Introduction.
Epilepsy is a common disorder with over a half million women of childbearing age having epilepsy in the USA alone.1 Antiseizure medications (ASMs) are the main therapeutic option for epilepsy and are among the most commonly prescribed teratogenic medications prescribed to women of childbearing age. The clinical dilemma is that drugs are generally contraindicated in pregnancy, but most women with epilepsy are unable to stop using ASMs due to risks of seizures with resultant consequences of injury, death, and loss of job or driving. Further, seizures during pregnancy can result in fetal injury, miscarriage or subsequent developmental delay. As a group, both somatic and functional neurodevelopment outcomes are reduced in children of women with epilepsy, but it is important to remember that the majority of children born to women with epilepsy are normal. The challenge to clinicians is to reduce these risks based on current knowledge. The challenge to basic and clinical researchers is to improve that inadequate evidence base.
Background History.
People with epilepsy have frequently suffered from stigma, which includes basic reproductive rights. In 1956, 17 states in the USA prohibited marriage in people with epilepsy; the last such state law in the USA was finally overturned in 1980.2 Similar laws existed in other countries in the mid 20th century. For example, a similar law existed in the UK until 1970. In addition, 18 states in the USA in 1956 provided for the sterilization of people with epilepsy.2 The justification of these sterilizations was very poorly conceived, based on eugenics, and violated basic human rights; they had nothing to do with teratogenic risks of ASMs which were not discovered until the 1960s.
Although the first modern ASM bromide was discovered in 1850’s,3 it would be over 100 years before the teratogenic potential of ASMs were recognized. Interestingly, this recognition was shortly after the thalidomide tragedy which highlighted the potential teratogenicity of pharmaceuticals. Thalidomide was introduced in the 1950’s leading to approximately 10,000 children were born with phocomelia (i.e., missing or underdeveloped limbs) in just a few years, and thalidomide was banned in most countries by 1961.4 In 1968, Meadow published a letter-to-the-editor describing six children with congenital malformations who had been exposed in utero to ASMs;5 however, he was cautious noting “before creating anxiety about useful drugs, it would be helpful to know if other people have encountered the association.” By 1974, multiple surveys were published, and Speidel and Meadow noted that most authors estimated the risk of malformations from fetal ASM exposure to be 2-3X the general population.6 Ironically, the letter by Meadow was a year after the discovery of the antiseizure effects of valproic acid which is now known to be the most teratogenic ASM.7 Spina bifida was linked to fetal valproate exposure in 1982,8 but it would not be until early in the 21st century that full scope of valproate’s risk would be recognized after the establishment of ASM pregnancy registries and other prospective studies. The risk could and should have been recognized earlier as a retrospective meta-analysis assessing the cumulative risk ratios for congenital malformations for each year beginning in 1983 showed risk as depicted by confidence intervals separated for valproate from other ASMs in 1990 never to overlap again.9 Unfortunately, this theme of many years passing as numerous fetal ASM exposures occur before the specific risks for specific ASMs are recognized continues today as the risks for the majority of ASMs used today are unknown.10
Anatomical Risks.
Children of women with epilepsy, who are exposed to ASMs during pregnancy, are at increased risk for congenital malformations. Children of women with epilepsy who did not take ASM during pregnancy and the children of fathers with epilepsy are not at increased risk.11 Thus, the risk appears to be due to fetal ASM exposure since ASMs as a class are known to be teratogenic and produce birth defects in animals if given in high enough dosages. Given that major organs are formed during the first trimester, exposure during the first part of pregnancy poses risk for major congenital malformations. Typical ASM-induced malformations include heart defects, orofacial clefts, skeletal deformities, urological abnormalities and neural tube defects.12 However, these risks vary across ASMs. Risks for polytherapy appear higher than monotherapy, but the risks for specific combinations are unclear.
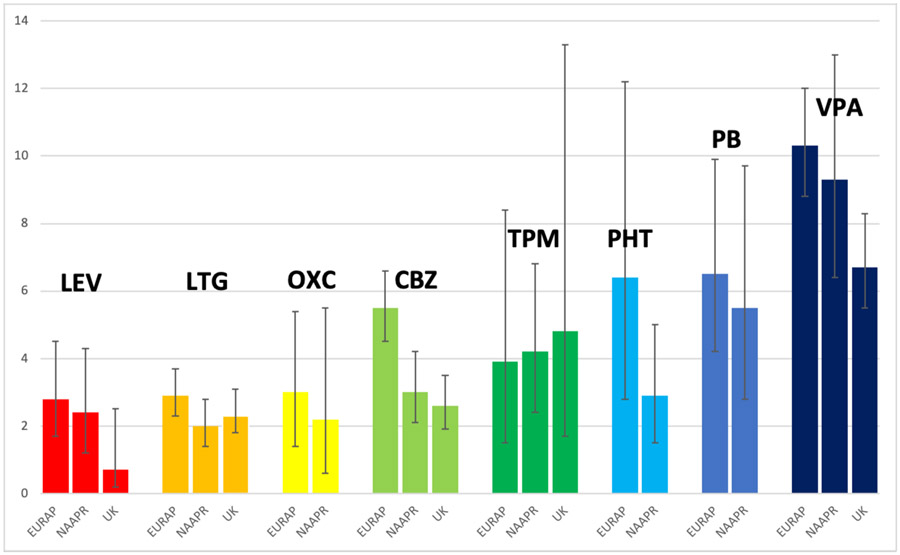
Multiple studies have consistently shown the increased risk of major congenital malformations (MCMs) from fetal valproate exposure, and demonstrated that the risk for valproate is higher than any other ASMs which have adequate data. Examples from the literature are offered. The North American Antiepileptic Drug Pregnancy Registry (NAAPR) has found that the risk for MCMs is 9.3% for valproate compared to phenobarbital 5.5%, topiramate 4.2%, phenytoin 2.9%, carbamazepine 3.0%, levetiracetam 2.4%, oxcarbazepine 2.2%, and lamotrigine 2.0%.13 Similarly, the EURAP noted the risk of MCMs was valproate is 10.3% compared to phenobarbital 6.5%, phenytoin 6.4%, topiramate 3.9%, carbamazepine 5.5%, oxcarbazepine 3.0%, lamotrigine 2.9%, and levetiracetam 2.8%.14 The same pattern has been seen in the UK-Ireland registry with MCMs occurring in 6.7% for valproate, 4.8% for topiramate, 2.6% for carbamazepine, 2.3% for lamotrigine, and 0.7% for levetiracetam.15-17 A case-control study across 19 registries in 14 countries from 1995-2005 disclosed specific malformation risks for valproate (spina bifida, atrial septal defect, cleft palate, hypospadias, polydactyly, and craniosynostosis) and for carbamazepine (spina bifida).18,19 A French national database study disclosed valproate was associated with 8 specific MCMs (spina bifida, ventricular, atrial, septal defects, pulmonary valve atresia, hypoplastic left heart syndrome, cleft palate, anorectal atresia, and hypospadias); specific defects were also seen for clonazepam (microcephaly), phenobarbital (ventricular septal defect), pregabalin (coarctation of aorta), and topiramate (cleft lip with or without cleft palate) but the findings for these other ASMs were based on a small number of MCMs.20 A recent Danish population-based cohort study of individual-linked data of births 1997-2014 reported the valproate risk of MCMs was aOR = 2.44, 95% CI = 1.80–3.30 and separated from lamotrigine as early as 1997;21 additionally, no significant differences were seen for lamotrigine, carbamazepine, oxcarbazepine or levetiracetam vs. unexposed.
Analyses of the United States Medicaid Analytic extract (MAX) dataset revealed no increased risk for gabapentin overall (RR=1.07; 95%CIs 0.94-1.21, but an increased risk for cardiac defects was seen when ≥2 dispensed gabapentin prescriptions (RR=1.40; 95%CIs 1.03–1.90), and an increased risk for pregabalin (1.80; 95%CIs 1.26–2.58) was seen for any use but not monotherapy (1.31; 95%CIs 0.80-2.14).22
Conclusions for Anatomical Risks:
Valproate poses an especially high risk for MCMs, and next highest for phenobarbital. The risks are intermediate for carbamazepine, gabapentin, phenytoin, pregabalin, and topiramate. The lowest risks of MCMs exists for levetiracetam, lamotrigine, and oxcarbazepine. See Figure 1 for overview. Data for other ASMs remains inadequate to accurately determination risks for MCMs (Table 1).
Figure 1. Malformation rates (mean %, 95% CIs) as function of ASM (adapted from references #13-17).
LEV=levetiracetam, LTG=lamotrigine, OXC=oxcarbazepine, CBZ=carbamazepine, TPM=topiramate, PHT=phenytoin, PB=phenobarbital, VPA=valproate
Table 1.
Risks of fetal ASM-induced major congenital malformations in humans based on current data.
| Data on Risks | Unknown Risks | |
|---|---|---|
| Highest Risks | Acetazolamide | Lacosamide |
| Phenobarbital | Briviracetam | Lorazepam |
| Valproate | Cannabidiol | Midazolam |
| Intermediate Risks | Cenobamate | Perampanel |
| Carbamazepine | Clobazam | Primidone |
| Gabapentin | Clonazepam | Rufinamide |
| Phenytoin | Diazepam | Stiripentol |
| Pregabalin | Eslicarbazepine | Sulthiame |
| Topiramate | Ethosuximide | Tiagabine |
| Lowest Risks | Everolimus | Vigabatrin |
| Lamotrigine | Felbamate | Zonisamide |
| Levetiracetam | Fenfluramine | |
| Oxcarbazepine | ||
ASMs = antiseizure medications.
Neuropsychological Outcomes.
Valproate:
Multiple studies have consistently shown the increased risk of neuropsychological impairments from fetal valproate exposure, and demonstrated that the risk for valproate is higher than any other ASMs which have adequate data.23-40 Examples from the literature are offered. The Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study prospectively enrolled women with epilepsy and evaluated their children who had fetal ASM exposure to carbamazepine, lamotrigine, phenytoin, or valproate monotherapy. At age 3 years old, children exposed to valproate had mean IQ 6-9 points lower than those exposed to the other ASMs.26 At 6 years old, children exposed to valproate had mean IQ 8-11 points lower than those exposed to the other ASMs, and the valproate children demonstrated a dose-dependent decremental effect across multiple cognitive domains.30 Other studies in the UK,23-25,27,34,36 Australia,28,29,38 and India40 have confirmed the adverse effects of feta valproate exposure on cognitive abilities. Also see population-based studies from Denmark, France, and Norway below. A Cochrane review in 2014 of 22 prospective cohort studies and six registry-based studies confirmed the findings adverse effects of valproate compared to unexposed children and to children exposed in utero to carbamazepine, lamotrigine and phenytoin, but those three ASMs did not differ.35 The review also noted valproate dose-dependent adverse effects in six studies. Given the dose-dependent effect, the question as to whether there is a safe dose has been raised, but valproate at <800mg/day is associated with impaired verbal abilities (−5.6, 95% CI −11.1 to −0.1; p=0.04) and a 6-fold increase in educational intervention (95% CI 1.4–18.0; p=0.01).36
Population-Based Studies:
Danish population studies have demonstrated an increased risk of fetal valproate exposure for autism spectrum disorder (adjusted HR, 2.9 [95% CI, 1.7-4.9]) and for childhood autism (adjusted HR, 5.2; CI, 2.7-10.0],41 an increased risk for attentional deficit hyperactivity disorder (adjusted hazard ratio, 1.48; 95%CI, 1.09-2.00),42 and an increased risk for of intellectual disability (aHR, 4.48; 95%CI, 2.97-6.76) and intellectual disability with delayed childhood milestones (aHR, 6.07; 95%CI, 4.67-7.89), but also found Increased risk of intellectual disability with prenatal exposure to maternal monotherapy use of carbamazepine (aHR, 3.84; 95%CI, 2.32-6.38), clonazepam (aHR, 2.41; 95%CI, 1.09-5.35), and oxcarbazepine (aHR, 3.70; 95%CI, 2.11-6.51) but not lamotrigine (aHR, 1.33; 95%CI, 0.71-2.48).43 Another Danish study found that valproate-exposed children scored worse on the sixth-grade language tests (adjusted difference, .0.27; 95%CI, .0.42 to .0.12) and sixth-grade mathematics tests (adjusted difference, .0.33; (95%CI, .0.47 to .0.19); they also found clonazepam-exposed children scored worse in the sixth-grade language tests (adjusted difference, .0.07; 95%CI, .0.12 to .0.02).39 Carbamazepine, lamotrigine, phenobarbital, and oxcarbazepine were not linked to poor school performance compared with unexposed children.
A French nationwide population-based cohort study found that fetal exposure to valproate was associated with increased risks of neurodevelopmental disorders (HR=2.7, 95% CI (1.8 to 4.0)), pervasive developmental disorders (HR=4.4 (2.1 to 9.3)), mental retardation (HR=3.1 (1.5 to 6.2)) and visits to speech therapists (HR=1.5 (1.1 to 1.9)), with a dose-response relationship.44 Among the other ASMs, pregabalin was associated with an increased risk of neurodevelopmental disorders (aHR: 1.5; 95% CI 1.0–2.1), and phenobarbital was associated with higher risk of behavioural and emotional disorders (aHR: 7.6, 95% CI 1.1 to 53.6).45
Other ASMs:
Although two studies have raised concerns,33,46 most studies have not found neuropsychological impairments after fetal lamotrigine exosure.26,28,30,32,34,36,37,39-44 Similarly, several studies have failed to find deficits from fetal levetiracetam exposure.47-49 The Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study, which is a continuation of the NEAD study with a new prospective cohort, recently reported age 2 year old cognitive outcomes for children who were mainly exposed to lamotrigine or levetiracetam or both.50 The cohort of children exposed to ASMs did not differ from children of healthy women. In addition, no blood level or dose effects were seen for the primary outcome of language abilities. A secondary analysis did disclose a level-dependent effect of levetiracetam on the motor domain, but additional studies are needed to confirm this observation.
Some studies have reported adverse effects for carbamazepine33,51 and phenytoin,52,53 but others have not found an adverse signal for fetal exposures.25,26,28,30,32,34,37,39,40,43,44 Similarly, there are mixed signals for oxcarbazepine.33,39,43
Several studies have demonstrated adverse effects of fetal phenobarbital exposure including two Danish studies with lower IQ scores on in two adult men exposed in utero suing different IQ measures,43 two studies from India with reduced IQ and language abilities,40,55 and a French population-based study with increased risk of intellectual disability.45
A Danish population-based study reported increased risk for intellectual disability in children with fetal clonazepam exposure,43 and another Danish study found reduced language and math performance on 6th grade assessments.39 In two small studies, fetal topiramate exposure has been reported mixed results on neurodevelopmental effects.49,66 A French nationwide population-based noted increased risk for neurodevelopmental disorders from pregabalin exposure (aHR, 1.5; 95%CI 1.0–2.1).45 Gabapentin showed a marginal adverse effect on psychomotor development (OR=9.03, 95%CIs 1.00-62.78) in a systematic review and network meta-analysis.67 It is obvious that data for some of the ASMs above are inadequate. Further, data for other ASMs are very inadequate or completely lacking.
Periconceptional Folate.
The NEAD study found that periconceptional folic acid supplementation was associated with higher IQ at age 6 in children with exposed to ASM in utero; adjusted means (95% CIs) were 108 (106,111) in those with folic acid exposure vs. 102 (98,104) in those not exposed to folic acid.30 The Norwegian Mother and Child Cohort Study found that periconceptional folic acid was associated with reduced risks for language delay and autism in children exposed to ASMs comprised primarily of lamotrigine, carbamazepine and valproate.68-70 Folate effects were related to exposure in the first 12 weeks of pregnancy. These researchers have also shown that higher ASM concentrations are correlated with higher concentrations of unmetabolized folic acid and inactive folate metabolites.71 Additional research is needed to define the optimum dose of folic acid as concerns have been raised for risks of high dose periconceptional folic acid.
Breastfeeding on ASMs.
Despite clear positive effects of breastfeeding for both mother and child in the general population,72 some have raised concerns for breastfeeding when taking ASMs. However, several studies in children who were previously exposed in utero have shown that breastfeeding while a woman is taking AMS is safe. The NEAD study and a Norweigen study found no adverse effects on cognition at 3 years old,73,74 and the NEAD study actually showed that children exposed in utero who then breastfed actually had a mean IQ 4 points higher than those who did not breastfeed.75 One reason why ASM exposure from breastmilk does not have adverse effects may be that ASM levels in breastfeeding children are much lower than their mothers in most cases.76
Conclusions for Neuropsychological Risks:
Valproate poses an especially high risk, and there is also clear evidence of risk from phenobarbital. Risks for carbamazepine and phenytoin are less than valproate. The greatest safety in regards to behavioral teratogenesis appears to exist for lamotrigine and levetiracetam. There are some concerning but uncertain signals for clonazepam, oxcarbazepine and pregabalin. Data for other ASMs is inadequate to accurately determination risks for cognitive and behavioral deficits from fetal exposure (Table 2). Periconceptional folate improves neuropsychological outcomes in children, and breastfeeding is safe while taking ASMs.
Table 2.
Risks of fetal ASM-induced neuropsychological developmental impairments in humans based on current data.
| Data on Risks | Unknown Risks | |
|---|---|---|
| Highest Risks | Acetazolamide | Lacosamide |
| Phenobarbital | Briviracetam | Lorazepam |
| Valproate | Cannabidiol | Midazolam |
| Intermediate Risks | Cenobamate | Perampanel |
| Carbamazepine | Clobazam | Primidone |
| Phenytoin | Diazepam | Rufinamide |
| Possible Risks | Eslicarbazepine | Stiripentol |
| Clonazepam | Ethosuximide | Sulthiame |
| Oxcarbazepine | Everolimus | Tiagabine |
| Pregabalin | Felbamate | Topiramate |
| Lowest Risks | Fenfluramine | Vigabatrin |
| Lamotrigine | Gabapentin | Zonisamide |
| Levetiracetam | ||
ASMs = antiseizure medications.
Mechanisms of ASM Teratogenicity.
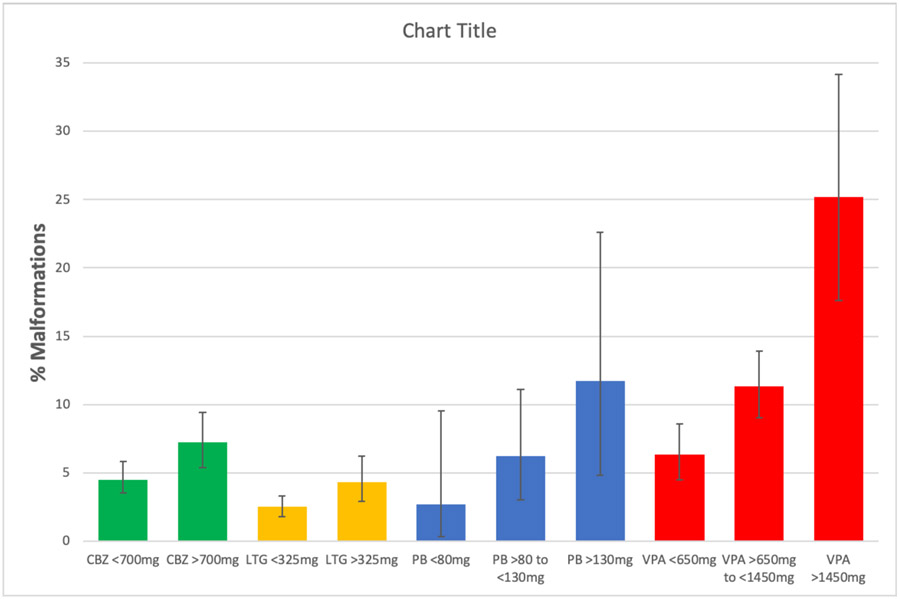
Several posited mechanisms for ASM teratogenic effects on fetal development have included suppression neuronal activity, folate related mechanisms, ischemia/hypoxia, reactive intermediates, free radicals, arene oxides, neuronal apoptosis and synaptic dysfunction, and antagonism of neutropins and signal proteins. Teratogens are known to act in a dose-dependent manner on a susceptible genotype. In regards to ASMs, the EURAP registry demonstrated dose-dependent effects for major congenital malformations across all the four of their most commonly used ASMs (i.e., carbamazepine, lamotrigine, phenobarbital, valproate), see Figure 2.14 Further, clear dose-dependent effects of valproate have been demonstrated across multiple cognitive domains for valproate.30 Although the genetic substrate for ASM-induced teratogenesis is unknow, there is evidence for genetic influences on ASM-induced risks in humans. If a woman taking ASM during a pregnancy has a child with a malformation, her risk of having another child with a malformation when taking the same ASM (16.8%) is higher than a woman whose first child did not have a malformation in a prior pregnancy.77,78
Figure 2. Malformation rates (mean %, 95% CIs) as function of ASM dose (adapted from reference #14).
CBZ=carbamazepine, LTG=lamotrigine, PB=phenobarbital, VPA=valproate. Dosages given as mg/day.
There likely to be multiple teratogenic mechanisms. The mechanisms for anatomical and behavioral teratogenesis may differ as major congenital malformations are the result of first trimester exposures while behavioral teratogenesis may be primarily due to third trimester exposures. The underlying mechanism for behavioral teratogenesis of ASMs may be similar to fetal alcohol effects on the immature brain which produces neuronal apoptosis and synaptic dysfunction in the surviving neurons.79 In fact, widespread neural apoptosis in the immature brains of animals has been demonstrated for clonazepam, diazepam, phenobarbital, phenytoin, valproate, and vigabatrin, but apoptosis was absent for carbamazepine, lamotrigine, levetiracetam, or topiramate in monotherapy exposures.80-87 However, these ASMs without apoptosis in monotherapy can enhance the apoptosis produced by phenytoin when given in combination, except for levetiracetam which did not enhance apoptosis. In addition to neuronal apoptosis, there is cell death in developing white matter,88 synaptic changes,89,90 and behavioral deficits.91-93 Unfortunately, most ASMs have not been tested in this model (Table 3).
Table 3.
ASMs tested in the apoptotic model.
| Data on Risks | Unknown Risks | |
|---|---|---|
| Higher Risks | Acetazolamide | Lorazepam |
| Clonazepam | Briviracetam | Midazolam |
| Diazepam | Cannabidiol | Oxcarbazepine |
| Phenobarbital | Cenobamate | Perampanel |
| Phenytoin | Clobazam | Pregabalin |
| Valproate | Diazepam | Primidone |
| Vigabatrin | Eslicarbazepine | Rufinamide |
| Ethosuximide | Stiripentol | |
| Lower Risks | Everolimus | Sulthiame |
| Carbamazepine | Felbamate | Tiagabine |
| Lamotrigine | Fenfluramine | Zonisamide |
| Levetiracetam | Gabapentin | |
| Topiramate | Lacosamide | |
Note that the model is predictive of effects in humans, but does not explore all possible mechanisms.
ASMs = antiseizure medications.
Future Directions.
Despite advances in our knowledge over the past two decades, information is lacking for most ASMs on risks for malformations, neuropsychological outcomes, and animal models. Recommendations to improve the situation have been made including a national reporting system for congenital malformations, routine meta-analyses of cohort studies to detect teratogenic signals, monitoring of ASM prescription practices for women, routine preclinical testing of all new ASMs for neurodevelopmental effects, and improved funding of basic and clinical research to fully delineate risks and underlying mechanisms for ASM-induced anatomical and behavioral teratogenesis.10 With such actions, future care in women requiring ASMs could be evidence-based and achieve precision medicine that focuses on early detection, prediction (e.g., via susceptible genotype), and targeted therapies.94
Summary.
ASMs pose both anatomical and behavioral teratogenetic risks to the developing immature brain. These risks vary across ASMs, but remain unknown for many ASMs. Based on present knowledge valproate and phenobarbital pose the greatest risks while lamotrigine and levetiracetam have the lowest risks.
Key Points.
Women with epilepsy are at increased risks for both poor anatomical and behavioral outcomes in their children, but most children born to these women are normal.
Teratogenic risks vary across antiseizure medications (ASMs).
Valproate poses a special risk for both anatomical and behavioral teratogenic risks compared to other ASMs.
The risks for many ASMs remain uncertain.
Women of childbearing potential taking ASMs should be taking folic acid.
Synopsis.
Most children born to women with epilepsy (WWE) are normal, but have increased risks for malformations and poor neuropsychological outcomes. Antiseizure medications (ASMs) are among the most commonly prescribed teratogenic medications in women of childbearing age. However, WWE typically cannot avoid using ASMs during pregnancy. Teratogenic risks vary across ASMs. Valproate poses a special risk for both anatomical and behavioral teratogenic risks compared to other ASMs. The risks for many ASMs remain uncertain. Women of childbearing potential taking ASMs should be taking folic acid. Breastfeeding while taking ASMs appears safe. WWE should receive informed consent outlining risks prior to conception.
Clinics Care Points.
Most children born to WWE are normal but have increased risks for malformations and poor neuropsychological outcomes.
WWE should receive informed consent outlining risks prior to conception.
Valproate is a poor 1st choice ASM for most WWE of childbearing potential.
WWE of childbearing potential should be taking folic acid.
Breastfeeding on ASMs appears safe.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement.
KJM has received research support from the National Institutes of Health, Eisai, and Medtronic Inc. The Epilepsy Study Consortium pays his university for his research consultant time related to Eisai, GW Pharmaceuticals, NeuroPace, Novartis, Supernus, Upsher-Smith Laboratories, UCB Pharma, and Vivus Pharmaceuticals. In addition, KJM is Co-I and Director of Cognitive Core of the Human Epilepsy Project for the Epilepsy Study Consortium. KJM is on the editorial boards for Neurology, Cognitive & Behavioral Neurology, Epilepsy & Behavior, and Epilepsy & Behavior Case Reports.
References.
- 1.Harden CL, Hopp J, Ting TY, et al. Practice parameter update: management issues for women with epilepsy—focus on pregnancy (an evidence-based review): obstetrical complications and change in seizure frequency: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology 2009;73(2):126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The history and stigma of epilepsy. 2003. https://onlinelibrary.wiley.com/doi/pdf/10.1046/j.1528-1157.44.s.6.2.x (accessed 12/26/2021).
- 3.Friedlander WJ. Who was 'the father of bromide treatment of epilepsy'? Arch Neurol 1986;43(5):505–7. [DOI] [PubMed] [Google Scholar]
- 4.Kim JH, Scialli AR. Thalidomide: the tragedy of birth defects and the effective treatment of disease. Toxicol Sci 2011;122(1):1–6. [DOI] [PubMed] [Google Scholar]
- 5.Meadow SR. Anticonvulsant drugs and congenital abnormalities. Lancet 1968;2(7581):1296. [DOI] [PubMed] [Google Scholar]
- 6.Speidel BD, Meadow SR. Epilepsy, anticonvulsants and congenital malformations. Drugs 1974;8(5):354–65. [DOI] [PubMed] [Google Scholar]
- 7.Tomson T, Battino D, Perucca E. The remarkable story of valproic acid. Lancet Neurology 2016:15(2):141–141. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control (CDC). Valproic acid and spina bifida: a preliminary report—France. MMWR 1982;31(42):565–6. [PubMed] [Google Scholar]
- 9.Tanoshima M, Kobayashi T, Tanoshima R, Beyene J, Koren G, Ito S. Risks of congenital malformations in offspring exposed to valproic acid in utero: A systematic review and cumulative meta-analysis. Clin Pharmacol Ther 2015;98(4):417–41. [DOI] [PubMed] [Google Scholar]
- 10.Meador KJ, Loring DW. Developmental effects of antiepileptic drugs and the need for improved regulations. Neurology. 2016. Jan 19;86(3):297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meador KJ. Cognitive effects of epilepsy and its treatments, Chapter 93. In: Wyllie E, Cascino GD, Gidal BE, Goodkin HP, Loddenkemper T, Sirven JI (eds). Wyllie’s Treatment of Epilepsy: Principles & Practice, 7th Edition. Philadelphia: Lippincott Williams & Wilkins, 2020: 1058–63. [Google Scholar]
- 12.Kellogg MA, Meador KJ. Neurodevelopmental effects of antiepileptic drugs. Neurochemical Research 2017;42(7):2065–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez-Diaz S, Smith CR, Shen A, et al. Comparative safety of antiepileptic drugs during pregnancy. Neurology 2012;78(21):1692–9. [DOI] [PubMed] [Google Scholar]
- 14.Tomson T, Battino D, Bonizzoni E, et al. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol 2018;17(6):530–538. [DOI] [PubMed] [Google Scholar]
- 15.Hunt S, Russell A, Smithson WH, et al. Topiramate in pregnancy: preliminary experience from the UK Epilepsy and Pregnancy Register. Neurology 2008;71(4):272–6. [DOI] [PubMed] [Google Scholar]
- 16.Mawhinney E, Craig J, Morrow J, et al. Levetiracetam in pregnancy: results from the UK and Ireland epilepsy and pregnancy registers. Neurology 2013;80(4):400–5. [DOI] [PubMed] [Google Scholar]
- 17.Campbell E, Kennedy F, Russell A, et al. Malformation risks of antiepileptic drug monotherapies in pregnancy: updated results from the UK and Ireland Epilepsy and Pregnancy Registers. J Neurol Neurosurg Psychiatry 2014;85(9):1029–34. [DOI] [PubMed] [Google Scholar]
- 18.Jentink J, Loane MA, Dolk H, et al. Valproic acid monotherapy in pregnancy and major congenital malformations. N Engl J Med 2010;362(23):2185–93. [DOI] [PubMed] [Google Scholar]
- 19.Jentink J, Dolk H, Loane MA, et al. Intrauterine exposure to carbamazepine and specific congenital malformations: systematic review and case-control study. BMJ 2010;341:c6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blotiere PO, Raguideau F, Weill A, et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology 2019;93(2):e167–e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christensen J, Trabjerg BB, Sun Y, et al. Prenatal exposure to valproate and risk of congenital malformations-Could we have known earlier? - A population-based cohort study. Epilepsia 2021;62(12):2981–2993. [DOI] [PubMed] [Google Scholar]
- 22.Patorno E, Hernandez-Diaz S, Huybrechts KF, et al. Gabapentin in pregnancy and the risk of adverse neonatal and maternal outcomes: A population-based cohort study nested in the US Medicaid Analytic extract dataset. PLoS Med 2020;17(9):e1003322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adab N, Kini U, Vinten J, et al. The longer term outcome of children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry 2004;75:1575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinten J, Adab N, Kini U, et al. Neuropsychological effects of exposure to anticonvulsant medication in utero. Neurology 2005;64:949–54. [DOI] [PubMed] [Google Scholar]
- 25.Bromley RL, Mawer G, J Clayton-Smith J, Baker GA, Liverpool and Manchester Neurodevelopment Group. Autism spectrum disorders following in utero exposure to antiepileptic drugs. Neurology 2008;71(23):1923–4. [DOI] [PubMed] [Google Scholar]
- 26.Meador KJ, Baker GA, Browning N, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med 2009;360:1597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bromley RL, Mawer G, Love J, et al. Early cognitive development in children born to women with epilepsy: a prospective report. Epilepsia 2010;51:2058–65. [DOI] [PubMed] [Google Scholar]
- 28.Nadebaum C, Anderson VA, Vajda F, et al. Language skills of school-aged children prenatally exposed to antiepileptic drugs. Neurology 2011;76:719–26. [DOI] [PubMed] [Google Scholar]
- 29.Nadebaum C, Anderson V, Vajda F, Reutens D, Barton S, Wood A. The Australian brain and cognition and antiepileptic drugs study: IQ in school-aged children exposed to sodium valproate and polytherapy. J Int Neuropsychol Soc 2011;17(1):133–42. [DOI] [PubMed] [Google Scholar]
- 30.Meador KJ, Baker GA, Browning N, et al. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol 2013;12:244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christensen J, Gronborg TK, Sorensen MJ, et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 2013;309:1696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen MJ, Meador KJ, Browning N, et al. Fetal antiepileptic drug exposure: adaptive and emotional/behavioral functioning at age 6 years. Epilepsy Behav 2013;29:308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veiby G, Daltveit AK, Schjolberg S, et al. Exposure to antiepileptic drugs in utero and child development: a prospective population-based study. Epilepsia 2013;54:1462–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bromley RL, Mawer GE, Briggs M, et al. The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J Neurol Neurosurg Psychiatry 2013;84:637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bromley R, Weston J, Adab N, et al. Treatment for epilepsy in pregnancy: neurodevelopmental outcomes in the child. Cochrane Database Syst Rev 2014:CD010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baker GA, Bromley RL, Briggs M, et al. IQ at 6 years after in utero exposure to antiepileptic drugs: a controlled cohort study. Neurology 2015;84:382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deshmukh U, Adams J, Macklin EA, et al. Behavioral outcomes in children exposed prenatally to lamotrigine, valproate, or carbamazepine. Neurotoxicol Teratol 2016;54:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barton S, Nadebaum C, Anderson VA, Vajda F, Reutens DC, Wood AG. Memory dysfunction in school-aged children exposed prenatally to antiepileptic drugs. Neuropsychology 2018;32(7):784–796. [DOI] [PubMed] [Google Scholar]
- 39.Elkjaer LS, Bech BH, Sun Y, et al. Association between prenatal valproate exposure and performance on standardized language and mathematics tests in school-aged children. JAMA Neurol 2018;75:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Unnikrishnan G, Jacob NS, Salim S, et al. Enduring language deficits in children of women with epilepsy and the potential role of intrauterine exposure to antiepileptic drugs. Epilepsia 2020. Nov;61(11):2442–2451. [DOI] [PubMed] [Google Scholar]
- 41.Christensen J, Grønborg TK, Sørensen MJ, et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 2013;309(16):1696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christensen J, Pedersen L, Sun Y, Dreier JW, Brikell I, Dalsgaard S. Association of prenatal exposure to valproate and other antiepileptic drugs with risk for attention-deficit/hyperactivity disorder in offspring. JAMA Netw Open 2019;2(1):e186606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daugaard CA, Pedersen L, Sun Y, Dreier JW, Christensen J. Association of prenatal exposure to valproate and other antiepileptic drugs with intellectual disability and delayed childhood milestones. JAMA Netw Open 2020;3(11):e2025570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blotière PO, Miranda S, Weill A, et al. Risk of early neurodevelopmental outcomes associated with prenatal exposure to the antiepileptic drugs most commonly used during pregnancy: a French nationwide population-based cohort study. BMJ Open 2020; 10(6):e034829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coste J, Blotiere PO, Miranda S, et al. Risk of early neurodevelopmental disorders associated with in utero exposure to valproate and other antiepileptic drugs: a nationwide cohort study in France. Sci Rep. 2020. Oct 22;10(1):17362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Husebye ESN, Gilhus NE, Spigset O, Daltveit AK, Bjørk MH. Language impairment in children aged 5 and 8 years after antiepileptic drug exposure in utero - the Norwegian Mother and Child Cohort Study. Eur J Neurol. 2020. Apr;27(4):667–675. [DOI] [PubMed] [Google Scholar]
- 47.Shallcross R, Bromley RL, Irwin B, et al. Child development following in utero exposure: levetiracetam vs sodium valproate. Neurology 2011;76:383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shallcross R, Bromley RL, Cheyne CP, et al. In utero exposure to levetiracetam vs valproate: development and language at 3 years of age. Neurology 2014;82:213–21. [DOI] [PubMed] [Google Scholar]
- 49.Bromley RL, Calderbank R, Cheyne CP, et al. Cognition in school-age children exposed to levetiracetam, topiramate, or sodium valproate. Neurology 2016;87:1943–53. [DOI] [PubMed] [Google Scholar]
- 50.Meador KJ, Cohen MJ, Loring DW, et al. Two-year-old cognitive outcomes in children of pregnant women with epilepsy in the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs Study. JAMA Neurol 2021;78(8):927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cummings C, Stewart M, Stevenson M, Morrow J, Nelson J. Neurodevelopment of children exposed in utero to lamotrigine, sodium valproate and carbamazepine. Arch Dis Child 2011;96(7):643–7. [DOI] [PubMed] [Google Scholar]
- 52.Scolnik D, Nulman I, Rovet J, et al. Neurodevelopment of children exposed in utero to phenytoin and carbamazepine monotherapy. JAMA 1994;271(10):767–70. [PubMed] [Google Scholar]
- 53.Verrotti A, Scaparrotta A, Cofini M, Chiarelli F, Tiboni GM. Developmental neurotoxicity and anticonvulsant drugs: a possible link. Reprod Toxicol 2014;48:72–80. [DOI] [PubMed] [Google Scholar]
- 54.Reinisch JM, Sanders SA, Mortensen EL, et al. In utero exposure to phenobarbital and intelligence deficits in adult men. JAMA. 1995;274:1518–1525. [PubMed] [Google Scholar]
- 55.Gopinath N, Muneer AK, Unnikrishnan S, Varma RP, Thomas SV. Children (10-12 years age) of women with epilepsy have lower intelligence, attention and memory: Observations from a prospective cohort case control study. Epilepsy Res 2015;117:58–62. [DOI] [PubMed] [Google Scholar]
- 66.Rihtman T, Parush S, Ornoy A. Preliminary findings of the developmental effects of in utero exposure to topiramate. Reprod Toxicol 2012;34:308–11. [DOI] [PubMed] [Google Scholar]
- 67.Veroniki AA, Rios P, Cogo E, et al. Comparative safety of antiepileptic drugs for neurological development in children exposed during pregnancy and breast feeding: a systematic review and network meta-analysis. BMJ Open 2017;7(7):e017248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Husebye ESN, Gilhus NE, Riedel B, Spigset O, Daltveit AK, Bjørk MH. Verbal abilities in children of mothers with epilepsy: Association to maternal folate status. Neurology 2018;91(9):e811–e821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bjørk M, Riedel B, Spigset O, et al. Association of folic acid supplementation during pregnancy with the risk of autistic traits in children exposed to antiepileptic drugs In utero. JAMA Neurol 2018;75(2):160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Husebye ESN, Gilhus NE, Spigset O, Daltveit AK, Bjork MH. Language impairment in children aged 5 and 8 years after antiepileptic drug exposure in utero - the Norwegian Mother and Child Cohort Study. Eur J Neurol 2020;27(4):667–675. [DOI] [PubMed] [Google Scholar]
- 71.Husebye ESN, Riedel B, Bjørke-Monsen AL, et al. Vitamin B status and association with antiseizure medication in pregnant women with epilepsy. Epilepsia 2021;62(12):2968–2980. [DOI] [PubMed] [Google Scholar]
- 72.Ip S, Chung M, Raman G, Trikalinos TA, Lau J. A summary of the Agency for Healthcare Research and Quality’s evidence report on breastfeeding in developed countries. Breastfeed Med 2009;4(suppl 1):S17–S30. [DOI] [PubMed] [Google Scholar]
- 73.Meador KJ, Baker GA, Browning N, et al. Effects of breastfeeding in children of women taking antiepileptic drugs. Neurology 2010;75(22): 1954–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Veiby G, Engelsen BA, Gilhus NE. Early child development and exposure to antiepileptic drugs prenatally and through breastfeeding: a prospective cohort study on children of women with epilepsy. JAMA Neurol 2013;70(11):1367–74. [DOI] [PubMed] [Google Scholar]
- 75.Meador KJ, Baker GA, Browning N, et al. Breastfeeding in children of women taking antiepileptic drugs: cognitive outcomes at age 6 years. JAMA Pediatr 2014; 168(8):729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Birnbaum AK, Meador KJ, Karanam A, et al. Antiepileptic drug exposure in infants of breastfeeding mothers with epilepsy. JAMA Neurol 2020;77(4):441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Campbell E, Devenney E, Morrow J, et al. Recurrence risk of congenital malformations in infants exposed to antiepileptic drugs in utero. Epilepsia 2013;54(1):165–171. [DOI] [PubMed] [Google Scholar]
- 78.Vajda FJ, O'Brien TJ, Lander CM, et al. Teratogenesis in repeated pregnancies in antiepileptic drug-treated women. Epilepsia 2013;54(1):181–186. [DOI] [PubMed] [Google Scholar]
- 79.Ikonomidou C, Bittigau P, Ishimaru MJ, et al. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science 2000;287(5455):1056–60. [DOI] [PubMed] [Google Scholar]
- 80.Bittigau P, Sifringer M, Genz K, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci U S A. 2002. Nov 12;99(23):15089–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bittigau P, Sifringer M, Ikonomidou C. Antiepileptic drugs and apoptosis in the developing brain. Ann N Y Acad Sci. 2003. May;993:103–14 [DOI] [PubMed] [Google Scholar]
- 82.Glier C, Dzietko M, Bittigau P, Jarosz B, Korobowicz E, Ikonomidou C. Therapeutic doses of topiramate are not toxic to the developing rat brain. Exp Neurol. 2004. Jun;187(2):403–9. [DOI] [PubMed] [Google Scholar]
- 83.Manthey D, Asimiadou S, Stefovska V, et al. Sulthiame but not levetiracetam exerts neurotoxic effect in the developing rat brain. Exp Neurol. 2005. Jun;193(2):497–503. [DOI] [PubMed] [Google Scholar]
- 84.Katz I, Kim J, Gale K, Kondratyev A. Effects of lamotrigine alone and in combination with MK-801, phenobarbital, or phenytoin on cell death in the neonatal rat brain. J Pharmacol Exp Ther. 2007. Aug;322(2):494–500. [DOI] [PubMed] [Google Scholar]
- 85.Kim J, Kondratyev A, Gale K. Antiepileptic drug-induced neuronal cell death in the immature brain: effects of carbamazepine, topiramate, and levetiracetam as monotherapy versus polytherapy. J Pharmacol Exp Ther. 2007. Oct;323(1):165–73. [DOI] [PubMed] [Google Scholar]
- 86.Kim JS, Kondratyev A, Tomita Y, Gale K. Neurodevelopmental impact of antiepileptic drugs and seizures in the immature brain. Epilepsia. 2007;48 Suppl 5:19–26. [DOI] [PubMed] [Google Scholar]
- 87.Forcelli PA, Kim J, Kondratyev A, Gale K. Pattern of antiepileptic drug-induced cell death in limbic regions of the neonatal rat brain. Epilepsia. 2011. Dec;52(12):e207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaushal S, Tamer Z, Opoku F, Forcelli PA. Anticonvulsant drug-induced cell death in the developing white matter of the rodent brain. Epilepsia 2016;57(5):727–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Forcelli PA, Janssen MJ, Vicini S, Gale K. Neonatal exposure to antiepileptic drugs disrupts striatal synaptic development. Ann Neurol. 2012. Sep;72(3):363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Al-Muhtasib N, Sepulveda-Rodriguez A, Vicini S, Forcelli PA. Neonatal phenobarbital exposure disrupts GABAergic synaptic maturation in rat CA1 neurons. Epilepsia. 2018. Feb;59(2):333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vorhees CV. Developmental neurotoxicity induced by therapeutic and illicit drugs. Environ Health Perspect 1994;102 Suppl 2(Suppl 2):145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Forcelli PA, Kozlowski R, Snyder C, Kondratyev A, Gale K. Effects of neonatal antiepileptic drug exposure on cognitive, emotional, and motor function in adult rats. J Pharmacol Exp Ther. 2012. Mar;340(3):558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gutherz SB, Kulick CV, Soper C, Kondratyev A, Gale K, Forcelli PA. Brief postnatal exposure to phenobarbital impairs passive avoidance learning and sensorimotor gating in rats. Epilepsy Behav. 2014. Aug;37:265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li Y, Zhang S, Snyder MP, Meador KJ. Precision medicine in women with epilepsy The challenge, systematic review, and future direction. Epilepsy Behav 2021;118:107928. [DOI] [PMC free article] [PubMed] [Google Scholar]