Abstract
The p36 protein of Mycoplasma hyopneumoniae is a cytosolic protein carrying species-specific antigenic determinants. Based on the genomic sequence of the reference strain ATCC 25934, primers were designed for PCR amplification of the p36-encoding gene (948 bp). These primers were shown to be specific to M. hyopneumoniae since no DNA amplicons could be obtained with other mycoplasma species and pathogenic bacteria that commonly colonize the porcine respiratory tract. The amplified p36 gene was subcloned into the pGEX-4T-1 vector to be expressed in Escherichia coli as a fusion protein with glutathione S-transferase (GST). The GST-p36 recombinant fusion protein was purified by affinity chromatography and cut by thrombin, and the enriched p36 protein was used to immunize female BALB/c mice for the production of anti-p36 monoclonal antibodies (MAbs). The polypeptide specificity of the nine MAbs obtained was confirmed by Western immunoblotting with cell lysates prepared from the homologous strain. Cross-reactivity studies of the anti-p36 MAbs towards two other M. hyopneumoniae reference strains (ATCC 25095 and J strains) and Quebec field strains that had been isolated in culture suggested that these anti-p36 MAbs were directed against a highly conserved epitope, or closely located epitopes, of the p36 protein. No reactivity was demonstrated against other mycoplasma species tested. Clinical signs and lesions suggestive of enzootic pneumonia were reproduced in specific-pathogen-free pigs infected experimentally with a virulent Quebec field strain (IAF-DM9827) of M. hyopneumoniae. The bacteria could be recovered from lung homogenates of pigs that were killed after the 3-week observation period by both PCR and cultivation procedures. Furthermore, the anti-p36 MAbs permitted effective detection by indirect immunofluorescence of M. hyopneumoniae in frozen lung sections from experimentally infected pigs. However, attempts to use the recombinant p36 protein as an antigen in an indirect enzyme-linked immunosorbent assay for the detection of antibodies in sera from convalescent pigs showed no correlation with clinical and pathological findings.
Mycoplasma hyopneumoniae is the causative agent of enzootic pig pneumonia (15, 21), a disease found on pig farms worldwide which is characterized by high morbidity and low mortality rates (19, 25). Coughing is the principal clinical sign, and retarded growth and poor food conversion may result in considerable economic losses. Furthermore, this agent predisposes the pigs to secondary pulmonary infections, hence, increasing the mortality among the herds and the financial problems associated with such losses (19, 25).
M. hyopneumoniae encodes several characterized immunodominant proteins, among which are the p36 cytosolic protein (28, 29), the p46, p65, and p74 membranous proteins (3, 17, 18, 22), and the p97 adhesin (31). The functions of these proteins have not been yet elucidated, but specific reactants may eventually be useful tools for the diagnosis of M. hyopneumoniae.
The cytosolic p36 protein has been cloned, expressed and characterized as a lactate dehydrogenase (16, 29). It has been shown to induce an early immune response in pigs that were experimentally and naturally infected by M. hyopneumoniae (10). Furthermore, p36 is apparently highly conserved among different M. hyopneumoniae strains from various parts of the world (28, 29). Hyperimmune sera that have been produced against the recombinant p36 protein showed no reactivity against other porcine mycoplasmas, including M. flocculare, M. hyorhinis and Acholeplasma laidlawii (29). Furthermore, no cross-reactivity was demonstrated against Mycoplasma or Acholeplasma species isolated from humans, other farm animals, cats, and dogs (29).
The diagnosis of M. hyopneumoniae is usually done by cultivation of the organism or by immunofluorescence tests performed on frozen thin lung sections using polyclonal antibodies (1, 7, 19, 20, 25). However, because of the fastidious nature of this microorganism, its culture and identification may take up to 1 month. The cultures are also often contaminated with M. hyorhinis, which is an opportunistic agent that invades the lower respiratory tract of pigs and overgrows M. hyopneumoniae in primary isolation attempts (9). Moreover, the overall efficacy of serological detection methods, such as enzyme-linked immunosorbent assays (ELISAs), is often hampered because of the potentially cross-reacting antigenic relationships that exist between M. hyopneumoniae, M. flocculare, and M. hyorhinis (2, 9).
The indirect immunofluorescence (IIF) assay is still widely used for the diagnosis of M. hyopneumoniae because it is a rapid and convenient technique and allows histopathological observations. However, the use of polyclonal antisera may result in the nonspecific detection of other pathogens, namely, M. flocculare and M. hyorhinis (2, 9, 24, 32). The use of monoclonal antibodies (MAbs) increases the specificity of serological and immunohistochemical tests (10, 26). This paper describes the cloning and Escherichia coli-expression of the p36 protein of M. hyopneumoniae, the production and characterization of anti-p36 MAbs, and their potential application in IIF for the detection of the agent in frozen sections of lung tissues.
(This report was taken in part from a dissertation to be submitted by J. Caron to the INRS-Institut Armand-Frappier, Université du Québec, in partial fulfillment of the requirements for the M.Sc. degree.)
MATERIALS AND METHODS
Microorganisms and growth conditions.
The ATCC 25934 strain of M. hyopneumoniae was used as the reference strain in this study. Other strains of M. hyopneumoniae (ATCC 25095 and J), as well as M. flocculare (ATCC 27399), M. argininii (ATCC 23838), M. hyorhinis (ATCC 17981), and A. laidlawii (ATCC 23206), were obtained from the American Type Culture Collection, Manassas, Va., and used for comparative antigenic studies. M. hyosynoviae was cultivated from a field case of polyarthritis and was kindly provided to us by Claude Montpetit, Ministère de l'Agriculture, des Pêcheries et de l'Alimentation du Québec. The mycoplasmas were grown in modified Friis medium (11), containing 20% porcine serum (Gibco-BRL), 5% fresh yeast extract (Gibco-BRL), methicillin (0.15 mg/ml; Sigma-Aldrich Canada, Oakville, Ontario, Canada), bacitracin (0.15 mg/ml; Sigma-Aldrich), and thallium acetate (0.08 mg/ml; Sigma-Aldrich). The cells were harvested by centrifugation at 12,000 × g for 30 min at 4°C, washed three times, and suspended in 0.1 M phosphate-buffered saline (PBS), pH 7.4.
Field isolates of M. hyopneumoniae were cultivated from lung homogenates of pigs suffering from acute or chronic respiratory problems that had been forwarded to our laboratory for confirmation by PCR of outbreaks of enzootic pneumonia in pig herds in Southern Quebec (4).
DNA extraction and PCR conditions.
Genomic DNA from M. hyopneumoniae was extracted and purified, as previously described (4). The oligonucleotide primers used for enzymatic amplification of the entire open reading frame (ORF) of the p36 gene (948 bp) of M. hyopneumoniae were selected from the previously published DNA sequence (16) of the strain ATCC 25934 (GenBank accession no. X67286). The sequence of the forward primer, FSp36, was 5′ GGG CCG ATG AAA CCT ATT AAA ATA GCT 3′, and that of the reverse primer, RSp36, was 5′ GCC GCG AAA TTA AAT ATT TTT AAT TGC ATC CTG 3′. The sequence analysis for the primer selection was performed using the McVector 3.5 (International Biothechnologies) and Gene Works 2.2 (IntelliGeneticsInc., Mountain View, Calif.) programs. The oligonucleotide primers were synthesized in an automated Gene Assembler DNA synthesizer (Pharmacia LKB). The PCR protocol used for amplification of the p36 gene was essentially similar to that described previously (4). The PCR amplifications were performed in a DNA Engine thermocycler (model PTC-100 with hot bonnet; MJ Research). Aliquots (10 μl) of the amplified products were analyzed by electrophoresis on 1% agarose gels (Boerhinger Mannheim) in TAE buffer (0.04 M Tris-acetate [pH 8.5], 0.002 M EDTA) in the presence of ethidium bromide at 100 V for 1 h and viewed under UV illumination.
Cloning, procaryotic expression, and purification of the GST-p36 recombinant fusion protein.
The complete ORF of the p36 gene (948 bp) was amplified by PCR as described above. The primers contained two restriction sites for EcoRI (sense primer) and BamHI (antisense primer) at their 5′ ends for directional cloning. The amplified products were purified by extraction with phenol and chloroform and precipitation with 100% ethanol, digested with EcoRI and BamHI, and ligated into the similarly digested pGEX-4T-1 procaryotic vector, according to the manufacturer's instructions (Pharmacia). The recombinant plasmid was used to transform competent E. coli, strain BL21, cells (Gibco-BRL) to produce the glutathione S-transferase (GST)-p36 fusion protein. Briefly, overnight cultures of the transformed bacteria were diluted 1:100 in a total volume of 1.2 liters of 2YT medium containing ampicillin (100 mg/ml) and 2% glucose, and grown at 37°C with shaking (250 rpm) to an A600 of 0.8 to 1.0. Protein expression was induced by the addition of 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and after a 5 h-incubation at 37°C with vigorous agitation (250 rpm), the cells were pelleted by centrifugation at 8,000 × g and resuspended in 75 ml of ice-cold 0.1 M PBS [pH 7.4] containing lysozyme (1 mg/ml) and 1 mM phenylmethylsulfonyl fluoride (PMSF; Boehringer Mannheim). Cells were disrupted by sonication, and Triton X-100 (Sigma-Aldrich) was added to a final concentration of 1%; this was followed by an incubation of 30 min at room temperature to aid the solubilization process. The soluble fusion protein was purified by affinity chromatography on glutathione-Sepharose 4B beads (Pharmacia) and either eluted with reduced glutathione (20 mM glutathione, 50 mM Tris-HCl [pH 8.0], 120 mM NaCl) (27) or directly cleaved through an overnight incubation with 18 U of thrombin to obtain the enriched p36 moiety. The expressed recombinant protein was analyzed by electrophoresis on sodium dodecyl sulfate (SDS)–12% polyacrylamide gels after solubilization in 4× Laemmli disruption buffer and boiling for 5 min.
Production of a monospecific anti-p36 rabbit hyperimmune serum.
Two New-Zealand Albino rabbits were inoculated by the intradermal route with 250 μg of the affinity-purified GST-p36 recombinant fusion protein emulsified in Freund's complete adjuvant (Gibco-BRL). The rabbits received boosters twice at 2-week intervals by the intramuscular route with the same amount of protein emulsified in incomplete Freund adjuvant. Reactivity of the antisera to the native p36 protein of M. hyopneumoniae was assessed by Western blotting for the complete antigen of the homologous strain.
Production and characterization of MAbs to the p36 gene of M. hyopneumoniae.
Female BALB/c mice were immunized intraperitoneally with 20 μg of affinity-purified p36 recombinant protein mixed with Freund's complete or incomplete adjuvant for the first and the three following injections, respectively. Injections were given at 2-week intervals, followed by an intravenous dose of the antigen without adjuvant 3 days prior to the fusion experiment. The fusion protocol for sensitized splenocytes with P3 × 63 Ag8.653 myeloma cells was essentially similar to that described previously (5). Hybrid cells were cultured in hypoxantine-aminopterine-thymidine medium containing 10% fetal calf serum. Hybridoma supernatants were screened for anti-p36 antibodies by an indirect ELISA (14), the optimal p36 antigen concentration, determined by checkerboard titration, corresponding to 0.15 μg of protein/well. In the ELISA, the washing buffer consisted of 0.1 M PBS [pH 7.4] containing 0.05% Tween 80 (PBS-T), whereas the saturation and dilution buffer consisted of PBS-T supplemented with 5% goat serum and 4% skim milk (Blotto). The secreting hybridoma cells were subcloned twice by serial dilutions. Immunoglobulin isotyping was done using a commercial enzyme immunoassay (Boehringer Mannheim). Ascitic fluids containing anti-p36 MAbs were obtained by intraperitoneally injecting 1 × 106 to 2 × 106 cloned hybrid cells into 16-week-old female BALB/c mice that had been primed 14 days before with 0.5 ml of pristane (2,6,10,14-tetramethyl pentadecane; Sigma-Aldrich) (14).
Western immunoblotting.
Mycoplasma proteins separated by SDS–12% polyacrylamide gel electrophoresis (PAGE) were electrotransferred to nitrocellulose membranes (0.45-μm pore size; Xymotech) for 2 h at 60 V, as previously described (5). Membranes were blocked overnight in 0.02 M Tris-HCl buffer (pH 8.6) containing 0.5% Tween 20 (TBS-T), 5% goat serum, and 5% skim milk. Then, the membranes were incubated for 2 h at room temperature in the presence of either a 1:1,000 dilution of rabbit hyperimmune serum, a 1:200 to 1:1,000 dilution of porcine hyperimmune serum, or a 1:1,000 dilution of mouse ascitic fluid in the blocking buffer. The immune reactions were revealed following incubation with a 1:2,000 dilution of the appropriate peroxidase-labeled anti-immunoglobulin G (IgG) conjugates and incubation in the enzyme substrate solution, as described elsewhere (5).
Experimental infection of SPF pigs.
Six crossbred F1 (Landrace × Yorkshire) castrated specific-pathogen-free (SPF) piglets 9-to-10 weeks of age were obtained from a breeding farm located in Southern Quebec, Canada. The breeding stock and piglets were tested and proven to be seronegative for porcine reproductive and respiratory syndrome virus, encephalomyocarditis virus, porcine parvovirus, hemagglutinating encephalomyelitis virus, transmissible gastroenteritis virus, and M. hyopneumoniae. The piglets were randomly divided into two groups, each group being allocated to separate isolation rooms in facilities equipped with a microorganism-free filtered in-flowing and out-flowing air system. They were fed commercial food and water ad libitum.
Two piglets kept as controls received 8 ml of fresh Friis medium given intratracheally. The remaining four piglets received by the same route 8 ml of a culture of the IAF-DM9827 strain of M. hyopneumoniae corresponding to an infectious dose of 107 color-changing units/ml (highest dilution of the culture that causes acidification of the medium within a 7-day incubation period). The animals were monitored clinically for a 3-week period, and then were euthanatized. Their lungs were aseptically collected and processed for histopathology, PCR, and cultivation attempts in modified Friis medium.
Histopathological examination.
Thin sections (5 μm thick) of formalin-fixed, paraffin-embedded tissues from the lungs of control and experimentally infected pigs were routinely processed for hematoxylin-eosin staining.
Indirect immunofluorescence staining.
Thin frozen lung sections showing typical enzootic pneumonia lesions were mounted on glass slides and fixed with 100% ice-cold acetone. Once the slides were dried, they were incubated with p36 MAbs at a dilution of 1:100, washed in PBS, and reacted with fluorescein-conjugated goat anti-mouse IgG (Boehringer Mannheim) diluted 1:40 in PBS. The fluorescent reaction was observed under a UV microscope.
RESULTS
PCR amplification, cloning, expression, and purification of the GST-p36 fusion protein.
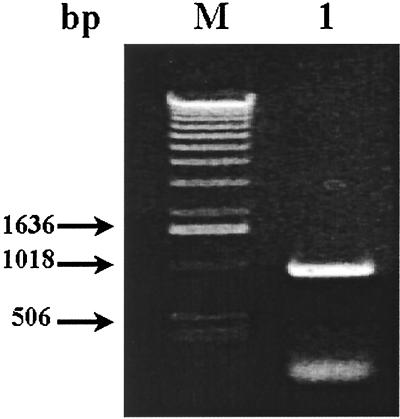
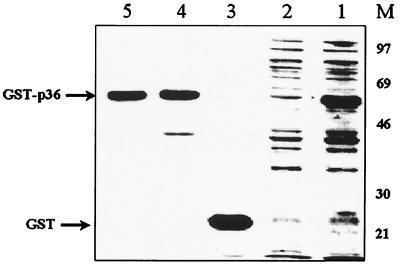
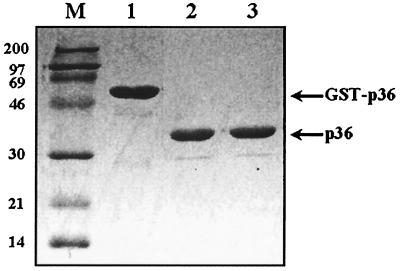
As described in the previous section, the PCR-amplified ORF (948 bp) of the p36 gene of M. hyopneumoniae (Fig. 1) and the procaryotic pGEX-4T-1 vector were both digested with EcoRI and BamHI and successfully ligated. The recombinant plasmid was used to transform competent E. coli cells, strain BL21, which were then induced by the addition of IPTG in the culture medium to express the fusion protein GST-p36. SDS-PAGE analysis of sonicates of IPTG-induced transformed E. coli cells revealed the presence of an additional 60-kDa protein species, absent in noninduced bacteria, corresponding to GST (molecular mass of 24 kDa) fused to the amino end of the cloned p36 protein (Fig. 2, lane 1). After bulk purification of the induced sonicates on glutathione-Sepharose beads, 3.0 to 3.5 mg of GST-p36 recombinant fusion protein was recovered from a culture of 1.2 liters of IPTG-induced and transformed bacteria (Fig. 2, lanes 4 and 5). To obtain purified recombinant p36 protein for immunization of mice and rabbits, the GST-p36 recombinant fusion protein was digested overnight with thrombin. The electrophoretic profile of the cleaved p36 portion of the fusion recombinant protein yielded a band migrating at the expected size (Fig. 3, lanes 2 and 3). Cleavage of the GST-p36 recombinant fusion protein was complete, with no residual uncut GST-p36 protein being detected by Western blotting analysis (Fig. 3, lanes 1 to 3).
FIG. 1.
PCR amplification of the ORF encoding the cytosolic p36 protein of M. hyopneumoniae (strain ATCC 25934). Lane 1, a 948-bp DNA amplicon corresponding to the entire p36 gene was obtained by PCR using the oligonucleotide primer pair FSp36 and RSp36; lane M, the molecular sizes of three fragments of the 1-kb ladder.
FIG. 2.
Expression of the recombinant GST-p36 protein in E. coli. Lane 1, IPTG-induced recombinant bacteria; lane 2, noninduced recombinant bacteria; lane 3, GST protein (24 kDa); lanes 4 and 5, purified GST-p36 recombinant fusion protein (60 kDa); lane M, low-molecular-weight markers.
FIG. 3.
Reactivity by Western immunoblotting of monospecific rabbit hyperimmune serum to the recombinant GST-p36 fusion protein prior to (lane 1) and after (lanes 2 and 3) cleavage by thrombin. Lane M, low-molecular-weight markers.
Reactivity and specificity of the anti-p36 rabbit hyperimmune serum.
As illustrated in Fig. 3, specific revelation of the GST-p36 recombinant fusion protein (60 kDa) and its cut p36 moiety was obtained by Western blotting with the monospecific anti-p36 rabbit hyperimmune serum. This antiserum showed only a weak reactivity with proteins of the nontransformed E. coli cells and failed to react against complete antigenic preparations of M. hyorhinis, M. flocculare, M. arginini, M. hyosynoviae, and A. laidlawii, thus confirming the species specificity of the p36 cytosolic protein of M. hyopneumoniae.
Production and characterization of anti-p36 MAbs.
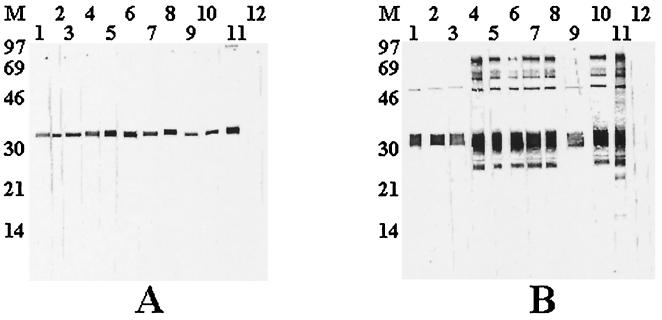
BALB/c mice that were injected with the purified p36 recombinant protein developed a high specific antibody response, with antibody titers in serum ranging from 12,800 to 51,200 as determined by indirect ELISA. Following one fusion experiment, 11 hybridoma cell lines could be established from which 9 were found to secrete anti-p36 antibodies. These 9 hybridomas were subcloned by a limiting dilution method and were subsequently expanded and retested before being injected to pristane-primed mice to produce ascitic fluids. Using a commercial isotyping kit (Boehringer Mannheim), all the MAbs were determined to be of the IgG1 subclass and to possess kappa light chains. In all cases, antibody titers above 51,200 were recovered from ascitic fluids. When tested by Western blotting against the native and the cleaved recombinant p36 protein, antibodies in ascitic fluids reacted very strongly and specifically to both antigens (Fig. 4).
FIG. 4.
Reactivity of the anti-p36 MAbs against whole M. hyopneumoniae antigen (A) and against the cleaved recombinant p36 protein (B). Lanes 1, MAb 11C4; lanes 2, MAb 13B2; lanes 3, MAb 13B7; lanes 4, MAb 13C9; lanes 5, MAb 13G3; lanes 6, MAb 15A3; lanes 7, MAb 15C2; lanes 8, MAb 15E10; lanes 9, 15H3; lanes 10, 15H3; lanes 11, prefusion mouse serum; lanes 12, normal mouse serum; lane M, low-molecular-weight markers.
Species specificity of the anti-p36 MAbs.
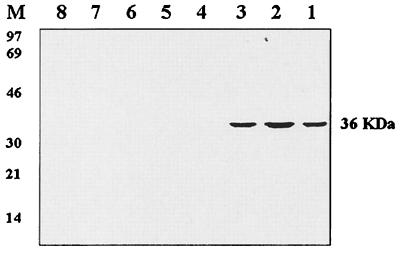
The MAbs were tested for their species specificity by Western blotting against complete antigenic preparations of three reference strains of M. hyopneumoniae (ATCC 25934, ATCC 25095, and J) and those of other porcine mycoplasma species, including A. laidlawii (Fig. 5). The antibodies reacted very specifically to the three M. hyopneumoniae strains and showed no reactivity with other mycoplasma species tested. Lack of reactivity to other mycoplasma species was also confirmed by indirect ELISA (data not shown).
FIG. 5.
Specificity of the anti-p36 MAbs as determined by Western immunoblotting. The anti-p36 MAbs were tested for their reactivity to three reference M. hyopneumoniae strains and other mycoplasma species most commonly found in pigs. Lane 1, M. hyopneumoniae strain ATCC 25934; lane 2, M. hyopneumoniae strain J; lane 3, M. hyopneumoniae strain ATCC 25095; lane 4, M. arginini; lane 5, M. flocculare; lane 6, M. hyorhinis; lane 7, A. laidlawii; lane 8, M. hyosynoviae; lane M, low-molecular-weight markers.
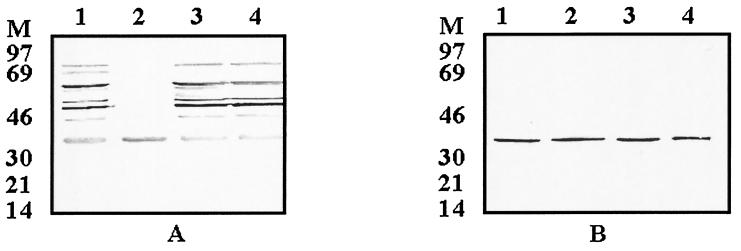
Three Quebec field strains of M. hyopneumoniae, IAF-DM9827, IAF-R202A, and IAF-20167, were cultivated in our laboratory, and the reactivity of the anti-p36 MAbs to these strains was also verified (Fig. 6B). The presence of the p36 protein in all of these three field strains was confirmed by Western blotting with hyperimmune mouse and rabbit sera (Fig. 6A). In all cases, a positive reaction was obtained with the nine anti-p36 MAbs, suggesting the presence of at least one highly conserved antigenic determinant (Fig. 6B, lanes 2 to 4).
FIG. 6.
Patterns of hyperimmune porcine serum (A) and anti-p36 MAbs (B) reactivity to the homologous and Quebec field strains of M. hyopneumoniae. Lanes 1, homologous strain ATCC 25934; lanes 2 to 4, Quebec field isolates IAF-DM9827, IAF-20167-20, IAF-R202A. The sizes of the low-molecular-weight markers are indicated to the left of each gel (lane M).
Clinical and pathological findings in experimentally infected pigs.
The four SPF pigs that were infected intratracheally with a culture of the IAF-DM9827 field strain of M. hyopneumoniae did not manifest obvious clinical signs over the 3-week observation period. By the end of the first week postinfection (p.i.), the infected pigs were apparently more apathic, reacted less to their environment, and preferred to lay on the ground rather than stand. Compared to the pigs in the control group, the infected pigs showed a little drop in feed consumption, with a transitory mild fever (39.5 to 40.5°C) that started by day 3 or 4 p.i. and persisted until the end of the observation period. After 10 to 14 days p.i., coughing could be elicited by exercising infected pigs around the pen, and it occurred with greater frequency in the period immediately following the exercise. Blood was collected from the control and infected pigs at 0, 7, 14, and 21 days p.i. to monitor the kinetics of p36 antibody production by indirect ELISA. The majority of the pigs did not produce significant antibody titers (<1:200) during the 3-week observation period.
Despite the low severity of the clinical signs, two infected pigs that were euthanatized at day 21 p.i. had gross lesions that were confined to the respiratory tract and thoracic cavity. The lung lesions were confined almost entirely to the apical and cardiac lobes and were clearly distinct from the normal lung tissue. Plum-colored or greyish areas of consolidation resembling lymphoid tissue were scattered along the ventral borders of the lobes. The mediastinal lymph nodes were enlarged and congested. There was also slight accumulation of a nonsuppurative exudate within the thoracic cavity (hydrothorax) and pericardium (hydropericardium). The other organs were apparently normal. To confirm an M. hyopneumoniae infection, PCR was conducted on lung homogenates and attempts were made to isolate the microorganism in culture. Expected DNA amplicons were obtained using specific p36 PCR primers for M. hyopneumoniae, but no reaction was observed using primer pairs designed to specifically amplify the p37 gene of M. hyorhinis (4). Following two serial passages in culture medium, growth of the microorganism was obtained as noted by changes of the acidity and turbidity of the medium. Confirmation that M. hyopneumoniae was the only microorganism growing in culture was obtained by PCR.
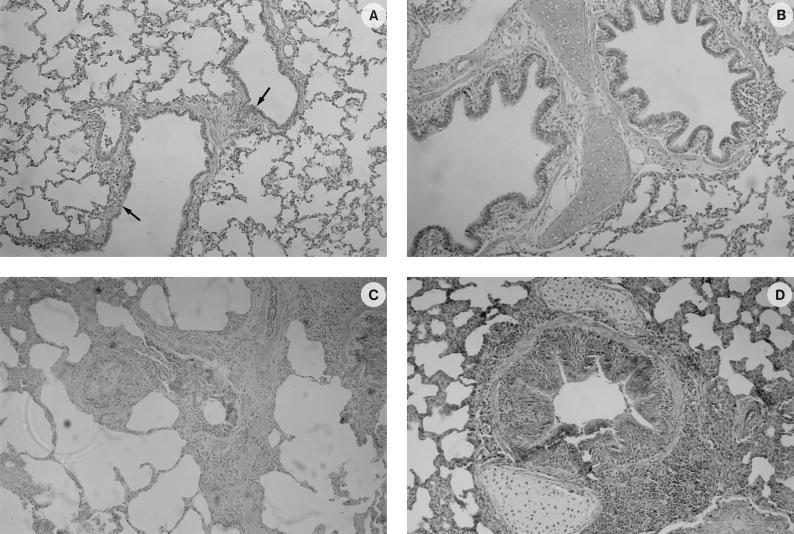
Microscopic lesions compatible with enzootic pneumonia were observed within the lungs of infected pigs (Fig. 7). Characteristic perivascular and peribronchiolar lymphomononuclear nodules of infiltration, often compressing the lumen of the bronchioles, could be observed in both euthanatized pigs. There were also pyknotic cell debris and few mononuclear cells present in the lumen of the bronchioles, with hyperplasia of the epithelial cells. The alveoli had a normal appearance, with only a mild lymphomononuclear infiltration of the interalveolar septae.
FIG. 7.
Histological findings in the lungs of control (A and B) and experimentally infected (C and D) SPF pigs as shown by HPS staining. (A) Spongiform aspect of the lung from a healthy pig, showing clear airway passages (bronchioli and alveolar duct indicated by arrows) and well-delineated interalveolar septae. (B) Normal appearance of a bronchiolus. (C) General aspect of the lung of a pig that has been infected with the IAF-DM9827 strain of M. hyopneumoniae, with characteristic perivascular and peribronchiolar mononuclear cells infiltration. No damage to the alveoli was observed, with only a mild mononuclear infiltration of the alveolar septae. (D) Peribronchiolar and perivascular accumulation of mononuclear cells with a mild hyperplasia of the bronchiolar epithelium.
Detection of anti-p36 circulating antibodies and detection of M. hyopneumoniae in lungs by indirect immunofluorescence.
In preliminary attempts to evaluate the p36 indirect ELISA as a serological test for M. hyopneumoniae infection, 45 field sera obtained from four farms in Southern Quebec having experienced typical outbreaks of enzootic pneumonia were tested in parallel using a commercial competitive ELISA (Dako Inc.), based on the detection of antibodies to the p74 membranous protein (8). Although percentages of competition with the anti-p74 MAbs varying from 35 to 90% were determined for most of the sera tested, only a few demonstrated some reactivity when tested against the p36 protein by indirect ELISA (data not shown). Therefore, no correlation was obtained between A450 of test serum/A450 negative serum ratios in the p36 indirect ELISA and percent competition values in the p74 ELISA.
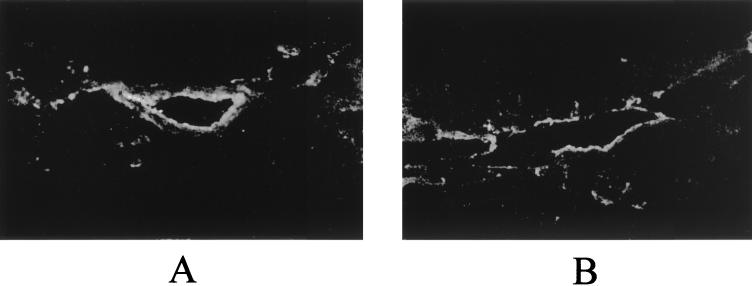
On the other hand, when frozen sections of lungs from pigs that were experimentally infected by M. hyopneumoniae (strain IAF-DM9827) were processed for IIF staining using a pool of anti-p36 MAbs, positive fluorescence was generally associated with the presence of microscopic lesions. Fluorescent cells were usually observed lining the bronchiolar epithelium and lining the wall of small alveolar ducts (Fig. 8) but not within the lamina propria or interstitial tissue surrounding those structures. No such fluorescence was observed when lung sections were incubated with normal mouse serum (data not shown).
FIG. 8.
Immunofluorescent staining with anti-p36 MAbs of frozen sections of lungs from experimentally infected pigs. (A) Fluorescence lining the bronchiolar epithelium; (B) fluorescence lining the wall of alveolar ducts or small airways.
DISCUSSION
The use of mycoplasma recombinant proteins or gene probes expressed in E. coli has been already reported either for the study of the genes or as diagnostic tools (12, 13, 17, 18, 29, 30). Different MAbs have been produced against M. hyopneumoniae proteins, namely, against the p46 (22, 23) and p74 (8) membranous proteins, and the respective MAbs have been used in double sandwich and competitive ELISA tests for serological diagnosis of the infection. In the present study, the pGEX-4T-1 expression vector was chosen for various reasons. First, this procaryotic expression system has been shown to easily and rapidly produce large quantities of pure proteins (24, 27) and because of the presence of a thrombin protease recognition site downstream of the GST coding sequence, which allows cleavage of the desired protein from the fusion partner. Different incubation temperatures and concentrations of IPTG used for the induction can be tested to increase the level of production of the recombinant protein and to avoid its accumulation in the form of inclusion bodies (14, 24). Furthermore, the GST protein is not present in E. coli; hence, pig sera should not possess any antibodies that would react against this protein and interfere with data obtained from serological tests.
The p36 protein was chosen since it has been previously reported to be highly specific and well conserved among the different strains of M. hyopneumoniae (4, 28), it is antigenic (12, 33), and there is no internal OPAL codon (TGA) in the sequence of the encoding gene that interferes with efficient translation of the entire protein (16). Recently, a single PCR assay, using the primer pair FSp36-RSp36 to amplify the entire encoding region of the p36 gene, was shown to be highly specific to M. hyopneumoniae, with no reactivity being observed with other mycoplasma species of swine (4). In the present study, the entire p36-encoding region (948 bp) could be amplified from genomic DNA of the reference ATCC strain 25934 of M. hyopneumoniae, cloned and expressed in E. coli, yielding relatively large amounts of GST-p36 recombinant fusion protein that could be easily purified by affinity chromatography on glutathione-Sepharose 4B beads. Efficient cleavage of the p36 moiety from the GST fusion partner was achieved following digestion with thrombin. Both the fusion protein and the cleaved p36 portion were stable at −80°C; barely any degradation could be observed by SDS-PAGE.
The GST-p36 recombinant fusion protein was used to produce monospecific polyclonal antiserum in rabbits. The latter was found to be specific to M. hyopneumoniae by Western blotting reacting strongly against the reference strains and all field strains cultivated in our laboratory (data not shown). This monospecific hyperimmune serum failed to recognize other mycoplasmas of the porcine species, as well as A. laidlawii, thus confirming the species specificity of the p36 cytosolic protein (4, 28). However, a mild reactivity could be noticed against E. coli proteins, a microorganism of the normal intestinal flora to which animals already have significant circulating antibody titers (4, 28). The production of anti-p36 MAbs was thus recognized to avoid such reactivity towards antigenic proteins of heterologous bacteria which may interfere or mask specific signals when using immunohistologic methods for the detection of Mycoplasma antigens in tissues of infected animals.
Nine secreting hybridoma cell lines producing MAbs to the p36 recombinant fusion protein were established and subcloned. From data obtained in Western blotting experiments, the anti-p36 MAbs obtained were apparently directed towards the same epitope or closely located epitopes of the p36 cytosolic protein, which was found to be highly preserved among all M. hyopneumoniae isolates tested, in agreement with previous findings (27). Since all anti-p36 MAbs reacted by Western blotting against the native mycoplasma protein, as well as the linear E. coli-expressed recombinant p36 protein, the anti-p36 MAbs obtained in the present study are probably directed against conformation-independent epitope(s).
IIF assay of frozen tissue sections is probably the most common diagnostic tool used for the detection of M. hyopneumoniae in tissues of infected pigs (7, 19, 25). The IIF test has many advantages compared to cultivation methods; it is rapid and sensitive and allows one to quantify damages or lesions caused by M. hyopneumoniae in the lungs or other tissues. However, polyclonal antibodies are currently used in the IIF test (1, 7, 19, 20, 25). Consequently, false-positive results may arise because of the cross-reactions existing between pathogenic (M. hyopneumoniae or M. hyosynoviae) and nonvirulent or less virulent (M. flocculare or M. hyorhinis) mycoplasma species (9). The use of MAbs which react to a specific immunodominant protein of M. hyopneumoniae was found to eliminate such undesirable cross-reactivities.
SPF pigs that were experimentally infected with a Quebec field isolate (IAF-DM9827) of M. hyopneumoniae developed pulmonary lesions compatible with a diagnosis of enzootic pneumonia, despite the absence of severe respiratory signs (19, 25). Confirmation of an M. hyopneumoniae infection was obtained by PCR performed on lung homogenates and isolation of the pathogen in culture. Furthermore, thin frozen sections of lung tissues were incubated in the presence of a pool of anti-p36 MAbs, and a specific fluorescent reaction that was limited to the epithelium lining bronchioles and small airways was observed. As expected, no fluorescence was observed within the interstitial tissues and lamina propria of the larger airways. In agreement with the immunofluorescent patterns obtained in the present study, M. hyopneumoniae has not been reported as a tissue invader but is rather considered an extracellular pathogen which associates very intimately with the ciliated epithelial cells of the porcine lower respiratory tract (19, 25).
The IIF technique described in the present study is time-efficient, taking 2 h to obtain the results once the sections are fixed. It only requires a small volume of diluted antiserum for any given reaction, and it is very specific. The p36 MAbs did not react with any other mycoplasma species commonly found in the pig's respiratory tract by Western blotting and ELISA. It is also a sensitive technique, although the intensity of the fluorescence was directly proportional to the quantity of lesions. Preliminary studies on tissues from naturally infected pigs also yielded very interesting results, but the use of the anti-p36 MAbs must be evaluated on a more representative number of clinical cases before being used routinely for diagnostic purposes.
However, preliminary attempts to use the recombinant p36 protein as an antigen in an indirect ELISA for the detection of antibodies in sera from experimentally and naturally infected pigs showed no correlation with clinical and pathological findings. The data obtained suggest that the p36 protein, which is a cytosolic protein rather than a membranous one, such as p46 and p74, is apparently weakly immunogenic and does not trigger an efficient humoral response in pigs, which may explain the unsuccessful results obtained when using the recombinant p36 protein as antigen in an ELISA.
ACKNOWLEDGMENTS
We thank Louise Wilson and Diane Rouleau for their excellent technical assistance. We also thank the following pathologists and microbiologists from the Laboratoire de pathologie animale, Ministère de l'Agriculture, des Pêcheries et de l'Alimentation du Québec (MAPAQ), for their assistance in obtaining clinical samples from sick pigs: Danielle Larochelle, René Sauvageau, and Claude Montpetit. We acknowledge the collaboration of José Daigneault, Pfizer Canda Inc., St-Hyacinthe, P.Q., for her clinical assistance.
This research was partly funded by the Conseil de Recherches en Pêche et Agro-Alimentaire du Québec (grant 4600); BioVet Research Inc., St-Hyacinthe, Quebec, Canada; and the Fédération des producteurs de porcs du Québec.
REFERENCES
- 1.Amanfu W, Weng C N, Ross R F, Barnes H J. Diagnosis of mycoplasmal pneumonia of swine: sequential study by direct immunofluorescence. Am J Vet Res. 1984;45:1349–1352. [PubMed] [Google Scholar]
- 2.Armstrong C H, Freeman M J, Sands-Freeman L. Cross-reactions between Mycoplasma hyopneumoniae and Mycoplasma flocculare: practical implications for the serodiagnosis of mycoplasmal pneumonia of swine. Isr J Med Sci. 1987;23:654–656. [PubMed] [Google Scholar]
- 3.Brooks E, Faulds D. The Mycoplasma hyopneumoniae 74.5kD antigen elicits neutralizing antibodies and shares sequence similarity with heat-shock proteins. Vaccine. 1989;89:265–269. [Google Scholar]
- 4.Caron J, Ouardani M, Dea S. Detection and differentiation of Mycoplasma hyopneumoniae and Mycoplasma hyorhinis by PCR amplification of the p36 and p46 genes. J Clin Microbiol. 2000;38:1390–1396. doi: 10.1128/jcm.38.4.1390-1396.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dea S, Tijssen P. Antigenic and polypeptide structure of turkey enteric coronaviruses as defined by monoclonal antibodies. J Gen Virol. 1989;70:1725–1741. doi: 10.1099/0022-1317-70-7-1725. [DOI] [PubMed] [Google Scholar]
- 6.Dea S, Bilodeau R, Sauvageau R, Martineau G P. Outbreaks of respiratory and reproductive problems associated with encephalomyocarditis virus in Quebec pig farms. J Vet Diagn Investig. 1991;3:275–282. doi: 10.1177/104063879100300401. [DOI] [PubMed] [Google Scholar]
- 7.Feenstra A A, Sörensen V, Friis N F, Jensen N E, Bille-Hansen V. Proceedings of the 13th International Pig Veterinary Society Congress. 1994. Experimental Mycoplasma hyopneumoniae infection in pigs. Bangkok, Thailand. [Google Scholar]
- 8.Feld N C, Qvist P, Ahrens P, Friis N F, Meyling A. A monoclonal blocking ELISA detecting serum antibodies to Mycoplasma hyopneumoniae. Vet Microbiol. 1992;30:35–46. doi: 10.1016/0378-1135(92)90092-8. [DOI] [PubMed] [Google Scholar]
- 9.Freeman M J, Armstrong C H, Freeman-Sands L L, Lopez-Osuna M. Serological cross-reactivity of porcine reference antisera to Mycoplasma hyopneumoniae, M. flocculare, M. hyorhinis and M. hyosynoviae indicated by the enzyme-linked immunosorbent assay, complement fixation and indirect hemagglutination tests. Can J Comp Med. 1984;48:202–207. [PMC free article] [PubMed] [Google Scholar]
- 10.Frey J, Haldimann A, Kobisch M, Nicolet J. Immune response against the l-lactate dehydrogenase of Mycoplasma hyopneumoniae in enzootic pneumonia of swine. Microb Pathog. 1994;17:313–322. doi: 10.1006/mpat.1994.1077. [DOI] [PubMed] [Google Scholar]
- 11.Friis N F. The pathogenicity of Mycoplasma flocculare. Acta Vet Scand. 1973;14:344–346. doi: 10.1186/BF03547455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frydenberg J, Lind K, Hu P C. Cloning of Mycoplasma pneumoniae DNA and expression of P1-epitopes in Escherichia coli. Isr J Med Sci. 1987;23:759–762. [PubMed] [Google Scholar]
- 13.Futo S, Seto Y, Okada M, Sato S, Suzuki T, Kawai K, Imada Y, Mori Y. Recombinant 46-kilodalton surface antigen (P46) of Mycoplasma hyopneumoniae expressed in Escherichia coli can be used for early specific diagnosis of mycoplasmal pneumonia of swine by enzyme-linked immunosorbent assay. J Clin Microbiol. 1995;33:680–683. doi: 10.1128/jcm.33.3.680-683.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonin P, Pirzadeh B, Gagnon C A, Dea S. Seroneutralization of porcine reproductive and respiratory syndrome virus correlates with antibody response to the GP5 major envelope glycoprotein. J Vet Diagn Investig. 1999;11:20–26. doi: 10.1177/104063879901100103. [DOI] [PubMed] [Google Scholar]
- 15.Goodwin R F W, Pomeroy A P, Whittlestone P. Production of enzootic pneumonia in pigs with mycoplasma. Vet Rec. 1965;77:1247–1249. [Google Scholar]
- 16.Haldimann A, Nicolet J, Frey J. DNA sequence determination and biochemical analysis of the immunogenic protein p36, the lactate dehydrogenase (LDH) of Mycoplasma hyopneumoniae. J Gen Microbiol. 1993;139:317–323. doi: 10.1099/00221287-139-2-317. [DOI] [PubMed] [Google Scholar]
- 17.Kim M F, Heidari M B, Stull S J, McIntosh M A, Wise K S. Identification and mapping of an immunogenic region of Mycoplasma hyopneumoniae p65 surface lipoprotein expressed in Escherichia coli from a cloned genomic fragment. Infect Immun. 1990;58:2637–2643. doi: 10.1128/iai.58.8.2637-2643.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klinkert M Q, Herrmann R, Schaller H. Surface proteins of Mycoplasma hyopneumoniae identified from an Escherichia coli expressed plasmid library. Infect Immun. 1985;49:329–335. doi: 10.1128/iai.49.2.329-335.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobish M, Friis N F. Swine mycoplasmoses. Rev Sci Off Int Epizootiol. 1996;15:1569–1606. doi: 10.20506/rst.15.4.983. [DOI] [PubMed] [Google Scholar]
- 20.Maes D, Verdonck M, Deluyker H, de Kruif A. Enzootic pneumonia in pigs. Vet Q. 1996;18:104–109. doi: 10.1080/01652176.1996.9694628. [DOI] [PubMed] [Google Scholar]
- 21.Maré C J, Switzer W P. Mycoplasma hyopneumoniae, a causative agent of virus pig pneumonia. Vet Med. 1965;60:841–845. [PubMed] [Google Scholar]
- 22.Mori Y, Hamaoka T, Sato S. Use of monoclonal antibody in an enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies against Mycoplasma hyopneumoniae. Isr J Med Sci. 1987;23:657–662. [PubMed] [Google Scholar]
- 23.Mori Y, Hamaoka T, Sato S, Takeuchi S. Immunoblotting analysis of antibody response in swine experimentally inoculated with Mycoplasma hyopneumoniae. Immunol Immunopathol. 1988;19:239–250. doi: 10.1016/0165-2427(88)90111-0. [DOI] [PubMed] [Google Scholar]
- 24.Nakano H, Yamazaki T, Ikeda M, Masai H, Miyatake S, Saito T. Purification of glutathione S-transferase fusion proteins as a non-degraded form by using a protease-negative E. coli strain, AD202. Nucleic Acids Res. 1993;22:543–544. doi: 10.1093/nar/22.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross R F. Mycoplasmal diseases. In: Leman A D, Straw B, Mengeling W, D'Allaire S, Taylor D, editors. Diseases of swine. 7th ed. Ames: Iowa State University Press; 1992. pp. 537–551. [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 27.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 28.Stipkovits L, Nicolet J, Haldimann A, Frey J. Use of antibodies against the p36 protein of Mycoplasma hyopneumoniae for the identification of M. hyopneumoniae strains. Mol Cell Probes. 1991;5:451–457. doi: 10.1016/s0890-8508(05)80017-9. [DOI] [PubMed] [Google Scholar]
- 29.Strasser M, Frey J, Bestetti G, Kobisch M, Nicolet J. Cloning and expression of a novel species-specific early immunogenic 36-kilodalton of Mycoplasma hyopneumoniae in Escherichia coli. Infect Immun. 1991;59:1217–1222. doi: 10.1128/iai.59.4.1217-1222.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trevino L B, Haldenwang W G, Baseman J B. Expression of Mycoplasma pneumoniae antigens in Escherichia coli. Infect Immun. 1986;53:129–134. doi: 10.1128/iai.53.1.129-134.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Q, Young T, Ross R F. Identification and characterization of a Mycoplasma hyopneumoniae adhesin. Infect Immun. 1995;63:1013–1019. doi: 10.1128/iai.63.3.1013-1019.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]