Abstract
Objective:
Research suggests that exposure to racism partially explains why African American women are 2 to 3 times more likely to deliver low birth weight and preterm infants. However, the physiological pathways by which racism exerts these effects are unclear. This study examined how lifetime exposure to racism, in combination with maternal blood pressure changes during pregnancy, was associated with fetal growth.
Methods:
African American pregnant women (n = 39) reported exposure to childhood and adulthood racism in several life domains (e.g., at school, at work), which were experienced directly or indirectly, meaning vicariously experienced when someone close to them was treated unfairly. A research nurse measured maternal blood pressure at 18 to 20 and 30 to 32 weeks gestation. Standardized questionnaires and trained interviewers assessed maternal demographics. Neonatal length of gestation and birth weight data were collected from medical charts.
Results:
Childhood racism interacted with diastolic blood pressure to predict birth weight. Specifically, women with two or more domains of indirect exposure to racism in childhood and increases in diastolic blood pressure between 18 and 32 weeks had lower gestational age adjusted birth weight than the other women. A similar pattern was found for direct exposure to racism in childhood.
Conclusions:
Increases in diastolic blood pressure between the second and third trimesters predicted lower birth weight, but only when racism exposure in childhood (direct or indirect) was relatively high. Understanding pregnant African American women’s lifetime direct and indirect experiences with racism in combination with prenatal blood pressure may improve identification of highest risk subgroups within this population.
Keywords: African American, blood pressure, fetal growth, racial disparities, racism
Relative to other races, African Americans’ disproportionate risk of having low birth weight (<2,500 g) infants is well documented but not well understood (Committee on Understanding Premature Birth and Assuring Healthy Outcomes, 2007; Giscombé & Lobel, 2005). Medical, behavioral, socioeconomic (Giscombé & Lobel, 2005; Goldenberg et al., 1996), and genetic (David & Collins, 2007; Fiscella, 2005) factors do not fully explain the persistent reproductive disparity. Differential exposure to racism has been shown to explain some of the racial differences in the prevalence and severity of adverse birth outcomes (Collins, David, Handler, Wall, & Andes, 2004; Dominguez, Dunkel-Schetter, Glynn, Hobel, & Sandman, 2008; Giscombé & Lobel, 2005; Hogue & Bremner, 2005). However, the physiological pathways by which racism exerts these effects are unclear.
Racism is a chronic stressor based on an individual’s race or ethnicity (Dominguez et al., 2008), and it is a unique stressor because it is a perceived threat based on an immutable personal characteristic central to a person’s identity (Landrine & Klonoff, 1994). It involves psychosocial challenges in the form of prejudice, denigration, and discrimination. These challenges occur over a lifetime in multiple domains including at school, at work, and at home (Krieger, Rowley, Herman, & Avery, 1993). Like other stressors, racism is correlated with measures of emotional distress (Dominguez et al., 2008). To assess the impact of racism, it is important to consider when a person was exposed to racism, the domains in which exposure occurred, and to account for the impact of other, nonracism-related stressors, as we have done in the present study.
Case-control studies indicate the adjusted odds of delivering a very low birth weight infant are 2.6 to 3.3 times greater among African Americans reporting exposure to racism events during pregnancy (Collins et al., 2000) and across a lifetime (Collins et al., 2004; Lespinasse, David, Collins, Handler, & Wall, 2004; Mustillo et al., 2004). Some studies have shown that African American women who report racism exposure in three or more life domains are at significant risk of low birth weight and preterm deliveries (Collins et al., 2004; Lespinasse et al., 2004; Mustillo et al., 2004). Prospective investigations also show 1.4 to 3.1 greater odds of preterm delivery (Dominguez, 2008; Giscombé & Lobel, 2005). In risk-adjusted models, exposure to racism partially explains Black–White differences in preterm delivery, low birth weight (Mustillo et al., 2004), and fetal growth (Dominguez et al., 2008). Less well studied are whether the form of racism (personal/direct or vicariously experienced/indirect) and timing of exposure (childhood or adulthood) matter, and the physiological pathways by which exposure to racism influences birth outcomes.
In the present study, we consider cardiovascular (CV) functioning as a potentially important factor in the link between racism and birth weight (Hilmert et al., 2008; Rosenthal & Lobel, 2011). The physiologic demands of pregnancy are substantial, requiring the maternal CV system to accommodate the hemodynamic needs of a growing fetus and to manage the challenges of labor and delivery. Typically, maternal blood pressure drops steadily until 24 to 32 weeks’ gestation, at which point it gradually rises about 8 mm|Hg diastolic blood pressure (DBP) and 10 mm|Hg systolic blood pressure (SBP) by parturition (MacGillivray, Rose, & Rowe, 1969; Thompson, Williams, & Miller, 2009).
Chronic and repeated psychosocial stress has been linked to CV dysfunction in general (Treiber et al., 2003). Exposure to severe psychosocial stressors such as racist events could evoke large physiological responses (Brondolo, Rieppi, Kelly, & Gerin, 2003) that result in wear and tear on the system. Over time, the wear and tear can accumulate and contribute to dysfunction in the cardiovascular and other stress-response systems (McEwen, 1998), resulting in adverse effects on fetal growth (Hilmert et al., 2008). One study of pregnant women found that racism exposure in African Americans predicted general perceived stress, which then predicted SBP between 32 and 36 weeks’ gestation (Stancil, Hertz-Picciotto, Schramm, & Watt-Morse, 2000). However, no studies have examined associations among racism, changes in blood pressure during pregnancy, and pregnancy outcomes all together.
Dominguez et al. (2008) reported that for pregnant African American women, lifetime and childhood exposure to racism predicted lower fetal growth (birth weight adjusted for gestation length). Using the same dataset, the present study extends that finding and tests a moderation model, hypothesizing that racism modifies the association between blood pressure adjustments to pregnancy and pregnancy outcome. In our past work, we found that the association between infant fetal growth and midgestation basal DBP varied depending on recent life stress in this cohort (Hilmert et al., 2008). Women who had a combination of relatively high stress and high resting DBP gave birth to smaller babies than women with only high stress, high blood pressure, or neither of these. Because blood pressure by itself did not predict birth weight, it is possible that some underlying components of DBP (e.g., cardiac output, vascular constriction) demarcate a pathway between stress and lower birth weight.
Past research on African American men and women has documented the prevalence of regular, lifelong exposure to racism (Nuru-Jeter et al., 2009). It is likely that African American women with the highest lifetime exposure to racism have accumulated more physiological wear and tear over their lifetimes resulting in stress-response dysfunction. During pregnancy, this dysfunction may manifest in many ways, one of which would be as increased blood pressure attributable to increased vascular resistance, which can lead to a decrease in uteroplacental blood flow and placental perfusion, thus compromising fetal growth (Easterling et al., 1991; Easterling, Carr, Brateng, Diederichs, & Schmucker, 2001; Khong, De Wolf, Robertson, & Brosens, 1986; Misra, Hobel, & Sing, 2009; Valero De Bernabe et al., 2004).
It is also possible that racism, if experienced during a critical period in one’s life, will result in adult physiological dysfunction. We know, for example, that childhood is a particularly sensitive time to experience racism (Krieger, Smith, Naishadham, Hartman, & Barbeau, 2005). A growing body of research has indicated that early life stress experiences can impact physiological and psychological responses to stress in adulthood (Hanson & Chen, 2010; Miller, Chen, & Parker, 2011; Seckl & Meaney, 2004; Taylor, Lerner, Sage, Lehman, & Seeman, 2004). Therefore, in terms of dysfunctions in physiological stress responding, childhood experiences of racism may be a particularly potent aspect of lifetime racism exposure.
Furthermore, past research has shown that both personal and collective experiences of racism cause significant distress during this period (Krieger et al., 2005). In fact, some studies have reported that childhood vicarious (indirect) racism exposures have stronger associations with pregnancy outcomes than direct, personal childhood exposures (Dominguez et al., 2008). This may be because direct experiences of racism are relatively rare in childhood or because children at young ages lack a developed understanding of racism, resulting in reports of few childhood direct experiences of racism. It is the reactions of older adults, usually the child’s parents, to racist behaviors that can be extremely vivid and life altering (Nuru-Jeter et al., 2009).
Based on the foregoing literature, we hypothesized that direct and indirect exposures to racism and prenatal changes in blood pressure would predict birth weight. We expected the most potent predictor to be exposure to racism in childhood. In addition, because past research has shown that higher daily DBP at 28 weeks’ gestation (Churchill, Perry, & Beevers, 1997) and higher average DBP during mid to late gestation were associated with lower birth weight (Hilmert et al., 2008) and SBP was not, we predicted an interaction such that larger increases in DBP combined with more racism exposure would predict lower birth weight. We consider why DBP is implicated and SBP is not in the discussion.
Method
Participants and Procedure
We examined data from (N = 42) U.S.-born African American pregnant women who participated in a study of stress and pregnancy at two major medical centers in southern California (Los Angeles and Orange counties). Women were recruited during a prenatal visit to their caregiver. Inclusion criteria were 18 years of age or older with a singleton, intrauterine pregnancy; no current or historical medical conditions related to CV, neuroendocrine, or immune function; and no self-reported use of cigarettes or controlled substances. Three women did not have Time 2 blood pressure data, and one did not have Time 1 blood pressure data. These women’s data showed no significant differences with the included sample on the variables of interest (all ps> .05), so they were omitted from further analyses, leaving N = 39. At each study visit, a research nurse measured the participant’s blood pressure, questionnaires were completed, and trained interviewers conducted interviews. The Institutional review boards of the institutions involved with data collection approved of the study. All participants provided informed consent.
Blood Pressure
Systolic blood pressure and DBP were assessed at each study visit by a Critikon Dinamap Vital Sign Monitor 2100 (GE Healthcare, Milwaukee, WI): 18 to 20 weeks’ (Time 1) and 30 to 32 weeks’ (Time 2) gestation. We focused on the period between 18 and 32 weeks’ gestation because blood pressure typically reaches a nadir during this time and then begins rising until parturition (MacGillivray et al., 1969; Thompson et al., 2009). We expected that blood pressure changes during this time would provide a relevant sample of cardiovascular adjustment during pregnancy. During blood pressure measurement, the participant was seated and two measures were taken 2 min apart. For correlation analyses, average blood pressure at Time 1 was subtracted from average blood pressure at Time 2 to calculate change in blood pressure. In regressions we used a residualized Time 2 blood pressure controlling for Time 1 blood pressure.
Racism Exposure
We assessed racism exposure at 22 to 24 weeks’ gestation with a standardized interview including measures adapted from Krieger, Smith, Naishadham, Hartman, and Barbeau (2005). To capture a comprehensive look at lifetime racism, our measure included subscales for direct and indirect exposure in childhood (age 16 or younger) and in adulthood (older than 16) across multiple domains (Dominguez et al., 2008). For instance, participants were asked what type or types of racism they experienced as a child. This was followed by four descriptions of racism in different domains, “personal discrimination, such as racial slurs or names, dirty looks, and so forth,” “educational discrimination, such as unfair treatment by teachers, or …,” “employment, hiring, or job related discrimination.” These options were used to assess exposure to racism in adulthood, and indirect racism experienced by close others in childhood and adulthood.
Yes or no responses were given for each domain, and yeses were summed to produce subscores for each type (direct or indirect) and timing (childhood or adulthood) of racism exposure. We also summed subscores into a lifetime score. Because the measure is based on domains of racism exposure, the scores represent pervasiveness rather than magnitude of exposure (Dominguez, Strong, Gillman, Krieger, & Rich-Edwards, 2009). This operationalization of racism as perceived exposure is common in studies of racism (Clark, Anderson, Clark, & Williams, 1999; Dominguez, 2008) and have been shown to be reliable and valid. Specifically, perceived exposure measures have been shown to have high test–retest reliability, to load on a single racism factor in confirmatory factor analyses, to correlate with other racism and discrimination measures, and to have the highest correlation with a latent racism variable in structural equation modeling (Klonoff & Landrine, 1999; Krieger et al., 2005; Landrine & Klonoff, 1996).
Other Psychosocial Stresses
To determine the unique influence of exposure to racism on birth outcomes, we controlled for psychosocial stress with a measure typically used in stress and pregnancy studies (Giscombé & Lobel, 2005). A Stressful Life Events (SLE) Inventory was administered at Time 1 and Time 2 to assess exposure (0 = no, 1 = yes) to 24 different stressful events in the 12 months preceding Time 1 and between Times 1 and 2. The inventory included items such as “a change in where you live,” “problems in your relationship,” and “someone close to you died.” None of the items referred to racism. Endorsed events were summed into an overall score (Dominguez, Dunkel Schetter, Mancuso, Rini, & Hobel, 2005).
Medical and Outcome Data
Medical data, including body mass index (BMI; kg/m2) and birth outcome data were collected from maternal charts. Length of gestation was determined by best obstetric estimate, using last menstrual period (LMP) and 15- to 18-week ultrasonography estimates (American College of Obstetricians and Gynecologists [ACOG], 2009). In our analyses of birth weight, we controlled for length of gestation. This allowed us to examine fetal growth rather than birth weight attributable to length of gestation.
Socioeconomic Status (SES)
Participants reported their household income and level of education (Table 1). To create an SES index, these values were transformed into z-scores, and an average SES score was computed.
Table 1.
Sample Characteristics (N = 39)
| Variable | M (SD)a |
|---|---|
| Age (years) | 28.74 (5.18) |
| Body mass index | 28.75 (8.04) |
| Nulliparous (%) | 41.0% |
| Adjusted income (×1,000/person/month) | 2.35 (1.67) |
| Education | |
| Less than high school | 35.9% |
| Some college | 46.2% |
| Bachelor’s degree | 15.4% |
| Graduate degree | 2.6% |
| Domains of racism exposure | |
| Childhood personalb | 0.59 (0.88) |
| Childhood indirectb | 0.84 (1.09) |
| Adult personalb | 0.87 (1.24) |
| Adult indirectb | 1.13 (1.30) |
| Totalc | 3.43 (3.26) |
| Stressful life events | 8.14 (4.99) |
| Time 1 DBP (mm|Hg) | 65.21 (8.56) |
| Time 2 DBP (mm|Hg) | 66.00 (8.04) |
| % Who increased in DBP | 66.6% |
| Time 1 SBP (mm|Hg) | 118.74 (12.33) |
| Time 2 SBP (mm|Hg) | 117.67 (11.19) |
| % Who increased in SBP | 51.3% |
| Birth weight (g) | 3212.85 (476.12) |
| Length of gestation (weeks) | 38.79 (1.96) |
Note. DBP = diabolic blood pressure; SBP = systolic blood pressure.
Values reported are M (SD) unless otherwise noted.
Racism subscale scores could range from 0 to 4 domains.
Total racism was the sum of the subscales with a possible range from 0 to 16.
Statistical Analyses
To assess the extent to which blood pressure changed during the study, a repeated measures analysis comparing Time 1 to Time 2 blood pressures was performed. Correlations among all study variables were calculated to determine the control variables to include in the primary interaction analyses. Our primary hypothesis was tested with multiple regression analyses.
Results
Sample Characteristics
Sample characteristics are presented in Table 1. This sample was financially stable and fairly well educated, with more than half having completed high school. On average, this sample of women reported having experienced racism in almost three and a half different domains, with most of them having occurred indirectly in adulthood and the fewest occurring directly in childhood. Average best obstetric estimate of gestational age at Time 1 was19.31 (SD = 0.72) weeks and at Time 2 was 30.82 (SD = 0.66) weeks. Gestational age within each 2-week time period was not associated with blood pressure or stress measures. Therefore, gestational age at visit is not included in the following analyses.
Changes in Blood Pressure
A within-subjects analysis showed that overall there was no significant change in DBP (M = 0.80 mm|Hg, SD = 7.87, p > .10) or SBP (M = −1.08 mm|Hg, SD = 11.25, p > .10) suggesting that there were increases and decreases occurring between Times 1 and 2. About half of the women had SBP decreases, and one-third had DBP decreases (Table 1). Two women (5.1%) had no change in DBP, and one woman (2.6%) had no change in SBP; 61.5% of the women had increases in DBP, and 46.2% had increases in SBP during the study period. To determine whether separately, increases and decreases in blood pressure were significant, we split the sample into subgroups of those who had blood pressure increases and those who had blood pressure decreases (women without changes in blood pressure were omitted from these analyses). Analyses showed that increases in SBP (M = 8.21 mm|Hg, SD = 6.15) and DBP (M = 5.27 mm|Hg, SD = 4.69) and decreases in SBP (M = −9.62 mm|Hg, SD = 6.95) and DBP (M = −7.86 mm|Hg, SD = 4.44) were significant (ps <.001) and relatively substantial considering the average change in SBP from nadir to parturition is 10 mm|Hg (Thompson et al., 2009).
Correlations
Adult indirect racism and total racism were significantly positively associated with BMI and SES (Table 2). There were significant positive correlations among the racism measures and stressful life events. Also, the stressful life events measure was significantly associated with decreases in SBP. A partial correlation analysis controlling for Time 1 SBP revealed that this significant association was primarily because of higher Time 1 SBP (p > .05). Greater exposure to childhood indirect, adulthood personal, and lifetime racism events were associated with lower birth weight (Table 2), as previously reported (Dominguez et al., 2008). Age was negatively associated with stressful life events, but was not significantly associated with the dependent or independent variables in this study. Thus, controls used in the following regression analyses were BMI, SES, and SLE.
Table 2.
Correlations Among Study Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participant characteristics | |||||||||||||
| 1. Maternal age | |||||||||||||
| 2. Body mass index | 0.22 | ||||||||||||
| 3. Nulliparity (= 1; multiparous = 0) | 0.19 | 0.19 | |||||||||||
| 4. Socioeconomic status | 0.20 | 0.08 | −0.42** | ||||||||||
| Racism experiences | |||||||||||||
| 5. Childhood personal | −0.26 | 0.15 | 0.03 | 0.22 | |||||||||
| 6. Childhood indirect | −0.19 | 0.23 | −0.27† | 0.13 | 0.49** | ||||||||
| 7. Adult personal | 0.11 | 0.14 | −0.30† | 0.29† | 0.24 | 0.42** | |||||||
| 8. Adult indirect | 0.13 | 0.48** | −0.08 | 0.36* | 0.23 | 0.36* | 0.40* | ||||||
| 9. Discrimination total | −0.04 | 0.35* | −0.23 | 0.36* | 0.62** | 0.77** | 0.75** | 0.73** | |||||
| 10. Stressful life events | −0.32* | 0.09 | −0.13 | 0.03 | 0.46** | 0.46** | 0.44** | 0.24 | 0.54** | ||||
| Blood pressure | |||||||||||||
| 11. Change in DBP | 0.29† | 0.13 | 0.17 | 0.02 | −0.06 | −0.15 | 0.05 | −0.12 | −0.10 | −0.28† | |||
| 12. Change in SBP | 0.25 | −0.12 | −0.03 | 0.14 | −0.21 | −0.30† | −0.11 | −0.21 | −0.28† | −0.46** | 0.23 | ||
| Outcome | |||||||||||||
| 13. Length of gestation | −0.18 | −0.12 | −0.09 | −0.27† | −0.12 | −0.22 | −0.13 | −0.20 | −0.23 | 0.08 | −0.27 | 0.07 | |
| 14. Birth weight | −0.07 | 0.15 | −0.04 | −0.27† | −0.14 | −0.42** | −0.43** | −0.24 | −0.44** | −0.25 | −0.13 | 0.06 | 0.62** |
p < .10.
p < .05.
p < .01.
Blood Pressure, Racism, and Adjusted Birth Weight
To examine the combined effects of racism exposure and change in blood pressure on birth weight adjusted for length of gestation, we first conducted regression analyses in which Step 1 included length of gestation and Time 1 blood pressure, Step 2 included Time 2 blood pressure and a racism variable, and in Step 3 we entered a racism by blood pressure change (Time 2 minus Time 1) interaction term. Then, to isolate the effects of exposure to racism and changes in blood pressure from potential confounds, we repeated the analyses controlling for BMI, SES, and SLE (see above). If an interaction was significant, we conducted simple slope analyses to examine the pattern of effects. We performed separate analyses with total racism exposure or a racism subscale and DBP or SBP change.
DBP change analyses.
In regression analyses, length of gestation consistently accounted for a significant amount of variance in birth weight, with all ps <.001. Consistent with correlations, initial analyses showed that childhood indirect, adulthood personal, and total racism exposure explained a significant amount of variance in birth weight (all ps <.05). The racism by change in DBP interactions were significant in the analyses involving childhood indirect racism (β = −.36, ΔR2 = 0.12, p < .01), and childhood personal racism (β = −.30, ΔR2 = 0.07, p < .05), both showing the same pattern of associations depicted in Figure 1.
Figure 1.
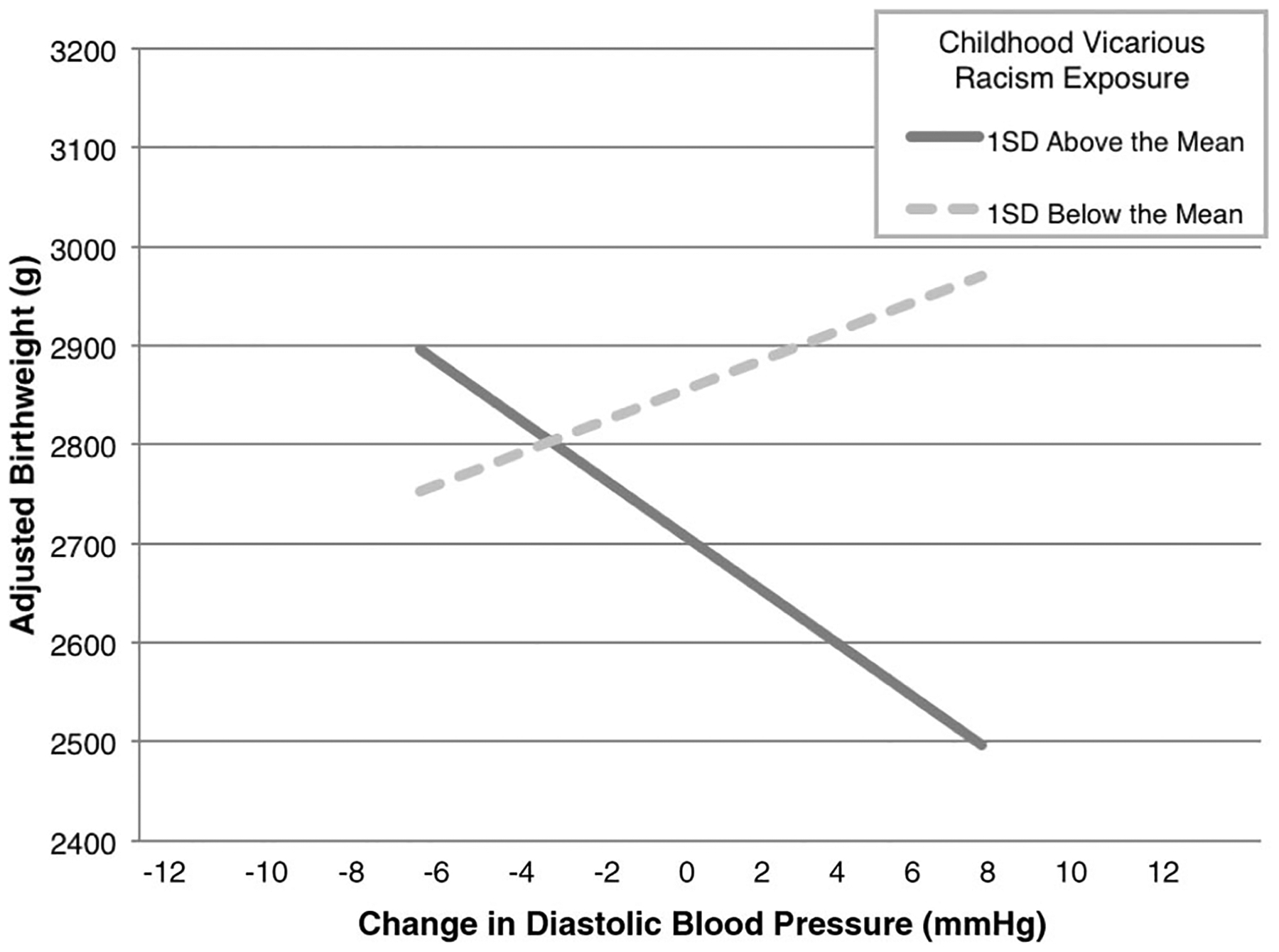
African American childhood indirect racism exposure and change in mid-to-late term diastolic (DBP) predicting adjusted birth weight. When no racism exposure was reported, the association between change in DBP and adjusted birth weight was not statistically significant. Values are predicted using methods described by Aiken and West (1991).
In regression analyses controlling for potential confounds, BMI and SLE explained a significant amount of variance in all analyses (all ps <.05), but SES did not (all ps >.10). With the inclusion of the control variables the association between adjusted birth weight and childhood indirect racism (β = −.24) was no longer significant (p > .10). An association between birth weight and total racism (β = −.27) was marginally significant (p < .10). Consistent with past analyses, adulthood personal racism accounted for a significant amount of variance in adjusted birth weight (β = −.26, p < .05; Dominguez et al., 2008). The interaction between change in DBP and childhood indirect racism exposure (β = −.25, ΔR2 = 0.04, p < .05) remained significant (Figure 1), and the interaction between change in DBP and childhood personal racism was marginally significant (β = −.22, ΔR2 = 0.03, p = .10).
Simple slope analyses confirmed that for African American women who reported approximately two (+1 SD = 1.93) domains of childhood indirect racism exposure, adjusted birth weight declined 19.98 g for every 1 mm|Hg increase in DBP (B = −160.65, p < .05). For African American women who reported no exposure to childhood indirect racism, the association between birth weight adjusted for gestational age and change in DBP was not statistically significant (B = 89.28, p > .30). Thus, for this sample of African American women, combinations of maternal childhood indirect racism exposure together with prenatal change in DBP predicted birth weight adjusted for length of gestation.
Because the racism distributions had positive skew (1.72) and high kurtosis (1.76), there was not an effective normalizing transform. Therefore, we repeated the analysis with a dichotomized racism variable. The recoded variable represented women who reported exposure to childhood indirect racism in two (+1 SD in racism) or more domains versus women who reported fewer than two domains of exposure. This analysis revealed a significant interaction (p < .05) and the same pattern of results, indicating that skew did not account for the previous results.
SBP change analyses.
Parallel analyses involving change in SBP revealed no statistically significant results (all ps >.05).
Discussion
Consistent with our hypothesis, African American women who reported greater exposure to racism, especially indirect childhood exposure in the context of increases in DBP between 18 and 32 weeks’ gestation, had infants of lower gestational-age adjusted birth weights. This effect appears to be because of restricted fetal growth rather than earlier delivery because timing of delivery was controlled. Consistent with our past research, this pattern was true for DBP, but not for SBP (Hilmert et al., 2008).
Larger increases in DBP were not statistically associated with lower adjusted birth weight when African American women reported little or no childhood indirect racism exposure. Thus, an increase in DBP by itself did not increase risk of lower birth weight, pointing to underlying mechanisms. For example, changes in blood pressure are determined by a combination of changes in cardiac stroke volume and heart rate (cardiac output), vascular resistance, and during pregnancy a blood volume increase of up to 50% (Edouard, Pannier, London, Cuche, & Safar, 1998). One possibility is that a history of racism exposure is related to how these underlying hemodynamic factors synthesize to produce an increase in blood pressure during pregnancy, consequently moderating how increases in DBP are associated with fetal growth.
The divergent DBP and SBP results suggest that vascular resistance may be a critical underlying factor. Although DBP and SBP are determined by both cardiac output and vascular resistance, as the lowest and highest points of pressure in the CV system, respectively, DBP may be primarily determined by vascular resistance whereas SBP may be primarily determined by cardiac output (Khurana, 2005). Therefore, we can speculate that increases in DBP indicated increases in vascular resistance, especially for women who experienced more racism. Greater resistance in the uterine arteries can cause a decrease in diastolic blood flow velocity that is not evident in systolic blood flow (Trudinger, Giles, & Cook, 1985b). A decrease in uterine blood flow during diastole has been associated with less placental perfusion and less umbilical blood flow to the fetus, resulting in restricted growth (Krebs et al., 1996; Trudinger, Giles, & Cook, 1985a).
It is possible that vascular resistance mediates the link between racism and racial disparities in birth weight. In general, African American blood pressure responses to stress involve more vascular resistance than Non-Hispanic White responses (Anderson, Mc-Neilly, & Myers, 1991). Blood pressure responses to threats, such as the experience of racism (Clark, 2000), involve more vascular resistance than reactions to challenging (nonthreatening) situations (Tomaka, Blascovich, Kelsey, & Leitten, 1993). Frequently repeated increases in vascular resistance, over time, can damage the CV system creating endothelial lesions that accumulate and contribute to the development of atherosclerosis (Rich-Edwards & Grizzard, 2005). Atherosclerosis in turn tends to exacerbate vascular resistance (Yeung et al., 1991), which can lead to more vascular damage and CV dysfunction.
Our findings provide moderate support for an accumulated damage pathway. Although there was a main effect of lifetime exposure to racism on adjusted birth weight, the interaction between lifetime racism and DBP change did not reach statistical significance (p = .15). The interaction between childhood indirect racism exposure and DBP change significantly predicted adjusted birth weight and the interaction between childhood direct racism and DBP change was a marginally significant predictor. This suggests that racism as a potent form of early life adversity especially affected adulthood physiology, an idea that has been rapidly gaining support (Buss, Davis, Muftuler, Head, & Sandman, 2010; Miller et al., 2011; Taylor et al., 2004).
Severe stress encountered during an early “sensitive period,” can have repercussions for birth outcomes, stress responses, and health later in life. For instance, Taylor et al. (2004) found that a harsh, nonnurturing early family environment is associated with dysfunctional physiological and psychological stress responses in adulthood. Furthermore, early life adversity in women who later become pregnant has been associated with prenatal, birth, and child outcomes (Astone, Misra, & Lynch, 2007; Cammack et al., 2011; Miller & Chen, 2007; Noll et al., 2007). It is possible that early, traumatic racism experiences are inordinately salient and are markers of a more toxic social environment during childhood. Thus, early exposure to racism could impact physiological responses to stress and adjustments to pregnancy in adulthood (Nuru-Jeter et al., 2009).
It is also possible that the influence of exposure to racism on adulthood physiology is initiated in the womb. The maternal-fetal environment has been associated with a variety of health-related outcomes (Davis, Glynn, Waffarn, & Sandman, 2011; Sandman, Davis, & Glynn, 2012). For example, large maternal cortisol responses to stress in pregnancy are associated with larger infant cortisol responses to a heel-stick (Davis et al., 2011). Frequent maternal vascular responses to racism may create a fetal environment that predisposes the fetus (and future mother) to such responses (Barker, 1990; Churchill et al., 1997).
Childhood exposure to racism that was indirect, that is, not directly experienced by the child had the most significant impact on DBP and adjusted birth weight associations. As noted, this may be because childhood direct experiences with racism are underreported or relatively rare. That the same effects were not statistically significant for childhood direct, adulthood, and total racism exposure must be interpreted with caution. The lack of significant results may have been due, at least in part, to a lack of statistical power in the current study. Future research on the health consequences of racism exposure should continue to consider racism exposure across the life span in multiple forms (e.g., direct and indirect).
In addition to racism exposure, other early major stressors may interact with blood pressure to impact birth outcomes. Although our analyses controlled for recent life events, we did not consider a wider range of early life stressors (e.g., child abuse, low childhood SES) that could have impacted adult physiology. Thus, it is not clear whether racism has a unique impact relative to other forms of early life adversity. It may be important to measure and test for a number of severe early life stressors to extend these results.
There were some potential limitations of the current study. Our sample size was not large and not representative of all African Americans. The participants were recruited at prenatal visits, had no clear behavioral risk factors for adverse birth outcomes, and no chronic health problems. This afforded us a unique opportunity to assess the effects of racism and blood pressure on birth outcomes absent the effects of these more well documented risk factors. Also, it is important to note that racism has an impact on health across the entire SES spectrum (Borrell, Kiefe, Williams, Diez-Roux, & Gordon-Larsen, 2006; D’Anna, Ponce, & Siegel, 2010; Williams & Mohammed, 2009). In a larger, more socioeconomically representative sample of pregnant African American women we might expect that associations among racism, blood pressure, and pregnancy outcomes would be stronger than those reported here.
Another limitation may be that in this study no clinically low birth weight (<2,500 g) babies were born. However, a growing body of research shows that lower birth weight in the normal range is an important outcome that can have developmental and adulthood health-related effects (Breslau, Chilcoat, DelDotto, Andreski, & Brown, 1996; Danziger, Silverwood, & Koupil, 2011; Kajantie et al., 2007; Matte, Bresnahan, Begg, & Susser, 2001; Pesonen et al., 2009; Sorensen et al., 1997).
Other potential limitations include the fact that the measure of racism used was not designed to detect the frequency or intensity of the individual’s exposure to racism, whereas that information may further explain how racism affects health (Utsey, 1998), and why certain forms of racism seem to have particular impacts on pregnancy outcomes. Also, time of day when blood pressure was measured was not recorded and may have added variability to these measures, the effect of which would be to minimize effects.
Our study provides evidence of one possible way in which racism affects birth outcomes. Specifically, childhood exposure to racism combined with an increase in DBP during 18 to 32 weeks’ gestation was associated with lower adjusted birth weight. It is possible that early racism stress impacted adulthood prenatal CV physiology thereby reducing the rate of fetal growth. Direct evidence of this pathway is still needed. With the present data, it is not clear whether childhood indirect racism contributed to CV dysfunction or not. For African American women with a history of racism, increases in prenatal DBP may indicate hormonal or immune dysfunction that affects fetal growth (Coussons-Read, Okun, Schmitt, & Giese, 2005; Davis et al., 2011). Future research should investigate a variety of direct and indirect pathways linking racism and prenatal maternal DBP changes to birth outcome.
Our results suggest that physiological adjustments to pregnancy must be considered within a psychosocial context. The association between prenatal change in DBP and adjusted birth weight depended on a woman’s past experiences with racism. Assessing maternal pregnancy physiology while considering maternal childhood and lifetime exposure to racism may be an important approach for identifying those African American women at greatest risk for adverse birth outcomes.
Acknowledgments
This project was supported by the National Institute for Child Health and Human Development Grant R01-HD28413 (awarded to Curt A. Sandman) and the first author received support from T32 MH15750 during early phases of the work. Tyan Parker Dominguez was supported in part with funding from the Larson Endowment for Innovative Research and Teaching, Hamovitch Center for Science in the Human Services, USC School of Social Work. We would like to acknowledge the contributions of Christine Rini, PhD, Sarah Roper Coleman, MA, Susan Jackman, RN, and Joan Herberg, who were members of the research team, and Linda Langley, PhD, and Siri Fiebiger, MD, for feedback on an earlier version of the article.
Contributor Information
Clayton J. Hilmert, North Dakota State University
Tyan Parker Dominguez, University of Southern California.
Christine Dunkel Schetter, University of California, Los Angeles.
Sindhu K. Srinivas, University of Pennsylvania
Laura M. Glynn, Chapman University and University of California, Irvine
Calvin J. Hobel, Cedars-Sinai Medical Center, Los Angeles, CA and University of California, Los Angeles
Curt A. Sandman, University of California, Irvine
References
- Aiken LS, & West SG (1991). Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA, US: Sage, Inc, 212. [Google Scholar]
- American College of Obstetricians and Gynecologists (ACOG). (2009). Ultrasonography in pregnancy. In American College of Obstetricians and Gynecologists (Ed.), ACOG practice bulletin (Vol. 101). Washington, DC: Author. [Google Scholar]
- Anderson NB, McNeilly M, & Myers H (1991). Autonomic reactivity and hypertension in blacks: A review and proposed model. Ethnicity and Disease, 1, 154–170. [PubMed] [Google Scholar]
- Astone NM, Misra D, & Lynch C (2007). The effect of maternal socio-economic status throughout the lifespan on infant birthweight. Paediatric and Perinatal Epidemiology, 21, 310–318. doi: 10.1111/j.1365-3016.2007.00821.x [DOI] [PubMed] [Google Scholar]
- Barker DJ (1990). The fetal and infant origins of adult disease. British Medical Journal, 301, 1111. doi: 10.1136/bmj.301.6761.1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell LN, Kiefe CI, Williams DR, Diez-Roux AV, & Gordon-Larsen P (2006). Self-reported health, perceived racial discrimination, and skin color in African Americans in the cardia study. Social Science and Medicine, 63, 1415–1427. doi: 10.1016/j.socscimed.2006.04.008 [DOI] [PubMed] [Google Scholar]
- Breslau N, Chilcoat H, DelDotto J, Andreski P, & Brown G (1996). Low birth weight and neurocognitive status at six years of age. Biological Psychiatry, 40, 389–397. doi: 10.1016/0006-3223(95)00399-1 [DOI] [PubMed] [Google Scholar]
- Brondolo E, Rieppi R, Kelly KP, & Gerin W (2003). Perceived racism and blood pressure: A review of the literature and conceptual and methodological critique. Annals of Behavioral Medicine, 25, 55–65. doi: 10.1207/S15324796ABM2501_08 [DOI] [PubMed] [Google Scholar]
- Buss C, Davis EP, Muftuler LT, Head K, & Sandman CA (2010). High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6–9-year-old children. Psychoneuroendocrinology, 35, 141–153. doi: 10.1016/j.psyneuen.2009.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammack AL, Buss C, Entringer S, Hogue CJ, Hobel CJ, & Wadhwa PD (2011). The association between early life adversity and bacterial vaginosis during pregnancy. American Journal of Obstetrics and Gynecology, 204, 431 e431–e438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill D, Perry IJ, & Beevers DG (1997). Ambulatory blood pressure in pregnancy and fetal growth. Lancet, 349, 7–10. doi: 10.1016/S0140-6736(96)06297-6 [DOI] [PubMed] [Google Scholar]
- Clark R (2000). Perceptions of interethnic group racism predict increased vascular reactivity to a laboratory challenge in college women. Annals of Behavioral Medicine, 22, 214–222. doi: 10.1007/BF02895116 [DOI] [PubMed] [Google Scholar]
- Clark R, Anderson NB, Clark VR, & Williams DR (1999). Racism as a stressor for African Americans. A biopsychosocial model. American Psychologist, 54, 805–816. doi: 10.1037/0003-066X.54.10.805 [DOI] [PubMed] [Google Scholar]
- Collins JW, David RJ, Handler A, Wall S, & Andes S (2004). Very low birthweight in African American infants: The role of maternal exposure to interpersonal racial discrimination. American Journal of Public Health, 94, 2132–2138. doi: 10.2105/AJPH.94.12.2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JW, David RJ, Symons R, Handler A, Wall SN, & Dwyer L (2000). Low-income African-American mothers’ perception of exposure to racial discrimination and infant birth weight. Epidemiology, 11, 337–339. doi: 10.1097/00001648-200005000-00019 [DOI] [PubMed] [Google Scholar]
- Committee on Understanding Premature Birth and Assuring Healthy Outcomes. (2007). Sociodemographic and community factors contributing to preterm birth. In Behrman RE & Butler AS (Eds.), Preterm birth: Causes, consequences, and prevention (pp. 124–147). Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Coussons-Read ME, Okun ML, Schmitt MP, & Giese S (2005). Prenatal stress alters cytokine levels in a manner that may endanger human pregnancy. Psychosomatic Medicine, 67, 625–631. doi: 10.1097/01.psy.0000170331.74960.ad [DOI] [PubMed] [Google Scholar]
- D’Anna LH, Ponce NA, & Siegel JM (2010). Racial and ethnic health disparities: Evidence of discrimination’s effects across the sep spectrum. Ethnicity & Health, 15, 121–143. doi: 10.1080/13557850903490298 [DOI] [PubMed] [Google Scholar]
- Danziger PD, Silverwood R, & Koupil I (2011). Fetal growth, early life circumstances, and risk of suicide in late adulthood. European Journal of Epidemiology, 26, 571–581. doi: 10.1007/s10654-011-9592-3 [DOI] [PubMed] [Google Scholar]
- David R, & Collins J Jr. (2007). Disparities in infant mortality: What’s genetics got to do with it? American Journal of Public Health, 97, 1191–1197. doi: 10.2105/AJPH.2005.068387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Waffarn F, & Sandman CA (2011). Prenatal maternal stress programs infant stress regulation. Journal of Child Psychology and Psychiatry and Allied Disciplines, 52, 119–129. doi: 10.1111/j.1469-7610.2010.02314.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez TP (2008). Race, racism, and racial disparities in adverse birth outcomes. Clinical Obstetrics and Gynecology, 51, 360–370. doi: 10.1097/GRF.0b013e31816f28de [DOI] [PubMed] [Google Scholar]
- Dominguez TP, Dunkel-Schetter C, Glynn LM, Hobel C, & Sandman CA (2008). Racial differences in birth outcomes: The role of general, pregnancy, and racism stress. Health Psychology, 27, 194–203. doi: 10.1037/0278-6133.27.2.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez TP, Dunkel Schetter C, Mancuso R, Rini CM, & Hobel C (2005). Stress in African American pregnancies: Testing the roles of various stress concepts in prediction of birth outcomes. Annals of Behavioral Medicine, 29, 12–21. doi: 10.1207/s15324796abm2901_3 [DOI] [PubMed] [Google Scholar]
- Dominguez TP, Strong EM, Gillman MW, Krieger N, & Rich-Edwards JW (2009). Differences in the self-reported racism experiences of us-born and foreign-born black pregnant women. Social Science & Medicine, 69, 258–265. doi: 10.1016/j.socscimed.2009.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterling TR, Benedetti TJ, Carlson KC, Brateng DA, Wilson J, & Schmucker BS (1991). The effect of maternal hemodynamics on fetal growth in hypertensive pregnancies. American Journal of Obstetrics and Gynecology, 165(4 Pt 1), 902–906. [DOI] [PubMed] [Google Scholar]
- Easterling TR, Carr DB, Brateng D, Diederichs C, & Schmucker B (2001). Treatment of hypertension in pregnancy: Effect of atenolol on maternal disease, preterm delivery, and fetal growth. Obstetrics and Gynecology, 98, 427–433. doi: 10.1016/S0029-7844(01)01477-6 [DOI] [PubMed] [Google Scholar]
- Edouard DA, Pannier BM, London GM, Cuche JL, & Safar ME (1998). Venous and arterial behavior during normal pregnancy. American Journal of Physiology, 274 (5 Pt 2), H1605–1612. [DOI] [PubMed] [Google Scholar]
- Fiscella K (2005). Race, genes and preterm delivery. Journal of theNational Medical Association, 97, 1516–1526. [PMC free article] [PubMed] [Google Scholar]
- Giscombé CL, & Lobel M (2005). Explaining disproportionately high rates of adverse birth outcomes among African Americans: The impact of stress, racism, and related factors in pregnancy. Psychological Bulletin, 131, 662–683. doi: 10.1037/0033-2909.131.5.662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg RL, Cliver SP, Mulvihill FX, Hickey CA, Hoffman HJ, Klerman LV, & Johnson MJ (1996). Medical, psychosocial, and behavioral risk factors do not explain the increased risk for low birth weight among black women. American Journal of Obstetrics and Gynecology, 175, 1317–1324. doi: 10.1016/S0002-9378(96)70048-0 [DOI] [PubMed] [Google Scholar]
- Hanson MD, & Chen E (2010). Daily stress, cortisol, and sleep: The moderating role of childhood psychosocial environments. Health Psychology, 29, 394–402. doi: 10.1037/a0019879 [DOI] [PubMed] [Google Scholar]
- Hilmert CJ, Schetter CD, Dominguez TP, Abdou C, Hobel CJ, Glynn L, & Sandman C (2008). Stress and blood pressure during pregnancy: Racial differences and associations with birthweight. Psychosomatic Medicine, 70, 57–64. doi: 10.1097/PSY.0b013e31815c6d96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogue CJ, & Bremner JD (2005). Stress model for research into preterm delivery among black women. American Journal of Obstetrics and Gynecology, 192(5 Suppl.), S47–S55. doi: 10.1016/j.ajog.2005.01.073 [DOI] [PubMed] [Google Scholar]
- Kajantie E, Feldt K, Raikkonen K, Phillips DI, Osmond C, Heinonen K, … Eriksson JG (2007). Body size at birth predicts hypothalamic-pituitary-adrenal axis response to psychosocial stress at age 60 to 70 years. Journal of Clinical Endocrinology and Metabolism, 92, 4094–4100. doi: 10.1210/jc.2007-1539 [DOI] [PubMed] [Google Scholar]
- Khong TY, De Wolf F, Robertson WB, & Brosens I (1986). Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. British Journal of Obstetrics and Gynaecology, 93, 1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x [DOI] [PubMed] [Google Scholar]
- Khurana I (2005). Textbook of medical physiology. Up, India: Elsevier. [Google Scholar]
- Klonoff EA, & Landrine H (1999). Cross-validation of the schedule of racist events. Journal of Black Psychology, 25, 231–254. doi: 10.1177/0095798499025002006 [DOI] [Google Scholar]
- Krebs C, Macara LM, Leiser R, Bowman AW, Greer IA, & Kingdom JC (1996). Intrauterine growth restriction with absent end-diastolic flow velocity in the umbilical artery is associated with maldevelopment of the placental terminal villous tree. American Journal of Obstetrics and Gynecology, 175, 1534–1542. doi: 10.1016/S0002-9378(96)70103-5 [DOI] [PubMed] [Google Scholar]
- Krieger N, Rowley DL, Herman AA, & Avery B (1993). Racism, sexism, and social class: Implications for studies of health, disease, and well-being. American Journal of Preventive Medicine, 9(6 Suppl.), 82–122. [PubMed] [Google Scholar]
- Krieger N, Smith K, Naishadham D, Hartman C, & Barbeau EM (2005). Experiences of discrimination: Validity and reliability of a self-report measure for population health research on racism and health. Social Science & Medicine, 61, 1576–1596. doi: 10.1016/j.socscimed.2005.03.006 [DOI] [PubMed] [Google Scholar]
- Landrine H, & Klonoff EA (1994). The African American acculturation scale: Development, reliability, and validity. Journal of Black Psychology, 20, 104–127. doi: 10.1177/00957984940202002 [DOI] [Google Scholar]
- Landrine H, & Klonoff EA (1996). The schedule of racist events: A measure of racial discrimination and a study of its negative physical and mental health consequences. Journal of Black Psychology, 22, 144–168. doi: 10.1177/00957984960222002 [DOI] [Google Scholar]
- Lespinasse AA, David RJ, Collins JW, Handler AS, & Wall SN (2004). Maternal support in the delivery room and birthweight among African-American women. Journal of the National Medical Association, 96, 187–195. [PMC free article] [PubMed] [Google Scholar]
- MacGillivray I, Rose G, & Rowe B (1969). Blood pressure survey during pregnancy. Clinical Science, 37, 395–407. [PubMed] [Google Scholar]
- Matte TD, Bresnahan M, Begg MD, & Susser E (2001). Influence of variation in birth weight within normal range and within sibships on IQ at age 7 years: Cohort study. BMJ: British Medical Journal, 323, 310–314. doi: 10.1136/bmj.323.7308.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (1998). Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840, 33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x [DOI] [PubMed] [Google Scholar]
- Miller GE, & Chen E (2007). Unfavorable socioeconomic conditions in early life presage expression of proinflammatory phenotype in adolescence. Psychosomatic Medicine, 69, 402–409. doi: 10.1097/PSY.0b013e318068fcf9 [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Parker KJ (2011). Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin, 137, 959–997. doi: 10.1037/a0024768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra VK, Hobel CJ, & Sing CF (2009). Placental blood flow and the risk of preterm delivery. Placenta, 30, 619–624. doi: 10.1016/j.placenta.2009.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustillo S, Krieger N, Gunderson EP, Sidney S, McCreath H, & Kiefe CI (2004). Self-reported experiences of racial discrimination and black-white differences in preterm and low-birthweight deliveries: The cardia study. American Journal of Public Health, 94, 2125–2131. doi: 10.2105/AJPH.94.12.2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll JG, Schulkin J, Trickett PK, Susman EJ, Breech L, & Putnam FW (2007). Differential pathways to preterm delivery for sexually abused and comparison women. Journal of Pediatric Psychology, 32, 1238–1248. doi: 10.1093/jpepsy/jsm046 [DOI] [PubMed] [Google Scholar]
- Nuru-Jeter A, Dominguez TP, Hammond WP, Leu J, Skaff M, Egerter S, … Braveman P (2009). “It’s the skin you’re in”: African-American women talk about their experiences of racism. An exploratory study to develop measures of racism for birth outcome studies. Maternal and Child Health Journal, 13, 29–39. doi: 10.1007/s10995-008-0357-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen AK, Raikkonen K, Matthews K, Heinonen K, Paavonen JE, Lahti J, … Strandberg T (2009). Prenatal origins of poor sleep in children. Sleep, 32, 1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich-Edwards JW, & Grizzard TA (2005). Psychosocial stress and neuroendocrine mechanisms in preterm delivery. American Journal of Obstetrics and Gynecology, 192(5 Suppl.),S30–S35. doi: 10.1016/j.ajog.2005.01.072 [DOI] [PubMed] [Google Scholar]
- Rosenthal L, & Lobel M (2011). Explaining racial disparities in adverse birth outcomes: Unique sources of stress for Black American women. Social Science & Medicine, 72, 977–983. doi: 10.1016/j.socscimed.2011.01.013 [DOI] [PubMed] [Google Scholar]
- Sandman CA, Davis EP, & Glynn LM (2012). Prescient human fetuses thrive. Psychological Science, 23, 93–100. doi: 10.1177/0956797611422073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckl JR, & Meaney MJ (2004). Glucocorticoid programming. Annals of the New York Academy of Sciences, 1032, 63–84. doi: 10.1196/annals.1314.006 [DOI] [PubMed] [Google Scholar]
- Sorensen HT, Sabroe S, Olsen J, Rothman KJ, Gillman MW, & Fischer P (1997). Birth weight and cognitive function in young adult life: Historical cohort study. British Medical Journal, 315, 401–403. doi: 10.1136/bmj.315.7105.401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancil TR, Hertz-Picciotto I, Schramm M, & Watt-Morse M (2000). Stress and pregnancy among African-American women. Paediatric and Perinatal Epidemiology, 14, 127–135. doi: 10.1046/j.1365-3016.2000.00257.x [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lerner JS, Sage RM, Lehman BJ, & Seeman TE (2004). Early environment, emotions, responses to stress, and health. Journal of Personality Special Emotions, Personality, and Health, 72, 1365–1393. [DOI] [PubMed] [Google Scholar]
- Thompson ML, Williams MA, & Miller RS (2009). Modelling the association of blood pressure during pregnancy with gestational age and body mass index. Paediatric and Perinatal Epidemiology, 23, 254–263. doi: 10.1111/j.1365-3016.2009.01027.x [DOI] [PubMed] [Google Scholar]
- Tomaka J, Blascovich J, Kelsey RM, & Leitten CL (1993). Subjective, physiological, and behavioral effects of threat and challenge appraisal. Journal of Personality and Social Psychology, 65, 248–260. doi: 10.1037/0022-3514.65.2.248 [DOI] [Google Scholar]
- Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, & Taylor T (2003). Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosomatic Medicine, 65, 46–62. [DOI] [PubMed] [Google Scholar]
- Trudinger BJ, Giles WB, & Cook CM (1985a). Flow velocity waveforms in the maternal uteroplacental and fetal umbilical placental circulations. American Journal of Obstetrics and Gynecology, 152, 155–163. [DOI] [PubMed] [Google Scholar]
- Trudinger BJ, Giles WB, & Cook CM (1985b). Uteroplacental blood flow velocity-time waveforms in normal and complicated pregnancy. British Journal of Obstetrics and Gynaecology, 92, 39–45. doi: 10.1111/j.1471-0528.1985.tb01046.x [DOI] [PubMed] [Google Scholar]
- Utsey SO (1998). Assessing the stressful effects of racism: A review of instrumentation. Journal of Black Psychology, 24, 269–288. doi: 10.1177/00957984980243001 [DOI] [Google Scholar]
- Valero De Bernabe J, Soriano T, Albaladejo R, Juarranz M, Calle ME, Martinez D, & Dominguez-Rojas V (2004). Risk factors for low birth weight: A review. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 116, 3–15. doi: 10.1016/j.ejogrb.2004.03.007 [DOI] [PubMed] [Google Scholar]
- Williams DR, & Mohammed SA (2009). Discrimination and racial disparities in health: Evidence and needed research. Journal of Behavioral Medicine, 32, 20–47. doi: 10.1007/s10865-008-9185-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung AC, Vekshtein VI, Krantz DS, Vita JA, Ryan TJ Jr., Ganz P, & Selwyn AP (1991). The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. New England Journal of Medicine, 325, 1551–1556. doi: 10.1056/NEJM199111283252205 [DOI] [PubMed] [Google Scholar]


