Abstract
Stroke represents a major cause of mortality and long-term disability among adult populations, leaving a devastating socioeconomic impact globally. Clinical manifestation of stroke is characterized by great diversity, ranging from minor disability to considerable neurological impairment interfering with activities of daily living and even death. Prognostic ambiguity has stimulated the interest for implementing stroke recovery biomarkers, including those provided by structural neuroimaging techniques, i.e., diffusion tensor imaging (DTI) and tractography for the study of white matter (WM) integrity. Considering the necessity of prompt and accurate prognosis in stroke survivors along with the potential capacity of DTI as a relevant imaging biomarker, the purpose of our study was to review the pertinent literature published within the last decade regarding DTI as a prognostic tool for recovery in acute and hyperacute stroke. We conducted a thorough literature search in two databases (MEDLINE and Science Direct) in order to trace all relevant studies published between 1 January 2012 and 16 March 2022 using predefined terms as key words. Only full-text human studies published in the English language were included. Forty-four studies were identified and are included in this review. We present main findings and by describing several methodological issues, we highlight shortcomings and gaps in the current literature so that research priorities for future research can be outlined. Our review suggests that DTI can track longitudinal changes and identify prognostic correlates in acute and hyperacute stroke patients.
Keywords: diffusion tensor imaging, tractography, acute stroke, hyperacute stroke, stroke prognosis, biomarkers
1. Introduction
Stroke represents a major cause of mortality and long-term disability among the adult population, leaving a devastating socioeconomic impact globally. More specifically, in Western countries, up to 4% of healthcare resources are expended on stroke. In the United States, the mean stroke-related cost per individual, including rehabilitation, is nearly USD 140,000 [1]. Taking into account that stroke incidence is highly age-dependent along with the continuously increasing life expectancy, a substantial increase in the numbers of stroke survivors is anticipated [2,3]. In this context, efficient prognostication is of paramount importance, as prompt and precise identification of recovery capabilities may optimize treatment strategies in poststroke patients.
Clinical manifestation of stroke is characterized by great diversity, ranging from minor disability to considerable neurological impairment interfering with activities of daily living (ADL) and even causing death. Prognostic ambiguity has stimulated an interest in implementing stroke recovery biomarkers. Ideally, prognostic markers hold high sensitivity and specificity, enabling appropriate management of healthcare resources and individualization of rehabilitation treatments. Efficiency of the selected biomarker is also based on its capacity to accurately depict underlying mechanisms of disease. Moreover, it should be non-invasive, readily accessible to patients and clinicians, easily interpreted by physicians, reproducible, and cost-effective [4,5].
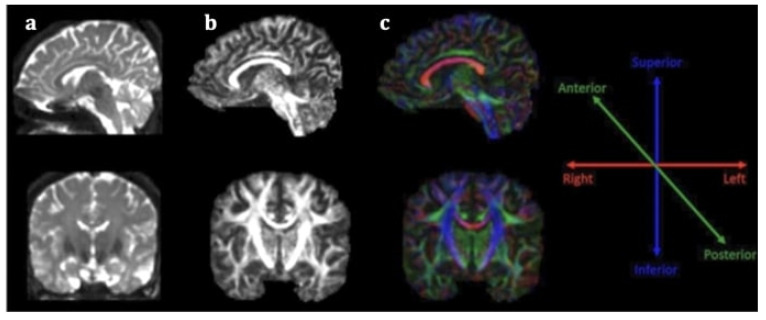
Stroke diagnosis has been facilitated by the incorporation of advanced neuroimaging modalities in clinical practice, especially diffusion weighted imaging (DWI), a technique based on the motion of water molecules. Nevertheless, conventional magnetic resonance imaging (MRI) is incapable of accurately illustrating microstructural impairment on white matter (WM) tracts, rendering its role in predicting stroke outcome limited. Conversely, diffusion tensor imaging (DTI) is an extension of this modality for in vivo mapping of white matter (WM) directionality and organization, allowing the qualitative and quantitative evaluation of major WM tracts and their microstructural integrity [6]. DTI is based on the random diffusion of water molecules [7]. In WM, water diffusion is slower perpendicular to the fibers, but it occurs faster along their longitudinal axis, producing anisotropic diffusion. The extent of anisotropy is influenced by integrity and organization of the WM tract and water diffusion mobility generated by axonal membranes and their myelin sheaths. Different computational algorithms are used to track different WM bundles and study WM organization in healthy participants and different disease samples [8]. The most widely employed DTI parameters are fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD). FA measures the preferential directionality of diffusion and is quantitatively expressed in numerical values between 0 and 1. High FA values indicate a greater degree of preferential directionality (anisotropic diffusion). Such a high degree of preferential directionality is commonly observed in highly organized WM tracts. On the other hand, low FA values indicate less preferential directionality of water molecules (isotropic diffusion). Low FA values close to 0 are observed in gray matter and cerebrospinal fluid. MD indicates the diffusion magnitude, AD describes the diffusivity along the dominant diffusion direction, and RD portrays the average diffusivity of two shorter eigenvectors [7]. It has been suggested that AD is mostly related to axonal degeneration whereas RD is mostly linked to demyelinating processes [9]. DTI offers directional information on water molecule diffusion and provides additional maps, including the fractional anisotropy (FA) map and the color-coded directional map (Figure 1). The color-coded directional maps are based on the convention that the blue color represents the water molecules that diffuse in an inferior–superior direction, the green color represents that water molecules that diffuse in an anteroposterior direction, and the red color represents the water molecules that diffuse in a left–right direction. Although DTI has not yet been incorporated in routine clinical care in stroke, a growing body of research suggests that it is a promising imaging biomarker for stroke recovery, owing to its ability to imprint white matter tract integrity in detail [9].
Figure 1.
Sagittal (first line) and coronal (second line) section of DTI data (a), fractional anisotropy map (b) and color fractional anisotropy map (c). The color-coding of the white matter tracts in the color fractional anisotropy map follows the assumption: red for left–right-oriented fibers, blue for superior–inferior-oriented fibers and green for anteroposterior-oriented fibers.
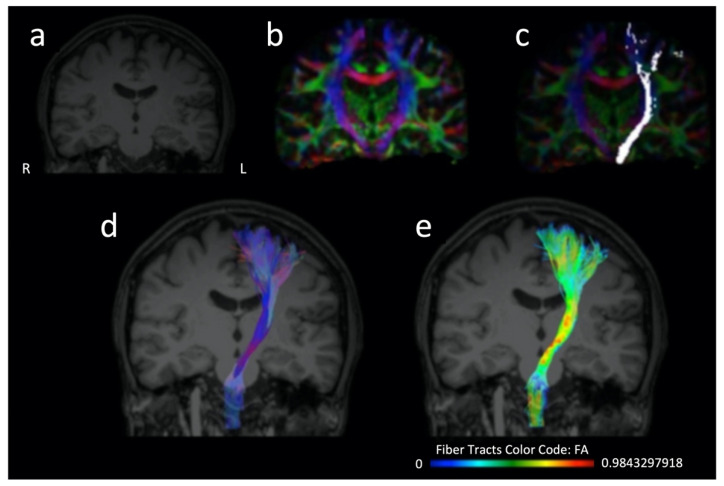
The majority of studies assessing DTI value as a stroke recovery biomarker have focused primarily on corticospinal tract (CST) integrity [6]. CST is a descending pathway (Figure 2) of great significance for motor function. Thus, it is reasonable that research on post-stroke rehabilitation has vastly focused on its assessment [10,11]. Among DTI parameters, FA is the most frequently utilized in research related to stroke recovery. Decrease of FA values of CST within the subacute stroke phase is widely associated with poorer motor outcomes [6,12,13]. Nevertheless, both increased and decreased FA values can be seen acutely [14]. Interestingly, it has been suggested that FA increase in the acute phase can be probably attributed to the more profound decrement in isotropic compared to anisotropic diffusion; therefore it cannot predict lesion age at the hyperacute stage [14]. Methods of FA of CST evaluation include measuring FA remotely at the stroke location, or the number of fibers passing through the stroke with tractography and calculating the ratio between the ipsilesional and contralesional pyramidal tracts [12]. Besides CST, DTI research has also expanded to other WM tracts, depending on the type of neurological deficit explored [15], including for example the common language-related WM network not only in the left but also in the right hemisphere [16,17,18,19,20].
Figure 2.
Three-dimensional T1 sequence (a), color fractional anisotropy map (b), distribution of the corticospinal tract fibers projected over a color fractional anisotropy map (c), three dimensional representation of the corticospinal tract (d,e) which is further color-coded according to the distribution of the fractional anisotropy values along the tract (e) and projected over a T1 sequence (d,e). The reconstruction of the corticospinal tract has been performed on a healthy subject (who provided written informed consent for the data acquisition, analysis and presentation) using the Brainance MD platform (Advantis Medical Imaging).
Research on DTI metrics as stroke outcome biomarker is not limited to the acute and subacute phases, as it is also implemented on chronic stroke patients [13,21]. Direct visualization of long tracts and their potential disruption provides insight into pathogenesis of functional deficits in stroke survivors as well as compensatory mechanisms on a microstructural level. Such knowledge may elucidate which group of patients is most likely to benefit from rehabilitation, and even help personalize treatment plans after the acute stroke phase according to each individual’s needs. Of note, it has been evidenced that rehabilitative processes induce microstructural alterations reflected on DTI and are compatible with neural integrity [13].
Considering the necessity of prompt and accurate prognosis in stroke survivors along with the potential capacity of DTI as a relevant imaging biomarker, the purpose of our study was to review the pertinent literature published within the last decade regarding DTI as a prognostic tool in acute and hyperacute stroke.
2. Materials and Methods
The Preferred Reporting Items for Systematic Reviews (PRISMA checklist) was used to guide this study. Our study’s methods were designed a priori.
2.1. Research Strategy
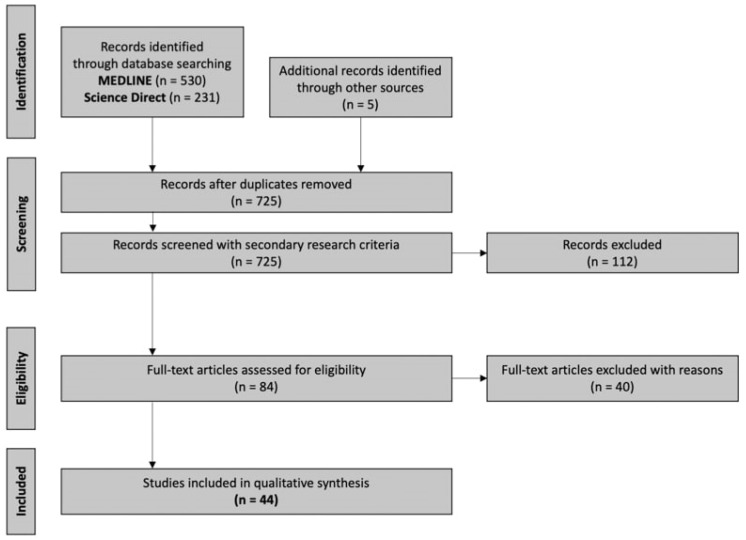
A literature research of two databases (MEDLINE and Science Direct) was conducted by one investigator in order to trace all relevant studies published between 1 January 2012 and 16 March 2022, using either “diffusion tensor imaging” as a keyword or related term “DTI” as a search criterion. Moreover, the terms “stroke prognosis” or “stroke outcome” or “stroke recovery” were used as a second search criterion. The retrieved articles were also hand-searched for any further potential eligible articles. Any disagreement regarding screening or selection process was solved by a second investigator until consensus was reached. Figure 3 presents the review flowchart.
Figure 3.
Study flow chart (PRISMA diagram).
2.2. Selection Criteria
Only full-text original articles published in the English language were included. Secondary analyses, reviews, case reports, guidelines, meeting summaries, comments, unpublished abstracts or studies conducted in animals were excluded. There was no restriction on study design or sample characteristics.
2.3. Data Extraction
Data extraction was performed using a predefined data form created in Excel. We recorded author, year of publication, number and age of participants, study design with regards to DTI acquisition (cross-sectional, longitudinal), type of stroke, time of DTI acquisition, main DTI parameters (MR field strength, diffusion directions, DTI analysis, and extracted parameters), anatomical regions examined, scales utilized, and main findings.
2.4. Data Analysis
No statistical analysis or meta-analysis was performed due to the high heterogeneity among studies. Thus, the data were only descriptively analyzed.
3. Results
3.1. Database Searches
Overall, 767 records were retrieved from the database search. Duplicates and irrelevant studies were excluded; hence, a total of 84 articles were selected. After screening the full text of the articles, 44 studies were eligible for inclusion.
3.2. Study Characteristics
Forty-four publications fulfilled our inclusion criteria. They were classified into three groups, according to the type of stroke. The first group comprised 29 studies focusing on ischemic stroke (Table 1), the second group consisted of 11 studies focusing on hemorrhagic stroke (Table 2), while the third group embodied 4 studies, assessing the prognostic value of DTI in a group comprising both ischemic stroke and hemorrhagic stroke patients (Table 3).
3.3. Study Design
Twenty-three studies were cross-sectional, and 21 studies were longitudinal with regards to the DTI acquisition. Of note, the majority of ischemic stroke studies were longitudinal (18/29 studies), the majority of hemorrhagic stroke studies were cross-sectional (8/11 studies) while all of the studies including both cohorts were cross-sectional (4/4 studies).
3.4. Stroke Patient Groups
The total number of stroke patients included in all studies ranged from n = 3 [22] to n = 165 [23]. Across the 44 studies, 6 studies had a disease sample size between 3–15 patients, 16 studies had a disease sample size between 16–30 patients, 11 had a disease sample size between 31–50 patients, and 11 studies had a disease sample size larger than 50 patients, with three of them including a disease sample size ≥100 patients [23,24].
3.5. Reference Groups
Across the 44 studies, the stroke patients were contrasted to demographically-matched healthy individuals in only 15 studies, with the rest of them (29/44 studies) not including a healthy control group (29/44 studies). None of the studies included a disease-control group other than stroke patients.
3.6. Demographic and Clinical Profiles
One study does did directly report participants’ age [23]. Mean/median patients’ age ranged from 47.5 years [25] to 72.7 years [25]. A comprehensive clinical description of participants’ demographic and clinical profiles is important for identifying meaningful imaging markers for stroke prognosis. The focus on clinical variables varied among the identified studies.
3.7. Target Brain Region
Except for three whole-brain studies that enabled the evaluation of WM changes in both motor and non-motor WM tracts [26,27,28], most studies focused on motor-related pathways, i.e., the pyramidal tract. Non-motor WM tracts were examined in six studies [18,26,29,30,31]. Interestingly, only two of them were dedicated non-motor WM studies that examined language-related pathways [29,30].
3.8. Supporting Imaging and Neurophysiological Modalities
Three studies included additional imaging modalities, i.e., resting-state functional MRI (rs-fMRI) [26,32,33]. With regards to additional neurophysiological measures, only one study examined the functional integrity of the pyramidal tract using transcranial magnetic stimulation (TMS) [34].
3.9. Field Strength, Acquisition Parameters (directions), Post-Processing Techniques and DTI Parameters
Seventeen studies used 1.5 T (10 studies were published between 2012–2017 and seven studies were published between 2018–2022) and 26 studies used 3 T (17 studies were published between 2012–2017 and 9 studies are published between 2018–2022). One study does did provide information regarding field strength [30]. With regards to the number of directions, three studies used 6 directions (two in 1.5 T [34,35] and one in 3.0 T [36]), eight studies used 12 directions (four in 1.5 T [37,38,39] and four in 3.0 T [40,41,42,43]), three studies used 15 directions (two in 1.5 T [44,45] and one in 3.0 T [23]), three studies used 16 directions (one in 1.5 T [46] and two in 3.0 T [24,47]), one study in 1.5 T used 20 directions [31], one study in 3.0 T used 25 directions [48], eight studies used 30 directions (two in 1.5 T [49,50] and six in 3.0 T [18,29,33,51,52,53]), three studies in 1.5 T used 32 directions [54,55,56], one study in 3.0 T used 55 directions [57], and seven studies in 3.0 T used 64 directions [25,26,27,32,58,59,60]). Six studies did not provide information regarding diffusion directions. Among the studies that applied tractography, four studies reported the use of probabilistic tractography/atlases [23,50,51,61]. With regards to the analysis software, 12 studies used manufacturer-provided software whereas almost half of them (22/44 studies) used non-commercial software packages for academic institutions (e.g., FSL, ExploreDTI, DTI Studio). With regards to the reporting metrics, the majority of studies focused on FA and FA ratio (rFA) (affected/unaffected tract or region of interest [ROI]), whilst additional information regarding diffusivity values (i.e., MD, AD, and RD and/or MD, AD, and RD ratio) were only provided in five studies [30,52,58,61,62]. Of note, fiber number (FN) and fiber number ration (rFN) was also reported in some studies. Three studies used qualitative characterization of reconstructed tracts (i.e., disrupted, displaced, preserved or non-disrupted, partially disrupted, and fully-disrupted) according to which they further categorize their stroke cohorts [37,38,39].
Table 1.
Basic characteristics and main findings of studies including patients with ischemic stroke.
| 1st Author (Year) | Type of Stroke, Study Design, Participants, Age (years) |
Time of DTI Acquisition, Field Strength, DTI Parameters, DTI Analysis/Metrics, Additional Imaging/Electrophysiology |
Anatomical Region Examined |
Outcome Scale Utilized | Main Findings |
|---|---|---|---|---|---|
| Ali (2012) [46] |
|
|
CST | NIHSS within 1 month. |
|
| Kwon (2012) [56] |
|
|
CST | MI at onset and at 6 months. |
|
| Puig (2013) [44] |
|
|
CST | NIHSS, MI at 2 years. |
|
| Forkel (2014) [29] |
|
|
Perisylvian language networks (long-segment, anterior segment, and posterior segment of the AF). |
WAB 14 days, 6 months. |
|
| Groisser (2014) [62] |
|
|
CST | Upper-limb section of the MI, NHPT 3 to 7 days (S1 acute), 1 to 2 months (S2, subacute), and 6 to 7 months (S3, chronic). |
|
| Maraka (2014) [57] |
|
|
CST | UE-FM, motor items of the [mNIHSS] 3–7 days, 30 days, and 90 days. |
|
| Rong (2014) [22] |
|
|
Medulla, CP, internal capsule, and CST. | FM, BI at each visit. |
|
| Takenobu (2014) [28] |
|
|
TBSS with ROI analysis for significant clusters found in the TBSS. |
FM within 2 weeks, and at 1 and 3 months. |
|
| Feng (2015) [51] |
|
|
CST | UE-FM Scale 2–7 days after stroke and 3 months. |
|
| Liu (2015) [25] |
|
|
CST | FM, NIHSS |
|
| Moulton (2015) [52] |
|
|
Subcortical WM of PrCG, corona radiata, PLIC, CP in ipsilesional and contralesional hemisphere; CCg as control region. |
NIHSS day 1, 7, and mRS at 3 months. |
|
| Zhang (2015) [60] |
|
|
ROIs: medulla, CP, internal capsule, CSO; tractography: CST. |
FM, mRS, and BI. |
|
| Bigourdan (2016) [24] |
|
|
CST | FMA score at 1 year. |
|
| Doughty (2016) [49] |
|
|
CP, a stretch of the CST caudal to each stroke lesion (Nearest-5-Slice, N5S). |
UE-FM assessment in the acute phase and at 3 months. |
|
| Jang (2016) [55] |
|
|
CST | MI, MBC, and FAC within 24 h and at 6 months. |
|
| Liu (2017) [27] |
|
|
Whole-brain WM analysis using TBSS. |
FM |
|
| Liu (2018) [58] |
|
|
Bilateral PMA and bilateral CP. | FM |
|
| Liu (2018) [59] |
|
|
Regions in corona radiata pathway: thalamus, corona radiata, and CSO. |
NIHSS, BI, and NIHSS8. |
|
| Etherton (2019) [61] |
|
|
WMH and NAWM contralateral to the acute infarct. |
NIHSS Admission, day 3–5 post-stroke. |
|
| Kulesh (2019) [63] |
|
|
CST (level of PLIC, pons), GIC, ALIC, CB, SLF, IFOF, SCC, infarction and the area within 3 cm from it. |
Measures on day 3, 10, and at discharge: NIHSS, Frenchay Arm Test, BBS, HAI, RMI, MoCA, FIM, and mRS. |
|
| Mahmoud (2019) [37] |
|
|
3D fiber tractography with multi-ROI technique and regions drawn in the unaffected portion of the WM tracts at the side of infarction and corresponding area at the contralateral hemisphere; degree of FA reduction of WM tracts at the site of infarction [mild (0.4), moderate (0.2–0.3), severe (0.1)]; and classification of WM tracts as disrupted, displaced, and preserved. |
NIHSS at admission and after 3 months. |
|
| Moulton (2019) [18] |
|
|
Second and third branches of the SLF (SLF-II and -III, respectively) and CST as part of the motor network in addition to the left and right AF, IFOF, ILF, and UF. |
NIHSS, JTT, and AHS at 3 months. |
|
| Keser (2020) [30] |
|
|
AF and FAT | BNT within 2 weeks and 6–12 months. |
|
| Berndt (2021) [23] |
|
|
CST (PLIC, PED) | NIHSS, mTICI, mRs at 90 days. |
|
| Darwish (2021) [54] |
|
|
CST (pons) | NIHSS at admission, and after 1, 6, and 9 months. |
|
| Liu (2021) [32] |
|
|
Bilateral inferior cerebellar peduncle (JHU-ICBM-DTI-81-WMPM-90p). | FM |
|
| Xia (2021) [33] |
|
|
CST | NIHSS, MMSE, FMA, and BI after each scan. |
|
| Li (2022) [26] |
|
|
ATR, CST, CCG, CH, FMAJ, FMIN, IFOF, ILF, SLF, UF and SLF-TP. |
UE-FM before and after each scan. |
|
| Shaheen (2022) [38] |
|
|
CST | NIHSS, MRC, mRS, and MI at baseline and 6 months. |
|
Table 2.
Basic characteristics and main findings of studies including patients with hemorrhagic stroke.
| 1st Author (Year) | Type of Stroke, Study Design, Participants, Age (years) |
Time of DTI Acquisition, Field Strength, DTI Parameters, DTI Analysis/Metrics, Additional Imaging/Electrophysiology |
Anatomical Region Examined | Outcome Scale Utilized | Main Findings |
|---|---|---|---|---|---|
| Kuzu (2012) [36] |
|
|
Bilateral CP | NIHSS at day 90. |
|
| Wang (2012) [64] |
|
|
CP | mRS, FIM, NIHSS, and PG at 6 monhts. |
|
| Koyama (2013a) [43] |
|
|
CP and the corona radiata/internal capsule. | MRC at 1 month. |
|
| Koyama (2013b) [41] |
|
|
CP | BRS, FIM-motor at 3–7 months. |
|
| Ma (2014) [35] |
|
|
CST | MFS on day 90. |
|
| Tao (2014) [50] |
|
|
CST | mRS at follow-up visits in the outpatient clinic. |
|
| Cheng (2015) [53] |
|
|
Pons, CP, perihaematoma oedema, and corona radiata. |
MI at admission, at 1 and 3 months. |
|
| Koyama (2015) [42] |
|
|
CP | BRS (shoulder/elbow/ forearm, hand, lower extremity), FIM-motor, and length of total hospital stay from admission to acute medical service to discharge from long-term rehabilitation facility (LOS). |
|
| Fragata (2017) [31] |
|
|
Frontal CSO, parietal CSO, lentiform nucleus, thalamus, PLIC, CCg, CCs, and mid-pons cerebellar peduncles. |
mRS, presence of DCI at 3 months. |
|
| Min (2020) [48] |
|
|
CST with ROIs at the pons. |
BMS, MBI, mRS, NIHSS, JHFT, and MI. |
|
| Gong (2021) [45] |
|
|
CST connecting the hand–knob area of the PrCG and the CP. |
BRS-H at post-stroke 3 weeks and 3 months. |
|
Table 3.
Basic characteristics and main findings of studies including patients with both ischemic and hemorrhagic stroke.
| 1st Author (Year) | Type of Stroke, Study Design, Participants, Age (years) |
Time of DTI Acquisition Field Strength, DTI Parameters, DTI Analysis/Metrics, Additional Imaging/Electrophysiology |
Anatomical Region Examined | Outcome Scale Utilized | Main Findings |
|---|---|---|---|---|---|
| Imura (2015) [47] |
|
|
CST | MI, BRS, BI, and FIM on the same data as DTI and at 1 month. |
|
| Nakashima (2017) [39] |
|
|
CP | FMA, MAL at 3 months. |
|
| Koyama (2018) [40] |
|
|
CP | BRS, FIM-motor monthly, and LOS. |
|
| Okamoto (2021) [34] |
|
|
PLIC | FMA, ARAT, and use or non-use of a short leg brace at discharge from the recovery rehabilitation unit. |
|
4. Discussion
A literature review over the last decade was carried out in order to delineate DTI prognostic value in post-stroke patients. Forty-four full-text original articles assessing the potential applicability of DTI on stroke prognosis were identified and classified into three groups based on the type of stroke evaluated (ischemic, hemorrhagic, or both).
4.1. Prediction of Recovery Using Different DTI Parameters in Studies with Ischemic Cohorts: The Role of FA
Regarding the role of FA, Berndt and colleagues [23] studied 165 large-vessel occlusion stroke patients who underwent mechanical thrombectomy and DTI within 7 days post-stroke. They reported reduced FA of posterior limbs of the internal capsule (PLIC) in the acute phase, reflecting impairment of CST integrity, and weak negative correlation with the National Institutes of Health Stroke Scale (NIHSS) at time of MRI. Furthermore, FA within PLIC in the acute phase was significantly correlated with clinical outcome at 90 days in peripheral infarcts, whereas no significant association was found for basal ganglia infarcts. Similarly, Xia and colleagues [33] reported a decline in FA values in the ipsilesional CST during the first week poststroke and then longitudinal increase over the next 12 weeks. Again, significant associations with clinical outcome were found after the acute phase. Increases in both interhemispheric functional connectivity (FC) and ipsilesional CST-FA were significantly correlated with the greater change of Fugl–Meyer Assessment (FMA) between weeks 1 and 4 post-stroke. In line with these findings, Mahmoud and colleagues [37] studied a cohort of 60 acute ischemic stroke patients within 2 days from onset. In patients with intact WM tracts, near-complete clinical recovery was noted. Moreover, the magnitude of FA reduction in the involved tracts was significantly associated with clinical score at admission and the 3-month clinical outcome. FA value in relation to motor recovery was also evaluated by Liu and colleagues [25] in their DTI study of 18 patients examined at weeks 1, 4, and 12 post-stroke. They reported positive correlations between FA values of contralesional medial frontal gyrus (MFG), thalamocortical connections, and FMA scores within 12 weeks following infarction. Besides CST, Takenobu and colleagues [28] studied the role of red nucleus in association with motor recovery in 10 patients who underwent DTI within 2 weeks and 1- and 3-months post-stroke. Significant positive correlation between the FA value of the red nucleus or dorsal pons cluster and Fugl–Meyer motor scale (FMMS) scores was observed, highlighting that microstructural alterations in the rubrospinal pathway may mediate motor recovery. With respect to upper extremity recovery, Doughty and colleagues [49] investigated the role of FA in the acute phase of stroke in relation to motor outcome at 3 months, using the Upper Extremity Fugl–Meyer (UE-FM) assessment. DTI was conducted in 58 patients within 80 h post-stroke, with ROIs located at the cerebral peduncle and a stretch of the CST caudal to each stroke lesion; the slope of the FA laterality and apparent diffusion coefficient (ADC) laterality indices of the nearest-5-slices (N5S) could predict 3-month UE-FM score weakly. In another study including 100 patients with ischemic stroke at the acute phase and applying DTI tractography to reconstruct both CST and commissural and associative WM tracts, Kulesh and colleagues found that the integrity (FA) of the integrity of the associative tracts of the affected hemisphere is more valuable than the microstructure of the intact hemisphere and the rFA for the prediction of global outcome whereas the integrity of the tracts of the intact hemisphere are important for the restoration of complex rehabilitation spheres, such as cognitive status and daily living and social skills [63].
Regarding aphasia recovery, Keser and colleagues [30] explored the integrity of arcuate fasciculus (AF) and frontal aslant tract (FAT) in 24 patients with left hemisphere ischemic stroke and consequent aphasia who underwent neuroimaging and language testing at acute and chronic time points. Acute right and left AF and FAT DTI values failed to correlate with recovery rate. On the contrary, longitudinal FA of the right AF was inversely correlated with naming recovery, providing evidence that reliance on left hemispheric components is linked with greater language recovery. With respect to perisylvian language networks, Forkel and colleagues [29] aimed to identify potential anatomic predictors of language recovery using DTI tractography. In their study, in the left hemisphere the only anatomical predictor of longitudinal aphasia was lesion size, whereas in the right hemisphere age and AF volume were associated with aphasia severity.
4.2. Prediction of Recovery Using Different DTI Parameters in Studies with Ischemic Cohorts: The Role of the FA Ratio
With regards to the FA ratio (rFA), Ali and colleagues [46] investigated 21 patients and demonstrated that an FA ratio between the affected and unaffected side under 0.8 at admission was associated with deficient motor recovery at discharge. Additionally, they visualized WM tracts using DTI tractography and found that patients with whole involvement of pyramidal tract exhibited higher NIHSS at discharge compared with the groups with intact and partial involvement. Similarly, Shaheen and colleagues [38] studied 45 middle cerebral artery (MCA) stroke patients and 17 controls and demonstrated that baseline FA and rFA were negatively associated with the NIHSS and Modified Rankin Score (mRS) and positively associated with Motricity Index (MI). In contrast to the aforementioned studies where DTI metrics at acute phase were predictive of outcome, Darwish and colleagues [54] examined 30 patients who underwent DTI on admission and 1 month post-stroke and found significant negative correlation between rFA at the rostral pons 1 month post-stroke and NIHSS score at 6 months. These findings also applied to FN in the CST ipsilateral to infarct. The prognostic value of rFA was also explored by Puig and colleagues [44] in a study of 89 MCA ischemic stroke patients who underwent DTI within 12 h, 3 days, and 30 days post-stroke onset. They illustrated that rFA at day 30 was the only independent predictor of long-term motor outcome. Similarly, Zhang and colleagues [60] studied 17 pontine infarct patients who underwent DTI at the acute phase and then consecutive examinations during a 6-month period and aimed to explore association with motor recovery. Positive correlation between the FMA scores on days 90 and 180 and rFA above the pons on day 14 were noted in line with these findings; Kwon and colleagues [56] sought to compare the predictability of early (1–14 days) and late (15–28 days) DTI by applying DTI tractography in relation to motor outcome at 6 months. Interestingly, CST integrity of the late scanning group could predict MI score, as opposed with the early group. Rong and colleagues [22] studied 3 medulla infarction patients with DTI and DTI tractography within 7, 14, and 30 days post-stroke onset and the FMA and Barthel index (BI) at each visit; two patients presented good motor recovery after 14 days and the FA values of their affected pyramidal tracts were slightly decreased, while they passed along periinfarct areas and their integrity was preserved in the medulla on DTI tractography. The most evident decrease in FA values along the affected CST was observed in the third patient, with right upper limb motor deficits presenting after 30 days. On DTI tractography, the affected pyramidal tract passed through the infarct and exhibited disruption in the medulla. Thus, it appears that the magnitude of impairment and sparing of periinfarct pyramidal tract compensation may constitute an important motor recovery mechanism.
4.3. Prediction of Recovery Using Different DTI Parameters in Studies with Ischemic Cohorts: The Role of MD
Regarding MD in stroke outcome prognosis, in the study of Liu and colleagues [32] 33 patients with acute subcortical stroke were investigated with DTI focused on the integrity of the inferior cerebellar peduncles (ICP) and lower limb FM assessment within 1 week, 4 weeks and 12 weeks post-stroke. Both MD and FA in contralesional ICP showed association with lower-limb FM score changes. Etherton and colleagues [61] studied a cohort of 42 patients to assess the role of DTI in early neurological improvement. Normal-appearing white matter (NAWM) MD was significantly lower in the group with early neurological improvement, and in multivariable logistic regression it was an independent predictor for early neurological improvement.
4.4. Prediction of Recovery Using Different DTI Parameters in Studies with Ischemic Cohorts: The Role of AD
With regards to the AD, Moulton and colleagues [52] examined 28 thrombolysed patients and found that the strongest independent predictor of clinical outcome was the corona radiata AD ratio (rAD), correlating with motor NIHSS scores on day 7 and with mRS at 3 months. Interestingly, FA values could not be correlated with clinical recovery. In another study of 45 patients under thrombolysis, Moulton and colleagues [18] reported that rAD in CST predicted long-term motor recovery, whereas rAD in AF independently predicted a 3-month aphasia outcome, thus highlighting its potential as an efficient biomarker in the acute phase of stroke. Similarly, Groisser and colleagues [62] recruited 10 acute stroke patients with severe upper-limb involvement and examined the association of CST injury with motor recovery; acute decrease of CST AD has a prognostic utility in strength and fine motor functions in both the subacute and the chronic phase. Liu and colleagues [58] studied 22 patients and 22 controls and highlighted that changes in the FM scores were greater in those patients with higher changes in AD of the ipsilesional primary motor area. However, the only predictor of motor improvement within 12 weeks post-stroke was initial impairment or lesion volume.
4.5. Prediction of Recovery Using Different DTI Parameters in Studies with Ischemic Cohorts: The Role of Fiber Number Ratio
As for the rFN, Jang and colleagues [55] studied 31 pontine infarct patients using DTI at days 7–28 and clinical evaluation at 6 months using MI, a modified Brunnstrom classification (MBC), and functional ambulation category (FAC). The rFN and the CST area ratio significantly correlated with all 6-month motor outcomes. Surprisingly, rFA failed to reach significant association with all 6-month motor functions. Maraka and colleagues [57] studied 23 ischemic stroke patients with DTI, UE-FM and mNIHSS at the acute, subacute and chronic phase reported that rFN (affected/unaffected CST) showed strong correlation with the UE-FM score, whereas it was negatively correlated with mNIHSS at each phase of ischemic stroke. The prognostic utility of early rFNR was explored by Bigourdan and colleagues [24] in 117 patients assessed for motor recovery using the FMA score. The rFN was evaluated at 24 to 72 h and 1 year post-stroke. It was demonstrated that the rFN in acute phase correlated with the measurement conducted at 1 year and it was strongly predictive of motor outcome.
4.6. Prediction of Recovery Using Different DTI Parameters in Studies with Hemorrhagic Cohorts: The Role of FA
Regarding DTI in prediction of recovery in hemorrhagic stroke, the majority of studies focus on FA in CST. Ma and colleagues [35] applied DTI imaging in a cohort of 23 hemorrhagic stroke patients at different time points, including day 0. They evaluated outcome with motor function score (MFS) 90 days post-stroke. Significant association was noted between initial FA of the affected cerebral peduncle and MFS of day 90. Additionally, the initial FA value over 0.45 was a motor outcome predictor with high sensitivity and specificity. Similarly, Kuzu and colleagues [36] conducted a study on 23 patients with intracerebral hemorrhage (ICH) who underwent DTI five times for the study of cerebral peduncles, with the first scanning being within 3 days post-onset and outcome of motor function being assessed on day 90. In the good recovery group, mean FA on day 3 was significantly higher than in the poor recovery group and this value in the pathological side could predict motor recovery with sensitivity of 100% and a specificity of 77.8%. Similarly, Min and colleagues [48] studied 12 putaminal hemorrhage patients with DTI and clinical assessment within day 1 and 3 weeks, 3 months, and 6 months after the initial treatment and found that mean FA at the level of the pons in the affected side on day 1 and on 6 months was higher in the good outcome group than in the poor outcome group. They also reported significant association between the initial mean FA value and the sum of Brunnstrom motor recovery stage scores at 6 months.
4.7. Prediction of Recovery Using Different DTI Parameters in Studies with Hemorrhagic Cohorts: The Role of FA Ratio
The rFA of cerebral peduncles (CP) was also investigated in the study of Wang and colleagues [64]. They demonstrated that within 3 days the ratio of rFA (affected/unaffected side) exhibited negative correlation with the paresis grading and mRS and positive correlation with the FIM scores at the end of follow-up. On the other hand, rFA at 2 weeks had positive correlation with the FIM and negative correlation with mRS scores and PG at the end of follow-up. Notably, as compared to the DTI within 3 days of ICH onset, the application of DTI at 2 weeks after ICH was superior to DTI within 3 days in terms of accurate prediction of motor outcome and daily living activities. In the study of Koyama and colleagues [43] including 32 patients with thalamic and putaminal hemorrhage, rFA values of the CP were significantly associated with Medical Research Council (MRC) scores, whereas the correlations with DTI values obtained for corona radiata/internal capsule were less significant. Similarly, another study Koyama and colleagues [41] reported a statistically significant association between rFA of CP and upper extremities function, which was stronger for the upper limb. Interestingly, rFA and FIM-motor scores were not correlated. On the other hand, in the study of Koyama and colleagues [42], rFA on cerebral peduncles were significantly associated with FIM-motor. The role of CP were also assessed by Tao and colleagues [50]; FA was measured within 4 days after onset at five slices below the level of the lesion on the affected and unaffected CST and in the CP along with rFA. Although rFA values at the CPs level were significantly lower in the poor functional outcome group, they were inferior to ICH score in predicting functional outcome. rFA was also explored in the prospective study of Cheng and colleagues [53] in 48 ICH patients who underwent DTI with a median time interval from onset of 7 days. Motor outcome was evaluated by MI at admission and after 1 and 3 months. It was found that lower rFA at the corona radiata predicted poor outcome at all time points of clinical evaluation.
4.8. Prediction of Recovery Using Different DTI Parameters in Studies with Hemorrhagic Cohorts: The Role of Qualitative Assessment of CST Integrity
The role of CST integrity in relation to hand function was explored by Gong and colleagues [45] in a study of 75 hypertensive hemorrhage patients receiving DTI in approximately 3 weeks following stroke. It was shown that degree of CST integrity was negatively correlated with the Brunnstrom Recovery Staging-Hand (BRS-H) at 3 weeks and 3 months. In particular, patients with intact or complete disruption of CST failed to present substantial improvement in BRS-H at 3 months. On the contrary, those having partial CST impairment based on DTI were remarkably improved at 3 months in comparison to 3 weeks post-stroke.
4.9. Prediction of Recovery Using Different DTI Parameters in Studies with Both Ischemic and Hemorrhagic Cohorts: The Role of FA and the FA Ratio
In a study including both hemorrhagic and ischemic stroke patients, Imura and colleagues [47] evaluated the most efficient DTI parameters in predicting motor outcomes and activities of daily living function. They employed FA, FN, and ADC within 10 days post-stroke. Clinical outcome was re-assessed at 1 month. Only FA of the affected CST exhibited significant correlation with the motor outcome and activities of daily living function within 10 days and at 1 month post-onset. In a similar cohort, Nakashima and colleagues [39] examined 17 patients with DTI and voxel-based morphometry and assessed clinical outcome at 3 months using FMA and the Motor Activity Log (MAL). In patients with incomplete CST disruption in tractography, rFA of the bilateral CP showed significant correlation with FMA, amount of use, and quality of movement. The rFA on CP was also examined in the study of Koyama and colleagues [40] who included 80 patients with hemorrhagic and ischemic stroke and DTI on days 14–21. Both the hemorrhagic and the infarct groups showed similar patterns of statistically significant correlations between rFA and outcome measures. Interestingly, such similarity did not apply to FIM-motor. In agreement with these findings, Okamoto and colleagues [34] investigated a cohort with both ischemic and hemorrhagic stroke patients and concluded that higher rFA values of the PLIC at admission to the recovery rehabilitation unit were associated with better outcomes of upper extremity function.
4.10. Methodological Considerations
The review of the published DTI studies reveals diverse methodological approaches. The majority of DTI studies, relying on 1.5 T or 3.0 T field strength, adopt a multi-ROI protocol for the reconstruction of WM tracts and only few capitalize on recent advances in whole-brain DTI. The vast majority of studies apply less than 30 gradient directions with only few studies using equal or more than 30 gradient directions. The anatomical accuracy of DTI tractography is inherently constrained since inferring information for fiber direction based on the water diffusion profile is a complex, undetermined inverse problem [65]. There are several pitfalls and sources of errors that may limit the accuracy of the tractography output. Of note, these limitations can emerge at any stage, including image acquisition, algorithms applied for tractography (deterministic vs. probabilistic, different deterministic algorithms), local voxel-wise reconstruction and tracking streamlines. Diffusion MRI is prone to artifacts that are related to susceptibility gradients affecting echo planar imaging acquisition, head motion, and eddy currents. All of these can affect the orientation estimates, diffusion indices, and geometric structure of the pathways which can result in anatomically incorrect tractography [66,67,68,69,70]. In addition, data acquisition features (e.g., magnetic field, signal-to-noise ratio, number of diffusion-encoding directions, b-values, voxel resolution) also affect fiber reconstruction [71,72,73]. Furthermore, each voxel can contain a large number of axons with many potential complex geometric configurations. Fibers with crossing, kissing, fanning and/or curving configurations are difficult to reconstruct specifically using early single-tensor deterministic algorithms (i.e., Fiber Assignment by Continuous Tracking), thus resulting in incorrect estimates of fiber orientation and false-positive or false-negative reconstructed fibers [74]. Of note, different algorithmic fiber tracking methodologies as well as the particularities of different WM fiber bundles affect the final reconstructed fibers, with some algorithms used for DTI tractography being less sensitive to the crossing and kissing fibers problem (e.g., reconstruction of CST, corpus callosum forceps major and forceps minor, and superior longitudinal fasciculus) [75]. Additionally, the tracking process is also subject to biases and/or inaccuracies due to the application of manual vs. automated tractography methods (with the former also being subject to raters’ knowledge of brain anatomy and experience regarding ROI selection and interpretation of false-positive and/or false-negative fibers) or choice of tracking parameters (e.g., seeding and stopping criteria for FA thresholds, curvature thresholds, step size, and fiber length) [76,77,78]. These methodological points have been well-documented [79] and their thorough consideration in stroke patients [80,81] explain different interpretations of DTI biomarkers both in acute and chronic stroke. The most evaluated WM tract is the pyramidal tract (either a single tract or specific regions located across the tract). Strikingly few studies have evaluated non-motor tracts, with most of them examining language-related tracts. Other WM tracts that might be affected in stroke and demonstrate a significant role in other cognitive functions (e.g., memory, perception) are not examined and clinical outcome with regards to these functions are not reported.
4.11. Common Shortcomings
The review of the published DTI studies also reveals common study limitations. Most of the published DTI studies in stroke patients only report single-modality changes; functional changes (i.e., fMRI or TMS) are not or only seldom evaluated. Some recent studies describe additional functional alterations but direct associations between structural and functional neuroimaging metrics are not consistently reported or not performed. Most hemorrhagic studies are cross-sectional, which preclude the assessment of longitudinal changes, ceiling, and flooring effects. The inclusion of multiple timepoints, although often difficult to apply in clinical practice, might also reveal the existence of linear and/or non-linear changes. Cross-sectional studies merely offer a snapshot of structural changes in WM tracts and patients are often in a different reorganization/regeneration stage of their post-stroke trajectory, which can be further complicated by pharmacological and non-pharmacological interventions. The latter are not comprehensively described in longitudinal studies and need to be addressed in future prospective studies. The admixing of patients with ischemic and hemorrhagic stroke should also be carefully considered when interpreting different findings. A requisite of future DTI studies is a prospective, multi-timepoint longitudinal design with a large and relatively homogenous clinical profile at enrolment and uniform follow-up intervals.
4.12. Future Directions
DTI and tractography need to be integrated into routine imaging protocols in stroke patients to establish their detection sensitivity, relative advantages, and monitoring and predictive potential. Future longitudinal studies should highlight the role of DTI as a viable clinical tool for monitoring and predicting patients’ functional outcomes not only with regards to motor and language functions but also other cognitive functions (e.g., memory), and detecting response to therapy, including different rehabilitation techniques [e.g., repetitive TMS (rTMS); transcranial direct current stimulation (tDCS)]. The practical demands of clinical imaging require relatively short acquisition times, transparent and reliable data interpretation processes, and ease of harmonizing protocols across clinical sites to conduct multicenter studies. There is a relative urgency to shift the emphasis from descriptive, qualitative studies to the development of protocols with practical clinical utility that can be applied in large sample sizes and provide accurate biomarkers for the prediction of stroke recovery. Finally, its potential as a putative monitoring biomarker for pharmacological and non-pharmacological interventions in stroke patients should be carefully evaluated to further identify both reorganization and degeneration processes.
5. Conclusions
In conclusion, the results of the present review are indicative of the utility of DTI and specific parameters (e.g., FA, FA ratio, diffusivity values, and fiber number ratio) for tracking longitudinal changes and identifying prognostic correlates in acute and hyperacute stroke patients. However, it is worth mentioning that additional efforts are needed to translate the insights gained from DTI studies in stroke patients into practical applications in clinical settings and routine clinical practice. Of note, ROC curves and various machine-learning frameworks in structural imaging in other neurological and psychiatric groups suggest that DTI may have a role in predicting patients’ outcomes with adequate accuracy at a single-patient level, thus providing valuable information for patients’ therapeutic management and both short- and long-term outcomes.
Acknowledgments
We acknowledge support of this work from the project “Study of the interrelationships between neuroimaging, neurophysiological and biomechanical biomarkers in stroke rehabilitation (NEURO-BIO-MECH in stroke rehab)” (MIS 5047286), which is implemented under the action “Support for Regional Excellence”, funded by the operational program “Competitiveness, Entrepreneurship and Innovation” (NSRFm2014–2020), and co-financed by Greece and the European Union (European Regional Development Fund).
Abbreviations
Action Research Arm Test (ARAT); activities of daily living (ADL); AD ratio (rAD); apparent diffusion coefficient (ADC); arcuate fasciculus (AF); axial diffusivity (AD); Barthel index (BI); Berg Balance Scale (BBS); Brunnstrom Recovery Stage (BR); Brunnstrom Recovery Staging-Hand (BRS-H); cerebral peduncles (CP); corticospinal tract (CST); diffusion tensor imaging (DTI); diffusion weighted imaging (DWI); magnetic resonance imaging (MRI); FA ratio (rFA); fiber number (FN); fiber number ration (rFN); fractional anisotropy (FA); frontal aslant tract (FAT); Fugl-Meyer Assessment (FMA); Fugl-Meyer motor scale (FMMS); functional ambulation category (FAC); functional connectivity (FC); Functional Independence Measure (FIM); Hauser Ambulation Index (HAI); inferior cerebellar peduncles (ICP); intracerebral hemorrhage (ICH); mean diffusivity (MD); medial frontal gyrus (MFG); Medical Research Council (MRC); middle cerebral artery (MCA); Modified Barthel Index (MBI); modified Brunnstrom classification (MBC); motor evoked potentials (MEP); motor items of National Institutes of Health Stroke Scale (mNIHSS); Modified Rankin Score (mRS); Motor Activity Log (MAL); motor component of the FIM (FIM-motor); motor function score (MFS); Motricity Index (MI); Montreal Cognitive Assessment Scale (MoCA); National Institutes of Health Stroke Scale (NIHSS); nearest 5 slices (N5S); normal appearing white matter (NAWM); posterior limbs of the internal capsule (PLIC); Preferred Reporting Items for Systematic Reviews (PRISMA); radial diffusivity (RD); Rivermead Mobility Index (RMI); region of interest (ROI); repetitive TMS (rTMS); resting-state functional MRI (rs-fMRI); transcranial direct current stimulation (tDCS); transcranial magnetic stimulation (TMS); Upper Extremity Fugl-Meyer (UE-FM); white matter (WM); length of hospital stay (LOS); Jebsen Hand Function Test (JHFT); centrum semiovale (CSO); genum of the corpus callosum (CCg); splenium of the corpus callosum (CCs); precentral gyrus (PrCG); corpus callosum (CC); cingulum bundle (CB); laterality index (LI); tract-based spatial statistics (TBSS); superior longitudinal fasciculus (SLF); inferior fronto-occipital fasciculus (IFOF); inferior longitudinal fasciculus (ILF); uncinate fasciculus (UF); Jebson-Taylor Test (JTT); Aphasia Handicap Score (AHS); Boston Naming Test (BNT); functional connectivity (FC); anterior thalamic radiation (ATR); cingulate gyrus (CCG); cingulate hippocampus (CH); forces major (FMAJ); forces minor (FMIN); superior longitudinal fasciculus (temporal part) (SLF-TP); Nine Hole Peg Test (NHPT).
Author Contributions
A.F. and D.T. reviewed the literature, screened the abstracts of the reference list, deleted duplicates and citations not meeting the inclusion criteria, and assessed the articles; K.V. solved any disagreement regarding screening or selection process; F.C. wrote the first manuscript; A.S., I.S., E.A.P., N.A., E.K. and K.V. reviewed the tables, the presentation of the data, and the methodology. The corrected version was discussed collegially. F.C., C.K., K.T., S.K. (Sofia Kitmeridou) and S.K. (Stella Karatzetzou) wrote the final version. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable. The presented DTI data (Introduction) were acquired from a healthy control subject who provided written informed consent for the scanning, analysis and presentation of the data.
Data Availability Statement
All data discussed within this manuscript are available on PubMed.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Katan M., Luft A. Global Burden of Stroke. Semin. Neurol. 2018;38:208–211. doi: 10.1055/s-0038-1649503. [DOI] [PubMed] [Google Scholar]
- 2.Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., Barengo N.C., Beaton A.Z., Benjamin E.J., Benziger C.P., et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin E.J., Virani S.S., Callaway C.W., Chamberlain A.M., Chang A.R., Cheng S., Chiuve S.E., Cushman M., Delling F.N., Deo R., et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 4.Karatzetzou S., Tsiptsios D., Terzoudi A., Aggeloussis N., Vadikolias K. Transcranial magnetic stimulation implementation on stroke prognosis. Neurol. Sci. 2022;43:873–888. doi: 10.1007/s10072-021-05791-1. [DOI] [PubMed] [Google Scholar]
- 5.Gkantzios A., Tsiptsios D., Karatzetzou S., Kitmeridou S., Karapepera V., Giannakou E., Vlotinou P., Aggelousis N., Vadikolias K. Stroke and Emerging Blood Biomarkers: A Clinical Prospective. Neurol. Int. 2022;14:65. doi: 10.3390/neurolint14040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moura L.M., Luccas R., de Paiva J.P.Q., Amaro E., Jr., Leemans A., Leite C.D.C., Otaduy M.C.G., Conforto A.B. Diffusion Tensor Imaging Biomarkers to Predict Motor Outcomes in Stroke: A Narrative Review. Front. Neurol. 2019;10:445. doi: 10.3389/fneur.2019.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mori S., Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Chung H.W., Chou M.C., Chen C.Y. Principles and limitations of computational algorithms in clinical diffusion tensor MR tractography. AJNR Am. J. Neuroradiol. 2011;32:3–13. doi: 10.3174/ajnr.A2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winklewski P.J., Sabisz A., Naumczyk P., Jodzio K., Szurowska E., Szarmach A. Understanding the Physiopathology Behind Axial and Radial Diffusivity Changes-What Do We Know? Front. Neurol. 2018;9:92. doi: 10.3389/fneur.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jang S.H. The corticospinal tract from the viewpoint of brain rehabilitation. J. Rehabil. Med. 2014;46:193–199. doi: 10.2340/16501977-1782. [DOI] [PubMed] [Google Scholar]
- 11.Jang S.H. The role of the corticospinal tract in motor recovery in patients with a stroke: A review. NeuroRehabilitation. 2009;24:285–290. doi: 10.3233/NRE-2009-0480. [DOI] [PubMed] [Google Scholar]
- 12.Puig J., Blasco G., Schlaug G., Stinear C.M., Daunis I.E.P., Biarnes C., Figueras J., Serena J., Hernandez-Perez M., Alberich-Bayarri A., et al. Diffusion tensor imaging as a prognostic biomarker for motor recovery and rehabilitation after stroke. Neuroradiology. 2017;59:343–351. doi: 10.1007/s00234-017-1816-0. [DOI] [PubMed] [Google Scholar]
- 13.Bhasin A., Srivastava P., Kumaran S.S. Correlation of DTI-Derived Measures to Therapy-Mediated Recovery after Stroke: Preliminary Findings. Neurol. India. 2021;69:1210–1216. doi: 10.4103/0028-3886.329584. [DOI] [PubMed] [Google Scholar]
- 14.Alegiani A.C., MacLean S., Braass H., Siemonsen S., Gerloff C., Fiehler J., Cho T.H., Derex L., Hermier M., Berthezene Y., et al. Comprehensive analysis of early fractional anisotropy changes in acute ischemic stroke. PLoS ONE. 2017;12:e0188318. doi: 10.1371/journal.pone.0188318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang S.H. Diffusion tensor imaging studies on arcuate fasciculus in stroke patients: A review. Front. Hum. Neurosci. 2013;7:749. doi: 10.3389/fnhum.2013.00749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breier J.I., Hasan K.M., Zhang W., Men D., Papanicolaou A.C. Language dysfunction after stroke and damage to white matter tracts evaluated using diffusion tensor imaging. AJNR Am. J. Neuroradiol. 2008;29:483–487. doi: 10.3174/ajnr.A0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kourtidou E., Kasselimis D., Angelopoulou G., Karavasilis E., Velonakis G., Kelekis N., Zalonis I., Evdokimidis I., Potagas C., Petrides M. The Role of the Right Hemisphere White Matter Tracts in Chronic Aphasic Patients After Damage of the Language Tracts in the Left Hemisphere. Front. Hum. Neurosci. 2021;15:635750. doi: 10.3389/fnhum.2021.635750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moulton E., Magno S., Valabregue R., Amor-Sahli M., Pires C., Lehericy S., Leger A., Samson Y., Rosso C. Acute Diffusivity Biomarkers for Prediction of Motor and Language Outcome in Mild-to-Severe Stroke Patients. Stroke. 2019;50:2050–2056. doi: 10.1161/STROKEAHA.119.024946. [DOI] [PubMed] [Google Scholar]
- 19.Zavanone C., Samson Y., Arbizu C., Dupont S., Dormont D., Rosso C. Critical brain regions related to post-stroke aphasia severity identified by early diffusion imaging are not the same when predicting short- and long-term outcome. Brain Lang. 2018;186:1–7. doi: 10.1016/j.bandl.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Kim S.H., Jang S.H. Prediction of aphasia outcome using diffusion tensor tractography for arcuate fasciculus in stroke. AJNR Am. J. Neuroradiol. 2013;34:785–790. doi: 10.3174/ajnr.A3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J.L., Schlaug G. Resting state interhemispheric motor connectivity and white matter integrity correlate with motor impairment in chronic stroke. Front. Neurol. 2013;4:178. doi: 10.3389/fneur.2013.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rong D., Zhang M., Ma Q., Lu J., Li K. Corticospinal tract change during motor recovery in patients with medulla infarct: A diffusion tensor imaging study. Biomed Res. Int. 2014;2014:524096. doi: 10.1155/2014/524096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berndt M.T., Purner D., Maegerlein C., Wunderlich S., Friedrich B., Zimmer C., Sepp D., Kaesmacher J., Boeckh-Behrens T. Basal Ganglia versus Peripheral Infarcts: Predictive Value of Early Fiber Alterations. AJNR Am. J. Neuroradiol. 2021;42:264–270. doi: 10.3174/ajnr.A6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bigourdan A., Munsch F., Coupe P., Guttmann C.R., Sagnier S., Renou P., Debruxelles S., Poli M., Dousset V., Sibon I., et al. Early Fiber Number Ratio Is a Surrogate of Corticospinal Tract Integrity and Predicts Motor Recovery After Stroke. Stroke. 2016;47:1053–1059. doi: 10.1161/STROKEAHA.115.011576. [DOI] [PubMed] [Google Scholar]
- 25.Liu G., Dang C., Chen X., Xing S., Dani K., Xie C., Peng K., Zhang J., Li J., Zhang J., et al. Structural remodeling of white matter in the contralesional hemisphere is correlated with early motor recovery in patients with subcortical infarction. Restor. Neurol. Neurosci. 2015;33:309–319. doi: 10.3233/RNN-140442. [DOI] [PubMed] [Google Scholar]
- 26.Li J., Rong D.D., Shan Y., Zhang M., Zhao C., Lu J. Brain Abnormalities in Pontine Infarction: A Longitudinal Diffusion Tensor Imaging and Functional Magnetic Resonance Imaging study. J. Stroke Cerebrovasc. Dis. 2022;31:106205. doi: 10.1016/j.jstrokecerebrovasdis.2021.106205. [DOI] [PubMed] [Google Scholar]
- 27.Liu G., Tan S., Dang C., Peng K., Xie C., Xing S., Zeng J. Motor Recovery Prediction With Clinical Assessment and Local Diffusion Homogeneity After Acute Subcortical Infarction. Stroke. 2017;48:2121–2128. doi: 10.1161/STROKEAHA.117.017060. [DOI] [PubMed] [Google Scholar]
- 28.Takenobu Y., Hayashi T., Moriwaki H., Nagatsuka K., Naritomi H., Fukuyama H. Motor recovery and microstructural change in rubro-spinal tract in subcortical stroke. Neuroimage Clin. 2014;4:201–208. doi: 10.1016/j.nicl.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forkel S.J., de Schotten M.T., Dell’Acqua F., Kalra L., Murphy D.G., Williams S.C., Catani M. Anatomical predictors of aphasia recovery: A tractography study of bilateral perisylvian language networks. Brain. 2014;137:2027–2039. doi: 10.1093/brain/awu113. [DOI] [PubMed] [Google Scholar]
- 30.Keser Z., Sebastian R., Hasan K.M., Hillis A.E. Right Hemispheric Homologous Language Pathways Negatively Predicts Poststroke Naming Recovery. Stroke. 2020;51:1002–1005. doi: 10.1161/STROKEAHA.119.028293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fragata I., Alves M., Papoila A.L., Nunes A.P., Ferreira P., Canto-Moreira N., Canhao P. Early Prediction of Delayed Ischemia and Functional Outcome in Acute Subarachnoid Hemorrhage: Role of Diffusion Tensor Imaging. Stroke. 2017;48:2091–2097. doi: 10.1161/STROKEAHA.117.016811. [DOI] [PubMed] [Google Scholar]
- 32.Liu G., Guo Y., Dang C., Peng K., Tan S., Xie C., Xing S., Zeng J. Longitudinal changes in the inferior cerebellar peduncle and lower limb motor recovery following subcortical infarction. BMC Neurol. 2021;21:320. doi: 10.1186/s12883-021-02346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia Y., Huang G., Quan X., Qin Q., Li H., Xu C., Liang Z. Dynamic Structural and Functional Reorganizations Following Motor Stroke. Med. Sci. Monit. 2021;27:e929092. doi: 10.12659/MSM.929092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okamoto Y., Ishii D., Yamamoto S., Ishibashi K., Wakatabi M., Kohno Y., Numata K. Relationship Between Motor Function, DTI, and Neurophysiological Parameters in Patients with Stroke in the Recovery Rehabilitation unit. J. Stroke Cerebrovasc. Dis. 2021;30:105889. doi: 10.1016/j.jstrokecerebrovasdis.2021.105889. [DOI] [PubMed] [Google Scholar]
- 35.Ma C., Liu A., Li Z., Zhou X., Zhou S. Longitudinal study of diffusion tensor imaging properties of affected cortical spinal tracts in acute and chronic hemorrhagic stroke. J. Clin. Neurosci. 2014;21:1388–1392. doi: 10.1016/j.jocn.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 36.Kuzu Y., Inoue T., Kanbara Y., Nishimoto H., Fujiwara S., Ogasawara K., Ogawa A. Prediction of motor function outcome after intracerebral hemorrhage using fractional anisotropy calculated from diffusion tensor imaging. Cerebrovasc. Dis. 2012;33:566–573. doi: 10.1159/000338904. [DOI] [PubMed] [Google Scholar]
- 37.Mahmoud B.E., Mohammad M.E., Serour D.K. What can DTI add in acute ischemic stroke patients? Egypt. J. Radiol. Nucl. Med. 2019;50:67. doi: 10.1186/s43055-019-0058-z. [DOI] [Google Scholar]
- 38.Shaheen H.A., Sayed S.S., Magdy M.M., Saad M.A., Magdy A.M., Daker L.I. Prediction of motor recovery after ischemic stroke: Clinical and diffusion tensor imaging study. J. Clin. Neurosci. 2022;96:68–73. doi: 10.1016/j.jocn.2021.12.029. [DOI] [PubMed] [Google Scholar]
- 39.Nakashima A., Moriuchi T., Mitsunaga W., Yonezawa T., Kataoka H., Nakashima R., Koizumi T., Shimizu T., Ryu N., Higashi T. Prediction of prognosis of upper-extremity function following stroke-related paralysis using brain imaging. J. Phys. Ther. Sci. 2017;29:1438–1443. doi: 10.1589/jpts.29.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koyama T., Koumo M., Uchiyama Y., Domen K. Utility of Fractional Anisotropy in Cerebral Peduncle for Stroke Outcome Prediction: Comparison of Hemorrhagic and Ischemic Strokes. J. Stroke Cerebrovasc. Dis. 2018;27:878–885. doi: 10.1016/j.jstrokecerebrovasdis.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 41.Koyama T., Marumoto K., Miyake H., Ohmura T., Domen K. Relationship between diffusion-tensor fractional anisotropy and long-term outcome in patients with hemiparesis after intracerebral hemorrhage. NeuroRehabilitation. 2013;32:87–94. doi: 10.3233/NRE-130825. [DOI] [PubMed] [Google Scholar]
- 42.Koyama T., Marumoto K., Uchiyama Y., Miyake H., Domen K. Outcome assessment of hemiparesis due to intracerebral hemorrhage using diffusion tensor fractional anisotropy. J. Stroke Cerebrovasc. Dis. 2015;24:881–889. doi: 10.1016/j.jstrokecerebrovasdis.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 43.Koyama T., Tsuji M., Nishimura H., Miyake H., Ohmura T., Domen K. Diffusion tensor imaging for intracerebral hemorrhage outcome prediction: Comparison using data from the corona radiata/internal capsule and the cerebral peduncle. J. Stroke Cerebrovasc. Dis. 2013;22:72–79. doi: 10.1016/j.jstrokecerebrovasdis.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 44.Puig J., Blasco G., Daunis I.E.J., Thomalla G., Castellanos M., Figueras J., Remollo S., van Eendenburg C., Sanchez-Gonzalez J., Serena J., et al. Decreased corticospinal tract fractional anisotropy predicts long-term motor outcome after stroke. Stroke. 2013;44:2016–2018. doi: 10.1161/STROKEAHA.111.000382. [DOI] [PubMed] [Google Scholar]
- 45.Gong Z., Zhang R., Jiang W., Fu Z. Integrity of The Hand Fibers of The Corticospinal Tract Shown by Diffusion Tensor Imaging Predicts Hand Function Recovery After Hemorrhagic Stroke. J. Stroke Cerebrovasc. Dis. 2021;30:105447. doi: 10.1016/j.jstrokecerebrovasdis.2020.105447. [DOI] [PubMed] [Google Scholar]
- 46.Ali G.G., Elhameed A.M.A. Prediction of motor outcome in ischemic stroke involving the pyramidal tract using diffusion tensor imaging. Egypt. J. Radiol. Nucl. Med. 2012;43:25–31. doi: 10.1016/j.ejrnm.2011.11.004. [DOI] [Google Scholar]
- 47.Imura T., Nagasawa Y., Inagawa T., Imada N., Izumi H., Emoto K., Tani I., Yamasaki H., Ota Y., Oki S., et al. Prediction of motor outcomes and activities of daily living function using diffusion tensor tractography in acute hemiparetic stroke patients. J. Phys. Ther. Sci. 2015;27:1383–1386. doi: 10.1589/jpts.27.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Min Y.S., Jang K.E., Park E., Kim A.R., Kang M.G., Cheong Y.S., Kim J.H., Jung S.H., Park J., Jung T.D. Prediction of Motor Recovery in Patients with Basal Ganglia Hemorrhage Using Diffusion Tensor Imaging. J. Clin. Med. 2020;9:1304. doi: 10.3390/jcm9051304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doughty C., Wang J., Feng W., Hackney D., Pani E., Schlaug G. Detection and Predictive Value of Fractional Anisotropy Changes of the Corticospinal Tract in the Acute Phase of a Stroke. Stroke. 2016;47:1520–1526. doi: 10.1161/STROKEAHA.115.012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tao W.D., Wang J., Schlaug G., Liu M., Selim M.H. A comparative study of fractional anisotropy measures and ICH score in predicting functional outcomes after intracerebral hemorrhage. Neurocritical Care. 2014;21:417–425. doi: 10.1007/s12028-014-9999-2. [DOI] [PubMed] [Google Scholar]
- 51.Feng W., Wang J., Chhatbar P.Y., Doughty C., Landsittel D., Lioutas V.A., Kautz S.A., Schlaug G. Corticospinal tract lesion load: An imaging biomarker for stroke motor outcomes. Ann. Neurol. 2015;78:860–870. doi: 10.1002/ana.24510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moulton E., Amor-Sahli M., Perlbarg V., Pires C., Crozier S., Galanaud D., Valabregue R., Yger M., Baronnet-Chauvet F., Samson Y., et al. Axial Diffusivity of the Corona Radiata at 24 Hours Post-Stroke: A New Biomarker for Motor and Global Outcome. PLoS ONE. 2015;10:e0142910. doi: 10.1371/journal.pone.0142910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng C.Y., Hsu C.Y., Huang Y.C., Tsai Y.H., Hsu H.T., Yang W.H., Lin H.C., Wang T.C., Cheng W.C., Yang J.T., et al. Motor outcome of deep intracerebral haemorrhage in diffusion tensor imaging: Comparison of data from different locations along the corticospinal tract. Neurol. Res. 2015;37:774–781. doi: 10.1179/1743132815Y.0000000050. [DOI] [PubMed] [Google Scholar]
- 54.Darwish H.S., ElShafey R., Kamel H. Prediction of Motor Recovery after Stroke by Assessment of Corticospinal Tract Wallerian Degeneration Using Diffusion Tensor Imaging. Indian J. Radiol. Imaging. 2021;31:131–137. doi: 10.1055/s-0041-1729671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jang S.H., Lee J., Lee M.Y., Park S.M., Choi W.H., Do K.H. Prediction of motor outcome using remaining corticospinal tract in patients with pontine infarct: Diffusion tensor imaging study. Somatosens. Mot. Res. 2016;33:99–103. doi: 10.1080/08990220.2016.1194821. [DOI] [PubMed] [Google Scholar]
- 56.Kwon Y.H., Jeoung Y.J., Lee J., Son S.M., Kim S., Kim C., Jang S.H. Predictability of motor outcome according to the time of diffusion tensor imaging in patients with cerebral infarct. Neuroradiology. 2012;54:691–697. doi: 10.1007/s00234-011-0972-x. [DOI] [PubMed] [Google Scholar]
- 57.Maraka S., Jiang Q., Jafari-Khouzani K., Li L., Malik S., Hamidian H., Zhang T., Lu M., Soltanian-Zadeh H., Chopp M., et al. Degree of corticospinal tract damage correlates with motor function after stroke. Ann. Clin. Transl. Neurol. 2014;1:891–899. doi: 10.1002/acn3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu G., Peng K., Dang C., Tan S., Chen H., Xie C., Xing S., Zeng J. Axial diffusivity changes in the motor pathway above stroke foci and functional recovery after subcortical infarction. Restor. Neurol. Neurosci. 2018;36:173–182. doi: 10.3233/RNN-170747. [DOI] [PubMed] [Google Scholar]
- 59.Liu Y., Chen L., Zeng J., Li W., Zeng S., Ye B., Liang Z. Proliferation of Bilateral Nerve Fibers Following Thalamic Infarction Contributes to Neurological Function Recovery: A Diffusion Tensor Imaging (DTI) Study. Med. Sci. Monit. 2018;24:1464–1472. doi: 10.12659/MSM.909071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang M., Lin Q., Lu J., Rong D., Zhao Z., Ma Q., Liu H., Shu N., He Y., Li K. Pontine infarction: Diffusion-tensor imaging of motor pathways-a longitudinal study. Radiology. 2015;274:841–850. doi: 10.1148/radiol.14140373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Etherton M.R., Wu O., Giese A.K., Lauer A., Boulouis G., Mills B., Cloonan L., Donahue K.L., Copen W., Schaefer P., et al. White Matter Integrity and Early Outcomes After Acute Ischemic Stroke. Transl. Stroke Res. 2019;10:630–638. doi: 10.1007/s12975-019-0689-4. [DOI] [PubMed] [Google Scholar]
- 62.Groisser B.N., Copen W.A., Singhal A.B., Hirai K.K., Schaechter J.D. Corticospinal tract diffusion abnormalities early after stroke predict motor outcome. Neurorehabilit. Neural Repair. 2014;28:751–760. doi: 10.1177/1545968314521896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kulesh A.A., Drobakha V.E., Sobyanin K.V., Kulikova S.P., Bykova A.Y., Kaileva N.A., Monak A.A., Shardakov I.N., Shestakov V.V. Role of cerebral reserve assessed using diffusion-weighted magnetic resonance imaging in determining the rehabilitation potential of acute ischemic stroke. Neurol. Neuropsychiatry Psychosom. 2019;11:26–34. doi: 10.14412/2074-2711-2019-3-26-34. [DOI] [Google Scholar]
- 64.Wang D.M., Li J., Liu J.R., Hu H.Y. Diffusion tensor imaging predicts long-term motor functional outcome in patients with acute supratentorial intracranial hemorrhage. Cerebrovasc. Dis. 2012;34:199–205. doi: 10.1159/000341857. [DOI] [PubMed] [Google Scholar]
- 65.Thomas C., Ye F.Q., Irfanoglu M.O., Modi P., Saleem K.S., Leopold D.A., Pierpaoli C. Anatomical accuracy of brain connections derived from diffusion MRI tractography is inherently limited. Proc. Natl. Acad. Sci. USA. 2014;111:16574–16579. doi: 10.1073/pnas.1405672111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alexander D.C., Barker G.J., Arridge S.R. Detection and modeling of non-Gaussian apparent diffusion coefficient profiles in human brain data. Magn. Reson. Med. 2002;48:331–340. doi: 10.1002/mrm.10209. [DOI] [PubMed] [Google Scholar]
- 67.Jones D.K. Determining and visualizing uncertainty in estimates of fiber orientation from diffusion tensor MRI. Magn. Reson. Med. 2003;49:7–12. doi: 10.1002/mrm.10331. [DOI] [PubMed] [Google Scholar]
- 68.Jones D.K., Cercignani M. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed. 2010;23:803–820. doi: 10.1002/nbm.1543. [DOI] [PubMed] [Google Scholar]
- 69.Wheeler-Kingshott C.A., Cercignani M. About "axial" and "radial" diffusivities. Magn. Reson. Med. 2009;61:1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- 70.Irfanoglu M.O., Walker L., Sarlls J., Marenco S., Pierpaoli C. Effects of image distortions originating from susceptibility variations and concomitant fields on diffusion MRI tractography results. Neuroimage. 2012;61:275–288. doi: 10.1016/j.neuroimage.2012.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alexander D.C., Barker G.J. Optimal imaging parameters for fiber-orientation estimation in diffusion MRI. Neuroimage. 2005;27:357–367. doi: 10.1016/j.neuroimage.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 72.Barrio-Arranz G., de Luis-Garcia R., Tristan-Vega A., Martin-Fernandez M., Aja-Fernandez S. Impact of MR Acquisition Parameters on DTI Scalar Indexes: A Tractography Based Approach. PLoS ONE. 2015;10:e0137905. doi: 10.1371/journal.pone.0137905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhan L., Mueller B.A., Jahanshad N., Jin Y., Lenglet C., Yacoub E., Sapiro G., Ugurbil K., Harel N., Toga A.W., et al. Magnetic resonance field strength effects on diffusion measures and brain connectivity networks. Brain Connect. 2013;3:72–86. doi: 10.1089/brain.2012.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tournier J.D. The biophysics of crossing fibers. In: Jones D.K., editor. Diffusion MRI: Theory, Methods, and Application. Oxford University Press; Oxford, UK: 2010. pp. 465–481. [Google Scholar]
- 75.Christidi F., Karavasilis E., Samiotis K., Bisdas S., Papanikolaou N. Fiber tracking: A qualitative and quantitative comparison between four different software tools on the reconstruction of major white matter tracts. Eur. J. Radiol. Open. 2016;3:153–161. doi: 10.1016/j.ejro.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dyrby T.B., Sogaard L.V., Parker G.J., Alexander D.C., Lind N.M., Baare W.F., Hay-Schmidt A., Eriksen N., Pakkenberg B., Paulson O.B., et al. Validation of in vitro probabilistic tractography. Neuroimage. 2007;37:1267–1277. doi: 10.1016/j.neuroimage.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 77.Knosche T.R., Anwander A., Liptrot M., Dyrby T.B. Validation of tractography: Comparison with manganese tracing. Hum. Brain Mapp. 2015;36:4116–4134. doi: 10.1002/hbm.22902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maier-Hein K.H., Neher P.F., Houde J.C., Cote M.A., Garyfallidis E., Zhong J., Chamberland M., Yeh F.C., Lin Y.C., Ji Q., et al. The challenge of mapping the human connectome based on diffusion tractography. Nat. Commun. 2017;8:1349. doi: 10.1038/s41467-017-01285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Soares J.M., Marques P., Alves V., Sousa N. A hitchhiker’s guide to diffusion tensor imaging. Front. Neurosci. 2013;7:31. doi: 10.3389/fnins.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kulikova S.P., Nikulin V.V., Dobrynina L.A., Nazarova M.A. A Possible Sensory Interpretation of Alternate Motor Fibers Relating to Structural Reserve during Stroke Recovery. Front. Neurol. 2017;8:355. doi: 10.3389/fneur.2017.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DiBella E.V.R., Sharma A., Richards L., Prabhakaran V., Majersik J.J., HashemizadehKolowri S.K. Beyond Diffusion Tensor MRI Methods for Improved Characterization of the Brain after Ischemic Stroke: A Review. AJNR Am. J. Neuroradiol. 2022;43:661–669. doi: 10.3174/ajnr.A7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data discussed within this manuscript are available on PubMed.