Abstract
Monoclonal antibodies (MAbs) against Ureaplasma urealyticum serotype 2, 5, 7, 8, 10, 11, 12, and 13 reference strains were developed. The reactivities of these MAbs with the 14 serotype reference strains was verified by colony immunofluorescence assay and Western blot assay. MAbs against serotypes 2, 7, 10, 11, and 12 were serotype specific, whereas MAbs against serotypes 5, 8, and 13 showed cross-reactivity. All MAbs against serotype 5 were cross-reactive with serotype 2, and one showed, in addition, cross-reactivity to serotypes 9 and 10. Mutual cross-reactivities were observed between MAbs against serotypes 8 and 13. The usefulness of the MAbs for the serotyping of U. urealyticum strains was evaluated by serotyping 21 selected clinical isolates. A complete set of MAbs (the newly developed MAbs and the previously described MAbs against serotypes 1, 3, 4, 6, 9, and 14) as well as a complete set of polyclonal antibodies (PAbs), PAbs 1 to 14, were used. MAbs were able to identify 18 of 21 isolates including 2 isolates with mixed serotypes. Polyreactivity, which occurred with 19 of the 21 isolates with PAbs, was not observed by the use of MAbs. MAbs seem to be a more valuable tool than PAbs for serotyping and could help in investigating a possible link between the expression or variability of the serotype-specific antigens and pathogenicity.
Ureaplasma urealyticum is a commensal organism of the human lower genital tract. It has been implicated in diseases of the genitourinary tract (30), unfavorable pregnancy outcome (3, 9, 10, 14), and infections of premature neonates (31). The high rate of isolation of U. urealyticum from the genital tract has made its role in genitourinary tract disease difficult to define. Since only a subpopulation of colonized individuals ultimately develops disease, it has been postulated that only certain subgroups of U. urealyticum are associated with disease. U. urealyticum comprises 14 serotypes divided into two biovars on the basis of DNA-DNA homology (6), restriction endonuclease DNA digestion (21, 22), polyacrylamide gel electrophoresis of proteins (12, 29), and sensitivity to manganese salts (25). The association of particular serotypes with disease is still controversial (11, 15, 17, 26, 27). This controversy could be due in part to the lack of a standardized method for serotyping. Until now, serotyping studies have been performed with polyclonal antibodies (PAbs). However, the use of PAbs for the serotyping of clinical isolates raised problems such as polyreactivity with clinical isolates and lack of reproducibility (15, 28). Of particular interest is that PAbs do not exhibit such polyreactivity with reference strains but they show cross-reactivity between serotype 2 and 5 reference strains. This cross-reactivity has been observed by many investigators (1, 2, 8, 18, 19, 20, 24, 27).
When clinical isolates were serotyped with a partial set of monoclonal antibodies (MAbs), good reproducibility was obtained and polyreactivity was not seen with clinical isolates (4, 5, 16). However, these promising preliminary results need to be confirmed with a study with a complete set of MAbs directed against the 14 serotypes of U. urealyticum.
In the study described in this paper we developed MAbs against serotypes 2, 5, 7, 8, 10, 11, 12, and 13 for which no serotype-specific MAbs were available, until now. These MAbs were used to identify serotype-specific antigens and were evaluated in a serotyping assay that included MAbs against the 14 serotypes.
MATERIALS AND METHODS
Antigen preparation.
Reference strains of U. urealyticum serotypes 1 to 10 were supplied by E. A. Freund (Institute of Medical Microbiology, University of Aarhus, Aarhus, Denmark), and those of serotypes 11 to 14 were supplied by J. A. Robertson (Department of Medical Microbiology and Infectious Diseases, University of Alberta, Edmonton, Alberta, Canada). U. urealyticum antigens were prepared by growing serotype reference strains in 1 liter of bromothymol blue broth (23). The cells were harvested by centrifugation at 25,000 × g for 30 min at 4°C. The pellet was washed three times with phosphate-buffered saline (PBS) and was resuspended in PBS before storage at −80°C. The aliquoted fractions of the antigenic preparations were used for MAb production as well as for the Western blot analysis.
MAb production procedure.
The MAbs against serotype 2 and 7 were produced as described previously (4). Immunization of mice was performed by intraperitoneal injection (4) for serotypes 2 and 7 and by footpad injection (7) for serotypes 5, 8, 10, 11, 12, and 13. Immunization of mice through footpad injection resulted in a shorter immunization period than that obtained by immunization through intraperitoneal injection: 2 weeks instead of 10 weeks. The fusion was performed as described previously (4). Screening of the hybridoma clones was performed by colony immunofluorescence assay (colony-IFA) (4). Positive clones were subcloned twice by limiting dilution. The immunoglobulin isotypes were determined with the Mouse Antibody Isotyping Kit (Life Technologies, Merelbeke, Belgium).
Characterization of the MAbs.
The serotype specificities of the MAbs were determined by colony-IFA (13). The Western blot assay (WBA) was performed as described previously (4).
Serotyping of clinical isolates.
Twenty-one U. urealyticum clinical isolates previously serotyped by colony-IFA with rabbit PAbs (15) were selected to evaluate the reactivities of the MAbs. The selection of clinical isolates was based on their reaction with PAbs 2, 5, 7, 8, 10, 11, 12, and 13 and on the presence of polyreactivity. The selected clinical isolates were reanalyzed by colony-IFA with PAbs 1 to 14 as well as with a complete set of MAbs: the newly developed MAbs against serotypes 2, 5, 7, 8, 10, 11, 12, and 13 and the existing set of MAbs against serotypes 1, 3, 4, 6, 9, and 14 (4, 5, 16).
A clinical isolate recognized by more than one specific antibody was defined as a polyreactive strain when each antibody reacted with more than 90% of the colonies or as a mixed serotype when each antibody reacted with a limited (complementary) number of the U. urealyticum colonies.
Cloning of clinical isolates.
The cloning of clinical isolates was performed as described previously (24).
RESULTS
Production and characterization of the MAbs.
For each serotype, one to five different MAb-secreting hybridomas were detected. The reactivities of these MAbs with the 14 serotype reference strains was verified by colony-IFA and WBA (Table 1 and Fig. 1). MAbs against serotypes 2, 7, 10, 11, and 12 do not demonstrate cross-reactivity with other serotypes and can be regarded as serotype-specific MAbs. MAbs against serotypes 5, 8, and 13 demonstrated cross-reactivity by colony-IFA or WBA, or by both techniques (Table 1).
TABLE 1.
Characterization of MAbs against serotypes 2, 5, 7, 8, 10, 11, 12, and 13
| Specificity (serotype) | MAb designation | Isotypea | Reactivity by colony-IFA [serotype(s)] | Antigen(s) recognized by WBA |
|---|---|---|---|---|
| 2 | 2-1B4 | IgG1 | 2 | Serotype 2, 63 kDa |
| 2-3A1 | IgG1 | 2 | Serotype 2, 63 kDa | |
| 5 | 5-16E1 | IgM | 2, 5 | Serotype 5, 61 and 49 kDa |
| 5-16E10 | IgM | 2, 5 | Serotype 5, 61 and 49 kDa | |
| 5-23D11 | IgM | 2, 5 | Serotype 5, 61 and 49 kDa | |
| 5-12H8 | IgM | 2, 5 | Serotype 5, 61 and 49 kDa | |
| 5-23G9 | IgM | 2, 5, 10 | Serotype 2, 63 kDa | |
| Serotype 5, 61 and 49 kDa | ||||
| Serotypes 9 and 10, 89 and 52 kDa | ||||
| 7 | 7-5C4 | IgG1 | 7 | Serotype 7, 53 kDa |
| 8 | 8-1C8 | IgM | 8 | Serotypes 8 and 13, 32 kDa |
| 8-11H11 | IgG2a | 8 | Serotypes 8 and 13, 32 kDa | |
| 8-14B8 | IgG1 | 8, 13 | Serotypes 8 and 13, 32 kDa | |
| 8-19F9 | IgG2b | 8 | Serotypes 8 and 13, 32 kDa | |
| 10 | 10-24A2 | IgG3 | 10 | Serotype 10, 62 kDa |
| 11 | 11-21C4 | IgG2b | 11 | Serotype 11, 56 kDa |
| 12 | 12-3F10 | IgG2b | 12 | Serotype 12, 87 kDa |
| 12-11G3 | IgG1 | 12 | Serotype 12, 87 kDa | |
| 12-16A3 | IgG2b | 12 | Serotype 12, 87 kDa | |
| 12-16H5 | IgG2a | 12 | Serotype 12, 87 kDa | |
| 13 | 13-1H7 | IgM | 13 | Serotypes 8 and 13, 32 kDa |
| 13-7A3 | IgM | 13 | Serotypes 8 and 13, 32 kDa |
Ig, immunoglobulin.
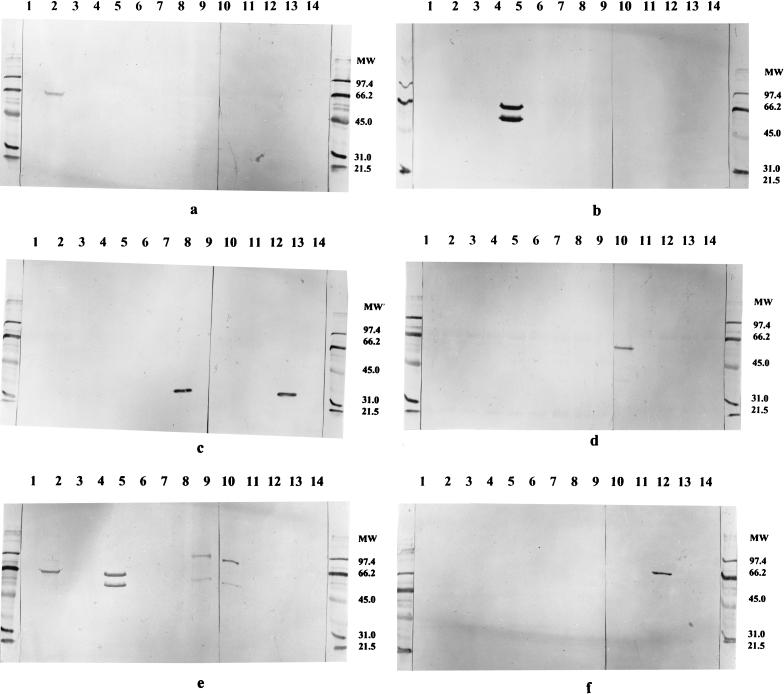
FIG. 1.
Western blot showing the reaction patterns of some of the newly developed MAbs against the 14 U. urealyticum serotypes. (a) MAb 2-3A1; (b) MAb 5-16E1; (c) MAb 8-19F9; (d) MAb 10-24A2; (e) MAb 5-23G9; (f) MAb 12-3F10. Lanes 1 to 14 contain antigenic preparations of the 14 serotype reference strains of U. urealyticum, respectively. Molecular weight (MW) markers are indicated on the right of the panels.
By colony-IFA all MAbs developed against serotype 5 were cross-reactive with serotype 2. One MAb (MAb 5-23G9) demonstrated, in addition, cross-reactivity with serotype 10. By WBA cross-reactivity was seen only for MAb 5-23G9. This MAb reacted with serotypes 2, 5, 9, and 10 (Fig. 1e). For the four other MAbs the cross-reactivity with serotype 2 was not seen by WBA (Fig. 1b). Among the four MAbs produced against serotype 8, one (MAb 8-14B8) showed cross-reactivity with serotype 13 by colony-IFA. However, by WBA all four MAbs recognized a 32-kDa protein from both serotypes 8 and 13 (Fig. 1c). Similar cross-reactivity was seen with the two MAbs developed against serotype 13; both MAbs were serotype specific by colony-IFA but showed cross-reactivity with serotype 8 by WBA, recognizing a 32-kDa protein.
Specificities of MAbs against all serotypes by colony-IFA.
Table 2 summarizes the results of the reactivities of all the available MAbs against the 14 serotypes of U. urealyticum (the newly developed MAbs and the already existing MAbs). Although for some MAbs heterospecificity was observed, the 14 U. urealyticum serotypes can be differentiated by colony-IFA with a combination of the MAbs described.
TABLE 2.
Specificities of the different MAbs against the U. urealyticum reference strains as determined by colony-IFA with the 14 U. urealyticum serotypes
| Serotype | Reactivities of MAbs to the following serotypea:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
| 1 | + | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 2 | − | + | − | − | + | − | − | − | − | − | − | − | − | − |
| 3 | − | − | + | − | − | − | − | − | − | − | − | − | − | + |
| 4 | − | − | − | + | − | − | − | − | − | − | − | − | − | − |
| 5 | − | − | − | − | + | − | − | − | − | − | − | − | − | − |
| 6 | − | − | − | − | − | + | − | − | − | − | − | − | − | − |
| 7 | − | − | − | − | − | − | + | − | − | − | − | − | − | − |
| 8 | − | − | − | − | − | − | − | + | − | − | − | − | − | − |
| 9 | − | − | − | − | − | − | − | − | + | − | − | − | − | − |
| 10 | − | − | − | − | − (+)b | − | − | − | − | + | − | − | − | − |
| 11 | − | − | − | − | − | − | − | − | − | − | + | − | − | − |
| 12 | − | − | − | − | − | − | − | − | − | − | − | + | − | − |
| 13 | − | − | − | − | − | − | − | − (+)c | − | − | − | − | + | − |
| 14 | − | − | − | − | − | − | − | − | − | − | − | − | − | + |
+, positive reaction; −, negative reaction.
This cross-reactivity was detected by only one of the five MAbs against serotype 5, MAb 5-23G9.
This cross-reactivity was detected by only one of the four MAbs against serotype 8, MAb 8-14B8.
Serotyping of clinical isolates with MAbs and PAbs.
The results of the serotyping assay for the 21 clinical isolates tested are summarized in Table 3. By use of PAbs, only two isolates (isolates S1 and S21) were recognized by a single PAb. The other 19 isolates tested showed polyreactivity. By use of PAbs, mixed serotypes could not be detected within these isolates.
TABLE 3.
Results of colony-IFA with U. urealyticum clinical isolates and MAbs
| Isolate | Serotype(s) by reaction with PAbs | Serotype(s) by reaction with MAbs
|
|
|---|---|---|---|
| Without cloning | After cloning | ||
| S1 | 2 | 2 | NDa |
| S2 | 2, 5, 10 | 2 | ND |
| S3 | 3, 10, 14 | 3 | ND |
| S4 | 2, 4, 5, 8, 10, 12 | 5 | ND |
| S5 | 2, 5, 10 | 5 | ND |
| S6 | 2, 3, 5, 10, 11, 12 | 5 | ND |
| S7 | 3, 10 | 6 | ND |
| S8 | 4, 5, 8, 10, 11 | 8 | ND |
| S9 | 4, 8, 10 | 8 | ND |
| S10 | 8, 9 | 9 | ND |
| S11 | 7, 8, 9, 10 | 9 | ND |
| S12 | 4, 5, 8, 11, 12 | 9 | ND |
| S13 | 4, 10, 12 | 12 | ND |
| S14 | 3, 4, 10, 11, 12 | 12 | ND |
| S15 | 4, 10, 12 | 12 | ND |
| S16 | 3, 4, 8, 10, 11, 12 | 12 | ND |
| S17 | 5, 8, 10, 11 | 5, 9, 11 | 5 |
| S18 | 4, 10 | 4, 5,b 10 | 5,b 10 |
| S19 | 1, 2 | NRc | ND |
| S20 | 1, 2 | NR | ND |
| S21 | 10 | NR | ND |
ND, not done.
Reaction with MAb 5-23G9 only.
NR, not reactive.
Three of 21 isolates were not typeable with the MAbs, despite their reactivity with at least one PAb. From the 18 isolates identified by the MAbs, 16 isolates were recognized by a single MAb. Two of these 16 isolates (isolates S7 and S12) were identified by a MAb that had a specificity different from those found with the PAbs. Two isolates (isolates S17 and S18) showed a fluorescent pattern of mixed serotypes. However, with the PAbs these same two isolates exhibited polyreactivity. In order to confirm the presence of mixed serotypes, these two isolates were cloned and reanalyzed by MAbs. After cloning, isolate S18 was recognized by MAbs 10-24A2 and 5-23G9 and lost its reactivity with MAb-4, and strain S17 was recognized only by MAb 5-23G9 and lost its reactivity with MAbs specific for serotypes 9 and 11.
DISCUSSION
In the present study we developed MAbs against several U. urealyticum reference strains in order to have a complete set of MAbs able to differentiate the 14 U. urealyticum serotypes. The MAbs were in general highly specific; however, some cross-reactivities with heterologous serotypes were observed either by colony-IFA or by WBA, or by both methods. For some MAbs (MAbs specific for serotypes 5, 8, and 13) a different cross-reactivity pattern was observed according to the technique used. Such differences in cross-reactivity between colony-IFA and WBA have been noted previously: a serotype 9-specific MAb that reacted with a strain by colony-IFA recognized a protein from serotype 2 by WBA (16), and a serotype 6-specific MAb reacted with a strain by colony-IFA, whereas it did not react with the strain by WBA (5). The differences in the reactivities of the MAbs by WBA and colony-IFA could be due to differences in antigenic exposure by the techniques used: by colony-IFA, in which freshly grown colonies are used, the extracellular native part of the antigen is presented, whereas by WBA denatured proteins are used. This could make the recognized epitope more accessible by one technique than by the other.
With the newly developed MAbs we could complete our set of MAbs. Evaluation of the MAbs with 21 selected clinical isolates revealed that clinical isolates and reference strains do not necessarily behave similarly: cross-reactivity between MAb 5-23G9 and serotype 10 was observed for reference strains as well as clinical isolate S18, whereas the cross-reactivity between serotype 5 MAbs and serotype 2 was observed only for reference strains and not for clinical isolates S1 and S2. The difference in reactivity between reference strains and clinical isolates may be due to the difference in expression of the serotype-specific antigens on the surfaces of some clinical isolates.
In this study 3 of 21 selected isolates (14%) could not be typed by our complete set of MAbs. This might be due to the failure of these MAbs to detect an occasional clinical isolate or to the loss of a serotype-specific epitope by a clinical isolate. Since this study was performed with selected clinical isolates (a selection of polyreactive isolates with reactivities to less common serotypes), this percentage should be interpreted with caution. A large study with randomly selected strains is necessary to evaluate the number of nontypeable isolates.
Polyreactivity is a problem frequently encountered when one is serotyping clinical isolates with PAbs (15, 28). Such a polyreactivity can interfere in the interpretation of the serotyping results and can be responsible for a lack of reproducibility of the serotyping assay. To evaluate whether such a polyreactivity is also encountered when one is using MAbs, we have serotyped selected clinical isolates that had already shown polyreactivity with PAbs in a previous assay. After retesting of these isolates in parallel with PAbs and MAbs, no polyreactivity was observed when MAbs were used, although polyreactivity with PAbs remains present in 19 of the 21 isolates.
In general, isolates that show polyreactivity with PAbs are recognized by a MAb that has the same specificity as one of the PAbs. Only two isolates (isolates S7 and S12) were identified by a MAb that had a specificity different from those found by the PAbs. Since polyreactivity and low reproducibility are the major problems of serotyping of clinical isolates with PAbs (15, 28), it is likely that the discordance observed between PAb and MAb typing for these two strains is due to the inaccuracy of PAb typing. Indeed, it has been observed previously for some strains that show polyreactivity with PAbs that repeated testing was sometimes associated with the disappearance of a positive reaction or with a shift from a negative to a positive reaction (15).
Two isolates (isolates S17 and S18) reacted with more than one MAb. The fluorescent patterns observed for these two isolates corresponded to those observed for mixed serotypes. Since these two isolates were polyreactive when serotyped with PAbs, we decided to confirm the presence of mixed serotypes in these isolates by a cloning procedure. If an isolate consists of more than one serotype, the cloning procedure will select only one serotype in the mixture and results in the loss of reactivity to one or more MAbs. The fluorescent pattern of a polyreactive strain will remain similar before and after cloning. Retesting of the two isolates with MAbs after cloning confirmed the presence of mixed serotypes: for both isolates only one serotype was recovered. For these isolates the presence of mixed serotypes would have been missed when only PAbs were used.
In summary, we have produced and characterized MAbs against all U. urealyticum serotypes. Although some cross-reactivities are found for some MAbs, the complete set of MAbs allows discrimination between the 14 U. urealyticum serotypes by colony-IFA. Serotyping with MAbs is not subject to polyreactivity, and mixed serotypes can be better identified with MAbs than with PAbs. Although clinical isolates may react differently than reference strains, the use of MAbs seems promising for the serotyping of clinical isolates. These MAbs are interesting for the further study of the pathogenicity of U. urealyticum. In order to investigate a possible link between serotypes and pathogenicity, large numbers of clinical isolates from different patients with and without pregnancy complications will be serotyped with these MAbs. The MAbs will also be used to study antigenic variations within U. urealyticum strains. For Mycoplasma pulmonis it has been demonstrated that variation in the size of the V1 antigen may play a role in the virulence of the different strains (32), and such a variation has also been observed in serotypes of U. urealyticum (4, 5). It is not impossible that antigenic variation plays an important role in invasive infections caused by U. urealyticum.
ACKNOWLEDGMENTS
This work was supported by a grant from the “Steunfonds Marguerite-Marie Delacroix.”
We thank Lea Brys from the Department of Cellular Immunology, Vrije Universiteit Brussel, and Françoise Cormaux from the Department of Experimental Immunology, Medical Institute, UCL, for technical advice.
REFERENCES
- 1.Black F T. Fifth International Congress on Infectious Diseases. Vol. 1. Vienna, Austria: Weiner Medizinische Akademie; 1970. Serological methods for classification of human T-mycoplasmas; pp. 407–411. [Google Scholar]
- 2.Black F T. Modifications of the growth inhibition test and its application to human T-mycoplasmas. Appl Microbiol. 1973;25:528–533. doi: 10.1128/am.25.4.528-533.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassell G H, Waites K B, Watson H L, Crouse D T, Harasawa R. Ureaplasma urealyticum intrauterine infection: role in prematurity and disease in newborns. Clin Microbiol Rev. 1993;6:69–87. doi: 10.1128/cmr.6.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng X, Naessens A, Lauwers S. Identification and characterization of serotype 4-specific antigens of Ureaplasma urealyticum by use of monoclonal antibodies. Infect Immun. 1993;61:2253–2256. doi: 10.1128/iai.61.5.2253-2256.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng X, Naessens A, Lauwers S. Identification of serotype 1-, 3-, and 6-specific antigens of Ureaplasma urealyticum by using monoclonal antibodies. J Clin Microbiol. 1994;32:1060–1062. doi: 10.1128/jcm.32.4.1060-1062.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christiansen C, Black F T, Feundt E A. Hybridization experiments with deoxyribonucleic acid from Ureaplasma urealyticum serovars I to VIII. Int J Syst Bacteriol. 1981;31:259–262. [Google Scholar]
- 7.Coyle P V, Wyatt D, McCaughey C, O'Neill H J. A simple standardised protocol for the production of monoclonal antibodies against viral and bacterial antigens. J Immunol Methods. 1992;153:81–84. doi: 10.1016/0022-1759(92)90308-g. [DOI] [PubMed] [Google Scholar]
- 8.Ford D K. Serum antibody levels against T-mycoplasmas in North American Indian populations. Arthritis Rheum. 1976;19:1328–1332. doi: 10.1002/art.1780190614. [DOI] [PubMed] [Google Scholar]
- 9.Foulon W, Naessens A, Dewaele M, Lauwers S, Amy J J. Chronic Ureaplasma urealyticum amnionitis associated with abruptio placentae. Obstet Gynecol. 1986;68:280–282. [PubMed] [Google Scholar]
- 10.Foulon W, Van Liedekerke D, Demanet C, Decatte L, Dewaele M, Naessens A. Markers of infection and their relationship to preterm delivery. Am J Perinatol. 1995;12:208–311. doi: 10.1055/s-2007-994454. [DOI] [PubMed] [Google Scholar]
- 11.Hewish M J, Birch D F, Fairley K F. Ureaplasma urealyticum serotypes in urinary tract disease. J Clin Microbiol. 1986;23:149–154. doi: 10.1128/jcm.23.1.149-154.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mouches C, Taylor-Robinson D, Stipkovits L, Bove J M. Comparison of human and animal ureaplasmas by one- and two-dimensional protein analysis on polyacrylamide slab gel. Ann Microbiol (Paris) 1981;132B:171–196. [PubMed] [Google Scholar]
- 13.Naessens A, Lauwers S. Modified indirect immunofluorescence test for serotyping large numbers of Ureaplasma urealyticum clinical isolates. J Clin Microbiol. 1987;25:191–192. doi: 10.1128/jcm.25.1.191-192.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naessens A, Foulon W, Cammu H, Goossens A, Lauwers S. Epidemiology and pathogenesis of Ureaplasma urealyticum in spontaneous abortion and early preterm labor. Acta Obstet Gynecol Scand. 1987;66:513–516. doi: 10.3109/00016348709015726. [DOI] [PubMed] [Google Scholar]
- 15.Naessens A, Foulon W, Breynaert J, Lauwers S. Serotypes of Ureaplasma urealyticum isolated from normal pregnant women and patients with pregnancy complications. J Clin Microbiol. 1988;26:319–322. doi: 10.1128/jcm.26.2.319-322.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naessens A, Cheng X, Lauwers S, Robertson J A. Development of a monoclonal antibody to a Ureaplasma urealyticum serotype 9 antigen. J Clin Microbiol. 1998;36:1125–1127. doi: 10.1128/jcm.36.4.1125-1127.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piot P. Distribution of eight serotypes of Ureaplasma urealyticum in cases of non-gonococcal urethritis and in healthy persons. Br J Vener Dis. 1976;52:266–268. doi: 10.1136/sti.52.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piot P. Comparison of growth inhibition and immunofluorescence tests in serotyping clinical strains of Ureaplasma urealyticum. Br J Vener Dis. 1977;53:186–189. doi: 10.1136/sti.53.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Purcell R H, Taylor-Robinson D, Wong D, Chanock R M. Color test for the measurement of antibody to T-strain mycoplasmas. J Bacteriol. 1966;92:6–12. doi: 10.1128/jb.92.1.6-12.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinn P A, Arshoff L U, Li H C. Serotyping of Ureaplasma urealyticum by immunoperoxidase assay. J Clin Microbiol. 1981;13:670–676. doi: 10.1128/jcm.13.4.670-676.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Razin S, Harasawa R, Barile M F. Cleavage patterns of the mycoplasma chromosome, obtained by using restriction endonucleases as indicators of genetic relatedness among strains. Int J Syst Bacteriol. 1983;33:201–206. [Google Scholar]
- 22.Razin S, Yogev D. Genetic relatedness among Ureaplasma urealyticum serotypes (serovars) Pediatr Infect Dis. 1986;5(6 Suppl):S300–S304. doi: 10.1097/00006454-198611010-00022. [DOI] [PubMed] [Google Scholar]
- 23.Robertson J A. Bromothymol blue broth: improved medium for detection of Ureaplasma urealyticum (T-strain mycoplasma) J Clin Microbiol. 1978;7:127–132. doi: 10.1128/jcm.7.2.127-132.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robertson J A, Stemke G W. Expanded serotyping scheme for Ureaplasma urealyticum strains isolated from humans. J Clin Microbiol. 1982;15:873–878. doi: 10.1128/jcm.15.5.873-878.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson J A, Chen M H. Effects of manganese on the growth of Ureaplasma urealyticum. J Clin Microbiol. 1984;19:857–864. doi: 10.1128/jcm.19.6.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robertson J A, Honore L H, Stemke G W. Serotypes of Ureaplasma urealyticum in spontaneous abortion. Pediatr Infect Dis. 1986;5(6 Suppl):S270–S272. doi: 10.1097/00006454-198611010-00014. [DOI] [PubMed] [Google Scholar]
- 27.Shepard M C, Runceford C D. Serological typing of Ureaplasma urealyticum isolates from urethritis patients by an agar growth inhibition method. J Clin Microbiol. 1978;8:566–574. doi: 10.1128/jcm.8.5.566-574.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stemke G W, Robertson J A. Problems associated with serotyping strains of Ureaplasma urealyticum. Diagn Microbiol Infect Dis. 1985;3:311–320. doi: 10.1016/0732-8893(85)90005-7. [DOI] [PubMed] [Google Scholar]
- 29.Swensen C E, Van Hamont J, Dunbar B S. Specific protein differences among strains of Ureaplasma urealyticum as determined by two-dimensional gel electrophoresis and a sensitive silver stain. Int J Syst Bacteriol. 1983;33:417–421. [Google Scholar]
- 30.Taylor-Robinson D, Csonka G W, Prentice M J. Human intra-urethral inoculation of ureaplasmas. Q J Med. 1977;46:309–326. [PubMed] [Google Scholar]
- 31.Waites K B, Crouse D T, Philips J B, Canupp K C, Cassell G H. Ureaplasmal pneumonia and sepsis associated with persistent pulmonary hypertension of the newborn. Pediatrics. 1989;83:79–85. [PubMed] [Google Scholar]
- 32.Watson H L, Zheng X, Cassel G H. Structural variations and phenotypic switching of mycoplasmal antigens. Clin Infect Dis. 1993;17(Suppl. I):S183–S186. doi: 10.1093/clinids/17.supplement_1.s183. [DOI] [PubMed] [Google Scholar]