Abstract
Vascular endothelial growth factor (VEGF) is the most important stimulator of endometrial tumor angiogenesis, a mechanism that may be a therapeutic target in the context of an incidence and persistent mortality of endometrial endometrial carcinomas (EEC). In this study, VEGF immunoexpression was analyzed for 50 cases of EEC in relation to the histopathological parameters of tumor aggressiveness. High VEGF scores have been associated with the high grade and advanced stage of EEC, but unrelated to the depth of myometrial invasion, the pattern of tumor invasion, or vascular invasion. VEGF may be useful for assessing EEC aggression, but also for tumor angiogenic potential, which recommends it as a possible mark for specific antitumor therapy.
Keywords: Endometrioid endometrial carcinomas, VEGF, tumor grade, tumor stage
Introduction
Endometrial carcinomas are common in peri-and postmenopausal age, are associated with numerous hormonal, metabolic and genetic risk factors, over 60% of cases being diagnosed after 60 years [1,2,3].
They represent 80-90% of uterine malignancies, of which around 60-75% are endometrial endometrioid carcinomas (EEC) [1,3,4].
The dualistic classification of endometrial carcinomas indicates EEC as estrogen-dependent tumors, with a better prognosis and mutations in specific genes and microsatellite instability [1,4,5].
Although the rate of EEC diagnosis has increased due to screening programs and accessibility to health care services, and surgical techniques and oncological protocols have evolved, the number of newly diagnosed cases and the mortality rate from these tumors remain constant.
In this context there is a permanent concern for the deep study of the biomolecular mechanisms involved in the progression and development of endometrial carcinomas [2,6].
One of these mechanisms is represented by tumor angiogenesis, which consists in the formation of new blood vessels from pre-existing ones and which ensures the survival and development of 1-2mm tumors [7].
The tumor angiogenic switch is essential for tumor survival, progression and metastasis, being acquired when the amount of antiangiogenic factors is exceeded by proangiogenic factors [8,9,10].
Among the proangiogenic factors, the most studied is the vascular endothelial growth factor (VEGF), specific for vascular endothelium, which binds to heparin and ensures local and distant growth and infiltration of tumor cells, by stimulating vascular endothelial cell proliferation and increasing vessel permeability [11].
The results obtained so far about the VEGF expression in relation to EEC histopathological parameters are heterogeneous and controversial, aspects that may be due to diagnostic criteria, working protocols or inclusion criteria in the study [1,7].
In this study, we analyzed VEGF expression in relation to EEC histological parameters of aggressiveness.
Material and Methods
In the study were included 50 cases of endometrioid endometrial carcinomas (EEC), which were investigated and surgical operated in Gynecology or General Surgery Departments of the Emergency County Hospital Craiova during four years (2017-2020).
The diagnosis was emitted in Pathology Department of the same hospital, in accordance with the latest criteria for the classification of malignant tumors of the uterine body recommended by the working group within the World Health Organization [3].
Surgical specimens were represented by total histerectomy samples, which were fixed in 10% formaldehyde and processed by the classical method of paraffin embedding and HE (Hematoxylin-Eosin) staining.
The study included only primitive EECs with no history of oncological/hormonal treatments, or the presence of malignant processes with other locations.
Subsequently, 4μm thick sections for immunohistochemical investigation (IHC) were obtained from the paraffin blocks, which were deparaffined with xylene and hydrated in alcohol solutions of decreasing concentrations; the sections were incubated 20 minutes at microwave in Tris-EDTA buffer solution pH 9 for the antigen retrieval.
Endogenous peroxidase blockage was done with 3% H2O2 (hydrogen peroxide) in PBS (phosphate-buffered saline), and for the non-specific sites we used 1% BSA (bovine serum albumin).
The incubation with primary antibody, represented by mouse monoclonal antihuman VEGF, clone VG1 (code M7273, Dako) in dilution of 1/40, was done overnight at 4ºC.
EnVision™ FLEX System (code K8002, Dako) was used for IHC reactions, and the signal detection was done by using DAB (3,3’-diaminobenzidine tetrahydrochloride).
IHC reactions were validated by external negative control, respectively the omission of primary antibody and by positive external control (kidney).
IHC reactions were assessed using a semiquantitative system and a final staining score (FSS), which was the result of multiplying of the score for positive labelled cells and the score for reactions intensity.
Depending on the average percent of positive cells counted in ten 40x microscopic fields for each case, the score was 1 (<25%), 2 (25-49%), 3 (50-74%) and 4 (≥75%).
Related with the intensity the score was 1 (mild reaction), 2 (moderate reaction) or 3 (strong reaction).
The FSS values were considered to be high for values of 8-12, and low for 1-4.
The absence of reaction lead to a negative FSS.
The statistical analysis was done by using comparison tests, respectively chi square (χ2), a function of the SPSS 10 (Statistical Package for the Social Sciences) software, the p<0.05 values being considered significant.
For publication of these data, the patients’ informed consents were obtained, and the study was approved by the Local Ethics Committee.
Results
In this study the age range for the diagnosis of EEC was between 40-72 years, with an average of 62.3±6.9 years.
Most of the tumors analyzed were well differentiated (G1) (52%), in stage IA (48%) and without vascular invasion (78%).
Histopathological analysis indicated the presence of histological differentiations in 40% of cases, the most common being squamous (20%) and secretory (8%), as well as a series of particular invasion patterns, especially irregular (64%) or pushing types (16%).
VEGF immunoexpression was identified in 44 cases (88%), the negative cases belonging to well/moderate differentiated EEC (G1/G2) with myometrial invasion (IA/IB).
Immunoreactions were present in the cytoplasm of tumor cells, as well as in vascular endothelium and stromal elements represented by macrophages, lymphocytes, fibroblasts.
For the whole analyzed group, the average number of positive tumor cells was 33±20.4, with variable reaction intensity and an average FSS value of 3.1.
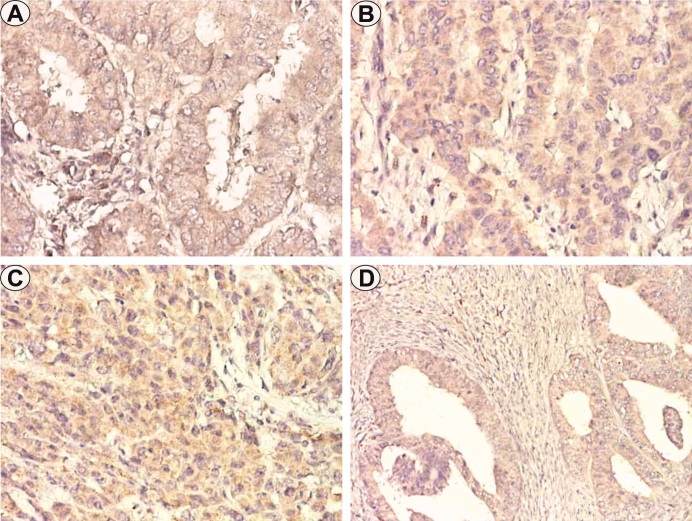
In relation to the degree of differentiation we found differences of VEGF expression, the lowest values being identified in the case of EEC G1, with an average number of positive cells of 23±16.3, mild/moderate reaction intensity and average FSS value of 1.6 (Table 1) (Figure 1A).
Table 1.
Cases distribution depending on the EECs histopathological parameters and VEGF FSS values
|
HP parameter/No. cases |
VEGF FSS (average value) |
p value (χ test) |
|
|
Tumor grade |
Well differentiated (G1)/26 |
1.6 |
p=0.007 |
|
|
Moderate differentiated (G2)/16 |
3.6 |
|
|
|
Poorly differentiated (G3)/8 |
6.7 |
|
|
Tumor stage |
IA/24 |
2.1 |
p=0.012 |
|
|
IB/19 |
3 |
|
|
|
II 4 |
7 |
|
|
|
IIIA/C1/3 |
6.3 |
|
|
Vascular invasion |
Absent/39 |
2.8 |
p=0.393 |
|
|
Present/11 |
3.8 |
|
Figure 1.
Endometrioid endometrial carcinoma (EEC), VEGF immunostaining. A. Well differentiated EEC, ×200. B. Moderate differentiated EEC, ×200. C. Poorly differentiated EEC, ×200. D. Well differentiated EEC, irregular invasive pattern, ×100
Comparatively, in the case of EEC G2, the number of labeled cells was 37.1±19, the intensity of mild/moderate reactions, with an FSS value of 3.6 (Figure 1B).
In the case of poorly differentiated EEC (G3), the mean number of labeled cells was 56.8±11.6, the intensity of moderate/ strong reactions and the FFS mean value of 6.7 (Table 1) (Figure 1C).
Regarding the tumor stage, the highest values of VEGF reactions were identified in the case of EEC in stages II and III1/C1, in which the average number of labeled cells was 60±14.7 and 55±8.5, with an intensity of predominantly moderate reactions with mean FSS values of 7 and respectively 6.3.
In comparison, in the case of EEC in stages IA and IB, the average number of labeled positive cells was 26±20.4 and 32.6±15.8, the intensity of the reactions being variable, and VEGF FSS having average values of 2.1 and 3 (Table 1).
In relation to vascular invasion, the values of VEGF reactions were higher in the case of EEC that presented this histological parameter, the tumors having an average number of labeled positive cells of 39±20.3, with a predominantly moderate intensity of reactions and an average FSS value of 3.8.
At the same time, tumors without vascular invasion had a mean number of labeled cells of 31.2±20.4, a variable intensity of reactions and a mean FSS value of 2.8 (Table 1).
Regarding invasion patterns, VEGF FSS high scores were present in the case of irregular, pushing or myoinvasive pattern, but the reactions were determined by tumor differentiation, rather than by depth of myometrial invasion or the presence of vascular invasion (Figure 1D).
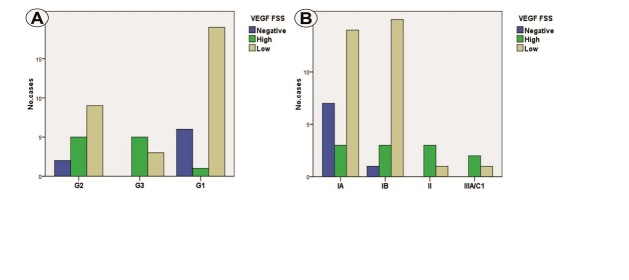
Statistical analysis indicated significant differences in VEGF FSS related to tumor grade (p=0.007, χ2 test) and tumor stage (p=0.012, χ2 test), with high scores being associated with G2/G3 carcinomas in advanced stages II/III (Figure 2A,2B).
Figure 2.
Distribution of cases depending on VEGF FSS scores and tumor grade (A) and tumor stage (B).
There were no differences in VEGF expression in relation to the depth of myometrial invasion, respectively between stages IA and IB (p=0.135, χ2 test).
The differences were non-significant in relation to the vascular invasion (p=0.393, χ2 test), the tumor differentiations (p=0.869, χ2 test) or the patterns of tumor invasion (p=0.575, χ2 test).
Discussions
Endometrial angiogenesis can be observed both in the physiological conditions of the menstrual cycle and in the development of EEC, being a process that is under the influence of steroid hormones [1].
Thus, because endothelial cells express ERα/β (estrogen receptor), estrogen stimulates or inhibits endothelial proliferation and can initiate/stop the production of new vessels directly, depending on the type of receptor to which it binds [12].
Intussusception, vascular elongation, sprouting and cooptation of progenitor endothelial cells are mechanisms by which angiogenesis is generally made, including in the endometrium [1,8,9].
These mechanisms are under the influence of many stimulants, including VEGF, PDGF (placental growth factor), FGF (fibroblast growth factor), TGF (transforming growth factor), EGF (endothelial growth factor), PDGF (platelet-derived growth factor), angiopoietins [1,7].
Of these factors, VEGF has been the most studied and has been considered the most important stimulator of endothelial cells and implicitly of tumor angiogenesis [1,8,9].
VEGF was identified in tumor cell lines by Dvorak H et al. in 1983, being called vascular permeability factor (VPF) and later on vascular endothelial growth factor (VEGF), which was isolated and established with essential role in angiogenesis [13,14,15].
The VEGF family includes types A-F and placental growth factor (PLGF), with effects mediated mainly by receptors 1 (FLT1) and 2 (KDR), existing also other compatible receptors, respectively VEGF-3 and neuropilin 1 and 2, as well and several protein isomorphs [1,16].
VEGF-A seems to be the most important for angiogenesis, by binding to receptor 1, the expression at the endometrium being higher in the epithelium compared to the stroma and in the secretory phase compared to the proliferative one [1,17].
In our study we used an antibody addressed to three of the five isomorphs of VEGF-A, respectively VEGF-121, VEGF-165, and VEGF-189.
VEGF was initially described as a multifunctional cytokine in angiogenesis, with a role in maintaining an inflammatory tumor microenvironment and stimulating paracrine and autocrine secretion [18].
In this context, in our study we observed the expression of VEGF, both in stromal inflammatory elements, vascular endothelium and fibroblasts.
Some studies indicate that immature stromal elements have a higher secretion capacity than the mature ones [18,19].
There are numerous studies in which VEGF immunoexpression has been analyzed in endometrial carcinomas, including EEC, with variable final results.
Thus, while some studies indicate the association of VEGF overexpression with myometrial invasion depth, degree of differentiation and advanced stage [7,20], others indicate the absence of any correlation of VEGF expression with EEC histopathological parameters, although it was superior in G1/G2 tumors versus non-tumor proliferative endometrium [7,21,22]; also, other studies indicated that VEGF was associated only with type 2 endometrial carcinomas, so that later the same authors on a group of EEC to indicate VEGF positivity in 42% of cases and a relation of overexpression with histological grade and clinical stage [7,2,3].
In this context, Laxmanan S et al. indicated that by binding to receptor 1, VEGF disrupts the immune response against tumor cells, which favors their distant extension [11,24].
The authors indicate increased VEGF expression in endometrial cancer compared with atypical hyperplasia or benign tumors [25,26].
There are other studies that have indicated that VEGF is not associated with the depth of myometrial invasion, being rather associated with advanced metastatic stages [27].
Within classical studies, increased VEGF expression is demonstrated in carcinomas with different localizations, including in the gynecological area, such as the endometrial or ovarian, and blocking of the associated receptors with specific antibodies inhibited the angiogenesis [28,29].
Also, in some studies, the increased VEGF expression in the EEC was associated with a poor prognosis and reduced survival [18].
In our study we found statistical associations of VEGF expression in relation to the tumor grade and stage, the highest values being observed in G2/G3 EEC, in advanced stages II/III, an aspect that supports some data in the literature.
Thus, in a cohort of 50 cases, Sanseverino F et al. indicates overexpression of VEGF in high-grade EEC [30], and in a recent study that analyzed VEGF as a prognostic factor of EEC, the authors indicated the correlation of high expression of the growth factor with high-grade tumors in advanced stages [31].
The variability of the results in the literature regarding the expression of VEGF, in addition to aspects related to techniques and inclusion criteria in studies, may also be the result of the influence of other growth factors and hormonal status.
Thus, on endometrial cancer cell lines, it was established that ERα overexpression significantly inhibits tumor growth, and subsequent of the overexpression, VEGF inhibition occurs in tumor xenografts and thus a blockade of tumor angiogenesis, which could explain the positive correlation of ER level with the rate of survival of patients with EEC [32].
Progesterone does not appear to be involved in stimulating endometrial VEGF expression, although in some animal models the stimulatory effect exists in the endometrium, while other studies indicate an inhibitory effect on growth factor [33].
In this study we did not find differences in VEGF expression in relation to the depth of myometrial invasion.
There are other studies that have indicated that VEGF is not associated with the depth of myometrial invasion, being rather associated with advanced metastatic stages [27].
In a recent study, the authors indicated an increase in VEGF expression in malignant lesions compared to the normal endometrium, without finding differences between stages IA and IB, but with differences between stages I and II-IV [11].
In contrast, other authors have indicated the association of increased VEGF expression with deep myometrial invasion [34,35,36].
Also, although in our study we did not identify statistical associations of VEGF expression with EEC invasion patterns, some studies indicate increased marker expression in the case of microcystic, elongated and fragmented (MELF) patterns, which together with the level of angiogenesis, it is considered a prognostic factor [18].
The analysis of the results obtained in the context of data from the literature indicates the need to continue investigating the factors that modulate endometrial angiogenesis based on a clear methodology and tumor groups as broad as possible, in order to validate possible therapeutic targets to improve EEC prognosis.
Conclusions
The study indicated the presence of VEGF expression in most EECs, suggesting the role of angiogenesis in tumor initiation and progression.
The highest scores of VEGF were associated with high grade tumors in advanced stages, but unrelated to vascular invasion, pattern of tumor invasion or depth of myometrial invasion.
VEGF immunoexpression can be considered an indicator of EEC aggressiveness and angiogenic potential, and may contribute to the design of effective therapies targeting endometrial protumoral mechanisms.
Conflict of interests
None to declare.
References
- 1.Żyła MM, Kostrzewa M, Litwińska E, Szpakowski A, Wilczyński JR, Stetkiewicz T. The role of angiogenic factors in endometrial cancer. Prz Menopauzalny. 2014;13(2):122–126. doi: 10.5114/pm.2014.42714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali AT. Reproductive factors and the risk of endometrial cancer. Int J Gynecol Cancer. 2014;24(3):384–393. doi: 10.1097/IGC.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 3.WHO Classification of Tumours Editorial Board, editor. Female genital tumours. WHO classification of tumours series. 5. Vol. 4 Lyon (France): International Agency for Research on Cancer; 2020. [Google Scholar]
- 4.López-Reig R, Fernández-Serra A, Romero I, Zorrero C, Illueca C, García-Casado Z, Poveda A, López-Guerrero JA. Prognostic classification of endometrial cancer using a molecular approach based on a twelve-gene NGS panel. Sci Rep. 2019;9(1):18093–18093. doi: 10.1038/s41598-019-54624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 6.Koskas M, Amant F, Mirza MR, Creutzberg CL. Cancer of the corpus uteri: 2021 update. Int J Gynaecol Obstet. 2021;155 Suppl 1:45–60. doi: 10.1002/ijgo.13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahecha AM, Wang H. The influence of vascular endothelial growth factor-A and matrix metalloproteinase-2 and-9 in angiogenesis, metastasis, and prognosis of endometrial cancer. Onco Targets Ther. 2017;10:4617–4624. doi: 10.2147/OTT.S132558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29(6 Suppl 16):15–8. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 9.Folkman J, Kalluri R. In: Cancer Medicine. 6. Holland JF, Frei E III, Bast RC, Kufe DW, Pollock RE, Weichselbaum RR, editors. Hamilton Ontario Canada: PC Decker Inc; 2002. Tumor Angiogenesis. [Google Scholar]
- 10.Yokoyama Y, Charnock-Jones DS, Licence D, Yanaihara A, Hastings JM, Holland CM, Emoto M, Sakamoto A, Sakamoto T, Maruyama H, Sato S, Mizunuma H, Smith SK. Expression of vascular endothelial growth factor (VEGF)-D and its receptor, VEGF receptor 3, as a prognostic factor in endometrial carcinoma. Clin Cancer Res. 2003;9(4):1361–1369. [PubMed] [Google Scholar]
- 11.Liu M, Cai L, Li Q, Chen X, Gao L, Jiang L. The Expression of VEGF and CD31 in Endometrial Lesions and Its Associations with Blood Flow Parameters of Transvaginal 3D Power Doppler Ultrasonography: A Preliminary Study. Cancer Manag Res. 2020;12:11211–11218. doi: 10.2147/CMAR.S277274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Critchley HO, Brenner RM, Henderson TA, Williams K, Nayak NR, Slayden OD, Millar MR, Saunders PT. Estrogen receptor beta, but not estrogen receptor alpha, is present in the vascular endothelium of the human and nonhuman primate endometrium. J Clin Endocrinol Metab. 2001;86(3):1370–1378. doi: 10.1210/jcem.86.3.7317. [DOI] [PubMed] [Google Scholar]
- 13.Ribatti D. The contribution of Harold F. Dvorak to the study of tumor angiogenesis and stroma generation mechanisms. Endothelium. 2007;14(3):131–135. doi: 10.1080/10623320701421651. [DOI] [PubMed] [Google Scholar]
- 14.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219(4587):983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 15.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 16.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 17.Smith SK. Angiogenesis, vascular endothelial growth factor and the endometrium. Hum Reprod Update. 1998;4(5):509–519. doi: 10.1093/humupd/4.5.509. [DOI] [PubMed] [Google Scholar]
- 18.Zinovkin DA, Achinovich SL, Zubritskiy MG, Whatmore JL, Pranjol MZI. High Expression of Galectin-1, VEGF and Increased Microvessel Density Are Associated with MELF Pattern in Stage I-III Endometrioid Endometrial Adenocarcinoma. J Pathol Transl Med. 2019;53(5):280–288. doi: 10.4132/jptm.2019.05.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li YL, Zhao H, Ren XB. Relationship of VEGF/VEGFR with immune and cancer cells: staggering or forward. Cancer Biol Med. 2016;13(2):206–214. doi: 10.20892/j.issn.2095-3941.2015.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Topolovec Z, Corusić A, Babić D, Mrcela M, Sijanović S, Müller-Vranjes A, Curzik D. Vascular endothelial growth factor and intratumoral microvessel density as prognostic factors in endometrial cancer. Coll Antropol. 2010;34(2):447–453. [PubMed] [Google Scholar]
- 21.Soufla G, Sifakis S, Porichis F, Spandidos DA. Prognostic value of tgfb1 protein in endometrioid adenocarcinoma. Eur J Clin Invest. 2013;43(1):79–90. doi: 10.1111/eci.12017. [DOI] [PubMed] [Google Scholar]
- 22.Saito M, Sato Y, Watanabe J, Kuramoto H, Kaba S, Fukuda T. Angiogenic factors in normal endometrium and endometrial adenocarcinoma. Pathol Int. 2007;57(3):140–147. doi: 10.1111/j.1440-1827.2006.02071.x. [DOI] [PubMed] [Google Scholar]
- 23.Dobrzycka B, Terlikowski SJ, Kwiatkowski M, Garbowicz M, Kinalski M, Chyczewski L. Prognostic significance of VEGF and its receptors in endometrioid endometrial cancer. Ginekol Pol. 2010;81(6):422–425. [PubMed] [Google Scholar]
- 24.Laxmanan S, Robertson SW, Wang E, Lau JS, Briscoe DM, Mukhopadhyay D. Vascular endothelial growth factor impairs the functional ability of dendritic cells through Id pathways. Biochem Biophys Res Commun. 2005;334(1):193–198. doi: 10.1016/j.bbrc.2005.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu H, Sun X, Sun WJ. Expression and clinical correlation of NGAL and VEGF in endometrial carcinoma. Eur Rev Med Pharmacol Sci. 2018;22(3):632–636. doi: 10.26355/eurrev_201802_14287. [DOI] [PubMed] [Google Scholar]
- 26.Sallinen H, Heikura T, Koponen J, Kosma VM, Heinonen S, Ylä-Herttuala S, Anttila M. Serum angiopoietin-2 and soluble VEGFR-2 levels predict malignancy of ovarian neoplasm and poor prognosis in epithelial ovarian cancer. BMC Cancer. 2014;14:696–696. doi: 10.1186/1471-2407-14-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Odeh M, Vainerovsky I, Grinin V, Kais M, Ophir E, Bornstein J. Three-dimensional endometrial volume and 3-dimensional power Doppler analysis in predicting endometrial carcinoma and hyperplasia. Gynecol Oncol. 2007;106(2):348–353. doi: 10.1016/j.ygyno.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 28.Holland CM, Day K, Evans A, Smith SK. Expression of the VEGF and angiopoietin genes in endometrial atypical hyperplasia and endometrial cancer. Br J Cancer. 2003;89(5):891–898. doi: 10.1038/sj.bjc.6601194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olson T, Mohanraj D, Ramakrishnan S. In vivo neutralization of vascular endothelial growth factor (VEGF) vascular permeability factor (VPF) inhibits ovarian carcinoma-associated ascites formation and tumor growth. Int J Oncol. 1996;8(3):505–511. doi: 10.3892/ijo.8.3.505. [DOI] [PubMed] [Google Scholar]
- 30.Sanseverino F, Santopietro R, Torricelli M, D'Andrilli G, Russo G, Cevenini G, Bovicelli A, Leoncini L, Scambia G, Petraglia F, Claudio PP, Giordano A. pRb2/p130 and VEGF expression in endometrial carcinoma in relation to angiogenesis and histopathologic tumor grade. Cancer Biol Ther. 2006;5(1):84–88. doi: 10.4161/cbt.5.1.2345. [DOI] [PubMed] [Google Scholar]
- 31.El Anwar NM, Amer AI. OCT4, Ki-67 and VEGF as Prognostic Factors in Endometrial Carcinoma and Their Role in The Differentiation between Atypical Endometrial Hyperplasia and Grade 1 Endometrial Carcinoma: an Immunohistochemical study. Int J Cancer Biomed Res. 2021;5(1):143–154. [Google Scholar]
- 32.Ali SH, O'Donnell AL, Balu D, Pohl MB, Seyler MJ, Mohamed S, Mousa S, Dandona P. Estrogen receptor-alpha in the inhibition of cancer growth and angiogenesis. Cancer Res. 2000;60(24):7094–7098. [PubMed] [Google Scholar]
- 33.Fujimoto J, Sakaguchi H, Hirose R, Ichigo S, Tamaya T. Progestins suppress estrogen-induced expression of vascular endothelial growth factor (VEGF) subtypes in uterine endometrial cancer cells. Cancer Lett. 1999;141(1-2):63–71. doi: 10.1016/s0304-3835(99)00073-7. [DOI] [PubMed] [Google Scholar]
- 34.Fujimoto J, Ichigo S, Hirose R, Sakaguchi H, Tamaya T. Expressions of vascular endothelial growth factor (VEGF) and its mRNA in uterine endometrial cancers. Cancer Lett. 1998;134(1):15–22. doi: 10.1016/s0304-3835(98)00232-8. [DOI] [PubMed] [Google Scholar]
- 35.Giatromanolaki A, Sivridis E, Brekken R, Thorpe PE, Anastasiadis P, Gatter KC, Harris AL, Koukourakis MI, Tumour and Angiogenesis Research Group The angiogenic "vascular endothelial growth factor/flk-1(KDR) receptor" pathway in patients with endometrial carcinoma: prognostic and therapeutic implications. Cancer. 2001;92(10):2569–2577. doi: 10.1002/1097-0142(20011115)92:10<2569::aid-cncr1609>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 36.Sunita BS, Sen A, Suhag V. To evaluate immunoreactivity of cyclooxygenase-2 in cases of endometrial carcinoma and correlate it with expression of p53 and vascular endothelial growth factor. J Cancer Res Ther. 2018;14(6):1366–1372. doi: 10.4103/0973-1482.202890. [DOI] [PubMed] [Google Scholar]