Abstract
Positron emission tomography (PET) represents molecular imaging for non-invasive phenotyping of physiological and biochemical processes in various oncological diseases. PET imaging with 18F-fluorodeoxyglucose (18F-FDG) for glucose metabolism evaluation is the standard imaging modality for the clinical management of lymphoma. One of the 18F-FDG PET applications is the detection and pre-treatment staging of lymphoma, which is highly sensitive. 18F-FDG PET is also applied during treatment to evaluate the individual chemo-sensitivity and accordingly guide the response-adapted therapy. At the end of the therapy regiment, a negative PET scan is indicative of a good prognosis in patients with advanced Hodgkin’s lymphoma and diffuse large B-cell lymphoma. Thus, adjuvant radiotherapy may be alleviated. Future PET studies using non-18F-FDG radiotracers, such as 68Ga-labeled pentixafor (a cyclic pentapeptide that enables sensitive and high-contrast imaging of C–X–C motif chemokine receptor 4), 68Ga-labeled fibroblast activation protein inhibitor (FAPI) that reflects the tumor microenvironment, and 89Zr-labeled atezolizumab that targets the programmed cell death-ligand 1 (PD-L1), may complement 18F-FDG and offer essential tools to decode lymphoma phenotypes further and identify the mechanisms of lymphoma therapy.
Keywords: Positron emission tomography (PET), Lymphoma, Glucose metabolism, Staging, Response assessment
Introduction
Positron emission tomography (PET) is a representative technique of molecular imaging that provides non-invasive phenotyping of biochemical processes at the molecular level in living subjects, based on the principle of tracing radioactivity. Since PET enables in vivo quantification of radioisotopes in nanomolar to picomolar level (Jacobs et al. 2003; Phelps 2000), it can provide whole-body phenotypic information and serve as a transpathological approach to precisely assess protein expression, receptor availability, transporter systems, signal transduction, gene mutation and histological profiles (Chen et al. 2017; Jacobs et al. 2003; Tian et al. 2021; Zhu et al. 2017). With the rapid development of equipment and probes, PET molecular imaging can detect the pathophysiological changes of various oncological diseases with high sensitivity, thus playing a vital role in diagnosing, evaluating, and treating many types of cancers (Jones and Price 2012; Rohren et al. 2004).
Lymphoma is a group of hematological malignancies categorized into Hodgkin’s lymphoma (HL) and non-Hodgkin’s lymphoma (NHL). NHL can be further subclassified into aggressive and indolent NHL, together accounting for more than 30 distinct subtypes with different diagnostic, prognostic, and therapeutic implications (Armitage et al. 2017). The definite diagnosis and molecular analysis of lymphoma rely predominantly on histopathological assessment. However, due to the high degree of clinical and biological heterogeneity of lymphoma, traditional single-biopsy may not provide a comprehensive and global characterization of the disease. PET/computed tomography (PET/CT) with the glucose analog 18F-fluorodeoxyglucose (18F-FDG) can provide whole-body glucose metabolism evaluation and be used extensively as an indispensable tool for pre-treatment staging, response assessment, and outcome prediction of lymphoma (Barrington and Kludge 2017; Barrington et al. 2014). More recently, with therapeutic advances in lymphoma, such as the advent of targeted therapies, additional novel PET tracers have been developed, which hold promise for visualizing other metabolic and receptor pathways at diagnosis and during treatment.
Although PET/CT has been widely applied in the diagnosis and treatment of lymphoma, the need for repeated examinations in which both PET and CT involve ionizing radiation is a concern for lymphoma patients, especially in younger individuals, and late risks of radiation exposure should be taken into account (Pearce et al. 2012). Recent advance in imaging hardware, such as the hybrid PET/magnetic resonance imaging (PET/MRI), offers the opportunity of gleaning the metabolic activity by PET and anatomic information by MRI while reducing the exposure to ionizing radiation compared to PET/CT (Torigian et al. 2013; Wehrl et al. 2015). Moreover, MRI also offers possibilities to evaluate tissue structure and metabolism not offered by CT, such as diffusion-weighed imaging (DWI) or MR spectroscopy (Mayerhoefer et al. 2015; Punwani et al. 2013).
In this review, we present an overview of the role of 18F-FDG PET/CT in managing patients with lymphoma, with an emphasis on HL, diffuse large B-cell lymphoma (DLBCL), and follicular lymphoma (FL). Furthermore, PET tracers other than 18F-FDG and their potential alternative applications, including PET image-guided therapy are discussed. We also discuss recent advances regarding the clinical application of 18F-FDG PET/MRI in lymphoma.
18F-FDG PET/CT in Pre-treatment Staging of Lymphoma
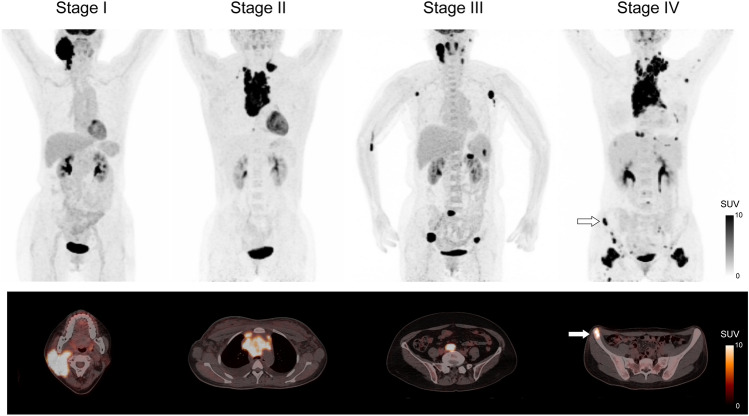
An accurate disease staging leads to better treatment planning and outcome. Current guidelines from the International Conference on Malignant Lymphoma recommend 18F-FDG PET/CT for staging in all lymphomas, except some indolent NHL subtypes such as small lymphocytic lymphoma and marginal zone lymphoma (Barrington et al. 2014; Cheson et al. 2014) (Fig. 1 and Table 1). 18F-FDG PET/CT shows superior staging accuracy than CT in HL and NHL, mainly due to improved detection of lymphoma in small lymph nodes and at extranodal sites. In the Response-Adapted Therapy in Hodgkin’s Lymphoma study (RATHL), 18F-FDG PET/CT led to upstaging in 14% of the patients and downstaging in 6% of the patients, with the extranodal disease in bone marrow, lung, or multiple sites accounting for the most discrepancies (Barrington et al. 2016). The superior sensitivity of 18F-FDG PET in lesion detection has made bone marrow biopsy (BMB) unnecessary for risk assessment in Hodgkin’s lymphoma. Importantly, increased sensitivity with FDG PET does not come at the cost of reduced specificity (Hutchings et al. 2006a). The focal FDG uptake in bone marrow could be detected by PET in 20% of newly diagnosed HL, compared to 5% detected by BMB (Barrington et al. 2016; El-Galaly et al. 2012a). Since BMB can rarely add more to diagnostic value to 18F-FDG PET/CT in staging HL, it is discouraged by the current guidelines (Cheson et al. 2014).
Fig. 1.
Pre-treatment 18F-FDG PET/CT for staging of lymphoma based on the Ann Arbor staging system. Stage I is defined as lymphomatous involvement confined to a single lymph node region or extranodal site. Stage II refers to lymphomatous involvement of more lymph node regions on one side of the diaphragm with or without limited contiguous extranodal involvement. Stage III is defined as lymph node involvement on both sides of the diaphragm. Stage IV refers to extensive extranodal involvement. White arrows denote bone marrow involvement. SUV indicates standardized uptake value
Table 1.
Evidence-based recommendations for the use of 18F-FDG PET at different treatment time points
| Time point | Indication | HLa | DLBCLa |
|---|---|---|---|
| Pre-treatment PET | Staging | +++ | +++ |
| Interim PET | Early response assessment | ++ | ++ |
| End-of-treatment PET | Remission assessment | ++ | ++ |
| PET during follow-up | Disease surveillance | ± | ± |
a+++: standard of care. ++: standard—depending on therapy regimen. ±: optional—recommended in selected cases, e.g., suspected relapse
FDG fluorodeoxyglucose, PET positron emission tomography, HL Hodgkin’s lymphoma, DLBCL diffuse large B-cell lymphoma
The use of 18F-FDG PET/CT is needed in staging both HL and DLBCL and can upstage 5–15% of patients, while downstaging is unusual (Elstrom et al. 2008; Fuertes et al. 2007). In patients with newly diagnosed DLBCL, 18F-FDG PET/CT is an accurate examination and complementary to BMB for the detection of focal bone marrow metastasis (Adams et al. 2014). The frequently observed diffuse increased BM skeletal FDG uptake in newly diagnosed BMB positive DLBCL patients may account for the relatively low PET sensitivity. The international guidelines recommend that positive 18F-FDG PET/CT findings prevent the need for BMB to detect bone marrow involvement in DLBCL patients. On the other hand, BMB might be the choice since negative 18F-FDG PET/CT findings cannot rule out bone marrow involvement. In clinical practice, however, BMB is performed whenever the presence or absence of bone marrow involvement affects the choice of therapy regiment, i.e., upstaging or downstaging.
For staging indolent lymphoma, 18F-FDG PET/CT is more accurate than CT alone, with high sensitivity of 94–98% and specificity of 88–100% (Karam et al. 2006; Wohrer et al. 2006). FDG PET upstages many, as high as 45%, patients with CT stage I/II FL altering their treatment strategy (Luminari et al. 2013; Wirth et al. 2008). The upstaging of patients have a profound impact on treatment planning, since such patients can no longer be considered for definitive radiotherapy (Dreyling et al. 2021). However, PET/CT has relatively low sensitivity and specificity for detecting bone marrow involvement. Because the increased BM FDG activity might be due to inflammation, infection, or stimulation of normal marrow rather than the lymphomatous infiltration (Dubreuil et al. 2017). In such patients, FDG PET/CT could help by navigating an accurate site for BMB selection, especially when the histologic transformation is clinically suspected (Barrington et al. 2014).
Role of Interim-Treatment 18F-FDG PET/CT
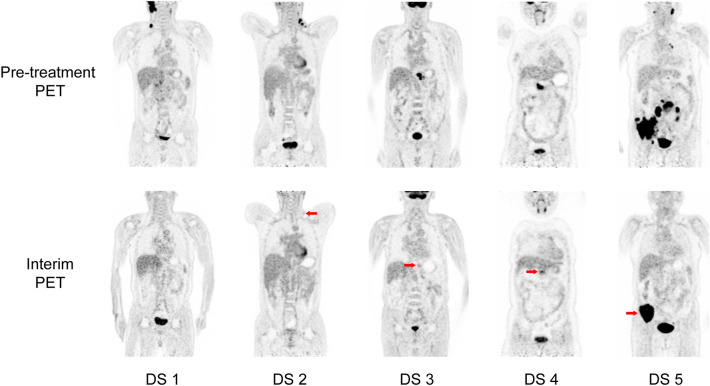
Interim 18F-FDG PET/CT is a promising approach for guiding response-adapted treatment, which aims to ensure the effectiveness of treatment and prohibit the possibility of progression. 18F-FDG PET shows metabolic response earlier than anatomic response and has the potential to replace CT. Studies have shown that the interim 18F-FDG PET is a robust prognostic indicator in HL (Biggi et al. 2013; Cerci et al. 2010a; Gallamini et al. 2007; Zinzani et al. 2012) and aggressive NHL (Mikhaeel et al. 2005; Terasawa et al. 2009; Yang et al. 2011), outperforming the International Prognostic Score (Gallamini et al. 2007) and International Prognostic Index (Mikhaeel et al. 2005) (Table 1). These findings highlight the potential of using interim 18F-FDG PET to tailor treatment according to individual response. The Deauville criteria (Table 2 and Fig. 2) and Lugano Classification criteria (Table 3) are currently recommended for 18F-FDG PET/CT based response assessment (Barrington et al. 2014; Cheson et al. 2014).
Table 2.
The Deauville criteria for response assessment of FDG-avid lymphomas
| Score | Criteria |
|---|---|
| 1 | No FDG uptake |
| 2 | FDG uptake ≤ mediastinum |
| 3 | FDG uptake > mediastinum but ≤ liver |
| 4 | FDG uptake moderately increased compared to the liver |
| 5 | FDG uptake markedly increased compared to the liver and/or new sites of disease |
FDG fluorodeoxyglucose
Fig. 2.
Coronal slices of pre-treatment and interim 18F-FDG PET images for response assessment in lymphoma, from left to right corresponding to Deauville score (DS) 1–5 with different FDG uptake (red arrows)
Table 3.
The Lugano classification criteria for response assessment of lymphoma
| Response and site | PET/CT-based criteria | CT-based criteria |
|---|---|---|
| CR | Complete metabolic response: Deauville score 1, 2, or 3 with or without a residual mass on 5PS | Complete radiologic response: target nodes/nodal masses must regress to ≤ 1.5 cm in LDi |
| PR | Partial metabolic response: deauville score 4 or 5 with reduced uptake compared with baseline and residual mass(es) of any size | Partial remission: ≥ 50% decrease in SPD of up to 6 target measurable nodes and extranodal sites |
| SD | No metabolic response: deauville score 4 or 5 with no significant change in FDG uptake from baseline at interim or end of treatment | Stable disease: < 50% decrease from baseline in SPD of up to 6 dominant, measurable nodes and extranodal sites; no criteria for progressive disease are met |
| PD | Progressive metabolic disease | Progressive disease |
| Individual target nodal masses | Deauville score 4 or 5 with an increase in intensity of uptake from baseline and/or |
An individual node/lesion must be abnormal with: LDi > 1.5 cm and increase by ≥ 50% from PPD nadir and an increase in LDi or SDi from nadir 0.5 cm for lesions ≤ 2 cm 1.0 cm for lesions > 2 cm In the setting of splenomegaly, the splenic length must increase by > 50% of the extent of its prior increase beyond baseline (e.g., a 15-cm spleen must increase to > 16 cm). If no prior splenomegaly, must increase by ≥ 2 cm from baseline New or recurrent splenomegaly |
| Extranodal lesions | New FDG-avid foci consistent with lymphoma at interim or end-of-treatment assessment | |
| Nonmeasured lesions | None | New or clear progression of preexisting nonmeasured lesions |
| New lesions | New FDG-avid foci consistent with lymphoma rather than another etiology (e.g., infection, inflammation). If uncertain regarding etiology of new lesions, biopsy or interval scan may be considered |
Regrowth of previously resolved lesions A new node > 1.5 cm in any axis A new extranodal site > 1.0 cm in any axis; if < 1.0 cm in any axis, its presence must be unequivocal and must be attributable to lymphoma Assessable disease of any size unequivocally attributable to lymphoma |
| Bone marrow | New or recurrent FDG-avid foci | New or recurrent involvement |
CR complete response, 5PS 5-point scale, LDi longest transverse diameter of a lesion, PR partial response, SPD sum of the product of the perpendicular diameters for multiple lesions, SD stable disease, FDG fluorodeoxyglucose, PD progression disease, PPD cross product of the LDi and perpendicular diameter, SDi shortest axis perpendicular to the LDi
Hodgkin’s Lymphoma (HL)
Since shrinkage of the tumor size takes a longer time compared to the observed decrease in radiotracer activity (function), conventional imaging with CT, especially in HL, lacked specificity in the setting of early treatment response assessment (Hutchings et al. 2006b). The negative predictive value (NPV) of PET during ongoing treatment depends on the cut-off for interim PET positivity and timing. Very early PET evaluation, such as after one cycle of chemotherapy, identifies fast responders; however, about 50% of patients with positive findings ultimately turn out negative after two cycles of chemotherapy. A meta-analysis involving more than 1300 patients with HL of all stages suggested that the interim treatment PET had a high NPV and moderate sensitivity of 67–71% (Adams et al. 2015). Interim-treatment PET showed consistently high NPV but a low positive predictive value (PPV) in early-stage (stage IA and stage IIA) HL, most likely due to the high performance of radiotherapy after chemotherapy with subsequent excellent prognosis (Radford et al. 2015).
The findings from several large prospective trials encourage interim PET for guiding treatment strategy in early-stage HL (André et al. 2017; Barrington et al. 2019; Fuchs et al. 2019). In the UK RAPID Trial, 571 patients with stage IA or IIA Hodgkin’s lymphoma without mediastinal bulk underwent interim PET after three cycles of ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine), of whom 426 (75%) patients had negative findings on interim PET (Deauville score 1 or 2) were randomly assigned to either involved-field radiotherapy (IFRT) or no further treatment, radiotherapy (Barrington et al. 2019). This study did not prove non-inferiority of chemotherapy alone, thus generally not recommending to omit consolidative radiotherapy in PET-negative patients after three cycles of ABVD. Radiotherapy omission appeared questionable in the H10 trial, which randomized 1950 patients with limited-stage favorable and unfavorable HL to PET-guided or standard treatment with 3–4 ABVD cycles plus involved-node radiotherapy (André et al. 2017). Furthermore, radiotherapy omission resulted in worse disease control in the HD16 research conducted by the German Hodgkin Study Group (GHSG) (Fuchs et al. 2019). Thus, generally, radiotherapy is recommended in limited-stage favorable HL after treatment with two cycles of ABVD. However, as revealed by the H10 trial, interim PET, after two cycles of chemotherapy, seems able to identify high-risk patients who might benefit from intensified therapy with escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) (André et al. 2017).
For advanced-stage HL, there is significant evidence regarding the use of FDG PET for tailoring treatment suggested by multiple trials, such as RATHL (Johnson et al. 2016), Italian HD 0607 (Gallamini et al. 2018), GHSG HD18 (Borchmann et al. 2017), and AHL2011 (Casasnovas et al. 2019). The RATHL trial used interim PET after two cycles of ABVD in 1214 patients, of whom 703 (59%) had the advanced-stage disease (Johnson et al. 2016). Patients with negative interim PET (Deauville score 1–3) were randomized to continue ABVD or therapeutic de-escalation by omitting bleomycin, namely AVD. With an updated follow-up of 52 months, the two groups had comparable 3-year progression-free survival (PFS), 85.4% versus 84.0%. However, patients receiving AVD had fewer infections, neutropenic fever events, and respiratory adverse events. Patients with positive interim PET (Deauville score 4 or 5) were assigned either four additional cycles of escalated BEACOPP or six other cycles of BEACOPP14, resulting in a 5-year PFS of 65.7%, comparing favorably with historic controls in which ABVD continued in interim PET-positive patients. Notably, the Deauville score among patients with negative interim PET was not predictive of outcome, suggesting that the omission of bleomycin in cycles 3–6 for patients with a Deauville score of 3 is safe as with a score of 1–2. The Italian HD 0607 study, including 782 patients with stage IIB–IV HL, showed similarly encouraging results (Gallamini et al. 2018). The 3-year PFS of PET-positive patients who were assigned escalated BEACOPP after initial ABVD was 57%. They also observed that irradiation of large nodal masses (> 5 cm) did not improve outcomes in PET-negative patients after six cycles of ABVD. In the GHSG HD18 study, treatment with four cycles of escalated BEACOPP was as effective as standard six cycles for PET-negative patients in 5-year PFS (90.8% versus 92.2%). Still, it was associated with significantly fewer infections and toxicities (Borchmann et al. 2017). Of note, in contrast to other studies limiting PET-positive to score 4 and 5, Deauville score 3–5 was defined as a positive interim PET in the GHSG HD18 study. Therefore, 12 weeks of escalated BEACOPP could be an attractive alternative to 24 weeks of ABVD treatment, especially for young patients. Importantly, PET-positive patients with a Deauville score of 4 who received continuing escalated BEACOPP still had an impressive 3-year PFS of 87.6%, indicating that the treatment strategy may influence the positive predictive value of interim FDG PET/CT. The AHL2011 study explored de-escalation to ABVD after initial escalated BEACOPP in PET-negative patients (Casasnovas et al. 2019). With a median follow-up of 50.4 months, patients in the full escalated BEACOPP group showed comparable 5-year PFS to those in the PET-driven de-escalation group (86.2% versus 85.7%, p = 0.65). Thus, for advanced-stage HL patients, negative interim PET can serve as an indication for treatment de-escalation. However, the insufficient initial response to ABVD should trigger the escalation to BEACOPP. Combining early response with molecular profiling and tumor burden assessment for prognostication needs to be explored for PET-adapted approaches (Agostinelli et al. 2016; Cottereau et al. 2018).
Diffuse Large B-Cell Lymphoma (DLBCL)
The current standard-of-care chemotherapy for DLBCL combines rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP), which is curative for approximately 70% of the patients (Coiffier et al. 2010; Pfreundschuh et al. 2011). Interim-PET is a possible biomarker for evaluating early response to R-CHOP chemotherapy in several studies (Casasnovas et al. 2017; Hertzberg et al. 2017; Mamot et al. 2015; Moskowitz et al. 2010; Pardal et al. 2014; Stewart et al. 2014; Swinnen et al. 2015). The rate of interim PET negativity varies widely, which may be due to variable timing of the interim-PET (reported as after 2, 3, or 4 cycles of chemotherapy), and various interpretation criteria with different definitions of FDG PET/CT positivity including internal visual point systems, International Harmonization Project criteria, Deauville criteria, and Lugano Classification criteria.
The prognostic value of interim-FDG PET/CT in DLBCL has been investigated in several prospective studies, which conducted serial FDG PET at baseline, interim, and end of treatment (Carr et al. 2014; Fan et al. 2017; Mamot et al. 2015; Schöder et al. 2020). Chemotherapy was not modified based on interim FDG PET/CT results unless there was clear disease progression. These studies demonstrated that interim PET negativity was strongly associated with end-of-treatment PET/CT negativity and favorable prognosis. However, in the International Atomic Energy Agency study, 13/205 (6%) patients with negative interim PET/CT developed a positive end-of-treatment PET/CT, of which 11 patients (85%) had the biopsy-proven refractory disease (Carr et al. 2014). The CALGB 50303 trial reported a similar result that 4/87 (5%) patients with negative interim-PET had a positive end-of-treatment PET (Schöder et al. 2020). In the SAKK 38/07, the International Atomic Energy Agency, and CALGB 50303 studies, more than half of the patients with positive interim PET eventually turned negative, with favorable 2-year event-free survival (EFS) and overall survival (OS) (86% and 92%, respectively) for this subgroup in the International Atomic Energy Agency study (Carr et al. 2014; Mamot et al. 2015; Schöder et al. 2020). These findings suggested that despite the very high negative predictive value of an interim-PET, false-positive results could occur in more than half of the cases. Therefore, treatment modification or escalation is likely unnecessary for patients with positive interim PET findings.
Several prospective trials have explored the role of interim FDG PET/CT in guiding treatment escalation for patients with positive interim PET findings (Casasnovas et al. 2017; Dührsen et al. 2018; Hertzberg et al. 2017; Kasamon et al. 2009; Moskowitz et al. 2010; Pardal et al. 2014; Stewart et al. 2014; Swinnen et al. 2015). However, these studies couldn't confirm a uniform relationship between treatment modification and outcome improvement. Furthermore, most of these studies were phase II investigations without a comparison arm, eliminating the ability to identify the actual contribution from the treatment escalation. For these reasons, current guidelines, outside of clinical trials, do not recommend treatment escalation based on a positive interim-FDG PET/CT. Future studies are required to incorporate other biomarkers for early treatment failure or novel regimens to improve outcomes for these individuals.
Follicular Lymphoma (FL)
The interim-PET is predictive of positive treatment response in patients with FL. A prospective study evaluated the prognostic value of interim and end-of-treatment PET in 121 FL patients treated with R-CHOP (Dupuis et al. 2012). Similar to the results reported in DLBCL, the power of interim-PET in discriminating between patients who responded and those who did not respond to the therapy regimen is not as effective as the end-of-treatment PET. However, in FL, the role of interim PET is less relevant to patient management because the assessment of response is a contiguous process throughout initial and maintenance therapy.
Role of End-of-Treatment 18F-FDG PET/CT
End-of-treatment PET/CT is more accurate than CT alone in remission assessment in patients with HL (Barnes et al. 2011; Cerci et al. 2010b), DLBCL (Micallef et al. 2011), and high-tumor-burden FL (Dupuis et al. 2012; Trotman et al. 2011). Unlike CT, end-of-treatment PET/CT can distinguish between the residual active tumor tissue and post-treatment fibrosis. Immediately after successful treatment regimens, including after immunotherapy, there might be relative increase in FDG activity of the tumor, which may cause a high false-positive rate. To minimize the risk of a high false-positive rate and in an attempt to eliminate the increase in nonspecific 18F-FDG uptake, the end-of-treatment PET should be performed optimally 4–6 weeks after completion of chemotherapy and at least eight weeks after radiotherapy (NCCN Clinical Practice Guidelines in Oncology 2021).
Hodgkin’s Lymphoma (HL)
Several studies have consistently suggested that achieving a negative end-of-treatment PET is associated with a high NPV and favorable outcomes in HL (De Wit et al. 2001; Engert et al. 2012; Kobe et al. 2008; Markova et al. 2012; Radford et al. 2015; Schaefer et al. 2007). For instance, the HD15 trial study reported that the NPV of FDG PET/CT with a Deauville score of 1–3 was 94% in patients with residual masses measuring ≥ 2.5 cm on CT (Engert et al. 2012). The prognosis of this subgroup is favorable, with a 4-year PFS of 92%. As a result, the current response criteria define a complete remission as a negative end-of-treatment PET regardless of the size of residual masses on CT.
In contrast to its high NPV, the PPV of end-of-treatment PET is generally lower and more variable, as a significant number of PET-positive patients do not develop refractory HL. In the HD15 trial, 191 patients with residual masses measuring ≥ 2.5 cm had a positive end-of-treatment PET with a Deauville score of 4–5 and received radiotherapy to the residual diseases (Engert et al. 2012). Even if their prognosis was worse than the PET-negative patients, the findings from this study demonstrated a high false-positive rate of end-of-treatment PET in a substantial proportion of patients. Furthermore, the study design did not permit an evaluation of the radiotherapy-related benefit (Engert et al. 2012). Based on these results, radiotherapy is limited to end-of-treatment PET-positive residual tissue in advanced-stage HL after escalated BEACOPP therapy. It could be omitted in end-of-treatment PET-negative cases. The findings of the randomized HD0607 trial supported this conclusion (Gallamini et al. 2020).
Diffuse Large B-Cell Lymphoma (DLBCL)
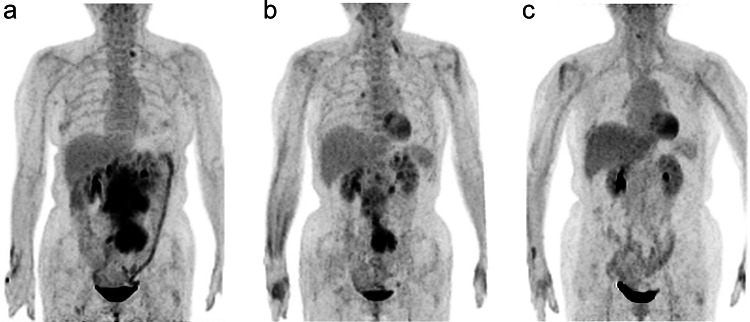
The current guidelines (NCCN Clinical Practice Guidelines in Oncology 2021) recommend the end-of-treatment PET as the standard of care for remission assessment in DLBCL. Importantly, in DLBCL, after R-CHOP therapy, the end-of-treatment PET positivity should be interpreted as a sign of unfavorable prognosis (Carr et al. 2014; Mamot et al. 2015). Several retrospective cohort studies evaluated the role of end-of-treatment PET in guiding consolidative radiotherapy to residual diseases and demonstrated improved outcomes (Dorth et al. 2012; Freeman et al. 2021; Held et al. 2014; Phan et al. 2010; Shi et al. 2013). Current guidelines state that a Deauville score of 4–5 with unchanged or increased intensity from baseline and appearance of new FDG-avid foci that is consistent with lymphoma deemed as treatment failure (Cheson et al. 2014) (Fig. 3). In the presence of remaining metabolically active tissue, one might consider a confirmatory biopsy with a subsequent salvage treatment (Tilly et al. 2015).
Fig. 3.
Serial 18F-FDG PET images of a 62-year-old woman with diffuse large B-cell lymphoma. a The initial staging PET image reveals lymphomatous involvement of para-aortic and iliac lymph nodes (defined as Stage II disease). b The PET image after six cycles of chemotherapy (R-CHOP) shows PET-positive residual uptake at the iliac lymph nodes (Deauville score 5). c The PET image after involved field radiotherapy indicates complete metabolic response
Follicular Lymphoma (FL)
In FL, PET predicts inferior outcomes in patients with high tumor burden who remain PET positive after first-line treatment (Trotman et al. 2018). In the GALLIUM study, patients with a complete metabolic response with a Deauville score of 1–3 had better 2.5-year PFS than those who did not have a complete metabolic response (87.4% versus 54.9%) (Trotman et al. 2018). The multivariate analysis showed that PET status at the end of induction therapy was the only independent predictor of OS. These findings have set the scene for exploring the need for maintenance therapy in FL patients with complete metabolic response and treatment escalation in patients classified as non-responding (Federico et al. 2019). Furthermore, the GALLIUM study demonstrated that at the end of induction therapy, 5/213 (2%) patients with a complete metabolic response had persistent bone marrow involvement, which implied the additional value of repeat biopsy in these patients (Rutherford et al. 2020).
The FOLL12 study investigated the role of PET at the end of induction therapy in treatment modifications in FL (Federico et al. 2019). Preliminary results from this study showed that even if a patient has a negative-PET at the end of induction therapy, the exclusion of the maintenance rituximab therapy results in an inferior PFS. On the other hand, PFS could be prolonged in PET-negative patients at the end of induction therapy when maintenance rituximab therapy is maintained. The ongoing UK-led PETReA trial (EudraCT number 2016-004010-10) may add further clarity to the effect of PET at the end of induction therapy on response-adapted treatment in FL.
18F-FDG PET/CT in Post-treatment Surveillance of Lymphomas
Although the initial first-line therapy could cure most aggressive lymphomas, depending on their histologic subtype and other prognostic factors, a subgroup of patients will experience relapse. Most relapses occur within the first two years after completing the primary therapy, which is usually not identifiable on routine CT imaging if at a subclinical stage (Thompson et al. 2014). Several retrospective and prospective studies explored the role of FDG PET/CT in the disease surveillance in such patients (Avivi et al. 2013; Cheah et al. 2013, 2014; Dann et al. 2014; El-Galaly et al. 2012b; Zinzani et al. 2009). For example, a prospective study of 421 patients with various lymphoma subtypes, including HL, aggressive NHL, and FL, who achieved complete remission after the initial therapy demonstrated that the rate of relapse detection by PET was higher among the subset of patients with the greatest a priori risk of recurrence (Zinzani et al. 2009). Another multicenter study of 161 patients with HL reported that PET/CT surveillance could detect 45% of subclinical relapses with a high false-positive rate (El-Galaly et al. 2012b). In a retrospective study of 116 DLBCL patients, PET/CT showed a limited role in disease surveillance for patients achieving complete remission after primary therapy (Cheah et al. 2013). For FL, a retrospective study examined the effect of surveillance imaging on relapse detection and OS and suggested that no OS benefit was associated with imaging-detected relapse (Goldman et al. 2021).
Owing to the lack of sufficient evidence demonstrating an improved outcome for the detection of relapse, the latest NCCN Guidelines do not recommend 18F-FDG PET as routine surveillance for patients with stage I–II disease who have achieved complete metabolic response after the initial therapy (NCCN Clinical Practice Guidelines in Oncology 2021). For patients with stage III–IV disease who achieve remission after initial treatment, the NCCN Guidelines recommend CT scans no more than every six months for up to two years after completion of the treatment, with no ongoing routine surveillance imaging beyond 2 years ( NCCN Clinical Practice Guidelines in Oncology 2021). In patients with primarily osseous presentations, PET/CT may be the preferable follow-up imaging modality. When the imaging studies reveal suspected recurrence, biopsy confirmation of the diagnosis is necessary before proceeding with a second-line therapy (Table 1). For these patients, response criteria are identical to those of first-line treatment evaluation.
18F-FDG PET/CT for Pre-transplantation Assessment
Up to 30% of patients with HL and DLBCL relapse after, or do not respond to, first-line treatment. A therapy choice for these patients is salvage chemotherapy followed by consolidation with high-dose therapy (HDT) and autologous stem-cell transplantation (ASCT) (NCCN Clinical Practice Guidelines in Oncology 2021). Several studies have investigated the prognostic impact of PET-assessed response to salvage chemotherapy before HDT-ASCT for relapsed or refractory lymphoma (Derenzini et al. 2008; Dickinson et al. 2010; Moskowitz et al. 2015; Sauter et al. 2015). In a phase II study of 129 patients with relapsed or refractory HL, PET-adapted sequential salvage chemotherapy followed by HDT resulted in a significant proportion of patients achieving negative PET results, potentially optimizing the chance of cure after ASCT (Moskowitz et al. 2015). Multiple studies focusing on relapsed or refractory DLBCL reported similar results (Derenzini et al. 2008; Dickinson et al. 2010; Sauter et al. 2015). These findings highlight both the importance of achieving negative PET status before ASCT and the potential benefit of PET-adapted salvage strategies to improve cure rates in relapsed disease.
PET/CT Lymphoma Imaging with Non-18F-FDG Radiotracers
Although 18F-FDG is not a malignant tumor-specific PET tracer, in the clinic, 18F-FDG is frequently used to follow the metabolic changes of the tumor tissue at a molecular level. In 18F-FDG PET imaging of tumors, myocardium, and inflammation, hypoxia may be one of the fundamental driving forces. The possible mechanism of 18F-FDG uptake caused by hypoxia may be related to metabolic changes, macrophage activation, and alterations in glycolysis-related enzyme activity. Most of the solid malignant tumors, nearly 95%, have some degree of hypoxia. 18F-FDG accumulates heavily in anoxic, hypoxic tumor cells with poor proliferation and low blood flow. However, its uptake decreases in well-oxygenated, oxygen-rich tumor cells with intense proliferation and high perfusion. This explanation describes the reason why some treatment-naive malignant lesions may have little or no 18F-FDG uptake. Furthermore, since well-oxygenated or necrotic cancer cells also have low 18F-FDG uptake, low 18F-FDG accumulation after anticancer therapy does not necessarily mean the total elimination of all viable cancer cells (Yao et al. 2021).
Despite the fact that 18F-FDG PET has accomplished remarkable progress in the diagnosis and treatment of lymphoma, related to the aforementioned issues, in clinical practice, there are a substantial demand for more accurate, non-18F-FDG radiotracers that can target other metabolic or receptor pathways, with the aim of being more specific in the setting of aggressive, highly FDG-avid lymphomas and more sensitive in low or variably FDG-avid subtypes. Table 4 shows potential targets and PET tracers for phenotyping the biochemical processes in lymphoma.
Table 4.
PET imaging for phenotyping the biochemical processes in lymphoma
| Target | Tracer |
|---|---|
| Substrate metabolism | 18F-FDG, 11C-choline, 11C-acetate |
| Protein synthesis | 11C-MET, 11C-tyrosine, 18F-FET |
| DNA synthesis | 11C-thymidine, 18F-FLT |
| Receptor affinity | 68Ga-pentixafor (targeting CXCR4), 89Zr-rituximab (targeting CD20), 68Ga-DOTATOC (targeting somatostatin receptor), 89Zr-atezolizumab (targeting PD-L1) |
| Tumor microenvironment | 68Ga-FAPI (targeting FAP) |
| Gene mutation | 89Zr-transferrin (reflecting myc status) |
| Angiogenesis | 18F-RGD-K5 (targeting integrin αvβ3) |
18F-FDG 18F-fluorodeoxyglucose, 11C-MET 11C-methionine, 18F-FET 18F-fluoroethyltyrosine, 18F-FLT 18F-fluorothymidine, CXCR4 C–X–C motif chemokine receptor 4, 68Ga-DOTATOC 68Ga-labeled dotatate-D-Phe-Tyr-octreotide, PD-L1 programmed cell death-ligand 1, 68Ga-FAPI 68Ga-labeled fibroblast activation protein inhibitor, FAP fibroblast activation protein, 18F-RGD-K5 18F-labeled arginine-glycine-aspartic
18F-Fluorothymidine
18F-Fluorothymidine (18F-FLT) is a radiolabeled thymidine analog that can reflect in vivo cell proliferation. 18F-FLT is less affected by post-treatment inflammatory changes caused by macrophages or monocyte infiltration and therefore expected to be more lymphoma-specific than 18F-FDG. A previous study compared the performance of interim FLT PET and FDG PET in response assessment in 48 patients with DLBCL (Minamimoto et al. 2016). The interim-FLT PET had a significantly higher PPV (91%) in predicting residual disease than interim FDG PET (range, 42–46%), regardless of the criteria used for FDG PET interpretation. The NPV of interim-FLT PET (94%) was higher than or similar to the NPV of interim-FDG PET (range, 82–95%). A recent multicenter study compared the prognostic value of interim-FLT PET and interim-FDG PET in 92 DLBCL patients (Minamimoto et al. 2021). The multivariate analysis showed that only interim-FLT PET status was an independent predictor for 3- and 5-year PFS. These findings suggest that interim-FLT PET may be more accurate than interim-FDG PET for therapy monitoring and prognostication of DLBCL and possibly other aggressive lymphomas.
68Ga-Pentixafor
C–X–C motif chemokine receptor 4 (CXCR4) is a widely studied transmembrane chemokine receptor involved in tumor growth, metastasis, and hematopoietic stem cell/progenitor homing and retention in hematopoietic sites (Jacobson and Weiss 2013). Due to overexpression in many solid and hematological malignancies, CXCR4 is an attractive target for cancer diagnosis and treatment. 68Ga-pentixafor is a radiolabeled CXCR4-targeted ligand developed for PET imaging. It is capable to non-invasively visualize CXCR4 expression in a variety of lymphomas, including DLBCL (Wester et al. 2015), lymphoplasmacytic lymphoma (Pan et al. 2020), and mantle cell lymphoma (MCL) (Mayerhoefer et al. 2021). Previous literature reported that 68Ga-pentixafor bound to CXCR4 with high affinity and selectivity in mouse models and lymphoma patients (Wester et al. 2015). Another study with 27 NHL patients investigated the CXCR4 expression by 68Ga-pentixafor PET, and demonstrated that CXCR4 expression varied in different types of non-Hodgkin’s lymphomas, such as lymphoplasmacytic lymphoma, marginal zone lymphoma, DLBCL, FL, and MCL (Pan et al. 2020). In a very recent study including 22 MCL patients, 68Ga-pentixafor PET showed higher detection rates and better tumor-to-background contrast ratios than 18F-FDG PET (Mayerhoefer et al. 2021). These results indicate that 68Ga-pentixafor PET holds potential for the non-invasive assessment of CXCR4 expression and may serve as an intriguing candidate for imaging lymphomas.
68Ga-FAPI
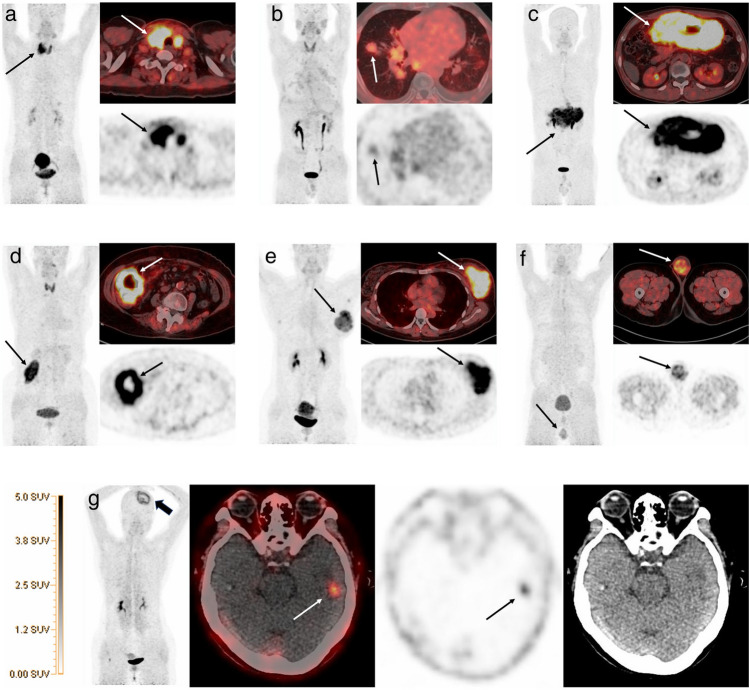
Cancer-associated fibroblasts (CAFs) that overexpress fibroblast activation protein (FAP) are abundant in many epithelial carcinomas and hematological neoplasms. Several previous lymphoma studies evaluated PET imaging with radiolabeled FAP inhibitor (FAPI) with promising results (Jin et al. 2021; Wang et al. 2021; Yang et al. 2021). A recent study identified FAP expression with 68Ga-FAPI PET in 73 patients with different lymphoma subtypes (Jin et al. 2021) (Fig. 4). Significantly elevated FAP uptake was observed in HL lesions, correlating with the intensity of FAP immunostaining. Aggressive NHL lesions exhibited intense to moderate 68Ga-FAPI uptake with moderate-to-strong FAP immunostaining, while indolent NHL lesions showed weak FAP staining and mild to moderate 68Ga-FAPI uptake. These encouraging data make 68Ga-FAPI PET an alternate imaging method for detecting FAP expression in lymphoma lesions and characterizing lymphoma profiles.
Fig. 4.
68Ga-FAPI PET/CT images of various extranodal sites in different subtypes of lymphoma patients. a Primary thyroid Burkitt lymphoma (arrow). b Hodgkin lymphoma with lung involvement (arrow). c Primary gastric diffuse large B-cell lymphoma (DLBCL, arrow). d Ileum DLBCL (arrow). e Left breast DLBCL (arrow). f Left testicle DLBCL (arrow). g Left temporal lobe DLBCL lesion (arrow) after left frontal lymphoma resection (thick arrow).
Adapted from Jin et al. (2021). Reprint permission was obtained
89Zr-Rituximab
CD20 is a well-studied transmembrane protein, which increases during the maturation of B-lineage lymphocytes and is lost upon differentiation towards plasma cells, making it a good target for treatment of B-cell lymphoma. Rituximab, an anti-CD20 monoclonal antibody, is incorporated in all first-line and subsequent treatment regimens for most B-cell lymphomas. An earlier study investigated the effectiveness of 89Zr-labeled rituximab in five patients with indolent lymphomas scheduled to receive 90Y-rituximab radioimmunotherapy (Muylle et al. 2015). This study showed that preloading with unlabeled rituximab had a marked effect on tracer biodistribution, with a higher tumor-to-background ratio compared with no preloading with unlabeled rituximab (Fig. 5). A later study investigated the use of 89Zr-rituximab in six patients with relapsed or refractory diffuse large B-cell lymphoma, revealing that tracer uptake corresponded with CD20 expression measured by immunohistochemistry in tumor biopsies (Jauw et al. 2017).
Fig. 5.
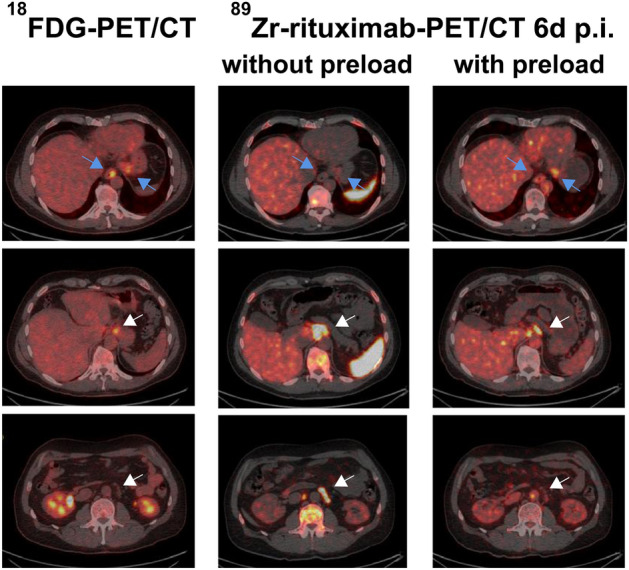
18F-FDG PET/CT and 89Zr-rituximab immuno-PET/CT images of a 51-year-old man with follicular lymphoma. 89Zr-rituximab immuno-PET/CT images obtained 6 days after injection with and without a preload of unlabelled rituximab show lower tracer uptake in involved lymph nodes with the preload (white arrows), but higher uptake in less accessible visceral lesions (esophagus and stomach; blue arrows) resulting in better tumor targeting.
Adapted from Muylle et al. (2015). Reprint permission was obtained
Furthermore, these two studies also observed uptake of anti-CD20 tracers in patients that had previously received rituximab and relapsed after anti-CD20 therapy. These data validated the feasibility of 89Zr-rituximab PET imaging in lymphoma. However, before the routine clinical use of 89Zr-rituximab PET imaging, several important issues, such as the influence of different treatment regimens, B-cell lymphoma subtypes, and preload doses of unlabeled rituximab on tracer uptake and clearance, should be addressed.
18F-FDG PET/MRI in Management of Lymphoma
18F-FDG PET/MRI has been successfully established as an alternative modality in initial staging of lymphoma. A retrospective study with 27 lymphoma patients suggested that 18F-FDG PET/MRI has high sensitivity and specificity for nodal involvement (Platzek et al. 2014). Similar results were observed in other studies which reported that 18F-FDG PET/MRI offers an equivalent whole-body staging examination as compared with 18F-FDG PET/CT (Atkinson et al. 2016; Heacock et al. 2015; Verhagen et al. 2021). A prospective study compared 18F-FDG PET/MRI with and without DWI to 18F-FDG PET/CT in lesion detection in 34 lymphoma patients. The authors observed that in indolent NHLs, which included those with a variable FDG avidity, the addition of DWI improved the sensitivity and accuracy of 18F-FDG PET/MRI and provided results that are superior to those of 18F-FDG PET/CT (Giraudo et al. 2016). However, another prospective study with 66 lymphoma patients indicated that DWI could not alter diagnostic accuracy (Afaq et al. 2017). Therefore, the added diagnostic value of DWI still requires further investigation.
To date, there are only a limited number of studies investigating the use of 18F-FDG PET/MRI in response assessment of lymphoma. In an early pilot study, 9 lymphoma patients who underwent pre- and post-treatment 18F-FDG PET/MRI were enrolled (Platzek et al. 2013). Despite the small number of patients and lack of follow-up data, the study demonstrated that 18F-FDG PET/MRI is feasible for response assessment in lymphoma. Another prospective study investigated the value of 18F-FDG PET/MRI for ultra-early response assessment in 58 lymphoma patients, and indicated that 18F-FDG PET/MRI can capture treatment-induced changes in glucose metabolism and cell density as early as 48–72 h after treatment initiation (Mayerhoefer et al. 2018). However, the above-mentioned two studies were limited by the lack of comparison between 18F-FDG PET/MRI and 18F-FDG PET/CT. In a very recent prospective study involving 24 patients with HL, 18F-FDG PET/MRI showed a comparable accuracy to 18F-FDG PET/CT for response evaluation (Verhagen et al. 2021) (Fig. 6). Besides, Deauville grading agreement between 18F-FDG PET/MRI and 18F-FDG PET/CT was excellent. These data suggested that 18F-FDG PET/MRI is a promising alternative to 18F-FDG PET/CT for response assessment in patients with HL. However, larger cohort studies including different subtypes of lymphoma are required to further illuminate the clinical implications of 18F-FDG PET/MRI.
Fig. 6.
Pre-treatment and interim 18F-FDG PET/MRI images of a 14-year-old male with Hodgkin’s lymphoma. Coronal a short tau inversion recovery (STIR), b 18F-FDG PET, and c 18F-FDG PET/MRI fused images demonstrate lymphomatous involvement of right supraclavicular and right paratracheal lymph nodes, and a right lung nodule (arrows). Early response assessment following 2 cycles of chemotherapy shows d residual right supraclavicular lymph node on coronal STIR, with no uptake on coronal e 18F-FDG PET and f fused 18F-FDG PET/MRI (arrows). The other sites of disease have not been detected.
Adapted from Verhagen et al. (2021). Reprint permission was obtained
Conclusion and Future Perspectives
18F-FDG PET is the established standard modality for both staging and therapy monitoring of FDG-avid lymphomas. 18F-FDG PET is highly sensitive for detecting lymphoma lesions and therefore plays a crucial role in current treatment strategies. However, it is noteworthy that the positive predictive value of PET is relatively moderate, with numerous non-malignant causes for 18F-FDG avidity potentially resulting in false-positive interpretations. Notably, undirected bone marrow biopsy can be safely omitted in patients with HL or DLBCL when 18F-FDG PET documents the disease involvement. The overall predictive value of interim-PET was exceptionally high in HL, and therefore its use was established earlier in this setting than in other lymphoma subtypes. In aggressive NHL, baseline and interim PET imaging provide essential information for disease prognosis as well. It is also necessary for response assessment at therapy completion since a positive end-of-treatment PET most likely demonstrates treatment failure. In contrast, a negative end-of-treatment PET is indicative of a good prognosis that no additional treatment is required, restricting the use of adjuvant radiotherapy in advanced HL and DLBCL and routine use of PET surveillance.
We anticipate precise imaging to improve our understanding of the dynamic tumor microenvironment and enable optimization of checkpoint inhibitor-based therapy strategies in solid tumors and also lymphoma. With therapeutic advances in lymphoma, novel PET tracers other than 18F-FDG are necessary to meet the growing demand for precision medicine. Currently, several studies in lymphoma are ongoing using non-18F-FDG PET tracers, including 68Ga-pentixafor for diagnosis and pre-therapy evaluation of CXCR4 expression in lymphoma (NCT03436342) and 89Zr-atezolizumab for quantification of programmed cell death-ligand 1 (PD-L1) expression in patients with DLBCL (NCT03850028). In the future, PET molecular imaging with novel tracers may serve as a promising approach for precise evaluation of metabolic change, functional alteration, gene mutation, and histological profile of lymphoma, thus providing opportunities for decoding lymphoma phenotypes and identifying treatment-related mechanisms. Recent advances in imaging hardware, such as the introduction of PET/MRI in clinical use, are also expected to improve our ability to achieve the goals of precise imaging, and aid us to better understand and manage lymphoma with much improved diagnostic, therapeutic, and prognostic outcomes (Civelek et al. 2021).
Authors' Contributions
MT, ACC, and HZ conceived and supervised the work. XZ and HJ drafted the paper. SW, JW, RZ, XH, SQ, SZ supported in gathering study data and made critical revisions. MT, ACC, and HZ made critical revisions and approved the final version.
Funding
This study was partially supported by National Natural Science Foundation of China (81761148029, 81725009, 82030049, 32027802), National Key R&D Program of China (2021YFA110004500, 2021YFE0108300) and Fundamental Research Funds for the Central Universities (2021FZZX002-05).
Data Availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Code Availability
Not applicable.
Declarations
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Ethics Approval
Patients included in this work were from a previous study which has been approved by the Institutional Review Board of The Second Affiliated Hospital of Zhejiang University School of Medicine (Approval No. 2019-350).
Consent to Participate
Informed consent was obtained from individual participant included in this study.
Consent to Publication
Not applicable.
Footnotes
Xiaohui Zhang and Han Jiang contributed equally to this paper.
Contributor Information
Hong Zhang, Email: hzhang21@zju.edu.cn.
Ali Cahid Civelek, Email: accivelek@gmail.com.
Mei Tian, Email: tianmei@fudan.edu.cn.
References
- Adams HJ, Kwee TC, de Keizer B, Fijnheer R, de Klerk JM, Nievelstein RA. FDG PET/CT for the detection of bone marrow involvement in diffuse large B-cell lymphoma: systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2014;41(3):565–574. doi: 10.1007/s00259-013-2623-4. [DOI] [PubMed] [Google Scholar]
- Adams HJ, Nievelstein RA, Kwee TC. Prognostic value of interim FDG-PET in Hodgkin lymphoma: systematic review and meta-analysis. Br J Haematol. 2015;170(3):356–366. doi: 10.1111/bjh.13441. [DOI] [PubMed] [Google Scholar]
- Afaq A, Fraioli F, Sidhu H, Wan S, Punwani S, Chen SH, et al. Comparison of PET/MRI with PET/CT in the evaluation of disease status in lymphoma. Clin Nucl Med. 2017;42(1):e1–e7. doi: 10.1097/RLU.0000000000001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostinelli C, Gallamini A, Stracqualursi L, Agati P, Tripodo C, Fuligni F, et al. The combined role of biomarkers and interim PET scan in prediction of treatment outcome in classical Hodgkin's lymphoma: a retrospective, European, multicentre cohort study. Lancet Haematol. 2016;3(10):e467–e479. doi: 10.1016/s2352-3026(16)30108-9. [DOI] [PubMed] [Google Scholar]
- André M, Girinsky T, Federico M, Reman O, Fortpied C, Gotti M, et al. Early positron emission tomography response-adapted treatment in stage I and II Hodgkin lymphoma: final results of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2017;35(16):1786–1794. doi: 10.1200/JCO.2016.68.6394. [DOI] [PubMed] [Google Scholar]
- Armitage JO, Gascoyne RD, Lunning MA, Cavalli F. Non-Hodgkin lymphoma. Lancet. 2017;390(10091):298–310. doi: 10.1016/s0140-6736(16)32407-2. [DOI] [PubMed] [Google Scholar]
- Atkinson W, Catana C, Abramson JS, Arabasz G, McDermott S, Catalano O, et al. Hybrid FDG-PET/MR compared to FDG-PET/CT in adult lymphoma patients. Abdom Radiol (NY) 2016;41(7):1338–1348. doi: 10.1007/s00261-016-0638-6. [DOI] [PubMed] [Google Scholar]
- Avivi I, Zilberlicht A, Dann EJ, Leiba R, Faibish T, Rowe JM, et al. Strikingly high false positivity of surveillance FDG-PET/CT scanning among patients with diffuse large cell lymphoma in the rituximab era. Am J Hematol. 2013;88(5):400–405. doi: 10.1002/ajh.23423. [DOI] [PubMed] [Google Scholar]
- Barnes JA, LaCasce AS, Zukotynski K, Israel D, Feng Y, Neuberg D, et al. End-of-treatment but not interim PET scan predicts outcome in nonbulky limited-stage Hodgkin's lymphoma. Ann Oncol. 2011;22(4):910–915. doi: 10.1093/annonc/mdq549. [DOI] [PubMed] [Google Scholar]
- Barrington SF, Kirkwood AA, Franceschetto A, Fulham MJ, Roberts TH, Almquist H, et al. PET-CT for staging and early response: results from the Response-Adapted Therapy in Advanced Hodgkin Lymphoma study. Blood. 2016;127(12):1531–1538. doi: 10.1182/blood-2015-11-679407. [DOI] [PubMed] [Google Scholar]
- Barrington SF, Kluge R. FDG PET for therapy monitoring in Hodgkin and non-Hodgkin lymphomas. Eur J Nucl Med Mol Imaging. 2017;44(Suppl 1):97–110. doi: 10.1007/s00259-017-3690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Mueller SP, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32(27):3048–3058. doi: 10.1200/JCO.2013.53.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrington SF, Phillips EH, Counsell N, Hancock B, Pettengell R, Johnson P, et al. Positron emission tomography score has greater prognostic significance than pretreatment risk stratification in early-stage Hodgkin lymphoma in the UK RAPID study. J Clin Oncol. 2019;37(20):1732. doi: 10.1200/JCO.18.01799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggi A, Gallamini A, Chauvie S, Hutchings M, Kostakoglu L, Gregianin M, et al. International validation study for interim PET in ABVD-treated, advanced-stage hodgkin lymphoma: interpretation criteria and concordance rate among reviewers. J Nucl Med. 2013;54(5):683–690. doi: 10.2967/jnumed.112.110890. [DOI] [PubMed] [Google Scholar]
- Borchmann P, Goergen H, Kobe C, Lohri A, Greil R, Eichenauer DA, et al. PET-guided treatment in patients with advanced-stage Hodgkin's lymphoma (HD18): final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet. 2017;390(10114):2790–2802. doi: 10.1016/s0140-6736(17)32134-7. [DOI] [PubMed] [Google Scholar]
- Carr R, Fanti S, Paez D, Cerci J, Gyorke T, Redondo F, et al. Prospective international cohort study demonstrates inability of interim PET to predict treatment failure in diffuse large B-cell lymphoma. J Nucl Med. 2014;55(12):1936–1944. doi: 10.2967/jnumed.114.145326. [DOI] [PubMed] [Google Scholar]
- Casasnovas R-O, Bouabdallah R, Brice P, Lazarovici J, Ghesquieres H, Stamatoullas A, et al. PET-adapted treatment for newly diagnosed advanced Hodgkin lymphoma (AHL2011): a randomised, multicentre, non-inferiority, phase 3 study. Lancet Oncol. 2019;20(2):202–215. doi: 10.1016/s1470-2045(18)30784-8. [DOI] [PubMed] [Google Scholar]
- Casasnovas RO, Ysebaert L, Thieblemont C, Bachy E, Feugier P, Delmer A, et al. FDG-PET-driven consolidation strategy in diffuse large B-cell lymphoma: final results of a randomized phase 2 study. Blood. 2017;130(11):1315–1326. doi: 10.1182/blood-2017-02-766691. [DOI] [PubMed] [Google Scholar]
- Cerci JJ, Pracchia LF, Linardi CC, Pitella FA, Delbeke D, Izaki M, et al. 18F-FDG PET after 2 cycles of ABVD predicts event-free survival in early and advanced Hodgkin lymphoma. J Nucl Med. 2010;51(9):1337–1343. doi: 10.2967/jnumed.109.073197. [DOI] [PubMed] [Google Scholar]
- Cerci JJ, Trindade E, Pracchia LF, Pitella FA, Linardi CC, Soares J, Jr, et al. Cost effectiveness of positron emission tomography in patients with Hodgkin's lymphoma in unconfirmed complete remission or partial remission after first-line therapy. J Clin Oncol. 2010;28(8):1415–1421. doi: 10.1200/JCO.2009.25.4367. [DOI] [PubMed] [Google Scholar]
- Cheah CY, Dickinson M, Hofman MS, George A, Ritchie DS, Prince HM, et al. Limited clinical benefit for surveillance PET-CT scanning in patients with histologically transformed lymphoma in complete metabolic remission following primary therapy. Ann Hematol. 2014;93(7):1193–1200. doi: 10.1007/s00277-014-2040-1. [DOI] [PubMed] [Google Scholar]
- Cheah CY, Hofman MS, Dickinson M, Wirth A, Westerman D, Harrison SJ, et al. Limited role for surveillance PET-CT scanning in patients with diffuse large B-cell lymphoma in complete metabolic remission following primary therapy. Br J Cancer. 2013;109(2):312–317. doi: 10.1038/bjc.2013.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Zhang Y, Hou H, Du F, Wu S, Chen L, et al. Neural correlates of the popular music phenomenon: evidence from functional MRI and PET imaging. Eur J Nucl Med Mol Imaging. 2017;44(6):1033–1041. doi: 10.1007/s00259-017-3614-7. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civelek AC, Niglio SA, Malayeri AA, Lin J, Gurram S, Chalfin HJ, et al. Clinical value of 18FDG PET/MRI in muscle-invasive, locally advanced, and metastatic bladder cancer. Urol Oncol. 2021;39(11):787.e17–787.e21. doi: 10.1016/j.urolonc.2021.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010;116(12):2040–2045. doi: 10.1182/blood-2010-03-276246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottereau AS, Versari A, Loft A, Casasnovas O, Bellei M, Ricci R, et al. Prognostic value of baseline metabolic tumor volume in early-stage Hodgkin lymphoma in the standard arm of the H10 trial. Blood. 2018;131(13):1456–1463. doi: 10.1182/blood-2017-07-795476. [DOI] [PubMed] [Google Scholar]
- Dann EJ, Berkahn L, Mashiach T, Frumer M, Agur A, McDiarmid B, et al. Hodgkin lymphoma patients in first remission: routine positron emission tomography/computerized tomography imaging is not superior to clinical follow-up for patients with no residual mass. Br J Haematol. 2014;164(5):694–700. doi: 10.1111/bjh.12687. [DOI] [PubMed] [Google Scholar]
- De Wit M, Bohuslavizki K, Buchert R, Bumann D, Clausen M, Hossfeld D. 18FDG-PET following treatment as valid predictor for disease-free survival in Hodgkin's lymphoma. Ann Oncol. 2001;12(1):29–37. doi: 10.1023/a:1008357126404. [DOI] [PubMed] [Google Scholar]
- Derenzini E, Musuraca G, Fanti S, Stefoni V, Tani M, Alinari L, et al. Pretransplantation positron emission tomography scan is the main predictor of autologous stem cell transplantation outcome in aggressive B-cell non-Hodgkin lymphoma. Cancer. 2008;113(9):2496–2503. doi: 10.1002/cncr.23861. [DOI] [PubMed] [Google Scholar]
- Dickinson M, Hoyt R, Roberts AW, Grigg A, Seymour JF, Prince HM, et al. Improved survival for relapsed diffuse large B cell lymphoma is predicted by a negative pre-transplant FDG-PET scan following salvage chemotherapy. Br J Haematol. 2010;150(1):39–45. doi: 10.1111/j.1365-2141.2010.08162.x. [DOI] [PubMed] [Google Scholar]
- Dorth JA, Prosnitz LR, Broadwater G, Diehl LF, Beaven AW, Coleman RE, et al. Impact of consolidation radiation therapy in stage III–IV diffuse large B-cell lymphoma with negative post-chemotherapy radiologic imaging. Int J Radiat Oncol Biol Phys. 2012;84(3):762–767. doi: 10.1016/j.ijrobp.2011.12.067. [DOI] [PubMed] [Google Scholar]
- Dreyling M, Ghielmini M, Rule S, Salles G, Ladetto M, Tonino SH, et al. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(3):298–308. doi: 10.1016/j.annonc.2020.11.008. [DOI] [PubMed] [Google Scholar]
- Dubreuil J, Salles G, Bozzetto J, Tordo J, Djaileb L, Berriolo-Riedinger A, et al. Usual and unusual pitfalls of 18F-FDG-PET/CT in lymphoma after treatment: a pictorial review. Nucl Med Commun. 2017;38(7):563–576. doi: 10.1097/MNM.0000000000000697. [DOI] [PubMed] [Google Scholar]
- Dührsen U, Müller S, Hertenstein B, Thomssen H, Kotzerke J, Mesters R, et al. Positron emission tomography-guided therapy of aggressive non-Hodgkin lymphomas (PETAL): a multicenter, randomized phase III trial. J Clin Oncol. 2018;36(20):2024–2034. doi: 10.1200/JCO.2017.76.8093. [DOI] [PubMed] [Google Scholar]
- Dupuis J, Berriolo-Riedinger A, Julian A, Brice P, Tychyj-Pinel C, Tilly H, et al. Impact of [18F] fluorodeoxyglucose positron emission tomography response evaluation in patients with high-tumor burden follicular lymphoma treated with immunochemotherapy: a prospective study from the Groupe d'Etudes des Lymphomes de l'Adulte and GOELAMS. J Clin Oncol. 2012;30(35):4317–4322. doi: 10.1200/JCO.2012.43.0934. [DOI] [PubMed] [Google Scholar]
- El-Galaly TC, d'Amore F, Mylam KJ, de Nully BP, Bogsted M, Bukh A, et al. Routine bone marrow biopsy has little or no therapeutic consequence for positron emission tomography/computed tomography-staged treatment-naive patients with Hodgkin lymphoma. J Clin Oncol. 2012;30(36):4508–4514. doi: 10.1200/JCO.2012.42.4036. [DOI] [PubMed] [Google Scholar]
- El-Galaly TC, Mylam KJ, Brown P, Specht L, Christiansen I, Munksgaard L, et al. Positron emission tomography/computed tomography surveillance in patients with Hodgkin lymphoma in first remission has a low positive predictive value and high costs. Haematologica. 2012;97(6):931–936. doi: 10.3324/haematol.2011.056010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstrom RL, Leonard JP, Coleman M, Brown RK. Combined PET and low-dose, noncontrast CT scanning obviates the need for additional diagnostic contrast-enhanced CT scans in patients undergoing staging or restaging for lymphoma. Ann Oncol. 2008;19(10):1770–1773. doi: 10.1093/annonc/mdn282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engert A, Haverkamp H, Kobe C, Markova J, Renner C, Ho A, et al. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin's lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet. 2012;379(9828):1791–1799. doi: 10.1016/s0140-6736(11)61940-5. [DOI] [PubMed] [Google Scholar]
- Fan Y, Zhang Y, Yang Z, Ying Z, Zhou N, Liu C, et al. Evaluating early interim fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography with the SUVmax-liver-based interpretation for predicting the outcome in diffuse large B-cell lymphoma. Leuk Lymphoma. 2017;58(9):1–9. doi: 10.1080/10428194.2016.1277384. [DOI] [PubMed] [Google Scholar]
- Federico M, Mannina D, Versari A, Ferrero S, Marcheselli L, Boccomini C, et al. Response oriented maintenance therapy in advanced Follicular Lymphoma. Results of the interim analysis of the FOLL 12 trial conducted by the Fondazione Italiana Linfomi. Hematol Oncol. 2019;37(S2):153–154. doi: 10.1002/hon.110_2629. [DOI] [Google Scholar]
- Freeman CL, Savage KJ, Villa DR, Scott DW, Srour L, Gerrie AS, et al. Long-term results of PET-guided radiation in patients with advanced-stage diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2021;137(7):929–938. doi: 10.1182/blood.2020005846. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Goergen H, Kobe C, Kuhnert G, Lohri A, Greil R, et al. Positron emission tomography-guided treatment in early-stage favorable Hodgkin lymphoma: final results of the international, randomized phase III HD16 trial by the German Hodgkin Study Group. J Clin Oncol. 2019;37(31):2835–2845. doi: 10.1200/JCO.19.00964. [DOI] [PubMed] [Google Scholar]
- Fuertes S, Setoain X, Lopez-Guillermo A, Montserrat E, Fuster D, Paredes P, et al. The value of positron emission tomography/computed tomography (PET/CT) in the staging of diffuse large B-cell lymphoma. Med Clin (barc) 2007;129(18):688–693. doi: 10.1157/13112510. [DOI] [PubMed] [Google Scholar]
- Gallamini A, Hutchings M, Rigacci L, Specht L, Merli F, Hansen M, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin's lymphoma: a report from a joint Italian-Danish study. J Clin Oncol. 2007;25(24):3746–3752. doi: 10.1200/JCO.2007.11.6525. [DOI] [PubMed] [Google Scholar]
- Gallamini A, Rossi A, Patti C, Picardi M, Romano A, Cantonetti M, et al. Consolidation radiotherapy could be safely omitted in advanced Hodgkin lymphoma with large nodal mass in complete metabolic response after ABVD: final analysis of the randomized GITIL/FIL HD0607 trial. J Clin Oncol. 2020;38(33):3905–3913. doi: 10.1200/JCO.20.00935. [DOI] [PubMed] [Google Scholar]
- Gallamini A, Tarella C, Viviani S, Rossi A, Patti C, Mulé A, et al. Early chemotherapy intensification with escalated BEACOPP in patients with advanced-stage Hodgkin lymphoma with a positive interim positron emission tomography/computed tomography scan after two ABVD cycles: long-term results of the GITIL/FIL HD 0607 trial. J Clin Oncol. 2018;36(5):454–462. doi: 10.1200/JCO.2017.75.2543. [DOI] [PubMed] [Google Scholar]
- Giraudo C, Raderer M, Karanikas G, Weber M, Kiesewetter B, Dolak W, et al. 18F-Fluorodeoxyglucose positron emission tomography/magnetic resonance in lymphoma: comparison with 18F-Fluorodeoxyglucose positron emission tomography/computed tomography and with the addition of magnetic resonance diffusion-weighted imaging. Invest Radiol. 2016;51(3):163–169. doi: 10.1097/RLI.0000000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman ML, Mao JJ, Strouse CS, Chen W, Rupji M, Chen Z, et al. Surveillance imaging during first remission in follicular lymphoma does not impact overall survival. Cancer. 2021;127(18):3390–3402. doi: 10.1002/cncr.33660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heacock L, Weissbrot J, Raad R, Campbell N, Friedman KP, Ponzo F, et al. PET/MRI for the evaluation of patients with lymphoma: initial observations. AJR Am J Roentgenol. 2015;204(4):842–848. doi: 10.2214/AJR.14.13181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held G, Murawski N, Ziepert M, Fleckenstein J, Poschel V, Zwick C, et al. Role of radiotherapy to bulky disease in elderly patients with aggressive B-cell lymphoma. J Clin Oncol. 2014;32(11):1112–1118. doi: 10.1200/JCO.2013.51.4505. [DOI] [PubMed] [Google Scholar]
- Hertzberg M, Gandhi MK, Trotman J, Butcher B, Taper J, Johnston A, et al. Early treatment intensification with R-ICE and 90Y-ibritumomab tiuxetan (Zevalin)-BEAM stem cell transplantation in patients with high-risk diffuse large B-cell lymphoma patients and positive interim PET after 4 cycles of R-CHOP-14. Haematologica. 2017;102(2):356–363. doi: 10.3324/haematol.2016.154039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings M, Loft A, Hansen M, Pedersen LM, Berthelsen AK, Keiding S, et al. Position emission tomography with or without computed tomography in the primary staging of Hodgkin's lymphoma. Haematologica. 2006;91(4):482–489. [PubMed] [Google Scholar]
- Hutchings M, Loft A, Hansen M, Pedersen LM, Buhl T, Jurlander J, et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood. 2006;107(1):52–59. doi: 10.1182/blood-2005-06-2252. [DOI] [PubMed] [Google Scholar]
- Jacobs AH, Li H, Winkeler A, Hilker R, Knoess C, Ruger A, et al. PET-based molecular imaging in neuroscience. Eur J Nucl Med Mol Imaging. 2003;30(7):1051–1065. doi: 10.1007/s00259-003-1202-5. [DOI] [PubMed] [Google Scholar]
- Jacobson O, Weiss ID. CXCR4 chemokine receptor overview: biology, pathology and applications in imaging and therapy. Theranostics. 2013;3(1):1–2. doi: 10.7150/thno.5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauw YW, Zijlstra JM, de Jong D, Vugts DJ, Zweegman S, Hoekstra OS, et al. Performance of 89Zr-labeled-rituximab-PET as an imaging biomarker to assess CD20 targeting: a pilot study in patients with relapsed/refractory diffuse large B cell lymphoma. PLoS ONE. 2017;12(1):e0169828. doi: 10.1371/journal.pone.0169828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Wei M, Wang S, Wang G, Lai Y, Shi Y, et al. Detecting fibroblast activation proteins in lymphoma using 68Ga-FAPI PET/CT. J Nucl Med (In Press). 2021 doi: 10.2967/jnumed.121.262134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P, Federico M, Kirkwood A, Fossa A, Berkahn L, Carella A, et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin's lymphoma. N Engl J Med. 2016;374(25):2419–2429. doi: 10.1056/NEJMoa1510093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T, Price P. Development and experimental medicine applications of PET in oncology: a historical perspective. Lancet Oncol. 2012;13(3):e116–e125. doi: 10.1016/s1470-2045(11)70183-8. [DOI] [PubMed] [Google Scholar]
- Karam M, Novak L, Cyriac J, Ali A, Nazeer T, Nugent F. Role of fluorine-18 fluoro-deoxyglucose positron emission tomography scan in the evaluation and follow-up of patients with low-grade lymphomas. Cancer. 2006;107(1):175–183. doi: 10.1002/cncr.21967. [DOI] [PubMed] [Google Scholar]
- Kasamon YL, Wahl RL, Ziessman HA, Blackford AL, Goodman SN, Fidyk CA, et al. Phase II study of risk-adapted therapy of newly diagnosed, aggressive non-Hodgkin lymphoma based on midtreatment FDG-PET scanning. Biol Blood Marrow Transpl. 2009;15(2):242–248. doi: 10.1016/j.bbmt.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobe C, Dietlein M, Franklin J, Markova J, Lohri A, Amthauer H, et al. Positron emission tomography has a high negative predictive value for progression or early relapse for patients with residual disease after first-line chemotherapy in advanced-stage Hodgkin lymphoma. Blood. 2008;112(10):3989–3994. doi: 10.1182/blood-2008-06-155820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luminari S, Biasoli I, Arcaini L, Versari A, Rusconi C, Merli F, et al. The use of FDG-PET in the initial staging of 142 patients with follicular lymphoma: a retrospective study from the FOLL05 randomized trial of the Fondazione Italiana Linfomi. Ann Oncol. 2013;24(8):2108–2112. doi: 10.1093/annonc/mdt137. [DOI] [PubMed] [Google Scholar]
- Mamot C, Klingbiel D, Hitz F, Renner C, Pabst T, Driessen C, et al. Final results of a prospective evaluation of the predictive value of interim positron emission tomography in patients with diffuse large B-cell lymphoma treated with R-CHOP-14 (SAKK 38/07) J Clin Oncol. 2015;33(23):2523–2529. doi: 10.1200/JCO.2014.58.9846. [DOI] [PubMed] [Google Scholar]
- Markova J, Kahraman D, Kobe C, Skopalova M, Mocikova H, Klaskova K, et al. Role of [18F]-fluoro-2-deoxy-D-glucose positron emission tomography in early and late therapy assessment of patients with advanced Hodgkin lymphoma treated with bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine and prednisone. Leuk Lymphoma. 2012;53(1):64–70. doi: 10.3109/10428194.2011.603444. [DOI] [PubMed] [Google Scholar]
- Mayerhoefer ME, Karanikas G, Kletter K, Prosch H, Kiesewetter B, Skrabs C, et al. Evaluation of diffusion-weighted magnetic resonance imaging for follow-up and treatment response assessment of lymphoma: results of an 18F-FDG-PET/CT-controlled prospective study in 64 patients. Clin Cancer Res. 2015;21(11):2506–2513. doi: 10.1158/1078-0432.CCR-14-2454. [DOI] [PubMed] [Google Scholar]
- Mayerhoefer ME, Raderer M, Jaeger U, Staber P, Kiesewetter B, Senn D, et al. Ultra-early response assessment in lymphoma treatment: [18F]FDG PET/MR captures changes in glucose metabolism and cell density within the first 72 hours of treatment. Eur J Nucl Med Mol Imaging. 2018;45(6):931–940. doi: 10.1007/s00259-018-3937-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerhoefer ME, Raderer M, Lamm W, Pichler V, Pfaff S, Weber M, et al. CXCR4 PET imaging of mantle cell lymphoma using [68Ga]Pentixafor: comparison with [18F]FDG-PET. Theranostics. 2021;11(2):567–578. doi: 10.7150/thno.48620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micallef IN, Maurer MJ, Wiseman GA, Nikcevich DA, Kurtin PJ, Cannon MW, et al. Epratuzumab with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy in patients with previously untreated diffuse large B-cell lymphoma. Blood. 2011;118(15):4053–4061. doi: 10.1182/blood-2011-02-336990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhaeel NG, Hutchings M, Fields PA, O'Doherty MJ, Timothy AR. FDG-PET after two to three cycles of chemotherapy predicts progression-free and overall survival in high-grade non-Hodgkin lymphoma. Ann Oncol. 2005;16(9):1514–1523. doi: 10.1093/annonc/mdi272. [DOI] [PubMed] [Google Scholar]
- Minamimoto R, Fayad L, Advani R, Vose J, Macapinlac H, Meza J, et al. Diffuse large B-cell lymphoma: prospective multicenter comparison of early interim FLT PET/CT versus FDG PET/CT with IHP, EORTC, Deauville, and PERCIST criteria for early therapeutic monitoring. Radiology. 2016;280(1):220–229. doi: 10.1148/radiol.2015150689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamimoto R, Fayad L, Vose J, Meza J, Advani R, Hankins J, et al. 18F-Fluorothymidine PET is an early and superior predictor of progression-free survival following chemoimmunotherapy of diffuse large B cell lymphoma: a multicenter study. Eur J Nucl Med Mol Imaging. 2021;48(9):2883–2893. doi: 10.1007/s00259-021-05353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz AJ, Schöder H, Yahalom J, McCall SJ, Fox SY, Gerecitano J, et al. PET-adapted sequential salvage therapy with brentuximab vedotin followed by augmented ifosamide, carboplatin, and etoposide for patients with relapsed and refractory Hodgkin's lymphoma: a non-randomised, open-label, single-centre, phase 2 study. Lancet Oncol. 2015;16(3):284–292. doi: 10.1016/s1470-2045(15)70013-6. [DOI] [PubMed] [Google Scholar]
- Moskowitz CH, Schöder H, Teruya-Feldstein J, Sima C, Iasonos A, Portlock CS, et al. Risk-adapted dose-dense immunochemotherapy determined by interim FDG-PET in advanced-stage diffuse large B-cell lymphoma. J Clin Oncol. 2010;28(11):1896. doi: 10.1200/JCO.2009.26.5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muylle K, Flamen P, Vugts DJ, Guiot T, Ghanem G, Meuleman N, et al. Tumour targeting and radiation dose of radioimmunotherapy with 90Y-rituximab in CD20+ B-cell lymphoma as predicted by 89Zr-rituximab immuno-PET: impact of preloading with unlabelled rituximab. Eur J Nucl Med Mol Imaging. 2015;42(8):1304–1314. doi: 10.1007/s00259-015-3025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q, Luo Y, Zhang Y, Chang L, Li J, Cao X, et al. Preliminary evidence of imaging of chemokine receptor-4-targeted PET/CT with [68Ga]pentixafor in non-Hodgkin lymphoma: comparison to [18F]FDG. EJNMMI Res. 2020;10(1):89. doi: 10.1186/s13550-020-00681-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardal E, Coronado M, Martin A, Grande C, Marin-Niebla A, Panizo C, et al. Intensification treatment based on early FDG-PET in patients with high-risk diffuse large B-cell lymphoma: a phase II GELTAMO trial. Br J Haematol. 2014;167(3):327–336. doi: 10.1111/bjh.13036. [DOI] [PubMed] [Google Scholar]
- Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380(9840):499–505. doi: 10.1016/s0140-6736(12)60815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfreundschuh M, Kuhnt E, Trümper L, Österborg A, Trneny M, Shepherd L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12(11):1013–1022. doi: 10.1016/s1470-2045(11)70235-2. [DOI] [PubMed] [Google Scholar]
- Phan J, Mazloom A, Jeffrey Medeiros L, Zreik TG, Wogan C, Shihadeh F, et al. Benefit of consolidative radiation therapy in patients with diffuse large B-cell lymphoma treated with R-CHOP chemotherapy. J Clin Oncol. 2010;28(27):4170–4176. doi: 10.1200/JCO.2009.27.3441. [DOI] [PubMed] [Google Scholar]
- Phelps ME. PET: the merging of biology and imaging into molecular imaging. J Nucl Med. 2000;41(4):661–681. [PubMed] [Google Scholar]
- Platzek I, Beuthien-Baumann B, Langner J, Popp M, Schramm G, Ordemann R, et al. PET/MR for therapy response evaluation in malignant lymphoma: initial experience. MAGMA. 2013;26(1):49–55. doi: 10.1007/s10334-012-0342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platzek I, Beuthien-Baumann B, Ordemann R, Maus J, Schramm G, Kitzler HH, et al. FDG PET/MR for the assessment of lymph node involvement in lymphoma: initial results and role of diffusion-weighted MR. Acad Radiol. 2014;21(10):1314–1319. doi: 10.1016/j.acra.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Punwani S, Taylor SA, Saad ZZ, Bainbridge A, Groves A, Daw S, et al. Diffusion-weighted MRI of lymphoma: prognostic utility and implications for PET/MRI? Eur J Nucl Med Mol Imaging. 2013;40(3):373–385. doi: 10.1007/s00259-012-2293-7. [DOI] [PubMed] [Google Scholar]
- Radford J, Illidge T, Counsell N, Hancock B, Pettengell R, Johnson P, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin's lymphoma. N Engl J Med. 2015;372(17):1598–1607. doi: 10.1056/NEJMoa1408648. [DOI] [PubMed] [Google Scholar]
- Recently updated NCCN clinical practice guidelines in oncology. https://www.nccn.org/guidelines/recently-published-guidelines. Accessed 18 Mar 2021
- Rohren EM, Turkington TG, Coleman RE. Clinical applications of PET in oncology. Radiology. 2004;231(2):305–332. doi: 10.1148/radiol.2312021185. [DOI] [PubMed] [Google Scholar]
- Rutherford SC, Herold M, Hiddemann W, Kostakoglu L, Marcus R, Martelli M, et al. Impact of bone marrow biopsy on response assessment in immunochemotherapy-treated lymphoma patients in GALLIUM and GOYA. Blood Adv. 2020;4(8):1589–1593. doi: 10.1182/bloodadvances.2019001261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter CS, Matasar MJ, Meikle J, Schoder H, Ulaner GA, Migliacci JC, et al. Prognostic value of FDG-PET prior to autologous stem cell transplantation for relapsed and refractory diffuse large B-cell lymphoma. Blood. 2015;125(16):2579–2581. doi: 10.1182/blood-2014-10-606939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer NG, Taverna C, Strobel K, Wastl C, Kurrer M, Hany TF. Hodgkin disease: diagnostic value of FDG PET/CT after first-line therapy–is biopsy of FDG-avid lesions still needed? Radiology. 2007;244(1):257–262. doi: 10.1148/radiol.2441060810. [DOI] [PubMed] [Google Scholar]
- Schöder H, Polley M-YC, Knopp MV, Hall N, Kostakoglu L, Zhang J, et al. Prognostic value of interim FDG-PET in diffuse large cell lymphoma: results from the CALGB 50303 Clinical Trial. Blood. 2020;135(25):2224–2234. doi: 10.1182/blood.2019003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Das S, Okwan-Duodu D, Esiashvili N, Flowers C, Chen Z, et al. Patterns of failure in advanced stage diffuse large B-cell lymphoma patients after complete response to R-CHOP immunochemotherapy and the emerging role of consolidative radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86(3):569–577. doi: 10.1016/j.ijrobp.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Stewart DA, Kloiber R, Owen C, Bahlis NJ, Duggan P, Mansoor A, et al. Results of a prospective phase II trial evaluating interim positron emission tomography-guided high dose therapy for poor prognosis diffuse large B-cell lymphoma. Leuk Lymphoma. 2014;55(9):2064–2070. doi: 10.3109/10428194.2013.862242. [DOI] [PubMed] [Google Scholar]
- Swinnen LJ, Li H, Quon A, Gascoyne R, Hong F, Ranheim EA, et al. Response-adapted therapy for aggressive non-Hodgkin's lymphomas based on early [18F] FDG-PET scanning: ECOG-ACRIN Cancer Research Group study (E3404) Br J Haematol. 2015;170(1):56–65. doi: 10.1111/bjh.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa T, Lau J, Bardet S, Couturier O, Hotta T, Hutchings M, et al. Fluorine-18-fluorodeoxyglucose positron emission tomography for interim response assessment of advanced-stage Hodgkin's lymphoma and diffuse large B-cell lymphoma: a systematic review. J Clin Oncol. 2009;27(11):1906–1914. doi: 10.1200/JCO.2008.16.0861. [DOI] [PubMed] [Google Scholar]
- Thompson CA, Ghesquieres H, Maurer MJ, Cerhan JR, Biron P, Ansell SM, et al. Utility of routine post-therapy surveillance imaging in diffuse large B-cell lymphoma. J Clin Oncol. 2014;32(31):3506–3512. doi: 10.1200/JCO.2014.55.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, He X, Jin C, He X, Wu S, Zhou R, et al. Transpathology: molecular imaging-based pathology. Eur J Nucl Med Mol Imaging. 2021;48(8):2338–2350. doi: 10.1007/s00259-021-05234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly H, Gomes da Silva M, Vitolo U, Jack A, Meignan M, Lopez-Guillermo A, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v116–125. doi: 10.1093/annonc/mdv304. [DOI] [PubMed] [Google Scholar]
- Torigian DA, Zaidi H, Kwee TC, Saboury B, Udupa JK, Cho Z-H, et al. PET/MR imaging: technical aspects and potential clinical applications. Radiology. 2013;267(1):26–44. doi: 10.1148/radiol.13121038. [DOI] [PubMed] [Google Scholar]
- Trotman J, Barrington SF, Belada D, Meignan M, MacEwan R, Owen C, et al. Prognostic value of end-of-induction PET response after first-line immunochemotherapy for follicular lymphoma (GALLIUM): secondary analysis of a randomised, phase 3 trial. Lancet Oncol. 2018;19(11):1530–1542. doi: 10.1016/s1470-2045(18)30618-1. [DOI] [PubMed] [Google Scholar]
- Trotman J, Fournier M, Lamy T, Seymour JF, Sonet A, Janikova A, et al. Positron emission tomography-computed tomography (PET-CT) after induction therapy is highly predictive of patient outcome in follicular lymphoma: analysis of PET-CT in a subset of PRIMA trial participants. J Clin Oncol. 2011;29(23):3194–3200. doi: 10.1200/JCO.2011.35.0736. [DOI] [PubMed] [Google Scholar]
- Verhagen MV, Menezes LJ, Neriman D, Watson TA, Punwani S, Taylor SA, et al. 18F-FDG PET/MRI for staging and interim response assessment in pediatric and adolescent Hodgkin lymphoma: a prospective study with 18F-FDG PET/CT as reference standard. J Nucl Med. 2021;62(11):1524–1530. doi: 10.2967/jnumed.120.260059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Jin X, Zhu H, Wang S, Ding J, Zhang Y, et al. 68Ga-NOTA-FAPI-04 PET/CT in a patient with primary gastric diffuse large B cell lymphoma: comparisons with [18F] FDG PET/CT. Eur J Nucl Med Mol Imaging. 2021;48(2):647–648. doi: 10.1007/s00259-020-04946-0. [DOI] [PubMed] [Google Scholar]
- Wehrl HF, Sauter AW, Divine MR, Pichler BJ. Combined PET/MR: a technology becomes mature. J Nucl Med. 2015;56(2):165–168. doi: 10.2967/jnumed.114.150318. [DOI] [PubMed] [Google Scholar]
- Wester HJ, Keller U, Schottelius M, Beer A, Philipp-Abbrederis K, Hoffmann F, et al. Disclosing the CXCR4 expression in lymphoproliferative diseases by targeted molecular imaging. Theranostics. 2015;5(6):618–630. doi: 10.7150/thno.11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth A, Foo M, Seymour JF, Macmanus MP, Hicks RJ. Impact of [18F] fluorodeoxyglucose positron emission tomography on staging and management of early-stage follicular non-hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2008;71(1):213–219. doi: 10.1016/j.ijrobp.2007.09.051. [DOI] [PubMed] [Google Scholar]
- Wohrer S, Jaeger U, Kletter K, Becherer A, Hauswirth A, Turetschek K, et al. 18F-fluoro-deoxy-glucose positron emission tomography (18F-FDG-PET) visualizes follicular lymphoma irrespective of grading. Ann Oncol. 2006;17(5):780–784. doi: 10.1093/annonc/mdl014. [DOI] [PubMed] [Google Scholar]
- Yang DH, Min JJ, Song HC, Jeong YY, Chung WK, Bae SY, et al. Prognostic significance of interim 18F-FDG PET/CT after three or four cycles of R-CHOP chemotherapy in the treatment of diffuse large B-cell lymphoma. Eur J Cancer. 2011;47(9):1312–1318. doi: 10.1016/j.ejca.2010.12.027. [DOI] [PubMed] [Google Scholar]
- Yang X, Hu Z, You Z, Chen Y, Liu H. Mediastinal diffuse large B-cell lymphoma mimicking invasive thymoma on 68Ga-FAPI PET/CT. J Nucl Cardiol (In Press) 2021 doi: 10.1007/s12350-021-02623-9. [DOI] [PubMed] [Google Scholar]
- Yao Y, Li Y-M, He Z-X, Civelek AC, Li X-F. Likely common role of hypoxia in driving 18F-FDG uptake in cancer, myocardial ischemia, inflammation and infection. Cancer Biother Radiopharm. 2021;36(8):624–631. doi: 10.1089/cbr.2020.4716. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Feng J, Ji J, Hou H, Chen L, Wu S, et al. Alteration of monoamine receptor activity and glucose metabolism in pediatric patients with anticonvulsant-induced cognitive impairment. J Nucl Med. 2017;58(9):1490–1497. doi: 10.2967/jnumed.116.189290. [DOI] [PubMed] [Google Scholar]
- Zinzani PL, Rigacci L, Stefoni V, Broccoli A, Puccini B, Castagnoli A, et al. Early interim 18F-FDG PET in Hodgkin's lymphoma: evaluation on 304 patients. Eur J Nucl Med Mol Imaging. 2012;39(1):4–12. doi: 10.1007/s00259-011-1916-8. [DOI] [PubMed] [Google Scholar]
- Zinzani PL, Stefoni V, Tani M, Fanti S, Musuraca G, Castellucci P, et al. Role of [18F]fluorodeoxyglucose positron emission tomography scan in the follow-up of lymphoma. J Clin Oncol. 2009;27(11):1781–1787. doi: 10.1200/JCO.2008.16.1513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Not applicable.