Abstract
Recently, we showed that the addition of physiological concentrations of ascorbic acid, a tear antioxidant, to the OptiSafe™ macromolecular eye irritation test reduced the OptiSafe irritation scores of false-positive (FP) chemicals that had reactive chemistries leading to formation of reactive oxygen species (ROS) and molecular crosslinking. The purpose of the current study was to 1) increase the number of chemicals tested to comprehensibly determine whether the antioxidant-associated reduction in optical density (OD) is specific to FP chemicals associated with ROS chemistries, and 2) determine whether the addition of antioxidants interferes with the detection of true positive (TP) and true negative (TN) ocular irritants. We report that when ascorbic acid is added to the test reagents, retesting of FP chemicals with reactive chemistries show significantly reduced OD values (P<0.05). Importantly, ascorbic acid had no significant effect on the OD values of TP or TN chemicals regardless of chemical reactivity. These findings suggest that supplementation of ascorbic acid in alternative ocular irritation test may help improve the detection of TN for those commonly misclassified reactive chemicals.
Keywords: Ocular Irritation, Antioxidant, Validation
Introduction
The in vivo rabbit eye test (Draize method) uses a clinical scoring system to assess the severity and duration of the ocular irritation response between 1 and 21 days after exposure to a test substance (Draize et al., 1944). The clinical scores and durations that result from the Draize test are then applied to either the United Nations Globally Harmonized System of classification and labeling (GHS; UN, 2011) or the U.S. Environmental Protection Agency (EPA) methods of eye irritation classification (ICCVAM, 2010). Since the use of live animals for routine product testing raises serious ethical and animal cruelty concerns, there is a movement toward the adoption of nonanimal, in vitro tests for the classification of eye area products and chemicals. While these alternative tests are accepted for the identification of the most severe level of ocular irritation (ocular corrosives, GHS Category 1) and the least irritating level [GHS Not Classified (NC) as an ocular irritant], detection of reversible irritants (GHS Category 2B and 2A) has been problematic because of the high false-positive (FP) and false-negative (FN) rate for detection of non-irritants and ocular corrosives leading to inaccurate predictions for the middle classification (reversible irritants) (Lebrun et al., 2019).
Recently, we reviewed the FP and FN rates for the currently accepted alternative eye irritation tests, including Bovine Corneal Opacity and Permeability, EpiOcular™, Isolated Chicken Eye, Ocular Irritection®, and OptiSafe™ tests. In this study, we identified that most if not all tests miss predicted the same group of chemicals as FP (GHS NC overpredicted as GHS Category 2 or 1), suggesting that current in vitro tests do not fully model the in vivo eye (Lebrun et al., 2020). To understand this deficiency, we evaluated the chemical properties of common FP chemicals by searching publication databases and identified that many exhibited chemistries that covalently bind molecules via electron transfer and redox cycling that can lead to the generation of reactive oxygen species (ROS) (van Amsterdam et al., 2001; Kovacic et al., 2002) or act as a chemical crosslinker (CL). We further noted that the eye contains high levels of antioxidants in the tear film, the first barrier to chemicals interacting with the eye. In a recent study, we found that the tear antioxidant, ascorbic acid, significantly reduced the OD for the macromolecular OptiSafe eye irritation test that were generated by several commonly misclassified FP chemicals (Patent Application No. 17/203467, 2021; Lebrun et al., 2021). In our study, five tear-related antioxidants were individually added to the OptiSafe™ formulation, and the effects on OD measurements used for irritant classification were determined. Ascorbic acid, the most abundant water-soluble antioxidant found in tears (Chen et al., 2009), was the most effective tear antioxidant that reduced both the OD and, consequently, the FP classification rate compared to the other tear antioxidants tested. Titration curves showed that this reduction occurred at physiologic tear concentrations for ascorbic acid and appeared specific for chemicals identified as producing ROS or acting as a CL. The purpose of this study was to expand upon these encouraging results by 1) increasing the number of chemicals tested to include prior chemicals used in the recently published OptiSafe validation study and determining whether the effect of ascorbic acid was specific to FP chemicals associated with reactive chemistries, and 2) establishing whether ascorbic acid interferes with the detection of true-positive (TP) and true-negative (TN) ocular irritants.
Methods
OptiSafe Eye Irritation Test
Details about the method and protocol used to perform the macromolecular OptiSafe eye irritation test have been previously published (Choksi et al., 2020; U.S. Patent No. 20160290982 A1, 2018). Briefly, OptiSafe measures the damage, that can be measured by the change in OD, to a solution of purified macromolecules in a test reagent mixture after interaction with test chemicals. The specific exposure of test chemicals to the reagent mix is determined using a defined approach (DA) wherein different physiochemical properties of the test chemical are measured, and the approach is modified accordingly. The change in OD is then measured and compared to a standard curve generated by known ocular irritants to give a final score that is then applied to a prediction model for irritation classification. To add ascorbic acid, the OptiSafe test reagent mix was placed in a beaker with a magnetic stir bar, and 0.1 mg/mL ascorbic acid (Sigma Aldrich, Milwaukee, WI; catalog number: A5960) was added and allowed to mix until the pH was stable (approximately 10 minutes). The pH was then adjusted following the OptiSafe procedure (see Choksi et al., 2020).
Test Chemicals
Test chemicals were selected from our prior validation test chemicals as previously reported (Choksi et al., 2020). Since we have made changes in the DA for testing some chemicals, only 62 of the original 78 chemicals used in the validation study were used in the current testing strategy and are listed in Table 1. All 62 chemicals were retested with the addition of ascorbic acid to the reagent mix and the OD measured. Chemicals included solids (powders and crystalline solids), liquids (viscous and nonviscous) and semisolids. Chemicals were identified as ROS or CL by searching the published literature from PubMed or Google Scholar. The name, CASRN, GHS classification, EPA classification, physical state, supplier, catalog number, and purity for chemicals tested are shown in Table 1. Of these, there were 36 GHS NC, 8 GHS Category 2B, 11 GHS Category 2A, and 7 GHS Category 1 chemicals.
Table 1.
Test Chemicals
| # | Name | CASRN | GHS | EPA | Phys. State | Supplier | Catalog No. | Purity (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | Cyclopentasiloxane | 541-02-6 | NC | NA | L | SiAl | 444278 | 97.0 |
| 2 | Glycerol | 56-81-5 | NC | IV | L | SiAl | G5516 | ≥99.0 |
| 3 | Hexane | 110-54-3 | NC | IV | L | SiAl | 270504 | ≥95.0 |
| 4 | Dodecane | 112-40-3 | NC | III | L | SiAl | 297879 | ≥99.0 |
| 5 | iso-Octyl acrylate | 29590-42-9 | NC | IV | L | SiAl | 437425 | ≥90.0 |
| 6 | Hexamethyldisiloxane | 107-46-0 | NC | IV | L | SiAl | 52630 | ≥98.5 |
| 7 | Hexyl cinnamic aldehyde | 101-86-0 | NC | IV | L | SiAl | W25690 | ≥95.0 |
| 8 | n-Hexyl bromide | 111-25-1 | NC | IV | L | SiAl | B68240 | 98.0 |
| 9 | 1,6-Dibromohexane | 629-03-8 | NC | IV | L | SiAl | D41007 | 96.0 |
| 10 | Di-iso-butyl ketone | 108-83-8 | NC | IV | L | SiAl | 273848 | 99.0 |
| 11 | Xylene | 1330-20-7 | NC | II | L | SiAl | 534056 | - |
| 12 | n-Octyl bromide | 111-83-1 | NC | IV | L | SiAl | 152951 | 99.0 |
| 13 | 3-Methoxy-1,2-propanediol | 623-39-2 | NC | IV | L | SiAl | 260401 | 98.0 |
| 14 | Propylene glycol | 57-55-6 | NC | IV | L | SiAl | 398039 | ≥99.5 |
| 15 | 1-Bromo-4-chlorobutane | 6940-78-9 | NC | IV | L | SiAl | B60800 | 99.0 |
| 16 | 1,2,6-Hexanetriol | 106-69-4 | NC | IV | L | SiAl | T66206 | 96.0 |
| 17 | 2-Ethylhexylthioglycolate | 7659-86-1 | NC | IV | L | SiAl | 88670 | ≥95.0 |
| 18 | 2,4-Pentanediol | 625-69-4 | NC | IV | L | SiAl | 156019 | 98.0 |
| 19 | p-Methyl thiobenzaldehyde | 3446-89-7 | NC | IV | L | SiAl | 222771 | 95.0 |
| 20 | n,n-Dimethylguanidine sulfate | 598-65-2 | NC | III | S | SiAl | 276669 | 97.0 |
| 21 | Ethyl acetate | 141-78-6 | NC | III | L | SiAl | 270989 | 99.8 |
| 22 | 3-Phenoxybenzyl alcohol | 13826-35-2 | NC | III | L | SiAl | 190284 | 98.0 |
| 23 | 2,4-Pentanedione | 123-54-6 | NC | III | L | SiAl | P7754 | ≥99.0 |
| 24 | Triphenyl phosphite | 101-02-0 | NC | IV | L | SiAl | T84654 | 97.0 |
| 25 | 1,4-Dibromobutane | 110-52-1 | NC | III | L | SiAl | 140805 | 99.0 |
| 26 | 1,5-Hexadiene | 592-42-7 | NC | III | L | SiAl | 128554 | 97.0 |
| 27 | iso-Propyl bromide | 75-26-3 | NC | IV | L | SiAl | B78114 | 99.0 |
| 28 | Triethylene glycol | 112-27-6 | NC | IV | L | SiAl | T59455 | 99.0 |
| 29 | 2,2-Dimethyl-3-pentanol | 3970-62-5 | NC | III | L | SiAl | D173622 | 97.0 |
| 30 | 2-(2-Ethoxyethoxy)ethanol | 111-90-0 | NC | III | L | SiAl | 537616 | 99.0 |
| 31 | Potassium tetrafluoroborate | 14075-53-7 | NC | IV | S | SiAl | 278955 | 96.0 |
| 32 | 1,9-Decadiene | 1647-16-1 | NC | IV | L | SiAl | 118303 | 97.0 |
| 33 | Ethylene glycol diethyl ether | 629-14-1 | NC | IV | L | SiAl | 224111 | 98.0 |
| 34 | Styrene | 100-42-5 | NC | III | L | SiAl | S4972 | ≥99.0 |
| 35 | 1,3-Di-iso-propylbenzene | 99-62-7 | NC | IV | L | SiAl | 113263 | 96.0 |
| 36 | 2-Ethoxyethyl methacrylate | 2370-63-0 | NC | IV | L | SiAl | 280666 | 99.0 |
| 37 | 2-Methyl-1-pentanol | 105-30-6 | 2B | III | L | SiAl | 214019 | 99.0 |
| 38 | Isobutyraldehyde | 78-84-2 | 2B | III | L | SiAl | 240788 | ≥99.0 |
| 39 | n,n-Diethyl-m-toluamide | 134-62-3 | 2B | III | L | SiAl | D100951 | 97.0 |
| 40 | 3-Chloropropionitrile | 542-76-7 | 2B | III | L | SiAl | C69101 | 98.0 |
| 41 | n-Butanal | 123-72-8 | 2B | III | L | SiAl | 418102 | 99.5 |
| 42 | Ethyl-2-methyl acetoacetate | 609-14-3 | 2B | III | L | SiAl | E35400 | 90.0 |
| 43 | Maneb (solid) | 12427-38-2 | 2B | III | S | SiAl | 45554 | 90.0 |
| 44 | 6-Methyl purine | 2004-03-7 | 2B | I | S | FiSc | 50-496-810 | - |
| 45 | Ammonium nitrate | 6484-52-2 | 2A | III | S | SiAl | A3795 | ≥99.5 |
| 46 | Isobutanol | 78-83-1 | 2A | II | L | SiAl | 33064 | ≥99.0 |
| 47 | Propasol solvent P | 1569-01-3 | 2A | II | L | SiAl | 484326 | ≥98.5 |
| 48 | Methyl cyanoacetate | 105-34-0 | 2A | II | L | SiAl | 108421 | 99.0 |
| 49 | Isopropanol | 67-63-0 | 2A | III | L | SiAl | I9516 | ≥99.5 |
| 50 | Allyl alcohol | 107-18-6 | 2A | III | L | SiAl | 240532 | ≥99.0 |
| 51 | Cyclopentanol | 96-41-3 | 2A | II | L | SiAl | C112208 | 99.0 |
| 52 | n-Hexanol | 111-27-3 | 2A | II | L | SiAl | 471402 | ≥99.0 |
| 53 | gamma-Butyrolactone | 96-48-0 | 2A | II | L | SiAl | B103608 | ≥99.0 |
| 54 | n-Octanol | 111-87-5 | 2A | II | L | SiAl | 297887 | ≥99.0 |
| 55 | Methyl acetate | 79-20-9 | 2A | II | L | SiAl | 296996 | 99.5 |
| 56 | n-Butanol | 71-36-3 | 1/2A | II | L | SiAl | B7906 | ≥99.0 |
| 57 | 3,4-Dichlorophenyl isocyanate | 102-36-3 | 1 | I | S | SiAl | 245607 | 97.0 |
| 58 | p-Tert-butylphenol | 98-54-4 | 1 | I | S | SiAl | B99901 | 99.0 |
| 59 | Methylthioglycolate | 2365-48-2 | 1 | II | L | SiAl | 108995 | 95.0 |
| 60 | Cyclohexanol | 108-93-0 | 1 | I | L | SiAl | 105899 | 99.0 |
| 61 | Protectol PP | 80-54-6 | 1 | I | L | SiAl | 43884 | ≥96.0 |
| 62 | Lauric acid | 143-07-7 | 1 | I | S | SiAl | W261408 | ≥98.0 |
Table 1. CASRN = Chemical Abstracts Service Registry Number; GHS = Globally Harmonized System of classification and labeling of chemicals; EPA = Environmental Protection Agency; NC = Not Classified; Phys. State = Physical State; L = Liquid; S = Solid; SiAl = Sigma Aldrich; FiSc = Fisher Scientific; Catalog No. = Catalog number.
Statistical analysis
All results are reported as the mean ± standard error (SE). Differences between groups were assessed by Chi Square and two-way analysis of variance (ANOVA) (Holm-Sidak method) for all pairwise multiple comparisons (Sigma Stat version 4.0, Systat Software Inc, Point Richmond, CA). All measurements were based on triplicate samples, and a P value of less than 0.05 was considered statistically significant.
Results
The averages and SEs for the triplicate OD measurements of the test chemicals with and without ascorbic acid are presented in Table 2. In addition, those chemicals that have been reported to have reactive chemical properties and capable of forming ROS or acting as CLs are identified along with the reporting sources. The average difference (ΔOD) between the measured OD before (OS I) and after (OS II) addition of ascorbic acid to the Optisafe (OS) test are also provided. Overall, of the 62 test chemicals, there were 18 TN chemicals, of which 5 were identified as reactive chemicals; 18 FN chemicals, of which 10 were identified as reactive chemicals; and 26 TP chemicals, of which 5 were identified as reactive chemicals. A Chi Square analysis indicated that there was a significant relationship between classification and chemistry with FP chemicals overly represented by reactive chemicals (P<0.05).
Table 2.
OD Comparison with/without Ascorbic Acid
| Chemical | Classification | ROS/CL | OS I | OS II | ΔOD | ||
|---|---|---|---|---|---|---|---|
| Avg. OD | SE | Avg. OD | SE | Avg. | |||
| 1 | True Negative | No evidence1 | 226.0 | 8.9 | 264.7 | 11.5 | 38.7 |
| 2 | True Negative | No evidence | 229.7 | 10.2 | 257.7 | 10.1 | 28.0 |
| 3 | True Negative | Y (Zhang, 2015)2 | 246.7 | 6.7 | 271.7 | 14.9 | 25.0 |
| 4 | True Negative | No evidence | 253.7 | 12.3 | 257.7 | 14.3 | 4.0 |
| 5 | True Negative | No evidence | 267.0 | 8.4 | 261.3 | 20.2 | −5.7 |
| 6 | True Negative | No evidence | 268.7 | 28.5 | 247.0 | 8.9 | −21.7 |
| 7 | True Negative | No evidence | 279.3 | 17.9 | 267.0 | 10.4 | −12.3 |
| 8 | True Negative | Y (Lee, 2010) | 286.3 | 15.1 | 298.0 | 9.5 | 11.7 |
| 9 | True Negative | Y (Metelko, 1989; Han, 2014) | 291.3 | 11.3 | 301.7 | 15.3 | 10.3 |
| 10 | True Negative | No evidence | 316.0 | 32.5 | 302.3 | 11.1 | −13.7 |
| 11 | True Negative | Y (Zhu, 2021) | 321.0 | 23.1 | 272.7 | 10.2 | −48.3 |
| 12 | True Negative | No evidence | 322.0 | 28.9 | 271.3 | 8.1 | −50.7 |
| 13 | True Negative | No evidence | 343.3 | 30.3 | 323.3 | 7.6 | −20.0 |
| 14 | True Negative | No evidence | 346.0 | 10.3 | 376.7 | 40.3 | 30.7 |
| 15 | True Negative | No evidence | 346.7 | 13.6 | 330.3 | 32.1 | −16.3 |
| 16 | True Negative | Y (Iza, 1998; Divakaran, 2014) | 354.3 | 31.8 | 331.7 | 13.7 | −22.7 |
| 17 | True Negative | No evidence | 350.3 | 10.4 | 325.7 | 15.7 | −24.7 |
| 18 | True Negative | No evidence | 444.3 | 14.4 | 416.3 | 14.8 | −28.0 |
| 19 | False Positive | No evidence | 323.3 | 11.2 | 354.0 | 8.5 | 30.7 |
| 20 | False Positive | No evidence | 543.3 | 4.3 | 478.7 | 40.5 | −64.7 |
| 21 | False Positive | No evidence | 798.0 | 6.9 | 674.3 | 17.3 | −123.7 |
| 22 | False Positive | No evidence | 1059.7 | 48.6 | 939.0 | 23.8 | −120.7 |
| 23 | False Positive | No evidence | 1325.0 | 31.7 | 1306.0 | 64.1 | −19.0 |
| 24 | False Positive | No evidence | 2888.0 | 20.5 | 2859.0 | 42.8 | −29.0 |
| 25 | False Positive | Y (Nishide, 1977; Sriram, 2001) | 377.7 | 11.8 | 292.7 | 11.3 | −85.0 |
| 26 | False Positive | Y (Lou, 2000; Zhao, 2006) | 453.3 | 27.9 | 253.7 | 5.0 | −199.7 |
| 27 | False Positive | Y (Wu, 2002) | 463.3 | 21.9 | 307.3 | 3.0 | −156.0 |
| 28 | False Positive | Y (Zhu, 2012; Mikulas, 2018) | 543.7 | 63.4 | 319.3 | 23.6 | −224.3 |
| 29 | False Positive | No evidence | 680.3 | 14.4 | 481.3 | 5.4 | −199.0 |
| 30 | False Positive | Y (Adedara, 2014; Bodin, 2003) | 734.7 | 7.3 | 433.3 | 2.2 | −301.3 |
| 31 | False Positive | No evidence | 740.3 | 3.5 | 419.0 | 25.7 | −321.3 |
| 32 | False Positive | Y (Palmlof, 2000; Smedburg, 1997) | 763.7 | 61.6 | 429.7 | 68.2 | −334.0 |
| 33 | False Positive | Y (Di Tommaso, 2011; Clark, 2001) | 785.7 | 18.2 | 583.0 | 17.0 | −202.7 |
| 34 | False Positive | Y (Zhang, 2017; Belvedere, 1981) | 1440.3 | 176.8 | 276.0 | 15.1 | −1164.3 |
| 35 | False Positive | Y (Cavalli, 1975; Baj, 1991) | 1579.7 | 25.2 | 845.7 | 40.5 | −734.0 |
| 36 | False Positive | Y (Chirila, 1991; Garcia, 2002) | 2953.0 | 29.5 | 377.0 | 16.3 | −2576.0 |
| 37 | True Positive | No evidence | 1032.3 | 20.3 | 930.0 | 7.8 | −102.3 |
| 38 | True Positive | No evidence | 2064.0 | 15.3 | 2075.7 | 46.5 | 11.7 |
| 39 | True Positive | No evidence | 1381.7 | 21.8 | 1365.0 | 66.0 | −16.7 |
| 40 | True Positive | No evidence | 1409.3 | 46.8 | 1261.0 | 25.2 | −148.3 |
| 41 | True Positive | Y (Shrager, 1969; Kuykendalll, 1992) | 1840.7 | 20.7 | 1473.7 | 73.7 | −367.0 |
| 42 | True Positive | Y (Hong, 2008) | 605.7 | 3.2 | 551.0 | 16.9 | −54.7 |
| 43 | True Positive | Y (Amara, 2015; Jaballi, 2017) | 1954.7 | 125.5 | 2412.7 | 76.3 | 458.0 |
| 44 | True Positive | No evidence | 796.3 | 49.0 | 604.0 | 94.0 | −192.3 |
| 45 | True Positive | No evidence | 640.7 | 35.0 | 630.3 | 19.6 | −10.3 |
| 46 | True Positive | No evidence | 1457.3 | 47.1 | 1335.7 | 26.2 | −121.7 |
| 47 | True Positive | No evidence | 1098.7 | 9.6 | 986.7 | 24.0 | −112.0 |
| 48 | True Positive | No evidence | 2091.7 | 11.6 | 1896.3 | 39.3 | −195.3 |
| 49 | True Positive | No evidence | 965.0 | 34.9 | 861.3 | 20.0 | −103.7 |
| 50 | True Positive | Y (Buonocore, 2010) | 1150.0 | 22.9 | 1236.0 | 20.2 | 86.0 |
| 51 | True Positive | Y (Brown, 1954) | 1701.7 | 30.9 | 1608.3 | 14.3 | −93.3 |
| 52 | True Positive | No evidence | 990.7 | 25.9 | 1053.0 | 81.5 | 62.3 |
| 53 | True Positive | No evidence | 909.7 | 12.7 | 880.0 | 63.3 | −29.7 |
| 54 | True Positive | No evidence | 638.0 | 21.4 | 614.3 | 34.9 | −23.7 |
| 55 | True Positive | No evidence | 840.0 | 67.9 | 634.0 | 10.0 | −206.0 |
| 56 | True Positive | No evidence | 1566.0 | 3.8 | 1420.7 | 27.7 | −145.3 |
| 57 | True Positive | No evidence | 2670.7 | 133.7 | 2739.7 | 146.4 | 69.0 |
| 58 | True Positive | No evidence | 2605.7 | 61.1 | 2023.3 | 22.1 | −582.3 |
| 59 | True Positive | No evidence | 1751.3 | 37.6 | 1691.3 | 58.4 | −60.0 |
| 60 | True Positive | No evidence | 1429.0 | 36.5 | 1386.7 | 17.7 | −42.3 |
| 61 | True Positive | No evidence | 1846.7 | 37.9 | 1672.7 | 15.1 | −174.0 |
| 62 | True Positive | No evidence | 2309.7 | 128.4 | 1616.0 | 125.3 | −693.7 |
Table 2. ROS = Reactive oxygen species; CL = Crosslinker; OS I = OptiSafe without Ascorbic acid; OS II = OptiSafe with Ascorbic acid; Avg. OD = Average optical density value; SE = Standard error; Avg. = Average; ΔOD = Change in OD value;
No Evidence was recorded for echemicals where a literature search failed to reveal evidence of the chemical forming ROS or crosslinks;
Yes (Y) and identifies those chemicals were a literature search identified a report of chemical reactivity to form ROS or crosslinks. Parenthesis identifies the reference source.
Table 3 provides a breakdown of the TN, FP, and TP chemicals into either the nonreactive or reactive (ROS/CL) subgroups and presents the average and SE of the OD measurements before (OS I) and after (OS II) the addition of ascorbic acid as well as the average and SE of the difference (OS II – OS I). A two-way ANOVA identified that ascorbic acid significantly lowered the OD values for FP chemicals with reactive chemistries but had no significant effect on the OD values of TNs or TPs, regardless of chemistry, or FPs with nonreactive chemistries. Further, ascorbic acid significantly lowered the OD values of the reactive FP chemicals compared to the nonreactive FP chemicals. Overall, the effect of ascorbic acid is best demonstrated in the scatter plots shown in Figure 1. Plotting of OD measurements with and without ascorbic acid for TN chemicals showed virtually no effect of ascorbic acid regardless of reactive chemistries (Figure 1A). Similarly, ascorbic acid showed no effect on the OD measurements of TPs, with and without reactive chemistries, and FPs without reactive chemistries (Figure 1B). However, ascorbic acid appeared to specifically lower all FP chemicals with reactive chemistries as shown by the red trendline (Figure 1B).
Table 3.
Effect of Ascorbic Acid by Chemical Classification and Reactivity
| Classification | Number | OS I | OS II | P-valule1 | OS II - OS I | P-value2 | |||
|---|---|---|---|---|---|---|---|---|---|
| Avg.OD | SE | Avg. OD | SE | Avg. ΔOD | SE | ||||
| True Negative | |||||||||
| Non Reactive | 13 | 16.9 | 14.5 | NS | 14.5 | NS | |||
| Reactive | 5 | 18.0 | 11.0 | NS | 6.0 | ||||
| False Positives | |||||||||
| Non Reactive | 8 | 266.2 | 277.4 | NS | 14.1 | <0.05 | |||
| Reactive | 10 | 251.6 | 57.4 | <0.05 | 76.9 | ||||
| True Positives | |||||||||
| Non Reactive | 21 | 136.0 | 124.2 | NS | 40.8 | NS | |||
| Reactive | 5 | 252.3 | 300.5 | NS | 134.8 | ||||
Table 3. OS I = OptiSafe without Ascorbic acid; OS II = OptiSafe with Ascorbic acid; Avg. OD = Average optical density value; SE = Standard error; Avg. ΔOD = Average change in OD value;
Effects of ascorbic acid on measured OD of chemicals;
Comparison of the effects of ascorbic acid on non-reactive versus reactive chemicals;
NS = Not significant.
Figure 1.
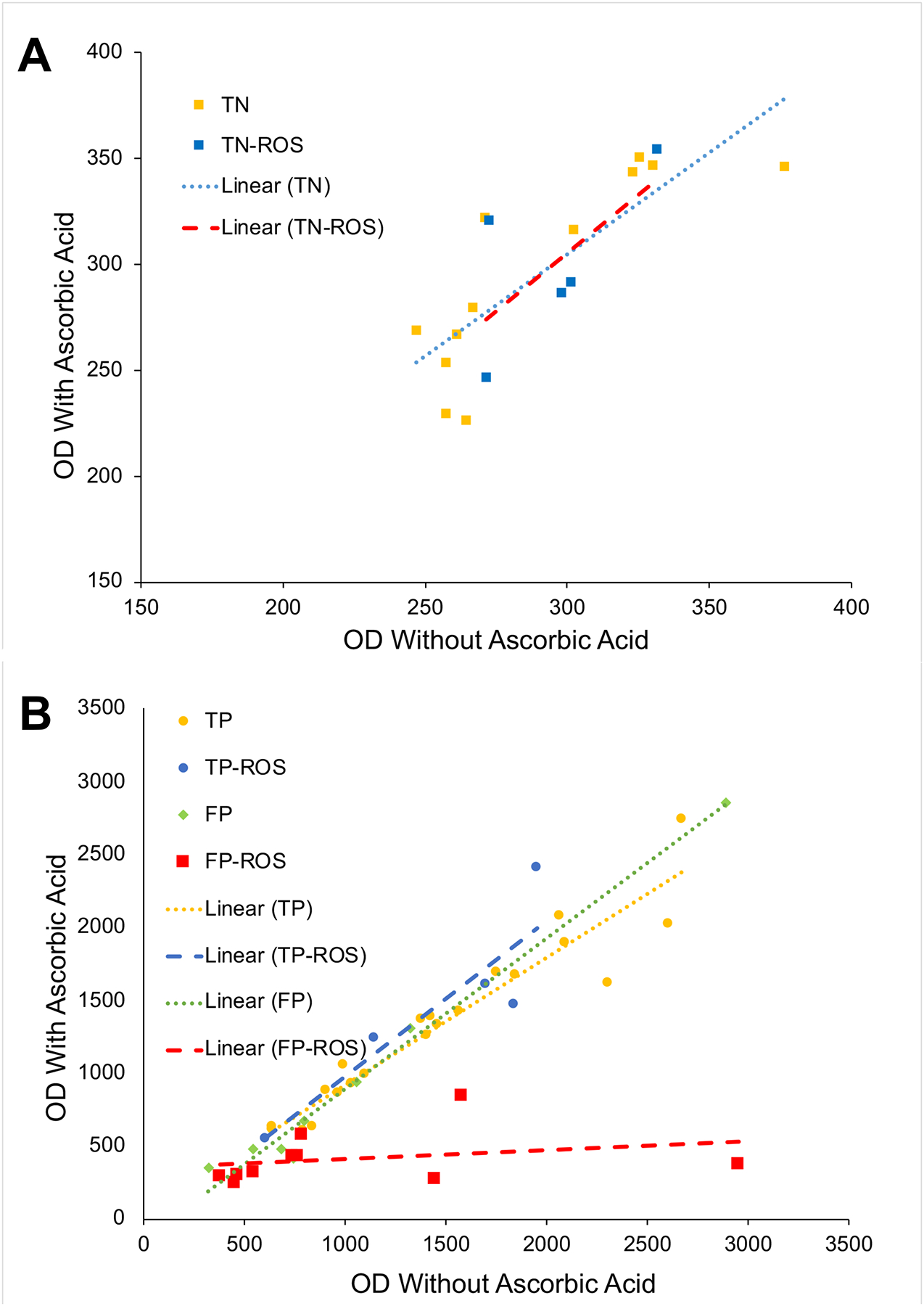
A. Scatter plots for true negatives test chemicals without (TN) and with (TN-ROS) reactive chemistry. Note that the trend lines show no effect of ascorbic acid. B. Scatter plots for true positives and false positives without (TP and FP, respectively) and with (TP-ROS and FP-ROS, respectively) ascorbic acid. Note that the trend line for the FP reactive chemicals (FP-ROS) shows a dramatic effect on the OD measurements. OD = Optical density value; TN = True negative; TP = True positive; FP = False positive; ROS = Reactive oxygen species.
Discussion
In our previous studies, we have shown that there is a group of chemicals that are generally misclassified by most, if not all, alternative ocular irritation tests (Lebrun et al., 2020). Analysis of these FP chemicals identified that many were associated with reactive chemistries, particularly those capable of forming ROS and acting as molecular CLs. The cornea is well known to have intracellular and extracellular defense mechanisms that protect against oxidative damage, particularly against UV injury (Chen et al., 2013). In a survey of corneal antioxidants, we identified that the ocular tear film also contains important antioxidants, including but not limited to ascorbic acid, which is in particularly high concentrations in the tears, the cornea, and aqueous humor. Recently, we tested the effects of ascorbic acid on a limited subset of reactive chemicals that showed FP classification using the macromolecular alternative ocular irritation test, OptiSafe (Lebrun et al., 2021). In that study, we showed that ascorbic acid significantly reduced the OptiSafe score for some FP reactive chemicals, while showing little effect on the OptiSafe score for a few chemicals classified as TP irritants or NC chemicals.
In this study, we confirm and extend our previous findings and show that the antioxidant, ascorbic acid, has a very specific effect on reactive chemicals that have been classified falsely as irritants/corrosives in the OptiSafe test. The study goes on to show using a validation test set, assembled by an outside source (NICETAM), that reactive chemicals are significantly more likely to be detected as FP chemicals compared to either TN or TP chemicals in our OptiSafe test. Since we have previously shown that many of these chemicals are also misidentified by other alternative tests, it is likely that this inability to correctly identify this set of chemicals has widespread implications for the design and predictability of alternative testing strategies.
First, our findings support the hypothesis that the ocular surface tear film plays an important role in modifying the properties of chemicals that are exposed to the eye. While in this specific case, ascorbic acid has been shown to significantly reduce the effects of FP reactive chemicals, other yet-to-be-identified tear components may have complementary or contrasting effects. These effects, if not taken into consideration in alternative tests, may explain other common mispredictions, particularly if shown to be consistent for the same chemicals between different alternative tests. To our knowledge, the effects of the tear film on the ocular irritation response has not been taken into consideration in modeling ocular irritation or the development of alternative ocular irritation tests and clearly requires further study.
Second, it was surprising to discover that ascorbic acid has such a specific effect on FP reactive chemicals, but not on other reactive chemicals that were correctly identified. Since ascorbic acid acts as a free radical scavenger, it was expected that ascorbic acid would similarly affect all chemicals with ROS or CL chemistries. Since this is not the apparent case, at least when concentrations of ascorbic acid are used at physiological levels, ascorbic acid must have a unique functional role within the tear film. It will therefore be important to assess the effect of ascorbic acid in other eye irritation testing strategies and determine whether similar effects on reducing the misclassification of reactive chemicals can be realized. This possibility also underscores the importance of the continued refinement of current eye irritation tests and the development of improved physiological models that more accurately recapitulate the intact eye.
Conclusion
The results of this study suggest that the tear-related antioxidant ascorbic acid specifically inactivates reactive molecules not associated with GHS ocular irritation before they damage macromolecules, offering an explanation for why some of these chemicals are FPs when tested with in vitro eye irritation tests.
Highlights:
A previous study found that a tear-related antioxidant (ascorbic acid) reduced the false-positive rate of the OptiSafe macromolecular eye irritation test, but only a limited number of chemicals were tested.
In the current study, chemicals from a prior validation study were retested with ascorbic acid.
Results indicate that the addition of ascorbic acid specifically reduced the false-positive rate.
Acknowledgments
Supported in part by NIEHS Small Business Innovative Research Grant, R44ES025501 (Lebrun), Unrestricted Grant from Research to Prevent Blindness, Inc. RPB-203478, and the Skirball program in Molecular Ophthalmology Research to Prevent Blindness, Inc.
Footnotes
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Stewart Lebrun reports financial support was provided by National Institute of Environmental Health Sciences. James V. Jester reports financial support was provided by Research to Prevent Blindness, Inc. and Skirball program in Molecular Ophthalmology Research to Prevent Blindness, Inc. Stewart Lebrun reports a relationship with Lebrun Labs LLC that includes: employment, equity or stocks, and funding grants. James V. Jester reports a relationship with Lebrun Labs LLC that includes: consulting or advisory. Sara Chavez reports a relationship with Lebrun Labs LLC that includes: employment. Roxanne Chan reports a relationship with Lebrun Labs LLC that includes: employment. Linda Nguyen reports a relationship with Lebrun Labs LLC that includes: employment. Stewart Lebrun has patent #“Biochemistry based ocular toxicity assay”. Issued Patent Number US 20160290982 A1 issued to Stewart Lebrun Stewart Lebrun has patent #“Formulations and Methods Related to Eye Irritation”. Patent Application Number 17/203467 pending to Lebrun Labs LLC Lebrun Labs LLC and Stewart Lebrun developed the OptiSafe test, sell the OptiSafe test as a kit and provide testing services for the OptiSafe test. The patent Biochemistry Based Ocular Toxicity Assay, Publication number: 20160290982 that covers the OptiSafe test and patent application Methods and Reagents to Improve the Specificity, Sensitivity and Accuracy of Nonanimal Eye Safety Tests, application number 63048112 that covers the use of antioxidants as described in this publication are owned by Stewart Lebrun.
Research reported in this publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under Small Business Innovative Research Grant Award Numbers R44ES025501. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Adedara IA, Farombi EO (2014). “Kolaviron protects against ethylene glycol monoethyl ether-induced toxicity in boar spermatozoa”. Andrologia. 46(4):399–407. doi: 10.1111/and.12095. [DOI] [PubMed] [Google Scholar]
- Amara IB, Saad HB, Hamdaoui L, Karray A, Boudawara T, Ali YB, Zeghal N (2015). “Maneb disturbs expression of superoxide dismutase and glutathione peroxidase, increases reactive oxygen species production, and induces genotoxicity in liver of adult mice”. Environmental Science and Pollution Research. 22: 12309–12322. doi: 10.1007/s11356-015-4434-6. [DOI] [PubMed] [Google Scholar]
- Baj S, Kulicki Z (1991). “High-performance liquid chromatographic determination of products of autoxidation of isopropylbenzene derivatives”. Journal of Chromatography A. 588(1–2): 33–39. doi: 10.1016/0021-9673(91)85004-Y. [DOI] [Google Scholar]
- Belvedere G, Tursi F (1981). “Styrene oxidation to styrene oxide in human blood erythrocytes and lymphocytes”. Research Communications in Chemical Pathology and Pharmacology. 33(2):273–282. [PubMed] [Google Scholar]
- Bodin A, Linnerbord M, Nilsson JLG, Karlberg AT (2003). “Structure elucidation, synthesis, and contact allergen activity of a major hydroperoxide formed at autoxidation of the ethoxylated surfactant C12E5”. Chem Res Toxicol. 16(5):575–82. doi: 10.1021/tx025609n. [DOI] [PubMed] [Google Scholar]
- Brown N, Anderson AW, Schweitzer CE (1954). “Cycloalkanone Peroxides. II. Nature of Peroxides Produced by Oxidation of Cyclopentanol”. J Am Chem Soc. 77(7): 1760–1761. doi: 10.1021/ja01612a011. [DOI] [Google Scholar]
- Buonocore G, Perrone S, Tataranno ML (2010). “Oxygen toxicity: chemistry and biology of reactive oxygen species”. Seminars in Fetal and Neonatal Medicine. 15(4): 186–190. doi: 10.1016/j.siny.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Cavalli L, Cancellieri G (1975). “Quantitative Evaluation of Peroxides, Hydroperoxides and Alcohols of m-Diisopropylbenzene by Nuclear Magnetic Resonance Spectroscopy”. Analyst. 100: 46–50. doi: 10.1039/AN9750000046. [DOI] [Google Scholar]
- Chen Y, Mehta G, Vasiliou V (2009). “Antioxidant defenses in the ocular surface”. Ocul Surf. 7(4):176–185. doi: 10.1016/s1542-0124(12)70185-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Thompson DC, Koppaka V, Jester JV, Vasiliou V (2013). “Ocular aldehyde dehydrogenases: protection against ultraviolet damage and maintenance of transparency for vision”. Prog Retin Eye Res. 33:28–39. doi: 10.1016/j.preteyeres.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirila TV, Walker LN, Constable IJ, Thompson DE, Barrett GD (1991). “Cytotoxic effects of residual chemicals from polymeric biomaterials for artificial soft intraocular lenses”. J Cataract Refract Surg. 17(2):154–62. doi: 10.1016/s0886-3350(13)80245-3. [DOI] [PubMed] [Google Scholar]
- Choksi N, Lebrun S, Nguyen M, Daniel A, DeGeorge G, Willoughby J, Layton A, Lowther D, Merrill J, Matheson J, Barroso K, Yozzo K, Casey W, Allen D (2020). “Validation of the OptiSafeTM eye irritation test”. Cutan Ocul Toxicol. 39(3):180–192. doi: 10.1080/15569527.2020.1787431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DE. (2001). “Peroxides and peroxide-forming compounds”. Chem. Health Saf 8(5):12–22. doi: 10.1021/acs.chas.8b08507. [DOI] [Google Scholar]
- Di Tommaso S, Rotureau P, Crescenzi O, Adamo C (2011). “Oxidation mechanism of diethyl ether: a complex process for a simple molecule”. Physical Chemistry Chemical Physics. 13:14636–14645. Doi: 10.1039/C1CP21357A. [DOI] [PubMed] [Google Scholar]
- Divakaran AV, Torris A, Lele AK, Badiger MV (2014). “Porous poly(ethylene glycol)-polyurethane hydrogels as potential biomaterials”. Polymer International. 64(3): 397–404. doi: 10.1002/pi.4802. [DOI] [Google Scholar]
- Draize JH, Woodard G, Calvery HO (1944). “Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes”. J. Pharmacol. And Exp. Therapeutics 82: 377–390. [Google Scholar]
- Garcia F, Garcia JM, Rubio F, de la Pena JL, Guzman J, Riande E (2002). “Reaction kinetics and gel effect on the polymerization of 2-ethoxyethyl methacrylate and 2(2-ethoxyethoxy) ethyl methacrylate”. Journal of Polymer Science Part A: Polymer Chemistry. 40(22). doi: 10.1002/pola.10480. [DOI] [Google Scholar]
- Han S, Zhang M, Shin J, Lee Y (2014). “A convenient crosslinking method for sulfonated poly(ether ether ketone) membranes via Friedel-crafts reaction using 1,6-dibromohexane and aluminum trichloride”. Journal of Applied Polymer Science. 131(17). doi: 10.1002/app.40695. [DOI] [Google Scholar]
- Hong Y, Hu H, Li F (2008). “Physiological and biochemical effects of allelochemical ethyl 2-methyl acetoacetate (EMA) on cyanobacterium Microcystis aeruginosa”. Exotoxicology and Environmental Safety. 71(2): 527–534. doi: 10.1016/j.ecoenv.2007.10.010. [DOI] [PubMed] [Google Scholar]
- ICCVAM. (2010). “ICCVAM Test Method Evaluation Report: Current Validation Status of a Proposed In Vitro Testing Strategy for U.S. Environmental Protection Agency Ocular Hazard Classification and Labeling of Antimicrobial Cleaning Products”. NIH Publication No. 10–7513 Research Triangle Park, NC: National Institute of Environmental Health Sciences. [Google Scholar]
- Iza M, Stoianovici G, Viora L, Grossiord JL, Courraze G (1998). “Hydrogels of poly(ethylene glycol): mechanical characterization and release of a model drug”. Journal of Controlled Release. 51(1–2): 41–51. doi: 10.1016/S0168-3659(97)00191-0. [DOI] [PubMed] [Google Scholar]
- Jaballi I, Saad HB, Bkhairia I, Kammoun I, Droguet M, Magne C, Boudawara T, Kallel C, Nasri M, Hakim A, Amara IB (2017). Increasing Maneb doses induces reactive oxgen species overproduction and nephrotoxicity in adult mice”. Toxicology Mechanisms and Methods. 27(5). doi: 10.1080/15376516.2017.1300617. [DOI] [PubMed] [Google Scholar]
- Kovacic P, Sacman A, Wu-Weis M (2002). “Nephrotoxins: Widespread Roles of Oxidative Stress and Electron Transfer”. Current Medicinal Chemistry. 9: 823–847. doi: 10.2174/0929867024606803. [DOI] [PubMed] [Google Scholar]
- Kuykendall JR, Bogdanffy MS (1992). “Efficiency of DNA-histone crosslinking induced by saturated and unsaturated aldehydes in vitro”. Mutation Research Letters. 283(2): 131–136. doi: 10.1016/0165-7992(92)90145-8. [DOI] [PubMed] [Google Scholar]
- Lebrun S, Chavez S, Chan R, Nguyen L, Jester JV (2021). “Modeling the Antioxidant Properties of the Eye Reduces the False-Positive Rate of a Nonanimal Eye Irritation Test (OptiSafe)”. Toxicology in Vitro; Accepted 29 June 2021, in press. Manuscript Number: TIV-D-21-00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun S, inventor. (2021). “Formulations and Methods Related to Eye Irritation”. Patent Application Number 17/203467. In review. United States Patent and Trademark Office. [Google Scholar]
- Lebrun S, inventor. (2018). “Biochemistry based ocular toxicity assay”. Issued Patent Number US 20160290982 A1. United States Patent and Trademark Office. [Google Scholar]
- Lebrun S, Nguyen L, Chavez S, Chan R, Le D, Nguyen M, Jester JV (2020). “Same-chemical comparison of nonanimal eye irritation test methods: Bovine corneal permeability, EpiOcular™, isolated chicken eye, ocular Irritection®, OptiSafe™, and short time exposure”. Toxicol In Vitro. 2020: 105070. doi: 10.1016/j.tiv.2020.105070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Lee DJ, Ha HW, Yoo JW, Ko GS, Kang MJ, Kang W, Jeong HG, Lee KB, Jeong TC (2010). “Hepatotoxicity and Immunotoxicity of 1-Bromohexane and Its Glutathione Conjugation in Female BALB/c Mice”. Journal of Health Science. 56(4): 434–441. doi: 10.1248/jhs.56.434. [DOI] [Google Scholar]
- Lou X, Dalton PD, Chirila TV (2000). “Hydrophilic spongers based on 2-hydroxyethyl methacrylate Part VII: Modulation of sponge characteristics by changes in reactivity and hydrophilicity of crosslinking agents”. Journal of Materials Science: Materials in Medicine. 11: 319–325. doi: 10.1023/A:1008977818135. [DOI] [PubMed] [Google Scholar]
- Meltelko M, Zupan M (1989). “Site-site Interactions in a Polymer Matrix. The Effect of Reaction Conditions on the Functionalization of Pyridine Rings in a Crosslinked Poly(Styrene-4-Vinylpyridine) with 1,6-Dibromohexane”. Journal of Macromolecular Science-Chemistry. 26(4): 715–726. doi: 10.1080/00222338908052004. [DOI] [Google Scholar]
- Mikulás K, Hermann P, Gera I, Komlódi T, Horváth G, Ambrus A, Tretter L (2018). “Triethylene glycol dimethacrylate impairs bioenergetic functions and induces oxidative stress in mitochondria via inhibiting respiratory Complex I”. Dent Mater. 34(7):e166–e181. doi: 10.1016/j.dental.2018.03.012. [DOI] [PubMed] [Google Scholar]
- Nishide H, Deguchi J, Tsuchida E (1977). “Adsorption of metal ions on crosslinked poly(4-vinylpyridine) resins prepared with a metal ion as template”. Journal of Polymer Science: Polymer Chemistry Edition. 15(2): 3023–3029. doi: 10.1002/pol.1977.170151216. [DOI] [Google Scholar]
- Palmlof M, Hjertbeg T (2000). “Chemical and mechanical changes in poly(ethylene-co-1,9-decadiene) following crosslinking induced by peroxides”. Polymer. 41(17): 6497–6505. doi: 10.1016/S0032-3861(99)00881-2. [DOI] [Google Scholar]
- Shrager PG, Strickholm A, Macey RI (1969). “Chemical modification of crayfish axons by protein crosslinking aldehydes”. Journal of Cellular Physiology. 74(1): 91–99. doi: 10.1002/jcp.1040740112. [DOI] [PubMed] [Google Scholar]
- Smedberg A, Hjertberg T, Gustafsson B (1997). “Crosslinking reactions in an unsaturated low density polyethylene”. Polymer. 38(16):4127–4138. doi: 10.1016/S0032-3861(96)00994-9. [DOI] [Google Scholar]
- Sriram V, Mahesh GN, Jeevan RG, Radhakrishnan G (2001). “Comparative studies on short-chain and long-chain crosslinking in polyurethane networks”. Macromolecular Chemistry and Physics. 201(18): 2799–2804. doi: . [DOI] [Google Scholar]
- UN (2011). United Nations Globally Harmonized System of Classification and Labelling of Chemicals (GHS), ST/SG/AC.10/30 Rev 4, Part 3 Health Hazards – Chapter 3.3 Serious eye damage / eye irritation New York & Geneva: United Nations Publications. pp. 133–144. Available: [http://www.unece.org/trans/danger/publi/ghs/ghs_rev04/04files_e.html]. [Google Scholar]
- van Amsterdam IMC, Ubbink M, Einsle O, Messerschmidt A, Merli A, Cavazzini D, Rossi GL, Canters GW (2001). “Dramatic modulation of electron transfer in protein complexes by crosslinking”. Nature Structural Biology. 9: 48–52. doi: 10.1038/nsb736. [DOI] [PubMed] [Google Scholar]
- Wu X, Faqi AS, Yang J, Pang B, Ding X, Jiang X, Chahoud I (2002). “2-Bromopropane induces DNA damage, impairs functional antioxidant cellular defenses, and enhances the lipid peroxidation process in primar cultures of rat Leydig cells”. Reproductive Toxicology. 16(4): 379–384. doi: 10.1016/S0890-6238(02)00039-4. [DOI] [PubMed] [Google Scholar]
- Zhang K, Banyon C, Togbe C, Dagaut P, Bugler J, Curren HJ (2015). “An experimental and kinetic modeling study of n-hexane oxidation”. Combustion and Flame. 162(11): 4194–4207. doi: 10.1016/j.combustflame.2015.08.001. [DOI] [Google Scholar]
- Zhang L, Zhang Z, He X, Zhang F, Zhang Z (2017). “Regulation of the products of styrene oxidation”. Chemical Engineering Research and Design. 120:171–178. doi: 10.1016/j.cherd.2017.02.012. [DOI] [Google Scholar]
- Zhao M, Ismayil N, Mi H, Maisur M, Song W (2006). “Synthesis of 1,5-hexadiene crosslinked alkene polymer and its shear stability. 35:364–368. [Google Scholar]
- Zhu Q, Zhou S, Wen Z, Li H, Huang B, Chen Y, Li X, Lin H, Wang Y, Ge R (2021). “Xylene delays the development of Leydig cells in pubertal rats by inducing reactive oxygen species”. Toxicology. 454: 152740. doi: 10.1016/j.tox.2021.152740. [DOI] [PubMed] [Google Scholar]
- Zhu T, Lim BS, Park HC, Son KM, Yang HC (2012). “Effects of the iron-chelating agent deferoxamine on triethylene glycol dimethacrylate, 2-hydroxylethyl methacrylate, hydrogen peroxide-induced cytotoxicity”. J Biomed Mater Res B Appl Biomater. 100(1):197–205. doi: 10.1002/jbm.b.31939. [DOI] [PubMed] [Google Scholar]


