Abstract
Background
To properly configure a treatment planning system, a measurement data set is needed, which consists of the values required for its configuration. The aim is to obtain a dosimetric model of the beam that is as compatible as possible with the measured values. The set of required data can be supplemented with optional values. The aim of the study was to assess the influence of optional measurement data on the compliance of the calculations with the measurements.
Materials and methods
Dosimetric measurements, model configuration and dose distribution calculations were performed for the photon radiation beams generated by the VMS TrueBeam® linear accelerator. Beams were configured on an Eclipse™ v. 15.6 system using the Acuros v. 15.6 algorithm. The measured and calculated data were entered into the Alfard™ software for comparison with the calculated dose distributions. In the last stage, the absolute dose values at the designated points were also compared. The obtained data were statistically analysed with Statistica™ v. 13.3.
Results
The work showed that the differences in the shape of the beam profile, depth dose and the dose value in points were not related to the use of optional data. Differences in dose distributions are within the tolerance. It cannot be determined under which conditions the use of optional data has a more favourable effect on the reflection of the actual dose values.
Conclusions
The use of optional data in modelling photon radiation beams does not significantly improve the compliance of the calculated and measured dose values.
Keywords: dosimetric verification, optional data, statistical significance
Introduction
Currently, the basic tool used in the preparation of external beam radiotherapy is a computerized treatment planning system. Its main task is to calculate the three-dimensional dose distribution in the patient’s body and to determine the number of monitor units needed for this purpose. Depending on the implemented calculation algorithm, a specific set of data is required to properly configure the system.
The purpose of the configuration is to obtain a dosimetric model of a specific beam of the configured apparatus. This model allows for a relatively fast preparation of the dose distribution and the number of monitor units in clinical cases. However, before clinical approval, the model must be thoroughly tested. In exchange for the aforementioned speed, the obtained results are subject to greater or lesser uncertainty. One of the tasks in the process of approving the system for clinical use is to study this uncertainty of the calculation algorithm.
There are international recommendations specifying the extent to which particular quantities should be checked [1, 2]. A few years ago, national recommendations were developed under the patronage of the Polish Society of Medical Physics [3]. The tests proposed in the literature concern various aspects of treatment planning: tests of computer equipment and peripheral devices (printers, plotters, etc.), data transfers between devices, methods of representing three-dimensional data in the software, orientation of coordinate systems, contouring tools, interpolation algorithms and many others. The scope and methodology used in the compliance assessment are detailed in [4]. The Acuros XB algorithm is based on the solution of the Linear Boltzmann Transport Equation (LBTE), which describes the interaction of radiation particles with matter. It is based on the so-called approximate numerical methods. By solving the Linear Boltzmann Transport Equation, the algorithm directly takes into account the effects of the occurrence of heterogeneity. LBTE is an equation that describes macroscopically the behaviour of radiation particles when interacting with matter. The obtained uncertainties of the results are comparable with the obtained Monte Carlo simulation methods [5]. The Acuros XB algorithm that calculates the dose distribution in three dimensions has already been quite comprehensively tested, as evidenced by the number of publications cited in the review [6]. It was compared both with its predecessor, the Anisotropic Analytical Algorithm (AAA) [7–10], and with the results obtained by Monte Carlo methods [11–13]. Its introduction to clinical use is also described [14, 15].
Modelling of algorithms in the Eclipse treatment planning system is performed under conditions strictly defined by the producer [16]. The set of entered measured values consists of required and optional data.
The required data are the values that must be entered to prepare the calculation model. Usually, it is a data set that does not take into account non-standard values, e.g. asymmetric fields, Percentage Depth Dose (PDD) changes in SSD function. Optional data are measurement quantities that can be entered into the configuration of the radiation beams but are not required by the model. These are: dosimetry data measured for different SSDs for beams with wedge filters and standard doses for asymmetric fields. Theoretically, the introduction of measurement data, e.g., different PDD for different SSD, should improve compatibility of calculations with measurements. The distribution of the radiation dose in the patient’s body is calculated by the algorithm for conditions other than measurement, e.g., during dosimetric measurements, the dimension of the radiation beam is formed by the base jaws. While during irradiation of the patient, the shape of the irradiation field is limited by the multi-leaf collimator. Therefore, a question arises: does the optional measurement data entered into the beam modelling algorithm improve the accuracy of the calculations, i.e., reduce the difference between the measured and calculated values? As the treatment planning system does not have the possibility of self-testing, it is necessary to use independent software.
To the best of the authors knowledge, the issue of the influence of optional data on the quality of dosimetric model fit has not been the subject of published studies so far.
The aim of the study
The aim of the study was to assess the impact of optional data for static fields in the configuration of the Acuros XB model of the Eclipse VMS v. 16.1 treatment planning system on compliance with the measured data.
Materials and methods
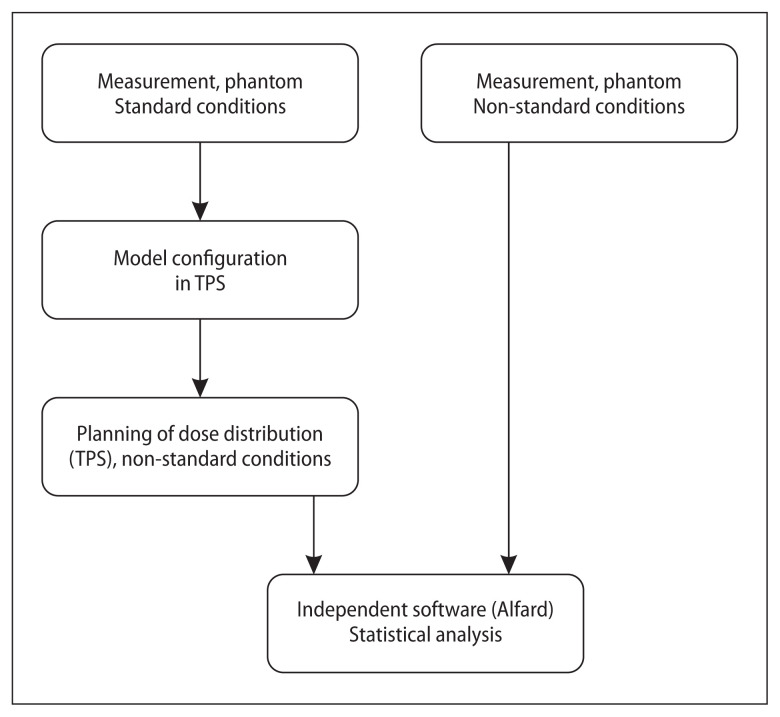
Percentage Depth Dose and Profile Function (PF) measurements were taken and entered into the treatment planning system. Modelling of the radiation beams, made with and without optional data, was performed according to the producer’s recommendations [17]. Based on the models prepared in this way, dose distributions were calculated using the Acuros XB algorithm in the phantom. The field of the beams was defined by a multi-leaf collimator. PDD and PFs, calculated by treatment planning system (TPS) Eclipse Acuros XB v 16.1.0 algorithm [13, 18], and measurement data were exported to the ‘Alfard’ [19]. Program ‘Alfard’ is a radiotherapy treatment planning system that has a module for reading and processing measurements from various different models of automatic phantoms [4]. It also allows you to present the loaded data in a graphic or text form and to compare large sets of measurement data with the calculation results of planning systems. PDD and PFs calculated by the treatment planning system were compared with the measurement data, in this program. The differences between the calculated and measured values were assessed on the basis of statistical tests for independent samples. The diagram of the procedure is presented in Figure 1.
Figure 1.
The method of comparing the measured data with the calculated data, used in the presented work [own study]. TPS — treatment planning system
Comparisons were made for the Varian Medical Systems TrueBeam® linear biomedical accelerator with the Millenium® multi-leaf collimator, for the X6FFF, X10FFF and X6MV, X15MV beams. For the beams with the flattening filter, physical wedge filters with angles of 15º, 30º, 45º and 60º were also used. Measurements for dosimetric modelling were made for SSD = 100.0 cm, and verification measurements [1] were made for SSD = 90.0 cm. Symmetrically, open fields were checked: 5 × 5 cm2, 10 × 10 cm2, 30 × 30 cm2, 5 × 20 cm2 and 20 × 5 cm2; asymmetrically open: 15 × 7.5 cm2, 10 × 20 cm2, 5 × 10 cm2 and 5 × 5 cm2. For beams with wedge filters, the following areas were checked: 10 × 10 cm2, 15 × 15 cm2, 20 × 20 cm2, 30 × 30 cm2 and the maximum rectangular area, i.e. 40 × 15 cm2 (for the 60º wedge), 40 × 20 cm2 (for the 45º wedge) and 40 × 30 cm2 (for the 30º and 15º wedges). Additionally, open fields 10 × 10 cm2 were also tested for three different SSD distances: 80, 100 and 120 cm.
The verification data set contained both the results of the absolute dose-point measurements and the relative dose distributions. Dosimetry equipment from PTW Freiburg (PTW-Freiburg, Freiburg, Germany) was used in all measurements, which includes: ionization chambers, dosimeter (Unidos) and an MP3 field analyser, i.e. a full-scatter 3D water phantom for measuring beam profiles with all the necessary equipment. The Mephysto v. 3.0 software allowed management and control of the analyser operation, enabling automatic collection of selected data.
All measurements were made according to report IAEA TRS-398 [20]. Various ionization chambers were used to measure the profiles and the percentage depth doses: Semiflex 3D (TM 31021) with an active volume of 0.07 cm3, positioned vertically for profiles measurements, and the Markus Advanced plane-parallel chamber (TM 34045), which is recommended for measurements of percentage depth dose curves. In all relative dose distributions, a reference chamber was used to control beam stability.
The absolute measurements were made with the calibrated Semiflex 0.125 cm3 ionization chamber and dedicated electrometer. The dose values were measured at a depth of 10.00 cm in the beam axis for symmetrical fields and at the geometric centre of the field for asymmetric fields.
For profile function, measurement data were collected at 2.0 mm intervals in the treatment areas and outside the field, and 1.0 mm in the high dose gradient area.
The profile functions were measured in two axes of the radiation beams: longitudinal “X” and lateral direction “Z”, at four depths: 1.5 cm for 6 MV, 2.3 cm for 10 MV and 2.5 cm for 15 MV, as well as 5, 10, and 20 cm for all nominal accelerating potentials. In the case of the PDD (measurement along the transversal ‘Y’ axis) the measurement time was 0.4 s per measurement point. The measuring step between the surface and the depth (dmax + 5 mm) was found to be 1.0 mm, and outside this range — 2.5 mm. PDD were measured from a depth of 30 cm by shifting the chamber towards the radiation source.
All raw measurement data were analysed and then processed [17], which is an important and necessary phase of obtaining measurement data. Smoothing and filtering procedures help remove unfavourable noise that always appears to a greater or lesser extent depending on the measurement system used. Using appropriate functions and filters (interpolations, Fourier transforms, etc.), the actual measurement data were extracted, and then smoothed and rebalanced (only in the case of symmetrical open fields).
The calculations in the TPS Eclipse were performed for the same set of fields. A set of beams with appropriate geometry was applied to the virtual phantom with dimensions of 50 × 50 × 50 cm3 generated in the system. The cubic virtual phantom had a given density equal to the density of water and reference points placed in strictly defined positions.
A computational grid of 2.5 mm was used, such as that used in non-stereotaxic clinical plans. During the calculations, the Calculate Dose with Preset Values [19] option was used, which allows for assigning a specified number of monitor units and determining the dose distribution for them. 100.0 monitor units were set for each beam.
The calculated PDD and profile functions were exported from the Eclipse treatment planning system to the Alfard software, into which the measurement data made for conditions identical to the calculations performed in TPS Eclipse were also entered.
In the case of profile functions, the first step was to change the resolution of both measurement and calculation curves to 1.0 mm for the same set of cutoffs. Next, they were normalized. Normalization was adopted to 100% in the beam axis for symmetrical open fields and 100% in the maximum dose value for wedge and off-axis fields. Then, the first derivative was determined from the measurement curve, treated as the reference curve. On its basis, the boundaries between small and large gradient values were determined 5.0 %/mm was assumed as the borderline value between the gradients. In the case of developing the calculated and measured PDD, the resolution was determined and the PDD curves were normalized to their maximum values.
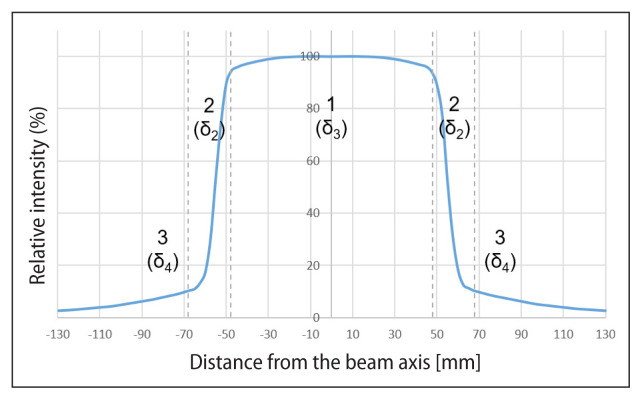
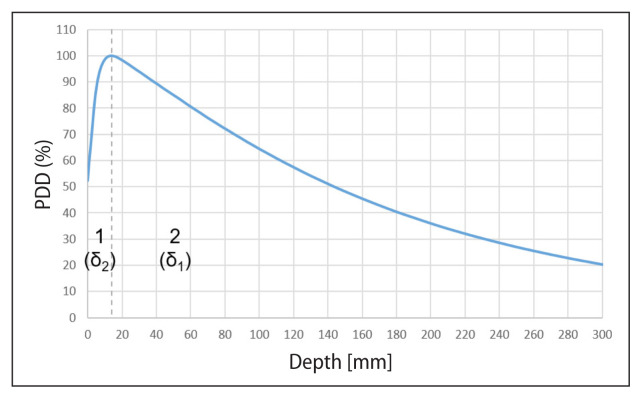
When comparing the beam profiles, the solution of the dose gradient limit value search was used [4], which divides the beam profile into three areas: (1) low dose and small gradient i.e. umbra area, (2) large gradient i.e. penumbra area, and (3) high dose and small gradient in the treatment area (Fig. 2). When comparing the PDD curves, two areas were identified: (1) above the depth of the maximum dose and (2) below the depth of the maximum dose (Fig. 3).
Figure 2.
Profile of the X-ray beam 6 MV, fields 10 x10 cm2, at a depth of 20.0 cm with marked areas: 1 — therapeutic; 2 — penumbra; 3 — umbra [own study]
Figure 3.
Percentage of deep beam dose × 6 MV of the field 10 × 10 cm2 with marked areas: 1 — dose increase; 2 — dose decrease [own study]
The permissible maximum values of the deviations between the compared doses for individual areas for the beam profile and the PDD curve were taken from the literature [3].
The comparisons were made point by point with a resolution of 1.0 mm for each interval according to the formula:
| (1) |
where:
δD — percentage of the difference,
DC — calculated dose,
DM — measured dose (reference dose)
The value in the beam axis for symmetrical and wedge field profiles was adopted as the reference dose, and the maximum value for off-axis profiles and PDD.
If the above-mentioned deviation values were exceeded, the Δ confidence limit was determined, in line with the formula [1]:
| (2) |
where:
δm — mean of the deviation results Δ,
SD — standard deviation of the results Δ.
The last stage was to compare the dose values at the designated points at a depth of 10.0 cm at a distance of SSD = 90.0 cm. In accordance with the recommendations [1], as a comparison of the calculated dose and the measured dose, the percentage criterion was used, which returns the deviations of the calculation results in relation to the standard measurement data for a given interval, according to the formula (1).
The maximum values of deviations between the compared doses for individual areas for the PF and the PDD, which allow to accept the conducted beam modelling, are summarized in Tables 1 and 2.
Table 1.
Permissible maximum values of deviations between the compared doses: measured and calculated for open fields and modified for the profile functions [1]
| Therapeutic area; high dose, low gradient (δ3) (%) | Penumbra area; large gradient (δ2) (%) | Umbra area; low dose, low gradient (δ4) (%) | |
|---|---|---|---|
| Homogeneous environment, simple geometry | 3 | 10 | 3 |
| Complex geometry (wedge filter, asymmetry, MLC) | 3 | 15 | 4 |
MLC — multileaf collimator
Table 2.
The permissible maximum values of deviations between the compared doses: measured and calculated, for open and modified fields for the percentage depth dose curve [1]
| Dose build-up area; large gradient (δ2) (%) | Dose drop area; little gradient (δ1) (%) | |
|---|---|---|
| Homogeneous environment, simple geometry | 10 | 2 |
| Complex geometry (wedge filter, asymmetry, MLC) | 15 | 3 |
MLC — multileaf collimator
The deviation between the measured dose value and the dose calculated by the treatment planning system, with and without optional data, was calculated according to formula 2. Using the non-parametric test for independent samples (Mann-Whitney U) it was assessed whether there were statistically significant differences between these sets. The significance level was 0.05 (Statistica™ v. 13.3 software, TIBCO Software Inc., Hillview Avenue, Palo Alto, USA). For the sample examined and for the parameters suggested by the producer of the algorithm the lack of a statistically significant difference between the sets proves that the introduction of optional data beams to modelling does not affect the difference between the measured and calculated values (PDD and PF).
In the case of wedge beams, the differences were examined separately for each axis, because the shapes of the wedge profiles along the breaking surface and perpendicular to it, differ from each other.
In order to analyse the dose values in points, the percentages of deviations were compared with each other. The smaller deviation value corresponds to the smaller difference between the calculated value and the measured value and thus to a better representation of the real value.
Results
Calculations and measurements were made for various beam geometries. Field dimensions:
symmetrically open fields: 5 × 5 cm2; 10 × 10 cm2; 30 × 30 cm2; 5 × 20 cm2; 20 × 5 cm2;
asymmetrically open fields: 15 × 7.5 cm2; 10 × 20 cm2; 5 × 10 cm2; 5 × 5 cm2;
wedge fields: 10 × 10 cm2; 15 × 15 cm2; 20 × 20 cm2; 30 × 30 cm2; 40 × 20 cm2; 40 × 15 cm2; 40 × 30 cm2; with wedges: W15, W30, W45, W60. The profile function was analysed at the depth:
1.5 cm; 5 cm; 10 cm; 20 cm for a ma × imum accelerating potential of 6 MV
2.3 cm; 5 cm; 10 cm; 20 cm for a ma × imum accelerating potential of 10 MV
2.5 cm; 5 cm; 10 cm; 20 cm for a ma × imum accelerating potential of 15 MV
Tables 3 and 4 show exemplary results of the comparison of the calculated and measured profile functions for the X6MV beam, open field (10 cm × 10 cm): symmetrical and asymmetrical.
Table 3.
Differences (%) between the measured values and those calculated in TPS, calculated according to formula (2), for the × 6 MV beam; jaws symmetrically open [own study].
| Field [cm2] | Depth (axis „Y”) [cm] | Axis | With optional data (%) | Without optional data [%] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δ4 | δ2 | δ3 | δ2 | δ4 | δ4 | δ2 | δ3 | δ2 | δ4 | |||
| 10 × 10 | 1.5 | X | 1.21 | 3.10 | 0.97 | 2.44 | 1.18 | 1.22 | 2.97 | 1.04 | 2.38 | 1.24 |
| 5 | 1.14 | 2.79 | 1.03 | 2.81 | 1.18 | 1.21 | 2.73 | 0.89 | 2.79 | 1.28 | ||
| 10 | 1.21 | 2.68 | 0.90 | 2.43 | 1.13 | 1.20 | 2.66 | 0.58 | 2.25 | 1.12 | ||
| 20 | 1.75 | 3.32 | 1.81 | 2.75 | 1.78 | 1.97 | 3.09 | 1.10 | 2.33 | 1.88 | ||
| 1.5 | Z | 1.21 | 2.65 | 1.13 | 2.71 | 1.34 | 1.21 | 2.62 | 1.26 | 2.55 | 1.34 | |
| 5 | 1.30 | 3.93 | 1.08 | 2.70 | 1.40 | 1.31 | 3.89 | 0.94 | 2.68 | 1.42 | ||
| 10 | 1.19 | 3.00 | 1.32 | 3.94 | 1.28 | 1.19 | 3.03 | 0.98 | 3.97 | 1.27 | ||
| 20 | 2.00 | 3.44 | 1.73 | 3.67 | 1.91 | 1.88 | 3.51 | 1.08 | 3.73 | 1.84 | ||
Table 4.
Differences (%) between the measured values and those calculated in TPS, calculated according to formula (2), for the × 6 MV beam; jaws asymmetrically open [own study]
| Field [cm2] | Depth (axis „Y”) [cm] | Axis | With optional data (%) | Without optional data (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δ4 | δ2 | δ3 | δ2 | δ4 | δ4 | δ2 | δ3 | δ2 | δ4 | |||
| 5 × 10 | 1.5 | X | 1.25 | 2.03 | 0.75 | 2.18 | 1.09 | 1.24 | 2.07 | 0.84 | 2.07 | 1.19 |
| 5 | 1.45 | 1.87 | 0.60 | 2.22 | 1.08 | 1.44 | 1.82 | 0.52 | 2.13 | 1.18 | ||
| 10 | 1.34 | 2.19 | 1.01 | 2.80 | 1.29 | 1.32 | 2.22 | 1.02 | 2.77 | 1.28 | ||
| 20 | 2.04 | 2.72 | 0.70 | 2.33 | 1.68 | 2.11 | 2.74 | 0.69 | 2.24 | 1.70 | ||
| 1.5 | Z | 0.62 | 3.06 | 1.49 | 3.57 | 0.70 | 0.64 | 3.03 | 1.61 | 3.51 | 0.72 | |
| 5 | 0.93 | 3.60 | 1.27 | 3.82 | 0.96 | 0.92 | 3.44 | 1.09 | 3.72 | 0.92 | ||
| 10 | 1.62 | 6.77 | 0.93 | 9.30 | 1.92 | 1.69 | 6.68 | 0.60 | 9.42 | 1.93 | ||
| 20 | 2.26 | 5.83 | 1.13 | 7.47 | 2.29 | 2.08 | 5.66 | 0.50 | 7.33 | 2.24 | ||
Thus, a comparison was made for all the above-mentioned beam conditions.
Table 5 presents exemplary (for the × 15 MV beam) results of the statistical analysis, statistical significance, differences between the sets with and without optional data.
Table 5.
Value of the “p” level (M-W U test) between sets of comparisons with and without optional data
| Geometry | Field [cm2] | p value | ||||
|---|---|---|---|---|---|---|
| δ4L | δ2L | δ3 | δ2R | δ4R | ||
| SO | 5 × 5 | 0.247 | 0.958 | 0.040 | 0.874 | 0.047 |
| 10 × 10 | 0.636 | 0.753 | 0.005 | 0.958 | 0.637 | |
| 30 × 30 | 0.958 | 0.156 | 0.793 | 0.227 | 0.673 | |
| 5 × 20 | 0.528 | 0.563 | 0.248 | 0.462 | 0.528 | |
| 20 × 5 | 0.635 | 0.958 | 0.792 | 0.713 | 0.269 | |
| 10 × 10 SSD100 | 0.713 | 0.753 | 0.018 | 0.599 | 0.563 | |
| 10 × 10 SSD80 | 1.000 | 0.793 | 0.002 | 0.599 | 0.792 | |
| 10 × 10 SSD120 | 0.171 | 0.495 | 0.040 | 0.431 | 0.189 | |
| AO | 15 × 7.5 | 0.317 | 0.958 | 0.834 | 0.834 | 0.599 |
| 10 × 20 | 0.753 | 0.875 | 0.317 | 1.000 | 0.599 | |
| 5 × 10 | 0.563 | 0.875 | 0.430 | 0.753 | 0.834 | |
| 5 × 5 | 0.636 | 0.793 | 0.318 | 0.713 | 0.875 | |
SO — geometry is symmetrical; AO — is asymmetric; L — the left side; R — the right side of the profile function [own study]
Statistically significant differences between comparisons with and without optional data are marked in red. In this analysis, for the × 15 MV beam without wedge filters, the differences are only in the area “Δ3” for a symmetrical field with sides 5, 10cm for SSD 100 cm, 80 cm and 120 cm, and in the area “Δ4” for a 5 × 5 cm2 field.
Only for sets showing statistical differences (p < 0.05), the mean deviations for modelling with and without measurement data were calculated (Tab. 4).
The analysis of the data presented in Table 6 shows that for all areas (Δi), the deviations of the differences between the measured and calculated values are greater for modelling with optional data than for beam modelling without optional data.
Table 6.
Mean deviations between the measured and calculated values (by TPS) for the × 15 MV symmetrical fields (SO) [own study]
| × 15 MV Geometry |
Field [cm2] | Area/side | Average with optional data (%) | Average without optional data (%) |
|---|---|---|---|---|
| SO | 5 × 5 | δ3 | 0.68 | 0.29 |
| δ4R | 1.05 | 0.94 | ||
| 10 × 10 | δ3 | 1.10 | 0.45 | |
| 10 × 10 SSD100 | δ3 | 1.16 | 0.52 | |
| 10 × 10 SSD80 | δ3 | 1.29 | 0.59 | |
| 10 × 10 SSD120 | δ3 | 1.28 | 0.54 |
It has been observed that the dose differences, both with and without optional data, are small, which means that the calculations are in good agreement with the measurements. For most of the sets, the results were within the given tolerance.
In the case of profile functions for X6MV beam, the results of the obtained differences above the tolerance were obtained mainly for large field dimensions and only for the direction along the breaking surface of wedged beams. For the 30° wedge with large fields: 30 × 30 cm2 and 40 × 30 cm2, using optional data, the calculated differences in the therapeutic area reached less than 4%. In the umbra area for the second field at the maximum dose depth, the difference was 4.17% using optional data. For the 45° wedge, optional data resulted in exceeding the tolerance in the umbra area for the maximum dose depth, reaching 4.31%. In the case of the 60° wedge, exceedances occurred for the 40 × 15 cm2 field, both for the model with and without optional data, in the umbra area. Using optional data, the differences were 4.65% for the maximum dose depth. When optional data was not used, the tolerance was exceeded at the level of 4.33%. The percentage depth dose for both open and wedge fields were within the tolerances advised by the PSMP recommendations [1].
In the model for the X6FFF, X10FFF and X15MV beams, no tolerances were exceeded in any of the tested cases concerning relative dosimetry (Tab. 1 and 2).
The comparison of the absolute dose at the point also did not give the values exceeding the set thresholds in each of the analysed cases.
Discussion
The obtained results, for analysed sets of parameters, do not justify the conclusion that modelling the beams with optional data reduces the differences between the measured and calculated values of deviations and dose.
Taking into account statistically significant differences and dose values in points, it cannot be stated whether the use of optional data has a more favourable effect on the reflection of the real dose values than the lack of optional data. Table 7 presents a summary of the obtained results. Cells for those cases of dosimetric models that better reflect the measurement values are marked in green.
Table 7.
List of data for which the difference was statistically significant. The cases that show a better fit to the measurements are marked in green [own study]
| With optional data | Without optional data | ||
|---|---|---|---|
| × 6 MV | |||
| Beam profile | Open fields | ||
| Wedge fields | |||
| PDD | Wedge fields | ||
| Dose in points | |||
| × 6FFF MV | |||
| Beam profile | Open fields | ||
| Dose in points | |||
| × 10FFF MV | |||
| Beam profile | Open fields | ||
| Dose in points | |||
| × 15 MV | |||
| Beam profile | Open fields | ||
| Dose in points | |||
Table 7 shows that in 40% of cases, the use of optional data better matches the beams calculated to the measured values. Failure to use optional data has a better effect on the representation of measurements in 60% of cases. Unfortunately, it cannot be said under which conditions the use of the data improves the fit. The available literature provides information on the testing of computational algorithms and their compliance with the measured values [15, 18, 19, 21], but there is no information about the influence of additional optional data on the results of comparisons. It may seem that the introduction of additional measurement data, which provide additional information on doses measured under non-standard conditions, e.g. for asymmetric fields, should reduce the differences between the measured and calculated values. Meanwhile, the conducted research does not confirm this. Perhaps one should consider introducing other verification conditions, e.g. comparing the fluency maps calculated with and without optional data.
Conclusions
The work has shown that there are differences between the measured and calculated values in the profile function, the PDD and the dose value in points depending on the application — or not — of the optional data. Statistical significance analysis has shown that most of all differences between measurements and calculations, both with and without optional data, are statistically insignificant. This means that statistically there is no difference whether optional data will be used or not. In cases where there are statistically significant differences, it can be seen that data calculated without the use of optional data better reflect the measured value, for the parameters recommended by the producer of the algorithm.
Footnotes
Conflict of interest
None declared.
Funding
None declared.
References
- 1.International Atomic Energy Agency. Commissioning and Quality Assurance of Computerized Planning Systems for Radiation Treatment of Cancer. Technical Reports Series no 430. IAEA; Vienna: 2004. [Google Scholar]
- 2.International Atomic Energy Agency. Commissioning of Radiotherapy Treatment Planning Systems: Testing for Typical External Beam Treatment Techniques. IAEA; Vienna: 2008. IAEA TECDOC – 1583. [Google Scholar]
- 3.Dybek M, Winiecki J, Iwanicki T, et al. Kontrola systemów planowania leczenia 3D w radioterapii wiązkami zewn¸trznymi fotonów i elektronów. Pol J Med Phys Eng. 2014;20(1):1–32. doi: 10.2478/pjmpe-2014-0001. [DOI] [Google Scholar]
- 4.Wendykier J, Bieniasiewicz M, Grządziel A, et al. Determination of bounds between ranges of high and low gradient of beam profile. Rep Pract Oncol Radiother. 2016;21(13):168–173. doi: 10.1016/j.rpor.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han T, Mikell JK, Salehpour M, et al. Dosimetric comparison of Acuros XB deterministic radiation transport method with Monte Carlo and model-based convolution methods in heterogeneous media. Med Phys. 2011;38(5):2651–2664. doi: 10.1118/1.3582690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ojala J. The accuracy of the Acuros XB algorithm in external beam radiotherapy — a comprehensive review. Int J Cancer Ther Oncol. 2014;2(4):020417. doi: 10.14319/ijcto.0204.17. [DOI] [Google Scholar]
- 7.Zaman A, Kakakhel M, Hussain A. A comparison of Monte Carlo, anisotropic analytical algorithm (AAA) and Acuros XB algorithms in assessing dosimetric perturbations during enhanced dynamic wedged radiotherapy deliveries in heterogeneous media. J Radiother Pract. 2018;18(1):75–81. doi: 10.1017/s1460396918000262. [DOI] [Google Scholar]
- 8.Krabch M, Chetaine A, Nourreddine A, et al. Comparative study between Acuros XB algorithm and Anisotropic Analytical Algorithm in the case of heterogeneity for the treatment of lung cancer. Pol J Med Phys Eng. 2018;24(3):115–119. doi: 10.2478/pjmpe-2018-0016. [DOI] [Google Scholar]
- 9.Kang SW, Chung JB, Lee JW, et al. Dosimetric accuracy of the Acuros XB and Anisotropic analytical algorithm near interface of the different density media for the small fields of a 6-MV flattening-filter-free beam. Int J Radiat Res. 2017;15(2):157–165. doi: 10.18869/acadpub.ijrr.15.2.157. [DOI] [Google Scholar]
- 10.Rana S, Rogers K. Radiobiological Impact of Acuros XB Dose Calculation Algorithm on Low-Risk Prostate Cancer Treatment Plans Created by RapidArc Technique. Austral– Asian J Cancer. 2012;11(4):261–269. [Google Scholar]
- 11.Ojala J, Kapanen M, Sipilä P, et al. The accuracy of Acuros XB algorithm for radiation beams traversing a metallic hip implant — comparison with measurements and Monte Carlo calculations. J Appl Clin Med Phys. 2014;15(5):4912. doi: 10.1120/jacmp.v15i5.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann L, Alber M, Söhn M, et al. Validation of the Acuros XB dose calculation algorithm versus Monte Carlo for clinical treatment plans. Med Phys. 2018;45(8):3909–3915. doi: 10.1002/mp.13053. [DOI] [PubMed] [Google Scholar]
- 13.Han T, Followill D, Mikell J, et al. Dosimetric impact of Acuros XB deterministic radiation transport algorithm for heterogeneous dose calculation in lung cancer. Med Phys. 2013;40(5):051710. doi: 10.1118/1.4802216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann L, Jørgensen MBK, Muren LP, et al. Clinical validation of the Acuros XB photon dose calculation algorithm, a grid-based Boltzmann equation solver. Acta Oncol. 2012;51(3):376–385. doi: 10.3109/0284186X.2011.629209. [DOI] [PubMed] [Google Scholar]
- 15.Yan C, Combine AG, Bednarz G, et al. Clinical implementation and evaluation of the Acuros dose calculation algorithm. J Appl Clin Med Phys. 2017;18(5):195–209. doi: 10.1002/acm2.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.EC LIPSE Photon and Electron Algorithms Reference Guide, P1020505-003-C. Varian Medical Systems; [Google Scholar]
- 17.Commissioning., CS505EU-Varian Applied Physics: Linac [Google Scholar]
- 18.Fogliata A, Nicolini G, Clivio A, et al. Dosimetric evaluation of Acuros XB Advanced Dose Calculation algorithm in heterogeneous media. Radiat Oncol. 2011;6:82. doi: 10.1186/1748-717X-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ALFARD. http://alfard.eu5.net/alfard/index.html .
- 20.International Atomic Energy Agency. Absorbed Dose Determination in External Beam Radiotherapy. Technical Reports Series no 430. IAEA; Vienna: 2000. [Google Scholar]
- 21.Hrbacek J, Lang S, Kloeck S. Commissioning of photon beams of a flattering filter-free linear accelerator and the accuracy of beam modeling using the anisotropic analytical algorithm. Int J Radiat Oncol Biol Phys. 2011;80(4):1228–1237. doi: 10.1016/j.ijrobp.2010.09.050. [DOI] [PubMed] [Google Scholar]