Abstract
Obesity is a strong predictor of heart disease, insulin resistance, and type II diabetes. Chronic, low-grade inflammation links obesity and insulin resistance through mitogen-activated protein kinase (MAPK) signaling pathways. Upstream kinases activate MAPK signaling, while MAPK-specific dual-specificity phosphatases (DUSPs) act as key modulators and controllers of MAPK deactivation (i.e. dephosphorylation). Using tumor necrosis factor α (TNFα) in 3 T3-L1 adipocytes as a model of inflammation, we report that TNFα-mediated induction of Dusp1, Dusp8 and Dusp16 modulated the transient regulation of MAPK (i.e., ERK, JNK, and p38) phosphorylation and subsequent inflammatory gene expression. All three MAPKs examined were phosphorylated in preadipocytes and adipocytes in response to TNFα, where signaling magnitude and duration were phenotype-specific. Moreover, TNFα increased mRNA abundance of DUSPs in preadipocytes and adipocytes in a phenotype-specific manner, concomitant with dephosphorylation of MAPKs. RNA interference (RNAi)-mediated knockdown of Dusp1, Dusp8 and Dusp16 increased signaling magnitude and duration of ERK, JNK, and p38 that subsequently resulted in significant increases in MAPK-dependent inflammatory gene expression of MCP-1, IL-6, and Cox-2 in response to TNFα. This study highlights important roles for DUSPs as integral components of MAPK signaling and adipocyte inflammatory gene expression.
1. Introduction
Obesity plays a causal role in the development of type 2 diabetes, which ensues from progressive loss of insulin sensitivity in adipose tissue, liver and skeletal muscle [1]. Numerous studies have highlighted localized inflammation and adipose tissue dysfunction as central mediators of the metabolic adaptations that couple the progression of obesity to the onset of insulin resistance [1,2]. Central to this argument, localized inflammation has been shown to elevate proinflammatory cytokine and free fatty acid release from adipose tissue that imparts peripheral insulin resistance and altered glucose homeostasis in both liver and muscle [1–3]. Molecular mechanisms that underlie the onset of obesity-induced inflammation include secretion of proinflammatory cytokines from fat-laden adipocytes, adipocyte precursor cells known as ‘preadipocytes’ as well as resident adipose tissue macrophages. As the severity of obesity progresses, tissue-derived chemokines, such as monocyte chemoattractant protein-1 (MCP-1), further promote macrophage infiltration into adipose tissue that exacerbates the secretion of proinflammatory mediators originating from adipose tissue, leading to the development of chronic inflammation and systemic insulin resistance [1–4].
Evidence highlights tumor necrosis factor α (TNFα) among the plethora of cytokines that perpetuate chronic inflammation leading to insulin resistance [3,5]. TNFα is expressed as a 26 kDa transmembrane monomer that is enzymatically cleaved to a 17 kDa soluble form. Membrane-bound and soluble forms of TNFα exert biological activity and impinge upon insulin sensitivity through autocrine, paracrine, or endocrine modalities [5,6]. Further, TNFα has been shown to suppress insulin signaling, alter glucose homeostasis, promote lipolysis, and inhibit lipogenesis in cultured adipocytes [1,3,6,7]. It has also been reported that TNFα is elevated in adipose tissue of obese subjects, while genetic ablation of TNFα can restore insulin sensitivity [1,6,8]. The contribution of adipose tissue to circulating levels of TNFα is a matter of debate. However, there is consensus that adipose tissue-derived TNFα can impact whole-body insulin sensitivity by impairing adipose tissue function through localized insulin resistance as well as through TNFα-mediated effects on proinflammatory gene expression [1,2].
The mitogen-activated protein kinases (MAPKs) play a critical role in linking metabolic effects of TNFα to the development of adipose insulin resistance and inflammation. The MAPKs are a family of serine/threonine kinases that include extracellular signal-regulated protein kinase (ERK), c-Jun N-terminal kinase (JNK), and p38. All three MAPKs are activated in adipose tissue in response to TNFα stimulation and serve as mediators of localized insulin resistance through transcriptional mechanisms involving adipocyte gene expression as well as the expression and secretion of other proinflammatory molecules [2,9–12]. In addition, MAPK activity has also been linked to cytosolic suppression of proximal insulin signaling through JNK phosphorylation of specific serine residues of insulin receptor substrate-1 (IRS-1) [12–14].
MAPKs are activated when both threonine and tyrosine residues are phosphorylated within the T-X-Y motif of the activation loop [10,15]. Upstream MAPK kinases (MAP2Ks) have historically been considered the principal regulators of MAPK activity. However, recent literature has shown that phosphatases act as pivotal regulators of many MAPK-dependent biological processes [16,17]. Dual specificity phosphatases (DUSPs) are a subclass of protein tyrosine phosphatases that specifically inhibit MAPK activity through dephosphorylation of ‘both’ threonine and tyrosine residues. There are ten known DUSPs that are MAPK-specific; these contain a MAP kinase-binding (MKB) domain that selectively imparts catalytic activity toward ERK, JNK, or p38 [17,18]. These MAPK-specific DUSPs are further classified based on gene structure, substrate specificity, and subcellular localization into three groups: 1) nuclear DUSPs (group I), 2) cytosolic ERK-specific DUSPs (group II) and 3) DUSPs that selectively inactivate the stress-activated MAPKs (group III). DUSPs are highly inducible at the level of gene expression in a cell-type and stimulus-dependent manner [17–20].
In this report, we examined the impact of DUSPs on MAPK dephosphorylation during TNFα-mediated inflammatory stress using 3T3-L1 murine adipocytes. This cell line afforded the opportunity to examine nearly homogenous populations of preadipocytes and adipocytes without macrophage contamination. This study showed that coordinated loss of DUSPs exacerbated MAPK phosphorylation and proinflammatory gene expression in adipocytes. Future studies investigating the in vivo physiological function of these DUSPs is likely to highlight potential therapeutic targets that control MAPK signaling and MAPK-dependent adipose tissue inflammation.
2. Materials and methods
2.1. Materials
Dulbecco’s Modified Eagle’s Medium (DMEM), calf bovine serum (CS), and trypsin-EDTA were purchased from Invitrogen. Fetal bovine serum (FBS) was obtained from HyClone. The following antibodies were used for western blot analysis: phospho-ERK, phosho-p38, phosphor-JNK, total JNK, total p38, α-Tubulin, and GAPDH (Cell Signaling). Pharmacological inhibitors of ERK (U0126), JNK (SP600125 and JNK-VIII), p38 (SB203580) were purchased from CalBiochem. Enhanced chemiluminescence (ECL) reagents were obtained from Perkin-Elmer Life Sciences. All Taqman primer probes used in this study (Supplementary Table S1) were purchased from Applied Biosystems. The murine 3 T3-L1 cell line was purchased from Howard Green, Harvard Medical School [21].
2.2. Cell culture
3T3-L1 preadipocytes were propagated in DMEM supplemented with 10% CS until density-induced growth arrest, as previously described [22]. At 2 days post-confluence, growth medium was replaced with DMEM supplemented with 10% FBS, 0.5 mM 1-methyl-3-isobutylxanthine, 1 μM dexamethasone, and 1.7 μM insulin (MDI). Throughout the study, ‘time 0’ refers to density arrested cells immediately before the addition of MDI to the culture medium. Experiments described herein were conducted in density-arrested preadipocytes (d0) and mature adipocytes (d8) stimulated in parallel with 100 pM TNFα. All experiments were repeated at least 3 times to validate results and ensure reliability.
2.3. Immunoblotting
Cell monolayers were washed with phosphate-buffered saline (PBS) and scraped into ice-cold lysis buffer containing 1.0 M Tris, pH 7.4, 150 mM NaCl, 1% Triton X, 0.5% Nonidet P-40 (NP40), 1 mM EDTA, 1 mM EGTA, and 10 mM N-ethylmaleimide. Phosphatase (20 mM β-glycerophosphate, 10 mM NaFl, and 2 μM sodium vanadate), as well as protease (0.3 μM aprotonin, 21 μM leupeptin, 1 μM pepstatin, 50 μM phenanthroline, and 0.5 μM phenylmethylsulfonyl fluoride) inhibitors were added to lysis buffer prior to cell harvest. Cell lysates were sonicated, centrifuged (13,000 ×g, 10 min, 4 °C), and the supernatant transferred to a fresh tube. Bicinchoninic acid assay (Pierce, Rockford, IL) was used to determine protein concentration. Cell lysates were resuspended in loading buffer containing 0.25 M Tris, pH 6.8, 4% sodium dodecyl sulfate (SDS), 10% glycerol, 10% dithiothreitol, and 0.01% bromophenol blue and heated for 5 min at 80 °C. Proteins were resolved on SDS-polyacrylamide gel electrophoresis gels (PAGE) and transferred to polyvinylidene fluoride membranes (Millipore Corp., Billerica, MA). After transfer, membranes were blocked with 4% milk and probed with indicated primary antibodies overnight at 4 °C. Membranes were subsequently probed with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Immunoblots were immersed in ECL and visualized by autoradiography using CL-XPosure film (Pierce).
2.4. Real-Time qRT-PCR
Total RNA was extracted and genomic DNA contamination was removed using the RNeasy Plus Mini Kit (Qiagen) following the manufacturer’s protocol. Total RNA was quantified with a Nanodrop ND-1000 spectrophotometer and reverse-transcribed to cDNA in a 10 μl reaction volume using a high capacity cDNA reverse transcription kit (Applied Biosystems). The reverse transcription (RT) master mix containing RT buffer, deoxyribonucleotide triphosphate (dNTP) mix, RT random primers, RNase inhibitor (1.0 U/μl), and MultiScribe RT was added to 1 μg RNA and RNase-free water. Reverse transcription reaction conditions followed the protocol (25 °C for 10 min, 37 °C for 120 min, 85 °C for 5 s, followed by 4 °C indefinitely/RT complete) and utilized the Gene Amp PCR System 9700 thermal cycler (Applied Biosystems) for cDNA synthesis.
PCR amplification was run utilizing the 7500 fast system (Applied Biosystems) that consisted of enzyme activation at 95 °C for 20 s, followed by 40 cycles of denaturation at 95 °C for 3 s combined with annealing/extension at 60 °C for 30 s. All data were analyzed with the ABI 7500 real time PCR system (Applied Biosystems). Data were recorded and analyzed with Sequence Detector Software (Applied Biosystems) and graphs visualized with SigmaPlot software. All data were presented as mean ± standard error of the mean (SEM) and representative of at least two experiments performed in duplicate. Data were normalized to 18S previously validated by this lab as a suitable reference gene under these experimental conditions [23]. Relative differences between treated and untreated control samples were analyzed by the method as previously described [23,24].
2.5. Statistical analyses were conducted using SPSS v18
Phenotypic differences were determined via student’s t-test where a p-value of < 0.05 was considered significant. Knockdown data were analyzed using ANOVA, with Tukey’s post-hoc analysis used when the p value for the respective parameter was statistically significant (p < 0.05). Inhibitor data were analyzed using analysis of variance, with Dunnett’s post-hoc analysis conducted to assess differences from controls (TNFα) when p < 0.05.
2.6. RNA interference
SMARTpools containing four different short interfering RNAs (siRNAs) for Dusp1, Dusp8, and Dusp16 specific sequences as well as non-targeting sequences were transfected using DharmaFect 3 transfection reagent according to manufacturer’s (Dharmacon) protocol. Briefly, 3 T3-L1 preadipocytes were propagated in 6-well culture dishes until reaching density-induced growth arrest. Growth medium was then replaced with DMEM supplemented with 10% CS, 3 μl DharmaFect 3 reagent and either 100 nM Dusp1, Dusp8, and/or Dusp16 specific siRNA or non-targeting siRNA for 72 h. Growth medium was subsequently switched to differentiation medium containing MDI as described above.
3. Results
3.1. TNFα-induced changes in MAPK phosphorylation magnitude and duration in 3T3-L1 adipocytes
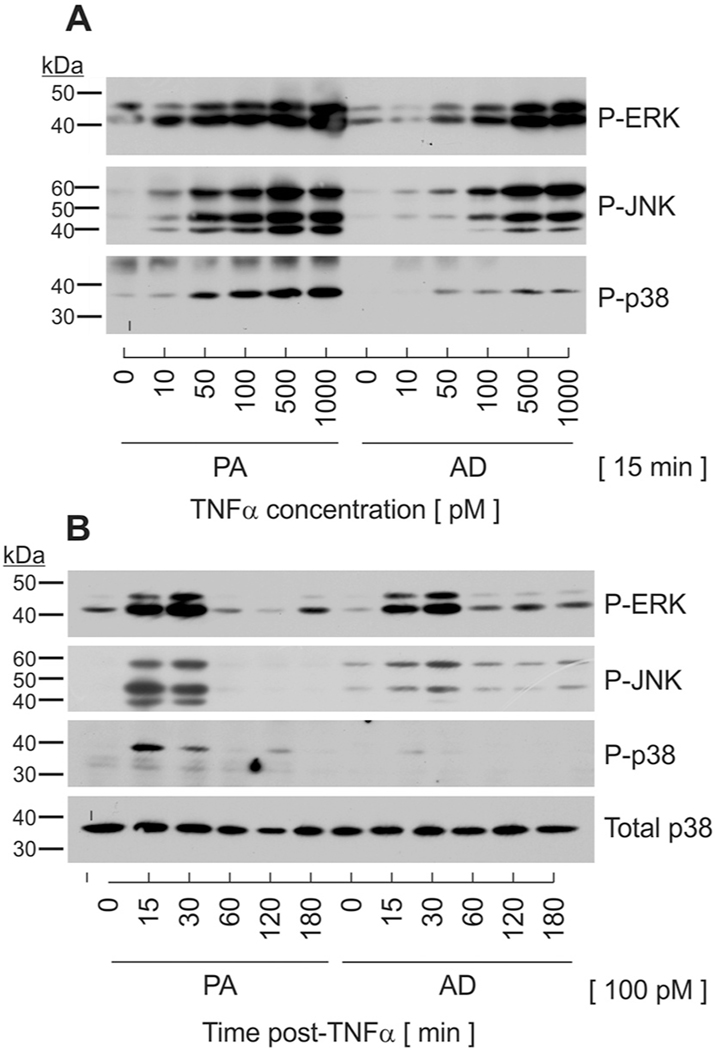
The objective of this investigation was to determine if MAPK-specific phosphatases play a role in modulating the biological outcome of TNFα activity in cells of adipocyte lineage. Preadipocytes and adipocytes serve unique roles during obesity-induced inflammation. As such, we began these studies by examining MAPK phosphorylation in each cell type following TNFα stimulation. For these studies, we used the 3T3-L1 murine adipocyte cell line that yielded quiescent preadipocytes prior to differentiation and > 90% conversion to mature adipocytes following differentiation. Cells were differentiated by established protocol for 8 days to yield functionally mature adipocytes and compared to undifferentiated, density-arrested preadipocytes of similar passage. Preadipocytes and adipocytes were stimulated concurrently with increasing concentrations of murine TNFα. Total cell lysates were harvested at 15 mins post-stimulation and immunoblotted with antibodies that recognize dual phosphorylation of p44-ERK1(T202/Y204), p42-Erk2(T185/Y187), p54-Jnk1(T183-Y185), p46-JNK2(T183/Y185) or p38(T180/Y182) on residues that are known to be both essential and sufficient for catalytic activity [10]. The magnitude of phosphospecific protein accumulation representing all three MAPKs increased in a concentration-dependent fashion in both preadipocytes and adipocytes, with an estimated ED50 of approximately 100 pM (Fig. 1A). This concentration was selected for further analyses as it approximates the concentration of TNFα in human and animal models of obesity and has been shown to suppress insulin signaling in culture [3,6]. Of note, the magnitude of MAPK phosphorylation was more robust in preadipocytes relative to adipocytes for any given concentration. This was especially noted for p38.
Fig. 1.
Changes in magnitude and duration of MAPK phosphorylation in response to TNFα stimulation. (A) Undifferentiated preadipocytes (PA) and mature adipocytes (AD) were stimulated in parallel with increasing concentrations of TNFα. Cell lysates were harvested at 15 min post-TNFα stimulation and immunoblotted on the same gel for phosphospecific P-ERK, P-JNK, and P-p38. (B) Preadipocytes and adipocytes were stimulated in parallel with TNFα (100 pM). Cell lysates were harvested over time following TNFα stimulation and immunoblotted for phosphospecific P-ERK, P-JNK, and P-p38 as well as total p38.
To compare differences in MAPK signaling duration, preadipocytes and adipocytes were stimulated with 100 pM TNFα in parallel and lysates harvested over time. TNFα stimulation resulted in a rapid, but transient activation of all three MAPKs in preadipocytes where robust phosphorylation was observed at 15 mins and complete dephosphorylation by 60 mins post-TNFα (Fig. 1B). Conversely, the same concentration of TNFα produced markedly lower magnitude, but sustained duration of JNK and ERK with minimal phosphorylation observed for p38 in mature adipocytes. The abundance of total p38 protein was not affected by TNFα. Thus, observed changes in MAPK phosphorylation likely reflect a balance of kinase/phosphatase mechanisms that were regulated in a phenotype-specific manner.
3.2. De novo mRNA synthesis regulated MAPK dephosphorylation
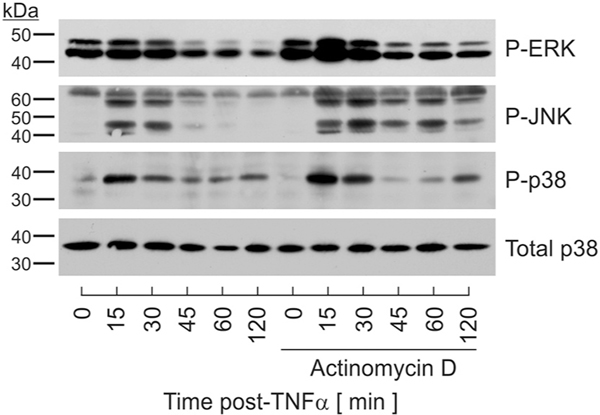
Data presented above demonstrated that TNFα stimulation resulted in robust and transient phosphorylation of all three MAPKs in a cell type-specific manner. As each kinase was rapidly dephosphorylated in the continued presence of TNFα, these data support the premise that MAPK phosphatases modulate magnitude and duration of MAPK activity. Many MAPK phosphatases are regulated by rapid changes in gene expression [17]. Therefore, we postulated that inducible DUSP expression regulated MAPK dephosphorylation in response to TNFα. To test this hypothesis, we initially determined if magnitude and/or duration of MAPK signaling were dependent on de novo mRNA synthesis. These determinations were performed in preadipocytes as all three MAPKs were robustly and transiently phosphorylated and dephosphorylated in this cell type. With this reasoning, preadipocytes were pretreated with and without actinomycin D for 30 mins at a concentration (1 ng/ml) previously shown to effectively block RNA synthesis [25,26] prior to TNFα stimulation. Cell lysates were harvested over time following 100 pM TNFα stimulation. Preventing de novo mRNA synthesis resulted in increased ERK and JNK phosphorylation extending from < 60 mins in the absence of actinomycin D to > 2 h when RNA synthesis was inhibited. While the magnitude of p38 phosphorylation was increased in the presence of actinomycin D, no effect on duration was observed (Fig. 2). These effects were not attributed to generalized stress as actinomycin D treatment for > 4 h in the absence of TNFα produced no increase in MAPK phosphorylation (data not shown).
Fig. 2.
Role for de novo mRNA synthesis in MAPK dephosphorylation. Preadipocytes were stimulated in the presence or absence of actinomcyin D (1 ng/ml) for 30 mins prior to stimulation with TNFα (100 pM). Cell lysates were collected over time and immunoblotted for phosphospecific P-ERK, P-JNK, P-p38, and total p38.
3.3. Phenotype-specific induction of DUSP gene expression following TNFα stimulation
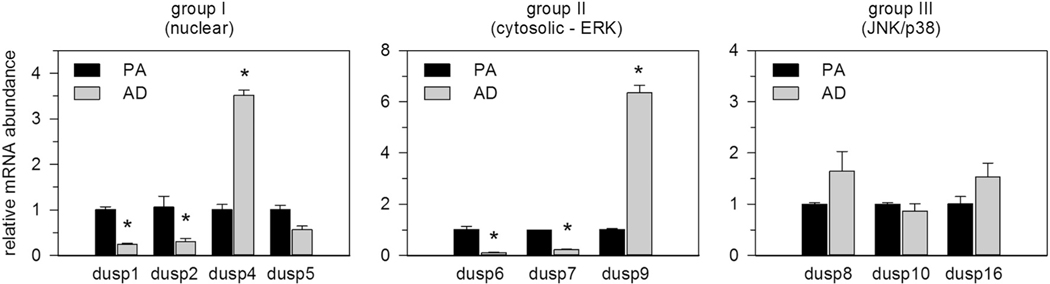
As the extent of MAPK phosphorylation was dependent on de novo mRNA synthesis, we tested our hypothesis that inducible DUSPs modulate MAPK activity following TNFα stimulation. For this, we initially screened unstimulated preadipocytes for mRNA abundance of all known DUSPs that contain MKB domains responsible for selective catalytic activity only toward MAPKs. All ten DUSPs were easily measured by qRT-PCR within the detectable limits of 36 threshold cycles (CT) with Dusp1, dusp6, dusp7 and dusp10 most abundantly and Dusp2, dusp8 and dusp9 least abundantly expressed (Table 1). This screen was extended to compare differences in basal DUSP expression between unstimulated preadipocytes and adipocytes. DUSPs were grouped based on gene structure, substrate specificity, and subcellular localization as group I (nuclear), group II (cytosolic, ERK-specific), and group III (JNK/p38-specific) phosphatases [17,19,20]. Relative mRNA abundance of Dusp1, dusp2, dusp6, and dusp7 was significantly elevated in preadipocytes compared to adipocytes whereas dusp4 and dusp9 were significantly elevated in adipocytes compared to preadipocytes. Dusp5, Dusp8, Dusp10, and Dusp16 showed no marked differences in basal expression between these cell types (Fig. 3).
Table 1.
DUSP and inflammatory genes analyzed in this study.
| Symbol | Name/alias | Accession | AB number | CT | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| GP | MKB | NLS | NES | EJP | ||||||
|
| ||||||||||
| Dual specificity phosphatases | Dusp1 | MKP-1 | I | ● | ● | NM_013642 | Mm00457274_g1 | 24 | ||
| Dusp2 | PAC-1 | I | ● | ● | NM_010090 | Mm00839675_g1 | 33 | |||
| Dusp4 | MKP-2 | I | ● | ● | NM_176933 | Mm00723761_m1 | 28 | |||
| Dusp5 | hVH3 | I | ● | ● | NM_001085390 | Mm01266104_m1 | 27 | |||
| Dusp6 | MKP-3 | II | ● | ● | E | NM_026268 | Mm00650255_g1 | 24 | ||
| Dusp7 | MKP-X | II | ● | ● | E | NM_153459 | Mm00463228_m1 | 25 | ||
| Dusp9 | MKP-4 | II | ● | ● | E | NM_029352 | Mm00512646_m1 | 31 | ||
| Dusp8 | M3/6 | III | ● | ● | ● | J/P | NM_008748 | Mm00456230_m1 | 32 | |
| Dusp10 | MKP-5 | III | ● | J/P | NM_022019 | Mm00517678_m1 | 24 | |||
| Dusp16 | MKP-7 | III | ● | ● | ● | J/P | NM_130447 | Mm00459935_m1 | 26 | |
| Inflammatory genes | IL-6 | interleukin-6 | NM_031168 | Mm99999064_m1 | 29 | |||||
| Cox-2 | cyclooxygenase-2 | NM_011198 | Mm00478372_m1 | 28 | ||||||
| TNFα | tumor necrosis factor-alpha | NM_013693 | Mm99999068_m1 | 35 | ||||||
| CCL2 | monocyte chemoattractant protein-1 (MCP-1) | NM_011333 | Mm00441242_m1 | 21 | ||||||
| CCL3 | macrophage inflammatory protein (MIP-1α) | NM_011337 | Mm99999057_m1 | 36 | ||||||
| CCL4 | MIP-1β | NM_013652 | Mm00443111_m1 | 35 | ||||||
| CCL5 | Rantes | NM_013653 | Mm01302428_m1 | 29 | ||||||
| CCL9 | MIP-1γ | NM_011338 | Mm00441260_m1 | 24 | ||||||
| CCL12 | MCP-5 | NM_011331 | Mm01617100_m1 | 34 | ||||||
| CCL20 | MIP-3A | NM_106960 | Mm00444228_m1 | 32 | ||||||
| Reference gene | 18S | 18 ribosomal RNA | X03205 | 4342930E | 9 | |||||
DUSP group (GP), MAPK binding domain (MKB), nuclear localization sequence (NLS), nuclear export sequence (NES), ERK (E), JNK (J), p38 (P), threshold cycle (CT) measured in unstimulated preadipocytes, Applied Biosystems (AB).
Fig. 3.
Cell type differences in DUSP mRNA expression. Total RNA was harvested from unstimulated preadipocytes and adipocytes. DUSP mRNA expression was measured by qRT-PCR. DUSPs were grouped as: nuclear (group I), cytosolic ERK-specific (group II), and DUSPs that selectively dephosphorylate stress activated MAPKs (group III). Data were normalized to 18S rRNA and expressed relative to untreated preadipocytes. Statistical significance was determined by Student’s t-test (* p < 0.05).
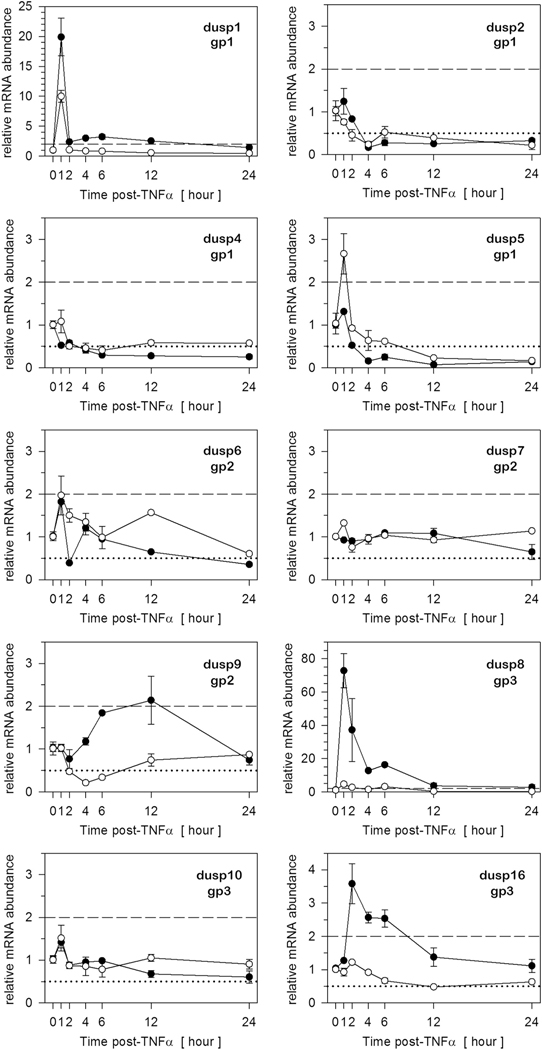
To determine which DUSPs were induced at the level of gene expression during inflammatory stress, preadipocytes and adipocytes were stimulated in parallel with 100 pM TNFα and relative mRNA abundance assessed by qRT-PCR over time. Prior to these studies, we set an arbitrary threshold of 2-fold change as a conservative measure of biological differences [27]. Thus, DUSP gene expression was only considered inducible when relative mRNA levels exceeded this threshold (dashed line). Determinations were examined over time to establish when specific DUSPs were induced as well as whether the induction was transient versus sustained for insight to functional roles. Dusp1 was the only group I phosphatase induced in preadipocytes with a 20-fold transient increase in relative gene expression peaking at 1 h post-TNFα, consistent with the rapid dephosphorylation of MAPK in Fig. 4. In addition, Dusp8 and Dusp16 (group III) were rapidly induced in preadipocytes, but in contrast to Dusp1, mRNA levels remained elevated ≥4 h following TNFα stimulation suggesting regulation of MAPK duration. Parallel determinations in adipocytes revealed that Dusp1, Dusp5, and Dusp8 were also induced within 2 h post-TNFα stimulation. However, while Dusp8 met our criterion for induction in both cellular phenotypes, the magnitude of induction was dramatically greater in preadipocytes versus adipocytes. No member of group II phosphatases was induced following TNFα stimulation. While there were similarities DUSP induction between cell types, there were also differences such as the induction of dusp5 in adipocytes, but not preadipocytes and the induction of Dusp16 in preadipocytes, but not adipocytes (Fig. 4).
Fig. 4.
Several DUSPs are induced in preadipocytes and adipocytes in response to TNFα. Total RNA was harvested over time from preadipocytes (closed circles) and adipocytes (open circles) stimulated concurrently with TNFα (100 pM). DUSP mRNA expression was assessed by qRT-PCR. DUSPs were grouped as: nuclear (gp1), cytosolic ERK-specific (gp2), and DUSPs that selectively dephosphorylate stress activated MAPKs (gp3). All data were normalized to 18S rRNA and expressed relative to untreated cells. Genes were selected as ‘inducible’ when upregulated above a 2-fold criterion indicated by the dashed line, while genes were considered suppressed that fell below a 0.5 criterion indicated by the dotted line on the graph.
While this study focused on inducible DUSPs during inflammatory stress, it should be noted that select DUSPs were suppressed following TNFα stimulation. Using a criterion of 0.5 fold changes (i.e., decrease) in mRNA abundance as an arbitrary threshold for gene suppression (dotted line), mRNA levels for Dusp2 and dusp4 of group I phosphatases were suppressed in both cell types where the degree of suppression was sustained for > 24 h. Dusp9 (group II) was also suppressed, but transiently and only in adipocytes, not preadipocytes (Fig. 4).
3.4. DUSPs regulate MAPK dephosphorylation in response to TNFα
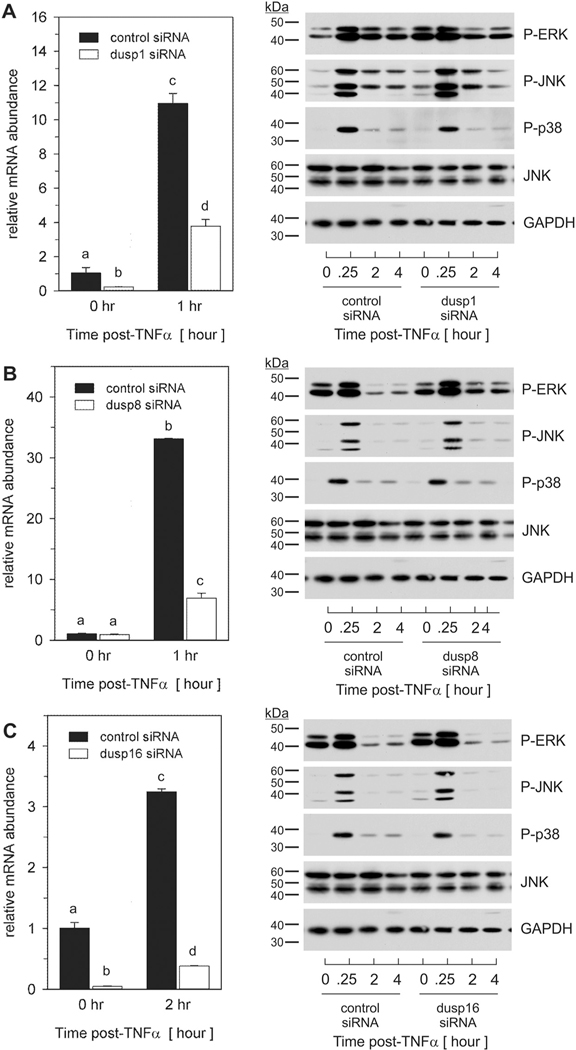
Data presented above demonstrated that select DUSPs were induced in a manner consistent with divergent regulatory roles in modulating the extent of MAPK phosphorylation in a cellular phenotype-specific manner. As Dusp1, Dusp8, and Dusp16 were induced in preadipocytes following transient phosphorylation of all three MAPKs, we focused the remainder of this study toward understanding the mechanistic role and regulation of these DUSPs in preadipocytes only. To address this question, preadipocytes were transfected with short interfering RNA (siRNA) individually targeted to each DUSP as well as non-targeting control siRNA for 72 h prior to stimulation with 100 pM TNFα. Total RNA and whole cell lysates were harvested over time and analyzed for relative mRNA abundance and phosphorylated MAPK protein, respectively. Targeted siRNA significantly inhibited TNFα-induced (A) Dusp1, (B) Dusp8, and (C) Dusp16 (Fig. 5). Suppression of Dusp1 mRNA increased basal levels of phosphorylated ERK and JNK as well as the magnitude of TNFα-mediated JNK phosphorylation through 1 h post-stimulation (Fig. 5A; right panel). While Dusp8 siRNA had no discernable effect on basal phosphorylation, targeted knockdown increased the duration of ERK and JNK through 4 h post-TNFα stimulation (Fig. 5B; right panel). Dusp16 siRNA yielded only a modest increase in magnitude of ERK and JNK (Fig. 5C; right panel). No discernable effects were noted regarding p38 phosphorylation for Dusp1, Dusp8, or Dusp16.
Fig. 5.
Dusp1, Dusp8 and Dusp16 regulate MAPK signaling in preadipocytes in response to TNFα. Preadipocytes were transfected with non-targeting control siRNA or siRNA specific for (A) Dusp1, (B) Dusp8, or (C) Dusp16 for 72 h prior to TNFα (100 pM) stimulation. Total RNA was harvested at the indicated times post-TNFα stimulation and qRT-PCR used to examine mRNA expression of each DUSP (left-panels). Data were normalized to 18S rRNA and changes in gene expression measured as fold differences relative to unstimulated control siRNA. Statistical differences were determined by ANOVA, with Tukey’s post-hoc analysis performed when the p value for the respective parameter was statistically significant (p < 0.05). Cell lysates were collected over time post-TNFα stimulation and immunoblotted for phospho-ERK, JNK, and p38 along with total JNK and GAPDH (right panels).
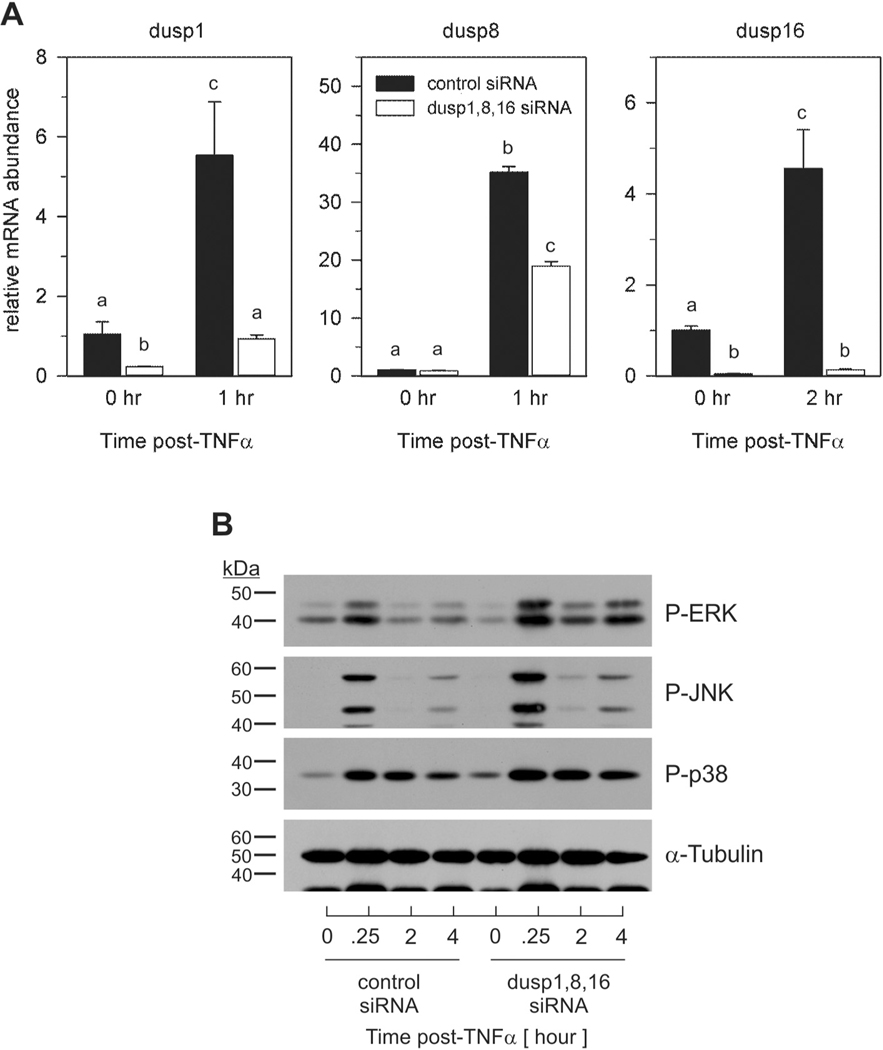
Individual knockdown demonstrated a role for DUSPs regarding MAPK signaling. As such, we further investigated the cooperative action of inducible DUSPs on MAPK dephosphorylation. For this, preadipocytes were transfected with pools of siRNAs for each DUSP prior to TNFα stimulation. Pooled siRNA for Dusp1, Dusp8 and Dusp16 significantly inhibited TNFα-induced DUSP mRNA expression similar to that shown above for individual DUSP siRNAs (Fig. 6A). Combined targeted knockdown of all three phosphatases resulted in an increase in ERK, JNK, and p38 phosphorylation when compared to control (Fig. 6B). These data demonstrate synergistic activity between phosphatases in the regulation of MAPK signaling. It should be noted that pooled siRNA had an additive effect on both magnitude and duration of all three MAPKs including p38 relative to that observed when each DUSP was targeted individually.
Fig. 6.
Combination DUSP knockdown exacerbates MAPK signaling magnitude and duration in preadipocytes in response to TNFα. Preadipocytes were transfected for 72 h with non-targeting control siRNA or pooled siRNAs for Dusp1, Dusp8, and Dusp16 prior to TNFα (100 pM) stimulation. (A) Total RNA was harvested at the indicated times post-TNFα stimulation and qRT-PCR used to assess mRNA expression. Data were normalized to 18S rRNA and changes in gene expression measured as fold differences relative to unstimulated control siRNA. Significant differences were determined by ANOVA with Tukey’s post-hoc analysis performed when the p value for the respective parameter was statistically significant (p < 0.05). (B) Cell lysates were collected over time post-TNFα (100 pM) stimulation and immunoblotted for phospho-ERK, JNK, and p38 as well as α-Tubulin.
3.5. DUSPs modulate proinflammatory gene expression in response to TNFα
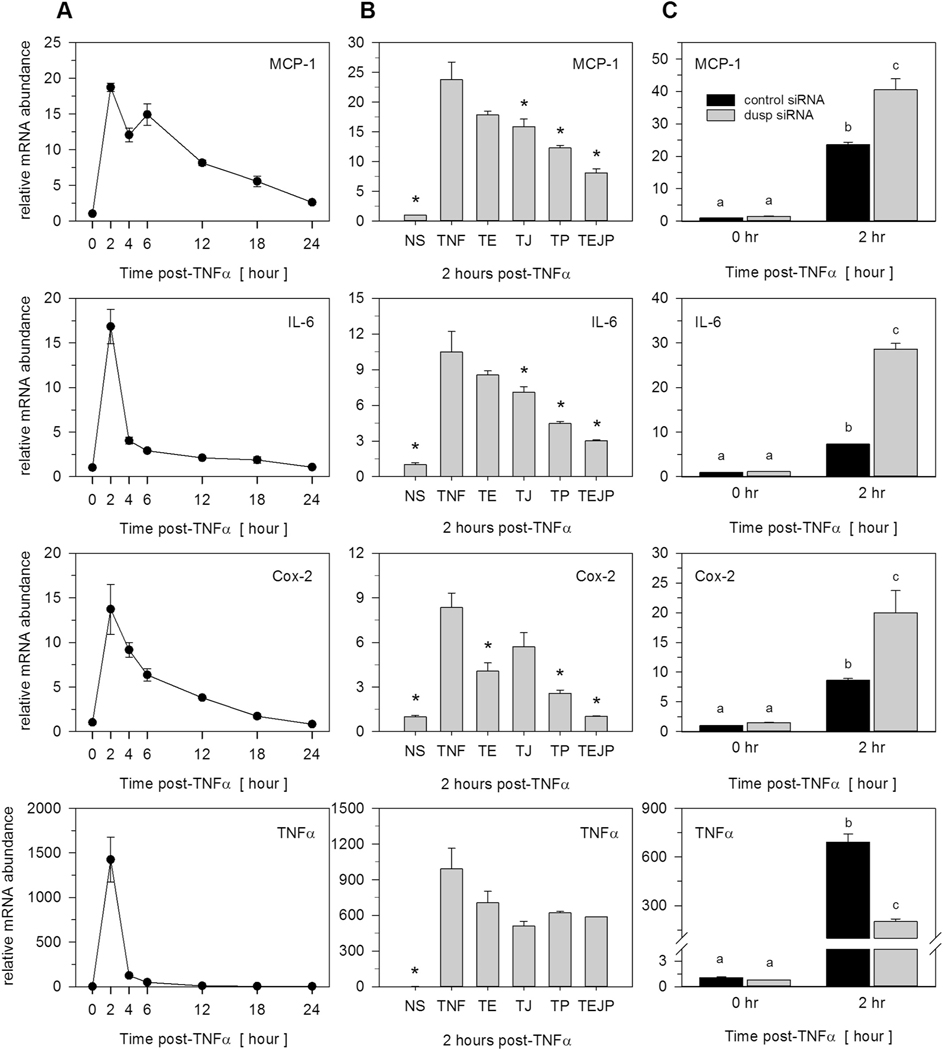
Data above demonstrated that specific inducible DUSPs regulate MAPK dephosphorylation in response to TNFα. As such, we postulated that DUSPs regulate the extent of TNFα-induced inflammatory gene expression. To test this, we initially examined changes in mRNA abundance over time following TNFα stimulation of four diverse, proinflammatory genes well known to be expressed in preadipocytes under conditions of inflammatory stress. TNFα stimulation resulted in rapid and transient changes in MCP-1, IL-6, Cox-2 and TNFα gene expression in density-arrested preadipocytes (Fig. 7A). To determine a role for MAPK activity regarding these changes in gene expression, preadipocytes were pretreated with U0126 (10 μM), SP600125 (20 μM), and SB203850 (10 μM), representing potent and selective inhibitors of ERK, JNK, and p38, respectively, for 1 h prior to stimulation with TNFα. Total RNA was harvested after 2 h post-TNFα stimulation corresponding to the time of peak induction for all four proinflammatory genes. TNFα induction of MCP-1, IL-6, and Cox-2, but not TNFα, was significantly suppressed by individual MAPK inhibitors; an effect that was amplified when all inhibitors were used in combination (Fig. 7B). Cell lysates were also immunoblotted under these conditions to confirm the efficacy of U0126 eradication of TNFα induced ERK phosphorylation (Supplementary Fig. S1). As SP600125 and SB203850 target catalytic activity and not phosphorylation state per se, efficacies for these inhibitors were judged by the effect on target gene expression (Fig. 7B).
Fig. 7.
TNFα-induced MAPK-dependent and MAPK-independent proinflammatory gene expression. (A) Total RNA was harvested over time from preadipocytes stimulated with 100 pM TNFα. Proinflammatory gene expression of MCP-1, IL-6, Cox-2, and TNFα was examined by qRT-PCR. Genes were selected as ‘inducible’ when upregulated above a 2-fold criterion. (B) Preadipocytes were pretreated (1 h) in the presence of inhibitors for ERK (U0126, 10 μM), JNK (SP600125, 20 μM), or p38 (SB203850, 10 μM) prior to TNFα stimulation. Total RNA was harvested 2 h post-TNFα stimulation and mRNA expression assessed by qRT-PCR. Nomenclature assigned as: T (TNFα), T + E (TNFα+ERK inhibitor), T + J (TNFα+JNK inhibitor), T + P (TNFα+p38 inhibitor), or TEJP (TNFα+ERK + JNK + p38 inhibitors). Data were expressed relative to unstimulated cells and normalized to the 18S rRNA. Statistical significance was determined by ANOVA with Dunnett’s post-hoc analysis conducted to assess differences from TNFα when p < 0.05. (C) Preadipocytes were transfected for 72 h with non-targeting control siRNA or combined pools of siRNAs for Dusp1, Dusp8, and Dusp16 prior to TNFα (100 pM) stimulation. Total RNA was collected at 0 h or 2 h post-TNFα stimulation and qRT-PCR used to examine MCP-1, IL-6, Cox-2, and TNFα. Significant differences were determined by ANOVA with Tukey’s post-hoc analysis performed when the p value for the respective parameter was statistically significant (p < 0.05).
To determine a role for DUSPs on MAPK-dependent changes in gene expression, preadipocytes were transfected with a combination of Dusp1, Dusp8, and Dusp16 siRNAs prior to TNFα stimulation. Combination knockdown of these DUSPs significantly elevated TNFα-induction of each proinflammatory gene that was shown above to be downstream of MAPK activity (Fig. 7C). In contrast, TNFα-mediated induction in TNFα gene expression was significantly suppressed following DUSP knockdown (Fig. 7C). While TNFα gene expression was marginally suppressed by MAPK inhibitors, the effects were not significant, suggesting that other non-MAPK signaling pathways (e.g., NFkB) played dominant roles in regulating the expression of this proinflammatory gene.
To confirm these effects on biological outcome, we further screened adipocytes for genes specifically in the chemokine family that were induced with TNFα and dependent on MAPK signaling. In a manner consistent with MCP-1 (CCL2) expression, this screen revealed that CCL3, CCL4, CCL5, CCL9, CCL12 and CCL20 were also detectable in unstimulated preadipocytes (Table 1), although phenotypic differences were noted between preadipocytes and adipocytes (Supplementary Fig. S2A). Chemokine mRNA abundance dramatically and rapidly increased following TNFα stimulation (Supplementary Fig. S2 B). While these chemokines fell equally into two groups based on kinetic profiles (i.e., rapid vs prolonged), the expression of all six genes was elevated within 2 h of stimulation. For all six genes, pretreatment with MAPK inhibitors significantly suppressed TNFα stimulation (Supplementary Fig. S3). As with other MAPK-dependent genes discussed above, combination knockdown of Dusp1, Dusp8, and Dusp16 significantly enhanced chemokine gene expression (Supplementary Fig. S4).
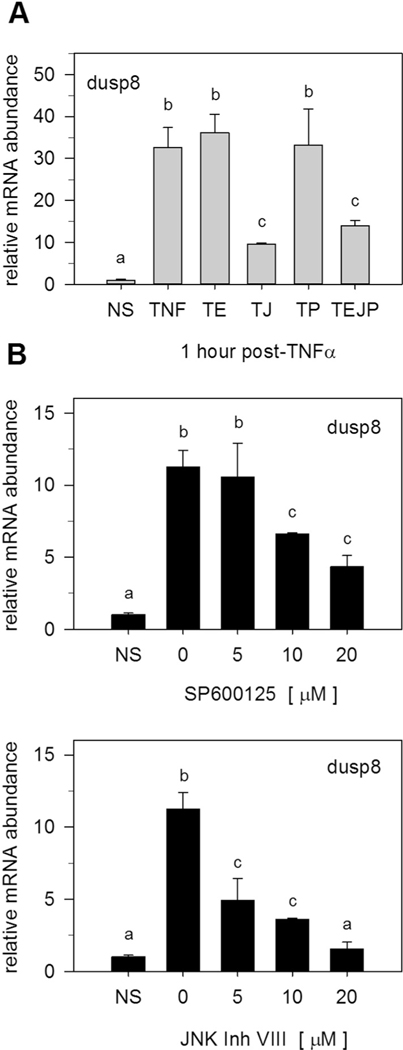
3.6. JNK activity is required for TNFα-mediated DUSP8 gene expression
Canonical roles for DUSPs entails MAPK-mediated induction of phosphatase gene expression that feedback inhibits MAPK activity [28,29]. To determine if MAPKs regulate TNFα-induced DUSP gene expression, preadipocytes were pretreated with pharmacological inhibitors for MAPK activity prior to TNFα stimulation. Inhibition of MAPK signaling had no discernable effect on Dusp1 or Dusp16 gene expression (Supplementary Fig. S5). However, suppression of JNK activity inhibited TNFα-induced changes in Dusp8 gene expression (Fig. 8A). These effects were confirmed by examining Dusp8 gene expression in response to two structurally independent inhibitors of JNK activity (SP600125 and JNK Inhibitor VII). Both inhibitors suppressed TNFα-mediated induction of Dusp8 gene expression in a similar, concentration-dependent manner (Fig. 8B). Collectively, these data demonstrate a regulatory feedback loop involving Dusp8 in the regulation of MAPK dephosphorylation. Signaling pathways coupling Dusp1 and Dusp16 with TNFα activity are still under investigation.
Fig. 8.
JNK activation is essential for Dusp8 gene expression. A) Preadipocytes were pretreated (1 h) in the presence of inhibitors for ERK (U0126, 10 μM), JNK (SP600125, 20 μM), or p38 (SB203850, 10 μM) prior to TNFα (100 pM) stimulation. Total RNA was harvested 1 h post-TNFα. Dusp1, Dusp8, and Dusp16 mRNA expression was examined by qRT-PCR. Nomenclature assigned as: T (TNFα), T + E (TNFα + ERK), T + J (TNFα + JNK), T + P (TNFα + p38), or TEJP (TNFα +ERK + JNK + p38). (B) Preadipocytes were pretreated (1 h) with increasing doses of two independent JNK inhibitors (SP600125 or JNK inhibitor VIII) prior to 100 pM TNFα stimulation (1 h treatment). Total RNA was harvested and qRT-PCR used to assess Dusp8 mRNA expression. All data were expressed relative to unstimulated cells (NS) and normalized to 18S rRNA. Statistical significance was determined by ANOVA with Dunnett’s post-hoc analysis conducted to assess differences from TNFα when p < 0.05.
4. Discussion
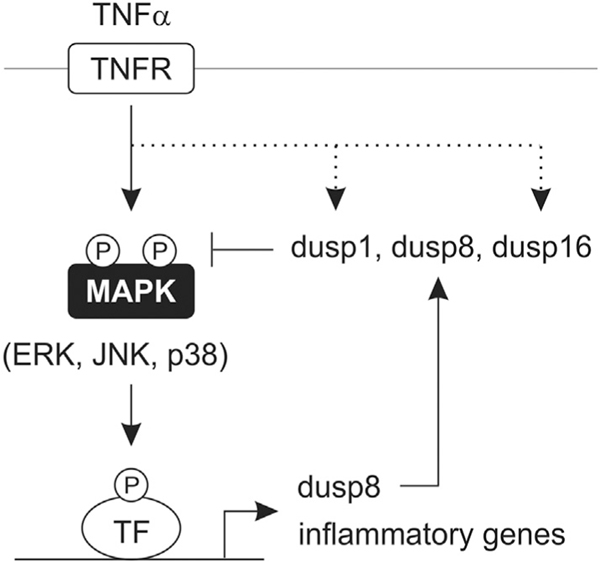
The findings of this study define DUSPs as negative regulators of preadipocyte inflammatory gene expression that function by modulating MAPK dephosphorylation. We propose a model in which JNK-dependent and MAPK-independent induction of Dusp8 and Dusp1/16, respectively, regulate the extent of ERK, JNK, and p38 signaling that links TNFα to inflammatory gene expression (Fig. 9). Collectively, these findings establish a previously unreported role for these three DUSPs in the control of preadipocyte MAPK signaling and inflammatory gene expression.
Fig. 9.
Working model for a role of DUSPs in adipocytes during inflammatory stress. Working model illustrating a role for Dusp1, Dusp8 and Dusp16 in modulating the magnitude and duration of MAPK signaling in response to TNFα stimulation in adipocytes. While Dusp8 was shown to be downstream of MAPK signaling, Dusp1 and Dusp16 were induced by MAPK-independent mechanisms (dotted line).
The etiology of most obesity-related comorbidities involves an adipose tissue-derived inflammatory response where preadipocytes, adipocytes, and macrophages produce and secrete numerous proinflammatory molecules in response to metabolic stress [30–32]. While both preadipocytes and adipocytes play critical roles in the initiation of adipose tissue inflammation, some reports have suggested that preadipocytes are generally more inflammatory than adipocytes [30,33]. Transcriptional profiling studies have further demonstrated that macrophages and preadipocytes are genetically related [34] and that preadipocytes may, in part, originate from adipose tissue macrophages [35]. Consistent with this premise, preadipocytes share many traits with macrophages such as phagocytosis and expression of membrane-bound NADPH oxidases and have been shown to transdifferentiate into macrophage-like cells [36]. We report here that the magnitude of ERK, JNK and p38 dual phosphorylation on residues that are necessary and essential for signaling activity was greater in preadipocytes than adipocytes when cells were stimulated concurrently with TNFα, suggesting that preadipocytes were more responsive to inflammatory stimuli.
Molecular mechanisms that underlie adipose tissue inflammation during the onset of obesity include the expression and release of various chemokines (i.e., MCP-1) and cytokines (i.e., TNFα and IL-6) that promote infiltration of macrophages and insulin resistance [1,3,4]. Previous reports demonstrate that MAPK activation results in increased inflammation, in part, through activation of downstream transcriptional programs (e.g. AP-1) involved in proinflammatory cytokine production [2,12,14,32]. Along these lines, we report that several proinflammatory cytokines and chemokines were induced at the level of gene expression in preadipocytes in response to TNFα. However, others have shown that the duration of MAPK phosphorylation is also important in controlling biological processes. For instance, prolonged phosphorylation of JNK inhibits insulin signaling through cytosolic regulation of IRS-1 signaling as well as inflammatory gene expression [12,13]. Thus, differences in magnitude and duration of MAPK phosphorylation that we observed between preadipocytes and adipocytes (Fig. 1A&B) may contribute to diverse inflammatory potential and ultimately lead to maladaptive processes responsible for metabolic inflammatory diseases.
MAPK-specific DUSPs constitute a structurally distinct family of 10 proteins characterized by a carboxy-terminal dual-specificity phosphatase domain and an amino-terminal MAPK binding domain [18,19]. DUSPs are further classified based on gene structure, substrate specificity, and subcellular localization into three groups: (i) nuclear DUSPs, (ii) cytosolic ERK-specific DUSPs, and (iii) DUSPs that selectively inactivate stress-activated MAPKs (i.e., JNK, p38).
Studies examining the functions of DUSPs in adipose tissue have emerged over the last several years. Of the inducible DUSPs, Dusp1 has been given considerable attention in the field of obesity, inflammation, and diabetes [37–42], where ablation of Dusp1 protects mice from diet-induced obesity [41] yet negatively correlates with MAPK-dependent proinflammatory status [39,40]. In addition, ectopic expression of Dusp1 attenuates MAPK signaling and stress-induced insulin resistance in adipocytes [37]. Similarly, ectopic expression of Dusp9 inhibits adipocyte differentiation and stress-induced insulin resistance in adipocytes. Further, Marcotorchino and colleagues [43] demonstrated that vitamin D-mediated suppression of inflammation in adipocytes was associated with increased expression of Dusp1, −10, and −16, although direct impacts for these DUSPs weren’t investigated. Recently, Dusp5 was implicated in adipose tissue inflammation, in which, we demonstrated that genetic loss of Dusp5 in vitro and in vivo exacerbated TNFα-induced inflammation [44]. Reports on Dusp6 deficient mice appear inconsistent, while one group reported protection from diet-induced obesity and glucose intolerance [45], another group observed minimal impact on obesity and impaired glucose intolerance [46].
Much more is known regarding the roles for DUSPs in the regulation of inflammation and the immune response [17]. The archetypal DUSP, Dusp1 has been shown to negatively regulate proinflammatory gene expression in bone marrow derived macrophages via inactivation of p38 MAPK [47]. Similarly, Dusp4 was shown to negatively regulate macrophage M1 activation via inactivation of JNK/p38 MAPK signaling; this resulted in attenuated inflammation in response to macrophage-adipocyte interactions [48]. Pharmacological and genetic loss of Dusp10 resulted in exacerbated p38 phosphorylation and proinflammatory gene expression in J774 murine macrophages [49]. In mice, Dusp6 ectopic expression has been shown to reduce parasite-mediated infection, in part, by regulating the CD40-redirected immune response [50]. Lastly, loss of function studies demonstrate an inverse relationship with Dusp8 and Dusp16 on JNK/p38-dependent inflammation in macrophages [17,51] as well as an essential role for Dusp16 on perinatal survival mediated by JNK-IL-12p40-depedent regulation [52]. Taken together, our findings reveal a role for Dusp1 and novel functions for Dusp8/16 as endogenous inhibitors of preadipocyte MAPK signaling and MAPK-dependent inflammatory gene expression.
While we did not examine the individual role for Dusps1, 8 and 16 on preadipocyte inflammatory gene expression, we did observe changes in MAPK signaling. Based on previous reports noted above, we would postulate that individual loss of DUSP expression would likely yield modest changes in proinflammatory gene expression; coordinated loss of function exacerbated this phenotype.
Canonical DUSP regulation involves feedback inhibition [19,28,29]; in which MAPKs induce DUSPs that, in turn, dephosphorylate MAPKs. However, recent investigations demonstrate that multiple signaling pathways such as protein kinase A (PKA) and PKC as well as NF-κB can regulate inducible DUSPs [53–56]. By consequence, it has been hypothesized that signaling cross-talk is mediated by MAPK-dependent and independent regulation of DUSPs. Indeed, studies demonstrate that MAPKs cross-talk with several signaling pathways involving cAMP, janus kinase–signal transducer and activator of transcription (JAKSTAT), and phosphatidylinositol 3-kinase (PI3K) [10,57,58]. Consistent with this notion, we report that only Dusp8 was downstream of JNK phosphorylation, while Dusp1/16 were regulated by MAPK-independent pathways (Fig. 9). Thus, MAPK-independent regulation of Dusp1/16 potentially contributes to intracellular signaling cross-talk, while JNK-dependent regulation of Dusp8 contributes cross-talk within the MAPK family. Moreover, DUSP-mediated amplification of MAPK signaling have the potential to alter other intracellular signaling pathway(s), potentially contributing to the regulation of MAPK-independent genes such as that observed for TNFα (Fig. 8). Consistent with our findings, others have shown that sustained JNK phosphorylation in response to mitogenic stimuli blocks ERK activation in COS-7 cells via transcriptional mediated processes [59], with recent reports suggesting a positive regulation of ERK from the JNK phosphatases Dusp10/16 [60]. Taken together, cross-talk within and between signaling modules potentially occur, in part, through the regulation of inducible DUSPs, prospectively contributing to MAPK-dependent and -independent regulation of inflammatory gene expression.
In summary, data outlined here highlight the importance of timely MAPK phosphorylation and dephosphorylation resulting from upstream kinases and downstream phosphatases in the regulation of inflammatory gene expression in preadipocytes. We demonstrate that MAPK-dependent and –independent regulation of DUSPs is essential for MAPK dephosphorylation. Moreover, we show that coordinated loss of DUSP function plays an essential role in the regulation of MAPK-dependent inflammatory gene expression. Further research is needed to determine if these actions are critical for linking obesity to metabolic inflammatory diseases. Future investigation of DUSPs in vivo may uncover novel therapeutic targets for the treatment and prevention of adipose tissue inflammation and obesity-induced insulin resistance.
Supplementary Material
Acknowledgments
The authors are grateful for superb technical assistance from Dr. Robin G. Hopkins (UNC Greensboro).
Grant funding
This work is supported by NIH-NIDDK funding (R15DK082799) to R.F.M and (F31DK084770) to B.S.F.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cellsig.2018.10.011.
Conflict of interest
The authors declare no conflicts of interest. The authors declare no financial conflicts of interest.
References
- [1].Gregor MF, Hotamisligil GS, Inflammatory mechanisms in obesity, Annu. Rev. Immunol 29 (2011) 415–445. [DOI] [PubMed] [Google Scholar]
- [2].Guilherme A, Virbasius JV, Puri V, Czech MP, Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes, Nat. Rev. Mol. Cell Biol 9 (5) (2008) 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hotamisligil GS, Shargill NS, Spiegelman BM, Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance, Science 259 (5091) (1993) 87–91. [DOI] [PubMed] [Google Scholar]
- [4].Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M, MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity, J. Clin. Invest 116 (6) (2006) 1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Xu H, Uysal KT, Becherer JD, Arner P, Hotamisligil GS, Altered tumor necrosis factor-alpha (TNF-alpha) processing in adipocytes and increased expression of transmembrane TNF-alpha in obesity, Diabetes 51 (6) (2002) 1876–1883. [DOI] [PubMed] [Google Scholar]
- [6].Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS, Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function, Nature 389 (6651) (1997) 610–614. [DOI] [PubMed] [Google Scholar]
- [7].Stephens JM, Pekala PH, Transcriptional repression of the C/EBP-alpha and GLUT4 genes in 3T3-L1 adipocytes by tumor necrosis factor-alpha. Regulations is coordinate and independent of protein synthesis, J. Biol. Chem 267 (19) (1992) 13580–13584. [PubMed] [Google Scholar]
- [8].Romanatto T, Roman EA, Arruda AP, Denis RG, Solon C, Milanski M, Moraes JC, Bonfleur ML, Degasperi GR, Picardi PK, Hirabara S, Boschero AC, Curi R, Velloso LA, Deletion of tumor necrosis factor-alpha receptor 1 (TNFR1) protects against diet-induced obesity by means of increased thermogenesis, J. Biol. Chem 284 (52) (2009) 36213–36222. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [9].Jain RG, Phelps KD, Pekala PH, Tumor necrosis factor-alpha initiated signal transduction in 3T3-L1 adipocytes, J. Cell. Physiol 179 (1) (1999) 58–66. [DOI] [PubMed] [Google Scholar]
- [10].Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH, Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions, Endocr. Rev 22 (2) (2001) 153–183. [DOI] [PubMed] [Google Scholar]
- [11].Ryden M, Dicker A, van Harmelen V, Hauner H, Brunnberg M, Perbeck L, Lonnqvist F, Arner P, Mapping of early signaling events in tumor necrosis factor-alpha -mediated lipolysis in human fat cells, J. Biol. Chem 277 (2) (2002) 1085–1091. [DOI] [PubMed] [Google Scholar]
- [12].Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS, A central role for JNK in obesity and insulin resistance, Nature 420 (6913) (2002) 333–336. [DOI] [PubMed] [Google Scholar]
- [13].Lee YH, Giraud J, Davis RJ, White MF, c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade, J. Biol. Chem 278 (5) (2003) 2896–2902. [DOI] [PubMed] [Google Scholar]
- [14].Fujishiro M, Gotoh Y, Katagiri H, Sakoda H, Ogihara T, Anai M, Onishi Y, Ono H, Abe M, Shojima N, Fukushima Y, Kikuchi M, Oka Y, Asano T, Three mitogen-activated protein kinases inhibit insulin signaling by different mechanisms in 3T3-L1 adipocytes, Mol. Endocrinol 17 (3) (2003) 487–497. [DOI] [PubMed] [Google Scholar]
- [15].Zhang Y, Dong C, Regulatory mechanisms of mitogen-activated kinase signaling, Cell. Mol. Life Sci 64 (21) (2007) 2771–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Reth M, Hydrogen peroxide as second messenger in lymphocyte activation, Nat. Immunol 3 (12) (2002) 1129–1134. [DOI] [PubMed] [Google Scholar]
- [17].Jeffrey KL, Camps M, Rommel C, Mackay CR, Targeting dual-specificity phosphatases: manipulating MAP kinase signalling and immune responses, Nat. Rev. Drug Discov 6 (5) (2007) 391–403. [DOI] [PubMed] [Google Scholar]
- [18].Kondoh K, Nishida E, Regulation of MAP kinases by MAP kinase phosphatases, Biochim. Biophys. Acta 1773 (8) (2007) 1227–1237. [DOI] [PubMed] [Google Scholar]
- [19].Dickinson RJ, Keyse SM, Diverse physiological functions for dual-specificity MAP kinase phosphatases, J. Cell Sci 119 (2006) 4607–4615 Pt 22. [DOI] [PubMed] [Google Scholar]
- [20].Keyse SM, Dual-specificity MAP kinase phosphatases (MKPs) and cancer, Cancer Metastasis Rev. 27 (2) (2008) 253–261. [DOI] [PubMed] [Google Scholar]
- [21].Djian P, Phillips M, Green H, The activation of specific gene transcription in the adipose conversion of 3T3 cells, J. Cell. Physiol 124 (3) (1985) 554–556. [DOI] [PubMed] [Google Scholar]
- [22].Morrison RF, Farmer SR, Role of PPARgamma in regulating a cascade expression of cyclin-dependent kinase inhibitors, p18(INK4c) and p21(Waf1/Cip1), during adipogenesis, J. Biol. Chem 274 (24) (1999) 17088–17097. [DOI] [PubMed] [Google Scholar]
- [23].Ferguson BS, Nam H, Hopkins RG, Morrison RF, Impact of reference gene selection for target gene normalization on experimental outcome using real-time qRT-PCR in adipocytes, PLoS One 5 (12) (2010) e15208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Livak KJ, Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method, Methods 25 (4) (2001) 402–408. [DOI] [PubMed] [Google Scholar]
- [25].Waltschewa LW, Interaction of actinomycin D with yeast ribosomal RNA, FEBS Lett. 111 (1) (1980) 179–180. [DOI] [PubMed] [Google Scholar]
- [26].Davis WR, Gabbara S, Hupe D, Peliska JA, Actinomycin D inhibition of DNA strand transfer reactions catalyzed by HIV-1 reverse transcriptase and nucleocapsid protein, Biochemistry 37 (40) (1998) 14213–14221. [DOI] [PubMed] [Google Scholar]
- [27].Gorzelniak K, Janke J, Engeli S, Sharma AM, Validation of endogenous controls for gene expression studies in human adipocytes and preadipocytes, Horm. Metab. Res 33 (10) (2001) 625–627. [DOI] [PubMed] [Google Scholar]
- [28].Teng CH, Huang WN, Meng TC, Several dual specificity phosphatases coordinate to control the magnitude and duration of JNK activation in signaling response to oxidative stress, J. Biol. Chem 282 (39) (2007) 28395–28407. [DOI] [PubMed] [Google Scholar]
- [29].Sprowles A, Robinson D, Wu YM, Kung HJ, Wisdom R, c-Jun controls the efficiency of MAP kinase signaling by transcriptional repression of MAP kinase phosphatases, Exp. Cell Res 308 (2) (2005) 459–468. [DOI] [PubMed] [Google Scholar]
- [30].Chung S, Lapoint K, Martinez K, Kennedy A, Boysen Sandberg M, McIntosh MK, Preadipocytes mediate lipopolysaccharide-induced inflammation and insulin resistance in primary cultures of newly differentiated human adipocytes, Endocrinology 147 (11) (2006) 5340–5351. [DOI] [PubMed] [Google Scholar]
- [31].Wellen KE, Hotamisligil GS, Inflammation, stress, and diabetes, J. Clin. Invest 115 (5) (2005) 1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hotamisligil GS, Erbay E, Nutrient sensing and inflammation in metabolic diseases, Nat. Rev. Immunol 8 (12) (2008) 923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Poulain-Godefroy O, Froguel P, Preadipocyte response and impairment of differentiation in an inflammatory environment, Biochem. Biophys. Res. Commun 356 (3) (2007) 662–667. [DOI] [PubMed] [Google Scholar]
- [34].Khazen W, M’Bika P, Tomkiewicz C, Benelli C, Chany C, Achour A, Forest C, Expression of macrophage-selective markers in human and rodent adipocytes, FEBS Lett. 579 (25) (2005) 5631–5634. [DOI] [PubMed] [Google Scholar]
- [35].Chazenbalk G, Bertolotto C, Heneidi S, Jumabay M, Trivax B, Aronowitz J, Yoshimura K, Simmons CF, Dumesic DA, Azziz R, Novel pathway of adipogenesis through cross-talk between adipose tissue macrophages, adipose stem cells and adipocytes: evidence of cell plasticity, PLoS One 6 (3) (2011) e17834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Charriere G, Cousin B, Arnaud E, Andre M, Bacou F, Penicaud L, Casteilla L, Preadipocyte conversion to macrophage. Evidence of plasticity, J. Biol. Chem 278 (11) (2003) 9850–9855. [DOI] [PubMed] [Google Scholar]
- [37].Bazuine M, Carlotti F, Tafrechi RS, Hoeben RC, Maassen JA, Mitogen-activated protein kinase (MAPK) phosphatase-1 and −4 attenuate p38 MAPK during dexamethasone-induced insulin resistance in 3T3-L1 adipocytes, Mol. Endocrinol 18 (7) (2004) 1697–1707. [DOI] [PubMed] [Google Scholar]
- [38].Sakaue H, Ogawa W, Nakamura T, Mori T, Nakamura K, Kasuga M, Role of MAPK phosphatase-1 (MKP-1) in adipocyte differentiation, J. Biol. Chem 279 (38) (2004) 39951–39957. [DOI] [PubMed] [Google Scholar]
- [39].Hammer M, Mages J, Dietrich H, Servatius A, Howells N, Cato AC, Lang R, Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS-induced genes and protects mice from lethal endotoxin shock, J. Exp. Med 203 (1) (2006) 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, Flavell RA, Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses, Proc. Natl. Acad. Sci. U. S. A 103 (7) (2006) 2274–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wu JJ, Roth RJ, Anderson EJ, Hong EG, Lee MK, Choi CS, Neufer PD, Shulman GI, Kim JK, Bennett AM, Mice lacking MAP kinase phosphatase-1 have enhanced MAP kinase activity and resistance to diet-induced obesity, Cell Metab. 4 (1) (2006) 61–73. [DOI] [PubMed] [Google Scholar]
- [42].Roth RJ, Le AM, Zhang L, Kahn M, Samuel VT, Shulman GI, Bennett AM, MAPK phosphatase-1 facilitates the loss of oxidative myofibers associated with obesity in mice, J. Clin. Invest 119 (12) (2009) 3817–3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Marcotorchino J, Gouranton E, Romier B, Tourniaire F, Astier J, Malezet C, Amiot MJ, Landrier JF, Vitamin D reduces the inflammatory response and restores glucose uptake in adipocytes, Mol. Nutr. Food Res 56 (12) (2012) 1771–1782. [DOI] [PubMed] [Google Scholar]
- [44].Habibian JS, Jefic M, Bagchi RA, Lane RH, McKnight RA, McKinsey TA, Morrison RF, Ferguson BS, DUSP5 functions as a feedback regulator of TNFalpha-induced ERK1/2 dephosphorylation and inflammatory gene expression in adipocytes, Sci. Rep 7 (1) (2017) 12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Feng B, Jiao P, Helou Y, Li Y, He Q, Walters MS, Salomon A, Xu H, Mitogen-activated protein kinase phosphatase 3 (MKP-3)-deficient mice are resistant to diet-induced obesity, Diabetes 63 (9) (2014) 2924–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pfuhlmann K, Pfluger PT, Schriever SC, Muller TD, Tschop MH, Stemmer K, Dual specificity phosphatase 6 deficiency is associated with impaired systemic glucose tolerance and reversible weight retardation in mice, PLoS One 12 (9) (2017) e0183488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Talwar H, Bauerfeld C, Bouhamdan M, Farshi P, Liu Y, Samavati L, MKP-1 negatively regulates LPS-mediated IL-1beta production through p38 activation and HIF-1alpha expression, Cell. Signal 34 (2017) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Jiao H, Tang P, Zhang Y, MAP kinase phosphatase 2 regulates macrophage-adipocyte interaction, PLoS One 10 (3) (2015) e0120755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hommo T, Pesu M, Moilanen E, Korhonen R, Regulation of Inflammatory Cytokine Production by MKP-5 in Macrophages, Basic Clin. Pharmacol. Toxicol 117 (2) (2015) 96–104. [DOI] [PubMed] [Google Scholar]
- [50].Srivastava N, Sudan R, Saha B, CD40-modulated dual-specificity phosphatases MAPK phosphatase (MKP)-1 and MKP-3 reciprocally regulate Leishmania major infection, J. Immunol 186 (10) (2011) 5863–5872. [DOI] [PubMed] [Google Scholar]
- [51].Matsuguchi T, Musikacharoen T, Johnson TR, Kraft AS, Yoshikai Y, A novel mitogen-activated protein kinase phosphatase is an important negative regulator of lipopolysaccharide-mediated c-Jun N-terminal kinase activation in mouse macrophage cell lines, Mol. Cell. Biol 21 (20) (2001) 6999–7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Niedzielska M, Bodendorfer B, Munch S, Eichner A, Derigs M, da Costa O, Schweizer A, Neff F, Nitschke L, Sparwasser T, Keyse SM, Lang R, Gene trap mice reveal an essential function of dual specificity phosphatase Dusp16/MKP-7 in perinatal survival and regulation of Toll-like receptor (TLR)-induced cytokine production, J. Biol. Chem 289 (4) (2014) 2112–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Short MD, Fox SM, Lam CF, Stenmark KR, Das M, Protein kinase Czeta attenuates hypoxia-induced proliferation of fibroblasts by regulating MAP kinase phosphatase-1 expression, Mol. Biol. Cell 17 (4) (2006) 1995–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zhang J, Wang Q, Zhu N, Yu M, Shen B, Xiang J, Lin A, Cyclic AMP inhibits JNK activation by CREB-mediated induction of c-FLIP(L) and MKP-1, thereby antagonizing UV-induced apoptosis, Cell Death Differ. 15 (10) (2008) 1654–1662. [DOI] [PubMed] [Google Scholar]
- [55].Cho IJ, Woo NR, Shin IC, Kim SG, H89, an inhibitor of PKA and MSK, inhibits cyclic-AMP response element binding protein-mediated MAPK phosphatase-1 induction by lipopolysaccharide, Inflamm. Res 58 (12) (2009) 863–872. [DOI] [PubMed] [Google Scholar]
- [56].Wang J, Ford HR, Grishin AV, NF-kappaB-mediated expression of MAPK phosphatase-1 is an early step in desensitization to TLR ligands in enterocytes, Mucosal Immunol. 3 (5) (2010) 523–534. [DOI] [PubMed] [Google Scholar]
- [57].Rommel C, Clarke BA, Zimmermann S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos GD, Glass DJ, Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt, Science 286 (5445) (1999) 1738–1741. [DOI] [PubMed] [Google Scholar]
- [58].Gerits N, Kostenko S, Shiryaev A, Johannessen M, Moens U, Relations between the mitogen-activated protein kinase and the cAMP-dependent protein kinase pathways: comradeship and hostility, Cell. Signal 20 (9) (2008) 1592–1607. [DOI] [PubMed] [Google Scholar]
- [59].Shen YH, Godlewski J, Zhu J, Sathyanarayana P, Leaner V, Birrer MJ, Rana A, Tzivion G, Cross-talk between JNK/SAPK and ERK/MAPK pathways: sustained activation of JNK blocks ERK activation by mitogenic factors, J. Biol. Chem 278 (29) (2003) 26715–26721. [DOI] [PubMed] [Google Scholar]
- [60].Caunt CJ, Armstrong SP, Rivers CA, Norman MR, McArdle CA, Spatiotemporal regulation of ERK2 by dual specificity phosphatases, J. Biol. Chem 283 (39) (2008) 26612–26623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.