Abstract
A simple and rapid method of simultaneously determining 15 Streptococcus pneumoniae serotypes was developed. Fifteen latex beads of different sizes and different red fluorescence levels were coated with 1 of 15 serotypes (1, 3, 4, 5, 6A, 6B, 7F, 9N, 9V, 14, 18C, 19A, 19F, 22F, and 23F) of pneumococcal capsular polysaccharide (PS). The bead mixture was incubated with individual pneumococcal lysate, a pool of rabbit antisera capable of binding the 15 serotypes, and fluorescein (green fluorescence)-conjugated anti-rabbit antibody. Bead size, red fluorescence, and green fluorescence were measured in a single flow cytometer run. The green fluorescence of the beads was inhibited only when there was a serotypic match between PS on the bead and PS in the pneumococcal lysate. This method distinguished cross-reactive serotypes and correctly identified the serotypes in 100% of 86 pneumococcal isolates tested.
Infections with Streptococcus pneumoniae are a significant problem for young children and older adults. Even with effective antibiotic treatment, patients with pneumococcal infections can have serious sequelae, and there is a rapid spread of antibiotic-resistant S. pneumoniae. Thus, the ability to prevent pneumococcal disease with vaccines remains critical to the optimal management of the pneumococcal disease. Although the 23-valent polysaccharide (PS) vaccine is currently available, it is not immunogenic in young children and fails to protect at least 30% of the immunized elderly (10). In an effort to prepare vaccines effective among young children and more effective in the elderly, conjugate vaccines containing PS linked to a protein moiety, analogous to the Haemophilus influenzae type b vaccine, are being developed with up to 11 PS serotypes in clinical trials (9).
Once the vaccine is licensed, its effectiveness will need to be monitored. Since the protection provided by the pneumococcal vaccines is serotype specific, the effective vaccine should selectively reduce the prevalence of S. pneumoniae expressing the vaccine serotypes without altering the prevalence of S. pneumoniae expressing the nonvaccine serotypes (1). In addition, S. pneumoniae has been shown to undergo in vivo transformation of capsular serotypes (8), and the vaccine may result in an increased frequency of strains with nonvaccine serotypes. The vaccine may therefore even force the appearance of new, virulent pneumococcal strains expressing some of the nonvaccine serotypes. Thus, serotypes of S. pneumoniae need to be monitored following the introduction of new pneumococcal vaccines.
Serotyping S. pneumoniae is not simple because over 20 serotypes are common among clinical isolates. Although several serotyping methods are available (2, 5, 7), the present typing system is laborious and slow and requires considerable technical experience. Consequently, all these methods are ill suited for efficacy studies of new vaccines, and there is a need for new techniques that can rapidly and reliably determine pneumococcal capsular serotypes of a large number of isolates. We report the development and evaluation of a simple and efficient flow cytometric method, with which a pneumococcal isolate could be simultaneously tested for 15 of the most common serotypes.
MATERIALS AND METHODS
Preparation of bead set coated with different pneumococcal capsular PS serotypes.
Latex beads of five different diameters ranging from 2 to 4.76 μm were obtained from Bangs Laboratories (Fisher, Ind.). They were dyed with Did oil (Molecular Probes, Eugene, Oreg.) to prepare them with three different levels (none, low, and high) of red fluorescence. To dye the beads with Did oil, they were mixed with the dye in dimethyl sulfoxide and incubated overnight with shaking at room temperature. The range of dye concentration for beads was 1 to 10 μg/ml for a low level of fluorescence and 1 to 5 mg/ml for a high level of fluorescence. The beads were washed with 0.25% Triton X-100 several times and then stored in the same solution.
Each bead was coated with 1 of 15 pneumococcal capsular PS serotypes (1, 3, 4, 5, 6A, 6B, 7F, 9N, 9V, 14, 18C, 19A, 19F, 22F, and 23F). Each bead preparation (about 1 to 200 μl depending on serotype) was washed with water (about 0.5 to 10 ml) by centrifugation and suspended in phosphate-buffered saline (PBS) (about 0.01 to 0.5 ml) containing capsular PS at 0.1% (wt/vol). The suspension was incubated overnight at room temperature with shaking. The beads were washed with 2 volumes of wash solution (0.05% Tween 20 in normal saline) and incubated with dilution buffer (1% bovine serum albumin, 0.05% Tween 20, PBS) for 30 min at room temperature. The 15 different bead preparations were then mixed together and used in the assay. Each batch permitted testing of about 100 samples.
Preparation of pneumococcal lysates.
Pneumococcal isolates used for this study were 65 laboratory strains stored in the University of Rochester and University of Alabama at Birmingham and 21 clinical isolates collected in Barnes-Jewish Hospital (St. Louis, Mo.) in 1998 from patients with pneumococcal sepsis and meningitis. All bacteria were serotyped prior to our study by agglutination reaction and/or Quellung reaction at the University of Alabama at Birmingham and the Centers for Disease Control and Prevention (Atlanta, Ga.). The bacteria were grown on sheep blood agar plates at 37°C in a candle jar overnight. A few colonies from each agar plate were applied to 100 μl of Todd-Hewitt broth (Difco Laboratories, Detroit, Mich.) supplemented with 0.5% yeast extract, 1% glucose, and 1% sheep erythrocytes (Colorado Serum Company, Denver, Colo.) in a well of a microtiter plate. After an overnight incubation at 37°C, the culture was mixed with an equal volume of lysis buffer (0.2% sodium deoxycholate, 0.02% sodium dodecyl sulfate, 0.3 M sodium citrate). After a 15-min incubation at 37°C, the cell lysate was centrifuged at 10,000 × g for 5 min. The supernatant was then mixed with 5 volumes of dilution buffer.
Multibead assay.
Fifty microliters of the latex bead mixture prepared as described above was added to the well of a U-bottomed 96-well microplate (Nalge Nunc International, Rochester, N.Y.). The beads were pelleted by centrifugation. To each pellet was added 30 μl of diluted bacterial culture lysate followed by 30 μl of rabbit antiserum Pool PT. The antiserum Pool PT was prepared by mixing equal volumes of rabbit pneumococcal antiserum pools P, Q, R, S, and T from Statens Seruminstitut (Copenhagen, Denmark). Before use, Pool PT was diluted 200-fold with dilution buffer containing 40 μg of pneumococcal cell wall PS per ml. After a 30-min incubation at room temperature, the beads were washed once by centrifugation and mixed with 60 μl of fluorescein-conjugated goat anti-rabbit immunoglobulin M (IgM) and IgG (Southern Biotechnology Associates, Birmingham, Ala.) diluted 50-fold in dilution buffer. After a 30-min incubation at room temperature, the beads were washed, resuspended in PBS containing 0.1% Tween 20, and analyzed in a flow cytometer (FACSCalibur; Becton Dickinson, San Jose, Calif.) by measuring forward scatter (size), red fluorescence by DiD oil, and green fluorescence by fluorescein isothiocyanate. The data were analyzed with CellQuest software (Becton Dickinson).
Analysis of serotype specificity of the multibead assay.
To assess the specificity of the multibead assay, a mouse monoclonal anti-6B antibody (Hyp6BM9) was examined in two different assay formats. First, its performance was studied in the multibead assay format with some modification. Instead of Pool PT, hybridoma supernatants of Hyp6BM9 were added to beads in the presence of various concentrations of 6A or 6B PS. Also, in place of fluorescent anti-rabbit antibody, fluorescein-conjugated anti-mouse immunoglobulins (Southern Biotechnology Associates) were used. Geometric mean values of green fluorescence of 6B-coated beads were used to calculate the percent inhibition of antibody bound. In the second assay, the ability of the Hyp6BM9 hybridoma antibody to bind 6A or 6B PS immobilized on the plastic surface was measured using a sandwich-type assay described before (13). Briefly, a serial dilution of the Hyp6BM9 antibody was added to microplate wells coated with 10 μg of 6A or 6B PS per ml. The wells were washed. Antibody bound to the wells was detected by alkaline phosphatase-conjugated anti-mouse IgM (Sigma) and p-nitrophenyl phosphate substrate.
RESULTS AND DISCUSSION
Development of the assay system.
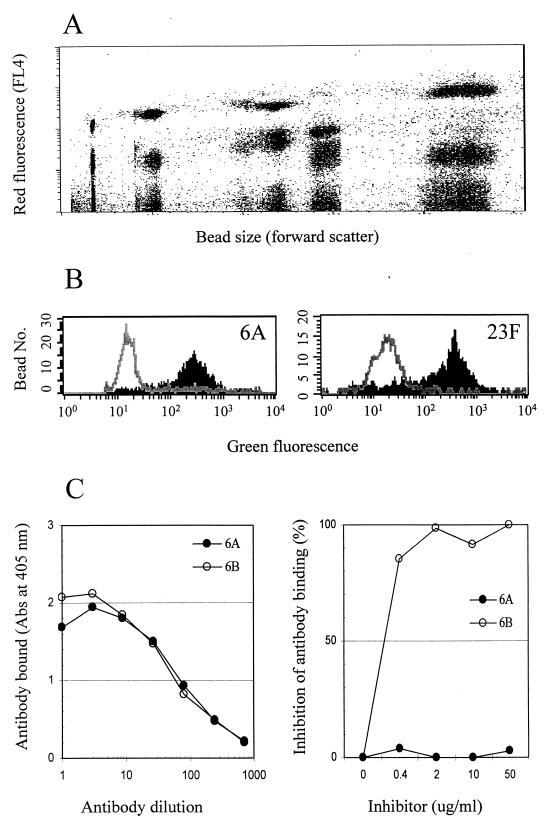
All assay parameters were optimized empirically in preliminary studies. By testing various concentrations of the Did oil and beads of different sizes, we produced 15 types of beads that were clearly distinguished from each other by flow cytometry, using their forward scatter and red fluorescence (Fig. 1A). Subsequently, each bead was coated with 1 of the 15 pneumococcal PS serotypes. When the assay was performed with the mixture of the 15 beads, green-fluorescence histograms for the 15 serotypes could be readily obtained by gating on individual beads in the plot of the forward scatter and red fluorescence (Fig. 1B). When there was a serotypic match between a pneumococcal isolate and a latex bead, the green fluorescence of that specific bead type was markedly reduced (Fig. 1B). In all cases we have tested so far, there was a decrease (>90%) in green fluorescence in only one bead type and no decrease (<10%) in any of the other bead types. Seventy percent inhibition was used as the cutoff. The assay thus permits a unique, unambiguous positive or negative assignment for each of the 15 serotypes.
FIG. 1.
Fluorescence analysis. (A) Red fluorescence and forward scatter of 15 different types of beads. (B) Histograms of green fluorescence for two types of beads. The shaded areas are histograms before inhibition; the open areas are histograms after inhibition. (C) The left panel shows that Hyp6BM9 can bind to 6A and 6B immobilized on the microtiter plate surface. The right panel shows inhibition of binding of Hyp6BM9 to 6B-coated latex particles in the presence of 6B PS or 6A PS in solution.
Increased assay specificity.
Our test panel contained pneumococci expressing several closely related serotypes (e.g., 6A versus 6B or 19A versus 19F). Initial studies unexpectedly suggested that our multibead assay was able to distinguish these serotypes (data not shown) even though the rabbit serum used for the assay was not designed to distinguish them. To confirm the increased specificity, Hyp6BM9 was tested for its reactivity to 6A and 6B in two different formats of the assay. This monoclonal antibody bound equally well to 6A and 6B immobilized on the microplate surface (Fig. 1C). However, the binding of Hyp6BM9 to 6B-coated beads was inhibited by 6B PS but not by 6A PS (Fig. 1C). Our multibead assay therefore can be more specific than other types of assays even when the same reagent is used.
Evaluation of the multibead assay.
The performance of the multibead assay was evaluated with a panel of 86 pneumococcal isolates of 26 serotypes (Table 1) common among clinical isolates. Blind testing of the 86 isolates correctly identified the serotypes of 83 of 86. When the three strains in question were checked by the Quellung test again, the new results agreed with the multibead assay results in all three cases. While it should be evaluated with additional samples in the future, our new method appears to have correctly identified the serotypes of the 86 isolates with 100% sensitivity and specificity. Since our multibead assay can identify all the serotypes included in the 11-valent conjugate vaccines currently under investigation, it should be useful for assessing their efficacy without any further modifications.
TABLE 1.
Serotyping results of 86 pneumococcal isolates with multibead assay
| Serotype | No. of isolates
|
|
|---|---|---|
| Assigneda | Multibead assay | |
| 1 | 2 | 2 |
| 3 | 4 | 4 |
| 4 | 6 | 6 |
| 5 | 2 | 2 |
| 6A | 10 | 10 |
| 6B | (7 − 1) | 6 |
| 7F | (3 − 1) | 2 |
| 9N | 2 | 2 |
| 9V | 3 | 3 |
| 14 | 8 | 8 |
| 18C | (5 − 1) | 4 |
| 19A | 5 | 5 |
| 19F | (4 + 1) | 5 |
| 22F | 3 | 3 |
| 23F | 3 | 3 |
| Others | (19 + 2) | 21 |
The numbers in parentheses reflect the adjustment in assignment that took place after the Quellung reaction, which was reperformed to address inconsistencies with the multibead assay results. For instance, seven isolates were originally labeled to be 6B. However, a repeat Quellung assay showed one isolate to have a different serotype. Thus, only six isolates were truly 6B.
Because of the clinical importance, various methods of serotyping pneumococci have been developed, which include the Quellung test (4), fluorescent antibody technique (14), capillary precipitin test, counterimmunoelectrophoresis (5), latex agglutination (7), coagglutination (11), and dot blot assay (2). Also, the use of serum pools has reduced the number of tests and reagents needed to characterize a clinical isolate (12). The multibead assay compares favorably to all these methods in terms of assay speed, reliability, specificity, and consumption of antisera. For instance, the Quellung test, still the reference method for serotyping pneumococci, requires a substantial amount of antisera, labor, and time. Agglutination and dot blot assay are popular since they are relatively simple and reliable and require small amounts of antisera. But they still require testing each pneumococcal isolate at multiple stages to minimize the amount of work and number of reagents. In contrast, the multibead assay is simple and rapid, and all the tests necessary for an isolate can be performed at one time, not at multiple stages. The one disadvantage of the multibead method, however, is the requirement for a flow cytometer.
In addition to serotyping pneumococci, this multibead assay should be useful for many analytical situations. More than 15 lysates could be analyzed simultaneously because the additional beads could be labeled with additional markers. Indeed, since Horan and Wheeless first described this analytical technology in the late 1970s (6), there have been gradual improvements. Up to 50 to 100 analytes can be measured in some commercial systems (3). While we used it to obtain qualitative information, the method may be able to quantitatively measure levels of antibodies to the different PSs or PS quantity in lysates. This assay system would drastically reduce the volume of samples needed and would be extremely useful in the analysis of sera from young children. For instance, with this method, the level in serum of antibodies to all pneumococcal serotypes included in the pneumococcal vaccines could be determined simultaneously.
ACKNOWLEDGMENTS
We acknowledge excellent technical help by Janice King. We also appreciate help by Joan Hoppe-Bauer, Ann Niles, and J. Besser for providing us with bacterial isolates.
This work was supported by grants from the National Institutes of Health (AI-85334) to M.H.N. and (AI-21548) to D.E.B. M.H.N. is partially supported by NIAID contract NO1 AI-45248.
REFERENCES
- 1.Butler J C, Breiman R F, Campbell J F, Lipman H B, Broome C V, Facklam R R. Pneumococcal polysaccharide vaccine efficacy. An evaluation of current recommendations. JAMA. 1993;270:1826–1831. [PubMed] [Google Scholar]
- 2.Fenoll A, Jado I, Vicioso D, Casal J. Dot blot assay for the serotyping of pneumococci. J Clin Microbiol. 1997;35:764–766. doi: 10.1128/jcm.35.3.764-766.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fulton R J, McDade R L, Smith P L, Kienker L J, Kettman J R., Jr Advanced multiplexed analysis with the FlowMetrix system. Clin Chem. 1997;43:1749–1756. [PubMed] [Google Scholar]
- 4.Henrichsen J, Berntsson E, Kaijser B. Comparison of counterimmunoelectrophoresis and the capsular reaction test for typing of pneumococci. J Clin Microbiol. 1980;11:589–592. doi: 10.1128/jcm.11.6.589-592.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holliday M G. Pneumococcal typing by polyvalent counterimmunoelectrophoresis. J Immunol Methods. 1981;46:243–249. doi: 10.1016/0022-1759(81)90140-x. [DOI] [PubMed] [Google Scholar]
- 6.Horan P K, Wheeless L L., Jr Quantitative single cell analysis and sorting. Science. 1977;198:149–157. doi: 10.1126/science.905822. [DOI] [PubMed] [Google Scholar]
- 7.Kaldor J, Asznowicz R, Dwyer R. Serotyping of Streptococcus pneumoniae by latex agglutination. Pathology. 1988;20:45–47. doi: 10.3109/00313028809085195. [DOI] [PubMed] [Google Scholar]
- 8.Nesin M, Ramirez M, Tomasz A. Capsular transformation of a multidrug-resistant Streptococcus pneumoniae in vivo. J Infect Dis. 1998;177:707–713. doi: 10.1086/514242. [DOI] [PubMed] [Google Scholar]
- 9.Rennels M B, Edwards K M, Keyserling H L, Reisinger K S, Hogerman D A, Madore D V, Chang I, Paradiso P R, Malinoski F J, Kimura A. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 in United States infants. Pediatrics. 1998;101:604–611. doi: 10.1542/peds.101.4.604. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro E D, Berg A T, Austrian R, Schroeder D, Parcells V, Margolis A, Adair R K, Clemens J D. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991;325:1453–1460. doi: 10.1056/NEJM199111213252101. [DOI] [PubMed] [Google Scholar]
- 11.Smart L E. Serotyping of Streptococcus pneumoniae strains by coagglutination. J Clin Pathol. 1986;39:328–331. doi: 10.1136/jcp.39.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sørensen U B S. Typing of pneumococci by using 12 pooled antisera. J Clin Microbiol. 1993;31:2097–2100. doi: 10.1128/jcm.31.8.2097-2100.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Y, Park M K, Kim J, Diamond B, Solomon A, Nahm M H. Repertoire of human antibodies against the polysaccharide capsule of Streptococcus pneumoniae serotype 6B. Infect Immun. 1999;67:1172–1179. doi: 10.1128/iai.67.3.1172-1179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wicher K, Kalinka C, Mlodozeniec P, Rose N R. Fluorescent antibody technic used for identification and typing of Streptococcus pneumoniae. Am J Clin Pathol. 1982;77:72–77. doi: 10.1093/ajcp/77.1.72. [DOI] [PubMed] [Google Scholar]