Abstract
A longitudinal study was carried out in Madagascar, the most important focus of chromoblastomycosis (P. Esterre, A. Andriantsimahavandy, E. Ramarcel, and J. L. Pecarrere, Am. J. Trop. Med. Hyg. 55:45–47, 1996), to investigate natural immunity to this disease. Sequential blood samples were obtained before, during, and at the end of a successful therapeutic trial with terbinafine, a new antifungal drug. Using enzyme-linked immunosorbent assay and immunoblot methods, detailed analyses of antibody concentration and antigen mapping were conducted for 136 serum samples and tentatively correlated to epidemiological and pathobiological data. Two different cytoplasmic antigens, corresponding to the two fungal species involved (Fonsecaea pedrosoi and Cladophialophora carrionii), were used to analyze the distribution of different classes of immunoglobulins. This was done with respect to the origin of the isolates, clinical and pathobiological. Although strong individual variations were noticed, some major antigens (one of 18.5 kDa specific for F. pedrosoi and two of 23.5 and 33 kDa, respectively, specific for C. carrionii) corresponded to high antibody prevalence and concentration. As some antigenic components were also detected by immunoglobulin M (IgM) and IgA antibodies, the role that these specific antibodies could play in the immune response is discussed.
The pigmented fungi belonging to the Dematiaceae family are considered an emerging group of pathogenic fungi, at least in westernized countries (22). This increasing medical problem is also linked with the AIDS epidemic (8), and a case of disseminated chromoblastomycosis evolving in an AIDS patient has been recently published (9). Chromoblastomycosis is a cosmopolitan chronic mycosis infecting humans after inoculation by trauma. In the absence of prompt medical intervention, typical cauliflower-like verrucous lesions develop, sometimes over a period of more than 30 years, and show a highly organized granulomatous reaction associated with an extensive fibrosis in the dermis and subcutaneous tissues (11, 12). The disease has a high morbidity, with Madagascar described as the most important focus in the world (12). Available drugs are not very effective, except for the new terbinafine drug which was recently tested in a multicentric therapeutic trial (supported by Novartis France and the Institut Pasteur de Madagascar) in two areas of endemicity in Madagascar (13, 15).
On that occasion, we monitored a cohort of 40 patients during 1 year of therapy and examined the specificity of their humoral immune responses by enzyme-linked immunosorbent assay (ELISA) and immunoblotting (Western blots). These techniques are particularly useful for the study of the host serological response during chromoblastomycosis, but no antigen with potential diagnostic value has ever been selected. In the present longitudinal study we examined the specificity of the human humoral immune response to the two main fungal species. For this purpose, immunoblots of Fonsecaea pedrosoi and Cladophialophora carrionii strains were analyzed with serum samples from chromoblastomycosis- and other fungal or parasitological disease-infected patients whose infections had been proven in the laboratory by, among other things, ELISA seropositivity. Results show that the antibody levels decreased during specific chemotherapy with the 18.5-kDa component restricted to F. pedrosoi-infected sera and the 23.5- and 33-kDa components restricted to C. carrionii-infected sera. Surprisingly, these results demonstrate, for the first time, immunoglobulin M (IgM) and IgA antibodies in the humoral immune response to this disease.
MATERIALS AND METHODS
Study area and patients.
A total of 40 patients (34 males and 8 females; mean age ± standard deviation [SD], 48.8 ± 15.3 years) presenting chronic lesions (mean evolution time of lesion, 10 years and 3 months) from two areas of chromoblastomycosis endemicity (12) were studied during a multicenter therapeutic trial (13). Thirty-five patients were infected with F. pedrosoi and followed during 1 year of specific therapy in the hospital of Andapa located in the rainy, northern part of Madagascar. Five patients were infected with C. carrionii and enrolled in a study of the same design organized in the hospital of Manambaro, located in the semidesertic southern region of Madagascar. For each patient, the two immunoassays were performed on the serum before (t0), during (at 4 [t4] and 8 [t8] months), and at the end (t12) of chemotherapy with terbinafine. The potential correlations of immunological results (levels of specific Igs and IgG, IgM, and IgA immunoblot profiles) with the different clinicopathological data collected during the therapeutic trial (15) were studied with this first group of 136 serum samples.
In addition, serum samples from 24 healthy controls (20 males and 4 females; mean age ± SD, 41.7 ± 13.8 years; P = 0.4 for controls versus patients) and 13 patients infected with diseases endemic to the area (one for each of the following diseases: candidiasis due to Candida albicans, Aspergillus fumigatus-associated aspergillosis, Trichophyton rubrum infection, fungal mycetoma, malaria, schistosomiasis mansoni and heamatobium, Wuchereria bancrofti-associated lymphatic filariasis, distomatosis, ascaridiasis, cysticercosis, Trichuris infection, hydatidosis, and taeniasis) were included in the analysis. All serum samples had been kept frozen (−80°C) and were examined under uniform laboratory conditions to avoid internal variations.
Fungal cultures and antigens.
Two reference strains, one of F. pedrosoi (IPM-A8) and one of C. carrionii (IPM-M8), were obtained from skin biopsies of two patients enrolled in the therapeutic trial. They were cultivated in 500 ml of Sabouraud's liquid medium, mechanically agitated (300 rpm for 10 to 15 days) in a roller-type cell culture system (Bellco New Technology, Ltd., Vineland, N.J.). Typical growth curves of the two fungi were obtained, and the antigens were prepared from the log phases (1, 19). We obtained two somatic antigens after 0.5% formaldehyde extraction, disintegration with a Polytron homogenizer (Kinematica, Ltd., Kriens, Switzerland), and sonication (20 kHz) with a Vibracell apparatus (Sonics & Materials Inc., Danbury, Conn.). The antigenic extracts were finally lyophilized (in 3-ml vials) and the protein contents were determined by the Bradford technique (Bio-Rad, Richmond, Va.) before and after the final step (4).
ELISA technique.
The ELISA technique was performed as previously described (1, 26), with only slight modifications in order to obtain optimal conditions with the fungal antigens: plates were coated with antigens (concentration, 1.0 μg/ml) and incubated for 1 h; serum dilutions were 1/200; the conjugate was peroxidase-labeled anti-human Ig (Sanofi Diagnostic-Pasteur, Marnes-la-Coquette, France) at a 1/8,000 dilution; and measurements of optical density at 492 nm were done with a UV spectrophotometer (Multiskan Plus; Labsystems, Helsinki, Finland) driven by a computer (Prolinea 486; Compaq Ltd., Houston, Tex.). Each assay was referenced by including a positive reference sample obtained from five pooled positive serum samples, and the results were expressed in arbitrarily defined immunoenzymatic units (IEU), as previously described (14, 26). Sera were classified as positive when the assay result was greater than 25 IEU, according to the normal parameters established by investigating serum samples from 24 healthy people from Antananarivo, where, due to the urban environment, chromoblastomycosis is absent. The reproducibility of the data was monitored by including on each ELISA plate one positive and one negative serum sample.
Immunoblotting.
The immunoblotting technique (26) was adapted for studying the humoral response in chromoblastomycosis (14). The optimal conditions for fungal antigens were as follows: sodium dodecyl sulfate-polyacrylamide gel electrophoresis with a 10 to 15% acrylamide gel was carried out for 4 h at a constant level of 35 mA in a discontinuous buffer system. Active protein transfer occurred with 36-V treatment overnight at 4°C, with a Transphor Power apparatus (Hoefer Scientific Instruments, San Francisco, Calif.). Incubation of nitrocellulose papers was performed with sera (dilution, 1/100) before the addition of alkaline phosphatase-labeled anti-human IgG (Protoblot AP II systems; Promega, Ltd., Madison, Wis.), IgM (Biosys, Compiegne, France), or IgA (Sigma, St. Louis, Mo.), incubated for 60 min at 1/6,000, 1/1,600 and 1/2,500 dilutions, respectively. Both antigen-stained electrophoresis and immunoblot nitrocellulose sheets were photographed while wet for reference laboratory records. In parallel, immunoblot profile thresholds were densitometrically determined using a video scanner (Scanjet IICX/T; Hewlett Packard, Boise, Idaho) and a specialized software (Deskcan 2.0; Hewlett Packard) based on a Bravo EL computer (AST Research Inc., Irvine, Calif.). Electronic recording of data allows us to organize a blind interpretation of immunoblot results by three investigators. After the precise definition of diagnosis bands, the band recognition frequencies and patterns were calculated. Samples showing one or more of the diagnosis bands were considered positive. An antigenic component recognized by more than 50% of the sera was identified as an immunodominant band.
Statistical analysis.
Comparison between patient and control ELISA data was done with the Student's t test and the Snedecor's F test. Comparisons of the values obtained with the same sample at different times were made with the t test of differences and the nonparametric Wilcoxon test for matched series. All P values of less than 0.05 were considered to indicate statistical significance.
RESULTS
We obtained two antigenic extracts, one of F. pedrosoi (IPM-A8 antigen; yield, 21 to 66 μg/ml) and the other one of C. carrionii (IPM-M8 antigen; yield, 19 to 35 μg/ml). The specificity was first tested on 19 historical serum samples studied by ELISA, with good reproducibility (86%) within this limited sample group (1). The results presented here are the results obtained with a larger collection of 136 serum samples, collected every 4 months from 42 patients monitored for 1 year after receiving specific antifungal chemotherapy (13, 15), and were compared with results obtained with serum samples from 24 healthy Malagasy people.
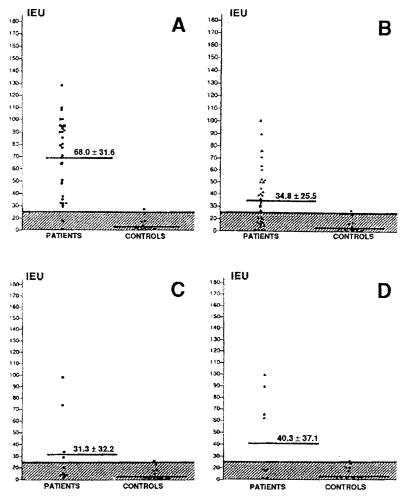
The ELISA technique was first developed for the purpose of diagnosis. Using mycological or histopathological examinations as reference tests for the diagnosis, we calculated a sensitivity of 86.9%, a specificity of 92.5%, and a global efficiency of 89.1% (14), results better than the ones obtained recently with double diffusion (25) and counterimmunoelectrophoresis (29). It is interesting to note that the best results were obtained with the F. pedrosoi antigen in all the combination experiments (Fig. 1).
FIG. 1.
Reactivity of sera from patients with F. pedrosoi-associated chromoblastomycosis (n = 35) tested with homologous (A8 antigen) and heterologous (M8 antigen) conditions (A and B, respectively) and from C. carrionii-infected patients (n = 5) tested with homologous (M8 antigen) and heterologous (A8 antigen) conditions (C and D, respectively). The ELISA IEU were calculated from the optical density values observed at 492 nm.
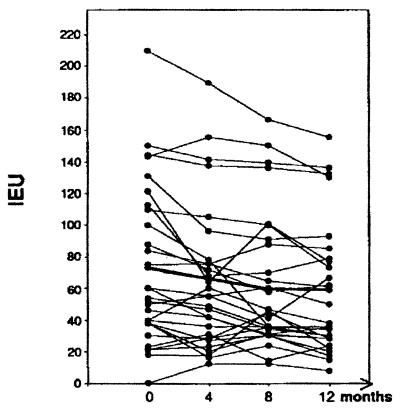
Concentrations of specific Igs in serum were measured before treatment (concentration at t0 ± SD, 72.9 ± 46.8 IEU), after 4 months (concentration at t4 ± SD, 66.1 ± 44.4 IEU) and 8 months (concentration at t8 ± SD, 59.4 ± 43.5 IEU), and at the end (concentration at t12 ± SD, 59.3.1 ± 40.7 IEU) of the trial (Fig. 2). The Ig levels gradually declined during the first 8 months of therapy and then remained stable until completion of the follow-up. It is interesting to confirm that the diagnosis can still be made by ELISA at the end of the therapy: even at t12, the levels of specific Igs were easily distinguishable (t = 6.22, P < 0.0001) from those found in normal controls. The patients with the most severe form of the disease, with multiple (at least three) lesions, presented higher titers of specific Igs compared with patients with localized lesions (99.6 ± 56.3 IEU versus 55.7 ± 32.2 IEU; P = 0.015). It is worth noting that the nine patients with a negative serology by ELISA generally presented a localized form (67% had only one lesion), sometimes complicated by a squamous cell carcinoma (22%), which tends to have appeared more recently, although the time difference is not statistically significant (mean duration of lesion ± SD, 7.8 ± 5.8 years, versus 10.2 ± 7.6 years in the whole sample; P = 0.4). No correlations could be found between Ig levels and tissue parasitism scores or circulating fibrosis markers levels (23).
FIG. 2.
Comparison of global antibody reactivity with F. pedrosoi A8 antigen, in sera from 35 F. pedrosoi-infected patients monitored during 1 year of specific therapy. The bold line shows the median IEU value before treatment (t0) and after 4 (t4), 8 (t8), and 12 (t12) months of treatment. The ELISA IEU were calculated from the optical density values observed at 492 nm.
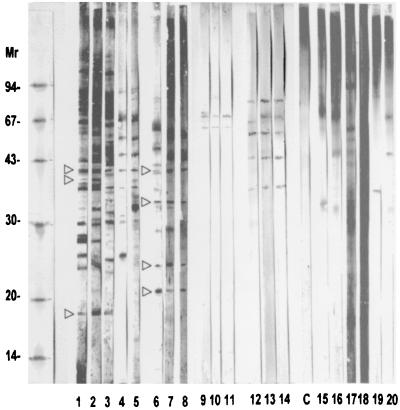
By Western immunoblotting, no band was revealed with the nonimmune sera but some cross-reactivities were clearly identified with other mycoses or parasitoses, leading to a clear-cut definition of diagnosis bands (Fig. 3). The IgG antibody pattern indicated that F. pedrosoi-infected patients recognized a wider range of antigenic molecules than the C. carrionii-infected group, a result parallel to the respective ELISA values. On 14 F. pedrosoi-specific (from 18- to 58-kDa) antigens, there was just one immunodominant band of 18.5 kDa (Fig. 3, lanes 1 to 5). On 10 C. carrionii-specific (from 23.5- to 94-kDa) antigens identified, two (corresponding to the 23.5- and 33-kDa bands) were immunodominant (Fig. 3, lanes 6 to 8). In addition, three antigens (corresponding to the 26-, 36-, and 40-kDa bands) were common to the two species. Parallel experiments confirmed that most of the IgG response was in fact IgG1 (A. Andriantsimahavandy, unpublished results). The detailed IgM and IgA (Fig. 3, lanes 9 to 14) mappings revealed that a limited number of antigenic determinants from the fungi were active for both IgG and IgM (the 64- and 68-kDa antigens), but no antigen was IgM specific, i.e., was not recognized by IgG or IgA antibodies. Four minor antigenic determinants were, irregularly, recognized by both IgG and IgA antibodies (36-, 44-, 50-, and 53-kDa antigens), while three bands (of 47-, 57-, and 84-kDa antigens) were obviously IgA specific. It is interesting to note that some immunoreactive components, including major 18.5- and 40-kDa bands, were found in ELISA-negative sera from chromoblastomycosis patients.
FIG. 3.
Immunoblot of F. pedrosoi and C. carrionii polypeptides with chromoblastomycosis-infected sera. Lanes 1 to 5, IgG response to F. pedrosoi-infected sera; lanes 6 to 8, IgG response to C. carrionii-infected sera; lanes 9 to 11, IgM response to F. pedrosoi-infected sera; lanes 12 to 14, IgA response to F. pedrosoi-infected sera. Note that one of the patient serum samples (lane 2) was completely ELISA negative. Lane C, pool of 10 negative sera; lanes 15 to 20, respectively, immunoblot of F. pedrosoi polypeptides with reference sera of candidosis, aspergillosis, trichophytosis, schistosomiasis mansoni, ascaridiosis, and lymphatic filariasis. Relative molecular masses (Mr) are given in kilodaltons. Arrowheads indicate the 18.5-, 36-, and 40-kDa bands on the F. pedrosoi antigen (lane 1) and the 23.5-, 26-, 33-, and 40-kDa bands on the C. carrionii antigen (lane 6).
The analysis of serum samples taken from seven patients during 1 year of specific therapy, in a trial designed to be a true time course experiment, revealed a decrease in the mean number of active macromolecules from seven bands identified at t0 to 2.5 at t12.
DISCUSSION
The demonstration of circulating antibodies in chromoblastomycosis was first made using double immunodiffusion (5, 25, 28, 29) or indirect immunofluorescence (6, 17) techniques, with inconsistent results. More recently, the antigen profile of F. pedrosoi was characterized by immunoprecipitation and iodination of surface proteins (19). Our laboratory developed an ELISA, the efficiency of which for diagnostic purposes was carefully evaluated on reference patients (1, 14). The availability of ELISA and Western immunoblotting (26) together with the partial characterization of fungal antigens (1, 19) led us to carry out, for the first time with regard to this disease, a longitudinal study on 40 patients, monitored during 1 year of antifungal therapy (13, 15).
As for most of fungal pathogens, the role of natural immunity in protection is uncertain, and this is especially true for the antibody response (7). In addition, a part of the population in areas of endemicity that has been exposed to the fungus will develop a specific humoral response but not the disease (2, 14). The time necessary for serology to become negative is variable in our experience, and some patients may have persistent positive results more than 1 year after the end of antifungal treatment. As there is no clear information on the evolution of the humoral immunity status of chromoblastomycosis patients receiving therapy, we monitored 40 reference patients during a 1-year therapeutic trial with terbinafine. In our sample and with the quantitative ELISA technique, it was always possible to make a diagnosis of infection after 12 months of successful therapy. Interestingly, the patients with the most severe form of the disease, that is, chronic multiple lesions, presented higher titers of specific Igs.
Western blotting with the two fungal lysates seems to be a powerful tool, since it allows a precise measurement of antibody pattern. Sera from people exposed to chromoblastomycosis-related fungal antigens responded to several bands in immunoblots, with wide intersubject variations in the pattern and in the number of bands observed. As has been done for other parasitic diseases (for a global overview, see references 20 and 21), we have documented heterogeneous humoral responses against species-specific antigens. Some antigenic determinants (so-called immunodominants) were strongly reactive in terms of high antibody frequencies: 18.5, 36, and 40 kDa for F. pedrosoi and 23.5, 26, 33, and 40 kDa for C. carrionii. Some minor antigens were recognized by both IgG and IgM or IgA. Surprisingly, three antigenic determinants, of 47-, 57-, and 84-kDa molecular masses, were only recognized by specific IgA. Both with ELISA and immunoblot techniques, C. carrionii-infected patients seemed to be worse responders than F. pedrosoi-infected patients.
The immunological parameters here presented (specific IgGs, most of them dealing with the IgG1 isotype; IgA and IgM levels; and their evolution during chemotherapy) seem to correlate with the clinical heterogeneity described for paracoccidioidomycosis, a two-pole disease with a Th2-like regulation of the humoral response (2, 3). The detailed Ig isotype mappings revealed a high degree of complexity for the stimulating antigen set, including high levels of IgM antibodies which may have been a consequence of constant antigenic stimulation due to continuously low background fungal degradation (11). Another explanation for the IgM binding is that many fungal proteins are actually glycoproteins, the glycosidic moiety evoking an IgM response. In the absence of any mucosal involvement in this pathology, IgA might represent only a potential marker of the immune status of patients with chromoblastomycosis. Even if the immune mechanisms involved in the control of the fungus seem to be of cellular origin, at least autoantibodies could be linked with the chronicity and the extent of the lesions in this pathology (16). In areas of endemicity, a part of the population that has been exposed to the fungus will not develop the disease but will have a significant level of circulating specific antibodies (14). At the moment, many factors relevant to susceptibility or resistance mechanisms in humans are still unknown. A next step would be to correlate our information on humoral immunity and immunopathology in chromoblastomycosis with in vivo studies of the CD40-CD40 ligand interactions (18, 27).
In summary, the ELISA and immunoblot techniques allowed the analysis of 136 serum samples taken from well-studied patients with chromoblastomycosis lesions. Large numbers of antigenic determinants with various molecular masses were observed in this humoral approach. The observed polymorphism corresponds to the well-known wide repertoire of F. pedrosoi and C. carrionii phenotypes that is due to dimorphic transformation from the saprophytic to the parasitic phase and associated fungal growth (19). Although natural immunity to fungal infections in general, and to chromoblastomycosis in particular, is not well understood, it is known to have no protective effect, as the disease has a very chronic and debilitating pattern of evolution. Our data confirm that high levels of specific antibodies are identified in the serum of patients and that IgG levels correlate positively, as do antineutrophilic antibody levels (16), with the chronicity and the extent of the lesions.
ACKNOWLEDGMENTS
We thank the Andapa (Mahatana Ratsioharana) and Manambaro (Emmanuelson Randrianiaina) hospital teams for technical assistance during serum collection and clinical examinations.
REFERENCES
- 1.Andriantsimahavandy A, Michel P, Rasolofonirina H, Roux J. Apport de l'immunologie au diagnostic de la chromoblastomycose à Madagascar. J Mycol Med. 1993;3:30–36. [Google Scholar]
- 2.Baida H, Biselli P J, Juvenale M, Del Negro G M B, Mendes-Giannini M J S, Durate J S, Bernard G. Differential antibody isotype expression to the major Paracoccidioides brasiliensis antigen in juvenile and adult form paracoccidioidomycosis. Microbes Infect. 1999;1:273–278. doi: 10.1016/s1286-4579(99)80022-7. [DOI] [PubMed] [Google Scholar]
- 3.Benard G, Hong M H, Del Negro G M B, Batista L, Shinakai-Yasuda M A, Duarte A J S. Antigen-specific immunosuppression in paracoccidioidomycosis. Am J Trop Med Hyg. 1996;54:7–12. doi: 10.4269/ajtmh.1996.54.7. [DOI] [PubMed] [Google Scholar]
- 4.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Buckley H, Murray I. Precipitating antibodies in chromoblastomycosis. Sabouraudia. 1966;5:78–80. doi: 10.1080/00362176785190121. [DOI] [PubMed] [Google Scholar]
- 6.Coulanges P, Mayoux A, Brygoo E R, Rakoto M. Utilisation de l'immunofluorescence indirecte dans la chromoblastomycose humaine et expérimentale àPhialophora pedrosoi et Cladosporium carrionii. Etude préliminaire. Arch Inst Pasteur Madagascar. 1970;39:65–76. [Google Scholar]
- 7.Dixon D M, Cox R, Cutler J, Deepe G. Researchers use molecular immunology and technology to combat fungal pathogens. ASM News. 1996;62:81–84. [Google Scholar]
- 8.Dromer F, Dupont B. The increasing problem of fungal infections in the immunocompromised host. J Mycol Med. 1996;6:1–6. [Google Scholar]
- 9.Duggan J M, Wolf M D, Kauffmann C A. Phialophora verrucosa infection in an AIDS patient. Mycoses. 1995;38:215–218. doi: 10.1111/j.1439-0507.1995.tb00052.x. [DOI] [PubMed] [Google Scholar]
- 10.Espinel-Ingroff A, Shadomy S, Dixon D, Goldson P. Exoantigen test for Cladosporium bantianum, Fonsecaea pedrosoi, and Phialophora verrucosa. J Clin Microbiol. 1986;23:305–310. doi: 10.1128/jcm.23.2.305-310.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esterre P, Peyrol S, Sainte-Marie D, Pradinaud R, Grimaud R. Granulomatous reaction and tissue remodelling in the cutaneous lesion of chromoblastomycosis. Virchows Arch. 1993;422:285–291. doi: 10.1007/BF01608337. [DOI] [PubMed] [Google Scholar]
- 12.Esterre P, Andriantsimahavandy A, Ramarcel E, Pecarrere J L. Forty years of chromoblastomycosis in Madagascar: a review. Am J Trop Med Hyg. 1996;55:45–47. doi: 10.4269/ajtmh.1996.55.45. [DOI] [PubMed] [Google Scholar]
- 13.Esterre P, Inzan C K, Ramarcel E, Andriantsimahavandy A, Ratsioharana M, Pecarrere J L, Roig P. Treatment of chromoblastomycosis with terbinafine: preliminary results of an open pilot study. Br J Dermatol. 1996;134:33–36. doi: 10.1111/j.1365-2133.1996.tb15658.x. [DOI] [PubMed] [Google Scholar]
- 14.Esterre P, Jahevitra M, Ramarcel E, Andriantsimahavandy A. Evaluation of the ELISA technique for the diagnosis and the seroepidemiology of chromoblastomycosis. J Mycol Med. 1997;7:137–141. [Google Scholar]
- 15.Esterre, P., C. K. Inzan, M. Ratsioharana, A. Andriantsimahavandy, C. Raharisolo, E. Randrianiaina, and P. Roig. A multicenter trial of terbinafine in patients with chromoblastomycosis: effects on clinical and biological criteria. J. Dermatol. Treat. 9:529–534.
- 16.Galperin C, Shoenfield Y, Bilburd B, Esterre P, Meroni L, Del Papa N, Halpern G M, Andriantsimahavandy A, Gershwin M E. Anti-neutrophilic cytoplasmic antibodies in patients with chromoblastomycosis. Clin Exp Rheumatol. 1996;14:479–483. [PubMed] [Google Scholar]
- 17.Gordon M A, Doory A. Application of fluorescent antibody procedures to the study of pathogenic dematiaceous fungi, II. Serological relationships of the genus Fonsecaea. J Bacteriol. 1965;89:551–556. doi: 10.1128/jb.89.3.551-556.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grewald I S, Flavell R A. A central role of CD40 ligand in the regulation of CD4+ T cell responses. Immunol Today. 1996;17:410–414. doi: 10.1016/0167-5699(96)10030-x. [DOI] [PubMed] [Google Scholar]
- 19.Ibrahim-Granet O, De Bièvre C, Jendoubi M. Immunochemical characterisation of antigens and growth inhibition of Fonsecaea pedrosoi by species-specific IgG. J Med Microbiol. 1988;26:217–222. doi: 10.1099/00222615-26-3-217. [DOI] [PubMed] [Google Scholar]
- 20.Langley J G, Kariuki H C, Hammersley A P, Ouma J H, Butterworth A E, Dunne D W. Human IgG subclass responses and subclass restriction to Schistosoma mansoni egg antigens. Immunology. 1994;83:651–658. [PMC free article] [PubMed] [Google Scholar]
- 21.Maizels R M, Bundy D A P, Selkirk M E, Smith D F, Anderson R M. Immunological modulation and evasion by helminth parasites in human populations. Nature. 1993;365:797–805. doi: 10.1038/365797a0. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto T, Matsuda T. Chromoblastomycosis and phaeohyphomycosis. Semin Dermatol. 1985;4:240–251. [Google Scholar]
- 23.Ricard-Blum S, Hartmann D, Esterre P. Monitoring of extracellular matrix metabolism and cross-linking in tissue, serum and urine of patients with chromoblastomycosis, a chronic skin fibrosis. Eur J Clin Investig. 1998;28:748–754. doi: 10.1046/j.1365-2362.1998.00335.x. [DOI] [PubMed] [Google Scholar]
- 24.Rolland-Burger L, Rolland X, Grieve C W, Monjour L. Immunoblot analysis of the humoral immune response to Leishmania donovani infantum polypeptides in human visceral leishmaniasis. J Clin Microbiol. 1991;29:1429–1435. doi: 10.1128/jcm.29.7.1429-1435.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero H, Guedez E, Magaldi S. Evaluation of immunoprecipitation technique in chromoblastomycosis. J Mycol Med. 1996;6:83–87. [Google Scholar]
- 26.Simac C, Michel P, Andriantsimahavandy A, Esterre P, Michault A. Use of ELISA and EITB for the diagnosis and monitoring of neurocysticercosis. Parasitol Res. 1995;81:132–136. doi: 10.1007/BF00931618. [DOI] [PubMed] [Google Scholar]
- 27.Stout R D, Suttles J. The many roles of CD40 in cell-mediated inflammatory responses. Immunol Today. 1996;17:487–492. doi: 10.1016/0167-5699(96)10060-i. [DOI] [PubMed] [Google Scholar]
- 28.Villalba E, Yegres J F. Detection of circulating antibodies in patients affected by chromoblastomycosis by Cladosporium carrionii using double immunodiffusion. Mycopathology. 1988;102:17–19. doi: 10.1007/BF00436247. [DOI] [PubMed] [Google Scholar]
- 29.Villalba E. Detection of antibodies in the sera of patients with chromoblastomycosis by counterimmunoelectrophoresis. Preliminary results. J Med Vet Mycol. 1988;26:73–74. doi: 10.1080/02681218880000091. [DOI] [PubMed] [Google Scholar]