Abstract
To determine whether trophozoites and lysates of pathogenic Acanthamoeba spp. induce apoptosis in primary-culture microglial cells, transmission electron microscopic (TEM) examinations, assessment of DNA fragmentation by agarose gel electrophoresis, and the TdT-mediated dUTP nick-end labeling assay were performed. When a trophozoite of pathogenic Acanthamoeba culbertsoni came in contact with a microglial cell, the digipodium was observed by TEM. Nuclear chromatin condensation was observed in 10% of microglial cells, while it was not revealed when they were cocultured with weakly pathogenic Acanthamoeba royreba trophozoites. DNA fragmentation in microglial cells cocultured with the A. culbertsoni lysate was detected by electrophoresis, showing DNA ladder formation, whereas it was hardly observed in microglial cells cocultured with A. royreba. DNA fragmentation of microglial cells was also confirmed by flow cytometry analysis. The fluorescence of TdT-stained apoptotic bodies became intensely visible with microglial cells cocultured with the A. culbertsoni lysate. In contrast, with microglial cells cocultured with the A. royreba lysate, only a background level of fluorescence of TdT-stained apoptotic bodies was detected. These results suggest that some rat microglial cells cocultured with pathogenic A. culbertsoni undergo cytopathic changes which show the characteristics of the apoptotic process, such as nuclear condensation and DNA fragmentation.
Acanthamoeba spp., free-living small limax amoebae, inhabit natural environments, such as soil, ponds, sewage, and air. Acanthamoeba culbertsoni causes chronic granulomatous meningoencephalitis (GME), and Acanthamoeba polyphaga and Acanthamoeba castellanii are causative agents of acanthamoebic keratitis (6, 17, 21, 24). In order to elucidate the pathogenicity of Acanthamoeba spp., experimental development of GME and study of its cytopathic effects (CPE) against target cells have been done with mice. A virulent amoeba which causes GME in mice is toxic for target cells (4, 14, 16). Recent studies have focused on the characterization of the CPE of A. castellanii with various established cell lines. Regarding the process of the penetration of A. castellanii into human corneal epithelium, it was suggested that cytolytic enzymes were released from trophozoites and subsequent phagocytosis was accomplished (11). Taylor et al. (20) demonstrated that the CPE caused by A. castellanii involve cytoskeletal elements which are necessary for phagocytosis, amoeba motility, and the formation of amoebastomes and pseudopodia. Alizadeh et al. (1) demonstrated that apoptosis was a mechanism in the cytolysis of murine neuroblastoma cells caused by A. castellanii and characterized by cell shrinkage, cell membrane blebbing, formation of apoptotic bodies, and nuclear condensation. Later, apoptosis was confirmed as a mechanism of CPE due to Acanthamoeba spp. in rat neuroblastoma cells (13).
In previous studies, established cell lines, such as rat neuroblastoma cells and corneal epithelial cells, were examined as target cells. Recently, the culture system of microglial cells, a kind of cell found in the brain and throughout the central nervous system (CNS), from rat and mouse became available, and the attempt to understand the pathogenicity of microorganisms against these cells was undertaken by several researchers (5, 22). Microglial cells originate from the monocyte/macrophage lineage (9) and are phenotypically identical to monocytes/macrophages (12). Microglial cells function as phagocytic cells and produce cytokines, such as interleukin-1, interleukin-6, and tumor necrosis factor alpha (3, 15). They have an amoeboid form during embryogenesis, a ramified shape in the mature normal brain, and a rod shape around inflammatory lesions in the CNS (18). Thus, it was suggested that microglial cells are involved in the protective immune response of the CNS, functioning as inflammatory or immunoregulatory cells (19).
The rationale for the present study was the possibility that microglial cells are involved in the development of GME due to infection by pathogenic Acanthamoeba and undergo in vitro cytopathic processes. The purpose of the present study was to determine whether primary-culture rat microglial cells show apoptosis induced by pathogenic Acanthamoeba trophozoites and lysates. In the present study, we compared the CPE of a high-virulence strain of Acanthamoeba with those of a very-low-virulence one.
A high-virulence strain of A. culbertsoni and a very-low-virulence strain of Acanthamoeba royreba (donated from J. B. Jardin of Belgium in 1977) were axenically cultured at 37°C in medium containing proteose peptone, yeast extract, and glucose (23). The degrees of virulence of the two Acanthamoeba spp. were described in a previous paper (7). An Acanthamoeba lysate was prepared by a previously described method, the so-called freezing-thawing method (7). The amoeba lysate was filtered with 0.22-μm-pore-size disk filters, and the protein concentration (adjusted to 10 mg/ml) was determined by the Bradford assay (2).
Microglial cells were prepared by the method of Guilian and Baker (5), with some modifications. Briefly, brain cortex cells were obtained from newborn rats (Sprague-Dawley, purchased from KIST in Daejeon, Korea) and homogenized by pumping with a 21-gauge syringe. The mixture was centrifuged at 300 × g for 10 min and resuspended in Eagle's minimal essential medium (EMEM) with 10% fetal bovine serum. The suspension was put into 75-cm3 tissue culture flasks pretreated with polylysine (Sigma Chemical Co.), in order to increase the adherence of cells, and was incubated at 37°C with 5% CO2 for 1 week. Microglial cells were harvested by vigorously flicking each culture flask, filtrated with nylon wool in order to remove any remaining astrocytes, and centrifuged at 300 × g for 10 min. The pellet was resuspended in EMEM with 10% fetal bovine serum and incubated at 37°C for 2 h. The supernatant was removed. Attached cells, mostly microglial cells, were harvested and counted at a concentration of 105 per well in a 24-well culture plate and 3 × 106 in a 75-cm3 tissue culture flask for the treatment of Acanthamoeba trophozoites or lysates.
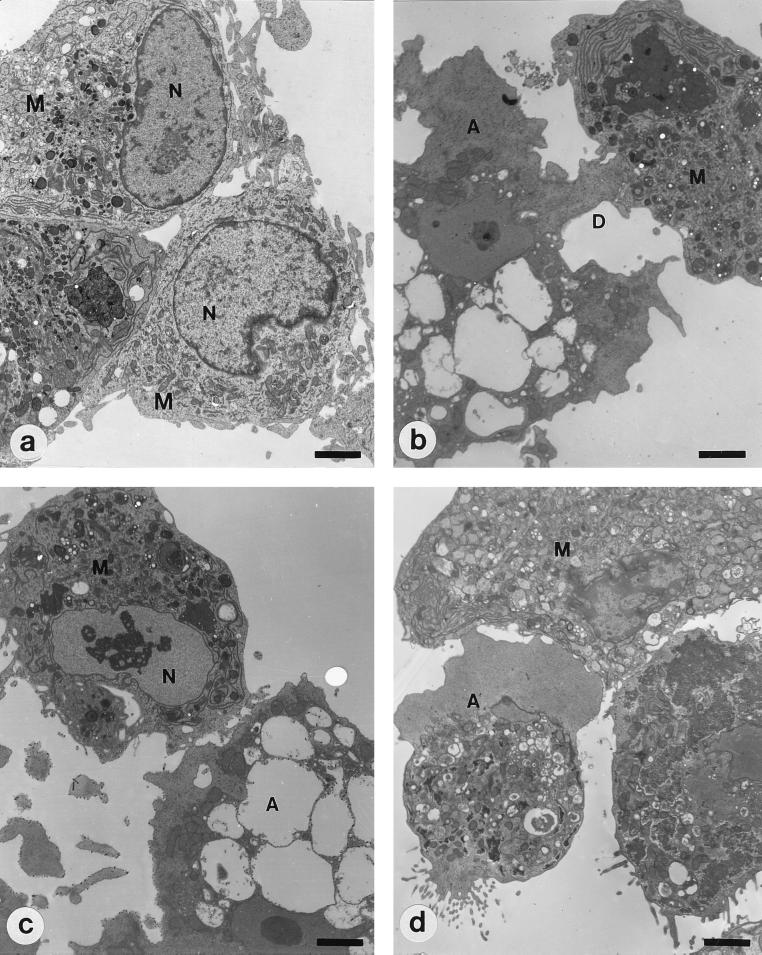
Transmission electron microscopy (TEM) was performed to observe the morphological changes of microglial cells that were cocultured with trophozoites of A. culbertsoni or A. royreba for 6 h. TEM was carried out according to the standard methods. The blocks were sectioned using a Reichert-Jung Ultracut S and stained with Ultrostain 1H and 2 (Leica, Vienna, Austria). Specimens were observed and photographed under a Zeiss EM 902 A electron microscope (Leo, Oberkohen, Germany). About 30 microglial cells per experimental group were observed by TEM. We observed the digipodium-like process, when a trophozoite of A. culbertsoni came in contact with a microglial cell (Fig. 1), by TEM. Nuclear chromatin condensations, evidence of apoptosis, were observed with three microglial cells, although they did not appear marginally (Fig. 1). In contrast, when microglial cells were cocultured with trophozoites of A. royreba, their morphology was almost normal, and they did not show nuclear condensation even though a trophozoite showed the vigorous pseudopodium process (Fig. 1).
FIG. 1.
TEM of microglia (M) and Acanthamoeba (A). (a) Primary cultured rat microglial cells show numerous pseudopodia and large nuclei (N) containing marginally scattered chromatin materials. (b and c) Microglial cells cocultured with A. culbertsoni trophozoites for 6 h. A trophozoite of A. culbertsoni produces a digipodium (D). At that time, condensed chromatin materials are visible in the nucleus of a microglial cell. (d) Microglial cell cocultured with A. royreba trophozoites for 6 h. Although the amoeba attached to the microglial cell with a vigorous pseudopodium, chromatin clumping was not observed in the nucleus of the microglial cell. Bars, 2.5 μm.
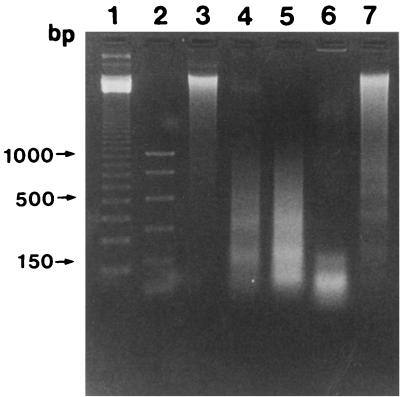
DNA extractions and agarose gel electrophoresis were performed to observe DNA fragmentation of microglial cells cocultured with lysates of A. culbertsoni and A. royreba. Microglial cells were harvested by sterile cell scrapers after cultivation with an amoeba lysate (1 mg/ml) for 6 or 18 h. Then, microglial cells were washed with phosphate-buffered saline (PBS) (pH 7.4) and suspended in 0.5 ml of TBE buffer (45 mM Tris-borate buffer, 1 mM EDTA [pH 8.0]) containing 0.25% Nonidet P-40 and 1 mg of RNase A per ml. After the mixture was incubated at 37°C for 30 min, 1 mg of proteinase K per ml was added. The mixture was incubated at 37°C for 30 min and resuspended in 0.1 ml of loading buffer (0.25% bromophenol blue, 0.25% xylene cyanol FF, 30% glycerol). The suspended volume of 25 μl was put on 1.5% agarose gel containing 10 ml of ethidium bromide per ml. Electrophoresis was carried out at 2 V/cm for 6 h. A 123-bp DNA ladder and PCR marker containing fragments of 1,000, 750, 500, 300, 150, and 50 bp (Promega Corporation, Madison, Wis.) were used as molecular size standards. DNA fragmentation was not observed with total genomic DNA of microglial cells treated with PBS, but the development of DNA ladders was shown in microglial cells cocultured with the A. culbertsoni lysate (1 mg/ml) for 3, 6, and 18 h, respectively (Fig. 2). As expected, DNA fragmentation was not observed with microglial cells cocultured with the A. royreba lysate for 3 and 6 h (data not shown), but it was visible as very faint DNA ladders after 18 h of incubation.
FIG. 2.
Electrophoresis for DNA fragmentation of microglial cells cocultured with lysates of A. culbertsoni and A. royreba. Lanes 1 and 2, standard marker; lane 3, intact genomic DNA of microglial cells; lanes 4, 5, and 6, DNA fragmentation of microglial cells cocultured with the A. culbertsoni lysate for 3, 6, and 18 h, respectively; lane 7, genomic DNA of microglial cells cocultured with the A. royreba lysate for 18 h.
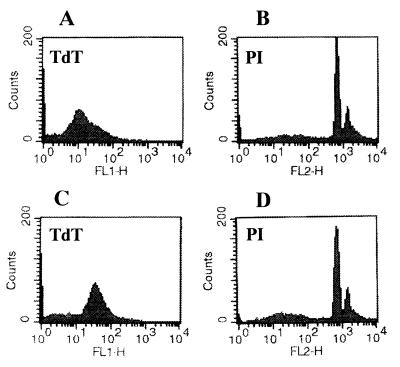
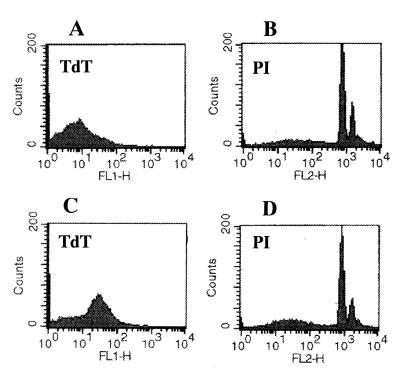
The fragmented DNAs of apoptotic bodies from microglial cells cocultured with the lysates of A. culbertsoni or A. royreba were measured with the TdT-mediated dUTP nick end labeling assay (Promega Corporation). Briefly, microglial cells cocultured for 6 or 18 h with 1 mg of each amoeba lysate per ml were harvested and washed twice with PBS (pH 7.4). After centrifugation at 300 × g at 4°C, the cells were resuspended in 0.5 ml of PBS. The cells were fixed by adding 5 ml of 1% methanol-free formaldehyde for 20 min on ice, centrifuged, and resuspended in 0.5 ml of PBS. The cell suspension was mixed with 5 ml of 70% ice-cold ethanol and kept at −20°C for 4 h. The mixture was centrifuged and resuspended in 1 ml of PBS. The suspended cells were adjusted to a concentration of 2 × 106 and transferred into a 1.5-ml microcentrifuge tube. After centrifugation, cells were resuspended in 80 μl of equilibration buffer (200 mM potassium cacodylate, 24 mM Tris-HCl, 0.2 mM dithiothreitol, 0.25 mg of bovine serum albumin/ml, 2.5 mM cobalt chloride [pH 6.6]). The buffer was removed by centrifugation, and then the cells were resuspended in 50 μl of TdT incubation buffer (45 μl of equilibration buffer, 5 μl of a nucleotide mixture, 1 μl of TdT enzyme). The nucleotide mixture used for TdT incubation buffer consisted of 50 μM fluorescein-12-dUTP, 100 μM dATP, 10 mM Tris-HCl (pH 7.6), and 1 mM EDTA. The suspended cells were incubated in a water bath for 60 min at 37°C. The reaction was terminated by adding 1 ml of 20 mM EDTA. Following centrifugation after the reaction, the pelleted cells were resuspended in 0.5 ml of PBS containing Triton X-100 and 5 mg of bovine serum albumin/ml. The cells were washed twice with PBS, centrifuged, and resuspended in 0.5 ml of propidium iodide (PI) solution (freshly diluted to 5 μg/ml in PBS) containing 250 μg of DNase-free RNase A. After the cells were incubated for 30 min in the dark, the green fluorescence of fluorescein-12-dUTP at 520 nm and the red fluorescence of PI at 620 nm were measured by FACScan flow cytometry (Becton Dickinson, Paramus, N.J.). PI staining demonstrated that some microglial cells cocultured for 6 h with the A. culbertsoni lysate (1 mg/ml) underwent apoptosis. This was confirmed in TdT staining, which showed increased intensity of fluorescence of the TdT-stained apoptotic bodies (Fig. 3). After 18 h of incubation, the same result was observed (Fig. 4). By contrast, microglial cells cocultured for 18 h with the A. royreba lysate did not show apparent changes in the intensity of fluorescence of the TdT-stained apoptotic cells, although the intensity of PI staining was weakly increased (Fig. 5).
FIG. 3.
Histograms of FACS analysis of microglial cells stained with TdT and PI. (A and B) Microglial cells were cultured on EMEM with PBS as a control group. (C and D) Microglial cells were cocultured with the A. culbertsoni lysate for 6 h.
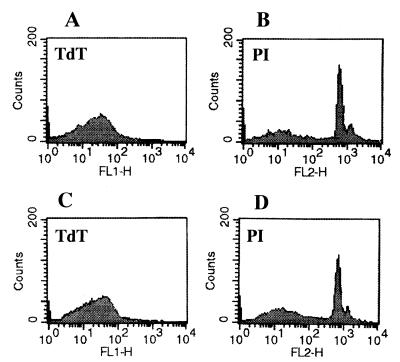
FIG. 4.
Histograms of FACS analysis of microglial cells stained with TdT and PI. (A and B) Microglial cells were cultured in EMEM with PBS as a control group. (C and D) Microglial cells were cocultured with the A. culbertsoni lysate for 18 h.
FIG. 5.
Histograms of FACS analysis of microglial cells stained with TdT and PI. (A and B) Microglial cells were cultured in EMEM with PBS as a control group. (C and D) Microglial cells were cocultured with the A. royreba lysate for 18 h.
It is well known that pathogenic Acanthamoeba spp. have CPE on various cells in vitro and in vivo. With the destruction of rat neuroblastoma cells by Acanthamoeba spp., the extension of a digipodium to target cells was observed after effector cells came in contact with target cells (13). A kind of contact-dependent cell lysis referred to as troglocytosis was also observed as a mechanism of the CPE by Naegleria fowleri, another pathogenic free-living amoeba (10). In this study, microglial cells, which may be concerned with protective activities during infection by Acanthamoeba, were cultured from newborn rat brain and used as the target cells. When a microglial cell came in contact with a trophozoite of A. culbertsoni, known as a high-virulence strain, the digipodium was shown, but not with the very weakly pathogenic A. royreba. Thus, the formation of a digipodium should be considered apparent evidence of the CPE of pathogenic Acanthamoeba.
Recently, necrosis via pore-forming lytic molecules and apoptosis, both of which disrupt cell membrane integrity, were found to be two fundamental mechanisms in the cytolysis of target cells by pathogenic free-living amoebae (1, 9, 13). In contrast to necrosis, apoptosis is characterized by various morphological features, such as cell shrinkage, cell membrane blebbing, the formation of apoptotic bodies, nuclear chromatin condensation, and DNA fragmentation. In previous studies, membrane blebbing of rat neuroblastoma cells cocultured with the pathogenic A. culbertsoni, A. castellanii, and A. polyphaga was observed by TEM and scanning electron microscopy and was not observed with nonpathogenic Acanthamoeba astronyxis (13). Otherwise, ladder formation of genomic DNA and apoptosis due to DNA fragmentation were observed by electrophoresis and flow cytometry analysis with PI-stained rat neuroblastoma cells that were cocultured with the A. castellanii trophozoites and lysate (1). Cell membrane blebbing and DNA fragmentation of target cells following exposure to Acanthamoeba cell extracts was observed with more than 70% of tumor cells. In contrast, only 7% of untreated control cells underwent DNA fragmentation. In our studies, nuclear condensation and DNA fragmentation were observed with about 10% of microglial cells cocultured with the pathogenic A. culbertsoni, although cell membrane blebbing was not clearly observed by TEM. With microglial cells cocultured with the weakly pathogenic A. royreba, nuclear condensation was not observed, but faint DNA ladders were observed only after 18 h of cultivation.
To confirm our observations that microglial cell death occurs by means of apoptosis, the TdT-mediated dUTP nick end labeling assay was performed. With untreated microglial cells, fragmented DNAs of apoptotic bodies were also observed. This phenomenon may be the result of the natural apoptotic process of cells. Nevertheless, when microglial cells were cocultured with the pathogenic A. culbertsoni lysate, the visual intensity of TdT-stained apoptotic bodies was remarkedly enhanced. On the other hand, with the A. royreba lysate, only slightly increased intensity was observed. Taken together, our results suggest that the CPE of Acanthamoeba, including the apoptotic process, vary depending on the degree of virulence, which was demonstrated by a previous study (13).
Finally, the results of this study demonstrate that a trophozoite of A. culbertsoni in contact with microglial cells produces a digipodium and that 10% of microglial cells show nuclear chromatin condensation. Some microglial cells cocultured with the pathogenic A. culbertsoni lysate underwent, in part, apoptotic processes, followed by DNA fragmentation. These findings are regarded as evidence of apoptosis of microglial cells that is induced by pathogenic Acanthamoeba and may provide important information for understanding the interaction of pathogenic Acanthamoeba with nerve cells in the development of GME.
Acknowledgments
We thank Hee-Sun Lee (Institute of Clinical Medicine, College of Medicine, University of Yonsei) for fluorescence-activated cell sorter (FACS) analyses.
This work was supported by a research grant of the Korea Science and Engineering Foundation (grant no. 981-0701-001-1).
REFERENCES
- 1.Alizadeh H, Pidherney M S, McCulley J P, Niederkorn J Y. Apoptosis as a mechanism of cytolysis of tumor cells by a pathogenic free-living amoeba. Infect Immun. 1994;62:1298–1303. doi: 10.1128/iai.62.4.1298-1303.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Chao C C, Hu S, Close K, Choi C S, Molitor T W, Novick W J, Peterson P K. Cytokine release from microglia: differential inhibition by pentoxifylline and dexamethasone. J Infect Dis. 1992;166:847–853. doi: 10.1093/infdis/166.4.847. [DOI] [PubMed] [Google Scholar]
- 4.Culbertson C G. The pathogenicity of soil amebas. Annu Rev Microbiol. 1971;25:231–254. doi: 10.1146/annurev.mi.25.100171.001311. [DOI] [PubMed] [Google Scholar]
- 5.Giulian D, Baker T J. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Im K I, Kim D S. Acanthamoebiasis in Korea: two new cases with clinical cases review. Yonsei Med J. 1998;39:478–484. doi: 10.3349/ymj.1998.39.5.478. [DOI] [PubMed] [Google Scholar]
- 7.Im K I, Shin H J, Seo D W, Jeon S H, Kim T E. Pathogenicity of Korean isolates of Acanthamoeba by observing the experimental infection and zymodemes of five isoenzymes. Korean J Parasitol. 1999;37:85–92. doi: 10.3347/kjp.1999.37.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ling E A. The origin and nature of microglia. In: Federoff S, Hertz L, editors. Advances in cellular neurobiology. New York, N.Y: Academic Press; 1981. pp. 33–82. [Google Scholar]
- 9.Lowrey D M, McLaughlin J. Activation of a heat-stable cytolytic protein associated with the surface membrane of Naegleria fowleri. Infect Immun. 1985;50:478–482. doi: 10.1128/iai.50.2.478-482.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marciano-Cabral F M, Zoghby K L, Bradley S G. Cytopathic action of Naegleria fowleri on rat neuroblastoma target cells. J Protozool. 1990;37:138–144. doi: 10.1111/j.1550-7408.1990.tb05884.x. [DOI] [PubMed] [Google Scholar]
- 11.Moore M B, Ubelaker J E, Martin J H, Silvany R, Dougherty J M, Meyer D R, McCulley J P. In vitro penetration of human corneal epithelium by Acanthameoba castellanii: a scanning and transmission electron microscopy study. Cornea. 1991;10:291–298. doi: 10.1097/00003226-199107000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Perry V H, Gordon S. Macrophages and microglia in the nervous system. Trends Neurosci. 1988;11:273–277. doi: 10.1016/0166-2236(88)90110-5. [DOI] [PubMed] [Google Scholar]
- 13.Pettit D A, Williamson J, Cabral G A, Marciano-Cabral F. In vitro destruction of nerve cell cultures by Acanthamoeba spp.: a transmission and scanning electron microscopy study. J Parasitol. 1996;82:769–777. [PubMed] [Google Scholar]
- 14.Pidherney M S, Alizadeh H, Stewart G L, McCulley J P, Niederkorn J Y. In vitro and in vivo tumorcidal properties of a pathogenic/free-living amoeba. Cancer Lett. 1993;72:91–98. doi: 10.1016/0304-3835(93)90016-3. [DOI] [PubMed] [Google Scholar]
- 15.Sawada M, Kondo N, Suzumura A, Marunouchi T. Production of tumor necrosis factor-alpha by microglia and astrocytes in culture. Brain Res. 1989;491:394–397. doi: 10.1016/0006-8993(89)90078-4. [DOI] [PubMed] [Google Scholar]
- 16.Shin H J, Ra M S, Im K I. Cytotoxicity of Acanthamoeba sp. YM-4 (Korean isolate) Yonsei Rep Trop Med. 1993;24:31–38. [Google Scholar]
- 17.Stehr-Green J K, Bailey T M, Visvesvara G S. The epidemiology of Acanthamoeba keratitis in the United States. Am J Ophthalmol. 1989;107:331–336. doi: 10.1016/0002-9394(89)90654-5. [DOI] [PubMed] [Google Scholar]
- 18.Suzumura A, Marrunouchi T, Yamamoto H. Morphological transformation of microglia in vitro. Brain Res. 1991;545:301–306. doi: 10.1016/0006-8993(91)91302-h. [DOI] [PubMed] [Google Scholar]
- 19.Suzumura A, Sawada M, Yamamoto H, Marunouchi T. Transforming growth factor-β suppresses activation and proliferation of microglia in vitro. J Immunol. 1993;151:2150–2158. [PubMed] [Google Scholar]
- 20.Taylor W M, Pidherney M S, Alizadeh H, Niederkorn Y J. In vitro characterization of Acanthamoeba castellanii cytopathic effect. J Parasitol. 1995;81:603–609. [PubMed] [Google Scholar]
- 21.van Klink F, Alizadeh H, Stewart G L, Pidherney M S, Silvany R E, He Y G, McCulley J P, Niederkorn J Y. Characterization and pathogenic potential of a soil isolate and an ocular isolate of Acanthamoeba castellanii in relation to Acanthamoeba keratitis. Curr Eye Res. 1992;12:1207–1220. doi: 10.3109/02713689208999546. [DOI] [PubMed] [Google Scholar]
- 22.Vela J M, Dalmau I, González B, Castellano B. Morphology and distribution of microglia cells in the young and adult mouse cerebellum. J Comp Neurol. 1995;361:602–616. doi: 10.1002/cne.903610405. [DOI] [PubMed] [Google Scholar]
- 23.Visvesvara G S, Balamuth W. Comparative studies on related free-living and pathogenic amoebae with special reference to Acanthamoeba. J Protozool. 1975;22:245–256. doi: 10.1111/j.1550-7408.1975.tb05860.x. [DOI] [PubMed] [Google Scholar]
- 24.Visvesvara G S, Stehr-Green J K. Epidemiology of free-living amoeba infections. J Protozool. 1990;37(Suppl.):25–33. doi: 10.1111/j.1550-7408.1990.tb01142.x. [DOI] [PubMed] [Google Scholar]