to MDI use [125]. Similar results were seen in a large T1D Swedish registry, which found pump use was associated with a 45% reduction in fatal coronary heart disease, 42% reduction in fatal CVD, and a 27% reduction in all-cause mortality as compared to MDI use over a mean follow-up period of 6.8 years. Authors hypothesize that the reduction in severe hypoglycemic episodes seen with insulin pump use in the study may have contributed to the reduction of cardiovascular mortality [126]. Similarly, a 2017 study in participants with T1D utilizing CSII found that longer duration of CSII use was related to longer duration of freedom from chronic diabetes complications, fewer cardiovascular events, and lower mortality [127].
Arterial stiffness is a marker of cardiovascular events, and pulse wave velocity (PWV) is the gold standard measure of arterial stiffness [128]. A prospective study found young adults with T1D of 10 or more years duration had increased PWV compared to healthy controls. After 5 years of follow-up, CSII use was associated with reduced PWV compared to MDI users [129]. These results align with previous literature which showed lower PWV in those with T1D using CSII as compared to MDI [130]. Endothelial dysfunction is suggested to play a role in development of atherosclerosis [131], and a recent study including 123 youth and adults with T1D found that pump use may impart cardiac benefit through improvements in endothelial function and overall myocardial performance. As compared to MDI use, CSII users had lower measures of carotid intima-media thickness and anteroposterior diameter of the infrarenal abdominal aorta via ultrasound assessment, and lower left and right Tei index and left E/e′ ratio [132].
Expert Commentary and Conclusions
For over 3 decades, the primary barometer for diabetes management has been HbA1c, based on established correlations between HbA1c and vascular complications. Over the past several years, however, TIR has emerged as a viable alternative to HbA1c. Analysis within the DCCT demonstrated that TIR derived from frequent SMBG measurements can hold similar correlations to T1D outcomes as those seen with HbA1c [133]. Additional analyses of correlations between HbA1c and average glucose concentrations have demonstrated wide ranges of average glucose at each HbA1c percentage, with potential bias for HbA1c tendencies across racial/ethnic groups [134, 135]. These observations have driven diabetes assessment to move “beyond HbA1c” to include use of other measures such as TIR, glucose management index (GMI), and glycemia risk index (GRI) [10, 136, 137]. During the quarantine period due to COVID-19, many practices managed PwD using CGM, with an emphasis on TIR and other CGM-derived metrics as patients were unable to obtain HbA1c measurements in a medical office or laboratory. With growth of telemedicine practices, it is expected that virtual visits will continue to require glycemic assessment via TIR.
AID research uses both HbA1c and TIR as pre-specified endpoints, though there is interest in the field to consider TIR as a primary glycemic outcome. Technology research moves at a rapid pace with new devices developed every year. Technology development studies frequently last 1–4 weeks and thus require a valid metric of glycemic control that can be assessed within that timeframe. Even within pivotal trials, the need for laboratory HbA1c assessments necessitates in-person visits and venipuncture, which may limit clinical trial participation for some populations. For these reasons, it is desirable for TIR- and CGM-based metrics to gain acceptance as valid endpoints.
A concern with fully equating CGM-derived metrics with established HbA1c targets is that little research exists to definitively correlate soft outcomes such as TIR, GMI, and GRI with hard outcomes such as diabetes-associated retinopathy, nephropathy, neuropathy, and cardiovascular disease. While HbA1c is clearly correlated with vascular hard endpoints, associations between CGM-derived metrics and vascular endpoints are limited to inferences made through associations with HbA1c rather than direct comparisons. This has been a major limitation for both regulatory agencies and payers accepting CGM-derived endpoints as fully validated surrogates for change in rates of vascular disease.
Next steps include combining data from large multicenter studies, registries, and national databases to clearly demonstrate these relationships with CGM metrics obtained over the past 5–10 years. Additionally, prospective longitudinal studies are needed to examine CGM-derived metrics, CGM and AID use, and the rates of vascular disease in order to move beyond dependence on HbA1c as the primary indicator of glycemic control in T1D.
Author Contribution
MEP and GPF contributed to the manuscript design. MEP performed literature search. All authors contributed to drafting and reviewing the manuscript and read and approved the final manuscript.
Funding
This project/publication is supported in part by NIH/NCATS Colorado CTSA Grant Number UL1 TR002535. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views. MEP receives salary and research support from the NIH/NIDDK T32 DK63687. KLT receives salary and research support from the NIH/NHLBI K23 HL159292, Children’s Hospital Colorado Research Scholar Award, University of Colorado Diabetes Research Center P30 DK116073, Ludeman Family Center for Women’s Health Research at the University of Colorado, and the Department of Pediatrics, Section of Endocrinology at the University of Colorado School of Medicine. JKSB receives salary support from the University of Colorado Diabetes Research Center P30 DK116073 and conducts research sponsored by NIH and JDRF. GPF conducts research sponsored by Medtronic, Dexcom, Abbott, Tandem, Insulet, Beta Bionics, and Lilly and has been a consultant, speaker, or advisory board member for Medtronic, Dexcom, Abbott, Tandem, Insulet, Beta Bionics, and Lilly.
Compliance with Ethical Standards
Conflict of Interest
MEP, KLT, and JKSB declare that they have no conflict of interest. GPF reports research support from Medtronic, Dexcom, Abbott, Tandem, Insulet, Beta Bionics, and Lilly; and has been a consultant/speaker/ad board member for Medtronic, Dexcom, Abbott, Tandem, Insulet, Beta Bionics, and Lilly.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Diabetes and Cardiovascular Disease
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report website. 2022. https://www.cdc.gov/diabetes/data/statistics-report/index.html. Accessed 2 July 2022.
- 2.Dabelea D, Mayer-Davis EJ, Saydah S, Imperatore G, Linder B, Divers J, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778–1786. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Assocation Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S17–s38. doi: 10.2337/dc22-S002. [DOI] [PubMed] [Google Scholar]
- 4.Holt RIG, DeVries JH, Hess-Fischl A, Hirsch IB, Kirkman MS, Klupa T, et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2021;64(12):2609–2652. doi: 10.1007/s00125-021-05568-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 6.Diabetes Control and Complications Trial Writing Group Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: diabetes control and complications trial. Diabetes Control and Complications Trial Research Group. J Pediatr. 1994;125(2):177–188. doi: 10.1016/S0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 7.Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, Freeman R, et al. 6. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S83–S96. doi: 10.2337/dc22-S006. [DOI] [PubMed] [Google Scholar]
- 8.Beck RW, Bergenstal RM, Cheng P, Kollman C, Carlson AL, Johnson ML, et al. The relationships between time in range, hyperglycemia metrics, and HbA1c. J Diabetes Sci Technol. 2019;13(4):614–626. doi: 10.1177/1932296818822496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vigersky RA, McMahon C. The relationship of hemoglobin A1C to time-in-range in patients with diabetes. Diabetes Technol Ther. 2019;21(2):81–85. doi: 10.1089/dia.2018.0310. [DOI] [PubMed] [Google Scholar]
- 10.Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beck RW, Bergenstal RM, Laffel LM, Pickup JC. Advances in technology for management of type 1 diabetes. Lancet. 2019;394(10205):1265–1273. doi: 10.1016/S0140-6736(19)31142-0. [DOI] [PubMed] [Google Scholar]
- 12.DeSalvo DJ, Miller KM, Hermann JM, Maahs DM, Hofer SE, Clements MA, et al. Continuous glucose monitoring and glycemic control among youth with type 1 diabetes: international comparison from the T1D Exchange and DPV Initiative. Pediatr Diabetes. 2018;19(7):1271–1275. doi: 10.1111/pedi.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster NC, Beck RW, Miller KM, Clements MA, Rickels MR, DiMeglio LA, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther. 2019;21(2):66–72. doi: 10.1089/dia.2018.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardona-Hernandez R, Schwandt A, Alkandari H, Bratke H, Chobot A, Coles N, et al. Glycemic outcome associated with insulin pump and glucose sensor use in children and adolescents with type 1 diabetes. Data From the International Pediatric Registry SWEET. Diabetes Care. 2021;44(5):1176–1184. doi: 10.2337/dc20-1674. [DOI] [PubMed] [Google Scholar]
- 15.van den Boom L, Karges B, Auzanneau M, Rami-Merhar B, Lilienthal E, von Sengbusch S, et al. Temporal trends and contemporary use of insulin pump therapy and glucose monitoring among children, adolescents, and adults with type 1 diabetes between 1995 and 2017. Diabetes Care. 2019;42(11):2050–2056. doi: 10.2337/dc19-0345. [DOI] [PubMed] [Google Scholar]
- 16.Bruttomesso D, Costa S, Baritussio A. Continuous subcutaneous insulin infusion (CSII) 30 years later: still the best option for insulin therapy. Diabetes Metab Res Rev. 2009;25(2):99–111. doi: 10.1002/dmrr.931. [DOI] [PubMed] [Google Scholar]
- 17.Laffel LM, Kanapka LG, Beck RW, Bergamo K, Clements MA, Criego A, et al. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323(23):2388–2396. doi: 10.1001/jama.2020.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riddlesworth T, Price D, Cohen N, Beck RW. Hypoglycemic event frequency and the effect of continuous glucose monitoring in adults with type 1 diabetes using multiple daily insulin injections. Diabetes Ther. 2017;8(4):947–951. doi: 10.1007/s13300-017-0281-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irace C, Cutruzzolà A, Nuzzi A, Assaloni R, Brunato B, Pitocco D, et al. Clinical use of a 180-day implantable glucose sensor improves glycated haemoglobin and time in range in patients with type 1 diabetes. Diabetes Obes Metab. 2020;22(7):1056–1061. doi: 10.1111/dom.13993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pease A, Szwarcbard N, Earnest A, Andrikopoulos S, Wischer N, Zoungas S. Glycaemia and utilisation of technology across the lifespan of adults with type 1 diabetes: results of the Australian National Diabetes Audit (ANDA) Diabetes Res Clin Pract. 2021;171:108609. doi: 10.1016/j.diabres.2020.108609. [DOI] [PubMed] [Google Scholar]
- 21.Forlenza GP, Kushner T, Messer LH, Wadwa RP, Sankaranarayanan S. Factory-calibrated continuous glucose monitoring: how and why it works, and the dangers of reuse beyond approved duration of wear. Diabetes Technol Ther. 2019;21(4):222–229. doi: 10.1089/dia.2018.0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klonoff DC. FDA approves new Glucose Monitoring System. Diabetes Technol Ther. 1999;1(3):349. doi: 10.1089/152091599317260. [DOI] [PubMed] [Google Scholar]
- 23.Meeting Report FDA advisory panel votes to recommend non-adjunctive use of Dexcom G5 Mobile CGM. Diabetes Technol Ther. 2016;18(8):512–516. doi: 10.1089/dia.2016.07252.mr. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez P, Ghosh-Dastidar S, Tweden KS, Kaufman FR. Real-world data from the first U.S. commercial users of an implantable continuous glucose sensor. Diabetes Technol Ther. 2019;21(12):677–681. doi: 10.1089/dia.2019.0234. [DOI] [PubMed] [Google Scholar]
- 25.Garg SK, Liljenquist D, Bode B, Christiansen MP, Bailey TS, Brazg RL, et al. Evaluation of accuracy and safety of the next-generation up to 180-day long-term implantable Eversense continuous glucose monitoring system: the PROMISE study. Diabetes Technol Ther. 2022;24(2):84–92. doi: 10.1089/dia.2021.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Laboudi AH, Godsland IF, Johnston DG, Oliver NS. Measures of glycemic variability in type 1 diabetes and the effect of real-time continuous glucose monitoring. Diabetes Technol Ther. 2016;18(12):806–812. doi: 10.1089/dia.2016.0146. [DOI] [PubMed] [Google Scholar]
- 27.Oskarsson P, Antuna R, Geelhoed-Duijvestijn P, Krӧger J, Weitgasser R, Bolinder J. Impact of flash glucose monitoring on hypoglycaemia in adults with type 1 diabetes managed with multiple daily injection therapy: a pre-specified subgroup analysis of the IMPACT randomised controlled trial. Diabetologia. 2018;61(3):539–550. doi: 10.1007/s00125-017-4527-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas MG, Avari P, Godsland IF, Lett AM, Reddy M, Oliver N. Optimizing type 1 diabetes after multiple daily injections and capillary blood monitoring: pump or sensor first? A meta-analysis using pooled differences in outcome measures. Diabetes Obes Metab. 2021;23(11):2521–2528. doi: 10.1111/dom.14498. [DOI] [PubMed] [Google Scholar]
- 29.Heinemann L, Freckmann G, Ehrmann D, Faber-Heinemann G, Guerra S, Waldenmaier D, et al. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet. 2018;391(10128):1367–1377. doi: 10.1016/S0140-6736(18)30297-6. [DOI] [PubMed] [Google Scholar]
- 30.Beck RW, Hirsch IB, Laffel L, Tamborlane WV, Bode BW, Buckingham B, et al. The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care. 2009;32(8):1378–1383. doi: 10.2337/dc09-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strategies to Enhance New CGM Use in Early Childhood (SENCE) Study Group A randomized clinical trial assessing continuous glucose monitoring (CGM) use with standardized education with or without a family behavioral intervention compared with fingerstick blood glucose monitoring in very young children with type 1 diabetes. Diabetes Care. 2021;44(2):464–472. doi: 10.2337/dc20-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck RW, Riddlesworth T, Ruedy K, Ahmann A, Bergenstal R, Haller S, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317(4):371–378. doi: 10.1001/jama.2016.19975. [DOI] [PubMed] [Google Scholar]
- 33.Munshi M, Slyne C, Davis D, Michals A, Sifre K, Dewar R, et al. Use of technology in older adults with type 1 diabetes: clinical characteristics and glycemic metrics. Diabetes Technol Ther. 2022;24(1):1–9. doi: 10.1089/dia.2021.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bratke H, Margeirsdottir HD, Assmus J, Njølstad PR, Skrivarhaug T. Does current diabetes technology improve metabolic control? A cross-sectional study on the use of insulin pumps and continuous glucose monitoring devices in a nationwide pediatric population. Diabetes Ther. 2021;12(9):2571–2583. doi: 10.1007/s13300-021-01127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roussel R, Riveline JP, Vicaut E, de Pouvourville G, Detournay B, Emery C, et al. Important drop in rate of acute diabetes complications in people with type 1 or type 2 diabetes after initiation of flash glucose monitoring in France: the RELIEF study. Diabetes Care. 2021;44(6):1368–1376. doi: 10.2337/dc20-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hilliard ME, Levy W, Anderson BJ, Whitehouse AL, Commissariat PV, Harrington KR, et al. Benefits and barriers of continuous glucose monitoring in young children with type 1 diabetes. Diabetes Technol Ther. 2019;21(9):493–498. doi: 10.1089/dia.2019.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cobry EC, Karami AJ, Meltzer LJ. Friend or foe: a narrative review of the impact of diabetes technology on sleep. Curr Diab Rep. 2022;22(7):283–290. doi: 10.1007/s11892-022-01468-x. [DOI] [PubMed] [Google Scholar]
- 38.Polonsky WH, Hessler D, Ruedy KJ, Beck RW. The impact of continuous glucose monitoring on markers of quality of life in adults with type 1 diabetes: further findings from the DIAMOND randomized clinical trial. Diabetes Care. 2017;40(6):736–741. doi: 10.2337/dc17-0133. [DOI] [PubMed] [Google Scholar]
- 39.Mulinacci G, Alonso GT, Snell-Bergeon JK, Shah VN. Glycemic outcomes with early initiation of continuous glucose monitoring system in recently diagnosed patients with type 1 diabetes. Diabetes Technol Ther. 2019;21(1):6–10. doi: 10.1089/dia.2018.0257. [DOI] [PubMed] [Google Scholar]
- 40.Patton SR, Noser AE, Youngkin EM, Majidi S, Clements MA. Early initiation of dabetes devices relates to improved glycemic control in children with recent-onset type 1 diabetes mellitus. Diabetes Technol Ther. 2019;21(7):379–384. doi: 10.1089/dia.2019.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, Freeman R, et al. 7. Diabetes technology: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S97–s112. doi: 10.2337/dc22-S007. [DOI] [PubMed] [Google Scholar]
- 42.Reddy M, Jugnee N, El Laboudi A, Spanudakis E, Anantharaja S, Oliver N. A randomized controlled pilot study of continuous glucose monitoring and flash glucose monitoring in people with Type 1 diabetes and impaired awareness of hypoglycaemia. Diabet Med. 2018;35(4):483–490. doi: 10.1111/dme.13561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hásková A, Radovnická L, Petruželková L, Parkin CG, Grunberger G, Horová E, et al. Real-time CGM is superior to flash glucose monitoring for glucose control in type 1 diabetes: the CORRIDA randomized controlled trial. Diabetes Care. 2020;43(11):2744–2750. doi: 10.2337/dc20-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Visser MM, Charleer S, Fieuws S, De Block C, Hilbrands R, Van Huffel L, et al. Comparing real-time and intermittently scanned continuous glucose monitoring in adults with type 1 diabetes (ALERTT1): a 6-month, prospective, multicentre, randomised controlled trial. Lancet. 2021;397(10291):2275–2283. doi: 10.1016/S0140-6736(21)00789-3. [DOI] [PubMed] [Google Scholar]
- 45.•• Sherr JL, Tauschmann M, Battelino T, de Bock M, Forlenza G, Roman R, et al. ISPAD clinical practice consensus guidelines 2018: diabetes technologies. Pediatr Diabetes. 2018;19 Suppl 27:302–25. Clinical practice guidelines regarding diabetes technology use in children, adolescents, and young adults with type 1 diabetes, developed by an international society for pediatric diabetes. Topics discussed include insulin pumps, continuous glucose monitors, sensor augmented pumps, and hybrid closed loop systems. [DOI] [PubMed]
- 46.Perez-Nieves M, Juneja R, Fan L, Meadows E, Lage MJ, Eby EL. Trends in U.S. insulin use and glucose monitoring for people with diabetes: 2009–2018. J Diabetes Sci Technol. 2021:19322968211028268. [DOI] [PMC free article] [PubMed]
- 47.Kesavadev J, Saboo B, Krishna MB, Krishnan G. Evolution of insulin delivery devices: from syringes, pens, and pumps to DIY artificial pancreas. Diabetes Ther. 2020;11(6):1251–1269. doi: 10.1007/s13300-020-00831-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karges B, Schwandt A, Heidtmann B, Kordonouri O, Binder E, Schierloh U, et al. Association of insulin pump therapy vs insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with type 1 diabetes. JAMA. 2017;318(14):1358–1366. doi: 10.1001/jama.2017.13994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marigliano M, Eckert AJ, Guness PK, Herbst A, Smart CE, Witsch M, et al. Association of the use of diabetes technology with HbA1c and BMI-SDS in an international cohort of children and adolescents with type 1 diabetes: the SWEET project experience. Pediatr Diabetes. 2021;22(8):1120–1128. doi: 10.1111/pedi.13274. [DOI] [PubMed] [Google Scholar]
- 50.Sherr JL, Hermann JM, Campbell F, Foster NC, Hofer SE, Allgrove J, et al. Use of insulin pump therapy in children and adolescents with type 1 diabetes and its impact on metabolic control: comparison of results from three large, transatlantic paediatric registries. Diabetologia. 2016;59(1):87–91. doi: 10.1007/s00125-015-3790-6. [DOI] [PubMed] [Google Scholar]
- 51.Miller KM, Beck RW, Foster NC, Maahs DM. HbA1c levels in type 1 diabetes from early childhood to older adults: a deeper dive into the influence of technology and socioeconomic status on HbA1c in the T1D exchange clinic registry findings. Diabetes Technol Ther. 2020;22(9):645–650. doi: 10.1089/dia.2019.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pala L, Dicembrini I, Mannucci E. Continuous subcutaneous insulin infusion vs modern multiple injection regimens in type 1 diabetes: an updated meta-analysis of randomized clinical trials. Acta Diabetol. 2019;56(9):973–980. doi: 10.1007/s00592-019-01326-5. [DOI] [PubMed] [Google Scholar]
- 53.Burckhardt MA, Smith GJ, Cooper MN, Jones TW, Davis EA. Real-world outcomes of insulin pump compared to injection therapy in a population-based sample of children with type 1 diabetes. Pediatr Diabetes. 2018;19(8):1459–1466. doi: 10.1111/pedi.12754. [DOI] [PubMed] [Google Scholar]
- 54.Haynes A, Hermann JM, Miller KM, Hofer SE, Jones TW, Beck RW, et al. Severe hypoglycemia rates are not associated with HbA1c: a cross-sectional analysis of 3 contemporary pediatric diabetes registry databases. Pediatr Diabetes. 2017;18(7):643–650. doi: 10.1111/pedi.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pickup JC, Sutton AJ. Severe hypoglycaemia and glycaemic control in Type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med. 2008;25(7):765–774. doi: 10.1111/j.1464-5491.2008.02486.x. [DOI] [PubMed] [Google Scholar]
- 56.Derosa G, Maffioli P, D'Angelo A, Salvadeo SA, Ferrari I, Fogari E, et al. Effects of insulin therapy with continuous subcutaneous insulin infusion (CSII) in diabetic patients: comparison with multi-daily insulin injections therapy (MDI) Endocr J. 2009;56(4):571–578. doi: 10.1507/endocrj.K08E-330. [DOI] [PubMed] [Google Scholar]
- 57.Jaser SS, Ellis D. Sleep in adolescents and young adults with type 1 diabetes: associations with diabetes management and glycemic control. Health Psychol Behav Med. 2016;4(1):49–55. doi: 10.1080/21642850.2015.1135293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maahs DM, Hermann JM, Holman N, Foster NC, Kapellen TM, Allgrove J, et al. Rates of diabetic ketoacidosis: international comparison with 49,859 pediatric patients with type 1 diabetes from England, Wales, the U.S., Austria, and Germany. Diabetes Care. 2015;38(10):1876–1882. doi: 10.2337/dc15-0780. [DOI] [PubMed] [Google Scholar]
- 59.Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311–320. doi: 10.1056/NEJMoa1002853. [DOI] [PubMed] [Google Scholar]
- 60.Hermanides J, Norgaard K, Bruttomesso D, Mathieu C, Frid A, Dayan CM, et al. Sensor-augmented pump therapy lowers HbA(1c) in suboptimally controlled Type 1 diabetes; a randomized controlled trial. Diabet Med. 2011;28(10):1158–1167. doi: 10.1111/j.1464-5491.2011.03256.x. [DOI] [PubMed] [Google Scholar]
- 61.Hermanides J, Devries JH. Sensor-augmented insulin pump more effective than multiple daily insulin injections for reducing HbA1C in people with poorly controlled type 1 diabetes. Evid Based Med. 2011;16(2):46–48. doi: 10.1136/ebm1159. [DOI] [PubMed] [Google Scholar]
- 62.O’Connell MA, Donath S, O'Neal DN, Colman PG, Ambler GR, Jones TW, et al. Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia. 2009;52(7):1250–1257. doi: 10.1007/s00125-009-1365-0. [DOI] [PubMed] [Google Scholar]
- 63.Coronel-Restrepo N, Blanco VM, Palacio A, Ramírez-Rincón A, Arbeláez S, Duque V, et al. Real-world effectiveness and safety of sensor-augmented insulin pump therapy in adults with type 1 diabetes: long-term follow-up. Endocrinol Diabetes Nutr (Engl Ed) 2021;68(8):567–572. doi: 10.1016/j.endien.2021.11.009. [DOI] [PubMed] [Google Scholar]
- 64.Tubili C, Pollakova D, Nardone MR, Di Folco U. Predictive low glucose suspend algorithm in real life: a five-year follow-up retrospective analysis. J Diabetes Sci Technol. 2021;15(6):1303–1307. doi: 10.1177/1932296820952107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garg S, Brazg RL, Bailey TS, Buckingham BA, Slover RH, Klonoff DC, et al. Reduction in duration of hypoglycemia by automatic suspension of insulin delivery: the in-clinic ASPIRE study. Diabetes Technol Ther. 2012;14(3):205–209. doi: 10.1089/dia.2011.0292. [DOI] [PubMed] [Google Scholar]
- 66.Forlenza GP, Li Z, Buckingham BA, Pinsker JE, Cengiz E, Wadwa RP, et al. Predictive low-glucose suspend reduces hypoglycemia in adults, adolescents, and children with type 1 diabetes in an at-home randomized crossover study: results of the PROLOG trial. Diabetes Care. 2018;41(10):2155–2161. doi: 10.2337/dc18-0771. [DOI] [PubMed] [Google Scholar]
- 67.Pinsker JE, Leas S, Müller L, Habif S. Real world improvements in hypoglycemia in an insulin-dependent cohort with diabetes mellitus pre/post tandem basal-IQ technology remote software update. Endocr Pract. 2020. [DOI] [PubMed]
- 68.Abraham MB, Nicholas JA, Smith GJ, Fairchild JM, King BR, Ambler GR, et al. Reduction in hypoglycemia with the predictive low-glucose management system: a long-term randomized controlled trial in adolescents with type 1 diabetes. Diabetes Care. 2018;41(2):303–310. doi: 10.2337/dc17-1604. [DOI] [PubMed] [Google Scholar]
- 69.Verbeeten KC, Perez Trejo ME, Tang K, Chan J, Courtney JM, Bradley BJ, et al. Fear of hypoglycemia in children with type 1 diabetes and their parents: effect of pump therapy and continuous glucose monitoring with option of low glucose suspend in the CGM TIME trial. Pediatr Diabetes. 2021;22(2):288–293. doi: 10.1111/pedi.13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cobelli C, Renard E, Kovatchev B. Artificial pancreas: past, present, future. Diabetes. 2011;60(11):2672–2682. doi: 10.2337/db11-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pease A, Lo C, Earnest A, Kiriakova V, Liew D, Zoungas S. Time in range for multiple technologies in type 1 diabetes: a systematic review and network meta-analysis. Diabetes Care. 2020;43(8):1967–1975. doi: 10.2337/dc19-1785. [DOI] [PubMed] [Google Scholar]
- 72.Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5(7):501–512. doi: 10.1016/S2213-8587(17)30167-5. [DOI] [PubMed] [Google Scholar]
- 73.Garg SK, Weinzimer SA, Tamborlane WV, Buckingham BA, Bode BW, Bailey TS, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2017;19(3):155–163. doi: 10.1089/dia.2016.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Forlenza GP, Pinhas-Hamiel O, Liljenquist DR, Shulman DI, Bailey TS, Bode BW, et al. Safety evaluation of the MiniMed 670G system in children 7–13 years of age with type 1 diabetes. Diabetes Technol Ther. 2018. [DOI] [PMC free article] [PubMed]
- 75.Forlenza GP, Ekhlaspour L, DiMeglio LA, Fox LA, Rodriguez H, Shulman DI, et al. Glycemic outcomes of children 2–6 years of age with type 1 diabetes during the pediatric MiniMed™ 670G system trial. Pediatr Diabetes. 2022. [DOI] [PMC free article] [PubMed]
- 76.Messer LH, Berget C, Vigers T, Pyle L, Geno C, Wadwa RP, et al. Real world hybrid closed loop discontinuation: predictors and perceptions of youth discontinuing the 670G system in the first 6 months. Pediatr Diabetes. 2019. [DOI] [PMC free article] [PubMed]
- 77.Berget C, Akturk HK, Messer LH, Vigers T, Pyle L, Snell-Bergeon J, et al. Real world performance of hybrid closed loop in youth, young adults, adults and older adults with type 1 diabetes: Identifying a clinical target for hybrid closed loop use. Diabetes Obes Metabol. 2021. [DOI] [PubMed]
- 78.Berget C, Messer LH, Vigers T, Frohnert BI, Pyle L, Wadwa RP, et al. Six months of hybrid closed loop in the real-world: an evaluation of children and young adults using the 670G system. Pediatr Diabetes. 2019. [DOI] [PMC free article] [PubMed]
- 79.Lal RA, Basina M, Maahs DM, Hood K, Buckingham B, Wilson DM. One year clinical experience of the first commercial hybrid closed-loop. Diabetes Care. 2019. [DOI] [PMC free article] [PubMed]
- 80.Carlson AL, Sherr JL, Shulman DI, Garg SK, Pop-Busui R, Bode BW, et al. Safety and glycemic outcomes during the MiniMed™ Advanced Hybrid Closed-Loop system pivotal trial in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2021. [DOI] [PubMed]
- 81.Place J, Robert A, Ben Brahim N, Keith-Hynes P, Farret A, Pelletier MJ, et al. DiAs web monitoring: a real-time remote monitoring system designed for artificial pancreas outpatient trials. J Diabetes Sci Technol. 2013;7(6):1427–1435. doi: 10.1177/193229681300700603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Keith-Hynes P, Mize B, Robert A, Place J. The diabetes assistant: a smartphone-based system for real-time control of blood glucose. Electronics. 2014;3(4):609. doi: 10.3390/electronics3040609. [DOI] [Google Scholar]
- 83.Brown SA, Kovatchev BP, Raghinaru D, Lum JW, Buckingham BA, Kudva YC, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381(18):1707–1717. doi: 10.1056/NEJMoa1907863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown SA, Kovatchev BP, Raghinaru D, Lum JW, Buckingham BA, Kudva YC, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019. [DOI] [PMC free article] [PubMed]
- 85.Breton MD, Kanapka LG, Beck RW, Ekhlaspour L, Forlenza GP, Cengiz E, et al. A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med. 2020;383(9):836–845. doi: 10.1056/NEJMoa2004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ekhlaspour L, Schoelwer MJ, Forlenza GP, DeBoer MD, Norlander L, Hsu LJ, et al. Safety and performance of the Tandem t:slim X2 with Control-IQ automated insulin delivery system in toddlers and preschoolers. Diabetes Technol Ther. 2020. [DOI] [PMC free article] [PubMed]
- 87.Tauschmann M, Thabit H, Bally L, Allen JM, Hartnell S, Wilinska ME, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet (London, England) 2018;392(10155):1321–1329. doi: 10.1016/S0140-6736(18)31947-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Forlenza GP, Lal RA. Current status and emerging options for automated insulin delivery systems. Diabetes Technol Ther. 2022. [DOI] [PMC free article] [PubMed]
- 89.Benhamou PY, Franc S, Reznik Y, Thivolet C, Schaepelynck P, Renard E, et al. Closed-loop insulin delivery in adults with type 1 diabetes in real-life conditions: a 12-week multicentre, open-label randomised controlled crossover trial. Lancet Digit Health. 2019;1(1):e17–e25. doi: 10.1016/S2589-7500(19)30003-2. [DOI] [PubMed] [Google Scholar]
- 90.Cobry EC, Berget C, Messer LH, Forlenza GP. Review of the Omnipod® 5 automated glucose control system powered by Horizon™ for the treatment of type 1 diabetes. Ther Deliv. 2020;11(8):507–519. doi: 10.4155/tde-2020-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brown SA, Forlenza GP, Bode BW, Pinsker JE, Levy CJ, Criego AB, et al. Multicenter trial of a tubeless, on-body automated insulin delivery system with customizable glycemic targets in pediatric and adult participants with type 1 diabetes. Diabetes Care. 2021. [DOI] [PMC free article] [PubMed]
- 92.Sherr JL, Bode BW, Forlenza GP, Laffel LM, Schoelwer MJ, Buckingham BA, et al. Safety and glycemic outcomes with a tubeless automated insulin delivery system in very young children with type 1 diabetes: a single-arm multicenter clinical trial. Diabetes Care. 2022. [DOI] [PMC free article] [PubMed]
- 93.Htay T, Soe K, Lopez-Perez A, Doan AH, Romagosa MA, Aung K. Mortality and cardiovascular disease in type 1 and type 2 diabetes. Curr Cardiol Rep. 2019;21(6):45. doi: 10.1007/s11886-019-1133-9. [DOI] [PubMed] [Google Scholar]
- 94.Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44(Suppl 2):S14–21. doi: 10.1007/PL00002934. [DOI] [PubMed] [Google Scholar]
- 95.Eeg-Olofsson K, Cederholm J, Nilsson PM, Zethelius B, Svensson AM, Gudbjornsdottir S, et al. Glycemic control and cardiovascular disease in 7,454 patients with type 1 diabetes: an observational study from the Swedish National Diabetes Register (NDR) Diabetes Care. 2010;33(7):1640–1646. doi: 10.2337/dc10-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lind M, Svensson AM, Kosiborod M, Gudbjornsdottir S, Pivodic A, Wedel H, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371(21):1972–1982. doi: 10.1056/NEJMoa1408214. [DOI] [PubMed] [Google Scholar]
- 97.Lind M, Svensson AM, Rosengren A. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2015;372(9):880–881. doi: 10.1056/NEJMc1415677. [DOI] [PubMed] [Google Scholar]
- 98.American Diabetes Association Cardiovascular disease and risk management: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S144–S174. doi: 10.2337/dc22-S010. [DOI] [PubMed] [Google Scholar]
- 99.Secrest AM, Becker DJ, Kelsey SF, LaPorte RE, Orchard TJ. All-cause mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes: the Allegheny County type 1 diabetes registry. Diabetes Care. 2010;33(12):2573–2579. doi: 10.2337/dc10-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Secrest AM, Becker DJ, Kelsey SF, Laporte RE, Orchard TJ. Cause-specific mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes. Diabetes. 2010;59(12):3216–3222. doi: 10.2337/db10-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Genuth SM, Backlund JY, Bayless M, Bluemke DA, Cleary PA, Crandall J, et al. Effects of prior intensive versus conventional therapy and history of glycemia on cardiac function in type 1 diabetes in the DCCT/EDIC. Diabetes. 2013;62(10):3561–3569. doi: 10.2337/db12-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lehto S, Ronnemaa T, Pyorala K, Laakso M. Poor glycemic control predicts coronary heart disease events in patients with type 1 diabetes without nephropathy. Arterioscler Thromb Vasc Biol. 1999;19(4):1014–1019. doi: 10.1161/01.ATV.19.4.1014. [DOI] [PubMed] [Google Scholar]
- 103.Foreman YD, van Doorn W, Schaper NC, van Greevenbroek MMJ, van der Kallen CJH, Henry RMA, et al. Greater daily glucose variability and lower time in range assessed with continuous glucose monitoring are associated with greater aortic stiffness: the Maastricht study. Diabetologia. 2021;64(8):1880–1892. doi: 10.1007/s00125-021-05474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gorst C, Kwok CS, Aslam S, Buchan I, Kontopantelis E, Myint PK, et al. Long-term glycemic variability and risk of adverse outcomes: a systematic review and meta-analysis. Diabetes Care. 2015;38(12):2354–2369. doi: 10.2337/dc15-1188. [DOI] [PubMed] [Google Scholar]
- 105.Bancks MP, Carson AP, Lewis CE, Gunderson EP, Reis JP, Schreiner PJ, et al. Fasting glucose variability in young adulthood and incident diabetes, cardiovascular disease and all-cause mortality. Diabetologia. 2019;62(8):1366–1374. doi: 10.1007/s00125-019-4901-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yapanis M, James S, Craig ME, O'Neal D, Ekinci EI. Complications of diabetes and metrics of glycemic management derived from continuous glucose monitoring. J Clin Endocrinol Metab. 2022;107(6):e2221–e2236. doi: 10.1210/clinem/dgac034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Snell-Bergeon JK, Roman R, Rodbard D, Garg S, Maahs DM, Schauer IE, et al. Glycaemic variability is associated with coronary artery calcium in men with Type 1 diabetes: the Coronary Artery Calcification in Type 1 Diabetes study. Diabet Med. 2010;27(12):1436–1442. doi: 10.1111/j.1464-5491.2010.03127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Frier BM. Hypoglycaemia in diabetes mellitus: epidemiology and clinical implications. Nat Rev Endocrinol. 2014;10(12):711–722. doi: 10.1038/nrendo.2014.170. [DOI] [PubMed] [Google Scholar]
- 109.Lung TW, Petrie D, Herman WH, Palmer AJ, Svensson AM, Eliasson B, et al. Severe hypoglycemia and mortality after cardiovascular events for type 1 diabetic patients in Sweden. Diabetes Care. 2014;37(11):2974–2981. doi: 10.2337/dc14-0405. [DOI] [PubMed] [Google Scholar]
- 110.Gimenez M, Lopez JJ, Castell C, Conget I. Hypoglycaemia and cardiovascular disease in Type 1 Diabetes. Results from the Catalan National Public Health registry on insulin pump therapy. Diabetes Res Clin Pract. 2012;96(2):e23–e25. doi: 10.1016/j.diabres.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 111.de Boer IH, Kestenbaum B, Rue TC, Steffes MW, Cleary PA, Molitch ME, et al. Insulin therapy, hyperglycemia, and hypertension in type 1 diabetes mellitus. Arch Intern Med. 2008;168(17):1867–1873. doi: 10.1001/archinternmed.2008.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nathan DM, Lachin J, Cleary P, Orchard T, Brillon DJ, Backlund JY, et al. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. 2003;348(23):2294–2303. doi: 10.1056/NEJMoa022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cleary PA, Orchard TJ, Genuth S, Wong ND, Detrano R, Backlund JY, et al. The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes. 2006;55(12):3556–3565. doi: 10.2337/db06-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.•• El Malahi A, Van Elsen M, Charleer S, Dirinck E, Ledeganck K, Keymeulen B, et al. Relationship between time in range, glycemic variability, HbA1c, and complications in adults with type 1 diabetes mellitus. J Clin Endocrinol Metab. 2022;107(2):e570-e81. Prospective multicenter observational cohort study assessing the impact of nationwide reimbursement of real-time continuous glucose monitors in adults with T1D who were already using insulin pumps, thereby advancing management to sensor augmented insulin pump therapy. Results found those with >70% time in range had lower A1c; time in range independently associated with microvascular complications and diabetes-related hospitalizations. [DOI] [PubMed]
- 116.de Oliveira LT, Cardoso JN, Lopes C, Carreira AR, Rodrigues-Barros S, Vide-Escada A, et al. The effect of insulin pump therapy in retinal vasculature in type 1 diabetic patients. Eur J Ophthalmol. 2021;31(6):3142–3148. doi: 10.1177/1120672121990576. [DOI] [PubMed] [Google Scholar]
- 117.Ferm ML, DeSalvo DJ, Prichett LM, Sickler JK, Wolf RM, Channa R. Clinical and demographic factors associated with diabetic retinopathy among young patients with diabetes. JAMA Netw Open. 2021;4(9):e2126126. doi: 10.1001/jamanetworkopen.2021.26126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marchand L, Kawasaki-Ogita Y, Place J, Fayolle C, Lauton D, Boulet F, et al. Long-term effects of continuous subcutaneous insulin infusion on glucose control and microvascular cin patients with type 1 diabetes. J Diabetes Sci Technol. 2017;11(5):924–929. doi: 10.1177/1932296817700161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ranjan AG, Rosenlund SV, Hansen TW, Rossing P, Andersen S, Nørgaard K. Improved time in range over 1 year is associated with reduced albuminuria in individuals with sensor-augmented insulin pump-treated type 1 diabetes. Diabetes Care. 2020;43(11):2882–2885. doi: 10.2337/dc20-0909. [DOI] [PubMed] [Google Scholar]
- 120.Reid LJ, Gibb FW, Colhoun H, Wild SH, Strachan MWJ, Madill K, et al. Continuous subcutaneous insulin infusion therapy is associated with reduced retinopathy progression compared with multiple daily injections of insulin. Diabetologia. 2021;64(8):1725–1736. doi: 10.1007/s00125-021-05456-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rosenlund S, Hansen TW, Andersen S, Rossing P. Effect of 4 years subcutaneous insulin infusion treatment on albuminuria, kidney function and HbA1c compared with multiple daily injections: a longitudinal follow-up study. Diabet Med. 2015;32(11):1445–1452. doi: 10.1111/dme.12950. [DOI] [PubMed] [Google Scholar]
- 122.Wysocka-Mincewicz M, Baszyńska-Wilk M, Gołębiewska J, Olechowski A, Byczyńska A, Hautz W, et al. Influence of metabolic parameters and treatment method on OCT angiography results in children with type 1 diabetes. J Diabetes Res. 2020;2020:4742952. doi: 10.1155/2020/4742952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zabeen B, Craig ME, Virk SA, Pryke A, Chan AK, Cho YH, et al. Insulin pump therapy is associated with lower rates of retinopathy and peripheral nerve abnormality. PLoS ONE. 2016;11(4):e0153033. doi: 10.1371/journal.pone.0153033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kamrath C, Tittel SR, Kapellen TM, von dem Berge T, Heidtmann B, Nagl K, et al. Early versus delayed insulin pump therapy in children with newly diagnosed type 1 diabetes: results from the multicentre, prospective diabetes follow-up DPV registry. Lancet Child Adolesc Health. 2021;5(1):17–25. doi: 10.1016/S2352-4642(20)30339-4. [DOI] [PubMed] [Google Scholar]
- 125.Derosa G, Catena G, Scelsi L, D'Angelo A, Raddino R, Cosentino E, et al. Glyco-metabolic control, inflammation markers, and cardiovascular outcomes in type 1 and type 2 diabetic patients on insulin pump or multiple daily injection (italico study) Diabetes Metab Res Rev. 2020;36(1):e3219. doi: 10.1002/dmrr.3219. [DOI] [PubMed] [Google Scholar]
- 126.Steineck I, Cederholm J, Eliasson B, Rawshani A, Eeg-Olofsson K, Svensson AM, et al. Insulin pump therapy, multiple daily injections, and cardiovascular mortality in 18,168 people with type 1 diabetes: observational study. BMJ. 2015;350:h3234. doi: 10.1136/bmj.h3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tubili C, Folco UD, Nardone MR, Clementi A. A single-center long-term continuous subcutaneous insulin infusion (CSII) experience: higher fractional use is associated with less diabetes complications. J Diabetes Sci Technol. 2017;11(5):1057–1058. doi: 10.1177/1932296817702170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 129.Heier M, Stensæth KH, Brunborg C, Seljeflot I, Margeirsdottir HD, Hanssen KF, et al. Increased arterial stiffness in childhood onset diabetes: a cardiovascular magnetic resonance study. Eur Heart J Cardiovasc Imaging. 2018;19(6):694–700. doi: 10.1093/ehjci/jex178. [DOI] [PubMed] [Google Scholar]
- 130.Rosenlund S, Theilade S, Hansen TW, Andersen S, Rossing P. Treatment with continuous subcutaneous insulin infusion is associated with lower arterial stiffness. Acta Diabetol. 2014;51(6):955–962. doi: 10.1007/s00592-014-0619-6. [DOI] [PubMed] [Google Scholar]
- 131.Gimbrone MA, Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118(4):620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Faienza MF, Scicchitano P, Lamparelli R, Zaza P, Cecere A, Brunetti G, et al. Vascular and myocardial function in young people with type 1 diabetes mellitus: insulin pump therapy versus multiple daily injections insulin regimen. Exp Clin Endocrinol Diabetes. 2022;130(6):415–422. doi: 10.1055/a-1523-7574. [DOI] [PubMed] [Google Scholar]
- 133.Beck RW, Bergenstal RM, Riddlesworth TD, Kollman C, Li Z, Brown AS, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2018. [DOI] [PMC free article] [PubMed]
- 134.Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The fallacy of average: how using HbA1c alone to assess glycemic control can be misleading. Diabetes Care. 2017;40(8):994–999. doi: 10.2337/dc17-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bergenstal RM, Gal RL, Connor CG, Gubitosi-Klug R, Kruger D, Olson BA, et al. Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med. 2017;167(2):95–102. doi: 10.7326/M16-2596. [DOI] [PubMed] [Google Scholar]
- 136.Bergenstal RM, Beck RW, Close KL, Grunberger G, Sacks DB, Kowalski A, et al. Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care. 2018;41(11):2275–2280. doi: 10.2337/dc18-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Klonoff DC, Wang J, Rodbard D, Kohn MA, Li C, Liepmann D, et al. A glycemia risk index (GRI) of hypoglycemia and hyperglycemia for continuous glucose monitoring validated by clinician ratings. J Diabetes Sci Technol. 2022:19322968221085273. [DOI] [PMC free article] [PubMed]
- 138.Messer LH, Berget C, Forlenza GP. A clinical guide to advanced diabetes devices and closed-loop systems using the CARES paradigm. Diabetes Technol Ther. 2019;21(8):462–469. doi: 10.1089/dia.2019.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Messer L, Berget C. PANTHER program. 2022. https://www.pantherprogram.org/. Accessed 12 July 2022.
- 140.Alva S BR, Castorino K, Liljenquist D, Liu H, Kipnes M. Performance of the FreeStyle Libre 3 System. Presented at ADA 82nd Scientific Sessions; New Orleans, LA. June 5, 2022.