Abstract
Background
Long-term hearing outcomes among children with symptomatic congenital cytomegalovirus (CMV) disease who received 6-week ganciclovir therapy early in life are unknown.
Methods
Longitudinal study of 76 children with symptomatic congenital CMV disease, born 1983-2005, who were categorized into three groups: group A treated with ganciclovir; group B untreated who had microcephaly, chorioretinitis, or sensorineural hearing loss (SNHL; ≥25 dB) diagnosed in the first month of life (congenital); and group C untreated who did not meet criteria for group B.
Results
Patients in groups A (n = 17), B (n = 27), and C (n = 32) were followed to median age of 13, 11, and 13 years, respectively. In group A, patients received ganciclovir for median of 40 (range, 11-63) days; 7 (41%) had grade 3 or 4 neutropenia. Congenital SNHL was diagnosed in 11 (65%) patients in group A, 15 (56%) in group B, and none in group C. Early-onset SNHL was diagnosed between ages ≥1-12 months in an additional 4 (24%), 6 (22%), and 8 (25%) patients in groups A, B, and C, respectively. By the end of follow-up, 12 (71%), 16 (59%), and 7 (22%) of patients in groups A, B, and C, respectively, had severe (>70 dB) SNHL in the better-hearing ear.
Conclusions
In this study, most patients with symptomatic congenital CMV disease and congenital or early-onset SNHL eventually developed hearing loss severe enough to have been potential candidates for cochlear implantation, with or without 6-week ganciclovir therapy. Understanding long-term hearing outcomes of patients treated with 6-month oral valganciclovir (current standard of care) is needed.
Keywords: congenital cytomegalovirus, ganciclovir, sensorineural hearing loss
This study indicated that most patients with symptomatic congenital CMV and congenital or early-onset sensorineural hearing loss developed severe loss, with or without ganciclovir therapy.
Approximately 4.5 per 1000 US children are born with congenital cytomegalovirus (CMV) infection annually [1]. Although a minority of infected children present with symptomatic congenital CMV disease at birth (10%-15%) [2, 3], 50%-70% of these children develop permanent sequelae, including intellectual disability and/or hearing, vision, and/or motor impairment [2]. Microcephaly, which has been reported in 2% of infected infants identified in newborn CMV screening studies [4–12], is an important predictor of poor cognitive outcomes and sensorineural hearing loss (SNHL) [13, 14].
Antiviral therapy for neonates with symptomatic congenital CMV disease with central nervous system involvement has been studied in phase I-II and phase III clinical trials conducted by the National Institute of Allergy and Infectious Disease (NIAID) Collaborative Antiviral Study Group (CASG) [15–19]. Open-label phase I-II clinical trials evaluated safety, tolerance, and pharmacodynamics of intravenous ganciclovir in this population. The first phase III randomized controlled trial (RCT), conducted during 1991-1999, found that 6-week therapy with intravenous ganciclovir was associated with improved hearing outcomes at 6 months of life and fewer developmental delays [18, 20], but also with a significant risk of neutropenia [18]. Another phase III RCT, conducted during 2008-2011, evaluated 6-month vs 6-week therapy with oral valganciclovir [19]. Although 6-month therapy did not improve hearing at 6 months of life, there was modest improvement in hearing and developmental outcomes at ages 12 and 24 months, and a lower risk of neutropenia during the first 6 weeks of valganciclovir therapy than observed in the ganciclovir RCT [19].
The American Academy of Pediatrics (AAP) currently recommends 6-month therapy with oral valganciclovir for infants with moderately to severely symptomatic congenital CMV disease who can start treatment within the first month of life [21]. Although 6-week ganciclovir therapy is no longer the standard of care for these infants, long-term hearing outcomes of children who received antiviral therapy early in life are not known. In this study, we describe hearing outcomes among children with symptomatic congenital CMV disease with and without 6-week intravenous ganciclovir therapy followed through 18 years of age.
METHODS
Our study included 76 children with symptomatic congenital CMV disease, born 1983-2005, who were enrolled in the Congenital CMV Longitudinal Study [11, 14, 22]. A symptomatic patient was a newborn with CMV infection detected by culture of urine samples collected within 3 weeks of life and microcephaly, unexplained neurological abnormality (ie, seizures, jitteriness, poor suck, hypo- or hypertonia), or signs of reticuloendothelial system involvement, characterized by purpura/petechiae, jaundice, hepatomegaly, splenomegaly, elevated alanine transaminase (>100 IU), hyperbilirubinemia (total bilirubin >3 mg/dL), hemolytic anemia, or thrombocytopenia (platelet count <75 000/mm3). For this study, we categorized symptomatic patients into three groups based on the eligibility for the ganciclovir phase III study [18]: group A treated with 6-week intravenous ganciclovir; group B untreated who had microcephaly, chorioretinitis, or SNHL diagnosed in the first month of life—intracranial calcifications were not included because they have also been observed in asymptomatic patients [23]; and group C untreated who did not meet criteria for group B but had signs of reticuloendothelial system involvement, other neurological abnormalities, or abnormal head computed tomography (CT) findings, or were born at ≤32 weeks’ gestation or weighing ≤1200 g [18]. The Institutional Review Board for Human Subject Research for Baylor College of Medicine and Affiliated Hospitals approved the study protocol.
Patients were categorized as microcephalic either by physician clinical assessment at birth or post hoc based on fronto-occipital head circumference ≤3rd percentile using Olsen’s intrauterine growth curves [24]. Neuroimaging evaluations by head CT scans were conducted within 4 months of age. We classified abnormal findings into three broad categories implying (1) tissue destruction (calcification, hemorrhage, stroke, encephalomalacia, white matter lucency, porencephaly/cysts, or atrophy); (2) attenuated growth (such as immaturity or hypoplasia); and (3) dysplastic growth (migrational abnormalities, such as lissencephaly or pachygyria). Volume loss was inferred by dilatation of cisterns and/or cerebrospinal fluid spaces [25].
Hearing evaluations included click and tone-burst auditory brainstem response (ABR), behavioral audiometry from 0.25 to 8 kHz, and tympanometry, conducted during infancy, preschool, elementary, middle, and high school years. We defined SNHL for each ear as ≥25 dB hearing level (HL) in the absence of middle ear disorder, as previously described [14]. We categorized SNHL as congenital (diagnosed in one or both ears within the first month of life), early-onset (detected in the first ABR assessment from ≥1 month to 12 months of life), or delayed-onset (detected after ≥1 assessments with normal hearing). We categorized SNHL as bilateral or unilateral, and HLs for each ear as follows: ≤20 dB as normal hearing, and 21-45 dB as mild, 46-70 dB as moderate, and >70 dB as severe hearing loss, based on the click ABR or average of the pure-tone thresholds at 0.5, 1, 2, and 4 kHz (four-frequency average) [18, 19]. The ear with higher thresholds at the initial assessment with SNHL was considered the poorer-hearing ear. We compared HL from baseline to follow-up assessment and categorized patients as having improved hearing (eg, from moderate to mild), maintained normal hearing, maintained same degree of hearing loss, or worsened hearing (eg, from moderate to severe).
Statistical Analyses
We used chi-square or Fisher exact test to assess maternal demographics, newborn clinical characteristics, head CT scan findings, and congenital SNHL among patients in groups A and B (treated and untreated groups but likely similar in disease severity), and among patients in groups B and C (both untreated groups but likely different in disease severity). We describe SNHL characteristics and HLs in the poorer- and better-hearing ears by patient groups; treated patients were included in group A regardless of treatment duration. We also assessed changes in HL from baseline to follow-up at ≤12 months, ≤24 months, and last assessment by better-hearing, poorer-hearing, and total ears. For all analyses, we used SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Description of Patient Groups by Maternal Demographics and Clinical Characteristics at Birth
All 76 patients followed in the longitudinal study were born before AAP recommended ganciclovir therapy. There were 17 (22%) patients in group A, 27 (36%) in group B, and 32 (42%) in group C (Table 1). In group A, 3 (18%) patients were treated in phase I-II clinical trials, 8 (47%) in the phase III RCT [18], and 6 (35%) were treated at physicians’ discretion. The median duration of ganciclovir administration among the 17 patients in group A was 40 (range, 11-63) days. During treatment, grade 3 or 4 neutropenia occurred in 7 (41%) patients.
Table 1.
Comparison of Demographic and Clinical Characteristics Among Children With Symptomatic Congenital CMV Disease by Patient Group (n = 76)
| Characteristics | Group A (n = 17), n (%) | Group B (n = 27), n (%) | P valuea | Group C (n = 32), n (%) | P valueb |
|---|---|---|---|---|---|
| Maternal demographics | |||||
| Age | |||||
| <25 years | 13 (76) | 16 (59) | .24 | 18 (56) | .81 |
| ≥25 years | 4 (24) | 21 (41) | 14 (44) | ||
| Race/Hispanic origin | |||||
| Non-Hispanic White | 9 (53) | 14 (52) | 1.00 | 20 (63) | .89 |
| Non-Hispanic Black | 3 (18) | 5 (19) | 4 (13) | ||
| Non-Hispanic Asian | 0 (0) | 1 (4) | 1 (3) | ||
| Hispanic | 5 (29) | 7 (26) | 7 (22) | ||
| Marital status (n = 73) | |||||
| Married | 7 (44) | 17 (65) | .17 | 20 (65) | .94 |
| Single | 9 (56) | 9 (35) | 11 (35) | ||
| Health insurance (n = 58) | |||||
| Private health insurance | 5 (31) | 11 (50) | .21 | 11 (55) | .52 |
| Medicaid | 7 (44) | 10 (45) | 6 (30) | ||
| None | 4 (25) | 1 (5) | 2 (10) | ||
| Education (n = 75) | |||||
| Up to high school | 12 (71) | 15 (56) | .32 | 12 (39) | .29 |
| Some college or more | 5 (29) | 12 (44) | 19 (61) | ||
| Newborn’s characteristics | |||||
| Year of birth | |||||
| 1983-1989 (before ganciclovir studies) | 0 (0) | 5 (19) | .16 | 13 (41) | .17 |
| 1990-1999 (enrollment phase of ganciclovir studies) | 14 (82) | 16 (59) | 15 (47) | ||
| 2000-2005 (after ganciclovir studies) | 3 (18) | 6 (22) | 4 (13) | ||
| Participation in ganciclovir studiesc | |||||
| Yes | 11 (65) | 4 (15) | <.01 | 1 (3) | .16 |
| No | 6 (35) | 23 (85) | 31 (97) | ||
| Gender | |||||
| Male | 8 (47) | 11 (41) | .68 | 16 (50) | .48 |
| Female | 9 (53) | 16 (59) | 16 (50) | ||
| Gestational age at birth | |||||
| Premature (<37 weeks) | 8 (47) | 6 (22) | .09 | 10 (31) | .43 |
| Term | 9 (53) | 21 (78) | 22 (69) | ||
| Smallforgestational age | |||||
| Physician clinical assessment at birth | 8 (47) | 10 (37) | .51 | 10 (31) | .64 |
| Birth weight <10th percentiled | 10 (59) | 19 (70) | .43 | 15 (47) | .07 |
| Reticuloendothelial system involvement at birth | 16 (94) | 24 (89) | 1.00 | 30 (94) | .65 |
| Purpura/petechiae | 14 (82) | 21 (78) | 1.00 | 20 (63) | .20 |
| Jaundice | 9 (53) | 10 (37) | .30 | 11 (34) | .83 |
| Hepatomegaly | 14 (82) | 16 (59) | .11 | 14 (44) | .24 |
| Splenomegaly | 13 (76) | 16 (59) | .24 | 10 (31) | .03 |
| Elevated alanine transaminase (>100 IU) (n = 70) | 6 (35) | 3 (13) | .13 | 3 (10) | 1.00 |
| Hyperbilirubinemia (total bilirubin >3 mg/dL) (n = 74) | 11 (65) | 6 (23) | <.01 | 11 (36) | .31 |
| Hemolytic anemia | 0 (0) | 2 (7) | .51 | 1 (3) | .59 |
| Thrombocytopenia (platelet count <75 000/mm3) (n = 53) | 11 (65) | 19 (76) | .50 | 20 (69) | .57 |
| >2 signs of RE system involvement | 14 (82) | 19 (70) | .48 | 16 (50) | .11 |
| Microcephaly | |||||
| Physician clinical assessment at birth | 12 (71) | 19 (70) | .99 | 0 (0) | <.01 |
| Head circumference ≤3rd percentiled (n = 73) | 11 (65) | 14 (54) | .48 | 0 (0) | <.01 |
| Either criteria | 14 (82) | 20 (74) | .71 | 0 (0) | <.01 |
| Other neurological abnormalitiese | 7 (41) | 9 (33) | .60 | 7 (22) | .32 |
| Seizures | 2 (12) | 3 (11) | 1.00 | 0 (0) | .09 |
| Poor suck | 1 (6) | 1 (4) | 1.00 | 2 (6) | 1.00 |
| Jitteriness | 0 (0) | 2 (7) | .51 | 2 (6) | 1.00 |
| Hypotonia | 5 (29) | 1 (4) | .03 | 4 (13) | .36 |
| Hypertonia | 2 (12) | 7 (26) | .45 | 3 (9) | .16 |
| Chorioretinitis | 3 (18) | 10 (37) | .20 | 1 (3)f | <.01 |
| Head CT findings (n = 74) | |||||
| Intracranial calcifications | 15 (88) | 19 (70) | .27 | 8 (27) | <.01 |
| Ventricular dilatation | 12 (71) | 18 (67) | .79 | 8 (27) | <.01 |
| White matter lucency | 2 (12) | 7 (26) | .45 | 6 (20) | .59 |
| Broad categories | |||||
| Tissue destruction | 15 (88) | 22 (81) | .68 | 13 (41) | <.01 |
| Volume loss | 12 (71) | 18 (67) | .79 | 8 (25) | <.01 |
| Attenuated growth | 5 (29) | 7 (26) | 1.00 | 9 (28) | .85 |
| Dysplastic growth | 3 (18) | 3 (11) | .66 | 0 (0) | .09 |
| Number of broad category findings | |||||
| 0 | 1 (6) | 2 (7) | .87 | 8 (27) | <.01 |
| 1 | 4 (23) | 5 (19) | 15 (50) | ||
| ≥2 | 12 (71) | 20 (74) | 7 (23) | ||
| Deathg | 2 (12) | 6 (22) | .46 | 0 (0) | <.01 |
Abbreviations: CMV, cytomegalovirus; CT, computed tomography; RCT, randomized controlled trial; SNHL, sensorineural hearing loss.
Group A: All patients received intravenous ganciclovir; all patients had at least one of the following signs: microcephaly, chorioretinitis, or sensorineural hearing loss diagnosed in the first month of life, except one who had multiple signs of reticuloendothelial system involvement (petechiae, jaundice, hepatomegaly, splenomegaly, elevated alanine transaminase, hyperbilirubinemia, thrombocytopenia), multiple intracranial calcifications, and diffuse cerebral white matter lucency suggesting undermyelination, and was treated in the phase III RCT.
Group B: Untreated patients who had at least one of the following signs: microcephaly, chorioretinitis, or sensorineural hearing loss diagnosed in the first month of life, and thus would have been eligible for ganciclovir therapy.
Group C: Untreated patients who did not meet criteria for group B but had signs of reticuloendothelial system involvement, other neurological abnormalities, or abnormal head CT findings, or were born ≤32 weeks’ gestation and weighing ≤1200 g.
a Comparison between groups A and B, P-values based on chi-square test or Fisher exact test.
b Comparison between groups B and C, P-values based on chi-square test or Fisher exact test.
c Of the 16 patients participating in ganciclovir studies, 11 were treated (two in phase I study, one in phase II study, and eight in phase III RCT) and five were untreated (phase III). Six patients were treated at physician’s discretion.
d Based on Olsen’s intrauterine growth charts.
e As noted in the patient chart.
f Birth weight <1200 g.
g Two (12%) patients in group A died; ages: 12 years (pneumonia) and 20 years (complications from spinal cord surgery). Six (22%) patients in group B died; ages: 2 months (sudden infant death syndrome), 2 years (one had respiratory failure and another had G-tube postoperative complications), 13 years (one had respiratory failure and another died in sleep at home), and 20 years (accidental drug overdose). All patients who died had SNHL in the last assessment, and 6 had intellectual disability.
All patients in group A had microcephaly, chorioretinitis, or congenital SNHL, except one (enrolled in the phase III RCT) who had multiple signs of reticuloendothelial system involvement and abnormal head CT findings. Four patients in group B and one in group C were enrolled in the phase III RCT (allocated to receive no treatment). Three patients in group C would not have been eligible to participate in the ganciclovir clinical trials: two were born ≤32 weeks’ gestation and one had birth weight of ≤1200 g.
There were no statistically significant differences by maternal demographics between groups A and B, nor between groups B and C. Purpura/petechiae was the most common sign of reticuloendothelial system involvement in all patient groups (82% in group A, 78% in group B, and 63% in group C), followed by hepatomegaly in group A (82%) and thrombocytopenia in groups B (70%) and C (60%). There were statistically significant differences in the proportions of patients with hyperbilirubinemia between group A and B (P = .01), and with splenomegaly between group B and C (P = .03).
Although the proportion of patients in groups A and B with microcephaly on the basis of physician clinical assessment at birth was similar (71% and 70%), a smaller proportion, particularly in group B, had head circumference ≤3rd percentile (65% and 51%); none in group C had microcephaly by definition. Neurological abnormalities in the clinical exam were noted for 41%, 33%, and 22% of patients in groups A, B, and C, respectively, and chorioretinitis in 18%, 37%, and 3% (one patient who had birth weight ≤1200 g).
Head CT Scan Findings
Head CT scans were performed at a median age of 6 (range, 0-146) days among 17 patients in group A, 9 (0-111) days among 27 patients in group B, and 8 (0-121) days among 30 patients in group C. There were no statistically significant differences in the proportions of patients in groups A and B regarding abnormal head CT scan findings, either specific findings such as intracranial calcifications (88% vs 70%), or broad categories such as tissue destruction (88% vs 81%) or volume loss (71% vs 67%); ≥2 broad category findings were noted in 71% of group A and 74% of group B (P > .05) (Table 1). Most abnormal findings were significantly more common in group B than group C. No patient in group C had findings suggesting dysplastic growth, which was also uncommon among the other groups (Table 1).
Hearing Outcomes
The median age at the first and last hearing assessments for group A was 0.3 (range, 0.1-4.8) months and 13 (0.4-18) years, respectively; 0.5 (0.1-10.3) months and 11 (0.1-18) years for group B; and 1.3 months (0.2 months-8 years) and 13 (0.0-18) years for group C.
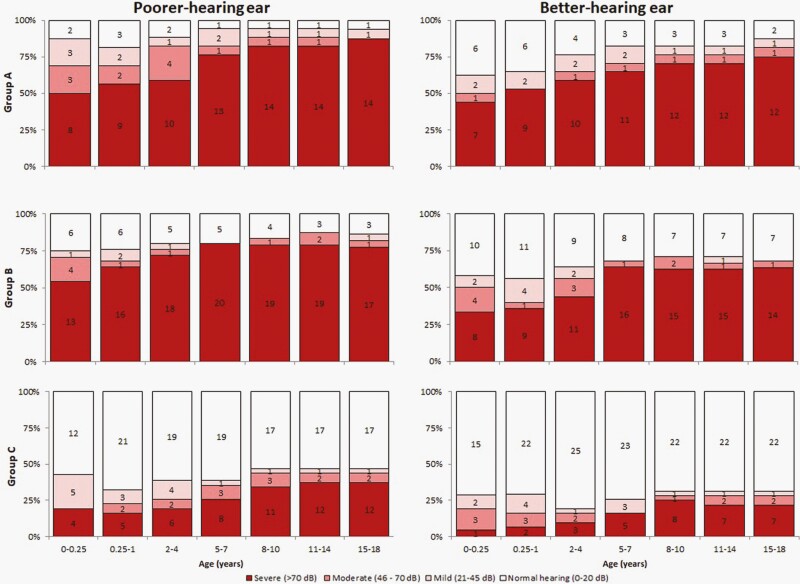
Overall, 12 (71%) patients in group A, 16 (59%) in group B, and 8 (25%) in group C were screened by ABR within the first month of life. Eleven (65%) and 15 (56%) patients in groups A and B, respectively, were diagnosed with congenital SNHL (P > .05); among whom 9/11 (81%) and 11/15 (73%), had bilateral loss (Table 2). Not all patients had an assessment within the first month of life, and an additional 4 (24%) in group A, 6 (22%) in group B, and 8 (25%) in group C were categorized as having early-onset SNHL; most had bilateral loss at initial diagnosis. Patients with normal hearing in both ears at the first assessment also developed SNHL. At the end of follow-up, a total of 16 (94%) in group A, 24 (89%) in group B, and 16 (50%) in group C had SNHL; most had bilateral SNHL. SNHL severity increased with age in all patient groups (Figure 1). At last assessment, 12 (71%) patients in group A, 16 (59%) in group B, and 7 (22%) in group C had severe hearing loss in the better-hearing ear (Figure 1). Comparisons in hearing from baseline to follow-up at ≤12 months, ≤24 months, and last assessment are described in Table 3.
Table 2.
Characteristics of Sensorineural Hearing Loss Among Children With Symptomatic Congenital CMV Disease by Patient Group (n = 76)
| SNHL Characteristics | Group A (n = 17), n (%) | Group B (n = 27), n (%) | Group C (n = 32), n (%) |
|---|---|---|---|
| Congenitala | 11 (65) | 15 (56) | 0 (0) |
| Bilateral | 9 (53) | 11 (40) | 0 (0) |
| Unilateral | 2 (12) | 4 (16) | 0 (0) |
| Early-onsetb | 4 (24) | 6 (22) | 8 (25) |
| Bilateral | 3 (18) | 6 (22) | 5 (16) |
| Unilateral | 1 (6) | 0 (0) | 3 (9) |
| Delayed-onsetc | 1 (6) | 3 (11) | 8 (25) |
| Bilateral | 1 (6) | 2 (7) | 5 (16) |
| Unilateral | 0 (0) | 1 (4) | 3 (9) |
| Total with SNHL at last assessmentd | 16 (94) | 24 (89) | 16 (50) |
| Bilaterale | 14 (82) | 22 (81) | 11 (34) |
| Unilateral | 2 (12) | 2 (7) | 5 (16) |
Abbreviations: ABR, auditory brainstem response; CMV, cytomegalovirus; SNHL, sensorineural hearing loss.
a Diagnosed in one or both ears within the first month of life; P = .55 for comparison between groups A and B.
b Detected in the first ABR assessment from >1 month to 12 months of life.
c Detected after ≥1 assessments with normal hearing.
d P = 1.00 for comparison between groups A and B, and <.01 for comparison between groups B and C.
e Some patients with unilateral congenital or early-onset SNHL developed bilateral loss later (1 in group A, 3 in group B, 1 in group C).
Figure 1.
Proportion of patients with normal hearing and mild, moderate, and severe hearing loss in the poorer- and better-hearing ears by patient group and age. Hearing level was categorized based on the click ABR or average of the pure-tone thresholds at 0.5, 1, 2, and 4 kHz (four-frequency average), as follows: ≤20 dB as normal hearing (white), and 21-45 dB as mild (light pink), 46-70 dB as moderate (dark pink), and >70 dB as severe (red) hearing loss; number of children in each category is shown inside the bars. We categorized the ear with higher thresholds at the initial assessment with SNHL as the poorer-hearing ear. Patients were not included until the first assessment, and patients who died were excluded from age intervals after death occurred. Patients with missing assessment for a given interval were considered to have hearing level of prior assessment. One patient whose first ABR showed severe and moderate hearing loss in the poorer- and better-hearing ears, had normal hearing bilaterally during follow-up. Five patients whose first ABR showed mild hearing loss in the poorer-hearing ears had normal hearing bilaterally during follow-up. One treated patient who retained normal hearing throughout the follow-up did not have microcephaly or chorioretinitis at birth, and if untreated would have been categorized as group C. Abbreviations: ABR, auditory brainstem response; SNHL, sensorineural hearing loss.
Table 3.
Comparison of Hearing at Baseline and Follow-up at ≤12 Months, 24 Months, and Last Assessment Among Patients With Symptomatic Congenital CMV Disease in Groups A, B, and C
| Comparison of Hearing at Baseline and Follow-up, n (%) | |||||||
|---|---|---|---|---|---|---|---|
| Group | Ear | Follow-up Assessment | n | Improved Hearing | Maintained Normal Hearing | Maintained Same Degree of Hearing Loss | Worsened Hearing |
| A | Better | ≤12 | 15 | 1 (6) | 5 (33) | 6 (40) | 3 (20) |
| 24 | 16 | 0 (0) | 4 (25) | 6 (38) | 6 (38) | ||
| Last | 16 | 0 (0) | 3 (19) | 5 (31) | 8 (50) | ||
| Poorer | ≤12 | 15 | 2 (13) | 2 (13) | 9 (60) | 2 (13) | |
| 24 | 16 | 1 (6) | 2 (13) | 5 (31) | 8 (50) | ||
| Last | 16 | 1 (6) | 1 (6) | 9 (56) | 5 (31) | ||
| Total | ≤12 | 30 | 3 (10) | 7 (23) | 15 (50) | 5 (17) | |
| 24 | 32 | 1 (3) | 6 (19) | 11 (34) | 14 (44) | ||
| Last | 32 | 1 (3) | 4 (13) | 14 (44) | 13 (41) | ||
| B | Better | ≤12 | 19 | 4 (21) | 2 (11) | 9 (47) | 4 (21) |
| 24 | 21 | 3 (14) | 2 (10) | 12 (57) | 4 (19) | ||
| Last | 25 | 4 (16) | 3 (12) | 9 (36) | 9 (36) | ||
| Poorer | ≤12 | 19 | 2 (11) | 1 (5) | 11 (58) | 5 (26) | |
| 24 | 20 | 1 (5) | 1 (5) | 10 (50) | 8 (40) | ||
| Last | 25 | 1 (4) | 2 (8) | 13 (52) | 9 (36) | ||
| Total | ≤12 | 38 | 6 (16) | 3 (8) | 20 (52) | 9 (24) | |
| 24 | 41 | 4 (10) | 3 (7) | 22 (54) | 12 (29) | ||
| Last | 50 | 5 (10) | 5 (10) | 22 (44) | 18 (36) | ||
| C | Better | ≤12 | 13 | 2 (15) | 8 (62) | 1 (8) | 2 (15) |
| 24 | 18 | 3 (17) | 11 (61) | 2 (11) | 2 (11) | ||
| Last | 29 | 5 (17) | 16 (55) | 2 (10) | 6 (21) | ||
| Poorer | ≤12 | 14 | 4 (29) | 6 (43) | 3 (21) | 1 (7) | |
| 24 | 18 | 5 (28) | 5 (28) | 3 (17) | 5 (28) | ||
| Last | 29 | 6 (21) | 9 (31) | 3 (10) | 11 (38) | ||
| Total | ≤12 | 27 | 6 (22) | 14 (52) | 4 (15) | 3 (11) | |
| 24 | 36 | 8 (22) | 16 (44) | 5 (14) | 7 (19) | ||
| Last | 58 | 11 (19) | 25 (43) | 5 (9) | 17 (29) | ||
Abbreviations: CMV, cytomegalovirus; SNHL, sensorineural hearing loss.
Comparisons include auditory brainstem response threshold elicited with either click or tone-burst stimuli at 2 and 4 kHz with average pure-tone levels for the same frequencies. The ear with higher thresholds at the initial assessment with SNHL was considered the poorer-hearing ear. Patients were categorized as having improved hearing (eg, from moderate to mild), maintained normal hearing, maintained same degree of hearing loss, or worsened hearing (eg, from moderate to severe). Median age at last assessment was 13 years for group A, 11 years for group B, and 13 years for group C.
DISCUSSION
This study included children with symptomatic congenital CMV disease with and without 6-week intravenous ganciclovir therapy. To our knowledge, this study reports the longest follow-up of children who received antiviral treatment [26–30]. Most treated patients had microcephaly and bilateral congenital or early-onset SNHL; about half had severe loss bilaterally at initial diagnosis. Patients in group B would have been considered for ganciclovir therapy but were born before, during, or just after the enrollment phases of clinical trials. Although our sample size was small, about 90% of patients in groups A and B, and 25% in group C developed SNHL. Severity of SNHL increased with age in all patient groups, such that the proportion with severe bilateral SNHL (as indicated by the better-hearing ear status) at the last assessment was 71%, 59%, and 22% among patients in groups A, B, and C, respectively. These patients would have been potential candidates for cochlear implants.
We describe a spectrum of symptomatic congenital CMV disease. Patients in groups A and B were likely comparable, but a greater proportion in group B was born at term and appeared to have had fewer signs of reticuloendothelial system involvement than treated patients (group A). Among patients in group C, at least half had >2 signs of reticuloendothelial system involvement, and would have met current criteria for moderately to severely symptomatic congenital CMV disease according to recent recommendations from an informal expert panel [31]; none had SNHL as a single sign. In a previous analysis of this cohort, we found that hepatosplenomegaly was associated with an increased risk of SNHL [14]. Microcephaly and head CT scan findings suggesting tissue destruction or dysplastic growth were also associated with an increased risk of SNHL [14]. Brain imaging abnormalities were found in over 90% of patients in groups A and B but were less common and less severe among patients in group C. By definitions used in this analysis, untreated patients with microcephaly, chorioretinitis, or congenital SNHL, but not with brain imaging abnormalities alone (eg, intracranial calcifications), were included in group B.
Among children with symptomatic congenital CMV disease, microcephaly is an important predictor of poor cognitive and hearing outcomes [13]. About 70% in either group A or B had microcephaly based on the physician’s assessment at birth. In the post hoc classification based on Olsen’s intrauterine growth curves [24] fewer but different patients were microcephalic. The differences between groups A and B were not significant. However, these data suggest that using standardized methods to assess microcephaly in infants with congenital CMV infection is important to inform clinical management decisions and prognosis.
Our study had limitations. The sample size was small and limited our ability to detect significant differences among treated and untreated patients; it is possible that patients in group A may have had more severe disease than patients in group B. They were prescribed 6-week intravenous ganciclovir therapy when it was first available decades ago; several treated and untreated patients were enrolled in the NIAID CASG clinical trials. However, this analysis lacks randomization, and some infants did not complete the 6-week therapy. Some patients with true congenital SNHL may have been misclassified as having early-onset SNHL because there was no assessment within the first month of life; most patients were born before the implementation of universal newborn hearing screening in Texas. However, the proportion with early-onset SNHL was similar in all patient groups. We may have also missed SNHL progression among patients lost to follow-up early in childhood. Furthermore, our findings cannot be extrapolated to infants who receive 6-month therapy with oral valganciclovir, which is the current standard of care [21].
Limited data exist on the long-term benefits and safety of more contemporary cohorts of patients treated with oral valganciclovir for 6 months, warranting further evaluation in future studies. Our study highlights that several factors may influence the findings of studies evaluating efficacy or (real-world) effectiveness of antiviral treatment. The heterogeneity of clinical presentation at birth, duration of therapy and follow-up, type of assessments, and outcome measures may produce selection and/or information biases that can affect the quality of evidence from clinical trials and observational studies.
Although all patients included in this study had symptomatic congenital CMV disease, most children with congenital CMV-associated SNHL will be asymptomatic at the newborn physical exam. Children with asymptomatic congenital CMV infection may also present with brain abnormalities, which have been associated with SNHL [23]. About half of the SNHL cases among children with asymptomatic congenital CMV infection are unilateral, progressing in severity with increasing age [14]. Thus, studies assessing the effect of valganciclovir therapy in these children might need to control for brain imaging findings, and include poorer-hearing outcomes to assess differences in the SNHL progression among treated and untreated children.
In the United States, antiviral therapy is increasingly being administered to infants with symptomatic congenital CMV disease [32]. In a majority of treated infants, administration of antivirals is likely in line with current AAP recommendations for treatment of infants with moderately to severely symptomatic congenital CMV disease, including infants with central nervous system disease [32]. However, the risk-benefit ratio of antiviral therapy is still debated due to risk of toxicity and possible carcinogenicity [33–35]. Data from additional clinical trials assessing the effect of valganciclovir therapy in children with SNHL who start treatment when they are older [36, 37], or those diagnosed with asymptomatic congenital CMV infection and SNHL within 6 months of life [38], will be important to inform the evidence base for different treatment regimens and target populations. In our study, most patients with congenital or early-onset SNHL eventually developed hearing loss severe enough to have been potential candidates for cochlear implantation, with or without 6-week ganciclovir therapy. The apparent lack of enduring benefits of early antiviral therapy in children with symptomatic congenital CMV disease makes prevention a priority strategy to reduce the disease burden.
Notes
Acknowledgments. We thank all the children who participated in the study, their families, and physicians for their lifetime of dedication and support for this study. We also thank Winnie Chung for reviewing patients’ audiological data.
The Congenital CMV Longitudinal Study Group through the years has included: Shahzad Ahmed, Hanna Baer, MD, Amit R. Bhatt, MD, Peggy Blum, AuD and Texas Children’s Hospital Audiology, Frank Brown, MD, Francis Catlin, MD, Alison C. Caviness, MD, PhD, MPH, David K. Coats, MD, Jane C. Edmonds, MD, Marily Flores, MS, Daniel Franklin, MD, Cindy Gandaria, Jewel Greer, Carol Griesser, RN, Mohamed A. Hussein, MD, Isabella Iovino, PhD, Allison Istas, MPH, Haoxing (Douglas) Jin, Mary K. Kelinske, OD, Joseph T. Klingen, Antone Laurente, PhD, Thomas Littman, PhD, Mary Murphy, MS, Jerry Miller, PhD, Christopher Nelson, MD, Daniel Noyola, MD, Evelyn A. Paysse, MD, Alan Percy, MD, Sara Reis, RN, Ann Reynolds, MD, Judith Rozelle, MS, O’Brien Smith, PhD, Paul Steinkuller, MD, Marie Turcich, MS, Sherry Sellers Vinson, MD, Robert G. Voigt, MD, Bethann Walmus, Jill Williams, MA, Daniel Williamson, MD, Kimberly G. Yen, MD, Martha D. Yow, MD, and Gail J. Demmler-Harrison, MD.
Disclaimer. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. The study was supported, in part, by the CMV Research Fund Donors at Baylor College of Medicine; the Woman’s Hospital of Texas Research Foundation; the Office of Research Resources and the General Clinical Research Center for Children at Texas Children’s Hospital and Baylor College of Medicine (NIH 5M0I RR00188-33); the Mental Retardation Research Center at Baylor College of Medicine (NIH-CHHD 5 P30 HD24064P); the Research to Prevent Blindness, Inc. New York, NY; the Deafness Foundation, Houston, TX; the Vale Ashe Foundation, Houston, TX; the Maddie’s Mission Foundation, Katy, TX; the Naymola Foundation, Beaumont, TX; the American Pediatric Society-Society for Pediatric Research Summer Student Research Program (NIH-CHHD); and the Centers for Disease Control and Prevention (Cooperative Agreement FOA IP 10-006).
Potential conflicts of interest. Dr. G. D. H.’s institution received funding from Merck Sharpe & Dohme Corporation since July 2016 to assist with salary support for further analysis on long-term outcomes of congenital CMV infection not included in this report. The other authors have no potential conflicts to disclose and also all authors have no financial relationships relevant to this article to disclose. All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Tatiana M Lanzieri, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Alison Chantal Caviness, Department of Pediatrics, Baylor College of Medicine, Houston, Texas, USA.
Peggy Blum, Texas Children’s Hospital, Houston, Texas, USA.
Gail Demmler-Harrison, Department of Pediatrics, Baylor College of Medicine, Houston, Texas, USA; Texas Children’s Hospital, Houston, Texas, USA.
Congenital Cytomegalovirus Longitudinal Study Group:
Shahzad Ahmed, Hanna Baer, Amit R Bhatt, Peggy Blum, Frank Brown, Francis Catlin, Alison C Caviness, David K Coats, Jane C Edmonds, Marily Flores, Daniel Franklin, Cindy Gandaria, Jewel Greer, Carol Griesser, Mohamed A Hussein, Isabella Iovino, Allison Istas, Haoxing (Douglas) Jin, Mary K Kelinske, Joseph T Klingen, Antone Laurente, Thomas Littman, Mary Murphy, Jerry Miller, Christopher Nelson, Daniel Noyola, Evelyn A Paysse, Alan Percy, Sara Reis, Ann Reynolds, Judith Rozelle, O’Brien Smith, Paul Steinkuller, Marie Turcich, Sherry Sellers Vinson, Robert G Voigt, Bethann Walmus, Jill Williams, Daniel Williamson, Kimberly G Yen, Martha D Yow, and Gail J Demmler-Harrison
References
- 1. Fowler KB, Ross SA, Shimamura M, et al. Racial and ethnic differences in the prevalence of congenital cytomegalovirus infection. J Pediatr 2018; 200:196–201.e1. [DOI] [PubMed] [Google Scholar]
- 2. Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol 2007; 17:355–63. [DOI] [PubMed] [Google Scholar]
- 3. Goderis J, De Leenheer E, Smets K, et al. Hearing loss and congenital CMV infection: a systematic review. Pediatrics 2014; 134:972–82. [DOI] [PubMed] [Google Scholar]
- 4. Ahlfors K, Ivarsson SA, Harris S. Report on a long-term study of maternal and congenital cytomegalovirus infection in Sweden. Review of prospective studies available in the literature. Scand J Infect Dis 1999; 31:443–57. [DOI] [PubMed] [Google Scholar]
- 5. Boppana SB, Fowler KB, Britt WJ, et al. Symptomatic congenital cytomegalovirus infection in infants born to mothers with preexisting immunity to cytomegalovirus. Pediatrics 1999; 104:55–60. [DOI] [PubMed] [Google Scholar]
- 6. Melish ME, Hanshaw JB. Congenital cytomegalovirus infection. Developmental progress of infants detected by routine screening. Am J Dis Child 1973; 126:190–4. [DOI] [PubMed] [Google Scholar]
- 7. Numazaki K, Fujikawa T. Chronological changes of incidence and prognosis of children with asymptomatic congenital cytomegalovirus infection in Sapporo, Japan. BMC Infect Dis 2004; 4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peckham CS, Chin KS, Coleman JC, et al. Cytomegalovirus infection in pregnancy: preliminary findings from a prospective study. Lancet 1983; 1:1352–5. [DOI] [PubMed] [Google Scholar]
- 9. Saigal S, Lunyk O, Larke RP, Chernesky MA. The outcome in children with congenital cytomegalovirus infection. A longitudinal follow-up study. Am J Dis Child 1982; 136:896–901. [DOI] [PubMed] [Google Scholar]
- 10. Starr JG, Bart RD Jr, Gold E. Inapparent congenital cytomegalovirus infection. Clinical and epidemiologic characteristics in early infancy. N Engl J Med 1970; 282:1075–8. [DOI] [PubMed] [Google Scholar]
- 11. Yow MD, Williamson DW, Leeds LJ, et al. Epidemiologic characteristics of cytomegalovirus infection in mothers and their infants. Am J Obstet Gynecol 1988; 158:1189–95. [DOI] [PubMed] [Google Scholar]
- 12. Koyano S, Inoue N, Oka A, et al. ; Japanese Congenital Cytomegalovirus Study Group. Screening for congenital cytomegalovirus infection using newborn urine samples collected on filter paper: feasibility and outcomes from a multicentre study. BMJ Open 2011; 1:e000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Noyola DE, Demmler GJ, Nelson CT, et al. ; Houston Congenital CMV Longitudinal Study Group. Early predictors of neurodevelopmental outcome in symptomatic congenital cytomegalovirus infection. J Pediatr 2001; 138:325–31. [DOI] [PubMed] [Google Scholar]
- 14. Lanzieri TM, Chung W, Flores M, et al. Hearing loss in children with asymptomatic congenital cytomegalovirus infection. Pediatrics 2017; 139(3):e20162610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trang JM, Kidd L, Gruber W, et al. Linear single-dose pharmacokinetics of ganciclovir in newborns with congenital cytomegalovirus infections. NIAID Collaborative Antiviral Study Group. Clin Pharmacol Ther 1993; 53:15–21. [DOI] [PubMed] [Google Scholar]
- 16. Zhou XJ, Gruber W, Demmler G, et al. Population pharmacokinetics of ganciclovir in newborns with congenital cytomegalovirus infections. NIAID Collaborative Antiviral Study Group. Antimicrob Agents Chemother 1996; 40:2202–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whitley RJ, Cloud G, Gruber W, et al. Ganciclovir treatment of symptomatic congenital cytomegalovirus infection: results of a phase II study. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. J Infect Dis 1997; 175:1080–6. [DOI] [PubMed] [Google Scholar]
- 18. Kimberlin DW, Lin CY, Sánchez PJ, et al. ; National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr 2003; 143:16–25. [DOI] [PubMed] [Google Scholar]
- 19. Kimberlin DW, Jester PM, Sánchez PJ, et al. ; National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med 2015; 372:933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oliver SE, Cloud GA, Sánchez PJ, et al. ; National Institute of Allergy, Infectious Diseases Collaborative Antiviral Study Group. Neurodevelopmental outcomes following ganciclovir therapy in symptomatic congenital cytomegalovirus infections involving the central nervous system. J Clin Virol 2009; 46(Suppl 4):S22–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. American Academy of Pediatrics. Cytomegalovirus infection. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. Red Book: 2018 Report of the Committee on Infectious Diseases. 31st ed. Itasca, IL: American Academy of Pediatrics; 2018; 310–7. [Google Scholar]
- 22. Williamson WD, Demmler GJ, Percy AK, Catlin FI. Progressive hearing loss in infants with asymptomatic congenital cytomegalovirus infection. Pediatrics 1992; 90:862–6. [PubMed] [Google Scholar]
- 23. Lanzieri TM, Chung W, Leung J, et al. ; Congenital Cytomegalovirus Longitudinal Study Group. Hearing trajectory in children with congenital cytomegalovirus infection. Otolaryngol Head Neck Surg 2018; 158:736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Olsen IE, Groveman SA, Lawson ML, et al. New intrauterine growth curves based on United States data. Pediatrics 2010; 125:e214–24. [DOI] [PubMed] [Google Scholar]
- 25. Lanzieri TM, Leung J, Caviness AC, et al. Long-term outcomes of children with symptomatic congenital cytomegalovirus disease. J Perinatol 2017; 37:875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Michaels MG, Greenberg DP, Sabo DL, Wald ER. Treatment of children with congenital cytomegalovirus infection with ganciclovir. Pediatr Infect Dis J 2003; 22:504–9. [DOI] [PubMed] [Google Scholar]
- 27. Amir J, Wolf DG, Levy I. Treatment of symptomatic congenital cytomegalovirus infection with intravenous ganciclovir followed by long-term oral valganciclovir. Eur J Pediatr 2010; 169:1061–7. [DOI] [PubMed] [Google Scholar]
- 28. Amir J, Attias J, Pardo J. Treatment of late-onset hearing loss in infants with congenital cytomegalovirus infection. Clin Pediatr (Phila) 2014; 53:444–8. [DOI] [PubMed] [Google Scholar]
- 29. Bilavsky E, Shahar-Nissan K, Pardo J, et al. Hearing outcome of infants with congenital cytomegalovirus and hearing impairment. Arch Dis Child 2016; 101:433–8. [DOI] [PubMed] [Google Scholar]
- 30. McCrary H, Sheng X, Greene T, Park A. Long-term hearing outcomes of children with symptomatic congenital CMV treated with valganciclovir. Int J Pediatr Otorhinolaryngol 2019; 118:124–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rawlinson WD, Boppana SB, Fowler KB, et al. Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect Dis 2017; 17:e177–88. [DOI] [PubMed] [Google Scholar]
- 32. Leung J, Dollard SC, Grosse SD, et al. Valganciclovir use among commercially and Medicaid-insured infants with congenital CMV infection in the United States, 2009-2015. Clin Ther 2018;40:430–9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Korndewal MJ, de Vries JJ, de Melker HE. Valganciclovir for congenital cytomegalovirus. N Engl J Med 2015; 372:2462–3. [DOI] [PubMed] [Google Scholar]
- 34. Natale F, De Curtis M. Valganciclovir for congenital cytomegalovirus. N Engl J Med 2015; 372:2462. [DOI] [PubMed] [Google Scholar]
- 35. Schornagel FA, Oudesluys-Murphy AM, Vossen AC. Valganciclovir for congenital cytomegalovirus. N Engl J Med 2015; 372:2462. [DOI] [PubMed] [Google Scholar]
- 36. Kimberlin D. Congenital CMV and Hearing Loss in Children up to 4 Years of Age: Treating with Valganciclovir Therapy. Accessed August 8, 2017. https://clinicaltrials.gov/ct2/show/NCT01649869
- 37. Assistance Publique - Hôpitaux de Paris. Evaluation of the Benefit of Antiviral Treatment with Valganciclovir on Congenital CMV Infection-Related Deafness on Hearing and Balance (GANCIMVEAR). Accessed August 8, 2017. https://clinicaltrials.gov/ct2/show/NCT02606266
- 38. Park A. Randomized Controlled Trial of Valganciclovir for Asymptomatic Cytomegalovirus Infected Hearing Impaired Infants (ValEAR). Accessed August 8, 2017. https://clinicaltrials.gov/ct2/show/NCT03107871