Abstract
In 2020, the highest rates of cervical cancer incidence and mortality were reported in Asian and African regions of the world. Across the globe, growing evidence confirms cancer disparities among racial and ethnic minorities, low socioeconomic status groups, sexual and gender minorities, uninsured individuals, and rural residents. Recognition of these stark disparities has led to increased global efforts for improving screening rates overall and, in medically underserved populations, highlighting the urgent need for research to inform the successful implementation of cervical cancer screening. Implementation science, defined as the study of methods to promote the integration of research evidence into health care practice, is well-suited to address this challenge. With a multilevel, implementation focus, we present key research directions that can help address cancer disparities in resource-limited settings. First, we describe several global feasibility studies that acknowledge the effectiveness of self-sampling as a strategy to improve screening coverage. Second, we highlight Project ECHO as a strategy to improve providers’ knowledge through an extended virtual learning community, thereby building capacity for health care settings to deliver screening. Third, we consider community health workers, who are a cornerstone of implementing public health interventions in global communities. Finally, we see tremendous learning opportunities that use contextually relevant strategies to advance the science of community engagement and adaptations that could further enhance the uptake of screening in resource-limited settings. These opportunities provide future directions for bidirectional exchange of knowledge between local and global resource-limited settings to advance implementation science and address disparities.
Keywords: Implementation Science, Cervical Cancer, Global Health, Rural Health
Introduction
The Asian and African regions of the world continue to report the highest cervical cancer incidence and mortality.1 The global recognition of these disparities and the growing evidence base around cervical cancer prevention has contributed to the World Health Organization’s (WHO) agenda for elimination of cervical cancer as a public health problem.2 This initiative, while important in promoting the adoption of evidence-based interventions (ie, vaccination, screening, and treatment), further highlights the urgent need for research to inform the successful implementation of cervical cancer screening. In a recent commentary, a WHO working group suggested the importance of implementation research with a specific focus on broadening stakeholder engagement, focusing on equity in reach and navigation throughout the continuum of care, and improving the readiness of health systems to increase adoption, implementation, scale-up, and sustainability of cervical cancer screening programs.3 In parallel to these foci, we highlight key research advances within the field of rural and resource-limited settings that could contribute toward enhancing the science of implementation around global cervical cancer screening efforts.
Throughout the world, cervical cancer continues to disproportionately affect individuals from racial and ethnic minorities, low socioeconomic status groups, rural residents, and those who lack access to screening, medical and oncology service.4,5 Biomedical research has resulted in a robust evidence base for the WHO-recommended interventions for cervical cancer. However, there continues to be a persistent underinvestment in cancer prevention and a failure to ensure that all population subgroups benefit from access to care, including diagnostics and treatment. Implementing cancer screening programs for populations requires the consideration of a series of steps beyond initial testing. Some of these steps may include screening and treatment approaches, further testing for diagnosis confirmation, and ensuring timely and adequate treatment for the screen-positives and re-testing for screen-negatives, as outlined in the WHO recommendations. Social, behavioral and health services research provides evidence that cancer screening efforts, including implementation and uptake, are affected by individual- and system-level contextual factors, such as economic status, place of residence, as well as health care facility resources and personnel. Implementation research, defined as the study of methods to promote the integration of research evidence into health care policy and practice, is well-suited to address this challenge.6
As a field of scientific study, implementation research is focused on: understanding the multilevel health care context; identifying strategies to help adopt, implement, and sustain interventions in health care; consider implementation outcomes (ie, acceptability, appropriateness, and feasibility, among others); along with effectiveness outcomes. Applied to cancer screening, an implementation research agenda can help ensure considerations across the cancer continuum (ie, screening, diagnostics, and treatment), target multiple ecological levels to promote implementation effectiveness of cancer screening programs, and make certain that key social and behavioral attributes of marginalized populations are incorporated into programming. As shown in Figure 1, we highlight key research directions, supported by evidence from resource-limited settings within a rural context for promoting the WHO cervical cancer elimination agenda, thereby urging bidirectional learning on promising implementation strategies from the global north and the global south.
Figure 1. Key research directions for advancing global implementation science around cervical cancer screening.
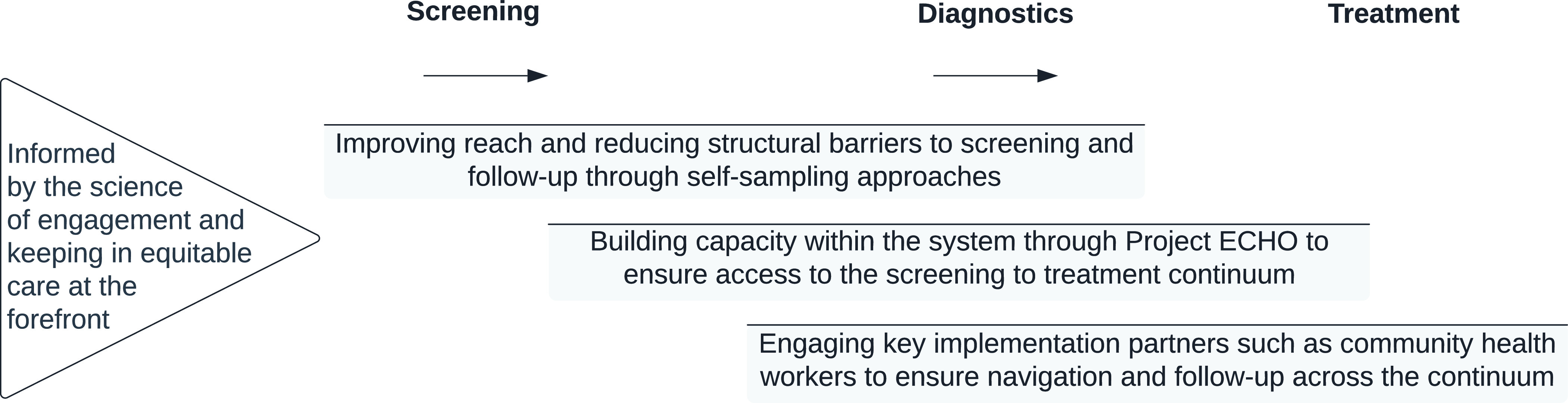
Improving Reach and Reducing Structural Barriers using Self-collected Samples for Screening
We acknowledge global feasibility studies that have created a substantial evidence base of effectiveness around using self-collected samples for cervical cancer screening. In resource-limited settings, self-sampling was perceived to be easy, painless, less embarrassing, and in some cases preferred over provider-sampling.7 Feasibility studies conducted in rural India for cervical cancer screening using home-based HPV self-sampling showed high screening compliance with reduced operational problems.8 In much of sub-Saharan Africa, screening uptake of Pap smear and visual inspection has remained low despite high willingness of women to screen, demonstrating the importance of addressing environmental constraints. HPV DNA-based testing, which offers the options for self-sampling, can shift screening from clinical to community settings thereby addressing environmental barriers to screening.9
This growing evidence for self-sampling from low- and middle-income countries (LMICs) is substantial and an important contribution toward elimination efforts across the globe, especially in the post-pandemic world.10 The recently launched “Last Mile” initiative, which is a public-private partnership, aims to validate self-sampling as a comparable, non-inferior alternative to provider-collected samples and seeks to receive regulatory approvals in the United States.11 Despite the growing interest and evidence surrounding self-sampling as a strategy to increase screening uptake,12 its implementation in resource-limited settings has been sub-optimal. With increasing evidence pointing toward the cost-effectiveness of this strategy when considering population impact,13 implementation studies, which evaluate ways in which delivery of such a strategy could be incorporated into routine services for resource-limited primary health care settings, are scarce. There is an immediate need to invest in research that is aimed at understanding how self-collected samples can be incorporated into routine clinical workflows in resource-limited health care settings, thereby reducing the burden for primary health care services and the patient.
As our global society emerges from the COVID-19 pandemic, approaches that reduce clinic visits, allow for screening outside the clinical environment, and utilize fewer clinical resources will be needed and have since gained broader acceptance.14 Self-sampling approaches can also be critical tools that improve uptake among individuals from medically underserved communities. Investing in implementation research for resource-limited settings, which serve low socioeconomic and racial/ethnic minority populations, can further contribute toward a health equity-oriented research agenda globally.15
Building Workforce Capacity using Project ECHO® (Extension for Community Healthcare Outcomes) as a Strategy to Reach and Train Providers
Building workforce capacity to deliver cervical cancer screening services remains a challenge globally,16-18 where only a limited number of physicians and nurses are appropriately trained to provide primary health care in rural and other medically underserved areas. Limited access to academic public health and medicine further reduces the ability of this workforce to develop or improve the competencies needed to deliver cancer prevention services in an effective and efficient manner. Here, we describe one program working to address these issues.
Project ECHO is a digital mentoring platform designed to increase capacity and improve providers’ knowledge.16 Proven effective in India, Project ECHO makes knowledge accessible for health care stakeholders.19 In response to the COVID-19 pandemic, such digital platforms to connect, coordinate, and overcome barriers to care have become essential. The ECHO model has been especially important in cancer control initiatives by bringing together experts and practitioners to promote implementation capacity.20 In India, the National Institute of Cancer Prevention supports a program that aims to reach and train gynecologists, primary care physicians, nurses, and community health workers to support the implementation of a national, population-based cancer screening program. Their goals are to increase clinical capacity in low-resource settings by empowering health care providers in medically underserved areas to screen and treat patients in their own communities, thereby reducing referrals. Studies from India have shown that Project ECHO is an effective tool to develop skills, confidence, and knowledge and contribute to building capacity for health care settings to deliver screening to address global disparities in health care access.19
The global reach of this strategy exemplifies the bidirectional learning needed for advancing global implementation science. Focused on cervical cancer screening, Project ECHO has been used to connect providers in medically underserved areas of Texas, including the Texas-Mexico border, with specialists at academic health centers such as MD Anderson to discuss follow-up of cases for patients who have abnormal cervical cancer screening tests.21,22 Additional programs based on this model have been developed and implemented in Africa including a program in Cameroon, Mozambique and Nepal. The potential for Project ECHO to truly contribute to implementation of cancer screening services in health care settings, however, remains under-explored.23 There is an immediate need to evaluate existing ECHO programs and examine how and when the ECHO programs contribute to supporting care delivery and what impact the program has on population outcomes. Combined with recent advances in health care apps24 and virtual visits,25 additional telehealth opportunities could also contribute toward building capacity by task-shifting to health workers who assist or conduct screening interventions in rural settings.
Community Health Workers: Helping Patients Navigate Health Care Systems to Access Cervical Cancer Screening
Community health workers (CHWs) are an important cornerstone for implementing public health interventions in global communities.26,27 To implement cervical screening efforts, CHWs serve in many roles, including: educating the community members about cancer prevention and navigating patients through the screening process from their homes or other community spaces to the health care system.28 In public health systems across the globe, CHWs form an important interface between the community and the health care system. In many cases, CHWs live in the community they serve and, therefore, they are familiar with the social and cultural practices of members in the community. Often, they are the first to be sought out for any health-related concerns among the medically underserved populations. In Africa, CHWs have been key to delivering maternal and child health, TB, HIV/AIDS, and malaria services, to name a few.29 Considering the fact that population-based cancer screening is primarily targeting asymptomatic individuals, the CHW’s ability to engage with the community can be leveraged for increased community awareness, screening coverage, and ultimately improved navigation across the continuum.27 In fact, there is growing evidence from African30,31 and Asian countries32 on the success of task-shifting from physicians to CHWs as a potentially effective and affordable strategy for improving access to health care.33
An important gap or challenge to the implementation of cervical cancer screening with self-sampling is the follow-up of women to share results and refer for further diagnostic testing, if needed.7 This demonstrated loss-to-follow-up has at times been cited as an argument against HPV testing and in favor of “screen-and-treat” approaches with other screening methods. CHWs can be an important player in the notification and tracking of women post-screening to ensure linkage to follow-up testing and services. A systematic review of the CHW’s role in cervical cancer screening in LMICs found that CHWs have been underutilized for follow-up, while they are most commonly involved in community education and awareness raising efforts.28 Another strategy has been to use Project ECHO in combination with patient navigators to provide community outreach and education, as well as to provide navigation services for women in need of screening and follow-up in clinical settings. This comprehensive or multi-level intervention was effective in increasing cervical cancer screening, diagnosis, and treatment for women in clinics near the Texas-Mexico Border.22
Contributions to and from the Science of Engagement and Health Equity
Finally, and most importantly, we see tremendous bidirectional learning opportunities for the science of community and partnership engagement that is rooted within the Global North and primarily emerges from an understanding of the implementation of interventions, often in well-resourced settings. We believe that developing such a scientific base equivalently across the globe could inform an in-depth understanding of disparities in screening uptake and begin to move toward an equity-orientation (ie, addressing the disparities for improving screening uptake). This will require being knowledgeable of Community-Based Participatory Research (CBPR) approaches, which aim to: recognize the community; build on the assets within the community; facilitate a collaborative and equitable partnership in all phases of research; foster co-learning and capacity building; achieve a balance between knowledge generation and intervention for the mutual benefit of partners; focus on the local relevance of public health problems; engage in an iterative process; disseminate results; address issues of social determinants of health; and commit to a long-term process toward sustainability.34
Using a CBPR approach can be beneficial for both the communities served in resource-limited settings and the providers involved in the implementation. To achieve effective and efficient implementation, both deliverers and recipients of the intervention have to perceive interventions and strategies used to implement these interventions, as acceptable, appropriate, and feasible.6 Engaging with the recipients and deliverers also provides the opportunity to examine intervention and implementation strategies (ie, methods or techniques used to implement interventions, such as those mentioned in this article) to describe the core components that lead to implementation outcomes and therefore population health outcomes.6 We note here that the specific strategies shared in this article are not stand-alone strategies or isolated from each other; instead, we believe that they must be used synergistically and in combination, keeping the implementation context at the forefront.
Conclusion
The path to achieving population-level benefit from existing evidence-based interventions, such as cervical cancer screening, is clear only if we are to see it through an implementation research perspective, and address complexities associated with screening in resource-limited settings across the globe. To achieve these goals, we will need to accelerate the bidirectional exchange of information, innovations, and lessons learned as we gain a deeper understanding of context surrounding resource-limited settings, begin to examine the implementation of screening as a process that includes not just the screening test but also ensures follow-up, and the sustainability of implementation efforts. We hope that the growing emphasis on studying the implementation of evidence-based interventions will inform next steps for cancer prevention and care as we continue to emerge from the global COVID-19 pandemic.
References
- 1.International Agency for Research on Cancer . Cervix Uteri - Globocan 2020 [report]. 2021. Last accessed August 22, 2022 from https://gco.iarc.fr/today/data/factsheets/cancers/23-Cervix-uteri-fact-sheet.pdf.
- 2.Canfell K. Towards the global elimination of cervical cancer. Papillomavirus Res. 2019;8:100170. https://doi.org/ 10.1016/j. pvr.2019.100170 PMID:31176807 [DOI] [PMC free article] [PubMed]
- 3.Broutet N, Jeronimo J, Kumar S, et al. Implementation research to accelerate scale-up of national screen and treat strategies towards the elimination of cervical cancer. Prev Med. 2022;155:106906. https://doi.org/ 10.1016/j. ypmed.2021.106906 PMID:34896155 [DOI] [PubMed]
- 4.Islami F, Guerra CE, Minihan A, et al. American Cancer Society’s report on the status of cancer disparities in the United States, 2021. CA Cancer J Clin. 2022;72(2):112-143. 10.3322/caac.21703 [DOI] [PubMed] [Google Scholar]
- 5.Buskwofie A, David-West G, Clare CA. A review of cervical cancer: incidence and disparities. J Natl Med Assoc. 2020;112(2):229-232. 10.1016/j.jnma.2020.03.002 [DOI] [PubMed] [Google Scholar]
- 6.Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38(2):65-76. 10.1007/s10488-010-0319-7 10.1007/s10488-010-0319-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamath Mulki A, Withers M. Human Papilloma Virus self-sampling performance in low- and middle-income countries. BMC Womens Health. 2021;21(1):12. 10.1186/s12905-020-01158-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adsul P, Srinivas V, Gowda S, et al. A community-based, cross-sectional study of hrHPV DNA self-sampling-based cervical cancer screening in rural Karnataka, India. Int J Gynaecol Obstet. 2019;146(2):170-176. 10.1002/ijgo.12859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lott BE, Trejo MJ, Baum C, et al. Interventions to increase uptake of cervical screening in sub-Saharan Africa: a scoping review using the integrated behavioral model. BMC Public Health. 2020;20(1):654. 10.1186/s12889-020-08777-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lozar T, Nagvekar R, Rohrer C, Dube Mandishora RS, Ivanus U, Fitzpatrick MB. Cervical cancer screening postpandemic: self-sampling opportunities to accelerate the elimination of cervical cancer. Int J Womens Health. 2021;13:841-859. 10.2147/IJWH.S288376 10.2147/IJWH.S288376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Cancer Institute . NCI Cervical Cancer ‘Last Mile’ Initiative. [webpage] 2022. Last accessed August 22, 2022 from https://prevention.cancer.gov/major-programs/nci-cervical-cancer-last-mile-initiative.
- 12.Yeh PT, Kennedy CE, de Vuyst H, Narasimhan M. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Glob Health. 2019;4(3):e001351. 10.1136/bmjgh-2018-001351 10.1136/bmjgh-2018-001351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malone C, Barnabas RV, Buist DSM, Tiro JA, Winer RL. Cost-effectiveness studies of HPV self-sampling: A systematic review. Prev Med. 2020;132:105953-105953. 10.1016/j.ypmed.2019.105953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah SK, McElfish PA. Cancer screening recommendations during the COVID-19 pandemic: scoping review. JMIR Cancer. 2022;8(1):e34392. 10.2196/34392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta S, Palmer C, Bik EM, et al. Self-sampling for human papillomavirus testing: increased cervical cancer screening participation and incorporation in international screening programs. Front Public Health. 2018;6:77. 10.3389/fpubh.2018.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhanasekaran K, Babu R, Kumar V, Mehrotra R, Hariprasad R. Capacity building of gynecologists in cancer screening through hybrid training approach. J Cancer Educ. 2020;35(6):1243-1249. 10.1007/s13187-019-01589-0 [DOI] [PubMed] [Google Scholar]
- 17.Dsouza JP, Van den Broucke S, Pattanshetty S, Dhoore W. Cervical cancer screening status and implementation challenges: report from selected states of India. Int J Health Plann Manage. 2022;37(2):824-838. 10.1002/hpm.3353 [DOI] [PubMed] [Google Scholar]
- 18.Jatho A, Mugisha NM, Kafeero J, et al. Capacity building for cancer prevention and early detection in the Ugandan primary healthcare facilities: working toward reducing the unmet needs of cancer control services. Cancer Med. 2021;10(2):745-756. 10.1002/cam4.3659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arora S, Kalishman S, Thornton K, et al. Project ECHO (Project Extension for Community Healthcare Outcomes): a national and global model for continuing professional development. J Contin Educ Health Prof. 2016;36(suppl 1):S48-S49. 10.1097/CEH.0000000000000097 [DOI] [PubMed] [Google Scholar]
- 20.Nethan ST, Hariprasad R, Babu R, Kumar V, Sharma S, Mehrotra R. Project ECHO: a potential best-practice tool for training healthcare providers in oral cancer screening and tobacco cessation. J Cancer Educ. 2020;35(5):965-971. https://doi.org 10.1007/s13187-019-01549-8. PMID:31124001Kumar, Sharma, Mehrotra, 2019”) [DOI] [PubMed]
- 21.Varon ML, Baker E, Byers E, et al. Project ECHO Cancer Initiative: a tool to improve care and increase capacity along the continuum of cancer care. J Cancer Educ. 2021;36(S1)(suppl 1):25-38. 10.1007/s13187-021-02031-0 10.1007/s13187-021-02031-0 [DOI] [PubMed] [Google Scholar]
- 22.Salcedo MP, Gowen R, Rodriguez AM, et al. Addressing high cervical cancer rates in the Rio Grande Valley along the Texas- Mexico border: a community-based initiative focused on education, patient navigation, and medical provider training/telementoring. Perspect Public Health. June 2021. [online]. https://doi.org/ 10.1177/1757913921994610 PMID:34130548 [DOI] [PubMed]
- 23.Dearing J, Cruz S, Kee K, Larson R, Rahm A. Project ECHO Review and Research Agenda [report]. Diffusion Associates; 2019. Last accessed August 22, 2022 from http://www.diffusionassociates.com/pdfs/echo.pdf
- 24.Arrossi S, Paolino M, Antelo VS, et al. ; ATICA Study team. Effectiveness of an mHealth intervention to increase adherence to triage of HPV DNA positive women who have performed self-collection (the ATICA study): A hybrid type I cluster randomised effectiveness-implementation trial. Lancet Reg Health Am. 2022;9:9. https://doi.org/ 10.1016/j. lana.2022.100199 PMID:35655914 [DOI] [PMC free article] [PubMed]
- 25.Quinley KE, Gormley RH, Ratcliffe SJ, et al. Use of mobile telemedicine for cervical cancer screening. J Telemed Telecare. 2011;17(4):203-209. 10.1258/jtt.2011.101008 10.1258/jtt.2011.101008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berini CR, Bonilha HS, Simpson AN. Impact of Community Health Workers on Access to Care for Rural Populations in the United States: A Systematic Review. J Community Health. 2022;47(3):539-553. 10.1007/s10900-021-01052-6 [DOI] [PubMed] [Google Scholar]
- 27.Kedar A, John A, Goala S, et al. Barriers and facilitators in implementing population based common cancer screening through community health workers. Ecancermedicalscience. 2021;15:1277. 10.3332/ecancer.2021.1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Donovan J, O’Donovan C, Nagraj S. The role of community health workers in cervical cancer screening in low-income and middle-income countries: a systematic scoping review of the literature. BMJ Glob Health. 2019;4(3):e001452. 10.1136/bmjgh-2019-001452 10.1136/bmjgh-2019-001452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehmann U, Friedman I, Sanders D. Review of the Utilisation and Effectiveness of Community-Based Health Workers in Africa, A Joint Learning Initiative: Human Resources for Health and Development. Cape Town: University of the Western Cape; 2004. [Google Scholar]
- 30.Awolude OA, Oyerinde SO, Akinyemi JO. Screen and triage by community extension workers to facilitate screen and treat: task-sharing strategy to achieve universal coverage for cervical cancer screening in Nigeria. J Glob Oncol. 2018;4(4):1-10. 10.1200/JGO.18.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mboineki JF, Wang P, Dhakal K, Getu MA, Chen C. The effect of peer-led navigation approach as a form of task shifting in promoting cervical cancer screening knowledge, intention, and practices among urban women in Tanzania: a randomized controlled trial. Cancer Control. 2022;29 [online]; https://doi. org/ 10.1177/10732748221089480. PMID: 35666651 [DOI] [PMC free article] [PubMed]
- 32.Poli UR, Muwonge R, Bhoopal T, Lucas E, Basu P. Feasibility, acceptability, and efficacy of a community health worker-driven approach to screen hard-to-reach Periurban women using self-sampled HPV detection test in India. JCO Glob Oncol. 2020;6(6):658-666. 10.1200/GO.20.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perry HB, Zulliger R, Rogers MM. Community health workers in low-, middle-, and high-income countries: an overview of their history, recent evolution, and current effectiveness. Annu Rev Public Health. 2014;35(1):399-421. 10.1146/annurev-publhealth-032013-182354 10.1146/annurev-publhealth-032013-182354 [DOI] [PubMed] [Google Scholar]
- 34.Wallerstein N, Duran B, Oetzel J, Minkler M, eds. Community-Based Participatory Research for Health: Advancing Social and Health Equity. 3rd ed. San Francisco: Jossey-Bass; 2018.proce [Google Scholar]


