Abstract
A four-component domino Michael–Mannich cyclocondensation of amines, dialkyl acetylenedicarboxylaes, and formaldehyde was utilized to develop a green technique for sans metal combination of polyfunctionalized dihydro-2-oxypyrroles. It involves visible light as an environmentally friendly power source and acridine yellow G (AYG) as a photo-induced electron transfer (PET) photocatalyst. The motivation behind this examination was to expand the utilization of a non-metal dye that is both reasonable and broadly accessible. Photochemically catalyzed AYG flaunts exceptional returns, energy effectiveness, and natural agreeableness, as well as extraordinary iota economy, efficient highlights, and comfort of purpose. Key abilities consist of an easy experimental setup, big substrate tolerance, finance-friendly, clean painting-up strategies within the absence of tedious separation techniques, and minimized the quantity of waste for each organic transformation. The type of yields is pretty uniform (85–97%, average 92.09%), and the shape of reaction times might be very speedy (15–30 min, average 21.59 min), and the factor stated inside the dialogue is that the method tolerates quite a number electron-donating and electron-withdrawing functional groups, while, however, giving extremely good yields. The response within the reason is insensitive to the person of the substituents. Subsequently, many compounds and natural factors can be followed over the course of time. Shockingly, gram-scale cyclization is conceivable, proposing that the strategy could be utilized in industry.
Keywords: acridine yellow G (AYG), photo-induced electron transfer (PET), renewable energy source, polyfunctionalized dihydro-2-oxypyrroles, photochemical synthesis
Introduction
Because of retaining light in the noticeable scope of the electromagnetic range, photo-redox catalyst fosters their stable photoexcited states (Patel et al., 2021). Various flow reactors (Politano and Oksdath-Mansilla, 2018) including atom inexpensive, green, and efficient processes, have been constructed using visible light and dual photosensitized electrochemical methods (Verschueren and de Borggraeve, 2019), (Patel et al., 2021). Acridine yellow G (Figure 1) is a highly fluorescent dye that is widely used in cytology to stain cells (Tangelder et al., 1995; Teuber et al., 2001), DNA staining in chromatography (Vincent and Goldstein, 1981), fluorescent markers (Goryacheva et al., 2000), spectrophotometry to identify residues of impurities (Pérez-Ruiz et al., 1997; Pérez-Ruíz et al., 2003), and photocatalysis (Takemura, 1962; Amat et al., 2007; Arques et al., 2009). AYG has been shown to have photosensitizing (Saint-Cricq et al., 2012) and photodynamic (antibacterial) (Webb et al., 1979) effects (Kostjukova et al., 2021).
FIGURE 1.
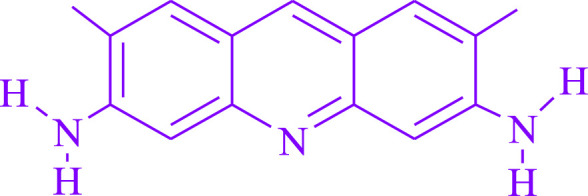
Acridine yellow G structure.
As a result of its enormous energy holds, modest expense, and sustainable power sources, green physicists believe noticeable light illumination to be a dependable innovation for harmless to the ecosystem compound combination (Mohamadpour, 2020a; Mohamadpour, 2021a; Mohamadpour, 2021b).
The designs that make up pyrrole subordinates have aroused the curiosity of chemists because of their organic and pharmacological impacts (Lampe et al., 1993; Shiraki et al., 1995; Singh et al., 1995; Borthwick et al., 2002; Chen et al., 2005; Alp et al., 2010). There are numerous options to synthesize polyfunctionalized dihydro-2-oxypyrroles, including I2 (Khan et al., 2012), glycine (Mohamadpour, 2020b), AcOH (Zhu et al., 2009), Cu(OAc)2.H2O (Lv et al., 2013), Fe3O4@nano-cellulose–OPO3H (Salehi and Mirjalili, 2017), tartaric acid (Mohamadpour et al., 2017), nano-Fe3O4@SiO2/SnCl4 (Mirjalili et al., 2019), glutamic acid (Mohamadpour, 2019a), graphene oxide (Bavadi and Niknam, 2018), caffeine (Mohamadpour, 2019b), 2,6-pyridinedicarboxylic acid (Khan et al., 2016), saccharin (Mohamadpour et al., 2016), BiFeO3 nanoparticles (Singh and Rajput, 2018), and CoFe2O4@SiO2@IRMOF-3 (Zhang et al., 2017). In order to manufacture heterocyclic compounds, we investigated photocatalysts (Mohamadpour, 2021c; Mohamadpour, 2021d; Mohamadpour, 2021e; Mohamadpour, 2022) in the green medium. This concentrate likewise tells the best way to utilize a photo-redox catalyst that is modest and broadly accessible. The photochemical mechanism by which AYG works as a photo-induced electron transfer (PET) photocatalyst has already been reported (Sengupta et al., 2018). Visible light facilitates the Michael–Mannich cyclocondensation of amines, dialkyl acetylenedicarboxylaes, and formaldehyde in ethanol at rt. This reaction was carried out at high speed and yielded.
Experimental
General procedure
A solution of amine 1 (1.0 mmol) and dialkyl acetylenedicarboxylate 2 (1.0 mmol) in EtOH (3 ml) was agitated for 15 min in the presence of AYG (1.5 mol%) under blue LED (12 W) irradiation at rt. The reaction mixture was then stirred at rt while the amine 3 (1.0 mmol) and formaldehyde 4 (1.5 mmol) were added. TLC was used to track the response. Thin layer chromatography (TLC) was carried out with silica gel as the stationary phase utilizing EtOAc/n-hexane (1:2) as an eluent. After the reaction, the resulting product was screened and washed with ethanol to produce the pure chemical without additional purification. Regardless of whether we could blend the previously mentioned synthetic substances utilizing gram-scale advancements, we needed to check whether we could increase to the level expected for drug process R&D. In one examination, 50 mmol aniline, 37.5 mmol formaldehyde, and 25 mmol diethyl acetylenedicarboxylate (DEAD) were used. The enormous scope response proceeded according to the plan, taking simply 20 min to complete, and then the item was accumulated utilizing standard filtration techniques. This substance’s 1HNMR range demonstrates that it is spectroscopically unadulterated. The items were ordered subsequently looking at spectroscopic information (1HNMR). For this composition, the spectroscopic information is given in the Supporting Information file.
Results and discussion
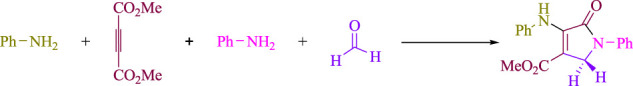
To start, the condensation of formaldehyde, aniline, and dimethyl acetylenedicarboxylate (DMAD) is investigated. The reaction was carried out at room temperature, in EtOH (3 ml), and utilizing LED light. In the absence of a photocatalyst, a trace amount of product was produced. Acridine yellow G, xanthene, riboflavin, 9H-xanthen-9-one, rhodamine B, alizarin, phenanthrenequinone, acenaphthenequinone, rose bengal, erythrosin B, and fluorescein were all attempted in the identical settings to improve the response. With yields ranging from 42 to 97%, this reaction generated the approved matching product 5a. As per the discoveries, AYG performed better in such a response. The yield was expanded to 97% utilizing 1.5 mol% AYG (Table 1, entry 3). H2O, DMF, DMSO, THF, DCM, toluene, and solvent-free conditions generally brought about lower item yields, as displayed in Table 2. In MeOH, EtOAc, and CH3CN, the yield and reaction rate improve. The reaction occurred in EtOH with a high rate and yield. Under indistinguishable circumstances, a yield of 97% was delivered, as displayed in Table 2. A variety of light sources were used to investigate how blue light influences yield. There was a minuscule measure of 5a without utilizing the light source, as per the test control. As per the discoveries, light and AYG are expected for fruitful amalgamation of item 5a. Blue LED intensity changes were also utilized to determine the ideal settings. The best outcomes, as indicated by the analysts, were obtained when blue LED (12 W) was utilized (Table 2, entry 10). Many substrates were tried under ideal circumstances (Table 3 and Scheme 1). It is vital to take note that the aniline substituent significantly affected the response’s result (Table 3). Both electron-donating and electron-withdrawing functional groups functioned admirably. The yield of all aliphatic and benzylic amines is incredibly high. The reaction patterns of dimethyl acetylenedicarboxylate (DMAD) and diethyl acetylenedicarboxylate (DEAD) were similar.
TABLE 1.
Photocatalyst optimization table is supplied for 5a production a .
| ||||
|---|---|---|---|---|
| Entry | Photocatalyst | Solvent (3 ml) | Time (min) | Isolated yield (%) |
| 1 |

|
EtOH | 60 | Trace |
| 2 | Acridine yellow G (1.0 mol%) | EtOH | 20 | 78 |
| 3 | Acridine yellow G (1.5 mol%) | EtOH | 20 | 97 |
| 4 | Acridine yellow G (2.0 mol%) | EtOH | 20 | 97 |
| 5 | Xanthene (1.5 mol%) | EtOH | 20 | 47 |
| 6 | Riboflavin (1.5 mol%) | EtOH | 20 | 61 |
| 7 | 9H-Xanthen-9-one (1.5 mol%) | EtOH | 20 | 49 |
| 8 | Rhodamine B (1.5 mol%) | EtOH | 20 | 57 |
| 9 | Alizarin (1.5 mol%) | EtOH | 20 | 42 |
| 10 | Phenanthrenequinone (1.5 mol%) | EtOH | 20 | 44 |
| 11 | Acenaphthenequinone (1.5 mol%) | EtOH | 20 | 46 |
| 12 | Rose bengal (1.5 mol%) | EtOH | 20 | 68 |
| 13 | Erythrosin B (1.5 mol%) | EtOH | 20 | 65 |
| 14 | Fluorescein (1.5 mol%) | EtOH | 20 | 67 |
Reaction conditions: at rt, formaldehyde (1.5 mmol), aniline (2 mmol), and dimethyl acetylenedicarboxylate (DMAD) (1 mmol) in EtOH, were utilized, alongside a blue LED (12 W) and an assortment of photocatalysts.
The bold parts are the results of the present study.
TABLE 2.
Table of solvent and visible light optimization is provided for 5a synthesis a .


| ||||
|---|---|---|---|---|
| Entry | Light source | Solvent (3 ml) | Time (min) | Isolated yield (%) |
| 1 | — | EtOH | 60 | Trace |
| 2 | Blue light (10 W) | EtOH | 20 | 91 |
| 3 | Blue light (18 W) | EtOH | 20 | 97 |
| 4 | White light (12 W) | EtOH | 20 | 84 |
| 5 | Green light (12 W) | EtOH | 20 | 89 |
| 6 | Blue light (12 W) | MeOH | 20 | 79 |
| 7 | Blue light (12 W) | H2O | 40 | 42 |
| 8 | Blue light (12 W) | — | 40 | 45 |
| 9 | Blue light (12 W) | DMF | 55 | 28 |
| 10 | Blue light (12 W) | EtOH | 20 | 97 |
| 11 | Blue light (12 W) | DMSO | 50 | 31 |
| 12 | Blue light (12 W) | EtOAc | 20 | 68 |
| 13 | Blue light (12 W) | THF | 55 | 36 |
| 14 | Blue light (12 W) | CH3CN | 20 | 65 |
| 15 | Blue light (12 W) | DCM | 55 | 12 |
| 16 | Blue light (12 W) | toluene | 40 | 38 |
Reaction conditions: formaldehyde (1.5 mmol), aniline (2 mmol), and dimethyl acetylenedicarboxylate (DMAD) (1 mmol) were added to AYG (1.5 mol%) at room temperature.
The bold parts are the results of the present study.
TABLE 3.
Photoexcited AYG produces polyfunctionalized dihydro-2-oxypyrroles.

| |
|---|---|

|

|

|

|

|

|


|


|


|


|


|


|

|

|

|

|
SCHEME 1.

Synthesis of polyfunctionalized dihydro-2-oxypyrroles.
Table 4 additionally remembers information for turnover frequency (TOF) and turnover number (TON). The higher the TON and TOF mathematical qualities, the less the catalyst is utilized and the more prominent the yield gets, and as the worth ascents, the impetus turns out to be more viable.
TABLE 4.
For polyfunctionalized dihydro-2-oxypyrroles, the determined turnover number (TON) and the turnover frequency (TOF).
| Entry | Product | TON | TOF | Entry | Product | TON | TOF |
|---|---|---|---|---|---|---|---|
| 1 | 5a | 64.6 | 3.23 | 12 | 5L | 62.6 | 2.50 |
| 2 | 5b | 62 | 3.1 | 13 | 5m | 64 | 2.56 |
| 3 | 5c | 57.3 | 2.29 | 14 | 5n | 62.6 | 3.13 |
| 4 | 5d | 64.6 | 4.30 | 15 | 5o | 63.3 | 3.16 |
| 5 | 5e | 58.6 | 2.34 | 16 | 5p | 58 | 1.93 |
| 6 | 5f | 63.3 | 4.22 | 17 | 5q | 63.3 | 4.22 |
| 7 | 5g | 61.3 | 3.06 | 18 | 5r | 56.6 | 1.88 |
| 8 | 5h | 63.3 | 4.22 | 19 | 5s | 61.3 | 3.06 |
| 9 | 5i | 64.6 | 4.30 | 20 | 5t | 60.6 | 3.03 |
| 10 | 5j | 60 | 2.4 | 21 | 5u | 59.3 | 2.37 |
| 11 | 5k | 56.6 | 2.26 | 22 | 5v | 62 | 2.48 |
The bold parts are the results of the present study.
To acquire knowledge of the response system of this noticeable light-advanced four-component condensation, various control tests were performed. As displayed in Scheme 2, the condensation of aniline 3) with formaldehyde 4) was performed under standard circumstances (AYG in EtOH under blue LED) with the end of H2O to get the related imine (I). When dimethyl acetylenedicarboxylate (DMAD) 2) was responded with formaldehyde 4) under indistinguishable response conditions, no item was created. For the condensation of imine (I) and enamine radical (II), the yield for 5a was 97%. A trace of the corresponding product 5a was obtained when the reaction was carried out in the dark. Scheme 3 provides a possible reaction route in the presence of AYG after reviewing the findings of this experiment.
SCHEME 2.
Significant control reads up for grasping the component of formaldehyde (4, 1.5 mmol), dimethyl acetylenedicarboxylate (DMAD) (2, 1 mmol), and aniline (1 and 3, 2 mmol) condensations.
SCHEME 3.
Manufacture of polyfunctionalized dihydro-2-oxypyrroles was described using a mechanistic process.
The recommended technique is depicted in Scheme 3. This broadly accessible AYG utilizes noticeable light as a wellspring of sustainable power to develop reactant frameworks that use the PET pathway. This process can be accelerated with visible light energy. Enamine (A) is produced through the Michael reaction between amine 1) and dialkyl acetylenedicarboxylate (2). To boost the visible-light-induced AYG*, the aniline radical (B) is produced utilizing a PET method and visible light irradiation. After that, the radical cation (B) reacts with formaldehyde 4) to create a radical cation (C). The electron transfer (ET) process between the radical adduct (C) and the AYG radical produces the intermediate (D) and ground-state AYG. Then, from (E), an H2O molecule is removed, leaving intermediate (F). To boost the visible-light-induced AYG*, the enamine radical (G) is produced utilizing a PET approach. A Mannich reaction occurs between an activated imine (F) and an enamine radical (G), resulting in an intermediate (H) that changes into a more stable tautomeric form (I). In the final phase, the intramolecular cyclization in intermediate (I) tautomerizes into polyfunctionalized dihydro-2-oxypyrroles (5).
Conclusion
A radical synthesis of polyfunctionalized dihydro-2-oxypyrroles utilizing AYG dye as a photo-induced electron transfer photocatalyst was studied. Visible light is used as a renewable energy source in an ethanol solution at room temperature in an air environment. The utilization of a minimal amount of photocatalyst, brilliant yields, a response with high proficiency, stable response conditions, and a renewable energy source, and a fast methodology without the utilization of harmful solvents or impetuses are the clearest benefits of this green convention. Chromatographic purging was not needed. As per a multigram scale response of model substrates, this response can be increased without compromising the result. Therefore, this innovation offers colossal advantages regarding both gathering modern requirements and settling ecological worries.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.1015330/full#supplementary-material
References
- Alp C., Ekinci D., Gültekin M. S., Şentürk M., Şahin E., Küfrevioğlu Ö. İ. (2010). A novel and one-pot synthesis of new 1-tosyl pyrrol-2-one derivatives and analysis of carbonic anhydrase inhibitory potencies. Bioorg. Med. Chem. 18, 4468–4474. 10.1016/j.bmc.2010.04.072 [DOI] [PubMed] [Google Scholar]
- Amat A. M., Arques A., Galindo F., Miranda M. A., Santos-Juanes L., Vercher R. F., et al. (2007). Acridine yellow as solar photocatalyst for enhancing biodegradability and eliminating ferulic acid as model pollutant. Appl. Catal. B Environ. 73, 220–226. 10.1016/j.apcatb.2006.12.003 [DOI] [Google Scholar]
- Arques A., Amat A. M., Santos-Juanes L., Vercher R. F., Marin M. L., Miranda M. A. (2009). Abatement of methidathion and carbaryl from aqueous solutions using organic photocatalysts. Catal. Today 144, 106–111. 10.1016/j.cattod.2008.11.013 [DOI] [Google Scholar]
- Bavadi M., Niknam K. (2018). Synthesis of functionalized dihydro-2-oxopyrroles using graphene oxide as heterogeneous catalyst. Mol. Divers. 22, 561–573. 10.1007/s11030-017-9809-9 [DOI] [PubMed] [Google Scholar]
- Borthwick A. D., Crame A. J., Ertl P. F., Exall A. M., Haley T. M., Hart G. J., et al. (2002). Design and synthesis of pyrrolidine-5, 5-trans-lactams (5-oxohexahydropyrrolo [3, 2-b] pyrroles) as novel mechanism-based inhibitors of human cytomegalovirus protease. 2. Potency and chirality. J. Med. Chem. 45, 1–18. 10.1021/jm0102203 [DOI] [PubMed] [Google Scholar]
- Chen Y., Zeng D. X., Xie N., Dang Y. Z. (2005). Study on photochromism of diarylethenes with a 2, 5-dihydropyrrole bridging unit: A convenient preparation of 3, 4-diarylpyrroles from 3, 4-diaryl-2, 5-dihydropyrroles. J. Org. Chem. 70, 5001–5005. 10.1021/jo050236r [DOI] [PubMed] [Google Scholar]
- Goryacheva I. Y., Mel’nikov G. V., Shtykov S. N. (2000). Acridine dyes in the triplet state as reagents for the selective luminescence determination of polycyclic aromatic hydrocarbons. J. Anal. Chem. 55, 874–878. 10.1007/BF02757853 [DOI] [Google Scholar]
- Khan A. T., Ghosh A., Khan M. M. (2012). One-pot four-component domino reaction for the synthesis of substituted dihydro-2-oxypyrrole catalyzed by molecular iodine. Tetrahedron Lett. 53, 2622–2626. 10.1016/j.tetlet.2012.03.046 [DOI] [Google Scholar]
- Khan M. M., Khan S., Iqbal S., Yousuf R. (2016). Synthesis of functionalized dihydro-2-oxypyrroles and tetrahydropyridines using 2, 6-pyridinedicarboxylic acid as an efficient and mild organocatalyst. New J. Chem. 40, 7504–7512. 10.1039/C6NJ01170E [DOI] [Google Scholar]
- Kostjukova L. O., Leontieva S. V., Kostjukov V. V. (2021). The vibronic absorption spectra and electronic states of acridine yellow in aqueous solution. J. Mol. Liq. 326, 115312. 10.1016/j.molliq.2021.115312 [DOI] [PubMed] [Google Scholar]
- Lampe J. W., Chou Y. L., Hanna R. G., Di Meo S. V., Erhardt P. W., Hagedorn A. A., et al. (1993). (Imidazolylphenyl) pyrrol-2-one inhibitors of cardiac cAMP phosphodiesterase. J. Med. Chem. 36, 1041–1047. 10.1021/jm00060a012 [DOI] [PubMed] [Google Scholar]
- Lv L., Zheng S., Cai X., Chen Z., Zhu Q., Liu S. (2013). Development of four-component synthesis of tetra-and pentasubstituted polyfunctional dihydropyrroles: Free permutation and combination of aromatic and aliphatic amines. ACS Comb. Sci. 15, 183–192. 10.1021/co300148c [DOI] [PubMed] [Google Scholar]
- Mirjalili B. B., Araqi R., Mohajeri S. A. (2019). A simple and green approach for the synthesis of substituted dihydro-2-oxypyrroles catalyzed by nano-Fe3O4@SiO2/SnCl4 superparamagnetic nanoparticles. Iran. J. Catal. 9, 11–20. [Google Scholar]
- Mohamadpour F. (2021c10.1016/j.jphotochem.20211134). A new role for photoexcited Na2 eosin Y as direct hydrogen atom transfer (HAT) photocatalyst in photochemical synthesis of dihydropyrano[2, 3-c]pyrazole scaffolds promoted by visible light irradiation under air atmosphere. J. Photochem. Photobiol. A Chem. 418, 113428. 10.1016/j.jphotochem.2021.113428 [DOI] [Google Scholar]
- Mohamadpour F. (2019b). Caffeine as a naturally green and biodegradable catalyst promoted convenient and expedient synthetic route for the synthesis of polysubstituted dihydro-2 oxypyrroles. Bull. Chem. Soc. Ethiop. 33, 149–158. 10.4314/bcse.v33i1.15 [DOI] [Google Scholar]
- Mohamadpour F. (2019a). Glutamic acid as green and bio-based α-amino acid catalyst promoted one-pot access to polyfunctionalized dihydro-2-oxypyrroles. J. Serbian Chem. Soc. 84, 1083–1092. 10.2298/JSC180720006M [DOI] [Google Scholar]
- Mohamadpour F., Maghsoodlou M. T., Heydari R., Lashkari M. (2016). Saccharin: A green, economical and efficient catalyst for the one-pot, multi-component synthesis of 3, 4-dihydropyrimidin-2-(1H)-one derivatives and 1H-pyrazolo[1, 2-b]phthalazine-5, 10-dione derivatives and substituted dihydro-2-oxypyrrole. J. Iran. Chem. Soc. 13, 1549–1560. 10.1007/s13738-016-0871-5 [DOI] [Google Scholar]
- Mohamadpour F., Maghsoodlou M. T., Heydari R., Lashkari M. (2017). Tartaric acid: A naturally green and efficient di-functional brønsted acid catalyst for the one-pot four-component synthesis of polysubstituted dihydropyrrol-2-ones at ambient temperature. Iran. J. Sci. Technol. Trans. Sci. 41, 843–849. 10.1007/s40995-016-0049-0 [DOI] [Google Scholar]
- Mohamadpour F. (2021d). New role for photoexcited organic dye, Na2 eosin Y via the direct hydrogen atom transfer (HAT) process in photochemical visible-light-induced synthesis of spiroacenaphthylenes and 1H-pyrazolo[1, 2-b]phthalazine-5, 10-diones under air atmosphere. Dyes Pigments 194, 109628. 10.1016/j.dyepig.2021.109628 [DOI] [Google Scholar]
- Mohamadpour F. (2021e). Photoexcited Na2 eosin Y as direct hydrogen atom transfer (HAT) photocatalyst promoted photochemical metal-free synthesis of tetrahydrobenzo[b]pyran scaffolds via visible light-mediated under air atmosphere. J. Taiwan Inst. Chem. Eng. 129, 52–63. 10.1016/j.jtice.2021.09.017 [DOI] [Google Scholar]
- Mohamadpour F. (2021b). Catalyst-free and solvent-free visible light irradiation-assisted Knoevenagel–Michael cyclocondensation of aryl aldehydes, malononitrile, and resorcinol at room temperature. Monatsh. Chem. 152, 507–512. 10.1007/s00706-021-02763-1 [DOI] [Google Scholar]
- Mohamadpour F. (2021a). Catalyst-free, visible light irradiation promoted synthesis of spiroacenaphthylenes and 1H-pyrazolo[1, 2-b]phthalazine-5, 10-diones in aqueous ethyl lactate. J. Photochem. Photobiol. A Chem. 407, 113041. 10.1016/j.jphotochem.2020.113041 [DOI] [Google Scholar]
- Mohamadpour F. (2020b). Imin-based synthesis of polyfunctionalized dihydro-2-oxypyrroles catalyzed by glycine amino acid via tandem Michael–Mannich cyclocondensation reaction under ambient temperature. Res. Chem. Intermed. 46, 1931–1940. 10.1007/s11164-019-04072-z [DOI] [Google Scholar]
- Mohamadpour F. (2022). Methylene blue as a photo-redox catalyst: The development synthesis of tetrahydrobenzo[b]pyran scaffolds via a single-electron transfer/energy transfer. Front. Chem. 10, 934781. 10.3389/fchem.2022.934781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamadpour F. (2020a). Visible light irradiation promoted catalyst-free and solvent-free synthesis of pyrano[2,3-d]pyrimidine scaffolds at room temperature. J. Saudi Chem. Soc. 24, 636–641. 10.1016/j.jscs.2020.06.006 [DOI] [Google Scholar]
- Patel R. I., Sharma A., Sharma S., Sharma A. (2021). Visible light-mediated applications of methylene blue in organic synthesis. Org. Chem. Front. 8, 1694–1718. 10.1039/D0QO01182G [DOI] [Google Scholar]
- Pérez-Ruiz T., Martínez-Lozano C., Tomás V., Fenoll J. (2003). Spectrofluorimetric determination of formaldehyde by a flow-injection method based on its catalytic effect on the acridine yellow-bromate reaction. Anal. Bioanal. Chem. 375, 661–665. 10.1007/s00216-003-1763-y [DOI] [PubMed] [Google Scholar]
- Pérez-Ruiz T., Martínez-Lozano C., Sanz A., San Miguel M. T. (1997). Flow extraction spectrophotometric method for the determination of diclofenac sodium in pharmaceutical preparations. J. Pharm. Biomed. Analysis 16, 249–254. 10.1016/S0731-7085(97)00028-9 [DOI] [PubMed] [Google Scholar]
- Politano F., Oksdath-Mansilla G. (2018). Light on the horizon: Current research and future perspectives in flow photochemistry. Org. Process Res. Dev. 22, 1045–1062. 10.1021/acs.oprd.8b00213 [DOI] [Google Scholar]
- Saint-Cricq P., Pigot T., Blanc S., Lacombe S. (2012). Selective oxidation with nanoporous silica supported sensitizers: An environment friendly process using air and visible light. J. Hazard. Mater. 211-212, 266–274. 10.1016/j.jhazmat.2011.09.066 [DOI] [PubMed] [Google Scholar]
- Salehi N., Mirjalili B. B. (2017). Synthesis of highly substituted dihydro-2-oxopyrroles using Fe3O4@nano-cellulose–OPO3H as a novel bio-based magnetic nanocatalyst. RSC Adv. 7, 30303–30309. 10.1039/C7RA04101B [DOI] [Google Scholar]
- Sengupta C., Mitra P., Chatterjee S., Bhattacharjee G., Satpati B., Basu S. (2018). Photoinduced electronic interactions between acridine derivatives and small gold nanoparticles: A spectroscopic insight. J. Mol. Liq. 272, 198–208. 10.1016/j.molliq.2018.09.080 [DOI] [Google Scholar]
- Shiraki R., Sumino A., Tadano K. I., Ogawa S. (1995). Total synthesis of PI-091. Tetrahedron Lett. 36, 5551–5554. 10.1016/0040-4039(95)01049-N [DOI] [Google Scholar]
- Singh H., Rajput J. K. (2018). Chelation and calcination promoted preparation of perovskite-structured BiFeO3 nanoparticles: A novel magnetic catalyst for the synthesis of dihydro-2-oxypyrroles. J. Mat. Sci. 53, 3163–3188. 10.1007/s10853-017-1790-2 [DOI] [Google Scholar]
- Singh S. B., Goetz M. A., Jones E. T., Bills G. F., Giacobbe R. A., Herranz L., et al. (1995). Oteromycin: A novel antagonist of endothelin receptor. J. Org. Chem. 60, 7040–7042. 10.1021/jo00126a071 [DOI] [Google Scholar]
- Takemura F. (1962). Dye-sensitized photopolymerization of vinyl monomers. II. Photobleaching of acridine yellow in some vinyl monomers. Bull. Chem. Soc. Jpn. 35, 1078–1086. 10.1246/bcsj.35.1078 [DOI] [Google Scholar]
- Tangelder G. J., Janssens C. J., Slaaf D. W., Oude Egbrink M. G., Reneman R. S. (1995). In vivo differentiation of leukocytes rolling in mesenteric postcapillary venules. Am. J. Physiology-Heart Circulatory Physiology 268, 909–915. 10.1152/ajpheart.1995.268.2.H909 [DOI] [PubMed] [Google Scholar]
- Teuber M., Rögner M., Berry S. (2001). Fluorescent probes for non-invasive bioenergetic studies of whole cyanobacterial cells. Biochimica Biophysica Acta - Bioenergetics 1506, 31–46. 10.1016/S0005-2728(01)00178-5 [DOI] [PubMed] [Google Scholar]
- Verschueren R. H., De Borggraeve W. M. (2019). Electrochemistry and photoredox catalysis: A comparative evaluation in organic synthesis. Molecules 24, 2122–2160. 10.3390/molecules24112122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent W. S., Goldstein E. S. (1981). Rapid preparation of covalently closed circular DNA by acridine yellow affinity chromatography. Anal. Biochem. 110, 123–127. 10.1016/0003-2697(81)90121-4 [DOI] [PubMed] [Google Scholar]
- Webb R. B., Hass B. S., Kubitschek H. E. (1979). Photodynamic effects of dyes on bacteria. Mutat. Research/Fundamental Mol. Mech. Mutagen. 59, 1–13. 10.1016/0027-5107(79)90190-8 [DOI] [PubMed] [Google Scholar]
- Zhang J. N., Yang X. H., Guo W. J., Wang B., Zhang Z. H. (2017). Magnetic metal–organic framework CoFe2O4@SiO2@IRMOF-3 as an efficient catalyst for one-pot synthesis of functionalized dihydro-2-oxopyrroles. Synlett 28, 734–740. 10.1055/s-0036-1588924 [DOI] [Google Scholar]
- Zhu Q., Jiang H., Li J., Liu S., Xia C., Zhang M. (2009). Concise and versatile multicomponent synthesis of multisubstituted polyfunctional dihydropyrroles. J. Comb. Chem. 11, 685–696. 10.1021/cc900046f [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.




