Abstract
Background
Central sleep apnoea (CSA) is characterised by abnormal patterns of ventilation during sleep due to a dysfunctional drive to breathe. Consequently, people with CSA may present poor sleep quality, sleep fragmentation, inattention, fatigue, daytime sleepiness, and reduced quality of life.
Objectives
To assess the effectiveness and safety of non‐invasive positive pressure ventilation (NIPV) for the treatment of adults with CSA.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and Scopus on 6 September 2021. We applied no restrictions on language of publication. We also searched clinical trials registries for ongoing and unpublished studies, and scanned the reference lists of included studies to identify additional studies.
Selection criteria
We included randomised controlled trials (RCTs) reported in full text, those published as abstract only, and unpublished data.
Data collection and analysis
Two review authors independently selected studies for inclusion, extracted data, and assessed risk of bias of the included studies using the Cochrane risk of bias tool version 1.0, and the certainty of the evidence using the GRADE approach. In the case of disagreement, a third review author was consulted.
Main results
We included 15 RCTs with a total of 1936 participants, ranging from 10 to 1325 participants. All studies had important methodological limitations. We assessed most studies (11 studies) as at high risk of bias for at least one domain, and all studies as at unclear risk of bias for at least two domains. The trials included participants aged > 18 years old, of which 70% to 100% were men, who were followed from one week to 60 months. The included studies assessed the effects of different modes of NIPV and CSA. Most participants had CSA associated with chronic heart failure. Because CSA encompasses a variety of causes and underlying clinical conditions, data were carefully analysed, and different conditions and populations were not pooled. The findings for the primary outcomes for the seven evaluated comparisons are presented below.
Continuous positive airway pressure (CPAP) plus best supportive care versus best supportive care in CSA associated with chronic heart failure
In the short term, CPAP plus best supportive care may reduce central apnoea hypopnoea index (AHI) (mean difference (MD) −14.60, 95% confidence interval (CI) −20.11 to −9.09; 1 study; 205 participants). However, CPAP plus best supportive care may result in little to no difference in cardiovascular mortality compared to best supportive care alone. The evidence for the effect of CPAP plus best supportive care on all‐cause mortality is very uncertain. No adverse effects were observed with CPAP, and the results for adverse events in the best supportive care group were not reported.
Adaptive servo ventilation (ASV) versus CPAP in CSA associated with chronic heart failure
The evidence is very uncertain about the effect of ASV versus CPAP on quality of life evaluated in both the short and medium term. Data on adverse events were not reported, and it is not clear whether data were sought but not found.
ASV versus bilevel ventilation in CSA associated with chronic heart failure
In the short term, ASV may result in little to no difference in central AHI. No adverse events were detected with ASV, and the results for adverse events in the bilevel ventilation group were not reported.
ASV plus best supportive care versus best supportive care in CSA associated with chronic heart failure
In the medium term, ASV plus best supportive care may reduce AHI compared to best supportive care alone (MD −20.30, 95% CI −28.75 to −11.85; 1 study; 30 participants). In the long term, ASV plus best supportive care likely increases cardiovascular mortality compared to best supportive care (risk ratio (RR) 1.25, 95% CI 1.04, 1.49; 1 study; 1325 participants). The evidence suggests that ASV plus best supportive care may result in little to no difference in quality of life in the short, medium, and long term, and in all‐cause mortality in the medium and long term. Data on adverse events were evaluated but not reported.
ASV plus best supportive care versus best supportive care in CSA with acute heart failure with preserved ejection fraction
Only adverse events were reported for this comparison, and no adverse events were recorded in either group.
ASV versus CPAP maintenance in CPAP‐induced CSA
In the short term, ASV may slightly reduce central AHI (MD −4.10, 95% CI −6.67 to −1.53; 1 study; 60 participants), but may result in little to no difference in quality of life. Data on adverse events were not reported, and it is not clear whether data were sought but not found.
ASV versus bilevel ventilation in CPAP‐induced CSA
In the short term, ASV may slightly reduce central AHI (MD −8.70, 95% CI −11.42 to −5.98; 1 study; 30 participants) compared to bilevel ventilation. Data on adverse events were not reported, and it is not clear whether data were sought but not found.
Authors' conclusions
CPAP plus best supportive care may reduce central AHI in people with CSA associated with chronic heart failure compared to best supportive care alone. Although ASV plus best supportive care may reduce AHI in people with CSA associated with chronic heart failure, it likely increases cardiovascular mortality in these individuals. In people with CPAP‐induced CSA, ASV may slightly reduce central AHI compared to bilevel ventilation and to CPAP.
In the absence of data showing a favourable impact on meaningful patient‐centred outcomes and defining clinically important differences in outcomes in CSA patients, these findings need to be interpreted with caution. Considering the level of certainty of the available evidence and the heterogeneity of participants with CSA, we could draw no definitive conclusions, and further high‐quality trials focusing on patient‐centred outcomes, such as quality of life, quality of sleep, and longer‐term survival, are needed to determine whether one mode of NIPV is better than another or than best supportive care for any particular CSA patient group.
Keywords: Adolescent; Adult; Female; Humans; Male; Continuous Positive Airway Pressure; Disorders of Excessive Somnolence; Heart Failure; Sleep Apnea, Central; Sleep Apnea, Central/therapy; Sleep Apnea, Obstructive; Sleep Apnea, Obstructive/therapy
Plain language summary
Non‐invasive ventilation for people with central sleep apnoea
What was studied in this review?
This review looked at the effect of non‐invasive ventilation (NIV) compared to other treatments or a different types of NIV in people with central sleep apnoea (CSA), a disorder where the person regularly stops breathing during sleep because their brain fails to tell their muscles to take in air. CSA more commonly affects men and people with chronic heart disease. People with CSA may wake up often during the night, feel very sleepy during the day, and not be able to exercise as much as usual. Sleep apnoea may also increase a person's risk of other conditions, such as heart attack and stroke, and death.
In NIV a machine is used to help the person breath. During NIV therapy, the person uses a face mask or nasal mask through which air is forced into their lungs to lessen the need of respiratory effort. Several types of NIV therapies are available, using different ways of delivering the breathing support; some examples are below.
• Continuous positive airway pressure (CPAP): air is forced into the lungs continuously (during inspiration and expiration). • Bilevel positive airway pressure (BiPAP): air is forced into the lungs more during inspiration than during expiration. • Adaptive servo ventilation (ASV): air is forced into the lungs mainly when the person stops breathing.
Non‐invasive ventilation may help the air get into the lungs. However, we do not know if NIV also helps the person live longer and better, or if it can cause any harm.
What was the aim of this review?
The aim of this review was to find out whether any type of NIV can be more helpful than harmful to people with CSA than other therapies or another type of NIV. We collected and analysed all currently available relevant studies to answer this question.
What were the main results of this review?
We included 15 studies dating from 1995 to 2019, involving a total of 1936 adult participants. Most studies included men who had chronic heart disease and CSA at the same time. The included studies looked at different comparisons, such as CPAP versus best supportive care, ASV versus best supportive care, ASV versus CPAP, and ASV versus BiPAP. In these studies, participants were assigned to receive the types of NIV or other therapies by chance (randomised controlled trials).
As we found studies that compared several modes of NIV, with mostly small numbers of participants to include in each comparison, the effects of these studies, even when study results were combined, were not precise. Also, the methods used to assign participants to one of two or more study treatments were described incompletely, thus it is unclear whether these methods were adequate. Furthermore, in several studies, the participants were evaluated by researchers who knew which treatments participants had received, which may have influenced their assessments and led to results that are biased. We are therefore not confident in the results of the currently available studies, and can draw no strong conclusions from them.
In people with CSA associated with chronic heart failure, using NIV (CPAP or ASV) seems to result in a lower frequency of episodes of apnoea (when the person stops breathing) than best supportive care alone in the short term (up to three months). However, it is not clear whether there are benefits in the long term (more than one year). In contrast, in the long‐term, ASV probably results in increased death related to heart conditions in people with CSA and chronic heart failure. Very few data were available regarding people with CSA that do not have chronic heart failure.
Key messages
Taken together, the results of the studies evaluated in this review do not allow us to be sure if one kind of NIV leads to more side effects than another, or whether one kind of NIV leads to more harm than benefit compared to best supportive care in people with CSA.
How up‐to‐date is this review?
This review is current to 6 September 2021.
Summary of findings
Summary of findings 1. Continuous positive airway pressure (CPAP) plus best supportive care versus best supportive care for central sleep apnoea associated with chronic heart failure.
| CPAP plus best supportive care versus best supportive care for CSA associated with chronic heart failure | ||||||
|
Participants: people with CSA associated with chronic heart failure Setting: outpatient Intervention: CPAP plus best supportive care Comparison: best supportive care | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| With best supportive care | With CPAP plus best supportive care | Difference | ||||
| Central AHI ‐ after 3 months Number of participants: 205 (1 RCT) |
‐ | The mean central AHI with best supportive care was 30.70. | The mean central AHI with CPAP was 16.10 (12.81 to 19.39). | MD 14.60 lower (20.11 lower to 9.09 lower) |
⊕⊕⊝⊝ Low1 2 |
CPAP plus best supportive care may reduce central AHI in the short term. |
| Cardiovascular mortality ‐ after 24 months Number of participants: 258 (1 RCT) |
RR 1.17 (0.68 to 2.02) | The mean death rate from cardiovascular events with best supportive care was 15.40%. | The mean death rate from cardiovascular events with CPAP was 18.0% (10.50 to 31.10). | 2.60% more deaths from cardiovascular events in the treatment group (4.90 fewer to 15.70 more) | ⊕⊕⊝⊝ Low 3 4 | CPAP plus best supportive care may result in little to no difference in cardiovascular mortality in the long term. |
| Serious adverse events | Not reported | |||||
| Quality of sleep | Not reported | |||||
| Quality of life | Not reported | |||||
| AHI ‐ after up to 3 months Number of participants: 264 (4 RCT) |
‐ | The mean AHI with best supportive care ranged across control groups from 19.00 to 37.60. | The mean AHI with CPAP ranged across intervention groups from 14.7 to 18.5. | MD 15.27 lower (22.44 lower to 8.11 lower) | ⊕⊕⊝⊝ Low 1 2 | CPAP plus best supportive care may reduce AHI in the short term. |
| All‐cause mortality ‐ after 24 months Number of participants: 287 (2 RCTs) |
RR 1.14 (0.83 to 1.56) | The mean death rate with best supportive care was 26.20%. | The mean death rate with CPAP was 29.90% (21.80 to 40.90). | 3.70% more deaths in the treatment group (4.50 fewer to 14.70 more) | ⊕⊝⊝⊝ Very low 5 6 | The evidence is very uncertain about the effect of CPAP plus best supportive care on all‐cause mortality in the long term. |
| Time to life‐saving cardiovascular intervention (assessed with: transplant‐free survival rate) ‐ after 18 months Number of participants: 258 (1 RCT) |
1 study reported an HR for transplantation‐free survival of 0.66; P = 0.06 (CANPAP 2006). | ⊕⊕⊝⊝ Low 3 4 | CPAP plus best supportive care may slightly increase time to life‐saving intervention in the long term. | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AHI: apnoea hypopnoea index; CI: confidence interval; CPAP: continuous positive airway pressure; CSA: central sleep apnoea; HR: hazard ratio; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1We downgraded one level due to methodological limitations (one study with high risk of bias for blinding of participants and personnel, incomplete outcome data, and selective reporting). 2We downgraded one level due to small sample size. 3We downgraded one level due to methodological limitations (one study with high risk of bias for incomplete outcome data and selective reporting). 4We downgraded one level due to the presence of few events. 5We downgraded one level due to methodological limitations (one study with high risk bias for incomplete outcome data and selective reporting and one study with unclear risk of bias for random sequence generation, allocation concealment, and selective reporting). 6We downgraded two levels due to the presence of few events and large CI (CI includes both important benefit and important harm).
Summary of findings 2. Adaptive servo ventilation (ASV) versus continuous positive airway pressure (CPAP) for central sleep apnoea associated with chronic heart failure.
| ASV versus CPAP for CSA associated with chronic heart failure | ||||||
|
Participants: people with CSA associated with chronic heart failure Setting: outpatient Intervention: ASV Comparison: CPAP | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| With CPAP | With ASV | Difference | ||||
| Central AHI | Not reported | |||||
| Cardiovascular mortality | Not reported | |||||
| Serious adverse events | Not reported | |||||
| Quality of sleep | Not reported | |||||
| Quality of life measured with MLHFQ ‐ after 6 months Number of participants: 17 (1 RCT) |
‐ | The mean difference in quality of life with CPAP was −12.52. | The mean difference in quality of life with ASV was −21.16 (−33.14 to −9.18). | MD 8.64 lower (25.56 lower to 8.28 higher) | ⊕⊝⊝⊝ Very low 1 2 | The evidence is very uncertain about the effect of ASV on quality of life in the medium term. |
| AHI ‐ after 6 months Number of participants: 17 (1 RCT) |
‐ | The mean difference in AHI with CPAP was −17.80. | The mean difference in AHI with ASV was −46.47 (−60.91 to −32.03). | MD 28.67 lower (48.28 lower to 9.06 lower) | ⊕⊕⊝⊝ Low 3 4 | ASV may reduce AHI in the medium term. |
| All‐cause mortality | Not reported | |||||
| Time to life‐saving cardiovascular intervention | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AHI: apnoea hypopnoea index; ASV: adaptive servo ventilation; CI: confidence interval; CPAP: continuous positive airway pressure; CSA: central sleep apnoea; MD: mean difference; MLHFQ: Minnesota Living with Heart Failure Questionnaire; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1We downgraded one level due to important methodological limitations (one study with high risk of bias for blinding of participants and personnel, blinding of outcome assessors, and incomplete outcome data and unclear risk of bias for random sequence generation, allocation concealment, and selective reporting). 2We downgraded two levels due to small sample size and large CI (CI includes both important benefit and important harm). 3We downgraded one level due to important methodological limitations (one study with high risk of bias for blinding of participants and personnel and incomplete outcome data and unclear risk of bias for random sequence generation, allocation concealment, and selective reporting). 4We downgraded one level due to small sample size.
Summary of findings 3. Adaptive servo ventilation (ASV) versus bilevel ventilation for central sleep apnoea associated with chronic heart failure.
| ASV versus bilevel ventilation for CSA associated with chronic heart failure | ||||||
|
Participants: people with CSA associated with chronic heart failure Setting: outpatient Intervention: ASV Comparison: bilevel ventilation | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| With bilevel ventilation | With ASV | Difference | ||||
| Central AHI ‐ after 6 weeks Number of participants: 30 (1 RCT) |
‐ | The mean central AHI with bilevel ventilation was 1.60. | The mean central AHI with ASV was 2.50 (0.06 to 4.94). | MD 0.90 higher (2.40 lower to 4.20 higher) | ⊕⊕⊝⊝ Low1 2 |
ASV may result in little to no difference in central AHI in the short term. |
| Cardiovascular mortality | Not reported | |||||
| Serious adverse events | Not reported | |||||
| Quality of sleep | Not reported | |||||
| Quality of life | Not reported | |||||
| AHI ‐ after 6 weeks Number of participants: 30 (1 RCT) |
‐ | The mean AHI with bilevel ventilation was 16.40. | The mean AHI with ASV was 11.20 (5.99 to 16.41). | MD 5.20 lower (14.63 lower to 4.23 higher) | ⊕⊝⊝⊝ Very low 1 3 | The evidence is very uncertain about the effect of ASV on AHI in the short term. |
| All‐cause mortality | Not reported | |||||
| Time to life‐saving cardiovascular intervention | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AHI: apnoea hypopnoea index; ASV: adaptive servo ventilation; CI: confidence interval; CSA: central sleep apnoea; MD: mean difference; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1We downgraded one level due to methodological limitations (all domains at unclear risk of bias except other potential sources of bias). 2We downgraded one level due to small sample size. 3We downgraded two levels due to small sample size and large CI (CI includes both important benefit and important harm).
Summary of findings 4. Adaptive servo ventilation (ASV) versus continuous positive airway pressure (CPAP) for CPAP‐induced central sleep apnoea.
| ASV versus CPAP for CPAP‐induced CSA | ||||||
|
Participants: people with CPAP‐induced CSA Setting: outpatient Intervention: ASV Comparison: CPAP | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| With CPAP | With ASV | Difference | ||||
| Central AHI ‐ after 3 months Number of participants: 60 (1 RCT) |
‐ | The mean central AHI with CPAP was 4.80. | The mean central AHI with ASV was 0.70 (−0.59 to 1.99) | MD 4.10 lower (6.67 lower to 1.53 lower) | ⊕⊕⊝⊝ Low1 2 |
ASV may slightly reduce central AHI in the short term. |
| Cardiovascular mortality | Not reported | |||||
| Serious adverse events | Not reported | |||||
| Quality of sleep | Not reported | |||||
| Quality of life ‐ after 3 months Number of participants: 61 (1 RCT) |
‐ | The mean difference in quality of life with CPAP was 0.10. | The mean difference in quality of life with ASV was 0.10 (−0.27 to 0.47). | MD 0.00 (0.46 lower to 0.46 higher) | ⊕⊕⊝⊝ Low 2 3 | ASV may result in little to no difference in quality of life in the short term. |
| AHI ‐ after 3 months Number of participants: 60 (1 RCT) |
‐ | The mean AHI with CPAP was 9.90. | The mean AHI with ASV was 4.40 (0.75 to 8.05). | MD 5.50 lower (10.74 lower to 0.26 lower) | ⊕⊕⊝⊝ Low 1 2 | ASV may slightly reduce AHI in the short term. |
| All‐cause mortality | Not reported | |||||
| Time to life‐saving cardiovascular intervention | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AHI: apnoea hypopnoea index; ASV: adaptive servo ventilation; CI: confidence interval; CPAP: continuous positive airway pressure; CSA: central sleep apnoea; MD: mean difference; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1We downgraded one level due to important methodological limitations (one study with high risk of bias for blinding of participants and personnel and incomplete outcome data and unclear risk of bias for random sequence generation, allocation concealment, and blinding of outcome assessors). 2We downgraded one level due to small sample size. 3We downgraded one level due to important methodological limitations (one study with high risk of bias for blinding of participants and personnel, blinding of outcome assessors, and incomplete outcome data and unclear risk of bias for random sequence generation and allocation concealment).
Summary of findings 5. Adaptive servo ventilation (ASV) versus bilevel ventilation for CPAP‐induced central sleep apnoea.
| ASV versus bilevel ventilation for CPAP‐induced CSA | ||||||
|
Participants: people with CPAP‐induced CSA Setting: outpatient Intervention: ASV Comparison: bilevel ventilation | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| With bilevel ventilation | With ASV | Difference | ||||
| Central AHI ‐ after 6 weeks Number of participants: 30 (1 RCT) |
‐ | The mean central AHI with bilevel ventilation was 10.20. | The mean central AHI with ASV was 1.50 (0.56 to 2.44). | MD 8.70 lower (11.42 lower to 5.98 lower) | ⊕⊕⊝⊝ Low 1 2 | ASV may slightly reduce central AHI in the short term. |
| Cardiovascular mortality | Not reported | |||||
| Serious adverse events | Not reported | |||||
| Quality of sleep | Not reported | |||||
| Quality of life | Not reported | |||||
| AHI ‐ after 6 weeks Number of participants: 30 (1 RCT) |
‐ | The mean AHI with bilevel ventilation was 16.50. | The mean AHI with bilevel ventilation was 7.40 (5.07 to 9.73). | MD 9.10 lower (13.67 lower to 4.53 lower) | ⊕⊕⊝⊝ Low 1 2 | ASV may slightly reduce AHI in the short term. |
| All‐cause mortality | Not reported | |||||
| Time to life‐saving cardiovascular intervention | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AHI: apnoea hypopnoea index; ASV: adaptive servo ventilation; CI: confidence interval; CPAP: continuous positive airway pressure; CSA: central sleep apnoea; MD: mean difference; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1We downgraded one level due to methodological limitations (one study with high risk of bias due to lack of blinding of outcome assessment and incomplete outcome data and unclear risk of bias for random sequence generation and allocation concealment). 2We downgraded one level due to small sample size.
Summary of findings 6. Adaptive servo ventilation (ASV) plus best supportive care versus best supportive care alone or inactive control for central sleep apnoea associated with chronic heart failure.
| ASV plus best supportive care versus best supportive care alone or inactive control for CSA associated with chronic heart failure | ||||||
|
Participants: people with CSA associated with chronic heart failure Setting: outpatient Intervention: ASV plus best supportive care Comparison: best supportive care alone or inactive control | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| With best supportive care | With ASV plus best supportive care | Difference | ||||
| Central AHI | Not reported | |||||
| Cardiovascular mortality ‐ up to 80 months Number of participants: 1325 (1 RCT) |
RR 1.25 (1.04 to 1.49) | 24.00% | 30.00% (24.90 to 35.70) | 6.00% more (1.00 more to 11.70 more) | ⊕⊕⊕⊝ Moderate 1 | ASV plus best supportive care likely increases cardiovascular mortality in the long term. |
| Serious adverse events | Not reported | |||||
| Quality of sleep | Not reported | |||||
| Quality of life ‐ after 48 months Number of participants: 1325 (1 RCT) |
‐ | The mean quality of life with best supportive care was 32.03. | The mean quality of life with ASV plus best supportive care was 32.86 (30.99 to 34.73). | MD 0.83 higher (1.81 lower to 3.47 higher) | ⊕⊕⊝⊝ Low 2 3 | ASV plus best supportive care may result in little to no difference in quality of life in the long term. |
| AHI ‐ after 6 months Number of participants: 30 (1 RCT) |
‐ | The mean difference in AHI with best supportive care was −0.50. | The mean difference in AHI with ASV plus best supportive care was 20.80 (−28.80 to −12.71). | MD 20.30 lower (28.75 lower to 11.85 lower) | ⊕⊕⊝⊝ Low 4 5 | ASV plus best supportive care may reduce AHI in the medium term. |
| All‐cause mortality ‐ up to 80 months Number of participants: 1355 (2 RCTs) |
RR 0.56 (0.06 to 4.93) | 29.20% | 16.40% (1.80 to 100.00) | 12.90% fewer (27.50 fewer to 114.90 more) | ⊕⊕⊝⊝ Low 3 6 | ASV plus best supportive care may result in little to no difference in all‐cause mortality in the long term. |
| Time to life‐saving cardiovascular intervention | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AHI: apnoea hypopnoea index; ASV: adaptive servo ventilation; CI: confidence interval; CSA: central sleep apnoea; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1We downgraded one level due to important methodological limitations (one study with high risk of bias for incomplete outcome data and unclear risk of bias for allocation concealment). 2We downgraded one level due to important methodological limitations (one study with high risk of bias for blinding of participants and personnel, blinding of outcome assessors, and incomplete outcome data and unclear risk of bias for allocation concealment). 3We downgraded one level due to large CI (CI includes both important benefit and important harm). 4We downgraded one level due to important methodological limitations (one study with unclear risk of bias for random sequence generation, allocation concealment, blinding of outcome assessors, and selective reporting). 5We downgraded one level due to small sample size. 6We downgraded one level due to important methodological limitations (one study with high risk of bias for incomplete outcome data and unclear risk of bias for allocation concealment, and one study with high risk of bias for incomplete outcome data and selective reporting and unclear risk of bias for random sequence generation).
Background
Description of the condition
Central sleep apnoea (CSA) is characterised by abnormal patterns of breathing during sleep due to a dysfunctional drive to breathe. These abnormal patterns of ventilation are compounded by hypoventilation (reduction of the air flow whilst breathing) and recurrent apnoeas (cessation of breathing) or hypopnoeas (overly shallow breathing or an abnormally low respiratory rate). Hypoventilation may lead to compromised gas exchange, hypercapnia (elevation in the arterial carbon dioxide levels), and sleep fragmentation (Eckert 2007). As a consequence, people with CSA may present with a variety of symptoms, such as poor sleep quality, inattention, fatigue, daytime sleepiness, and reduced quality of life.
Overall prevalence is not as established for CSA as it is for obstructive sleep apnoea (OSA), but in one recent population‐based study of adults over 40 years old undergoing polysomnography, the observed prevalence of CSA was 0.9% (Donovan 2016). Prevalence appears to be increased amongst men compared with women (Bixler 2001; Donovan 2016), as well as in older age, particularly in those over 65 years old (Bixler 2001; Donovan 2016). CSA is particularly common in a few high‐risk groups including individuals with heart failure (Donovan 2016), stroke (Johnson 2010), renal failure (Hanly 2001), and atrial fibrillation (Sin 1999; Stevenson 2008), and people receiving long‐term opioid therapy (Correa 2015; Wang 2005).
The International Classification of Sleep Disorders (ICSD), 3rd edition, presents several types of central sleep apnoea syndromes (CSAS) in adults, defined by the pattern of abnormal breathing, existence of comorbidity, or other features from a person's history, such as substance abuse or exposure to high altitude (American Academy of Sleep Medicine 2014). The categories of CSAS described in the ICSD 3rd edition thus include CSA with Cheyne‐Stokes breathing; CSA due to a medical disorder without Cheyne‐Stokes breathing; CSA due to high‐altitude periodic breathing; CSA due to a medication or substance; primary CSA; and treatment‐emergent CSA. Clinicians can diagnose CSAS on the basis of polysomnographic findings and diagnostic criteria conjointly.
CSA with Cheyne‐Stokes breathing occurs in 40% to 50% of people with chronic heart failure (Peer 2010), and is considered to be a marker of chronic heart failure severity (Peer 2010); however, this combined disorder may also be responsible for aggravated heart failure (Hanly 1996). This dysfunctional breathing pattern is not exclusive of chronic heart failure and may be found in other conditions, such as stroke and renal failure. Cheyne‐Stokes breathing is characterised by a cyclical breathing pattern compounded by periods of 20 to 30 seconds of hyperventilation alternating with central apnoeas typically lasting from 10 to 40 seconds, in a crescendo‐decrescendo pattern (Naughton 2012). Periodic breathing is also observed in CSA due to high altitude, which may occur after ascent to altitudes as high as 4000 metres.
People with neurological disease or other medical conditions may manifest central apnoeas comprising other patterns of ventilatory dysfunction typically distinct from Cheyne‐Stokes breathing. Congenital central hypoventilation syndrome, also known as Ondine's curse, is an example. The ventilatory dysfunction in congenital central hypoventilation syndrome is characterised by reduced tidal volume, with a relatively preserved breathing frequency, manifested mainly in non‐rapid eye movement sleep (Muzumdar 2008).
CSA may also arise after the start of treatment of OSA with positive pressure ventilation or, rarely, after resolution of obstructive apnoea by other modalities of treatment, such as the use of oral appliances (Kuźniar 2011). Lastly, the co‐existence of CSA and OSA in the same individual is frequently found. Common mechanistic traits may explain such overlap (Eckert 2007).
Description of the intervention
Non‐invasive positive pressure ventilation (NIPV) encompasses several non‐invasive ventilatory therapies that employ different ways of delivering positive airway pressure through nasal or facial masks.
Continuous positive airway pressure (CPAP) therapy consists of the deliverance of continuous levels of positive pressure during both inspiration and expiration, aimed at maintenance of airway patency. CPAP is considered the gold‐standard treatment for people with moderate to severe OSA (Epstein 2009), but its role in the management of central apnoeas is controversial, as trials have reported limited benefits of CPAP for some people with CSA‐Cheyne‐Stokes breathing (CANPAP 2006).
Other types of non‐invasive positive pressure employ more sophisticated technology with the intention of delivering positive pressure more effectively. Bilevel positive airway pressure (BiPAP) supplies positive pressure at two distinct levels, according to inspiratory and expiratory phases of the breathing cycle. In addition to promoting airway patency, BiPAP aims to mitigate hypoventilation by delivering higher positive pressure during inspiration and by providing a ventilatory backup rate. Adaptive servo ventilation (ASV) is a modality of non‐invasive ventilation in which pressure is preset and volume or flow is cycled. ASV provides dynamic adjustment of inspiratory pressure support to normalise breathing patterns according to a prespecified target. ASV aims to mitigate hyperventilation and consequent hypocapnia by delivering preset minute ventilation (Aurora 2012).
How the intervention might work
The underlying mechanism for CSA falls into one of two main categories: hyperventilation or hypoventilation. Hyperventilation‐related CSA is characterised by periods of hyperpnoea during sleep that induce hypocapnia, which in turn causes central apnoea. This is due in part to a drop in partial pressure of carbon dioxide (PaCO2) level to below the central chemoreceptor threshold for ventilatory drive. Onset of central apnoea subsequently leads to increased PaCO2, which may restore normal ventilation or lead to another phase of hyperventilation, reinitiating the cycle. CSA due to hypoventilation is associated with impaired ventilation due to central nervous system disease, central nervous system‐depressing drugs, neuromuscular disorder, or abnormality in chest wall mechanics (such as kyphoscoliosis). In this patient group, sleep leads to a general decline in ventilatory drive with profound hypoventilation or prolonged apnoea. These periods are terminated by phases of arousal from deep sleep but recommence once deeper sleep is re‐established.
NIPV may improve ventilation by reducing oscillations of PaCO2 and by compensating central apnoeas. CPAP and BiPAP provide a pneumatic splint effect in the airways. BiPAP may further improve ventilation via backup ventilation frequency (spontaneous timed (ST) mode) (Kuźniar 2008b). ASV automatically calculates the ventilatory support needed during inspiration through continuous analysis of the breathing pattern in an attempt to compensate for hypoventilation periods (Bitter 2010).
The co‐existence of central and obstructive apnoeas in the same patient is not rare, as both conditions are relatively common in people with chronic heart failure. Also, abnormal ventilatory control may induce airway obstruction in people with vulnerable pharyngeal anatomy, and the inverse is also observed (Eckert 2007). Given that NIPV is highly effective in treating individuals with OSA, it is logical to assume that by counteracting airway obstruction, NIPV may positively influence ventilatory control.
Why it is important to do this review
Treatment of people with CSAS may rely on treating or eliminating the underlying mechanism (e.g. withdrawal of opioids). However, treatment of the underlying condition is not always possible or effective. Clinical management of chronic heart failure via β‐blockers and angiotensin‐converting enzyme inhibitors does not reduce the prevalence of Cheyne‐Stokes breathing (MacDonald 2008). Specific treatment strategies to mitigate CSAS are therefore needed.
Different types of non‐invasive positive pressure treatment have been developed for the treatment of CSAS, such as ASV, which aims to compensate the pattern of hyperventilation and hypoventilation found in Cheyne‐Stokes breathing. However, there are safety issues related to ASV treatment of people with Cheyne‐Stokes breathing. The Treatment of Sleep‐Disordered Breathing with Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients with Heart Failure (SERVE‐HF) trial came to the unexpected result that all‐cause and cardiovascular mortality are increased in people with very low ejection fraction treated with ASV (SERVE HF 2015). It is not entirely clear whether these results can be extrapolated to all people with chronic heart failure with a low ejection fraction. The same level of uncertainty applies to other non‐invasive positive pressure devices and to CSAS of different causes, given the significant heterogeneity noted amongst patient groups and their response to different treatment modalities. Synthesis of evidence identifying the best treatment options for different types of CSAS has the potential to aid clinicians, patients, and policymakers.
Objectives
To assess the effectiveness and safety of non‐invasive positive pressure ventilation for treatment of adults with central sleep apnoea syndromes.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) with a parallel design that employed individual allocation. We considered studies reported in full text, those published as abstract only, and unpublished data for inclusion in the review.
Types of participants
We included participants older than 18 years of age for whom one of the following CSAS had been diagnosed, as defined by the ICSD, 3rd edition (American Academy of Sleep Medicine 2014).
CSA with Cheyne‐Stokes breathing.
CSA due to a medical disorder without Cheyne‐Stokes breathing.
CSA due to a medication or substance.
Primary CSA.
Treatment‐emergent CSA.
Types of interventions
We included studies comparing any type of non‐invasive positive pressure versus prespecified comparators, as listed below.
Types of non‐invasive positive pressure ventilation included the following.
Continuous positive airway pressure (CPAP).
Auto‐set positive airway pressure.
Bilevel positive airway pressure (BiPAP).
Adaptive servo ventilation (ASV).
Types of comparators included the following.
Sham therapy (subtherapeutic positive pressure).
Another type of non‐invasive positive pressure.
No treatment.
Usual care defined as treatment for underlying disease.
We planned to make comparisons involving different types of interventions versus active controls or inactive controls, as follows:
CPAP versus another type of non‐invasive positive pressure
CPAP versus inactive control (subtherapeutic positive pressure, no treatment, or usual care).
Auto‐set positive airway pressure versus another type of non‐invasive positive pressure.
Auto‐set positive airway pressure versus inactive control (subtherapeutic positive pressure, no treatment, or usual care).
BiPAP versus another type of non‐invasive positive pressure.
BiPAP versus inactive control (subtherapeutic positive pressure, no treatment, or usual care).
ASV versus another type of non‐invasive positive pressure.
ASV versus inactive control (subtherapeutic positive pressure, no treatment, or usual care).
For each comparison, we performed distinct meta‐analyses for each type of population, according to the type of health condition associated with CSA.
Types of outcome measures
Primary outcomes
Central apnoea hypopnoea index (cAHI)
We defined cAHI as the number of central apnoea hypopnoeas per hour of sleep, measured objectively by polysomnography or assessed by analysis of data from non‐invasive positive pressure devices, registered in smart cards, or transmitted by modem, or via Web‐based methods.
Cardiovascular mortality
We defined cardiovascular mortality as the number of deaths attributable to myocardial ischaemia and infarction, heart failure, cardiac arrest because of other or unknown cause, or cerebrovascular accident (Carrero 2011).
Serious adverse events
We defined serious adverse events as life‐threatening events and those leading to death, hospitalisation, disability or permanent damage, congenital anomaly, or that required intervention to prevent permanent impairment or damage.
Secondary outcomes
Quality of sleep
We planned to include studies that assessed quality of sleep using validated scales or questionnaires, such as the Pittsburgh Sleep Quality Index (Buysse 1989).
Quality of life
We included studies that assessed quality of life using validated scales or questionnaires, such as the 36‐item Short Form Health Survey (SF‐36) (Jenkinson 1996).
Apnoea hypopnoea index (AHI)
We included studies evaluating total AHI, defined as the number of events of obstructive, mixed, or central apnoea/hypopnoea per hour of sleep, measured objectively by polysomnography or assessed by analysis of data from non‐invasive positive pressure devices, registered in smart cards, or transmitted by modem, or via Web‐based methods.
All‐cause mortality, defined as number of deaths irrespective of cause.
Time to life‐saving cardiovascular intervention (cardiac transplantation, implantation of cardioverter‐defibrillator).
Adverse events/side effects, such as nasal congestion, upper airway dryness, mask‐induced pressure ulcer.
We assessed outcomes at all time points reported in primary studies and planned to pool short‐, medium‐, and long‐term data, defined as follows.
Short‐term: up to three months.
Medium‐term: three months to one year.
Long‐term: longer than one year.
Search methods for identification of studies
Electronic searches
We identified studies from the following sources:
Cochrane Airways Trials Register, through the Cochrane Register of Studies (CRS 2022), from inception to 6 September 2021;
Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies (CRS 2022), on 6 September 2021 (2021, Issue 9);
MEDLINE (Ovid SP) ALL from 1946 to 6 September 2021;
Embase (Ovid SP) from 1974 to 6 September 2021;
Scopus from inception to 6 September 2021.
Search strategies are listed in Appendix 1. We designed the initial search strategy in MEDLINE and adapted this for use in the other databases. We searched all databases from their inception to 6 September 2021, and applied no restrictions on language of publication. We handsearched conference abstracts and grey literature through the CENTRAL database.
In addition, we searched the following trials registries on 6 September 2021.
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov/).
World Health Organization International Clinical Trials Registry Platform (trialsearch.who.int/).
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references. We searched relevant manufacturers' websites for study information.
We searched for errata or retractions from the included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed), and planned to report within the review the date this was done.
Data collection and analysis
Selection of studies
Two review authors independently (ACPNP, AR, or DVP) screened the titles and abstracts of studies identified by the search, coding studies as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports of all potentially eligible studies, and two review authors (ACPNP, AR, or DVP) independently screened these for inclusion and recorded the reasons for exclusion of ineligible studies. Any disagreements were resolved through discussion or by consulting a third person/review author (ACPNP, AR, or DVP) when required. We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and 'Characteristics of excluded studies' tables (Moher 2009).
Data extraction and management
We used a data collection form that had been piloted on at least one study in the review to record study characteristics and outcomes, adapted from EPOC 2013.
One review author (ACPNP or DVP) extracted the following study characteristics from the included studies.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and locations, study setting, withdrawals, and dates of study.
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, and exclusion criteria.
Interventions: intervention, comparison, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for studies and notable conflicts of interest of trial authors.
Two review authors (ACPNP, AR, or DVP) independently extracted outcome data from the included studies. We noted in the 'Characteristics of included studies' tables if outcome data were not reported in an useable way. Any disagreements were resolved through consensus or by involving a third person/review author (AR or DVP). One review author (DVP) transferred data into the Review Manager 5 file (Review Manager 2020). We double‐checked that data had been entered correctly by comparing the data presented in the systematic review with information provided in the study reports. A second review author (ACPNP) spot‐checked the study characteristics for accuracy against the study report.
Assessment of risk of bias in included studies
Two review authors (ACPNP, AR) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreements were resolved by discussion or through consultation with another review author (DVP). We assessed risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We judged each risk of bias domain as low, high, or unclear risk of bias, and provided a quote from the study report together with a justification for our judgement in the risk of bias table. We summarised risk of bias judgements across different studies for each of the domains listed.
We considered the domains 'blinding of participants and personnel' and 'blinding of outcome assessment' differently according to the type of outcome. For subjective outcomes, such as quality of life and quality of sleep, we judged any deviation from blinding procedures as introducing a high risk of bias. For objective outcomes, such as mortality, we did not judge absence or inadequacy of blinding as imposing risk of bias. For other outcomes, including laboratory outcomes such as AHI, we required at least blinding of outcome assessors to permit a judgement of low risk of bias for this domain.
Where required, we attempted to contact study authors to check information on risk of bias. When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol (Pachito 2017).
Measures of treatment effect
We analysed dichotomous data as risks ratios (RRs) and continuous data as mean differences (MDs) or standardised mean differences (SMDs). If we combined data from rating scales in a meta‐analysis, we ensured that the data were entered with a consistent direction of effect (e.g. lower scores always indicate improvement).
We undertook meta‐analyses only when this was meaningful, that is if treatments, participants, and the underlying clinical question were similar enough for pooling to make sense. We planned to describe skewed data narratively (e.g. as medians and interquartile ranges for each group).
When multiple trial arms were reported in a single study, we included only the relevant arms. If two comparisons (e.g. intervention A versus placebo and intervention B versus placebo) were combined in the same meta‐analysis, we would combine the active arms, or halve the control group to avoid double‐counting.
If adjusted analyses were available (analysis of variance (ANOVA) or analysis of covariance (ANCOVA)), we used these as items of preference in our meta‐analyses. If both change‐from‐baseline and endpoint scores were available for continuous data, we used change from baseline. If a study reported outcomes at multiple time points, we used all time points. We used intention‐to‐treat (ITT) or 'full analysis set' analyses when these were reported (i.e. analyses in which data have been imputed for participants who were randomly assigned but did not complete the study) instead of completer or per‐protocol analyses.
Unit of analysis issues
For dichotomous outcomes, we used participants, rather than events, as the unit of analysis (i.e. number of participants admitted to hospital, rather than number of admissions per participant). However, if rate ratios were reported in a study, we planned to analyse them on this basis.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data when possible. When this was not possible, and missing data were thought to introduce serious bias, we took this into consideration in the GRADE rating for the affected outcomes, and planned to explore the impact of including such studies in the overall assessment of results by sensitivity analysis. We planned that if outcome data such as standard deviations or correlation coefficients were not available and could not be obtained from the trial authors, we would calculate these values from other available statistics such as P values, according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022).
Assessment of heterogeneity
We used the I2 statistic to measure heterogeneity in each analysis. If we identified substantial heterogeneity, we would report this and explore possible causes by performing prespecified subgroup analyses. We considered heterogeneity as substantial for values of I2 equal to or above 50% (Higgins 2011), although we recognise that uncertainty surrounds the I2 measurement when a meta‐analysis includes few studies. We used a significance level of P < 0.1 to indicate whether we observed a problem with heterogeneity.
Assessment of reporting biases
We planned that if we were able to pool more than 10 studies, we would create and examine a funnel plot to explore possible small‐study and publication biases.
Data synthesis
We used a random‐effects model to summarise pooled data.
Subgroup analysis and investigation of heterogeneity
If we found substantial heterogeneity and there were sufficient data, we would investigate possible causes for the heterogeneity by exploring the impact of the condition of participants using subgroup analyses. We planned to carry out the following subgroup analyses.
Severity of CSA based on cAHI, with two prespecified subgroups, namely mild CSA, defined as fewer than 15 central apnoeas per hour of sleep, and moderate to severe CSA, defined as equal or more than 15 central apnoeas per hour of sleep.
The rationale for this subgroup analysis was based on the assumption that treatment for CSA may have variable impact according to the severity of CSA.
Severity of chronic heart failure based on the functional classification of the New York Heart Association (Class IV versus others) (NYHA 1994), or based on the ejection fraction (< 30% versus other levels).
The rationale for scrutinising intervention effects regarding severity of chronic heart failure was based on findings of higher mortality rates amongst people with severe chronic heart failure treated with servo ventilation (SERVE HF 2015).
Cause of central sleep apnoea as defined by the ICSD, 3rd edition (American Academy of Sleep Medicine 2014), for comparisons involving heterogeneous populations.
We planned to use the following outcomes in subgroup analyses.
AHI.
Cardiovascular mortality.
We planned to use the formal test for subgroup interactions provided in Review Manager 5 (Review Manager 2020). However, we included few studies regarding these outcomes in meta‐analyses, with insufficient data to be explored, therefore these subgroup analyses could not be performed.
Sensitivity analysis
We planned to carry out the following sensitivity analyses whilst removing the following from analyses of primary outcomes.
For assessment of risk of bias (excluding studies with high risk of bias), we planned to consider studies as having a high risk of bias if they fulfilled criteria for high or unclear risk of bias in at least two risk of bias domains. However, these analyses were not possible because no studies included in the meta‐analyses were at low risk of bias.
For comparisons involving any type of NIPV versus inactive control (sham therapy, no treatment, or usual care), we planned to perform sensitivity analysis by including only studies that used sham therapy as the comparator, in order to account for the placebo effect. However, these analyses were not possible because none of the studies included in the meta‐analyses used sham therapy.
We assessed the role of industry sponsorship in identified studies.
We compared the results derived from a fixed‐effect model versus those obtained from a random‐effects model.
Summary of findings and assessment of the certainty of the evidence
We created summary of findings tables using the following outcomes.
AHI
Cardiovascular mortality
Quality of sleep
Quality of life
All‐cause mortality
Time to life‐saving cardiovascular intervention
Serious adverse events
We planned to include quality of sleep and serious adverse events; however, no studies evaluated these outcomes. When outcomes were reported at more than one time point, we presented the longer time point in the summary of findings table.
We used the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to studies that contribute data for the prespecified outcomes. We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), employing GRADEpro GDT software (GRADEpro GDT). We justified all decisions to downgrade the certainty of the evidence using footnotes, and made comments to aid the reader's understanding of the review when necessary.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
Our search conducted on 6 September 6 2021 yielded 2541 records. After excluding duplicate publications and irrelevant records, we identified 15 studies (29 reports) for inclusion in the review. A study flow diagram is presented in Figure 1.
1.
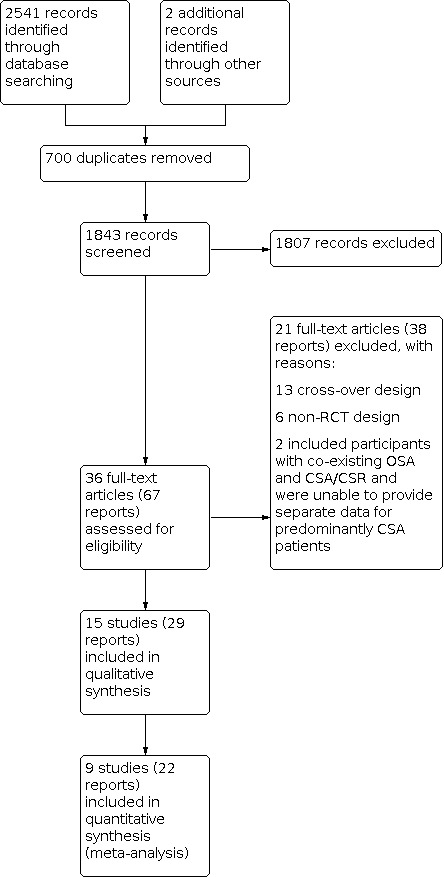
Study flow diagram.
Included studies
This review is based on a published protocol (Pachito 2017). An overview of the characteristics of the included studies is provided in Table 7. We identified three ongoing studies (see Characteristics of ongoing studies).
1. Study characteristics.
| Study | Participants | Intervention | Comparator | ||||||
| N, condition | CSA or CSR criteria | Age, mean (SD) | AHI, mean (SD) per hour/cAHI, mean % (SD) | BMI, mean (SD) | LVEF, mean % (SD) | Men n (%) | |||
| CANPAP 2006 | 258 participants with CSA associated with chronic heart failure | 15 or more episodes of apnoea and hypopnoea per hour of sleep, more than 50% of which were determined to be central | 63 (10) | 40 (16)/89a | 29.05a | 24.50 (7.70) | 248 (96.12) |
CPAP plus best supportive care | Best supportive care |
| Granton 1996 | 17 participants with CSA associated with chronic heart failure | CSR‐CSA with a cAHI of at least 10/h of sleep, alternating with a crescendo‐decrescendo pattern of hyperpnoea; at least 85% of apnoeas and hypopnoeas had to be central | 58.15a | 42a/NR | 27.15a | 22.30a | 17 (100) | CPAP plus best supportive care | Best supportive care |
| Naughton 1995a | 29 participants with CSA associated with chronic heart failure | CSR‐CSA, with a crescendo‐decrescendo pattern of hyperpnoea alternating with central apnoeas or hypopnoeas at a rate of > 10/h of sleep | 58.80a | 38.15a/NR | 26.55a | 20.45a | 29 (100) | CPAP plus best supportive care | Best supportive care |
| Naughton 1995b | 18 participants with CSA associated with chronic heart failure | CSR‐CSA, with a crescendo‐decrescendo pattern of hyperpnoea alternating with central apnoeas or hypopnoeas at a rate of > 10/h of sleep | 60.50 (1.40)b | 42.70 (4.60)b/NR | 25.80 (1.00)b | 18.30 (2.00)b | 18 (100) | CPAP plus best supportive care | Best supportive care |
| Sin 2000 | 29 participants with CSA associated with chronic heart failure | CSR‐CSA, with a crescendo‐decrescendo pattern of hyperpnoea, alternating with central apnoeas or hypopnoeas at a rate ≥ 15 per hour of sleep of which > 75% of events were central | 57.90 (9.70) | 39.20 (21.90)/NR | NR | 20.20 (10.00) | 29 (100) | CPAP plus best supportive care | Best supportive care |
| Kasai 2013 | 23 participants with CSA associated with chronic heart failure | AHI 15 events per hour, of which ≥ 50% were central | 65.05a | 47.85a/81.60a | 26.60a | 32.45a | 23 (100) | ASV | CPAP |
| Philippe 2006 | 25 participants with CSA associated with chronic heart failure | CSR, with a crescendo–decrescendo periodic ventilation and CSA with an AHI of 15/h of which more than 80% were central | 62.25a | 43.75a/NR | 27a | 29.50a | 25 (100) | ASV | CPAP |
| Fietze 2008 | 37 participants with CSA associated with chronic heart failure |
CSR with an RDI > 15/h, with less than 20% obstructive respiratory events | 58.90 (10.40) | 33.30a,c/ 32.20a,d | 28 (3.90) | 25.05a | 34 (91.89) | ASV | Bilevel ventilation |
| Morgenthaler 2014 | 66 participants with CPAP‐induced CSA | CPAP titration eliminating events defining OSA, but had a residual CAI ≥ 10 events/hour or residual CSR pattern that was predominant and disruptive during PSG | 59.20 (12.90) | 34.45a/ 23.70a,e | 35.00 (8.00) | NR | 56 (84.84) | ASV | CPAP |
| Dellweg 2013 | 37 participants with CPAP‐induced CSA | AHI ≥ 15 during the initial PSG with a predominance of obstructive events according to AASM criteria and AHI ≥ 15 on CPAP therapy after 6 weeks of CPAP treatment during a follow‐up PSG with a predominance of central events according to AASM criteria | 62.50a | 28.15a/ 17.45a,e | 30a | NR | 21 (70) | ASV | Bilevel ventilation |
| SERVE HF 2015 | 1325 participants with CSA associated with chronic heart failure | cAHI of ≥ 10 events/hour and AHI of ≥ 15 events/hour, of which > 50% were central | 69.45a | 31.45a/ 81.30a | 28.50a | 32.35a | 1198 (90.42) | ASV plus best supportive care | Best supportive care |
| Hetland 2013 | 51 participants with CSA associated with chronic heart failure | CSR of > 25%; CSB with a minimum of 3 consecutive cycles of a crescendo‐decrescendo pattern in the breathing signal with periods of hyperventilation separated by central apnoeas or hypopnoeas | 70.50a | 29.65a/65.50a,f | 27.15a | 31.80a | 47 (92.16) | ASV plus best supportive care | Best supportive care |
| Pepperell 2003 | 30 participants with CSA associated with chronic heart failure | > 50% of apnoeas or hypopnoea events were central | 71.15a | 24a/NR | 26.20a | 34.75a | 29 (96.67) | ASV plus best supportive care | Best supportive care |
| Toyama 2016 | 31 participants with CSA associated with chronic heart failure | CSR‐CSA, with at least 3 consecutive cycles of a cyclic crescendo‐decrescendo change in breathing and an AHI ≥ 5/h, or persistence of a cyclic crescendo‐decrescendo breathing pattern for at least 10 consecutive minutes | 68 (9) | 25.25a/37.85a,g | 24.10a | 26.50a | 28 (93.33) | ASV plus best supportive care | Best supportive care |
| D'Elia 2019 | 10 participants with CSA associated with acute heart failure | AHI > 15/h, of which > 50% were central | 69.90a | 36.90a/28a,e | 26.65a | 50.80a | 10 (100) | ASV plus best supportive care | Best supportive care |
Abbreviations: AASM: American Academy of Sleep Medicine; AHI: apnoea hypopnoea index; ASV: adaptive servo ventilation; BMI: body mass index; cAHI: central apnoea hypopnoea index; CAI: central apnoea index; CPAP: continuous positive airway pressure; CSA: central sleep apnoea; CSB: Cheyne‐Stokes breathing; CSR: Cheyne‐Stokes respiration; LVEF: left ventricular ejection fraction; NR: not reported; OSA: obstructive sleep apnoea; PSG: polysomnography; RDI: respiratory disturbance index; SD: standard deviation
aAs the study did not provide total measures, we calculated an average between means of experimental and control groups. bData expressed in standard error. cRespiratory disturbance index per hour calculated by the sum of Cheyne‐Stokes respiration index, obstructive apnoea index, and mixed apnoea index. dCheyne‐Stokes respiration index per hour. eCentral sleep apnoea index, events per hour. fCheyne‐Stokes respiration %. gCSA %.
We assessed 17 studies as awaiting classification (see Characteristics of studies awaiting classification). Six of these studies evaluated the effects of NIPV in people with sleep apnoea (Arzt 2013; CAT‐HF 2016; Egea 2004; Ushijima 2014; Yoshihisa 2013; Zhao 2006), and we contacted the authors to check the availability of separate data for CSA. Seven studies evaluated the effects of NIPV in people with CSA, but did not present results for the outcomes of interest in this review; we contacted the authors of these studies to check whether they assessed and could provide results for the outcomes of interest in this review (Alter 2013; Goldberg 2007; Miyata 2012; Noda 2007; Tkacova 1997; Toepfer 2003; Yoshihisa 2009). There was insufficient information to judge whether the remaining four studies fulfilled the eligibility criteria of this review. We contacted the authors to check whether these studies were eligible (De Michelis 2008; Murase 2016; Teschler 2000; Vogt‐Ladner 2002), but did not receive any responses.
Methods
We included 15 randomised, parallel‐group, two‐arm studies. The studies were performed from 1995 to 2019. We found protocols for only five of the 15 RCTs (CANPAP 2006; Dellweg 2013; Hetland 2013; Morgenthaler 2014; SERVE HF 2015), therefore for the majority of studies it was difficult to determine whether data were sought but not reported. Only four studies performed sample size calculations (CANPAP 2006; Dellweg 2013; Morgenthaler 2014; SERVE HF 2015). In five trials (CANPAP 2006; Dellweg 2013; Hetland 2013; Naughton 1995a; Philippe 2006), there were important losses of participants during follow‐up, with the following dropout: CANPAP 2006 (40/258), Dellweg 2013 (7/37), Hetland 2013 (21/51), Naughton 1995a (5/29), and Philippe 2006 (8/25). Only two of these studies performed an ITT analysis (CANPAP 2006; Morgenthaler 2014).
Setting
The trials included outpatient participants aged > 18 years old who were followed from one week to 60 months. The studies were conducted in Canada (Granton 1996; Naughton 1995a; Naughton 1995b; Sin 2000), the USA (Morgenthaler 2014), Japan (Kasai 2013; Toyama 2016), the UK (Pepperell 2003), Italy (D'Elia 2019), Germany (Dellweg 2013; Fietze 2008), France (Philippe 2006), and Norway (Hetland 2013); one multicentric study was conducted in Canada and Germany (CANPAP 2006), and the largest study was conducted in several countries including Australia, Switzerland, the Czech Republic, Germany, Denmark, Finland, France, the UK, the Netherlands, Norway, and Sweden (SERVE HF 2015).
Participants
We included 15 RCTs with a total of 1936 participants, of which 70% to 100% were men. The smallest study included 10 adult participants (D'Elia 2019), and the largest study included 1325 adult participants (mean 129 participants) (SERVE HF 2015). Most studies included people with CSA associated with chronic heart failure (CANPAP 2006; Fietze 2008; Granton 1996; Hetland 2013; Kasai 2013; Naughton 1995a; Naughton 1995b; Pepperell 2003; Philippe 2006; SERVE HF 2015; Sin 2000; Toyama 2016); one study included people with acute heart failure and preserved ejection fraction (D'Elia 2019); and two studies included people with CPAP‐induced CSA (Dellweg 2013; Morgenthaler 2014).
Interventions
The variability of participants, intervention types, controls, and follow‐up periods made it difficult to carry out a comprehensive meta‐analysis. For summarising the available evidence, we formulated different comparisons, being careful not to pool different populations, interventions, and follow‐up periods. We therefore performed seven separate comparisons, as follows.
-
Continuous positive airway pressure versus inactive control (subtherapeutic positive pressure, no treatment, or usual care):
CPAP plus best supportive care versus best supportive care alone (or inactive control) in CSA associated with chronic heart failure (five studies: CANPAP 2006; Granton 1996; Naughton 1995a; Naughton 1995b; Sin 2000).
-
Adaptive servo ventilation versus another type of non‐invasive positive pressure:
ASV versus CPAP in CSA associated with chronic heart failure (two studies: Kasai 2013; Philippe 2006);
ASV versus bilevel ventilation in CSA associated with chronic heart failure (one study: Fietze 2008);
ASV versus CPAP in CPAP‐induced CSA (one study: Morgenthaler 2014);
ASV versus bilevel ventilation in CPAP‐induced CSA (one study: Dellweg 2013).
-
Adaptive servo ventilation versus inactive control (subtherapeutic positive pressure, no treatment, or usual care):
ASV plus best supportive care versus best supportive care alone (or inactive control) in CSA associated with chronic heart failure (four studies: Hetland 2013; Pepperell 2003; SERVE HF 2015; Toyama 2016);
ASV plus best supportive care versus best supportive care alone (or inactive control) in people with CSA and acute heart failure with preserved ejection fraction (one study: D'Elia 2019).
Conflicts of interest and study funding
In D'Elia 2019, the authors declared that they did not have any conflict of interest. In Naughton 1995a and Naughton 1995b, the authors reported they did not receive grants from industry. In Toyama 2016, the authors also indicated that they had no financial conflict of interest. In nine studies (CANPAP 2006; Dellweg 2013; Granton 1996; Kasai 2013; Morgenthaler 2014; Pepperell 2003; Philippe 2006; SERVE HF 2015; Sin 2000), at least one author received grants from industry. In one manuscript (Fietze 2008), we found no report regarding possible author conflict of interest. In Hetland 2013, although the study received funding from industry, the authors reported that the sponsors had no role in study design, data collection and interpretation, manuscript writing, or the decision to submit for publication, and therefore they did not have any conflicts of interest.
Regarding study funding, eight of the 15 studies received financial support from an industry sponsor (CANPAP 2006; Fietze 2008; Hetland 2013; Morgenthaler 2014; Naughton 1995a; Pepperell 2003; Philippe 2006; SERVE HF 2015). Four studies received other support: Granton 1996 and Naughton 1995b were supported by operating grants from both the Ontario Ministry of Health and the Medical Research Council of Canada; Sin 2000 was supported by an operating grant from the Medical Research Council of Canada only; and Kasai 2013 was funded by grants from the Okinaka Memorial Institute for Medical Research in Tokyo, Japan. The authors of Dellweg 2013 declared that their study was not funded by industry sponsors but did not report whether it had received other support. In the other two manuscripts (D'Elia 2019; Toyama 2016), we found no report regarding possible funding sources.
Outcomes
Regarding our primary outcomes, only four trials evaluated central AHI (CANPAP 2006; Dellweg 2013; Fietze 2008; Morgenthaler 2014); two trials evaluated cardiovascular mortality (CANPAP 2006; SERVE HF 2015); and only the authors of the CANPAP 2006 trial reported on serious adverse effects, finding that none occurred in participants randomised to CPAP within one month of its initiation; however, results for the best supportive care study arm were not reported.
Regarding our secondary outcomes, most trials (11/15) evaluated AHI (CANPAP 2006; Dellweg 2013; Fietze 2008; Granton 1996; Kasai 2013; Morgenthaler 2014; Naughton 1995a; Naughton 1995b; Pepperell 2003; Philippe 2006; Toyama 2016). However, only three trials evaluated quality of life (Hetland 2013; Morgenthaler 2014; SERVE HF 2015); four evaluated all‐cause mortality (CANPAP 2006; Hetland 2013; SERVE HF 2015; Sin 2000); and one evaluated time to life‐saving intervention (CANPAP 2006). Although SERVE HF 2015 reported the composite endpoint of time to first event of death from any cause, life‐saving cardiovascular intervention, or unplanned hospital admission for worsening heart failure, the results of time to life‐saving cardiovascular intervention were not reported separately. Regarding non‐serious adverse events, although Fietze 2008 and Naughton 1995a mention that they did not detect any adverse events of treatment, they did not provide the data for the control group. Only one study evaluated the effects of ASV plus best supportive care versus best supportive care alone in 10 consecutive acute heart failure with preserved ejection fraction (HFpEF) (left ventricle ejection fraction (LVEF) ≥ 45%) patients with CSA (D'Elia 2019). The authors reported that no adverse events were recorded in either group. No studies evaluated quality of sleep.
Excluded studies
We excluded 21 full‐text articles; the reasons for their exclusion are described in the Characteristics of excluded studies table. Six studies did not have an RCT design (Arzt 2005; Hetland 2014; Hetland 2017; Hetzenecker 2016; Krachman 2003; Priefert 2017); two studies included people with co‐existing OSA and CSA/Cheyne‐Stokes respiration and did not separate data for predominantly CSA patients (Kasai 2010; Randerath 2012); and 13 studies were cross‐over studies (Campbell 2012; Cao 2014; Galetke 2014; Hu 2005; Javaheri 2011; Kohnlein 2002; Krachman 1999; Liu 2015; Morgenthaler 2007; Oldenburg 2015; Shapiro 2015; Szollosi 2006; Teschler 2001).
Risk of bias in included studies
Our risk of bias evaluations for each included study are presented in the risk of bias tables in Characteristics of included studies. Review authors' judgements on each risk of bias domain of the included studies are presented in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Only two studies described their randomisation sequence generation methods (CANPAP 2006; SERVE HF 2015); we assessed the remaining studies as at unclear risk of bias for this domain. Only two studies clearly described their methods of concealing the allocation (CANPAP 2006; Pepperell 2003); we assessed the remaining studies as at unclear risk of bias for allocation concealment.
Blinding
In nine studies, participants and treating physicians were not blinded (CANPAP 2006; D'Elia 2019; Hetland 2013; Kasai 2013; Morgenthaler 2014; Naughton 1995a; Naughton 1995b; Philippe 2006; SERVE HF 2015); we assessed these studies as at high risk of performance bias. We assessed the remaining studies as at low risk of performance bias.
For detection bias, we judged subjective or self‐reported outcomes separately from other outcomes. Only nine trials evaluated subjective outcomes (CANPAP 2006; D'Elia 2019; Hetland 2013; Kasai 2013; Morgenthaler 2014; Naughton 1995a; Pepperell 2003; Philippe 2006; SERVE HF 2015); we assessed all of these trials as having a high risk of bias because blinding was not performed, and the lack of blinding was likely to influence the outcome results.
Regarding other outcomes, one study did not have blind outcome assessors, and we considered the lack of blinding likely to influence the results (Dellweg 2013). Four trials provided insufficient information to permit a judgement of whether outcome assessors were blind; we therefore assessed these studies as at unclear risk of detection bias (Fietze 2008; Granton 1996; Morgenthaler 2014; Toyama 2016). In all other trials, the outcome assessors were blind, and the trials were judged as having a low risk of bias.
Incomplete outcome data
In six trials (CANPAP 2006; Dellweg 2013; Hetland 2013; Naughton 1995a; Philippe 2006; SERVE HF 2015), there were important losses of participants during follow‐up; we assessed these trials as at high risk of attrition bias. Three studies provided insufficient information to permit a judgement of whether there were important losses, so these studies were classified as having an unclear risk of bias (D'Elia 2019; Fietze 2008; Granton 1996). In the remaining trials, there were no large numbers of losses, and when there were losses, dropouts were reported, along with the reasons for the dropouts. We therefore assessed these trials as at low risk of bias.
Selective reporting
One trial registered the protocol retrospectively (CANPAP 2006), and one trial did not describe the results for all outcomes planned in the protocol (Hetland 2013); we assessed these trials as at high risk of reporting bias. Three trials reported all prespecified outcomes and were therefore assessed as at low risk of reporting bias (Dellweg 2013; Morgenthaler 2014; SERVE HF 2015). The protocols were not available for the remaining 10 trials, therefore we assessed these studies as at unclear risk of bias.
Other potential sources of bias
We noted no other potential sources of bias in any of the included studies, therefore we rated all studies as at low risk of other bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
The results for the outcomes of interest for this review are reported below. We planned to perform subgroup analyses considering the severity of CSA, severity of chronic heart failure, and cause of CSA, using AHI and cardiovascular mortality outcomes. However, as few studies were meta‐analysed for these outcomes, and the studies were homogeneous regarding these characteristics, it was not possible to perform any of the planned subgroup analyses.
Continuous positive airway pressure versus inactive control (subtherapeutic positive pressure, no treatment, or usual care)
CPAP plus best supportive care versus best supportive care alone (or inactive control) in CSA associated with chronic heart failure
Primary outcomes
Central apnoea hypopnoea index ‐ Short term
CPAP plus best supportive care may reduce cAHI (mean difference (MD) −14.60, 95% confidence interval (CI) −20.11 to −9.09; 1 study; 205 participants) (CANPAP 2006). The certainty of evidence was low due to serious risk of bias and imprecision (Table 1).
Cardiovascular mortality ‐ Long term
In the long term, CPAP plus best supportive care may result in little to no difference in cardiovascular mortality compared to best supportive care alone (risk ratio (RR) 1.17, 95% CI 0.68 to 2.02; 1 study; 258 participants; low‐certainty evidence due to serious risk of bias and imprecision) (CANPAP 2006).
Serious adverse events ‐ Short term
The authors of CANPAP 2006 state in their trial that no serious adverse effects occurred in participants randomised to CPAP within one month of its initiation; however, results for the best supportive care study arm are not reported.
Secondary outcomes
Quality of sleep
This outcome was not reported.
Quality of life
This outcome was not reported.
Apnoea hypopnoea index ‐ Short term
CPAP plus best supportive care may reduce AHI (MD −15.27, 95% CI −22.44 to −8.11; 4 studies; 264 participants; I2 = 24%, favouring CPAP plus best supportive care; Analysis 1.1). As planned, we performed a sensitivity analysis to compare the results derived from a fixed‐effect model versus those obtained from a random‐effects model, and the results remained similar (MD −17.23, 95% CI −22.25 to −12.22; 4 studies; 264 participants; I2 = 24%). We also performed a sensitivity analysis to compare the results derived from industry‐sponsored trials. We found different results between industry‐sponsored (MD −18.86, 95% CI −24.25 to −13.48; 2 studies; 229 participants; I2 = 0%) and non‐industry‐sponsored trials (MD −6.56, 95% CI −20.34 to 7.22; 2 studies; 35 participants; I2 =0%; Analysis 1.1). Whilst the results of industry‐sponsored trials show that CPAP plus best supportive care may reduce AHI, the results of non‐industry‐sponsored trials show that CPAP plus best supportive care may result in little to no difference in AHI in participants with CSA associated with chronic heart failure.
1.1. Analysis.

Comparison 1: CPAP plus best supportive care versus best supportive care (or inactive control) in CSA associated with chronic heart failure, Outcome 1: AHI ‐ short term
Apnoea hypopnoea index ‐ Long term
In the long term, we found low‐certainty evidence (due to serious risk of bias and imprecision) that CPAP plus best supportive care may reduce AHI compared to best supportive care alone (MD −15.99, 95% CI −23.06 to −8.92; 1 study; 258 participants) (CANPAP 2006).
All‐cause mortality ‐ Long term
The evidence is very uncertain (due to serious risk of bias and very serious imprecision) about the effect of CPAP plus best supportive care on all‐cause mortality in the long term (RR 1.14, 95% CI 0.83 to 1.56; 2 studies; 287 participants; Analysis 1.2).
1.2. Analysis.

Comparison 1: CPAP plus best supportive care versus best supportive care (or inactive control) in CSA associated with chronic heart failure, Outcome 2: All‐cause mortality ‐ long term
Time to life‐saving cardiovascular intervention
In CANPAP 2006, after six months there was an early divergence in the event rates that favoured the control group (hazard ratio (HR) for transplantation‐free survival, 1.5; P = 0.02) that was altered after 18 months to favour the CPAP group (HR for transplantation‐free survival, 0.66; P = 0.06). Due to the early divergence in the event rates, enrolment at only 50% of the predicted rate, and a falling rate of death and heart transplantation that precluded the ability to detect a difference in the primary outcome between the two groups, the data and safety monitoring committee recommended termination of the study. The executive committee therefore stopped the trial on 21 May 2004.
Adverse events/side effects
Only one trial mentioned that no immediate or long‐term adverse effects of nasal CPAP were observed (Naughton 1995a); however, results for the best supportive care study arm are not reported.
Adaptive servo ventilation versus another type of non‐invasive positive pressure
ASV versus CPAP in CSA associated with chronic heart failure
Primary outcomes
Central apnoea hypopnoea index ‐ Short term
This outcome was not reported.
Cardiovascular mortality
This outcome was not reported.
Serious adverse events
This outcome was not reported, and it is not clear whether these data were sought but not found.
Secondary outcomes
Quality of sleep
This outcome was not reported.
Quality of life
This outcome was not reported.
Quality of life ‐ Short and medium term
The evidence is very uncertain (due to serious risk of bias and very serious imprecision) (Table 2) about the effect of ASV versus CPAP on quality of life evaluated in the short term (MD 1.20, 95% CI −11.47 to 13.87; 1 study; 20 participants) and medium term (MD −8.64, 95% CI −25.56 to 8.28; 1 study; 17 participants) (Philippe 2006).
Apnoea hypopnoea index ‐ Short term
In the short term, ASV may reduce AHI compared to CPAP ventilation (MD −20.37, 95% CI −25.32 to −15.41; Analysis 2.1).
2.1. Analysis.

Comparison 2: ASV versus CPAP in CSA associated with chronic heart failure, Outcome 1: AHI ‐ short term
Apnoea hypopnoea index ‐ Medium term
Only one study evaluated AHI in the medium term (Philippe 2006). We found low‐certainty evidence (due to serious risk of bias and imprecision) that ASV may reduce AHI compared to CPAP (MD −28.67, 95% CI −48.28 to −9.06; 1 study; 17 participants) (Philippe 2006).
All‐cause mortality
This outcome was not reported.
Time to life‐saving intervention
This outcome was not reported.
Adverse events/side effects
This outcome was not reported, and it is not clear whether these data were sought but not found.
ASV versus bilevel ventilation in CSA associated with chronic heart failure
Primary outcomes
Central apnoea hypopnoea index ‐ Short term
We found low‐certainty evidence (due to serious risk of bias and imprecision) (Table 3) of no difference between ASV and bilevel ventilation in cAHI in the short term (MD 0.90, 95% CI −2.40 to 4.20; 1 study; 30 participants) (Fietze 2008).
Cardiovascular mortality
This outcome was not reported.
Serious adverse events
The authors of one study reported that they did not detect any adverse events of treatment, but did not provide data for the control group (Fietze 2008).
Secondary outcomes
Quality of sleep
This outcome was not reported.
Quality of life
This outcome was not reported.
Apnoea hypopnoea index ‐ Short term
In the short term, the evidence is very uncertain (due to serious risk of bias and very serious imprecision) about the effect of ASV on total AHI (MD −5.20, 95% CI −14.63 to 4.23; 1 study; 30 participants) (Fietze 2008).
All‐cause mortality
This outcome was not reported.
Time to life‐saving intervention
This outcome was not reported.
Adverse events/side effects
The authors of one study reported that they did not detect any adverse events of treatment, but did not provide data for the control group (Fietze 2008).
ASV versus CPAP maintenance in CPAP‐induced CSA
Primary outcomes
Central apnoea hypopnoea index ‐ Short term
We found low‐certainty evidence (due to serious risk of bias and imprecision) (Table 4) that ASV may slightly reduce cAHI compared to CPAP ventilation in the short term (MD −4.10, 95% CI −6.67 to −1.53; 1 study; 60 participants) (Morgenthaler 2014).
Cardiovascular mortality
This outcome was not reported.
Serious adverse events
This outcome was not reported, and it is not clear whether these data were sought but not found.
Secondary outcomes
Quality of sleep
This outcome was not reported.
Quality of life ‐ Short term
In the short term, ASV may result in little to no difference in quality of life (MD 0.00, 95% CI −0.46 to 0.46; 1 study; 61 participants; low‐certainty evidence due to serious risk of bias and imprecision) (Morgenthaler 2014).
Apnoea hypopnoea index ‐ Short term
We found low‐certainty evidence (due to serious risk of bias and imprecision) that ASV may slightly reduce AHI in the short term (MD −5.50, 95% CI −10.74 to −0.26; 1 study; 60 participants) (Morgenthaler 2014).
All‐cause mortality
This outcome was not reported.
Time to life‐saving intervention
This outcome was not reported.
Adverse events/side effects
This outcome was not reported, and it is not clear whether these data were sought but not found.
ASV versus bilevel ventilation in CPAP‐induced CSA
Primary outcomes
Central apnoea hypopnoea index ‐ Short term
We found low‐certainty evidence (due to serious risk of bias and imprecision) (Table 5) that ASV may slightly reduce cAHI compared to bilevel ventilation in the short term (MD −8.70, 95% CI −11.42 to −5.98; 1 study; 30 participants) (Dellweg 2013).
Cardiovascular mortality
This outcome was not reported.
Serious adverse events
This outcome was not reported, and it is not clear whether these data were sought but not found.
Secondary outcomes
Quality of sleep
This outcome was not reported.
Quality of life
This outcome was not reported.
Apnoea hypopnoea index ‐ Short term
We found low‐certainty evidence (due to serious risk of bias and imprecision) that ASV may slightly reduce AHI compared to bilevel ventilation in the short term (MD −9.10, 95% CI −13.67 to −4.53; 1 study; 30 participants) (Dellweg 2013).
All‐cause mortality
This outcome was not reported.
Time to life‐saving intervention
This outcome was not reported.
Adverse events/side effects
This outcome was not reported, and it is not clear whether these data were sought but not found.
Adaptive servo‐ventilation versus inactive control (subtherapeutic positive pressure, no treatment, or usual care)
ASV plus best supportive care versus best supportive care alone (or inactive control) in CSA associated with chronic heart failure
Primary outcomes
Central apnoea hypopnoea index
This outcome was not reported.
Cardiovascular mortality ‐ Long term
We found moderate‐certainty evidence (due to serious risk of bias) (Table 6) that ASV plus best supportive care likely increases cardiovascular mortality compared to best supportive care in the long term (RR 1.25, 95% CI 1.04 to 1.49; 1 study; 1325 participants) (SERVE HF 2015).
Serious adverse events
This outcome was evaluated but not reported.
Secondary outcomes
Quality of sleep
This outcome was not reported.
Quality of life ‐ Short, medium, and long term
ASV plus best supportive care may result in little to no difference in quality of life in the short term (MD −0.04, 95% CI −2.31 to 2.23; 2 studies; 1355 participants; Analysis 3.1; Hetland 2013; SERVE HF 2015) and medium term (MD 0.34, 95% CI −2.11 to 2.79; 1 study; 1325 participants; SERVE HF 2015). ASV plus best supportive care may also result in little to no difference in quality of life in the long term (MD 0.83, 95% CI −1.81 to 3.47; 1 study; 1325 participants; low‐certainty evidence; SERVE HF 2015).
3.1. Analysis.

Comparison 3: ASV plus best supportive care versus best supportive care alone (or inactive control) in CSA associated with chronic heart failure, Outcome 1: Quality of life ‐ short term
Apnoea hypopnoea index ‐ Short term
We found low‐certainty evidence (due to serious risk of bias and imprecision) that ASV plus best supportive care may reduce AHI compared to best supportive care alone in the short term (MD −9.30, 95% CI −16.50 to −2.10; 1 study; 30 participants; Pepperell 2003).
Apnoea hypopnoea index ‐ Medium term
We found low‐certainty evidence (due to serious risk of bias and imprecision) that ASV plus best supportive care may reduce AHI compared to best supportive care alone in the medium term (MD −20.30, 95% CI −28.75 to −11.85; 1 study; 30 participants; Toyama 2016).
All‐cause mortality ‐ Medium term
In the medium term, ASV plus best supportive care may result in little to no difference in all‐cause mortality (RR 0.33, 95% CI 0.01 to 7.58; 1 study; 30 participants; Hetland 2013).
All‐cause mortality ‐ Long term
In the long term, ASV plus best supportive care may result in little to no difference in all‐cause mortality (RR 0.56, 95% CI 0.06 to 4.93; 2 studies; 1355 participants; low‐certainty evidence (due to serious risk of bias and imprecision); Analysis 3.2) (Hetland 2013; SERVE HF 2015). Of note, I2 for this analysis was 63%. As only two studies evaluated this outcome, no subgroup analysis could be performed to investigate causes of heterogeneity.
3.2. Analysis.

Comparison 3: ASV plus best supportive care versus best supportive care alone (or inactive control) in CSA associated with chronic heart failure, Outcome 2: All‐cause mortality ‐ long term
Time to life‐saving cardiovascular intervention
The results of time to life‐saving cardiovascular intervention were not reported separately.
Adverse events/side effects
This outcome was evaluated but not reported.
ASV plus best supportive care versus best supportive care alone (or inactive control) in CSA with acute heart failure with preserved ejection fraction
Primary outcomes
Central apnoea hypopnoea index
This outcome was not reported.
Cardiovascular mortality
This outcome was not reported.
Serious adverse events
The authors of one study reported that no adverse events were recorded in either group (D'Elia 2019).
Secondary outcomes
Quality of sleep
This outcome was not reported.
Quality of life
This outcome was not reported.
Apnoea hypopnoea index
This outcome was not reported.
All‐cause mortality
This outcome was not reported.
Time to life‐saving intervention
This outcome was not reported.
Adverse events/side effects
The authors of one study reported that no adverse events were recorded in either group (D'Elia 2019).
Discussion
Summary of main results
In this systematic review, we summarised the available evidence on the effects of non‐invasive positive pressure ventilation in people with CSA. We found 15 RCTs, including a wide range of treatment techniques using non‐invasive ventilation, with several configurations of parameters, and few data that could be pooled. Most participants were men and had CSA associated with chronic heart failure.
When comparing CPAP plus best supportive care versus best supportive care (or inactive control), we found that CPAP plus best supportive care may reduce cAHI in the short term, and may reduce AHI in the short and long term. However, CPAP plus best supportive care may result in little to no difference in cardiovascular mortality, and the evidence is very uncertain about its effects on all‐cause mortality in the long term. One trial mentioned that no immediate or long‐term adverse effects of nasal CPAP were observed (Naughton 1995a); however, no results for the best supportive care study arm were reported. Of note, whilst the results of industry‐sponsored trials showed that CPAP plus best supportive care may reduce AHI, the results of non‐industry‐sponsored trials showed that CPAP plus best supportive care may result in little to no difference in AHI in participants with CSA associated with chronic heart failure. As few studies were included in this analysis, there may be important imprecision in the effect estimates. The inclusion of further trials in future updates of this review may therefore clarify whether industry sponsorship can influence the results.
In people with CSA associated with chronic heart failure, ASV may result in little to no difference in cAHI compared to bilevel ventilation. We are uncertain about the effect of ASV on total AHI when compared to bilevel ventilation. However, in people with CPAP‐induced CSA, ASV may slightly reduce cAHI and AHI in the short term compared to bilevel ventilation. Adverse events were also not reported in these participants. No trials compared the effect of ASV on cardiovascular mortality versus bilevel ventilation.
Compared to CPAP, ASV may reduce AHI in the short and medium term in people with CSA associated with chronic heart failure. However, the evidence is uncertain about the effect of ASV versus CPAP on quality of life in the short and medium term. Of note, the reduction in AHI for ASV is probably a consequence of the back‐up functionality inherent to this mode, which aims to assure a target minute ventilation. This finding should therefore be interpreted with caution, as it may not reflect a long‐lasting physiological effect. In CPAP‐induced CSA, we found that ASV may slightly reduce cAHI and AHI, but may result in little to no difference in quality of life in the short term. CPAP‐induced CSA may be caused by unstable ventilatory control with a change of ventilatory drive (Moura 2001). This condition appears to resolve spontaneously over time in some but not all patients during continued CPAP therapy (Javaheri 2009; Kuźniar 2008a)
Interestingly, although we found that ASV plus best supportive care may reduce AHI in the short and medium term compared to best supportive care alone, we found moderate‐certainty evidence indicating that ASV likely increases cardiovascular mortality compared to best supportive care alone in people with CSA associated with chronic heart failure. Additionally, ASV plus best supportive care may result in little to no difference in quality of life in the short, medium, and long term, and in all‐cause mortality in the medium and long term. It should be mentioned that the finding regarding cardiovascular mortality was derived from a single study, although with a large sample size, and that all other outcomes for the comparison ASV plus best supportive care versus best supportive care alone were also largely influenced by this trial (SERVE HF 2015). Also, only people with low ejection fraction were included in the one study that assessed cardiovascular mortality (SERVE HF 2015), and some methodological limitations related to this study reduced our certainty in the evidence. Nevertheless, SERVE HF 2015 was a multicentre RCT with a long‐term follow‐up, and probably provides precise effects estimates for the outcomes assessed in this subgroup of patients.
Of note, the differences favouring NIPV are related to potential improvements in surrogate markers only, such as laboratory measurements, but not to patient‐centred outcomes, such as quality of sleep, quality of life, or longer‐term survival. For example, a mean difference of 15 hypoventilation episodes per hour between CPAP plus best supportive care and best supportive care in people with CSA and chronic heart failure, that is one event every four minutes, as found in this review, could be interpreted by some clinicians as a dramatic clinical improvement. However, there are currently no data to define minimal clinically important differences for AHI in CSA patients. In the absence of data showing a favourable impact on meaningful patient‐centred outcomes, these findings need to be interpreted with caution.
Overall completeness and applicability of evidence
As most of the studies included in this systematic review included men and people with CSA associated with chronic heart failure, the generalisability of our results is mostly limited to individuals with these characteristics. Although we found some evidence on people with CSA associated with other conditions, such as CPAP‐induced CSA, further trials are needed including these individuals.
In this review, we were cautious about analysing data and did not pool results of studies comparing different modes of NIPV and including participants with CSA with different causes. Despite this, we believe not only the mode, but also the parameters of NIPV and the interface used to perform the NIPV therapy may also influence the effect estimates of the interventions. Due to several methodological limitations in the included studies, a number of questions still persist, and the evidence found in this review is therefore of moderate to very low certainty.
Quality of the evidence
We included 15 RCTs with a total of 1936 participants in this review. The evidence found in the review was of moderate to very low certainty, therefore results should be interpreted in light of the confidence in the effects estimated for each outcome. For example, we found moderate‐certainty evidence suggesting that ASV plus best supportive care likely increases cardiovascular mortality in people with CSA associated with chronic heart failure. For this result, the true effect is likely to be close to the effect we estimated, but there is a possibility that it is substantially different. For evidence of low certainty, our confidence in the effect estimate is limited, and the true effect may be substantially different from the effect we estimated. For evidence of very low certainty, we have very little confidence in the effect estimate, and the true effect is likely to be substantially different from the estimate of effect.
We downgraded the certainty of evidence due to methodological limitations and imprecision in the effects estimated. As most of the included studies were assessed as unclear and high risk of bias for several domains, we downgraded the certainty of evidence due to methodological limitations in the included studies for all outcomes. Finally, clinical heterogeneity precluded a large meta‐analysis, and where the effects estimated were derived from small sample sizes or had large confidence intervals, we also downgraded the certainty of evidence for imprecision. Nevertheless, we believe this scientifically rigorous systematic review provides critical data to support further trials as well as to expose literature gaps.
Potential biases in the review process
We conducted this review according to our prespecified protocol (Pachito 2017), and following the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We performed sensitive searches in the most important databases and clinical trial registers and manual searches to identify and collect all relevant RCTs. Although we identified several trials, including conference abstracts, some studies lacked sufficient data to permit inclusion or exclusion; we assessed these trials as awaiting classification. The body of the available evidence may therefore be larger than the evidence presented in our systematic review. We also could not design a funnel plot to assess publication bias due to an insufficient number of RCTs included in the meta‐analyses. Many data were only available in incomplete formats in graphs, and our attempts to contact several authors did not receive a response in most cases.
Agreements and disagreements with other studies or reviews
We found a previous systematic review that evaluated the effects of NIPV in people with CSA and heart failure (Nakamura 2015). Besides not including participants without heart failure, which we did, Nakamura’s review also included studies with both OSA and CSA patients, which we did not include in our review. Nakamura 2015 suggested that NIPV therapy significantly reduced mortality compared to control. In our systematic review, the certainty of evidence prevented any conclusions as to whether any type of NIPV leads to clinically important benefits in people with CSA. Conversely, we found that the addition of ASV to best supportive care may lead to greater harm than benefit. Besides being more up‐to‐date, our searches were broader, included more databases, and included studies published irrespective of language or publication status. The Nakamura 2015 authors only included trials published in English. We therefore provide a more comprehensive, rigorously conducted, up‐to‐date review on the effects of NIPV in people with CSA with or without heart failure, which could justify the differences between our results and those reported by Nakamura.
Authors' conclusions
Implications for practice.
We found low‐certainty evidence suggesting that continuous positive airway pressure (CPAP) plus best supportive care may reduce central apnoea hypopnoea index (AHI) in people with central sleep apnoea (CSA) associated with chronic heart failure compared to best supportive care alone. Low‐certainty evidence suggests that ASV plus best supportive care may also reduce AHI in these individuals compared to best supportive care alone. Despite this, the results on the effects of adaptive servo ventilation (ASV) in cardiovascular mortality in people with CSA associated with chronic heart failure are controversial and indicate that ASV plus best supportive care likely increases cardiovascular mortality in these patients. In people with CPAP‐induced CSA, low‐certainty evidence suggests that ASV may slightly reduce central AHI and AHI compared to bilevel ventilation and to CPAP. In light of the existing evidence and the heterogeneity of participants with CSA, we cannot draw any conclusions as to whether any single ventilatory strategy is advantageous over other interventions for any particular CSA patient group. Non‐invasive positive pressure ventilation (NIPV) treatment approaches need to be individualised with appropriate follow‐up arrangements and evaluation of clinical response and potential adverse effects. Further high‐quality trials with long‐term follow‐up are needed to determine whether one mode of NIPV is safer or more effective than another or than best supportive care for the treatment of people with CSA.
Implications for research.
As most trials included in this review used different modes of NIPV, in people with different causes of CSA, we were not able to perform a comprehensive meta‐analysis. More high‐quality randomised controlled trials are needed, using similar NIPV sets and modes, for treating participants with predominantly CSA. Additionally, studies with large samples or approximately 200 more events in the experimental intervention group and the comparator intervention group are required. These trials should not include people with obstructive sleep apnoea (OSA) or, if people with OSA are included, data from participants with CSA should be provided separately from those with OSA. Additionally, trials should preferably not mix the results of people with and without chronic heart failure. Furthermore, there is a special need for future studies to focus on patient‐centred outcomes, such as quality of life, quality of sleep, and longer‐term survival. Finally, a thorough, systematic evaluation of adverse effects in the short, medium, and long term is needed.
What's new
| Date | Event | Description |
|---|---|---|
| 24 October 2022 | Amended | Republished to correct error in author order. |
History
Protocol first published: Issue 11, 2017 Review first published: Issue 10, 2022
Acknowledgements
We sincerely thank Elizabeth Stovold for developing and running part of the searches embedded in this review.
We would also like to thank Emma Jackson, Emma Dennett, and the Cochrane Airways Editorial Group for their assistance in the development of this review. The authors and Cochrane Airways’ Editorial Team are grateful to Kris Bauchmuller (UK) and Alastair Glossop (UK) for their peer‐review comments.
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
This project was supported by the National Institute for Health and Care Research (NIHR), via Cochrane Infrastructure funding to Cochrane Airways. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health and Social Care.
Appendices
Appendix 1. Database search strategies
| Database/search platform/date of last search | Search strategy |
| Airways Register (via Cochrane Register of Studies) Date of most recent search: 6 September 2021 | #1 MESH DESCRIPTOR Sleep Apnea, Central #2 (central NEAR sleep NEAR (apnea* or apnoea*)) #3 central NEAR alveolar NEAR hypoventilation* #4 central NEAR sleep disordered breathing #5 (ondine* NEAR (syndrome or curse)) #6 MESH DESCRIPTOR Cheyne‐Stokes Respiration #7 Cheyne* Stokes #8 (periodic NEAR (breathing or respiration)) #9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 #10 MESH DESCRIPTOR Continuous Positive Airway Pressure #11 CPAP:ti,ab,kw #12 bipap:ti,ab,kw #13 nippv:ti,ab,kw #14 nppv:ti,ab,kw #15 niv:ti,ab,kw #16 aprv:ti,ab,kw #17 ippv:ti,ab,kw #18 nCPAP:ti,ab,kw #19 airway pressure release ventilation:ti,ab,kw #20 servo ventilation:ti,ab,kw #21 servoventilation:ti,ab,kw #22 MESH DESCRIPTOR Noninvasive Ventilation #23 noninvasive ventilation:ti,ab,kw #24 non‐invasive ventilation:ti,ab,kw #25 positive‐pressure:ti,ab,kw #26 positive airway pressure:ti,ab,kw #27 #26 OR #25 OR #24 OR #23 OR #22 OR #21 OR #20 OR #18 OR #19 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 OR #10 #28 #27 AND #9 #29 INREGISTER #30 #29 AND #28 |
| CENTRAL (via Cochrane Register of Studies) Date of most recent search: 6 September 2021 | 1 MESH DESCRIPTOR Sleep Apnea, Central AND CENTRAL:TARGET 2 (central NEAR sleep NEAR (apnea* or apnoea*)) AND CENTRAL:TARGET 3 central NEAR alveolar NEAR hypoventilation* AND CENTRAL:TARGET 4 central NEAR sleep disordered breathing AND CENTRAL:TARGET 5 (ondine* NEAR (syndrome or curse)) AND CENTRAL:TARGET 6 MESH DESCRIPTOR Cheyne‐Stokes Respiration AND CENTRAL:TARGET 7 Cheyne* Stokes AND CENTRAL:TARGET 8 (periodic NEAR (breathing or respiration)) AND CENTRAL:TARGET 9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 10 MESH DESCRIPTOR Continuous Positive Airway Pressure AND CENTRAL:TARGET 11 CPAP:ti,ab,kw AND CENTRAL:TARGET 12 bipap:ti,ab,kw AND CENTRAL:TARGET 13 nippv:ti,ab,kw AND CENTRAL:TARGET 14 nppv:ti,ab,kw AND CENTRAL:TARGET 15 niv:ti,ab,kw AND CENTRAL:TARGET 16 aprv:ti,ab,kw AND CENTRAL:TARGET 17 ippv:ti,ab,kw AND CENTRAL:TARGET 18 nCPAP:ti,ab,kw AND CENTRAL:TARGET 19 airway pressure release ventilation:ti,ab,kw AND CENTRAL:TARGET 20 servo ventilation:ti,ab,kw AND CENTRAL:TARGET 21 servoventilation:ti,ab,kw AND CENTRAL:TARGET 22 MESH DESCRIPTOR Noninvasive Ventilation AND CENTRAL:TARGET 23 noninvasive ventilation:ti,ab,kw AND CENTRAL:TARGET 24 non‐invasive ventilation:ti,ab,kw AND CENTRAL:TARGET 25 positive‐pressure:ti,ab,kw AND CENTRAL:TARGET 26 positive airway pressure:ti,ab,kw AND CENTRAL:TARGET 27 #26 OR #25 OR #24 OR #23 OR #22 OR #21 OR #20 OR #18 OR #19 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 OR #10 28 #27 AND #9 |
| MEDLINE (Ovid) ALL Date of most recent search: 6 September 2021 | 1. Sleep Apnea, Central/ 2. (central adj2 sleep adj2 (apnea$ or apnoea$)).tw. 3. (central adj2 alveolar adj2 hypoventilation$).tw. 4. (central adj2 sleep disordered breathing).tw. 5. (ondine$ adj2 (syndrome or curse)).tw. 6. Cheyne‐Stokes Respiration/ 7. Cheyne$ Stokes.tw. 8. (periodic adj2 (breathing or respiration)).tw. 9. or/1‐8 10. Continuous Positive Airway Pressure/ 11. CPAP.tw. 12. bipap.tw. 13. nippv.tw. 14. nppv.tw. 15. niv.tw. 16. aprv.tw. 17. ippv.tw. 18. nCPAP.tw. 19. airway pressure release ventilation.tw. 20. servo ventilation.tw. 21. servoventilation.tw. 22. Noninvasive Ventilation/ 23. noninvasive ventilation$.tw. 24. non‐invasive ventilation$.tw. 25. positive‐pressure$.tw. 26. positive airway pressure.tw. 27. or/10‐26 28. 9 and 27 29. (controlled clinical trial or randomized controlled trial).pt. 30. (randomized or randomised).ab,ti. 31. placebo.ab,ti. 32. dt.fs. 33. randomly.ab,ti. 34. trial.ab,ti. 35. groups.ab,patency. 36. or/29‐35 37. Animals/ 38. Humans/ 39. 37 not (37 and 38) 40. 36 not 39 41. 28 and 40 |
| Embase (Ovid) Date of most recent search: 6 September 2021 | 1. central sleep apnea syndrome/ 2. (central adj2 sleep adj2 (apnea$ or apnoea$)).tw. 3. (central adj2 alveolar adj2 hypoventilation$).tw. 4. (central adj2 sleep disordered breathing).tw. 5. (ondine$ adj2 (syndrome or curse)).tw. 6. Cheyne Stokes breathing/ 7. Cheyne$ Stokes.tw. 8. (periodic adj2 (breathing or respiration)).tw. 9. or/1‐8 10. positive end expiratory pressure/ 11. CPAP.tw. 12. bipap.tw. 13. nippv.tw. 14. nppv.tw. 15. niv.tw. 16. aprv.tw. 17. ippv.tw. 18. nCPAP.tw. 19. airway pressure release ventilation.tw. 20. servo ventilation.tw. 21. servoventilation.tw. 22. noninvasive ventilation/ 23. noninvasive ventilation$.tw. 24. non‐invasive ventilation$.tw. 25. positive‐pressure$.tw. 26. positive airway pressure.tw. 27. or/10‐26 28. 9 and 27 29. Randomized Controlled Trial/ 30. randomization/ 31. controlled clinical trial/ 32. Double Blind Procedure/ 33. Single Blind Procedure/ 34. Crossover Procedure/ 35. (clinica$ adj3 trial$).tw. 36. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (mask$ or blind$ or method$)).tw. 37. exp Placebo/ 38. placebo$.ti,ab. 39. random$.ti,ab. 40. ((control$ or prospectiv$) adj3 (trial$ or method$ or stud$)).tw. 41. (crossover$ or cross‐over$).ti,ab. 42. or/29‐41 43. exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 44. human/ or normal human/ or human cell/ 45. 43 and 44 46. 43 not 45 47. 42 not 46 48. 28 and 47 |
|
Scopus Date of most recent search: 6 September 2021 |
#1 "sleep apnoea central" #2 "central sleep (apnoea* OR apnea*)" #3 "central alveolar hipoventilation*" #4 "central sleep disordered breathing" #5 "ondine* (syndrome OR curse)" #6 "cheyne‐stokes respiration" #7 "cheyne* stokes" #8 "periodic (breathing OR respiration)" #9 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 #10 "continuous positive airway pressure" #11 cpap #12 bipap #13 nippv #14 nppv #15 niv #16 aprv #17 ippv #18 ncpap #19 "airway pressure release ventilation" #20 "servo ventilation" #21 servoventilation #22 "noninvasive ventilation*" #23 "non‐invasive ventilation*" #24 "positive‐pressure*" #25 "positive airway pressure" #26 #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 #27 #9 and #26 |
| ClinicalTrials.gov Date of most recent search: 6 September 2021 | Study type: Interventional
Condition: central sleep apnea Intervention: CPAP OR bipap OR nippv OR nppv OR niv OR aprv OR ippv OR Ncpap OR ventilation OR servoventilation OR servo ventilation OR positive pressure OR positive airway |
| WHO trials portal Date of most recent search: 6 September 2021 | Condition: central sleep apnea Intervention: CPAP OR bipap OR nippv OR nppv OR niv OR aprv OR ippv OR Ncpap OR ventilation OR servoventilation OR servo ventilation OR positive pressure OR positive airway |
Data and analyses
Comparison 1. CPAP plus best supportive care versus best supportive care (or inactive control) in CSA associated with chronic heart failure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 AHI ‐ short term | 4 | 264 | Mean Difference (IV, Random, 95% CI) | ‐15.27 [‐22.44, ‐8.11] |
| 1.1.1 Industry sponsored | 2 | 229 | Mean Difference (IV, Random, 95% CI) | ‐18.86 [‐24.25, ‐13.48] |
| 1.1.2 Not industry sponsored | 2 | 35 | Mean Difference (IV, Random, 95% CI) | ‐6.56 [‐20.34, 7.22] |
| 1.2 All‐cause mortality ‐ long term | 2 | 287 | Risk Ratio (IV, Random, 95% CI) | 1.14 [0.83, 1.56] |
Comparison 2. ASV versus CPAP in CSA associated with chronic heart failure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 AHI ‐ short term | 2 | 41 | Mean Difference (IV, Random, 95% CI) | ‐20.37 [‐25.32, ‐15.41] |
Comparison 3. ASV plus best supportive care versus best supportive care alone (or inactive control) in CSA associated with chronic heart failure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Quality of life ‐ short term | 2 | 1355 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐2.31, 2.23] |
| 3.2 All‐cause mortality ‐ long term | 2 | 1355 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.06, 4.93] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
CANPAP 2006.
| Study characteristics | ||
| Methods | Multicentric randomised controlled trial | |
| Participants |
Assigned participants: 258 participants (EG: 128; CG: 130) aged 18 to 79 years with HF and functional class II to IV, stabilised with best supportive care for at least 1 month Age: 63 (SD 10); EG: 63.2 (SD 9.1); CG: 63.5 (SD 9.8) (years, mean, SD) Gender: men = 248 (96.12%); women = 10 (3.88%) (number; %) BMI: 29.05; EG: 28.8 (SD 5.5); CG: 29.3 (SD 6.5) LVEF: 24.5% (SD 7.7%); EG: 24.8 (SD 7.9); CG: 24.2 (SD 7.6) AHI: 40 (SD 16); EG:40 (SD 15); CG: 40 (SD 17) cAHI: 89; EG: 91 (SD 12); CG: 87 (SD 14) |
|
| Interventions | CPAP plus best supportive care versus best supportive care
|
|
| Outcomes | All‐cause mortality, cardiovascular mortality, rate of transplantation, AHI, cAHI, quality of life Oucomes not included in this review: LVEF, 6MWD, polysomnographic and laboratory outcomes Time points: 1, 3, 6, and 24 months |
|
| Notes |
Conflicts of interest: the authors received grant support from Respironics, ResMed, and Tyco Healthcare for participation in the CANPAP trial. In addition, Dr Ferguson reports receiving lecture fees from GlaxoSmithKline, Respironics Inc, and VitalAire. Dr Pfeifer reports receiving grant support from Respironics. Dr Hanly reports having equity ownership in Tyco Healthcare. Funding sources: this study received funds from an industry sponsor. One‐third of the funding was provided by the CIHR, and two‐thirds was provided by the industry partners. 4 industry partners each agreed to provide 25% of the industry contribution, including donation of its brand of CPAP device. Other notes: we contacted the authors to check quality of life data, who replied that they could not provide the needed information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed using a computer‐generated method. |
| Allocation concealment (selection bias) | Low risk | Treatment assignment was communicated to the study centres by the data management centre. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Blinding of participants and personnel was not performed. |
| Blinding of outcome assessment (detection bias) Subjective or self‐reported outcomes | High risk | For the self‐reported outcome (quality of life), blinding was not possible. |
| Blinding of outcome assessment (detection bias) Other outcomes | Low risk | Outcome assessors were blind. "CANPAP was a prospective randomized, open‐label trial with blinded evaluation of outcomes" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was a substantial dropout rate (15.5%). |
| Selective reporting (reporting bias) | High risk | The protocol was retrospectively registered (ISRCTN25258560). |
| Other bias | Low risk | No other sources of bias were found. |
D'Elia 2019.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants |
Assigned participants: 10 participants (EG: 5; CG: 5) aged > 18 years old with history of SDB with an AHI > 15/h and prevalence (> 50%) of CSA with acute HF and preserved EF (LVEF > 45%) Age: 69.9; EG: 69 (SD 4); CG: 70.8 (SD 5) (years, mean, SD) Gender: men = 10 (100%); women = 0 (0%) (number, %) BMI: 26.65; EG: 27.0 (SD 1); CG: 26.3 (SD 1.5) LVEF: 50.8; EG: 49.0 (SD 3.8); CG: 52.6 (SD 5.5) AHI: 36.9; EG: 39.6 (SD 3.2); CG: 34.2 (SD 5.1) cAHI: 28; EG: 31.2 (SD 1.6); CG: 24.8 (SD 4.7) (central sleep apnoea index) |
|
| Interventions | Enhanced adaptive servo ventilation (adaptive servo ventilation with auto‐adjustment of expiratory positive airway pressure) associated with best supportive care versus best supportive care alone
|
|
| Outcomes | Adverse events Outcomes not included in this review: BNP, echocardiographic parameters Time point: 7 days |
|
| Notes |
Conflicts of interest: the authors declare that they have no conflict of interest. Funding sources: no report regarding possible funding sources was found in the manuscript. Other notes: we contacted the authors to check the availability of other outcomes results, and they informed us that they performed polysomnography after 7 days only in the ASV group and did not have numbers on AHI and CSA after 7 days of best supportive care without ASV. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for the generation of the allocation sequence were not described. |
| Allocation concealment (selection bias) | Unclear risk | Details on allocation concealment were not provided. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Blinding of participants and personnel was not performed. |
| Blinding of outcome assessment (detection bias) Subjective or self‐reported outcomes | High risk | Blinding was not performed, and the outcome could have been influenced by the lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It was not clear if there were losses. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol was available. |
| Other bias | Low risk | No other sources of bias were found. |
Dellweg 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants |
Assigned participants: 37 (EG: 18; CG: 17) participants aged 18 to 80 years with CPAP‐induced CSA (complex sleep apnoea syndrome) Age: 62.5; EG: 61 (SD 11); CG: 64 (SD 11) (years, mean, SD) Gender: men = 21 (70%); women = 9 (30%) (number; %) (completers) BMI: 30; EG: 30.3 (SD 4.3); CG: 29.7 (SD 4.2) LVEF: NR AHI: 28.15; EG: 27.7 (SD 9.7); CG: 28.6 (SD 6.5) cAHI: 17.45; EG: 18.2 (SD 7.1); CG: 16.7 (SD 5.4) (central sleep apnoea index) |
|
| Interventions | Dynamic servo ventilator (Somnovent CR, Weinmann, Hamburg, Germany) versus bilevel ventilation
|
|
| Outcomes | cAHI, total AHI Outcomes not included in this review: other polysomnographic parameters (e.g. percentage slow wave sleep, percentage REM sleep, arousal index, sleeptime, sleep efficacy) Time point: 6 weeks |
|
| Notes |
Conflicts of interest: Dr Dellweg has consulted for Weinmann (Hamburg, Germany) and has participated in speaking engagements for ResMed (Martinried, Germany). Dr Kerl has participated in speaking engagements for Weinmann. The other authors indicated no financial conflicts of interest. Funding sources: this was not an industry‐supported study. Other notes: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for the generation of the allocation sequence were not described. |
| Allocation concealment (selection bias) | Unclear risk | Details on allocation concealment were not provided. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Blinding was not performed, but these outcomes were unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | Outcome assessors were not blind. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was a substantial loss rate (18%). |
| Selective reporting (reporting bias) | Low risk | The trial protocol was available (NCT01609244), and all planned outcomes were analysed. |
| Other bias | Low risk | No other sources of bias were found. |
Fietze 2008.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants |
Assigned participants: 37 participants (EG: 17; CG: 20) with stable, pharmacologically treated chronic heart failure (LVEF < 45%, NYHA functional class II to III), and CSB with a RDI > 15/h, with less than 20% obstructive respiratory events Age: 58.9 (SD 10.4); EG: 61.9 (SD 9.1); CG: 56.4 (SD 10.9); (years, mean, SD) Gender: men = 34 (91.89%); women = 3 (8.11%) (number, %) BMI: 28 (SD 3.9); EG: 26.9 (SD 2.4); CG: 28.9 (SD 4.8) LVEF: 25.05; EG: 24.6 (SD 7.9); CG: 25.5 (SD 9.2) AHI: 33.3; EG: 31.7 (SD 9.8); CG: 34.9 (SD 20.4) (RDI per hour calculated by the sum of Cheyne‐Stokes respiration index, obstructive apnoea index, and mixed apnoea index) cAHI: 32.2; EG: 31.0 (SD 10.0); CG: 33.4 (SD 20.5) (Cheyne‐Stokes respiration index per hour) |
|
| Interventions | ASV mode device (AutoSetCS; ResMed, Sydney, Australia) versus bilevel S/T mode device (VPAPII‐ST; ResMed, Sydney, Australia)
|
|
| Outcomes | cAHI, total AHI Outcomes not included in this review: LVEF, blood pressure, heart rate, and other polysomnography parameters Time point: 6 weeks |
|
| Notes |
Conflicts of interest: no report regarding possible conflicts of interest was found in the manuscript. Funding sources: this study was supported in part by an unrestricted grant from ResMed, Germany. Other notes: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for the generation of the allocation sequence were not described. |
| Allocation concealment (selection bias) | Unclear risk | Details on allocation concealment were not provided. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Blinding was not performed, but these outcomes were unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Other outcomes | Unclear risk | It was not clear whether AHI assessors were blind. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It was not clear if there were losses. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol was available. |
| Other bias | Low risk | No other sources of bias were found. |
Granton 1996.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants |
Assigned participants: 17 participants (EG: 9; CG: 8) aged < 75 years old with chronic heart failure and CSA with CSB with a history of chronic heart failure of at least 6 months duration as defined by at least 1 prior episode of symptomatic heart failure characterised by dyspnoea at rest or on exertion and radiographic evidence of cardiomegaly and pulmonary congestion; with continued dyspnoea (NYHA class II to III) despite best supportive care; with an LVEF < 45 at rest (lower limit of normal for resting LVEF in the laboratory was 55%); clinically stable for at least 1 month prior to randomisation on best supportive care, including maximally tolerated doses of angiotensin‐converting enzyme inhibitors, digoxin, and diuretics as clinically appropriate; and with CSB‐CSA during the baseline sleep study Age: 58.15; EG: 58.3 (SD 2.2); CG: 58.0 (SD 2.0) (years, mean, SD) Gender: men = 17 (100%); women = 0 (0%) (number, %) BMI: 27.15; EG: 28.9 (SD 1.9); CG: 25.4 (SD 1.8) LVEF: 22.3; EG: 24.0 (SD 4.0); CG: 20.6 (SD 3.2) AHI: 42; EG: 49 (SD 11); CG: 35 (SD 11) cAHI: NR |
|
| Interventions | CPAP plus best supportive care versus best supportive care
|
|
| Outcomes | AHI Outcomes not included in this review: maximal inspiratory and expiratory pressures, LVEF, dyspnoea, fatigue Time points: 1 and 3 months |
|
| Notes |
Conflicts of interest: MT Naughton was supported by Research Fellowships from the Medical Research Council of Canada and Respironics Inc. PP Uu is a recipient of a Career Investigator Award from the Heart and Stroke Foundation of Ontario. TD Bradley is a recipient of a Career Scientist Award from the Ontario Ministry of Health. Funding sources: this study was supported by operating grants from the Ontario Ministry of Health and the Medical Research Council of Canada (MT 11607). Other notes: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for the generation of the allocation sequence were not described. |
| Allocation concealment (selection bias) | Unclear risk | Details on allocation concealment were not provided. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Blinding was not confirmed, but the outcome was unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Other outcomes | Unclear risk | It was not clear whether the AHI assessor was blind. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It was not clear if there were losses. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol was available. |
| Other bias | Low risk | No other sources of bias were found. |
Hetland 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants |
Assigned participants: 51 participants (EG: 27; CG: 24) aged 57 to 81 years old with chronic heart failure and CSA, LVEF < 40%, and/or NYHA functional class III or IV Age: 70.5; EG: 71.1 (SD 7.6); CG: 69.9 (SD 6.7) (years, mean, SD) Gender: men = 47 (92.16%); women = 4 (7.84%) (number; %) BMI: 27.15; EG: 27.4 (SD 5.4); CG: 26.9 (SD 3.4) LVEF: 31.8; EG: 31.9 (SD 11.0); CG: 31.7 (SD 9.4) AHI: 29.65; EG: 32.2 (SD 12.9); CG: 27.1 (SD 18.0) cAHI: 131; EG: 68.2 (SD 16.8); CG: 62.8 (SD 21.6) (Cheyne‐Stokes respiration %) |
|
| Interventions | ASV plus best supportive care versus best supportive care
|
|
| Outcomes | Quality of life ‐ MLHFQ Outcomes not included in this review: LVEF, NYHA, 6MWD Time point: 1 week |
|
| Notes |
Conflicts of interest: the authors report that the sponsors had no role in the study design, data collection and interpretation, manuscript writing, or the decision to submit for publication. The authors declare no conflicts of interest. Funding sources: this study was funded by Østfold Hospital Trust and University of Oslo. ResMed Norway A/S, Høvik, Norway supplied the adaptive servo ventilation machines used in this study. Other notes: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for the generation of the allocation sequence were not described. |
| Allocation concealment (selection bias) | Unclear risk | Details on allocation concealment were not provided. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Blinding of participants and personnel was not performed. |
| Blinding of outcome assessment (detection bias) Subjective or self‐reported outcomes | High risk | For the self‐reported outcome (quality of life), blinding was not possible. |
| Blinding of outcome assessment (detection bias) Other outcomes | Low risk | Outcome assessors were blind: "Reanalysis were performed blinded to clinical outcome" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was a substantial loss rate: 12 participants in the ASV group and 9 participants in the usual care group did not complete the study. |
| Selective reporting (reporting bias) | High risk | Not all planned outcomes were reported according to the protocol (NCT00563693). |
| Other bias | Low risk | No other sources of bias were found. |
Kasai 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants |
Assigned participants: 23 participants (EG: 12; CG: 11) with history of persistent systolic HF (even after CPAP initiation), with moderate to severe CSA Age: 65.05; EG: 64.3 (SD 8.8); CG: 65.8 (SD 8.7) (years, mean, SD) Gender: men = 23 (100%); women = 0 (0%) (number, %) BMI: 26.6; EG: 26.3 (SD 4.2); CG: 26.9 (SD 5.2) LVEF: 32.45; EG: 32.0 (SD 7.9); CG: 32.9 (SD 5.9) AHI: 47.85; EG: 48.3 (SD 11.3); CG: 47.4 (SD 8.1) cAHI: 81.6; EG: 84.4 (SD 12.0); CG: 78.8 (SD 15.1) |
|
| Interventions | ASV versus CPAP
|
|
| Outcomes | AHI, quality of life Outcomes not included in this review: other polysomnography parameters, sleepiness, and arterial blood gas data, plasma BNP, 6MWD, cardiac function variables Time point: 3 months |
|
| Notes |
Conflicts of interest: Dr Kasai has received an unrestricted research fellowship from Fuji‐Respironics Inc. All other authors reported that they have no relationships relevant to the contents of this paper to disclose. Funding sources: this research was funded by grants from the Okinaka Memorial Institute for Medical Research, Tokyo, Japan. Other notes: we contacted the authors to check availability of quality of life data, but have received no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for the generation of the allocation sequence were not described. |
| Allocation concealment (selection bias) | Unclear risk | Details on allocation concealment were not provided. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Blinding of participants was probably not performed effectively. "Although subjects were blinded to their treatment modes, they may have been aware of the assigned treatment mode because of the perception of pressure support or backup ventilation while awakening after sleep onset. Assumptions about treatment assignment may have affected treatment compliance and/or QOL responses" |
| Blinding of outcome assessment (detection bias) Subjective or self‐reported outcomes | High risk | For the self‐reported outcome (quality of life), blinding was not possible. |
| Blinding of outcome assessment (detection bias) Other outcomes | Low risk | Sonographers were blind to treatment assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses occurred. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol was available. |
| Other bias | Low risk | No other sources of bias were found. |
Morgenthaler 2014.
| Study characteristics | ||
| Methods | Multicentric randomised controlled trial | |
| Participants |
Assigned participants: 66 participants (EG: 33; CG: 33) with complex sleep apnoea syndrome who during polysomnography had a CPAP titration eliminating events defining OSA, but had a residual CAI ≥ 10 events/hour or residual CSB pattern that was predominant and disruptive Age: 59.2 (SD 12.9); EG: 59.1 (SD 14.2); CG: 59.4 (SD 11.7) (years, mean, SD) Gender: men = 56 (84.84%); women = 10 (15.16%) (number; %) BMI: 34.95; EG: 34.8 (SD 7.5); CG: 35.1 (SD 8.5) LVEF: NR AHI: 34.45; EG: 35.7 (SD 23.6); CG: 33.2 (SD 17.9) cAHI: 23.7; EG: 25.2 (SD 19.1); CG: 22.2 (SD 12.6) (central sleep apnoea index, events per hour) |
|
| Interventions | ASV versus CPAP
Titration on assigned therapy was performed over a full night of PSG, and participants began therapy at best settings. |
|
| Outcomes | AHI, cAHI, quality of life Outcomes not included in this review: other polysomnographic variables, sleepiness Time points: 30 and 90 days |
|
| Notes |
Conflicts of interest: other than this work, Drs Morgenthaler, Kuzniar, McLain, and Goldberg have indicated no other financial conflicts of interest. Dr Wolfe has served as a consultant for ResMed Inc and Hill‐Rom. Leslee Willes has served as a consultant for ResMed Inc, and was contracted to perform the statistical analysis for this work. Funding sources: this work was supported by a grant from the ResMed Science Center, ResMed Corp. Other notes: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for the generation of the allocation sequence were not described. |
| Allocation concealment (selection bias) | Unclear risk | Details on allocation concealment were not provided. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Although titration procedures were blind, participants may have been aware of the assigned treatment mode because of the perception of pressure. |
| Blinding of outcome assessment (detection bias) Subjective or self‐reported outcomes | High risk | For the self‐reported outcome (quality of life), blinding was not possible. |
| Blinding of outcome assessment (detection bias) Other outcomes | Unclear risk | It was not clear whether AHI assessors were blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was a low dropout rate (9%), and reasons for dropout were provided. An intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | Protocol was available (NCT00915499), and all planned outcomes were analysed. |
| Other bias | Low risk | No other sources of bias were found. |
Naughton 1995a.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants |
Assigned participants: 29 participants (EG: 14; CG: 15) with chronic heart failure and CSA Age: 58.8; EG: 61.0 (SD 3.2); CG: 56.6 (SD 3.2) (years, mean, SD) Gender: men = 29 (100%); women = 0 (0%) (number; %) BMI: 26.55; EG: 26.0 (SD 1.5); CG: 27.1 (SD 1.5) LVEF: 20.45; EG: 21.2 (SD 3.8); CG: 19.7 (SD 2.7) AHI: 76.3; EG: 43.2 (SD 4.9); CG: 33.1 (SD 7.1) cAHI: NR |
|
| Interventions | CPAP plus best supportive care versus best supportive care
|
|
| Outcomes | AHI, quality of life (chronic heart failure questionnaire) Outcomes not included in this review: polysomnographic variables, LVEF, NYHA, cardiovascular function variables, adverse cardiac events Time points: 1 and 3 months |
|
| Notes |
Conflicts of interest: Dr Bradley is a Career Scientist of the Ontario Ministry of Health. Funding sources: this work was supported by research fellowships from the Respironics Corporation and the Medical Research Council of Canada, and by an Operating grant from the Ontario Ministry of Health. Other notes: we contacted the authors to check quality of life data, but have received no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for the generation of the allocation sequence were not described. |
| Allocation concealment (selection bias) | Unclear risk | Details on allocation concealment were not provided. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Blinding of participants was not performed. “Patients were unblinded to their treatment" |
| Blinding of outcome assessment (detection bias) Subjective or self‐reported outcomes | High risk | For the self‐reported outcome (quality of life), blinding was not possible. |
| Blinding of outcome assessment (detection bias) Other outcomes | Low risk | Outcome assessors were blind. “Sleep studies were reported by a polysomnographer blind to the patients’ treatment” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2 participants in the CPAP group and 3 participants in the usual care group did not complete the study. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol was available. |
| Other bias | Low risk | No other sources of bias were found. |
Naughton 1995b.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants |
Assigned participants: 18 participants (EG: 9; CG: 9) with chronic heart failure and CSA Age: 60.5 (1.4); EG: NR; CG: NR (years, mean, SD) Gender: men = 18 (100%); women = 0 (0%) (number; %) BMI: 25.8 (SD 1.0); EG: NR; CG: NR LVEF: 18.2; EG: 17.0 (3.0); CG: 19.4 (4.0) AHI: 42.7 (SD 4.6); EG: 48.1 (SD 4.9); CG: 37.3 (SD 7.6) cAHI: NR |
|
| Interventions | CPAP plus best supportive care versus best supportive care
|
|
| Outcomes | AHI Outcomes not included in this review: polysomnographic variables, LVEF, NYHA, cathecholamine, cardiovascular function variables Time point: 1 month |
|
| Notes |
Conflicts of interest: MT Naughton is a Fellow of the Medical Research Council of Canada. PP Liu is the recipient of a Career Investigator award from the Heart and Stroke Foundation of Ontario. TD Bradley is the recipient of a Career Scientist award from the Ontario Ministry of Health. Funding sources: this research was supported by operating grants from the Medical Research Council of Canada (MT 11607) and the Ontario Ministry of Health. Other notes: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for the generation of the allocation sequence were not described. |
| Allocation concealment (selection bias) | Unclear risk | Details on allocation concealment were not provided. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Participants were unblinded to their treatment assignment. |
| Blinding of outcome assessment (detection bias) Other outcomes | Low risk | Outcome assessors were blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses occurred. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol was available. |
| Other bias | Low risk | No other sources of bias were found. |
Pepperell 2003.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants |
Assigned participants: 30 participants (EG: 15; CG: 15) with HF (NYHA class II to IV) and CSA with CSB Age: 71.15; EG: 71.4 (SD 8.6); CG: 70.9 (SD 7.9) (years, mean, SD) Gender: men = 29 (96.67%); women = 1 (3.33%) (number; %) BMI: 26.2; EG: 26.8 (SD 4.6); CG: 25.6 (SD 4.1) LVEF: 34.75; EG: 36.5 (SD 11.5); CG: 33.0 (SD 11.3) AHI: 24; EG: 24.7 (SD 11.3); CG: 23.3 (SD 13.3) cAHI: NR |
|
| Interventions | Therapeutic ASV versus subtherapeutic ASV
|
|
| Outcomes | AHI, quality of life Outcomes not included in this review: other polysomnography parameters, sleepiness, serum BNP, urinary catecholamine excretion Time point: 4 weeks |
|
| Notes |
Conflicts of interest: JCTP was reimbursed by ResMed for travel expenses (GBP 480) to attend the American Thoracic Society Annual Conference 2002; NAM has declared no conflict of interest; DRJ has declared no conflict of interest; BAL‐W has declared no conflict of interest; NC has declared no conflict of interest; JRS has declared no conflict of interest; RJOD has declared no conflict of interest. Funding sources: this research was supported by charitable trust funds including a non‐commercial donation made by ResMed UK to support research in the Oxford Sleep Unit in 1998. Other notes: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for the generation of the allocation sequence were not described. |
| Allocation concealment (selection bias) | Low risk | Details on allocation concealment were provided. "Treatment with therapeutic or subtherapeutic ASV was allocated by the selection of a pre sealed and numbered envelope." |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Blinding of participants was performed. "Both trial groups received treatments that felt similar prior to sleep onset with the therapeutic and sub‐therapeutic treatments only becoming substantially different after sleep onset, when the subject is unaware of the machines behaviour" |
| Blinding of outcome assessment (detection bias) Subjective or self‐reported outcomes | High risk | For the self‐reported outcome (quality of life), blinding was not possible. |
| Blinding of outcome assessment (detection bias) Other outcomes | Low risk | Outcome assessors were blind. "The research staff performing the outcome assessments were unaware of the randomisation status of the patients and were not involved with machine set up, maintenance or patient support" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was a low loss rate (13%), and reasons for losses were provided. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol was available. |
| Other bias | Low risk | No other sources of bias were found. |
Philippe 2006.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants |
Assigned participants: 25 participants (EG: 12; CG: 13) with stable chronic heart failure and CSA with CSB Age: 62.25; EG: 64.2 (SD 15.5); CG: 60.3 (SD 11.5) (years, mean, SD) Gender: men = 25 (100%); women = 0 (0%) (number; %) BMI: 27; EG: 25.2 (SD 3.3); CG: 28.8 (SD 6.3) LVEF: 29.5; EG: 29 (SD 9); CG: 30 (SD 9) AHI: 43.75; EG: 47 (SD 18.6); CG: 40.5 (SD 13.9) cAHI: NR |
|
| Interventions | ASV versus CPAP
|
|
| Outcomes | AHI, quality of life Outcomes not included in this review: other polysomnographic parameters, daytime sleepiness, respiratory function, cardiovascular assessment Time point: 3 and 6 months |
|
| Notes |
Conflicts of interest: CP and M‐P d’O were reimbursed by ResMed for travel expenses to attend the American Thoracic Society Annual Conference 2004; SR is employed by ADEP Assistance, which is a nonprofit organisation for home care. Other authors have declared no conflict of interest. Funding sources: the study was supported by nonprofit organisation funds (ADEP Assistance) and a non‐commercial donation made by ResMed France to support research in the Créteil Sleep Laboratory in 2001. Other notes: we contacted the authors to check AHI and quality of life values, but have received no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for the generation of the allocation sequence were not described. |
| Allocation concealment (selection bias) | Unclear risk | Details on allocation concealment were not provided. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Participants and personnel were not blind. |
| Blinding of outcome assessment (detection bias) Subjective or self‐reported outcomes | High risk | For the self‐reported outcome (quality of life), blinding was not possible. |
| Blinding of outcome assessment (detection bias) Other outcomes | Low risk | Outcome assessors were blind. "Study personnel who took polysomnography readings and LVEF measurements were blinded to the treatment assigned to patients." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was a substantial loss rate (32%). |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol was available. |
| Other bias | Low risk | No other sources of bias were found. |
SERVE HF 2015.
| Study characteristics | ||
| Methods | Randomised controlled trial with parallel design | |
| Participants |
Assigned participants: 1325 participants (EG: 666; CG: 659) with symptomatic chronic HF and reduced EF ≤ 45%, under stable treatment, and predominantly CSA Age: 69.45; EG: 69.6 (SD 9.5); CG: 69.3 (SD 10.4) (years, mean, SD) Gender: men = 1198 (90.42%); women = 127 (9.58%) (number; %) BMI: 28.5; EG: 28.4 (SD 4.7); CG: 28.6 (SD 5.1) LVEF: 32.35; EG: 32.2 (SD 7.9); CG: 32.5 (SD 8.0) AHI: 31.45; EG: 31.2 (SD 12.7); CG: 31.7 (SD 13.2) cAHI: 81.3; EG: 80.8 (SD 15.5); CG: 81.8 (SD 15.7) |
|
| Interventions | ASV (PaceWave, Auto Set CS; ResMed) plus best supportive care versus best supportive care alone
|
|
| Outcomes | Quality of life (EQ‐5D, MLHFQ), mortality, cardiovascular death, time to first event of the composite of death from any cause, a life‐saving cardiovascular intervention, or an unplanned hospitalisation for worsening chronic heart failure, time to death from any cause, time to death from cardiovascular causes, and change in NYHA class Outcomes not included in this review: change in 6MWD, sleepiness, blood variables, cardiac function variables, weight, BMI Time points/follow‐up: up to 60 months |
|
| Notes |
Conflicts of interest: Dr Cowie reports receiving consulting fees from Servier, Novartis, Pfizer, St Jude Medical, Boston Scientific, Respicardia, and Medtronic and grant support through his institution from Bayer; Dr Woehrle, being an employee of ResMed; Dr Wegscheider, receiving grant support from ResMed; Dr Angermann, receiving fees for serving on advisory boards from ResMed, Servier, Boehringer Ingelheim, and Vifor Pharma, fees for serving on a steering committee from ResMed, lecture fees from Servier and Vifor Pharma, grant support from ResMed, Thermo Fisher Scientific, Boehringer Ingelheim, Lundbeck, and Vifor Pharma, financial support for statistical analyses from Thermo Fisher Scientific, and study medication from Lundbeck; Dr d’Ortho, receiving fees for serving on advisory boards from
ResMed and IP Santé, lecture fees from ResMed, Philips, IP Santé, and VitalAire, grant support from Fisher and Paykel Healthcare, ResMed, Philips, ADEP Assistance, and IP Santé, and small material donations from VitalAire; Dr Somers, receiving consulting fees from PricewaterhouseCoopers, Sorin, GlaxoSmithKline, Respicardia, uHealth, Ronda Grey, and ResMed, working with Mayo Medical Ventures on intellectual property related to sleep and cardiovascular disease, and having a pending patent (12/680073) related to biomarkers of sleep apnoea; Dr Zannad, receiving fees for serving on steering committees from Janssen Pharmaceutica, Bayer, Pfizer, Novartis, Boston Scientific, ResMed, and Takeda Pharmaceutical, receiving consulting fees from Servier, Stealth Peptides, Amgen, and CVRx, and receiving lecture fees from Mitsubishi; and Dr Teschler, receiving consulting fees, grant support, and hardware and software for the development of devices from ResMed. No other potential conflict of interest relevant to this article was reported. Funding sources: this trial was supported by ResMed and by grants from the National Institute for Health Research (NIHR) Cardiovascular Biomedical Research Unit (to Dr Cowie), the NIHR Respiratory Biomedical Research Unit (to Dr Simonds), and the National Institutes of Health (R01HL065176, to Dr Somers). Other notes: we contacted the authors and the sponsor to check quality of life values. The sponsor answered that due to budget restrictions they did not have sufficient funds to pay a person to extract the data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Details on the random sequence generation were provided: "Randomisation was done using codes generated by a central computer" |
| Allocation concealment (selection bias) | Unclear risk | Details on allocation concealment were not provided. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Blinding of participants and personnel was not performed. |
| Blinding of outcome assessment (detection bias) Subjective or self‐reported outcomes | High risk | This was an open‐label study. For the self‐reported outcome (quality of life), blinding was not possible. |
| Blinding of outcome assessment (detection bias) Other outcomes | Low risk | This was an open‐label study, but the outcomes measured were unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Substantial losses occurred in the intervention group (12%) and the control group (11%). |
| Selective reporting (reporting bias) | Low risk | The trial protocol was available (NCT00733343), and all planned outcomes were analysed. |
| Other bias | Low risk | No other sources of bias were found. |
Sin 2000.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants |
Assigned participants: 29 participants (EG: 14; CG: 15) with chronic heart failure and CSA with CSB Age: 57.9 (SD 9.7); EG: 57.6 (SD 10.4); CG: 62.2 (SD 11.7) (years, mean, SD) Gender: men = 29 (100%); women = 0 (0%) (number; %) BMI: NR LVEF: 20.2 (SD 10.0); EG: 20.6 (SD 11.3); CG: 19.8 (SD 9.0) AHI: 39.2 (SD 21.9); EG: 46.4 (SD 18.9); CG: 33.4 (SD 22.2) cAHI: NR |
|
| Interventions | CPAP plus best supportive care versus best supportive care
|
|
| Outcomes | Mortality Outcomes not included in this review: LVEF, polysomnography parameters Time point: 3 months |
|
| Notes |
Conflicts of interest: Dr Sin is supported by a research fellowship from the Alberta Heritage Foundation for Medical Research. P Liu is the Heart and Stroke/Polo Chair in Cardiovascular Research. Drs Bradley and Logan are investigators in a multicentre trial of CPAP to treat chronic heart failure, which is jointly sponsored by the Medical Research Council of Canada and 3 manufacturers of CPAP (Malinkrodt, ResMed, and Respironics). Funding sources: this research was supported by operating grant MA‐12422 from the Medical Research Council of Canada. Other notes: we contacted the authors to check availability of mortality data in CSR‐CSA patients who either received or did not receive CPAP, but have received no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for the generation of the allocation sequence were not described. |
| Allocation concealment (selection bias) | Unclear risk | Details on allocation concealment were not provided. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Blinding was not performed, but the outcome of interest could not be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was a low loss rate, and reasons for losses were provided. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol was available. |
| Other bias | Low risk | No other sources of bias were found. |
Toyama 2016.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants |
Assigned participants: 31 participants (EG: 16; CG: 15) with dilated or ischaemic cardiomyopathy and CSR‐CSA with chronic heart failure Age: 68 (9); EG: 69 (9); CG: 68 (10) (years, mean, SD) Gender: men = 28 (93.33%); women = 2 (6.67%) (number; %) BMI: 24.1; EG: 23.4 (SD 4.5); CG: 24.8 (SD 4.0) LVEF: 26.5; EG: 26 (SD 9); CG: 27 (SD 9) AHI: 25.25; EG: 25.5 (SD 14.7); CG: 25.0 (SD 13.5) cAHI: 37.85; EG: 36.7 (SD 25.4); CG: 39.0 (SD 28.6) (CSA %) |
|
| Interventions | ASV versus best supportive care
|
|
| Outcomes | AHI Outcomes not included in this review: other polysomnography parameters, NYHA, ANP, and LVEF Time point: 6 months |
|
| Notes |
Conflicts of interest: the authors have indicated that they have no financial conflict of interest. Funding sources: no report regarding possible funding sources was found in the manuscript. Other notes: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for the generation of the allocation sequence were not described. |
| Allocation concealment (selection bias) | Unclear risk | Details on allocation concealment were not provided. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Blinding was not confirmed, but the outcome was unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Other outcomes | Unclear risk | It was not clear whether the AHI assessor was blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was a low loss rate, and reasons for losses were provided. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol was available. |
| Other bias | Low risk | No other sources of bias were found. |
6MWD: 6‐minute walk distance; AHI: apnoea hypopnoea index; ANP: atrial natriuretic peptide; ASV: adaptive servo ventilation; BMI: body mass index; BNP: brain natriuretic peptide; cAHI: central apnoea hypopnoea index; CAI: central apnoea index; CG: control group; CIHR: Canadian Institutes of Health Research; CPAP: continuous positive airway pressure; CSA: central sleep apnoea; CSB: Cheyne‐Stokes breathing; CSR: Cheyne‐Stokes respiration; EF: ejection fraction; EG: experimental group; EPAP: expiratory positive airway pressure; HF: heart failure; IPAP: inspiratory positive airway pressure; LVEF: left ventricular ejection fraction; MLHFQ: Minnesota Living with Heart Failure Questionnaire; NR: not reported; NYHA: New York Heart Association; OSA: obstructive sleep apnoea; PSG: polysomnography; RDI: respiratory disturbance index; REM: rapid eye movement; SD: standard deviation; SDB: sleep‐disordered breathing
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arzt 2005 | Non‐RCT design |
| Campbell 2012 | Cross‐over design |
| Cao 2014 | Cross‐over design |
| Galetke 2014 | Cross‐over design |
| Hetland 2014 | Non‐RCT design |
| Hetland 2017 | Non‐RCT design |
| Hetzenecker 2016 | Non‐RCT design |
| Hu 2005 | Cross‐over design |
| Javaheri 2011 | Cross‐over design |
| Kasai 2010 | Participants with co‐existing OSA and CSA/CSR; no separate data for predominantly CSA patients |
| Kohnlein 2002 | Cross‐over design |
| Krachman 1999 | Cross‐over design |
| Krachman 2003 | Non‐RCT design |
| Liu 2015 | Cross‐over design |
| Morgenthaler 2007 | Cross‐over design |
| Oldenburg 2015 | Cross‐over design |
| Priefert 2017 | Inappropriate RCT because of subanalysis |
| Randerath 2012 | Participants with co‐existing OSA and CSA/CSR; no separate data for predominantly CSA patients |
| Shapiro 2015 | Cross‐over design |
| Szollosi 2006 | Cross‐over design |
| Teschler 2001 | Cross‐over design |
CSA: central sleep apnoea; CSR: Cheyne‐Stokes respiration; OSA: obstructive sleep apnoea; RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
Alter 2013.
| Methods | Randomised controlled trial |
| Participants | People with chronic, medically treated heart failure (LVEF 25 ± 9%) and SDB (AHI 27 ± 14, CSA ≥ 50%) (n = 6) |
| Interventions | CPAP versus control |
| Outcomes | LVEF, end systolic wall stress, end diastolic wall stress |
| Notes | We contacted the authors to check whether they had assessed any of the review outcomes, but have received no response. |
Arzt 2013.
| Methods | Randomised controlled trial |
| Participants | People with HF (age 66 years SD 9, LVEF < 40%) and SDB (AHI 48 ± 19/h, 51% CSA) n = 42 |
| Interventions | Auto‐servo ventilation (ASV, BiPAP‐ASV, Philips Respironics) compared to best supportive care alone |
| Outcomes | AHI, central AHI, serious adverse events, quality of life Outcomes not included in this review: arousal index, sleep stage 1, fragmentation index (actigraphy), sleep efficiency and daytime activity duration (actigraphy) |
| Notes | We contacted the authors to check availability of separate data for CSA patients, but have received no response. |
CAT‐HF 2016.
| Methods | Multicentric randomised controlled trial |
| Participants | Acute decompensated heart failure patients, with either preserved ejection fraction or reduced ejection fraction and moderate to severe SDB (obstructive and central) (n = 126) |
| Interventions | MV‐targeted ASV in addition to optimised medical therapy versus optimised medical therapy alone. Duration of treatment = 6 months |
| Outcomes | Composite global rank endpoint (survival free from CV hospitalisation and improvement in functional capacity measured by 6MWD); functional capacity; biomarkers; quality of life; sleep parameters; imaging parameters; NYHA class; recurrent hospitalisations or urgent clinic visits, cardiovascular and all‐cause death, and days alive and out of the hospital |
| Notes | We contacted the authors to check availability of separate data for CSA patients, but have received no response. |
De Michelis 2008.
| Methods | Unclear |
| Participants | Congestive heart failure patients with CSA, Cheyne‐Stokes respiration, and AHI > 30 (n = 16) |
| Interventions | Adaptive servo ventilation compared with dynamic bilevel positive airways pressure ventilation Treatment duration was 3 months. |
| Outcomes | AHI, ODI, desaturations, LVEF, 6MWT |
| Notes | We contacted the authors to check whether this is a randomised controlled trial, but have received no response. |
Egea 2004.
| Methods | Randomised controlled trial |
| Participants | People with chronic heart failure (LVEF < 45%) and an AHI greater than 10 (n = 60) |
| Interventions | CPAP compared to sham CPAP |
| Outcomes | AHI, LVEF, hypertension, daytime sleepiness (ESS), quality of life (SF‐36), NYHA score, dyspnoea (using the Borg scale), and exercise tolerance (6MWD) |
| Notes | Included mixed population of OSA and CSA patients. No separate data reported. We contacted the authors to check availability of separate data for CSA patients, but have received no response. |
Goldberg 2007.
| Methods | Multicentric randomised controlled trial |
| Participants | 60 participants with HF and CSA |
| Interventions | ASV versus oxygen therapy |
| Outcomes | Quality of life (SF‐36 or MLHFQ): results not reported Outcomes not included in this review: rehospitalisation rates, LVEF, 6MWD |
| Notes | We contacted the authors to check the availability of the results for the outcomes of interest in this review, but have received no response. |
Miyata 2012.
| Methods | Randomised controlled trial |
| Participants | People with CSA, CSB, and chronic heart failure and who had implanted CRT with defibrillator |
| Interventions | ASV versus non‐ASV group |
| Outcomes | All‐cause rehospitalisations, LVEF, and BNP |
| Notes | We contacted the authors to check whether they had assessed any of the review outcomes, but have received no response. |
Murase 2016.
| Methods | Randomised controlled trial |
| Participants | People with SDB and chronic heart failure (LVEF ≤ 50%) |
| Interventions | ASV versus oxygen therapy |
| Outcomes | AHI, NT‐proBNP level, echocardiographic parameters, 6MWD, MLHFQ score, compliance, systolic blood pressure, diastolic blood pressure, heart rate, changes in body composition |
| Notes | We contacted the authors to check eligibility, but have received no response. |
Noda 2007.
| Methods | Randomised controlled trial |
| Participants | People with idiopathic dilated cardiomyopathy who underwent both cardiac catheterisation and standard polysomnography and had an AHI > 20 episodes per hour were included; individuals with OSA were excluded. |
| Interventions | Medical therapy either alone (n = 11) or with BiPAP (n = 10) |
| Outcomes | LVEF, concentration of BNP |
| Notes | We contacted the authors to check whether they had assessed any of the review outcomes, but have received no response. |
Teschler 2000.
| Methods | Unclear |
| Participants | Central sleep apnoea in brainstem stroke |
| Interventions | Autoset CS servo‐ventilation |
| Outcomes | Unclear |
| Notes | We contacted the authors to check eligibility, but have received no response. |
Tkacova 1997.
| Methods | Randomised controlled trial |
| Participants | Chronic heart failure patients with CSA and CSB |
| Interventions | Nocturnal CPAP plus best supportive care or best supportive care alone |
| Outcomes | Outcomes not included in this review: NYHA, ANP, and LVEF |
| Notes | We contacted the authors to check whether they had assessed any of the review outcomes, but have received no response. |
Toepfer 2003.
| Methods | Randomised controlled trial |
| Participants | People with severe central sleep apnoea due to HF |
| Interventions | ASV versus placebo |
| Outcomes | Outcomes not included in this review: maximal oxygen uptake (VO2max), anaerobic threshold (VO2), 6MWD, and ventilatory response to carbon dioxide |
| Notes | We contacted the authors to check whether they had assessed any of the review outcomes, but have received no response. |
Ushijima 2014.
| Methods | Randomised controlled trial |
| Participants | 57 people with HF (ejection fraction < 0.45), 40 with sleep apnoea |
| Interventions | CPAP and ASV |
| Outcomes | Haemodynamic parameters, respiratory parameters, and muscle sympathetic nerve activity |
| Notes | We contacted the authors to check the availability of separate data for CSA patients, but have received no response. |
Vogt‐Ladner 2002.
| Methods | Randomised controlled trial |
| Participants | People with stable chronic heart failure (age range 39 to 74 years); NYHA III; LEVF < 40% |
| Interventions | Nocturnal oxygen therapy versus ASV |
| Outcomes | Oxygen saturation, AHI, number of arousals/sleep time, 6MWD, and maximal oxygen uptake (VO2max) |
| Notes | We contacted the authors to check eligibility, but have received no response. |
Yoshihisa 2009.
| Methods | Randomised controlled trial |
| Participants | 18 participants with CSB and HF |
| Interventions | ASV plus best supportive care versus best supportive care |
| Outcomes | Oucomes not included in this review: plasma BNP levels, cardiac function variables |
| Notes | We contacted the authors to check whether they had assessed any of the review outcomes, but have received no response. |
Yoshihisa 2013.
| Methods | Randomised controlled trial |
| Participants | 36 participants with moderate to severe SDB (AHI > 15/h) and HF with preserved ejection fraction (LVEF > 50%) |
| Interventions | ASV plus best supportive care versus best supportive care |
| Outcomes | Oucomes not included in this review: NYHA, plasma BNP levels, cardiac function variables, troponin T |
| Notes | Included mixed population of OSA and CSA patients. No separate data were reported. We contacted the authors to check the availability of separate data for CSA patients, but have received no response. |
Zhao 2006.
| Methods | Randomised controlled trial |
| Participants | People with sleep apnoea and chronic heart failure |
| Interventions | Positive pressure ventilation or control |
| Outcomes | Plasma amino NT‐proBNP levels |
| Notes | We contacted the authors to check the availability of separate data for CSA patients and data on outcomes of interest, but have received no response. |
6MWD: 6‐minute walk distance; 6MWT: 6‐minute walk test; AHI: apnoea hypopnoea index; ANP: atrial natriuretic peptide; ASV: adaptive servo ventilation; BiPAP: bilevel positive airway pressure; BNP: B‐type natriuretic peptide; CPAP: continuous positive airway pressure; CRT: cardiac resynchronisation therapy; CSA: central sleep apnoea; CSB: Cheyne‐Stokes breathing; CV: cardiovascular; ESS: Epworth Sleepiness Scale; HF: heart failure; LVEF: left ventricular ejection fraction; MLHFQ: Minnesota Living with Heart Failure Questionnaire; MV: mechanical ventilation; NT‐proBNP: N‐terminal pro‐brain natriuretic peptide; NYHA: New York Heart Association; ODI: oxygen desaturation index; OSA: obstructive sleep apnoea; SD: standard deviation; SDB: sleep‐disordered breathing; SF‐36: 36‐item Short Form Health Survey; VO2: volume of oxygen
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐TRC‐12003881.
| Study name | Multicenter study on diagnosis of central sleep apnea complicated with heart failure by diaphragm electromyogram and efficacy of bi‐level positive airway pressure ventilation |
| Methods | Randomised controlled trial |
| Participants | People from 18 to 75 years, diagnosis of stable chronic heart failure (NYHA class I to III, LVEF < 40%, and serum NT‐proBNP > 450 pg/mL); people that have accepted best supportive care for at least 1 month; AHI of central sleep apnoea tested by diaphragm electromyogram more than 15/h |
| Interventions | BiPAP versus control |
| Outcomes | AHI, NT‐proBNP, LVEF, 6‐minute walk test, life quality score |
| Starting date | 1 January 2014 |
| Contact information | liujiannan@163.com, lugan0121@163.com |
| Notes |
NCT01128816.
| Study name | Advent‐HF |
| Methods | Multicentric, randomised controlled trial |
| Participants | Stable outpatients with a history of heart failure with preserved ejection fraction (LVEF ≤ 45%) of at least 3‐month duration and SDB, both central and obstructive types. Sleep apnoea is defined as an AHI ≥ 15, and is divided into predominantly OSA (≥ 50% of events obstructive) or predominantly CSA (> 50% of events central). |
| Interventions | ASV associated with standard medical therapy for heart failure alone compared to standard medical therapy for heart failure alone |
| Outcomes | The primary outcome is the cumulative incidence rate of the composite of all‐cause mortality, first hospitalisation for CV diseases, new‐onset atrial fibrillation/flutter requiring anticoagulation but not hospitalisation, and delivery of an appropriate discharge from an implantable cardioverter‐defibrillator (ICD) not resulting in hospitalisation. Secondary outcomes:
|
| Starting date | 22 September 2010 |
| Contact information | douglas.bradley@utoronto.ca |
| Notes |
NCT01212705.
| Study name | Effect of adaptive servoventilation on cardiac function in chronic heart failure and Cheyne‐Stokes respiration |
| Methods | Clinical trial |
| Participants | Adults with chronic heart failure with ejection fraction ≤ 45%, with best supportive care for at least 1 month and clinical diagnosis of CSB |
| Interventions | Adaptive servo ventilation |
| Outcomes | Cardiac function parameters |
| Starting date | 1 October 2010 |
| Contact information | annakazimierczak@poczta.onet.pl, krystian.krzyzanowski@gmail.com |
| Notes |
6MWD: 6‐minute walk distance; AHA/ACC: American Heart Association/American College of Cardiology; AHI: apnoea hypopnoea index; ASV: adaptive servo ventilation; BiPAP: bilevel positive airway pressure; CSA: central sleep apnoea; CSB: Cheyne‐Stokes breathing; CV: cardiovascular; ESS: Epworth Sleepiness Scale; HF: heart failure; LV: left ventricular; LVEF: left ventricular ejection fraction; MLHFQ: Minnesota Living with Heart Failure Questionnaire; NT‐proBNP: N‐terminal pro‐brain natriuretic peptide; NYHA: New York Heart Association; OSA: obstructive sleep apnoea; SDB: sleep‐disordered breathing
Differences between protocol and review
We decided not to include studies focusing on central sleep apnoea (CSA) due to periodic breathing at high altitudes because this condition is usually triggered by environmental exposure and constitutes a very different condition compared to other types of CSA studies in this review. Although we did not find studies including people with CSA due to periodic breathing at high altitudes, we plan to exclude these studies in future updates of this review. This review was otherwise performed in accordance with our protocol (Pachito 2017).
Contributions of authors
ACPNP: co‐ordination of the review; search and selection of studies for inclusion in the review; data collection; risk of bias assessment; data analysis; GRADE assessment; interpretation of data; writing of the review.
AR: search and selection of studies for inclusion in the review; data collection; risk of bias assessment; data analysis; GRADE assessment; interpretation of data; writing of the review.
DVP: conception of the review; design of the review; co‐ordination of the review; search and selection of studies for inclusion in the review; data collection; risk of bias assessment; data analysis; GRADE assessment; interpretation of data; writing of the review.
LFD: writing of the review.
GLF: writing of the review.
Contributions of editorial team
Sally Spencer (Co‐ordinating Editor) edited the review; advised on methodology, interpretation, and content; approved the review prior to publication.
Teresa Anna Cantisani (Contact Editor): edited the review; advised on methodology, interpretation, and content.
Rebecca Fortescue (Co‐ordinating Editor): checked the data in the review.
Emma Dennett (Deputy Co‐ordinating Editor): advised on methodology, interpretation, and content; edited the review.
Emma Jackson (Managing Editor): co‐ordinated the editorial process; conducted peer review and edited the review.
Elizabeth Stovold (Information Specialist): designed the search strategy; ran the searches; edited the Search methods section.
Sources of support
Internal sources
-
Cochrane Brazil, Brazil
Institutional support for DVP, ALCM, COCL, RLP, RR
-
Universidade Federal da São Paulo, Brazil
Institutional support for DVP, ALCM, COCL, RLP, RR
-
Universidade de São Paulo ‐ Instituto do Coração (USP ‐ INCOR), Brazil
Institutional support for LD, GLF
External sources
-
National Institute for Health and Care Research, UK
Cochrane infrastructure funding to the Airways group
Declarations of interest
ACPNP: none known.
AR: none known.
DVP: ResMed Brasil ‐ consultancy activities for economic studies related to continuous positive airway pressure therapy.
LFD: ResMed Foundation ‐ scientific consultant for real world data.
GLF: Biologix ‐ ownership of stocks of a startup of a simple device for sleep apnoea diagnosis.
Edited (no change to conclusions)
References
References to studies included in this review
CANPAP 2006 {published data only}
- Arzt M, Floras JS, Logan AG, Kimoff RJ, Series F, Morrison D, et al. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007;115 C(25):3173-80. [DOI: 10.1161/CIRCULATIONAHA.106.683482] [CRSREF: 19581355] [DOI] [PubMed] [Google Scholar]
- Arzt M, Floras JS, Logan AG, Ryan C, Tomlinson G, Douglas BT. The CANPAP trial: effects of CPAP on central sleep apnea, cardiovascular function and mortality in heart failure [Abstract]. Sleep Medicine 2006;7 C:S19 [142]. [DOI: 10.1016/j.sleep.2006.07.046] [DOI] [Google Scholar]
- Arzt M, Ryan C, Logan AG, Floras JS, Bradley TD. Transplant free survival of heart failure patients according to effect of CPAP on central sleep apnea in the CANPAP trial [Abstract]. Proceedings of the American Thoracic Society 2006;3:A516. [Google Scholar]
- Bradley TD, Logan AG, Kimoff RJ, Series F, Morrison D, Ferguson K, et al. Continuous positive airway pressure for central sleep apnea and heart failure. New England Journal of Medicine 2005;353(19):2025-33. [DOI: 10.1056/NEJMoa051001] [DOI] [PubMed] [Google Scholar]
- Ruttanaumpawan P, Logan AG, Floras JS, Bradley TD, CANPAP Investigators. Effect of continuous positive airway pressure on sleep structure in heart failure patients with central sleep apnea. Sleep 2009;32 C(1):91-8. [PMC free article] [PubMed] [Google Scholar]
- Ruttanaumpawan P, Logan AG, Floras JS, Bradley TD, CANPAP Investigators. Effect of continuous positive airways pressure on sleep structure in heart failure patients with central sleep apnea [Abstract]. In: American Thoracic Society International Conference; 2007 May 18-23; San Francisco. 2007.
D'Elia 2019 {published data only}
- D'elia E, Ferrero P, Vittori C, Baio P, Duino V, Grosu A, et al. Enhanced adaptive servo-ventilation improves echocardiographic parameters of diastolic and right ventricle function in patients with sleep apnea and heart failure with preserved ejection fraction. European Journal of Heart Failure 2016;18:474. [Google Scholar]
- D’Elia E, Ferrero P, Vittori C, Iacovoni A, Grosu A, Gori M, et al. Beneficial effects of adaptive servo-ventilation on natriuretic peptides and diastolic function in acute heart failure patients with preserved ejection fraction and sleep-disordered breathing. Sleep and Breathing 2019;23:287-91. [DOI: 10.1007/s11325-018-1681-z] [DOI] [PubMed] [Google Scholar]
Dellweg 2013 {published data only}
- Dellweg D, Kerl J, Hoehn E, Wenzel M, Koehler D. Randomized controlled trial of noninvasive positive pressure ventilation (NPPV) versus servoventilation in patients with CPAP-induced central sleep apnea (complex sleep apnea). Sleep 2013;36(8):1163-71. [DOI: 10.5665/sleep.2878] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fietze 2008 {published data only}
- Fietze I, Blau A, Glos M, Theres H, Baumann G, Penzel T. Bi-level positive pressure ventilation and adaptive servo ventilation in patients with heart failure and Cheyne-Stokes respiration. Sleep Medicine 2008;9:652-9. [DOI: 10.1016/j.sleep.2007.09.008] [DOI] [PubMed] [Google Scholar]
Granton 1996 {published data only}
- Granton JT, Naughton MT, Benard DC, Liu PP, Goldstein RS, Bradley TD. CPAP improves inspiratory muscle strength in patients with heart failure and central sleep apnea. American Journal of Respiratory and Critical Care Medicine 1996;153(1):277-82. [DOI: 10.1164/ajrccm.153.1.8542129] [DOI] [PubMed] [Google Scholar]
Hetland 2013 {published data only}
- Hetland A, Haugaa KH, Olseng M, Gjesdal O, Ross S, Saberniak J, et al. Three months treatment with adaptive servo-ventilation improves cardiac function and physical activity in patients with chronic heart failure and Cheyne-Stokes respiration in a prospective randomized controlled trial. Circulation 2011;124(21 Suppl):A11007. [DOI: 10.1161/circ.124.suppl_21.A11007] [DOI] [PubMed] [Google Scholar]
- Hetland A, Haugaa KH, Olseng M, Gjesdal O, Ross S, Saberniak J, et al. Three-month treatment with adaptive servoventilation improves cardiac function and physical activity in patients with chronic heart failure and Cheyne-Stokes respiration: a prospective randomized controlled trial. Cardiology 2013;126(C(2)):81-90. [DOI: 10.1159/000350826] [DOI] [PubMed] [Google Scholar]
- Olseng MW, Olsen BF, Hetland A, Fagermoen S, Jacobsen M. Quality of life improves in patients with chronic heart failure and Cheyne-Stokes respiration treated with adaptive servo-ventilation in a nurse-led heart failure clinic. Journal of Clinical Nursing 2017;26(9-10):1226-33. [DOI: 10.1111/jocn.13416] [DOI] [PubMed] [Google Scholar]
Kasai 2013 {published data only}
- Kasai T, Kasagi S, Maeno K-I, Dohi T, Kawana F, Kato M, et al. Adaptive servo-ventilation in cardiac function and neurohormonal status in patients with heart failure and central sleep apnea nonresponsive to continuous positive airway pressure. JACC: Heart Failure 2013;1(1):58-63. [DOI: 10.1016/j.jchf.2012.11.002] [DOI] [PubMed] [Google Scholar]
Morgenthaler 2014 {published data only}
- Morgenthaler TI, Kuzniar TJ, Wolfe LF, Willes L, McLain WC, Goldberg R. The Complex Sleep Apnea Resolution Study: a prospective randomized controlled trial of continuous positive airway pressure versus adaptive servoventilation therapy. Sleep 2014;37(5):927-34. [DOI: 10.5665/sleep.3662] [DOI] [PMC free article] [PubMed] [Google Scholar]
Naughton 1995a {published data only}
- Naughton MT, Liu PP, Bernard DC, Goldstein RS, Bradley TD. Treatment of congestive heart failure and Cheyne-Stokes respiration during sleep by continuous positive airway pressure. American Journal of Respiratory and Critical Care Medicine 1995;151(1):92-7. [DOI: 10.1164/ajrccm.151.1.7812579] [DOI] [PubMed] [Google Scholar]
- Naughton MT, Lui PP, Benard DC, Goldstein RS, Bradley TD. A randomized controlled clinical trial of CPAP in patients with heart failure and Cheyne-Stokes respiration. American Review of Respiratory Disease 1993;147(7):A687. [Google Scholar]
Naughton 1995b {published data only}
- Naughton MT, Benard DC, Liu PP, Rutherford R, Rankin F, Bradley TD. Effects of nasal CPAP on sympathetic activity in patients with heart failure and central sleep apnea. American Journal of Respiratory and Critical Care Medicine 1995;152(2):473-9. [DOI: 10.1164/ajrccm.152.2.7633695] [DOI] [PubMed] [Google Scholar]
Pepperell 2003 {published data only}
- Pepperell JCT, Maskell NA, Jones DR, Langford-Wiley BA, Crosthwaite N, Stradling JR, et al. A randomized controlled trial of adaptive ventilation for Cheyne-Stokes breathing in heart failure. American Journal of Respiratory and Critical Care Medicine 2003;168(9):1109-14. [DOI: 10.1164/rccm.200212-1476OC] [DOI] [PubMed] [Google Scholar]
Philippe 2006 {published data only}
- Philippe C, Stoica-Herman M, Drouot X, Raffestin B, Escourrou P, Hittinger L, et al. Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne-Stokes respiration in heart failure over a six month period. Heart 2006;92(3):337-42. [DOI: 10.1136/hrt.2005.060038] [DOI] [PMC free article] [PubMed] [Google Scholar]
SERVE HF 2015 {published data only}
- Cowie M, Woehrle H, Wegscheider K, Angermann C, D'Ortho MP, Erdmann E, et al. Treatment of predominant central sleep apnoea with ASV in patients with chronic heart failure: SERVE-HF primary results. European Respiratory Journal 2015;46:OA3475. [DOI: 10.1183/13993003.congress-2015.OA3475] [DOI] [Google Scholar]
- Cowie MR, Woehrle H, Wegscheider K, Angermann C, D'Ortho M-P, Erdmann E, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. New England Journal of Medicine 2015;373(12):1095-105. [DOI: 10.1056/NEJMoa1506459] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie MR, Woehrle H, Wegscheider K, Vettorazzi E, Lezius S, Koenig W, et al. Adaptive servo-ventilation for central sleep apnoea in systolic heart failure: results of the major substudy of SERVE-HF. European Journal of Heart Failure 2018;20:536-44. [DOI: 10.1002/ejhf.1048] [DOI] [PubMed] [Google Scholar]
- Eulenburg C, Wegscheider K, Woehrle H, Angermann C, D'Ortho M-P, Erdmann E, et al. Understanding SERVE-HF: a multistate analysis to explain mechanisms underlying increased mortality risk in patients randomised to adaptive servo-ventilation (ASV). European Respiratory Journal 2016;48:OA1985. [DOI: 10.1183/13993003.congress-2016.OA1985] [DOI] [Google Scholar]
- Tamisier R, Pepin JL, Cowie M, Wegscheider K, Vettorazzi E, Suling A, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure: PSG data from the SERVE-HF major substudy. American Journal of Respiratory and Critical Care Medicine 2017;195:A4513. [DOI: 10.1164/ajrccm-conference.2017.B80-G] [DOI] [Google Scholar]
- Tamisier R, Pepin J-L, Salvat M, Woehrle H, Barone-Rochette G, Rocca C, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure does not improve muscle sympathetic nerve activity: a SERVE-HF substudy. European Respiratory Journal 2016;48:PA2083. [DOI: 10.1183/13993003.congress-2016.PA2083] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sin 2000 {published data only}
- Sin DD, Logan AG, Fitzgerald FS, Liu PP, Bradley TD. Effects of continuous positive airway pressure on cardiovascular outcomes in heart failure patients with and without Cheyne-Stokes respiration. Circulation 2000;102 C(1):61-6. [DOI: 10.1161/01.cir.102.1.61] [DOI] [PubMed] [Google Scholar]
Toyama 2016 {published data only}
- Toyama T, Hoshizaki H, Kasama S, Miyaishi Y, Kan H, Yamashita E, et al. Adaptive servo-ventilation therapy improves cardiac sympathetic nerve activity, cardiac function, exercise capacity, and symptom in patients with chronic heart failure and Cheyne-Stokes respiration. Journal of Nuclear Cardiology 2016;24(6):1-12. [DOI: 10.1007/s12350-016-0529-9] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arzt 2005 {published data only}
- Arzt M, Schulz M, Wensel R, Montalvan S, Blumberg FC, Riegger GA, et al. Nocturnal continuous positive airway pressure improves ventilatory efficiency during exercise in patients with chronic heart failure. Chest 2005;127(3):794-802. [DOI: 10.1378/chest.127.3.794] [DOI] [PubMed] [Google Scholar]
Campbell 2012 {published data only}
- Campbell A, Ferrier K, Neill A. Oxygen versus adaptive pressure support servoventilation (Autoset CS) in patients with central sleep apnoea-Cheyne Stokes respiration (CSA-CSR) and congestive heart failure (CHF). Internal Medicine Journal 2005;35:A43. [DOI] [PubMed] [Google Scholar]
- Campbell AJ, Ferrier K, Neill AM. Effect of oxygen versus adaptive pressure support servo-ventilation in patients with central sleep apnoea-Cheyne Stokes respiration and congestive heart failure. Internal Medicine Journal 2012;42(10):1130-6. [DOI: 10.1111/j.1445-5994.2011.02623.x] [DOI] [PubMed] [Google Scholar]
Cao 2014 {published data only}
- Cao M, Kushida C, Cardell C, Willes L, Mendoza J, Benjaield A. A novel adaptive servo ventilation (ASVAuto) for the treatment of central sleep apnea associated with chronic use of opioids. Sleep 2014;37:A143. [DOI: 10.5664/jcsm.3954] [DOI] [PMC free article] [PubMed] [Google Scholar]
Galetke 2014 {published data only}
- Galetke W, Anduleit N, Funke N, Kuhnel J, Richter K, Stieglitz S. Continuous positive airway pressure versus adaptive servoventilation in patients with combined obstructive sleep apnea and Cheyne-Stokes respiration. American Thoracic Society International Conference 2009;179:A5339. [Google Scholar]
- Galetke W, Ghassemi BM, Priegnitz C, Stieglitz S, Anduleit N, Richter K, et al. Anticyclic modulated ventilation versus continuous positive airway pressure in patients with coexisting obstructive sleep apnea and Cheyne-Stokes respiration: a randomized crossover trial. Sleep Medicine 2014;15(8):874-9. [DOI: 10.1016/j.sleep.2014.02.012] [DOI] [PubMed] [Google Scholar]
- Galetke W, Kietzmann I, Richter K, Randerath W. Continuous positive airway pressure versus adaptive servoventilation in patients with obstructive sleep apnea and Cheyne-stokes respiration - a randomized cross-over trial. In: European Respiratory Society 19th Annual Congress; 2009 Sep 12-15; Vienna. 2009:[362].
Hetland 2014 {published data only}
- Hetland A, Haugaa K, Vistnes M, Liland KH, Olseng M, Jacobsen MB, et al. Adaptive servo-ventilation decreased mortality and admission rates in heart failure patients with Cheyne-Stokes respiration in a 18 months prospective study. Journal of the American College of Cardiology 2014;63(12 Suppl 1):A757. [Google Scholar]
- Hetland A, Haugaa KH, Vistnes M, Liland KH, Olseng M, Jacobsen MB, et al. A retrospective analysis of cardiovascular outcomes in patients treated with ASV. Scandinavian Cardiovascular Journal 2017;51(2):106-13. [DOI: 10.1080/14017431.2016.1262546] [DOI] [PubMed] [Google Scholar]
Hetland 2017 {published data only}
- Hetland A, Lerum TV, Haugaa KH, Edvardsen T. Patients with Cheyne Stokes respiration and heart failure: patient tolerance after three-month discontinuation of treatment with adaptive servo-ventilation. Heart and Vessels 2017;32(8):909-15. [DOI: 10.1007/s00380-017-0951-1] [DOI] [PubMed] [Google Scholar]
Hetzenecker 2016 {published data only}
- Hetzenecker A, Roth T, Birner C, Maier LS, Pfeifer M, Arzt M. Adaptive servo-ventilation therapy of central sleep apnoea and its effect on sleep quality. Clinical Research in Cardiology 2016;105(3):189-95. [DOI: 10.1007/s00392-015-0904-6] [DOI] [PubMed] [Google Scholar]
Hu 2005 {published data only}
- Hu K, Yang J, Chen XQ, Yu CP, Zhao JL. Comparison of oxygen therapy with nasal continuous positive airway pressure on Cheyne-Stokes respiration in patients with chronic congestive heart failure. Chinese Journal of Internal Medicine 2005;44(10):759-63. [PubMed] [Google Scholar]
Javaheri 2011 {published data only}
- Javaheri S, Goetting MG, Goodwin JL, Wylie P, Khayat R. Auto servo ventilation successfully treats complex central sleep apnea (CompCSA) and Hunter-Cheyne-Stokes breathing (HCSB). Sleep 2010;33(Suppl):A146-7. [Google Scholar]
- Javaheri S, Goetting MG, Khayat R, Wylie PE, Goodwin JL, Parthasarathy S. The performance of two automatic servo-ventilation devices in the treatment of central sleep apnea. Sleep 2011;34(12):1693-8. [DOI: 10.5665/sleep.1438] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaheri S, Khayat R, Goodwin J, Wylie P, Goetting M. Complex central sleep apnea (compcsa) treatment with auto servo ventilation. Chest 2009;136(4):43S-f. [DOI: 10.1378/chest.136.4_MeetingAbstracts.43S-f] [DOI] [Google Scholar]
Kasai 2010 {published data only}
- Kasai T, Usui Y, Yoshioka T, Yanagisawa N, Takata Y, Narui K, et al. Effect of flow-triggered adaptive servo-ventilation compared with continuous positive airway pressure in patients with chronic heart failure with coexisting obstructive sleep apnea and Cheyne-Stokes respiration. Circulation: Heart Failure 2010;3(1):140-8. [DOI: 10.1161/CIRCHEARTFAILURE.109.868786] [DOI] [PubMed] [Google Scholar]
Kohnlein 2002 {published data only}
- Kohnlein T, Welte T, Tan LB, Elliott MW. Assisted ventilation for heart failure patients with Cheyne-Stokes respiration. European Respiratory Journal 2002;20 C(4):934-41. [DOI: 10.1183/09031936.00.02622001] [DOI] [PubMed] [Google Scholar]
- Kohnlein T, Welte T, Tan LB, Elliott MW. Treatment of central sleep apnea in patients with chronic heart failure with CPAP or bilevel ventilation. American Journal of Respiratory and Critical Care Medicine 2002;165 C(Suppl 8):A247. [Google Scholar]
Krachman 1999 {published data only}
- Krachman SL, D'Alonzo GE, Berger TJ, Eisen HJ. Comparison of oxygen therapy with nasal continuous positive airway pressure on Cheyne-Stokes respiration during sleep in congestive heart failure. Chest 1999;116 C(6):1550-7. [DOI: 10.1378/chest.116.6.1550] [DOI] [PubMed] [Google Scholar]
Krachman 2003 {published data only}
- Krachman SL, Crocetti J, Berger TJ, Chatila W, Eisen HJ, D'Alonzo GE. Effects of nasal continuous positive airway pressure on oxygen body stores in patients with Cheyne-Stokes respiration and congestive heart failure. Chest 2003;123(1):59-66. [DOI: 10.1378/chest.123.1.59] [DOI] [PubMed] [Google Scholar]
Liu 2015 {published data only}
- Liu J, Lu Z, Zhu X, Zhang D, Sun M, Zhang X, et al. Beneficial effect of bilevel positive airway pressure on Cheyne-Stokes respiration in patients with congestive heart failure. Sleep Medicine 2015;16:S190. [Google Scholar]
Morgenthaler 2007 {published data only}
- Gay PC, Morgenthaler TI, Brown LK, Fedorovich CL. A multi-center randomized trial of adaptive servo-ventilation (ASV) vs bilevel nasal positive pressure ventilation (NPPV) treatment for central apnea/Cheyne-Stokes respiration (CSA/CSR) and complex sleep apnea syndromes (CompSAS) [Abstract]. In: American Thoracic Society International Conference; 2006 May 19-21; San Diego. 2006.
- Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep 2007;30(4):468-75. [DOI: 10.1093/sleep/30.4.468] [DOI] [PubMed] [Google Scholar]
Oldenburg 2015 {published data only}
- Oldenburg O, Bitter T, Prib N, Lohse M, Koerber B, Fischbach T, et al. Performance of adaptive servoventilation and enhanced adaptive servoventilation in heart failure patients with central sleep apnea. Sleep 2012;35 C:A176. [Google Scholar]
- Oldenburg O, Spiesshofer J, Fox H, Prib N, Horstkotte D. Performance of conventional and enhanced adaptive servoventilation (ASV) in heart failure patients with central sleep apnea who have adapted to conventional ASV. Sleep and Breathing 2015;19(3):795-800. [DOI: 10.1007/s11325-014-1083-9] [DOI] [PubMed] [Google Scholar]
Priefert 2017 {published data only}
- Priefert HJ, Hetzenecker A, Escourrou P, Luigart R, Series F, Lewis K, et al. Effects of adaptive servo-ventilation on ventricular arrhythmias in patients with stable chronic heart failure and sleep-disordered breathing: subanalysis of a randomized controlled trial. Somnologie 2016;20(2):96-105. [DOI: 10.1007/s11818-016-0072-6] [DOI] [Google Scholar]
- Priefert H-J, Hetzenecker A, Escourrou P, Luigart R, Series F, Lewis K, et al. Effects of adaptive servoventilation on ventricular arrhythmias in patients with stable congestive heart failure and sleep-disordered breathing: subanalysis of a randomized controlled trial. Somnologie 2017;Suppl 1(21):S19–27. [DOI: 10.1007/s11818-016-0072-6] [DOI] [Google Scholar]
Randerath 2012 {published data only}
- Randerath W, Galetke W, Stiegliitz S, Priegnitz C, Schafer T. Adaptive servoventilation in coexisting obstructive sleep apnoea/hypopnoea and Cheyne-Stokes respiration [Abstract]. In: European Respiratory Society 18th Annual Congress; 2008 Oct 3-7; Berlin. 2008.
- Randerath W, Nothofer G, Anduleit N, Tremi M, Schafer T, Galetke W. Longterm efficacy of adaptive servo-ventilation (ASV) in patients with co-existing obstructive sleep apnea (OSAS) and Cheyne-stokes respiration (CSR). A randomized CPAP-controlled trial [Abstract]. In: European Respiratory Society 19th Annual Congress; 2009 Sep 12-15; Vienna. 2009:[359].
- Randerath W, Nothofer G, Anduleit N, Treml M, Priegnitz C, Galetke W. Effect of auto servo-ventilation (ASV) and continuous positive airway pressure (CPAP) on B-type natriuretic peptide (NT-proBNP) in patients with co-existing obstructive (OSA) and central sleep apnea (CSA) in heart failure (HF). In: European Respiratory Society Annual Congress; 2011 Sep 24-28; Amsterdam. 2011 . [299s [P1744]]
- Randerath W, Nothofer G, Anduleit N, Treml M, Priegnitz C, Galetke W. Efficacy of auto servo-ventilation (ASV) in patients with co-existing obstructive sleep apnea (OSAS) and Cheyne-Stokes respiration (CSR). A randomized CPAP-controlled trial [Abstract]. In: European Respiratory Society 20th Annual Congress; 2010 Sep 18-22; Barcelona. 2010:[P924].
- Randerath WJ, Galetke W, Domanski U, Gillissen A, Ruhle KH. Efficacy of adaptive servo-ventilation versus constant positive airways pressure therapy in patients with heart failure and Cheyne-Stokes respiration [Abstract]. In: American Thoracic Society 100th International Conference; 2004 May 21-26; Orlando. 2004:B87.
- Randerath WJ, Nothofer G, Priegnitz C, Anduleit N, Treml M, Kehl V, et al. Long-term auto-servoventilation or constant positive pressure in heart failure and coexisting central with obstructive sleep apnea. Chest 2012;142(2):440-7. [DOI: 10.1378/chest.11-2089] [DOI] [PubMed] [Google Scholar]
Shapiro 2015 {published data only}
- Pegram V, Wylie PE, Rosenberg R, Holle R, Shapiro CM, Chung SA, et al. Servo ventilation versus CPAP therapy in chronic pain patients with sleep disordered breathing. Sleep 2013;36 C:A150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro CM, Chung SA, Wylie PE, Hossain NK, Holle RH, Rosenberg RP, et al. Home-use servo-ventilation therapy in chronic pain patients with central sleep apnea: initial and 3-month follow-up. Schlaf und Atmung [Sleep and Breathing] 2015;19 C(4):1285-92. [DOI: 10.1007/s11325-015-1161-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie PE, Pegram V, Rosenberg R, Holle R, Shapiro CM, Hossain NK, et al. Home-use servo ventilation therapy in chronic pain patients with sleep disordered breathing. Sleep 2013;36 C:A149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Szollosi 2006 {published data only}
- Szollosi I, O'Driscoll DM, Dayer MJ, Coats AJ, Morrell MJ, Simonds AK. Adaptive servo-ventilation and deadspace: effects on central sleep apnoea. Journal of Sleep Research 2006;15(2):199-205. [DOI: 10.1111/j.1365-2869.2006.00515.x] [DOI] [PubMed] [Google Scholar]
Teschler 2001 {published data only}
- Teschler H, Dohring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. American Journal of Respiratory and Critical Care Medicine 2001;164 C(4):614-9. [DOI: 10.1164/ajrccm.164.4.9908114] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Alter 2013 {published data only}
- Alter P, Wagner J, Apelt S, Rupp H, Heitmann J. Influences of continuous positive airway pressure (CPAP) ventilation on cardiac function and left ventricular wall stress in dilative heart failure as assessed by cardiac magnetic resonance imaging. Somnologie 2013;17:75. [Google Scholar]
Arzt 2013 {published data only}
- Artz M, Series F, Lewis K, Escourrou P, Obermeier R, Keh IV, et al. Effects of auto-servo ventilation on cardiovascular function in patients with congestive heart failure and sleep-disordered breathing - a multicenter randomised controlled trial. European Respiratory Journal 2011;38:1743. [Google Scholar]
- Arzt M, Schroll S, Series F, Lewis K, Benjamin A, Escourrou P, et al. Auto-servoventilation in heart failure with sleep apnoea: a randomised controlled trial. European Respiratory Journal 2013;42:1244-54. [DOI: 10.1183/09031936.00083312] [DOI] [PubMed] [Google Scholar]
- Arzt M, Series F, Lewis K, Benjamin A, Escourrou P, Luigart R, et al. Treatment of central and obstructive sleep apnea in stable heart failure patients with auto-servo ventilation reduces sleep fragmentation - a randomized controlled trial. European Respiratory Journal 2012;40(C Suppl 56):588s [3289]. [Google Scholar]
- Birner C, Series F, Lewis K, Benjamin A, Escourrou P, Luigart R, et al. Treatment with auto-servo ventilation of patients with sleep-disordered breathing, stable systolic heart failure and concomitant diastolic dysfunction - a randomized controlled pilot study. European Respiratory Journal 2012;40:3467. [Google Scholar]
- Birner C, Series F, Lewis K, Benjamin A, Wunderlich S, Escourrou P, et al. Effects of auto-servo ventilation on patients with sleep-disordered breathing, stable systolic heart failure and concomitant diastolic dysfunction: subanalysis of a randomized controlled trial. Respiration 2014;87(1):54-62. [DOI: 10.1159/000351797] [DOI] [PubMed] [Google Scholar]
- Hetzenecker A, Escourrou P, Kuna ST, Series F, Lewis K, Birner C, et al. Treatment of sleep apnea in chronic heart failure patients with auto-servo ventilation improves sleep fragmentation: a randomized controlled trial. Sleep Medicine 2016;17:25-31. [DOI: 10.1016/j.sleep.2015.08.020] [DOI] [PubMed] [Google Scholar]
CAT‐HF 2016 {published data only}
- Daubert MA, Whellan DJ, Woehrle H, Tasissa G, Anstrom KJ, Lindenfeld J, et al. Treatment of sleep-disordered breathing in heart failure impacts cardiac remodeling: insights from the CATHF Trial. American Heart Journal 2018;201:40-8. [DOI: 10.1016/j.ahj.2018.03.026] [DOI] [PubMed] [Google Scholar]
- Fiuzat M, Oldenberg O, Whellan DJ, Woehrle H, Punjabi NM, Anstrom KJ, et al. Lessons learned from a clinical trial: design, rationale, and insights from The Cardiovascular Improvements with Minute Ventilation-targeted Adaptive Sero-Ventilation (ASV) Therapy in Heart Failure (CAT-HF) Study. Contemporary Clinical Trials 2016;47:158-64. [DOI: 10.1016/j.cct.2016.01.001] [DOI] [PubMed] [Google Scholar]
- O'connor C, Whellan D, Fiuzat M, Benjafield A, Woehrle H, Punjabi N, et al. Adaptive servo-ventilation in heart failure: results of a randomized, controlled clinical trial. European Journal of Heart Failure 2016;18:318. [DOI: 10.1002/ejhf.539] [DOI] [Google Scholar]
- O'Connor CM, Whellan DJ, Fiuzat M, Punjabi NM, Tasissa G, Anstrom KJ, et al. Cardiovascular outcomes with minute ventilation-targeted adaptive servo-ventilation therapy in heart failure: the CAT-HF trial. Journal of the American College of Cardiology 2017;69(12):1577-87. [DOI: 10.1016/j.jacc.2017.01.041] [DOI] [PubMed] [Google Scholar]
- Piccini JP, Pokorney SD, Anstrom KJ, Oldenburg O, Punjabi NM, Fiuzat M, et al. Adaptive servo-ventilation reduces atrial fibrillation burden in patients with heart failure and sleep apnea. Heart Rhythm 2019;16:91-7. [DOI: 10.1016/j.hrthm.2018.07.027] [DOI] [PubMed] [Google Scholar]
De Michelis 2008 {published data only}
- De Michelis C, Prest G, Perretta E, Riva L, Zoccali P. Congestive heart failure and Cheyne-Stokes periodic respiration breathing: new modalities of positive airways pressure ventilation alternative to CPAP. Rassegna di Patologia dell'Apparato Respiratorio 2008;23(1):17-25. [Google Scholar]
Egea 2004 {published and unpublished data}
- Egea C, Aizpuru F, Pinto J, Ayuela J, Ballester E, Zamarron C, et al. Cardiac function after CPAP therapy in chronic heart failure and sleep apnea. A multicenter study. In: American Thoracic Society International Conference; 2007 May 18-23; San Francisco. [A296]
- Egea C, Pinto J, Ayuela J, Ballester E, Zamarron C, Sojo A, et al. Eficacy of the treatment with CPAP in patients with chronic heart failure and sleep apnoea. European Respiratory Journal 2004;24 C(Suppl 48):564s. [Google Scholar]
- Egea CJ, Aizpuru F, Pinto JA, Ayuela JM, Ballester E, Zamarron C, et al. Cardiac function after CPAP therapy in patients with chronic heart failure and sleep apnea: a multicenter study. Sleep Medicine 2008;9 C(6):660-6. [DOI: 10.1016/j.sleep.2007.06.018] [DOI] [PubMed] [Google Scholar]
Goldberg 2007 {published data only}
- Goldberg LR, Horstkotte D, Shakar SF, Lowes BD, Walker JM, Cloward TV, et al. Long term results of a randomized multicenter trial comparing adaptive servo ventilation positive airway pressure to nasal oxygen in heart failure patients with central sleep apnea. Journal of the American College of Cardiology 2007;49(9):94A-5A. [Google Scholar]
Miyata 2012 {published data only}
- Miyata M, Yoshihisa A, Suzuki S, Sugimoto K, Yamaki T, Kunii H, et al. Adaptive servo ventilation improves cardiac function and reduces re-hospitalization in chronic heart failure patients with Cheyne-Stokes respiration after cardiac resynchronization therapy. European Heart Journal 2012;33:332. [DOI: 10.1016/j.jjcc.2012.01.021] [DOI] [Google Scholar]
- Miyata M, Yoshihisa A, Suzuki S, Yamada S, Kamioka M, Kamiyama Y, et al. Adaptive servo ventilation improves cardiac function and prognosis in chronic heart failure patients with Cheyne-Stokes respiration after cardiac resynchronization therapy. In: American Heart Association (AHA) Scientific Sessions; 2011 Nov 12-16; Orlando. 2011.
Murase 2016 {published data only}
- Murase K, Ono K, Akao M, Miki S, Nohara R, Mishima M, et al. The clinical effects of adaptive servo ventilation versus nocturnal oxygen therapy for sleep disordered breathing in heart failure patients: a randomized trial. American Journal of Respiratory and Critical Care Medicine 2015;191:A6109. [Google Scholar]
- Murase K, Ono K, Yoneda T, Iguchi M, Yokomatsu T, Mizoguchi T, et al. Adaptive servoventilation versus oxygen therapy for sleep disordered breathing in patients with heart failure: a randomised trial. Open Heart 2016;3(1):e000366. [DOI: 10.1136/openhrt-2015-000366] [DOI] [PMC free article] [PubMed] [Google Scholar]
Noda 2007 {published data only}
- Noda A, Izawa H, Asano H, Nakata S, Hirashiki A, Murase Y, et al. Beneficial effect of bilevel positive airway pressure on left ventricular function in ambulatory patients with idiopathic dilated cardiomyopathy and central sleep apnea-hypopnea: a preliminary study. Chest 2007;131(C(6)):1694-701. [DOI: 10.1378/chest.06-2271] [DOI] [PubMed] [Google Scholar]
Teschler 2000 {published data only}
- Teschler H, Dohring J, Wessendorf TE, Wang YM, Thilmann A, Berthon Jones M. Effect of autoset CS servo-ventilation on central sleep apnea in brainstem stroke. American Journal of Respiratory and Critical Care Medicine 2000;161 C(3 Suppl):A361. [Google Scholar]
Tkacova 1997 {published data only}
- Tkacova R, Liu PP, Naughton MT, Bradley TD. Effect of continuous positive airway pressure on mitral regurgitant fraction and atrial natriuretic peptide in patients with heart failure. Journal of the American College of Cardiology 1997;30(3):739-45. [DOI] [PubMed] [Google Scholar]
Toepfer 2003 {published data only}
- Toepfer V, El-Sebai MA, Schulz R, Wessendorf TE, Teschler H. Six weeks adaptive servo-ventilation increases exercise capacity and reduces sympathetic activity in central sleep apnea due to heart failure [Abstract]. In: American Thoracic Society 99th International Conference; 2003 May 16-21; Seattle. 2003.
Ushijima 2014 {published data only}
- Ushijima R, Joho S, Akabane T, Oda Y, Inoue H. Differing effects of adaptive servoventilation and continuous positive airway pressure on muscle sympathetic nerve activity in patients with heart failure. Circulation Journal 2014;78(6):1387-95. [DOI: 10.1253/circj.cj-13-1468] [DOI] [PubMed] [Google Scholar]
Vogt‐Ladner 2002 {published data only}
- Vogt Ladner G, Schacher C, Ditterich W, Vogt M, Teschler H, Worth H. Nocturnal oxygen therapy (NOY) versus adaptive servo-ventilation (ASV) in patients with severe chronic heart failure (CHF) and Cheyne Stokes respiration (CSR). American Journal of Respiratory and Critical Care Medicine 2002;165(C Suppl 8):A247. [Google Scholar]
Yoshihisa 2009 {published data only}
- Yoshihisa A, Sato T, Suzuki H, Saito S, Ishibashi T, Takeishi Y. Adaptive servo ventilation restores cardiac dysfunction in heart failure patients with Cheyne-Stokes respiration. European Journal of Heart Failure 2009;8:ii75. [Google Scholar]
Yoshihisa 2013 {published data only}
- Yoshihisa A, Suzuki S, Takeishi Y. Impact of adaptive servo ventilation on cardiovascular function and prognosis in patients with HFpEF and sleep-disordered breathing. Journal of Cardiac Failure 2014;20(10):S140. [Google Scholar]
- Yoshihisa A, Suzuki S, Yamaki T, Sugimoto K, Kunii H, Nakazato K, et al. Impact of adaptive servo-ventilation on cardiovascular function and prognosis in heart failure patients with preserved leC ventricular ejection fraction and sleep disordered breathing. European Journal of Heart Failure 2013;15:543-50. [DOI: 10.1093/eurjhf/hfs197] [DOI] [PubMed] [Google Scholar]
Zhao 2006 {published data only}
- Zhao Z, Liu Z, Luo Q. The effect of positive pressure ventilation in patients with heart failure and sleep apnea. In: American Thoracic Society International Conference; 2008 May 16-21; Toronto. 2008:A480.
- Zhao ZH, Liu ZH, Luo Q, Xiong CM, Ni XH, Zhang J, et al. Positive pressure ventilation treatment reduces plasma levels of amino terminal-pro brain natriuretic peptide in congestive heart failure patients with sleep apnea. Circulation Journal 2006;70(5):572-4. [DOI: 10.1253/circj.70.572] [DOI] [PubMed] [Google Scholar]
- Zhao ZH, Liu ZH, Luo Q, Zhang J, Xiong CM, Ni XH, et al. The effects of noninvasive positive pressure ventilation treatment on plasma concentration of amino terminal-pro brain natriuretic peptide in congestive heart failure in patients with sleep apnea. Zhonghua nei ke za zhi [Chinese Journal of Internal Medicine] 2006;45(5):386-8. [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR‐TRC‐12003881 {published data only}
- ChiCTR-TRC-12003881. Multicenter study on diagnosis of central sleep apnea complicated with heart failure by diaphragm electromyogram and efficacy of bi-level positive airway pressure ventilation. www.chictr.org.cn/hvshowproject.aspx?id=8123 (first received 1 January 2014).
NCT01128816 {published data only}
- Lyons OD, Floras JS, Logan AG, Beanlands R, Cantolla JD, Fitzpatrick M, et al. Design of the effect of adaptive servo-ventilation on survival and cardiovascular hospital admissions in patients with heart failure and sleep apnoea: the ADVENT-HF trial. European Journal of Heart Failure 2017;19(4):579-87. [DOI: 10.1002/ejhf.790] [DOI] [PubMed]
- NCT01128816. Effect of adaptive servo ventilation (ASV) on survival and hospital admissions in heart failure [A multi-centre, randomized study to assess the effects of adaptive servo ventilation (ASV) on survival and frequency of hospital admissions in patients with heart failure (HF) and sleep apnea (SA) - The ADVENT-HF Trial]. clinicaltrials.gov/show/NCT01128816 (first received 24 May 2010).
NCT01212705 {published data only}
- NCT01212705. Effect of adaptive servoventilation on cardiac function in chronic heart failure and Cheyne-Stokes respiration [Effect of adaptive servoventilation on cardiac function, exercise tolerance and quality of life in patients with chronic heart failure and Cheyne-Stokes respiration]. clinicaltrials.gov/show/NCT01212705 (first received 1 October 2010).
Additional references
American Academy of Sleep Medicine 2014
- American Academy of Sleep Medicine. International Classification of Sleep Disorders. Darien: American Academy of Sleep Medicine, 2014. [Google Scholar]
Aurora 2012
- Aurora RN, Chowdhuri S, Ramar K, Bista SR, Casey KR, Lamm CI, et al. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses. Sleep 2012;35(1):17-40. [DOI: 10.5665/sleep.1580] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bitter 2010
- Bitter T, Westerheide N, Faber L, Hering D, Prinz C, Langer C, et al. Adaptive servoventilation in diastolic heart failure and Cheyne-Stokes respiration. European Respiratory Journal 2010;36(2):385-92. [DOI: 10.1183/09031936.00045609] [DOI] [PubMed] [Google Scholar]
Bixler 2001
- Bixler EO, Vgontzas AN, Lin H, Ten Have T, Rein J, Vela-Bueno A, et al. Prevalence of sleep-disordered breathing in women: effects of gender. American Journal of Respiratory and Critical Care Medicine 2001;163:608-13. [DOI: 10.1164/ajrccm.163.3.9911064] [DOI] [PubMed] [Google Scholar]
Buysse 1989
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research 1989;28(2):193-213. [DOI: 10.1016/0165-1781(89)90047-4] [DOI] [PubMed] [Google Scholar]
Carrero 2011
- Carrero JJ, De Jager DJ, Verduijn M, Ravani P, De Meester J, Heaf JG, et al. Cardiovascular and noncardiovascular mortality among men and women starting dialysis. Clinical Journal of the American Society of Nephrology 2011;6(7):1722-30. [DOI: 10.2215/CJN.11331210] [DOI] [PubMed] [Google Scholar]
Correa 2015
- Correa D, Farney RJ, Chung F, Prasad A, Lam D, Wong J. Chronic opioid use and central sleep apnea. Anesthesia and Analgesia 2015;120(6):1273-85. [DOI: 10.1213/ANE.0000000000000672] [DOI] [PubMed] [Google Scholar]
CRS 2022
- CRS (Cochrane Register of Studies). community.cochrane.org/help/tools-and-software/crs-cochrane-register-studies (accessed 4 February 2022).
Donovan 2016
- Donovan LM, Kapur VK. Prevalence and characteristics of central compared to obstructive sleep apnea: analyses from the Sleep Heart Health Study cohort. Sleep 2016;39(7):1353-9. [DOI: 10.5665/sleep.5962] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eckert 2007
- Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest 2007;131(2):595-607. [DOI: 10.1378/chest.06.2287] [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOC 2013
- EPOC. Data collection form. EPOC Resources for Review Authors. epoc.cochrane.org/epoc-specific-resources review-authors (accessed prior to 24 July 2017).
Epstein 2009
- Epstein LJ, Kristo D, Strollo PJ, Friedman N, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. Journal of Clinical Sleep Medicine 2009;5(3):263-76. [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 24 July 2017. Hamilton (ON): McMaster University (developed by Evidence Prime). Available from gradepro.org.
Hanly 1996
- Hanly PJ, Zuberi-Khokhar NS. Increased mortality associated with Cheyne-Stokes respiration in patients with congestive heart failure. American Journal of Respiratory and Critical Care Medicine 1996;153(1):272-6. [DOI: 10.1164/ajrccm.153.1.8542128] [DOI] [PubMed] [Google Scholar]
Hanly 2001
- Hanly PJ, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. New England Journal of Medicine 2001;344(2):102-7. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2022
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Javaheri 2009
- Javaheri S, Smith J, Chung E. The prevalence and natural history of complex sleep apnea. Journal of Clinical Sleep Medicine 2009;5:205-11. [PMC free article] [PubMed] [Google Scholar]
Jenkinson 1996
- Jenkinson C, Layte R, Wright L, Coulter A. Manual and Interpretation Guide for the UK SF-36. Oxford: Health Services Research Unit, 1996. [Google Scholar]
Johnson 2010
- Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. Journal of Clinical Sleep Medicine 2010;6(2):131-7. [DOI: 10.5664/jcsm.27760] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuźniar 2008a
- Kuzniar TJ, Pusalavidyasagar S, Gay PC, Morgenthaler TI. Natural course of complex sleep apnea - a retrospective study. Sleep and Breathing 2008;12:135-9. [DOI: 10.1007/s11325-007-0140-z] [DOI] [PubMed] [Google Scholar]
Kuźniar 2008b
- Kuźniar TJ, Morgenthaler TI. Treatment of complex sleep apnea syndrome. Current Treatment Options in Neurology 2008;10(5):336-41. [DOI: 10.1007/s11940-008-0036-7] [DOI] [PubMed] [Google Scholar]
Kuźniar 2011
- Kuźniar TJ, Kovacevic-Ristanovic R, Freedom T. Complex sleep apnea unmasked by the use of a mandibular advancement device. Sleep and Breathing 2011;15(2):249-52. [DOI: 10.1007/s11325-010-0459-8] [DOI] [PubMed] [Google Scholar]
MacDonald 2008
- MacDonald M, Fang J, Pittman SD, White DP, Malhotra A. The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. Journal of Clinical Sleep Medicine 2008;4(1):38-42. [DOI: 10.5664/jcsm.27076] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI: 10.1136/bmj.b2535] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moura 2001
- Moura SMT, Bittencourt LR, Bagnato MC, Lucas SR, Tufik S, Nery LE, et al. Acute effect of nasal continuous positive air pressure on the ventilatory control of patients with obstructive sleep apnea. Respiration 2001;68(3):243-9. [DOI: 10.1159/000050505] [DOI] [PubMed] [Google Scholar]
Muzumdar 2008
- Muzumdar H, Arens R. Central alveolar hypoventilation syndromes. Sleep Medicine Clinics 2008;3(4):601-15. [DOI: 10.1016/j.jsmc.2008.08.006] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakamura 2015
- Nakamura S, Asai K, Kubota Y, Murai K, Takano H, Tsukada YT, et al. Impact of sleep-disordered breathing and efficacy of positive airway pressure on mortality in patients with chronic heart failure and sleep-disordered breathing: a meta-analysis. Clinical Research in Cardiology 2015;104(3):208-16. [DOI: 10.1007/s00392-014-0774-3] [DOI] [PubMed] [Google Scholar]
Naughton 2012
- Naughton MT. Cheyne's Stokes respiration: friend or foe? Thorax 2012;67(4):357-60. [DOI: 10.1136/thoraxjnl-2011-200927] [DOI] [PubMed] [Google Scholar]
NYHA 1994
- New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th revised edition. Boston: Lippincott Williams and Wilkins, 1994. [Google Scholar]
Peer 2010
- Peer A, Lorber A, Suraiya S, Malhotra A, Pillar G. The occurrence of Cheyne-Stokes respiration in congestive heart failure: the effect of age. Frontiers in Psychiatry 2010;1:1-6. [DOI: 10.3389/fpsyt.2010.00133] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Sin 1999
- Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. American Journal of Respiratory and Critical Care Medicine 1999;160(4):1101-6. [DOI: 10.1164/ajrccm.160.4.9903020] [DOI] [PubMed] [Google Scholar]
Stevenson 2008
- Stevenson IH, Teichtahl H, Cunnington D, Ciavarella S, Gordon I, Kalman JM. Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. European Heart Journal 2008;29(13):1662-9. [DOI: 10.1093/eurheartj/ehn214] [DOI] [PubMed] [Google Scholar]
Wang 2005
- Wang D, Teichtahl H, Drummer O, Goodman C, Cherry G, Cunnington D, et al. Central sleep apnea in stable methadone maintenance treatment patients. Chest 2005;128(3):1348-56. [DOI: 10.1378/chest.128.3.1348] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Pachito 2017
- Pachito DV, Martimbianco ALC, Latorraca COC, Pacheco RL, Drager LF, Lorenzi‐Filho G, et al. Non‐invasive positive pressure ventilation for central sleep apnoea in adults. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD012889. [DOI: 10.1002/14651858.CD012889] [DOI] [PMC free article] [PubMed] [Google Scholar]


