Abstract
In the present study, we demonstrate that recombinant human secretory leukocyte protease inhibitor (rhSLPI) inhibits infection of lymphocyte- and monocyte-derived tumor cell lines and peripheral blood lymphocytes with laboratory-adapted isolates and with the primary isolate, NDK, of free human immunodeficiency virus type 1 (HIV-1). In contrast, rhSLPI did not exhibit inhibitory activity toward transcytosis of cell-associated HIV-1 through a tight monolayer of endometrial epithelial cells. These observations indicate that the inhibitory effect of SLPI is restricted to free HIV-1 in corporal fluids.
Human secretory leukocyte protease inhibitor (SLPI) is an 11.7-kDa single-chain polypeptide serine protease inhibitor, produced in epithelial cells (20), that concentrates in extravascular fluids, lining the mucosal surfaces. The physiological role of SLPI is primarily believed to ensure the protection of connective tissues from degradation by endogenous proteolytic enzymes of inflammatory leukocytes (15). SLPI may also exhibit additional functions as a host defense factor, consistent with its location in areas of the body routinely exposed to potential pathogens (e.g., the oral cavity, upper respiratory tract, and genital tract). In particular, SLPI is expressed at high concentrations in the lower female genital tract (6, 7), as shown in Fig. 1.
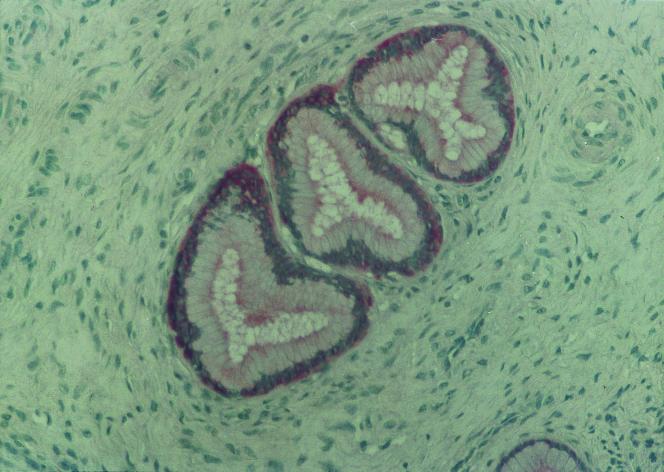
FIG. 1.
Immunohistochemical localization of SLPI in the endocervix. A sample of cervix tissue was obtained from a 35-year-old HIV-seronegative woman. Serial sections were saturated by treatment with 3% bovine serum albumin in phosphate-buffered saline (PBS) and further incubated with rabbit IgG anti-human SLPI (3 μg/ml) in PBS–0.1% bovine serum albumin. The sections were washed with PBS and revealed with biotinylated mouse Ig to rabbit Fcγ. After the sections had been washed, streptavidin-biotin-phosphatase alkaline complex was added for 30 min, and the slides were incubated with a chromogenic solution containing fast red. After the slides had been washed, they were counterstained with hematoxylin. Lung tissues were used as a positive control (not shown). The negative control included normal rabbit serum IgG instead of the anti-SLPI antibody. The secretory epithelium and glandular structures of the endocervix, but not the exocervix (not shown), showed marked immunoreactivities for SLPI (in red). Magnification, ×200.
A specific role for SLPI as an inhibitor of human immunodeficiency virus type 1 (HIV-1) infection has been suggested (12). Thus, the high concentrations of salivary SLPI could contribute to the antiviral activity of saliva that is associated with the infrequent oral transmission of HIV-1 (12, 13, 22, 23). SLPI was reported to inhibit the infection of purified monocytes and T cells and of peripheral blood mononuclear cells (PBMCs) with monocytotropic and lymphocytotropic HIV-1 isolates in vitro (18, 22). The antiviral activity of SLPI was shown to reside in the disruption of the infection process soon after virus binding (13). Thus, SLPI could prevent the transmucosal penetration of HIV-1 by inhibiting infection of mononuclear cells that are target cells for HIV in the mucosa (11). However, attempts to document the anti-HIV activity of SLPI unexpectedly failed in the hands of another group (21). This discrepancy has not been explained. In the present study, we reevaluated the anti-HIV-1 activity of SLPI toward lymphocytes and monocytes and further investigated its function by exploring its ability to inhibit viral transcytosis through the mucosal barrier.
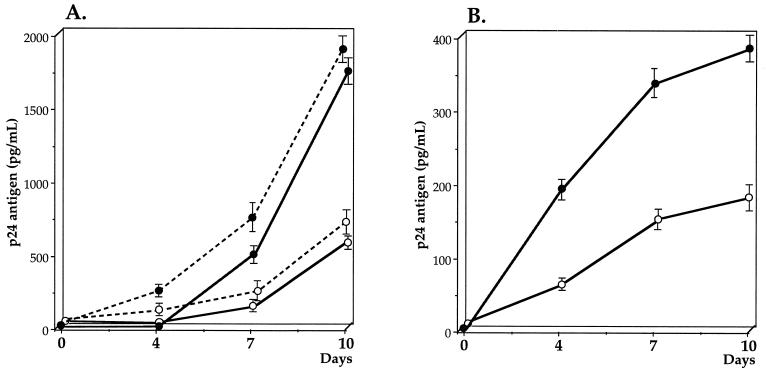
We have assessed the activity of recombinant human SLPI (rhSLPI) against free HIV-1 in cultures of the human lymphocyte-derived tumor cell line SupT1 and in peripheral blood lymphocytes (PBLs). A primary isolate of HIV-1 subtype A was obtained by culturing PBMCs of an African HIV-1-infected patient and further propagating the virus in the SupT1 cell line, as described previously (17). The strain was of the syncytium-inducing phenotype in a conventional assay with MT2 cells. Replication of HIV was quantitated by measuring p24 antigen released in culture supernatants with an immunocapture enzyme-linked immunosorbent assay (DuPont de Nemours, Wilmington, Del.). Purified rhSLPI was purchased from R & D Systems, Minneapolis, Minn. The biological activity of rhSLPI was measured by its ability to inhibit trypsin-catalyzed hydrolysis of tosyl-Gly-Pro-Lys-4-nitroanilide acetate at a 1:1 molar ratio of rhSLPI to activated trypsin. Free HIV-1 in an amount corresponding to 6,000 pg of p24 antigen was incubated with 20 μg of rhSLPI in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), under a final volume of 500 μl, or with culture medium alone, for 60 min at 37°C. The virus was then added to 2 × 106 SupT1 cells and incubated in RPMI 1640, in the presence of 10% FCS and antibiotics (penicillin and streptomycin) at 5% CO2 for 2 h at 37°C. The cells were washed three times with RPMI 1640 and further cultured for 10 days. The cultures were fed twice a week by replacement of culture medium. The concentration of p24 antigen was measured in culture supernatants at 0, 4, 7, and 10 days. As shown in Fig. 2, the exposure of free virus to rhSLPI resulted in decreases in HIV replication to 28% at day 4 and 33% at day 10, as compared with replication in control SupT1 cells that had been incubated with untreated virus. Similar results were obtained when the human promonocyte-derived tumor cell line U937 infected by HIV-1LAI was used (Fig. 2). In a control experiment, infectivity of free HIV collected in the basal chamber after transcytosis was conserved. We further assessed the inhibitory activity of rhSLPI on PBLs as an HIV-1 carrier. PBLs (8 × 105) were obtained by stimulating PBMCs of healthy donors with phytohemagglutinin (2 μg/ml) for 3 days prior to interleukin 2 stimulation (10 IU/ml) for 24 h. The primary HIV-1 NDK isolate (19) (5 ng of p24 antigen per ml) was incubated for 1 h at 37°C with rhSLPI (20 μg) or with RPMI 1640 before being used for infection of PBLs. The cells were further processed as described above. As shown in Fig. 2, exposure of free virus to rhSLPI resulted in decreases in HIV replication of 64% at day 4 and 47% at day 10, as compared with replication in control PBLs that had been incubated with untreated virus. The possible toxic effect of rhSLPI on cell line SupT1, promonocyte-derived tumor cell line U937, and PBLs was evaluated according to Trypan blue coloration at 4, 7, and 10 days of incubation. The percentages of dead cells were strictly similar in cells incubated with medium alone or with medium plus rhSLPI. These observations demonstrate that rhSLPI inhibits infection of both lymphocyte- and monocyte-derived tumor cell lines and PBLs with laboratory-adapted and primary isolates of HIV-1. Our observations are in accordance with previously reported findings of anti-HIV activity of rhSLPI in cultures of human monocytes (12) and lymphocytes (22).
FIG. 2.
Effect of rhSLPI on HIV-1 infection of lymphocyte tumor cell line SupT1, promonocyte-derived tumor cell line U937, and PBLs. (A) Free laboratory-adapted HIV-1 subtype A was incubated with rhSLPI (20 μg) (open circles) or RPMI 1640 (solid circles) for 1 h prior to addition to SupT1 cells (solid line) and further incubation for 2 h. The cells were then washed and cultured for 10 days. Cell supernatants were removed at 3- to 4-day intervals, and p24 antigen was quantitated in supernatants. Free HIV-1LAI was incubated with rhSLPI (open circles) or RPMI 1640 (closed circles) prior to addition to U937 cells (dotted line), as described above in the case of SupT1 cells. (B) Free primary isolate HIV-1 NDK was incubated with rhSLPI (20 μg) (open circles) or RPMI 1640 (solid circles) for 1 h at 37°C before infection of PBLs, as described above. Data are expressed as the mean ± standard error from the same experiment performed in duplicate.
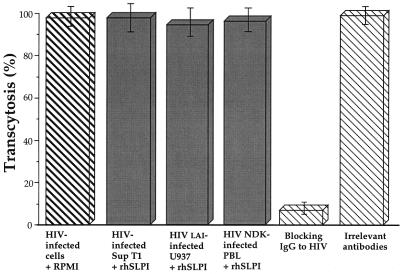
Several mechanisms are involved in the penetration of HIV-1 through the mucosal barrier. Both free and cell-associated HIV (11, 16) could reach submucosal CD4+ cells (i.e., T cells, monocytes/macrophages, and Langerhans' cells), provided that the integrity of the mucosa is compromised. In the presence of an intact mucosal surface, free HIV could enter the mucosa by infecting a susceptible cell harboring the CD4 receptor, such as Langerhans' cells, or by penetrating the mucosal epithelium through a cyclic AMP-dependent transport mechanism involving the interaction of the lectin-like domain of gp120 and mannosyl residues on glycoproteins on the mucosal surface (9). In addition, mononuclear cell-associated HIV may cross the genital mucosa, as shown in appropriate in vitro models of transcytosis (4). In order to test whether SLPI interferes with the latter mechanism of transmucosal penetration of HIV, in addition to its inhibitory effect on viral infection of lymphocytes and monocytes, we assessed the effect of rhSLPI on transcytosis of cell-associated HIV-1 in vitro. We used cells of the previously described model of the endometrial epithelial cell line HEC1 (3, 4) grown as a monolayer on a permeable support (4, 5, 8, 14). HEC1 cells do not express CD4, but express galactosylceramide (5) and CXCR4 coreceptor, as assessed by immunofluorescence staining with antihuman CXCR4 monoclonal antibody (R & D Systems) and reverse transcription-PCR (RT-PCR) for mRNA of CXCR4 by using previously described primers (2; data not shown). We verified that HEC1 cells do not produce SLPI, as assessed by negativity of immunofluorescence staining with polyclonal antibody to SLPI, as previously described (10), and lack of detection of SLPI mRNA in HEC1 cells by RT-PCR, as previously described (1). In brief, HEC1 cells were grown as a tight polarized monolayer on a permeable support of 0.4-μm-pore-diameter polycarbonate (Transwell; Costar, Cambridge, Mass.). The tightness of the monolayer of HEC-1 cells was monitored by measuring resistivity at the apical and basolateral poles of the cells, which was above 200 Ω/cm2. SupT1 cells were infected with HIV-1 subtype A, as described above. A total of 2 × 106 infected SupT1 cells were preincubated with RPMI 1640 medium supplemented with 10% FCS alone or in the presence of 1, 10, or 20 μg of rhSLPI, under a final 300-μl volume, prior to being deposited on the apical pole of the HEC1 monolayer. The cells were allowed to interact for 180 min at 37°C. A positive control for inhibition of HIV transcytosis consisted of specific anti-HIV immunoglobulin G (IgG) antibodies purified from pooled HIV-seropositive sera. HIV transcytosis was monitored in the basolateral chamber by measuring the concentration of p24 antigen. As shown in Fig. 3, at all concentrations of rhSLPI that we used, preincubation of infected cells with rhSLPI did not result in inhibition of transcytosis. HIV transcytosis was markedly inhibited, however, by anti-HIV IgG antibodies purified from pooled HIV-seropositive serum (1 μg/ml), but not by purified HIV-seronegative IgG. rhSLPI did not inhibit transcytosis when preincubated with epithelial cells before the addition of HIV-infected cells. Similar results were obtained with PBLs infected with the primary isolate HIV-1 NDK (Fig. 3). The lack of inhibitory activity of rhSLPI on transcytosis was also observed with U937 monocytes infected with the HIV-1LAI isolate (Fig. 3).
FIG. 3.
Lack of inhibitory activity of rhSLPI on transcytosis through a tight epithelial monolayer of SupT1 cell-associated HIV-1 subtype A, of U937 cell-associated HIVLAI, and of the PBL-associated primary HIV-1 NDK isolate. Transcytosis was expressed as the percentage of p24 antigen recovered in the basolateral chamber in the presence of rhSLPI compared with the amount of p24 antigen recovered in the presence of irrelevant IgG. HIV transcytosis was markedly inhibited by immunopurified human polyclonal anti-gp160 IgG antibodies (positive control). No inhibition of transcytosis was observed after preincubation of HIV-infected cells with HIV-seronegative IgG, RPMI 1640 alone, or rhSLPI. Data are expressed as the mean percentage ± standard error in the same experiment performed in duplicate. Only data obtained with SLPI (20 μg) are shown.
Our findings confirm that rhSLPI expresses antiviral activity against free HIV in a model using monocyte- and lymphocyte-derived tumor cell lines and PBLs and document the lack of inhibitory activity of rhSLPI on transcytosis of cell-associated HIV through a monolayer of epithelial cells. We have previously evaluated the cervicovaginal production of SLPI under normal conditions (unpublished data). Cervicovaginal secretion lavage samples from 15 healthy HIV-negative women without genital symptoms were evaluated for SLPI by enzyme-linked immunosorbent assay (R & D Systems). The cervicovaginal SLPI concentration (mean ± standard error) in healthy HIV-negative women was 8.76 ± 0.85 μg/ml. Thus, the concentration of rhSLPI we used in the present study was close to the physiological cervicovaginal concentrations of SLPI.
Taken together with the results of previously published studies (4, 5, 18, 22), these observations suggest that the inhibitory effect of SLPI is restricted to free HIV-1 in corporal fluids, such as saliva, cervicovaginal secretions, or breast milk. The latter effect may be particularly relevant to hamper infection of both intraepithelial Langerhans' cells and submucosal CD4-expressing cells reached by the virus through lesions in the epithelium layer. The lack of inhibition of HIV transcytosis by rhSLPI suggests that SLPI has no effect on cell-mediated infection of mucosae. These observations may be of significance, given the fact that SLPI is largely expressed in the endocervix and the endometrium (6, 7). Our data indicate that the relevance of SLPI in decreasing the infectiousness of the index partner or the susceptibility to HIV of an exposed individual should be considered in the context of the multiple pathways that may be used by HIV to cross the mucosae.
Acknowledgments
We are indebted to Véronique Marchand for providing anti-SLPI antibodies for immunochemistry.
Grant support was provided by Agence Nationale de Recherches contre le SIDA (ANRS FFC007) and Sidaction, France.
REFERENCES
- 1.Abe T, Kobayashi N, Yoshimura K, Trapnell B C, Kim H, Hubbard R C, Brewer M T, Thompson R C, Crystal R G. Expression of the secretory leukoprotease inhibitor gene in epithelial cells. J Clin Investig. 1991;87:2207–2215. doi: 10.1172/JCI115255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amara A, Gall S L, Schwartz O, Salamero J, Montes M, Loetscher P, Baggiolini M, Virelizier J-L, Arenzana-Seisdedo F. HIV coreceptor downregulation as viral principle: SDF-1alpha-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bal J M, Moldoveanu Z, Melsen L R, Koslowsky P A, Jackson S, Mulligan M J, Mestecky J F, Compans R W. A polarized endometrial cell line that binds and transports polymeric IgA. In Vitro Cell Dev Biol. 1995;31:196–206. doi: 10.1007/BF02639434. [DOI] [PubMed] [Google Scholar]
- 4.Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat Med. 1997;3:42–47. doi: 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- 5.Bomsel M, Heyman M, Hocini H, Lagaye S, Belec L, Dupont C, Desgranges C. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope dIgA or IgM. Immunity. 1998;9:277–287. doi: 10.1016/s1074-7613(00)80610-x. [DOI] [PubMed] [Google Scholar]
- 6.Casslen B, Rosengren M, Ohlsson K. Localization and quantitation of a low molecular weight proteinase inhibitor, antileukoprotease, in the human uterus. Hoppe-Seylers Z Physiol Chem. 1981;362:953–958. doi: 10.1515/bchm2.1981.362.2.953. [DOI] [PubMed] [Google Scholar]
- 7.Franken C, Meijer C J L M, Dijkman J H. Tissue distribution of antileukoprotease and lysozyme in humans. J Histochem Cytochem. 1989;37:493–498. doi: 10.1177/37.4.2926127. [DOI] [PubMed] [Google Scholar]
- 8.Hocini H, Belec L, Iscaki S, Garin B, Pillot J, Becquart P, Bomsel M. High-level ability of secretory IgA to block HIV type 1 transcytosis: contrasting secretory IgA and IgG responses to glycoprotein 160. AIDS Res Hum Retrovir. 1997;13:1179–1185. doi: 10.1089/aid.1997.13.1179. [DOI] [PubMed] [Google Scholar]
- 9.Kage A, Shoolian E, Rokos K, Özel M, Nuck R, Reutter W, Köttgen E, Pauli G. Epithelial uptake and transport of cell-free human immunodeficiency virus type 1 and gp120-coated microparticles. J Virol. 1998;72:4231–4236. doi: 10.1128/jvi.72.5.4231-4236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchand V, Tournier J-M, Polette M, Nawrocki B, Fuchey C, Pierrot D, Burlet H, Puchelle E. The elastase-induced expression of secretory leukocyte protease inhibitor is decreased in remodelled airway epithelium. Eur J Pharmacol. 1997;336:187–196. doi: 10.1016/s0014-2999(97)01222-3. [DOI] [PubMed] [Google Scholar]
- 11.Mayer K, Anderson D. Heterosexual HIV transmission. Infect Agents Dis. 1995;4:273–284. [PubMed] [Google Scholar]
- 12.McNeely T B, Dealy M, Dripps D J, Orenstein J M, Eisenberg S P, Wahl S M. Secretory leucocyte protease inhibitor: a human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J Clin Investig. 1995;96:456–462. doi: 10.1172/JCI118056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNeely T B, Shugars D C, Rosendahl M, Tucker C, Eisenberg S P, Wahl S M. Inhibition of human immunodeficiency virus type 1 infectivity by secretory leukocyte protease inhibitor occurs prior to viral reverse transcription. Blood. 1997;90:1141–1149. [PubMed] [Google Scholar]
- 14.Phillips D M, Bourinbaiar A S. Mechanism of HIV spread from lymphocytes to epithelia. Virology. 1992;186:261–273. doi: 10.1016/0042-6822(92)90080-9. [DOI] [PubMed] [Google Scholar]
- 15.Rice W G, Weiss S J. Regulation of proteolysis at the neutrophil-substrate interface by secretory leukoprotease inhibitor. Science. 1990;249:178–181. doi: 10.1126/science.2371565. [DOI] [PubMed] [Google Scholar]
- 16.Royce R A, Sena A, Cates W, Cohen M S. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 17.Sato H, Orenstein J, Dimitrov D, Martin M. Cell-to-cell spread of HIV-1 occurs within minutes and may not involve the participation of virus particles. Virology. 1992;186:712–724. doi: 10.1016/0042-6822(92)90038-q. [DOI] [PubMed] [Google Scholar]
- 18.Shugars D C, Fauls D L, Weinberg J B. Secretory leukocyte protease inhibitor blocks infectivity of primary monocytes and mononuclear cells with both monocytotropic and lymphocytotropic strains of human immunodeficiency virus type 1. Oral Dis. 1997;3(Suppl. 1):S70–S72. doi: 10.1111/j.1601-0825.1997.tb00379.x. [DOI] [PubMed] [Google Scholar]
- 19.Spire B, Sire J, Zachar V, Rey F, Barré-Sinoussi F, Galibert F, Hampe A, Chermann J-C. Nucleotide sequence of HIV-1 NDK, a highly cytopathic strain of the human immunodeficiency virus HIV-1. Gene. 1989;81:275–284. doi: 10.1016/0378-1119(89)90188-1. [DOI] [PubMed] [Google Scholar]
- 20.Thompson R C, Ohlsson K. Isolation, properties, and complete amino acid sequence of human secretory leukocyte protease inhibitor, a potent inhibitor of leukocyte elastase. Proc Natl Acad Sci USA. 1986;83:6692–6696. doi: 10.1073/pnas.83.18.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turpin J A, Schaeffer C A, Bu M, Graham L, Buckheit R W, Clanton D, Rice W G. Human immunodeficiency virus type 1 (HIV-1) replication is unaffected by human secretory leukocyte protease inhibitor. Antivir Res. 1996;29:269–277. doi: 10.1016/0166-3542(95)00907-8. [DOI] [PubMed] [Google Scholar]
- 22.Wahl S M, McNeely T B, Janoff E N, Shugars D, Worley P, Tucker C, Orenstein J M. Secretory leukocyte protease inhibitor (SLPI) in mucosal fluids inhibits HIV-1. Oral Dis. 1997;3(Suppl. 1):S64–S69. doi: 10.1111/j.1601-0825.1997.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 23.Wahl S M, Worley P, Jin W W, McNeely T B, Eisenberg S, Fasching C, Orenstein J M, Janoff E D. Anatomic dissociation between HIV-1 and its endogenous inhibitor in mucosal tissues. Am J Pathol. 1997;150:1275–1284. [PMC free article] [PubMed] [Google Scholar]