Abstract
Purpose
Data specific to the epidemiology, clinical features, and management of chronic urticaria (CU) in the geriatric population remain limited and not well understood. We aim to systematically review the prevalence, clinical manifestations, treatment, and clinical course of elderly patients with CU.
Patients and methods
Original articles that included data of elderly (aged >60 years) with CU that were published until February 2021 were searched in PubMed, Scopus, and Embase using predfefined search terms. Related articles were evaluated according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses recommendations.
Results
Among the included 85 studies and 1,112,066 elderly CU patients, most (57.4%) were women. The prevalence of elderly CU in the general population ranged from 0.2–2.8%, and from 0.7–33.3% among all CU patients. Compared to adult CU, elderly CU patients had a higher percentage of wheal alone (73.9%), and lower rate of positive autologous serum skin test and atopy. Gastrointestinal diseases were the most common comorbidity (71.9%), and there was a high rate of malignancies and autoimmune diseases. Second generation H1-antihistamines were commonly used, and achievement of complete control was most often reported. Omalizumab was prescribed in 59 refractory patients, and a significant response to treatment was reported in most patients. The treatment of comorbidities also yielded significant improvement in CU.
Conclusion
Elderly CU was found to be different from adult CU in both clinical and laboratory aspects. H1- antihistamines are effective as first-line therapy with minimal side-effects at licensed doses. Treatment of secondary causes is important since the elderly usually have age-related comorbidities.
Keywords: prevalence, clinical manifestations, treatment, chronic urticaria, elderly, systematic review
Introduction
People are now living longer due to new innovations in both technology and modern medicine.1 The result has been a doubling of global life expectancy over the past century, and an increase in the aging population worldwide.2 The World Health Organization and the United Nations define elderly as age ≥60 years and age ≥65 years, respectively.3,4 Thus, elderly-specific medical care has become and will continue to be a top priority of global public health.
Chronic urticaria (CU) is one of the most common pruritic conditions in the older population.5,6 CU is characterized by the presence of recurrent wheal, with or without angioedema, occurring at least twice a week for longer than 6 weeks.7 CU can be classified into two subtypes: chronic spontaneous urticaria (CSU) and chronic inducible urticaria (CIndU).7 The pathogenesis of CU is still unclear, but it is thought to be related to histamine, other mediators, and cytokines that are released from activated mast cells by degranulation.7–9 Among all patient with CU, 4.1–5.5% are elderly.10−12 Moreover, several systemic and autoimmune diseases have been reported to be associated with CU in the elderly population, including hypertension, chronic kidney disease, diabetes mellitus, thyroid disease, atopic dermatitis and other allergic diseases, cardiac and cerebral vascular disease, and cancer.11,13–20 CU can also affect various aspects of patient quality of personal and social life, including sleep disorders, anxiety and depression, sexual dysfunction, and decreased work performance.21–23
Our current understanding of CU in the elderly is still limited since the number of studies describing the clinical manifestations and responses to treatment of CU in the geriatric population with CU remains comparatively small. The International EAACI/GA2 LEN/EuroGuiDerm/APAAACI Guideline for the Definition, Classification, Diagnosis and Management of Urticaria recommends second generation H1-antihistamine (sgAH1) as the first-line treatment for CU.7 If disease control is inadequate after 2–4 weeks of treatment, increasing the dose up to 4-fold of the standard dose of sgAH1s is recommended. For antihistamine-refractory patients, omalizumab and cyclosporine (CsA) are the treatments of choice.7 However, the use of some antihistamines and other medications to treat older patients with CU can be limited due to several factors. In recalcitrant cases, other differential diagnoses related to underlying medical conditions should be considered. In an effort to bridge this knowledge gap, this systematic review was conducted to investigate the reported epidemiology, clinical features, treatments, and clinical course in elderly CU from all available studies.
Methods
Protocol and Registration
The protocol of this systematic review has been reviewed and approved by the Siriraj Institutional Review Board (SIRB), Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand with SIRB Protocol No. 107/2564 (Exempt), and followed the standard protocol of Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA).24 Studies published until February 2021 were searched in the PubMed, Scopus, and Embase databases. The search terms were “urticaria and elderly”, “urticaria and aging”, and “urticaria and geriatric”.
Eligibility Criteria for Systematic Review
Case reports, case series, randomized controlled trials (RCTs), prospective cohort, retrospective cohort, and other types of studies that reported the epidemiology and clinical manifestation of CU in patients aged equal to or greater than 60 years were included. Due to the relatively limited number of studies of CU in the elderly, we included the studies performed in patients aged equal to or greater than 60 years, case reports and case series, aiming to collect data from available published evidence as much as possible. Treatment data was also extracted, but it was not part of the inclusion criteria (ie, studies that described only epidemiology and clinical manifestation without a description of treatment were eligible). Five investigators (KK, CR, KM, ST, and SP) independently screened all titles and abstracts of all retrieved articles. Potentially eligible articles were reviewed in full-text to determine their final eligibility. That process was also independently conducted by the same five reviewers. Any disagreement was resolved by discussion and consensus among the five reviewers.
Data Extraction
The following data were independently extracted by the same five investigators (KK, CR, KM, ST, and SP): 1) first author’s name and the year of publication; 2) number of reported patients; 3) epidemiology; 4) clinical manifestations; 5) laboratory investigations; and 6) treatment and clinical course. Response to treatment was classified into four groups, as follows: i) complete control was defined as free of symptoms on continuation of treatment; ii) marked improvement was defined as symptoms having improved considerably, but that some symptoms were still present during treatment; iii) partial improvement was defined as partial reduction of severity of symptoms during treatment; and iv) no improvement was defined as no improvement of symptoms while on medications.
Statistical Analysis
Descriptive statistics, including mean plus/minus standard deviation and number and percentage, were used to describe demographic data, clinical manifestation, prevalence, laboratory findings, treatment, and clinical course. All data were analyzed using PASW Statistics for Windows (version 18.0; SPSS, Inc., Chicago, IL).
Results
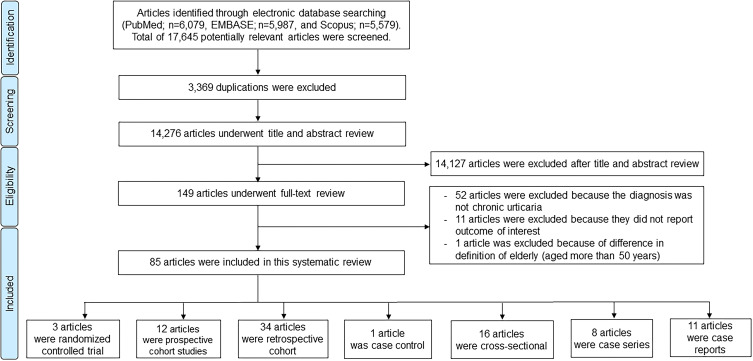
From the three databases that were searched, 17,645 articles were identified (6,079 from PubMed, 5,579 from Scopus, and 5,987 from Embase). Of those, 3,369 duplicate articles were excluded. The remaining 14,276 articles underwent title and abstract review. This process eliminated 14,127 articles that did not meet the inclusion criteria. The remaining 149 articles underwent full-text review. Of those, 85 articles (three randomized controlled trials, 12 prospective cohort studies, 34 retrospective cohort studies, one case control study, 16 cross-sectional studies, eight cases series, and 11 case reports) fulfilled the inclusion criteria and were included for systematic review (Figure 1).
Figure 1.
Flow diagram of the literature review process in this systematic review. Eighty-five articles were suitable for the inclusion criteria and were included in our systemic review. There were three randomized controlled trials, 12 prospective cohorts, 34 retrospective cohorts, one case-control, 16 cross-sectional, eight case series, and 11 case reports.
Proportion of the Elderly Among All Patients with CU, and the Prevalence of CU Among the Elderly
As shown in Table 1, the percentage of elderly among all CU patients from a single-center cohort ranged from 0.7% to 18.0%,10,12,20,25–28 while the reported percentage in general population ranged from 14.1% to 33.3%.19,29–34 Only two studies reported the percentage of elderly among all CSU patients in the general population (15.6% and 31.5%),34,35 while the percentage of elderly among CSU patients from single-center studies ranged from 6.7% to 21.7%.20,36–42 The percentage of elderly CIndU patients was reported in five studies.32,34,43–45 The highest proportion was described in a general population study (16.3%).32 The prevalence of elderly CU in the general population was reported to range from 0.2% to 2.8%29,33,35,46 (Table 2).
Table 1.
The Reported Prevalence of Chronic Urticaria, Chronic Spontaneous Urticaria, and Chronic Inducible Urticaria in Elderly Patients Relative to All Reported Cases of These Disorders
| Study (year) | Study design | Country | Population | Elderly patients among all reported patients |
|---|---|---|---|---|
| N/Total (%) | ||||
| Chronic urticaria | ||||
| Juhlin (1981)12 | Retrospective cohort | Sweden | University Hospital | 18/330 (5.5) |
| Greene et al (1985)244 | Prospective cohort | USA | Department of Dermatology Mayo Clinic |
9/50 (18.0) |
| Mekkes et al (1986)25 | Retrospective cohort | Netherland | Dermatology clinic University Hospital |
3/109 (2.8) |
| Barlow et al (1993)26 | Retrospective cohort | UK | Urticaria clinic St John’s Dermatology Centre |
1/135 (0.7) |
| Hashiro et al (1994)27 | Cross-sectional | Japan | Outpatient clinic Hospital |
5/30 (16.7) |
| Gaig et al a (2004)29 | Cross-sectional | Spain | Spanish population National telephone directory survey |
10/30 (33.3) |
| Chen et al b (2012)19 | Retrospective cohort | Taiwan | Taiwan population National Health Insurance Research Database |
3,615/12,720 (28.4) |
| Krupashankar et al (2012)28 | Prospective cohort | India | University Hospital | 5/80 (6.3) |
| Magen et al (2013)63 | Retrospective cohort | Israel | Allergy consultation Secondary and tertiary care |
124/1,319 (9.4) |
| Ban et al (2014)11 | Retrospective cohort | South Korea | Allergy clinic University Hospital |
37/837 (4.4) |
| Chuamanochan et al (2016)10 | Retrospective cohort | Thailand | Urticaria clinic University Hospital |
67/1,622 (4.1) |
| Chu b (2017)30 | Retrospective cohort | Taiwan | Taiwan population National Health Insurance Research Database |
40,816/177,879 (23.0) |
| Eun et al (2018)31 | Prospective cohort | Korea | Korean Population National Health Insurance Service - National Sample Cohort |
447/2,980 (15.0) |
| Seo et al c (2019)32 | Retrospective cohort | Korea | Korean Population Health Insurance Review and Assessment Service database |
41,882/174,579 (24.0) |
| Wertenteil et al d (2019)33 | Cross-sectional | USA | Multihealth system data analytics and research platform | 22,900/69,570 (32.9) |
| Jankowska-Konsur et ale (2019)34 | Cross-sectional | Poland | Poland Population Recruitment from multi centers all over the country |
154/1,091 (14.1) |
| Chung et al (2020)74 | Retrospective cohort | Korea | Outpatient clinic University Hospital |
26/329 (7.9) |
| Napolitano et al (2021)45 | Retrospective cohort | Italy | Dermatology unit University Hospital |
153/1,970 (7.8) |
| Chronic spontaneous urticaria | ||||
| Yang et al (2005)41 | Cross-sectional | Taiwan | Dermatological Clinics University Hospital |
5/75 (6.7) |
| Hiragun et al (2013)38 | Retrospective cohort | Japan | University Hospital | 22/117 (18.8) |
| Magen et al (2013)63 | Retrospective cohort | Israel | Allergy consultation secondary and tertiary care | 92/1,051 (8.8) |
| Vikramkumar et al (2014)42 | Cross-sectional | India | Department of Dermatology | 5/48 (10.4) |
| Lapi et al f (2016)35 | Retrospective cohort | Italy | Italian population The Health Search IMS Health Longitudinal Patient Database |
4,242/13,479 (31.5) |
| Curto-Barredo et al (2018)36 | Retrospective cohort | Spain | Urticaria unit Hospital |
119/549 (21.7) |
| Nettis et al (2018)40 | Retrospective cohort | Italy | Secondary care centers | 32/322 (9.9) |
| Curto-Barredo et al (2019)37 | Retrospective cohort | Spain | Urticaria unit Hospital |
99/549 (18.0) |
| Jankowska-Konsur et al e (2019)34 | Cross-sectional | Poland | Poland Population Recruitment from multi centers all over the country |
104/667 (15.6) |
| Jo et al (2019)39 | Retrospective cohort | South Korea | University Hospital | 79/970 (8.1) |
| Chronic inducible urticaria | ||||
| Dover et al c (1988)43 | Retrospective cohort | England | Dermatology Hospital | 1/44 (2.3) |
| Katsarou-Katsari et al (2008)44 | Retrospective cohort | Greece | Skin allergy division Hospital |
10/62 (16.1) |
| Seo et al g (2019)32 | Retrospective cohort | Korea | Korean Population Health Insurance Review and Assessment Service database |
3,290/20,191 (16.3) |
| Jankowska-Konsur et al e (2019)34 | Cross-sectional | Poland | Poland Population Recruitment from multi centers all over the country |
46/383 (12.0) |
| Napolitano et al (2021)45 | Retrospective cohort | Italy | Dermatology Unit University Hospital |
26/451 (5.8) |
Notes: aGaig et al conducted a population-based study among adults in Spain. Population sample was randomly selected from a national telephone directory. The phone survey was performed with each individual employing the Computer-assisted Telephone Interview technique. bThe database from National Health Insurance Research of Taiwan represented approximately 99.9% of Taiwan’s population. cDover et al reported the prevalence of delayed pressure urticaria in hospital for diseases of the skin and the dermatology institute. dElectronic health records data for a demographically heterogenous population-based sample of >55 million patients. The database is from 27 participating integrated health care organizations, representing over 55 million unique persons (17% of the population across all four census regions of the United States). eThis nationwide, multi-center, cross-sectional questionnaire-based study was performed under the auspices of the Polish Dermatological Society. Ten chronic urticaria patients were recruited by each of 102 dermatologists and allergists from different regions of Poland to achieve a good representation of patients from the whole country. fThe database from the Health Search IMS Health Longitudinal Patient Database (HSD) contained the computer-based patient records from about 1,000 general practitioners (GPs) throughout Italy. Included in this study were almost 1 million electronic patient records which met standard quality criteria. They were selected on a geographical basis to represent the whole Italian population. gThe database from Health Insurance Review and Assessment Service covers 97.0% of the Korean population. Not all types of urticaria were included in the study of Seo et al. The reported subtypes of chronic inducible urticaria were cholinergic urticaria and cold/heat urticaria, as shown in this table. Prevalence of dermographism was also reported but not included in this current study, as it was not symptomatic dermographism, which is chronic inducible urticaria subtypes.
Table 2.
The Reported Prevalence of Chronic Urticaria in the Elderly Population
| Study (year) | Study design | Country | Population | Reported chronic urticaria among all elderly patients N/Total (%) |
|---|---|---|---|---|
| Chronic urticaria | ||||
| Gaig et ala (2004)29 | Cross-sectional | Spain | Spanish population National telephone directory survey |
10/1,047 (1.0) |
| Lapi et alb (2016)35 | Retrospective cohort | Italy | Italian population The Health Search IMS Health Longitudinal Patient Database |
13,476/488,145 (2.8) |
| Wertenteil et alc (2019)33 | Cross-sectional study | USA | Multihealth system data analytics and research platform | 22,900/9,757,210 (0.2) |
| Gaber et al (2020)46 | Prospective cohort | Egypt | Outpatient clinic University Hospital |
2/260 (0.8) |
Notes: aGaig et al conducted a population-based study among adults in Spain. Population sample was randomly selected from a national telephone directory. The phone survey was performed with each individual employing the Computer-assisted Telephone Interview technique. bThe database from the Health Search IMS Health Longitudinal Patient Database (HSD) contained the computer-based patient records from about 1,000 general practitioners (GPs) throughout Italy. Included in this study were almost 1 million electronic patient records which met standard quality criteria. They were selected on a geographical basis to represent the whole Italian population. cElectronic health records data for a demographically heterogenous population-based sample of >55 million patients. The database is from 27 participating integrated health care organizations, representing over 55 million unique persons (17% of the population across all four census regions of the United States).
Epidemiological Data
Clinical features and demographic data of the elderly with CU are summarized in Table 3. Women accounted for 57.4%, 63.9%, and 57.9% of elderly CU, CSU, and CIndU, respectively. The mean age at presentation among all CU patients was 70.4±6.2 years. Most presented with wheal alone (73.9%), followed by wheal with angioedema (25.9%). Only 0.2% presented with wheal and anaphylaxis. The average duration of disease prior to diagnosis was 1.9±3.6 years. Allergic rhinitis, asthma, and allergic dermatitis were the three most common associated atopic diseases. Cold urticaria, symptomatic dermographism, and cholinergic urticaria were found in 10.9%, 7.3%, and 3.5% of elderly CU patients, respectively.
Table 3.
The Reported Demographic and Clinical Characteristics of Elderly Patients with Chronic Urticaria (CU), and Compared Between the Two Subtypes of CU – Chronic Spontaneous Urticaria and Chronic Inducible Urticaria
| Clinical features: N/Total (%) | CUa | CSU | CIndU |
|---|---|---|---|
| (N=1,112,066) | (N=891) | (N=1,568) | |
| Gender | |||
| Female | 61,170/106,669 (57.4) | 276/432 (63.9) | 873/1,509 (57.9) |
| Age at presentation, mean±SD, years | 70.4±6.2 | 71.6±6.7 | 69.9±3.8 |
| Symptoms | |||
| Wheal alone | 305/413 (73.9) | 236/312 (75.6) | NA |
| Wheal with angioedema | 107/413 (25.9) | 76/312 (24.4) | NA |
| Wheal with anaphylaxis | 1/413 (0.2) | 0/312 (0.0) | 1/1 (100.0) |
| Duration of disease prior diagnosis, mean±SD, years | 1.9±3.6 | 1.9±3.7 | NA |
| Personal history of atopyb | |||
| Allergic rhinitis | 44/233 (18.9) | 9/100 (9.0) | 9/27 (33.3) |
| Asthma | 84,519/982,862 (8.6) | 4/101 (4.0) | 2/27 (7.4) |
| Atopic Dermatitis | 57,163/985,228 (5.8) | 15/162 (9.3) | 4/27 (14.8) |
| Allergic conjunctivitis | 2/73 (2.7) | 0/7 (0.0) | 1/27 (3.7) |
| Unspecified atopy | 18/144 (12.5) | 8/105 (7.6) | 0/1 (0.0) |
| Family history of atopy | |||
| Allergic rhinitis | 5/74 (6.8) | 0/1 (0.0) | 0/2 (0.0) |
| Asthma | 2/74 (2.7) | 0/1 (0.0) | 0/2 (0.0) |
| Atopic Dermatitis | 1/74 (1.4) | 0/1 (0.0) | 0/2 (0.0) |
| Types of chronic inducible urticaria | |||
| Cold urticaria | 18/165 (10.9) | NA | 7/154 (4.6) |
| Symptomatic dermographism | 25/344 (7.3) | NA | 25/344 (7.3) |
| Cholinergic urticaria | 1,468/42,006 (3.5) | NA | 3/124 (2.4) |
| Delayed pressure urticaria | 4/126 (3.2) | NA | 2/124 (1.6) |
| Heat urticaria | 3/154 (2.0) | NA | 2/153 (1.3) |
| Solar urticaria | 2/125 (1.6) | NA | 1/124 (0.8) |
| Aquagenic urticaria | 1/153 (0.7) | NA | 1/153 (0.7) |
| Comorbidityb,c | |||
| Gastrointestinal diseases | 708,417/985,284 (71.9) | 1/5 (20.0) | NA |
| Coronary and other vascular diseases | |||
| Cardiac/cerebral vascular diseases | 72/196 (36.7) | 52/169 (30.8) | 19/26 (73.1) |
| Atrial fibrillation | 1/5 (20.0) | 1/5 (20.0) | NA |
| Metabolic diseases | |||
| Dyslipidemia | 3/7 (42.9) | 2/6 (33.3) | 1/1 (100.0) |
| Hypertension | 183,473/986,035 (18.6) | 103/264 (39.0) | 20/27 (74.1) |
| Obesity | 5/30 (16.7) | 0/4 (0.0) | 5/26 (19.2) |
| Diabetes Mellitus | 34/271 (12.6) | 34/271 (12.6) | NA |
| Unspecified metabolic syndrome | 21/67 (31.3) | 21/67 (31.3) | NA |
| Musculoskeletal diseases | |||
| Osteoporosis | 3/7 (42.9) | 3/7 (42.9) | NA |
| Gout | 1/5 (20.0) | 0/4 (0.0) | NA |
| Avascular hip necrosis | 1/5 (20.0) | 1/5 (20.0) | NA |
| Thyroid diseases | |||
| Hyperthyroidism | 1/1 (100.0) | NA | NA |
| Hypothyroidism | 5/9 (55.6) | 1/5 (20.0) | NA |
| Grave’s disease | 4/9 (44.4) | 2/6 (33.3) | NA |
| Hashimoto’s thyroid diseases | 22/106 (20.8) | 21/105 (20.0) | NA |
| Parathyroid adenoma | 1/5 (20.0) | 1/5 (20.0) | NA |
| Unspecified thyroid diseases | 28/142 (19.7) | 24/116 (20.7) | 4/26 (15.4) |
| Systemic diseases | |||
| Raynaud phenomena | 2/6 (33.3) | 0/4 (0.0) | NA |
| Systemic lupus erythematosus | 1/5 (20.0) | 1/5 (20.0) | NA |
| Anemia | 1/5 (20.0) | 0/4 (0.0) | NA |
| Unspecified autoimmune diseases | 8/70 (11.4) | 8/69 (11.6) | 0/1 (0.0) |
| High myopia | 1/5 (20.0) | 0/4 (0.0) | NA |
| Genitourinary disorders | |||
| Benign prostate hyperplasia | 3/30 (10.0) | 3/30 (10.0) | 3/26 (11.5) |
| Chronic kidney diseases | 6/96 (6.3) | 6/96 (6.3) | NA |
| Chronic obstructive pulmonary diseases | 2/31 (6.5) | 1/5 (20.0) | 1/26 (3.8) |
| Psychiatric problems | |||
| Dementia | 4/67 (6.0) | 4/67 (6.0) | NA |
| Unspecified psychiatric problems | 5/130 (3.9) | 2/103 (1.9) | 2/26 (7.7) |
| Dermatologic diseases | |||
| Psoriasis | 4/96 (4.2) | 4/96 (4.2) | NA |
| Contact dermatitis | 3/96 (3.1) | 3/96 (3.1) | NA |
| Malignancy | |||
| Gastrointestinal cancer | 6/10 (60.0) | 0/4 (0.0) | NA |
| Genitourinary cancer | 2/6 (33.3) | 0/4 (0.0) | NA |
| Bronchioalveolar cancer | 2/6 (33.3) | 0/4 (0.0) | NA |
| Thyroid cancer | 2/6 (33.3) | 2/6 (33.3) | NA |
| Malignant melanoma | 1/5 (20.0) | 0/4 (0.0) | NA |
| Hematologic malignancy | 35/3,625 (1.0) | 0/8 (0.0) | NA |
| Unspecified malignancy | 459/3,714 (12.4) | 11/99 (11.1) | NA |
| Possible causes of urticaria | |||
| Stress | 27/99 (27.3) | 27/99 (27.3) | NA |
| Aspirin intolerance | 8/42 (2.0) | 1/5 (20.0) | 0/4 (0.0) |
| Parasitic infection | 6/129 (4.7) | 0/4 (0.0) | NA |
| Collagen vascular disease | 4/124 (3.2) | NA | NA |
| Insect bite | 3/126 (2.4) | 1/1 (100.0) | 1/27 (3.7) |
| Paronychia | 1/1 (100.0) | NA | NA |
| Unspecified drug allergy | 12/132 (9.1) | 1/8 (12.5) | NA |
| Unspecified food allergy | 1/33 (3.0) | 0/7 (0.0) | 1/26 (3.9) |
| Laboratory investigations | |||
| Positive ASST | 125/263 (47.5) | 107/195 (54.9) | 1/3 (33.3) |
| Positive SPT | 1/9 (11.1) | 0/4 (0.0) | 1/1 (100.0) |
| Positive Basophil histamine release test | 5/13 (38.5) | 4/9 (44.4) | NA |
| Leukocytosis | 4/82 (4.9) | 4/70 (5.7) | 0/1 (0.0) |
| Positive HBsAg | 8/75 (10.7) | 8/68 (11.8) | 0/1 (0.0) |
| Positive anti-HCV | 0/70 (0.0) | 0/68 (0.0) | 0/2 (0.0) |
| Total serum IgE | |||
| Elevated IgE | 8/19 (42.1) | 7/16 (43.8) | 1/1 (100.0) |
| IgE level, mean±SD, kU/L | |||
| ImmunoCAP method (normal range 0–119 kU/L) d | 477.3±288.8 | 477.3±288.8 | NA |
| Pharmacia CAP System IgE FEIA methode (normal range 0–100 kU/L) | 164.9±210.4 | 194.5±269.7 | NA |
| Nephelometry methodf (normal range 0–100 kU/L) | 125 | 125 | NA |
| Elevated erythrocyte sedimentation rate | 22/82 (26.8) | 18/71 (25.4) | 0/1 (0.0) |
| Elevated D-dimer | 2/4 (50.0) | NA | NA |
| Elevated prothrombin fragment | 3/4 (75.0) | NA | NA |
| Abnormal C3 | 0/18 (0.0) | 0/7 (0.0) | 0/1 (0.0) |
| Abnormal C4 | 2/18 (11.1) | 0/7 (0.0) | 0/1 (0.0) |
| Abnormal CH50 | 1/11 (0.0) | 0/5 (0.0) | NA |
| Abnormal C1-INH | 0/13 (0.0) | 0/7 (0.0) | NA |
| Positive antinuclear antibodies | 13/81 (16.0) | 13/69 (18.8) | 0/2 (0.0) |
| Positive anticentromere antibodies | 2/2 (100.0) | NA | NA |
| Positive Anti-FcεRI antibodies | 2/3 (66.7) | 2/3 (66.7) | NA |
| Abnormal free T3 | 0/22 (0.0) | 0/9 (0.0) | 0/1 (0.0) |
| Abnormal free T4 | 4/31 (12.9) | 3/20 (15.0) | 0/1 (0.0) |
| Abnormal TSH | 6/33 (18.2) | 3/19 (15.8) | 0/2 (0.0) |
| Positive antithyroid peroxidase antibodies | 40/150 (26.7) | 25/110 (22.7) | 1/2 (50.0) |
| Positive antithyroglobulin antibodies | 42/124 (33.9) | 31/86 (36.1) | 0/1 (0.0) |
| Abnormal urinalysis | 10/64 (15.6) | 10/63 (15.9) | NA |
| Abnormal stool examination | 6/85 (7.1) | 4/77 (5.2) | NA |
Notes: aIt should be noted that the CU group included all CU patients aged above 60 years. Studies that reported specifically for CSU or CIndU subtypes were also included in the subgroups of CSU and CIndU. bOne patient could have more than one personal history of atopy or one comorbidity. Comorbidity and history of atopy were only showed information from papers which mentioned about each disease. cIt should be noted that the studies of Urbach, Lindelof et al, and Chen et al reported only the number of patients with malignancy, other comorbidities were not identified. dTotal IgE level measured by ImmunoCAP method were reported in three studies, Ban et al (n=37), Romano et al (n=1), and Nettis et al (n=32), which reported about CU, CSU, and CSU, respectively. Referring to the National Center for Health Statistics, Centers for Disease Control and Prevention, the normal range of total IgE based on ImmunoCAP method is 0–119 kU/L. eTotal IgE level measured by Pharmacia CAP method was reported in only one study, Staubach et al (n=4), which reported about CSU. fTotal IgE level measured by Nephelometry method was reported in only one study, Kulthanan et al (n=1), which reported about CSU.
Abbreviations: Anti-FcεRI, anti-FcepsilonRI; ASST, autologous serum skin test; C3, complement C3; C4, complement C4; CH50, total hemolytic complement; C1-INH, complement 1 esterase inhibitor; CIndU, chronic inducible urticaria; CSU, chronic spontaneous urticaria; CU, chronic urticaria; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; Ig, immunoglobulin; NA, not available/not applicable; SD, standard deviation; SPT, skin prick test; TSH, thyroid stimulating hormone.
Severity of CU was reported in 19 studies, mostly moderate-to-severe disease activity.11,20,40,47–62 Urticaria activity score (weekly total score 42) was used in 13 studies, and the average score among all studies was 22.1±12.2.20,40,48,53–55,57–63 The other scores used to report severity were Visual Analog Scale (VAS; total score 10),54 Urticaria Activity Score (UAS; total score 9),50 Urticaria Activity Score (UAS; total score 15),11 Urticaria Severity Score (USS; total score 93),51 and Treatment Score (TS; total score 5).49,53 Twelve CSU studies reported severity using Urticaria Activity Score (UAS; weekly total score 42) with an average score among studies of 26.1±12.2.40,53–55,57–60,62 Severity of CIndU was reported in heat urticaria, which showed a temperature threshold of 38°C, and in cold urticaria which showed 22 mm for the wheal and 40 mm for the flare by cold stimulation test.52,56
Elderly CU Patients Suffer from Various Age-Related Comorbidities
The reported comorbidities of study patients are shown in Table 3. Unspecified gastrointestinal (GI) disease was the most commonly reported comorbidity among elderly CU patients (71.9%), with the majority of cases collected from a large national database (Korean Health Insurance Review and Assessment Service: HIRA).64 The reported prevalence of coronary and cerebral vascular disease were also high at 36.7%. The prevalence of dyslipidemia, hypertension, obesity, and diabetes mellitus in elderly CU patients was 42.9%, 18.6%, 16.7%, and 12.6%, respectively. Thyroid diseases were reported in 20 studies,10,20,37,45,49–51,53,61,62,65–74 and some of them were related to autoimmune disorders. For example, Grave’s disease and Hashimoto’s disease was reported in 44.4% and 20.8% of aging CU, respectively. Other common comorbidities were osteoporosis (42.9%), Raynaud phenomena (33.3%), gout (20.0%), avascular hip necrosis (20.0%), systemic lupus erythematosus (20.0%), and anemia (20.0%). Malignancies were also reported at a high rate. Most malignancies were unspecified but, among those that were specified, GI cancer was the most prevalent (60.0%). Other possible causes or aggravating factors of CU were paronychia (100.0%), stress (27.3%), unspecified drug allergy (9.1%), parasitic infection (4.7%), collagen vascular disease (3.2%), unspecified food allergy (3.0%), insect bite (2.4%), and aspirin intolerance (2.0%).
Laboratory results in Elderly with CU
As shown in Table 3, a positive autologous serum skin test (ASST) was found in 47.5% of elderly CU patients, which was less than in elderly CSU patients (54.9%). A Basophil histamine release test was reported in six studies,50,61,75–78 and the result was positive in five of 13 tested patients (38.5%). There were 22 studies that reported the level of total serum IgE, and 16 of those studies reported the IgE value. The average level among those 16 studies was higher than the normal upper limit.11,20,37,40,49,50,52,55,57,61,62,76,79–82 The other six studies reported only whether the level was elevated or not. The value was elevated in 42.1% of patients,58,69,75,83–85 and this rate was similar to the 43.8% rate reported in elderly CSU. Erythrocyte sedimentation rate (ESR) was increased in 26.8% and 25.4% of elderly CU and CSU, respectively. Positive D-dimer was found in 50.0% of elderly CU patients, and elevated prothrombin fragment was found in 75.0%.86 Antinuclear antibody (ANA) was reported in 13 studies10,45,51,56,58,63,65,69,79,82,83,85,87 with an average positivity rate of 16.0% among those studies. Anti-FcεRI antibody was reported in one study (66.7% positive).72 Abnormal thyroid hormone was common since it was reported in five of 21 studies.65,71–73,77 No study reported abnormal free T3, but 13.0% of elderly CU patients had abnormal free T4 hormone, and 18.2% had abnormal thyroid stimulating hormone. Twenty-four studies reported thyroid autoantibodies with a positivity rate of antithyroid peroxidase antibodies of 26.4%, and a positivity rate of antithyroglobulin antibodies of 15.6%.10,11,40,50,51,55,56,60,63,65–67,69–73,77,78,82,83,85,88,89
Treatments for CU
Among the elderly who achieved complete control with the use of AH1, sgAH1 was most often used at a regular dose (24 of 34 patients), whereas first generation H1-antihistamine (fgAH1) was prescribed at a high dose (2 of 2 patients). Side-effects of antihistamines were reported in one study. A combination of multiple high-dose fgAH1, which were hydroxyzine (dose: 25–200 mg/day), diphenhydramine (dose: 25–200 mg/day), and doxepin (dose: 25–125 mg/day), showed no additional benefit and caused severe sedation. Treatment in those cases was later changed to omalizumab.60 Omalizumab was prescribed in 15 studies. Complete control was observed in 59 of 89 patients, and the prescribed dose ranged from 150 to 300 mg every 2–4 weeks. Fifty patients from five studies received omalizumab alone.48,76,81,90,91 Others received omalizumab in combination with other treatments, including with H1-antihistamine (AH1) in seven patients from five studies,49,50,57,58,61 and systemic corticosteroid in two patients from one study.55,60 Side-effects of omalizumab were reported in three studies.62,81,90 Two patients experienced nausea, two patients reported asthenia that spontaneously resolved within 48 hours, and one patient had pain at the injection site.
Treatment of Secondary Causes Should Be Considered a Strategy for Controlling CU
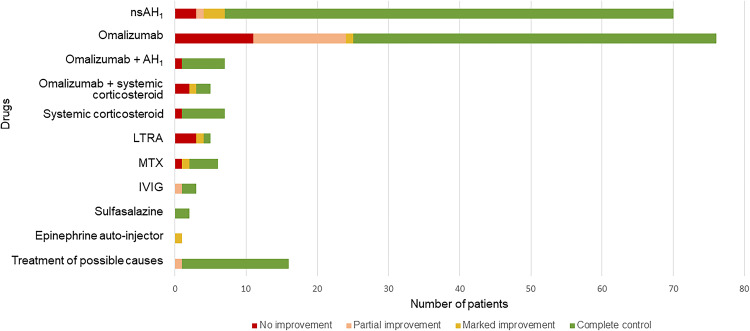
Treatment of secondary causes was also effective for controlling CU in the elderly. Thirty-nine studies described the treatment of secondary causes and the outcomes of treatment (Figure 2 and Table 4). More specifically, the following treatments, prescriptions, or procedures improved CU symptoms in the elderly: treatment for Helicobacter pylori (H. pylori) infection,63 treatment for Strongyloides infection,92 treatment for thyroid diseases,73,88 prescription of immunosuppressants for malignancies,79,83,93 prescription of intravenous immunoglobulin (IVIG)53,54,60 or sulfasalazine to treat recalcitrant CSU,94 and surgical removal of adenoma/neoplasms.69,84,85,89,95–97
Figure 2.
Treatments and responses to treatment among elderly with chronic urticaria.
Notes: Some patients received more than one type of treatment.
Abbreviations: AH1,H1-antihistamine; fgAH1, first generation H1-antihistamine; IVIG, intravenous immunoglobulin; LTRA, leukotriene receptor antagonist; MTX, methotrexate; NA, not available/not applicable; sgAH1, second generation H1-antihistamine.
Table 4.
The Reported Treatment for and Clinical Course of Chronic Urticaria in the Elderly
| Study (year) | N = Elderly CU/ Total | Treatment | Duration of treatment | Treatment response | Follow-up after treatment and outcome | Side-effects after treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AH1 | Corticosteroids | LTRA | Omalizumab | Immunosuppressant | Others | |||||||||
| fgAH1 | sgAH1 | Unspecified AH1 | Systemic | Topical | ||||||||||
| Prospective Cohort Studies | ||||||||||||||
| Leznoff et al (1983)67 | N = 1 of 17 | NA | NA | NA | NA | NA | NA | NA | NA | - Levothyroxine (dose: 0.2 mg/d) for euthyroid patient who had autoimmune thyroiditis | NA | Partial improvement | NA | NA |
| Rumbyrt et al (1995)88 | N = 1 of 7 | NA | NA | NA | Previous use of - Prednisolone (dose: NA) with no improvement | NA | NA | NA | NA | Previous use of - Famotidine (dose: NA) with no improvement - Doxepin (dose: NA) with no improvement -Thyroxine (0.05 mg/d) for euthyroid patient who had autoimmune thyroiditis |
At least 4 weeks | Complete control then discontinued thyroxine | Longer than 1 year of rare hive, with use of Hydroxyzine (dose: NA, as needed) | NA |
| O’Donnell et al (1998)54 | N = 1 of 10 | NA | - Cetirizine (dose: 10 mg twice daily) | Previous use of 0 (dose: NA) with no improvement | NA | NA | NA | NA | No | - IVIG (dose: 0.4 g/kg/d for 5 days) | 5 days | Partial improvement | 6 months | Headache |
| Sanada et al (2005)242 | N = 5 of 25 | NA | Previous use of - Ebastine (dose: 20 mg/d) with no improvement - Ebastine (dose: 20 mg/d) |
NA | NA | NA | - All cases: Montelukast (dose: 10 mg/d) | NA | NA | NA | NA | Complete control | NA | NA |
| NA | Previous use of - Ebastine (dose: 20 mg/d) with no improvement - Ebastine (dose: 20 mg/d) |
Previous use of - Betamethasone (dose: 0.5 mg/d orally) with no improvement |
Marked improvement | |||||||||||
| Previous use of - Hydroxyzine (dose: 50 mg/d) with no improvement |
Previous use of - Homochlorcyclizine (dose: 30 mg/d) with no improvement - Homochlorcyclizine (dose: 30 mg/d) |
NA | No improvement | |||||||||||
| NA | Previous use of - Olopatadine (dose: 20 mg/d) with no improvement - Olopatadine (dose: 20 mg/d) |
Previous use of - Betamethasone (dose: 0.5 mg/d orally) with no improvement |
No improvement | |||||||||||
| NA | Previous use of - Loratadine (dose: 10 mg/d) - Ebastine (dose: 10 mg/d) with no improvement - Loratadine (dose: 10 mg/d) - Ebastine (dose: 10 mg/d) |
NA | No improvement | |||||||||||
| Kaplan et al a (2008)50 | N = 2 of 12 | - Hydroxyzine (dose: 25–50 mg every 6 hours as needed, total 100–175 mg/d) | NA | NA | NA | NA | NA | - All cases: Omalizumab 150 mg sc every 2 weeks or every 4 weeks | No | NA | 4 months | No improvement | NA | NA |
| - Hydroxyzine (dose: 25–50 mg every 6 hours as needed, total 175 mg/d in the first 4 week then tapered dose until stop at week 8) | Complete control | |||||||||||||
| Uysal et al (2014)61 | N = 3 of 27 | NA | All cases: - Desloratadine or fexofenadine; (dose: recommended dose for 3–4 times) |
NA | NA | NA | NA | - Omalizumab 150 mg sc every 2 weeks then extend to 5 weeks | Previous use of - MTX (dose: NA) with no improvement |
NA | 112 days | Complete control then discontinued omalizumab | NA | NA |
| - Omalizumab 150 mg sc every 2 weeks then extend to 7 weeks | Previous use of - AZA (dose: NA) with no improvement |
78 days | ||||||||||||
| - Omalizumab 150 mg sc every 2 weeks then extend to 7 weeks | Previous use of - AZA (dose: NA) with no improvement |
62 days | ||||||||||||
| Retrospective cohort studies | ||||||||||||||
| McGirt et al (2006)94 | N = 2 of 19 | Previous use of - Hydroxyzine (dose: maximum 25 mg nightly) with no improvement |
Previous use of - Cetirizine (dose: maximum 10 mg/d) with no improvement |
NA | Previous use of - Prednisolone (dose: 10 mg/d for 3 days; 2–3 times) with no improvement - Prednisolone (dose: 10 mg/d once for 5 months) |
NA | NA | NA | NA | - Sulfasalazine (dose: start at 500 mg/d then increased by 500 mg each week until 2 g/d) for total 12 months | 12 months | Complete control then tapered off of sulfasalazine, sgAH1 | 3 months | NA |
| NA | Previous use of - Cetirizine (dose: 10 mg/d) with no improvement |
Previous use of - Prednisolone dose pack (dose: NA) for 5 courses with no improvement |
- Sulfasalazine (dose: start at 500 mg/d then increased by 500 mg each week until 2 g/d) for total 11 months | 11 months | Complete control then discontinued sulfasalazine | NA | ||||||||
| Perez et al (2010)241 | N = 1 of 16 | NA | Previous use of - sgAH1 (unmentioned name, dose: above the recommended dose) with no improvement |
NA | Previous use of - Prednisolone (dose: 20 mg/d) with no improvement | NA | NA | NA | Previous use of - CsA (dose: NA) with no improvement - MTX (dose: 5 mg weekly) |
Previous use of 0 (unmentioned name, dose: NA) with no improvement - Folic acid (dose: 5 mg weekly) |
NA | Marked improvement | NA | NA |
| Mitzel-Kaoukhov et al (2010)53 | N = 2 of 6 | NA | Previous use of - sgAH1 (unmentioned name, dose: 4-fold of the recommended dose) with no improvement |
NA | NA | NA | All cases: previous use of - LTRA (unmentioned name, dose: NA) with no improvement |
NA | All cases: previous use of - CsA (dose: NA) with no improvement |
Previous use of - Histaglobin (dose: NA) with no improvement - IVIG (dose: 2 mg/kg every 4 weeks) for 11 cycles |
10 months | Complete control then discontinued | 14 months | No |
| Previous use of - sgAH1 (unmentioned name, dose: 8-fold of the recommended dose) with no improvement |
Previous use of - Systemic corticosteroid (unmentioned name, dose: high dose) with no improvement |
NA | Previous use of - Dapsone (dose: NA) with no improvement - IVIG (dose: 2 mg/kg every 4 weeks) for 4 cycles then remission but 4 months later |
11 months | Complete control then discontinued | 2 months then relapse occurred so IVIG was reinitiated for other 5 cycles | Impairment of pre-existing HT, disappeared after extending the treatment period of IVIG from 2 to 3 days | |||||||
| Sagi et al (2011)83 | N = 5 of 8 | All cases: previous use of - fgAH1 (unmentioned name, high dose) with no improvement |
All cases: previous use of - sgAH1 (unmentioned name, high dose) with no improvement |
NA | Previous use of - Systemic corticosteroid (unmentioned name, dose: NA) with no improvement - Systemic corticosteroid (dose: 30–40 mg/d) for multiple courses then tapered down (dose: NA) - Systemic corticosteroid (dose: 30–40 mg/d) for multiple courses then tapered down (dose: NA) - Systemic corticosteroid (dose: 30–40 mg/d) for multiple courses then tapered down until off |
NA | NA | NA | - MTX (dose: 15 mg weekly) for 1 month then tapering down to 10 and 5 mg oral weekly for 1 and 1 month, respectively) | - Folic acid (dose: 5 mg weekly) | 3 months | Complete control then discontinued MTX and folic acid | 8 months | No |
| - MTX (dose: 15 mg weekly) for 3 months then currently in the process of tapering down to 10 mg oral weekly for 2 months, without recurrence of urticaria) | 5 months | Complete control (still in the MTX tapering process) | NA | Elevated liver enzyme (Twice the normal values) – resolved after reducing MTX dosage | ||||||||||
| - MTX (dose: 15 mg oral weekly) for 3 months | 3 months | Complete control then discontinued MTX and folic acid | 2 months | No | ||||||||||
| - MTX (dose: 7.5 mg oral weekly) for 2 months | 2 months | No improvement then discontinued MTX and folic acid due to poor compliance | 2 months | Fatigue | ||||||||||
| - MTX (dose: 15 mg oral weekly) for 1 month then change to 15 mg IM weekly for 4 months | 5 months | Complete control then tapering MTX down but relapse occurred and required a constant dose of MTX 15 mg/week | NA | Gastrointestinal discomfort – resolved after changing to MTX IM route | ||||||||||
| Magen et al (2013)20 | N = 49 of 92 | - fgAH1 (unmentioned name; dose: NA) in 8 of 46 patients |
- sgAH1 (unmentioned name; dose: NA) in 49 of 49 patients |
NA | - Systemic corticosteroid (unmentioned name, dose: NA) in 2 of 49 patients | NA | NA | NA | NA | NA | 12 months | Complete control in 34 of 46 patients | NA | NA |
| Magen et al (2013)63 | N = 1 of 9 | NA | NA | NA | NA | NA | NA | NA | NA | - Amoxicillin (dose: 2 g/d) - Clarithromycin (dose: 1 g/d) - Omeprazole (dose: 40 mg/d) for treatment of H. pylori infection |
2 weeks | Complete control then discontinued H. pylori infection treatment | NA | NA |
| Song et al (2013)55 | N = 4 of 16 | NA | All cases: previous use of - Cetirizine (dose: 60–80 mg/d) with no improvement |
NA | Previous use of - Prednisolone (dose: 15 mg/d) for 10 courses with no improvement - Prednisolone (dose: NA) for short courses Previous use of - Prednisolone (dose: 10 mg/d) for >20 courses with no improvement - Prednisolone (dose: NA) Previous use of - Prednisolone (dose: 5–20 mg/d) for >20 courses with no improvement - Prednisolone (dose: NA) tapered dose then off shortly after start omalizumab Previous use of - Prednisolone (dose: 5–10 mg/d) for >20 courses with no improvement - Prednisolone (dose: NA) tapered dose then off shortly after start omalizumab |
NA | NA | All cases: - Omalizumab 150 mg sc every 4 weeks |
NA | NA | 24 months | Complete control and continued with omalizumab 150 mg sc every 4–8 weeks | NA | No |
| 2 months | No improvement then discontinued omalizumab and went into spontaneous remission then discontinued prednisolone |
Flare of urticaria after first dose of omalizumab injection | ||||||||||||
| 2 months | No improvement then discontinued omalizumab | No | ||||||||||||
| 24 months | Complete control and continued with omalizumab 150 mg sc every 4–8 weeks | No | ||||||||||||
| Romano et al (2015)62 | N= 1 of 9 | Previous use of - Cinnarizine (dose: NA) with no improvement |
NA | Previous use of -AH1 (unmentioned name, dose: NA) with no improvement |
Previous use of - Systemic steroid (unmentioned name, dose: NA) with no improvement |
NA | Previous use of - LTRA (unmentioned name, dose: NA) with no improvement |
- Omalizumab 150 mg sc every 4 weeks | Previous use of - CsA (dose: NA) with no improvement |
NA | 5 months | No improvement then discontinued omalizumab | 42 months | Pain at injected site |
| Sugiyama et al (2015)73 | N = 2 of 40 | NA | Previous use of - Olopatadine (dose: 5 mg/d) with partial improvement - Olopatadine (dose: 5 mg/d) NA |
NA | NA | NA | NA | NA | NA | All cases: - Triiodothyronine (dose: 25 g/d) for Hashimoto’s disease |
3 months | Complete control then discontinued triiodothyronine | >10 months of complete control then recurrence occurred after triggered by upper respiratory tract infection; symptom was well-controlled with olopatadine 2.5 mg/d | NA |
| NA | ||||||||||||||
| Kulthanan et al (2017)57 | N = 1 of 13 | NA | Previous use of - Desloratadine (dose: 20 mg/d) - Levocetirizine (10 mg/d) with no improvement - Desloratadine (dose: 5–10 mg/d) |
NA | Previous use of - Prednisolone (5–10 mg/d) with no improvement |
NA | Previous use of - Montelukast (dose: NA) with no improvement |
- Omalizumab 150 mg sc every 4 weeks | Previous use of - CsA (dose: NA) - HCQ (dose: NA) with no improvement |
Previous use of 0 (unmentioned name, dose: NA) with no improvement |
4 months | Complete control then discontinued omalizumab | NA | No |
| Napolitano et al (2018)93 | N = 1 of 1,493 | NA | NA | Previous use of 0 (dose: 4 times of licensed dose) with no improvement |
Previous use of - Prednisolone (dose: NA) with partial improvement |
NA | NA | NA | NA | - Chemotherapy for small cell lung cancer | NA | Complete control | NA | NA |
| Napolitano et al (2021)45 | N = 26 of 451 | NA | - sgAH1 (unmentioned name, recommended dose) in 23 of 26 patients - sgAH1 (unmentioned name, double dose) in 3 of 26 patients with SD |
NA | NA | NA | NA | NA | NA | NA | NA | Complete control in 26 of 26 patients | NA | No |
| Martina et al (2021)90 | N = 62 of 62 | NA | NA | NA | NA | NA | NA | - Omalizumab 300 mg sc every 4 weeks | NA | NA | 3 months | - Complete control in 44 of 62 patients - Partial improvement in 11 of 62 patients - No improvement in 7 of 62 patients |
NA | asthenia; spontaneously resolved within 48 hours (2 patients) |
| Case Series | ||||||||||||||
| Manganoni et al (2007)89 | N = 1 of 4 | Previous use of - Oxatomide (dose: 60 mg/d) with no improvement |
NA | NA | Previous use of - Betamethasone (dose: 2 mg/d orally) with no improvement |
NA | NA | NA | NA | - Surgery: total thyroidectomy for papillary thyroid carcinoma | NA | Complete control | 60 months | NA |
| Godse (2011)48 | N = 1 of 5 | NA | Previous use of - sgAH1 (unmentioned name, dose: 4 times of recommended dose) with no improvement |
NA | Previous use of - Systemic corticosteroid (dose: NA) with no improvement |
NA | NA | - Omalizumab 300 mg sc every 4 weeks | NA | NA | 4 months | Complete control then discontinued omalizumab | NA | NA |
| Groffik et al (2011)49 | N = 1 of 9 | NA | Previous use of - sgAH1 (unmentioned name, dose: 4 times of recommended dose) with no improvement |
NA | Previous use of - Systemic corticosteroid (dose: NA) for long-term with no improvement |
NA | NA | - Omalizumab 300 mg sc every 2 weeks | NA | NA | 2 months | Complete control then discontinued omalizumab | NA | NA |
| Metz et al (2011)52 | N = 1 of 7 | NA | Previous use of - Loratadine (recommended dose) - Cetirizine (recommended dose) - Desloratadine (2–6 fold of recommended dose) - Ebastine (recommended dose) - Rupatadine (2–6 fold of recommended dose) - Levocetirizine (recommended dose) with no improvement |
NA | NA | NA | Previous use of - Montelukast (dose: NA) |
- Omalizumab 300 mg sc every 2 weeks | NA | Previous use of - Ranitidine (dose: NA) - Antibiotics (unmentioned name, dose: NA) with no improvement |
3 months | No improvement then discontinued omalizumab | NA | NA |
| Kirkpatrick et al (2012)51 | N = 1 of 6 | NA | NA | Previous use of 0 (dose: NA) with no improvement |
Previous use of - Systemic corticosteroid (unmentioned name, dose: NA) with no improvement |
NA | NA | NA | No | - Levothyroxine (dose: 150 g/d) for hypothyroidism due to post I131 for Grave’s disease | 1 month | Complete control, then continue levothyroxine same dose | 24 months. After that, levothyroxine was tapered to 125 g/d but relapsed occurred within 3 weeks, so dose was increased to 150 g/d again; complete control | NA |
| Ivyanskiy et al (2012)76 | N = 3 of 19 | NA | NA | All cases: previous use of 0 (dose: NA) with no improvement |
NA | NA | NA | All cases: - Omalizumab 150 mg sc every 2 weeks |
No Previous use of - CsA (dose: NA) with no improvement Previous use of - CsA (dose: NA) - AZA (dose: NA) - MMF (dose: NA) with no improvement in all treatment |
No Previous use of - TNF-α inhibitor (dose: NA) with no improvement Previous use of - TNF-α inhibitor (dose: NA) with no improvement |
6 months | Complete control then discontinued omalizumab | NA | No |
| 9 months | Complete control then discontinued omalizumab | |||||||||||||
| 4 months | Partial improvement then discontinued omalizumab | |||||||||||||
| Armengot-Carbo et al (2013)81 | N = 5 of 15 | NA | NA | Previous use of 0 (dose: NA) with no improvement |
Previous use of - Systemic corticosteroid (unmentioned name, dose: NA) with no improvement NA Previous use of - Systemic corticosteroid (unmentioned name, dose: NA) with no improvement NA Previous use of - Systemic corticosteroid (unmentioned name, dose: NA) with no improvement |
NA | NA | - Omalizumab 150 mg sc every 4 weeks for 3 months then 300 mg sc every 4 weeks for other 3 months | Previous use of - CsA (dose: NA) with no improvement |
Previous use of -AH2 (unmentioned name, dose: NA) with no improvement Previous use of -AH2 (unmentioned name, dose: NA) with no improvement Previous use of -AH2 (unmentioned name, dose: NA) with no improvement NA Previous use of -AH2 (unmentioned name, dose: NA) with no improvement |
6 months | Partial improvement |
NA | Nausea |
| - Omalizumab 150 mg sc every 4 weeks for 3 months | 3 months | No improvement then discontinued omalizumab | Nausea | |||||||||||
| - Omalizumab 150 mg sc every 2 weeks for 3 months then 150 mg sc every 4 weeks for other 3 months | 6 months | Complete control | No | |||||||||||
| - Omalizumab 300 mg sc every 4 weeks for 6 months | 6 months | Complete control | No | |||||||||||
| - Omalizumab 150 mg sc every 4 weeks for 3 months | 3 months | No improvement then discontinued omalizumab | No | |||||||||||
| Zubrinich et al (2019)92 |
N = 1 of 4 |
NA | NA | Previous use of - unspecified AH1 (dose: NA) with partial improvement |
Previous use of - Prednisolone (dose: NA) with partial improvement |
NA | NA | NA | NA | - Ivermectin (dose: NA) for treatment of Strongyloides infection | NA | Complete control | 10 months | NA |
| Case reports | ||||||||||||||
| Urbach (1942)97 | N = 1 of 1 | NA | NA | NA | NA | NA | NA | NA | NA | - Surgery: neoplasm removal for rectal carcinoma | NA | Complete control | NA | NA |
| Anderson et al (1991)95 | N = 1 of 1 | Previous use of - Hydroxyzine (dose: NA) with no improvement |
- Terfenadine (dose: NA) | NA | Previous use of - Systemic corticosteroid (unmentioned name, dose: NA) for short course with no improvement |
Previous use of - Hydrocortisone cream (dose: NA) with no improvement |
NA | NA | NA | - Surgery: neoplasm removal for colon carcinoma | NA | Complete control | 60 months | NA |
| Amoroso et al (1997)69 | N = 1 of 1 | NA | NA | NA | Previous use of - Betamethasone (dose: 4 mg IV) - Betamethasone (dose: 0.5 mg/d orally) with no improvement |
NA | NA | NA | NA | - Surgery: total thyroidectomy for Hashimoto’s thyroiditis | NA | Complete control | 18 months | NA |
| Zhang et al (2004)79 | N = 1 of 1 | Previous use of - Chlorpheniramine (dose: 12 mg/d) with no improvement |
NA | NA | - Prednisolone (dose: 10 mg/d) for 3 months and 1 week | NA | NA | NA | - Melphalan (dose: 2 mg) for 1 week followed by - Cyclophosphamide (dose: 50 mg/d) for 3 months for IgA Myeloma |
NA | 3.25 months | Complete control then discontinued prednisolone, melphalan, and cyclophosphamide | NA, symptom relapsed when myeloma relapsed | NA |
| Wong et al (2010)56 | N = 1 of 1 | Previous use of - Diphenhydramine (dose: 50 mg once) with complete control |
- Cetirizine (dose: 10 mg/d) for prophylaxis | NA | NA | NA | NA | NA | NA | - Epinephrine auto-injector (dose: NA) | 24 months | Marked improvement but 2 months later she acquired another hymenoptera sting, and within 2 weeks developed systemic urticaria when exposing to cold temperature | NA | NA |
| Baroni et al (2012)85 | N = 1 of 1 | NA | NA | Previous use of 0 (dose: NA) with no improvement |
Previous use of - Systemic corticosteroid (unmentioned name, dose: NA) with no improvement |
Previous use of - Topical corticosteroid (unmentioned name, dose: NA) with no improvement |
NA | NA | NA | - Surgery: radical prostatectomy for prostate adenocarcinoma | NA | Complete control | 24 months | NA |
| Hui-Hui et al (2012)84 | N = 1 of 1 | NA | Previous use of - Loratadine (dose: NA, taken once every other day) for 4 months with partial improvement |
NA | NA | NA | NA | NA | NA | - Surgery: right middle lobectomy for lung cancer removal | NA | Complete control | 6 months | NA |
| Zimmer et al (2016)82 | N = 1 of 1 | NA | NA | Previous use of -AH1 (unmentioned name, dose: up to 4 times of licensed dose) with no improvement |
NA | NA | NA | - Omalizumab 300 mg sc every 4 weeks | NA | NA | 4 months | Marked improvement then discontinued omalizumab | 5 months, then relapse occurred | No |
| Sussman et al (2016)60 | N = 1 of 1 | Previous use of - Hydroxyzine (dose: 25–200 mg/d) - Diphen-hydramine (dose: 25–200 mg/d) - Doxepin (dose: 25–125 mg/d) with no improvement in all treatment but caused sedation |
Previous use of - Cetirizine (dose: 10–40 mg/d) - Loratadine (dose: 10 mg/d) with no improvement in all treatment - Cetirizine (dose: 20 mg/d) |
Previous use of 0 (dose: NA dosage as needed) with no improvement |
Previous use of - Prednisolone (dose: 5–40 mg/d) with no improvement - Prednisolone (dose: tapering doses from before study until discontinued) |
NA | Previous use of - Montelukast (dose: 10 mg/d) with no improvement |
- Omalizumab 150 mg sc every 4 weeks | Previous use of - HCQ (dose: 400 mg/d) for 2 months - CsA (dose: 300 mg/d) for 2 months with no improvement in all treatment |
Previous use of - Ranitidine (dose: 300 mg/d) with no improvement - IVIG (dose: NA, discontinued due to hemolytic reaction) Both with partial improvement |
36 months | Marked improvement after 1 week then continued same dose of omalizumab but stopped taking prednisolone, resulting in low daily UAS7 scores. After 36 months, symptoms became severe, required longer courses and doses of prednisolone. Moreover, omalizumab was increased to 300 mg sc every 4 weeks to maintain low UAS7. | NA | NA |
| Kasperska-Zajac et al (2016)91 | N = 1 of 1 | NA | NA | Previous use of 0 (high dose) with no improvement |
Previous use of - Prednisolone (dose: up to 15 mg) for the past 3–10 years with no improvement |
NA | NA | - Omalizumab 300 mg sc | NA | NA | NA | Complete control after 1 dose of omalizumab then continued with omalizumab 150–300 mg every 5–6 weeks | NA | No |
| Aldasouqi et al (2018)96 | N = 1 of 1 | NA | NA | NA | NA | NA | NA | NA | NA | - Surgery: parathyroidectomy for primary hyperparathyroidism caused by large parathyroid adenoma | NA | Complete control | NA | NA |
| Pannofino (2018)58 | N = 1 of 1 | NA | - Rupatadine (dose: 10 mg twice daily) for 20 days then continue with 10 mg/d for 6 months then discontinued | Previous use of - unspecified AH1 (dose: NA) with no improvement |
Previous use of - Oral corticosteroid (unmentioned name, dose: NA) with no improvement | NA | NA | - Omalizumab 300 mg sc every 4 weeks | NA | NA | 6 months | Complete control then discontinued omalizumab | 12 months | NA |
Notes: aIt should be noted that the study of Kaplan et al included 12 CU patients (with 2 elderly patients) to be received placebo for 4 weeks and then omalizumab for 16 weeks. Omalizumab was injected every 2 weeks or every 4 weeks, dosed according to the patient’s body weight, and serum IgE at the screening visit.
Abbreviations: AH1, H1-antihistamine; AH2, H2-antihistamine; AZA, azathioprine; CsA, cyclosporine; d, day; fgAH1, first generation antihistamine; HCQ, hydroxychloroquine; IM, intramuscular; IV, intravenous; IVIG, intravenous immunoglobulin; LTRA, Leukotriene-receptor antagonist; mg, milligram; MMF, mycophenolate mofetil; MTX, methotrexate; NA, not available/not applicable; sc, subcutaneous; SD, symptomatic dermographism; sgAH1, second generation antihistamine; TNF-α inhibitor, tumor necrosis factor-α inhibitors; UAS7, Weekly Urticarial Activity Score.
Follow-Up Time, Tapering, Relapse, and Mean Duration of Treatment
The follow-up time after completion of treatment was mentioned in 16 studies,51,53,54,58,62,69,73,82–85,88,89,92,94,95 and the average follow-up time was 17.5 months. Some patients who had already achieved complete control continued their previous medication during the follow-up period, such as sulfasalazine and sgAH1, until they could be tapered off.94 Methotrexate (MTX) was tapered off in two patients, but one of them relapsed.83 Four patients continued to receive omalizumab maintenance at the same dose with an attempt to increase the interval between doses.55,60,91 One patient was prescribed fgAH1 as needed, but there was no report of the actual frequency of use.88 Another patient continued levothyroxine for 2 years before tapering, but relapse occurred. The dose was increased back to the initial dose and complete control was re-established.51 The average duration of treatment in this study was 205.8 days (6.9 months).
Discussion
The results of this systematic review revealed some similarities and differences between adult CU and elderly CU. Previously reported prevalence of CU in adult population ranges from 0.1% to 3.4%, which is relatively similar to the 0.2% to 2.8% prevalence of CU in the elderly.33,98 Our review also showed variation between prevalence in various geographic areas. As shown in Table 1, large population and nationwide studies showed a relatively higher prevalence of elderly in the CU population than smaller studies. However, larger studies and smaller studies reported a similar prevalence of CU in the overall elderly population (Table 2).
Even if women formed the majority of this study, which was similar to previous elderly and adult CU reports,10,11,23,99,100 some clinical presentations of elderly patients differed from adult CU. Comparison of the reported demographic and clinical characteristics of elderly patients with CU and those of non-elderly is shown in Table 5. Although the majority of both groups presented with wheal alone, its proportions in the elderly were higher than in adults, ranging from 33% to 87% across the studies, while the prevalence of concurrent angioedema was less.9,10,12,20,37,40,90 Wheal with anaphylaxis in our review was found only in one case report of the elderly with cold urticaria, which was the type that could have concurrent anaphylaxis up to 3.7–38.0%.44,101–107 CSU was the most common subtype among the elderly, similar to adult CU.10,20,26,45,108–111 Concerning CIndU, symptomatic dermographism (SD) was reported as the most common CIndU in both groups.10,20,45,74,108,112 Similar to the report of Ban et al,11 history of atopy, which is known to be associated with CU, was found at a relatively lower rate in this study than adult CU,10,11,20,37,45,64,74,90 in contrast with some previous studies.10,11,18 Regarding comorbidities, Lapi et al35 reported the risk of developing CU to be related to numerous factors. Gastrointestinal diseases, being the most common concomitant disease, together with coronary heart diseases, cerebrovascular diseases, metabolic syndrome, autoimmune diseases, thyroid diseases, psychological problems, and malignancies, were all reported at high rates in elderly CU. These findings were consistent with previous studies that reported CU to be associated with increased risk of having metabolic syndrome in both adults and the elderly.20,113–115 Moreover, the risk of developing metabolic syndrome was also found to increase with age.116,117 As reported by Zbiciak-Nylec et al118 that later onset of urticaria symptoms can result from obesity. Similar to previous studies, autoimmune diseases including autoimmune thyroid diseases, rheumatoid arthritis, and systemic lupus erythematosus had been reported in high rates in all age groups of CU patients, but much more in the elderly.17,70,90,119–123 This can be a result from increasing production of autoantibodies with aging, as Ramos-Casals et al124 proposed. In addition, a previous nationwide study reported depression to be common in adult CU, while elderly CU was reported mainly in dementia and other non-specific psychological problems.100
Table 5.
Comparison of the Reported Demographic and Clinical Characteristics of Elderly Patients with Chronic Urticaria (CU) with Those of Non-Elderly
| Clinical features: N/Total (%) | Elderly (our systematic review) | Non-elderly |
|---|---|---|
| Demographic Data | ||
| Prevalence of CU in population | 22,900/9,757,210 (0.2) – 13,476/488,145 (2.8)29,33,35,46 | 6019/7,555,991 (0.1) – 90/2613 (3.4)9,19,29,30,33,98,134–140 |
| CSU proportion in CU | 127/153 (83.0) – 63/65 (96.9)10,20,45 | 145/220 (66.0) – 215/231 (93.1)26,34,74,108–111,141–146 |
| CIndU proportion in CU | 2/65 (3.1) – 26/153 (17.0)10,20,32,45 | 17/329 (5.2) – 75/220 (34.0)26,74,108–111,141–146 |
| Sex ratio (Male: Female) | 1: 0.9–3.310–12,20–25,30–33,37,39,40,45,90 | 1: 1.0–5.710,12,19,28–30,33,34,36–38,40,41,74,100,109,114,130,131,134,136,139,142–144,147–163 |
| Clinical presentation | ||
| Wheal alone | 10/30 (33.3) – 86/99 (86.9)10,12,20,37,40,90 | 96/330 (29.1) – 77/102 (75.5)12,20,40,107,109,136,141,143,147,149,163–168 |
| Wheal with angioedema | 13/99 (13.1) – 20/30 (66.7)10,12,20,37,40,90 | 17/248 (6.9) – 152/199 (76.4)12,28,36–40,74,109,111,120,136,141,143,147,149,158,159,163,165–173 |
| Wheal with anaphylaxis a | 1/1 (100.0)56 | 0/2,175 (0.0)28,174,175 |
| Personal history of atopy | 2/92 (2.2) – 9/26 (34.6)10,11,20,37,45,64,90 | 171/13,479 (1.3) −101/147(68.7)11,12,28,34–38,74,100,106,114,147,159,160,163,176–179 |
| Comorbidities | ||
| Gastrointestinal diseases | 5/104 (4.8) – 708,415/985,278 (71.9)20,64 | 127/12,185 (1.0) – 145/330 (44.0)12,28,100,111,131,178,180–182 |
| Metabolic syndrome | 21/63 (33.3) – 44/92 (47.8)20,90 | 276/12/185 (2.3) – 1,741/11,261 (15.5)100,114,116 |
| Thyroid diseases | 1/67 (1.5) – 19/99 (19.2)10,20,37,45 | 34/13,479 (0.3) – 20/47 (42.5)12,28,30,35–37,67,70,100,153,159,165,170–172,178,183,184 |
| Autoimmune diseases | 8/63 (12.7)90 | 40/12,185 (0.3) –25/209 (12.0)30,100,153,159,170,172 |
| Psychiatric problems | ||
| Anxiety disorders | NA | 266/13,479 (2.0) – 24/30 (80.0)35,139,185–189 |
| Depression & other psychiatric problems | 2/99 (2.0) – 2/26 (7.7)37,45,65 | 121/12,185 (1.0) – 21/30 (70.0)12,30,36,37,100,139,148,154,159,185–187,189–192 |
| Malignancies | ||
| Hematologic malignancy b | 33/3,615 (0.9)19 | 80/36,910 (0.2) – 25/9,105 (0.3)18,19 |
| Other | 415/3,615 (11.5) – 11/92 (12.0)19,20 | 231/9,105 (0.3) – 330/13,479 (2.5)18,19,35,148 |
| Most common subtype of CIndU | Symptomatic dermographism10,20,45 | Symptomatic dermographism10,36,37,74,108,111,193–195 |
| Laboratory investigations | ||
| Positive antinuclear antibodies | 13/63 (20.6) of CSU10 | 248/12,778 (1.9) – 131/195 (67.2) of CSU10,120,155,158,160,196,197 |
| Elevated erythrocyte sedimentation rate | 18/63 (28.6)10 | 3/184 (1.6) – 65/133 (48.9)10,74,155,160,165,172,178,198 |
| Elevated total serum IgE c | NA | 14/330 (4.2) – 34/62 (54.8)12,28,36,74,165,199–201 |
| Positive ASST | 11/61 (18.0) – 3/5 (60.0) of CSU20,37,40,42 | 12/45 (26.7) – 49/67 (73.1) of CSU10,28,36,37,40,74,108,111,130,142,150,155,156,160,168,196,202–211 |
| Abnormal thyroid function test c | NA | 20/330 (6.1) – 20/66 (30.3)12,38,67,70,78,198,212,213 |
| Abnormal free T3 c | NA | 1/56 (1.8) – 99/165 (60.0)74,214 |
| Abnormal free T4 c | NA | 97/165 (58.8)74 |
| Abnormal TSH c | NA | 2/56 (3.6) – 99/167 (59.3)74,165,171,183,214,215 |
| Positive thyroid autoantibodies | 3/24 (12.5) – 21/63 (33.3)10,11,40 | 3/79 (3.8) – 27/47 (57.5)10,11,36,40,67,70,71,73,74,106,109,120,130,153,155–157,160,161,163,165,168,170–173,183,184,196,198,199,201–203,207,209–237 |
| Positive HBsAg | 8/63 (12.7)10 | 0/121 (0.0) – 2/56 (3.6)10,28,111,128,129,192,238 |
| Duration of disease prior to diagnosis (years) | 0.2–2.039,60,67,69,79,85,87 | 3.2–6.387,172 |
| Treatment | ||
| Response to 1st line (standard dose AH1) | 17.045/99 (45.5) – 23/26 (88.5)37,38,45 | 164/516 (31.8) – 163/248 (65.9)36–39,160,170 |
| Needed 2nd line | 3/26 (11.5) – 24/96 (25.0)37,45 | 36/335 (10.8) – 199/569 (34.9)36,37,170,172 |
| Needed 3rd line | 5/32 (15.6) – 28/95 (29.5)37,40 | 36/361 (10.0) – 93/329 (28.3)36,37,40,74,108,159,172,178 |
Notes: aWheal with anaphylaxis in elderly was found in only one case report of cold urticaria. bIt should be noted that the only retrospective study which reported malignancy in population was from Chen et al cProportion of elevated IgE, abnormal thyroid function test, free T3, free T4, and TSH in elderly patients were reported in only case reports and case series. No prospective or retrospective cohort study was found.
Abbreviations: ASST, autologous serum skin test; CIndU, chronic inducible urticaria; CSU, chronic spontaneous urticaria; CU, chronic urticaria; HBsAg, hepatitis B surface antigen; NA, not available/not applicable; TSH, thyroid stimulating hormone.
The high rate of malignancies, both hematologic and non-hematologic, in the present study may be explained by the advanced age. Most studies reported CU patients to be at high risk of developing cancers, and the incidence of cancer also increased with age.19,89,125,126 A possible mechanism is alteration of the immune system by the tumor.126 Age-appropriate malignancy screening is, therefore, strongly encouraged for early detection and treatment, which will improve the outcomes of both cancer and urticaria.89,93,97,126
The high prevalence of thyroid autoantibodies in both geriatric and adult CU suggests the relationship between CU and thyroid autoimmunity,10,11,40,67,70,120,123,127 even though this study and the previous report showed no difference of thyroid autoantibodies between the two groups.11 Focusing on infections, hepatitis B virus was the only infection in this study that was reported at higher prevalence (12.7%) than in previously reported general CU patients (0–3.6%).128,129 There was no difference in other laboratory findings, such as ESR, ANA, and total serum IgE levels. However, elderly CSU was reported to have a relatively lower proportion of positive ASST than adult CSU, as in the study by Magen et al.20
Treatment of CU in elderly patients usually follows the same guidelines as the general population. SgAH1 is recommended as the first-line treatment for elderly CU. The regular dose of SgAH1 is generally sufficient to achieve complete control in most patients, with a higher proportion of response in elderly CU than adults. This was in line with the finding of a lower rate of ASST in the elderly. As ASST positivity correlates with higher severity and longer duration of disease of CSU,127,130–132 geriatric patients may have less severe CU symptoms than adult CU, resulting in fewer associated angioedema and good response to standard treatment. Updosing to a higher dose or 4-times was also reported the good efficacy in SgAH1. For patients who fail on antihistamines, successful symptom control has been achieved by the use of omalizumab 150–300 mg every 2–4 weeks.
Some patients with autoimmune thyroiditis and hypothyroid were treated by levothyroxine, which also helps in improving urticaria.51,67,73 The risks and benefits of these third-line drugs have not been sufficiently explored and additional studies are needed.7,83 Another treatment strategy that significantly improved CU symptoms was treatment of secondary causes concurrent with standard treatments, especially in aging patients in whom autoimmune disorders, malignancies and infections are more common. A systematic review by Kolkhir et al133 found CSU to be quite common in patients with strongyloidiasis. Its pathogenesis may be due to eosinophil and complement activation leading to skin mast cell activation. Magen et al63 and Zubrinich et al92 reported an association between H. Pylori infection, Strongyloides infection, and CU. Treatment with standard antiparasitic drugs yielded complete control.63,92,133 Therefore, treatment of these associated comorbidities, including infection, might result in a better CU control.
Limitations
Most of the included articles were retrospective studies, case reports, and case series, which are inherently classified as having a lower level of evidence (Table 6). Only three randomized controlled trials were eligible to be included in the analysis, hence, the number of control groups was low. Furthermore, only a few studies had a study population consisting only of elderly patients. These limitations further underscore the potential value of this study and make clinicians more aware that more prospective studies are needed on cases of CU in the elderly.
Table 6.
Quality and Risk of Bias Assessment of Included Articles in Systematic Review
| A. Randomized controlled trials | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study, year Ref | Random sequence generation (selection bias) | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | ||||||||||||||||||
| Staubach et al, 2016239 | + | + | + | + | + | + | ||||||||||||||||||
| Kaplan et al, 2005240 | ? | ? | + | + | + | + | ||||||||||||||||||
| Goldsobel et al, 198668 | + | + | + | + | + | + | ||||||||||||||||||
| B. Non-randomized controlled trials | ||||||||||||||||||||||||
| Study, year Ref | Criteria | Additional criteria in the case of comparative study | ||||||||||||||||||||||
| A stated aim of the study | Inclusion of consecutive patients | Prospective collection of data | End point appropriate to the study aim | Unbiased evaluation of end points | Follow-up period appropriate | Loss to follow-up not exceeding 5% | Prospective calculation of the study size | A control group having the criterion standard intervention | Contemporary groups | Baseline equivalence of groups | Prospective calculation of the sample size | Statistical analyses adapted to the study design | Total | |||||||||||
| Martina et al, 202190 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Napolitano et al, 202145 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Gaber et al, 202046 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | - | - | - | - | - | 12 | ||||||||||
| Chung et al, 202074 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Seo et al, 201932 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Zubrinich et al, 201992 | 1 | 1 | 1 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 7 | ||||||||||
| Wertenteil et al, 201933 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | - | - | - | - | - | 8 | ||||||||||
| Jankowska-Konsur et al, 201934 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | - | - | - | - | - | 8 | ||||||||||
| Jo et al, 201939 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Curto-Barredo et al, 201937 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Eun et al, 201831 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | - | - | - | - | - | 12 | ||||||||||
| Nettis et al, 201840 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Napolitano et al, 201893 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Chanprapaph et al, 2018160 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Curto-Barredo et al, 201836 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Pannofino, 201858 | 2 | 0 | 2 | 1 | 0 | 2 | 0 | 0 | - | - | - | - | - | 7 | ||||||||||
| Aldasouqi, 201896 | 2 | 0 | 2 | 1 | 0 | 1 | 0 | 0 | - | - | - | - | - | 6 | ||||||||||
| Kulthanan et al, 201757 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Lee et al, 201764 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Chu et al, 201730 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Ali, 2016152 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 0 | 2 | 2 | 0 | 2 | 20 | ||||||||||
| Chuamanochan et al, 201610 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Kasperska-Zajac et al, 201691 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Lapi et al, 201635 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Zimmer et al, 201682 | 1 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 5 | ||||||||||
| Sussman et al, 201660 | 1 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 5 | ||||||||||
| Romano et al, 201562 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Sugiyama et al, 201573 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Uysal et al, 201461 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | - | - | - | - | - | 12 | ||||||||||
| Ban et al, 201411 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Vikramkumar et al, 201442 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | - | - | - | - | - | 8 | ||||||||||
| Magen et al, 201320 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Song et al, 201355 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Magen et al, 201320 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Lefevre et al, 201375 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Hiragun et al, 201238 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Armengot-Carbo et al, 201381 | 1 | 1 | 1 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 7 | ||||||||||
| Kirkpatrick et al, 201251 | 2 | 1 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 9 | ||||||||||
| Chen et al, 201219 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Krupashankar et al, 201228 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | - | - | - | - | - | 12 | ||||||||||
| Ivyanskiy et al, 201276 | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 0 | - | - | - | - | - | 6 | ||||||||||
| Hui-hui et al, 201284 | 2 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 6 | ||||||||||
| Baroni et al, 201285 | 1 | 1 | 1 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 7 | ||||||||||
| Groffik et al, 201149 | 1 | 1 | 1 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 7 | ||||||||||
| Godse, 201148 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Asero et al, 201186 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | - | - | - | - | - | 8 | ||||||||||
| Sagi et al, 201183 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Metz et al, 201152 | 1 | 1 | 1 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 7 | ||||||||||
| Mitzel-Kaoukhov et al, 201053 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | - | - | - | - | - | 12 | ||||||||||
| Mozena et al, 201072 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | - | - | - | - | - | 8 | ||||||||||
| Perez et al, 2010241 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Wong et al, 201056 | 1 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 5 | ||||||||||
| Staubach et al, 200980 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | - | - | - | - | - | 8 | ||||||||||
| Kaplan et al, 200850 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 0 | 0 | 2 | 2 | 0 | 2 | 19 | ||||||||||
| Katsarou-Katsari et al, 200844 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Feibelmann, 200771 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | - | - | - | - | - | 8 | ||||||||||
| Manganoni et al, 200789 | 1 | 1 | 1 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 7 | ||||||||||
| Cebeci et al, 200670 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | - | - | - | - | - | 8 | ||||||||||
| McGirt et al, 200694 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Sanada et al, 2005242 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | - | - | - | - | - | 12 | ||||||||||
| Yang et al, 200541 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | - | - | - | - | - | 8 | ||||||||||
| O’Donnell, 200577 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | - | - | - | - | - | 8 | ||||||||||
| Gaig et al, 200429 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | - | - | - | - | - | 8 | ||||||||||
| Zhang et al, 200479 | 1 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | - | - | - | - | - | 4 | ||||||||||
| Asero et al, 200378 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | - | - | - | - | - | 8 | ||||||||||
| O’Donnell, 199854 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | - | - | - | - | - | 12 | ||||||||||
| Amoroso, 199769 | 1 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 5 | ||||||||||
| Rumbyrt et al, 199588 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | - | - | - | - | - | 12 | ||||||||||
| Hashiro et al, 199427 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | - | - | - | - | - | 8 | ||||||||||
| Barlow et al, 199326 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Anderson, 199195 | 1 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | - | - | - | - | - | 4 | ||||||||||
| Lindelof et al, 1990148 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Reisman et al, 1989243 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | - | - | - | - | - | 12 | ||||||||||
| Dover, 198843 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Lanigan et al, 198766 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | - | - | - | - | - | 3 | ||||||||||
| Mekkes et al, 198625 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Greene et al, 1985244 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 2 | 2 | 0 | 2 | 20 | ||||||||||
| Lanigan et al, 198465 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | - | - | - | - | - | 6 | ||||||||||
| Leznoff et al, 198367 | 1 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | - | - | - | - | - | 11 | ||||||||||
| Vaida et al, 198387 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | - | - | - | - | - | 10 | ||||||||||
| Juhlin, 198112 | 1 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | - | - | - | - | - | 5 | ||||||||||
| Urbach, 194297 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | - | - | - | - | - | 2 | ||||||||||
Notes: +, low risk of bias; -, high risk of bias; ?, unclear risk of bias; 0, not reported; 1, reported but inadequate; 2, reported and adequate.
Conclusions
This systematic review found that the prevalence CU ranges between 0.2–2.8% in the elderly population. CSU was still the most common type, and exhibited a female predominance. Compared with adult CU, a lower rate of atopy, more age-related comorbidities including metabolic syndrome, autoimmune disorders, and malignancies, a lower rate of associated angioedema, and lower ASST positivity, were reported in elderly CU. The use of antihistamines often yielded good results as first-line treatment. Omalizumab was effective in AH1-resistant cases, and other differential diagnosis should be considered in patients refractory to standard treatment. More prospective studies are necessary to further elucidate the characteristics of the disease in this age group.
Acknowledgment
The authors gratefully acknowledge Saowalak Hunnangkul, PhD for her assistance with statistical analysis, and for her advice regarding the systematic review process.
Abbreviations
AH1, H1-antihistamine; CIndU, Chronic inducible urticaria; CSU, Chronic spontaneous urticaria; CsA, Cyclosporine; CU, Chronic urticaria; ESR, Erythrocyte sedimentation rate; fgAH1, First generation H1-antihistamine; GI, Gastrointestinal; H. pylori, Helicobacter pylori; IVIG, Intravenous immunoglobulin; MTX, Methotrexate; RCT, Randomized controlled trial; SD, Symptomatic dermographism; sgAH1, Second generation H1-antihistamine.
Disclosure
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors for the present study. All of the authors report no conflicts of interest in this work.
References
- 1.Wamble DE, Ciarametaro M, Dubois R. The effect of medical technology innovations on patient outcomes, 1990-2015: results of a physician survey. J Manag Care Spec Pharm. 2019;25(1):66–71. doi: 10.18553/jmcp.2018.18083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buxbaum JD, Chernew ME, Fendrick AM, Cutler DM. Contributions of public health, pharmaceuticals, and other medical care To US life expectancy changes, 1990-2015. Health Aff. 2020;39(9):1546–1556. [DOI] [PubMed] [Google Scholar]
- 3.United Nations Department of Economic and Social Affairs Population Division. World Population Ageing 2020 Highlights: living arrangements of older persons. New York: United Nations Publication; 2020:1. Available from: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/undesa_pd-2020_world_population_ageing_highlights.pdf. Accessed Oct 12, 2021. [Google Scholar]
- 4.World Health Organization (WHO). Decade of healthy ageing: baseline report: summary. Geneva: World Health Organization; 2021:20. Available from: https://www.who.int/publications/i/item/9789240023307. Accessed Oct 12, 2021. [Google Scholar]
- 5.Ward JR, Bernhard JD. Willan’s itch and other causes of pruritus in the elderly. Int J Dermatol. 2005;44(4):267–273. [DOI] [PubMed] [Google Scholar]
- 6.Thaipisuttikul Y. Pruritic skin diseases in the elderly. J Dermatol. 1998;25(3):153–157. [DOI] [PubMed] [Google Scholar]
- 7.Zuberbier T, Abdul latiff AH, Abuzakouk M, et al. The International EAACI/GA2LEN/EuroGuiDerm/APAAACI Guideline for the Definition, Classification, Diagnosis and Management of Urticaria. Allergy. 2022;77(3):734–766. [DOI] [PubMed] [Google Scholar]
- 8.Maurer M, Eyerich K, Eyerich S, et al. Urticaria: collegium Internationale Allergologicum (CIA) Update 2020. Int Arch Allergy Immunol. 2020;181(5):321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saini S, Shams M, Bernstein JA, Maurer M. Urticaria and Angioedema Across the Ages. J Allergy Clin Immunol Pract. 2020;8(6):1866–1874. [DOI] [PubMed] [Google Scholar]
- 10.Chuamanochan M, Kulthanan K, Tuchinda P, Chularojanamontri L, Nuchkull P. Clinical features of chronic urticaria in aging population. Asian Pac J Allergy Immunol. 2016;34(3):201–205. doi: 10.12932/AP0708 [DOI] [PubMed] [Google Scholar]
- 11.Ban G-Y, Kim M-Y, Yoo H-S, et al. Clinical features of elderly chronic urticaria. Korean J Intern Med. 2014;29(6):800–806. doi: 10.3904/kjim.2014.29.6.800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juhlin L. Recurrent urticaria: clinical investigation of 330 patients. Br J Dermatol. 1981;104(4):369–381. doi: 10.1111/j.1365-2133.1981.tb15306.x [DOI] [PubMed] [Google Scholar]
- 13.Al-Thani MH, Al-Thani AAM, Cheema S, et al. Prevalence and determinants of metabolic syndrome in Qatar: results from a National Health Survey. BMJ Open. 2016;6(9):e009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonomini F, Rodella LF, Rezzani R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 2015;6(2):109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Syed MA, Alnuaimi AS, Zainel AJ, Qotba HA. Prevalence of non-communicable diseases by age, gender and nationality in publicly funded primary care settings in Qatar. BMJ Nutr Prev Health. 2019;2(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gunalan P, Indradevi R, Oudeacoumar P, et al. Pattern of skin diseases in geriatric patients attending tertiary care centre. J Evol Med Dent Sci. 2017;6:1566–1570. [Google Scholar]
- 17.Kolkhir P, Metz M, Altrichter S, Maurer M. Comorbidity of chronic spontaneous urticaria and autoimmune thyroid diseases: a systematic review. Allergy. 2017;72(10):1440–1460. [DOI] [PubMed] [Google Scholar]
- 18.Kim BR, Yang S, Choi JW, Choi CW, Youn SW. Epidemiology and comorbidities of patients with chronic urticaria in Korea: a nationwide population-based study. J Dermatol. 2018;45(1):10–16. [DOI] [PubMed] [Google Scholar]
- 19.Chen YJ, Wu CY, Shen JL, Chen TT, Chang YT. Cancer risk in patients with chronic urticaria: a population-based cohort study. Arch Dermatol. 2012;148(1):103–108. [DOI] [PubMed] [Google Scholar]
- 20.Magen E, Mishal J, Schlesinger M. Clinical and laboratory features of chronic idiopathic urticaria in the elderly. Int J Dermatol. 2013;52(11):1387–1391. [DOI] [PubMed] [Google Scholar]
- 21.Flohr C, Hay R. Putting the burden of skin diseases on the global map. Br J Dermatol. 2021;184(2):189–190. [DOI] [PubMed] [Google Scholar]
- 22.Maurer M, Ortonne J-P, Zuberbier T. Chronic urticaria: an internet survey of health behaviours, symptom patterns and treatment needs in European adult patients. Br J Dermatol. 2009;160(3):633–641. [DOI] [PubMed] [Google Scholar]
- 23.Gonçalo M, Gimenéz-Arnau A, Al-Ahmad M, et al. The global burden of chronic urticaria for the patient and society. Br J Dermatol. 2021;184(2):226–236. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mekkes JR, van der Schaar, WW, Bos JD. Anamnese en diagnostiek van chronische urticaria. Ned Tijdschr Geneeskd. 1986;130:1801–1805. [PubMed] [Google Scholar]
- 26.Barlow RJ, Warburton F, Watson K, Black AK, Greaves MW. Diagnosis and incidence of delayed pressure urticaria in patients with chronic urticaria. J Am Acad Dermatol. 1993;29(6):954–958. [DOI] [PubMed] [Google Scholar]
- 27.Hashiro M, Okumura M. Anxiety, depression, psychosomatic symptoms and autonomic nervous function in patients with chronic urticaria. J Dermatol Sci. 1994;8(2):129–135. [DOI] [PubMed] [Google Scholar]
- 28.Krupashankar DS, Shashikala K, Madala R. Clinical and investigative assessment of patients with positive versus negative autologous serum skin test: a study of 80 South Indian patients. Indian J Dermatol. 2012;57(6):434–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaig P, Olona M, Muñoz Lejarazu D, et al. Epidemiology of urticaria in Spain. J Investig Allergol Clin Immunol. 2004;14(3):214–220. [PubMed] [Google Scholar]
- 30.Chu CY, Cho YT, Jiang JH, Lin EI, Tang CH. Epidemiology and comorbidities of patients with chronic urticaria in Taiwan: a nationwide population-based study. J Dermatol Sci. 2017;88(2):192–198. [DOI] [PubMed] [Google Scholar]
- 31.Eun SJ, Lee JY, Kim DY, Yoon HS. Natural course of new-onset urticaria: results of a 10-year follow-up, nationwide, population-based study. Allergol Int. 2019;68(1):52–58. [DOI] [PubMed] [Google Scholar]
- 32.Seo JH, Kwon JW. Epidemiology of urticaria including physical urticaria and angioedema in Korea. Korean J Intern Med. 2019;34(2):418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wertenteil S, Strunk A, Garg A. Prevalence estimates for chronic urticaria in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2019;81(1):152–156. [DOI] [PubMed] [Google Scholar]
- 34.Jankowska-Konsur A, Reich A, Szepietowski J. Polish Chronic Urticaria Working G. Clinical characteristics and epidemiology of chronic urticaria: a nationwide, multicentre study on 1091 patients. Postepy Dermatol Alergol. 2019;36(2):184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lapi F, Cassano N, Pegoraro V, et al. Epidemiology of chronic spontaneous urticaria: results from a nationwide, population-based study in Italy. Br J Dermatol. 2016;174(5):996–1004. [DOI] [PubMed] [Google Scholar]
- 36.Curto-Barredo L, Archilla LR, Vives GR, Pujol RM, Giménez-Arnau AM. Clinical features of chronic spontaneous urticaria that predict disease prognosis and refractoriness to standard treatment. Acta Derm Venereol. 2018;98(7):641–647. [DOI] [PubMed] [Google Scholar]
- 37.Curto-Barredo L, Pujol RM, Roura-Vives G, Gimenez-Arnau AM. Chronic urticaria phenotypes: clinical differences regarding triggers, activity, prognosis and therapeutic response. Eur J Dermatol. 2019;29(6):627–635. [DOI] [PubMed] [Google Scholar]
- 38.Hiragun M, Hiragun T, Mihara S, Akita T, Tanaka J, Hide M. Prognosis of chronic spontaneous urticaria in 117 patients not controlled by a standard dose of antihistamine. Allergy. 2013;68(2):229–235. [DOI] [PubMed] [Google Scholar]
- 39.Jo YH, Yoo HW, Kim SH, Kim YM, Kim HY. Clinical characteristics and treatment response of chronic spontaneous urticaria according to age: a single-center Korean study. Asian Pac J Allergy Immunol. 2019. doi: 10.12932/AP-050719-0594 [DOI] [PubMed] [Google Scholar]
- 40.Nettis E, Cegolon L, Di Leo E, Canonica WG, Detoraki A. Omalizumab in elderly patients with chronic spontaneous urticaria: an Italian real-life experience. Ann Allergy Asthma Immunol. 2018;120(3):318–323. [DOI] [PubMed] [Google Scholar]
- 41.Yang HY, Sun CC, Wu YC, Wang JD. Stress, insomnia, and chronic idiopathic urticaria–a case-control study. J Formos Med Assoc. 2005;104(4):254–263. [PubMed] [Google Scholar]
- 42.Vikramkumar AG, Kuruvila S, Ganguly S. Autologous serum skin test as an indicator of chronic autoimmune urticaria in a tertiary care hospital in South India. Indian Dermatol Online J. 2014;5(Suppl 2):S87–S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dover JS, Black AK, Ward AM, Greaves MW. Delayed pressure urticaria. Clinical features, laboratory investigations, and response to therapy of 44 patients. J Am Acad Dermatol. 1988;18(6):1289–1298. [DOI] [PubMed] [Google Scholar]
- 44.Katsarou-Katsari A, Makris M, Lagogianni E, Gregoriou S, Theoharides T, Kalogeromitros D. Clinical features and natural history of acquired cold urticaria in a tertiary referral hospital: a 10-year prospective study. J Eur Acad Dermatol Venereol. 2008;22(12):1405–1411. [DOI] [PubMed] [Google Scholar]
- 45.Napolitano M, Fabbrocini G, Stingeni L, Patruno C. Prevalence of Chronic Inducible Urticaria in Elderly Patients. J Clin Med. 2021;10(2):247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaber M, Hasanin AZ. Skin diseases in elderly. Menoufia Medl J. 2020;33(1):272–276. [Google Scholar]
- 47.Dávila I, Del Cuvillo A, Mullol J, et al. Use of second generation H1 antihistamines in special situations. J Investig Allergol Clin Immunol. 2013;23(Suppl 1):S1–S16. [PubMed] [Google Scholar]
- 48.Godse KV. Omalizumab in treatment-resistant chronic spontaneous urticaria. Indian J Dermatol. 2011;56(4):444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Groffik A, Mitzel-Kaoukhov H, Magerl M, Maurer M, Staubach P. Omalizumab–an effective and safe treatment of therapy-resistant chronic spontaneous urticaria. Allergy. 2011;66(2):303–305. [DOI] [PubMed] [Google Scholar]
- 50.Kaplan AP, Joseph K, Maykut RJ, Geba GP, Zeldin RK. Treatment of chronic autoimmune urticaria with omalizumab. J Allergy Clin Immunol. 2008;122(3):569–573. [DOI] [PubMed] [Google Scholar]
- 51.Kirkpatrick CH. A mechanism for urticaria/angioedema in patients with thyroid disease. J Allergy Clin Immunol. 2012;130(4):988–990. [DOI] [PubMed] [Google Scholar]
- 52.Metz M, Altrichter S, Ardelean E, et al. Anti-immunoglobulin E treatment of patients with recalcitrant physical urticaria. Int Arch Allergy Immunol. 2011;154(2):177–180. [DOI] [PubMed] [Google Scholar]
- 53.Mitzel-Kaoukhov H, Staubach P, Müller-Brenne T. Effect of high-dose intravenous immunoglobulin treatment in therapy-resistant chronic spontaneous urticaria. Ann Allergy Asthma Immunol. 2010;104(3):253–258. [DOI] [PubMed] [Google Scholar]
- 54.O’Donnell BF, Barr RM, Black AK, et al. Intravenous immunoglobulin in autoimmune chronic urticaria. Br J Dermatol. 1998;138(1):101–106. [DOI] [PubMed] [Google Scholar]
- 55.Song CH, Stern S, Giruparajah M, Berlin N, Sussman GL. Long-term efficacy of fixed-dose omalizumab for patients with severe chronic spontaneous urticaria. Ann Allergy Asthma Immunol. 2013;110(2):113–117. [DOI] [PubMed] [Google Scholar]
- 56.Wong CG, Borici-Mazi R. Delayed-onset cold anaphylaxis after hymenoptera sting. Ann Allergy Asthma Immunol. 2012;109(1):77–78. [DOI] [PubMed] [Google Scholar]
- 57.Kulthanan K, Tuchinda P, Chularojanamontri L, Likitwattananurak C, Ungaksornpairote C. Omalizumab therapy for treatment of recalcitrant chronic spontaneous urticaria in an Asian population. J Dermatolog Treat. 2017;28(2):160–165. [DOI] [PubMed] [Google Scholar]
- 58.Pannofino A. Recurrent oedema of the uvula in a patient with chronic spontaneous urticaria successfully treated with omalizumab. J Dermatolog Treat. 2018;29(Suppl 4):S8–S9. [DOI] [PubMed] [Google Scholar]
- 59.Staubach P, Metz M, Chapman-Rothe N, et al. Omalizumab rapidly improves angioedema-related quality of life in adult patients with chronic spontaneous urticaria: x-ACT study data. Allergy. 2018;73(3):576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sussman GL, Hebert J, Simons FER. A 63-year-old man with chronic spontaneous urticaria. Cmaj. 2016;188(4):279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Uysal P, Eller E, Mortz CG, Bindslev-Jensen C. An algorithm for treating chronic urticaria with omalizumab: dose interval should be individualized. J Allergy Clin Immunol. 2014;133(3):914–915. [DOI] [PubMed] [Google Scholar]
- 62.Romano C, Sellitto A, De Fanis U, et al. Omalizumab for difficult-to-treat dermatological conditions: clinical and Immunological features from a retrospective real-life experience. Clin Drug Investig. 2015;35(3):159–168. [DOI] [PubMed] [Google Scholar]
- 63.Magen E, Schlesinger M, Hadari I. Chronic urticaria can be triggered by eradication of Helicobacter pylori. Helicobacter. 2013;18(1):83–87. [DOI] [PubMed] [Google Scholar]
- 64.Lee N, Lee JD, Lee HY, Kang DR, Ye YM. Epidemiology of Chronic Urticaria in Korea Using the Korean Health Insurance Database, 2010-2014. Allergy Asthma Immunol Res. 2017;9(5):438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lanigan SW, Adams SJ, Gilkes JJ, Robinson TW. Association between urticaria and hypothyroidism. Lancet. 1984;1(8392):1476. [DOI] [PubMed] [Google Scholar]
- 66.Lanigan SW, Short P, Moult P. The association of chronic urticaria and thyroid autoimmunity. Clin Exp Dermatol. 1987;12(5):335–338. [DOI] [PubMed] [Google Scholar]
- 67.Leznoff A, Josse RG, Denburg J, Dolovich J. Association of chronic urticaria and angioedema with thyroid autoimmunity. Arch Dermatol. 1983;119(8):636–640. [PubMed] [Google Scholar]
- 68.Goldsobel AB, Rohr AS, Siegel SC, et al. Efficacy of doxepin in the treatment of chronic idiopathic urticaria. J Allergy Clin Immunol. 1986;78(5 Pt 1):867–873. [DOI] [PubMed] [Google Scholar]
- 69.Amoroso A, Garzia P, Pasquarelli C, Sportelli G, Afeltra A. Hashimoto’s thyroiditis associated with urticaria and angio-oedema: disappearance of cutaneous and mucosal manifestations after thyroidectomy. J Clin Pathol. 1997;50(3):254–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cebeci F, Tanrikut A, Topcu E, Onsun N, Kurtulmus N, Uras AR. Association between chronic urticaria and thyroid autoimmunity. Eur J Dermatol. 2006;16(4):402–405. [PubMed] [Google Scholar]
- 71.Feibelmann TC, Gonçalves FT, Daud MS, Jorge Ade S, Mantese SA, Jorge PT. Avaliação da associação entre doença auto-imune de tireóide e urticária crônica idiopática [Assessment of association between autoimmune thyroid disease and chronic urticaria]. Arq Bras Endocrinol Metabol. 2007;51(7):1077–1083. [DOI] [PubMed] [Google Scholar]
- 72.Mozena JD, Tiñana A, Negri J, Steinke JW, Borish L. Lack of a role for cross-reacting anti-thyroid antibodies in chronic idiopathic urticaria. J Invest Dermatol. 2010;130(7):1860–1865. [DOI] [PubMed] [Google Scholar]
- 73.Sugiyama A, Nishie H, Takeuchi S, Yoshinari M, Furue M. Hashimoto’s disease is a frequent comorbidity and an exacerbating factor of chronic spontaneous urticaria. Allergol Immunopathol. 2015;43(3):249–253. [DOI] [PubMed] [Google Scholar]
- 74.Chung BY, Um JY, Kang SY, Kim HO, Park CW. Natural History of Chronic Urticaria in Korea. Ann Dermatol. 2020;32(1):38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lefévre AC, Deleuran M, Vestergaard C. A long term case series study of the effect of omalizumab on chronic spontaneous urticaria. Ann Dermatol. 2013;25(2):242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ivyanskiy I, Sand C, Thomsen SF. Omalizumab for chronic urticaria: a case series and overview of the literature. Case Rep Dermatol. 2012;4(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O’Donnell BF, Francis DM, Swana GT, Seed PT, Kobza black A, Greaves MW. Thyroid autoimmunity in chronic urticaria. Br J Dermatol. 2005;153(2):331–335. [DOI] [PubMed] [Google Scholar]
- 78.Asero R, Lorini M, Tedeschi A. Association of chronic urticaria with thyroid autoimmunity and Raynaud phenomenon with anticentromere antibodies. J Allergy Clin Immunol. 2003;111(5):1129–1130. [PubMed] [Google Scholar]
- 79.Zhang Y, Morita E, Matsuo H, Ueda D, Dekio S. Urticarial erythema associated with IgA myeloma. J Dermatol. 2004;31(8):661–665. [DOI] [PubMed] [Google Scholar]
- 80.Staubach P, Vonend A, Burow G, Metz M, Magerl M, Maurer M. Patients with chronic urticaria exhibit increased rates of sensitisation to Candida albicans, but not to common moulds. Mycoses. 2009;52(4):334–338. [DOI] [PubMed] [Google Scholar]
- 81.Armengot-Carbo M, Velasco-Pastor M, Rodrigo-Nicolas B, Pont-Sanjuan V, Quecedo-Estebanez E, Gimeno-Carpio E. Omalizumab in chronic urticaria: a retrospective series of 15 cases. Dermatol Ther. 2013;26(3):257–259. [DOI] [PubMed] [Google Scholar]
- 82.Zimmer S, Peveling-Oberhag A, Weber A, Gilfert T, Rady-Pizarro U, Staubach P. Unique coexistence of cold and solar urticaria and its efficient treatment. Br J Dermatol. 2016;174(5):1150–1152. [DOI] [PubMed] [Google Scholar]
- 83.Sagi L, Solomon M, Baum S, Lyakhovitsky A, Trau H, Barzilai A. Evidence for methotrexate as a useful treatment for steroid-dependent chronic urticaria. Acta Derm Venereol. 2011;91(3):303–306. [DOI] [PubMed] [Google Scholar]
- 84.Hu HH, Ying KJ, Wu XH, Chai Y. Urticaria as the initial presentation of early stage bronchioloalveolar carcinoma: a case report. Chin Med J. 2012;125(11):2065–2066. [PubMed] [Google Scholar]
- 85.Baroni A, Faccenda F, Russo T, Piccolo V. Figurate paraneoplastic urticaria and prostate cancer. Ann Dermatol. 2012;24(3):366–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Asero R, Cugno M, Tedeschi A. Activation of blood coagulation in plasma from chronic urticaria patients with negative autologous plasma skin test. J Eur Acad Dermatol Venereol. 2011;25(2):201–205. [DOI] [PubMed] [Google Scholar]
- 87.Vaida GA, Goldman MA, Bloch KJ. Testing for hepatitis B virus in patients with chronic urticaria and angioedema. J Allergy Clin Immunol. 1983;72(2):193–198. [DOI] [PubMed] [Google Scholar]
- 88.Rumbyrt JS, Katz JL, Schocket AL. Resolution of chronic urticaria in patients with thyroid autoimmunity. J Allergy Clin Immunol. 1995;96(6 Pt 1):901–905. [DOI] [PubMed] [Google Scholar]
- 89.Manganoni AM, Tucci G, Venturini M, Farisoglio C, Baronchelli C, Calzavara Pinton PG. Chronic urticaria associated with thyroid carcinoma: report of 4 cases. J Investig Allergol Clin Immunol. 2007;17(3):192–195. [PubMed] [Google Scholar]
- 90.Martina E, Damiani G, Grieco T, Foti C, Pigatto PDM, Offidani A. It is never too late to treat chronic spontaneous urticaria with omalizumab: real-life data from a multicenter observational study focusing on elderly patients. Dermatol Ther. 2021;34(2):e14841. [DOI] [PubMed] [Google Scholar]
- 91.Kasperska-Zajac A, Jarząb J, Żerdzińska A, Bąk K, Grzanka A. Effective treatment of different phenotypes of chronic urticaria with omalizumab: case reports and review of literature. Int J Immunopathol Pharmacol. 2016;29(2):320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zubrinich CM, Puy RM, O’Hehir RE, Hew M. Strongyloides infection as a reversible cause of chronic urticaria. J Asthma Allergy. 2019;12:67–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Napolitano M, Patruno C. Chronic urticaria can be caused by cancer and resolves with its cure. Allergy. 2018;73(8):1750–1751. [DOI] [PubMed] [Google Scholar]
- 94.McGirt LY, Vasagar K, Gober LM, Saini SS, Beck LA. Successful treatment of recalcitrant chronic idiopathic urticaria with sulfasalazine. Arch Dermatol. 2006;142(10):1337–1342. [DOI] [PubMed] [Google Scholar]
- 95.Anderson MH, Wray BB, Hooks VH 3rd. Urticaria in a 68-year-old man. Ann Allergy. 1991;66(3):207–211. [PubMed] [Google Scholar]
- 96.Aldasouqi S, Satoh P, Cardona Z, Alrasheed T, McLeod M, Mohan M. Chronic Autoimmune Urticaria and Cognitive Impairment as Unusual Presenting Symptoms of Primary Hyperparathyroidism. Endocr Pract. 2018;24(Suppl 1):S134. [Google Scholar]
- 97.Urbach E. Endogenous Allergy. Arch Dermatol Syphilol. 1942;45(4):697–722. [Google Scholar]
- 98.Fricke J, Ávila G, Keller T, et al. Prevalence of chronic urticaria in children and adults across the globe: systematic review with meta-analysis. Allergy. 2020;75(2):423–432. [DOI] [PubMed] [Google Scholar]
- 99.Cassano N, Colombo D, Bellia G, Zagni E, Vena GA. Gender-related differences in chronic urticaria. G Ital Dermatol Venereol. 2016;151(5):544–552. [PubMed] [Google Scholar]
- 100.Ghazanfar MN, Kibsgaard L, Thomsen SF, Vestergaard C. Risk of comorbidities in patients diagnosed with chronic urticaria: a nationwide registry-study. World Allergy Organ J. 2020;13(1):100097. doi: 10.1016/j.waojou.2019.100097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Deza G, Brasileiro A, Bertolín-Colilla M, Curto-Barredo L, Pujol RM, Giménez-Arnau AM. Acquired cold urticaria: clinical features, particular phenotypes, and disease course in a tertiary care center cohort. J Am Acad Dermatol. 2016;75(5):918–924. doi: 10.1016/j.jaad.2016.06.017 [DOI] [PubMed] [Google Scholar]
- 102.Horton BT. Hypersensitiveness to cold: with local and systemic manifestations of a histamine-like character: its amenability to treatment. J Am Med Assoc. 1936;107(16):1263–1269. doi: 10.1001/jama.1936.02770420001001 [DOI] [Google Scholar]
- 103.Jain SV, Mullins RJ. Cold urticaria: a 20-year follow-up study. J Eur Acad Dermatol Venereol. 2016;30(12):2066–2071. doi: 10.1111/jdv.13841 [DOI] [PubMed] [Google Scholar]
- 104.Kulthanan K, Tuchinda P, Chularojanamontri L, Kiratiwongwan R. Cold Urticaria: clinical Features and Natural Course in a Tropical Country. Allergy Asthma Immunol Res. 2019;11(4):538–547. doi: 10.4168/aair.2019.11.4.538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mathelier-Fusade P. Clinical predictive factors of severity in cold urticaria. Arch Dermatol. 1998;134(1):106–107. doi: 10.1001/archderm.134.1.106 [DOI] [PubMed] [Google Scholar]
- 106.Neittaanmäki H. Cold urticaria. Clinical findings in 220 patients. J Am Acad Dermatol. 1985;13(4):636–644. doi: 10.1016/S0190-9622(85)70208-3 [DOI] [PubMed] [Google Scholar]
- 107.Sánchez-Borges M, Ansotegui IJ, Baiardini I, et al. The challenges of chronic urticaria part 1: epidemiology, immunopathogenesis, comorbidities, quality of life, and management. World Allergy Organ J. 2021;14(6):100533. doi: 10.1016/j.waojou.2021.100533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ferrer M. Epidemiology, healthcare, resources, use and clinical features of different types of urticaria. Alergológica 2005. J Investig Allergol Clin Immunol. 2009;19(Suppl 2):S21–S26. [PubMed] [Google Scholar]
- 109.Kulthanan K, Jiamton S, Thumpimukvatana N, Pinkaew S. Chronic idiopathic urticaria: prevalence and clinical course. J Dermatol. 2007;34(5):294–301. doi: 10.1111/j.1346-8138.2007.00276.x [DOI] [PubMed] [Google Scholar]
- 110.Small P, Barrett D, Biskin N, Champlin E. Chronic urticaria and angioedema. Clin Allergy. 1982;12(2):131–136. doi: 10.1111/j.1365-2222.1982.tb01631.x [DOI] [PubMed] [Google Scholar]
- 111.Zhong H, Song Z, Chen W, et al. Chronic urticaria in Chinese population: a hospital-based multicenter epidemiological study. Allergy. 2014;69(3):359–364. doi: 10.1111/all.12338 [DOI] [PubMed] [Google Scholar]
- 112.Dressler C, Werner RN, Eisert L, Zuberbier T, Nast A, Maurer M. Chronic inducible urticaria: a systematic review of treatment options. J Allergy Clin Immunol. 2018;141(5):1726–1734. doi: 10.1016/j.jaci.2018.01.031 [DOI] [PubMed] [Google Scholar]
- 113.Chang H-W, Cheng H-M, Yen H-R, et al. Association between chronic idiopathic urticaria and hypertension: a population-based retrospective cohort study. Ann Allergy Asthma Immunol. 2016;116(6):554–558. doi: 10.1016/j.anai.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 114.Shalom G, Magen E, Babaev M, et al. Chronic urticaria and the metabolic syndrome: a cross-sectional community-based study of 11 261 patients. J Eur Acad Dermatol Venereol. 2018;32(2):276–281. doi: 10.1111/jdv.14548 [DOI] [PubMed] [Google Scholar]
- 115.Chung S-D, Wang K-H, Tsai M-C, Lin H-C, Chen C-H, Taniyama Y. Hyperlipidemia is associated with chronic urticaria: a population-based study. PLoS One. 2016;11(3):e0150304. doi: 10.1371/journal.pone.0150304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ye YM, Jin HJ, Hwang EK, et al. Co-existence of chronic urticaria and metabolic syndrome: clinical implications. Acta Derm Venereol. 2013;93(2):156–160. doi: 10.2340/00015555-1443 [DOI] [PubMed] [Google Scholar]
- 117.Suastika K, Dwipayana P, Ratna Saraswati IM, et al. Relationship between age and metabolic disorders in the population of Bali. Journal of Clinical Gerontology and Geriatrics. 2011;2(2):47–52. doi: 10.1016/j.jcgg.2011.03.001 [DOI] [Google Scholar]
- 118.Zbiciak-Nylec M, Wcisło-Dziadecka D, Kasprzyk M, et al. Overweight and obesity may play a role in the pathogenesis of chronic spontaneous urticaria. Clin Exp Dermatol. 2018;43(5):525–528. doi: 10.1111/ced.13368 [DOI] [PubMed] [Google Scholar]
- 119.Kolkhir P, Pogorelov D, Olisova O, Maurer M. Comorbidity and pathogenic links of chronic spontaneous urticaria and systemic lupus erythematosus - A systematic review. Clin Exp Allergy. 2016;46(2):275–287. doi: 10.1111/cea.12673 [DOI] [PubMed] [Google Scholar]
- 120.Verneuil L, Leconte C, Ballet JJ, et al. Association between chronic urticaria and thyroid autoimmunity: a prospective study involving 99 patients. Dermatology. 2004;208(2):98–103. doi: 10.1159/000076480 [DOI] [PubMed] [Google Scholar]
- 121.Pan X-F, Gu J-Q, Shan Z-Y. The prevalence of thyroid autoimmunity in patients with urticaria: a systematic review and meta-analysis. Endocrine. 2015;48(3):804–810. doi: 10.1007/s12020-014-0367-y [DOI] [PubMed] [Google Scholar]
- 122.Kasumagic-Halilovic E, Beslic N, Ovcina-Kurtovic N. Thyroid Autoimmunity in Patients with Chronic Urticaria. Med Arch. 2017;71(1):29–31. doi: 10.5455/medarh.2017.71.29-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kolkhir P, Borzova E, Grattan C, Asero R, Pogorelov D, Maurer M. Autoimmune comorbidity in chronic spontaneous urticaria: a systematic review. Autoimmun Rev. 2017;16(12):1196–1208. doi: 10.1016/j.autrev.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 124.Ramos-Casals M, García-Carrasco M, Brito MP, López-Soto A, Font J. Autoimmunity and geriatrics: clinical significance of autoimmune manifestations in the elderly. Lupus. 2003;12(5):341–355. doi: 10.1191/0961203303lu383ed [DOI] [PubMed] [Google Scholar]
- 125.White MC, Holman DM, Goodman RA, Richardson LC. Cancer risk among older adults: time for cancer prevention to go silver. Gerontologist. 2019;59(Suppl 1):S1–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Larenas-Linnemann D, Saini SS, Azamar-Jácome AA, Jensen-Jarolim E, Maurer M. Very rarely chronic urticaria can be caused by cancer and if so, resolves with its cure. Allergy. 2018;73(9):1925–1926. [DOI] [PubMed] [Google Scholar]
- 127.Staubach P, Onnen K, Vonend A, et al. Autologous whole blood injections to patients with chronic urticaria and a positive autologous serum skin test: a placebo-controlled trial. Dermatology. 2006;212(2):150–159. [DOI] [PubMed] [Google Scholar]
- 128.Yeh JW, Yang HS, Yang CC. Dermatological diseases associated with Hepatitis B virus infection. Dermatol Sin. 2020;38(3):142–150. [Google Scholar]
- 129.Kolkhir P, Pereverzina N, Olisova O, Maurer M. Comorbidity of viral hepatitis and chronic spontaneous urticaria: a systematic review. Allergy. 2018;73(10):1946–1953. [DOI] [PubMed] [Google Scholar]
- 130.Caproni M, Volpi W, Giomi B, et al. Chronic idiopathic and chronic autoimmune urticaria: clinical and immunopathological features of 68 subjects. Acta Derm Venereol. 2004;84(4):288–290. [DOI] [PubMed] [Google Scholar]
- 131.Sabroe RA, Seed PT, Francis DM, Barr RM, Black AK, Greaves MW. Chronic idiopathic urticaria: comparison of the clinical features of patients with and without anti-FcepsilonRI or anti-IgE autoantibodies. J Am Acad Dermatol. 1999;40(3):443–450. [DOI] [PubMed] [Google Scholar]
- 132.Metz M, Giménez-Arnau A, Borzova E, Grattan CE, Magerl M, Maurer M. Frequency and clinical implications of skin autoreactivity to serum versus plasma in patients with chronic urticaria. J Allergy Clin Immunol. 2009;123(3):705–706. [DOI] [PubMed] [Google Scholar]
- 133.Kolkhir P, Balakirski G, Merk HF, Olisova O, Maurer M. Chronic spontaneous urticaria and internal parasites–a systematic review. Allergy. 2016;71(3):308–322. [DOI] [PubMed] [Google Scholar]
- 134.Vázquez-Nava F, Martinez-Burnes J. Prevalence and factors associates to chronic urticaria. Analysis of the allergic rhinitis and symptoms related to asthma as a factors associated to chronic urticaria in a urban area of northeastern of Mexico. Alergologia e inmunologia clinica. 2004;19:16–24. [Google Scholar]
- 135.Zazzali JL, Broder MS, Chang E, Chiu MW, Hogan DJ. Cost, utilization, and patterns of medication use associated with chronic idiopathic urticaria. Ann Allergy Asthma Immunol. 2012;108(2):98–102. [DOI] [PubMed] [Google Scholar]
- 136.Zuberbier T, Balke M, Worm M, Edenharter G, Maurer M. Epidemiology of urticaria: a representative cross-sectional population survey. Clin Exp Dermatol. 2010;35(8):869–873. [DOI] [PubMed] [Google Scholar]
- 137.Broder MS, Raimundo K, Antonova E, Chang E. Resource use and costs in an insured population of patients with chronic idiopathic/spontaneous urticaria. Am J Clin Dermatol. 2015;16(4):313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Balp MM. The Burden of Chronic Urticaria from Brazilian Patients’ Perspective. Dermatol Ther. 2017;7(4):535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Balp MM, Vietri J, Tian H, Isherwood G. The Impact of Chronic Urticaria from the Patient’s Perspective: a Survey in Five European Countries. Patient. 2015;8(6):551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vietri J, Turner SJ, Tian H, Isherwood G, Balp MM, Gabriel S. Effect of chronic urticaria on US patients: analysis of the National Health and Wellness Survey. Ann Allergy Asthma Immunol. 2015;115(4):306–311. [DOI] [PubMed] [Google Scholar]
- 141.Sibbald RG, Cheema AS, Lozinski A, Tarlo S. Chronic urticaria. Evaluation of the role of physical, immunologic, and other contributory factors. Int J Dermatol. 1991;30(6):381–386. [DOI] [PubMed] [Google Scholar]
- 142.Giménez-Arnau AM, Ferrer M, Peter H-J, Maurer M, Pujol RM. Urticaria crónica: estudio etiológico prospectivo e importancia del síndrome autoinmune. Actas Dermo-Sifiliográficas. 2004;95(9):560–566. [Google Scholar]
- 143.Kozel MM, Mekkes JR, Bossuyt PM, Bos JD. Natural course of physical and chronic urticaria and angioedema in 220 patients. J Am Acad Dermatol. 2001;45(3):387–391. [DOI] [PubMed] [Google Scholar]
- 144.van der Valk PG, Moret G, Kiemeney LA. The natural history of chronic urticaria and angioedema in patients visiting a tertiary referral centre. Br J Dermatol. 2002;146(1):110–113. [DOI] [PubMed] [Google Scholar]
- 145.Humphreys F, Hunter JA. The characteristics of urticaria in 390 patients. Br J Dermatol. 1998;138(4):635–638. [DOI] [PubMed] [Google Scholar]
- 146.Schnyder B, Helbling A, Pichler WJ. Chronic idiopathic urticaria: natural course and association with Helicobacter pylori infection. Int Arch Allergy Immunol. 1999;119(1):60–63. [DOI] [PubMed] [Google Scholar]
- 147.Vázquez Nava F, Almeida Arvizu VM, Sánchez Nuncio HR, Villanueva Carreto Mde L, Guidos Fogelbach GA. Prevalence and potential triggering factors of chronic urticaria and angioedema in an urban area of northeastern Mexico. Rev Alerg Mex. 2004;51(5):181–188. [PubMed] [Google Scholar]
- 148.Lindelöf B, Sigurgeirsson B, Wahlgren CF, Eklund G. Chronic urticaria and cancer: an epidemiological study of 1155 patients. Br J Dermatol. 1990;123(4):453–456. [DOI] [PubMed] [Google Scholar]
- 149.Quaranta JH, Rohr AS, Rachelefsky GS, et al. The natural history and response to therapy of chronic urticaria and angioedema. Ann Allergy. 1989;62(5):421–424. [PubMed] [Google Scholar]
- 150.Nettis E, Dambra P, D’Oronzio L, et al. Reactivity to autologous serum skin test and clinical features in chronic idiopathic urticaria. Clin Exp Dermatol. 2002;27(1):29–31. [DOI] [PubMed] [Google Scholar]
- 151.Asero R. Sex differences in the pathogenesis of chronic urticaria. J Allergy Clin Immunol. 2003;111(2):425–426. [DOI] [PubMed] [Google Scholar]
- 152.Kim A. Association of chronic urticaria with Helicobacter Pylori infection in Erbil: a case-control study. Zanco J Med Sci. 2016;20:1376–1384. [Google Scholar]
- 153.Confino-Cohen R, Chodick G, Shalev V, Leshno M, Kimhi O, Goldberg A. Chronic urticaria and autoimmunity: associations found in a large population study. J Allergy Clin Immunol. 2012;129(5):1307–1313. [DOI] [PubMed] [Google Scholar]
- 154.Ozkan M, Oflaz SB, Kocaman N, et al. Psychiatric morbidity and quality of life in patients with chronic idiopathic urticaria. Ann Allergy Asthma Immunol. 2007;99(1):29–33. [DOI] [PubMed] [Google Scholar]
- 155.Campos V, Yassumoto L, Filho O, Antunes R, Calamita Z. Chronic spontaneous urticaria: cutaneous reaction and laboratory aspects. Jornal Brasileiro de Patologia e Medicina Laboratorial. 2016;52:84–90. [Google Scholar]
- 156.Guttman-Yassky E, Bergman R, Maor C, Mamorsky M, Pollack S, Shahar E. The autologous serum skin test in a cohort of chronic idiopathic urticaria patients compared to respiratory allergy patients and healthy individuals. J Eur Acad Dermatol Venereol. 2007;21(1):35–39. [DOI] [PubMed] [Google Scholar]
- 157.Najib U, Bajwa ZH, Ostro MG, Sheikh J. A retrospective review of clinical presentation, thyroid autoimmunity, laboratory characteristics, and therapies used in patients with chronic idiopathic urticaria. Ann Allergy Asthma Immunol. 2009;103(6):496–501. [DOI] [PubMed] [Google Scholar]
- 158.Viswanathan RK, Biagtan MJ, Mathur SK. The role of autoimmune testing in chronic idiopathic urticaria. Ann Allergy Asthma Immunol. 2012;108(5):337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ertaş R, Hawro T, Altrichter S, et al. Antinuclear antibodies are common and linked to poor response to omalizumab treatment in patients with CSU. Allergy. 2020;75(2):468–470. [DOI] [PubMed] [Google Scholar]
- 160.Chanprapaph K, Iamsumang W, Wattanakrai P, Vachiramon V. Thyroid Autoimmunity and Autoimmunity in Chronic Spontaneous Urticaria Linked to Disease Severity, Therapeutic Response, and Time to Remission in Patients with Chronic Spontaneous Urticaria. Biomed Res Int. 2018;2018:9856843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kim JH, Oh TS, Lee SG, Kim IH. Prognostic significance of thyroid autoantibodies in urticaria. Korean J Dermatol. 2011;49(10):872–876. [Google Scholar]
- 162.Lee KR, Lee EG, Lee HJ, Yoon MS. Assessment of treatment efficacy and sebosuppressive effect of fractional radiofrequency microneedle on acne vulgaris. Lasers Surg Med. 2013;45(10):639–647. [DOI] [PubMed] [Google Scholar]
- 163.Kayastha AK. Chronic Idiopathic Urticaria and its association with antithyroglobulin antibody. Postgrad Med J NAMS. 2011;11(2):24–27. [Google Scholar]
- 164.Sánchez-Borges M, Asero R, Ansotegui IJ, et al. Diagnosis and treatment of urticaria and angioedema: a worldwide perspective. World Allergy Organ J. 2012;5(11):125–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tarbox JA, Gutta RC, Radojicic C, Lang DM. Utility of routine laboratory testing in management of chronic urticaria/angioedema. Ann Allergy Asthma Immunol. 2011;107(3):239–243. [DOI] [PubMed] [Google Scholar]
- 166.Jiamton S, Swad-Ampiraks P, Kulthanan K, Suthipinittharm P. Urticaria and angioedema in Siriraj medical students. J Med Assoc Thai. 2003;86(1):74–81. [PubMed] [Google Scholar]
- 167.Champion RH, Roberts SO, Carpenter RG, Roger JH. Urticaria and angio-oedema. A review of 554 patients. Br J Dermatol. 1969;81(8):588–597. [DOI] [PubMed] [Google Scholar]
- 168.Toubi E, Kessel A, Avshovich N, et al. Clinical and laboratory parameters in predicting chronic urticaria duration: a prospective study of 139 patients. Allergy. 2004;59(8):869–873. [DOI] [PubMed] [Google Scholar]
- 169.Prosty C, Gabrielli S, Le M, et al. Prevalence, Management, and Anaphylaxis Risk of Cold Urticaria: a Systematic Review and Meta-Analysis. J Allergy Clin Immunol Pract. 2022;10(2):586–596.e584. [DOI] [PubMed] [Google Scholar]
- 170.Magen E, Waitman DA, Dickstein Y, Davidovich V, Kahan NR. Clinical-laboratory characteristics of ANA-positive chronic idiopathic urticaria. Allergy Asthma Proc. 2015;36(2):138–144. [DOI] [PubMed] [Google Scholar]
- 171.Zauli D, Deleonardi G, Foderaro S, et al. Thyroid autoimmunity in chronic urticaria. Allergy Asthma Proc. 2001;22(2):93–95. [DOI] [PubMed] [Google Scholar]
- 172.Amin P, Levin L, Holmes SJ, Picard J, Bernstein JA. Investigation of patient-specific characteristics associated with treatment outcomes for chronic urticaria. J Allergy Clin Immunol Pract. 2015;3(3):400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Missaka RF, Penatti HC, Silvares MR, Nogueira CR, Mazeto GM. Autoimmune thyroid disease as a risk factor for angioedema in patients with chronic idiopathic urticaria: a case-control study. Sao Paulo Med J. 2012;130(5):294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rujitharanawong C, Tuchinda P, Chularojanamontri L, Chanchaemsri N, Kulthanan K. Cholinergic Urticaria: clinical Presentation and Natural History in a Tropical Country. Biomed Res Int. 2020;2020:7301652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Vollono L, Piccolo A, Lanna C, et al. Omalizumab for chronic spontaneous urticaria in “complex” patients: data from real-life clinical practice. Drug Des Devel Ther. 2019;13:3181–3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chiu HY, Muo CH, Sung FC. Associations of chronic urticaria with atopic and autoimmune comorbidities: a nationwide population-based study. Int J Dermatol. 2018;57(7):822–829. [DOI] [PubMed] [Google Scholar]
- 177.Cascone N, Nee TL, Boeckman JE, Petrov AA, Fajt ML. The Impact of Allergic Disease in Adults with Chronic Idiopathic Urticaria. J Allergy Clin Immunol. 2019;143(2 suppl):AB75. [Google Scholar]
- 178.Miller DA, Freeman GL, Akers WA. Chronic urticaria: a clinical study of fifty patients. Am J Med. 1968;44(1):68–86. [DOI] [PubMed] [Google Scholar]
- 179.Lee HC, Hong JB, Chu CY. Chronic idiopathic urticaria in Taiwan: a clinical study of demographics, aggravating factors, laboratory findings, serum autoreactivity and treatment response. J Formos Med Assoc. 2011;110(3):175–182. [DOI] [PubMed] [Google Scholar]
- 180.Gallo C, Vighi G, Schroeder J, et al. Chronic urticaria atopic dermatitis and celiac disease. Am J Gastroenterol. 1992;87(11):1684. [PubMed] [Google Scholar]
- 181.Aitella E, De Bartolomeis F, Savoia A, Fabiani M, Romano M, Astarita C. The overlap syndrome of urticaria and gastroesophageal reflux disease. PLoS One. 2018;13(11):e0207602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Doong JC, Chichester K, Oliver ET, Schwartz LB, Saini SS. Chronic Idiopathic Urticaria: systemic Complaints and Their Relationship with Disease and Immune Measures. J Allergy Clin Immunol Pract. 2017;5(5):1314–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fernandez Romero DS, Malbran A. Chronic urticaria with alterations of the thyroid function and thyroid peroxidase antibodies. Medicina. 2005;65(3):231–234. [PubMed] [Google Scholar]
- 184.Aamir IS, Tauheed S, Majid F, Atif A. Frequency of autoimmune thyroid disease in chronic urticaria. J Coll Physicians Surg Pak. 2010;20(3):158–161. [PubMed] [Google Scholar]
- 185.Staubach P, Dechene M, Metz M, et al. High prevalence of mental disorders and emotional distress in patients with chronic spontaneous urticaria. Acta Derm Venereol. 2011;91(5):557–561. [DOI] [PubMed] [Google Scholar]
- 186.Uguz F, Engin B, Yilmaz E. Axis I and Axis II diagnoses in patients with chronic idiopathic urticaria. J Psychosom Res. 2008;64(2):225–229. [DOI] [PubMed] [Google Scholar]
- 187.Staubach P, Eckhardt-Henn A, Dechene M, et al. Quality of life in patients with chronic urticaria is differentially impaired and determined by psychiatric comorbidity. Br J Dermatol. 2006;154(2):294–298. [DOI] [PubMed] [Google Scholar]
- 188.Barbosa F, Freitas J, Barbosa A. Chronic idiopathic urticaria and anxiety symptoms. J Health Psychol. 2011;16(7):1038–1047. [DOI] [PubMed] [Google Scholar]
- 189.Najmosadat A, Mahbooobeh R, Shadi P, Shadi G. Psychological status in patients with chronic urticaria. Med J Islam Repub Iran. 2011;25(4):200–204. [Google Scholar]
- 190.Pulimood S, Rajagopalan B, Rajagopalan M, Jacob M, John JK. Psychiatric morbidity among dermatology inpatients. Natl Med J India. 1996;9(5):208–210. [PubMed] [Google Scholar]
- 191.Calikuşu C, Yücel B, Polat A, Baykal C. The relation of psychogenic excoriation with psychiatric disorders: a comparative study. Compr Psychiatry. 2003;44(3):256–261. [DOI] [PubMed] [Google Scholar]
- 192.Lindelöf B, Wahlgren CF. Chronic urticaria associated with internal malignancy. Int J Dermatol. 1990;29(5):384. [DOI] [PubMed] [Google Scholar]
- 193.Breathnach SM, Allen R, Ward AM, Greaves MW. Symptomatic dermographism: natural history, clinical features laboratory investigations and response to therapy. Clin Exp Dermatol. 1983;8(5):463–476. [DOI] [PubMed] [Google Scholar]
- 194.Magerl M, Altrichter S, Borzova E, et al. The definition, diagnostic testing, and management of chronic inducible urticarias - The EAACI/GA(2) LEN/EDF/UNEV consensus recommendations 2016 update and revision. Allergy. 2016;71(6):780–802. [DOI] [PubMed] [Google Scholar]
- 195.Kontou-Fili K, Borici-Mazi R, Kapp A, Matjevic LJ, Mitchel FB. Physical urticaria: classification and diagnostic guidelines. An EAACI position paper. Allergy. 1997;52(5):504–513. [DOI] [PubMed] [Google Scholar]
- 196.Calamita Z, Pelá calamita AB. Chronic spontaneous urticaria: epidemiological characteristics focusing on the histocompatibility profile and presence of antibodies. Inflamm Allergy Drug Targets. 2013;12(1):8–11. [DOI] [PubMed] [Google Scholar]
- 197.Viswanathan RK, Biagtan MJ, Mathur SK. Autoimmune profiling in chronic idiopathic urticaria – is there utility or futility? J Allergy Clin Immunol. 2012;129(2suppl):AB224. [Google Scholar]
- 198.Kim DH, Sung NH, Lee AY. Effect of Levothyroxine Treatment on Clinical Symptoms in Hypothyroid Patients with Chronic Urticaria and Thyroid Autoimmunity. Ann Dermatol. 2016;28(2):199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kessel A, Helou W, Bamberger E, et al. Elevated serum total IgE–a potential marker for severe chronic urticaria. Int Arch Allergy Immunol. 2010;153(3):288–293. [DOI] [PubMed] [Google Scholar]
- 200.Stutes SA, Cho CB, Altrich M, Ardoin SP, Ogbogu PU. Auto-antibodies in Chronic Idiopathic Urticaria (CIU) and Non-urticarial Systemic Autoimmune Disorders. J Allergy Clin Immunol. 2012;129(2 suppl):AB224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Krupa Shankar DS, Ramnane M, Rajouria EA. Etiological approach to chronic urticaria. Indian J Dermatol. 2010;55(1):33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Abd El-Azim M, Abd El-Azim S. Chronic autoimmune urticaria: frequency and association with immunological markers. J Investig Allergol Clin Immunol. 2011;21(7):546–550. [PubMed] [Google Scholar]
- 203.Sajedi V, Movahedi M, Aghamohammadi A, et al. Comparison between sensitivity of autologous skin serum test and autologous plasma skin test in patients with Chronic Idiopathic Urticaria for detection of antibody against IgE or IgE receptor (FcεRIα). Iran J Allergy Asthma Immunol. 2011;10(2):111–117. [PubMed] [Google Scholar]
- 204.George M, Balachandran C, Prabhu S. Chronic idiopathic urticaria: comparison of clinical features with positive autologous serum skin test. Indian J Dermatol Venereol Leprol. 2008;74(2):105–108. [DOI] [PubMed] [Google Scholar]
- 205.Godse KV. Autologous serum skin test in chronic idiopathic urticaria. Indian J Dermatol Venereol Leprol. 2004;70(5):283–284. [PubMed] [Google Scholar]
- 206.Kulthanan K, Jiamton S, Gorvanich T, Pinkaew S. Autologous serum skin test in chronic idiopathic urticaria: prevalence, correlation and clinical implications. Asian Pac J Allergy Immunol. 2006;24(4):201–206. [PubMed] [Google Scholar]
- 207.Lunge SB, Borkar M, Pande S. Correlation of serum antithyroid microsomal antibody and autologous serum skin test in patients with chronic idiopathic urticaria. Indian Dermatol Online J. 2015;6(4):248–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Marasoğlu Çelen O, Kutlubay Z, Aydemir EH. Usefulness of the autologous serum test for the diagnosis of chronic idiopathic urticaria. Ann Dermatol. 2014;26(5):592–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Curto-Barredo L, Yelamos J, Gimeno R, Mojal S, Pujol RM, Giménez-Arnau A. Basophil Activation Test identifies the patients with Chronic Spontaneous Urticaria suffering the most active disease. Immun Inflamm Dis. 2016;4(4):441–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Magen E, Zueva E, Mishal J, Schlesinger M. The clinical and laboratory characteristics of acute spontaneous urticaria and its progression to chronic spontaneous urticaria. Allergy Asthma Proc. 2016;37(5):394–399. [DOI] [PubMed] [Google Scholar]
- 211.Rojo-Gutiérrez MI, Flores-Ruvalcaba CN, Mellado-ábrego J, Castillo-Narváez G, Ramírez-Rojo DP. Usefulness of studies looking for autoimmunity in patients with spontaneous chronic urticaria. Rev Alerg Mex. 2015;62(3):175–181. [PubMed] [Google Scholar]
- 212.Turktas I, Gokcora N, Demirsoy S, Cakir N, Onal E. The association of chronic urticaria and angioedema with autoimmune thyroiditis. Int J Dermatol. 1997;36(3):187–190. [DOI] [PubMed] [Google Scholar]
- 213.Díaz-Angulo S, López-Hoyos M, Muñoz cacho P, et al. Prevalence of thyroid autoimmunity in Spanish patients with chronic idiopathic urticaria: a case-control study involving 343 subjects. J Eur Acad Dermatol Venereol. 2016;30(4):692–693. [DOI] [PubMed] [Google Scholar]
- 214.Palma-Carlos AG, Palma-Carlos ML. Chronic urticaria and thyroid auto-immunity. Eur Ann Allergy Clin Immunol. 2005;37(4):143–146. [PubMed] [Google Scholar]
- 215.Chomiciene A, Jurgauskiene L, Blaziene A. Chronic urticaria and thyroid autoimmunity markers. Cent Eur J Med. 2012;7(6):736–741. [Google Scholar]
- 216.Alcaraz Calderón L, Escárcega Barbosa D, Castrejón Vázquez MI, et al. Presence of anti-Helicobacter pylori, antithyroid, and high-affinity IgE receptor antibodies in patients with chronic urticaria. Rev Alerg Mex. 2003;50(3):96–102. [PubMed] [Google Scholar]
- 217.Czarnecka-Operacz M, Sadowska-Przytocka A, Jenerowicz D, Szeliga A, Adamski Z, Łącka K. Thyroid function and thyroid autoantibodies in patients with chronic spontaneous urticaria. Postepy Dermatol Alergol. 2017;34(6):566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Leznoff A, Sussman GL. Syndrome of idiopathic chronic urticaria and angioedema with thyroid autoimmunity: a study of 90 patients. J Allergy Clin Immunol. 1989;84(1):66–71. [DOI] [PubMed] [Google Scholar]
- 219.Collet E, Petit JM, Lacroix M, Bensa AF, Morvan C, Lambert D. Chronic urticaria and autoimmune thyroid diseases. Ann Dermatol Venereol. 1995;122(6–7):413–416. [PubMed] [Google Scholar]
- 220.Ryhal B, DeMera RS, Shoenfeld Y, Peter JB, Gershwin ME. Are autoantibodies present in patients with subacute and chronic urticaria? J Investig Allergol Clin Immunol. 2001;11(1):16–20. [PubMed] [Google Scholar]
- 221.Kikuchi Y, Fann T, Kaplan AP. Antithyroid antibodies in chronic urticaria and angioedema. J Allergy Clin Immunol. 2003;112(1):218. [DOI] [PubMed] [Google Scholar]
- 222.Atta AM, Rodrigues MZ, Sousa CP, Medeiros Júnior M, Sousa-Atta ML. Autoantibody production in chronic idiopathic urticaria is not associated with Helicobacter pylori infection. Braz J Med Biol Res. 2004;37(1):13–17. [DOI] [PubMed] [Google Scholar]
- 223.Caproni M, Giomi B, Volpi W, et al. Chronic idiopathic urticaria: infiltrating cells and related cytokines in autologous serum-induced wheals. Clin Immunol. 2005;114(3):284–292. [DOI] [PubMed] [Google Scholar]
- 224.Fusari A, Colangelo C, Bonifazi F, Antonicelli L. The autologous serum skin test in the follow-up of patients with chronic urticaria. Allergy. 2005;60(2):256–258. [DOI] [PubMed] [Google Scholar]
- 225.Gangemi S, Saitta S, Lombardo G, Patafi M, Benvenga S. Serum thyroid autoantibodies in patients with idiopathic either acute or chronic urticaria. J Endocrinol Invest. 2009;32(2):107–110. [DOI] [PubMed] [Google Scholar]
- 226.Ye YM, Park JW, Kim SH, et al. Prognostic Factors for Chronic Spontaneous Urticaria: a 6-Month Prospective Observational Study. Allergy Asthma Immunol Res. 2016;8(2):115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lee SY, Song WJ, Jung JW, et al. Thyroid autoantibodies and the prognosis of chronic idiopathic urticaria. Allergy Asthma Respir Dis. 2013;1(2):151–156. [Google Scholar]
- 228.Dionigi PC, Menezes MC, Forte WC. A prospective ten-year follow-up of patients with chronic urticaria. Allergol Immunopathol. 2016;44(4):286–291. [DOI] [PubMed] [Google Scholar]
- 229.Oguz Topal I, Kocaturk E, Gungor S, Durmuscan M, Sucu V, Yıldırmak S. Does replacement of vitamin D reduce the symptom scores and improve quality of life in patients with chronic urticaria? J Dermatolog Treat. 2016;27(2):163–166. [DOI] [PubMed] [Google Scholar]
- 230.Okba AM, Sheha DS, Moustafa AS, El-Sherbeny AA, Mohamed NA, Aglan MF. Association between thyroid autoimmunity and chronic urticaria in patients versus healthy controls. Egypt J Obes Diabetes Endocrinol. 2015;1(2):84. [Google Scholar]
- 231.Chaykivska Z, Antoszczyk G, Czarnobilska E. The elevated level of anti-thyroid antibodies aTPO in chronic spontaneous urticaria. Przegl Lek. 2015;72(12):736–738. [PubMed] [Google Scholar]
- 232.Shin YS, Suh DH, Yang EM, Ye YM, Park HS. Serum Specific IgE to Thyroid Peroxidase Activates Basophils in Aspirin Intolerant Urticaria. J Korean Med Sci. 2015;30(6):705–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Arshi S, Babaie D, Nabavi M, et al. Circulating level of CD4+ CD25+ FOXP3+ T cells in patients with chronic urticaria. Int J Dermatol. 2014;53(12):e561–566. [DOI] [PubMed] [Google Scholar]
- 234.Boonpiyathad T, Pradubpongsa P, Sangasapaviriya A. Vitamin d supplements improve urticaria symptoms and quality of life in chronic spontaneous urticaria patients: a prospective case-control study. Dermatoendocrinol. 2014;6:125. doi: 10.4161/derm.29727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Irani C, Jammal M, Asmar G, Hajj H, Halaby G. Chronic urticaria and autoimmune thyroiditis. J Med Liban. 2012;60(2):88–90. [PubMed] [Google Scholar]
- 236.Concha LB, Chang CC, Szema AM, Dattwyler RJ, Carlson HE. IgE antithyroid antibodies in patients with Hashimoto’s disease and chronic urticaria. Allergy Asthma Proc. 2004;25(5):293–296. [PubMed] [Google Scholar]
- 237.Al-Balbeesi AO. Significance of antithyroid antibodies and other auto-antibodies in Saudi patients with chronic urticaria. Possible parameters in predicting chronic over three years disease. J Dermatol Dermatol Surg. 2011;15(2):47–51. [Google Scholar]
- 238.Buss YA, Garrelfs UC, Sticherling M. Chronic urticaria–which clinical parameters are pathogenetically relevant? A retrospective investigation of 339 patients. J Dtsch Dermatol Ges. 2007;5(1):22–29. [DOI] [PubMed] [Google Scholar]
- 239.Staubach P, Metz M, Chapman-Rothe N, et al. Effect of omalizumab on angioedema in H1 -antihistamine-resistant chronic spontaneous urticaria patients: results from X-ACT, a randomized controlled trial. Allergy. 2016;71(8):1135–1144. [DOI] [PubMed] [Google Scholar]
- 240.Kaplan AP, Spector SL, Meeves S, Liao Y, Varghese ST, Georges G. Once-daily fexofenadine treatment for chronic idiopathic urticaria: a multicenter, randomized, double-blind, placebo-controlled study. Ann Allergy Asthma Immunol. 2005;94(6):662–669. [DOI] [PubMed] [Google Scholar]
- 241.Perez A, Woods A, Grattan CE. Methotrexate: a useful steroid-sparing agent in recalcitrant chronic urticaria. Br J Dermatol. 2010;162(1):191–194. [DOI] [PubMed] [Google Scholar]
- 242.Sanada S, Tanaka T, Kameyoshi Y, Hide M. The effectiveness of montelukast for the treatment of anti-histamine-resistant chronic urticaria. Arch Dermatol Res. 2005;297(3):134–138. [DOI] [PubMed] [Google Scholar]
- 243.Reisman RE, Livingston A. Late-onset allergic reactions, including serum sickness, after insect stings. J Allergy Clin Immunol. 1989;84(3):331–337. [DOI] [PubMed] [Google Scholar]
- 244.Greene SL, Reed CE, Schroeter AL. Double-blind crossover study comparing doxepin with diphenhydramine for the treatment of chronic urticaria. J Am Acad Dermatol. 1985;12(4):669–675. [DOI] [PubMed] [Google Scholar]