During spring and summer, the habitats of children and ticks overlap. It is therefore not surprising that the full spectrum of tick-borne diseases occurs in children and that some tick-borne agents infect children disproportionately. For example, two-thirds of all cases of Rocky Mountain spotted fever (RMSF) occur in children less than 15 years of age, and the highest attack rate is in 5- to 9-year-olds (12). The fact that an outbreak of arthritis in children led to the first clinical description of Lyme disease is a fitting illustration of the intersection between children and tick-borne diseases (48).
The pediatrician is challenged by the fact that symptoms of many tick-borne infections mimic those seen in common childhood illnesses. A history of tick exposure can be helpful, but its absence does not exclude tick-borne disease (20, 56). Diagnosis requires a high index of suspicion, knowledge of common clinical manifestations, and familiarity with seasonal and geographic features.
LYME DISEASE
Lyme disease, the most common vector-borne infection in the United States, is caused by the spirochete Borrelia burgdorferi. Although cases of Lyme disease have been reported from 48 states, the vast majority occur in the northeastern coastal regions, the Upper Midwest (Wisconsin and Michigan), and northern California (46). The distribution of cases corresponds to the geographic distribution of the two principal tick vectors, Ixodes scapularis (Northeast and Upper Midwest) and Ixodes pacificus (California). Reservoirs for the organism include the white-footed mouse and the white-tailed deer.
In areas of endemicity, Lyme disease enters the differential diagnosis for children with an expanding circular rash, a swollen knee, facial palsy, or even fatigue and declining school performance. In areas where the illness is not endemic, children with these same symptoms are unlikely to have Lyme disease, and alternative diagnoses should be considered (unless there has been recent travel to an area of endemicity).
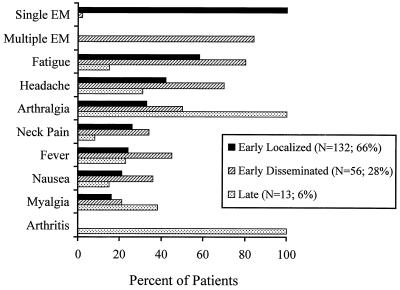
Lyme disease presents in three clinical stages, i.e., early localized, early disseminated, and late disseminated disease, the features of which are summarized in Fig. 1 (46). Early localized disease, seen in 66% of patients, consists of erythema migrans (EM), an erythematous, annular skin lesion that begins as a macule or papule and enlarges centrifugally, sometimes leaving an area of central clearing (6, 20, 36, 39). A central papule with erythema, hyperpigmentation, or scale may persist at the site of the bite, and rarely central vesiculation may occur. Lesions may be pruritic, mildly painful, or asymptomatic and usually occur 1 to 2 weeks after exposure. Enlargement by 20 cm2 per day occurs as organisms migrate outward from the inoculation site. Mild systemic complaints, such as fever, fatigue, myalgias, arthralgias, headache, and regional lymphadenopathy, may accompany EM (6, 20, 43, 49, 56).
FIG. 1.
Clinical manifestations of Lyme disease in children (from reference 20).
Almost one-third of pediatric patients present with early disseminated Lyme disease, occurring weeks after inoculation with B. burgdorferi (20). As spirochetes spread via the blood or lymphatics, nearly every organ system can be affected, and systemic complaints are common (50). Multiple EM lesions occur from dissemination to the skin in approximately 90% of cases. Unilateral or bilateral palsy of cranial nerve VII is the most common neurologic finding and is frequently accompanied by aseptic meningitis (7, 20, 56). Other manifestations include fatigue, headache, arthralgias, myalgias, conjunctivitis, and carditis (20, 50).
Arthritis is the cardinal finding in late disseminated disease, occurring months after infection. Lyme arthritis is oligoarticular, subacute, and only mildly painful. The knee is affected in more than 90% of cases, followed by the elbow, ankle, hip, and wrist (21, 56). Episodes of arthritis generally persist for less than 7 days but may be recurrent. Chronic arthritis is rare (21, 43).
Also rare in children are late central nervous system manifestations. Reported findings of neuroborreliosis include memory impairment, headache, fatigue, poor school performance, behavioral changes, and deficits in auditory or visual sequential processing (5, 8, 52).
Lyme disease has similar manifestations in adults and children, with a few exceptions. Children are more likely than adults to present with fever and joint complaints (56). Children are less likely to experience temporomandibular and sternoclavicular arthritis (21), and radiculopathy and peripheral neuropathy are rarely seen (7).
EM must be differentiated from bacterial cellulitis, eczema, tinea corporis, and granuloma annulare (18). Care must be taken to distinguish EM from local reactions to spider or other insect envenomations as well as hypersensitivity reactions to tick bites (56). Lyme arthritis can mimic septic arthritis, postinfectious arthritis, and rheumatologic disease. Seventh nerve paralysis can be confused with idiopathic Bell's palsy. Lyme meningitis shares clinical features with viral meningitis but can be differentiated by longer duration of headache; absence of fever, accompanying cranial neuropathy, or papilledema; and fewer white blood cells in the cerebrospinal fluid (17). Chronic fatigue syndrome and fibromyalgia are sometimes misdiagnosed as neuroborreliosis (51).
To make things more complicated, not all EM is caused by B. burgdorferi. In 1994 and 1995, 14 attendees at a camp in North Carolina developed EM and mild constitutional symptoms after tick bites (33). None of the patients developed antibodies to B. burgdorferi, and spirochetes were not identified in skin biopsies. Amblyomma americanum, the Lone Star tick, was found on campers and in the surrounding vegetation. Interestingly, sequences of a putative new agent, Borrelia lonestari, have been identified using molecular techniques in A. americanum ticks collected in New Jersey, New York, Missouri, and Texas (3). It is possible that EM-like rash illnesses occurring outside regions where Lyme disease is endemic are due to this organism or related organisms.
Lyme disease should be considered when a child presents with compatible clinical symptoms and probable tick exposure in an area of endemicity. Most physicians practicing in areas where Lyme disease is not endemic are faced with many more cases of “Lyme anxiety” than true Lyme disease. The former stems in part from popular misconceptions perpetuated by the news media and internet-based “experts”. The use of Lyme disease serology to screen individuals who are unlikely to be infected results in false-positive tests, and treatment of these individuals leads to much-publicized treatment failures, inspiring fear in an increasingly tick-exposed public and contributing to the development of a Lyme disease counterculture (47). A willingness to overdiagnose creates the false notion that any constellation of vague, chronic symptoms may be due to Lyme disease.
Many early cases are treated without laboratory confirmation of infection. In other cases, positive antibody screening tests should be confirmed by Western blotting. Molecular diagnosis using techniques such as PCR should be considered investigational at this time. Amoxicillin or doxycycline is recommended for treatment of early localized and early disseminated disease. Cefrtiaxone is preferred for Lyme meningitis and late disseminated disease, and prolonged courses of amoxicillin or doxycycline are used for arthritis. A vaccine for use in high-risk individuals over 15 years of age was recently approved.
RMSF
Rickettsia rickettsii, the etiologic agent of RMSF, is a small, gram-negative, intracellular bacillus. Ticks serve as both the reservoir for infection and the vector for transmission. R. rickettsii is found in Dermacentor variabilis (the dog tick) in southeastern and western states and in Dermacentor andersoni (the wood tick) in Rocky Mountain states.
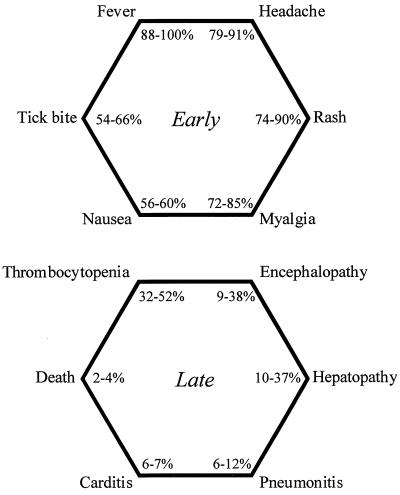
RMSF must be considered in children presenting with fever and rash in the summer; rare patients present with overwhelming shock and prostration. There is disagreement in the literature about what constitutes the classic RMSF triad. For Helmick and colleagues (27), it is fever, rash, and tick bite, which are present in 67% of patients. For Dalton and colleagues (12), it is fever, rash, and headache, which are noted in 55% of patients. In reality, there are six principal features of early RMSF: fever, headache, rash, myalgia, nausea, and a history of a tick bite (Fig. 2) (27, 31, 55).
FIG. 2.
Clinical manifestations of early and late RMSF (from references 27, 31, and 55).
Symptoms of RMSF typically occur after an incubation period of 2 to 12 days. Following a prodrome of malaise, headache, and myalgia (calf pain is common), patients experience the abrupt onset of high, oscillating fever. While recognition of the characteristic rash of RMSF is helpful in early diagnosis, this is absent in 50% of patients during the first 3 days and never develops in 10% (27, 45). The rash often begins as discrete, blanching macules concentrated on the peripheral extremities. Lesions on the palms and soles occur in 49 to 82% of cases (41, 55). The rash spreads centripetally, usually sparing the face, and may become petechial, progressing to ecchymosis and distal necrosis in some cases (1, 24). Conjunctival suffusion may occur.
The six principal features of late RMSF are also shown in Fig. 2. Encephalopathy has been associated with seizures and coma. Other laboratory findings include elevated hepatic transaminases, hyponatremia, anemia, hyperbilirubinemia, elevated creatine kinase, and mononuclear cerebrospinal fluid pleocytosis. Long-term sequelae in survivors include hearing loss, paraparesis, peripheral neuropathy, bladder and bowel dysfunction, vestibular and motor disorders, behavioral disturbances, and learning disabilities (1, 23, 54).
Children are more likely than adults to manifest classic findings of RMSF, including rash. This fact may lead to earlier diagnosis in children and helps to explain the lower mortality rate (3 versus 6%). Adults are nearly four times more likely than children to suffer hearing loss, arrhythmias, and pneumonia (27).
Early RMSF may be difficult to differentiate from common febrile viral illnesses, especially those due to enteroviruses and Epstein-Barr virus. RMSF may also mimic meningococcemia and toxic shock syndrome. When nausea, vomiting, diarrhea, and abdominal pain are prominent, early RMSF may be confused with infectious gastroenteritis or even acute surgical abdomen. Skin manifestations can resemble idiopathic thrombocytopenic purpura, drug hypersensitivity, and secondary syphilis. Prolonged fever, rash, and peripheral edema might also suggest Kawasaki disease.
Because serologic confirmation is generally not possible before the second week of illness and since delays in initiation of antibiotic therapy beyond the seventh day of illness are associated with increased mortality, empiric treatment of suspected RMSF with doxycycline is essential (32). Short courses of doxycycline in young children are unlikely to cause staining of dental enamel (35).
TULAREMIA
Francisella tularensis, the etiologic agent of tularemia, is a small, nonmotile, gram-negative aerobic coccobacillus. Rabbits, hares, and ticks serve as natural reservoirs for the organism; ticks are also vectors, as are fleas and deerflies. Infection follows contact with infected animal tissue or the bite of an infected arthropod. Ticks capable of transmitting disease include D. andersoni, D. variabilis, and A. americanum. Currently, most cases of tularemia are tick related (53).
Tularemia should be considered in children presenting with fever and regional lymphadenopathy, although other clinical syndromes can occur depending on the site of inoculation. Ulceroglandular and glandular syndromes account for well over half of reported cases and result from skin inoculation by ticks (Table 1) (28, 42). Oropharyngeal and gastrointestinal infection occurs following ingestion of contaminated, inadequately cooked meat or infected milk or water. Oculoglandular disease (Parinaud's syndrome), characterized by nodular conjunctivitis and painful preauricular lymphadenopathy, generally arises from self-inoculation by contaminated fingers. Pneumonic tularemia is caused by inhalation of aerosolized F. tularensis, an occurrence most commonly encountered in laboratories. Typhoidal tularemia occurs when organisms disseminate to the bloodstream, resulting in a sepsis-like picture.
TABLE 1.
Clinical forms of Tularemia
Two to 12 days following the bite of an infected tick, fever, chills, headache, myalgias, and arthralgias ensue. An erythematous, nonhealing ulcer develops at the site of the bite in as many as 45% of infected children. Large, tender lymphadenopathy develops proximal to the eschar; nodes may suppurate and drain spontaneously. Pneumonia, manifested by cough, pleuritic chest pain, and radiographic infiltrates, may be present in 10 to 15% of infected children (29).
Children, who are more likely than adults to sustain tick bites to the head and neck, commonly present with cervical lymphadenopathy, while adults present with inguinal or femoral lymphadenopathy (29, 37). Constitutional symptoms, including fever, chills, and malaise, are more common in children, as are gastrointestinal symptoms. While tularemia is more common in males overall, a gender discrepancy is less apparent in the pediatric population (42, 53).
The differential diagnosis of ulceroglandular and glandular tularemia includes bacterial lymphadenitis caused by Staphylococcus aureus or group A streptococcus, Bartonella henselae, and nontuberculous mycobacteria. Oculoglandular syndromes can be seen in cat scratch disease as well. Oropharyngeal tularemia can mimic diphtheria. A 7-day course of intramuscular streptomycin or intravenous gentamicin is the preferred therapy for tularemia.
BABESIOSIS
Babesiosis is caused by intraerythrocytic protozoa, principally Babesia microti. Because disease in the northeastern United States is spread by I. scapularis, the same vector that harbors B. burgdorferi, Lyme disease and babesiosis may occur simultaneously in the same patient. As with B. burgdorferi, the white-footed mouse is an important reservoir for this parasite.
In North America, most cases of babesiosis are asymptomatic, but a malaria-like illness may occur, with fever and intravascular hemolysis. One to 4 weeks after the bite of an infected tick, nonspecific symptoms, including fever, diaphoresis, rigors, fatigue, myalgia, headache, nausea, and abdominal pain appear (9). Emotional lability and depression may also be present. Physical findings include hepatomegaly with a petechial rash or ecchymoses. Anemia and thrombocytopenia are common, along with elevated levels of serum bilirubin and liver transaminases. Proteinuria and hemoglobinuria are also observed.
Differences between pediatric and adult infection are not well described. Serosurveys on Block Island, Rhode Island, where babesiosis is endemic, indicate that B. microti is at least as prevalent in children as it is in adults (34). However, more severe disease appears to occur in individuals over 40 years of age, asplenic patients, and immunocompromised hosts (38). Treatment is reserved for severely affected patients or those at risk for severe disease and consists of oral quinine and clindamycin for 7 days.
EHRLICHIOSIS
Ehrlichia are small, intracellular, gram-negative coccobacilli that resemble rickettsiae. The organisms are characterized by their trophism for phagocytic cells and the formation of cytoplasmic morulae. Two species cause the majority of clinically apparent disease in the United States. Ehrlichia chaffeensis infects monocytes and is the agent responsible for human monocytic ehrlichiosis (HME) (14). Most reported cases of HME infection occur in the South and Southeast (Texas, Oklahoma, Arkansas, Missouri, and Georgia). A. americanum and D. variabilis are the principal tick vectors. Reservoirs of disease are not well defined but are believed to include deer and other large mammals.
Human granulocytic ehrlichiosis (HGE), distinguished by the presence of morulae in neutrophils, is caused by a species very closely related to Ehrlichia equi and Ehrlichia phagocytophilia (14). HGE has been reported in Wisconsin, Minnesota, Connecticut, California, Maryland, New York, Florida, Rhode Island, Pennsylvania, and Arkansas. Given the geographic overlap with Lyme disease, it is not surprising that Ixodes ticks are thought to be the principal vectors and that white-tailed deer serve as a reservoir.
Recently, four patients from Missouri who were infected with Ehrlichia ewingii, an organism that causes granulocytic ehrlichiosis in dogs, were reported. At this point, little is known about the mode of transmission to humans (10).
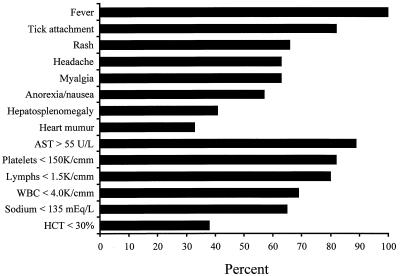
Children with ehrlichiosis may present with symptoms similar to those seen in RMSF, or they may have an undifferentiated febrile illness (Fig. 3) (30). Hepatosplenomegaly, a systolic heart murmur, and rash are common physical findings. Although HME has been called “spotless” RMSF, macular, maculopapular, and petechial rashes are reported in 66% of infected, symptomatic children (30). Less common findings include nuchal rigidity, conjunctivitis, pharyngitis, lymphadenopathy, peripheral edema, and oral or genital ulcers (4, 15, 16, 22, 25, 26, 40, 44). Thrombocytopenia, leukopenia, and elevated liver transaminases are common, and mild anemia can be present. Severe HME in children, with shock, disseminated intravascular coagulation, and encephalitis, has been described (13, 44).
FIG. 3.
Clinical and laboratory features of HME in children (from reference 30). AST, alanine aminotransferase; WBC, white blood cells; HCT, hematocrit.
Data on the clinical manifestations of HGE in children are sparse. Fever, sweats, rigors, myalgias, and headache are commonly reported symptoms in adults (2). In contrast to patients with HME, those with HGE generally lack physical findings, such as rash and hepatomegaly, but laboratory abnormalities are similar to those reported with HME (30).
Reports from Connecticut and New York suggest that clinical ehrlichiosis is a disease of older adults (11). However, seroprevalence rates may exceed disease incidence rates in children (G. S. Marshall, unpublished observations), suggesting that ehrlichia infection may be underrecognized. Symptomatic children with HME are more likely than adults to present with rash, hepatomegaly, splenomegaly, and lymphadenopathy; they are also more likely to have a history of a tick bite (16). Elderly patients are at the greatest risk for severe disease and death (19).
Ehrlichiosis may be indistinguishable from RMSF, particularly when a rash is present. The differential diagnosis for these two diseases is similar. When fever, hepatomegaly, and heart murmur are present, bacterial endocarditis also becomes a consideration. As for RMSF, doxycycline is the treatment of choice for ehrlichiosis and must be initiated before results of serologic tests are known.
ACKNOWLEDGMENT
K.A.B. is supported by the Kosair Charities Fellowship in Pediatric Infectious Diseases.
REFERENCES
- 1.Archibald L K, Sexton D J. Long-term sequelae of Rocky Mountain spotted fever. Clin Infect Dis. 1995;20:1122–1125. doi: 10.1093/clinids/20.5.1122. [DOI] [PubMed] [Google Scholar]
- 2.Bakken J S, Krueth J, Wilson-Nordskog C, Tilden R L, Asanovich K, Dumler J S. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA. 1996;275:199–205. [PubMed] [Google Scholar]
- 3.Barbour A G, Maupin G O, Teltow G J, Carter C J, Piesman J. Identification of an uncultivatable Borrelia sp. in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. Clin Infect Dis. 1995;20:1118–1121. doi: 10.1093/infdis/173.2.403. [DOI] [PubMed] [Google Scholar]
- 4.Barton L L, Dawson J E, Letson G W, Luisiri A, Scalzo A J. Simultaneous ehrlichiosis and Lyme disease. Pediatr Infect Dis J. 1990;9:127–129. doi: 10.1097/00006454-199002000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Belman A L, Iyer M, Copyle P K, Dattwyler R. Neurologic manifestations in children with North American Lyme disease. Neurology. 1993;43:2609–2614. doi: 10.1212/wnl.43.12.2609. [DOI] [PubMed] [Google Scholar]
- 6.Berger B W. Dermatologic manifestations of Lyme disease. Rev Infect Dis. 1989;11:S1475–S1481. doi: 10.1093/clinids/11.supplement_6.s1475. [DOI] [PubMed] [Google Scholar]
- 7.Bingham P M, Galetta S L, Athreya B, Sladky J. Neurologic manifestations in children with Lyme disease. Pediatrics. 1995;96:1053–1056. [PubMed] [Google Scholar]
- 8.Bloom B J, Wykcoff P M, Meissner H C, Steere A C. Neurocognitive abnormalities in children after classic manifestations of Lyme disease. Pediatr Infect Dis J. 1998;17:189–196. doi: 10.1097/00006454-199803000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Boustani M R, Gelfand J A. Babesiosis. Clin Infect Dis. 1996;22:611–615. doi: 10.1093/clinids/22.4.611. [DOI] [PubMed] [Google Scholar]
- 10.Buller R S, Arens M, Hmiel S P, Paddock C D, Sumner J W, Rikihisa Y, Unver A, Gaudreault-Keener M, Manian F A, Liddell A M, Schmulewitz N, Storch G A. Ehrlichia ewingii: a newly recognized agent of human ehrlichiosis. N Engl J Med. 1999;341:148–155. doi: 10.1056/NEJM199907153410303. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Statewide surveillance for ehrlichiosis—Connecticut and New York, 1994–1997. Morb Mortal Wkly Rep. 1998;47:476–480. [PubMed] [Google Scholar]
- 12.Dalton M J, Clarke M J, Holman R C, et al. National surveillance for Rocky Mountain spotted fever, 1981–1992: epidemiologic summary and evaluation of risk factors for fatal outcome. Am J Trop Med Hyg. 1995;52:404–413. doi: 10.4269/ajtmh.1995.52.405. [DOI] [PubMed] [Google Scholar]
- 13.Doran T I, Parmley R T, Logas P C, Chamblin S. Infection with Ehrlichia canis in a child. J Pediatr. 1989;114:809–812. doi: 10.1016/s0022-3476(89)80143-x. [DOI] [PubMed] [Google Scholar]
- 14.Dumler J S, Bakken J S. Ehrlichial diseases of humans: emerging tick-borne infections. Clin Infect Dis. 1995;20:1102–1110. doi: 10.1093/clinids/20.5.1102. [DOI] [PubMed] [Google Scholar]
- 15.Edwards M S, Jones J E, Leass D L, Whitmore J W, Dawson J E, Fishbein D B. Childhood infection caused by Ehrlichia canis or a closely related organism. Pediatr Infect Dis J. 1988;7:651–654. doi: 10.1097/00006454-198809000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Eng T R, Harkess J R, Fishbein D B, Dawson J E, Greene C N, Redus M A, Satalowich F T. Epidemiologic, clinical and laboratory findings of human ehrlichiosis in the United States. JAMA. 1988;264:2251–2258. [PubMed] [Google Scholar]
- 17.Eppes S C, Nelson D K, Lewis L L, Klein J D. Characterization of Lyme meningitis and comparison with viral meningitis in children. Pediatrics. 1999;103:957–960. doi: 10.1542/peds.103.5.957. [DOI] [PubMed] [Google Scholar]
- 18.Feder H M, Hunt M S. Pitfalls in the diagnosis and treatment of Lyme disease. JAMA. 1995;274:66–68. [PubMed] [Google Scholar]
- 19.Fishbein D B, Dawson J E, Robinson L E. Human ehrlichiosis in the United States, 1985–1990. Ann Intern Med. 1994;120:736–743. doi: 10.7326/0003-4819-120-9-199405010-00003. [DOI] [PubMed] [Google Scholar]
- 20.Gerber M A, Shapiro E D, Burke G S, Parcells V J, Bell G L the Pediatric Lyme Disease Study Group. Lyme disease in children in southeastern Connecticut. N Engl J Med. 1996;335:1270–1274. doi: 10.1056/NEJM199610243351703. [DOI] [PubMed] [Google Scholar]
- 21.Gerber M A, Zemel L S, Shapiro E D. Lyme arthritis in children: Clinical epidemiology and long-term outcomes. Pediatrics. 1998;102:905–908. doi: 10.1542/peds.102.4.905. [DOI] [PubMed] [Google Scholar]
- 22.Golden S E. Aseptic meningitis associated with Ehrlichia canis infection. Pediatr Infect Dis J. 1989;8:335–337. [PubMed] [Google Scholar]
- 23.Gorman R J, Saxon S, Sneed O C., III Neurologic sequelae of Rocky Mountain spotted fever. Pediatrics. 1981;67:354–357. [PubMed] [Google Scholar]
- 24.Griffith G L, Luce E A. Massive skin necrosis in Rocky Mountain spotted fever. South Med J. 1978;71:1337–1340. doi: 10.1097/00007611-197811000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Hammill W W, Wilson M B, Reigart J R, Flick J T, Laver J. Ehrlichia canis infection in a child in South Carolina. Clin Pediatr. 1992;31:432–434. doi: 10.1177/000992289203100710. [DOI] [PubMed] [Google Scholar]
- 26.Harkess J R, Ewing S A, Brunit T, Mettry C R. Ehrlichiosis in children. Pediatrics. 1991;87:199–203. [PubMed] [Google Scholar]
- 27.Helmick C G, Bernard K W, D'Angelo L J. Rocky Mountain spotted fever: clinical, laboratory, and epidemiological features of 262 cases. J Infect Dis. 1984;150:480–488. doi: 10.1093/infdis/150.4.480. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs R F, Narain J P. Tularemia in children. Pediatr Infect Dis J. 1983;83:487–491. doi: 10.1097/00006454-198311000-00019. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs R F, Condrey Y M, Yamauchi T. Tularemia in adults and children: a changing presentation. Pediatrics. 1985;76:818–822. [PubMed] [Google Scholar]
- 30.Jacobs R F, Schutze G E. Ehrlichiosis in children. J Pediatr. 1997;131:184–192. doi: 10.1016/s0022-3476(97)70152-5. [DOI] [PubMed] [Google Scholar]
- 31.Kirk J L, Fine D P, Sexton D J, Muchmore H G. Rocky Mountain spotted fever: a clinical review based on 48 confirmed cases, 1943–1986. Medicine. 1990;69:35–45. [PubMed] [Google Scholar]
- 32.Kirkland, K. B., W. E. Wilkinson, and D. J. Sexton. 1995. Therapeutic delay and mortality in cases of Rocky Mountain spotted fever. 20:1118–1121. [DOI] [PubMed]
- 33.Kirkland K B, Klimko T B, Merriweather R A, Schreifer M, Levin M, Levine J, MacKenzie W R, Dennis D T. Erythema migrans-like rash illness at a camp in North Carolina: a new tick-borne disease? Arch Intern Med. 1997;157:2635–2641. [PubMed] [Google Scholar]
- 34.Krause P J, Telford III S R, Pollack R J, Ryan R, Brassard P, Zemel L, Speilman A. Babesiosis: an underdiagnosed disease of children. Pediatrics. 1992;89:1045–1048. [PubMed] [Google Scholar]
- 35.Lochary M E, Lockhart P B, Williams W T., Jr Doxycycline and staining of the permanent teeth. Pediatr Infect Dis J. 1998;17:429–431. doi: 10.1097/00006454-199805000-00019. [DOI] [PubMed] [Google Scholar]
- 36.Malane M S, Grant-Kels J M, Feder H M, Luger S W. Diagnosis of Lyme disease based on clinical manifestations. Ann Intern Med. 1991;114:490–498. doi: 10.7326/0003-4819-114-6-490. [DOI] [PubMed] [Google Scholar]
- 37.Markowitz L E, Hynes N A, De la Cruz P, et al. Tick-borne tularemia: an outbreak of lymphadenopathy in children. JAMA. 1985;254:2922–2925. doi: 10.1001/jama.254.20.2922. [DOI] [PubMed] [Google Scholar]
- 38.Meldrum S C, Birkhead G S, White D J, Benach J L, Morse D L. Human babesiosis in New York State: an epidemiological description of 136 cases. Clin Infect Dis. 1992;15:1019–1023. doi: 10.1093/clind/15.6.1019. [DOI] [PubMed] [Google Scholar]
- 39.Nadelman R B, Wormser G P. Erythema migrans and early Lyme disease. Am J Med. 1995;98:15S–23S. doi: 10.1016/s0002-9343(99)80040-0. [DOI] [PubMed] [Google Scholar]
- 40.Rathore M H. Infection due to Ehrlichia canis in children. South Med J. 1992;85:703–705. doi: 10.1097/00007611-199207000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Rein G N. Rocky Mountain spotted fever appears in Michigan. J Mich State Med Soc. 1948;47:182–187. [PubMed] [Google Scholar]
- 42.Rohrbach B W, Westerman E, Istre G R. Epidemiology and clinical characteristics of tularemia in Oklahoma, 1979–1985. South Med J. 1991;84:1091–1096. doi: 10.1097/00007611-199109000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Salazar J C, Gerber M A, Goff C W. Long-term outcome of Lyme disease in children. J Pediatr. 1993;122:591–593. doi: 10.1016/s0022-3476(05)83541-3. [DOI] [PubMed] [Google Scholar]
- 44.Schutze G E, Jacobs R F. Human monocytic ehrlichiosis in children. Pediatrics. 1997;100:1–5. doi: 10.1542/peds.100.1.e10. [DOI] [PubMed] [Google Scholar]
- 45.Sexton J J, Corey G R. Rocky Mountain “spotless” and “almost spotless” fever: a wolf in sheep's clothing. Clin Infect Dis. 1992;15:439–448. doi: 10.1093/clind/15.3.439. [DOI] [PubMed] [Google Scholar]
- 46.Shapiro E D. Lyme disease. Semin Pediatr Infect Dis. 1994;5:112–120. [Google Scholar]
- 47.Sigal L H. Anxiety and persistence of Lyme disease. Am J Med. 1995;98(Suppl. 4A):74S–78S. doi: 10.1016/s0002-9343(99)80048-5. [DOI] [PubMed] [Google Scholar]
- 48.Steere A C, Malawista S E, Snydman D R, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum. 1977;20:7–17. doi: 10.1002/art.1780200102. [DOI] [PubMed] [Google Scholar]
- 49.Steere A C, Bartenhagen N H, Craft J E, et al. The early clinical manifestations of Lyme disease. Ann Intern Med. 1983;99:76–82. doi: 10.7326/0003-4819-99-1-76. [DOI] [PubMed] [Google Scholar]
- 50.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 51.Steere A C, Taylor E, McHugh G L, Logigan E L. The overdiagnosis of Lyme disease. JAMA. 1993;269:1812–1816. [PubMed] [Google Scholar]
- 52.Szer I S, Taylor E, Steere A C. The long-term course of Lyme arthritis in children. N Engl J Med. 1991;325:159–163. doi: 10.1056/NEJM199107183250304. [DOI] [PubMed] [Google Scholar]
- 53.Taylor J P, Istre G R, McChesney T C, Satalowich F T, Parker R L, McFarland L M. Epidemiologic characteristics of human tularemia in the southwest central states, 1981–1987. Am J Epidemiol. 1991;133:1032–1038. doi: 10.1093/oxfordjournals.aje.a115812. [DOI] [PubMed] [Google Scholar]
- 54.Thomas M H, Berlin L. Neurologic sequelae of Rocky Mountain spotted fever. Arch Neurol Psychiat. 1948;60:574–583. [PubMed] [Google Scholar]
- 55.Thorner A R, Walker D H, Petri W A., Jr Rocky Mountain spotted fever. Clin Infect Dis. 1998;27:1353–1360. doi: 10.1086/515037. [DOI] [PubMed] [Google Scholar]
- 56.Williams C L, Strobino B, Lee A, Curran A S, Benach J L, Inamar S, Cristofaro R. Lyme disease in childhood: clinical and epidemiologic features of ninety cases. Pediatr Infect Dis J. 1990;9:10–14. [PubMed] [Google Scholar]