Abstract
Sugars are fundamental metabolites synthesized in leaves and further delivered to fruit in fruit crops. They not only provide “sweetness” as fruit quality traits, but also function as signaling molecules to modulate the responses of fruit to environmental stimuli. Therefore, the understanding to the molecular basis for sugar metabolism and transport is crucial for improving fruit quality and dissecting responses to abiotic/biotic factors. Here, we provide a review for molecular components involved in sugar metabolism and transport, crosstalk with hormone signaling, and the roles of sugars in responses to abiotic and biotic stresses. Moreover, we also envisage the strategies for optimizing sugar metabolism during fruit quality maintenance.
Keywords: Abiotic and biotic stress, Fruit quality maintenance, Postharvest diseases, Sugar metabolism, Transporter
Introduction
Sugars are primary metabolites providing building blocks and energy for cells (Dai et al. 2013; Ruan 2014). Previous studies have indicated that sugars are not only crucial for maintaining cellular turgor and promoting cell expansion, but also function as signaling molecules to modulate fruit development (Ruan et al. 2014; Chen et al. 2020). In fruit, sugar content is often measured as the total soluble solid content, which determines fruit sweetness and ultimately fruit quality (Ruan et al. 2012; Chen et al. 2021). In fruit crops, sucrose, sorbitol and occasionally trehalose or raffinose are synthesized and delivered from leaves (source organs) to fruit, tuber and seeds (sink organs) (Ruan et al. 2014; Li et al. 2018), thereby involving in metabolic activities of plants. Moreover, sugar transport is synergistically mediated by transporters from different superfamilies, which are elaborately tuned at transcriptional, posttranscriptional and posttranslational levels. A part of the sugars transported to fruit is utilized in physiological processes, whereas the other part is stored in vacuoles. Therefore, a comprehensive understanding of these processes is important for uncovering important players in physiologically relevant processes (Wipf et al. 2021).
Major sugar-related metabolic activities in fruit
Sugar metabolic and transport processes function differentially among fruit species. In apple, sorbitol and sucrose are synthesized in leaves and further delivered and metabolized in fruit (Fig. 1), whereas sucrose is the major translocated sugar in many citrus varieties (Shangguan et al. 2014; Sadka et al. 2019). As compared with leaf photosynthesis, fruit photosynthesis seems not important for primary metabolism in tomato fruit, as suppression of glutamate 1-semialdehyde aminotransferase (GSA), a rate-limiting enzyme involved in chlorophyll biosynthesis, did not obviously affect normal fruit development (Lytovchenko et al. 2011). Moreover, there seems no strict relationship between leaf photosynthesis rate and fruit yield, as overexpression of maize sucrose-6-phosphate synthase in tomato showed increased photosynthetic activity and sucrose synthesis in leaves but did not influence the final fruit yield (Micallef et al. 1995). These results implied that sugar-related metabolic activities in fruit are modulated by specific mechanisms.
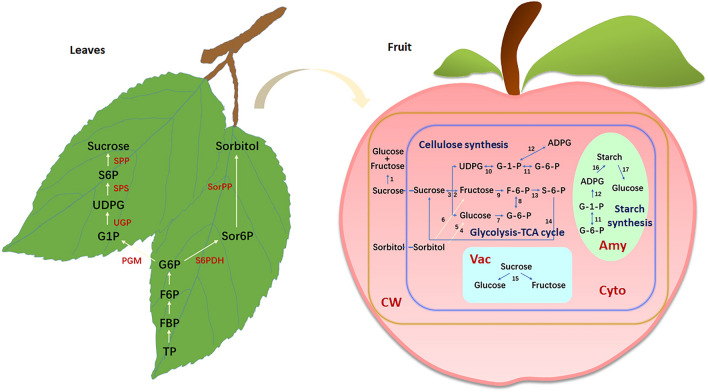
Fig. 1.
A simplified model for sugar metabolism in leaves and fruit. Taking apple as an example, sorbitol and sucrose are synthesized in leaves mainly by the enzymes as follows: F6P fructose-6-phosphate; FBP fructose-1,6-bisphosphate; G1P glucose-1-phosphate; G6P glucose-6-phosphate; S6P sucrose-6-phosphate; Sor6P sorbitol-6-phosphate; TP triose phosphate; PGM phosphoglucomutase; S6PDH sorbitol-6-phosphate dehydrogenase; SorPP sorbitol-6-phosphate phosphatase. Sorbitol and sucrose are further transported to fruit. Major enzymes involved in sugar metabolism in fruit: 1. apoplastic invertase; 2. cytosolic invertase; 3. sucrose synthase; 4. NADP+-sorbitol dehydrogenase; 5. sorbitol oxidase; 6. NAD+-sorbitol dehydrogenase; 7. hexokinase; 8. phosphoglucose isomerase; 9. fructokinase; 10. UDP-glucose pyrophosphorylase; 11. phosphoglucomutase; 12. ADP-glucose pyrophosphorylase; 13. sucrose phosphate synthase; 14. sucrose phosphate phosphatase; 15. soluble acidic invertase; 16. granule-bound starch synthases and other enzymes; 17. α-amylase and other enzymes (refer to Li et al. 2012b). Note: Amy amyloplasts, Cyto cytosol, CW cell wall, Vac vacuoles
Sucrose metabolism has pivotal functions in normal development and stress responses of plants. The sucrose cycle plays a central role in sugar metabolism in fruit (Nguyen-quoc and Foyer 2001; Wind et al. 2010), substantially connecting sugar metabolic activities with other primary metabolic processes (including starch synthesis, glycolysis, tricarboxylic acid cycle, and cell wall biosynthesis). This metabolic cycle consists of several steps: sucrose degradation by sucrose synthase and cytosolic invertase in the cytosol or acidic invertase in the vacuole/apoplastic space, subsequent phosphorylation of the resulted hexoses, transformation between UDP-glucose and hexose phosphates, and ultimately sucrose re-generation catalyzed by sucrose-6-phosphate phosphatase and sucrose-6-phosphate synthase (SPS). Upon antisense suppression of aldose-6-phosphate reductase, the transgenic apple fruit showed lower sorbitol level, much higher glucose level but comparable concentrations of fructose, sucrose, and starch to the control, indicating that the sucrose cycle and the sugar translocation system function effectively in maintaining the fructose level in apple fruit (Li et al. 2018).
Sucrose utilization depends on its degradation into glucose and fructose by sucrose synthase and invertases in fruit (Zhang et al. 2004; Ruan et al. 2014). Sucrose synthase is a cytosolic glycosyl transferase converting sucrose into UDP-glucose and fructose, thus is closely related to the growth rate and the quantity of starch in fruit (Yelle et al. 1988). Invertases hydrolyze sucrose into glucose and fructose, which are crucial for normal development and responses to biotic and abiotic stresses in plants (Qin et al. 2016). According to their specific sites of action, invertases are categorized into three groups, i.e., cell wall (apoplastic) invertases (CWIN), vacuolar invertases (VIN), and cytoplasmic invertases (CIN) (Li et al. 2012b). Invertases have crucial roles in fruit set and ripening (Palmer et al. 2015; Qin et al. 2016). LIN5 is one of the CWIN enzymes responsible for sugar uptake into tomato fruit (Zanor et al. 2009; Vallarino et al. 2017). A study demonstrated that both CWIN and INH (the gene encoding LIN5 inhibitor) mRNAs displayed a phloem-specific pattern during tomato fruit development. The CWIN LIN5 specifically functions in the walls of sieve elements with increasing activity, thereby facilitating phloem unloading and generating sugar signaling to regulating cell division (Palmer et al. 2015).
Upon phloem unloading into sink cells apoplasmically or symplasmically (Li et al. 2018), sucrose is hydrolyzed by apoplastic invertase into glucose and fructose before being transported into cytosol. It has been reported in model plants that apoplastic glucose may be perceived by Regulator of G protein Signaling 1 (RGS1) coupled with heterotrimer G protein (Liu et al. 2019), whereas extracellular glucose may promote the endocytosis of RGS1 and thus release RGS1 from the coupling machinery with G protein. Further efforts should be made to examine whether the sensing of apoplastic glucose may be involved in the regulations on development and responses to biotic factors in fruit (Urano et al. 2012; Liang et al. 2018). In contrast, the sucrose transported via plasmodesmata or by sucrose transporters is degraded by cytosolic invertase and sucrose synthase. The intracellular hexose is utilized for glycolysis as well as synthesis of starch, cellulose and other carbohydrate polymers (Ruan et al. 2014). Since the vacuole is a storage organelle for sugars, vacuolar invertase is supposed to modulate the sucrose/hexose ratio in fruit. Antisense suppression of TIV1 in tomato resulted in lower hexose accumulation (Klann et al. 1996). Similar results were also reported in grape berry and muskmelon (Davies and Robinson 1996; Yu et al. 2008). In contrast, vacuolar invertase activity is tightly regulated by various signals, including corresponding invertase inhibitors. Qin et al. (2016) reported that RIPENING-INHIBITOR (RIN) transcriptionally activated the expression of SlVIF (a gene encoding a vacuolar invertase inhibitor in tomato), and the color transition of the transgenic SlVIF-RNAi fruit was retarded as compared to wild type, further suggesting a role for vacuolar invertase in regulating tomato fruit development (Qin et al. 2016).
Being the major photosynthate in Rosacea species, sorbitol accounts for about 80% of soluble carbohydrates synthesized in leaves, but only 3%–8% of soluble carbohydrates in fruit, suggesting that sorbitol is rapidly metabolized in fruit (Loescher 1987; Cheng et al. 2005). Both sorbitol and sucrose are translocated to and utilized in apple fruit, in which sorbitol accounts for about 60%–70% of the photosynthates synthesized in leaves (Li et al. 2012b). The enzymes related to sorbitol metabolism in apple are mainly sorbitol-6-phosphate dehydrogenase (S6PDH), sorbitol dehydrogenase (SDH) and sorbitol oxidase (SOX). S6PDH mainly catalyzes sorbitol biosynthesis in leaves, whereas SDH and SOX are involved in sorbitol metabolism in apple fruit. Their functions have also been defined. NAD+-SDH reversibly catalyzes the production of fructose from sorbitol (NAD+ functions as a co-enzyme) and SOX catalyzes the irreversible conversion from sorbitol to glucose (Li et al. 2012b; Shangguan et al. 2014).
Sugar transport
Sugar transporters are indispensable for sugar export, phloem loading and unloading (Wei et al. 2014; Durán-Soria et al. 2020; Li et al. 2020a). As the main transporter families, sorbitol transporters (SOTs), sucrose transporters (SUTs), monosaccharide transporters (MSTs) [including hexose transporters, tonoplast transporters, vacuolar glucose transporters, ERD six-like transporters, and plastid glucose transporters], and Sugar Will Eventually be Exported Transporters (SWEETs) function coordinately in sugar transport (Jeena et al. 2019) (Fig. 2). Occurring either apoplasmically, symplasmically, or synergistically, SWEETs and SUTs are involved in sucrose export during phloem unloading. SUTs are energy-requiring sucrose/H+ symporters (Chen et al. 2010) and SWEETs function as facilitated diffusers in four phylogenetic clades for transmembrane delivery of sugars (Chen et al. 2012; Braun et al. 2014; Eom et al. 2015). These transporters are elaborately regulated at transcriptional, translational and epigenetic levels. The overexpression of sucrose transporter gene MdSUT2.2 promoted sugar accumulation and drought tolerance in apple plants, which depended on the phosphorylation by MdCIPK22 upon drought treatment (Ma et al. 2019). In another Rosacea species Pyrus ussuriensis, PuSWEET15, a clade III member transporting sucrose, showed a high expression level in the bud sport variety of ‘Nanguo’ fruit, which was attributed to the activation by PuWRKY31 and the high acetylation level in PuWRKY31 promoter (Li et al. 2020b).
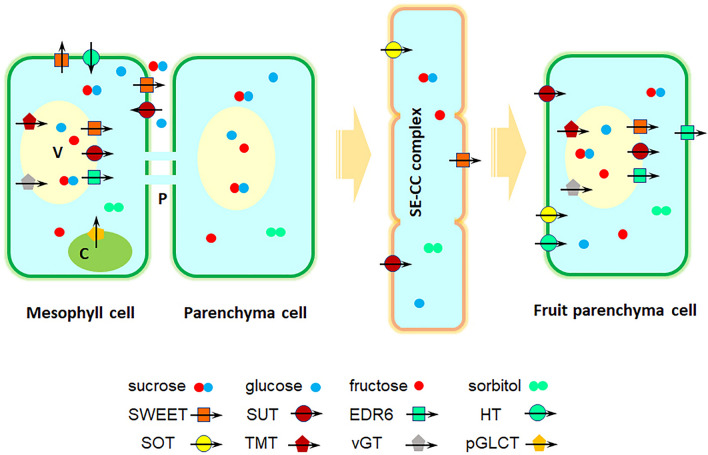
Fig. 2.
Sugar transport from leaves to fruit in apple. Sorbitol and sucrose are loaded into phloem and then unloaded into fruit parenchyma cells (Ruan et al. 2014; Wei et al. 2014). In apples, sorbitol transporters (SOTs), sucrose transporters (SUTs), SWEETs, monosaccharide transporters (MSTs) [including hexose transporters (HTs), tonoplast membrane transporters (TMTs), vacuolar glucose transporters (vGTs), ERD six-like transporters (EDR6), and plastid glucose transporters (pGLCT)] are major enzymes involved in sugar transport. SWEETs are uniporters mainly mediating the export of sugars. SUTs, SOTs, and HTs are H+/sugar importers importing Sor, Suc, and hexose. vGTs and TMTs function as sugar/H+ antiporters mobilizing sugars into vacuoles, while EDR6 and SWEET IV subfamilies are involved in sugar efflux from vacuoles. In addition, pGLCTs modulate sugar efflux from plastids. Note: C chloroplasts, P plasmodesmata, SE-CC complex sieve element/companion cell complex, V vacuoles
After cell wall invertases degrade sucrose into hexoses in apoplasmic unloading (Ruan et al. 2014), sugar transporters translocate sucrose, fructose, and glucose in the vacuole (Durán-Soria et al. 2020). In this process, tonoplast sugar transporters (TSTs) play important roles (Liu et al. 2016b; Ren et al. 2018). ClTSaT2 expression was positively correlated with tonoplast uptake and sugar accumulation in fruit flesh cells in watermelon, which was regulated by a sugar-induced transcription factor SUSIWM1 (Ren et al. 2018). In addition, raffinose family oligosaccharides are also major sugars translocated in the vascular bundle in cucurbits. ClAGA2, ClSWEET3 (a plasma membrane-localized clade I hexose transporter) and ClTST2 function in raffinose hydrolysis, transmembrane sugar transport and sugar storage in vacuoles, respectively, thereby contributing to the derivation of sweet watermelon from non-sweet ancestors during domestication (Ren et al. 2021). Moreover, it was reported recently that MdERDL6-1, a tonoplast glucose/H+ symporter, exports glucose to cytosol and up-regulates MdTST1 and MdTST2 expression, thus facilitating sugar accumulation from cytosol to vacuole in apples (Zhu et al. 2021). In contrast, the glucose, fructose and sucrose contents decreased during apple fruit senescence during storage, which was partly explained by the differential expression of ERD six-like transporter and tonoplast monosaccharide transporter 1, suggesting a efflux of sugars from the vacuole during storage (Zhu et al. 2013).
Crosstalk between sugar metabolism and hormones during fruit development and ripening
Crosstalk between sucrose and abscisic acid
Sugar signaling is closely related to the function of ABA in climacteric fruit ripening. Prior to the onset of tomato fruit ripening, an ABA content peak appears ahead of the ethylene peak, suggesting that both hormones function in promoting fruit ripening in climacteric fruit (Li et al. 2016; Chen et al. 2020). ABA positively modulates sugar accumulation in apple fruit by integrating multiple pathways. Besides the induction in the transcript levels of the tonoplast monosaccharide transporters MdTMT1 and MdSUT2, ABA also triggered the CIPK22-dependent phosphorylation of an ABA-responsive transcription factor MdAREB2 (Ma et al. 2017a). MdAREB2 directly activates the expression of amylase and sugar transporter genes to promote soluble sugar accumulation in M. domestica (Ma et al. 2017b). The crosstalk between sucrose and abscisic acid has also been reported in nonclimacteric fruit. FaSUT1 overexpression increased endogenous sucrose level, FaNCED expression of and ABA level in strawberry fruit, suggesting a synergistic action between ABA and sucrose (Jia et al. 2013). RNAi knockdown of 9-cis-epoxycarotenoid dioxygenase (NCED), a key enzyme regulating ABA biosynthesis, significantly delayed the ripening process of strawberry (Jia et al, 2011). Moreover, simultaneous application with sucrose and ABA exerted a more pronounced effect to accelerate the ripening of strawberry fruit, suggesting coordination between ABA and sucrose signaling in promoting ripening (Jia et al. 2016). However, Siebeneichler et al. reported that ABA and sucrose application on harvested strawberry fruit resulted in altered chemical composition and firmness, implying that postharvest ripening of strawberry fruit induced by ABA and sucrose may differ from that in natural ripening (Siebeneichler et al. 2020). Further investigations are still required to ascertain the underlying mechanisms.
Crosstalk between sugars and other hormones
Sugar metabolism and transport are also intersected with the signaling of other hormones, including auxin, ethylene, jasmonic acid and others. Besides the contribution of ABA and sucrose to strawberry fruit ripening, auxin (indole acetic acid, IAA) showed a negative role in sucrose accumulation as it suppresses the expression of genes related to sugar accumulation during tomato and strawberry fruit ripening (Jia et al. 2016). Moreover, in young pear fruit, auxin functioned in preventing fruit abscission by accumulating in the peduncle and allowing sucrose transport to sink cells in which it is degraded by CWIN (Murayama et al. 2015). Consequently, the produced glucose may inhibit the incidence of programmed cell death (PCD) at the abscission site and further guarantee fruit development (Ruan 2012). As a gas hormone, ethylene has been known for its function in fruit ripening, particularly in climacteric fruit (Chen et al. 2020). Upon the onset of fruit ripening for tomato, apple and grapes, the expression of ethylene receptor genes displayed increasing patterns, which was in accordance with the increase in sugar accumulation (Li et al. 2016). Moreover, ethylene promoted sucrose accumulation in kiwifruit and grapes during fruit ripening (Farcuh et al. 2018), while sucrose can also promote ethylene production and tomato fruit ripening at postharvest stage (Li et al. 2016), suggesting the existence of potential modulators involved in sugar and ethylene signaling. Moreover, evidence has been shown that methyl jasmonate (MeJA) promoted ethylene biosynthesis by regulating MdERFs and ethylene biosynthetic genes via MdMYC2 (Li et al. 2017). However, it has also been reported that JA may inhibit ethylene biosynthesis and delay fruit ripening in other fruit species, including sweet cherries and pears (Kondo et al. 2000; Lindo-Garcia et al. 2020). Therefore, it seems that the relationship between sugars and hormones depend on fruit species and environmental conditions, which requires in-depth investigations in specific contexts.
Sugar metabolism involved in responses to abiotic stress
Adverse environmental conditions often result in the accumulation of excess reactive oxygen species (ROS) and ultimately oxidative stress, during which sugar metabolism has its contribution to the responses of fruit crops to abiotic stress (Tian et al. 2013; Wang et al. 2019). RNA sequencing (RNA-seq) profiling for the antisense-A6PR apple line showed that sorbitol had important roles in the responses to abiotic and biotic stresses by modulating the genes involved in ABA, salicylic acid and JA signaling as well as nucleotide-binding site leucine-rich repeat (NBS-LRR) resistance (Wu et al. 2015). Moreover, as glucose contributes to the pentose phosphate pathway and the production of non-enzymatic antioxidants (including phenolics and flavonoids), sugar metabolism may function in maintaining fruit quality through modulating intracellular redox homeostasis and preventing oxidative stress under unfavorable conditions (Liu et al. 2013).
It has been documented that fruit development are sensitive to temperature (Zhang et al. 2010; Wang et al. 2019). After measuring CWIN, VIN, and sucrose synthase activities among the tomato cultivars showing differential heat sensitivity at normal and high temperature, Li et al. (2012a) found that that both CWIN and VIN activities were higher in the heat-tolerant cultivar (CLN2413R), suggesting that these two enzymes were involved in the responses of reproductive organs to heat stress. In accordance with the phenotype for CWIN overexpression (Liu et al. 2016a), the transcript level of PLDa1, a PCD regulator, was higher in the heat-sensitive cultivar (T9246), which may be related to invertase activities. The authors hypothesized that the suppression on the expression of PLDa1 and other PCD regulators may be attributed to the higher hexose content in the heat-tolerant cultivar (Li et al. 2012a). Taken together, these findings confirm that invertases have crucial roles in correlating sugar signaling and heat tolerance of fruit. Moreover, sucrose metabolism also functions in the responses to low temperature. Exogenous JA alleviated chilling injury in peach fruit by preventing the decrease in soluble sugar content, which was resulted from the scavenging of reactive oxygen species and the stabilization of membranes (Zhao et al. 2021). Similarly, the chilling resistant ‘Ninghaibai’ loquat fruit showed higher hexokinase and fructokinase activities, higher hexose contents, and higher sucrose degrading activities. These results suggest that the higher hexose contents and hexose sensor activity may also contribute to the chilling tolerance of loquat fruit (Cao et al. 2013). In addition, high salinity also interferes with sugar metabolism in tomato fruit, leading to lower sink activity and yield loss (Albacete et al. 2014). Overexpression of CIN1, a CWIN gene, alleviated the impairment of high salinity on fruit yield. Therefore, the authors proposed that globally activated hormonal signaling had positive roles in this process. However, the underlying mechanisms still require further clarifications.
Besides hexose contents and sensor activity, the trehalose pathway also contributes to the responses of fruit to abiotic stress (Durán-Soria et al. 2020). The trehalose precursor trehalose-6-phosphate (T6P) has been proposed to function via the sucrose non-fermenting-related kinase-1 (SnRK1) pathway, in which SnRK1 acts as a key modulator activated by stress conditions, such as hypoxia and submerging. It was reported that heterologous overexpression of SnRK1 from Malus hupehensis and Prunus persica in tomato accelerated fruit ripening, which was attributed to the interaction with RIN (Yu et al. 2018; Wang et al. 2012). In the presence of abundant sucrose, T6P inhibited SnRK1 activity but activated the expression of growth-promoting genes, whereas SnRK1 functioned as a growth repressor to regulate normal growth and stress responses (O’Hara et al. 2013). Nevertheless, the exact roles of T6P and SnRK1 in abiotic responses of fruit still require further exploration.
Sugar metabolism involved in responses to biotic stress
Sugar metabolism and transport have important roles in the interaction between fruit and pathogens. Exogenous sucrose increased endogenous sucrose, glucose and fructose levels, further stimulated the phenylpropanoid metabolic activity, resulting in higher levels of isoflavone glycosides and defense responses to Fusarium oxysporum in lupine (Morkunas et al. 2005). Interestingly, Sigha et al. reported that mitogen-activated protein kinase (MAPK) activities and LIN6 expression were substantially induced by non-metabolizable sugars, which were similar to the variations substantially triggered by fungal elicitors from F. oxysporum (Sigha et al. 2002). The authors proposed that non-metabolizable sugars may be perceived as stress signals. In addition to trehalose synthesis, pathogens may synthesize trehalose or trehalose-6-phosphate (T6P), thereby triggering metabolic reprogramming of plant cells. An interesting study showed that Snf1-related kinase (SnRK) 2.8, a member in the SnRK-CDPK module, may be hijacked by pathogens to phosphorylate bacterial effectors (Lei et al. 2020). These data identify a strategy co-opted by pathogens to promote disease incidence, which may be utilized for genetic breeding to improve resistance. Sugar transporters also play roles in fruit-pathogen interaction. The transcript level of VvSWEET4 (a clade II member) was strongly stimulated upon the invasion of the necrotrophic fungus Botrytis cinerea (Chong et al. 2014), which may be explained by the manipulation on sugars in hosts by B. cinerea. Coincidently, the knock-out mutants for AtSWEET4 (a gene encoding a clade II SWEET) displayed cell death phenotype and higher resistance to B. cinerea (van Kan 2006). Another line of evidence showed that B. cinerea infection induced SlSWEET15 (a clade III SWEET) but down-regulated VvSWEET7 (a clade II SWEET), thereby providing sugars to facilitate mycelial growth of B. cinerea (Asai et al. 2016; Breia et al. 2020), suggesting that sugar transporters in the same superfamily may respond differentially to pathogen invasion.
Pathogens compete for sugars as nutrient sources to gain access to their hosts, whereas host sugar metabolism may contribute to the variations in host environment, thereby affecting the consequence for interactions between fruit and pathogens (Prusky and Yakoby 2003; Tian et al. 2016; Wang et al. 2019). It was reported that excessive sugar content in fruit contributed to pH modulation in host tissues by oxidation of glucose to gluconic acid by glucose oxidase 2 in acidifying pathogens. In contrast, the deamination reaction catalyzed by glutamate dehydrogenase 2 led to ammonia production, indicating that fungus-induced pH modulation was affected by sugar content in hosts (Bi et al. 2016). Similarly, in the analysis on the interactions of Colletotrichum gloeosporioides and Penicillium expansum with different tomato cultivars, P. expansum showed enhanced colonization in the high sugar content cultivar, whereas C. gloeosporioides showed enhanced colonization in the low sugar content cultivar. Moreover, the specific gene responsive profiles for the two host lines differed significantly, which depended on the sugar level. The authors suggested that such differential response patterns may contribute to the differential host ranges for both pathogens (Ziv et al. 2020).
Sugar may also function as a signaling molecule to modulate the resistance of plants to pathogens (Meng et al. 2018). Antisense suppression of A6PR led to reduced sorbitol synthesis in M. domestica leaves, downregulation of Nucleotide-binding Leucine-rich Repeat (NLR) genes, and ultimately susceptible responses to Alternaria alternata. Overexpression of MdNLR16 in A6PR lines improved the resistance, whereas sorbitol promoted the transcriptional activation of MdNLR16 by MdWRKY79, suggesting sorbitol could modulate the resistance to A. alternata through the interaction between MdNLR16 and fungal effector in a classical gene-for-gene manner in apple (Meng et al. 2018). To our knowledge, this is the only report on the signaling roles of sugars in modulating disease resistance to fungal pathogens up to now.
Strategies for metabolic engineering on sugar content
Sweeter fruit with higher sugar content is often favored by consumers. A gene co-expression analysis on citrus fruit transcriptome showed that most the sugar/acid ratio-related genes are involved in transport, signaling, transcription and metabolic enzymes, only few genes are weakly correlated with sugar level and none corresponded to acid-associated genes, suggesting that a fruit-specific dissection for sugar metabolic processes may be necessary for genetic engineering (Qiao et al. 2017). Latest studies on gene regulatory elements have provided new targets for future work. An interesting study generated the transgenic tomato lines harboring the main open reading frames (ORFs) of SlbZIP1 and SlbZIP2 (driven by the fruit-specific E8 promoter), whereas the sucrose-induced repression of translation (SIRT)-responsive uORFs were removed (Sagor et al. 2016). The contents of sugar (sucrose/glucose/fructose) and several amino acids (such as asparagine and glutamine) were significantly higher in the transgenic fruit. This strategy is promising as it substantially increases the sweetness of fruit without substantially affecting normal growth of fruit crops. Similar results have also been reported to modulate the growth/immunity tradeoff of plants in cereal crops (Xu et al. 2017).
In return, the genes involved in sugar metabolism, such as some SWEET genes, may be co-opted by fungal pathogens, as revealed in the gray mold caused by B. cinerea (Chen et al. 2010). SWEET genes play a major role in growth and development of plants, but also act as susceptibility (S) genes in response to pathogen invasion. Therefore, these dual-function genes modulating the balance between growth and defense should be further explored, and their functions as S genes in regulating pathogen susceptibility might be elucidated. The use of SWEET genes may potentially enable the improvements for fruit crops, including tomato and banana, as their substantial functions as S genes has already been demonstrated (Gupta et al. 2020). However, the clustering of a SWEET in a specific clade does not guarantee a definite physiological process for the protein, which requires in-depth studies on the exact functions (Eom et al. 2015). In addition, during the production of botrytized wines using some white-skinned grape berries, noble rot reprograms the metabolism of cv. Sémillon berries by inducing stress responses and fruit ripening, resulting in accumulation of sugars, aroma substances, and flavor compounds, as well as enhancement of phenylpropanoid metabolism and anthocyanin biosynthesis (Vannini and Chilosi 2013; Blanco-Ulate et al. 2015). Although the underlying mechanisms have not been elucidated so far, this represents another example for the engineering of primary and secondary metabolism of fruit utilizing “beneficial” fungal pathogens.
Concluding remarks and future perspectives
In conclusion, metabolic and transport activities synergistically contribute to composition and concentrations of sugars in various fruit species, ultimately determining fruit quality and commodity. Sugars may function coordinately with ABA and other phytohormones. Moreover, sugars also act as crucial modulators of stress responses and disease resistance to fungal pathogens. Importantly, the development of different fruits is underpinned by different transport and metabolic processes, and transporters or catalytic enzymes also changes substantially during development of different fruit crops. Therefore, an in-depth understanding in the molecular networks involving sugar metabolism and transport in specific biological context may greatly intensify and accelerate future metabolic engineering of fruit crops.
Acknowledgements
We sincerely thank the two anonymous reviewers for their comments on the manuscript and apologize for the omission of any pertinent original reference due to space limitations.
Author contribution
ST conceives, writes the original manuscript, revises and approves the final version; TC, ZZ, BL and GQ discuss on the data and write the original manuscript.
Funding
This work was supported by National Natural Science Foundation of China (31930086, 32072637).
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Compliance with ethical standards
Not applicable.
References
- Albacete A, Cantero-Navarro E, Balibrea ME, Großkinsky DK, De La Cruz Gonzalez M, Martinez-Andujar C, Smigocki AC, Roitsch T, Pérez-Alfocea F. Hormonal and metabolic regulation of tomato fruit sink activity and yield under salinity. J Exp Bot. 2014;65(20):6081–6095. doi: 10.1093/jxb/eru347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai Y, Kobayashi Y, Kobayashi I. Increased expression of the tomato SISWEET15 gene during grey mold infection and the possible involvement of the sugar efflux to apoplasm in the disease susceptibility. J Plant Pathol Microbiol. 2016;7(1):329. doi: 10.4172/2157-7471.1000329. [DOI] [Google Scholar]
- Bi F, Barad S, Ment D, Luria N, Dubey A, Casado V, Glam N, Mínguez JD, Espeso EA, Fluhr R, Prusky D. Carbon regulation of environmental pH by secreted small molecules that modulate pathogenicity in phytopathogenic fungi. Mol Plant Pathol. 2016;17(8):1178–1195. doi: 10.1111/mpp.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Ulate B, Amrine KCH, Collins TS, Rivero RM, Vicente AR, Morales-Cruz A, Doyle CL, Ye Z, Allen G, Heymann H, Ebeler SE, Cantu D. Developmental and metabolic plasticity of white-skinned grape berries in response to Botrytis cinerea during noble rot. Plant Physiol. 2015;169(4):2422–2443. doi: 10.1104/pp.15.00852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun DM, Wang L, Ruan YL. Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security. J Exp Bot. 2014;65(7):1713–1735. doi: 10.1093/jxb/ert416. [DOI] [PubMed] [Google Scholar]
- Breia R, Conde A, Pimentel D, Conde C, Fortes AM, Granell A, Gerós H. VvSWEET7 is a mono- and disaccharide transporter up-regulated in response to Botrytis cinerea infection in grape berries. Front Plant Sci. 2019;10:1753. doi: 10.3389/fpls.2019.01753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Yang Z, Zheng Y. Sugar metabolism in relation to chilling tolerance of loquat fruit. Food Chem. 2013;136(1):139–143. doi: 10.1016/j.foodchem.2012.07.113. [DOI] [PubMed] [Google Scholar]
- Chen LQ, Hou BH, Lalonde S, Takanaga H, Hartung ML, Qu XQ, Guo W-J, Kim J-G, Underwood W, Chaudhuri B, Chermak D, Antony G, White FF, Somerville SC, Mudgett MB, Frommer WB. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature. 2010;468(7323):527–532. doi: 10.1038/nature09606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LQ, Qu XQ, Hou BH, Sosso D, Osorio S, Fernie AR, Frommer WB. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science. 2012;335(6065):207–211. doi: 10.1126/science.1213351. [DOI] [PubMed] [Google Scholar]
- Chen T, Qin G, Tian S. Regulatory network of fruit ripening: current understanding and future challenges. New Phytol. 2020;228(4):1219–1226. doi: 10.1111/nph.16822. [DOI] [PubMed] [Google Scholar]
- Chen T, Ji D, Zhang Z, Li B, Qin G, Tian S. Advances and strategies for controlling the quality and safety of postharvest fruit. Engineering. 2021 doi: 10.1016/j.eng.2020.07.029. [DOI] [Google Scholar]
- Cheng L, Zhou R, Reidel EJ, Sharkey TD, Dandekar AM. Antisense inhibition of sorbitol synthesis leads to up-regulation of starch synthesis without altering CO2 assimilation in apple leaves. Planta. 2005;220:767–776. doi: 10.1007/s00425-004-1384-5. [DOI] [PubMed] [Google Scholar]
- Chong J, Piron MC, Meyer S, Merdinoglu D, Bertsch C, Mestre P. The SWEET family of sugar transporters in grapevine: VvSWEET4 is involved in the interaction with Botrytis cinerea. J Exp Bot. 2014;65(22):6589–6601. doi: 10.1093/jxb/eru375. [DOI] [PubMed] [Google Scholar]
- D’Aoust MA, Yelle S, Nguyen-Quoc B. Antisense inhibition of tomato fruit sucrose synthase decreases fruit setting and the sucrose unloading capacity of young fruit. Plant Cell. 1999;11(12):2407–2418. doi: 10.1105/tpc.11.12.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai ZW, Léon C, Feil R, Lunn JE, Delrot S, Gomès E. Metabolic profiling reveals coordinated switches in primary carbohydrate metabolism in grape berry (Vitis vinifera L.), a non-climacteric fleshy fruit. J Exp Bot. 2013;64(5):1345–1355. doi: 10.1093/jxb/ers396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C, Robinson SP. Sugar accumulation in grape berries. Cloning of two putative vacuolar invertase cDNAs and their expression in grapevine tissues. Plant Physiol. 1996;111(1):275–283. doi: 10.1104/pp.111.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durán-Soria S, Pott DM, Osorio S, Vallarino JG. Sugar signaling during fruit ripening. Front Plant Sci. 2020;11:564917. doi: 10.3389/fpls.2020.564917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom J-S, Chen L-Q, Sosso D, Julius BT, Lin IW, Qu X-Q, Braun DM, Frommer WB. SWEETs, transporters for intracellular and intercellular sugar translocation. Curr Opin Plant Biol. 2015;25:53–62. doi: 10.1016/j.pbi.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Farcuh M, Rivero RM, Sadka A, Blumwald E. Ethylene regulation of sugar metabolism in climacteric and non-climacteric plums. Postharvest Biol Technol. 2018;139:20–30. doi: 10.1016/j.postharvbio.2018.01.012. [DOI] [Google Scholar]
- Gambetta GA, Matthews MA, Shaghasi TH, McElrone AJ, Castellarin SD. Sugar and abscisic acid signaling orthologs are activated at the onset of ripening in grape. Planta. 2010;232(1):219–234. doi: 10.1007/s00425-010-1165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PK. SWEET genes for disease resistance in plants. Trends Genet. 2020;36(12):901–904. doi: 10.1016/j.tig.2020.08.007. [DOI] [PubMed] [Google Scholar]
- Jeena GS, Kumar S, Shukla RK. Structure, evolution and diverse physiological roles of SWEET sugar transporters in plants. Plant Mol Biol. 2019;100(4–5):351–365. doi: 10.1007/s11103-019-00872-4. [DOI] [PubMed] [Google Scholar]
- Jia H, Chai Y, Li CL, Lu D, Luo J, Qin L, Shen Y. Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol. 2011;157(1):188–199. doi: 10.1104/pp.111.177311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Wang Y, Sun M, Li B, Han Y, Zhao Y, Li X, Ding N, Li C, Ji W, Jia W. Sucrose functions as a signal involved in the regulation of strawberry fruit development and ripening. New Phytol. 2013;198(2):453–465. doi: 10.1111/nph.12176. [DOI] [PubMed] [Google Scholar]
- Jia H, Jiu S, Zhang C, Wang C, Tariq P, Liu Z, Wang B, Cui L, Fang J. Abscisic acid and sucrose regulate tomato and strawberry fruit ripening through the abscisic acid-stress-ripening transcription factor. Plant Biotechnol J. 2016;14(10):2045–2065. doi: 10.1111/pbi.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Ni DA, Ruan Y-L. Posttranslational elevation of cell wall invertase activity by silencing its inhibitor in tomato delays leaf senescence and increases seed weight and fruit hexose level. Plant Cell. 2009;21(7):2072–2089. doi: 10.1105/tpc.108.063719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klann EM, Hall B, Bennett AB. Antisense acid invertase (TIV1) gene alters soluble sugar composition and size in transgenic tomato fruit. Plant Physiol. 1996;112(3):1321–1330. doi: 10.1104/pp.112.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Tomiyama A, Seto H. Changes of endogenous jasmonic acid and methyl jasmonate in apples and sweet cherries during fruit development. J Am Soc Hortic Sci. 2000;125(3):282–287. doi: 10.21273/jashs.125.3.282. [DOI] [Google Scholar]
- Lei L, Stevens DM, Coaker G. Phosphorylation of the Pseudomonas effector AvrPtoB by Arabidopsis SnRK2.8 is required for bacterial virulence. Mol Plant. 2020;13(10):1513–1522. doi: 10.1016/j.molp.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Feng F, Cheng L. Expression patterns of genes involved in sugar metabolism and accumulation during apple fruit development. PLoS ONE. 2012;7(3):e33055. doi: 10.1371/journal.pone.0033055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Palmer WM, Martin AP, Wang R, Rainsford F, Jin Y, Patrick JW, Yang Y, Ruan Y-L. High invertase activity in tomato reproductive organs correlates with enhanced sucrose import into, and heat tolerance of, young fruit. J Exp Bot. 2012;63(3):1155–1166. doi: 10.1093/jxb/err329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Mou W, Wang Y, Li L, Mao L, Ying T, Luo Z. Exogenous sucrose treatment accelerates postharvest tomato fruit ripening through the influence on its metabolism and enhancing ethylene biosynthesis and signaling. Acta Physiol Plant. 2016;38:225. doi: 10.1007/s11738-016-2240-5. [DOI] [Google Scholar]
- Li T, Xu Y, Zhang L, Ji Y, Tan D, Yuan H, Wang A. The jasmonate-activated transcription factor MdMYC2 regulates ETHYLENE RESPONSE FACTOR and ethylene biosynthetic genes to promote ethylene biosynthesis during apple fruit ripening. Plant Cell. 2017;29(6):1316–1334. doi: 10.1105/tpc.17.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Li P, Ma F, Dandekar AM, Cheng L. Sugar metabolism and accumulation in the fruit of transgenic apple trees with decreased sorbitol synthesis. Hortic Res. 2018;5:60. doi: 10.1038/s41438-018-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Meng D, Piñeros MA, Mao Y, Dandekar AM, Cheng L. A sugar transporter takes up both hexose and sucrose for sorbitol-modulated in vitro pollen tube growth in apple. Plant Cell. 2020;32(2):449–469. doi: 10.1105/tpc.19.00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Guo W, Li J, Yue P, Bu H, Jiang J, Liu W, Xu Y, Yuan H, Li T, Wang A. Histone acetylation at the promoter for the transcription factor PuWRKY31 affects sucrose accumulation in pear fruit. Plant Physiol. 2020;182(4):2035–2046. doi: 10.1104/pp.20.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Ma M, Zhou Z, Wang J, Yang X, Rao S, Bi G, Li L, Zhang X, Chai J, Chen S, Zhou JM. Ligand-triggered de-repression of Arabidopsis heterotrimeric G proteins coupled to immune receptor kinases. Cell Res. 2018;28(5):529–543. doi: 10.1038/s41422-018-0027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindo-Garcia V, Munoz P, Larrigaudière C, Munne-Bosch S, Gine-Bordonaba J. Interplay between hormones and assimilates during pear development and ripening and its relationship with the fruit postharvest behaviour. Plant Sci. 2020;291:110339. doi: 10.1016/j.plantsci.2019.110339. [DOI] [PubMed] [Google Scholar]
- Liu YH, Offler CE, Ruan YL. Regulation of fruit and seed response to heat and drought by sugars as nutrients and signals. Front Plant Sci. 2013;4:282. doi: 10.3389/fpls.2013.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YH, Offler CE, Ruan YL. Cell wall invertase promotes fruit set under heat stress by suppressing ROS-independent cell death. Plant Physiol. 2016;172(1):163–180. doi: 10.1104/pp.16.00959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Wang Y, Qin G, Tian S. iTRAQ-based quantitative proteomic analysis reveals the role of the tonoplast in fruit senescence. J Proteomics. 2016;146:80–89. doi: 10.1016/j.jprot.2016.06.031. [DOI] [PubMed] [Google Scholar]
- Liu C, Fan W, Zhu P, Xia Z, Hu J, Zhao A. Mulberry RGS negatively regulates salt stress response and tolerance. Plant Signal Behav. 2019;14(12):1672512. doi: 10.1080/15592324.2019.1672512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loescher WH. Physiology and metabolism of sugar alcohols in higher plants. Physiol Plantarum. 1987;70(3):553–557. doi: 10.1111/j.1399-3054.1987.tb02857.x. [DOI] [Google Scholar]
- Lytovchenko A, Eickmeier I, Pons C, Osorio S, Szecowka M, Lehmberg K, Arrivault S, Tohge T, Pineda B, Anton MT, Hedtke B, Lu Y, Fisahn J, Bock R, Stitt M, Grimm B, Granell A, Fernie AR. Tomato fruit photosynthesis is seemingly unimportant in primary metabolism and ripening but plays a considerable role in seed development. Plant Physiol. 2011;157(4):1650–1663. doi: 10.1104/pp.111.186874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q-J, Sun M-H, Lu J, Liu Y-J, You C-X, Hao Y-J. An apple CIPK protein kinase targets a novel residue of AREB transcription factor for ABA-dependent phosphorylation. Plant Cell Environ. 2017;40(10):2207–2219. doi: 10.1111/pce.13013. [DOI] [PubMed] [Google Scholar]
- Ma Q-J, Sun M-H, Lu J, Liu Y-J, Hu D-G, Hao Y-J. Transcription factor AREB2 is involved in soluble sugar accumulation by activating sugar transporter and amylase genes. Plant Physiol. 2017;174(4):2348–2362. doi: 10.1104/pp.17.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q-J, Sun M-H, Lu J, Kang H, You C-X, Hao Y-J. An apple sucrose transporter MdSUT2.2 is a phosphorylation target for protein kinase MdCIPK22 in response to drought. Plant Biotechnol J. 2019;17(3):625–637. doi: 10.1111/pbi.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng D, Li C, Park H-J, González J, Wang J, Dandekar AM, Turgeon BG, Cheng L. Sorbitol modulates resistance to Alternaria alternata by regulating the expression of an NLR resistance gene in apple. Plant Cell. 2018;30(7):1562–1581. doi: 10.1105/tpc.18.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micallef BJ, Haskins KA, Vanderveer PJ, Roh KS, Shewmaker CK, Sharkey TD. Altered photosynthesis, flowering, and fruiting in transgenic tomato plants that have an increased capacity for sucrose synthesis. Planta. 1995;196:327–334. doi: 10.1007/BF00201392. [DOI] [Google Scholar]
- Morkunas I, Marczak Ł, Stachowiak J, Stobiecki M. Sucrose-induced lupine defense against Fusarium oxysporum Sucrose-stimulated accumulation of isoflavonoids as a defense response of lupine to Fusarium oxysporum. Plant Physiol Biochem. 2005;43(4):363–373. doi: 10.1016/j.plaphy.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Murayama H, Sai M, Oikawa A, Itai A. Inhibitory factors that affect the ripening of pear fruit on the tree. Hortic J. 2015;84(1):14–20. doi: 10.2503/hortj.MI-015. [DOI] [Google Scholar]
- Nguyen-quoc B, Foyer CH. A role for ‘futile cycles’ involving invertase and sucrose synthase in sucrose metabolism of tomato fruit. J Exp Bot. 2001;52(358):881–889. doi: 10.1093/jexbot/52.358.881. [DOI] [PubMed] [Google Scholar]
- O’Hara LE, Paul MJ, Wingler A. How do sugars regulate plant growth and development? New insight into the role of trehalose-6-phosphate. Mol Plant. 2013;6(2):261–274. doi: 10.1093/mp/sss120. [DOI] [PubMed] [Google Scholar]
- Palmer WM, Ru L, Jin Y, Patrick JW, Ruan Y-L. Tomato ovary-to-fruit transition is characterized by a spatial shift of mRNAs for cell wall invertase and its inhibitor with the encoded proteins localized to sieve elements. Mol Plant. 2015;8(2):315–328. doi: 10.1016/j.molp.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Prusky D, Yakoby N. Pathogenic fungi: leading or led by ambient pH? Mol Plant Pathol. 2003;4(6):509–516. doi: 10.1046/j.1364-3703.2003.00196.x. [DOI] [PubMed] [Google Scholar]
- Prusky DB, Bi F, Moral J, Barad S. How does host carbon concentration modulate the lifestyle of postharvest pathogens during colonization? Front Plant Sci. 2016;7:1306. doi: 10.3389/fpls.2016.01306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L, Cao M, Zheng J, Zhao Y, Zheng Z-L. Gene coexpression network analysis of fruit transcriptomes uncovers a possible mechanistically distinct class of sugar/acid ratio-associated genes in sweet orange. BMC Plant Biol. 2017;17(1):186. doi: 10.1186/s12870-017-1138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin G, Zhu Z, Wang W, Cai J, Chen Y, Li L, Tian S. A tomato vacuolar invertase inhibitor mediates sucrose metabolism and influences fruit ripening. Plant Physiol. 2016;172(3):1596–1611. doi: 10.1104/pp.16.01269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Guo S, Zhang J, He H, Sun H, Tian S, Gong G, Zhang H, Levi A, Tadmor Y, Xu Y. A tonoplast sugar transporter underlies a sugar accumulation QTL in watermelon. Plant Physiol. 2018;176(1):836–850. doi: 10.1104/pp.17.01290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Li M, Guo S, Sun H, Zhao J, Zhang J, Liu G, He H, Tian S, Yu Y, Gong G, Zhang H, Zhang X, Alseekh S, Fernie AR, Scheller HV, Xu Y. Evolutionary gain of oligosaccharide hydrolysis and sugar transport enhanced carbohydrate partitioning in sweet watermelon fruits. Plant Cell. 2021 doi: 10.1093/plcell/koab055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan YL. Signaling role of sucrose metabolism in development. Mol Plant. 2012;5(4):763–765. doi: 10.1093/mp/sss046. [DOI] [PubMed] [Google Scholar]
- Ruan YL. Sucrose metabolism: gateway to diverse carbon use and sugar signaling. Annu Rev Plant Biol. 2014;65:33–67. doi: 10.1146/annurev-arplant-050213-040251. [DOI] [PubMed] [Google Scholar]
- Sadka A, Shlizerman L, Kamara I, Blumwald E. Primary metabolism in citrus fruit as affected by its unique structure. Front Plant Sci. 2019;10:1167. doi: 10.3389/fpls.2019.01167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagor GHM, Berberich T, Tanaka S, Nishiyama M, Kanayama Y, Kojima S, Muramoto K, Kusano T. A novel strategy to produce sweeter tomato fruits with high sugar contents by fruit-specific expression of a single bZIP transcription factor gene. Plant Biotechnol J. 2016;14(4):1116–1126. doi: 10.1111/pbi.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shangguan L, Song C, Leng X, Kayesh E, Sun X, Fang J. Mining and comparison of the genes encoding the key enzymes involved in sugar biosynthesis in apple, grape, and sweet orange. Sci Hortic. 2014;165:311–318. doi: 10.1016/j.scienta.2013.11.026. [DOI] [Google Scholar]
- Siebeneichler TJ, Crizel RL, Camozatto GH, Paim BT, da Silva MR, Rombaldi CV, Galli V. The postharvest ripening of strawberry fruits induced by abscisic acid and sucrose differs from their in vivo ripening. Food Chem. 2020;317:126407. doi: 10.1016/j.foodchem.2020.126407. [DOI] [PubMed] [Google Scholar]
- Sinha AK, Hofmann MG, Römer U, Köckenberger W, Elling L, Roitsch T. Metabolizable and non-metabolizable sugars activate different signal transduction pathways in tomato. Plant Physiol. 2002;128(4):1480–1489. doi: 10.1104/pp.010771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S, Qin G, Li B. Reactive oxygen species involved in regulating fruit senescence and fungal pathogenicity. Plant Mol Biol. 2013;82(6):593–602. doi: 10.1007/s11103-013-0035-2. [DOI] [PubMed] [Google Scholar]
- Tian S, Torres R, Ballester AR, Li B, Vilanova L, Gonzalez-Candelas L. Molecular aspects in pathogen-fruit interactions: Virulence and resistance. Postharvest Biol Tech. 2016;122:11–21. doi: 10.1016/j.postharvbio.2016.04.018. [DOI] [Google Scholar]
- Urano D, Phan N, Jones JC, Yang J, Huang J, Grigston J, Taylor JP, Jones AM. Endocytosis of the seven-transmembrane RGS1 protein activates G-protein-coupled signalling in Arabidopsis. Nat Cell Biol. 2012;14:1079–1088. doi: 10.1038/ncb2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallarino JG, Yeats TH, Maximova E, Rose JK, Fernie AR, Osorio S. Postharvest changes in LIN5-down-regulated plants suggest a role for sugar deficiency in cuticle metabolism during ripening. Phytochemistry. 2017;142:11–20. doi: 10.1016/j.phytochem.2017.06.007. [DOI] [PubMed] [Google Scholar]
- Vannini A, Chilosi G. Botrytis infection: grey mould and noble rot. In: Mencarelli F, Tonutti P, editors. Sweet, reinforced and fortified wines: grape biochemistry, technology and vinification. Wiley-Blackwell; 2013. pp. 159–169. [Google Scholar]
- Wang X, Peng F, Li M, Yang L, Li G. Expression of a heterologous SnRK1 in tomato increases carbon assimilation, nitrogen uptake and modifies fruit development. J Plant Physiol. 2012;169(12):1173–1182. doi: 10.1016/j.jplph.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ji D, Chen T, Li B, Zhang Z, Qin G, Tian S. Production, signaling, and scavenging mechanisms of reactive oxygen species in fruit-pathogen interactions. Int J Mol Sci. 2019;20(12):2994. doi: 10.3390/ijms20122994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Liu F, Chen C, Ma F, Li M. The Malus domestica sugar transporter gene family: identifications based on genome and expression profiling related to the accumulation of fruit sugars. Front Plant Sci. 2014;5:569. doi: 10.3389/fpls.2014.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wind J, Smeekens S, Hanson J. Sucrose: metabolite and signaling molecule. Phytochemistry. 2010;71(14–15):1610–1614. doi: 10.1016/j.phytochem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Wipf D, Pfister C, Mounier A, Leborgne-Castel N, Frommer WB, Courty P-E. Identification of putative interactors of Arabidopsis sugar transporters. Trends in Plant Sci. 2021;26(1):13–22. doi: 10.1016/j.tplants.2020.09.009. [DOI] [PubMed] [Google Scholar]
- Wu T, Wang Y, Zheng Y, Fei Z, Dandekar AM, Xu K, Han Z, Cheng L. Suppressing sorbitol synthesis substantially alters the global expression profile of stress response genes in apple (Malus domestica) leaves. Plant Cell Physiol. 2015;56(9):1748–1761. doi: 10.1093/pcp/pcv092. [DOI] [PubMed] [Google Scholar]
- Xu G, Yuan M, Ai C, Liu L, Zhuang E, Karapetyan S, Wang S, Dong X. uORF-mediated translation allows engineered plant disease resistance without fitness cost. Nature. 2017;545(7655):491–494. doi: 10.1038/nature22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelle S, Hewitt JD, Robinson NL, Damon S, Bennett AB. Sink metabolism in tomato fruit: III. analysis of carbohydrate assimilation in a wild species. Plant Physiol. 1988;87(3):737–740. doi: 10.1104/pp.87.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Wang X, Zhang W, Qian T, Tang G, Guo Y, Zheng C. Antisense suppression of an acid invertase gene (MAI1) in muskmelon alters plant growth and fruit development. J Exp Bot. 2008;59(11):2969–2977. doi: 10.1093/jxb/ern158. [DOI] [PubMed] [Google Scholar]
- Yu W, Peng F, Xiao Y, Wang G, Luo J. Overexpression of PpSnRK1a in tomato promotes fruit ripening by enhancing RIPENING INHIBITOR regulation pathway. Front Plant Sci. 2018;871:1856. doi: 10.3389/fpls.2018.01856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanor MI, Osorio S, Nunes-Nesi A, Carrarib F, Lohse M, Usadel B, Kühn C, Bleiss W, Giavalisco P, Willmitzer L, Sulpice R, Zhou Y-H, Fernie AR. RNA interference of LIN5 in Solanum lycopersicum confirms its role in controlling Brix content, uncovers the influence of sugars on the levels of fruit hormones and demonstrates the importance of sucrose cleavage for normal fruit development and fertility. Plant Physiol. 2009;150(3):1204–1218. doi: 10.1104/pp.109.136598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L-Y, Peng Y-B, Pelleschi-Travier S, Fan Y, Lu Y-F, Lu Y-M, Gao X-P, Shen Y-Y, Delrot S, Zhang D-P. Evidence for apoplasmic phloem unloading in developing apple fruit. Plant Physiol. 2004;135(1):574–586. doi: 10.1104/pp.103.036632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CF, Ding ZS, Xu XB, Wang Q, Qin GZ, Tian SP. Crucial roles of membrane stability and its related proteins in the tolerance of peach fruit to chilling injury. Amino Acids. 2010;39(1):181–194. doi: 10.1007/s00726-009-0397-6. [DOI] [PubMed] [Google Scholar]
- Zhao YY, Song CC, Brummell DA, Qia SN, Lin Q, Duan YQ. Jasmonic acid treatment alleviates chilling injury in peach fruit by promoting sugar and ethylene metabolism. Food Chem. 2021;338:128005. doi: 10.1016/j.foodchem.2020.128005. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Liu RL, Li BQ, Tian SP. Characterisation of genes encoding key enzymes involved in sugar metabolism of apple fruit in controlled atmosphere storage. Food Chem. 2013;141(4):3323–3328. doi: 10.1016/j.foodchem.2013.06.025. [DOI] [PubMed] [Google Scholar]
- Zhu L, Li B, Wu L, Li H, Wang Z, Wei X, Ma B, Zhang Y, Ma F, Ruan Y-L, Li M. MdERDL6-mediated glucose efflux to the cytosol promotes sugar accumulation in the vacuole through up-regulating TSTs in apple and tomato. Proc Natl Acad Sci U S A. 2021;118(1):e2022788118. doi: 10.1073/pnas.2022788118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv C, Kumar D, Sela N, Itkin M, Malitsky S, Schaffer AA, Prusky DB. Sugar-regulated susceptibility of tomato fruit to Colletotrichum and Penicillium requires differential mechanisms of pathogenicity and fruit responses. Environ Microbiol. 2020;22(7):2870–2891. doi: 10.1111/1462-2920.15031. [DOI] [PubMed] [Google Scholar]