Abstract
Mendel’s laws state that each of the two alleles would segregate during gamete formation and show the same transmission ratio in the next generation. However, an unexpected biased allele transmission was first detected in Drosophila a century ago, and was subsequently observed in other animals, plants, and microorganisms. Such segregation distortion (SD) shows substantial effects in population structure and fitness of the progenies, which would ultimately lead to reproductive isolation and speciation. Here, we trace the early investigations on the violation of Mendelian genetic principle, which appears as a wide-existence phenomenon rather than a case of exception. The occurence of SD in the whole genome was observed in a number of plant species at the single- and multi-locus level. Biased transmission ratio might occur at meiosis stage due to asymmetric movement of the chromosome; transmission ratio advantage is also caused by interaction and battle between the alleles from respective genomes at the genetic and molecular level. The origin of a SD system is likely to be determined by coevolution of the killer and protector via recurrent breakdown or rebalance loop. These updated understandings also promote genetic improvement of hybrid crops.
Keywords: Segregation distortion, Killer-protector system, Breakdown and rebalance model, Crop genetic improvement
Introduction
Species tried to improve its adaptation to the environment during the evolution. The common strategy is to increase the frequencies of genes with higher fitness, because these genes might increase the ability of their hosts in survival or reproduction. However, a special group of genes, i.e., genes causing segregation distortion (SD), can spread in the population through transmission advantage despite being harmful to the fitness of the offspring. Generally speaking, based on law of segregation and law of independent assortment, alleles for each gene would segregate independently during meiosis, resulting in euqal transmission of each allele and 1:2:1 genotype ratio over generations. Different from such normal case, SD shows a selective advantage to one allele or a certain genotype for its own favor, but results in a cost of another allele or the other genotypes. The favored allele or genotype can spread in the populations over time regardless of its fitness, which would affect the composition of a population. Therefore, the evolution of SD changes the the allele frequency and genotype frequency from generation to generation, which would ultimately show substantial effects on population structure. In addition, biased transmission ratio is always accompanied with reduced adaptation or fitness in the progenies, resulting in genetic differentiation and even speciation.
First glimpse towards SD: violation of Mendelian genetic principle
Back in 1928, Gershenson gave his first view of sex-ratio abnormality by a gametic lethal via a sex-linked SD gene in Drosophila obscura (Gershenson 1928). Strong deviation of sex distribution was observed in the progeny lines of D. obscura, leading to 96% of females and only 4% of males. Such sex-linked biased transmission ratio was a universal phenomenon observed in different fly species such as D. pseudoobscura, D. affinis, D. athabasca, and D. azteca (Sturtevant and Dobzhansky 1936). Sandler and Novitski summarized that heterozygotes might fail to produce gametes with equal frequency in the next generation (Sandler and Novitski 1957). Such unusual pattern of segregation depends on the process of meiotic divisions and the driving force is thus defined as meiotic drive. Systematic studies in mouse also detected that males heterozygous for the t haplotype preferentially transmitted the t chromosome to their offspring, forming significant departures from normal segregation (Braden 1958; Dunn 1939; Dunn and Bennett 1968; Hammer et al. 1989; Lyon et al. 1988). In addition to understandings in fly and mouse, numerous cases in SD were also observed in a number of species such as mosquito (Hickey and Craig 1966), flour beetles (Beeman et al. 1992), and Neurospora (Raju 1979; Turner and Perkins 1979).
Less attention had been paid on SD in plants in the early days, and the first case was detected in maize showing distorted genetic ratios due to preferential segregation in megasporogenesis (Rhoades 1942; Rooades and Vilkomerson 1942). Such biased transmission ratio was likely caused by an abnormal terminal knob on chromosome 10, which might affect chromosome movement and thus non-random segregation. Subsequently, reduction in male transmission was observed in wheat and thereby changed the expected 3:1 ratio to about 9:7 in F2 generation (Loegering and Sears 1963), whereas in tomato, both male and female gametes with Gec genotype were preferentially aborted (Rick 1966, 1971).
Taken together, early studies had described SD as transmission ratio distortion, sex ratio distortion, or non-mendelian segregation, and proposed that the driving force of SD might be meiotic drive or genetical drive. It was realized that such violation of Mendelian genetic principle, i.e., significant biased allele frequency or genotype frequency in the offspring, was not simply a case of exception. And although SD had been observed in a range of species from animals to plants, understanding in the occurrence of SD at the population level remained a mystery in those days.
How often does SD occur among different plant species?
SD frequently occurs in a wide range of plant crosses including monocots and dicots; it may also affect the development of gametes in offspring and thus showing effect in reproductive isolation.
A good system for investigation of deviation of Mendelian segregation might be inter-subspecific crosses between indica (also referred to as xian rice) and japonica rice (also referred to as geng rice) in Oryza sativa, which also show postzygotic reproductive barriers such as hybrid sterility and hybrid weakness between these two subspecies (Ouyang et al. 2010; Ouyang and Zhang 2013, 2018; Yamamoto et al. 2010). Numerous SD loci were mapped covering the entire genome in F2 progeny of indica-japonica crosses (Harushima et al. 2001, 2002). Wide distribution of SD was also observed from reciprocal F2 and BC1F1 mapping populations derived from indica and japonica parents (Reflinur et al. 2014). The connection between SD and its effect on hybrid fertility was extensively investigated using three pairwise indica-japonica crosses, which quantified the effect of SD in the whole genome at the single- and multi-locus level (Li et al. 2017). A framework for SD was established from nine inter-subspecific rice crosses at the whole genome level, which further confirmed the high occurrence of SD in rice (Li et al. 2019). A total of 61 single-locus quantitative trait loci (QTLs) and 194 digenic interactions were identified showing biased transmission ratio, and the majority (72.13%) of these effective loci for SD were repeatedly detected in multiple populations. In Arabidopsis thaliana, the occurrence of SD was only detected in a quarter of the surveyed 583 segregating F2 populations (Seymour et al. 2019). Besides, most Arabidopsis populations only contained a single SD locus, which revealed a very distinct pattern relative to that in rice. In addition, SD was widely observed in a number of other plant species such as hop (Zhang et al. 2017), maize (Sibov et al. 2003), barley (Bélanger et al. 2016), oak (Bodénès et al. 2016), poplar (Zhou et al. 2015), senecio (Brennan et al. 2014), watermelon (Ren et al. 2015), alfalfa (Li et al. 2011), and triticale (Alheit et al. 2011).
An interesting finding is that one genome always wins the battle under the competition after meiosis. Gametes from indica varieties were preferred (77.05%) after meiosis in inter-subspecific crosses, suggesting that the japonica alleles were always defeated under competition. Similarly, a genome-wide identification of SD in interspecific lettuce hybrids detected that all distorted regions were skewed towards Lactuca sativa alleles, leading to hybrid incompatibility (Giesbers et al. 2019).
It was observed that two-locus interaction contributed significantly to the deviation of genotype frequencies in rice in the whole genome (Li et al. 2017, 2019). Likewise, digenic interactions were also responsible for SD among Lactuca species (Giesbers et al. 2019). Evidence for SD caused by epistatic interactions was also observed in triticale across the whole genome (Alheit et al. 2011). Therefore, epistasis might represent important and general mechanism driving SD and genetic differentiation in plants; this suggested a rather distinct pattern, such that the importance of epistasis in SD was largely weakened in animals such as Drosophila (Corbett-Detig et al. 2013).
How can SD genes ensure preferential transmission of its gamete?
There had long been a paradox to understand the conflict role of SD genes, i.e., the reduced fitness of the progeny and increased frequencies of themselves. One solution was the widely existed killer-protector system, which ensured the preferential transmission of the killer at the cost of the gametes without the protector (Table 1). A well-studied case was the S5 system in rice, which regulated both SD and embryo-sac fertility in the offspring between indica and japonica subspecies (Chen et al. 2008; Yang et al. 2012; Zhu et al. 2017). Three linked genes, ORF3, ORF4, and ORF5, were identified at the S5 locus; they had differentiated into distinct alleles with divergent functions in respective rice populations, including the killer ORF5 + , the partner of the killer ORF4 + , and the protector ORF3 + . The indica subspecies contained the genotype of ORF3 + ORF4-ORF5 + , whereas the japonica rice group carried a completely reversed genotype of ORF3-ORF4 + ORF5-. Therefore, the killer ORF5 + and its partner ORF4 + would meet in the hybrid, which triggered ER stress and subsequently premature programmed cell death in the female gametes. Such detrimental signal would lead to selective abortion of the developing megaspores without the protector ORF3 + , thus resulting in biased transmission of the gametes with indica allele and hybrid sterility in the progeny.
Table 1.
Genes or loci causing segregation distortion (SD) in plants and other organisms
| Stage for SD | Species | Genes or loci | Alleles/Genotypes | Reason for fixation | Evolutionary model | Molecular mechanism | References |
|---|---|---|---|---|---|---|---|
| During meiosis | Zea mays | Ab10 |
Kindr/knob –/– |
Linkage | – | The kinesin encoded by Kindr could bind to the neocentromere (knob), thus making the Ab10 chromosome move faster at anaphase during meiosis | Dawe et al. (2018); Yu et al. (1997) |
| Mus musculus | CDC42 signaling |
CDC42 – |
– | – | CDC42 can tyrosinate α-tubulin, the uneven distribution of tyrosinated α-tubulin and detyrosinated α-tubulin on a chromosome will lead to spindle assymetry | Akera et al. (2017) | |
| After meiosis | Oryza sativa L. | Sa |
SaF+/SaM+ SaF–/SaM– |
Linkage | Breaking down of ancestral killer-protector balance | SaM+ and SaF+ selectively kill male gemetes without SaM+ in hybrids | Long et al. (2008) |
| Oryza sativa L. | S5 |
ORF3+/ORF4-/ORF5+ ORF3-/ORF4+/ORF5- |
Linkage | Breaking down of ancestral killer-protector balance | ORF4+ and ORF5+ selectively kill female gametes without ORF3+ in hybrids |
Chen et al. (2008, Yang et al. (2012), Zhu et al. (2017) |
|
| Oryza sativa L. and Oryza meridionalis | qHMS7 |
ORF2D/ORF3 ORF2M/− |
Linkage | Formation of killer-protector balance | ORF2D in O. sativa selectively kills male gametes without ORF3 in hybrids | Yu et al. (2018) | |
| Oryza glaberrima Steud. and Oryza sativa L. | S1 |
TPR/A4/A6 TP/–/– |
Linkage | Formation of killer-protector balance | TPR, A4 and A6 (SSP) result in preferential transmission of gametes containing TPR |
Koide et al. (2018), Xie et al. (2017b), Xie et al. (2019) |
|
| Caenorhabditis elegans | sup-35/pha-1 |
sup-35/pha-1 –/– |
Linkage and inversion | Breaking down of ancestral killer-protector balance | Maternally expressed sup-35 kills diploids without pha-1 | Ben-David et al. (2017) | |
| Caenorhabditis elegans | zeel-1/peel-1 |
zeel-1Bristol/peel-1Bristol zeel-1Hawaii/peel-1Hawaii |
Linkage | – | Paternally expressed peel-1Bristol kills embryos with zeel-1Hawaii | Seidel et al. (2008) | |
| Drosophila melanogaster | SD |
SD/Rspi SD+/Rsps |
Linkage (near the centromere) | – | SD selectively kills gametes containing Rsps |
Kusano et al. (2003) Larracuente and Presgraves (2012) Merrill et al. (1999) |
|
| Mus musculus | t-haplotype |
Tcr/Tcd1-3 Tcrwt/Tcdwt |
Linkage and inversion | – | The preferential transmission of the t-haplotype is caused by interaction between Tcd and Tcr |
Bauer et al. (2005), Bauer et al. (2007), Charron et al. (2019), Herrmann et al. (1999) |
|
| Oryza sativa L. | Sc |
Sc-i Sc-j |
– | – | Sc-i selectively kills Sc-j male gametes | Shen et al. (2017) | |
| Schizosaccharomyces pombe | wtf13/wtf18-2 |
wtf13poison/wtf18-2 wtf13antidote/wtf18-2 |
– | – | wtf13poison kills spores that do not carry it, whereas wtf18-2 gains a transmission advantage by suppressing wtf13poison | Bravo Núñez et al. (2018) |
Note that the gametes with the functional killer ensured a transmission advantage at the cost of the ones without a protector allele, and such SD pattern was extensively supported by a number of cases in rice (Table 1). For instance, the qHMS7 locus with two tightly linked genes ORF2 and ORF3 was identified for SD in progeny from the wild and cultivated rice species. Selected pollen grains without qHMS7-ORF3 were eliminated by the killer allele qHMS7-ORF2D from the cultivated rice (Yu et al. 2018). Likewise, three tightly linked alleles in African rice at S1 locus, S1A4, S1TPR, and S1A6 (SSP), constituted a functional killer in meiotic cells of hybrids between Asian and African cultivated rice; it triggered selective abortion of both male and female gametes from Asian cultivated rice due to lack of a dual-role allele S1TPR (acting as a protector as well), thus leading to SD and reduced fertility in the offspring (Koide et al. 2018; Xie et al. 2017b, 2019). Transmission ratio distortion was also observed at a male sterility locus Sa with two adjacent genes SaF and SaM (Long et al. 2008). Although the components at Sa locus were not defined as killer and protector, it was observed that the pollen grains with SaM- were selectively killed due to deleterious interaction between SaF + , SaM + , and SaM-. Such egoism by the killer gave a general solution to SD even in a wide range of animals, such as the t-haplotype in mouse (Bauer et al. 2005, 2007, 2012; Charron et al. 2019; Herrmann et al. 1999), the segregation distorter system in Drosophila (Larracuente and Presgraves 2012), and the zeel-1/peel-1 (Seidel et al. 2008) and sup-35/pha-1 (Ben-David et al. 2017) cases in Caenorhabditis elegans.
Another strategy for gaining transmission ratio advantage might be the asymmetric movement of the chromosome at meiosis stage (Table 1). A classic example was the preferential transmission mediated by abnormal chromosome 10 (Ab10) in maize, which converted heterochromatic regions (knobs) into motile neocentromeres that moved faster toward spindle poles during meiosis (Dawe et al. 2018; Yu et al. 1997). A Kinesin driver (Kindr) complex on Ab10 comprised a functional minus-end-directed kinesin with 180 bp repeats, which was responsible for neocentromere motility and thus changed segregation patterns for alleles linked to knobs. This case represented a selfish role of centromeric sequences in SD for their own transmission advantage. In mouse, spindle asymmetry was detected in female meiosis leading to non-Mendelian segregation driven by CDC42 signaling (Akera et al. 2017). Therefore, asymmetry at meiosis stage might be able to bias transmission ratio in a broad spectrum of species including both plants and animals.
How did the SD genes originate and evolve?
What are the evolutionary scenarios for the spread of SD genes? The formation of closely linked killer and protector might be a general distortion system favored by natural selection (Fig. 1). Two critical prerequisites are required for the establishment of such SD system. First of all, at least two tightly linked killer and protector were involved, and recombination was thus prohibited from each other to avoid collapse of the system by the suicidal killer/non-protector genotype (Burt and Trivers 2006). For example, a number of cases involved in SD might be composed of physically adjacent genes, such as the participants in S5 (Yang et al. 2012), Sa (Long et al. 2008), S1 (Xie et al. 2019), qHMS7 (Yu et al. 2018), and zeel-1/peel-1 (Seidel et al. 2008) as well as sup-35/pha-1 (Ben-David et al. 2017) locus-pair. The recombination might also be largely suppressed by inversions or locations near the centromere like t-haplotype in mouse (Hammer et al. 1989), SD locus in Drosophila (Kusano et al. 2003), and sup-35/pha-1 in Caenorhabditis (Ben-David et al. 2017). Therefore, such genetical linkage of the killer and protector is a favored characteristic of the SD system, which facilitates the spread of the killer.
Fig. 1.
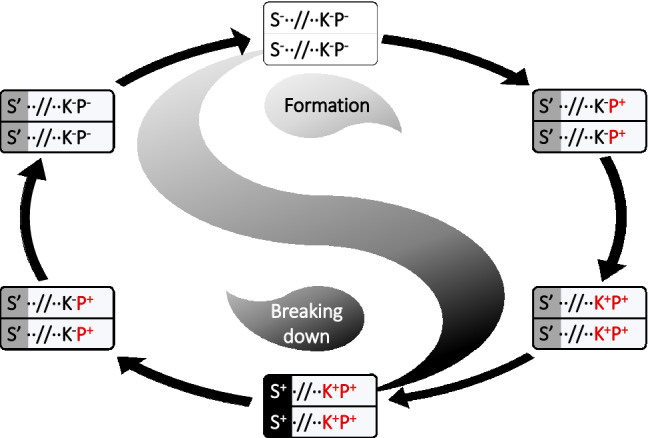
Evolutionary dynamics for origin of segregation distortion via a recurrent breakdown and rebalance loop. Three components, K for the killer, P for the protector, and S for the suppressor, are involved in segregation distortion, with + for functional allele, – for non-functional allele, and ’ for intermediate allele. The red color is used to highlight the evolutionary dynamics. The killer and protector are linked, whereas the suppressor exists in the background
Second, the origin of a SD system might be determined by either breakdown or formation of the killer-protector balance, which was caused by two or more sequential steps during the evolution (Fig. 1) (Ouyang and Zhang 2013, 2018; Ouyang 2019). The origin of S5 complex under parallel-sequential divergence model was well studied, which revealed the co-existence of the killer and protector in ancestral populations (Du et al. 2011; Mi et al. 2020; Ouyang et al. 2016). The loss-of-function mutations occurred in the killer first, which gave the permission to the protector to evolve freely. Thus, the nonfunctional mutations occurred in the protector subsequently, and such combination of nonfunctional-killer plus nonfunctional protector would be selectively killed by the ancestral combination with functional killer and protector. The similar breakdown processes were also found in systems under sequential divergence model, such as the Sa locus in rice (Long et al. 2008) and sup35/pha-1 in Caenorhabditis (Ben-David et al. 2017). However, a converse case of S1 locus provided a genetic background with a functional protector first, which allowed the emergence of a killer without any decrease in fitness (Koide et al. 2018; Ouyang 2019; Xie et al. 2017b, 2019). The newly evolved killer and protector would lead to preferential transmission of its genotype when meet the ones lacking the protector. The evolutionary dynamics of this formation pattern was also observed in a qHMS7 case (Yu et al. 2018), and a buffered genetic background for the killer would be the key step for establishment of a SD system.
Once the SD system was established, the frequency of the killer alleles would increase rapidly and might even be fixed in a species owing to their transmission advantage. An alternative solution might be the emergence of an unlinked suppressor during the evolution, which could balance the distortion of the allele frequency due to the spread of the killer (Fig. 1). Investigation of unlinked modifiers remains preliminary in plants. In rice, it was reported that transmission ratio distortion determined by S6 locus was largely dependent on the genetic background (Koide et al. 2012). Besides, SD due to S24 locus was counteracted by an unlinked locus EFS in indica genetic background (Kubo et al. 2016). A SD case in Schizosaccharomyces pombe provided a better understanding. It was observed that a wtf13 gene killed spores that did not inherit it, and its killer effect was suppressed by another wtf gene, wtf18-2, which allowed the transmission advantage of the spores with wtf18-2. Therefore, the severe transmission ratio distortion by wtf13 might be mitigated by an unlinked suppressor wtf18-2 (Bravo Núñez et al. 2018).
Significance of SD in crop genetic improvement
SD and sterility are closely related phenotypes in crop hybrids. They are important evolutionary forces for reproductive isolation and even speciation, but they are troublesome obstacles for utilization of intersubspecific or interspecific heterosis especially in rice. Distorted transmission ratio and reduced fertility in hybrids might be rescued by manipulating of SD systems, either by breaking down the killer, introducing the protector, or transferring a suppressor in the genetic background.
The CRISPR/Cas9 technology might greatly speed up the breeding process via breakdown of the killer components in the SD system. In this way, SaF and SaM alleles were knocked out at the Sa locus, which restored male fertility and SD in intersubspecific hybrids (Xie et al. 2017a). Likewise, disruption of the killer alleles SSP or OgTPR1 in O. glaberrima had been applied in rice genetic improvement (Koide et al. 2018; Xie et al. 2017b). Molecular marker-assisted backcross breeding strategy might be more applicable to improvement of SD and fertility by introgression of a protector. In addition, it was proposed that the suppressors in indica genetic background might provide an additional strategy for breaking the obstacle of inter-subspecific hybrid sterility (Mi et al. 2019). And further understanding in biochemical pathways and interaction networks of SD would allow a more precise editing of the genomes in crops.
Concluding remarks and future perspective
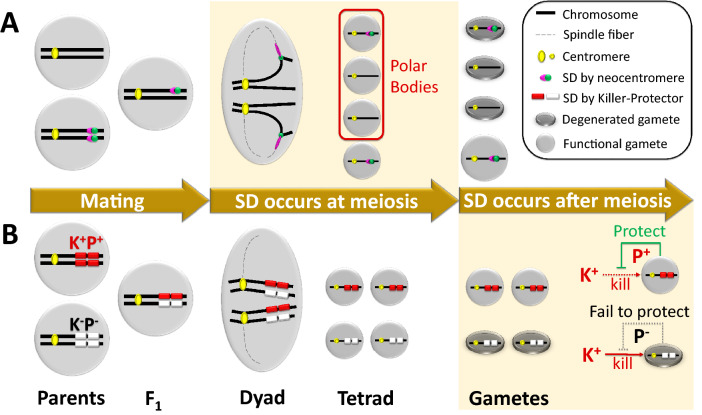
It has been nearly a century since the discovery of the first case of SD. Since then a range of cases are unveiled, although only a handful of the advances in this field have been achieved at the molecular and evolutionary level. Based on these well-studied cases, we realize that the intrinsic force driving SD might either occur at meiotic stage or at post-meiotic stage (Fig. 2). The former situation always results in biased transmission ratio of female gametophyte in higher plants and animals, because only the megaspore that occupies a specific position (e.g., chalazal megaspore for embryo sac of Polygonum type) is functional due to megaspore tetrad degeneration. Therefore, when a sister chromatid gains advantage by asymmetric strategy such as asymmetric movement or spindle asymmetry in meiotic division, preferential transmission is awarded to the winner. We detect more for the latter type of SD, which acts on developing gametes after the meiosis, by means of selective killing of the gametes without the selfish genes. Such post-meiotic SD strategy is guaranteed by the balance of linked killer and protector, leading to transmission ratio distortion of either or both male and female gametophytes.
Fig. 2.
Intrinsic driving force for segregation distortion at either meiotic stage or post-meiotic stage. A Biased transmission ratio of female gametophyte caused by asymmetric strategy in meiotic division. This figure is modified from Dawe et al. 2018. B Preferential transmission ratio due to selective killing of male or/and female gametes without the functional protector after meiosis. K indicates the killer and P indicates the protector, with + for functional allele and – for non-functional allele.
(This figure is modified from Dawe et al. 2018)
The phenomenon of SD is caused by competition between diverged genomes; SD is also largely affected by suppressors or epistatic interaction in background that made it difficult for understanding. An efficient solution is the construction of near-isogenic lines, which avoid interference from the genetic background. Comparative genomics against candidate regions and transcriptome for developing gametes would greatly accelerate future investigation of causal genes for SD.
The evolution of SD increases the frequency of favored allele or genotype from generation to generation, which may change the structure and divergence of sibling populations. Therefore, it is important to understand SD in the perspective of evolution in further work, based on a wide range of germplasm with extensive geographic distributions and closely related species in different time scales.
In some cases, SD might lead to hybrid sterility due to preferential abortion of the gametes, which becomes a major obstacle of hybrid crop breeding, a key strategy for promoting yield in many crop species such as rice and maize. Therefore, overcoming SD and intersubspecific or interspecific hybrid sterility is an essential strategy allowing further increase of crop yield. The difficulty is that a number of SD loci are distributed across the whole genome, and removal of a single distorted region will not meet the required fertility. Future mechanistic understanding of SD and identification of the selfish alleles would allow precise editing of a specific target, and advanced technology of CRISPR/Cas editing may facilitate manipulating of multiple SD loci simultaneously. Knocking-out of the killer and introgression of the protector at SD loci have proven to be a promising strategy for recovering fertility in rice hybrids. And we anticipate utilization of distant hybridization for other crops in the near future.
Acknowledgements
This research was supported by grants from the National Natural Science Foundation of China (31991223), the Hubei Provincial Natural Science Foundation of China (2019CFA061), the Fundamental Research Funds for the Central Universities (2662020SKPY005), and the National Program for Support of Top-notch Young Professionals.
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interests to declare.
References
- Akera T, Chmátal L, Trimm E, Yang K, Aonbangkhen C, Chenoweth DM, Janke C, Schultz RM, Lampson MA. Spindle asymmetry drives non-mendelian chromosome segregation. Science. 2017;358:668–672. doi: 10.1126/science.aan0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheit KV, Reif JC, Maurer HP, Hahn V, Weissmann EA, Miedaner T, Würschum T. Detection of segregation distortion loci in triticale (× Triticosecale Wittmack) based on a high-density DArT marker consensus genetic linkage map. BMC Genom. 2011;12:380. doi: 10.1186/1471-2164-12-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer H, Willert J, Koschorz B, Herrmann BG. The t complex-encoded GTPase-activating protein Tagap1 acts as a transmission ratio distorter in mice. Nat Genet. 2005;37:969–973. doi: 10.1038/ng1617. [DOI] [PubMed] [Google Scholar]
- Bauer H, Veron N, Willert J, Herrmann BG. The t-complex-encoded guanine nucleotide exchange factor Fgd2 reveals that two opposing signaling pathways promote transmission ratio distortion in the mouse. Genes Dev. 2007;21:143–147. doi: 10.1101/gad.414807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer H, Schindler S, Charron Y, Willert J, Kusecek B, Herrmann BG. The nucleoside diphosphate kinase gene Nme3 acts as quantitative trait locus promoting non-Mendelian inheritance. PLoS Genet. 2012;8:e1002567. doi: 10.1371/journal.pgen.1002567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeman RW, Friesen KS, Denell RE. Maternal-effect selfish genes in flour beetles. Science. 1992;256:89–92. doi: 10.1126/science.1566060. [DOI] [PubMed] [Google Scholar]
- Bélanger S, Clermont I, Esteves P, Belzile F. Extent and overlap of segregation distortion regions in 12 barley crosses determined via a Pool-GBS approach. Theor Appl Genet. 2016;129:1393–1404. doi: 10.1007/s00122-016-2711-5. [DOI] [PubMed] [Google Scholar]
- Ben-David E, Burga A, Kruglyak L. A maternal-effect selfish genetic element in Caenorhabditis elegans. Science. 2017;356:1051–1055. doi: 10.1126/science.aan0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodénès C, Chancerel E, Ehrenmann F, Kremer A, Plomion C. High-density linkage mapping and distribution of segregation distortion regions in the oak genome. DNA Res. 2016;23:115–124. doi: 10.1093/dnares/dsw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden AW. Influence of time of mating on the segregation ratio of alleles at the T locus in the house mouse. Nature. 1958;181:786–787. doi: 10.1038/181786a0. [DOI] [PubMed] [Google Scholar]
- Bravo Núñez MA, Lange JJ, Zanders SE. A suppressor of a wtf poison-antidote meiotic driver acts via mimicry of the driver's antidote. PLoS Genet. 2018;14:e1007836. doi: 10.1371/journal.pgen.1007836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan AC, Hiscock SJ, Abbott RJ. Interspecific crossing and genetic mapping reveal intrinsic genomic incompatibility between two Senecio species that form a hybrid zone on Mount Etna, Sicily. Heredity (Edinb) 2014;113:195–204. doi: 10.1038/hdy.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt A, Trivers R. Genes in conflict: the biology of selfish genetic elements. London: The Belknap Press of Harvard University Press; 2006. [Google Scholar]
- Charron Y, Willert J, Lipkowitz B, Kusecek B, Herrmann BG, Bauer H. Two isoforms of the RAC-specific guanine nucleotide exchange factor TIAM2 act oppositely on transmission ratio distortion by the mouse t-haplotype. PLoS Genet. 2019;15:e1007964. doi: 10.1371/journal.pgen.1007964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Ding JH, Ouyang YD, Du HY, Yang JY, Cheng K, Zhao J, Qiu SQ, Zhang XL, Yao JL, Liu KD, Wang L, Xu CG, Li XH, Xue YB, Xia M, Ji Q, Lu JF, Xu ML, Zhang QF. A triallelic system of S5 is a major regulator of the reproductive barrier and compatibility of indica-japonica hybrids in rice. Proc Natl Acad Sci USA. 2008;105:11436–11441. doi: 10.1073/pnas.0804761105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett-Detig RB, Zhou J, Clark AG, Hartl DL, Ayroles JF. Genetic incompatibilities are widespread within species. Nature. 2013;504:135–137. doi: 10.1038/nature12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe RK, Lowry EG, Gent JI, Stitzer MC, Swentowsky KW, Higgins DM, Ross-Ibarra J, Wallace JG, Kanizay LB, Alabady M, Qiu WH, Tseng KF, Wang N, Gao Z, Birchler JA, Harkess AE, Hodges AL, Hiatt EN. A kinesin-14 motor activates neocentromeres to promote meiotic drive in maize. Cell. 2018;173:839–850. doi: 10.1016/j.cell.2018.03.009. [DOI] [PubMed] [Google Scholar]
- Du HY, Ouyang YD, Zhang CJ, Zhang QF. Complex evolution of S5, a major reproductive barrier regulator, in the cultivated rice Oryza sativa and its wild relatives. New Phytol. 2011;191:275–287. doi: 10.1111/j.1469-8137.2011.03691.x. [DOI] [PubMed] [Google Scholar]
- Dunn LC. The inheritance of taillessness (anury) in the house mouse. III. taillessness in the balanced lethal line 19. Genetics. 1939;24:728–731. doi: 10.1093/genetics/24.5.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LC, Bennett D. A new case of transmission ratio distortion in the house mouse. Proc Natl Acad Sci USA. 1968;61:570–573. doi: 10.1073/pnas.61.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershenson S. A new sex-ratio abnormality in Drosophila obscura. Genetics. 1928;13:488–507. doi: 10.1093/genetics/13.6.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesbers AKJ, den Boer E, Ulen J, van Kaauwen MPW, Visser RGF, Niks RE, Jeuken MJW. Patterns of transmission ratio distortion in interspecific lettuce hybrids reveal a sex-independent gametophytic barrier. Genetics. 2019;211:263–276. doi: 10.1534/genetics.118.301566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer MF, Schimenti J, Silver LM. Evolution of mouse chromosome 17 and the origin of inversions associated with t haplotypes. Proc Natl Acad Sci USA. 1989;86:3261–3265. doi: 10.1073/pnas.86.9.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harushima Y, Nakagahra M, Yano M, Sasaki T, Kurata N. A genome-wide survey of reproductive barriers in an intraspecific hybrid. Genetics. 2001;159:883–892. doi: 10.1093/genetics/159.2.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harushima Y, Nakagahra M, Yano M, Sasaki T, Kurata N. Diverse variation of reproductive barriers in three intraspecific rice crosses. Genetics. 2002;160:313–322. doi: 10.1093/genetics/160.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann BG, Koschorz B, Wertz K, McLaughlin KJ, Kispert A. A protein kinase encoded by the t complex responder gene causes non-mendelian inheritance. Nature. 1999;402:141–146. doi: 10.1038/45970. [DOI] [PubMed] [Google Scholar]
- Hickey WA, Craig GB., Jr Genetic distortion of sex ratio in a mosquito, Aedes aegypti. Genetics. 1966;53:1177–1196. doi: 10.1093/genetics/53.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide Y, Shinya Y, Ikenaga M, Sawamura N, Matsubara K, Onishi K, Kanazawa A, Sano Y. Complex genetic nature of sex-independent transmission ratio distortion in Asian rice species: the involvement of unlinked modifiers and sex-specific mechanisms. Heredity (Edinb) 2012;108:242–247. doi: 10.1038/hdy.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide Y, Ogino A, Yoshikawa T, Kitashima Y, Saito N, Kanaoka Y, Onishi K, Yoshitake Y, Tsukiyama T, Saito H, Teraishi M, Yamagata Y, Uemura A, Takagi H, Hayashi Y, Abe T, Fukuta Y, Okumoto Y, Kanazawa A. Lineage-specific gene acquisition or loss is involved in interspecific hybrid sterility in rice. Proc Natl Acad Sci USA. 2018;115:E1955–E1962. doi: 10.1073/pnas.1711656115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T, Yoshimura A, Kurata N (2016) Pollen killer gene S35 function requires interaction with an activator that maps close to S24, another pollen killer gene in rice. G3 (Bethesda) 6:1459–1468 [DOI] [PMC free article] [PubMed]
- Kusano A, Staber C, Ganetzky B. Nuclear mislocalization of enzymatically active RanGAP causes segregation distortion in Drosophila. Dev Cell. 2001;1:351–361. doi: 10.1016/S1534-5807(01)00042-9. [DOI] [PubMed] [Google Scholar]
- Kusano A, Staber C, Ganetzky B. Segregation distortion induced by wild-type RanGAP in Drosophila. Proc Natl Acad Sci USA. 2002;99:6866–6870. doi: 10.1073/pnas.102165099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano A, Staber C, Chan HY, Ganetzky B. Closing the (Ran)GAP on segregation distortion in Drosophila. BioEssays. 2003;25:108–115. doi: 10.1002/bies.10222. [DOI] [PubMed] [Google Scholar]
- Larracuente AM, Presgraves DC. The selfish segregation distorter gene complex of Drosophila melanogaster. Genetics. 2012;192:33–53. doi: 10.1534/genetics.112.141390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XH, Wang XJ, Wei YL, Brummer EC. Prevalence of segregation distortion in diploid alfalfa and its implications for genetics and breeding applications. Theor Appl Genet. 2011;123:667–679. doi: 10.1007/s00122-011-1617-5. [DOI] [PubMed] [Google Scholar]
- Li GW, Li XT, Wang Y, Mi JM, Xing F, Zhang DH, Dong QY, Li XH, Xiao JH, Zhang QF, Ouyang YD. Three representative inter and intra-subspecific crosses reveal the genetic architecture of reproductive isolation in rice. Plant J. 2017;92:349–362. doi: 10.1111/tpj.13661. [DOI] [PubMed] [Google Scholar]
- Li GW, Jin JY, Zhou Y, Bai XF, Mao DH, Tan C, Wang GW, Ouyang YD. Genome-wide dissection of segregation distortion using multiple inter-subspecific crosses in rice. Sci China Life Sci. 2019;62:507–516. doi: 10.1007/s11427-018-9452-8. [DOI] [PubMed] [Google Scholar]
- Loegering WQ, Sears ER. Distorted inheritance of stem-rust resistance of timstein wheat caused by a pollen-killing gene. Can J Genet Cytol. 1963;5:65–72. doi: 10.1139/g63-010. [DOI] [Google Scholar]
- Long YM, Zhao LF, Niu BX, Su J, Wu H, Chen YL, Zhang QY, Guo JX, Zhuang CX, Mei MT, Xia JX, Wang L, Wu HB, Liu YG. Hybrid male sterility in rice controlled by interaction between divergent alleles of two adjacent genes. Proc Natl Acad Sci USA. 2008;105:18871–18876. doi: 10.1073/pnas.0810108105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon MF, Zenthon J, Evans EP, Burtenshaw MD, Willison KR. Extent of the mouse t complex and its inversions shown by in situ hybridization. Immunogenetics. 1988;27:375–382. doi: 10.1007/BF00395134. [DOI] [PubMed] [Google Scholar]
- Merrill C, Bayraktaroglu L, Kusano A, Ganetzky B. Truncated RanGAP encoded by the Segregation Distorter locus of Drosophila. Science. 1999;283:1742–1745. doi: 10.1126/science.283.5408.1742. [DOI] [PubMed] [Google Scholar]
- Mi JM, Lei Y, Kim SR, Prahalada SD, Ouyang YD, Mou TM. An effective strategy for fertility improvement of indica-japonica hybrid rice by pyramiding S5-n, f5-n, and pf12-j. Mol Breeding. 2019;39:138. doi: 10.1007/s11032-019-1044-x. [DOI] [Google Scholar]
- Mi JM, Li GW, Xu CH, Yang JY, Yu HH, Wang GW, Li XH, Xiao JH, Song HZ, Zhang QF, Ouyang YD. Artificial selection in domestication and breeding prevents speciation in rice. Mol Plant. 2020;13:650–657. doi: 10.1016/j.molp.2020.01.005. [DOI] [PubMed] [Google Scholar]
- Ouyang YD. Understanding and breaking down the reproductive barrier between Asian and African cultivated rice: a new start for hybrid rice breeding. Sci China Life Sci. 2019;62:1114–1116. doi: 10.1007/s11427-019-9592-6. [DOI] [PubMed] [Google Scholar]
- Ouyang YD, Zhang QF. Understanding reproductive isolation based on the rice model. Annu Rev Plant Biol. 2013;64:111–135. doi: 10.1146/annurev-arplant-050312-120205. [DOI] [PubMed] [Google Scholar]
- Ouyang YD, Zhang QF. The molecular and evolutionary basis of reproductive isolation in plants. J Genet Genom. 2018;45:613–620. doi: 10.1016/j.jgg.2018.10.004. [DOI] [PubMed] [Google Scholar]
- Ouyang YD, Liu YG, Zhang QF. Hybrid sterility in plant: stories from rice. Curr Opin Plant Biol. 2010;13:186–192. doi: 10.1016/j.pbi.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Ouyang YD, Li GW, Mi JM, Xu CH, Du HY, Zhang CJ, Xie WB, Li XH, Xiao JH, Song HZ, Zhang QF. Origination and establishment of a trigenic reproductive isolation system in rice. Mol Plant. 2016;9:1542–1545. doi: 10.1016/j.molp.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Raju NB. Cytogenetic behavior of spore killer genes in neurospora. Genetics. 1979;93:607–623. doi: 10.1093/genetics/93.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reflinur, Kim B, Jang SM, Chu SH, Bordiya Y, Akter MB, Lee J, Chin JH, Koh HJ. Analysis of segregation distortion and its relationship to hybrid barriers in rice. Rice. 2014;7:3. doi: 10.1186/s12284-014-0003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren RS, Ray R, Li PF, Xu JH, Zhang M, Liu G, Yao XF, Kilian A, Yang XP. Construction of a high-density DArTseq SNP-based genetic map and identification of genomic regions with segregation distortion in a genetic population derived from a cross between feral and cultivated-type watermelon. Mol Genet Genom. 2015;290:1457–1470. doi: 10.1007/s00438-015-0997-7. [DOI] [PubMed] [Google Scholar]
- Rhoades MM. Preferential segregation in maize. Genetics. 1942;27:0395–0407. doi: 10.1093/genetics/27.4.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick CM. Abortion of male and female gametes in the tomato determined by allelic interaction. Genetics. 1966;53:85–96. doi: 10.1093/genetics/53.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick CM. The tomato ge locus: linkage relations and geographic distribution of alleles. Genetics. 1971;67:75–85. doi: 10.1093/genetics/67.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooades MM, Vilkomerson H. On the anaphase movement of chromosomes. Proc Natl Acad Sci USA. 1942;28:433–436. doi: 10.1073/pnas.28.10.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler L, Novitski E. Meiotic drive as an evolutionary force. Am Nat. 1957;91:105–110. doi: 10.1086/281969. [DOI] [Google Scholar]
- Seidel HS, Rockman MV, Kruglyak L. Widespread genetic incompatibility in C. elegans maintained by balancing selection. Science. 2008;319:589–594. doi: 10.1126/science.1151107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour DK, Chae E, Arioz BI, Koenig D, Weigel D. Transmission ratio distortion is frequent in Arabidopsis thaliana controlled crosses. Heredity. 2019;122:294–304. doi: 10.1038/s41437-018-0107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen RX, Wang L, Liu XP, Wu J, Jin WW, Zhao XC, Xie XR, Zhu QL, Tang HW, Li Q, Chen LT, Liu YG. Genomic structural variation-mediated allelic suppression causes hybrid male sterility in rice. Nat Commun. 2017;8:1310. doi: 10.1038/s41467-017-01400-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibov ST, De Souza Jr CL, Garcia AAF, Silva AR, Garcia AF, Mangolin CA, De Souza AP. Molecular mapping in tropical maize (Zea mays L.) using microsatellite markers. 1 Quantitative trait loci (QTL) for grain yield, plant heigth, ear height and grain moisture. Hereditas. 2003;139(2):107–115. doi: 10.1111/j.1601-5223.2003.01667.x. [DOI] [PubMed] [Google Scholar]
- Sturtevant AH, Dobzhansky T. Geographical distribution and cytology of “sex ratio” in Drosophila pseudoobscura and related species. Genetics. 1936;21:473–490. doi: 10.1093/genetics/21.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BC, Perkins DD. Spore killer, a chromosomal factor in neurospora that kills meiotic products not containing it. Genetics. 1979;93:587–606. doi: 10.1093/genetics/93.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie YY, Niu BX, Long YM, Li GS, Tang JT, Zhang YL, Ren D, Liu YG, Chen LT. Suppression or knockout of SaF/SaM overcomes the Sa-mediated hybrid male sterility in rice. J Integr Plant Biol. 2017;59:669–679. doi: 10.1111/jipb.12564. [DOI] [PubMed] [Google Scholar]
- Xie YY, Xu P, Huang JL, Ma SJ, Xie XR, Tao DY, Chen LT, Liu YG. Interspecific hybrid sterility in rice is mediated by OgTPR1 at the S1 locus encoding a peptidase-like protein. Mol Plant. 2017;10:1137–1140. doi: 10.1016/j.molp.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Xie YY, Tang JT, Xie XR, Li XJ, Huang JL, Fei Y, Han JL, Chen SF, Tang HW, Zhao XC, Tao DY, Xu P, Liu YG, Chen LT. An asymmetric allelic interaction drives allele transmission bias in interspecific rice hybrids. Nat Commun. 2019;10:2501. doi: 10.1038/s41467-019-10488-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto E, Takashi T, Morinaka Y, Lin S, Wu J, Matsumoto T, Kitano H, Matsuoka M, Ashikari M. Gain of deleterious function causes an autoimmune response and Bateson–Dobzhansky–Muller incompatibility in rice. Mol Genet Genom. 2010;283:305–315. doi: 10.1007/s00438-010-0514-y. [DOI] [PubMed] [Google Scholar]
- Yang JY, Zhao XB, Cheng K, Du HY, Ouyang YD, Chen JJ, Qiu SQ, Huang JY, Jiang YH, Jiang LW, Ding JH, Wang J, Xu CG, Li XH, Zhang QF. A killer-protector system regulates both hybrid sterility and segregation distortion in rice. Science. 2012;337:1336–1340. doi: 10.1126/science.1223702. [DOI] [PubMed] [Google Scholar]
- Yu HG, Hiatt EN, Chan A, Sweeney M, Dawe RK. Neocentromere-mediated chromosome movement in maize. J Cell Biol. 1997;139:831–840. doi: 10.1083/jcb.139.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XW, Zhao ZG, Zheng XM, Zhou JW, Kong WY, Wang PR, Bai WT, Zheng H, Zhang H, Li J, Liu JF, Wang QM, Zhang L, Liu K, Yu Y, Guo XP, Wang JL, Lin QB, Wu FQ, Ren YL, Zhu SS, Zhang X, Cheng ZJ, Lei CL, Liu SJ, Liu X, Tian YL, Jiang L, Ge S, Wu CY, Tao DY, Wang HY, Wan JM. A selfish genetic element confers non-Mendelian inheritance in rice. Science. 2018;360:1130–1132. doi: 10.1126/science.aar4279. [DOI] [PubMed] [Google Scholar]
- Zhang D, Easterling KA, Pitra NJ, Coles MC, Buckler ES, Bass HW, Matthews PD. Non-Mendelian single-nucleotide polymorphism inheritance and atypical meiotic configurations are prevalent in hop. Plant Genome. 2018 doi: 10.3835/plantgenome2017.04.0032. [DOI] [PubMed] [Google Scholar]
- Zhou W, Tang Z, Hou J, Hu N, Yin T. Genetic map construction and detection of genetic loci underlying segregation distortion in an intraspecific cross of Populus deltoides. PLoS One. 2015;10:e0126077. doi: 10.1371/journal.pone.0126077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YF, Yu YM, Cheng K, Ouyang YD, Wang J, Gong L, Zhang QH, Li XH, Xiao JH, Zhang QF. Processes underlying a reproductive barrier in indica-japonica rice hybrids revealed by transcriptome analysis. Plant Physiol. 2017;174:1683–1696. doi: 10.1104/pp.17.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]