The pairing of coagulation factors containing a gamma-carboxyglutamate (Gla) domain on a phosphatidylserine-rich surface in the presence of divalent cations, such as calcium, enhances the rate of conversion of a zymogen to an active species by 1 to 2 million fold. This principle underpins the use of warfarin to prevent the vitamin K dependent post-translational conversion of specific Glu to Gla residues in coagulation factors [1]. Yet, one coagulation protease, factor XI (FXI), does not contain a Gla domain and escapes regulation by warfarin. Rather, FXI is comprised of a trypsin-like catalytic domain and four 90 to 91 amino acid repeats termed apple domains, due to their resemblance to the forbidden fruit. The apple domain structure is shared only with its monomeric homolog prekallikrein [2] and has formed a shroud of mystery over the mechanisms by which FXI binds to cells and partners with other proteins of the coagulation and plasma kallikrein-kinin systems. As FXI has enjoyed a renewed interest as a druggable target for thrombosis and other inflammatory disorders [3], this curtain is being slowed pulled back.
Thrombotic diseases present a challenge to the field of medicine as therapeutics need to maintain the haemostatic balance between clotting and anticoagulation. Proteins of the coagulation cascade have been targeted for antithrombotic therapeutics to treat deep vein thrombosis, acute myocardial infarction, and ischemic stroke. Inhibition of the activation or activity of coagulation factors, including FX and thrombin, successfully prevents thrombosis yet come with a risk for bleeding, which limits their clinical utility. FXI plays the role of bad apple in thromboinflammatory diseases including sepsis and atherosclerosis [4, 5], while playing a minor role in haemostasis, making it a promising therapeutic target [6]. As such, a pharmacopoeia of FXI inhibitors have been developed, including small molecule active site inhibitors [7], antisense oligonucleotides [8], and monoclonal antibodies [9]. An additional therapeutic tool being explored herein is the development of nanobodies. Nanobodies are derived from naturally occurring heavy-chain-only antibodies that contain a single variable domain [10]. They are versatile tools for basic research, disease diagnosis, molecular imaging, drug delivery and therapy [11]. Traditional antibody therapeutics are widely used but have many limitations due to their large size and poor penetration of tissue [12]. Repeated administration of antibody therapeutics can cause adverse immune responses through antidrug antibody production. This has the potential to diminish or totally cancel the antibody therapeutic effect [13]. The small size increased stability, and pharmacokinetic properties of nanobodies make them a promising therapeutic tool.
In this issue of Journal of Thrombosis and Haemostasis, Bar Barroeta et al. identify five nanobodies that inhibit FIX activation through binding to the apple 3 domain of FXI. A new interaction between FXI and high molecular weight kininogen (HK) was discovered through characterization of a sixth nanobody that competed with FXI-HK binding to indirectly inhibit FIX activation [14]. Bar Barroeta et al. developed FXI nanobodies through subcutaneous injection of recombinant FXI and FXIa into two llamas. The nanobodies were isolated and screened for FXI binding. As FIX binds the apple 3 domain of FXI [15], six nanobodies that bind this domain were evaluated for their potential to inhibit clotting times, FXIa activation of FIX, FXIa activity, and thrombin generation. They found that the clones 1H9 and 1D4 exhibited near complete inhibition of FXIa-mediated FIX activation in a purified system. Five of the six nanobodies showed a dose-dependent inhibition of FXIa activity, suggesting that these nanobodies compete with the FIX binding site. Consistently, these five nanobodies increased clotting time initiated by the contact pathway while reducing thrombin generation and FXIa activity, as well as FXIIa-mediated activation of FXI.
The unexpected apple fell with the observation that clone 2G3 increased clotting time initiated by the contact pathway and reduced FXIa activity, yet did not affect FXIa activation of FIX nor thrombin generation when initiated through the extrinsic pathway. Based on these puzzling observations, Bar Barroeta et al. tested whether 2G3 influenced the binding of substrates that bind to FXI, namely FXII and HK. While 2G3 did not interfere with the activation of FXI by FXIIa, the surprising observation was that 2G3 blocked HK binding to FXI. If indeed 2G3 mapped to the apple 3 domain, this observation suggests a novel interaction site between FXI and HK. Moreover, this suggests an ill-defined function for this interaction in thrombin generation, adding to the established role of FXI-HK interactions in mediating FXI binding to endothelial cells and platelets, as well as negatively regulating FXI activation in the presence of DNA and polyphosphates [16].
The nanobodies were characterized through HDX MS to unravel how they inhibited FXI function. Perhaps not surprising at this point in the story, 2G3 did not bind the FIX binding epitope on FXI, but rather largely bound to a distinct region within the second, fourth and fifth beta strands of the apple 3 domain (Ala193-Ala205 and Leu239-Phe260). Conversely, the five nanobodies that functionally inhibited FXIa-mediated FIX activation shared a binding epitope within the apple 3 domain overlapping with the established FIX binding epitope (Figure) [17]. Yet, this still doesn’t explain how these same apple 3 domain-specific nanobodies inhibited FXIIa mediated activation of FXI, as FXIIa interacts with FXI via the apple 2 and apple 4 domains [18]. Bar Barroeta et al., speculate the nanobodies sterically interfere with FXIIa activation of FXI or that FXIIa interacts with the apple 3 domain in addition to its main binding epitope. A parallel mechanism of action has been observed for the anti-FXI monoclonal antibody, 1A6, which binds to the apple 3 domain and inhibits FXI activation by FXIIa and FIX activation by FXIa [19], suggesting that perhaps the mechanistic apple doesn’t fall far from the tree. In conclusion, the study by Bar Barroeta and colleagues uncovered a novel interaction site between FXI and HK within the apple 3 domain of FXI, which may represent an ideal site for targeted drug develop for the prevention or treatment of thromboinflammation.
Figure 1.
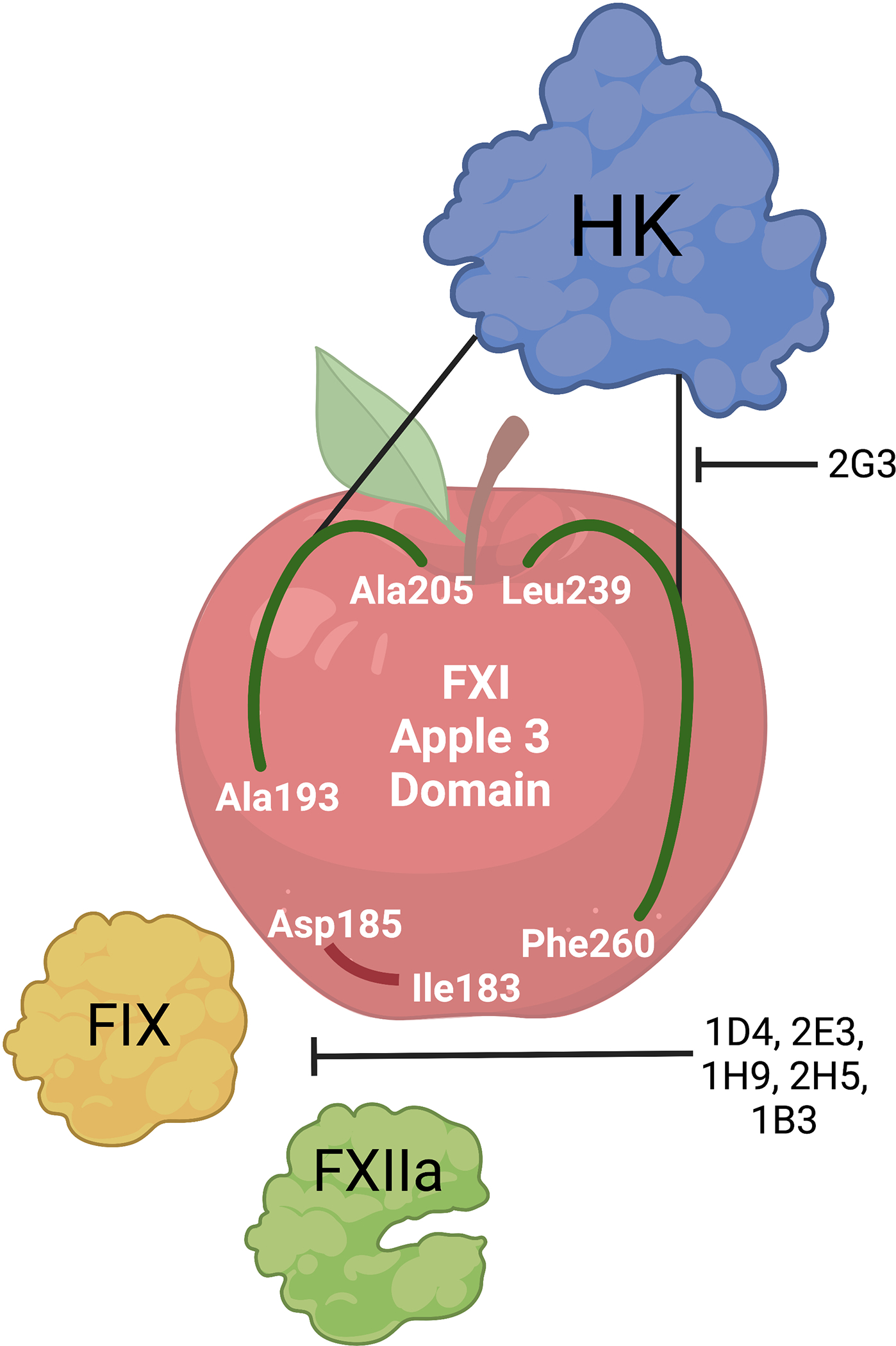
The nanobodies identified by Bar Barreota et al interact with the apple 3 domain of the coagulation factor XI through two distinct pathways. Five of the six nanobodies share a binding epitope with FIX (Asp186-Ile183) and thus block and functionally inhibit FIX activation by activated FXI; they also inhibit the activation of FXI by FXIIa. Alternatively, nanobody 2G3 binds to a unique region on FXI (Ala193-Ala205 and Leu239-Phe260) and blocks high molecular weight kininogen (HK)-FXI binding, preventing clotting induced by the contact activation system but not interfering with the enzymatic step of FIX activation by FXIa.
Funding
This work was supported by the National Institutes of Health [R01HL101972, R01AI157037, and R01HL144113].
Footnotes
CONFLICT OF INTEREST
None.
Invited Editorial to accompany: Nanobodies against FXI apple 3 domain inhibit binding of FIX and reveal a novel binding site for high molecular weight kininogen
REFERENCES
- 1.de Lara DV, de Melo DO, Araujo Silva LC, Goncalves TS, Junior Lima Santos PC. Pharmacogenetics of clopidogrel and warfarin in the treatment of cardiovascular diseases: an overview of reviews. Pharmacogenomics. 2022;23:443–52. [DOI] [PubMed] [Google Scholar]
- 2.Visser M, Heitmeier S, Ten Cate H, Spronk HMH. Role of Factor XIa and Plasma Kallikrein in Arterial and Venous Thrombosis. Thromb Haemost. 2020;120:883–993. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava P, Gailani D. The rebirth of the contact pathway: a new therapeutic target. Curr Opin Hematol. 2020;27:311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ngo ATP, Jordan KR, Mueller PA, Hagen MW, Reitsma SE, Puy C, Revenko AS, Lorentz CU, Tucker EI, Cheng Q, Hinds MT, Fazio S, Monia BP, Gailani D, Gruber A, Tavori H, McCarty OJT. Pharmacological targeting of coagulation factor XI mitigates the development of experimental atherosclerosis in low-density lipoprotein receptor-deficient mice. J Thromb Haemost. 2021;19:1001–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silasi R, Keshari RS, Lupu C, Van Rensburg WJ, Chaaban H, Regmi G, Shamanaev A, Shatzel JJ, Puy C, Lorentz CU, Tucker EI, Gailani D, Gruber A, McCarty OJT, Lupu F. Inhibition of contact-mediated activation of factor XI protects baboons against S aureus-induced organ damage and death. Blood Adv. 2019;3:658–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Horani RA. Factor XI(a) inhibitors for thrombosis: an updated patent review (2016-present). Expert Opin Ther Pat. 2020;30:39–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollack CV Jr., Kurz MA, Hayward NJ. EP-7041, a Factor XIa Inhibitor as a Potential Antithrombotic Strategy in Extracorporeal Membrane Oxygenation: A Brief Report. Crit Care Explor. 2020;2:e0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Younis HS, Crosby J, Huh JI, Lee HS, Rime S, Monia B, Henry SP. Antisense inhibition of coagulation factor XI prolongs APTT without increased bleeding risk in cynomolgus monkeys. Blood. 2012;119:2401–8. [DOI] [PubMed] [Google Scholar]
- 9.Koch AW, Schiering N, Melkko S, Ewert S, Salter J, Zhang Y, McCormack P, Yu J, Huang X, Chiu YH, Chen Z, Schleeger S, Horny G, DiPetrillo K, Muller L, Hein A, Villard F, Scharenberg M, Ramage P, Hassiepen U, et al. MAA868, a novel FXI antibody with a unique binding mode, shows durable effects on markers of anticoagulation in humans. Blood. 2019;133:1507–16. [DOI] [PubMed] [Google Scholar]
- 10.Jovcevska I, Muyldermans S. The Therapeutic Potential of Nanobodies. BioDrugs. 2020;34:11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Fan Z, Shao L, Kong X, Hou X, Tian D, Sun Y, Xiao Y, Yu L. Nanobody-derived nanobiotechnology tool kits for diverse biomedical and biotechnology applications. Int J Nanomedicine. 2016;11:3287–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol. 2009;157:220–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Brummelen EM, Ros W, Wolbink G, Beijnen JH, Schellens JH. Antidrug Antibody Formation in Oncology: Clinical Relevance and Challenges. Oncologist. 2016;21:1260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bar Barroeta A, Marquart JA, Bakhtiari K, Meijer AB, Urbanus RT, Meijers JCM. Nanobodies against factor XI apple 3 domain inhibit binding of factor IX and reveal a novel binding site for high molecular weight kininogen. J Thromb Haemost. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Y, Gailani D. Identification of a factor IX binding site on the third apple domain of activated factor XI. J Biol Chem. 1996;271:29023–8. [DOI] [PubMed] [Google Scholar]
- 16.Mohammed BM, Matafonov A, Ivanov I, Sun MF, Cheng Q, Dickeson SK, Li C, Sun D, Verhamme IM, Emsley J, Gailani D. An update on factor XI structure and function. Thromb Res. 2018;161:94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bar Barroeta A, van Galen J, Stroo I, Marquart JA, Meijer AB, Meijers JCM. Hydrogen-deuterium exchange mass spectrometry highlights conformational changes induced by factor XI activation and binding of factor IX to factor XIa. J Thromb Haemost. 2019;17:2047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng Q, Tucker EI, Pine MS, Sisler I, Matafonov A, Sun MF, White-Adams TC, Smith SA, Hanson SR, McCarty OJ, Renne T, Gruber A, Gailani D. A role for factor XIIa-mediated factor XI activation in thrombus formation in vivo. Blood. 2010;116:3981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tucker EI, Marzec UM, White TC, Hurst S, Rugonyi S, McCarty OJ, Gailani D, Gruber A, Hanson SR. Prevention of vascular graft occlusion and thrombus-associated thrombin generation by inhibition of factor XI. Blood. 2009;113:936–44. [DOI] [PMC free article] [PubMed] [Google Scholar]


