Abstract
Staphylococcus aureus, a common pulmonary pathogen in cystic fibrosis (CF), produces exotoxins that are extremely potent superantigens. A number of animal studies have shown that superantigens cause pulmonary inflammation, but the possible role of superantigens in CF has not been investigated. The present study assessed possible differences between control and CF B cells in presenting superantigens to T cells. Immortalized B-cell lines were used as superantigen-presenting cells to avoid environmental influences (e.g., infection or antibiotics) common to freshly isolated cells. The results show that CF B-cell lines presented a staphylococcal superantigen to the immortalized T-cell line (Jurkat) as effectively as did control B-cell lines as measured by interleukin-2 production. However, in contrast to the case for control B-cell lines, dexamethasone did not inhibit CF B-cell lines from presenting superantigen. The resistance of superantigen-presenting CF B cells to corticosteroids suggests that the pulmonary response to superantigens may be poorly regulated in CF, leading to an exaggerated inflammatory response to S. aureus.
Chronic bacterial infection of the lung accounts for the majority of the mortality and morbidity observed in cystic fibrosis (CF) (11, 17). Staphylococcus aureus is frequently the initial colonizing microbe (1). S. aureus is well known for production of a family of exotoxins that are very potent activators of the immune system (reviewed in reference 14). These exotoxins are called superantigens, in part because they stimulate large numbers of T cells without a requirement for prior sensitization. The end result is excessive production of T-cell and proinflammatory cytokines.
Staphylococcal superantigens are highly heat- and enzyme-resistant proteins, notorious for causing food poisoning and toxic shock syndrome (14). The possible role of staphylococcal superantigens in pulmonary diseases has not received much attention until recently. Systemic or intratracheal administered superantigens elicit pulmonary inflammation in animals (12, 15, 22, 23). In addition, staphylococcal enterotoxins stimulate interleukin-8 (IL-8) production by cultures of bronchial epithelial cells (2) and by human lung microvascular endothelial cells (13). IL-8 is a suspected major proinflammatory cytokine in CF (27).
The present study compared the ability of CF and normal B-cell lines to present a staphylococcal superantigen to T cells. In addition, based on a suspected abnormality of a number of CF cell types in regulation by corticosteroids (6, 7, 18, 29, 32), including B cells (10), the effect of dexamethasone on presentation of superantigen by CF B-cell lines was also examined. Endogenous corticosteroids and exogenous analogues, e.g., dexamethasone, are potent inhibitors of proinflammatory cytokine production and inflammation (5, 30).
A simple assay system was employed, using the immortalized Jurkat T-cell line as a source of T cells and immortalized control and CF B cells as presenting cells (24, 33). B cells are one of several cell types that can present superantigens (14, 24, 33), owing to their relatively high surface density of major histocompatibility complex (MHC) class II antigens. They also express other surface substances, e.g., adhesion molecules, and produce cytokines that facilitate T-cell stimulation (34). The use of immortalized T- and B-cell lines to perform these studies avoided possible extrinsic variables (antibiotic treatment or chronic infection of patient, etc.).
MATERIALS AND METHODS
Culture medium.
RPMI 1640 (Irvine Scientific, Irvine, Calif.) with HEPES buffer (10 mM), 10% heat-inactivated fetal calf serum, penicillin (100 U/ml), streptomycin (100 μg/ml), gentamicin (20 μg/ml), glutamine (2 mM), and 2-mercaptoethanol (5 × 10−5 M) was used.
Chemicals and cytokines.
Phorbol myristate acetate (PMA) (Sigma, St. Louis, Mo.) was dissolved at 200 μg/ml in dimethyl sulfoxide and stored at −20°C. Staphylococcal enterotoxin E (SEE) (Toxin Technology, Sarasota, Fla.) was dissolved in saline containing 0.1 mg of bovine serum albumin per ml to a stock concentration of 100 μg/ml. Further dilutions were made in culture medium. Dexamethasone (LyphoMed Inc., Rosemont, Ill.) was dissolved in dimethyl sulfoxide and diluted in culture medium. Recombinant human interleukins were obtained from Collaborative Research, Boston, Mass. (IL-1); Cetus Corp., Emeryville, Calif. (IL-2); Genzyme, Cambridge, Mass. (IL-4); and Amgen, Thousand Oaks, Calif. (IL-6).
B-cell lines.
Epstein-Barr virus (EBV)-transformed B-cell lines were established from the peripheral blood of children with and without CF (31). Informed consent was obtained prior to blood collection, and none of the children was on steroids. Control B-cell lines were from age-matched children lacking known CF mutations. All of the B-cell lines were homozygous for the delta 508 mutation except one line, CF-4, that had an unidentified mutation. In brief, to establish the B-cell lines, lymphocytes were purified from heparinized blood by density gradient centrifugation (33). The purified lymphoid cells were cultured in 13-mm-diameter plastic tissue culture tubes at 1 × 106 to 4 × 106/0.5 ml of culture medium. EBV (5 × 107 transforming units) (B95-8; Tampa Bay Research Institute, St. Petersburg, Fla.) and cyclosporin A (1 μg/ml) were added. The B-cell lines were used at least 2 months after transformation.
Superantigen presentation assay.
Jurkat T cells (clone E6-1, TIB 152; American Type Culture Collection, Manassas, Va.) were cultured with CF, and control B-cell lines with and without SEE added. The Jurkat T-cell line does not produce IL-2 spontaneously but responds to SEE presented by HLA-DR-bearing cells by producing IL-2 (24, 33). In brief, 5 × 104 Jurkat T cells and 2.5 × 103 B cells in 0.2 ml of culture medium containing 6 ng of PMA per ml were added to flat-bottomed culture wells of a 96-well plate (Falcon Plastics, Oxnard, Calif.). SEE was added at a final concentration of 0.01 μg/ml. The number of B cells and concentration of SEE used were determined by titration to be the lowest required for optimal production of IL-2. After 24 h, supernatant fluids were assayed for IL-2.
Stimulation of IL-6 production.
B-cell lines were cultured in round-bottomed wells of a 96-well plate at 2 × 104 cells per well in 0.2 ml of medium. The cocktail used to stimulate IL-6 production was PMA (6 ng/ml) and recombinant cytokines IL-1 (40 ng/ml), IL-2 (100 U/ml), and IL-4 (1 ng/ml).
Cytokine assays. (i) IL-2.
The murine T-cell line CTLL-2, which is dependent on IL-2 for growth, was used for assay of supernatant IL-2 as described previously (24, 33). One unit of IL-2 was the dilution of supernatant that yielded one-half-maximal proliferation relative to a standard curve. Results were expressed as the mean ± standard deviation of three values. The assay sensitivity was 0.1 U of IL-2/ml.
(ii) IL-6.
Il-6 was measured as described previously using the murine IL-6-dependent hybridoma cell line B9.9 (16). Polyclonal antiserum to IL-6 (Genzyme) neutralized the supernatant activity. Results were expressed as the mean ± standard deviation of three values. The assay sensitivity was 1 pg/ml.
RESULTS
Presentation of SEE by CF and control B-cell lines.
EBV-transformed CF and control B-cell lines were assessed for their ability to present SEE to the Jurkat T-cell line, clone E6-1, which was selected for high IL-2 production following activation (35). In the presence of MHC class II-presenting cells, SEE is a potent activator of Jurkat T cells, with other superantigens being significantly less active (24, 33). IL-2 production is greatly enhanced by the presence of PMA, a potent accessory signal for T cells that have bound antigen or superantigens (34, 35). The extent of activation of the Jurkat T cells is quantified by measuring the IL-2 concentration in supernatant fluids approximately 1 day after activation (34, 35).
Table 1 shows the results of presentation of SEE to the Jurkat line by CF and control B-cell lines. The B-cell lines were effective presenters of SEE, with IL-2 production increased from <1 to >10 U/ml in all cases except when presenting cells were omitted. There were no significant differences in IL-2 production (range, 11 and 15 U/ml) relative to the B-cell line used to present SEE. The B cells themselves made undetectable levels of IL-2, with the Jurkat T cells being the sole source of IL-2 (data not shown).
TABLE 1.
Presentation of superantigen to T cells by CF and control B-cell linesa
| Presenting cells added to Jurkat T cells | IL-2, U/ml (mean ± SD)
|
|
|---|---|---|
| Without SEE | With SEE | |
| None | <1 | <1 |
| CF-2 | <1 | 15 ± 3 |
| CF-3 | <1 | 12 ± 0.3 |
| CF-4 | <1 | 13 ± 0.3 |
| CF-9 | <1 | 13 ± 0.2 |
| CF-11 | <1 | 11 ± 1 |
| Control 1 | <1 | 15 ± 1 |
| Control 2 | <1 | 13 ± 0.5 |
| Control 3 | <1 | 11 ± 1 |
B-cell lines (or none as a negative control) were added to the Jurkat T-cell line with and without SEE added. After 24 h, supernatant fluids were assayed for IL-2. B-cell lines alone without the T-cell line added did not make detectable IL-2 (<1 U/ml) (data not shown).
It should be noted that to increase the likelihood of finding differences between CF and control B-cell lines in presentation of SEE, the lowest concentration of SEE and the fewest B cells that could induce optimal IL-2 production by the T-cell line were used. The B-cell number and concentration of SEE were varied in other experiments not shown here, and, in all cases, CF B cells presented SEE to the Jurkat T-cell line no differently than control B-cell lines.
Presentation of SEE after preincubation of B-cell lines with dexamethsone.
Next, it was determined if there might be differences in superantigen-presenting ability after preincubation of the B-cell lines with corticosteroids. Corticosteroids are well-known inhibitors of immune activity (5, 30), and a number of CF cell types have been shown to be resistant to regulation by corticosteroids (6, 7, 10, 18, 29, 32). Control and CF B-cell lines were preincubated for 3 days with a range of concentrations of dexamethasone, washed three times to remove dexamethasone, counted, and then added to the T-cell line with and without addition of SEE. After 24 h, supernatant fluids were assayed for IL-2.
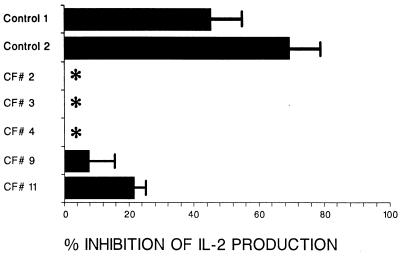
Figure 1 shows that treatment with 10−7 M dexamethasone inhibited control B-cell lines, but not the CF B-cell lines, from presenting superantigen (58% ± 13% [mean of two values] versus 6% ± 2% [mean of five values]). These results are representative of those from three other experiments. A concentration of 10−7 M dexamethasone was the highest concentration that did not inhibit cell growth or reduce cell viability; lower concentrations of dexamethasone were ineffective (data not shown).
FIG. 1.
Effect of dexamethasone pretreatment of B-cell lines on subsequent presentation of SEE to the Jurkat T-cell line. CF and control B-cell lines were preincubated with dexamethasone (10−7 M) for 3 days. After three washes by centrifugation to remove the dexamethasone, the B-cell lines were assessed for superantigen presentation ability by addition to Jurkat T cells. SEE was added at a final concentration of 0.01 μg/ml. After 24 h, supernatant fluids were quantified for IL-2. Results are expressed as the mean (± SD) percent inhibition of IL-2 production relative to IL-2 produced in response to SEE presented by non-dexamethasone-treated B cells. The asterisks indicate no inhibition.
Mechanism of dexamethasone-mediated suppression of superantigen presentation by control B-cell lines but not CF B-cell lines.
Because superantigens must bind to MHC class II molecules to be presented, MHC class II expression on the dexamethasone-treated control and CF B cells was compared. Fluorescence-activated cell sorter analysis showed that dexamethasone had no effect on surface expression of HLA class II molecules; no differences were detected between control and CF B-cell lines with or without incubation with dexamethasone (data not shown). The expression of another prerequisite surface molecule, intracellular adhesion molecule 1 (ICAM-1) (34), also was not affected (data not shown).
Because corticosteroids are known to inhibit proinflammatory cytokine synthesis (5), it was next determined if dexamethasone affected the synthesis of IL-6 by the B-cell lines. The IL-6 gene has corticosteroid response elements (25), and IL-6 is also made by the B-cell lines in large amounts upon stimulation with a PMA-cytokine cocktail, as shown in Table 2. Two of the five CF B-cell lines made significantly more IL-6 than the others, but on the whole, control and CF B cells made similar amounts of IL-6.
TABLE 2.
Production of IL-6 by stimulated B-cell linesa
| B-cell line | IL-6, pg/ml (mean ± SD)
|
|
|---|---|---|
| Without stimulation | With stimulation | |
| None | <10 | <10 |
| CF-2 | <10 | 315 ± 21 |
| CF-3 | <10 | 313 ± 23 |
| CF-4 | <10 | 2,850 ± 636 |
| CF-9 | <10 | 286 ± 30 |
| CF-11 | <10 | 1,290 ± 304 |
| Control 1 | <10 | 300 ± 64 |
| Control 2 | <10 | 775 ± 163 |
| Control 3 | <10 | 647 ± 92 |
B-cell lines (or none as a background control) were stimulated for 2 days with a PMA-cytokine cocktail. Supernatant fluids were then assayed for IL-6.
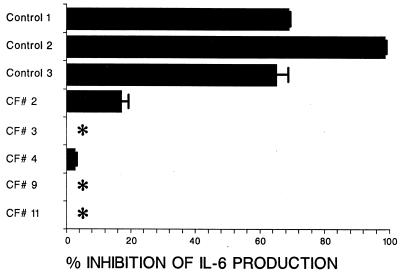
To study the effects of dexamethasone on IL-6 production, the B-cell lines were first preincubated with dexamethasone for 3 days. The cells were then washed three times, and the PMA-cytokine cocktail was added to stimulate IL-6 production. Two days later the supernatant fluids were assayed for IL-6. Figure 2 shows that dexamethasone preincubation inhibited subsequent IL-6 production by the control B-cell lines but not the CF B-cell lines. The control B-cell lines were inhibited by 77% ± 15% (mean of 68, 99, and 65%), while the CF B-cell lines were inhibited by only 4% ± 7% (mean of 17, 0, 4, 0 and 0%).
FIG. 2.
Effect of dexamethasone pretreatment on subsequent stimulation of IL-6 production by CF and control B-cell lines. CF and control B cell lines were preincubated with dexamethasone (10−7 M) for 3 days. After three washes by centrifugation to remove dexamethasone, the B cells were stimulated with and without a cytokine-PMA cocktail (see Materials and Methods). Supernatant fluids were quantified for IL-6 by bioassay. Results are presented as the mean (± SD) percent inhibition of IL-6 production. The asterisks indicate no inhibition.
DISCUSSION
Despite the fact that S. aureus produces the majority of superantigens known, the possible role of superantigens in CF has not been studied. The present study found that CF B cells were resistant to inhibition of superantigen presentation by dexamethasone. If, as the present study suggests, the immune response to superantigens is poorly regulated in CF due to endogenous resistance to corticosteroids, superantigens could play an important role in pulmonary injury.
The mechanism of inhibition of the control B-cell lines with dexamethasone was briefly examined. Inhibition was not explained by cytotoxicity, as the concentration of dexamethasone used in these studies was not cytotoxic. Possible down-regulation by dexamethasone of surface molecules required for superantigen presentation, i.e., HLA-DR and ICAM-1, was ruled out. Dexamethasone treatment was found, however, to inhibit IL-6 production by control, but not CF B cells (Fig. 2), suggesting that inhibition of IL-6 might be the mechanism of inhibition of superantigen presentation. IL-6, however, is not required for the Jurkat cells to produce IL-2 in response to SEE due to the presence of PMA, which is itself a potent accessory signal for T cells (reference 35 and unpublished observation). Rather, these preliminary mechanistic studies suggest that dexamethasone inhibits an as-yet-unknown factor(s) that has corticosteroid response elements (20, 25) similar to IL-6.
The mechanism for the resistance of the CF B-cell lines to regulation by dexamethasone was not addressed in the present study. The relationship between a defective CFTR gene product and an abnormal response to corticosteroids remains speculative despite a number of studies showing resistance of various CF cell types to corticosteroids; these include freshly isolated B cells (10), bronchial submucosal gland cultures (32), tracheal serous gland cultures (18), and fibroblasts (6). CF peripheral blood lymphocytes are resistant to corticosteroid-induced inhibition of arachidonic acid release (7) and leukotriene production (29). On the other hand, the mitogen-stimulated proliferation of peripheral blood control and CF T cells is equally inhibited by corticosteroids (8, 21), showing that not all CF cell types are resistant to corticosteroids. Because the CFTR gene product not only is responsible for chloride ion transport but also indirectly affects regulation of other ion channels (28), a defective CFTR gene product could have a broader effect on cell functions than chloride ion transport alone.
Although it is difficult to extrapolate from in vitro studies to possible clinical significance, the abnormal response of many CF cell types to corticosteroids suggests that corticosteroids may not be effective in controlling inflammation in CF. In an early trial with anti-inflammatory agents, long-term treatment with systemic corticosteroids delayed progressive pulmonary deterioration (3); however, subsequent studies (9, 26) failed to demonstrate a therapeutic response sufficient to justify the significant side effects. Inhaled corticosteroids primarily benefited patients with hyperreactive airways (4). A less toxic anti-inflammatory treatment than corticosteroids, high-dose ibuprofen, was shown to lower the rate of decline in pulmonary function in children with CF compared to controls over a 4-year period (19).
The use of immortalized T- and B-cell lines in the present study facilitated detection of an intrinsic resistance of CF B cells to dexamethasone. Immortalized cell lines are free of possible confounding extrinsic variables, e.g., the clinical state of the patient, common to freshly purified lymphoid cells. Moreover, CF B-cell lines provided a pure population of B lymphoid cells, uncontaminated by other cell types. The immortalized B-cell lines provide a convenient source of CF cells for future molecular analysis of the relationship between the defective CFTR gene product and corticosteroid regulation of superantigen-presenting cells.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the H. N. and Frances C. Berger Foundation and by the Southern California Chapter of the Arthritis Foundation. Josef Ben-Ari is a recipient of an American Physician Fellowship for Medicine in Israel, Inc., and David Gozal is a recipient of a Parker B. Francis Fellowship in Pulmonary Research.
REFERENCES
- 1.Armstrong D S, Grimwood K, Carlin J B, Carzino R, Gutierrez J P, Hull J, Olinsky A, Phelan E M, Robertson C F, Phelan P D. Lower airway inflammation in infants and young children with cystic fibrosis. Am J Respir Crit Care Med. 1997;156:1197–1204. doi: 10.1164/ajrccm.156.4.96-11058. [DOI] [PubMed] [Google Scholar]
- 2.Aubert V, Schneeberger D, Sauty A, Sperisen P, Aubert J D, Spertini F. Induction of tumor necrosis factor alpha and interleukin-8 gene expression in bronchial epithelial cells by toxic shock syndrome toxin 1. Infect Immun. 2000;68:120–124. doi: 10.1128/iai.68.1.120-124.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auerbach H S, Williams M, Kirkpatric J A, Colten H R. Alternate-day prednisone reduces morbidity and improves pulmonary function in cystic fibrosis. Lancet. 1985;ii:686–688. doi: 10.1016/s0140-6736(85)92929-0. [DOI] [PubMed] [Google Scholar]
- 4.Bisgaard H, Pedersen S S, Nielsen K G, Skov M, Laussen E M, Kronborg G, Hoiby N, Koch C. Controlled trial of inhaled budesonide in patients with cystic fibrosis and chronic bronchopulmonary Pseudomonas aeruginosa infection. Am J Respir Crit Care Med. 1997;156:1190–1196. doi: 10.1164/ajrccm.156.4.9612044. [DOI] [PubMed] [Google Scholar]
- 5.Braun C M, Huang S K, Bashian G G, Kagley-Sobotka A, Lichtenstein L M, Essayan E M. Corticosteroid modulation of human, antigen-specific Th1 and Th2 responses. J Allergy Clin Immunol. 1997;100:400–407. doi: 10.1016/s0091-6749(97)70255-0. [DOI] [PubMed] [Google Scholar]
- 6.Breslow J L, Epstein J, Fontaine J H, Forbes G B. Enhanced dexamethasone resistance in cystic fibrosis cells: potential use for heterozygote detection and prenatal diagnosis. Science. 1978;201:180–182. doi: 10.1126/science.663650. [DOI] [PubMed] [Google Scholar]
- 7.Carlstedt-Duke J, Bronnegard M, Strandvik B. Pathological regulation of arachidonic acid release in cystic fibrosis: the putative basic defect. Proc Natl Acad Sci USA. 1986;83:9202–9206. doi: 10.1073/pnas.83.23.9202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Distelhorst C W, Benutto B M, Corry J M. Effect of mitogen concentration on glucocorticoid suppression of normal and cystic fibrosis lymphocyte activation. Cell Immunol. 1983;75:188–192. doi: 10.1016/0008-8749(83)90318-0. [DOI] [PubMed] [Google Scholar]
- 9.Eigen H, Rosenstein B J, FitzSimmons S, Schidlow D V. A multicenter study of alternate-day prednisone therapy in patients with cystic fibrosis. J Pediatr. 1995;126:515–523. doi: 10.1016/s0022-3476(95)70343-8. [DOI] [PubMed] [Google Scholar]
- 10.Emilie D, Crevon M C, Chicheportiche R, Auffredou M T, Barot-Ciorbaru R, Lenoir G, Dayer J M, Galanaud P. Cystic fibrosis patients' B-lymphocyte response is resistant to the in vitro enhancing effect of corticosteroids. Eur J Clin Invest. 1990;20:620–626. doi: 10.1111/j.1365-2362.1990.tb01910.x. [DOI] [PubMed] [Google Scholar]
- 11.Fick R B., Jr Pathogenesis of the pseudomonas lung lesion in cystic fibrosis. Chest. 1989;96:158–164. doi: 10.1378/chest.96.1.158. [DOI] [PubMed] [Google Scholar]
- 12.Fujiki M, Shinbori T, Suga M, Miyakawa H, Ando M. Role of T cells in bronchoalveolar space in the development of interstitial pneumonia induced by superantigen in autoimmune-prone mice. Am J Respir Cell Mol Biol. 1999;21:675–683. doi: 10.1165/ajrcmb.21.6.3498. [DOI] [PubMed] [Google Scholar]
- 13.Fujisawa N, Hayashi S, Kurdowska A, Noble J M, Naitoh K, Miller E J. Staphylococcal enterotoxin A-induced injury of human lung endothelial cells and IL-8 accumulation are mediated by TNF-α. J Immunol. 1998;161:5627–5632. [PubMed] [Google Scholar]
- 14.Herman, Kappler A J W, Marrack P, Pullen A M. Superantigens: mechanism of T-cell stimulation and role in immune responses. Ann Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- 15.Herz U, Rückert R, Wollenhaupt D, Tschernig T, Neuhaus-Steinmetz U, Pabst R, Renz H. Airway exposure to bacterial superantigen (SEB) induces lymphocyte-dependent airway inflammation associated with increased airway responsiveness—a model for non-allergic asthma. Eur J Immunol. 1999;29:1021–1031. doi: 10.1002/(SICI)1521-4141(199903)29:03<1021::AID-IMMU1021>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Hofman F M, Wright A D, Dohadwala M M, Wong-Stall F, Walker S M. Exogenous tat protein activates human endothelial cells. Blood. 1993;82:2774–2780. [PubMed] [Google Scholar]
- 17.Hudson V L, Wielinski C L, Regelmann W E. Prognostic implications of initial oropharyngeal bacterial flora in patients with cystic fibrosis diagnosed before the age of two years. J Pediatr. 1993;122:854–860. doi: 10.1016/s0022-3476(09)90007-5. [DOI] [PubMed] [Google Scholar]
- 18.Kammouni W, Figarella C, Marchand S, Merten M. Altered cytokine production by cystic fibrosis tracheal gland serous cells. Infect Immun. 1997;65:5176–5183. doi: 10.1128/iai.65.12.5176-5183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konstan M W, Byard P J, Hoppel C L, Davies P B. Effect of high-dose ibuprofen in patients with cystic fibrosis. N Engl J Med. 1995;332:848–854. doi: 10.1056/NEJM199503303321303. [DOI] [PubMed] [Google Scholar]
- 20.Konty E, Ziolkowska M, Ryzewska A, Malinski W. Protein kinase c-dependent pathway is critical for the production of pro-inflammatory cytokines (TNF-alpha, IL-1 beta, IL-6) Cytokine. 1999;11:839–848. doi: 10.1006/cyto.1998.0496. [DOI] [PubMed] [Google Scholar]
- 21.Langhoff E, Pedersen P S, Koch C. Methylprednisolone resistance of cystic fibrosis lymphocytes. Pediatr Res. 1984;18:488–489. doi: 10.1203/00006450-198405000-00019. [DOI] [PubMed] [Google Scholar]
- 22.Miller E J, Cohen A B, Peterson B T. Peptide inhibitor of interleukin-8 (IL-8) reduces staphylococcal enterotoxin-A (SEA) induced neutrophil trafficking to the lung. Inflamm Res. 1996;45:393–397. doi: 10.1007/BF02252934. [DOI] [PubMed] [Google Scholar]
- 23.Neumann B, Emmanuilidis K, Stadler M, Holzmann B. Distinct functions of interferon-gamma for chemokine expression in models of acute lung inflammation. Immunology. 1998;95:512–521. doi: 10.1046/j.1365-2567.1998.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osusky R, Dorio R J, Arora Y K, Ryan S J, Walker S M. MHC class II positive retinal pigment epithelial (RPE) cells can function as antigen-presenting cells for microbial superantigen. Occular Immunol Inflam. 1997;5:43–50. doi: 10.3109/09273949709085049. [DOI] [PubMed] [Google Scholar]
- 25.Ray A, LaForge S, Sehgal P B. On the mechanism for efficient repression of the interleukin-6 promoter by glucocorticoids: enhancer, TATA box, and RNA start site (Inr motif) occlusion. Mol Cell Biol. 1990;10:5736–5746. doi: 10.1128/mcb.10.11.5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenstein B J, Eigen H. Risk of alternate-day prednisone in patients with cystic fibrosis. Pediatrics. 1991;87:245–246. [PubMed] [Google Scholar]
- 27.Schuster A, Haarmann A, Wahn V. Cytokines in neutrophil-dominated airway inflammation in patients with cystic fibrosis. Eur Arch Oto-Rhino-Laryng. 1995;1(Suppl.):S59–S60. doi: 10.1007/BF02484436. [DOI] [PubMed] [Google Scholar]
- 28.Schwiebert E M, Benos D J, Egan M E, Stutts M J, Guggino W B. CFTR is a conductance regulator as well as a chloride channel. Physiol Rev. 1999;79(Suppl. 1):S145–S166. doi: 10.1152/physrev.1999.79.1.S145. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu T, Hansson C, Strandvik B. Defective inhibition by dexamethasone of leukotriene B4 and C4 production by leukocytes in patients with cystic fibrosis. Prostaglandins Leukotrienes Essen Fatty Acids. 1994;51:407–410. doi: 10.1016/0952-3278(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 30.Snyder D S, Unanue E R. Corticosteroids inhibit murine macrophage Ia expression and interleukin 1 production. J Immunol. 1982;129:1803–1805. [PubMed] [Google Scholar]
- 31.Sugden B. An intricate route to immortality. Cell. 1989;57:5–7. doi: 10.1016/0092-8674(89)90165-7. [DOI] [PubMed] [Google Scholar]
- 32.Tabary O, Zahm J M, Hinnrasky J, Couetil J P, Cornillet P, Guenounou M, Gaillard D, Puchelle E, Jacquot J. Selective up-regulation of chemokine IL-8 expression in cystic fibrosis bronchial gland cells in vivo and in vitro. Am J Pathol. 1998;153:921–930. doi: 10.1016/S0002-9440(10)65633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takei S, Arora Y, Walker S M. Intravenous immunoglobulin contains antibodies inhibitory to activation of T cells by staphylococcal toxin superantigens. J Clin Invest. 1993;91:602–607. doi: 10.1172/JCI116240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Seventer G A, Newman W, Shimizu Y, Nutman T B, Tanaka Y, Horgan K J, Gopal T V, Ennis E, O'Sullivan D, Grey H, Shaw S. Analysis of T cell stimulation by superantigen plus major histocompatibility complex class II molecules or by CD3 monoclonal antibody: costimulation by purified adhesion ligands VCAM-1, ICAM-1, but not ELAM-1. J Exp Med. 1991;174:901–913. doi: 10.1084/jem.174.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss A, Shields R, Newton M, Manger B, Imboden J. Ligand-receptor interactions required for commitment to the activation of the interleukin-2 gene. J Immunol. 1987;138:2169–2178. [PubMed] [Google Scholar]