Abstract
Background
Between people with and without inflammatory bowel disease (IBD), there was no statistically significant difference in the probability of contracting the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). However, the risk of adverse outcomes in IBD patients after virus infection remains unclear.
Methods
Eligible studies conducted from January 1, 2020 to March 17, 2022 were obtained by searching PubMed, Embase, and Web of Science. Information was collected in tables from the included studies. Random-effects and fixed-effects models were used as measures for the pooled estimates. All data were estimated by R version 4.1.3.
Results
Twenty-four studies were included. The risk ratio (RR) of adverse outcomes in COVID-19 patients with IBD increased by 32% (RR 1.32; 95% CI 1.06–1.66) relative to COVID-19 patients without IBD. The RR of mortality was higher in COVID-19 patients with IBD from Europe (RR 1.72; 95% CI 1.11–2.67) than in those that were not from Europe (RR 1.00; 95% CI 0.79–1.26; χ2 = 4.67; P = 0.03). Patients with ulcerative colitis were at higher risk of adverse outcomes after SARS-CoV-2 infection than patients with Crohn’s disease patients (RR1.38; 95% CI 1.27–1.50). The IBD drugs treatment was associated with the risk of adverse outcomes, the pooled odds ratio (OR) of mesalazine (1.79; 95% CI 1.59–2.02), immunomodulators (1.30; 95% CI 1.10–1.53), and anti-TNF (0.47; 95% CI 0.41–0.53) were assessed.
Conclusion
COVID-19 patients with IBD had an increased risk of adverse outcomes than those without IBD, whereas anti-TNF treatment might reduce the risk.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00384-022-04265-w.
Keywords: SARS-CoV-2, Adverse outcome, IBD, IBD drug, Meta-analysis
Introduction
The coronavirus disease 2019 (COVID-19) has exerted the most significant impact on human health among the epidemics in the last 100 years [1, 2]. As of May 29, 2022, more than 526 million people had been infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and over six million died due to the virus [3]. Preliminary studies have shown that advanced age, being male, high BMI, and pre-existing chronic diseases increase the risk of developing adverse forms and fatal outcomes [4, 5]. The entry of SARS-CoV-2 into host cells depend on the interactions of viral spike protein and angiotensin-converting enzyme 2 (ACE-2) [6, 7]. Thus, high ACE-2 expression levels in intestinal epithelial cells and SARS-CoV-2 may cause intestinal symptoms or results in poor prognosis in patients with chronic intestinal diseases [8–11].
Inflammatory bowel disease (IBD) refers to a group of disabling chronic and immune-mediated inflammatory disorders including ulcerative colitis (UC) and Crohn’s disease (CD) and is associated with human immune system [12]. In 2017, approximately 6.8 million patients with IBD were recorded worldwide [13], including 2 million from Europe and 1.5 million from North America [14]. Notably, ACE-2 expression increases in patients with IBD, particularly in the colonic tissue of patients with UC [8, 15], which might enable SARS-CoV-2 infection and cause poor outcomes [16]. The intestine might serve as an entry point for serious COVID-19 complications, such as endotoxemia and thrombosis [17]. In addition, a significant proportion of patients with IBD are treated with IBD drugs, including mesalazine, corticosteroids, immunomodulators (IMs), and anti-TNF, which may be associated with low immunity in patients and increased risk of COVID-19 infection and adverse outcomes [18–21].
Given these premises, a much-debated question is whether patients with IBD are at increased risk of being infected by COVID-19 and developing adverse outcomes [22–27]. Currently, the world is going through massive waves of infections by the omicron and delta variants of SARS-CoV-2, and the vast majority of people seem to be susceptible to the omicron variant [28]. Although the virulence of this variant has weakened and disease severity has been reduced through vaccination [29], the vast waves of omicron infections have indicated increasing number of adverse outcomes [28], especially in people with underlying diseases [30–32]. Therefore, focusing on adverse outcomes, such as hospitalization, intensive care unit (ICU), and mortality in COVID-19 patients with IBD in the context of high infectivity of SARS-CoV-2 is critical.
To date, the risk of adverse outcomes in patients with IBD after SARS-CoV-2 infection is contradictory in different studies [25–27, 33], and a meta-analysis assessed this risk in COVID-19 patients with and without IBD has not been conducted. Therefore, we performed the meta-analysis. Then, the association between adverse outcomes and IBD drug treatment in COVID-19 patients with IBD was explored.
Materials and methods
Search strategy
We systematically searched electronic databases (PubMed, Embase, and Web of Science) from January 1, 2020 to March 17, 2022 by three independent authors (CL, HK, and CC). The following combined free-text terms and MeSH terms with no language limitation were used: COVID-19 (such as “COVID-19,” “SARS-CoV-2,” “2019 Novel Coronavirus,” “2019-nCoV,” “Coronavirus Disease-19,” “2019-nCoV Disease,” or “severe COVID-19”) and IBD (such as “inflammatory bowel disease,” “ulcerative colitis,” “Crohn disease,” “enteritides,” “bowel disease,” “IBD,” “UC,” or “CD”) were adopted in the search strategies. In addition, we manually searched the lists of references of relevant articles to prevent omission. This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Selection criteria
We used the PECO strategy (patient, exposure, comparison, outcome) in constructing research questions and searching evidence. The meta-analysis adopted the following inclusion criteria: (a) prevalence of adverse outcomes in COVID-19 patients with and without IBD can be calculated; (b) prevalence of adverse outcomes in patients suffering from different types of IBD (UC and CD) and infected with SARS-CoV-2 can be calculated; or (c) provided medication status (mesalazine, corticosteroids, IMs, and anti-TNF) in adverse and mild cases.
Adverse outcomes were defined as requiring hospitalization, invasive ventilation, or intensive care unit (ICU) admission, or death [34], and mild outcomes were defined as presenting with mild or no symptoms of COVID-19 and without adverse outcomes. The study included cross-sectional, cohort, case–control, and case series studies. Animal experiments, literature without complete original data and no access to original data, and single case reports were excluded.
Data extraction and quality assessment
First, two authors (CL and HK) independently analyzed the titles and abstracts to exclude irrelevant studies. Subsequently, the full texts of the included studies were further reviewed. In the case of any disagreement, a third reviewer was consulted (CC).
The following pieces of information were extracted from the included studies: first author, study name, type of study design, publication year, country, number of COVID-19 patients with IBD, number of adverse outcomes in patients with IBD, number of comparators (COVID-19 patients without IBD), number of comparators with adverse outcomes, type of IBD (UC and CD), demographic information (age, gender, and comorbidity), and ongoing IBD treatments, including mesalazine, corticosteroids, IMs (including azathioprine, mercaptopurine, and methotrexate), and anti-TNF. The Newcastle–Ottawa scale (NOS) was used in evaluating the quality of eligible studies [35]. Each study has a maximum score of nine (highest quality), and a NOS score of ≥ 6 indicated high quality.
Data analysis
The RR was used as a unified effect size for assessing the risk of adverse outcomes in COVID-19 patients with IBD and those without and in patients with UC or CD. And the odds ratio (OR) was used in estimating the association between IBD drugs and adverse outcomes. Random-effects models (I2 > 50%) and common-effects models (I2 ≤ 50%) were used in estimating the pooled adjusted effect, and Q test and I2 statistics were used in assessing heterogeneity among the studies. An I2 value of < 25% demonstrated no heterogeneity among the studies, 25–50% indicated low heterogeneity, and > 50% indicated moderate-to-high heterogeneity. For subgroup analyses, the studies were stratified by region, the source of the population, gender, age, disease type, and sample size. We further conducted sensitivity analysis by sequentially eliminating each study to assess the stability of the results. Egger’s test and funnel plots were used in evaluating publication bias.
A two-tailed P < 0.05 was considered statistically significant in all the analyses, which were performed with R version 4.1.3 and RStudio (the integrated development environment of R) with meta-packages.
Results
Study selection and characteristics
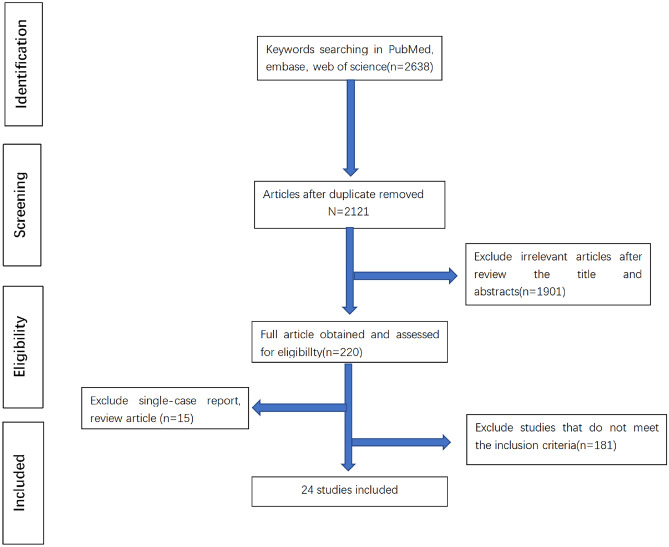
The exclusion and inclusion processes for articles are presented in Fig. 1. A total of 2638 articles were identified in the databases. After duplicates were excluded, titles and abstracts of 2121 articles were screened, and full-text reading was performed in 223 studies. Finally, 24 articles met the inclusion criteria, and data from the SECURE-IBD registry were included (date of last update: January 25, 2022). Nine studies evaluated the risk of adverse outcomes in patients with IBD and COVID-19 and comparative population, and 14 studies evaluated the risk in patients with UC or CD. a total of 15 studies analyzed IBD drug exposure in adverse and mild cases. Among these studies, 13 were conducted in European countries, seven in North American countries (six in the USA and one in Canada), three in Asia, and one in multiple healthcare organizations.
Fig. 1.
Study selection flowchart. A total of 2638 studies were obtained from three databases: PubMed (N = 886), Embase (N = 901), and Web of Science (N = 871), by keyword search
Table 1 provides the included studies’ main characteristics, including type of research, location, publication date, number of subjects, use of IBD drugs, comorbidities, types of inclusion criteria (a, b, and c), and NOS score. Among the included studies, 20 respected the NOS for good-quality research, and three case series and one cross-sectional study had unclear answers.
Table 1.
Demographics of the patients in the included studies
| Authors | Location | Type of study | COVID-19 patients with IBD, N | Age(years) | Female, N (%) | Compare population, N | Patients with adverse outcomes, N | IBD drugs | Comorbidities, N | Inclusion criteria | NOS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IBD Comparators | ||||||||||||
| Ardizzone et al. [18] | Italy | Retrospective Cohort | 7 (4 UC, 3 CD) | 56 (26–78) | 4(57.1) | 85,481 |
4 (2 UC, 2 CD) Death: 2 |
42,942 Death:15,597 |
5-ASA 1 Steroid 1 Biologicals 7 |
2 | a and c | 7 |
| Maconi et al. [22] | Italy | Case control | 2 | NA | NA | 10 | 1 | 6 | NA | NA | a | 6 |
| Singh et al. [25] | multiple health care organizations (HCOs) globally | Retrospective Cohort | 232 | 51.2 ± 18.1 | 147 (63.4) | 232 | 56 | 60 |
5-ASA 32 Steroid 111 IMS 62 Biologicals 37 |
Essential hypertension: 121 COPD and asthma: 91 DM: 62 |
a | 7 |
| Attauabi et al. [26] | Denmark | Prospective Cohort | 516 (319 UC, 197 CD) |
UC 48 (35–61) CD 44 (30–59) |
270 (52.3) | 230,087 |
70 (46 UC, 24 CD) Death:15 |
13,306 Death: 516 |
NA | 365 | a and c | 6 |
| Curtis et al. [37] | USA | Retrospective cohort | 811 | 52 (18–89) | 428 (52.8) | 311,563 |
155 Death: 23 |
48,423 Death: 7937 |
Steroid 198 Anti-TNF 76 JAK 11 Tofacitinib 11 |
Hypertension: 1088 Hyperlipidemia:827 DM: 479 Coronary artery disease: 356 COPD: 288 |
a | 7 |
| Hadi et al. [38] | USA | Retrospective cohort | 4310 (2082 UC, 2190 CD) | 49.7 ± 18.19 | 2503 (58.1) | 4310 |
515 (272 UC,235 CD) Death: 90 |
441 Death: 95 |
NA |
Hypertension: 1934 Heart failure: 464 Chronic lower: 1499 DM: 868 |
a and c | 7 |
| Ludvigsson et al. [39] | Sweden | Prospective cohort | 811 | NA | NA | 2890 |
IBD 202 Death:53 |
558 Death: 122 |
NA | NA | a | 7 |
| Attauabi et al. [36] | Denmark | Prospective cohort | 76 (45 UC, 31 CD) |
UC 51 (39–70) CD 54 (38–62) |
31 (40.8) | 7945 | Death: 4 | Death: 460 |
5-ASA 37 Steroid 6 IMS 16 Biologicals 18 |
26 | a | 6 |
| Sima et al. [40] | Iran | Prospective cohort | 84 (60 UC, 24 CD) | 43.35 ± 14.1 | 35 (41.6) | 49 |
36 (28 UC, 8 CD) Death: 1 |
8 Death: 1 |
5-ASA 59 Steroid 13 IMS 28 Anti-TNF 20 |
Hypertension: 11 Chronic Liver disease:8 DM: 7 COPD: 6 |
a, b and c | 7 |
| Allocca et al. [23] | France, Italy | Retrospective cohort | 15 (6 UC,9 CD) | 39 (26–61) | 11 (73.3) | NA | 5 (3 UC, 2 CD) | NA |
5-ASA 1 Steroid 2 IMS 3 Biologicals 11 |
9 | b and c | 6 |
| Axelrad et al. [41] | USA | Case series | 84 (27 UC, 56 CD) | 35 (27–45) | 39 (47) | NA | 5 (1 UC, 4 CD) | NA |
5-ASA 13 Steroid 10 IMS 6 Biologicals 58 (anti-TNF 44) |
Organ transplantation: 2 Kidney disease: 1 Hypertension: 3 DM: 1 COPD: 1 |
b and c | NA |
| Bezzio et al. [50] | Italy | Prospective cohort | 11 | NA | 101 (41.6) | NA | 2 | NA |
Steroid 9 Biologicals 2 |
93 | c | 7 |
| Burke et al. [42] | USA | Retrospective cohort | 39 (22 UC, 17 CD) | 45.6 ± 18.8 | 24 (62) | NA | 7 (5 UC, 2 CD) | NA |
5-ASA 12 IMS 3 Biologicals 20 (anti-TNF 13) |
Obesity: 11 DM: 3 Hypertension: 7 Asthma: 4 |
b and c | 7 |
| Conley et al. [48] | UK | Prospective cohort | 42 (28 UC, 14 CD) | NA | NA | NA | 0 | NA | NA | NA | b | 6 |
| Kornbluth et al. [43] | USA | Retrospective cohort | 65 (24 UC, 41 CD) | 39 (17–71) | NA | NA | 3 (3 UC, 0 CD) | NA |
5-ASA 5 Steroid 2 IMS 1 Biologicals 37 Antibiotics 2 |
NA | b | 6 |
| Lamb et al. [44] | UK | Retrospective cohort | 211 (109UC, 86 CD) | NA | 94 (44.6) | NA | 56 (37UC,16 CD) | NA |
5-ASA 91 Steroid 10 IMS 34 Biologicals 95 (Anti-TNF 32) |
COPD: 15 Hypertension: 52 DM: 31 Obesity: 11 |
b and c | 7 |
| Lee et al. [49] | South Korea | Case series | 9 (7 UC, 2 CD) | 42 (21–64) | 3 (33.3) | NA | 0 | NA |
5-ASA 7 Steroid 1 IMS 2, Biologicals 2 |
1 | b | NA |
| Rizzello et al. [46] | Italy | Cross-sectional | 26 (11 UC, 15 CD) | 49 (24–86) | 14 (53.8) | NA | 7 (4 UC, 3 CD) | NA |
5-ASA 19 Steroid 4 IMS 1 Biologicals 4 |
10 | b and c | NA |
| Taxonera et al. [33] | Spain | Case series | 12 (5 UC, 7 CD) | 51 (20–76) | 9 (75.0) | NA | 8 (5 UC, 3 CD) | NA |
5-ASA 4 IMS 6 Biologicals 5 |
5 | b and c | NA |
| Wetwittayakhlang et al. [47] | Canada | Retrospective cohort | 82 (19 UC, 63 CD) | 39 (27–48) | 41 (50.0) | NA | 6 (2 UC, 4 CD) | NA |
5-ASA 18 Steroid 9 IMS 3 Biologicals 59 Antibiotics 3 |
CVD: 8 Chronic lung disease: 7 DM: 5 Obesity (BMI > 30 kg/m2): 14 Malignancies: 2 |
b and c | 6 |
| Nakase et al. [45] | Japan | Retrospective cohort | 187 (104 UC, 74 CD) | 42.0 ± 15.6 | 72 (38.5) | NA | 12 (11 UC,1 CD) | NA |
5-ASA 144 Steroid 14 IMS 57 Anti-TNF 74 |
DM: 5 CKD: 4 Liver diseases: 8 CVD: 4 All: 56 |
b and c | 7 |
| Bezzio et al. [34] | Italy | Prospective cohort | 937 (446 UC, 491 CD) | 44 (10–86) | 424 (45.3) | NA | 165 (83 UC, 82 CD) | NA |
5-ASA 492 Steroid 122 IMS 101 Biologicals 512 (anti-TNF 346) |
376 | b | 7 |
| Khan et al. [51] | USA | Retrospective cohort | 649 | NA | NA | NA | 149 | NA |
5-ASA 247 Steroid 61 IMS 92 Biologicals 173 |
NA | c | 6 |
| Zabana et al. [52] | Spain | Prospective cohort | 482 (221 UC, 247 CD) | 52 (42–61) | 231 (48) | NA | 167 | NA |
5-ASA 202 Steroid 26 IMS 113 Biologicals 117 (anti-TNF 117) |
NA | b | 7 |
The values of age are median (interquartile range, IQR) or mean ± standard deviation (SD)
NA data not available, 5-ASA mesalazine, IMS immunomodulators including azathioprine, mercaptopurine and methotrexate, JAK JAK inhibitor, DM diabetes mellitus, CKD chronic kidney disease, BMI body mass index, COPD chronic obstructive pulmonary disease, CVD cardiovascular disease, NOS Newcastle–Ottawa scale
Risk of adverse outcomes in COVID-19 patients with IBD versus comparative population
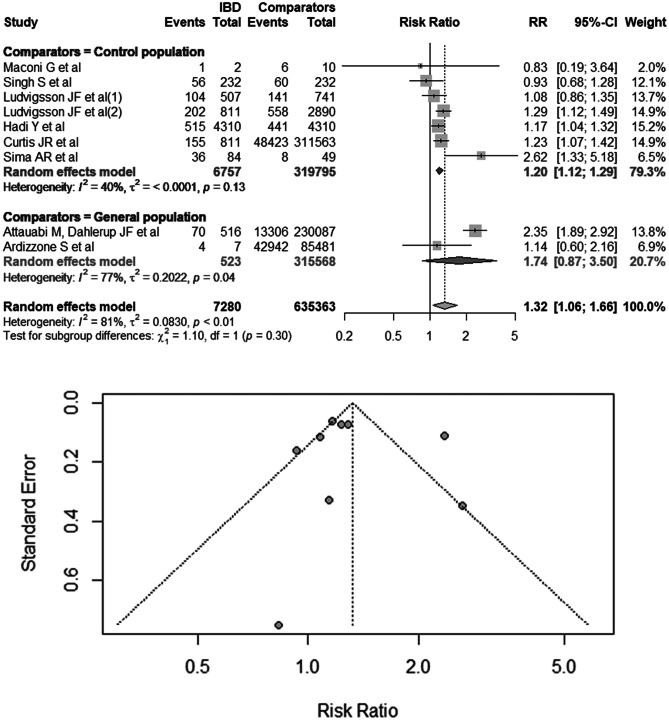
Nine studies regarded IBD as the exposure factor in COVID-19 patients and adverse effects as outcomes [18, 22, 25, 26, 36–40]. A total of 7280 COVID-19 patients with IBD and 635,363 COVID-19 patients without IBD served as the comparative populations, including a matched population adjusted for age, gender, and comorbidities and the general population in the same period. In the comparison of the risk of adverse outcomes in COVID-19 patients with IBD and comparators, the pooled RR was 1.32 (95% CI 1.06–1.66), and heterogeneity was high (I2 = 81%; P < 0.01; Fig. 2). The results of Egger’s test indicated no evidence of publication bias (P = 0.72). In subgroup analyses performed according to the source of comparators (matched and general population), the pooled RRs of adverse outcomes were 1.20 (95% CI 1.12–1.29; I2 = 40%; P = 0.13) in the control population group and 1.74 (95% CI 0.87–3.50; I2 = 77%; P = 0.04; Fig. 2) in the general population group.
Fig. 2.
Risk of adverse outcomes in COVID-19 patients with IBD versus comparative population. The comparison population includes the control population infected with SARS-CoV-2 adjusted for age, gender, and comorbidities and the general population infected with SARS-CoV-2 during the same period
In the analysis of the risk of mortality in COVID-19 patients with IBD, the pooled RR values were 1.35 (95% CI 0.95–1.92; I2 = 63%; P = 0.01), 1.72 (95% CI 1.11–2.67) with mild heterogeneity (I2 = 47%; P = 0.13) in the European studies, and 1.00 (95% CI 0.79–1.26; I2 = 0%; Supplementary Fig. 1) in the non-European studies. The difference in the risk of mortality between the two groups was statistically significant (χ2 = 4.67; P = 0.03). The RR of mortality in European patients with IBD was higher than that in non-European patients with IBD after SARS-CoV-2 infection.
Risk of adverse outcomes between UC and CD patients infected with SARS-CoV-2
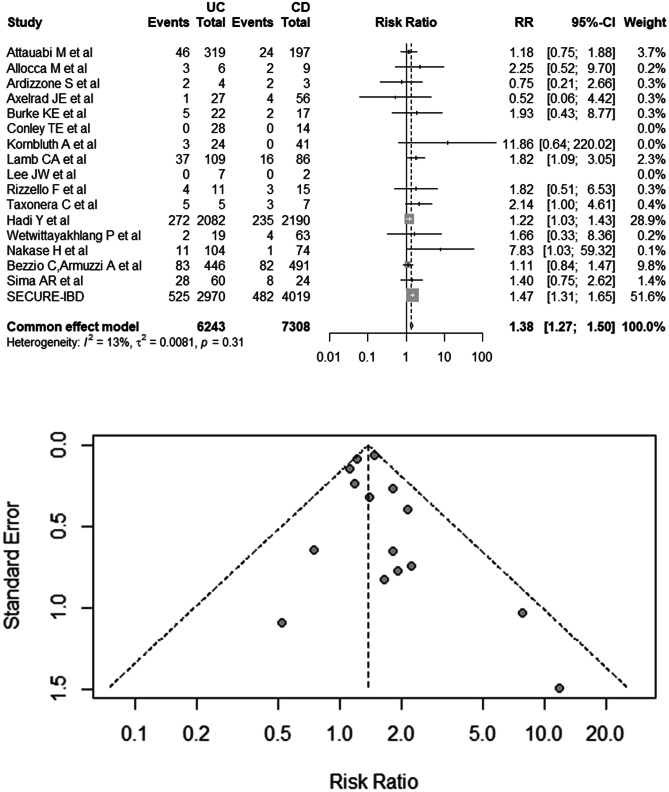
Information from 16 studies and the SECURE-IBD registry were used in evaluating the risk of adverse outcomes in UC and CD patients infected with SARS-CoV-2, including 6243 UC patients and 7308 CD patients [18, 23, 33, 34, 36, 38, 40–49]. The pooled RR was 1.38 (95% CI 1.27–1.50), with no evidence of heterogeneity (I2 = 13%; P = 0.31; Fig. 3) or publication bias (Egger’s test, P = 0.36). On the risk of mortality in UC and CD patients infected with SARS-CoV-2, the summary RR was 1.35 (95% CI 1.04–1.75; I2 = 0%; Supplementary Fig. 2).
Fig. 3.
Risk of adverse outcomes in COVID-19 patients with UC and CD
Association between adverse outcomes and IBD drugs in COVID-19 patients with IBD
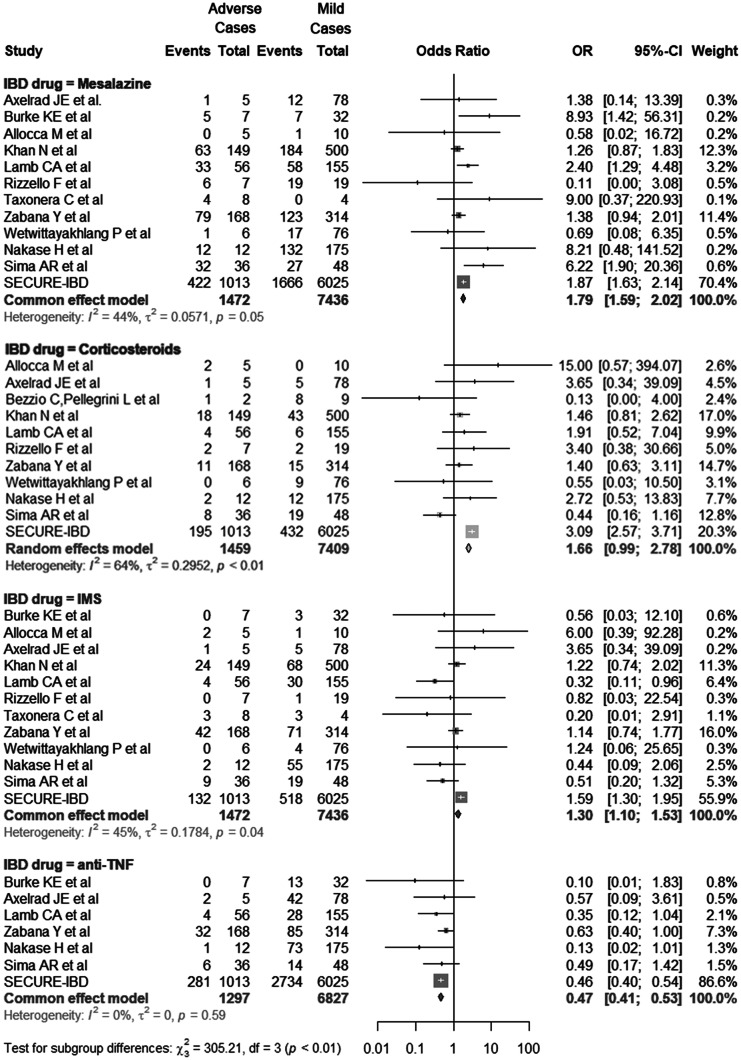
Data used in evaluating the association between adverse outcomes and IBD drugs were obtained from the 12 included studies and the SECURE-IBD registry, including 1474 adverse and 7445 mild cases [23, 33, 41, 42, 44–47, 50–52]. The pooled OR of mesalazine (1.79; 95% CI 1.59–2.02; I2 = 44%; P = 0.05), corticosteroids (1.66; 95% CI 0.99–2.78; I2 = 64%; P < 0.01), IMS (1.30; 95% CI 1.10–1.53; I2 = 45%; P = 0.04), anti-TNF (0.47; 95% CI 0.41–0.53; I2 = 0%; P = 0.59) are shown in Fig. 4). No publication bias was observed (Egger’s test, P mesalazine = 0.83, P corticosteroids = 0.11, P IMS = 0.09, P anti-TNF = 0.46).
Fig. 4.
Exposure to IBD drugs in adverse and mild cases. The study compared exposure to IBD drugs, including mesalazine; corticosteroids; immunomodulators (IMS), including azathioprine, mercaptopurine, and methotrexate; and anti-TNF in adverse and mild cases
Subgroup and sensitivity analyses
Subgroup analyses defined by age, region, sample size, source of comparators (control and general population), gender, and type of IBD were associated with the risk of adverse outcomes (Table 2). In subgroup analyses of the source of the comparators, the pooled RR was 1.20 (95% CI 1.12–1.29) with mild heterogeneity (I2 = 40%; P = 0.13) in the control population group, and the pooled RR was 1.74 (95% CI 0.87–3.50) with high heterogeneity (I2 = 77%; P = 0.04) in the general population group. Therefore, the different sources of comparators may account for the high heterogeneity.
Table 2.
Subgroup analysis on the risk of adverse COVID-19 outcomes in IBD patients
| Subgroup | Studies, n | RR (95%CI) | I2(%) | P |
|---|---|---|---|---|
| Source of the comparators | ||||
| Comparators = matched population | 7 | 1.20 (1.12–1.29) | 40 | 0.13 |
| Comparators = general population | 2 | 1.74 (0.87–3.50) | 77 | 0.04 |
| Geographic area | ||||
| Europe | 5 | 1.38 (0.97–1.98) | 86 | < 0.01 |
| Non-Europe | 4 | 1.19 (1.09–1.29) | 61 | 0.05 |
| Sample size | ||||
| ≥ 100 | 6 | 1.29 (1.01–1.64) | 87 | < 0.01 |
| < 100 | 3 | 1.52 (0.77–3.00) | 48 | 0.14 |
| Gender (male, %) | ||||
| ≥ 55 | 1 | 2.62 (1.33–5.18) | ||
| < 55 | 5 | 1.31 (0.95–1.81) | 89 | < 0.01 |
| NA | 3 | 1.20 (1.01–1.43) | 4 | 0.35 |
| Type of IBD (UC, %) | ||||
| ≥ 55 | 3 | 1.44 (0.94–2.21) | 57 | 0.10 |
| < 55 | 3 | 1.37 (0.80–2.36) | 94 | < 0.01 |
| NA | 3 | 1.20 (1.01–1.43) | 4 | 0.35 |
| Age | ||||
| ≥ 50 | 3 | 1.13 (0.92–1.39) | 18 | 0.29 |
| < 50 | 3 | 1.84 (1.09–3.10) | 94 | < 0.01 |
| NA | 3 | 1.20 (1.01–1.43) | 4 | 0.35 |
NA data not available, RR risk ratio
In sensitivity analysis, none of the individual studies led to a substantial change in pooled risk in the leave-one-out analysis removing one study in turn (Supplementary Fig. 3).
Discussion
During the SARS-CoV-2 pandemic, IBD patients, as immune-mediated disease patients, should be treated more carefully than the general population. Until now, many patients with IBD did not receive or complete vaccines because of concerns about adverse reactions to vaccines, and the effectiveness of vaccines may wane more rapidly in patients with IBD [53–55]. Accumulating evidence of poor prognosis in patients with other diseases accompanied by IBD and increased risk of developing malignancies in these patients has been obtained, such as myocardial infarction and hematological malignancies [56–58]. Therefore, the risk of hospitalization, death, and other adverse outcomes in patients suffering from IBD and infected with SARS-CoV-2 should be an ongoing concern.
To the best of our knowledge, this study is the first meta-analysis to evaluate the risk of adverse outcomes between COVID-19 patients with and without IBD. In this study, we found that COVID-19 patients with IBD were at increased risk for adverse outcomes than those without IBD. Furthermore, patients with UC have an increased risk than those with CD. Moreover, COVID-19 may intersect with the pathogenesis of IBD and extend treatment. As a result, mesalazine (5-ASA) and IMS treatment might be risk factors for adverse outcomes in COVID-19 patients with IBD. By contrast, anti-TNF treatment might provide protection against the development of negative outcomes.
On subgroup analyses of the source of comparators (control and general population group), there is a pooled RR with low heterogeneity in control population group adjusted for age, gender, and comorbidities. Inconsistency in the distribution of these confounders may account for the heterogeneity.
The increased risk of adverse outcomes in patients with IBD may be associated with increased SARS-CoV-2 replication and imbalance of ACE-2 levels in the intestine. On the one hand, the intestines of IBD patients with high ACE-2 expression may provide favorable sites for virus replication. On the other hand, ACE-2 not only is a SARS-CoV-2 binding receptor but also acts as an enzyme in the renin–angiotensin system to reduce inflammatory response [59, 60]. The renin–angiotensin system functions in inflammation, fibrosis, and cell proliferation in opposite roles regulated through two complementary pathways (classical and alternative) [61, 62]. The ACE-2/Ang 1–7/MasR axis can reduce proinflammatory response and cytokine storm in the renin–angiotensin system [63]. Recent studies have revealed that the key enzymes of the system (ACE and ACE-2) were expressed and active in the human intestine [62, 64]. As a result of binding to virus, ACE-2 in the guts of patients with IBD may be severely depleted [65], and this effect may result in an imbalance in the renin–angiotensin system that promotes fibrosis and inflammatory response and has negative effects.
Similarly, differences in ACE-2 expression levels in the guts of patients with UC or CD may account for differences in the risk of developing adverse outcomes. In contrast to the results of our study, UC patients without COVID-19 were not at increased risk of developing adverse outcomes in contrast to CD patients without COVID-19 [66, 67]. These findings were consistent with the meta-analysis results. In addition to the above reason, UC patients may prefer 5-ASA [68], an IBD drug associated with high risk of developing adverse outcomes. In our study, 5-ASA and IMS treatments might be risk factors for adverse outcomes in COVID-19 patients with IBD. By contrast, anti-TNF treatment might protect against the development of negative outcomes. Notably, reduced small bowel but elevated colonic ACE-2 levels in IBD patients were associated with adverse outcomes but returned to normal after anti-TNF therapy [69]. Although evidence showing the risk of adverse outcomes in IBD patients treated wtih corticosteroids is insufficient, previous studies have shown that corticosteroids should be selected carefully [70, 71].
Our study has some limitations. Nevertheless, it has offered a comprehensive review of the risk of adverse outcomes in IBD patients after being infected with SARS-CoV-2, in patients with UC or CD, and in patients using different IBD treatment drugs. First, many small case series were included in our meta-analysis, including four studies with quality not evaluated using the NOS. Second, heterogeneity in our meta-analysis was high, which is a general limitation of all published COVID-19 studies. Third, some studies showed data duplication in reporting adverse outcomes, such as hospitalization, ICU admission, and death. In these studies, the number of patients hospitalized or admitted to ICUs was the number of patients with adverse outcomes, and some patients who died but were not hospitalized may have been not included.
In conclusion, this systematic review and meta-analysis shows that COVID-19 patients with IBD have a higher risk of developing adverse outcomes than patients without IBD. The 5-ASA and IMS treatments may be associated with high risk of adverse outcomes in COVID-19 patients with IBD, whereas anti-TNF treatment can reduce this risk.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Fig. 1. Risk of mortality in COVID-19 patients with IBD and without IBD. (EPS 83342 KB)
Supplementary Fig. 2. Risk of mortality in COVID-19 patients with UC and CD. (EPS 79816 KB)
Supplementary Fig. 3. Sensitivity analysis. Leave-one-out analysis removed one study in turn. (EPS 66436 KB)
Author contribution
LC and KH were responsible for the conception and design of the work and the drafting of the manuscript; LC, KH, and CC analyzed the data; QH, LZ, TA, YG, SC, GD revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82073618) and Epidemic Prevention and Control Research and Development projects in Henan Province (211100310900).
Declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Long Chen and Kai Hu these authors contributed equally.
References
- 1.Rader B, Scarpino SV, Nande A, Hill AL, Adlam B, Reiner RC, et al. Crowding and the shape of Covid-19 epidemics. Nat Med. 2020;26(12):1829–1834. doi: 10.1038/s41591-020-1104-0. [DOI] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Who. Coronavirus disease 2019. https://www.Who.Int/Emergencies/Diseases/Novel-Coronavirus-2019. Accessed 29 May 2022
- 4.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to Covid-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 5.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with Covid-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. Sars-Cov-2 cell entry depends on Ace2 and Tmprss2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Potdar AA, Dube S, Naito T, Li K, Botwin G, Haritunians T, et al. Altered intestinal Ace2 levels are associated with inflammation, severe disease, and response to anti-cytokine therapy in inflammatory bowel disease. Gastroenterology. 2021;160(3):809-22e7. doi: 10.1053/j.gastro.2020.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suarez-Farinas M, Tokuyama M, Wei G, Huang R, Livanos A, Jha D, et al. Intestinal inflammation modulates the expression of Ace2 and Tmprss2 and potentially overlaps with the pathogenesis of Sars-Cov-2-related disease. Gastroenterology. 2021;160(1):287–301 e20. doi: 10.1053/j.gastro.2020.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toyonaga T, Araba KC, Kennedy MM, Keith BP, Wolber EA, Beasley C, et al. Increased colonic expression of Ace2 associates with poor prognosis in Crohn’s disease. Sci Rep. 2021;11(1):13533. doi: 10.1038/s41598-021-92979-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang B, Zhang L, Wang Y, Dai T, Qin Z, Zhou F, et al. Alterations in microbiota of patients with Covid-19: potential mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2022;7(1):143. doi: 10.1038/s41392-022-00986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saez A, Gomez-Bris R, Herrero-Fernandez B, Mingorance C, Rius C, Gonzalez-Granado JM (2021) Innate lymphoid cells in intestinal homeostasis and inflammatory bowel disease. Int J Mol Sci 22(14). 10.3390/ijms22147618 [DOI] [PMC free article] [PubMed]
- 13.GBD 2017 Inflammatory Bowel Disease Collaborators (2020) The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol 5(1):17–30. 10.1016/s2468-1253(19)30333-4. Epub 20191021 [DOI] [PMC free article] [PubMed]
- 14.Jairath V, Feagan BG. Global burden of inflammatory bowel disease. Lancet Gastroenterol Hepatol. 2020;5(1):2–3. doi: 10.1016/s2468-1253(19)30358-9. [DOI] [PubMed] [Google Scholar]
- 15.Verstockt B, Verstockt S, Abdu Rahiman S, Ke BJ, Arnauts K, Cleynen I, et al. Intestinal receptor of Sars-Cov-2 in inflamed ibd tissue seems downregulated by Hnf4a in ileum and upregulated by interferon regulating factors in colon. J Crohns Colitis. 2021;15(3):485–498. doi: 10.1093/ecco-jcc/jjaa185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suárez-Fariñas M, Tokuyama M, Wei G, Huang R, Livanos A, Jha D, et al. Intestinal Inflammation Modulates the expression of Ace2 and Tmprss2 and potentially overlaps with the pathogenesis of Sars-Cov-2-related disease. Gastroenterology. 2021;160(1):287–301.e20. doi: 10.1053/j.gastro.2020.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alpers DH. Is the intestine a portal of entry for the serious Covid-19 complications of endotoxemia and thrombosis? Clin Transl Gastroenterol. 2021;12(6):e00367. doi: 10.14309/ctg.0000000000000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ardizzone S, Ferretti F, Monico MC, Carvalhas Gabrielli AM, Carmagnola S, Bezzio C, et al. Lower incidence of Covid-19 in patients with inflammatory bowel disease treated with non-gut selective biologic therapy. J Gastroenterol Hepatol. 2021;36(11):3050–3055. doi: 10.1111/jgh.15591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilissen LPL, Heinen SGH, Rijpma-Jacobs L, Schoon E, Schreuder RM, Wensing AM et al (2021) Neither inflammatory bowel disease nor immunosuppressants are associated with an increased risk of severe Covid-19: an observational dutch cohort study. Clin Exp Med 1–12. 10.1007/s10238-021-00755-3. Epub 20210920 [DOI] [PMC free article] [PubMed]
- 20.Iborra I, Puig M, Marín L, Calafat M, Cañete F, Quiñones C, et al. Treatment adherence and clinical outcomes of patients with inflammatory bowel disease on biological agents during the Sars-Cov-2 pandemic. Dig Dis Sci. 2021;66(12):4191–4196. doi: 10.1007/s10620-020-06807-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kjeldsen S, Nielsen J, Mertz Norgard B, Kjeldsen J (2021) Mesalazine in inflammatory bowel disease and Covid-19: hospitalization and adverse in-hospital outcomes based on nationwide data. Inflamm Bowel Dis. 10.1093/ibd/izab299. Epub 20211124 [DOI] [PMC free article] [PubMed]
- 22.Maconi G, Bosetti C, De Monti A, Boyapati RK, Shelton E, Piazza N, et al. Risk of Covid 19 in patients with inflammatory bowel diseases compared to a control population. Dig Liver Dis. 2021;53(3):263–270. doi: 10.1016/j.dld.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allocca M, Fiorino G, Zallot C, Furfaro F, Gilardi D, Radice S, et al. Incidence and patterns of Covid-19 among inflammatory bowel disease patients from the Nancy and Milan cohorts. Clin Gastroenterol Hepatol. 2020;18(9):2134–2135. doi: 10.1016/j.cgh.2020.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh AK, Jena A, Kumar MP, Sharma V, Sebastian S. Risk and outcomes of coronavirus disease in patients with inflammatory bowel disease: a systematic review and meta-analysis. United European Gastroenterol J. 2021;9(2):159–176. doi: 10.1177/2050640620972602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh S, Khan A, Chowdhry M, Bilal M, Kochhar GS, Clarke K. Risk of severe coronavirus disease 2019 in patients with inflammatory bowel disease in the United States: a multicenter research network study. Gastroenterology. 2020;159(4):1575-8 e4. doi: 10.1053/j.gastro.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Attauabi M, Poulsen A, Theede K, Pedersen N, Larsen L, Jess T, et al. Prevalence and outcomes of Covid-19 among patients with inflammatory bowel disease-a Danish prospective population-based cohort study. J Crohns Colitis. 2021;15(4):540–550. doi: 10.1093/ecco-jcc/jjaa205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Creemers RH, Rezazadeh Ardabili A, Jonkers DM, Leers MPG, Romberg-Camps MJ, Pierik MJ, et al. Severe Covid-19 in inflammatory bowel disease patients in a population-based setting. PLoS ONE. 2021;16(10):e0258271. doi: 10.1371/journal.pone.0258271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray CJL. Covid-19 will continue but the end of the pandemic is near. Lancet. 2022;399(10323):417–419. doi: 10.1016/s0140-6736(22)00100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson MG, Burgess JL, Naleway AL, Tyner H, Yoon SK, Meece J, et al. Prevention and attenuation of Covid-19 with the Bnt162b2 and Mrna-1273 vaccines. N Engl J Med. 2021;385(4):320–329. doi: 10.1056/NEJMoa2107058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao YD, Ding M, Dong X, Zhang JJ, Kursat Azkur A, Azkur D, et al. Risk factors for severe and critically ill Covid-19 patients: a review. Allergy. 2021;76(2):428–455. doi: 10.1111/all.14657. [DOI] [PubMed] [Google Scholar]
- 31.Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM et al (2020) Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J 55(5). 10.1183/13993003.00547-2020. Epub 20200514 [DOI] [PMC free article] [PubMed]
- 32.Singh S, Khan A. Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the United States: a multicenter research network study. Gastroenterology. 2020;159(2):768–71.e3. doi: 10.1053/j.gastro.2020.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taxonera C, Sagastagoitia I, Alba C, Manas N, Olivares D, Rey E. 2019 novel coronavirus disease (Covid-19) in patients with inflammatory bowel diseases. Aliment Pharmacol Ther. 2020;52(2):276–283. doi: 10.1111/apt.15804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bezzio C, Armuzzi A, Furfaro F, Ardizzone S, Milla M, Carparelli S, et al. Therapies for inflammatory bowel disease do not pose additional risks for adverse outcomes of Sars-Cov-2 infection: an Ig-Ibd study. Aliment Pharmacol Ther. 2021;54(11-12):1432–1441. doi: 10.1111/apt.16663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wells G (ed) (2004) The Newcastle-Ottawa scale (Nos) for assessing the quality of non-randomised studies in meta-analyses. Symposium on Systematic Reviews: Beyond the Basics
- 36.Attauabi M, Dahlerup JF, Poulsen A, Hansen MR, Verner-Andersen MK, Eraslan S et al (2021) Outcomes and long-term effects of Covid-19 in patients with inflammatory bowel diseases - a Danish prospective population-based cohort study with individual-level data. J Crohns Colitis. 10.1093/ecco-jcc/jjab192. Epub 20211110 [DOI] [PMC free article] [PubMed]
- 37.Curtis JR, Zhou X, Rubin DT, Reinisch W, Yazdany J, Robinson PC, et al. Characteristics, comorbidities, and outcomes of Sars-Cov-2 infection in patients with autoimmune conditions treated with systemic therapies: a population-based study. J Rheumatol. 2022;49(3):320–329. doi: 10.3899/jrheum.210888. [DOI] [PubMed] [Google Scholar]
- 38.Hadi Y, Dulai PS, Kupec J, Mohy-Ud-Din N, Jairath V, Farraye FA, et al. Incidence, outcomes, and impact of Covid-19 on inflammatory bowel disease: propensity matched research network analysis. Aliment Pharmacol Ther. 2022;55(2):191–200. doi: 10.1111/apt.16730. [DOI] [PubMed] [Google Scholar]
- 39.Ludvigsson JF, Axelrad J, Halfvarson J, Khalili H, Larsson E, Lochhead P, et al. Inflammatory bowel disease and risk of severe Covid-19: a nationwide population-based cohort study in Sweden. United European Gastroenterol J. 2021;9(2):177–192. doi: 10.1002/ueg2.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sima AR, Saberzadeh-Ardestani B, Vahedi H, Fakheri H, Mansour-Ghanaei F, Maleki I, et al. Outcomes of Covid-19 in patients with inflammatory bowel disease: comparison with household members and the role of Ibd medications. Arch Iran Med. 2022;25(1):17–25. doi: 10.34172/aim.2022.04. [DOI] [PubMed] [Google Scholar]
- 41.Axelrad JE, Malter L, Hong S, Chang S, Bosworth B, Hudesman D. From the American epicenter: coronavirus disease 2019 in patients with inflammatory bowel disease in the New York City metropolitan area. Inflamm Bowel Dis. 2021;27(5):662–666. doi: 10.1093/ibd/izaa162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burke KE, Kochar B, Allegretti JR, Winter RW, Lochhead P, Khalili H, et al. Immunosuppressive therapy and risk of Covid-19 infection in patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2021;27(2):155–161. doi: 10.1093/ibd/izaa278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kornbluth A, Kissous-Hunt M, George J, Legnani P. Management of inflammatory bowel disease and Covid-19 in New York City 2020: the epicenter of Ibd in the first epicenter of the global pandemic. Inflamm Bowel Dis. 2020;26(11):1779–1785. doi: 10.1093/ibd/izaa212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamb CA, Sebastian S, Kent AJ, Segal JP, Gonzalez HA, Brookes MJ, et al. Letter: Risk of severe Covid-19 outcomes associated with inflammatory bowel disease medications-reassuring insights from the United Kingdom prepare-Ibd multicentre cohort study. Aliment Pharmacol Ther. 2021;53(11):1236–1240. doi: 10.1111/apt.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakase H, Hayashi Y, Hirayama D, Matsumoto T, Matsuura M, Iijima H, et al. Interim analysis of a multicenter registry study of Covid-19 patients with inflammatory bowel disease in Japan (J-Cosmos) J Gastroenterol. 2022;57(3):174–184. doi: 10.1007/s00535-022-01851-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rizzello F, Calabrese C, Salice M, Calandrini L, Privitera H, Melotti L, et al. Covid-19 in Ibd: the experience of a single tertiary Ibd center. Dig Liver Dis. 2021;53(3):271–276. doi: 10.1016/j.dld.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wetwittayakhlang P, Albader F, Golovics PA, Hahn GD, Bessissow T, Bitton A, et al. Clinical outcomes of Covid-19 and impact on disease course in patients with inflammatory bowel disease. Can J Gastroenterol Hepatol. 2021;2021:7591141. doi: 10.1155/2021/7591141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Conley TE, Probert C, Subramanian S. Prevalence of Covid-19 symptoms among inflammatory bowel disease patients treated with biological agents. J Crohns Colitis. 2020;14(12):1794–1795. doi: 10.1093/ecco-jcc/jjaa187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee JW, Song EM, Jung SA, Jung SH, Kim KW, Koh SJ, et al. Clinical course of Covid-19 in patients with inflammatory bowel disease in Korea: a Kasid multicenter study. J Korean Med Sci. 2021;36(48):e336. doi: 10.3346/jkms.2021.36.e336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bezzio C, Pellegrini L, Manes G, Arena I, Picascia D, Della Corte C, et al. Biologic Therapies may reduce the risk of Covid-19 in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2020;26(10):e107–e109. doi: 10.1093/ibd/izaa242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khan N, Mahmud N, Trivedi C, Reinisch W, Lewis JD. Risk factors for Sars-Cov-2 infection and course of Covid-19 disease in patients with Ibd in the Veterans Affair Healthcare System. Gut. 2021;70(9):1657–1664. doi: 10.1136/gutjnl-2021-324356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zabana Y, Marin-Jimenez I, Rodriguez-Lago I, Vera I, Martin-Arranz MD, Guerra I et al (2022) Nationwide Covid-19-Eii study: incidence, environmental risk factors and long-term follow-up of patients with inflammatory bowel disease and Covid-19 of the Eneida Registry. J Clin Med 11(2). 10.3390/jcm11020421. Epub 20220114 [DOI] [PMC free article] [PubMed]
- 53.Crispino F, Brinch D, Carrozza L, Cappello M. Acceptance of Sars-Cov-2 vaccination among a cohort of Ibd patients from Southern Italy: a cross-sectional survey. Inflamm Bowel Dis. 2021;27(11):e134–e135. doi: 10.1093/ibd/izab133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jena A, Mishra S, Deepak P, Kumar MP, Sharma A, Patel YI, et al. Response to Sars-Cov-2 vaccination in immune mediated inflammatory diseases: systematic review and meta-analysis. Autoimmun Rev. 2022;21(1):102927. doi: 10.1016/j.autrev.2021.102927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kennedy NA, Lin S, Goodhand JR, Chanchlani N, Hamilton B, Bewshea C, et al. Infliximab is associated with attenuated immunogenicity to Bnt162b2 and Chadox1 Ncov-19 Sars-Cov-2 vaccines in patients with Ibd. Gut. 2021;70(10):1884–1893. doi: 10.1136/gutjnl-2021-324789. [DOI] [PubMed] [Google Scholar]
- 56.Panhwar MS, Mansoor E, Al-Kindi SG, Sinh P, Katz J, Oliveira GH, et al. Risk of myocardial infarction in inflammatory bowel disease: a population-based national study. Inflamm Bowel Dis. 2019;25(6):1080–1087. doi: 10.1093/ibd/izy354. [DOI] [PubMed] [Google Scholar]
- 57.Wang LH, Yang YJ, Cheng WC, Wang WM, Lin SH, Shieh CC. Higher risk for hematological malignancies in inflammatory bowel disease: a nationwide population-based study in Taiwan. Am J Gastroenterol. 2016;111(9):1313–1319. doi: 10.1038/ajg.2016.239. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y, Li Y, Liu Y, Zhang Y, Ke Z, Zhang Y, et al. Patients with Ibd receiving methotrexate are at higher risk of liver injury compared with patients with non-Ibd diseases: a meta-analysis and systematic review. Front Med (Lausanne) 2021;8:774824. doi: 10.3389/fmed.2021.774824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Menikdiwela KR, Ramalingam L, Rasha F, Wang S, Dufour JM, Kalupahana NS, et al. Autophagy in metabolic syndrome: breaking the wheel by targeting the renin-angiotensin system. Cell Death Dis. 2020;11(2):87. doi: 10.1038/s41419-020-2275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Santos RAS, Oudit GY, Verano-Braga T, Canta G, Steckelings UM, Bader M. The Renin-angiotensin system: going beyond the classical paradigms. Am J Physiol Heart Circ Physiol. 2019;316(5):H958–H970. doi: 10.1152/ajpheart.00723.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khajah MA, Fateel MM, Ananthalakshmi KV, Luqmani YA. Anti-inflammatory action of angiotensin 1–7 in experimental colitis. PLoS ONE. 2016;11(3):e0150861. doi: 10.1371/journal.pone.0150861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garg M, Angus PW, Burrell LM, Herath C, Gibson PR, Lubel JS. Review Article: The pathophysiological roles of the renin-angiotensin system in the gastrointestinal tract. Aliment Pharmacol Ther. 2012;35(4):414–428. doi: 10.1111/j.1365-2036.2011.04971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simões e Silva AC, Silveira KD, Ferreira AJ, Teixeira MM. Ace2, angiotensin-(1–7) and Mas receptor axis in inflammation and fibrosis. Br J Pharmacol. 2013;169(3):477–492. doi: 10.1111/bph.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A Human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275(43):33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 65.Rojas A, Schneider I, Lindner C, Gonzàlez I, Morales MA. Receptor for advanced glycation end-products axis and coronavirus disease 2019 in inflammatory bowel diseases: a dangerous liaison? World J Gastroenterol. 2021;27(19):2270–2280. doi: 10.3748/wjg.v27.i19.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Odes S, Vardi H, Friger M, Wolters F, Russel MG, Riis L, et al. Cost analysis and cost determinants in a European inflammatory bowel disease inception cohort with 10 years of follow-up evaluation. Gastroenterology. 2006;131(3):719–728. doi: 10.1053/j.gastro.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 67.Zhao M, Gonczi L, Lakatos PL, Burisch J. The burden of inflammatory bowel disease in Europe in 2020. J Crohns Colitis. 2021;15(9):1573–1587. doi: 10.1093/ecco-jcc/jjab029. [DOI] [PubMed] [Google Scholar]
- 68.Ungaro RC, Brenner EJ, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, et al. Effect of Ibd medications on Covid-19 outcomes: results from an international registry. Gut. 2021;70(4):725–732. doi: 10.1136/gutjnl-2020-322539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Potdar AA, Dube S, Naito T, Li K, Botwin G, Haritunians T, et al. Altered intestinal Ace2 levels are associated with inflammation, severe disease, and response to anti-cytokine therapy in inflammatory bowel disease. Gastroenterology. 2021;160(3):809–22.e7. doi: 10.1053/j.gastro.2020.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meyer A, Semenzato L, Zureik M, Weill A, Carbonnel F, Dray-Spira R. Risk of Severe Covid-19 in patients treated with Ibd medications: a French nationwide study. Aliment Pharmacol Ther. 2021;54(2):160–166. doi: 10.1111/apt.16410. [DOI] [PubMed] [Google Scholar]
- 71.Alrashed F, Battat R, Abdullah I, Charabaty A, Shehab M (2021) Impact of medical therapies for inflammatory bowel disease on the severity of Covid-19: a systematic review and meta-analysis. BMJ Open Gastroenterol 8(1). 10.1136/bmjgast-2021-000774 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Risk of mortality in COVID-19 patients with IBD and without IBD. (EPS 83342 KB)
Supplementary Fig. 2. Risk of mortality in COVID-19 patients with UC and CD. (EPS 79816 KB)
Supplementary Fig. 3. Sensitivity analysis. Leave-one-out analysis removed one study in turn. (EPS 66436 KB)