Abstract
Background
There has been growing evidence of the benefits of high-intensity aerobic interval training (HIIT) and resistance training (RES) for populations with cancer. However, these two modalities have not yet been performed alone in rectal cancer patients undergoing neoadjuvant chemoradiotherapy (NACR T). Therefore, this study aimed to determine the feasibility of HIIT and RES in rectal cancer patients undergoing NACR T.
Materials and methods
Rectal cancer patients set to undergo NACRT were randomly assigned to HIIT intervention, RES intervention, or the usual care. Feasibility of HIIT and RES was assessed by measuring recruitment rate, adherence (retention rate, attendance rate, and exercise sessions duration and intensity), and adverse events. Endpoints (changes in fatigue, health-related quality of life, depression, daytime sleepiness, insomnia, sleep quality, functional exercise capacity, and executive function) were assessed at baseline and at week 5.
Results
Among the 20 eligible patients, 18 subjects were enrolled and completed the study, yielding a 90% recruitment rate and 100% retention rate. Attendance at exercise sessions was excellent, with 92% in HIIT and 88% in RES. No exercise-related adverse events occurred.
Conclusion
This study demonstrated that HIIT and RES are feasible in rectal cancer patients undergoing NACR T.
Trial registration
www.clinicaltrials.gov, NCT03252821 (date of registration: March 30, 2017)
Keywords: exercise, high-intensity interval training, neoadjuvant chemoradiotherapy, rectal cancer, resistance training
Introduction
The current standard treatment for locally advanced rectal cancer is neoadjuvant chemoradiotherapy (NACRT) followed by surgery [1]. Its introduction in the management of rectal cancer has reduced rates of local recurrence and has facilitated surgical resection [2]. Despite these benefits, patients experience physical and psychological impairments related to NACRT, such as fatigue, reduced exercise capacity and sleep disturbances [3–5] that negatively affect health-related quality of life [6].
A growing number of studies have shown that exercise training is an effective way to mitigate adverse effects during cancer treatment [7–9]. An expert scientific roundtable recognized aerobic and resistance exercise intervention as significant strategies to counter toxicities from cancer treatment [8]. During radiotherapy, resistance training (RES) has been shown to improve fatigue, muscle strength and sleep disturbance in breast cancer patients [10–12]. Moderate-intensity continuous aerobic training has been reported to improve fatigue, functional exercise capacity, and muscle strength in men undergoing radiation therapy for prostate cancer [13, 14], and its feasibility has been reported in rectal cancer patients during and after NACRT [15]. However, previous studies showed the superiority of high-intensity aerobic interval training (HIIT) to improve cardiorespiratory fitness and body composition compared to moderate-intensity aerobic training in colorectal cancer survivors [16, 17]. In addition, HIIT is a time-efficient and more motivating training modality [18, 19]. The comparison of HIIT and RES with a usual care control group (UC) in men with prostate cancer undergoing radiotherapy showed that HIIT and RES were effective to counteract increase in fatigue and improve functional exercise capacity compared to UC, while no additional benefit of HIIT was found compared to RES [20]. Although there has been growing evidence of the benefits of RES and HIIT for populations with cancer, the feasibility, safety, and efficacy of these two exercise modalities are unknown in rectal cancer patients undergoing NACRT as well as the optimal exercise prescription.
Therefore, the objective of this randomized controlled trial was to determine the feasibility of HIIT and RES during NACRT in rectal cancer patients. In addition, we investigated whether the benefits of one of the two modalities are superior to UC to improve the physical and psychological impairments related to NACRT.
Materials and methods
Study design
The present study was a single-center, three-arm, randomized controlled study. Ethical approval was granted by the regional Ethics Committee of the Cliniques universitaires Saint-Luc and Université catholique de Louvain in Brussels (B403201732718). The study was conducted in accordance with the Good Clinical Practice and the Declaration of Helsinki. Written informed consent was obtained from all participants before enrollment. This study was prospectively registered at clinicaltrials.gov (NCT03252821) and reported using the CONSORT guidelines [21].
Recruitment and randomization
Localized rectal cancer patients scheduled to undergo NACRT were recruited between November 2017 and September 2019 from the radiotherapy department at the Cliniques universitaires Saint-Luc in Brussels. The study coordinator consecutively screened patients and invited eligible patients to participate in the study at the radiation simulation. After a baseline assessment, the study coordinator randomly allocated consenting subjects to either the HIIT, the RES, or the UC. Allocation was on a 1:1:1 basis, using computer-generated numbers (JMP Pro 12 software). The study coordinator (E.P.) was responsible for conducting evaluations and exercise supervision. Participants were not blinded due to the study design. As a safety precaution, subjects allocated to the HIIT were required to complete a cardiac stress test prior to starting the high-intensity physical training to check for potential contraindications to high-intensity training.
Eligibility criteria
Eligibility criteria included: subject (1) diagnosed with a localized rectal cancer; (2) planned to receive long-course NACRT; (3) aged over 18 years; and (4) able to understand and speak French or English. Exclusion criteria included: (1) any conditions that could prevent participation in both assessments and exercise training, such as uncontrolled cardiac disease, uncontrolled pulmonary disease, uncontrolled insulin-dependent diabetes mellitus, or any other physical or mental disorder; (2) participation in a regular exercise program; and (3) an abnormal cardiac stress test.
Chemoradiotherapy treatment
Radiotherapy treatment was delivered using an intensity-modulated radiation therapy. Patients received a total dose of 45.0 Gy in 25 fractions on all weekdays over five weeks with concurrent oral capecitabine (dose of 1500 mg/m2 twice daily on days of radiotherapy) or continuous intravenous infusions of 5-fluorouracil (5-FU) (dose of 225 mg/m2 daily, five days per week).
Intervention
Both exercise interventions (HIIT and RES) were performed thrice weekly for five weeks, starting the same day as the radiation therapy and ending on the penultimate day. Exercise sessions took place in the department of Physical Medicine and Rehabilitation after the radiotherapy fraction and was supervised one-on-one by a trained physiotherapist. Each participant received a training journal in which exercise attendance, the intensity and duration of the exercise session, the reason for missing a session, or any exercise-related adverse events were reported.
Participants allocated to the HIIT intervention performed training on a cycle ergometer with a work-recovery ratio of 1:1 with a 60-s work interval at a cadence range of 90–100 revolutions per min at ≥ 85% of the theoretical maximal heart rate (THRmax = 220 – age) interspersed by 60-s active rest, unloaded, at a cadence range of 50–60 revolutions per minute. Warm-up and cool-down were done at 65–70% THRmax for 5 min each. Each training session lasted between 26 and 40 min, including warm-up and cool-down. During high-intensity bouts, the power output was enhanced until the required exercise intensity was reached. The number of intervals performed was set to eight for the first session and then increased week by week until 15 intervals, based on target heart rate and effort perception. Heart rate was monitored throughout the session using a Polar heart rate monitor (Polar, FT7, Electro Oy, Kempele, Finland).
Participants allocated to the RES intervention performed resistance training in which major muscle groups (abdominal, pectoral, deltoid, trapezius, latissimus dorsi, erector spinae, biceps, triceps, quadriceps, hamstrings, gastrocnemius, soleus, and gluteus) were targeted for 30 to 40 min. Subjects performed 1–3 sets of 8–12 repetitions of eight exercises at a rating of perceived exertion of 4–6 on the modified Borg scale [22]. Resistance training equipment included body weight, resistance bands, or dumbbells. The physiotherapist provided individual adjustments and progression throughout the intervention based on the perceived exertion to prevent overload.
Participants randomized to UC received information about physical activity and health according to the World Health Organization’s recommendations at the baseline assessment.
Endpoints
Feasibility of HIIT and RES was determined by calculating the recruitment, adherence, and safety. Recruitment rate was defined as the percentage of the number of recruited patients out of the number of eligible subjects. Adherence was determined by measuring the retention rate, attendance rate, and exercise session duration and intensity [23]. Retention rate was defined as the percentage of patients who complete intervention and assessments out of the number of enrolled patients. Exercise session attendance was calculated as the percentage of the number of exercise sessions attended out of the prescribed number of sessions. Duration and intensity adherence were based on adherence to predefined minutes and prescribed exercise intensity. Safety was determined as any exercise-related adverse events.
Self-reported and objective measure outcomes were assessed 10 days before NACRT treatment start (T0) and the last fraction of RT (T1). Self-report outcomes were used to assess fatigue, QoL, depressive symptoms, daytime sleepiness, insomnia, and sleep quality. Functional exercise capacity and cognitive function were measured with objective tests.
Self-report outcomes
Fatigue was evaluated with the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT–F) [24]. FACIT–F is a 13-item instrument and uses a 5-point Likert scale ranging from 0 (not at all) to 4 (very much). The FACIT–F score range is from 0 to 52, with a high score indicating a low level of fatigue.
Quality of life was measured by the Functional Assessment of Cancer Therapy–General (FACT–G) questionnaire [25]. The FACT–G includes 27 items divided into four domains: physical well-being, social/family well-being, emotional well-being, and functional well-being. Ratings were based on a 5-point Likert scale ranging from 0 to 4, with a total score ranging from 0 to 102. On all scales, the higher the score, the better the QoL.
Depressive symptoms was evaluated using the 20-item Center for Epidemiologic Studies Depression Scale (CES-D) [26]. Respondents were asked to rate, on a 4-point scale (0–3), how often they had experienced different symptoms/feelings over the past week. The total score ranges from 0 to 60, with a higher score denoting more depressive symptoms.
Daytime sleepiness was measured using the Epworth Sleepiness Scale (ESS), an 8-item questionnaire used to identify the respondent’s tendency to fall asleep during daytime [27]. The ESS score varies from 0 to 24, with higher scores indicating more daytime sleepiness.
The Insomnia Severity Index (ISI) is a 7-item questionnaire assessing the patient’s perception of both nocturnal and daytime symptoms of insomnia over the two previous weeks [28]. The total score ranges from 0 to 28 and is interpreted as follows: no clinically significant insomnia (0–7), sub-threshold insomnia (8–14), moderate insomnia (15–21), and severe insomnia (22–28).
The Pittsburgh sleep quality index (PSQI) is a self-reported questionnaire that assesses sleep quality and disturbances over the past month [29]. PSQI consists of 19 individual items yielding seven components (subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction), each ranging from 0 to 3. The total score, obtained by adding the seven component scores, varies from 0 to 21.
Objective measured outcomes
Functional exercise capacity was evaluated by the total distance walked during a 6-min walking test (6MWT) in a 30-m hallway [30]. This submaximal test is a valid and reliable instrument in cancer patients [31].
Cognitive function was measured with the trail-making test (TMT), a standardized, reliable, and valid neuropsychological tool [32, 33]. The test consists of two parts (A and B) in which the subject is instructed to connect numbers and/or letters in a logical order. The test was administered according to the guidelines presented by Spreen and Strauss. The total time needed to complete each part was recorded, with higher values indicating a worse cognitive function.
Statistical analysis
Since there are no specific sample size recommendations for feasibility aims in healthcare research [34], it was arbitrarily decided to obtain a sample size of 18 subjects (six per group). Categorical and dichotomous data were presented as numbers (percentages) and continuous data were expressed as median [interquartile range]. Non-parametric tests were applied because of the small number of subjects per group. Chi-square and Kruskal-Wallis tests were used to investigate differences in sociodemographic and medical variables between the three groups. The Kruskal-Wallis test was employed to detect differences in changes and percentage changes from pre- to post-intervention in self-reported questionnaires and objective measures between the three groups. Where appropriate, Mann-Whitney tests were applied to identify any significant difference between groups. Data were analyzed with an intention-to-treat approach using IBM SPSS software, version 25.0 (IBM Corp., Armonk, NY, USA). The statistical significance for all analyses was set to p < 0.05.
Results
Participant characteristics
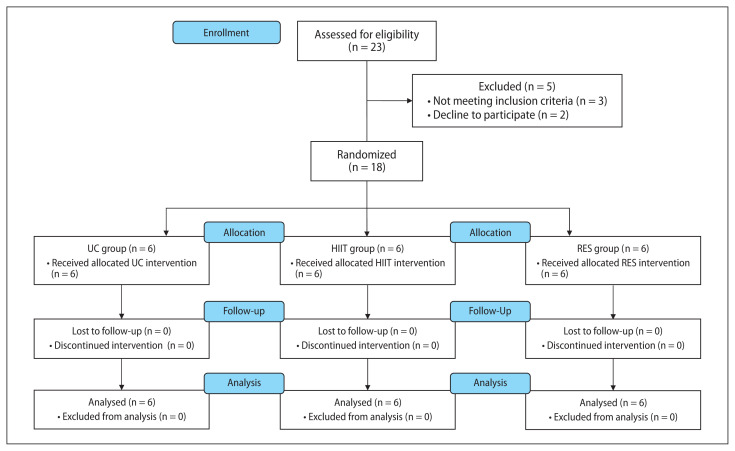
Figure 1 provides the CONSORT flow diagram of the study.21 Eighteen subjects were recruited and randomized to the study: six to the HIIT group, six to the RES group and six to the UC group. Baseline participant characteristics were comparable between the three groups (Tab. 1). Most patients were male (72%) with a median age of 62.0 (59.8 to 68.8) years, married/cohabiting (83%), still working (56%), and nonsmokers (72%). All participants received a total radiation dose of 45 Gy in 25 fractions concurrent with capecitabine (83%) or 5-Fu (17%).
Figure 1.
CONSORT flow diagram. Abbreviation: HIIT: high-intensity aerobic interval training; RES — resistance training; UC — usual care
Table 1.
Participant characteristics [median (interquartile range 25–75) or n (%)]
| Variables | UC (n = 6) | HIIT (n = 6) | RES (n = 6) | p-value |
|---|---|---|---|---|
| Age [years] | 64.5 (62.5; 72.5) | 61.0 (54.5; 65.3) | 61.5 (52.8; 73.5) | 0.233 |
| BMI [kg/m2] | 25.3 (23.3; 28.3) | 27.0 (25.4; 31.0) | 27.0 (24.4; 30.1) | 0.519 |
| Gender | 0.758 | |||
| Male | 4 (66.6%) | 5 (83.3%) | 4 (66.6%) | |
| Female | 2 (33.3%) | 1 (16.7%) | 2 (33.3%) | |
| Employment status | 0.407 | |||
| Working | 2 (33.3%) | 4 (66.6%) | 4 (66.6%) | |
| Retired | 4 (66.6%) | 2 (33.3%) | 2 (33.3%) | |
| Marital status | 0.301 | |||
| Married/cohabitant | 4 (16.6%) | 6 (100%) | 5 (83.3%) | |
| Divorced/separated | 2 (33.3%) | 0 (0.0%) | 1 (16.7%) | |
| Ethnicity | 1.000 | |||
| Caucasian | 6 (100%) | 6 (100%) | 6 (100%) | |
| Highest education level | 0.407 | |||
| Secondary school | 2 (33.3%) | 2 (33.3%) | 4 (66.6%) | |
| Higher education | 4 (66.6%) | 4 (66.6%) | 2 (33.3%) | |
| Smoking status | 0.188 | |||
| Non-smoker | 4 (66.6%) | 4 (66.6%) | 5 (83.3%) | |
| Current smoker | 2 (33.3%) | 0 (0.0%) | 0 (0.0%) | |
| Ex-smoker | 0 (0.0%) | 2 (33.3%) | 1 (16.7%) | |
| Comorbidities* (no.) | 1.0 (0.0; 2.3) | 1.0 (0.0; 1.3) | 2.0 (0.0; 2.8) | 0.562 |
| Chemotherapy regimen | 1.000 | |||
| 5-FU | 1 (16.7%) | 1 (16.7%) | 1 (16.7%) | |
| Capecitabine | 5 (83.3%) | 5 (83.3%) | 5 (83.3%) | |
| Cancer stage | 0.472 | |||
| II | 2 (33.3%) | 3 (50.0%) | 1 (16.7%) | |
| III | 4 (66.6%) | 3 (50.0%) | 5 (83.3%) | |
5-FU — 5-fluorouracil; BMI — body mass index; HIIT — high-intensity aerobic interval training; RES — resistance training; UC — usual care;
obesity, high blood pressure, cardiac disease, hyperlipidemia
Feasibility
Of the 23 subjects assessed for eligibility, three did not meet the inclusion criteria (due to a language disorder, orthopedic problem, and postoperative chemoradiotherapy, respectively) (Fig. 1). Among the 20 eligible patients, two refused to participate, citing a lack of time and interest. Therefore, 18 subjects were enrolled in the study, yielding a 90% recruitment rate (18 subjects out of 20). No patient enrolled in the HIIT group was excluded because of an abnormal cardiac stress test. All randomized patients completed the study, yielding a 100% retention rate (18 subjects out of 18). Attendance at exercise sessions was 92% (77 exercise sessions out of 84) in HIIT and 88% in RES (74 exercise sessions out of 84), from which four and two subjects, respectively, attended 100% of the prescribed sessions. The most common reasons for missing a session were intense diarrhea and fatigue. Mean resistance training duration was 31.1 ± 6.7 min per session (RPE = 4.2 ± 1.1). Mean HIIT duration increased from 26.3 ± 3.2 per session to 38.5 ± 6.3 per session. Regarding HIIT intensity, all the participants in the HIIT reached the target heart rate during high-intensity bouts. In both groups, no exercise-related adverse events occurred.
Outcome measures
Self-reported outcomes
Baseline self-reported questionnaire scores were comparable between the three groups (Tab. 2). Changes in FACIT–F, FACT–G, CES-D, ESS, ISI, and PSQI were not different between groups (p > 0.05), except for the social/family well-being subscale of the FACT–G (p = 0.022), which increased in RES compared to HIIT [+2.0 (−0.5; 4.3) vs. −3.1 (−5.5; −1.5), p = 0.017].
Table 2.
Differences between groups at baseline and (%) change differences from pre- to post-intervention between groups for the measured outcomes [median (interquartile range 25–75)]
| Variables | UC (n = 6) | HIIT (n = 6) | RES (n = 6) | p-value |
|---|---|---|---|---|
| FACIT-F | ||||
| T0 | 42.5 (20.6; 49.5) | 45.0 (37.5; 51.3) | 45.0 (31.8; 48.0) | 0.684 |
| T1 | 36.2 (22.9; 48.3) | 39.5 (29.8; 41.3) | 40.5 (22.0; 47.0) | |
| Change from T0 to T1 | −1.6 (−6.8; 1.6) | −11.0 (−16.3; 3.8) | 0.0 (−9.5; 2.5) | 0.610 |
| FACT-G | ||||
| T0 | 85.6 (57.6; 92.3) | 87.2 (73.2; 96.0) | 76.9 (64.7; 95.7) | 0.767 |
| T1 | 79.6 (60.6; 92.0) | 75.0 (71.5; 84.0) | 83.5 (68.0; 94.3) | |
| Change from T0 to T1 | 0.6 (−9.6; 7.6) | −10.8 (−17.6; 0.0) | 7.3 (−6.5; 11.1) | 0.316 |
| Physical well-being | ||||
| T0 | 26.5 (13.0; 28.0) | 26.3 (21.3; 28.0) | 24.7 (21.5; 27.0) | 0.750 |
| T1 | 21.5 (16.8; 26.3) | 22.5 (17.8; 23.3) | 23.0 (18.3; 26.0) | |
| Change from T0 to T1 | −1.5 (−5.0; 3.3) | −3.8 (−7.3; 1.5) | −1.5 (−3.5; 0.7) | 0.649 |
| Social/family well-being | ||||
| T0 | 25.9 (22.1; 28.0) | 23.0 (21.5; 28.0) | 21.5 (15.2; 28.0) | 0.534 |
| T1 | 24.2 (23.0; 25.6) | 19.9 (17.8; 22.8) | 24.3 (18.8; 26.5) | |
| Change from T0 to T1 | −1.2 (−3.0; 1.2) | −3.1 (−5.5; −1.5)(RES)* | 2.0 (−0.5; 4.3)(HIIT)* | 0.022 |
| Emotional well-being | ||||
| T0 | 15.0 (12.8; 19.3) | 19.0 (17.0; 23.0) | 20.0 (14.3; 21.3) | 0.249 |
| T1 | 19.5 (14.5; 21.2) | 20.5 (19.3; 22.0) | 22.0 (18.8; 22.3) | |
| Change from T0 to T1 | 1.8 (0.0; 6.0) | 0.5 (−2.3; 4.5) | 1.5 (0.0; 6.8) | 0.608 |
| Functional well-being | ||||
| T0 | 18.5 (5.5; 22.3) | 15.5 (13.3; 24.0) | 14.5 (8.8; 26.0) | 0.932 |
| T1 | 13.5 (6.3 ; 20.3) | 14.5 (11.8; 17.3) | 15.0 (10.8; 22.3) | |
| Change from T0 to T1 | −2.0 (−6.3 ; 3.6) | −3.5 (−6.5; 2.5) | −0.5 (−4.8; 4.0) | 0.794 |
| CES-D | ||||
| T0 | 14.5 (7.5; 31.3) | 7.5 (4.8; 21.5) | 6.5 (4.5; 14.0) | 0.215 |
| T1 | 15.0 (4.8 ; 26.3) | 12.0 (10.0; 14.3) | 9.5 (7.3; 17.5) | |
| Change from T0 to T1 | −3.0 (−6.5; 1.5) | 4.0 (−7.3; 5.5) | 3.5 (−2.0; 6.5) | 0.377 |
| ESS | ||||
| T0 | 4.0 (2.8; 7.0) | 5.5 (3.8; 6.3) | 5.5 (3.5; 7.5) | 0.800 |
| T1 | 3.0 (2.5; 8.8) | 6.5 (4.5; 7.3) | 6.0 (2.8; 9.0) | |
| Change from T0 to T1 | 0.5 (−2.0; 1.8) | 1.0 (0.0; 1.3) | 0.0 (−2.3; 1.3) | 0.363 |
| ISI | ||||
| T0 | 9.5 (5.5; 20.8) | 5.5 (3.0; 9.3) | 8.5 (3.0; 12.8) | 0.303 |
| T1 | 11.5 (3.8; 16.5) | 9.0 (6.8; 10.8) | 9.5 (6.0; 12.8) | |
| Change from T0 to T1 | 0.5 (−6.3; 3.5) | 3.0 (1.5; 4.0) | 1.0 (−2.0; 4.0) | 0.367 |
| PSQI | ||||
| T0 | 6.0 (5.5; 16.5) | 5.0 (2.5; 8.3) | 6.5 (4.0; 9.0) | 0.527 |
| T1 | 8.5 (6.6; 13.5) | 6.5 (4.3; 10.0) | 6.5 (4.5; 9.0) | |
| Change from T0 to T1 | 1.0 (−2.5; 3.1) | 1.0 (1.0; 2.3) | 0.5 (−0.8; 2.0) | 0.612 |
| 6MWT [m] | ||||
| T0 | 463.0 (375.0; 530.8) | 581.5 (535.0; 612.8) | 498.0 (445.0; 534.0) | 0.028 |
| T1 | 498.0 (388.0; 574.3) | 609.0 (552.3; 636.5) | 522.5 (463.0; 547.5) | |
| % change from T0 to T1 | 9.9 (2.2; 16.6) | 2.5 (−3.7; 6.7) | 4.2 (−8.6; 8.3) | 0.236 |
| TMT-A [s] | ||||
| T0 | 45.2 (33.7; 55.2) | 35.8 (27.4; 43.3) | 42.0 (23.9; 59.7) | 0.459 |
| T1 | 41.8 (33.7; 52.0) | 32.6 (26.3; 37.2) | 31.1 (21.1; 45.1) | |
| % change from T0 to T1 | −14.9 (−33.6; 7.6) | −6.2 (−17.5; 4.6) | −10.8 (−17.7; 8.4) | 0.593 |
| TMT-B [s] | ||||
| T0 | 102.4 (100.2; 124.0) | 80.2 (69.6; 98.2) | 86.0 (55.1; 101.5) | 0.050 |
| T1 | 83.1 (70.5; 130.7) | 59.8 (54.9; 79.0) | 73.3 (46.4; 99.1) | |
| % change from T0 to T1 | −18.1 (−20.7; −1.7) | −26.3 (−29.2; −11.8) | −16.0 (−33.6; 8.1) | 0.484 |
| TMT B-A [s] | ||||
| T0 | 64.9 (48.5; 85.2) | 46.4 (37.1; 55.6) | 33.1 (29.4; 54.0) | 0.038 |
| T1 | 42.9 (32.3; 82.3) | 31.3 (23.1; 40.7) | 34.8 (26.0; 65.1) | |
| % change from T0 to T1 | −5.0 (−25.0; 31.9) | −33.4 (−50.9; −15.2) | −32.4 (−48.2; 18.0) | 0.302 |
6MWT — 6-minute walk test; CES-D — epidemiological studies depression scale; ESS — epworth sleepiness scale; FAC IT–F — functional assessment of chronic illness therapy-fatigue; FAC T–G — functional assessment of cancer therapy-general; HIIT — high-intensity aerobic interval training; ISI — insomnia severity index; PSQI — pittsburg sleep quality index; RES — resistance training; T0 — assessment 10 days before NACRT treatment start; T1 — assessment after intervention; TMT — trail-making-test; UC — usual care;
statistically significant differences between RES and HIIT (p = 0.017) for social/family well-being
Objective measured outcomes
TMT-A (p = 0.459) and TMT-B (p = 0.050) were similar between groups at the baseline (Tab. 2). In contrast, significant differences between the three groups were observed at the baseline for 6MWT (p = 0.028) and TMT B-A (p = 0.038). The gains in 6MWT, TMT-A, TMT-B, and TMT B-A were not significantly different between the three groups (p > 0.05).
Discussion
The primary purpose of this study was to investigate the feasibility of HIIT and RES in rectal cancer patients undergoing NACRT. Our findings demonstrated that both exercise modalities are feasible, safe, and well tolerated during NACRT in these patients, as they reported keen interest in participating in exercise programs, adhered to the intervention, and had no exercise-related adverse events.
All eligible patients were invited to participate in the study. Most rectal cancer patients were willing and able to participate in an exercise program: we had a recruitment rate of 90%. The eighteen subjects recruited were generally representative of the rectal cancer patients undergoing NACRT in terms of age, sex and disease diagnosis stage [2]. The present recruitment rate is higher than the 56–75% rates observed in studies performing supervised exercise training during NACRT in rectal cancer patients [35–37]. The interest in participating in the current study might be attributable to patient education by a certified physiotherapist about the importance and benefits of performing exercise training during cancer treatment performed. An additional reason may be that exercise sessions were held in the rehabilitation department, close to the radiotherapy department and immediately following the radiotherapy session, which reduced the travel burden and minimized wasted time for participants. Indeed, time and distance to the training site are frequently cited reasons for declining to participate.
The study retention rate was also maximal (100%). Indeed, all randomized participants in the three groups completed the study. This was consistent with the study of Egegaard et al. who performed HIIT in NSCLC patients during NACRT [38], but was higher (69–78%) than in trials including rectal cancer patients who participated in exercise training during NACRT [35–37]. Overall attendance at HIIT training sessions was 92% versus 88% for RES, which is similar to a study that performed the same exercise design in prostate cancer patients undergoing radiotherapy (94% for HIIT and 92% for RES) [20]. Other supervised programs in studies recruiting rectal cancer patients undergoing NACRT showed a lower or similar attendance at exercise sessions (74–95%). As mentioned above for the recruitment rate, the location and timing of the exercise sessions are also likely to contribute to the retention rate and attendance at exercise sessions. The proximity between the rehabilitation center and the radiotherapy department was appreciated by the patients, indicating that performing the exercise intervention in the same area as the radiotherapy department can maximize attendance at exercise training. In addition, the supervision, the adjustment of the training contents to individual needs, and flexibility to reschedule training sessions at an opportune time for the patient may have also contributed to the high retention and attendance rate. Furthermore, given the fact that enjoyment is a predictor of exercise adherence [39], we set up a HIIT program with a work–recovery ratio of 1:1 with 60-s intervals of work interspersed by 60-s intervals of rest, which has previously been shown to result in positive affective responses [18, 40]. In addition, Martinez et al. showed greater enjoyment and positive affect for shorter intervals of 30 s and 60 s than for longer intervals [41]. These results suggest that intervals ≤ 60 s with a work–recovery ration of 1:1 could be the optimal setting of HIIT to have a high adherence to exercise. Resistance training has also been shown to elicit feelings of pleasure in healthy populations [42].
Regarding safety, no exercise-related adverse events occurred throughout the NACRT, similar to other studies in this field [35–37, 43]. However, it is interesting to note that gastrointestinal perturbation is a known side effect that occurs throughout NACRT in rectal cancer patients and this perturbation negatively influences tolerance of exercise programs [44]. In this study, diarrhea was one of the most frequently reported reasons for missing an exercise session, but no patients withdrew from the study for this reason. We observed that the diarrhea frequency gradually increased throughout NACRT, but the intensity varied from one day to the next. In general, gastrointestinal disturbances were present before the radiotherapy fraction and did not worsen directly after the radiotherapy session. Therefore, the exercise session performed after the radiotherapy session was well tolerated and it does not seem necessary to perform the exercise session prior to an RT session, as proposed by Singh et al. [36], which would be more uncomfortable for patients who were treated with a full bladder. The high intensity of the HIIT did not seem to influence bowel activation more than RES as the number of missed sessions due to diarrhea was similar between the two groups.
Both exercise interventions in the present study were performed throughout the neoadjuvant treatment. By implementing exercise training during this period, we sought to evaluate the effects of exercise on treatment-related adverse events. We observed no significant change in any measured outcome between the three groups except for the social/family well-being subscale of FACT–G, which increased in RES compared to HIIT. Regarding functional exercise capacity, one patient in the HIIT group and two patients in the resistance group decreased walking capacity from preto post-intervention. Interestingly, these patients were the only ones to have an attendance rate to exercise sessions of less than 70%, underlining that effectiveness of exercise training is related to the amount of exercise performed. The lack of significant results may be attributed to the small number of patients in each group. Similar to our findings, Egegaard et al., who randomized non-small-cell lung cancer patients undergoing NACRT either to a daily moderate-to-high-intensity interval training group or to UC did not show between-group differences after intervention for physiological outcomes [38]. In contrast, a recent study used the same RCT design with a power sample size in prostate cancer patients undergoing radiotherapy and showed that HIIT and RES were effective for counteracting fatigue and improving functional exercise capacity compared to UC, while no additional benefit of HIIT was found compared to RES.20 Other studies with a supervised exercise program in rectal cancer patients undergoing NACRT reported interesting but limited results because of the single-arm design [35–37, 43].
This study, to our knowledge, is the first to perform an HIIT or a RES alone for rectal cancer patients undergoing NACRT. Both exercise interventions were conducted using a patient-centered approach, in which sessions were supervised, tailored to individual patient abilities, performed close to the radiation department and immediately after the radiation session, and could be rescheduled at a time convenient to the patient. In addition, this study directly compared two different exercise training interventions. However, certain limitations to this study have to be highlighted. Firstly, although the sample size is considered appropriate for a feasibility study [34], this study was underpowered to evaluate the effects of exercise on the physical and psychological variables. In addition, neither the participants nor the evaluator were blinded to the intervention, which affect the internal validity of the study.
This study showed that the majority of rectal cancer patients were interested and able to engage in HIIT or RES during NACRT and both exercise training programs were feasible in this population. It is essential that the exercise intervention take a patient-centered approach to maximize adherence to the program. Although this study found that both interventions are feasible in this population, adequately powered randomized controlled trials are needed to determine their effectiveness on the physical and psychological impairments related to NACRT. Furthermore, more high-quality studies directly comparing different exercise training are warranted in order to develop specific exercise intervention for rectal cancer patients undergoing NACRT.
Conclusion
In conclusion, this study showed that HIIT and RES are feasible in rectal cancer patients undergoing NACRT. A high recruitment rate and adherence were demonstrated, with no exercise-related adverse events. In view of our results, none of the two exercise programs was superior to UC to improve the physical and psychological impairments related to NACRT. Future randomized controlled trials are needed to determine the effect of both exercise programs on physical and psychological outcomes in these patients.
Acknowledgements
The authors acknowledge the technical support of Clement Carbonnelle and Pierre Delbecque.
Footnotes
Conflict of interest
None declared.
Funding
X is funded by a grant from the Fonds National de la Recherche Scientifique (FRIA-FNRS). X is funded by a grant from the Institut de Recherche Expérimentale et Clinique (Université catholique de Louvain, Brussels, Belgium).
References
- 1.Benson AlB, Venook AP, Al-Hawary MM, et al. Anal Carcinoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(7):852–871. doi: 10.6004/jnccn.2018.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosset JF, Collette L, Calais G, et al. EORTC Radiotherapy Group Trial 22921. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 3.Wang XS, Janjan NA, Guo H, et al. Fatigue during preoperative chemoradiation for resectable rectal cancer. Cancer. 2001;92(6 Suppl):1725–1732. doi: 10.1002/1097-0142(20010915)92:6+<1725::aid-cncr1504>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 4.West MA, Loughney L, Lythgoe D, et al. Effect of prehabilitation on objectively measured physical fitness after neoadjuvant treatment in preoperative rectal cancer patients: a blinded interventional pilot study. Br J Anaesth. 2015;114(2):244–251. doi: 10.1093/bja/aeu318. [DOI] [PubMed] [Google Scholar]
- 5.Dhruva A, Paul SM, Cooper BA, et al. A longitudinal study of measures of objective and subjective sleep disturbance in patients with breast cancer before, during, and after radiation therapy. J Pain Symptom Manage. 2012;44(2):215–228. doi: 10.1016/j.jpainsymman.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herman JM, Narang AK, Griffith KA, et al. The quality-of-life effects of neoadjuvant chemoradiation in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2013;85(1):e15–e19. doi: 10.1016/j.ijrobp.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belloni S, Arrigoni C, Caruso R. Effects from physical exercise on reduced cancer-related fatigue: a systematic review of systematic reviews and meta-analysis. Acta Oncol. 2021;60(12):1678–1687. doi: 10.1080/0284186X.2021.1962543. [DOI] [PubMed] [Google Scholar]
- 8.Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc. 2019;51(11):2375–2390. doi: 10.1249/MSS.0000000000002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schumacher O, Luo H, Taaffe DR, et al. Effects of Exercise During Radiation Therapy on Physical Function and Treatment-Related Side Effects in Men With Prostate Cancer: A Systematic Review and Meta-Analysis. Int J Radiat Oncol Biol Phys. 2021;111(3):716–731. doi: 10.1016/j.ijrobp.2021.06.034. [DOI] [PubMed] [Google Scholar]
- 10.Steindorf K, Schmidt ME, Klassen O, et al. Randomized, controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: results on cancer-related fatigue and quality of life. Ann Oncol. 2014;25(11):2237–2243. doi: 10.1093/annonc/mdu374. [DOI] [PubMed] [Google Scholar]
- 11.Steindorf K, Wiskemann J, Ulrich CM, et al. Effects of exercise on sleep problems in breast cancer patients receiving radiotherapy: a randomized clinical trial. Breast Cancer Res Treat. 2017;162(3):489–499. doi: 10.1007/s10549-017-4141-8. [DOI] [PubMed] [Google Scholar]
- 12.Wiskemann J, Schmidt ME, Klassen O, et al. Effects of 12-week resistance training during radiotherapy in breast cancer patients. Scand J Med Sci Sports. 2017;27(11):1500–1510. doi: 10.1111/sms.12777. [DOI] [PubMed] [Google Scholar]
- 13.Windsor PM, Nicol KF, Potter J. A randomized, controlled trial of aerobic exercise for treatment-related fatigue in men receiving radical external beam radiotherapy for localized prostate carcinoma. Cancer. 2004;101(3):550–557. doi: 10.1002/cncr.20378. [DOI] [PubMed] [Google Scholar]
- 14.Monga U, Garber SL, Thornby J, et al. Exercise prevents fatigue and improves quality of life in prostate cancer patients undergoing radiotherapy. Arch Phys Med Rehabil. 2007;88(11):1416–1422. doi: 10.1016/j.apmr.2007.08.110. [DOI] [PubMed] [Google Scholar]
- 15.Latrille M, Buchs NC, Ris F, et al. Physical activity programmes for patients undergoing neo-adjuvant chemoradiotherapy for rectal cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2021;100(51):e27754. doi: 10.1097/MD.0000000000027754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devin JL, Jenkins DG, Sax AT, et al. Cardiorespiratory Fitness and Body Composition Responses to Different Intensities and Frequencies of Exercise Training in Colorectal Cancer Survivors. Clin Colorectal Cancer. 2018;17(2):e269–e279. doi: 10.1016/j.clcc.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Devin JL, Sax AT, Hughes GI, et al. The influence of high-intensity compared with moderate-intensity exercise training on cardiorespiratory fitness and body composition in colorectal cancer survivors: a randomised controlled trial. J Cancer Surviv. 2016;10(3):467–479. doi: 10.1007/s11764-015-0490-7. [DOI] [PubMed] [Google Scholar]
- 18.Thum JS, Parsons G, Whittle T, et al. High-Intensity Interval Training Elicits Higher Enjoyment than Moderate Intensity Continuous Exercise. PLoS One. 2017;12(1):e0166299. doi: 10.1371/journal.pone.0166299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mugele H, Freitag N, Wilhelmi J, et al. High-intensity interval training in the therapy and aftercare of cancer patients: a systematic review with meta-analysis. J Cancer Surviv. 2019;13(2):205–223. doi: 10.1007/s11764-019-00743-3. [DOI] [PubMed] [Google Scholar]
- 20.Piraux E, Caty G, Renard L, et al. Effects of high-intensity interval training compared with resistance training in prostate cancer patients undergoing radiotherapy: a randomized controlled trial. Prostate Cancer Prostatic Dis. 2021;24(1):156–165. doi: 10.1038/s41391-020-0259-6. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Hopewell S, Schulz KF, et al. CONSORT, Consolidated Standards of Reporting Trials Group. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340(8):c869–37. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 23.Hawley-Hague H, Horne M, Skelton DA, et al. Review of how we should define (and measure) adherence in studies examining older adults’ participation in exercise classes. BMJ Open. 2016;6(6):e011560. doi: 10.1136/bmjopen-2016-011560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yellen SB, Cella DF, Webster K, et al. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FAC T) measurement system. J Pain Symptom Manage. 1997;13(2):63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 25.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 26.Radloff L. The CES-D Scale. Appl Psychol Meas. 2016;1(3):385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- 27.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 28.Morin CM. Insomnia: Psychological Assessment and Management. Guilford Press; New York: 1993. [Google Scholar]
- 29.Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 30.Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428–1446. doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt K, Vogt L, Thiel C, et al. Validity of the six-minute walk test in cancer patients. Int J Sports Med. 2013;34(7):631–636. doi: 10.1055/s-0032-1323746. [DOI] [PubMed] [Google Scholar]
- 32.Sánchez-Cubillo I, Periáñez JA, Adrover-Roig D, et al. Construct validity of the Trail Making Test: role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J Int Neuropsychol Soc. 2009;15(3):438–450. doi: 10.1017/S1355617709090626. [DOI] [PubMed] [Google Scholar]
- 33.Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol. 1955;19(5):393–394. doi: 10.1037/h0044509. [DOI] [PubMed] [Google Scholar]
- 34.Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008;31(2):180–191. doi: 10.1002/nur.20247. [DOI] [PubMed] [Google Scholar]
- 35.Morielli AR, Usmani N, Boulé NG, et al. A Phase I Study Examining the Feasibility and Safety of an Aerobic Exercise Intervention in Patients With Rectal Cancer During and After Neoadjuvant Chemoradiotherapy. Oncol Nurs Forum. 2016;43(3):352–362. doi: 10.1188/16.ONF.352-362. [DOI] [PubMed] [Google Scholar]
- 36.Singh F, Galvão DA, Newton RU, et al. Feasibility and Preliminary Efficacy of a 10-Week Resistance and Aerobic Exercise Intervention During Neoadjuvant Chemoradiation Treatment in Rectal Cancer Patients. Integr Cancer Ther. 2018;17(3):952–959. doi: 10.1177/1534735418781736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heldens AF, Bongers BC, de Vos-Geelen J, et al. Feasibility and preliminary effectiveness of a physical exercise training program during neoadjuvant chemoradiotherapy in individual patients with rectal cancer prior to major elective surgery. Eur J Surg Oncol. 2016;42(9):1322–1330. doi: 10.1016/j.ejso.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 38.Egegaard T, Rohold J, Lillelund C, et al. Pre-radiotherapy daily exercise training in non-small cell lung cancer: A feasibility study. Rep Pract Oncol Radiother. 2019;24(4):375–382. doi: 10.1016/j.rpor.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jekauc D. Enjoyment during Exercise Mediates the Effects of an Intervention on Exercise Adherence. Psychology. 2015;06(01):48–54. doi: 10.4236/psych.2015.61005. [DOI] [Google Scholar]
- 40.Bartlett JD, Close GL, MacLaren DPM, et al. High-intensity interval running is perceived to be more enjoyable than moderate-intensity continuous exercise: implications for exercise adherence. J Sports Sci. 2011;29(6):547–553. doi: 10.1080/02640414.2010.545427. [DOI] [PubMed] [Google Scholar]
- 41.Martinez N, Kilpatrick MW, Salomon K, et al. Affective and Enjoyment Responses to High-Intensity Interval Training in Overweight-to-Obese and Insufficiently Active Adults. J Sport Exerc Psychol. 2015;37(2):138–149. doi: 10.1123/jsep.2014-0212. [DOI] [PubMed] [Google Scholar]
- 42.Cavarretta D, Hall E, Bixby W. The acute effects of resistance exercise on affect, anxiety, and mood — practical implications for designing resistance training programs. Int Rev Sport Exercise Psych. 2018;12(1):295–324. doi: 10.1080/1750984x.2018.1474941. [DOI] [Google Scholar]
- 43.Singh F, Newton RU, Baker MK, et al. Feasibility and Efficacy of Presurgical Exercise in Survivors of Rectal Cancer Scheduled to Receive Curative Resection. Clin Colorectal Cancer. 2017;16(4):358–365. doi: 10.1016/j.clcc.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 44.Piraux E, Caty G, Aboubakar Nana F, et al. Effects of exercise therapy in cancer patients undergoing radiotherapy treatment: a narrative review. SAGE Open Med. 2020;8:2050312120922657. doi: 10.1177/2050312120922657. [DOI] [PMC free article] [PubMed] [Google Scholar]