Abstract
Duchenne muscular dystrophy (DMD) is the most common hereditary progressive muscular disease. It was recently reported that symptoms of anxiety and depression are frequent in patients with DMD, and psychosocial support is important for their quality of life. We reported 3 patients (2 patients with DMD and 1 patient with muscular dystrophy of an unknown etiology) with panic disorder and treated them successfully with neurofeedback (NFB). The NFB protocols were slow motor rhythm at T4 for 2 patients, beta training at F3 for 1 patient, and alpha/theta training at Pz for all patients. All patients had significantly improved anxiety symptoms, including panic attacks, after NFB therapy. NFB can be considered a safe therapeutic alternative without causing even minor side effects and without drug interactions for DMD patients with panic or anxiety disorders.
Keywords: Muscular dystrophies, panic disorder, neurofeedback
MAIN POINTS.
We reported 3 patients who experienced panic attacks in the course of deteriorating Duchenne muscular dystrophy (DMD) with panic disorder and treated them successfully with neurofeedback.
The neurofeedback protocols were slow motor rhythm at T4 for 2 patients, beta training at F3 for 1 patient, and alpha/theta at Pz for all patients.
Neurofeedback treatment is suggested as a safe and effective complimentary treatment for panic disorder patients who are intolerant to pharmacotherapy because of medical conditions, such as DMD.
Introduction
Duchenne muscular dystrophy (DMD), an X-chromosome recessive inheritance form of muscular dystrophy (MD), occurs in nearly 1 in 3600–6000 live male births.1 It is manifested as respiratory, orthopedic, and cardiac complications.
Panic disorder is commonly comorbid with medical diseases, including cardiovascular, cerebrovascular, and respiratory diseases.2 Dyspnea or breathlessness, a common complication in patients with DMD, is physically debilitating and emotionally distressing. Furthermore, negative beliefs about breathlessness increase panic in patients with chronic respiratory diseases, such as DMD.3
In general, the primary treatment for panic disorder is medication and cognitive-behavioral therapy. However, in DMD patients, psychopharmacological treatment is not preferred because of medical concerns, interaction with other drugs, and nonpharmacological treatment, which is considered first. Neurofeedback (NFB) is an operant conditioning procedure that improves the brain’s functional activity.4,5 Functional brain diseases such as psychiatric disorders can derive from pathological brain networks, which can be detected by electroencephalogram (EEG).5 NFB uses a brain–computer interface that enables the users to know the real-time activity of their brain and learn to control it by feedback signals.4,5
Here, we report 3 patients who experienced panic attacks in the course of deteriorating MD. All patients gave written informed consent, and the study was approved by an institutional review board (YUMC 2016-07-023).
Case Presentation
Case 1
A 17-year-old man with DMD was consulted for psychiatric evaluation after complaints of recurrent panic attacks. Because of his medical condition, physicians and doctors of rehabilitation medicine recommended nonpharmacological treatment. NFB treatment was initiated in addition to supportive psychotherapy. The patient was treated with slow motor rhythm (SMR) at T4 and alpha/theta (α/θ) at Pz, 1–2 times a week. We assessed patient’s panic symptoms by the panic and agoraphobia scale6 at baseline and after the NFB treatment. His panic attacks subsided rapidly, and his sleep quality also improved. After 12 sessions, the patient did not experience any panic attacks and had no relapse after discontinuing the NFB treatment.
Case 2
A 20-year-old man with DMD was suggested psychiatric treatment because of recurrent panic attacks and depressed mood. We could not prescribe antidepressants because of his medical condition and therefore initiated NFB treatment 1–2 times a week. The protocol was beta (β) training at F3 and α/θ training at Pz. The patient showed improvements in insomnia and low energy after 4 sessions of the treatment and a reduction in panic attacks after 12 sessions. His painful physical symptoms persisted, but his preoccupation with physical pain was decreased.
Case 3
A 40-year-old man with MD and unknown etiology was referred because of recurrent panic attacks and anticipatory anxiety. To control his anxiety, we first prescribed paroxetine and alprazolam. However, we had to withdraw medications due to intolerable side effects and medical concerns. Therefore, NFB treatment was initiated. The protocol included SMR at T4 and α/θ at Pz. His panic attacks decreased immediately after the initiation of the NFB treatment. After 7 treatment sessions, he did not experience any panic attacks.
NFB Apparatus and Protocol
The neurocybernetics model (Neurocybernetics) used NFB training. The computer game was introduced to patients in the SMR or β training protocol, and reward feedback was represented with achievement scores during and after training (Table 1).5,7 In the α/θ training protocol in the Pz, patients sat in a chair with their eyes closed and provided only audio feedback. For SMR training, the reward band ranged from 12 to 15 Hz, and for β training, it ranged from 15 to 18 Hz. For the α/θ training, theta (5–8 Hz) and alpha (8–12 Hz) were rewarded bands.5,7
Table 1.
States of Neurofeedback and Clinical Assessment by the Panic and Agoraphobia Scale
| Subjects | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Frequency | 12 | 12 | 7 |
| Protocol | SMR at T4 alpha/theta at Pz | beta at F3 alpha/theta at Pz | SMR at T4 alpha/theta at Pz |
| P&A | |||
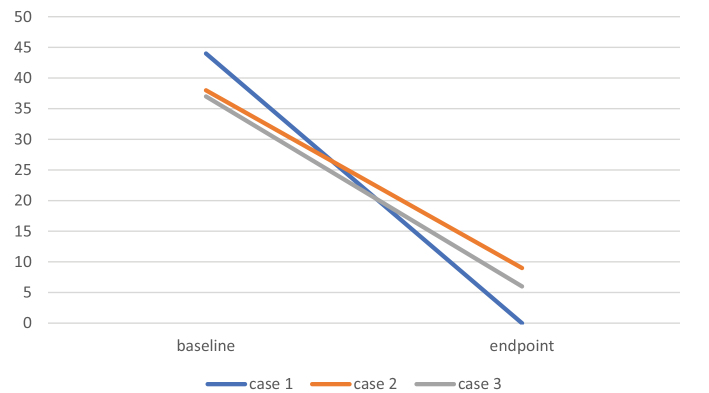
| Baseline | 44 | 38 | 37 |
| Endpoint | 0 | 9 | 6 |
P&A, panic and agoraphobia scale; SMR, slow motor rhythm.
Discussion
Although NFB treatments for panic disorder have been reported, most studies that consider anxiety disorders are case reports with a small number of subjects, and no large-scale, well-managed studies have been conducted so far.8 To the best of our knowledge, this study is the first case report of the treatment of panic symptoms in DMD patients with NFB.
Patients with anxiety disorders need to be relaxed and calm. Individuals in meditative states show high-amplitude alpha activity. 5,9 Accordingly, previous studies have considered training with NFB to increase alpha activity in patients with anxiety disorders. 10 However, Thoma and Sattelberger9 reported that training to increase alpha waves was only beneficial for patients with low amplitude alpha. These previous results indicated that using NFB to decrease alpha activity and increase beta activity also reduced anxiety. Based on these results, we found that treatment plans should be formulated for each case individually based on the patient’s medical history and baseline EEG pattern.5,9
In this study, the NFB protocol was determined by an NFB-certified psychiatrist during each patient’s NFB team meeting. The patients’ most severe symptoms were considered for preferential treatment. Cases 1 and 3 were treated with SMR at T4. Their main symptoms were panic attacks and anxiety. Major depressive disorder (MDD) patients, consistent with a key role of the right prefrontal cortex in anxiety and anxiety disorders.11,12 T4 SMR might help to reduce right prefrontal hyperactivity. In contrast, Case 2 was provided NFB treatment with β training at F3. Enhancing left frontal activity with NFB also alleviated depressive symptoms.5,13 In this study, all 3 patients received α/θ training. The effectiveness of NFB α/θ training can be explained by the ability to cope with anxiety and situations that induce anxiety.14 A study has also suggested that NFB targeting lower frequencies, such as alpha–theta wave, may directly affect core neurocognitive networks, thereby improving symptoms.15 Neuroanatomical circuits include the ascending mesencephalic arousal system and the limbic circuits. These include the connections between the frontal and posterior cortices and illustrate the role of theta and alpha waves in mediating interactions between distal and widely distributed connections.7
NFB treatment is suggested as a safe and effective complimentary treatment for panic disorder patients who are intolerant to pharmacotherapy because of medical conditions, such as DMD.
Figure 1.
Neurofeedback effectiveness was assessed by the panic and agoraphobia scale.
Footnotes
Cite this article as: Kim HG, Koo BH, Lee SW, Cheon EJ. Management of panic disorder with neurofeedback in patients with muscular dystrophy: a case report. Alpha Psychiatry. 2021;22(4):219-221.s
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer Review: Externally peer-reviewed.
Author Contributions: Concept - H.G.K., E.J.C.; Design - H.G.K., S.W.L., E.J.C.; Supervision - B.H.K., E.J.C.; Resources - H.G.K., B.H.K., S.W.L., E.J.C.; Materials - H.G.K., S.W.L.; Data Collection and/or Processing - H.G.K., S.W.L.; Analysis and/or Interpretation - H.G.K., S.W.L.; Literature Search - H.G.K., S.W.L.; Writing Manuscript - H.G.K., E.J.C.; Critical Review - B.H.K., E.J.C.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9(1):77–93. 10.1016/S1474-4422(09)70271-6 [DOI] [PubMed] [Google Scholar]
- 2.Chen YH, Lin HC. Patterns of psychiatric and physical comorbidities associated with panic disorder in a nationwide population-based study in Taiwan. Acta Psychiatr Scand. 2011;123(1):55–61. 10.1111/j.1600-0447.2010.01541.x [DOI] [PubMed] [Google Scholar]
- 3.Hallas CN, Howard C, Theadom A, Wray J. Negative beliefs about breathlessness increases panic for patients with chronic respiratory disease. Psychol Health Med. 2012;17(4):467–477. 10.1080/13548506.2011.626434 [DOI] [PubMed] [Google Scholar]
- 4.Weiskopf N, Scharnowski F, Veit R, et al. Self-regulation of local brain activity using real-time functional magnetic resonance imaging (fMRI). J Physiol Paris. 2004;98(4–6):357–373. 10.1016/j.jphysparis.2005.09.019 [DOI] [PubMed] [Google Scholar]
- 5.Cheon EJ, Koo BH, Seo WS, et al. Effects of neurofeedback on adult patients with psychiatric disorders in a naturalistic setting. Appl Psychophysiol Biofeedback. 2015;40(1):17–24. 10.1007/s10484-015-9269-x [DOI] [PubMed] [Google Scholar]
- 6.Bandelow B. Assessing the efficacy of treatments for panic disorder and agoraphobia. II. The Panic and agoraphobia Scale. Int Clin Psychopharmacol. 1995;10(2):73–81. 10.1097/00004850-199506000-00003 [DOI] [PubMed] [Google Scholar]
- 7.Cheon EJ, Koo BH, Choi JH. The efficacy of neurofeedback in patients with major depressive disorder: an open labeled prospective study. Appl Psychophysiol Biofeedback. 2016;41(1):103–110. 10.1007/s10484-015-9315-8 [DOI] [PubMed] [Google Scholar]
- 8.Moore NC. A review of EEG biofeedback treatment of anxiety disorders. Clin Electroencephalogr. 2000;31(1):1–6. 10.1177/155005940003100105 [DOI] [PubMed] [Google Scholar]
- 9.Thomas JE, Sattlberger E. Treatment of chronic anxiety disorder with neurotherapy. J Neurother. 1997;2(2):14–19. 10.1300/J184v02n02_03 [DOI] [Google Scholar]
- 10.Schwartz MS. Biofeedback : A Practitioner’s Guide. New York: Guilford Press; 1987. [Google Scholar]
- 11.Bruder GE, Fong R, Tenke CE, et al. Regional brain asymmetries in major depression with or without an anxiety disorder: a quantitative electroencephalographic study. Biol Psychiatry. 1997;41(9):939–948. 10.1016/S0006-3223(96)00260-0 [DOI] [PubMed] [Google Scholar]
- 12.Wiedemann G, Pauli P, Dengler W, et al. Frontal brain asymmetry as a biological substrate of emotions in patients with panic disorders. Arch Gen Psychiatry. 1999;56(1):78–84. 10.1001/archpsyc.56.1.78 [DOI] [PubMed] [Google Scholar]
- 13.Rosenfeld JP. An EEG biofeedback protocol for affective disorders. Clin Electroencephalogr. 2000;31(1):7–12. 10.1177/155005940003100106 [DOI] [PubMed] [Google Scholar]
- 14.Peniston EG, Kulkosky PJ. Alpha-theta brainwave training and betaendorphin levels in alcoholics. Alcohol Clin Exp Res. 1989;13(2):271–279. 10.1111/j.1530-0277.1989.tb00325.x [DOI] [PubMed] [Google Scholar]
- 15.Niv S. Clinical efficacy and potential mechanisms of neurofeedback. Pers Individ Dif. 2013;54(6):676–686. 10.1016/j.paid.2012.11.037 [DOI] [Google Scholar]