ABSTRACT
Electronic cigarettes (ECs) are purported to be tobacco harm-reduction products whose degree of harm has been highly debated. EC use is considered less hazardous than smoking but is not expected to be harmless. Following the banning of e-liquid flavors in countries such as the US, Finland, Ukraine, and Hungary, there are growing concerns regarding the safety profile of e-liquid flavors used in ECs. While these are employed extensively in the food industry and are generally regarded as safe (GRAS) when ingested, GRAS status after inhalation is unclear. The aim of this review was to assess evidence from 38 reports on the adverse effects of flavored e-liquids on the respiratory system in both in vitro and in vivo studies published between 2006 and 2021. Data collected demonstrated greater detrimental effects in vitro with cinnamon (9 articles), strawberry (5 articles), and menthol (10 articles), flavors than other flavors. The most reported effects among these investigations were perturbations of pro-inflammatory biomarkers and enhanced cytotoxicity. There is sufficient evidence to support the toxicological impacts of diacetyl- and cinnamaldehyde-containing e-liquids following human inhalation; however, safety profiles on other flavors are elusive. The latter may result from inconsistencies between experimental approaches and uncertainties due to the contributions from other e-liquid constituents. Further, the relevance of the concentration ranges to human exposure levels is uncertain. Evidence indicates that an adequately controlled and consistent, systematic toxicological investigation of a broad spectrum of e-liquid flavors may be required at biologically relevant concentrations to better inform public health authorities on the risk assessment following exposure to EC flavor ingredients.
KEYWORDS: Electronic cigarette, e-liquid, flavors, lung, toxicity
Introduction
Tobacco smoking remains the leading cause of preventable deaths globally (World Health Organization 2019). Tobacco smoke contains approximately 7000 different chemicals, including nicotine (National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health 2014), of which 93 chemicals of concern are proposed to produce direct/ indirect harm through inhalation, ingestion, or absorption into the body (FDA 2012; Hoffmann and Hoffmann 1997). Some of these toxicants are responsible for the onset of life-threatening medical conditions that affect the cardiovascular (Frost-Pineda et al. 2011), respiratory (Buist, Vollmer, and McBurnie 2008; Vestbo et al. 2013), and digestive (Doll et al. 2005; El-Zayadi 2006) systems. Nicotine is responsible for tobacco dependence; thus, nicotine replacement therapies (NRT), void of most of the 93 chemicals of concern, are used as smoking cessation aids. It is noteworthy that addition smoking cessation aids also contain low levels of volatile organic compounds (VOCs) which may initiate adverse health effects (Kim, Kim, and Shin 2022). Further, traditional NRTs have protracted nicotine absorption profiles that take several min to reach peak plasma concentrations, creating an unpopular choice for cigarette smoking cessation aids. Alternatively, electronic cigarettes (ECs) or electronic nicotine delivery systems (ENDS) are battery-powered devices designed to vaporize a nicotine-containing solution (known as e-liquid) for a relatively rapid and efficient nicotine delivery system to the brain, which is more comparable to traditional cigarettes. E-liquids contain propylene glycol (PG) and/or vegetable glycerin (VG) and may contain nicotine and/or flavors. ECs, provide reinforcing sensory and behavioral cues in addition to nicotine, which (1) aids in alleviating withdrawal symptoms, (2) reduce nicotine craving, and (3) decreases relapse to smoking (Wadgave and Nagesh 2016).
Nicotine in e-liquids may exist in two distinctive forms: free-base nicotine or nicotine salts, and the state of one or the other is determined by the pH of the e-liquid (Duell, Pankow, and Peyton 2020). Interestingly, the pharmacokinetics of ECs with nicotine salts typically match the absorption profile of a traditional pyrogenic cigarette, thus making them the most utilized non-tobacco nicotine delivery systems in the UK (ASH 2021).
There are many variations of EC devices on the market which are characterized into 4 generations based upon the device’s structure, reusability, and voltage (Figure 1). First-generation ECs are disposable devices that resemble cigarettes. Certain types resemble pens, screwdrivers, or hookah tips, aiming to imitate the conventional way of smoking (Hiemstra and Bals 2016). Compared to the first generation, the second generation is slightly larger in shape with a higher voltage lithium battery, and comprised of a heating element called an atomizer used to heat e-liquids into vapor (i.e. aerosols) and a replaceable cartridge or tank to hold refill solutions (Talih et al. 2015). Third-generation EC devices are comparable to second-generation; however, these provide the user more freedom to customize components of the device (i.e., low resistance ‘sub-ohm’ heating coils) and adjust temperature and power. Low resistance, high power settings enable vapers to ‘cloud-chase’ – consuming greater volumes of e-liquid to generate higher amounts of aerosol. Finally, the fourth generation of ECs is referred to as pod mods, which resemble some of the first EC devices in shape; however, these items are refillable and rechargeable using a USB cable. These e-liquids predominantly contain nicotine salts permitting a quicker nicotine delivery to the brain.
Figure 1.
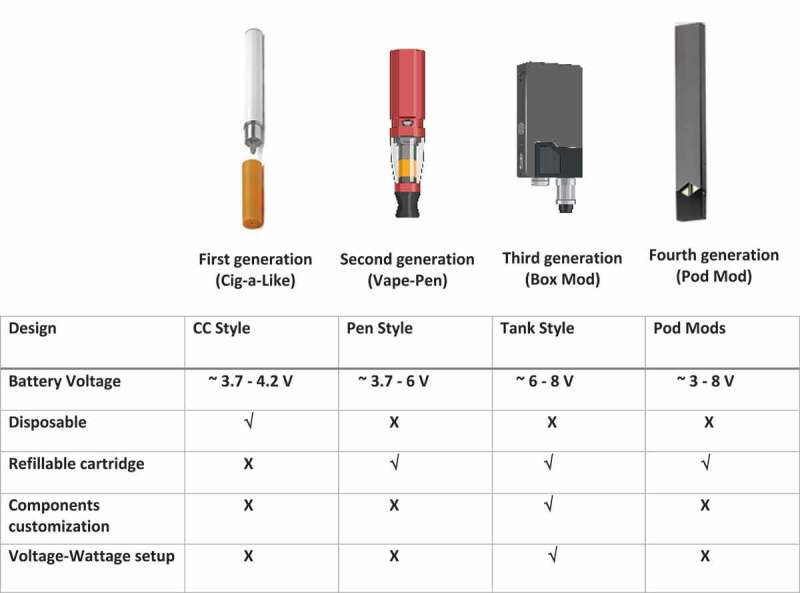
Different generations of EC devices and their main differences. The figure was produced with Biorender.com.
Electronic cigarette use, recent trends, effectiveness & potential hazards
In 2021, after 16 years of commercial availability, an estimated 3.6 million adults comprising ex-smokers (64.6%) or dual users (30.5%) and relatively few never-smokers (4.9%) were EC consumers in Great Britain (ASH 2021). An increase in the proportion of ex-smokers and a fall in current smokers using EC over time is thought to be due to the general perception that ECs are a safer alternative to tobacco smoking and therefore used as a smoking cessation aid (ASH 2020). A review commissioned by Public Health England of available evidence suggested that ECs are at least 95% less harmful to health than tobacco smoking (McNeill et al. 2018). Therefore, smokers who switched completely to EC-only use are expected to improve their long-term health compared with those that continue to smoke (McNeill et al. 2018).
Although ECs are considered less hazardous than cigarettes, it is less clear how harmful EC use as evidenced by oncogenicity (Bracken-Clarke et al. 2021) is compared with not using EC, especially in former smokers. EC users avoid or reduce exposure to most of the 93 toxicants in combusted tobacco smoke. It is considered that approximately 80% of cigarette smoke-induced cardiovascular toxicity is attributed to the first 1–3 cigarettes per day (Gallucci et al. 2020). This is likely in contrast to vaping because carbon monoxide (CO) and many other toxicants are not generated during vaping. However, in addition to nicotine, EC users are also exposed to PG, VG, flavor compounds, VOCs and both degradation and condensation byproducts formed during the heating of e-liquid to generate an inhalable aerosol (Kim, Kim, and Shin 2022; Schick et al. 2017). It appears that the composition of the e-liquid and repeated dosing or vaping “behavior” may be responsible for the observed toxicity. Burgwardt et al. (2020) reported that Valsalva vaping may enhance exposure to harmful constituents such as flavoring in the EC aerosol vaper. Valsalva vaping is achieved when EC users over-inhale and then forcefully exhale to eliminate the vapor, which is usually produced in greater quantities than that generated by a conventional cigarette. Concerns exist that EC use may be associated with the onset of various adverse health effects related to the cardiovascular (Bhatnagar 2016; Schweitzer, Wills, and Behner 2017) and lung diseases (Chun et al. 2017; Moazed and Calfee 2017). Data in animals suggest that short-term or long-term exposures to EC aerosols might result in cardiovascular remodeling driven by PG/VG vehicles, with nicotine potentially playing a protective role (Neczypor et al. 2022). Analyses of the variety and concentrations of EC aerosol toxicants infer that EC aerosol is less harmful than tobacco smoke (Azzopardi et al. 2016); however, the presence of lower concentrations of tobacco-related toxicants indicates these substances are not likely to be risk-free.
Several investigators reported the presence of hazardous carbonyl compounds such as formaldehyde, acetaldehyde, glyoxal, and acrolein in EC aerosol (Bekki et al. 2014; Etter, Zäther, and Svensson 2013; Ogunwale et al. 2017; Schober et al. 2014) and tobacco-specific N-nitrosamines (TSNA) in e-liquids and their aerosols (Farsalinos et al. 2015). Acetaldehyde and formaldehyde are recognized carcinogens (Feron et al. 1991; Roe and Wood 1992), and acrolein is a cardiovascular toxicant (Arman and İşisağ Üçüncü 2020; DeJarnett et al. 2014). Tobacco-specific N-nitrosamines are carcinogenic compounds formed from the curing and processing of tobacco leaves through nitrosation of nicotine and related tobacco alkaloids (Bush et al. 2001; Wang et al. 2017). Conflicting evidence exists from (Farsalinos et al. 2015; Goniewicz et al. 2014a; Kim and Shin 2013; Laugesen 2008; U.S. Food and Drug Administration 2009) to suggest that ECs are a source of TSNA exposures; however, it is physiologically possible that endogenous formation might originate from nicotine through nitrite exposure in the saliva (Knezevich et al. 2013), acidic stomach, or microbiome-mediated nitrosation at different sites (Ziebarth 1997). Traces of toxic metals such as cadmium, nickel, and lead originating from EC devices (Goniewicz et al. 2014a) are also found in the blood and urine of EC users (Aherrera et al. 2017; Badea et al. 2018; Goniewicz et al. 2018; Jain 2019;). Exposure to these metals is known to induce nasal and lung cancers and developmental defects (Hsueh et al. 2017; Jaishankar et al. 2014; Son 2020). Further, EC refills and aerosols contain volatile organic chemical compounds like benzene, toluene, xylene, and styrene (BTXS) (Schober et al. 2014), albeit at substantially lower concentrations than in tobacco smoke (Margham et al. 2016). Previous epidemiological studies found that individuals who are chronically exposed occupationally to BTXS (individuals or mixtures) exhibit a lower peak expiratory flow [PEF, litter per second (L/s)] and 25–75% of forced expiratory flow (FEF25∼75%, L/s) (Liao et al. 2022). In addition, occupational exposure to BTXS was linked to risks of prostate (Blanc-Lapierre, Sauvé, and Parent 2018) and lung cancer (Warden et al. 2018). In addition to this evidence, concerns over increasing utilization by young individuals and the potential for youth EC use to progress to tobacco smoking led to regulations restricting their use in many countries. In the UK alone, there has been a 3% rise in EC use among young people between the ages of 11–17 from 2020 to 2022 (ASH 2022). Although there are some regulations regarding the use of EC in the EU, the Tobacco Products Directive 2014/14/EU (TPD) introduced new restrictions on nicotine concentration and size of EC refill containers, the government needs to ensure existing laws are enforced and identify where regulations could be extended.
Electronic cigarette flavorings
The flavoring compounds used in EC e-liquids are generally regarded as safe (GRAS) for ingestion and employed extensively in the food industry. However, there are concerns regarding EC flavoring toxicity following inhalation using EC as evidence for safety following has not been extensively investigated (Farsalinos et al. 2013; Khlystov and Samburova 2016; Leigh et al. 2016; Sundar et al. 2016). In 2014, there were an estimated 8000 flavored e-liquids on the market, which has likely increased significantly (Zhu et al. 2014). Initially, the most popular EC flavors in the UK mimicked the flavors of tobacco products including cigarettes, cigars, or pipe tobacco and menthol (ASH 2021). However, preferences have shifted more recently to fruity flavors, and the popularity of other flavors such as desserts, candy or beverages has also risen (ASH 2021). Unfortunately, some product names and flavors provide little information regarding the flavor category such as unicorn blood, truth serum or snake oil let alone their likely constituents. The vast number of products and presence of hard-to-categorize product names led the Dutch National Institute for Public Health and the Environment (RIVM) to develop a flavor wheel (Krüsemann et al. 2019),which groups e-liquids into 13 flavor categories: candy, dessert, other sweets, fruit, alcohol, coffee/tea, other beverages, spices, nuts, menthol/mint, tobacco, and unflavored, based upon the chemical composition of the e-liquid. Table 1 presents the main flavoring chemicals in several flavors in e-liquids.
Table 1.
Flavors in e-liquid and some of the main flavoring chemicals they are composed of.
| Flavoring chemical | Common flavors with chemical agents | References |
|---|---|---|
| Cinnamaldehyde, 2-methoxycinnamaldehyde | Cinnamon | (Behar et al., 2014; Gerloff et al., 2017) |
| 3,4-Dimethoxybenzaldehyde | Cherry | (Kavvalakis et al., 2015) |
| 2,5-dimethylpyrazine | Chocolate | (Kavvalakis et al., 2015) |
| Maltol, ethyl maltol | Caramel, Vanilla | (Gerloff et al., 2017; Tierney et al.,2016) |
| Menthol, Menthone | Mint | (Berkelhamer et al., 2019) |
| p-Anisaldehyde | Anise | (Behar et al., 2018a; Berkelhamer et al., 2019; Noël et al. 2020b) |
| Benzaldehyde | Almond | (Behar et al., 2018a; Berkelhamer et al., 2019) |
| Vanillin, O-vanillin | Vanilla | (Behar et al., 2014; Gerloff et al., 2017; Muthumalage et al. 2018) |
| Hexyl acetate, 3-Hexen-1-ol |
Apple | (Kerasioti et al., 2020; Kim et al., 2018) |
| Isoamyl acetate | Banana | (Fetterman et al., 2018) |
| Methyl cinnamate | Balsamic, Strawberry | (Berkelhamer et al., 2019; Leigh et al.,2016) |
| Methyl anthranilate | Berry | (Tierney et al. 2016) |
| Other flavoring agents less known: Damascenone (α or β), 3-methyl-1,2- cyclopentanedione, acetamide, linalool, terpineol, citral, corylon, anisaldehyde, trimethylpyrazine, eugenol, benzaldehyde, limonene | Miscellaneous | (Gerloff et al., 2017; Tierney et al., 2016) |
Flavor additives in food may undergo enzymatic metabolism, which might produce fewer toxic metabolites due to phase I and II metabolism (Del Olmo, Calzada, and Nuñez 2017). In contrast, exposure to GRAS-like substances by inhalation after thermal degradation may produce pharmacologically active compounds that produce severe adverse health effects (Fedan et al. 2006). Rose (2017) reported that occupational diacetyl inhalation exposure might lead to bronchiolitis obliterans (BO), more commonly referred to as “popcorn lung.” Bronchiolitis obliterans induced severe coughing, wheezing, and shortness of breath, symptoms resembling chronic obstructive pulmonary disease (COPD) (Aguilar, Michelson, and Isakow 2016). van Rooy et al. (2007) conducted an epidemiological study in the Netherlands suggesting a causal link between BO and chemical workers producing diacetyl for food flavorings. The British Medical Association (BMA) believes flavors’ safety should be closely monitored as evidence for potential adverse effects are emerging after heating and inhalation of e-liquid aerosol (Sassano et al. 2018). The medicines and healthcare products regulatory agency (MHRA) collects UK reports of EC’s harmful effects and safety concerns through their yellow-card scheme (https://yellowcard.mhra.gov.uk/).
The EU TPD established a priority list of 15 potentially harmful additives (Havermans et al. 2022) that needs to not be contained in EC based upon the published literature. These include flavoring additives and chemicals such as guaiacol, geraniol, menthol, ethyl maltol, and diacetyl. Since 2016, diacetyl has also been banned from e-liquids in the UK, whereas flavors containing menthol and ethyl maltol are still available.
Flavors in e-liquids, as mentioned earlier, may contain pharmacologically active compounds (Clapp et al. 2019). Several investigators found that mint flavors contain menthol or menthone (Pereira et al. 2013), and candy flavors including vanillin and cinnamaldehyde (Krüsemann et al. 2019). Fruit flavors might contain 3-hexen-1-ol (apple) or methyl anthranilate (berry), and others (Tierney et al. 2016) (Table 1). Menthone is the ketone analog of menthol. Menthol (l-menthol) is a naturally-occurring agonist for the cold transient receptor potential melastatin 8 (TRPM8), which is functionally expressed in the airways (Sabnis et al. 2008). Due to the efficacy of menthol in inhibiting cigarette-induced cough via the TRPM8, it was added to tobacco as a ‘flavoring’ (Lin et al. 2018). Moreover, the masking of the harshness and irritancy of tobacco underpins the initiation of more intense and protracted smoking (Wickham 2020). This effect contributes to the inclusion of menthol in the EU TPD list of potentially harmful tobacco additives, yet there is a widespread use of menthol in e-liquids.
Vanillin and cinnamaldehyde are TRPV1 and TRPA1 agonists, respectively, which are also functional in the airways (Wang et al. 2019). Therefore, activation of TRPM8, TRPA1, and TRPV1 through EC usage may alter normal physiological responses of the airways. The effects of other chemical constituents of flavors, such as 3-hexen-1-ol and methyl anthranilate, warrant further investigation. Unsurprisingly, the largest category on the flavor wheel, given its popularity in the UK, is fruit, which the National Institute for Public Health and Environment (RIVM) in the Netherlands subcategorized into groups (tropical, citrus, berries, and others) in the flavor wheel (Krüsemann et al. 2019). Not explicitly covered by the flavor wheel, tobacco e-liquids may be sub-divided into those that extract flavor compounds directly from tobacco and those using synthetic flavorings to simulate tobacco taste.
There is little to no direct evidence of the toxicological effects of EC flavors in humans. To date, studies of EC flavor-induced are primarily in vitro studies in human cell lines, with a handful of investigations conducted in vivo. Therefore, this review aimed to systematically synthesize and critically evaluate published data on flavored e-liquids and their effects on the respiratory system to identify EC flavors and flavor chemicals most associated with adverse respiratory effects. A secondary aim was to investigate whether the outcomes observed in vitro are reflected by adverse effects reported in vivo. Finally, a potential ‘gold standard’ approach is proposed for future EC investigations regarding the experimental method, which may help reduce inter studies discrepancies. This is the first systematic review to focus specifically on the toxicity of EC flavors on the respiratory system. It may help researchers identify the essential EC flavors and chemicals that need to be the focus of further investigations, including risk assessments that may advise regulators on meaningful and appropriate e-liquid ingredient regulation.
We acknowledge that Stefaniak et al. (2021) published a systematic review with a search engine dating only 8 months prior to our search commencement. Although their review contained a comprehensive description of EC and of published evidence on flavored-e-liquid toxicity across different in vitro biological models and in vivo systems, our review focuses on the effects specifically on the pulmonary system. Although the Stefaniak et al. (2021) review summarized the published data, our aim was to be more critical and questioning of the published data, including 1) contributions of nicotine and inconsistencies across studies, 2) effect of vehicle/PG/VG, and lack of appropriate controls in many studies 3) potential additive effects of nicotine 4) although a mention of primary cells being more sensitive than immortalized cell lines was made, no differentiation between normal and cancer-derived cell lines was done 5) there was no discussion on the appropriate dose and minimal discussion on the dose-response relationship and improvement this gives to overcoming uncertainty 6) there were little interstudy comparisons 7) there was little explanation for differences in observations between air-liquid interface (ALI) and submerged cultures. It is appreciated that several of these points were raised in their knowledge gaps section, but a critique of the current evidence regarding these gaps was not provided. Therefore, a critical assessment of the literature was undertaken and where the results allow for a misleading interpretation of flavor toxicity, this has been discussed this in detail, which separates our approach significantly from that of Stefaniak et al. (2021).
Methods
Data sources and searches
A literature search was completed in November 2020, which was updated in November 2021, using Scopus and healthcare database advanced search (HDAS) (including CINAHL, EMBASE, MEDLINE, PsychINFO, PubMed. Retrieved articles on in vitro and in vivo studies were published between 2006 and 2021 Keywords included terms to capture concepts associated with e-cigarettes, flavors, and the respiratory system. The search stream here pasted was used for HDAS search – (((((electronic cigarette OR electronic nicotine delivery system OR electronic non-nicotine delivery system OR e cigarette OR e-cigarette OR e-cig OR e cig) AND (flavor OR flavor OR flavoring OR flavoring OR flavoring agents OR Flavoring agents OR Flavored OR Flavored)) AND (lung OR alveolar OR bronchial OR pulmonary)).title, abstract, key. Whereas the search stream below was used for our Scopus search – ((TITLE-ABS-KEY (“electronic cigarette”)) OR (TITLE-ABS-KEY (“electronic nicotine delivery system”)) OR (TITLE-ABS-KEY (“electronic (non) nicotine delivery system”)) OR (TITLE-ABS-KEY (“e cigarette”)) OR (TITLE-ABS-KEY (“e-cigarette”)) OR (TITLE-ABS-KEY (“e-cig”)) OR (TITLE-ABS-KEY (“e cig”))) AND ((TITLE-ABS-KEY (flavor)) OR (TITLE-ABS-KEY (flavor)) OR (TITLE-ABS-KEY (flavoring)) OR (TITLE-ABS-KEY (flavoring)) OR (TITLE-ABS-KEY (“Flavouring agents”)) OR (TITLE-ABS-KEY (“Flavoring agents”)) OR (TITLE-ABS-KEY (“Flavoured”)) OR (TITLE-ABS-KEY (“Flavored”)) AND (TITLE-ABS-KEY (lung)) OR (TITLE-ABS-KEY (alveolar))) AND ((TITLE-ABS-KEY (lung)) OR (TITLE-ABS-KEY (alveolar)) OR (TITLE-ABS-KEY (bronchial)) OR (TITLE-ABS-KEY (pulmonary))) AND (LIMIT-TO (DOCTYPE, “ar”)) AND (LIMIT-TO (LANGUAGE, “English”)))))).
Selection and exclusion criteria
Retrieved articles were screened, duplicates were eliminated, and remaining citations were organized in Zotero (George Mason University, Virginia) following Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines. Only primary articles published in English were included. After a manual screening of titles and abstracts within the 225 and 74 records from HDAS and Scopus, respectively, 38 primary articles (one paper outside the search string) met the inclusion criteria for this systematic review (Figure 2).
Figure 2.
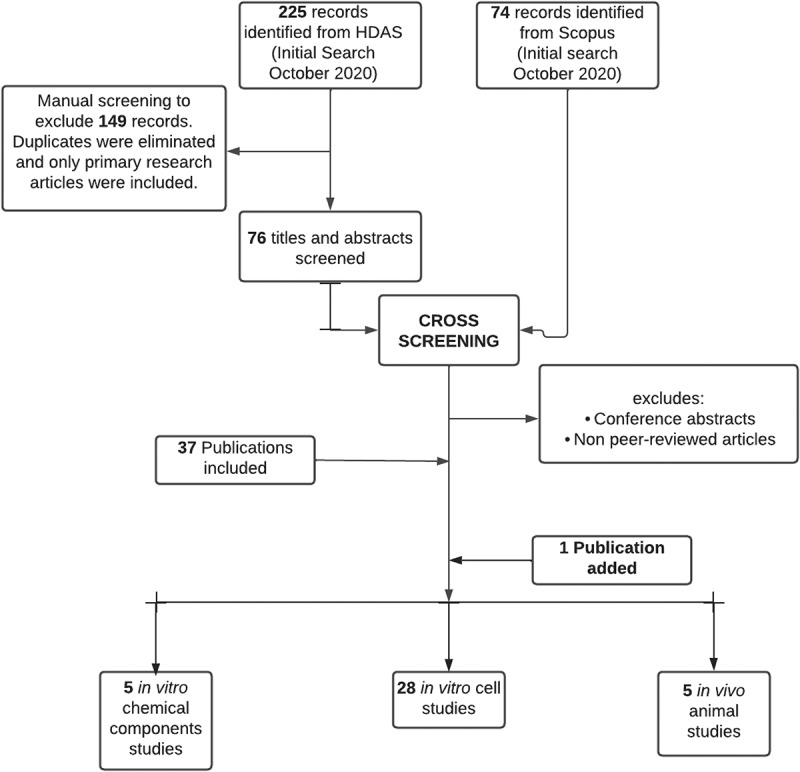
PRISMA flowchart. Articles were retrieved from HDAS and Scopus databases. Primary research articles that met the selection criteria were included irrespective of the year of publication. The figure was created with Lucidchart.com.
Results
The search stream included 28 in vitro studies where human cell lines were exposed to flavored e-liquids, 5 articles were e-liquid chemical composition studies, and 5 reported studies where rodents (mice and rats) were exposed to flavored e-liquids. Analysis of the 38 included reports and their reported adverse effects of flavors are summarized in Tables 2, 3 , 4 , 5 , and 6.
Table 2.
In vitro studies where toxicological effects of flavored e-liquids were investigated with primary epithelial cells alone or together with immortalized normal pulmonary cells.
| Cell type | Flavors or flavoring chemicals investigated | Exposure (Time & flavor dose) | E-liquid Nicotine Dose | Nicotine Control | Main findings | Reference |
|---|---|---|---|---|---|---|
| Primary human bronchial epithelial (HBECs), immortalized bronchial epithelial cells (BEAS-2B) |
(Each containing cinnamaldehyde)
|
0, 20, 40, 60, 80, 100, 120 min exposure to aerosol at ALI | No nicotine | N/A |
|
(Clapp et al. 2019) |
| Human bronchial epithelial cells (hBE) |
|
Puff duration of 2.6 s, 50 × 3 s puffs with 5 s in between were bubbled through 10 mL of culture medium to create EC extract. Cells were exposed to 100% of this media. |
|
|
|
(Ween et al. 2020a) |
| Primary human 24-h alveolar macrophages, neutrophils, and natural killer (NK) cells. |
|
Adherent alveolar macrophages were challenged in triplicate
|
|
|
|
(Clapp et al. 2017) |
| Primary human bronchial epithelial cells |
|
|
|
|
exposure. |
(McGraw et al., 2020) |
| 16HBE14o- airway epithelial cell line (16HBE), Normal human bronchial epithelial cells (NHBE), THP-1 differentiated macrophages & primary alveolar macrophages (AM). |
|
|
|
|
Chocolate > Banana > nicotine induced increase in IL-8 secretion by NHBE. Decrease in IL-1β
|
(Ween et al. 2020b) |
| Human bronchial epithelial cells of COPD models, immortalized 16HBE cells |
|
Intense puffing regime of 55 mL over 4 s, with 30s rest interval) for 30 min at ALI | 5 mg/ml | No nicotine alone control |
|
(O’Farrell et al. 2021) |
Table 3.
In vitro studies where toxicological effects of flavored e-liquids were carried out on immortalized pulmonary normal epithelial cells alone or together with carcinoma pulmonary cells.
| Cell type | Flavors or flavoring chemicals investigated | Exposure (Time & flavor dose) | E-liquid Nicotine Dose | Nicotine Control | Main findings | Reference |
|---|---|---|---|---|---|---|
| Human bronchial epithelial cell line (16HBE14o) |
|
24-h direct exposure (flavoring chemical) | No nicotine | N/A |
|
(Sherwood and Boitano, 2016) |
| Immortalized human bronchial epithelial cells (16-HBE, BEAS-2B) |
|
66 puffs during 22 minutes with a three-second puff duration at 1.6 L/min flow rate and an inter puff interval of approximately 17s. | 50 mg/mL nicotine | No nicotine control |
|
(Muthumalage et al. 2019) |
| Normal human bronchial epithelial (NHBE) cells |
|
24-h chemical exposure after media dilution in an ALI model | No nicotine | N/A |
|
(Park et al., 2019) |
| BEAS-2B and human pulmonary fibroblast (HPF) |
|
48-h chemical (diluted liquid) direct exposure | No nicotine | N/A |
|
(Omaiye et al. 2019) |
| BEAS-2B |
|
30 min x 3 (Each one after 12-h) aerosol exposure | 5% nicotine (Specific mg/ml of nicotine not specified) |
No nicotine control |
|
(Lamb, Muthumalage, and Rahman 2020) |
| Human pulmonary fibroblasts (HPF) |
|
48-h e-liquid direct exposure to 1% and 0.3% doses. | Different concentrations of nicotine, but not specified. | No nicotine control |
|
(Behar et al. 2014b) |
| Human lung fibroblasts (HPF) |
|
48-h direct e-liquid exposure to 0.001%, 0.01%, 0.03%, 0.1%, 0.3%, and 1% v/v. | 0 to 24 mg/ml | No nicotine alone control |
|
(Bahl et al. 2012) |
| Human airway epithelial cells (H292) & human lung fibroblast cells (HFL-1 cell line) |
|
30 s interval with a 4 s pulse for different time durations 5, 10, and 15 minutes at ALI | 0, 6, 16, 18 and 24 mg | No nicotine alone control present |
|
(Lerner et al. 2015) |
| Normal human bronchial epithelial (NHBE) cells |
|
24-h direct e-liquid exposure | Different dilutions (0.03125%, 0.0625%, 0.125%, 0.25%, 0.5%, 1%, 2% & 3% of 24 mg/ml e-liquids. | No Nicotine control |
|
(Czekala et al. 2019) |
| Pulmonary fibroblasts (HFL-1) |
|
24-h direct e-liquid exposure 0.1%, 0.25%, 0.5% & 1%. | 3 mg/ml (No nicotine-free group included) |
Nicotine alone control present at 0.25% and 0.5%. |
|
(Lucas et al. 2020) |
| Human bronchial epithelial cell lines (BEAS-2B, H292) |
|
5s duration every 30s for 1-h to aerosol at ALI | 50 mg/mL | No nicotine control |
|
(Pinkston et al. 2020) |
| BEAS-2B, Human cystic fibrosis cell line (IB3-1), and Calu3 |
|
24-h exposure to condensates (50%, 25%, and 12.5% diluted with media) | 0, 6, 12 and 18 mg/mL | No nicotine alone control |
|
(Leslie et al. 2017) |
| Human pulmonary fibroblasts (HPF), alveolar epithelial cells (A549) |
|
E-fluid (0.001%, 0.01%, 0.03%, 0.1%, 0.3%, 1.0%) and aerosol exposures. For each batch of aerosol, 24 puffs were collected into 4 mL of culture medium | 18 mg/ml | No nicotine control |
|
(Behar et al., 2018) |
Table 4.
In vitro studies where toxicological effects of flavored e-liquids were investigated in pulmonary carcinoma cells alone or together with primary pulmonary cells.
| Cell type | Flavors or flavoring chemicals investigated | Exposure (Time & flavor dose) | E-liquid Nicotine Dose | Nicotine Control | Main findings | Reference |
|---|---|---|---|---|---|---|
| Human bronchial epithelial cells (H292) |
|
3 s puff duration, every 30s, with a 55 mL puff volume. A period of 30 min was used as this was the minimum exposure at ALI. | 0, 6, 12, 18, or 24 mg/mL of nicotine | Nicotine alone control present. |
|
(Leigh et al. 2016) |
| Primary bronchial epithelial cells and human distal lung epithelial cells (NCI-H441) |
|
40 ml/puff, 3s puff duration, 30s puff interval, 10 puffs/ session at ALI | 0, 3 mg/ml | No nicotine alone control present | Exposure to mix-1 (nicotine-free) significantly increased transcript expression of pro-inflammatory cytokines CXCL8, IL6, NFKB1, TNF (not specified whether α or β
The presence of nicotine in mix-2 increased the transcript of TNF (not specified whether α or β) |
(Ganguly et al. 2020) |
| H292 cells |
|
|
|
No nicotine control |
than butter-flavored e‑cig aerosol.
|
(Noël et al. 2020b) |
| A549 (3D) co-culture of alveolar and H441 cells |
|
24-h exposure to condensed aerosol (puff regime was set to 55 mL puff volume, 3 s draw, 60s 200 puff) | 0 mg/ mL & 18 mg/ mL | No Nicotine control present |
|
(Bengalli et al. 2017) |
| CALU-3 airway epithelial cells |
|
24-h direct e-liquid exposure (0%, 0.1%, 0.3%, 1%, 3.0%, 6.0%, 10% (v/v)) | 0 mg/mL and 12 mg/mL of nicotine | Nicotine alone control present |
|
(Rowell et al. 2017) |
| Human adenocarcinoma alveolar basal epithelial cells (A549 cells) |
|
50 min aerosol exposure followed by analysis at 2-h, 6-h, 24-h. | Nicotine concentration not specified | No nicotine alone control present |
|
(Cervellati et al., 2014) |
| Human airway epithelial cells (H292) AND human lung fibroblast cells (HFL-1) |
|
30 s interval with a 4 s pulse for different time durations 5, 10, and 15 minutes | 0, 6, 16, 18 and 24 mg | No nicotine alone control present |
|
(Lerner et al., 2015) |
| Human adenocarcinoma alveolar basal epithelial cells (A549) |
|
Six puffs were dissolved per 1 mL of A549 culture medium, referred to as 6 total-puff- equivalents (TPE) of aerosol, and grown in control or treatment medium for 3–8 days. | 48 mg/ml (No nicotine free e-liquids present) |
No nicotine control |
|
(Zahedi et al. 2018) |
| Primary human epithelial cells (HBEC), cancerous human bronchial epithelial cells CALU-3 |
|
5–25 puffs | 0 and 12 mg/ml nicotine | No nicotine alone control, but 0 mg/mL BP |
|
(Rowell et al., 2020) |
Table 5.
Chemical composition analyses of flavored EC.
| E-liquid flavor | Experimental approach | E-liquid Nicotine Dose | Main findings | Reference |
|---|---|---|---|---|
| Menthol, Vanillin, Maltol | Particle size distribution | 18 mg/ mL of nicotine |
|
(Lechasseur et al., 2019) |
| Menthol | GC−MS Analysis of Liquid, Vapor, and Particulate Phases, FTIR Spectrometry | 18 mg nicotine/mL |
|
(Ooi et al., 2019) |
| Strawberry, Cinnamon, Sweet cream | Hydroxyl radicals in reaction mixes were measured based on the formation of 2-hydroxyterephthalic acid (2OHTA) | 3, 12, 24 and 36 mg/mL |
|
(Son et al., 2019) |
| 51 flavors | Mass spectrometry | 5% nicotine |
|
(Allen et al., 2016) |
| Mango, Banana, Tobacco, Cappuccino, Chocolate, Bubblegum, Peppermint, Cinnamon, Cherry, Apple | GC-MS |
|
(Ween et al. 2020b) |
Table 6.
In vivo studies of toxicological effects of EC flavors.
| Strain/sex | Exposure | E-liquid Nicotine Dose | Flavor(s) investigated. | Main findings | Reference |
|---|---|---|---|---|---|
| Adult Balb/c/ (male and female) | 30 min twice daily, 6 days/week exposed for 18 days (aerosol exposure) |
0 mg/mL &12 mg/mL |
|
|
(Chapman et al. 2019) |
| C57BL/6 /female | 2 h a day, 7 days a week, for 6 weeks | No nicotine |
|
|
(Szafran et al. 2020) |
| Balb/c/Pregnant and offspring | 14–31 days exposure. Vaping topography profile of 3-s puff duration and a 55-mL puff volume every 30s. | 36 mg/ml Nic (No 0 mg/mL available) |
|
offspring birth weight and body length compared to non-exposed controls
|
(Noël et al. 2020a) |
| C57BL6/male | Whole-body exposure for 3 days or 4 weeks. Puff volume was 20 ml. Mice were exposed to CS or EC 4 times a day with 30-min smoke-free intervals. |
18 mg/ml (No 0 mg/mL control) |
|
|
(Glynos et al. 2018) |
| Crl:CD (Sprague-Dowley) rats/male and female | 90-day nose-only inhalation to 3.2, 9.6 and 32 mg/kg/day of aerosol, which are referred to as low, mid, and high, respectively. | 20 mg Nic |
|
|
(Werley et al. 2016) |
Most in vitro investigations on cells were carried out on at least two different cell lines belonging to one of the three classes of the cell line: primary (5 articles), immortalized normal (15 articles), and cancerous pulmonary cells (13 articles). Therefore, we have subdivided the reporting of these papers based on the cell lines used in Tables 2, 3 and 4. The mint/menthol (10 articles) was the most frequently reported to demonstrate harmful effects, followed by cinnamon flavor (9 articles) and strawberry (5 articles) in cytotoxicity assays, metabolic activity assays, and pro-inflammatory biomarkers assays.
Similarly, cinnacide (cinnamon-contained), black licorice, banana pudding, vanilla, and tobacco-induced differential toxicity in vivo (Table 6). Toxic effects observed in vivo include but are not limited to increased levels of pro-inflammatory cells in bronchial alveolar lavage (BAL), MUC5AC (the predominant mucin glycoprotein produced by respiratory epithelium that normally protects against infection but when over-expressed is associated with respiratory diseases like asthma and COPD) production, and oxidative stress markers. There were no human studies where the effects of flavors on the respiratory system were investigated.
Discussion
This systematic review screened literature to determine which e-liquid flavors and flavoring agents (if any) elicit adverse effects in the airways. The studies identified several flavor categories demonstrating toxicity (Tables 2, 3 and 4), suggesting that several flavor chemicals might adversely affect respiratory cells. However, the most frequently reported e-liquids eliciting adverse responses were cinnamon, menthol, and strawberry flavors. It is unclear whether these flavors are the most frequently reported toxic as these are the most investigated. Further, it is unclear which flavoring chemical plays the critical role in the toxicity of strawberry-induced pulmonary cells; nonetheless, cinnamaldehyde and menthol are the primary components of cinnamon and menthol flavors, respectively (Clapp et al. 2017; Omaiye et al. 2019), which were found to activate TRPS. Several investigators showed induction of oxidative stress, inflammation and disruption of pulmonary barrier functionalities following e- liquids flavor exposure (Behar et al. 2014; Clapp et al. 2019, 2017; Lamb, Muthumalage, and Rahman 2020; Muthumalage et al. 2019, 2018). The mode of toxicological actions for many of the flavors in e-liquids is elusive. Still, evidence suggests that flavors such as cinnamon, menthol, strawberry, tobacco, and many others either induce one or more of the following adverse effects: mitochondrial dysfunction, cell death, ROS production, and dysregulation of inflammatory cytokines (Tables 2, 3 , 4 and 6). Given that cinnamaldehyde and menthol might activate TRPA1 and TRPM8, respectively, the adverse effects observed attributed to cinnamon and menthol may occur via the TRPs. Nonetheless, a few omissions were identified such as (1) absence of appropriate controls, (2) lack of validation for the experimental model, and (3) justification for concentrations used, which may impact inter-study comparisons and variability and hinder accurate interpretation of results. As such, it is recommended that experimental approaches be employed to reduce discrepancies in the toxicity testing of EC flavorings in future studies.
Adverse effects of flavored e-liquids
The evidence presented in this review suggests that flavors in e-liquids are not non-hazardous. Evidence regarding the risk of e-liquids might be biased or affected due to the limited number of appropriate toxicological studies. The most frequently reported adverse effects are reduced cell count and viability, altered pro-inflammatory biomarkers, cytokine release, and increased oxidative stress (Figure 3 for a schematic overview). These effects mentioned are consistent across pulmonary cell types, including normal and cancerous cells, large and small airway epithelial cells, alveolar epithelial cells, pulmonary smooth muscle, fibroblasts, and alveolar macrophages. Although there are no apparent studies where the sensitivity to different flavored e-liquids across primary, normal immortalized, and cancerous cells are simultaneously probed; nonetheless, in this review, primary cells and normal immortalized cells are seemingly more susceptible to the adverse effects of flavored e-liquid exposures than cancerous cell lines (Tables 3 and 4) (Leslie et al. 2017; Pinkston et al. 2020).
Figure 3.
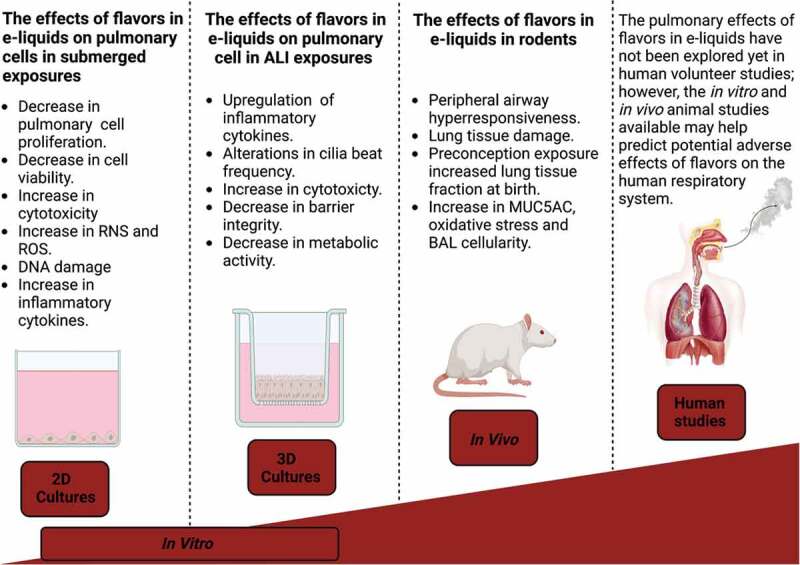
The effects of flavors in e-liquids reported in different experimental models. The figure was produced with Biorender.com.
Immortalized human cell lines are normal cells artificially manipulated to proliferate indefinitely. In contrast, cancer cell lines are transformed cells with mutations in oncogenes and tumor suppressor genes. There is evidence to suggest that cancer cells have increased levels of antioxidant enzymes such as superoxide dismutase and catalases compared to healthy cells, potentially as a defense mechanism against oxidative stress or to promote DNA damage and cancer progression (Asaduzzaman Khan et al. 2010; Chung-man Ho et al. 2001; Sun et al. 1989). Given the differential expression of antioxidant enzymes in cancer cells vs. primary and normal cells, this may contribute to the inter-studies variability conducted on EC flavors toxicity. Therefore, caution needs to be taken in interpreting and drawing conclusions regarding findings obtained from experimental models that may not reflect normal human cellular physiology.
Currently, there is no standardized protocol for physiologically relevant EC exposure dosage ranges in vitro or in vivo. Most studies carried out dose-response investigations (Behar et al. 2018a; Bengalli et al. 2017; Ganguly et al. 2020; Leslie et al. 2017; Lucas et al. 2020; Rowell et al. 2017). Decrease in cell proliferation and viability are indicative of increased cytotoxicity. A concentration-dependent decrease in cell proliferation and cell viability was observed (Tables 2, 3 and 4) with a mixture of tobacco, coconut, vanilla, and cookie (Lucas et al. 2020), hot cinnamon, menthol tobacco, black cigar (Rowell et al. 2017), butter finger and caramel (Behar et al. 2018a) following cellular exposure. Further, a combination of single-dose and multiple-dose studies reported that menthol (Czekala et al. 2019; Lamb, Muthumalage, and Rahman 2020; Muthumalage et al. 2019), blueberry and tobacco (Czekala et al. 2019), cinnamon (Behar et al. 2014; Rowell et al. 2017), menthol tobacco and kola (Rowell et al. 2017), and creamy/buttery and caramel (Behar, Wang, and Talbot 2018b) demonstrated the most significant effects were mitochondrial dysfunction in airway epithelial cells suggesting some role for induction of mitochondrial-induced apoptosis. In addition, cinnamon (Noël et al. 2020b), menthol (Muthumalage et al. 2019), and crème brûlée flavor (Pinkston et al. 2020) showed the most significant increase in production of markers of oxidative stress such as reactive oxygen species (ROS) and reactive nitrogen species (RNS) in bronchial epithelial cells (Figure 2). ROS and RNS are highly reactive chemical molecules intrinsic to cellular functioning when maintained at low normal cell levels. Still, excess ROS and RNS formation induces damage to cellular macromolecules, including DNA affecting normal cell functions (O’Farrell et al. 2021).
Several studies investigated the ability of flavored e-liquids to induce differential expression of pro-inflammatory cytokines in pulmonary epithelial cells (Clapp et al. 2019; Pinkston et al. 2020; Ween et al. 2020b), basal cells (Zahedi et al. 2018), and lung fibroblasts (Behar et al. 2014; Lucas et al. 2020; Omaiye et al. 2019b). Although Bahl et al. (2012); Leigh et al. (2016); Lucas et al. (2020); Rowell et al. (2017) explored different ranges of flavored e-liquid concentration, none of these justified the relevance of the applied dosages concerning human exposure. Lucas et al. (2020) investigated the concentration-dependent effects on IL-8 release but found that the highest concentrations of flavor e-liquid (0.5% or 1%) did not induce a marked change in IL-8 whereas 0.25% elevated the levels. It is unclear why this ‘bell-shaped’ effect was noted with a mixture of tobacco, coconut, vanilla, and cookie. However, it might be that higher concentrations failed to induce a rise in IL-8 as a consequence of enhanced cytotoxicity (Lucas et al. 2020). IL-8 is a potent chemokine that is critical in leukocyte infiltration during inflammation. IL-8 levels were elevated by chocolate, banana, cinnamon, tobacco, and a mixture of tobacco, coconut, vanilla, and cookie flavors in lung epithelial cells and pulmonary fibroblast. In Tables 2, 3 and 4, menthol, cinnamon, cool cucumber, classic menthol, and caffe’ latte flavored e-liquids produced increased release of pro-inflammatory biomarkers (IL-1ß, IFN-γ, IL-17) in lung epithelial cells. Coffee enhanced the release of IL-6, CXCL1, and CXCL2, whereas strawberry elevated the release of IL-1ß, IL-10, CYCL1, CXCL2, and CXCL10. IL-1ß, CXCL1, and CXCL2 which are pro-inflammatory cytokines (Leigh et al. 2016). IL-10 is an anti-inflammatory cytokine, and IL-6 functions as either pro- or anti-inflammatory depending upon the phase of inflammation at which it is released. In contrast, in a separate study, apple flavor reduced the pro-inflammatory cytokines secretion of TNF-α, IL-6, IP-10, MIP-1α, and MIP-1β (Ween et al. 2020b). Tables 2, 3 and 4 show that EC liquid flavors increased pro-inflammatory biomarkers, predominantly in pulmonary epithelial and fibroblast cells. The release of cytokines by the lung epithelium leads to recruitment, activation, and differentiation of innate and adaptive inflammatory cells: macrophages, neutrophils, B cells, and T cells. Although not many investigators assessed flavor-induced pro-inflammatory cytokines in immune cells in vitro, cinnamon suppressed alveolar macrophage phagocytosis in vitro (Clapp et al. 2017), suggesting prolonged exposure to cinnamon flavor may impact the resolution of lung inflammation. The proposed mechanism for this effect is elusive but likely via might involve one its predominant constituents, cinnamaldehyde. The latter was found to inhibit the toll-like receptor (TLR) subtype 4 activation (Youn et al. 2008), which subsequently might play a key role in macrophage phagocytosis (Skjesol et al. 2019). In alveolar macrophages, phagocytosis is critical for (1) defense against invading pathogens, (2) removal of dead cells or foreign particles, and (3) resolution of inflammatory responses and tissue remodeling, processes that are mediated by various surface receptors, including TLRs (Micera et al. 2016; Paradowska-Gorycka 2015). Evidence suggests that the resolution of respiratory inflammation may be altered in users of cinnamon flavors.
In vitro observations following flavored-e-liquid exposures were mirrored in the few in vivo animal studies on EC-mediated toxicity. Cinnacide, whose active flavor ingredient is cinnamaldehyde, and black licorice produced alterations in airways inflammation in vivo (Chapman et al. 2019) (Table 6) by lowering numbers of eosinophils and increasing number of macrophages, respectively, in BAL in allergenic airways disease utilizing adult Balb/c mouse model (males and females). In contrast, vanilla elevated number of dendritic cells, CD4+ cytotoxic T cells, and CD19 + B cells infiltration in the C57BL/6 mouse (female) lungs (Szafran et al. 2020). Further, the effects of cinnamon-flavor exposure in Balb/c pregnant mice and their offspring showed that preconception flavor exposure increases lung tissue fraction at birth compared to non-exposed groups (Noël et al. 2020a), suggesting that cinnamaldehyde may be teratogenic. Glynos et al. (2018) noted a flavor effect on broncoalveolar lavage fluid (BALF) markers, such as elevation in malondialdehyde and BALF protein carbonyls levels, increased BALF macrophages number, elevated IL-1β and IL-6 levels, and rise in BALF total cell count in an acute C57BL/6 male mice exposure. Werley et al. (2016) demonstrated that addition of flavor (flavor type unknown) did not impact pulmonary toxicity in a chronic Sprague-Dawley rat (female and male) exposure study. However, the effects observed were driven by the PG/VG vehicle (Table 6). Taken together, the reported in vivo animal studies suggest potential adverse lung effects for at least some flavored-e-liquids on the pulmonary system in rodents irrespective of strain and/or gender.
It is recognized that caution needs to be taken in interpreting the in vivo studies on EC-initiated toxicity (Marczylo 2020) where whole-body exposures were performed. Animal experimentation does not mimic human exposure models as these rodents are exposed through inhalation and the oral route via grooming. Further in vivo studies examining the influence of different flavors are warranted to enable for comparisons between flavors. The above evidence points toward potential harm from exposure to EC flavors. Some e-liquids induce oxidative stress, release of pro-inflammatory mediators, and cytotoxicity (Figure 3), which may exert implications for healthy lungs and might exacerbate inflammatory-mediated lung diseases such as COPD (O’Farrell et al. 2021), emphysema, and pneumonia. However, some uncertainties need addressing. Some studies did not provide sufficient detail on exposure dose, nicotine concentrations, and exposure durations to enable investigations to be reproduced. In addition, most studies (Table 2, 3 , 4 , 5 and 6) were not designed to include appropriate controls (PG/VG, nicotine) to enable an assessment of the specific contributions of flavor components to observed toxicity. These findings are important because studies, where PG/VG and nicotine controls were included, showed that these alone exert some adverse consequences that may account for at least some of the observed toxicities of the flavored e-liquids.
Additive effects of nicotine and PG/VG on flavor-induced toxicity
Nicotine is a potent stimulator of cell proliferation and may stimulate cancer development and growth (Dasgupta 2006; Khalil et al. 2013; Lee et al. 2005; Mravec et al. 2020). Nicotine is an agonist for the nicotinic acetylcholine receptors (nAChRs) (Dani 2015; Victoria et al. 2022), which are functionally expressed in the non-neuronal tissues of the lung (Chernyavsky et al. 2015; Improgo, Tapper, and Gardner 2011;). There are more than a dozen different nAChR subunit proteins, subdivided into α and β subfamilies, which form pentameric ion channels consisting of either a single type of α subunit (homopentamers) or a combination of α and β subunits (heteropentamers) (Shahsavar et al. 2016). As ligand-gated ion channels, nAChRs undergo complex allosteric changes in response to binding either the endogenous ligand acetylcholine (Ach) or exogenous ligands, including nicotine. nAChRs are classically linked to the plasma membrane depolarization required for neurotransmission; however, non-neuronal nAChRs in the lung act most frequently as calcium channels and were reported to activate numerous cellular pathways upon binding to either adrenergic receptors, nicotinic receptors, or by direct action within the cytoplasm (Wen et al. 2011) which regulate cell proliferation. Nicotine alone is not a carcinogen but is a tumor promoter (Ping Wu 2019). High doses of nicotine induce multi-organ toxicity and perhaps death from paralysis of respiratory muscles via the nAchRs (Mishra et al. 2015).
Most commercially available e-liquids contain nicotine; therefore, it is understandable that most studies report using nicotine-containing products (Voos, Goniewicz, and Eissenberg 2019). There is conflicting evidence regarding the additive effects of nicotine to flavor-induced toxicity. Only 4 (Bahl et al. 2012; Leigh et al. 2016; Rowell et al. 2017; Ween et al. 2020a) out of the 28 in vitro studies in Tables 2, 3 and 4 that reported adverse flavor effects in nicotine-containing e-liquids had included appropriate controls for both nicotine content and PG/VG to isolate flavor-effects. (Leigh et al. 2016) and (Bahl et al. 2012) showed that nicotine exerted no additive effects on strawberry, coffee (Leigh et al. 2016), menthol, and cinnamon (Bahl et al. 2012) flavor-dependent toxicity in H929 cells and HPF cells, respectively. In contrast, (Rowell et al. 2017; Ween et al. 2020a) noted the effects of nicotine-alone controls on cell proliferation inhibition, decreased cell viability flavor (Rowell et al. 2017a), and necrosis by Sytox Green (Ween et al. 2020a) in Calu3 and NHBE cells, respectively. These discrepancies may arise from 1) variability in nicotine concentrations and/or 2) differential expression of nAchRs or β-adrenergic receptors in the submerged models used. Because nAChR activation often leads to a positive feedback loop that induces receptor expression, high-concentration stimulation of nAChRs might lead to channel desensitization and decreased activity. Thus, elucidating functional roles for nAChRs is particularly complex and requires consideration of subunit composition, dose response, and duration of ligand stimulation. Further, undifferentiated bronchial epithelial cells in submerged cultures may be considered uniformly basal. In contrast, validated ALI models of bronchial epithelium represent a model of differentiated airway cells, including ciliated epithelial cells and goblet cells. Therefore, the lack of a nicotine effect in submerged cell cultures may be attributed to low expression levels or absence of cell surface receptors, including nAChRs and β-adrenergic receptors. Ganguly et al. (2020) reported additive effects of nicotine in reducing inflammatory levels of cytokines of IL-1ß, 1 L-10, IL-13, and IL-6 in a validated NCI-H441 ALI model, which probably expressed nAChRs. In contrast, (Wang et al. 2020) showed that in vivo EC exposure with or without nicotine affected lung inflammation, repair responses, and extracellular matrix remodeling mediated by the α7 nicotinic acetylcholine receptor (nAChRα7) in a gender-dependent manner. In addition, Glynos et al. (2018) detected no nicotine-dependent effects on inflammatory markers (IL-6, IL-1β, TNF-α) release and MUC5AC score in acute or chronic C57BL/6 mouse exposure models. However, Glynos et al. (2018) noted a nicotine plus flavor-dependent effect suggesting that e-liquid toxicity may not be associated with nicotine but rather the flavor content. No nicotine-related adverse pulmonary effects were observed in male or female Sprague-Dawley rats following chronic exposures, but a PG/VG effect was observed. Although there is contrasting evidence on the adverse health effects of nicotine in vitro against in vivo EC studies, the non-harmful effects of nicotine via inhalation were proven in a two-year survey by Waldum et al. (1996). Therefore, evidence indicates that nicotine may promote EC-related adverse pulmonary effects when these are initiated by the flavor or vehicle component of the e-liquid.
Four studies enabled assessment of PG/VG components alone by including that as vehicle control. Evidence showed that PG/VG-alone decreased metabolic activity in H929 (Leigh et al. 2016), H441, and HBECs (Woodall et al. 2020) via a mechanism that likely involves the blockade of glucose transporter uptake. Woodall et al. (2020) showed that PG/VG reduced glucose uptake and metabolism via the glucose transporters (GLUT1, GLUT2, and GLUT10) in human bronchial epithelial cells. In comparison, Bahl et al. (2012), Leigh et al. (2016), and Ween et al. (2020a) reported that PG/VG exerted no marked adverse effects on the pulmonary cells. Therefore, based on the contrasting evidence provided above, potentially stemming from methodological differences including the choice of cell line and dosage, attributing the adverse effects observed with flavored nicotine-contained e-liquids to the presence of nicotine by comparison to their nicotine-free e-liquid equivalents (Table 2, 3 , 4 , 5 and 6), without appropriate nicotine- and PG/VG-alone control may be misleading.
As such, future studies on flavors and/or flavoring ingredients of e-liquids might be improved by including appropriate controls: (1) cell-only for submerged exposures and air-only controls for air-liquid interface (ALI) exposures, (2) PG/VG/vehicle alone controls, and (3) PG/VG + nicotine controls to better inform on the contributions of the flavor compounds. In addition, justifications for e-liquid dosages might improve the interpretation of the relevance of the findings for health of individuals that vaper. These would lead to a better understanding of the relative contributions of flavor compounds, vehicles, and nicotine to the observed adverse effects of e-liquids and aid the ongoing debate over the safety and banning of flavored-e-liquids.
Recommended approach to reduce uncertainty in EC investigation in vitro and in vivo models
This review note the following: 1) lack of appropriate controls in many studies to enable reliable interpretation of data for identification of hazards and risks due to flavor components, 2) ambiguity over physiologically relevant doses of e-liquid/flavor used, and 3) lack of assessments of multiple dosing to establish the presence of dose-dependent relationships. Therefore, this section proposes an approach to reduce discrepancies among EC investigations, whether in vitro or in vivo.
In vitro submerged culture models are extensively employed in EC-induced toxicity screening. Evidence suggests that cytotoxicity of e-liquids in submerged models accurately predicts aerosol cytotoxicity 74% of the time (Behar, Wang, and Talbot 2018b; Sassano et al. 2018). Behar, Wang, and Talbot (2018b) conducted a pattern of cytotoxicity study on 35 e-liquids. The patterns were characterized as follows: (1) both the refill fluid and its aerosol were non-cytotoxic (7 of 35 = 20%) (2) both the refill fluid and its aerosol were cytotoxic (19 of 35 = 54%) (3) the refill fluid was cytotoxic, but the aerosol was not (1 of 35 = 3%) (4) the aerosol was cytotoxic, but the refill fluid was not (8 of 35 = 23%) Collectively when combining patterns (1) and (2), 74% of the 35 unheated refill fluids correctly predicted cytotoxicity of their corresponding aerosol. The remaining 26% did not reliably predict cytotoxicity because: (1) certain flavors (i.e., menthol artic) were cytotoxic in submerged cultures but not in their aerosol exposures in the ALI model (2) other flavors are cytotoxic in their aerosolized form (i.e., butter finger and caramel) but not in their liquid form (Behar, Wang, and Talbot 2018b). Although primary human bronchial epithelial cells in submerged cultures are basal cells and do not recapitulate the microenvironment of in vivo lung epithelial structures, using e-liquid with submerged models with the inclusion of appropriate controls: nicotine-alone, PG/VG-alone, and physiologically relevant range of flavor doses may help high-throughput screening of the extensive library of flavored e-liquids for toxicity. Subsequently, the most revealing e-liquids might be progressed into aerosol exposures using air-liquid interface (ALI) systems with standardized puff profiles suggested by CORESTA (CORESTA 2015) to eliminate potential variability within EC vapor toxicity.
In contrast to the submerged models, ALI lung cell models that have been validated for adequate transepithelial membrane resistance (TEER) measurements, expression of differential markers for goblet cells (MUC5AC), ciliated cells (FOXJ1), Clara cells (SCGB1A1), and basal cells (KrT5) may be used to investigate physiological and pathophysiological responses of the respiratory tract, molecular events, modes of action and interactions of different cell types. Potentially, these cell models mimic the in vivo microenvironment of cells in the respiratory tract more closely, namely, being apically exposed to air. In addition, these cells generate airway surface liquid (ASL), and the apical surface may be exposed directly to EC vapor. It is probably a better model than adding e-liquid in submerged basal cell culture. Such ALI models focus on anatomical regions of the lung or molecular pathways and aim to model relevant processes in vivo. Pairing this with analytical approaches to identify potential chemical compositions and concentrations (especially in flavors where toxicity is observed) for further investigation may eventually lead to limits on specific flavor chemical concentrations or outright bans from e-liquids. In addition, studies assessing the usefulness of ECs in risk and harm reduction needs to consider comparing e-cigarette data against conventional cigarettes before concluding the relative safety of these devices. This could be a gold-standard protocol to screen e-liquids toxicity that minimizes uncertainties.
To date, no apparent human studies have explored the toxicological effects of flavors in e-liquids on the respiratory system. Nonetheless, results such as inflammatory biomarkers, gene expression and cytotoxicity from either in vitro studies where the experimental model used to assess the safety of EC flavors closely recapitulates the microenvironments of the 3D human lung (ALI, or lung-on-chip) or in vivo animal testing may help discuss the potential extrapolation of EC toxicity to humans, taking into consideration dosage and exposure times required to mimic human exposures (Figure 3).
Conclusions
Some flavors in e-liquids may elicit pulmonary toxicity. However, these flavors are generally believed to attract long-time tobacco cigarette smokers to switch to EC use. As such, imposing restrictions on the use of flavors based upon reported evidence may be counterproductive. This is because 1) several in vitro studies demonstrated that EC-mediated toxicity to be related to PG/VG and nicotine rather than flavors per se, 2) not all flavors produce toxicity, and 3) more evidence-based risk assessments need to be used for those flavors that induced some toxicity to set concentration limits to reduce the potential for causing harm in humans. Although no apparent human studies data accounted for potential adverse pulmonary effects of flavored e-liquids, in vitro and in vivo studies reported toxicity attributed to cinnamon, strawberry, menthol, and other flavors. Therefore, public health authorities need to extrapolate data from these studies to predict risks posed by flavors to human health.
Acknowledgments
This paper is based on independent research commissioned and funded by the National Institute for Health Research (NIHR). Tim Marczylo and Felix Effah are part-funded by the NIHR Health Protection Research Unit (HPRU) in Health Impacts of Environmental Hazards at Imperial College London.
Funding Statement
This work was supported by the National Institute for Health Research
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
References
- Aguilar, P. R., Michelson A. P., and Isakow W.. 2016. Obliterative bronchiolitis. Transplantation 100:272–83. doi: 10.1097/TP.0000000000000892. [DOI] [PubMed] [Google Scholar]
- Aherrera, A., Olmedo P., Grau-Perez M., Tanda S., Goessler W., Jarmul S., Chen R., Cohen J. E., Rule A. M., and Navas-Acien A.. 2017. The association of e-cigarette use with exposure to nickel and chromium: A preliminary study of non-invasive biomarkers. Environ. Res 159:313–20. doi: 10.1016/j.envres.2017.08.014. [DOI] [PubMed] [Google Scholar]
- Allen, J. G., Flanigan S. S., LeBlanc M., Vallarino J., MacNaughton P., Stewart J. H., and Christiani D. C.. 2016. Flavoring Chemicals in E-Cigarettes: Diacetyl, 2,3-Pentanedione, and Acetoin in a Sample of 51 Products, Including Fruit-, Candy-, and Cocktail-Flavored E-Cigarettes. Environ. Health Perspect. 124 (6):733–39. doi: 10.1289/ehp.1510185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arman, S., and İşisağ Üçüncü S.. 2020. Cardiac toxicity of acrolein exposure in embryonic zebrafish (Danio rerio). Environ. Sci. Pollut. Res 27 (18):22423–33. doi: 10.1007/s11356-020-08853-7. [DOI] [PubMed] [Google Scholar]
- Asaduzzaman Khan, M., Tania M., Zhang D., and Chen H.. 2010. Antioxidant enzymes and cancer. Chin. J. Cancer Res. 22 (2):87–92. doi: 10.1007/s11670-010-0087-7. [DOI] [Google Scholar]
- ASH . 2020. E-cigarette use decreases as evidence as evidence shows they increase smokers’ chances of quitting. https://ash.org.uk/media-centre/news/press-releases/e-cigarette-use-decreases-as-evidence-shows-they-increase-smokers-chances-of-quitting
- ASH . 2021. Use of e-cigarettes (vapes) among adults in Great Britain. https://ash.org.uk/uploads/Use-of-e-cigarettes-vapes-among-adults-in-Great-Britain-2021.pdf
- ASH . 2022. Fears of growth in children vaping disposables backed up by new national survey. https://ash.org.uk/uploads/Use-of-e-cigarettes-among-young-people-in-Great-Britain-2022.pdf?v=1661866458
- Azzopardi, D., Patel K., Jaunky T., Santopietro S., Camacho O. M., McAughey J., and Gaça M.. 2016. Electronic cigarette aerosol induces significantly less cytotoxicity than tobacco smoke. Toxicol. Mech. Meth 26 (6):477–91. doi: 10.1080/15376516.2016.1217112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea, M., Luzardo O. P., González-Antuña A., Zumbado M., Rogozea L., Floroian L., Alexandrescu D., Moga M., Gaman L., Radoi M.. 2018. Body burden of toxic metals and rare earth elements in non-smokers, cigarette smokers and electronic cigarette users. Environ. Res 166:269–75. doi: 10.1016/j.envres.2018.06.007. [DOI] [PubMed] [Google Scholar]
- Bahl, V., Lin S., Xu N., Davis B., Wang Y., and Talbot P.. 2012. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod. Toxicol 34 (4):529–37. doi: 10.1016/j.reprotox.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Behar, R. Z., Davis B., Wang Y., Bahl V., Lin S., and Talbot P.. 2014. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol. in Vitro 28 (2):198–208. doi: 10.1016/j.tiv.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Behar, R. Z., Luo W., McWhirter K. J., Pankow J. F., and Talbot P.. 2018a. Analytical and toxicological evaluation of flavor chemicals in electronic cigarette refill fluids. Sci. Rep 8 (1):8288. doi: 10.1038/s41598-018-25575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar, R. Z., Wang Y., and Talbot P.. 2018b. Comparing the cytotoxicity of electronic cigarette fluids, aerosols and solvents. Tob. Control 27 (3):325–33. doi: 10.1136/tobaccocontrol-2016-053472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekki, K., Uchiyama S., Ohta K., Inaba Y., Nakagome H., and Kunugita N.. 2014. Carbonyl compounds generated from electronic cigarettes. Int. J. Environ. Res. Public Health 11 (11):11192–200. doi: 10.3390/ijerph111111192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengalli, R., Ferri E., Labra M., and Mantecca P.. 2017. Lung toxicity of condensed aerosol from E-CIG liquids: Influence of the flavor and the in vitro model used. Int. J. Environ. Res Public Health 14 (10):1254. doi: 10.3390/ijerph14101254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkelhamer, S. K., Helman J. M., Gugino S. F., Leigh N. J., Lakshminrusimha S., and Goniewicz M. L.. 2019. In vitro consequences of electronic-cigarette flavoring exposure on the immature lung. Int J Environ Res Public Health 16 (19):3635. doi: 10.3390/ijerph16193635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar, A. 2016. E-Cigarettes and cardiovascular disease risk: Evaluation of evidence, policy implications, and recommendations. Curr. Cardiovasc. Risk Rep 10 (7):24. doi: 10.1007/s12170-016-0505-6. [DOI] [Google Scholar]
- Blanc-Lapierre, A., Sauvé J.-F., and Parent M.-E.. 2018. Occupational exposure to benzene, toluene, xylene and styrene and risk of prostate cancer in a population-based study. Occup. Environ. Med 75 (8):562–72. doi: 10.1136/oemed-2018-105058. [DOI] [PubMed] [Google Scholar]
- Bracken-Clarke, D., Kapoor D., Baird A. M., Buchanan P. J., Gately K., Cuffe S., and Finn S. P.. 2021. Vaping and lung cancer-A review of current data and recommendations. Lung Cancer 153:11–20. doi: 10.1016/j.lungcan.2020.12.030. [DOI] [PubMed] [Google Scholar]
- Buist, A. S., Vollmer W. M., and McBurnie M. A.. 2008. Worldwide burden of COPD in high- and low-income countries. Part I. The burden of obstructive lung disease (BOLD) initiative. Int. J. Tuberc. Lung Dis 12 (7):703–08. [PubMed] [Google Scholar]
- Burgwardt, S., Huskic A., Schwartz G., Mason D. P., Tapias L., and Podgaetz E.. 2020. Spontaneous pneumomediastinum secondary to electronic cigarette use. Baylor Univ Med Center Proc 33 (2):229–30. doi: 10.1080/08998280.2020.1717407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush, L. P., Cui M., Shi H., Burton H. R., Fannin F., Lei L., and Dye N.. 2001. Formation of tobacco-specific nitrosamines in air-cured tobacco. Rec. Adv. Tob. Sci 27:23–46. [Google Scholar]
- Cervellati, F., Muresan X. M., Sticozzi C., Gambari R., Montagner G., Forman H. J., Torricelli C., Maioli E., and Valacchi G.. 2014. Comparative effects between electronic and cigarette smoke in human keratinocytes and epithelial lung cells. Toxicology in Vitro 28 (5):999–1005. doi: 10.1016/j.tiv.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, D. G., Casey D. T., Ather J. L., Aliyeva M., Daphtary N., Lahue K. G., van der Velden J. L., Janssen-Heininger Y. M. W., and Irvin C. G.. 2019. The effect of flavored e-cigarettes on murine allergic airways disease. Sci. Rep 9 (1):13671. doi: 10.1038/s41598-019-50223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyavsky, A. I., Shchepotin I. B., Galitovkiy V., and Grando S. A.. 2015. Mechanisms of tumor-promoting activities of nicotine in lung cancer: Synergistic effects of cell membrane and mitochondrial nicotinic acetylcholine receptors. BMC Cancer 15 (1):152. doi: 10.1186/s12885-015-1158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung-man Ho, J., Zheng S., Comhair S. A., Farver C., and Erzurum S. C.. 2001. Differential expression of manganese superoxide dismutase and catalase in lung cancer. Cancer Res. 61 (23):8578–85. [PubMed] [Google Scholar]
- Chun, L. F., Moazed F., Calfee C. S., Matthay M. A., and Gotts J. E.. 2017. Pulmonary toxicity of e-cigarettes. Am. J. Physiol Lung Cell. Mol. Physiol 313 (2):L193–L206. doi: 10.1152/ajplung.00071.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp, P. W., Lavrich K. S., van Heusden C. A., Lazarowski E. R., Carson J. L., and Jaspers I.. 2019. Cinnamaldehyde in flavored e-cigarette liquids temporarily suppresses bronchial epithelial cell ciliary motility by dysregulation of mitochondrial function. Am. J. Physiol Lung Cell. Mol. Physiol 316 (3):L470–L486. doi: 10.1152/ajplung.00304.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp, P. W., Pawlak E. A., Lackey J. T., Keating J. E., Reeber S. L., Glish G. L., and Jaspers I.. 2017. Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. Am. J. Physiol Lung Cell. Mol. Physiol 313 (2):L278–L292. doi: 10.1152/ajplung.00452.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORESTA . 2015. E-cigarette task force technical report, 2014 electronic cigarette aerosol parameters study. https://www.coresta.org/2014-electronic-cigarette-aerosol-parameters-study-29232.html
- Czekala, L., Simms L., Stevenson M., Trelles-Sticken E., Walker P., and Walele T.. 2019. High content screening in NHBE cells shows significantly reduced biological activity of flavoured e-liquids, when compared to cigarette smoke condensate. Toxicol. in Vitro 58:86–96. doi: 10.1016/j.tiv.2019.03.018. [DOI] [PubMed] [Google Scholar]
- Dani, J. A. 2015. Neuronal and nicotinic acteylcholine receptor structure and function and response to nicotine. Int. Rev. Neurobiol. 124:3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta, P. 2006. Nicotine induces cell proliferation by β-arrestin-mediated activation of Src and Rb-Raf-1 pathways. J. Clin Invest 116 (8):2208–17. doi: 10.1172/JCI28164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJarnett, N. D. J. C., Riggs D. W., Myers J. A., O’Toole T. E., Wagner S., Wagner S., Wagner S., Chugh A., Ramos K. S., Srivastava S.. 2014. Acrolein exposure is associated with increased cardiovascular disease risk. J. Am. Heart Association 3 (4):e000934. doi: 10.1161/JAHA.114.000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Olmo, A., Calzada J., and Nuñez M.. 2017. Benzoic acid and its derivatives as naturally occurring compounds in foods and as additives: Uses, exposure, and controversy. Crit Rev. Food Sci. Nutri 57 (14):3084–103. doi: 10.1080/10408398.2015.1087964. [DOI] [PubMed] [Google Scholar]
- Doll, R., Peto R., Boreham J., and Sutherland I.. 2005. Mortality from cancer in relation to smoking: 50 years observations on British doctors. Br. J. Cancer 92 (3):426–29. doi: 10.1038/sj.bjc.6602359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duell, A. K., Pankow J. F., and Peyton D. H.. 2020. Nicotine in tobacco product aerosols: ‘It’s déjà vu all over again. Tob. Control 29 (6):656–62. doi: 10.1136/tobaccocontrol-2019-055275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Zayadi, A.-R. 2006. Heavy smoking and liver. World J. Gastroenterol. 12 (38):6098–101. doi: 10.3748/wjg.v12.i38.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter, J.-F., Zäther E., and Svensson S.. 2013. Analysis of refill liquids for electronic cigarettes: E-liquids. Addiction 108 (9):1671–79. doi: 10.1111/add.12235. [DOI] [PubMed] [Google Scholar]
- Farsalinos, K., Gillman G., Poulas K., and Voudris V.. 2015. Tobacco-specific nitrosamines in electronic cigarettes: Comparison between liquid and aerosol levels. Int. J. Environ. Res. Public Health 12 (8):9046–53. doi: 10.3390/ijerph120809046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos, K., Romagna G., Tsiapras D., Kyrzopoulos S., Spyrou A., and Voudris V.. 2013. Impact of flavour variability on electronic cigarette use experience: An internet survey. Int. J. Environ. Res. Public Health 10 (12):7272–82. doi: 10.3390/ijerph10127272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA . 2012. Reporting harmful and potentially harmful constituents in tobacco products and tobacco smoke under section 904(a)(3) of the Federal food, drug, and cosmetic act. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/reporting-harmful-and-potentially-harmful-constituents-tobacco-products-and-tobacco-smoke-under
- Fedan, J. S., Dowdy J. A., Fedan K. B., and Hubbs A. F.. 2006. Popcorn worker’s lung: In vitro exposure to diacetyl, an ingredient in microwave popcorn butter flavoring, increases reactivity to methacholine. Toxicol. Appl. Pharmacol 215 (1):17–22. doi: 10.1016/j.taap.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Feron, V. J., Til H. P., de Vrijer F., Woutersen R. A., Cassee F. R., and van Bladeren P. J.. 1991. Aldehydes: Occurrence, carcinogenic potential, mechanism of action and risk assessment. Mutat. Res 259 (3–4):363–85. doi: 10.1016/0165-1218(91)90128-9. [DOI] [PubMed] [Google Scholar]
- Fetterman, J. L., Weisbrod R. M., Feng B., Bastin R., Tuttle S. T., Holbrook M., Baker G., Robertson R. M., Conklin D. J., Bhatnagar A., et al. 2018. Flavorings in tobacco products induce endothelial cell dysfunction. Arterioscler. Thromb. Vasc. Biol. 38 (7):1607–15. doi: 10.1161/ATVBAHA.118.311156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost-Pineda, K., Liang Q., Liu J., Rimmer L., Jin Y., Feng S., Kapur S., Mendes P., Roethig H., and Sarkar M.. 2011. Biomarkers of potential harm among adult smokers and nonsmokers in the total exposure study. Nicotine Tob. Res 13 (3):182–93. doi: 10.1093/ntr/ntq235. [DOI] [PubMed] [Google Scholar]
- Gallucci, G., Tartarone A., Lerose R., Lalinga A. V., and Capobianco A. M.. 2020. Cardiovascular risk of smoking and benefits of smoking cessation. J. Thorac. Dis 12 (7):3866–76. doi: 10.21037/jtd.2020.02.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly, K., Nordström A., Thimraj T. A., Rahman M., Ramström M., Sompa S. I., Lin E. Z., O’Brien F., Koelmel J., Ernstgård L.. 2020. Addressing the challenges of E-cigarette safety profiling by assessment of pulmonary toxicological response in bronchial and alveolar mucosa models. Sci. Rep 10 (1):20460. doi: 10.1038/s41598-020-77452-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff, J., Sundar I. K., Freter R., Sekera E. R., Friedman A. E., Robinson R., Pagano T., and Rahman I.. 2017. Inflammatory response and barrier dysfunction by different e-cigarette flavoring chemicals identified by gas chromatography–mass spectrometry in e-liquids and e-vapors on human lung epithelial cells and fibroblasts. Applied in vitro Toxicology 3 (1):28–40. doi: 10.1089/aivt.2016.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynos, C., Bibli S.-I., Katsaounou P., Pavlidou A., Magkou C., Karavana V., Topouzis S., Kalomenidis I., Zakynthinos S., and Papapetropoulos A.. 2018. Comparison of the effects of e-cigarette vapor with cigarette smoke on lung function and inflammation in mice. Am. J. Physiol Lung Cell. Mol. Physiol 315 (5):L662–L672. doi: 10.1152/ajplung.00389.2017. [DOI] [PubMed] [Google Scholar]
- Goniewicz, M. L., Knysak J., Gawron M., Kosmider L., Sobczak A., Kurek J., Prokopowicz A., Jablonska-Czapla M., Rosik-Dulewska C., Havel C.. 2014a. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control 23 (2):133–39. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz, M. L., Smith D. M., Edwards K. C., Blount B. C., Caldwell K. L., Feng J., Wang L.. 2018. Comparison of nicotine and toxicant exposure in users of electronic cigarettes and combustible cigarettes. J. Am. Medical Association Netw Open 1:e185937. doi: 10.1001/jamanetworkopen.2018.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havermans, A., Mallock N., Zervas E., Caillé-Garnier S., Mansuy T., Michel C., Pennings J.. 2022. Review of industry reports on EU priority tobacco additives Part A: Main outcomes and conclusions. Tob. Prev. Cessation 8:1–18. doi: 10.18332/tpc/151529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiemstra, P. S., and Bals R.. 2016. Basic science of electronic cigarettes: Assessment in cell culture and in vivo models. Respir. Res. 17 (1):127. doi: 10.1186/s12931-016-0447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, D., and Hoffmann I.. 1997. The changing cigarette, 1950-1995. J Toxicol Environ Health 50 (4):307–64. doi: 10.1080/009841097160393. [DOI] [PubMed] [Google Scholar]
- Hsueh, Y.-M., Lee C.-Y., Chien S.-N., Chen W.-J., Shiue H.-S., Huang S.-R., Lin M.-L., Mu S.-C., and Hsieh R.-L.. 2017. Association of blood heavy metals with developmental delays and health status in children. Sci Rep 7 (1):43608. doi: 10.1038/srep43608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Improgo, M. R., Tapper A. R., and Gardner P. D.. 2011. Nicotinic acetylcholine receptor-mediated mechanisms in lung cancer. Biochem. Pharmacol 82 (8):1015–21. doi: 10.1016/j.bcp.2011.05.020. [DOI] [PubMed] [Google Scholar]
- Jain, R. B. 2019. Concentrations of cadmium, lead, and mercury in blood among US cigarettes, cigars, electronic cigarettes, and dual cigarette-e-cigarette users. Environ. Pollut 251:970–74. doi: 10.1016/j.envpol.2019.05.041. [DOI] [PubMed] [Google Scholar]
- Jaishankar, M., Tseten T., Anbalagan N., Mathew B. B., and Beeregowda K. N.. 2014. Toxicity, mechanism, and health effects of some heavy metals. Interdiscip. Toxicol 7 (2):60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavvalakis, M. P., Stivaktakis P. D., Tzatzarakis M. N., Kouretas D., Liesivuori J., Alegakis A. K., Vynias D., and Tsatsakis A. M.. 2015. Multicomponent analysis of replacement liquids of electronic cigarettes using chromatographic techniques. J Anal Toxicol 39 (4):262–69. doi: 10.1093/jat/bkv002. [DOI] [PubMed] [Google Scholar]
- Kerasioti, E., Veskoukis A. S., Skaperda Z., Zacharias A., Poulas K., Lazopoulos G., and Kouretas D.. 2020. The flavoring and not the nicotine content is a decisive factor for the effects of refill liquids of electronic cigarette on the redox status of endothelial cells. Toxicology Reports 7:1095–102. doi: 10.1016/j.toxrep.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil, A. A., Jameson M. J., Broaddus W. C., Lin P. S., and Chung T. D.. 2013. Nicotine enhances proliferation, migration, and radioresistance of human malignant glioma cells through EGFR activation. Brain Tumor Pathol 30 (2):73–83. doi: 10.1007/s10014-012-0101-5. [DOI] [PubMed] [Google Scholar]
- Khlystov, A., and Samburova V.. 2016. Flavoring compounds dominate toxic aldehyde production during E-cigarette vaping. Environ. Sci. Technol 50 (23):13080–85. doi: 10.1021/acs.est.6b05145. [DOI] [PubMed] [Google Scholar]
- Kim, -Y.-Y., Kim M.-K., and Shin H.-S.. 2022. Determination of volatile organic compounds(VOCs) levels from various smoking cessation aids by using gas chromatography-mass spectrometry methodology. J. Toxicol. Environ. Health Part A 85 (3):110–20. doi: 10.1080/15287394.2021.1979436. [DOI] [PubMed] [Google Scholar]
- Kim, H.-J., and Shin H.-S.. 2013. Determination of tobacco-specific nitrosamines in replacement liquids of electronic cigarettes by liquid chromatography–tandem mass spectrometry. J Chromatog A 1291:48–55. doi: 10.1016/j.chroma.2013.03.035. [DOI] [PubMed] [Google Scholar]
- Kim, S. A., Smith S., Beauchamp C., Song Y., Chiang M., Giuseppetti A., Frukhtbeyn S., Shaffer I., Wilhide J., Routkevitch D., et al. 2018. Cariogenic potential of sweet flavors in electronic-cigarette liquids. PLoS ONE. 13(9):e0203717. doi: 10.1371/journal.pone.0203717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knezevich, A., Muzic J., Hatsukami D. K., Hecht S. S., and Stepanov I.. 2013. Nornicotine nitrosation in saliva and its relation to endogenous synthesis of N’-nitrosonornicotine in humans. Nicotine Tob. Res 15 (2):591–95. doi: 10.1093/ntr/nts172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüsemann, E. J. Z., Boesveldt S., de Graaf K., and Talhout R.. 2019. An e-liquid flavor wheel: A shared vocabulary based on systematically reviewing e-liquid flavor classifications in literature. Nicotine Tob. Res 21 (10):1310–19. doi: 10.1093/ntr/nty101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb, T., Muthumalage T., and Rahman I.. 2020. Pod-based menthol and tobacco flavored e-cigarettes cause mitochondrial dysfunction in lung epithelial cells. Toxicol. Lett 333:303–11. doi: 10.1016/j.toxlet.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugesen, M. 2008. Safety Report on the Ruyan® e-cigarette cartridge and inhaled aerosol. http://www.healthnz.co.nz/RuyanCartridgeReport30-Oct-08.pdf
- Lechasseur, A., Altmejd S., Turgeon N., Buonanno G., Morawska L., Brunet D., Duchaine C., and Morissette M. C.. 2019. Variations in coil temperature/power and e-liquid constituents change size and lung deposition of particles emitted by an electronic cigarette. Physiological Reports 7 (10):e14093. doi: 10.14814/phy2.14093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H.-J., Guo H.-Y., Lee S.-K., Jeon B.-H., Jun C.-D., Lee S.-K., Park M.-H., and Kim E.-C.. 2005. Effects of nicotine on proliferation, cell cycle, and differentiation in immortalized and malignant oral keratinocytes. J. Oral Pathol. Med 34 (7):436–43. doi: 10.1111/j.1600-0714.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- Leigh, N. J., Lawton R. I., Hershberger P. A., and Goniewicz M. L.. 2016. Flavourings significantly affect inhalation toxicity of aerosol generated from electronic nicotine delivery systems (ENDS). Tob. Control 25 (Suppl 2):ii81. doi: 10.1136/tobaccocontrol-2016-053205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner, C. A., Sundar I. K., Yao H., Gerloff J., Ossip D. J., McIntosh S., Robinson R., Rahman I., and Khan M. F.. 2015. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS ONE 10 (2):e0116732. doi: 10.1371/journal.pone.0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie, L. J., Vasanthi Bathrinarayanan P., Jackson P., Mabiala Ma Muanda J. A., Pallett R., Stillman C. J. P., and Marshall L. J.. 2017. A comparative study of electronic cigarette vapor extracts on airway-related cell lines in vitro. Inhal. Toxicol 29 (3):126–36. doi: 10.1080/08958378.2017.1318193. [DOI] [PubMed] [Google Scholar]
- Liao, Q., Du R., Ma R., Liu X., Zhang Y., Zhang Z., Ji P., Xiao M., Cui Y., Xing X.. 2022. Association between exposure to a mixture of benzene, toluene, ethylbenzene, xylene, and styrene (BTEXS) and small airways function: A cross-sectional study. Environ. Res 212:113488. doi: 10.1016/j.envres.2022.113488. [DOI] [PubMed] [Google Scholar]
- Lin, A.-H., Liu M.-H., Ko H.-K., Perng D.-W., Lee T.-S., and Kou Y. R.. 2018. Menthol cigarette smoke induces more severe lung inflammation than non-menthol cigarette smoke does in mice with subchronic exposure – Role of trpm8. Front. Physiol 9:1817. doi: 10.3389/fphys.2018.01817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas, J. H., Muthumalage T., Wang Q., Friedman M. R., Friedman A. E., and Rahman I.. 2020. E-Liquid containing a mixture of coconut, vanilla, and cookie flavors causes cellular senescence and dysregulated repair in pulmonary fibroblasts: Implications on premature aging. Front. Physiol 11:924. doi: 10.3389/fphys.2020.00924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczylo, T. 2020. How bad are e‐cigarettes? What can we learn from animal exposure models? J. Physiol 598 (22):5073–89. doi: 10.1113/JP278366. [DOI] [PubMed] [Google Scholar]
- Margham, J., McAdam K., Forster M., Liu C., Wright C., Mariner D., and Proctor C.. 2016. Chemical composition of aerosol from an e-cigarette: A quantitative comparison with cigarette smoke. Chem. Res. Toxicol 29 (10):1662–78. doi: 10.1021/acs.chemrestox.6b00188. [DOI] [PubMed] [Google Scholar]
- McGraw, M. D., Kim S.-Y., Reed C., Hernady E., Rahman I., Mariani T. J., and Finkelstein J. N.. 2020. Airway basal cell injury after acute diacetyl (2,3-butanedione) vapor exposure. Toxicol. Lett. 325:25–33. doi: 10.1016/j.toxlet.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill, A. D., Brose L. S., Calder R., Bauld L., and Robson D. J.. 2018. Evidence review of e-cigarettes and heated tobacco products 2018 : A report commissioned by public health England.
- Micera, A., Balzamino B. O., Zazzo A. D., Biamonte F., Sica G., and Bonini S.. 2016. Toll-like receptors and tissue remodeling: The pro/cons recent findings: TLRs and tissue remodeling. J. Cell. Physiol 231 (3):531–44. doi: 10.1002/jcp.25124. [DOI] [PubMed] [Google Scholar]
- Mishra, A., Chaturvedi P., Datta S., Sinukumar S., Joshi P., and Garg A.. 2015. Harmful effects of nicotine. Indian J. Med. Paediatr. Oncol 36 (1):24–31. doi: 10.4103/0971-5851.151771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed, F., and Calfee C. S.. 2017. The canary in the coal mine is coughing: Electronic cigarettes and respiratory symptoms in adolescents. Am. J. Respir. Crit. Care Med 195 (8):974–76. doi: 10.1164/rccm.201611-2259ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mravec, B., Tibensky M., Horvathova L., and Babal P.. 2020. E-cigarettes and cancer risk. Cancer Prev Res (Phila) 13 (2):137–44. doi: 10.1158/1940-6207.CAPR-19-0346. [DOI] [PubMed] [Google Scholar]
- Muthumalage, T., Lamb T., Friedman M. R., and Rahman I.. 2019. E-cigarette flavored pods induce inflammation, epithelial barrier dysfunction, and DNA damage in lung epithelial cells and monocytes. Sci. Rep 9 (1):19035. doi: 10.1038/s41598-019-51643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthumalage, T., Prinz M., Ansah K. O., Gerloff J., Sundar I. K., and Rahman I.. 2018. Inflammatory and oxidative responses induced by exposure to commonly used e-cigarette flavoring chemicals and flavored e-liquids without nicotine. Front. Physiol 8:1130. doi: 10.3389/fphys.2017.01130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health , 2014. The health consequences of smoking—50 years of progress. a report of the surgeon general. Centers for Disease Control and Prevention (US). [PubMed] [Google Scholar]
- Neczypor, E. W., Saldaña T. A., Mears M. J., Aslaner D. M., Escobar Y.-N. H., Gorr M. W., and Wold L. E.. 2022. E-cigarette aerosol reduces left ventricular function in adolescent mice. Circulation 145 (11):868–70. doi: 10.1161/CIRCULATIONAHA.121.057613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noël, A., Hansen S., Zaman A., Perveen Z., Pinkston R., Hossain E., Xiao R., and Penn A.. 2020a. In utero exposures to electronic-cigarette aerosols impair the Wnt signaling during mouse lung development. Am. J. Physiol Lung Cell. Mol. Physiol 318 (4):L705–L722. doi: 10.1152/ajplung.00408.2019. [DOI] [PubMed] [Google Scholar]
- Noël, A., Hossain E., Perveen Z., Zaman H., and Penn A. L.. 2020b. Sub-ohm vaping increases the levels of carbonyls, is cytotoxic, and alters gene expression in human bronchial epithelial cells exposed at the air–liquid interface. Respir. Res 21 (1):305. doi: 10.1186/s12931-020-01571-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Farrell, H. E., Brown R., Brown Z., Milijevi B., Ristovski Z. D., Bowman R. V., Fong K. M., Vaughan A., and Yang I. A.. 2021. E-cigarettes induce toxicity comparable to tobacco cigarettes in airway epithelium from patients with COPD. Toxicol. in Vitro 75:105204. doi: 10.1016/j.tiv.2021.105204. [DOI] [PubMed] [Google Scholar]
- Ogunwale, M. A., Li M., Ramakrishnam Raju M. V., Chen Y., Nantz M. H., Conklin D. J., and Fu X.-A.. 2017. Aldehyde detection in electronic cigarette aerosols. Am. Chem Soc Omega 2:1207–14. doi: 10.1021/acsomega.6b00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omaiye, E. E., McWhirter K. J., Luo W., Pankow J. F., and Talbot P.. 2019b. High-Nicotine electronic cigarette products: Toxicity of JUUL fluids and aerosols correlates strongly with nicotine and some flavor chemical concentrations. Chem. Res. Toxicol. 32 (6):1058–69. doi: 10.1021/acs.chemrestox.8b00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omaiye, E. E., McWhirter K. J., Luo W., Tierney P. A., Pankow J. F., and Talbot P.. 2019. High concentrations of flavor chemicals are present in electronic cigarette refill fluids. Sci. Rep 9 (1):2468. doi: 10.1038/s41598-019-39550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi, B. G., Dutta D., Kazipeta K., and Chong N. S.. 2019. Influence of the e-cigarette emission profile by the ratio of glycerol to propylene glycol in e-liquid composition. ACS Omega 4 (8):13338–48. doi: 10.1021/acsomega.9b01504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradowska-Gorycka, A. 2015. U1-RNP and Toll-like receptors in the pathogenesis of mixed connective tissue disease Part II. Endosomal TLRs and their biological significance in the pathogenesis of mixed connective tissue disease. Reumatologia 53:143–51. doi: 10.5114/reum.2015.53136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, H.-R., O'Sullivan M., Vallarino J., Shumyatcher M., Himes B. E., Park J.-A., Christiani D. C., Allen J., and Quan L.. 2019. Transcriptomic response of primary human airway epithelial cells to flavoring chemicals in electronic cigarettes. Sci Rep 9. doi: 10.1038/s41598-018-37913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira, E. J., Sim L., Driver H. S., Parker C. M., and Fitzpatrick M. F.. 2013. The effect of inhaled menthol on upper airway resistance in humans: A randomized controlled crossover study. Can. Respir. J 20 (1):e1–e4. doi: 10.1155/2013/383019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping Wu, J. 2019. Nicotine is insufficient as a carcinogen, it’s functions as a tumor promoter on purpose. Am. J. Biomed. Sci. Res 5 (1):47–48. doi: 10.34297/AJBSR.2019.05.000872. [DOI] [Google Scholar]
- Pinkston, R., Zaman H., Hossain E., Penn A. L., and Noël A.. 2020. Cell-specific toxicity of short-term JUUL aerosol exposure to human bronchial epithelial cells and murine macrophages exposed at the air–liquid interface. Respir. Res 21 (1):269. doi: 10.1186/s12931-020-01539-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe, F. J. C., and Wood D.. 1992. Review: Acetaldehyde and formaldehyde: Is there a cancer risk for man? Indoor Environ 1:8–15. doi: 10.1177/1420326X9200100103. [DOI] [Google Scholar]
- Rose, C. S. 2017. Early detection, clinical diagnosis, and management of lung disease from exposure to diacetyl. Toxicology 388:9–14. doi: 10.1016/j.tox.2017.03.019. [DOI] [PubMed] [Google Scholar]
- Rowell, T. R., Keating J. E., Zorn B. T., Glish G. L., Shears S. B., and Tarran R.. 2020. Flavored e-liquids increase cytoplasmic Ca 2+ levels in airway epithelia. American Journal of Physiology-Lung Cellular and Molecular Physiology 318 (2):L226–L241. doi: 10.1152/ajplung.00123.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell, T. R., Reeber S. L., Lee S. L., Harris R. A., Nethery R. C., Herring A. H., Glish G. L., and Tarran R.. 2017. Flavored e-cigarette liquids reduce proliferation and viability in the CALU3 airway epithelial cell line. Am. J. Physiol Lung Cell. Mol. Physiol 313 (1):L52–L66. doi: 10.1152/ajplung.00392.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabnis, A. S., Shadid M., Yost G. S., and Reilly C. A.. 2008. Human lung epithelial cells express a functional cold-sensing TRPM8 variant. Am. J. Respir. Cell Mol. Biol 39 (4):466–74. doi: 10.1165/rcmb.2007-0440OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassano, M. F., Davis E. S., Keating J. E., Zorn B. T., Kochar T. K., Wolfgang M. C., Glish G. L., Tarran R., and Khosla C.. 2018. Evaluation of e-liquid toxicity using an open-source high-throughput screening assay. PLoS Biol. 16 (3):e2003904. doi: 10.1371/journal.pbio.2003904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick, S. F., Blount B. C., Jacob P., Saliba N. A., Bernert J. T., El Hellani A., Jatlow P.. 2017. Biomarkers of exposure to new and emerging tobacco delivery products. Am. J. Physiol. Lung Cell. Mol. Physiol 313 (3):L425–L452. doi: 10.1152/ajplung.00343.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober, W., Szendrei K., Matzen W., Osiander-Fuchs H., Heitmann D., Schettgen T., Jörres R. A., and Fromme H.. 2014. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int. J. Hyg. Environ. Health 217 (6):628–37. doi: 10.1016/j.ijheh.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Schweitzer, R. J., Wills T. A., and Behner J. D.. 2017. E-cigarette use and indicators of cardiovascular disease risk. Curr. Epidemiol. Rep 4 (3):248–57. doi: 10.1007/s40471-017-0118-8. [DOI] [Google Scholar]
- Shahsavar, A., Gajhede M., Kastrup J. S., and Balle T.. 2016. Structural studies of nicotinic acetylcholine receptors: Using acetylcholine-binding protein as a structural surrogate. Basic Clin. Pharmacol. Toxicol 118 (6):399–407. doi: 10.1111/bcpt.12528. [DOI] [PubMed] [Google Scholar]
- Sherwood, C. L., and Boitano S.. 2016. Airway epithelial cell exposure to distinct e-cigarette liquid flavorings reveals toxicity thresholds and activation of CFTR by the chocolate flavoring 2,5-dimethypyrazine. Respir. Res. 17 (1):57. doi: 10.1186/s12931-016-0369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjesol, A., Yurchenko M., Bösl K., Gravastrand C., Nilsen K. E., Melsæther Grøvdal L., Agliano F., Patane, F., Lentini, G., Kim, H. et al . 2019. The TLR4 adaptor TRAM controls the phagocytosis of Gram-negative bacteria by interacting with the Rab11-family interacting protein 2. PLoS Pathog. 15 (3):e1007684. doi: 10.1371/journal.ppat.1007684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son, Y.-O. 2020. Molecular mechanisms of nickel-induced carcinogenesis. Endocr Metab Immune Disord Drug Targets 20 (7):1015–923. doi: 10.2174/1871530319666191125112728. [DOI] [PubMed] [Google Scholar]
- Son, Y., Mishin V., Laskin J. D., Mainelis G., Wackowski O. A., Delnevo C., Schwander S., Khlystov A., Samburova V., and Meng Q.. 2019. Hydroxyl radicals in e-cigarette vapor and e-vapor oxidative potentials under different vaping patterns. Chem. Res. Toxicol. 32 (6):1087–95. doi: 10.1021/acs.chemrestox.8b00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefaniak, A. B., LeBouf R. F., Ranpara A. C., and Leonard S. S.. 2021. Toxicology of flavoring- and cannabis-containing e-liquids used in electronic delivery systems. Pharmacol. Ther. 224:107838. doi: 10.1016/j.pharmthera.2021.107838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar, I. K., Javed F., Romanos G. E., and Rahman I.. 2016. E-cigarettes and flavorings induce inflammatory and pro-senescence responses in oral epithelial cells and periodontal fibroblasts. Oncotarget 7 (47):77196–204. doi: 10.18632/oncotarget.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Y., Oberley L. W., Elwell J. H., and Sierra-Rivera E.. 1989. Antioxidant enzyme activities in normal and transformed mouse liver cells. Int. J. Cancer 44 (6):1028–33. doi: 10.1002/ijc.2910440615. [DOI] [PubMed] [Google Scholar]
- Szafran, B. N., Pinkston R., Perveen Z., Ross M. K., Morgan T., Paulsen D. B., Penn A. L., Kaplan B. L. F., and Noël A.. 2020. Electronic-cigarette vehicles and flavoring affect lung function and immune responses in a murine model. Int. J. Mol. Sci 21 (17):6022. doi: 10.3390/ijms21176022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talih, S., Balhas Z., Eissenberg T., Salman R., Karaoghlanian N., El Hellani A., Baalbaki R., Saliba N., and Shihadeh A.. 2015. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: Measurements and model predictions. Nicotine Tob. Res 17 (2):150–57. doi: 10.1093/ntr/ntu174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney, P. A., Karpinski C. D., Brown J. E., Luo W., and Pankow J. F.. 2016. Flavour chemicals in electronic cigarette fluids. Tob. Control 25 (e1):e10–e15. doi: 10.1136/tobaccocontrol-2014-052175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration . 2009. Summary of results: Laboratory analysis of electronic cigarettes conducted by FDA. http://www.fda.gov/NewsEvents/PublicHealthFocus/ucm173146.htm
- van Rooy, F. G. B. G. J., Rooyackers J. M., Prokop M., Houba R., Smit L. A. M., and Heederik D. J. J.. 2007. Bronchiolitis obliterans syndrome in chemical workers producing diacetyl for food flavorings. Am. J. Respir. Crit. Care Med 176 (5):498–504. doi: 10.1164/rccm.200611-1620OC. [DOI] [PubMed] [Google Scholar]
- Vestbo, J., Hurd S. S., Agustí A. G., Jones P. W., Vogelmeier C., Anzueto A., Barnes P. J., Fabbri L. M., Martinez F. J., Nishimura M.. 2013. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med 187 (4):347–65. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- Victoria, S., Hein M., Harrahy E., and King-Heiden T. C.. 2022. Potency matters: Impacts of embryonic exposure to nAChR agonists thiamethoxam and nicotine on hatching success, growth and neurobehavior in larval zebrafish. J. Toxicol. Environ. Health Part A 85 (18):767–82. doi: 10.1080/15287394.2022.2081641. [DOI] [PubMed] [Google Scholar]
- Voos, N., Goniewicz M. J., and Eissenberg T.. 2019. What is the nicotine delivery profile of electronic cigarettes. Expert Opin Drug Deliv 16 (11):1193–203. doi: 10.1080/17425247.2019.1665647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadgave, U., and Nagesh L.. 2016. Nicotine replacement therapy: An overview. Int. J. Health Sci. (Qassim) 10 (3):425–35. [PMC free article] [PubMed] [Google Scholar]
- Waldum, H. L., Nilsen O. G., Nilsen T., Rørvik H., Syversen U., Sandvik A. K., Haugen O. A., Torp S. H., and Brenna E.. 1996. Long-term effects of inhaled nicotine. Life Sci. 58 (16):1339–46. doi: 10.1016/0024-3205(96)00100-2. [DOI] [PubMed] [Google Scholar]
- Wang, Q., Sundar I. K., Li D., Lucas J. H., Muthumalage T., McDonough S. R., and Rahman I.. 2020. E-cigarette-induced pulmonary inflammation and dysregulated repair are mediated by nAChR α7 receptor: Role of nAChR α7 in SARS-CoV-2 Covid-19 ACE2 receptor regulation. Respir. Res 21 (1):154. doi: 10.1186/s12931-020-01396-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J., Yang H., Shi H., Zhou J., Bai R., Zhang M., and Jin T.. 2017. Nitrate and nitrite promote formation of tobacco-specific nitrosamines via nitrogen oxides intermediates during postcured storage under warm temperature. J. Chem. 2017:1–11. doi: 10.1155/2017/6135215. [DOI] [Google Scholar]
- Wang, M., Zhang Y., Xu M., Zhang H., Chen Y., Chung K. F., Adcock I. M., and Li F.. 2019. Roles of TRPA1 and TRPV1 in cigarette smoke -induced airway epithelial cell injury model. Free Radic. Biol. Med 134:229–38. doi: 10.1016/j.freeradbiomed.2019.01.004. [DOI] [PubMed] [Google Scholar]
- Warden, H., Richardson H., Richardson L., Siemiatycki J., and Ho V.. 2018. Associations between occupational exposure to benzene, toluene and xylene and risk of lung cancer in Montréal. Occup. Environ. Med 75:696–702. doi: 10.1136/oemed-2017-104987. [DOI] [PubMed] [Google Scholar]
- Ween, M. P., Hamon R., Macowan M. G., Thredgold L., Reynolds P. N., and Hodge S. J.. 2020b. Effects of e‐cigarette e‐liquid components on bronchial epithelial cells: Demonstration of dysfunctional efferocytosis. Respirology 25 (6):620–28. doi: 10.1111/resp.13696. [DOI] [PubMed] [Google Scholar]
- Ween, M. P., Moshensky A., Thredgold L. L., Bastian N. A., Hamon R., Badiei A., Nguyen P. T., Herewane K., Jersmann H., Bojanowski C. M.. 2020a. E-cigarettes and health risks: More to the flavour than just the name. Am. J. Physiol Lung Cell. Mol. Physiol 320 (4):L600–L614. doi: 10.1152/ajplung.00370.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, J., Fu J.-H., Zhang W., and Guo M.. 2011. Lung carcinoma signaling pathways activated by smoking. Chin. J. Cancer 30 (8):551–58. doi: 10.5732/cjc.011.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werley, M. S., Kirkpatrick D. J., Oldham M. J., Jerome A. M., Langston T. B., Lilly P. D., Smith D. C., and McKinney W. J.. 2016. Toxicological assessment of a prototype e-cigarette device and three flavor formulations: A 90-day inhalation study in rats. Inhal. Toxicol 28 (1):22–38. doi: 10.3109/08958378.2015.1130758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham, R. J. 2020. The biological impact of menthol on tobacco dependence. Nicotine Tob. Res 22 (10):1676–84. doi: 10.1093/ntr/ntz239. [DOI] [PubMed] [Google Scholar]
- Woodall, M., Jacob J., Kalsi K. K., Schroeder V., Davis E., Kenyon B., Khan I., Garnett J. P., Tarran R., and Baines D. L.. 2020. E-cigarette constituents propylene glycol and vegetable glycerin decrease glucose uptake and its metabolism in airway epithelial cells in vitro. Am. J. Physiol Lung Cell. Mol. Physiol 319 (6):L957–L967. doi: 10.1152/ajplung.00123.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2019. WHO report on the global tobacco epidemic 2019: Offer help to quit tobacco use. https://www.who.int/publications/i/item/9789241516204
- Youn, H. S., Lee J. K., Choi Y. J., Saitoh S. I., Miyake K., Hwang D. H., and Lee J. Y.. 2008. Cinnamaldehyde suppresses toll-like receptor 4 activation mediated through the inhibition of receptor oligomerization. Biochem. Pharmacol 75 (2):494–502. doi: 10.1016/j.bcp.2007.08.033. [DOI] [PubMed] [Google Scholar]
- Zahedi, A., Phandthong R., Chaili A., Remark G., and Talbot P.. 2018. Epithelial-to-mesenchymal transition of A549 lung cancer cells exposed to electronic cigarettes. Lung Cancer 122:224–33. doi: 10.1016/j.lungcan.2018.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, S.-H., Sun J. Y., Bonnevie E., Cummins S. E., Gamst A., Yin L., and Lee M.. 2014. Four hundred and sixty brands of e-cigarettes and counting: Implications for product regulation. Tob. Control 23 (suppl 3):iii3–iii9. doi: 10.1136/tobaccocontrol-2014-051670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebarth, D. 1997. N-nitrosation of medicinal drugs catalysed by bacteria from human saliva and gastro-intestinal tract, including Helicobacter pylori. Carcinogenesis 18 (2):383–89. doi: 10.1093/carcin/18.2.383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.


