Abstract
Purpose
This study was to explore the influence of flax seeds on the gut microbiota of elderly patients with functional constipation.
Patients and Methods
Sixty elderly patients (68.68±8.73 years) with functional constipation were recruited between January 2018 and March 2018. They received oral flax seeds (50 g/d) for one month. Bowel habits and adverse events were recorded before and after treatment. Fresh stool was collected before and after treatment and the amplification product of 16S rRNA V5 region was sequenced using the next-generation sequencing technique on the Ion Torrent PGM platform. The gut microbiota were analyzed before and after flax seeds treatment in the same subject.
Results
Flax-seed treatment significantly increased the frequency of defecation and decreased abdominal distension in elderly patients with chronic constipation. The majority of gut bacteria belonged to the phyla of Firmicutes, Bacteroidetes, and Proteobacteria, accounting for 98.71%. After flax seeds treatment, the diversity of bacterial clusters significantly increased with increases of Roseburia_hominis, Pseudomonas_azotoformans, uncultured_Clostridiales_bacterium, Blautia_obeum, Ruminococcus_sp._16442, Pyramidobacter_piscolens, Acinetobacter_lwoffii, Prevotella_melaninogenica. The abundance of Blautia in patients with chronic constipation was significantly lower than healthy controls, while Blautia_obeum increased significantly after flax seed treatment. Blautia_obeum might be the predominant genus accounting for the therapeutic effect of flax seeds.
Conclusion
Flax seeds may improve the defecation in elderly patients with chronic constipation and change intestinal microecological structure. Thus, flax seeds may serve as an effective diet supplement in the management of chronic constipation.
Keywords: constipation, flax seeds, high-throughput sequencing, gut microbiota
Introduction
Functional constipation is a syndrome characterized by decreased frequency of defecation, wet stool, and laborious defecation,1 but no morphology and biochemistry abnormalities can be observed in functional constipation patient.2 Meanwhile, the symptoms are incompatible with the diagnostic criteria of irritable bowel syndrome (IBS).3 The incidence of constipation has been increasing, and it is more common in middle-aged and elderly populations as well as women.4 Some treatments have been developed for the management of functional constipation, including medical and non-medical therapies and the lifestyle modulation.5
Among non-medical therapies, flax seeds have been increasingly used. They are rich in linolenic acid, lignan and other bioactive compounds that have pharmacological effects such as lowering blood lipids, body weight, blood pressure and blood glucose, improving immunity, ameliorating inflammation and preventing cardiocerebrovascular diseases.6,7 They also have fibers that can improve the gut motility and shorten the time of defecation. It has been reported that the gut microbiota significantly change in healthy male adults after consuming flax seeds for a week.8 However, whether flax seeds can ameliorate chronic constipation in the elderly population is still unclear. Therefore, the present study aimed to assess the influence of flax seeds on the chronic constipation in elderly population and explore the potential mechanism, especially the role of gut microbiota. Our findings will provide a new way for the management of chronic constipation.
Patients and Methods
Participants
A total of 60 patients (54 males and 6 females) with chronic constipation were admitted from the gastrointestinal outpatient clinic of Huadong Hospital of Fudan University between January 2018 and March 2018. The average age was 68.68±8.73 years (range: 65–75 years) and the duration of constipation was 168.2±20.8 months. Twenty age-matched healthy controls (3 males and 17 females) were included as controls. The average age was 70.68±8.73 years. The inclusion criteria of patients were as follows: (1) chronic constipation confirmed by the Roman IV standard,9 and the following criteria were simultaneously met: (i) there were at least two of following manifestations: a. straining at more than 25% defecations; b. lumpy or hard stools (Bristol Stool Form10 Scale type 1–2) at more than 25% defecations; c. sensation of incomplete evacuation at more than 25% of defecations; d. sensation of anorectal obstruction/blockage at more than 25% defecations; e. manual maneuvers to facilitate more than 25% defecations; f. fewer than 3/week spontaneous bowel movements. (ii) loose stools were hardly observed in the absence of laxatives; (iii) irritable bowel disease was excluded. The above symptoms lasted at least 6 months and the above manifestations were present in the past 3 months. Symptoms were assessed after discontinuing medications that influence defecation. (2) There was no use of probiotics, antibiotics or medications that may cause constipation in the prior month. The exclusion criteria were as follows: (1) there were concomitant severe heart, brain, liver, or hemopoietic system diseases, or mental illnesses; (2) there was allergic disease or they were allergic to multiple drugs; (3) there were severe gastrointestinal, abdominal, or pelvic diseases; (4) there was disturbance of consciousness or impaired cognitive function that influences the questionnaire survey; (5) they participated in other nutritional intervention projects; (6) they were active smokers; (7) they were vegans. This present study was approved by the Human Research Ethics Committee of Huadong Hospital (ID: 2017K066) and registered in the Chinese Clinical Trials Registry (www.chictr.org.cn, ID: ChiCTR1800014882). Written informed consent was obtained from all participants before study. This study complies with the Declaration of Helsinki.
Treatments
Flax seed powder (TAFOOD) was provided by Suzhou Huidong Biotech and imported from TAFOODS LTD (Canada). There was no restriction regarding the timing and method of flax seed consumption as long as the daily dose of 50 g was reached. It was recommended to mix flax seed powder with hot liquid (water, milk, soymilk, etc.), which facilitated dissolution of the flax seed powder. It could also be taken along with wheat flour or rice preparations as the main food, with varied cooking methods based on personal preference. It was not recommended to take a large amount of flax seed powder on an empty stomach to avoid any stomach discomfort; it was advisable to take flax seed powder 2–3 times daily by dividing the dose for 4 weeks. Adverse reactions were recorded before and after flax seed treatment.
Parameters for Comparison Before and After Treatment
Symptoms of constipation: The Wexner grading scale, developed by Agachan et al,11 was used to assess symptoms of constipation. This scale rated the frequency of constipation, straining, emptying, pain, defecation time, assisted defecation, failed defecation, and medical history with a score ranging from 0 to 30.
Grading based on Bristol Stool Form Scale: 1. Isolated hard lumps like nuts; 2. Lumps like sausages; 3. Lumps like sausages with crackles; 4. Soft and smooth like sausages or a snake; 5. Soft lumps with clear borders; 6. Velvet or pasty material without clear borders; 7. Watery stool, completely liquid without any solid material.
Frequency of defecation: the frequency of defecation in one day.
Rating of therapeutic effect: clinically effective, effective, ineffective.
(i) Clinically effective: decrease of Wexner scale by ≥50% after 4-week treatment; (Wexner scale before treatment-Wexner scale after treatment)/Wexner scale before treatment ≥50%; (ii) Effective: decrease of Wexner scale by ≥25–50% after 4-week treatment; 25%≤ (Wexner scale before treatment–Wexner scale after treatment)/Wexner scale before treatment <50%; (iii) Ineffective: decrease of Wexner scale by <25% (Wexner scale before treatment–Wexner scale after treatment)/Wexner scale before treatment <25%.
Collection of Samples and DNA Extraction
Stool samples (5 g for each subject) of 60 elderly patients with chronic constipation and 20 healthy controls were collected and stored at −80°C within 2 h. No stabilizers were added before further analysis. Types of gut bacteria were identified using 16S rRNA detection technique according to the manufacturer’s instructions (Rapid DNA extraction kit, MP Biomedicals, CA, USA). They were then compared before and after treatment for each individual. In this assay, the quality of DNA was determined by its concentration and purity. A concentration >5 ng/μL indicated good-quality DNA, and the purity was determined by detecting the absorbance at 260 nm and 280 nm (A260/280) with a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, MA, USA).
Amplification of 16S rRNA and PCR Assay
Polymerase chain reaction was conducted by amplifying the gene sequence of V4-V5 of bacterial 16S rRNA. The primers were: 515 forward 5’-GTGCCAGCMGCCGCGGTAA-3’, 926, Reverse: 5’-CCGTCAATTCMTTTGAGTTT-3’. The PCR mixture included Q5 reaction buffer (10 μL, 5×), Q5 high-fidelity DNA polymerase (1 U), dNTPs (1 μL 2.5 mM), primers (1 μL of 10 μM each), DNA template (50 ng), and ddH2O (40 μL). The amplification conditions were as follows: initial degeneration at 94℃ for 2 min, 22 cycles of 94℃ for 30s, 55℃ for 30s, and 72°C for 30s and a final extension for 5 min and heat preservation for 10 min. The PCR mixture with ddH2O was used as the negative control. Amplification products were electrophoresed in 2% agarose gel, and DNA bands were collected for purification using the AxyPrepDNA Gel DNA Extraction Kit (AP-GX-250, Axygen, CA, USA). Extracted DNA was amplified again under the following conditions: initial degeneration at 94℃ for 2 min, 8 cycles of 94℃ for 30s, 56℃ for 30s, and 72℃ for 30s and a final extension for 5 min and heat preservation for 10 min. All PCR amplification products were extracted using the AxyPrepDNA extraction kit, and the concentrations of fluorophore-labeled DNA samples were detected using an FTC-3000TM real-time PCR instrument (ABI9700, Thermo, USA). These samples were then mixed at the same molar concentration to construct the DNA library for sequencing. Amplicons were maintained in equal amounts and were sequenced by the Illumina MiSeq platform in conjunction with the MiSeq Reagent at Shanghai Personal Biotechnology Co, Ltd (Shanghai, China).
Bioinformatic Analysis
An Illumina platform library was constructed for high-throughput sequencing and bioinformatic analysis.12 Briefly, readings of primary sequences were allocated to the origin of samples through matching their bar codes and denoted as valid sequences. The screening criteria for low-quality sequences were as follows: length <150 bp, average of Phred scores <20, and fuzzy bases and highly repeated single bases >8 bp.13,14 Singletons (single sequence for matching reads) in the long-spliced reads were filtered, and only Operational Taxonomic Units (OTU) data with a similarity of 97% were used for bioinformatic analysis. Subsequently, the clean tags were assigned to OTUs depending on the species of bacteria identified after deciphering OTUs with the software USEARCH.15 Those were excluded and the remaining high-quality sequences were assigned to OTUs using UCLUST based on the 97% clustering consistency.13 Representative sequences were searched in the Greengenes database using the best hit in BLAST according to their OTUs. As a result, an OUT list was produced with the abundance and categories of OTUs of each sample. For all samples, only less than 0.001% of sequences were deleted. To minimize the sequencing depth between different samples, an average circular list with further divided OTUs was produced. Data analysis was performed at 90% sequencing depth after averaging 100 OTUs. The QIIME software was used to analyze α diversity indices such as abundance-based coverage estimate, Chao1 index, estimated number of OTUs, Simpson index, and Shannon index, and relative abundance of gut microbiota at the phylum and family levels. Abundance and evenness of OTUs between samples were compared after producing an OTU-level-based abundance curve. Structural changes of gut microbiota in different samples were assessed by analyzing beta diversity. With UniFrac measurements, visualization was completed through the principal coordinate analysis (PCoA).16 In the violin plot, comparisons were made between groups at levels of phylum, class, order, family, genus, and species.17
Statistical Analysis
Data are expressed as mean ± standard deviation (SD). After assessing the distribution of continuous variables using the Kolmogorov–Smirnov test, Student’s t-test was used for comparisons of data with normal distribution, and Mann–Whitney test was used for comparisons without normal distribution. A value of P<0.05 was considered statistically significant.
Results
Clinical Features
The baseline characteristics of subjects in two groups (healthy control group and flax-seed group) are shown in Table 1. Patients with chronic constipation showed significantly increased frequency in defecation, alleviated abdominal distension, anorexia and anxiety, and decreased dependence on laxatives when compared with symptoms before treatment with flax seeds (all P > 0.05) (Table 2). No adverse reactions were observed during the period of treatment.
Table 1.
Baseline Characteristics of Patients with Chronic Constipation and Healthy Controls
| Variables | Healthy Control Group (n=20) | Flax-Seed Group (n=60) | P value |
|---|---|---|---|
| Age (years) | 70.54±8.76 | 68.68±8.73 | 0.671 |
| Sex (M/F) | 3/17 | 6/54 | 0.918 |
| Body mass index (kg/m2) | 23.14±2.68 | 23.14±2.61 | 0.999 |
| Diet, exercise | |||
| Exercise (per week) | 2.75±2.24 | 2.75±2.24 | 0.790 |
| Drink (mL) | 1210.7±532.2 | 1271.4±528.3 | 0.323 |
| Vegetables (g/d) | 308.9±119.5 | 322.7±135.6 | 0.611 |
| Fruits (g/d) | 182.1±98.3 | 219.6±151.7 | 0.928 |
| Whole cereal (per week) | 3.57±1.91 | 3.07±0.04 | 0.660 |
| Spicy food (per week) | 0.84±0.10 | 0.89±0.12 | 0.924 |
| Deep fried food (per week) | 0.91±1.03 | 0.77±0.80 | 0.587 |
| Soft drink (per week) | 1.18±0.14 | 1.14±0.14 | 0.709 |
Table 2.
Comparison of Gut Microbiota Between Patients with Chronic Constipation and Healthy Controls at Phylum Level (%)
| Group | Bacteroidetes | Firmicutes | Proteobacteria |
|---|---|---|---|
| Patients (flax seeds) | 59.90±1.26 | 33.97±1.30 | 4.84±0.48 |
| Healthy controls (H) | 60.25±1.74 | 34.70±1.81 | 4.44±0.49 |
| P value | 0.31 | 0.06 | 0.91 |
In this study, Wexner score was used to evaluate the efficacy of flax-seed treatment in patients with constipation based on the frequency of defecation, degree of difficulty in defecation and defecation time. In the present study, marked effectiveness was observed in 27 (45.0%) subjects, effectiveness in 26 (43.3%) subjects, and ineffectiveness in 7 (11.7%) subjects. The total effectiveness rate was 88.3%.
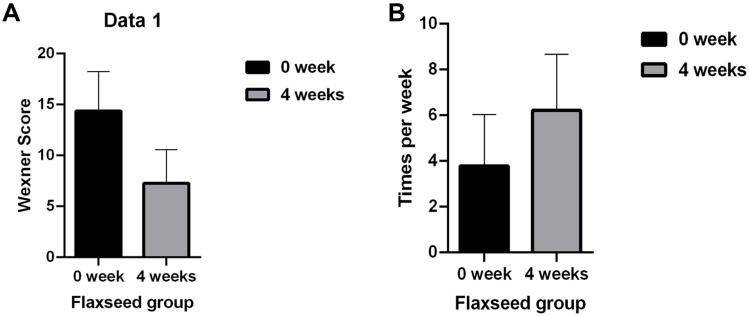
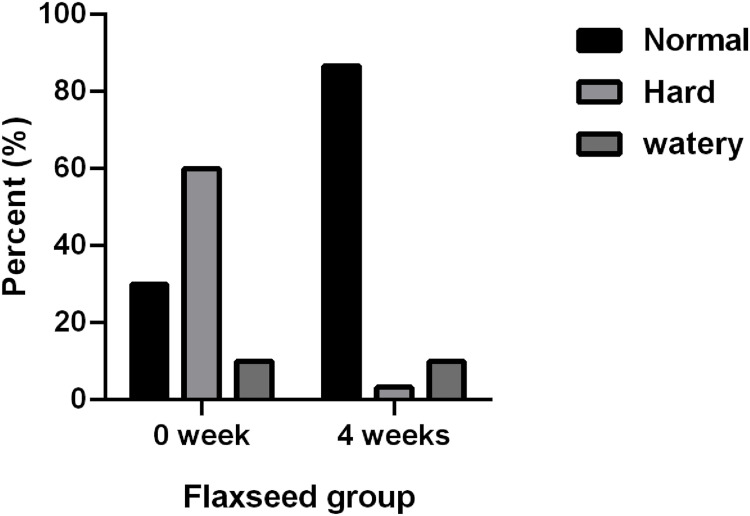
After flax seeds treatment, the total Wexner score in constipation patients decreased from 14.38 ± 3.84 to 7.28 ± 3.29 (P<0.05, Figure 1A), and the frequency of defecation (times/week) increased significantly from 3.79 ± 2.24 to 6.22 ± 2.44 (P<0.05, Figure 1B). After flax-seed intervention, 2 subjects had hard stool (3.33%), 52 had normal stool (86.6%) and 6 had sparse stool (10.0%). The proportion of subjects with hard stool decreased from 60% to 3.33%, the proportion of subjects with normal stool increased from 30% to 86.6%, and the proportion of subjects with loose stool remained unchanged. These results indicated that flax seeds improved the stool characteristics of patients with constipation and made the stool easier to discharge (Figure 2).
Figure 1.
Influence of flax seeds on Wexner score (A) and frequency of defecation (B).
Figure 2.
Influence of flax seeds on stool characteristics.
Evaluation of the Alpha Diversity in FC Patients
In order to analyze the species diversity and evenness in the FC group after flax seeds treatment, we estimated the community species richness using the abundance-based coverage estimator (ACE) index. All the sequenced read pools showed a tendency to reach a plateau. In all cases, so the retrieved sequencing data were considered sufficient to cover most of the biodiversity contained in the sample. The ACE indexes of FC patients before and after flax seeds treatment were not significantly different (p = 0.537).
Structural Analysis of Gut Microbiota
Comparison of Gut Microbiota Between Healthy Controls and Constipation Subjects
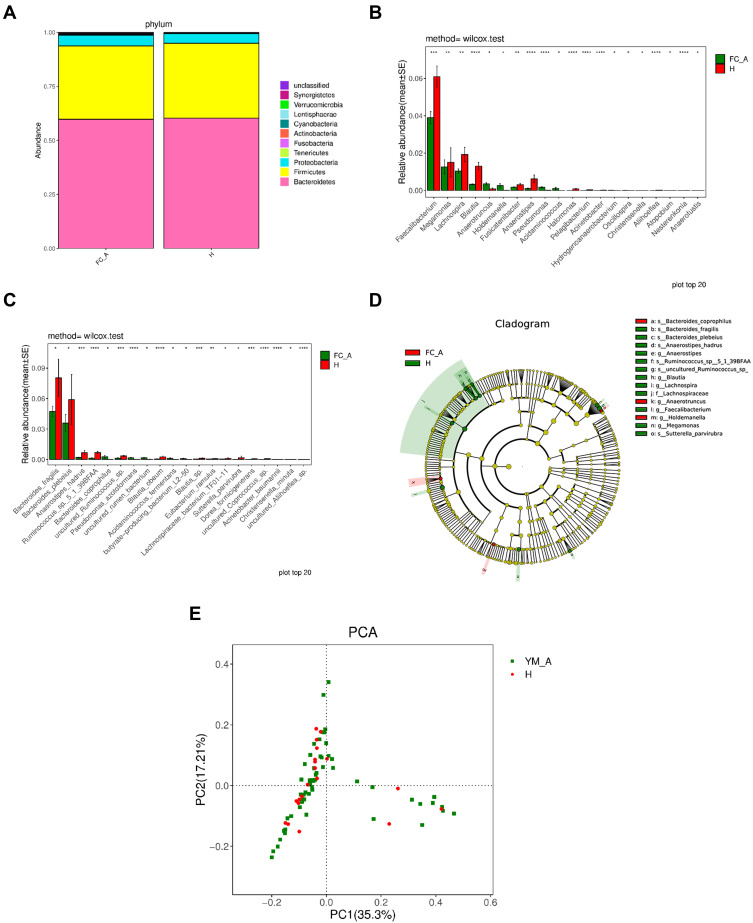
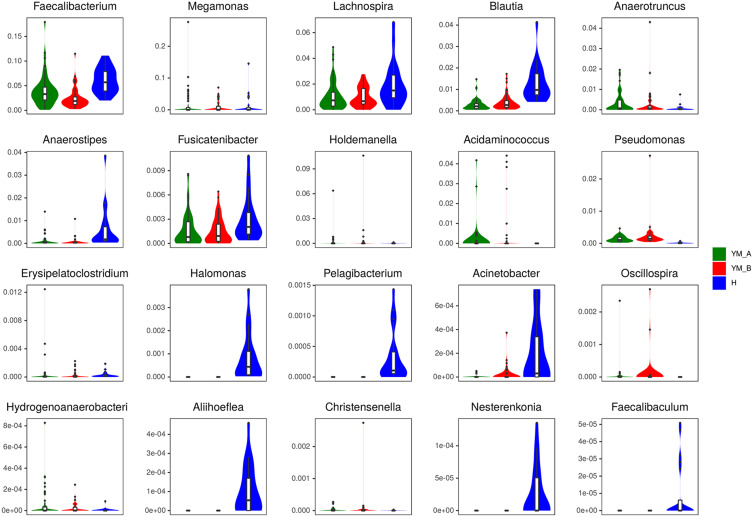
No significant difference was observed in gut microbiota at level of phylum between healthy controls and constipation patients. The compositions of gut bacteria in both groups are presented in Figure 3A. Bacteroidetes was the predominant phylum, accounting for 60.25% and 59.90% of total bacteria (P=0.31) in healthy controls and constipation subjects, respectively, followed by Firmicutes (34.70% and 33.97%, respectively; P=0.06) and proteobacteria (4.44% and 4.84%, respectively; P=0.91). These three phyla accounted for over 97% of total bacteria in both groups, and no significant difference was observed between two groups (Table 2). At the genus level, significant difference was observed in gut microbiota between two groups (Figure 3B). Patients with chronic constipation had less Faecalibacterium, Lachnospira, Blautia, Anaerostipes, Fusicatenibacter, Halomonas, Pelagibacterium, Acinetobacter, Aliihoeflea, Nesterenkonia, and Faecalibaculum than healthy controls (P < 0.05), but had more Megamonas, Anaerotruncus, Holdemanella, Acidaminococcus, Erysipelatoclostridium, Oscillospira, Hydrogenoanaerobacterium, Christensenella and Pseudomonas (P<0.05). At the species level, significant difference was observed in the gut microbiota between two groups (Figure 3C). Patients with chronic constipation had less Bacteroides_fragilis, Anaerostipes, hadrus, Ruminococcus_sp._5_1_39BFAA, Ruminococcus_sp._YE281, uncultured_Ruminococcus_sp, uncultured_Aliihoeflea_sp, uncultured_Mesorhizobium_sp, uncultured_Xanthomonas_sp, uncultured_Coprococcus_sp, Blautia_obeum, Blautia_sp, Eubacterium_ramulus, Lachnospiraceae_bacterium_TF01-11, Acinetobacter_baumannii, Prevotella_disiens, Eubacterium_sulci, Pseudomonas_fragi, and Dorea_formicigenerans than healthy controls, but had more Bacteroides_coprophilus, Acidaminococcus_fermentans, butyrate-producing_bacterium_L2-50, uncultured_rumen_bacterium, Pseudomonas_azotoformans, Erysipelatoclostridium_ramosum and Christensenella_ minuta.
Figure 3.
Comparison of gut microbiota between elderly healthy controls and patients with chronic constipation. (A). Predominant phyla in both groups. (B). The relative abundance of genera that could distinguish chronic constipation from controls is plotted on a logarithmic scale, and values of zero are assigned 1e-06. *P < 0.05,**P < 0.001,***P < 0.0001,****P < 0.00001 (C). The relative abundance of species that could distinguish chronic constipation from controls is plotted on a logarithmic scale, and values of zero are assigned 1e-06.*P < 0.05, **P < 0.001,***P < 0.0001,****P < 0.00001 (D). Histogram of linear discriminant analysis scores for differently abundant genera. (E). PCoA diagram based on relative abundance (97% similarity). Each symbol represents a sample. Green symbol indicates patients with chronic constipation and red symbol represents healthy controls.
In the LEfSe analysis, g_Megamonas, s_Bacteroides_Coprophilus, and g_Anaerotruncus were enriched in patients with constipation, which might be positively correlated with constipation. s_Ruminococcus_sp,_o_Erysipelotrichales, f_Erysipelotrichaceae, c_Erysipelotrichia, s_Ruminococcus_sp_5_1_39BFAA, g_Anaerostopes_hadrus, g_Anaerostipes, g_Lachnpspira, g_Blautia, g_Faecalibacterium, s_Bacteroides_ fragilis, and f_Lachnospiraceae were enriched in healthy controls, which was expected to be negatively correlated with constipation (Figure 3D). PCoA revealed two principal coordinates that were able to differentiate patients with constipation from healthy controls, corresponding to 35.4% for PC1 and 17.21% for PC2 (Figure 3E).
Comparison of Gut Microbiota of Elderly Patients with Chronic Constipation Before and After Treatment
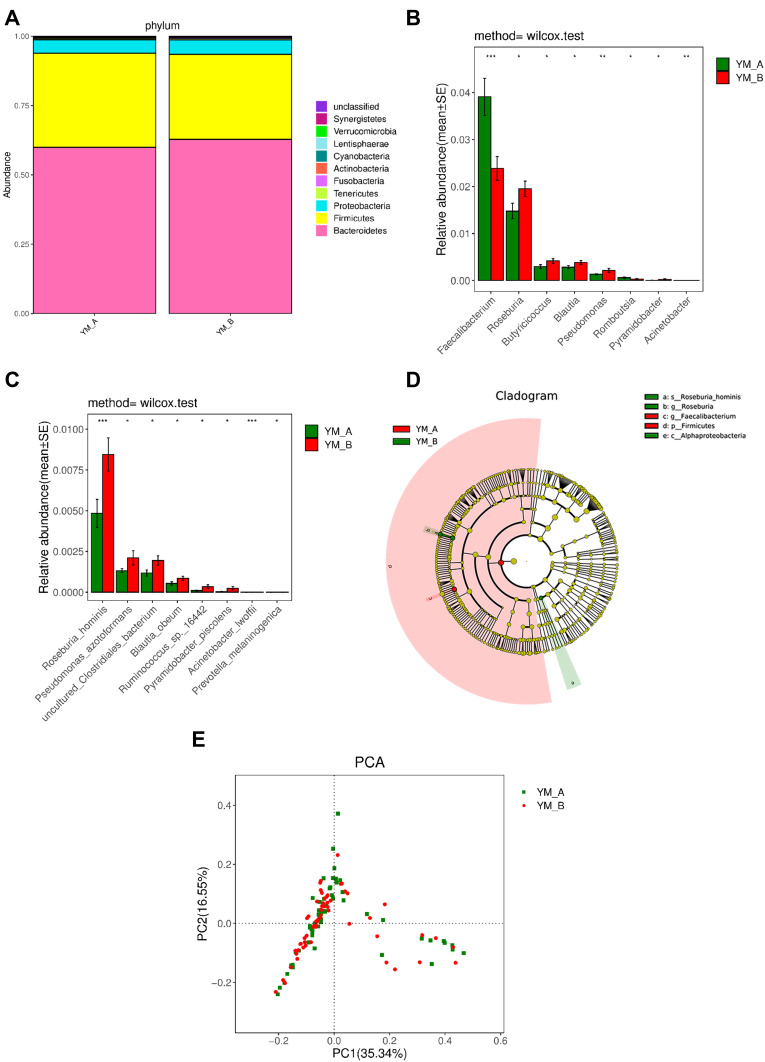
At phylum level, a significant decrease in Firmicutes and a significant increase in Synergistetes were observed after flax seeds treatment (P=0.03 and P=0.03) (Figure 4A). At genus level, significant decreases in Faecalibacterium and Romboutsia were observed after flax seeds treatment, whereas significant increases in Roseburia, Butyricicoccus, Blautia, Pseudomonas, Pyramidobacter, and Acinetobacter were observed after flax seeds treatment (Figure 4B). At species level, significant increases were noted in Roseburia_hominis, Pseudomonas_ azotoformans, uncultured_Clostri- diales_bacterium, Blautia_obeum, Ruminococcus_ sp._16442, Pyramidobacter_piscolens, Acinetobacter_lwoffii, and Prevotella_melaninogenica after flax seeds treatment (Figure 4C), indicating that there was an increased abundance of gut microbiota.
Figure 4.
Comparison of gut microbiota in elderly patients with chronic constipation before and after treatment. (A). Predominant phyla in both groups. (B). The relative abundance of genera that could distinguish chronic constipation from before and after flaxseeds treatment is plotted on a logarithmic scale, and values of zero are assigned 1e-06.*P < 0.05,**P < 0.001, ***P < 0.0001. (C). The relative abundance of species that could distinguish chronic constipation from before and after flaxseeds treatment is plotted on a logarithmic scale, and values of zero are assigned 1e-06.*P < 0.05,***P < 0.0001. (D). Histogram of linear discriminant analysis scores for differently abundant genera. (E). PCoA diagram based on relative abundance (97% similarity). Each symbol represents a sample. Green symbol indicates patients with chronic constipation before treatment and red symbol indicates those after treatment.
In the LEfSe analysis, p_Firmicutes and g_Faecalibacterium were enriched in patients with constipation, but s_Roseburia_hominis, g_Roseburia, and c_Alp haproteobacteria were more enriched after flax seeds treatment. This might be related to improved constipation (Figure 4D). After flax seeds treatment, PCoA revealed the two principal coordinates that were able to differentiate subjects before treatment from those after treatment, corresponding to 35.34% for PC1 and 16.55% for PC2 (Figure 4E).
Comparison of Gut Microbiota Among Three Groups
The top 20 bacteria were compared among the three groups: health control group, before flax seed treatment group (0 week), and after flax seed treatment group (4 weeks) at genus level, and a significant increase in Blautia was observed in patients with chronic constipation after flax seeds treatment, which was similar to that in healthy controls (P<0.05, Figure 5). Enrichment of Megamonas and Anaerotruncus increased in patients with chronic constipation as compared to healthy controls, but decreased after flax seeds treatment, which was similar to that in healthy controls. However, this association was not statistically significant.
Figure 5.
Comparison of top 20 bacterial genera with significant difference among three groups: health control group, before flax seed treatment group (0 week) and after flax seed treatment group (4 week).
Discussion
Chronic constipation is a common disease, manifested as difficulty in defecation and/or reduced frequency of defecation and hard stools.18 Difficulty in defecation refers to straining during defecation, difficulty in expelling the stool, sensation of incomplete evacuation or rectal blockage, longer time to complete defecation, and need for manual maneuvers to facilitate evacuation. The currently available treatments for constipation include non-pharmacological treatments, pharmacological treatments, transplantation of fecal bacteria, and surgery.19 Owing to the challenges associated with long-term medication in middle-aged or elderly populations and the high incidence of complications and trauma after surgery, these treatments are limited in these patients in clinical practice.20
Transplantation of fecal bacteria has been reported to be effective,21 but it is still in its infancy in clinical trials. Several problems associated with transplantation of fecal bacteria influence the wide clinical use of this treatment. Lifestyle intervention including diet intervention is the foundation in the management of constipation.22 As a type of food, flax seeds are rich in fibers and other bioactive compounds, and are able to improve constipation by modulating the gut microbiota.23,24 Guo et al reported that elderly patients with chronic constipation had unique gut microbiome,25 which was consistent with our findings given the significant difference in the gut microbiota between chronic constipation subjects and healthy controls. In another study, flax seeds were found to increase the frequency of defecation and improve bowel movement and quality of life, and its therapeutic efficacy was better than that of lactulose.26 However, the underlying mechanism underlying the therapeutic effect of flax seeds on the constipation is still poorly understood. Lagkouvardos et al found that healthy male adults showed significant change in their gut microbiota after flax seeds treatment (0.3 g/kg/d) for a week. Furthermore, the types of predominant bacteria were closely correlated with the content of short chain fatty acids (SCFA).8 In studies on mammals, it has been reported that flax seeds decrease the number of harmful bacteria such as Clostridium perfringens and increase the content of SCFA, thus lowering the likelihood of developing colitis or colon cancer. However, the influences of flax seeds on constipation in the elderly and on the gut microbiota have never been reported. In an animal study about the influence of flax seeds on gut microbiota, mice were fed with high-fat diet, and results showed the intestinal probiotics Akkerman and Bifidobacterium increased after flax seeds intervention. The increases of propionate and butyrates might account for the correction of altered metabolism owing to the high-fat diet.27 These indicate that flax seeds might regulate gut microbiota as a probiotic.
In the present study, the frequency of defecation increased, and the symptoms were significantly relieved with correction of disturbed gut microbiota after flax seeds intervention for 4 weeks. At phylum level, there was a significant decrease in Firmicutes and a marked increase in Synergistetes. At genus level, there were significant decreases in Faecalibacterium, Lachnospira, Blautia, and Anaerostipes in patients with constipation as compared to healthy controls, but Blautia significantly increased after flax seeds treatment for 4 weeks, which was similar to that in healthy controls. Blautia might be the predominant genus accounting for the therapeutic effect of flax seeds.
Another study has reported that a low-fat diet increased the level of Blautia-containing butyrates. This bacterium is known for its anti-inflammatory and immune-regulating effect, as well as its correlation with low level of cholesterol.28 It is also known that butyric acid inhibits the activity of histone-deacetylase in the intestinal epithelial cells (IECs) of colon and consequently downregulates proinflammatory cytokines such as IL-6 and IL-12. Thus, inflammation is suppressed and the risk of colon cancer decreased.29 In addition, our results showed the abundances of Megamonas and Anaerotruncus were higher in patients with constipation than in healthy controls. Flax seeds treatment downregulated these two phyla to different extents. Although no statistical difference was observed, a decreasing trend was noted in these two phyla, which might be related to the short duration of flax seed treatment.
At genus level, Roseburia_hominis, Pseudomonas_ azotoformans, uncultured_Clostri-diales_bacterium, Blautia_obeum, Ruminococcus_sp._16442, Pyramidobacter_Piscolens, Acinetobacter_lwoffii, and Prevotella_melani nogenica significantly increased after flax seeds treatment in patients with constipation, indicating that flax seeds increased the abundance of these genera in the gut. In a previous study, patients with slow transit constipation had different gut microbiota as compared to patients with other types of constipation and healthy controls. Faecalibacterium, Lactococcus, and Roseburia can increase the gut motility.30 This study revealed that flax seeds improved symptoms of patients with constipation, which might be attributed to the increased abundance of Roseburia. Another study showed that xylan in the wheat bran increased the content of butyric acid in the stool and decreased the body weight, and the abundances of Bifidobacterium longum, Prevotella_melaninogenica, and Blautia obeum increased.31 Similar findings were observed in the present study after flax seeds intervention for 4 weeks: both Prevotella_melaninogenica and Blautia obeum significantly increased. This might be related to the abundance of fibers in flax seeds. Because of the evident heterogeneity in the gut microbiota between individuals, in the present study, the gut microbiota were compared before and after flax seeds intervention in each subject.
There were still limitations in the present. First, there were only 60 patients with chronic constipation and the sample size was relatively small. Second, the elderly patients with chronic constipation were predominantly females. Third, the diet, lifestyles and medications were not taken into account, which may bias our results. Therefore, more studies with large sample size are needed to confirm our findings and further elucidate the underlying mechanisms.
It is known that flax seeds are a safe, effective and convenient food supplement without causing side effects. Therefore, subjects had a high compliance to the flax seeds intervention without loss to follow-up. Flax seed powder can be easily incorporated in the diet in many ways. It has been reported that flax seeds can decrease blood glucose, blood lipids as well as body weight, and prevent colon tumors.32–36 This may be another reason for the high compliance in the present study. This also suggests that flax seeds are acceptable by the general population. In summary, further insights into functional constipation in the elderly have increased clinicians’ attention to flax seeds given the therapeutic effect it shows on improving the symptoms of patients with constipation, and optimizing the gut microbiota. The findings from the present study will likely accelerate the clinical use of flax seeds for patients with constipation.
Conclusion
Flax seeds may improve the defecation and gut microbiota in elderly patients with chronic constipation and increase the diversity of gut microbiota. Flax seeds were safe to take during the trial. Thus, flax seeds may serve as an effective diet supplement in the management of chronic constipation.
Acknowledgments
This work was supported by Shanghai Shenkang Hospital Development Center (No. SHDC12013123 and SHDC12015107).
Data Sharing Statement
We intend to share all clinical trial data. The individual deidentified participant data and other study documents could be available from the corresponding author upon reasonable request. They will be made available for three years.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Huang L, Zhu Q, Qu X, Qin H. Microbial treatment in chronic constipation. Sci China Life Sci. 2018;61:744–752. doi: 10.1007/s11427-017-9220-7 [DOI] [PubMed] [Google Scholar]
- 2.Baffy N, Foxx-Orenstein AE, Harris LA, Sterler S. Intractable constipation in the elderly. Curr Treat Options Gastroenterol. 2017;15(3):363–381. doi: 10.1007/s11938-017-0142-2 [DOI] [PubMed] [Google Scholar]
- 3.Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150(6):1393–1407. [DOI] [PubMed] [Google Scholar]
- 4.Forootan M, Bagheri N, Darvishi M. Chronic constipation. Medicine. 2018;97(20):e10631. doi: 10.1097/MD.0000000000010631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emmanuel A, Mattace-Raso F, Neri MC, Petersen KU, Rey E, Rogers J. Constipation in older people: a consensus statement. Int J Clin Pract. 2017;71:e12920. [DOI] [PubMed] [Google Scholar]
- 6.Hanif Palla A, Gilani AH. Dual effectiveness of Flaxseed in constipation and diarrhea: possible mechanism. J Ethnopharmacol. 2015;169:60–68. doi: 10.1016/j.jep.2015.03.064 [DOI] [PubMed] [Google Scholar]
- 7.Winnik S, Lohmann C, Richter EK, et al. Dietary α-linolenic acid diminishes experimental atherogenesis and restricts T cell-driven inflammation. Eur Heart J. 2011;32(20):2573–2584. doi: 10.1093/eurheartj/ehq501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lagkouvardos I, Kläring K, Heinzmann SS, et al. Gut metabolites and bacterial community networks during a pilot intervention study with flaxseeds in healthy adult men. Mol Nutr Food Res. 2015;59(8):1614–1628. doi: 10.1002/mnfr.201500125 [DOI] [PubMed] [Google Scholar]
- 9.Drossman DA. Rome IV–functional GI disorders: disorders of gut-brain interaction. Gastroenterology. 2016;150(6):1257–1261. [DOI] [PubMed] [Google Scholar]
- 10.Lane MM, Czyzewski DI, Chumpitazi BP, Shulman RJ. Reliability and validity of a modified Bristol stool form scale for children. J Pediatr. 2011;159:437–441.e431. doi: 10.1016/j.jpeds.2011.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agachan F, Chen T, Pfeifer J, Reissman P, Wexner SD. A constipation scoring system to simplify evaluation and management of constipated patients. Dis Colon Rectum. 1996;39(6):681–685. doi: 10.1007/BF02056950 [DOI] [PubMed] [Google Scholar]
- 12.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–336. doi: 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72(7):5069–5072. doi: 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill SR, Pop M, Deboy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355–1359. doi: 10.1126/science.1124234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altschul SF, Madden TL, Schäffer AA, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paliy O, Shankar V. Application of multivariate statistical techniques in microbial ecology. Mol Ecol. 2016;25(5):1032–1057. doi: 10.1111/mec.13536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buttigieg PL, Ramette A. A guide to statistical analysis in microbial ecology: a community-focused, living review of multivariate data analyses. FEMS Microbiol Ecol. 2014;90(3):543–550. doi: 10.1111/1574-6941.12437 [DOI] [PubMed] [Google Scholar]
- 18.Corsetti M, Brown S, Chiarioni G, et al. Chronic constipation in adults: contemporary perspectives and clinical challenges. 2: conservative, behavioural, medical and surgical treatment. Neurogastroenterol Motil. 2021;33(7):e14070. [DOI] [PubMed] [Google Scholar]
- 19.Bharucha AE, Lacy BE. Mechanisms, evaluation, and management of chronic constipation. Gastroenterology. 2020;158(5):1232–1249.e1233. doi: 10.1053/j.gastro.2019.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucak S, Lunsford TN, Harris LA. Evaluation and treatment of constipation in the geriatric population. Clin Geriatr Med. 2021;37(1):85–102. doi: 10.1016/j.cger.2020.08.007 [DOI] [PubMed] [Google Scholar]
- 21.Tian Y, Zuo L, Guo Q, et al. Potential role of fecal microbiota in patients with constipation. Therap Adv Gastroenterol. 2020;13:1756284820968423. doi: 10.1177/1756284820968423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duan M, Sun X, Ma N, et al. Polysaccharides from Laminaria japonica alleviated metabolic syndrome in BALB/c mice by normalizing the gut microbiota. Int J Biol Macromol. 2019;121:996–1004. doi: 10.1016/j.ijbiomac.2018.10.087 [DOI] [PubMed] [Google Scholar]
- 23.Xu J, Zhou X, Chen C, et al. Laxative effects of partially defatted flaxseed meal on normal and experimental constipated mice. BMC Complement Altern Med. 2012;12(1):14. doi: 10.1186/1472-6882-12-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soltanian N, Janghorbani M. A randomized trial of the effects of flaxseed to manage constipation, weight, glycemia, and lipids in constipated patients with type 2 diabetes. Nutr Metab. 2018;15(1):36. doi: 10.1186/s12986-018-0273-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo M, Yao J, Yang F, et al. The composition of intestinal microbiota and its association with functional constipation of the elderly patients. Future Microbiol. 2020;15(3):163–175. doi: 10.2217/fmb-2019-0283 [DOI] [PubMed] [Google Scholar]
- 26.Sun J, Bai H, Ma J, et al. Effects of flaxseed supplementation on functional constipation and quality of life in a Chinese population: a randomized trial. Asia Pac J Clin Nutr. 2020;29(1):61–67. doi: 10.6133/apjcn.202003_29(1).0009 [DOI] [PubMed] [Google Scholar]
- 27.Yang C, Xu Z, Deng Q, Huang Q, Wang X, Huang F. Beneficial effects of flaxseed polysaccharides on metabolic syndrome via gut microbiota in high-fat diet fed mice. Food Res Int. 2020;131:108994. doi: 10.1016/j.foodres.2020.108994 [DOI] [PubMed] [Google Scholar]
- 28.Wan Y, Wang F, Yuan J, et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: a 6-month randomised controlled-feeding trial. Gut. 2019;68(8):1417–1429. doi: 10.1136/gutjnl-2018-317609 [DOI] [PubMed] [Google Scholar]
- 29.Verma MS, Fink MJ, Salmon GL, et al. A common mechanism links activities of butyrate in the colon. ACS Chem Biol. 2018;13(5):1291–1298. doi: 10.1021/acschembio.8b00073 [DOI] [PubMed] [Google Scholar]
- 30.Parthasarathy G, Chen J, Chen X, et al. Relationship between microbiota of the colonic mucosa vs feces and symptoms, colonic transit, and methane production in female patients with chronic constipation. Gastroenterology. 2016;150(2):367–379.e361. doi: 10.1053/j.gastro.2015.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen NK, Deehan EC, Zhang Z, et al. Gut microbiota modulation with long-chain corn bran arabinoxylan in adults with overweight and obesity is linked to an individualized temporal increase in fecal propionate. Microbiome. 2020;8(1):118. doi: 10.1186/s40168-020-00887-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolodziejczyk AA, Zheng D, Elinav E. Diet–microbiota interactions and personalized nutrition. Nat Rev Microbiol. 2019;17(12):742–753. doi: 10.1038/s41579-019-0256-8 [DOI] [PubMed] [Google Scholar]
- 33.DeLuca JAA, Garcia-Villatoro EL, Allred CD. Flaxseed bioactive compounds and colorectal cancer prevention. Curr Oncol Rep. 2018;20(8):59. doi: 10.1007/s11912-018-0704-z [DOI] [PubMed] [Google Scholar]
- 34.Ahmad N, Manzoor MF, Shabbir U, et al. Health lipid indices and physicochemical properties of dual fortified yogurt with extruded flaxseed omega fatty acids and fibers for hypercholesterolemic subjects. Food Sci Nutr. 2020;8(1):273–280. doi: 10.1002/fsn3.1302 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Parikh M, Kura B, O’Hara KA, et al. Cardioprotective effects of dietary flaxseed post-infarction are associated with changes in MicroRNA expression. Biomolecules. 2020;10(9):1297. doi: 10.3390/biom10091297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han Y, Deng X, Zhang Y, Wang X, Zhu X. Antidepressant‐like effect of flaxseed in rats exposed to chronic unpredictable stress. Brain Behav Immun. 2020;10:e01626. [DOI] [PMC free article] [PubMed] [Google Scholar]