Abstract
Birth weight (BW) is a key determinant of infant mortality. Previous studies have reported seasonal fluctuation of BW. However, the responsible environmental factors remain disputable. High-altitude environment provides a great opportunity to test the current hypotheses due to its distinctive climate conditions. We collected BW data of ~ 9000 Tibetan singletons born at Lhasa (elevation: 3660 m) from 2014 to 2018. Using regression models, we analyzed BW seasonality of highland Tibetans. Multivariate models with meteorological factors as independent variables were employed to examine responsible environmental factors accounting for seasonal variation. We compared BW, low-BW prevalence and sex ratio between highland and lowland populations, and we observed a significant seasonal pattern of BW in Tibetans, with a peak in winter and a trough in summer. Notably, there is a marked sex-biased pattern of BW seasonality (more striking in males than in females). Sunlight exposure in the 3rd trimester and barometric pressure exposure in the 2nd trimester are significantly correlated with BW, and the latter can be explained by seasonal change of oxygen partial pressure. In particular, due to the male-biased BW seasonality, we found a more serious BW reduction and higher prevalence of low-BW in males, and a skewed sex ratio in highlanders. The infant BW of highland Tibetans has a clear pattern of seasonality. The winter BW is larger than the summer BW, due to the longer sunlight exposure during the late-trimester. Male infants are more sensitive to hypoxia than female infants during the 2nd trimester, leading to more BW reduction and higher mortality.
Supplementary Information
The online version contains supplementary material available at 10.1007/s43657-021-00038-7.
Keywords: Birth weight, Seasonality, Sex-bias, High altitude, Tibetans
Introduction
Birth weight (BW) is an important index for measuring neonatal health, and it is closely related with infant morbidity and mortality (Kramer 1987; Wilcox 2001). Previous studies have reported that BW variation is associated to health status and disease risk in infancy, childhood and adulthood. These observations were proposed as “fetal origins of adult disease hypothesis”, indicating that intra-uterine exposures may impact health outcomes many years later (Byrne and Phillips 2000; Fall 2013). For example, a low BW usually correlates with a high risk of type 2 diabetes, coronary heart disease and even cancers in adults (Forsen et al. 2000; Tibblin et al. 1995). However, the causality underlying such correlation are yet to be dissected.
Intriguingly, according to the published data, BW exhibits significant monthly and seasonal fluctuations, which was taken as a direct support to the “fetal origins of adult disease hypothesis”. However, different studies reported inconsistent monthly/seasonal patterns of BW (Chodick et al. 2009; Hemati et al. 2021). Some studies reported that winter and spring births tend to be slightly heavier compared to summer and autumn births (Murray et al. 2000; Selvin and Janerich 1971), while other studies found the paradoxically heavier BW in summer births (Chodick et al. 2007; Day et al. 2015). In addition, there were studies showing no monthly or seasonal changes in BW (Bakwin and Bakwin 1931; Schreier et al. 2013).
Various mechanisms have been proposed to explain BW seasonality, including maternal exposures of meteorological factors, such as sunlight, rainfall, temperature and air pollution (Chodick et al. 2009; Day et al. 2015; Murray et al. 2000), as well as other factors such as food supply, dietary habit, physical activity and infectious diseases (Bantje 1987; Rayco-Solon et al. 2005; Steketee et al. 1996). These reported controversial findings about the effect of environmental factors on BW were recently summarized as two main competing factors: temperature and sunlight. The temperature theory proposes that the thermodynamics of pregnancy plays an important role in birth outcome. Exposure to low temperature increases the plasma level of fibrinogen and blood viscosity, and it induces vascular constriction in the placenta, thus diminishing utero-placental blood flow and reducing fetal growth (Keatinge et al. 1984; Murray et al. 2000; Neild et al. 1994). The sunlight theory suggests that sunlight directly promotes the production of maternal vitamin D and influences fetal vitamin D exposure in utero (Thorne-Lyman and Fawzi 2012). Given that vitamin D is important for bone development and fetal growth, and maternal circulating 25-hydroxyvitamin D (25OHD) levels have a marked seasonal change, sunlight is regarded as the causal factor for BW seasonality (Haggarty et al. 2013).
The discrepancy between these two main hypotheses on BW seasonality is largely due to the synchronized periodicity of temperature and sunlight in seasonal changes of the studied populations mostly living in lowland regions. Consequently, it was impossible to analyze each environmental factor separately. Fortunately, this hurdle can be circumvented by studying highland regions because the distinctive climate conditions at high altitude can clearly separate the seasonal fluctuation curves of temperature and sunlight.
In this study, we investigated BW seasonality and its causal environmental factors of highland Tibetans in Lhasa (elevation: 3660 m), China, where the peaks of temperature and sunlight can be clearly separated. This is the first systematic BW analysis of highlanders, which will provide new insights into fetal growth and mortality of human populations living in extreme environmental conditions.
Methods
Data Collection
We collected 9105 births during 2014–2018 in Lhasa (elevation = 3660 m) of Tibetan Autonomous Region, China. Informed consents were obtained from the legal guardians of all participants. We filtered samples based on the following criteria: (1) singleton birth; (2) all pregnancy periods occurred in Lhasa; (3) mothers had lived in Lhasa for at least a year; (4) nonsmoking by self-report; (5) at least 36-week gestational time; (6) healthy babies.
After filtering, 8918 individuals were included in our analysis (8463 Tibetans and 455 Han Chinese), with information of BW, maternal age, gestational age, infant sex, ethnicity, parity and data of birth. Only Tibetans were included in seasonality analysis of BW. Han Chinese were only used in comparison analysis of sex difference in BW, low-BW ratio and sex ratio.
Data on meteorological factors of Lhasa were obtained from the National Meteorological Information Centre of China (http://data.cma.cn/en) for the same period (2013–2018). The mean values of five meteorological factors of each month during 2013–2018 were obtained, including barometric pressure (kPa), temperature (°C), relative humidity (%), daily rainfall capacity ≥ 0.1 mm days (day) and percentage of sunlight per month (%). The mean monthly value of each meteorological factor was calculated for the first, second, and third trimesters of each pregnancy by using the gestational age. Sex-specific models were constructed by considering the infant sex as a covariate.
Statistical Analysis
Univariate comparisons of average BW between the months and seasons were conducted by using analysis of variance (ANOVA) tests. The adjusted mean BW of each month and season were calculated using the effects package of R. Linear regression models were employed to test the association between birth month/season and each of the meteorological factors. All models were adjusted for maternal age, gestational age, infant sex and parity. Analysis of each trimester was adjusted by the corresponding factor in the other two trimesters using a conditional model.
Multiple test corrections were conducted by raising the significance threshold according to the situation: p-value (0.05/12 months) = 0.004 is considered significant for BW monthly change; p-value (0.05/4 seasons) = 0.01 is considered significant for BW seasonal change; p-value (0.05/(5 factors × 3 trimesters × 2 sex)) = 0.0002 is considered a significant association between BW and meteorological factors. All p values involving multiple comparisons were adjusted by the Bonferroni test.
The births with BW < 2500 g were designated as low birth weight (low-BW) in accordance with the classification of the World Health Organization (Bramer 1988).
Results
BW Seasonality and Sex-biased Fluctuation Pattern in Highland Tibetans
We collected BW information of 8463 Tibetan singleton live births during the study period (2014–2018) in Lhasa after data filtering (see “Methods”). We also collected meteorological data of Lhasa (2013 to 2018), where the seasonal peaks of sunlight and temperature can be clearly separated (Fig. 1 and Supplementary Fig. 1). By contrast, no peak separation was detected in the lowland cities of Chongqing and Wuhan of China at similar latitudes during the time period (Supplementary Fig. 1).
Fig. 1.
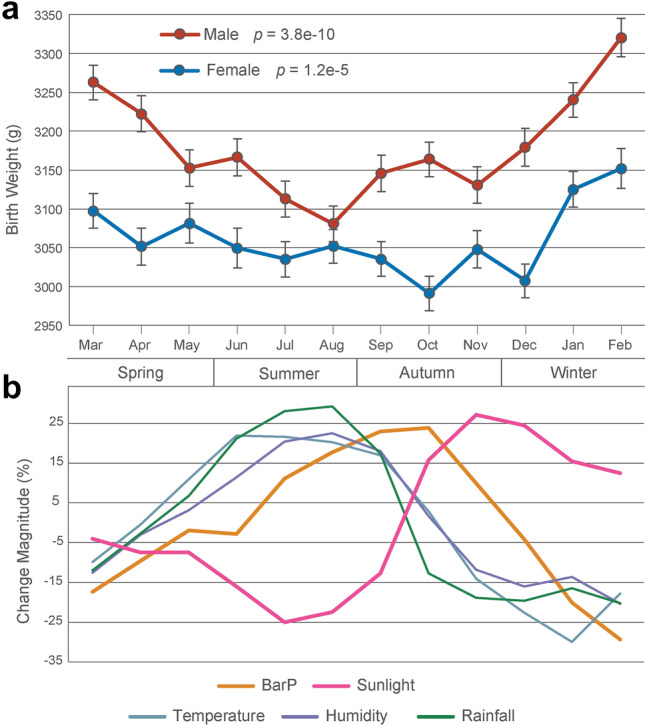
Monthly birth weight changes of Tibetans and fluctuation of meteorological factors in Lhasa. a Mean birth weight (sex separately) by month of birth after adjusted by maternal age, gestational age and parity. ANOVA is used to evaluate statistical significance. b Seasonal change of five meteorological factors in Lhasa (elevation: 3660 m)
Among the 8463 Tibetan live births, 50.8% are boys. The stats of basic information of these births are presented in Supplementary Table 1. Figure 1a shows the monthly BW of males and females, respectively (adjusted by maternal age, gestational age and parity). As expected, males are heavier than females for all monthly BW counts. There are significant between-month fluctuations for both males and females (p = 3.8e−10 for males and p = 1.2e−5 for females). For males, the heaviest BW is in February (3321 g) and the lightest in August (3081 g) (OR = 1.08, p = 1.9e−8). For females, the heaviest also occurs in February (3152 g) while the lightest occurs in October (2991 g) (OR = 1.05, p = 1.1e−4). When compared by four seasons, a clear seasonal pattern for BW is observed (Fig. 2a). In winter, BW is the heaviest for both males (3258 g) and females (3107 g), while the lightest for males (3120 g) is in summer (OR = 1.05; p = 3.8e−10) and the lightest for females (3046 g) is in autumn (OR = 1.03; p = 1.2e−5) (Supplementary Table 2).
Fig. 2.
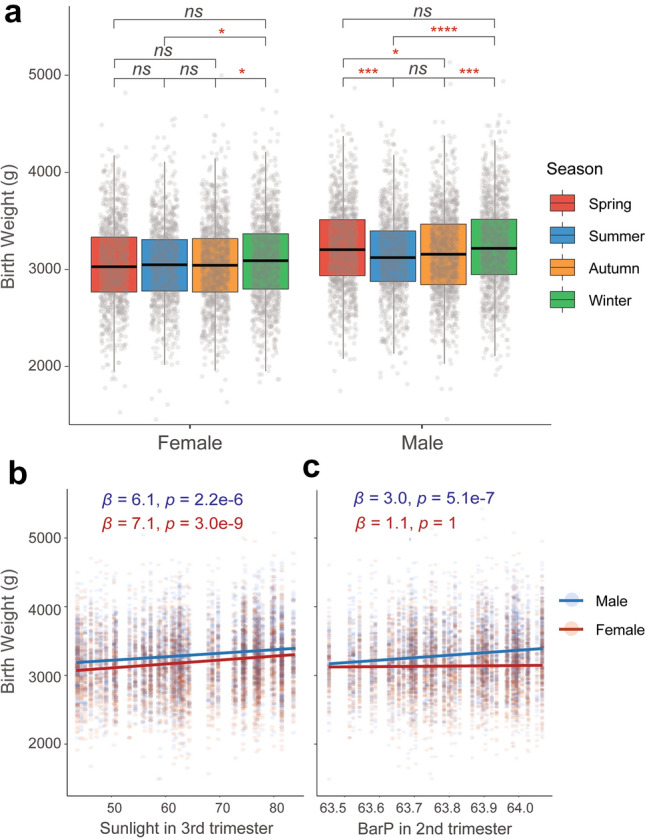
Seasonal fluctuation of BW and its correlation with multiple meteorological parameters. a Mean birth weight comparisons among the four birth seasons after adjusted by maternal age, gestational age and parity. ANOVA is used to evaluate statistical significance. Bonferroni corrections are conducted to adjust multiple comparisons. *p < 0.05; ***p < 0.001; ****p < 0.0001. b, c The correlations of sunlight and BarP exposure in the 3rd and the 2nd trimester with BW, respectively. Linear regression models are used to test the direction and significance of the associations (see “Methods”). β: regression coefficient; p value adjusted by covariates and Bonferroni corrections
Notably, we saw a sex-biased pattern of monthly and seasonal changes of BW (Figs. 1a and 2a), which was not reported in previous lowland BW studies. Male infants exhibit more dramatic BW changes than females. In other words, BW seasonality is more obvious in males than in females. In particular, no significant BW differences were detected in females among spring, summer and autumn (Fig. 2a). A similar pattern was observed when considering the maternal BMI (body mass index) as a confounding factor. This sex-biased pattern implies the potential sex-biased influence of high-altitude-specific environmental factors, such as hypobaric hypoxia.
Sunlight and Barometric Pressure Account for BW Seasonality in Tibetans
To test the effects of climate factors on monthly/seasonal fluctuation of BW, we then conducted detailed analyses of exposures to various meteorological parameters (barometric pressure (BarP), sunlight, temperature, humidity and rainfall) during each trimester of pregnancy (see “Methods”). As expected, we found a strong positive correlation between BW and sunlight (Fig. 2b). Both males and females exhibit significant associations. However, unlike the previous report of the 2nd trimester being the most significant stage (Day et al. 2015), we showed that the sunlight exposure in the 3rd trimester was the most significant (p = 2.2e−6 for males and p = 3.0e−9 for females, after Bonferroni corrections) for both sexes, while no significant correlations were seen for the 2nd trimester (p = 1 for males and females, after Bonferroni corrections; Table 1). In addition, there was a significant association with sunlight in the 1st trimester for females (p = 4.3e−5, after Bonferroni correction), but not for males (Table 1).
Table 1.
Associations of birth weight with five key meteorological factors during each trimester of pregnancy
| Factors | Trimesters | Male | Female | ||
|---|---|---|---|---|---|
| Effect (β)§ | p-value* | Effect (β)§ | p-value* | ||
| BarP | 1st | 0.27 | 1 | – 1.11 | 1 |
| 2nd | 2.97 | 5.1e−07 | 1.14 | 1 | |
| 3rd | – 1.37 | 1 | – 2.16 | 1 | |
| Sunlight | 1st | 1.42 | 1 | 5.94 | 4.3e−05 |
| 2nd | 0.83 | 1 | 0.85 | 1 | |
| 3rd | 6.06 | 2.2e-06 | 7.14 | 3.0e−09 | |
| Temperature | 1st | – 7.58 | 1 | – 11.46 | 1 |
| 2nd | 4.93 | 0.07 | 1.45 | 1 | |
| 3rd | – 18.34 | 0.21 | – 17.96 | 0.24 | |
| Humidity | 1st | 0.75 | 1 | – 2.98 | 1 |
| 2nd | 2.16 | 0.03 | 0.46 | 1 | |
| 3rd | – 2.79 | 1 | – 4.52 | 1 | |
| Rainfall | 1st | 3.45 | 1 | – 5.37 | 1 |
| 2nd | 2.38 | 1 | – 0.22 | 1 | |
| 3rd | – 4.92 | 1 | – 8.90 | 0.06 | |
Analysis of each trimester is adjusted by the corresponding factor in the other two trimesters using a conditional model
*All p-values are adjusted by Bonferroni corrections, and the p-values over the significant threshold are in bold
§The effect size is indicated by regression coefficient (β) of linear model
Importantly, we also observed a positive association of BW with BarP in males, but not in females during the 2nd trimester of pregnancy (male: p = 5.1e−7 and female: p = 1 after Bonferroni correction; Table 1 and Fig. 2c). Since BarP linearly reflects oxygen partial pressure (PO2), the observed sex-biased association hints that males are more sensitive to hypoxia than females during the 2nd trimester of pregnancy. No significant associations were observed with other factors (temperature, humidity and rainfall) after multiple test corrections (Table 1).
Sex-biased Sensitivity to Hypoxia Decreases Male BW and Survival Rate
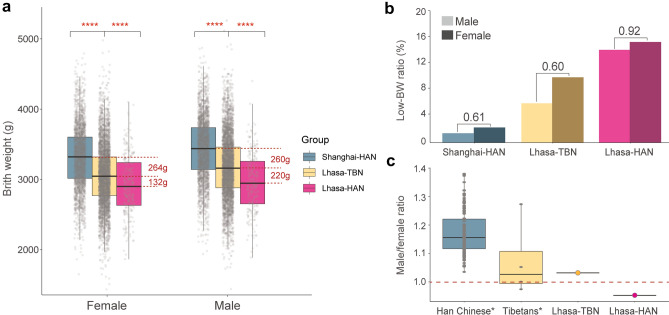
To further test the proposed hypoxia sensitivity of males, as reference, we collected BW data of 455 Han Chinese living at high altitude (all pregnancy periods occurred in Lhasa). We also obtained BW data of 4883 Han Chinese in Shanghai (sea level elevation) from a previous study (Du et al. 2010). We observed significant reductions of BW for both Tibetans and Han Chinese living in Lhasa when compared to those in Shanghai, indicating that high altitude environments indeed affect fetal development. Compared to Lhasa-Han, Lhasa-Tibetans show less BW reduction because they are genetically adaptive to such extreme conditions (Julian et al. 2009; Peng et al. 2011) (Fig. 3a and Supplementary Table 3). For Tibetans, the reduced weights are similar for males (260 g, 7.57%) and females (264 g, 7.95%). However, when comparing Han Chinese infants born in Lhasa with those in Shanghai, the BW reduction is more serious in males (480 g, 13.97%) than in females (396 g, 11.93%), suggesting that the Han Chinese male infants born in Lhasa experience more BW reduction than females (Fig. 3a and Supplementary Table 3).
Fig. 3.
Sex-biased BW reduction and sex ratio comparison among multiple datasets. a Comparisons of BW among Shanghai Han Chinese (HAN), Lhasa Tibetans (TBN) and Lhasa Han Chinese. ANOVA is used to test the significance. Bonferroni corrections are conducted to adjust multiple comparisons. ****p < 0.0001. b Comparison of low birth weight (low-BW) ratio among three populations. Male/female low-BW ratios are indicated, respectively. c Comparison of sex ratios (male/female) of Lhasa highlanders with other populations.*Sex ratio of lowland Han Chinese and highland Tibetans in China are from published data (Jiang et al. 2020)
Next, we checked the prevalence of low birth weight (low-BW, < 2500 g) (Bramer 1988) in these three populations, and we also saw a significant increase of low-BW ratio in males than in females in Han Chinese infants born in Lhasa (M/F low-BW ratio = 0.92), which is much higher than the ratio of Tibetan infants (0.60) and Han Chinese infants born in Shanghai (0.61) (Fig. 3b and Supplementary Table 4). It is known that low-BW is a dominant determinant of infant mortality. A drop of sex ratio (males/females) at birth would be expected in Han Chinese infants born in Lhasa if the observed male-biased BW reduction and the high low-BW incidence were true. We calculated sex ratio at birth of lowland Han Chinese and highland Tibetans since 2000 (Jiang et al. 2020) and compared it with our Lhasa data. The result shows that the sex ratio of Han Chinese infants born in Lhasa (M/F ratio = 0.95) is indeed lower than the ratios of Tibetan infants (1.03) and lowland Han Chinese infants (1.15) (Fig. 3c). Collectively, these data support the proposal that under high altitude conditions, male fetuses are likely more sensitive to hypobaric hypoxia than females during the 2nd trimester of pregnancy.
Discussion
In this study, we analyzed BW data of ~ 9000 Tibetan highlanders and delineated the seasonality pattern and the underlying environmental factors. This is the first report of BW seasonality of highland populations with a large sample size.
Previously, many studies were reported in geographic regions at different latitudes, but the findings were contradictory in view of seasonality pattern of BW and the underlying mechanisms. Currently, there are two major competing factors for BW seasonality: temperature and sunlight exposure. However, for lowland populations, it is hard to separate these two factors since they usually synchronously fluctuate with monthly periodicity. For example, summer is always at the peak of both sunlight exposure and high temperature, and winter at the trough of these two factors. Hence, highland areas such as the Tibetan plateau provide an ideal climatic system to study BW seasonality, where the peaks of temperature and sunlight exposure can be clearly separated (Fig. 1b and Supplementary Fig. 1). In addition, the unique environmental factors at high altitude, such as hypobaric hypoxia, serve as extra variables that have not been evaluated before.
Consistent with most of the published data, the Tibetan BW also shows a clear pattern that seasonality and sunlight exposure serve as the key environmental factors. Interestingly, unlike the previously reported 2nd trimester being the most significant stage (Day et al. 2015), the 3rd trimester was the most significant stage in Tibetans in our study. This is consistent with the view that the 3rd trimester has a higher incidence of severe deficiency of vitamin D than the 1st and the 2nd trimesters (Murad et al. 2019), and the concentration of infant 25OHD mainly depends on the maternal circulating plasma 25OHD level during the 3rd trimester (Thomas et al. 2011), implying that supplement vitamin D in late trimester of pregnancy might play a key role in fetal growth and BW.
The most interesting observation in this study is the male-biased pattern of BW seasonality in Tibetans, which has not been reported in previous studies. Compared to female infants, the Tibetan male infants exhibit more striking BW seasonal fluctuation (Figs. 1a and 2a). Given that other factors (maternal age, gestational age and parity) were already adjusted in our analysis, we speculate that the highland-specific factors are responsible. Among the five meteorological factors we investigated, BarP shows a male-biased association with BW, and only males show a positive association with BarP during the 2nd trimester of pregnancy. It was reported that low BarP (namely hypobaric) is closely related to hypoxia exposure (Beall 2007; Grocott et al. 2009). Hypobarism indicates low PO2 and low ambient oxygen levels. There is a seasonal periodicity of BarP in Lhasa with a peak in summer and autumn and a trough in spring and winter (Supplementary Fig. 1), meaning that the ambient oxygen is less in spring/winter than in summer/autumn. Therefore, hypobaric hypoxia at high altitude is the most likely explanation for the observed male-specific association for BarP in the 2nd trimester, and male fetuses are more likely to be sensitive than female fetuses.
Several potential mechanisms could explain the observed male-biased sensitivity to hypoxia. First of all, between-sex differences of lung function may provide clues. It was reported that compared with females, males have poorer respiratory function and more respiratory illnesses in infants, children and adults, leading to a higher premature birth rate and lower BW (Horiuchi et al. 2019; Schwartz et al. 1988; Thomas et al. 2006). In animal experiments, male rats show severer and more prevalent hypoxia response than female rats with lower ventilation and oxygen saturation during hypoxia (Holley et al. 2012).
Secondly, the second trimester (namely mid-trimester) is a period of rapid fetal weight gain, and reportedly associated with placental insufficiency and fetal growth restriction (Kennedy et al. 2020). Given the male-biased association of BarP is observed in the 2nd trimester, we propose that there is a “sensitive period” with a male-biased hypoxic response. This proposal is consistent with the previous observation that male fetuses suffer more than female fetuses in an adverse maternal environment, and a higher expression of ADAM12 was detected in mothers who carry male fetuses in the mid-trimester than those carrying female fetuses, reflecting a sex-biased placental response, resulting in a higher prevalence of preeclampsia for male-carrying mothers (Myers et al. 2015). In addition, for adults, there are sex differences in high-altitude adaptation (Murphy 2014; Nishimura et al. 2020), which is in line with the proposed male-biased hypoxic response. Although the molecular mechanism remains unclear, a rat study found sex hormones could impact ventilator response to hypoxia during critical periods of respiratory development, and male rats show severer and more prevalent hypoxia response than female rats (Holley et al. 2012). Also, sex hormones can act on carotid body (Joseph et al. 2002), the key arterial oxygen-tension-sensing organ, which may play a potential role in high-altitude hypoxic adaptation (Wu and Kayser 2006). Besides the male-biased hypoxic response, other factors may also be relevant. It was reported that the maternal vitamin D status might affect the sex ratio of offspring (Purdue-Smithe et al. 2021). Since we did not have vitamin D data of the studied Tibetans, whether vitamin D contributes to the observed sex-bias pattern is yet to be tested in future studies.
It should be noted that there are some limitations in this study: (1) although our observation of positive association of BW with sunlight exposure supports the vitamin D hypothesis, we did not collect maternal/fetal vitamin D data to establish a causal mechanism; (2) other potential factors need to be evaluated in the future, such as infectious diseases, physical activities and maternal sociodemographic characteristics and so on; (3) for the sex difference of BW and mortality under hypoxia, independent samples and larger sample size are needed to validate the results; (4) we have no direct evidence to support the impact of sex hormone on the sex-biased BW seasonality, which calls for further physiological and functional tests.
In summary, we identified a clear seasonality of highlander BW by analyzing ~ 9000 births in Lhasa. We provided evidence that sunlight exposure during the 3rd trimester is the major factor underlying BW variation among seasons, and our results support the vitamin D hypothesis. In addition, we discovered a clear sex-biased pattern in BW seasonality, where males show more striking seasonal changes than females. We propose that hypoxia is likely the cause of the male-biased BW seasonality due to the sex-biased hypoxic response during the 2nd trimester of pregnancy. To our knowledge, this is the first large sample study that can clearly separate the two main environmental factors, temperature and sunlight exposure, and also it is the first analysis of BW seasonality of highland populations, shedding more light on the environmental impacts on fetal development and health.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to all participants and their legal guardians in this study. This work was supported by grants from the National Natural Science Foundation of China (NSFC) (91631306 to B.S; 3217040584 and 32000390 to Y.H.; 32070578 to X.Q and 32170629 to H.Z.), the Youth Innovation Promotion Association of CAS (to Y.H.), the Science and Technology General Program of Yunan Province (202001AT070110 to Y.H.), the Provincial Natural Science Foundation of Tibet Autonomous Region (XZ2018ZRG-130 to J.L.) and Tibetan Fukang Hospital (2017-04 to J.L.).
Authors’ contributions
BS, YH, XQ and Ouzhuluobu conceived the project. XQ, JL, Ouzhuluobu, WZ, YG, HZ, LC, CL, HL and CC collected the data. YH and TY conducted the statistical analysis. YH, BS and XQ wrote the article with inputs from all authors.
Funding
This study was funded by grants from the National Natural Science Foundation of China (NSFC) (91631306 to B.S; 3217040584 and 32000390 to Y.H.; 32070578 to X.Q and 32170629 to H.Z.), the Youth Innovation Promotion Association of CAS (to Y.H.), the Science and Technology General Program of Yunnan Province (202001AT070110 to Y.H.), the Provincial Natural Science Foundation of Tibet Autonomous Region (XZ2018ZRG-130 to J.L.) and Tibetan Fukang Hospital (2017–04 to J.L.).
Declarations
Conflict of Interest
The authors declare no conflict of interest.
Ethics Approval
This study was approved by the Internal Review Board of Kunming Institute of Zoology, Chinese Academy of Sciences (Approval ID: SMKX-20160311–45) and the research scheme is in accordance with Regulations of the People's Republic of China on the Administration of Human Genetic Resources.
Consent to Participate
All the legal guardians of the participants signed the informed consents.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Consent for Publication
All the legal guardians of the participants approved to publish.
Footnotes
Yaoxi He, Jun Li, Tian Yue, Wangshan Zheng, and Yongbo Guo have contributed equally to this work.
Contributor Information
Ouzhuluobu, Email: tbciyang@163.com.
Xuebin Qi, Email: qixuebin@mail.kiz.ac.cn.
Bing Su, Email: sub@mail.kiz.ac.cn.
References
- Bakwin H, Bakwin RM. Body bulid in infants: III. Body build in disease. J Clin Invest. 1931;10(2):395–403. doi: 10.1172/JCI100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantje H. Seasonality of births and birthweights in Tanzania. Soc Sci Med. 1987;24(9):733–739. doi: 10.1016/0277-9536(87)90110-9. [DOI] [PubMed] [Google Scholar]
- Beall CM. Two routes to functional adaptation: Tibetan and Andean high-altitude natives. Proc Natl Acad Sci USA. 2007;104(Suppl 1):8655–8660. doi: 10.1073/pnas.0701985104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramer GR. International statistical classification of diseases and related health problems. Tenth Rev World Health Stat Q. 1988;41(1):32–36. [PubMed] [Google Scholar]
- Byrne CD, Phillips DI. Fetal origins of adult disease: epidemiology and mechanisms. J Clin Pathol. 2000;53(11):822–828. doi: 10.1136/jcp.53.11.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodick G, Flash S, Deoitch Y, Shalev V. Seasonality in birth weight: review of global patterns and potential causes. Hum Biol. 2009;81(4):463–477. doi: 10.3378/027.081.0405. [DOI] [PubMed] [Google Scholar]
- Chodick G, Shalev V, Goren I, Inskip PD. Seasonality in birth weight in Israel: new evidence suggests several global patterns and different etiologies. Ann Epidemiol. 2007;17(6):440–446. doi: 10.1016/j.annepidem.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Day FR, Forouhi NG, Ong KK, Perry JRB. Season of birth is associated with birth weight, pubertal timing, adult body size and educational attainment: a UK Biobank study. Heliyon. 2015;1(2):e00031. doi: 10.1016/j.heliyon.2015.e00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Xu H, Qin M, Tan J, Zhang L, Jin H, Zhu L. An investigation of birth weight of neonates and its influencing factors in a period from 1999 to 2008 in Shanghai city. Chin J Woman Child Health Res. 2010;21(1):4–7. [Google Scholar]
- Fall CH. Fetal malnutrition and long-term outcomes. Nestle Nutr Inst Worksh Ser. 2013;74:11–25. doi: 10.1159/000348384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsen T, Eriksson J, Tuomilehto J, Reunanen A, Osmond C, Barker D. The fetal and childhood growth of persons who develop type 2 diabetes. Ann Intern Med. 2000;133(3):176–182. doi: 10.7326/0003-4819-133-3-200008010-00008. [DOI] [PubMed] [Google Scholar]
- Grocott MP, Martin DS, Levett DZH, McMorrow R, Windsor J, Montgomery HE. Arterial blood gases and oxygen content in climbers on Mount Everest. N Engl J Med. 2009;360(2):140–149. doi: 10.1056/NEJMoa0801581. [DOI] [PubMed] [Google Scholar]
- Haggarty P, Campbell DM, Knox S, Horgan GW, Hoad G, Boulton E, McNeill G, Wallace AM. Vitamin D in pregnancy at high latitude in Scotland. Br J Nutr. 2013;109(5):898–905. doi: 10.1017/s0007114512002255. [DOI] [PubMed] [Google Scholar]
- Hemati Z, Keikha M, Riahi R, Daniali SS, Goudarzi M, Kelishadi R. A systematic review on the association of month and season of birth with future anthropometric measures. Pediatr Res. 2021;89(1):31–45. doi: 10.1038/s41390-020-0908-4. [DOI] [PubMed] [Google Scholar]
- Holley HS, Behan M, Wenninger JM. Age and sex differences in the ventilatory response to hypoxia and hypercapnia in awake neonatal, pre-pubertal and young adult rats. Respir Physiol Neurobiol. 2012;180(1):79–87. doi: 10.1016/j.resp.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi M, Kirihara Y, Fukuoka Y, Pontzer H. Sex differences in respiratory and circulatory cost during hypoxic walking: potential impact on oxygen saturation. Sci Rep. 2019;9(1):9550. doi: 10.1038/s41598-019-44844-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Ge T, Tai X. Change in China’s sex ratio at birth since 2000: a decomposition at the provincial level. Appl Spat Anal Policy. 2020;13(3):547–574. doi: 10.1007/s12061-019-09317-3. [DOI] [Google Scholar]
- Joseph V, Soliz J, Soria R, Pequignot J, Favier R, Spielvogel H, Pequignot JM. Dopaminergic metabolism in carotid bodies and high-altitude acclimatization in female rats. Am J Physiol Regul Integr Comp Physiol. 2002;282(3):R765–R773. doi: 10.1152/ajpregu.00398.2001. [DOI] [PubMed] [Google Scholar]
- Julian CG, Wilson MJ, Moore LG. Evolutionary adaptation to high altitude: a view from in utero. Am J Hum Biol. 2009;21(5):614–622. doi: 10.1002/ajhb.20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keatinge WR, Coleshaw SR, Cotter F, Mattock M, Murphy M, Chelliah R. Increases in platelet and red cell counts, blood viscosity, and arterial pressure during mild surface cooling: factors in mortality from coronary and cerebral thrombosis in winter. Br Med J (clin Res Ed) 1984;289(6456):1405–1408. doi: 10.1136/bmj.289.6456.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy LM, Tong S, Robinson AJ, Hiscock RJ, Hui L, Dane KM, Middleton AL, Walker SP, MacDonald TM. Reduced growth velocity from the mid-trimester is associated with placental insufficiency in fetuses born at a normal birthweight. BMC Med. 2020;18(1):395. doi: 10.1186/s12916-020-01869-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;65(5):663–737. [PMC free article] [PubMed] [Google Scholar]
- Murad R, Mahboob T, Rehman R, Baig R. Comparison of serum levels of vitamin D and vitamin D-binding protein in normal, osteopenic and osteoporotic postmenopausal women. Pak J Med Sci. 2019;35(2):543–548. doi: 10.12669/pjms.35.2.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy WG. The sex difference in haemoglobin levels in adults—mechanisms, causes, and consequences. Blood Rev. 2014;28(2):41–47. doi: 10.1016/j.blre.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Murray LJ, O’Reilly DPJ, Betts N, Patterson CC, Smith GD, Evans AE. Season and outdoor ambient temperature: effects on birth weight. Obstet Gynecol. 2000;96(5 Pt 1):689–695. doi: 10.1016/s0029-7844(00)01022-x. [DOI] [PubMed] [Google Scholar]
- Myers JE, Thomas G, Tuytten R, Herrewege Y, Djiokep RO, Roberts CT, Kenny LC, Simpson NAB, North RA, Baker PN. Mid-trimester maternal ADAM12 levels differ according to fetal gender in pregnancies complicated by preeclampsia. Reprod Sci. 2015;22(2):235–241. doi: 10.1177/1933719114537713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neild PJ, Syndercombe-Court D, Keatinge WR, Donaldson GC, Mattock M, Caunce M. Cold-induced increases in erythrocyte count, plasma cholesterol and plasma fibrinogen of elderly people without a comparable rise in protein C or factor X. Clin Sci (lond) 1994;86(1):43–48. doi: 10.1042/cs0860043. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Ugarte J, Ohnishi M, Nishihara M, Alvarez G, Yasukochi Y, Fukuda H, Arima K, Watanuki S, Mendoza V, Aoyagi K. Individual variations and sex differences in hemodynamics with percutaneous arterial oxygen saturation (SpO2) in young Andean highlanders in Bolivia. J Physiol Anthropol. 2020;39(1):31. doi: 10.1186/s40101-020-00240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Yang Z, Zhang H, Cui C, Qi X, Luo X, Tao X, Wu Y, Ouzhuluobu B, Ciwangsangbu D, Chen H, Shi H, Su B. Genetic variations in Tibetan populations and high-altitude adaptation at the Himalayas. Mol Biol Evol. 2011;28(2):1075–1081. doi: 10.1093/molbev/msq290. [DOI] [PubMed] [Google Scholar]
- Purdue-Smithe AC, Kim K, Nobles C, Schisterman EF, Schliep KC, Perkins NJ, Sjaarda LA, Freeman JR, Robinson SL, Radoc JG, Mills JL, Silver RM, Ye A, Mumford SL. The role of maternal preconception vitamin D status in human offspring sex ratio. Nat Commun. 2021;12(1):2789. doi: 10.1038/s41467-021-23083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayco-Solon P, Fulford AJ, Prentice AM. Differential effects of seasonality on preterm birth and intrauterine growth restriction in rural Africans. Am J Clin Nutr. 2005;81(1):134–139. doi: 10.1093/ajcn/81.1.134. [DOI] [PubMed] [Google Scholar]
- Schreier N, Moltchanova E, Forsén T, Kajantie E, Eriksson JG. Seasonality and ambient temperature at time of conception in term-born individuals—influences on cardiovascular disease and obesity in adult life. Int J Circumpolar Health. 2013;72:21466. doi: 10.3402/ijch.v72i0.21466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J, Katz SA, Fegley RW, Tockman MS. Sex and race differences in the development of lung function. Am Rev Respir Dis. 1988;138(6):1415–1421. doi: 10.1164/ajrccm/138.6.1415. [DOI] [PubMed] [Google Scholar]
- Selvin S, Janerich DT. Four factors influencing birth weight. Br J Prev Soc Med. 1971;25(1):12–16. doi: 10.1136/jech.25.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee RW, Wirima JJ, Hightower AW, Slutsker L, Heymann DL, Breman JG. The effect of malaria and malaria prevention in pregnancy on offspring birthweight, prematurity, and intrauterine growth retardation in rural Malawi. Am J Trop Med Hyg. 1996;55(1 Suppl):33–41. doi: 10.4269/ajtmh.1996.55.33. [DOI] [PubMed] [Google Scholar]
- Thomas MR, Marston L, Rafferty GF, Calvert S, Marlow N, Peacock JL, Greenough A. Respiratory function of very prematurely born infants at follow up: influence of sex. Arch Dis Child Fetal Neonatal Ed. 2006;91(3):F197–201. doi: 10.1136/adc.2005.081927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SD, Fudge AN, Whiting M, Coates PS. The correlation between third-trimester maternal and newborn-serum 25-hydroxy-vitamin D in a selected South Australian group of newborn samples. BMJ Open. 2011;1(2):e000236. doi: 10.1136/bmjopen-2011-000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne-Lyman A, Fawzi WW. Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Paediatr Perinat Epidemiol. 2012;26(Suppl 1):75–90. doi: 10.1111/j.1365-3016.2012.01283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibblin G, Eriksson M, Cnattingius S, Ekbom A. High birthweight as a predictor of prostate cancer risk. Epidemiology. 1995;6(4):423–424. doi: 10.1097/00001648-199507000-00017. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ. On the importance—and the unimportance—of birthweight. Int J Epidemiol. 2001;30(6):1233–1241. doi: 10.1093/ije/30.6.1233. [DOI] [PubMed] [Google Scholar]
- Wu T, Kayser B. High altitude adaptation in Tibetans. High Alt Med Biol. 2006;7(3):193–208. doi: 10.1089/ham.2006.7.193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.