Abstract
Asparagine-linked glycosylation protein 1 homolog (ALG1) participates in the initial stage of protein N-glycosylation and N-glycosylation has been implicated in the process of hepatocellular carcinoma (HCC) progression. However, whether ALG1 plays a role in human HCC remains unknown. In this study, the expression profile of ALG1 in tumorous and corresponding adjacent non-tumor tissues was analyzed. The relationship of ALG1 expression with clinical features and prognosis of HCC patients was also evaluated using immuno-histochemical method. Here we found ALG1 decreased in HCC tissues compared with adjacent normal liver tissues, which predicted an unfavorable prognosis. Combined with RNA interference, nascent proteome and glycoproteome were determined systematically in Huh7 cell line. Bioinformatics analysis indicated that the differentially expressed proteins participating in the response of ALG1 knockdown were most significantly associated with cell–cell adhesion. Functional studies confirmed that knockdown of ALG1 reduced cell adhesion capacity, and promoted cell migration. Furthermore, down-regulation of H8N2 (on N-glycosite N651) and H5N4S2F1 (on N-glycosite N692) from N-cadherin was identified as a feature of ALG1 knockdown. Our findings revealed that ALG1 controlled the expression of glycosylated N-cadherin and played a role in HCC migration, with implications for prognosis.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s43657-022-00050-5.
Keywords: Nascent proteome, ALG1, Glycosylation, N-cadherin, Hepatocellular carcinoma
Introduction
As one of the most common malignant tumors, hepatocellular carcinoma (HCC) is the fourth leading cause of cancer death in the world (Bray et al. 2018). China has the greatest number of patients worldwide, due to an elevated incidence rate and the world’s largest population (McGlynn et al. 2021). Since hepato-carcinogenesis is a highly precisely regulated molecular biological process, integrative multi-omics analyses can help determine the complex process during the progression of this fatal disease (Wu et al. 2020). Recent progress in glyco-proteomics enables people to have a more comprehensive and in-depth understanding of HCC-related glycosylation events (Zhang et al. 2017).
Glycosylation is a key modification that helps proteins fold and maintain stability. The most common type of glycosylation, Asn-linked glycosylation, plays crucial roles in cell trafficking, signaling, adhesion and immune response (Rexer et al. 2018). In eukaryotic cells, the maturation of Asn-linked oligosaccharides needs the trimming of nascent glycan chains before extending to the complex-type structures on the surfaces of cells or secreted glycoproteins (Xiang et al. 2016). The biosynthesis of Asn-linked oligosaccharides consists of three steps: first, the assembly of the lipid-linked oligosaccharides precursor in endoplasmic reticulum (ER) membrane; second, the fully assembled lipid-linked oligosaccharides are transferred to the selected Asn residues on nascent glycoproteins; third, the Asn-linked oligosaccharides are further processed in rough ER and Golgi by glycosidases and glycosyltransferases (Gao et al. 2004; Takahashi et al. 2000).
Asparagine-linked glycosylation protein 1 homolog (ALG1), encodes an ER localized β-1,4-mannosyltransferase that catalyzes the synthesis of the lipid-linked oligosaccharide precursor which becomes the core of all Asn-linked glycans (Lee et al. 1997; Ng et al. 2016). Besides, ALG1 mutations cause a rare autosomal-recessive disorder, along with serious systemic disease (Ng et al. 2016; Rohlfing et al. 2014). Notably, congenital disorders of glycosylation (CDG), including ALG1–CDG, have been reported to be associated with liver disease, although it is not a unique or predominant feature (Lipinski et al. 2021; Marques-da-Silva et al. 2017). So far, the function of ALG1 has been gradually unveiled in higher eukaryotes (Li et al. 2017; Lombard 2016; Ramirez et al. 2017). However, the role of ALG1 in the liver or hepatocellular carcinoma remains unknown. Understanding how and to what extent the liver is affected by glycosylation or glycosyltransferases will improve the diagnosis, management, and treatment of liver cancer. In this study, we performed immunohistochemistry, real-time quantitative PCR (RT-qPCR) and Western blotting to profile ALG1 expression in HCC samples and explore its relationship with the clinical characteristics and prognosis of patients with HCC.
Cells respond to interference or environmental cues by changing protein expression levels (Ma et al. 2018). Measuring the immediate proteome response is the key to understand the mechanisms involved, which makes it possible to find new therapeutic targets (van Bergen et al. 2021). A previously developed method introducing bio-orthogonal tagging into proteins is applicable for labeling, enrichment and measurement of de novo-synthesized proteins, which can more distinctly reflect protein changes due to interference (Ma et al. 2017; McClatchy et al. 2018; Shao et al. 2021). With the elimination of distractions from the overwhelming static proteome, the characterization of newly synthesized proteins is more reliable than that of the whole proteome to some extent (Ma et al. 2018; McClatchy et al. 2018). Here, the nascent proteome and glycoproteome were combined to analyze the potential roles of ALG1 in HCC cells. This study aimed to provide novel insights into the clinical significance and molecular function of ALG1 in hepatocellular carcinoma.
Materials and Methods
Patients and Tissue Specimens
Cancerous tissues and surrounding non-cancerous hepatic parenchyma were obtained from 42 primary HCC patients who underwent surgical resection at First Affiliated Hospital of Guangxi Medical University. Informed consent was obtained from all patients before the study began. All specimens were stored at – 80 ℃. In addition, 267 HCC samples for immuno-histochemical staining were routinely formalin-fixed and paraffin-embedded. The clinical data of the patients are provided in Table S1. This study was approved by the Research Ethics Committee.
Western Blotting
Paired tumor and non-tumor liver tissues from six HCC patients were homogenated in RIPA lysis buffer (Beyotime, Shanghai, China). Protein concentration was quantified using a BCA kit (Beyotime). Equal amounts of protein were loaded onto 10% SDS-PAGE. After transferring and blocking, the membranes were incubated overnight with ALG1 antibody (Invitrogen, USA) and N-cadherin antibody (Proteintech, China). To confirm equal loading, β-actin antibody (Multisciences, Hangzhou, China) was served as a control.
Real-Time Quantitative PCR (RT-qPCR)
Frozen tissues were harvested in TRIzol (Invitrogen). Reverse transcription was performed with 2 μg of total RNA. Primers for β-actin and ALG1 were prepared as follows: ALG1, forward: AGCTCGTCATTGACTGGCACA, reverse: GCATTGGTAACACACAGGTTCAGG; β-actin, forward: TGGCACCCAGCACAATGAA, reverse: CTAAGTCATAGTCCGCCTAGAAGCA. Real-time PCR was performed in triplicate for each sample using TaKaRa TB Green™ Premix Ex Taq ™ II Real-time PCR system. Relative changes in transcript were calculated with the 2 −ΔΔCt method (Schmittgen and Livak 2008).
Immunohistochemistry
Paraffin sections were de-paraffinized and rehydrated. After antigen retrieval and washing, the sections were treated in a 3% hydrogen peroxide solution. Then the overnight incubation was performed with ALG1 antibody (Invitrogen). After being washed with TBST, the sections were reacted with HRP and visualized with di-amino-benzidine. Slides were counterstained with hematoxylin, dehydrated, and mounted. Two experienced pathologists evaluated staining independently.
Cell Culture and RNA Interference
The Huh7 cells were obtained from the Chinese Academy of Sciences and cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco, USA) containing 10% fetal bovine serum (FBS, Biological Industries) at 37℃ with 5% CO2 atmosphere. All experiments were performed with mycoplasma-free cells. The small interfering RNA (siRNA) targeting human ALG1 (5’-CAGAGGACGAAGACUUCUCUATT-3’) was commercially synthesized (Sangon Biotech, Shanghai, China). Along with scrambled RNAi oligonucleotides (siCtrl), the siRNA targeting ALG1 (siALG1) was transfected using transfection reagent (BBI life sciences, Shanghai, China). For membrane protein extraction, the Minute™ Plasma Membrane Protein Isolation Kit (Invent Biotechnologies, Minnesota, USA) was used.
Chemical Metabolic Labeling by AHA
Cells were cultured to about 70–80% confluence and were transferred into L-methionine-free DMEM (Gibco) with dialyzed FBS (Gibco) to deplete methionine reserves. Combined with transient transfection, azidohomoalanine (AHA, Cambridge Isotope Laboratories, Tewksbury, MA, USA) at 500 μM was added to metabolically label the newly synthesized proteins for 24 h. At last, cells were harvested and lysed with 0.5% SDS (Sigma-Aldrich, St Louis, MO, USA) with a cocktail of protease inhibitors (Roche, Basel, Switzerland). The cell suspension was sonicated with 5-s pulses for five minutes. After centrifugation, the supernatant fraction was collected and the protein concentration was determined using the BCA assay before being stored at – 80 ℃.
Click Reaction
After chemical metabolic labeling, a click reaction between AHA and alkyne–biotin was performed as previously published (Dieterich et al. 2007). A cocktail of 500 μM alkyne–biotin reagent (Sigma-Aldrich), 1 mM Copper Sulfate (CuSO4, Sigma-Aldrich), 6 mM 3-(4-((bis((1-tert-butyl-1H-1,2,3-triazol-4-yl)methyl)amino)methyl)-1H-1,2,3-triazol-1-yl)propanol (BTTP, Click Chemistry Tools, Scottsdale, AZ, USA) and 6 mM Ascorbate (Sigma-Aldrich) was added to 500 μg cell lysate. The samples were placed at room temperature on shaker for three hours.
Enrichment and Digestion of Newly Synthesized Protein
Newly synthesized proteins were enriched as previously described with minor modifications (Ma et al. 2017, 2018). The newly synthesized proteins were enriched with NeutrAvidin Agarose Resins (GenScript, Piscataway, NJ, USA). Then, the beads were washed in solutions of 6 M urea (Sigma), 20% ACN and PBS respectively. The washed beads were reconstituted and reduced with incubation of 10 mM di-thio-threitol at 37 ℃ for one hour. The samples were further alkylated with 25 mM iodo-acetamide in the dark at room temperature for 30 min to block cysteine residues. Sequencing-grade modified trypsin (Promega, Madison, WI, USA) was used for overnight digestion at 37 ℃. The digested peptides were desalted via Zip-Tip (Millipore, Billerica, MA, USA) and stored at – 20 ℃ until use. For glyco-peptide enrichment, the tryptic peptides were applied to Glycopeptide Enrichment Kit (Novagen, Darmstadt, Germany) following the instructions.
LC–MS/MS
The desalted peptides were analyzed on the Orbitrap Exploris 480 MS. Peptides were separated using a C18 column (75 μm × 500 mm column, ThermoFisher) on an Easy nLC 1200 high-pressure liquid chromatography system (Thermo Fisher Scientific, San Jose, CA, USA) operating at 300 nL/min. Buffer A (0.1% formic acid) and buffer B (0.1% formic acid in 80% ACN) were used. Peptides were separated by a linear gradient from 8% B to 23% B in 90 min followed by a linear increase to 50% B in 27 min. For data-dependent acquisition experiments, the full MS resolutions were set to 120,000 and the full MS AGC target was 300%. Mass range was set to 350–1200 Da. AGC target value for fragment spectra was set at 75% with a resolution of 15,000. Isolation width was set at 1.6 m/z. The normalized collision energy was set at 29%. All data were acquired in profile mode using positive polarity.
Data Analysis
Data file analysis was performed in PEAKS Studio software (Bioinformatics Solutions Inc., Waterloo, Canada) for de novo analysis. The Homo sapiens protein database was from Swiss-Prot (20,431 entries, date July 2019). The input parameters were: 10 ppm precursor mass tolerance, 0.02 Da fragment mass tolerance. The maximum false discovery rates for protein and peptides were set at 1%. A gene ontology (GO) analysis of the altered proteins was performed to annotate biological process (BP) using the David Functional Annotation Tool (https://david.ncifcrf.gov/home.jsp) (Huang et al. 2009). For glyco-peptides, the details about the database search and mass spectrometric analysis can be found in our previous study (Cao et al. 2021; Zhang et al. 2019).
Cell Adhesion and Migration Assays
Cell adhesion assays (BestBio Science, Shanghai, China) were conducted according to the manufacturer’s instructions. Cell migration was determined by Transwell assay as described (Noor et al. 2021). Cells deprived of FBS for 24 h were seeded in the upper chambers at a density of 5 × 104 cells/well in a total volume of 200 μL of FBS-free media. The lower chamber was filled with 600 μL of complete medium. The number of cells on the underside of the membrane was counted in five different fields with a light microscope at × 200 magnification. Each experiment was repeated at least three times.
Statistical Analysis
Calculations were performed using the statistical package IBM SPSS Statistics software (SPSS Inc., Chicago, IL, USA). Figures were generated using GraphPad Prism (version 8.0, GraphPad Software Inc., San Diego, CA, USA) and Hiplot (https://hiplot.com.cn). The numeric data are presented as means ± standard deviation. Statistical comparisons were calculated using a two-tailed Student’s t test. The χ2 test was performed to assess associations between ALG1 expression and clinico-pathological parameters. Survival curves were calculated via the Kaplan–Meier method and compared by the log-rank test. The Cox proportional hazard model was utilized for univariate and multivariate analyses to explore the effects of the clinico-pathological parameters and ALG1 expression on survival. p < 0.05 was considered to be statistically significant. N-glycan structures were drawn with GlycoWorkbench.
Results
The Expression Level of ALG1 in Paired HCC Tissues
We examined six HCC samples and corresponding non-cancerous hepatic tissues for ALG1 protein expression using Western blotting. The results showed that ALG1 was significantly reduced in HCC compared to non-cancerous tissues (Fig. 1a, p < 0.01). The same six cases of HCC tumorous and paired adjacent normal tissues were detected for ALG1 mRNA expression using RT-qPCR. The average ALG1/β-actin level in HCC was significantly lower than that in adjacent normal tissues (Fig. 1b, p < 0.01), which was consistent with protein expression. To further confirm this result, an immuno-histochemical study containing 36 patients was performed. ALG1 staining was observed in both cancer and non-cancerous tissues (Fig. 1c) and was significantly decreased in almost all tumorous tissues, compared with the adjacent normal tissues (Fig. 1d, p < 0.01).
Fig. 1.
a–b Relative expression of ALG1 in six paired HCC tumorous tissues and corresponding adjacent normal tissues; the Student’s paired t test was used c–d Immuno-histochemical analysis of ALG1 in HCC tumorous and the adjacent non-tumorous tissues (Original magnifications: × 200; × 400) .e Overall survival rates of patients with HCC. f Recurrence-free survival rates of patients with HCC; Kaplan–Meier analysis of overall survival and recurrence-free survival in HCC patients according to the results of immunohistochemistry
Prognostic Value of ALG1 Expression in Liver Cancer Patients
To investigate the prognostic value of ALG1, immunohistochemistry was performed in another 267 paraffin-embedded HCC tissue blocks. The demographics and clinical characteristics of the participants are shown in Table S1. Correlations between ALG1 expression and clinico-pathological features in HCC are listed in Table S2. The decreased expression of ALG1 was significantly associated with serum HBsAg positive (p = 0.043) and tumor differentiation (p = 0.003), but not with gender, age, serum AFP, cirrhosis, tumor size, tumor number and vascular invasion. Moreover, patients with low expression of ALG1 had a significantly shorter overall survival and recurrence-free survival than those with ALG1 high expression (Fig. 1e, p = 0.041; Fig. 1f, p = 0.040). Representative images were presented in Fig. S1. Univariate Cox regression analysis showed that ALG1 expression, serum AFP, pathology grade, cirrhosis status, tumor size, tumor number, tumor differentiation and vascular invasion were significantly correlated with overall survival and recurrence-free survival (Table S3). ALG1 expression and cirrhosis status were independent predictors for overall survival and recurrence-free survival of HCC patients in multivariate analysis.
ALG1-Related Changes of the Nascent Proteome in Huh7 Cell Line
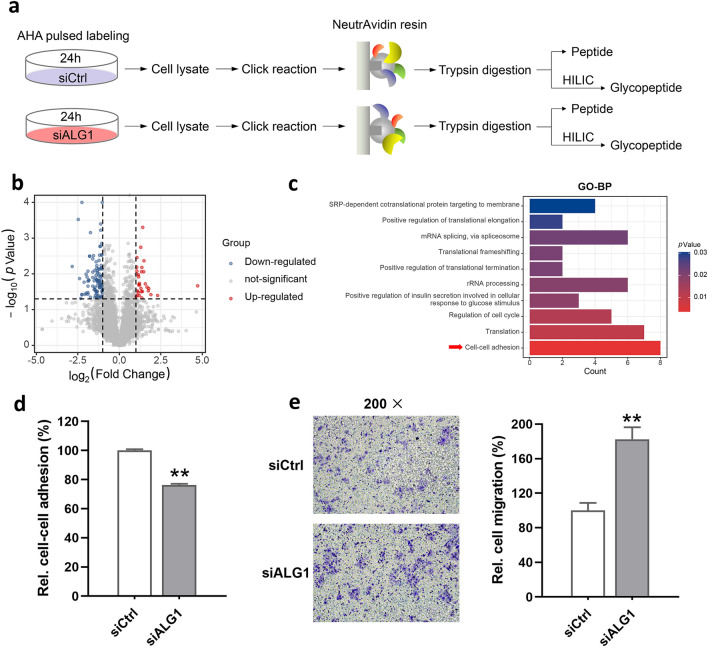
To gain insight into the functional implications of ALG1, quantitative nascent proteome analyses based on metabolic labeling were applied as described (Ma et al. 2018). Together with transient transfection, the cells were labeled with AHA in a methionine-free medium to specifically label de novo proteins for 24 h with three biological replicates for each group (Fig. 2a). Then the cells were harvested, lysed and reacted with alkyne–biotin, followed by enrichment via biotin–avidin affinity interaction in parallel. After thorough washing and trypsin digestion, ten percent enzymolysis products were utilized for desalination, and the rest were used for glyco-peptide enrichment. The obtained peptides and glyco-peptides were sent to LC–MS/MS for further identification and quantification. As the cutoff criterion was set as fold change > 2 or < 0.5, a total of 134 newly synthesized proteins (32 up- and 102 down-regulated) were differentially expressed (Fig. 2b). Cell–cell adhesion was most enriched according to GO-BP analysis (Fig. 2c). To further confirm the molecular events, cell–cell adhesion and migration capacities were analyzed. As expected, relative cell–cell adhesion of ALG1-deficient cells was significantly down-regulated (Fig. 2d). Consistent with the decrease of cell–cell adhesion, the migration capacity of Huh7 cells was increased by ALG1 knockdown when compared with control (Fig. 2e).
Fig. 2.
a Workflow for analyses of nascent proteome and glyco-proteome; three biological repetitions were conducted b Proteins with statistical significance after a t test are shown in red (up-regulated) or blue (down-regulated) on a volcano plot; the vertical lines correspond to two-fold up and down (log2 ratio), and the horizontal line represents a p value of 0.05; each point in the plot represents a different protein c GO biological process categories enriched during knockdown of ALG1. d Relative cell–cell adhesion of siCtrl and siALG1 cells to cell monolayers as indicated; cells deprived of FBS were seeded on confluent monolayers of cells and allowed to adhere for one hour at 37 ℃; the absorbance of each well to which cells were added was normalized against the mean absorbance of wells, where no cells were added; at least three biological repetitions were done e A transwell migration assay was performed to estimate the migration capacity (left panel); cells migrated to the lower surface of the eight-μm membrane were stained with crystal violet, counted, and represented graphically (right panel); an independent sample t test was used; data are representative of three biological replicates (**p < 0.01)
Knockdown of ALG1 Inhibited the Expression of Glycosylated N-cadherin
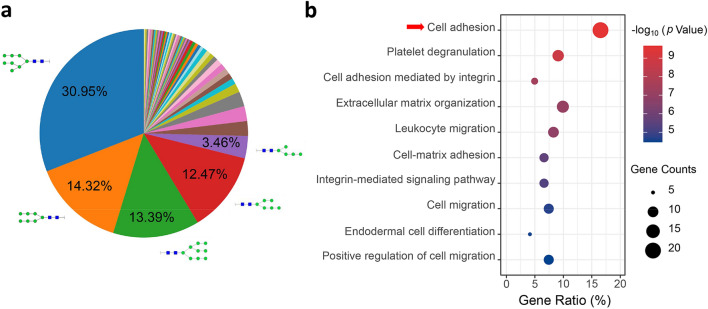
In the glycoproteome, a total of 432 newly synthesized N-glyco-peptides were identified. To simplify the glycan annotation, a four-digit nomenclature in HNSF order was utilized (H, Hexose; N, N-acetyl-hexosamine; S, Sialic acid; F, Fucose). Comparing the distribution of N-glycan compositions, H8N2 and H7N2 represented the most common compositions in the identification (Fig. 3a). And the de novo glycoproteins were selectively enriched for GO biological process categories linking to cell adhesion and platelet degranulation (Fig. 3b).
Fig. 3.
a Distribution of the identified N-glycan compositions. b Gene ontology categories of the identified newly synthesized glycoproteins
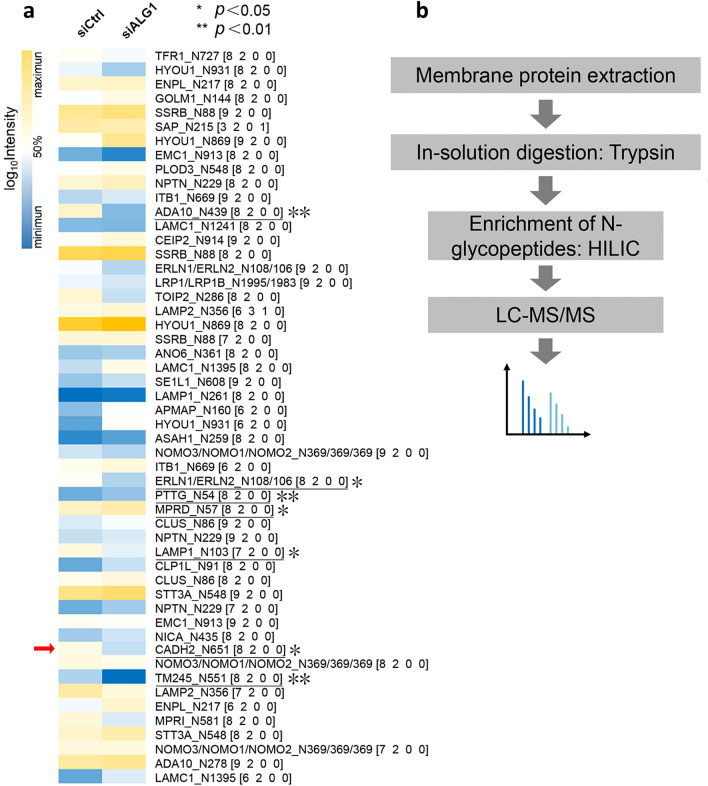
With the help of quantitation software termed pQuant (Liu et al. 2014), the peak area was determined for each glyco-peptide. And 52 N-glyco-peptides passed the strict filtering criteria (FDR < 1%) (Cao et al. 2021; Zhang et al. 2019) and obtained quantitative information (Fig. 4a). Compared with the control group, five differentially expressed intact N-glyco-peptides belonging to ADA10, ERLN1/ERLN2, LAMP1, CADH2 and TM245 were considerably decreased under the condition of ALG1 knockdown, while two glyco-peptides from PTTG and MPRD were significantly increased (Table S4). N-cadherin (CADH2) is a well-known cell adhesion protein, whose glycosylation was considered to be related to protein stability and cell adhesion (Xu et al. 2017). To confirm the down-regulation of glycosylated N-cadherin, and to increase the identification efficiency of the target protein, we applied selective membrane proteome enrichment to improve the sensitivity of LC–MS/MS analysis (Fig. 4b). Specifically, we observed that H8N2 (on N-glycosite N651) and H5N4S2F1 (on N-glycosite N692) of N-cadherin were significantly down-regulated in siALG1 cells (Fig. 5a). Overall, these results suggested that knockdown of ALG1 could partially inhibit the expression of glycosylated N-cadherin. Representative annotation spectra were shown in Fig. 5b–c. The protein level of N-cadherin was also evaluated using Western blotting (Fig. S2), and the result showed there was no significant difference between ALG1 knockdown and control. However, there was a mild downregulatory tendency of N-cadherin expression under the condition of ALG1 knockdown, implying the alteration of CADH2 might be attributed to the joint contribution of protein expression and glycosylation.
Fig. 4.
a Heat map displayed the relative abundance of the quantified newly synthesized intact glyco-peptides in Huh7 cells during transient transfection; p values were determined using two-tailed t tests (*p < 0.05, **p < 0.01). b Membrane proteins were extracted for further validation
Fig. 5.
a CADH2_N651 (H8N2) and CADH2_N692 (H5N4S2F1) represent significant differences between siCtrl and siALG1; three biological repeats were shown (*p < 0.05, **p < 0.01). b-c pGlyco annotations of N651 (H8N2) and N692 (H5N4S2F1); “J” represents the glycosylation site “N”; green circle: hexose (H); blue square: N-acetyl-glucosamine (N); purple rhombus: sialic acid (S); red triangle: fucose (F); the upper frame of each spectrum is designed to annotate peptide sequence and glycan composition; the mass deviations of the annotated peaks are shown in the box below
Discussion
Clinically, the diagnosis and staging of liver cancer depend on various conventional imaging techniques and serum-based biomarkers such as α-fetoprotein (Bargellini et al. 2014; Natu et al. 2021). The difficulty of early diagnosis and tumor heterogeneity are obstacles to effective treatment, especially for advanced HCC (Hu et al. 2022). As a cancer with very low 5-year relative survival rate (18%), it is essential to develop novel and effective biomarkers for early diagnosis and prognostic prediction to guide clinical treatment of liver cancer (Siegel et al. 2020). The liver is a major place of glycosylation in the body, which produces most of the glycosylated serum proteins. Disturbances in glycosylation can reflect the progression of liver diseases, including hepatic fibrosis, tumorigenesis and metastasis (Duan et al. 2018; Zhang et al. 2017). So far, several enzymes involved in N-glycan processing have been identified as candidate markers for the prognosis of HCC patients (Takayama et al. 2020).
As a member of the mannosyl-transferases family, ALG1 participates in the formation of the lipid-linked oligosaccharide precursor in the initial stage of protein glycosylation (Lombard 2016; Takahashi et al. 2000). ALG1 adds the first mannose onto GlcNAc2-PP-Dol to produce a core trisaccharide Man1GlcNAc2-PP-Dol, which is essential for assembling the dolichol-pyrophosphate-GlcNAc2-Man5 intermediate on the cytoplasmic surface of the endoplasmic reticulum (Li et al. 2017; Ng et al. 2016). Defective ALG1 causes ALG1-CDG, accompanied by severe symptoms including coagulation abnormalities, ascites, hepatomegaly, nephrotic syndrome (Rohlfing et al. 2014). However, little is known about the clinical implications and biological functions of ALG1 in liver cancer.
In the present study, the expression level of ALG1 in HCC was first examined. Compared with adjacent normal tissues, the expression of ALG1 decreased significantly at the transcription and translation levels. Besides, low expression of ALG1 in HCC was significantly associated with HBsAg status and tumor differentiation, indicating that ALG1 might be involved in the virus infection and the differentiation of cells. Multivariate Cox regression analysis showed that the expression level of ALG1 was an independent factor affecting the survival of HCC patients, which might constitute a prognostic predictor in liver cancer. For a deeper understanding of the underlying mechanisms, we applied an AHA-mediated protein labeling technique, to quantitate the newly synthesized protein changes during the down-regulation of ALG1 (Ma et al. 2017, 2018; McClatchy et al. 2018). Consistent with a previous study, our data showed that cellular adhesion was disturbed in response to ALG1 RNA interference, which potentially reflected the early stress response of cells under N-glycosylation inhibition (Shu et al. 2019).
It was speculated that the changes of the related functional glycoproteins might reflect the intracellular biological processes when the N-glycosylation synthesis pathway was blocked by ALG1 RNA interference. A novel finding in this study was that the two glyco-peptides from N-cadherin were significantly decreased by knockdown of ALG1, suggesting that the fully glycosylated N-cadherin was blocked up. As a highly glycosylated membrane protein, N-cadherin is known as a key protein involved in tumor invasion and metastasis (Xu et al. 2017). Notably, decreased expression of N-cadherin correlated with higher histopathological grade and a marked increase in invasive and migrative behavior (Asano et al. 1997, 2004). Moreover, removal of site-specific N-glycan dramatically affected the molecular organization and stability of cadherin, accompanied by elevated cell migration (Liwosz et al. 2006; Xu et al. 2017; Zhao et al. 2008). Current knowledge suggested that incomplete N-glycan biosynthesis suppressed the expression of glycosylated N-cadherin, resulting in damage to cell adhesion. Furthermore, studies in animal models are also necessary to clarify whether the expression of ALG1 affects tumor progression.
In summary, our results indicated for the first time that the expression of ALG1 was down-regulated in HCC compared to normal hepatic tissues and might serve as a prognostic indicator for HCC patients. Additionally, knockdown of ALG1 inhibited the expression of glycosylated N-cadherin, suppressed cell–cell adhesion and promoted cell migration. Our findings implied that the control of cell migration capacity by regulation of glycosyltransferases was important in hepatocellular carcinoma progression and therefore indicated that modulating glycoprotein biosynthesis might represent a new treatment strategy.
Conclusion
Immuno-histochemical analysis identified the clinical significance of glycosyltransferase ALG1 in primary hepatocellular carcinoma. Knockdown of ALG1 by siRNA in cell cultures and nascent proteome/glyco-proteome methodologies provided direct evidence for the involvement of ALG1 in tumor migration. Moreover, site-specific changes in the N-glycan structures of N-cadherin were detected by regulating ALG1 expression. The findings indicate that ALG1 may act as a prognostic marker in hepatocellular carcinoma and the impact of changes in glycosylation on tumor metastasis has been underestimated.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank openbiox community and Hiplot team (https://hiplot.com.cn) for providing technical assistance and valuable tools for data analysis and visualization. The authors thank Shu Zhang from Zhongshan Hospital, Fudan University, Shanghai, China, for her generous support. We acknowledge the financial support of the National Key Research and Development Program of China (2017YFA0505100) and NSF of China (Grants 21974025 and 82121004) for this work.
Abbreviations
- ALG1
Asparagine-linked glycosylation protein 1 homolog
- HCC
Hepatocellular carcinoma
- ER
Endoplasmic reticulum
- CDG
Congenital disorders of glycosylation
- RT-qPCR
Real-time quantitative PCR
- HRP
Horseradish peroxidase
- DMEM
Dulbecco's modified Eagle's medium
- FBS
Fetal bovine serum
- siRNA
Small interfereing RNA
- AHA
Azidohomoalanine
- GO
Gene ontology
- BP
Biological process
- H
Hexose
- N
N-Acetylhexosamine
- S
Sialic acid
- F
Fucose
Authors’ Contributions
Conceptualization: XC. Methodology: YS and PM. Visualization: ZC. Investigation: GY, JY, XZ. Software: CL. Data curation: LZ. Writing—original draft: HS. Writing—reviewing and editing: HL.
Data Availability
Partial mass spectrometric data and analyzed result datasets have been deposited in iProX (http://www.iprox.org), which is an official member of ProteomeXchange Consortium. The project ID is IPX0003587000.
Code Availability
Not applicable.
Declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
The studies involving human participants were reviewed and approved by the Research Ethics Committee of First Affiliated Hospital of Guangxi Medical University. Approval number was 2019(KY-E-086). The privacy rights of human subjects were also observed. No animal experimentation was performed.
Consent to Participate
Each participant provided written informed consent.
Consent to publish
Not applicable.
Footnotes
Xinyi Cao and Yuyin Shao have contributed equally.
Contributor Information
Hong Shu, Email: shuhong@gxmu.edu.cn.
Haojie Lu, Email: luhaojie@fudan.edu.cn.
References
- Asano K, Duntsch CD, Zhou Q, Weimar JD, Bordelon D, Robertson JH, Pourmotabbed T. Correlation of N-cadherin expression in high grade gliomas with tissue invasion. J Neurooncol. 2004;70:3–15. doi: 10.1023/b:neon.0000040811.14908.f2. [DOI] [PubMed] [Google Scholar]
- Asano K, Kubo O, Tajika Y, Huang MC, Takakura K, Ebina K, Suzuki S. Expression and role of cadherins in astrocytic tumors. Brain Tumor Pathol. 1997;14:27–33. doi: 10.1007/BF02478865. [DOI] [PubMed] [Google Scholar]
- Bargellini I, Battaglia V, Bozzi E, Lauretti DL, Lorenzoni G, Bartolozzi C. Radiological diagnosis of hepatocellular carcinoma. J Hepatocell Carcinoma. 2014;1:137–148. doi: 10.2147/JHC.S44379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Cao X, Cao Z, Shao Y, Liu C, Yan G, Meng X, Zhang L, Chen C, Huang G, Shu H, Lu H. Analysis of serum paraoxonase 1 using mass spectrometry and lectin immunoassay in patients with alpha-fetoprotein negative hepatocellular carcinoma. Front Oncol. 2021;11:651421. doi: 10.3389/fonc.2021.651421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich DC, Lee JJ, Link AJ, Graumann J, Tirrell DA, Schuman EM. Labeling, detection and identification of newly synthesized proteomes with bioorthogonal non-canonical amino-acid tagging. Nat Protoc. 2007;2:532–540. doi: 10.1038/nprot.2007.52. [DOI] [PubMed] [Google Scholar]
- Duan F, Wu H, Jia D, Wu W, Ren S, Wang L, Song S, Guo X, Liu F, Ruan Y, Gu J. O-GlcNAcylation of RACK1 promotes hepatocellular carcinogenesis. J Hepatol. 2018;68:1191–1202. doi: 10.1016/j.jhep.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Gao XD, Nishikawa A, Dean N. Physical interactions between the Alg1, Alg2, and Alg11 mannosyltransferases of the endoplasmic reticulum. Glycobiology. 2004;14:559–570. doi: 10.1093/glycob/cwh072. [DOI] [PubMed] [Google Scholar]
- Hu X, Chen R, Wei Q, Xu X. The Landscape of alpha fetoprotein in hepatocellular carcinoma: Where are we? Int J Biol Sci. 2022;18:536–551. doi: 10.7150/ijbs.64537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Lee SK, Li G, Yu SL, Alexander H, Alexander S. The Dictyostelium discoideum beta-1,4-mannosyltransferase gene, mntA, has two periods of developmental expression. Gene. 1997;204:251–258. doi: 10.1016/s0378-1119(97)00553-2. [DOI] [PubMed] [Google Scholar]
- Li ST, Wang N, Xu S, Yin J, Nakanishi H, Dean N, Gao XD. Quantitative study of yeast Alg1 beta-1, 4 mannosyltransferase activity, a key enzyme involved in protein N-glycosylation. Biochim Biophys Acta Gen Subj. 2017;1861:2934–2941. doi: 10.1016/j.bbagen.2016.09.023. [DOI] [PubMed] [Google Scholar]
- Lipinski P, Bogdanska A, Socha P, Tylki-Szymanska A. Liver involvement in congenital disorders of glycosylation and deglycosylation. Front Pediatr. 2021;9:696918. doi: 10.3389/fped.2021.696918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Song CQ, Yuan ZF, Fu Y, Chi H, Wang LH, Fan SB, Zhang K, Zeng WF, He SM, et al. pQuant improves quantitation by keeping out interfering signals and evaluating the accuracy of calculated ratios. Anal Chem. 2014;86:5286–5294. doi: 10.1021/ac404246w. [DOI] [PubMed] [Google Scholar]
- Liwosz A, Lei T, Kukuruzinska MA. N-glycosylation affects the molecular organization and stability of E-cadherin junctions. J Biol Chem. 2006;281:23138–23149. doi: 10.1074/jbc.M512621200. [DOI] [PubMed] [Google Scholar]
- Lombard J. The multiple evolutionary origins of the eukaryotic N-glycosylation pathway. Biol Direct. 2016;11:36. doi: 10.1186/s13062-016-0137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, McClatchy DB, Barkallah S, Wood WW, Yates JR., 3rd HILAQ: A novel strategy for newly synthesized protein quantification. J Proteome Res. 2017;16:2213–2220. doi: 10.1021/acs.jproteome.7b00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, McClatchy DB, Barkallah S, Wood WW, Yates JR., 3rd Quantitative analysis of newly synthesized proteins. Nat Protoc. 2018;13:1744–1762. doi: 10.1038/s41596-018-0012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-da-Silva D, Dos Reis FV, Monticelli M, Janeiro P, Videira PA, Witters P, Jaeken J, Cassiman D. Liver involvement in congenital disorders of glycosylation (CDG). A systematic review of the literature. J Inherit Metab Dis. 2017;40:195–207. doi: 10.1007/s10545-016-0012-4. [DOI] [PubMed] [Google Scholar]
- McClatchy DB, Ma Y, Liem DA, Ng DCM, Ping P, Yates JR., 3rd Quantitative temporal analysis of protein dynamics in cardiac remodeling. J Mol Cell Cardiol. 2018;121:163–172. doi: 10.1016/j.yjmcc.2018.07.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(Suppl 1):4–13. doi: 10.1002/hep.31288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natu A, Singh A, Gupta S. Hepatocellular carcinoma: understanding molecular mechanisms for defining potential clinical modalities. World J Hepatol. 2021;13:1568–1583. doi: 10.4254/wjh.v13.i11.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng BG, Shiryaev SA, Rymen D, Eklund EA, Raymond K, Kircher M, Abdenur JE, Alehan F, Midro AT, Bamshad MJ, et al. ALG1-CDG: clinical and molecular characterization of 39 unreported patients. Hum Mutat. 2016;37:653–660. doi: 10.1002/humu.22983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor SI, Hoffmann M, Rinis N, Bartels MF, Winterhalter PR, Hoelscher C, Hennig R, Himmelreich N, Thiel C, Ruppert T, et al. Glycosyltransferase POMGNT1 deficiency strengthens N-cadherin-mediated cell-cell adhesion. J Biol Chem. 2021;296:100433. doi: 10.1016/j.jbc.2021.100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez AS, Boilevin J, Lin CW, Ha Gan B, Janser D, Aebi M, Darbre T, Reymond JL, Locher KP. Chemo-enzymatic synthesis of lipid-linked GlcNAc2Man5 oligosaccharides using recombinant Alg1, Alg2 and Alg11 proteins. Glycobiology. 2017;27:726–733. doi: 10.1093/glycob/cwx045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexer TFT, Schildbach A, Klapproth J, Schierhorn A, Mahour R, Pietzsch M, Rapp E, Reichl U. One pot synthesis of GDP-mannose by a multi-enzyme cascade for enzymatic assembly of lipid-linked oligosaccharides. Biotechnol Bioeng. 2018;115:192–205. doi: 10.1002/bit.26454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfing AK, Rust S, Reunert J, Tirre M, Du Chesne I, Wemhoff S, Meinhardt F, Hartmann H, Das AM, Marquardt T. ALG1-CDG: a new case with early fatal outcome. Gene. 2014;534:345–351. doi: 10.1016/j.gene.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Shao Y, Bao H, Ma L, Yuan W, Zhang L, Yao J, Meng P, Peng Y, Zhang S, Cao T, Lu H. Enhancing comprehensive analysis of newly synthesized proteins based on cleavable bioorthogonal tagging. Anal Chem. 2021;93:9408–9417. doi: 10.1021/acs.analchem.1c00965. [DOI] [PubMed] [Google Scholar]
- Shu J, Dang L, Zhang D, Shah P, Chen L, Zhang H, Sun S. Dynamic analysis of proteomic alterations in response to N-linked glycosylation inhibition in a drug-resistant ovarian carcinoma cell line. FEBS J. 2019;286:1594–1605. doi: 10.1111/febs.14811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Honda R, Nishikawa Y. Cloning of the human cDNA which can complement the defect of the yeast mannosyltransferase I-deficient mutant alg 1. Glycobiology. 2000;10:321–327. doi: 10.1093/glycob/10.3.321. [DOI] [PubMed] [Google Scholar]
- Takayama H, Ohta M, Iwashita Y, Uchida H, Shitomi Y, Yada K, Inomata M. Altered glycosylation associated with dedifferentiation of hepatocellular carcinoma: a lectin microarray-based study. BMC Cancer. 2020;20:192. doi: 10.1186/s12885-020-6699-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bergen W, Heck AJR, Baggelaar MP. Recent advancements in mass spectrometry-based tools to investigate newly synthesized proteins. Curr Opin Chem Biol. 2021 doi: 10.1016/j.cbpa.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Liu Z, Xu X. Molecular subtyping of hepatocellular carcinoma: a step toward precision medicine. Cancer Commun (lond) 2020;40:681–693. doi: 10.1002/cac2.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Karaveg K, Moremen KW. Substrate recognition and catalysis by GH47 alpha-mannosidases involved in Asn-linked glycan maturation in the mammalian secretory pathway. Proc Natl Acad Sci U S A. 2016;113:E7890–E7899. doi: 10.1073/pnas.1611213113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Chang R, Xu F, Gao Y, Yang F, Wang C, Xiao J, Su Z, Bi Y, Wang L, Zha X. N-Glycosylation at Asn 402 stabilizes N-cadherin and promotes cell-cell adhesion of glioma cells. J Cell Biochem. 2017;118:1423–1431. doi: 10.1002/jcb.25801. [DOI] [PubMed] [Google Scholar]
- Zhang S, Cao X, Gao Q, Liu Y. Protein glycosylation in viral hepatitis-related HCC: characterization of heterogeneity, biological roles, and clinical implications. Cancer Lett. 2017;406:64–70. doi: 10.1016/j.canlet.2017.07.026. [DOI] [PubMed] [Google Scholar]
- Zhang S, Cao X, Liu C, Li W, Zeng W, Li B, Chi H, Liu M, Qin X, Tang L, et al. N-glycopeptide signatures of IgA2 in serum from patients with hepatitis B virus-related liver diseases. Mol Cell Proteomics. 2019;18:2262–2272. doi: 10.1074/mcp.RA119.001722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Liang Y, Xu Z, Wang L, Zhou F, Li Z, Jin J, Yang Y, Fang Z, Hu Y, et al. N-glycosylation affects the adhesive function of E-cadherin through modifying the composition of adherens junctions (AJs) in human breast carcinoma cell line MDA-MB-435. J Cell Biochem. 2008;104:162–175. doi: 10.1002/jcb.21608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Partial mass spectrometric data and analyzed result datasets have been deposited in iProX (http://www.iprox.org), which is an official member of ProteomeXchange Consortium. The project ID is IPX0003587000.
Not applicable.